The Impact of Exposure Profile on the Efficacy of Dual Amylin and Calcitonin Receptor Agonist Therapy
Abstract
:1. Background
2. Methods
2.1. Peptide Therapy
2.2. Animal Experiments
2.3. Study (1) Infusion vs. Injection of KBP-042 in HFD-Fed Sprague–Dawley Rats
2.4. Study (2) Infusion vs. Injection of KBP-088 in Diabetic ZDF Rats
2.5. Study (3) Comparison of KBP-088 and KBP-088A in HFD-Fed Sprague–Dawley Rats
2.6. Study (4) Intravenous Glucose Tolerance Test in HFD-Fed Sprague–Dawley Rats
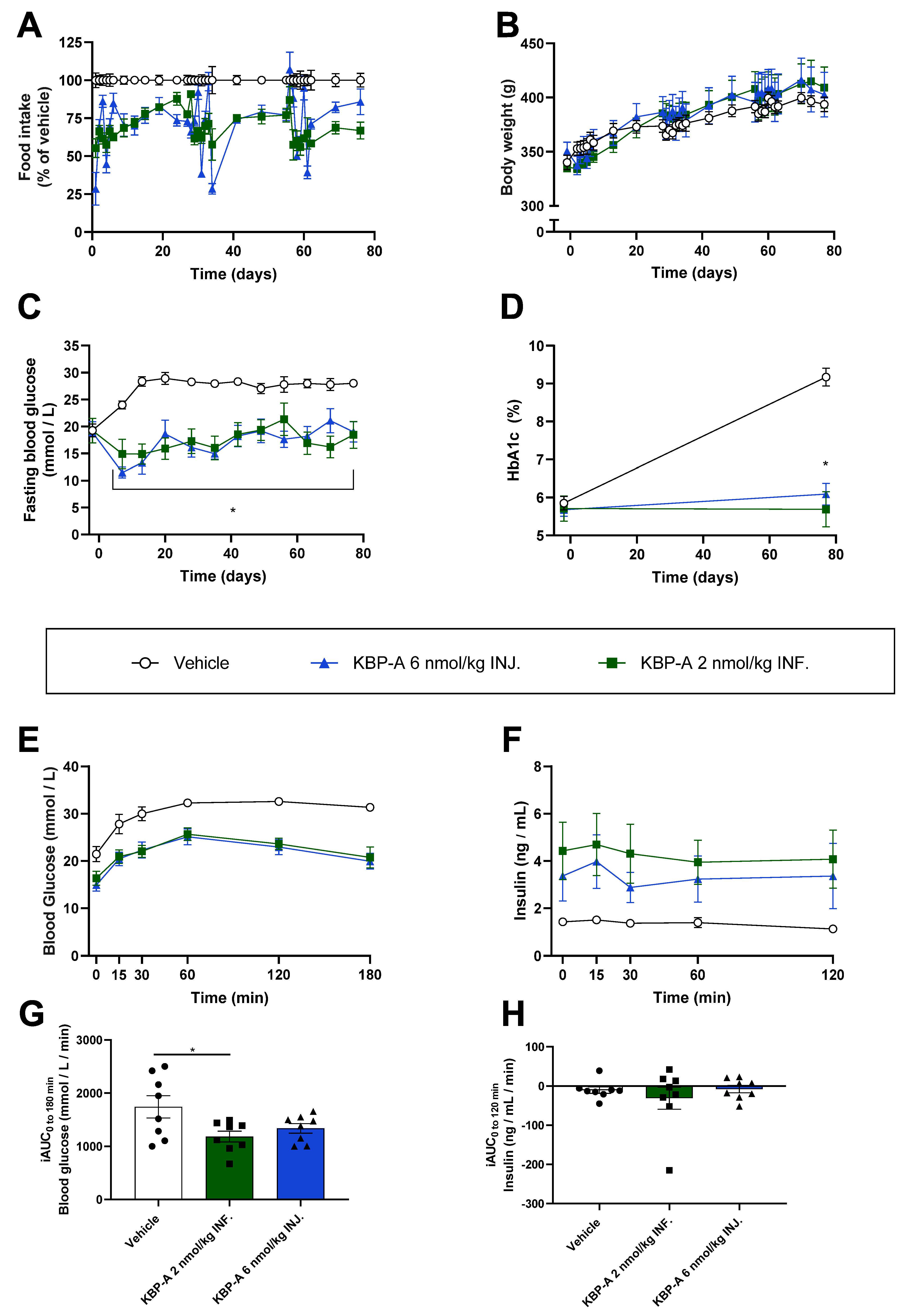
2.7. Study (5) Infusion vs. Injection of KBP-066A in Diabetic ZDF Rats
2.8. Oral Glucose Tolerance Test
2.9. Intravenous Glucose Tolerance Test (Only Study (4))
2.10. Biochemical Analysis
2.11. Statistical Analysis
3. Results
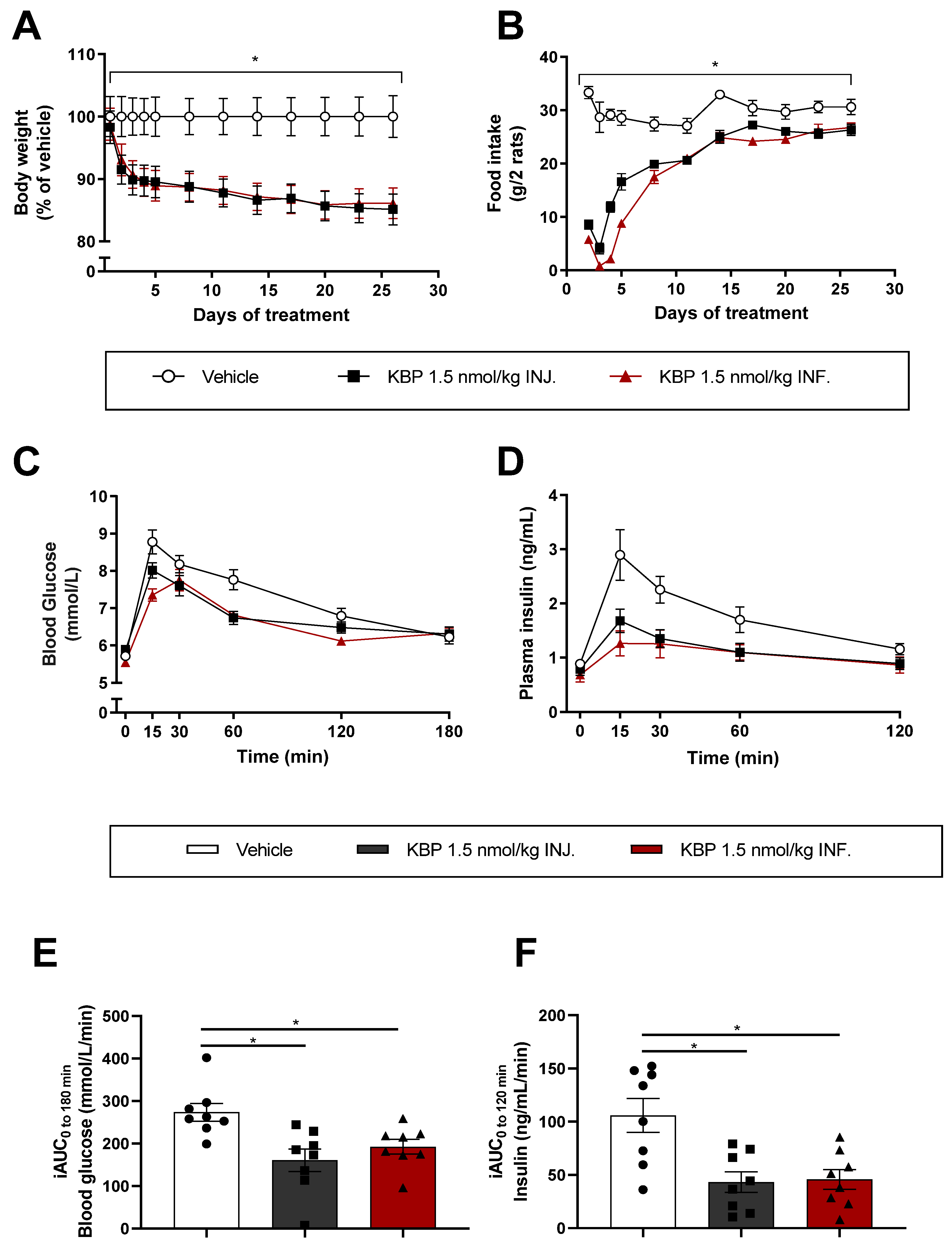
3.1. Infusion and Injection of KBP Was Equally Efficient on Weight Loss and Glucose Tolerance in HFD-Fed
Sprague–Dawley Rats (Study (1))
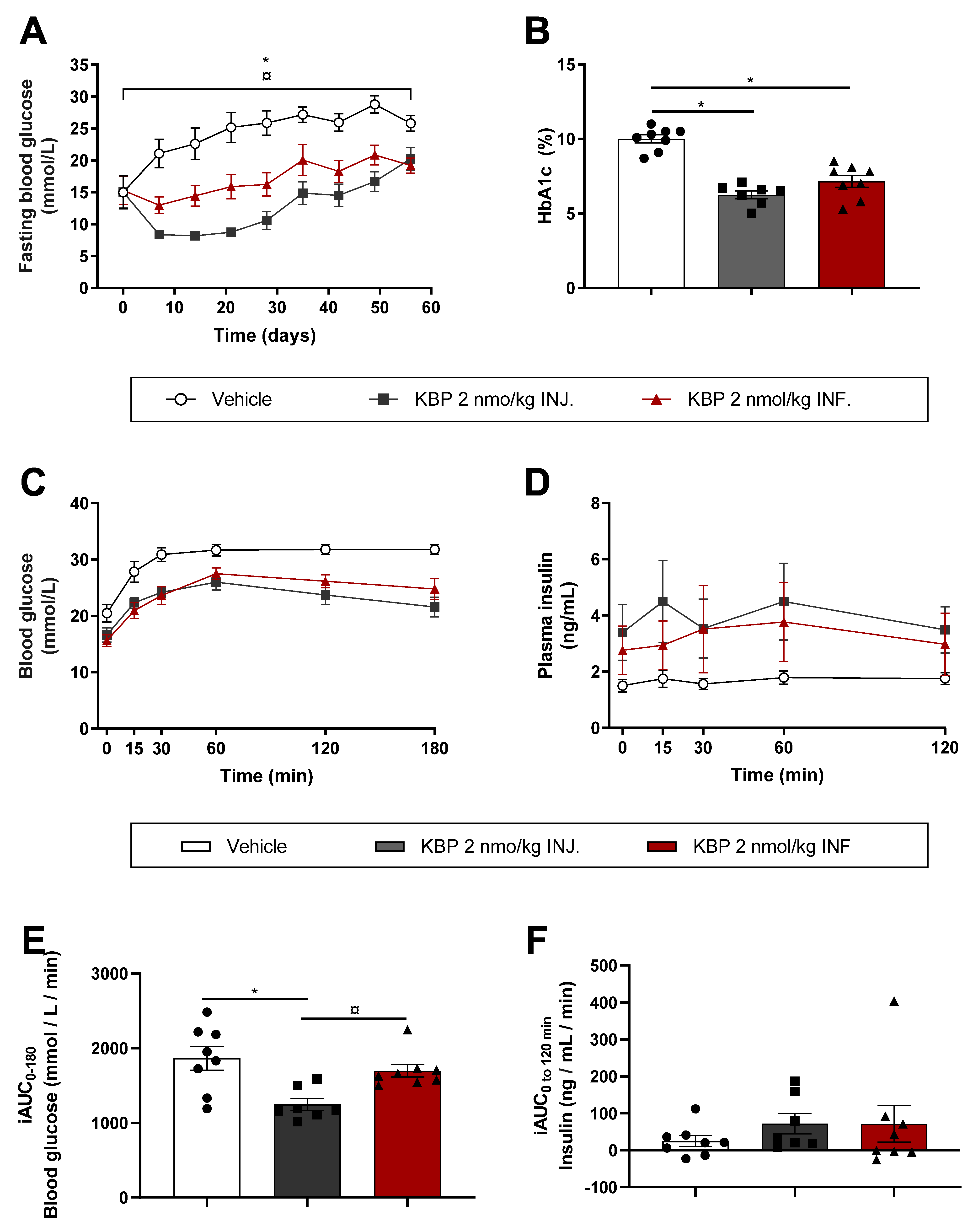
3.2. Injection of KBP Was Superior to Infusion on Glucose Control in Diabetic ZDF Rats (Study (2))
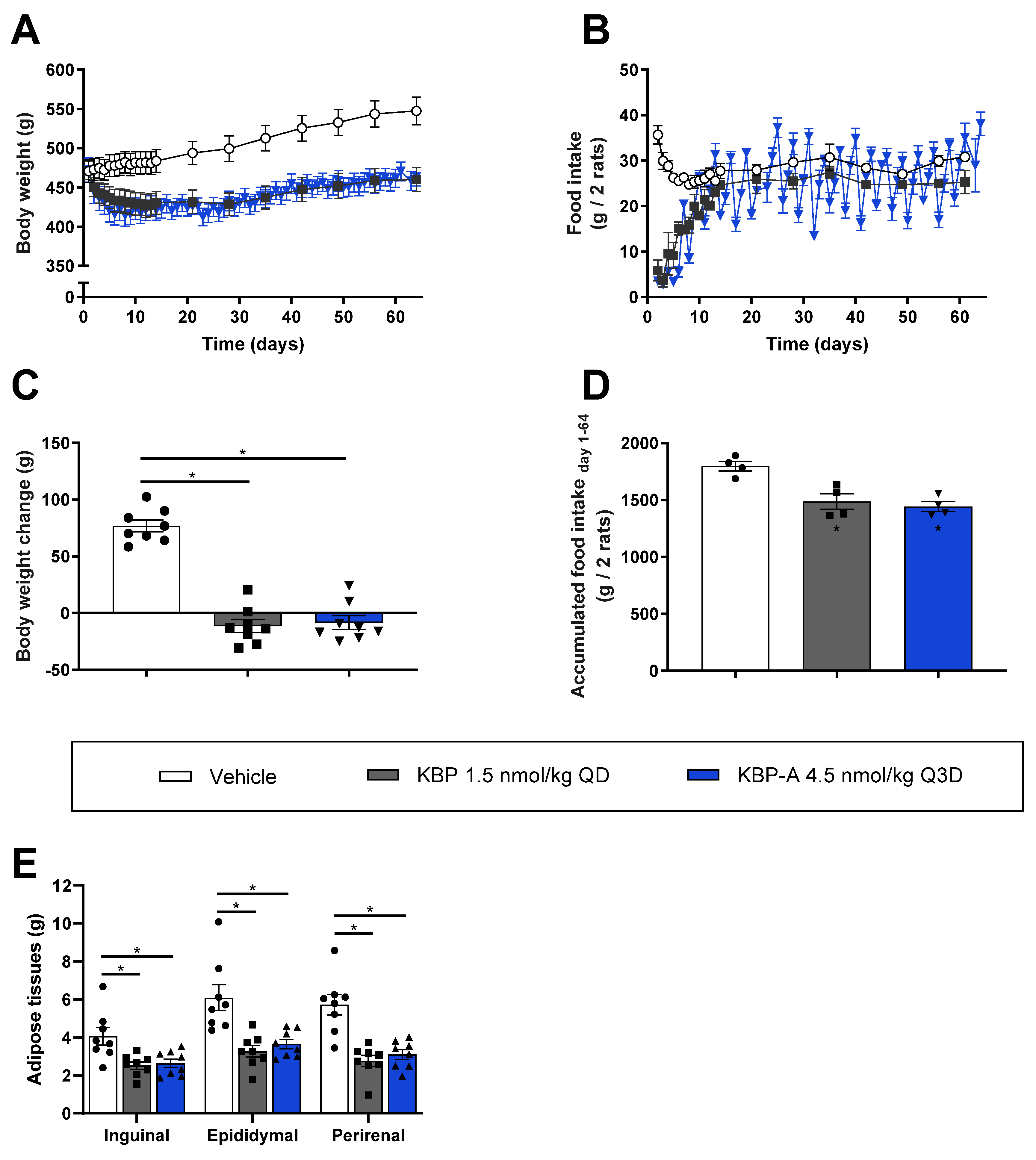
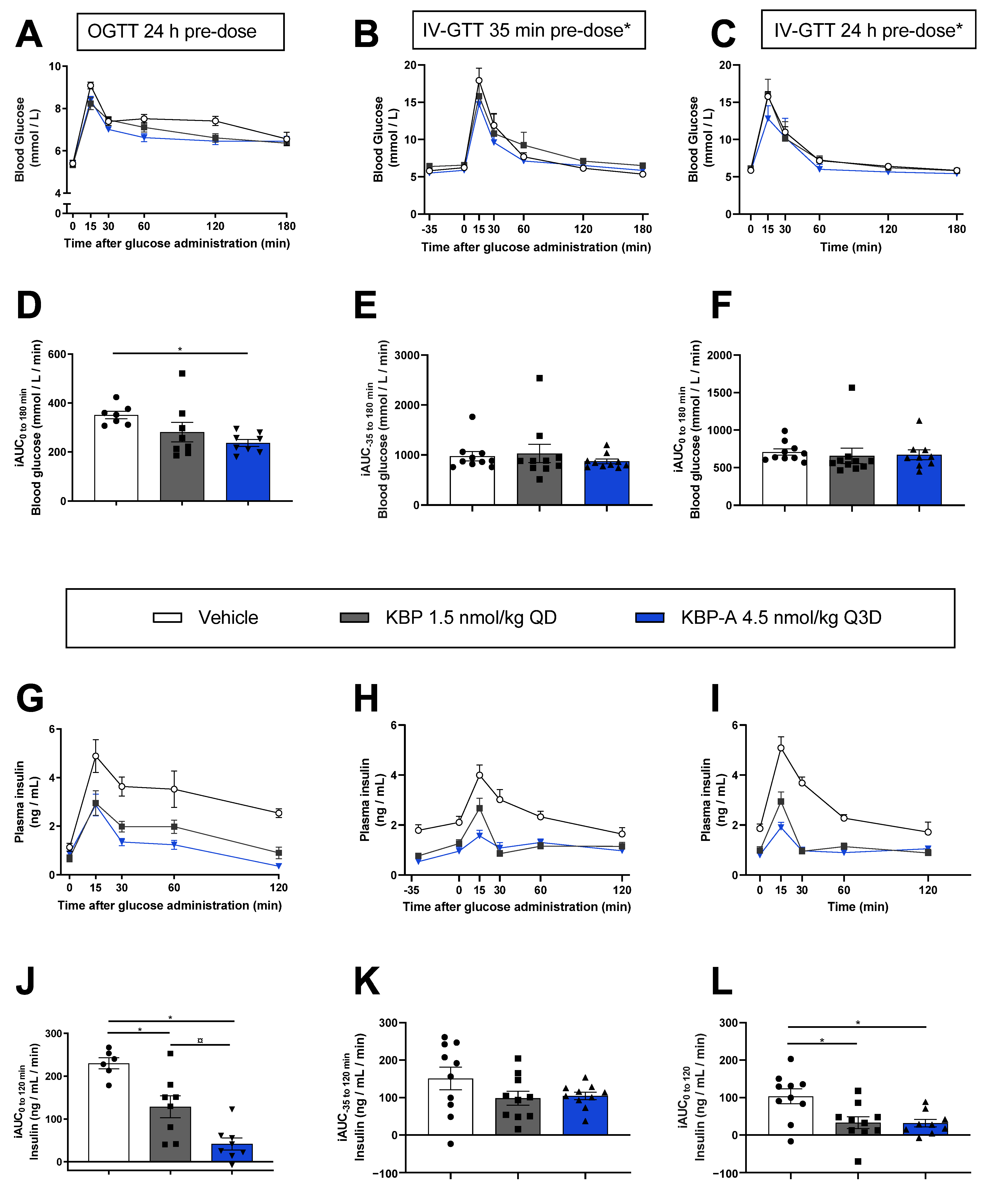
3.3. Both KBP and KBP-A Improve Body Weight and Glucose Tolerance in HFD-Fed Sprague–Dawley Rats (Study (3) and (4))
3.4. KBP-A Improves Glucose Control in Diabetic ZDF Rats Independent of Dosing Profile (Study (5))
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMY-R: | amylin receptor |
| CTR: | calcitonin receptor |
| DACRA: | Dual amylin and calcitonin receptor agonist |
| FBG: | fasting blood glucose |
| GPCR: | G protein-coupled receptor |
| KBP: | KeyBiosciencePeptide |
| HFD: | high-fat diet |
| INF.: | (drug delivery by) infusion |
| INJ.: | (drug delivery by) injection |
| IV: | intravenous |
| IV-GTT: | intravenous glucose tolerance test |
| OGTT: | oral glucose tolerance test |
| QD: | once daily |
| Q2D: | once every 2nd day/once every other day |
| Q3D: | once every 3rd day |
| RAMP: | receptor activity-modifying protein |
| s.c.: | subcutaneous |
| ZDF: | Zucker Diabetic Fatty (rat) |
References
- Lukinius, A.; Wilander, E.; Westermark, G.T.; Engström, U.; Westermark, P. Co-localization of islet amyloid polypeptide and insulin in the B cell secretory granules of the human pancreatic islets. Diabetologia 1989, 32, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Reidelberger, R.D.; Haver, A.C.; Arnelo, U.; Smith, D.D.; Schaffert, C.S.; Permert, J.; Roger, D.; Haver, A.C.; Arnelo, U.; Smith, D.; et al. Amylin receptor blockade stimulates food intake in rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2004, 287, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Rushing, P.A.; Hagan, M.M.; Seeley, R.J.; Lutz, T.A.; D’Alessio, D.A.; Air, E.L.; Woods, S.C. Inhibition of Central Amylin Signaling Increases Food Intake and Body Adiposity in Rats. Endocrinology 2001, 142, 5035–5038. [Google Scholar] [CrossRef] [PubMed]
- Dunn-Meynell, A.A.; Foll, C.L.; Johnson, M.D.; Lutz, T.A.; Hayes, M.R.; Levin, B.E. Endogenous VMH amylin signaling is required for full leptin signaling and protection from diet-induced obesity. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 310, R355–R365. [Google Scholar] [CrossRef] [PubMed]
- Gedulin, B.R.; Jodka, C.M.; Herrmann, K.; Young, A.A. Role of endogenous amylin in glucagon secretion and gastric emptying in rats demonstrated with the selective antagonist, AC187. Regul. Pept. 2006, 137, 121–127. [Google Scholar] [CrossRef]
- Roth, J.D.; Hughes, H.; Kendall, E.; Baron, A.D.; Anderson, C.M. Antiobesity effects of the β-cell hormone amylin in diet-induced obese rats: Effects on food intake, body weight, composition, energy expenditure, and gene expression. Endocrinology 2006, 147, 5855–5864. [Google Scholar] [CrossRef]
- Larsen, A.T.; Sonne, N.; Andreassen, K.V.; Gehring, K.; Karsdal, M.A.; Henriksen, K. The dual amylin and calcitonin receptor agonist KBP-088 induces weight loss and improves insulin sensitivity superior to chronic amylin therapy. J. Pharmacol. Exp. Ther. 2019, 370, 35–43. [Google Scholar] [CrossRef]
- Andreassen, K.V.; Hjuler, S.T.; Furness, S.G.; Sexton, P.M.; Christopoulos, A.; Nosjean, O.; Karsdal, M.A.; Henriksen, K. Prolonged calcitonin receptor signaling by salmon, but not human calcitonin, reveals ligand bias. PLoS ONE 2014, 9, e92042. [Google Scholar] [CrossRef]
- Gydesen, S.; Andreassen, K.V.; Hjuler, S.T.; Christensen, J.M.; Karsdal, M.A.; Henriksen, K. KBP-088, a novel DACRA with prolonged receptor activation, is superior to davalintide in terms of efficacy on body weight. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E821–E827. [Google Scholar] [CrossRef]
- Gydesen, S.; Hjuler, S.T.; Freving, Z.; Andreassen, K.V.; Sonne, N.; Hellgren, L.I.; Karsdal, M.A.; Henriksen, K. A novel Dual Amylin and Calcitonin Receptor Agonist (DACRA), KBP-089, induces weight loss through a reduction in fat, but not lean mass, while improving food preference. Br. J. Pharmacol. 2017, 174, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Andreassen, K.V.; Feigh, M.; Hjuler, S.T.; Gydesen, S.; Henriksen, J.E.; Beck-Nielsen, H.; Christiansen, C.; Karsdal, M.A.; Henriksen, K. A novel oral dual amylin and calcitonin receptor agonist (KBP-042) exerts antiobesity and antidiabetic effects in rats. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E24–E33. [Google Scholar] [CrossRef]
- Hjuler, S.T.; Gydesen, S.; Andreassen, K.V.; Karsdal, M.A.; Henriksen, K. The Dual Amylin- and Calcitonin Receptor Agonist KBP-042 works as Adjunct to Metformin on Fasting Hyperglycaemia and HbA1c in a rat model of Type 2 Diabetes. J. Pharmacol. Exp. Ther. 2017, 362, 24–30. [Google Scholar] [CrossRef]
- Larsen, A.T.; Sonne, N.; Andreassen, K.V.; Karsdal, M.A.; Henriksen, K. The Calcitonin Receptor Plays a Major Role in Glucose Regulation as a Function of Dual Amylin and Calcitonin Receptor Agonist Therapy. J. Pharmacol. Exp. Ther. 2020, 374, 74–83. [Google Scholar] [CrossRef]
- Hjuler, S.T.; Andreassen, K.V.; Gydesen, S.; Karsdal, M.A.; Henriksen, K. KBP-042 improves bodyweight and glucose homeostasis with indices of increased insulin sensitivity irrespective of route of administration. Eur. J. Pharmacol. 2015, 762, 229–238. [Google Scholar] [CrossRef]
- Sonne, N.; Larsen, A.T.; Andreassen, K.V.; Karsdal, M.A.; Henriksen, K. The Dual Amylin and Calcitonin Receptor Agonist, KBP-066, induces an equally potent weight loss across a broad dose range while higher doses may further improve insulin action. J. Pharmacol. Exp. Ther. 2020, 373, 92–102. [Google Scholar] [CrossRef]
- Andreassen, K.V.; Larsen, A.T.; Sonne, N.; Mohamed, K.E.; Karsdal, M.A.; Henriksen, K. KBP-066A, a Long-Acting Dual Amylin and Calcitonin Receptor Agonist, Induces Weight Loss and Improves Glycemic Control in Obese and Diabetic Rats. Mol. Metab. 2021, 53, 101282. [Google Scholar] [CrossRef]
- Karageorgos, V.; Venihaki, M.; Sakellaris, S.; Pardalos, M.; Kontakis, G.; Matsoukas, M.T.; Gravanis, A.; Margioris, A.; Liapakis, G. Current understanding of the structure and function of family B GPCRs to design novel drugs. Hormones 2018, 17, 45–59. [Google Scholar] [CrossRef]
- Alexander, S.P.H.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; et al. THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein-coupled receptors. Br. J. Pharmacol. 2019, 176, S21–S141. [Google Scholar] [CrossRef]
- Barwell, J.; Gingell, J.J.; Watkins, H.A.; Archbold, J.K.; Poyner, D.R.; Hay, D.L. Calcitonin and calcitonin receptor-like receptors: Common themes with family B GPCRs? Br. J. Pharmacol. 2012, 166, 51–65. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Brandi, M.L.; Rubin, M.; Silverberg, S.J. Primary hyperparathyroidism: New concepts in clinical, densitometric and biochemical features. J. Intern. Med. 2005, 257, 6–17. [Google Scholar] [CrossRef]
- Bellido, T.; Ali, A.A.; Plotkin, L.I.; Fu, Q.; Gubrij, I.; Roberson, P.K.; Weinstein, R.S.; O’Brien, C.A.; Manolagas, S.C.; Jilka, R.L. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts: A putative explanation for why intermittent administration is needed for bone anabolism. J. Biol. Chem. 2003, 278, 50259–50272. [Google Scholar] [CrossRef] [PubMed]
- Iida-Klein, A.; Lu, S.S.; Kapadia, R.; Burkhart, M.; Moreno, A.; Dempster, D.W.; Lindsay, R. Short-term continuous infusion of human parathyroid hormone 1-34 fragment is catabolic with decreased trabecular connectivity density accompanied by hypercalcemia in C57BL/J6 mice. J. Endocrinol. 2005, 186, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A.T.; Sonne, N.; Andreassen, K.V.; Karsdal, M.A.; Henriksen, K. Dose frequency optimization of the dual amylin and calcitonin receptor agonist KBP-088—Long-lasting improvement of food preference and body weight loss. J. Pharmacol. Exp. Ther. 2020, 373, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Coskun, T.; Sloop, K.W.; Loghin, C.; Alsina-Fernandez, J.; Urva, S.; Bokvist, K.B.; Cui, X.; Briere, D.A.; Cabrera, O.; Roell, W.C.; et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol. Metab. 2018, 18, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Usborne, A.; Byrd, R.A.; Meehan, J.; Blackbourne, J.L.; Sullivan, J.; Poitout-Belissent, F.; Prefontaine, A.; Martin, J.A.; Vahle, J.L. An investigative study of pancreatic exocrine biomarkers, histology, and histomorphometry in male Zucker Diabetic Fatty (ZDF) rats given dulaglutide by subcutaneous injection twice weekly for 13 weeks. Toxicol. Pathol. 2015, 43, 1093–1102. [Google Scholar] [CrossRef]
- Hjuler, S.T.; Gydesen, S.; Andreassen, K.V.; Lund, S.; Pedersen, K.; Hellgren, L.I.; Karsdal, M.A.; Henriksen, K. The Dual Amylin- and Calcitonin-Receptor Agonist KBP-042 Increases Insulin Sensitivity and Induces Weight Loss in Rats with Obesity. Obesity 2016, 24, 1712–1722. [Google Scholar] [CrossRef]
- Henry, R.R.; Rosenstock, J.; Logan, D.K.; Alessi, T.R.; Luskey, K.; Baron, M.A. Randomized trial of continuous subcutaneous delivery of exenatide by ITCA 650 versus twice-daily exenatide injections in metformin-treated type 2 diabetes. Diabetes Care 2013, 36, 2559–2565. [Google Scholar] [CrossRef]
- Novo Nordisk. Novo Nordisk Successfully Completes AM833 Phase 2 Trial and Phase 1 Combination Trial with AM833 and Semaglutide in Obesity. 2020. Available online: https://www.globenewswire.com/news-release/2020/06/18/2050266/0/en/Novo-Nordisk-successfully-completes-AM833-phase-2-trial-and-phase-1-combination-trial-with-AM833-and-semaglutide-in-obesity.html (accessed on 16 August 2022).
- Enebo, L.B.; Berthelsen, K.K.; Kankam, M.; Lund, M.T.; Rubino, D.M.; Satylganova, A.; Lau, D.C.W. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2.4 mg for weight management: A randomised, controlled, phase 1b trial. Lancet 2021, 397, 1736–1748. [Google Scholar] [CrossRef]
- Capehorn, M.S.; Catarig, A.M.; Furberg, J.K.; Janez, A.; Price, H.C.; Tadayon, S.; Vergès, B.; Marre, M. Efficacy and safety of once-weekly semaglutide 1.0 mg vs once-daily liraglutide 1.2 mg as add-on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020, 46, 100–109. [Google Scholar] [CrossRef]
- Drucker, D.J.; Buse, J.B.; Taylor, K.; Kendall, D.M.; Trautmann, M.; Zhuang, D.; Porter, L. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: A randomised, open-label, non-inferiority study. Lancet 2008, 372, 1240–1250. [Google Scholar] [CrossRef]
- Buse, J.B.; Drucker, D.J.; Taylor, K.L.; Kim, T.; Walsh, B.; Hu, H.; Wilhelm, K.; Trautmann, M.; Shen, L.Z.; Porter, L.E. DURATION-1: Exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care 2010, 33, 1255–1261. [Google Scholar] [CrossRef]
- Blevins, T.; Pullman, J.; Malloy, J.; Yan, P.; Taylor, K.; Schulteis, C.; Trautmann, M.; Porter, L. DURATION-5: Exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2011, 96, 1301–1310. [Google Scholar] [CrossRef]
- Wysham, C.H.; Rosenstock, J.; Vetter, M.L.; Dong, F.; Öhman, P.; Iqbal, N. Efficacy and tolerability of the new autoinjected suspension of exenatide once weekly versus exenatide twice daily in patients with type 2 diabetes. Diabetes Obes. Metab. 2018, 20, 165–172. [Google Scholar] [CrossRef] [Green Version]

| KBP-042: Ac-CSNLSTCVLGKLSQELHKLQTYPRTDVGANAP-NH2 KBP-088: Ac-CSNLSTCMLGRLSQELHRLQTFPKTDVGANAP-NH2 KBP-088A: Ac-CSNLSTCMLGKLSQELHRLQTFPKTDVGANAP-NH2 KBP-066A: Ac-CSNLSTCXLGKLSQDLHRLQTYPKTDVGANAP-NH2 |
| Ac: Acetylation of the N-terminus X: AiB (2-Aminoisobutyric acid) K: -PEG-PEG-γGlu-C18 diacid acylation complex conjugated to the lysine side chain NH2: Amidation of the C-terminal COOH group |
PEG-PEG-yGlu-C18 diacid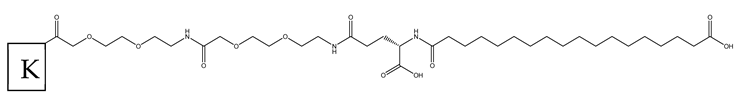 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonne, N.; Larsen, A.T.; Karsdal, M.A.; Henriksen, K. The Impact of Exposure Profile on the Efficacy of Dual Amylin and Calcitonin Receptor Agonist Therapy. Biomedicines 2022, 10, 2365. https://doi.org/10.3390/biomedicines10102365
Sonne N, Larsen AT, Karsdal MA, Henriksen K. The Impact of Exposure Profile on the Efficacy of Dual Amylin and Calcitonin Receptor Agonist Therapy. Biomedicines. 2022; 10(10):2365. https://doi.org/10.3390/biomedicines10102365
Chicago/Turabian StyleSonne, Nina, Anna Thorsø Larsen, Morten Asser Karsdal, and Kim Henriksen. 2022. "The Impact of Exposure Profile on the Efficacy of Dual Amylin and Calcitonin Receptor Agonist Therapy" Biomedicines 10, no. 10: 2365. https://doi.org/10.3390/biomedicines10102365
APA StyleSonne, N., Larsen, A. T., Karsdal, M. A., & Henriksen, K. (2022). The Impact of Exposure Profile on the Efficacy of Dual Amylin and Calcitonin Receptor Agonist Therapy. Biomedicines, 10(10), 2365. https://doi.org/10.3390/biomedicines10102365





