Arterial Thrombotic Complications in COVID-19: A Case of Renal Infarction
Abstract
:1. Introduction
2. Case Report
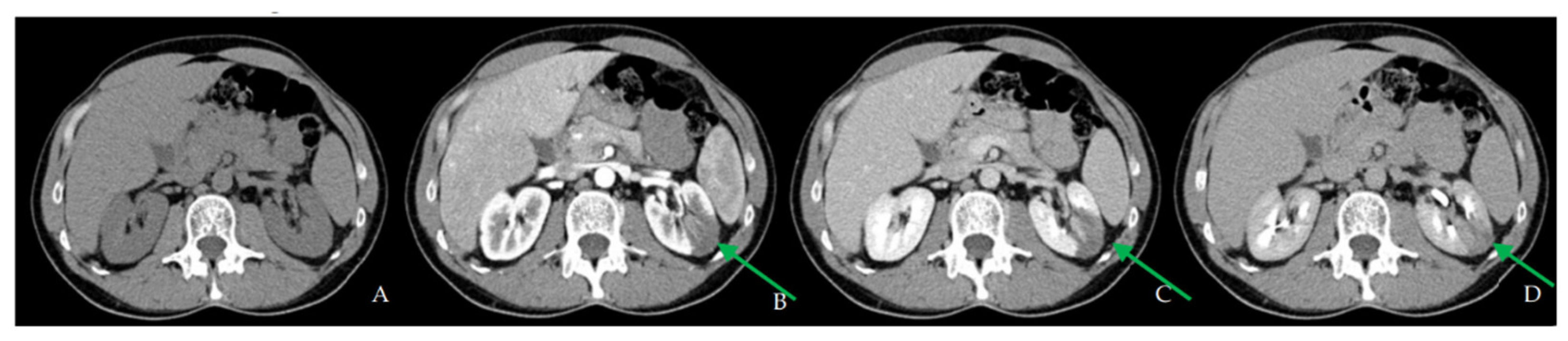
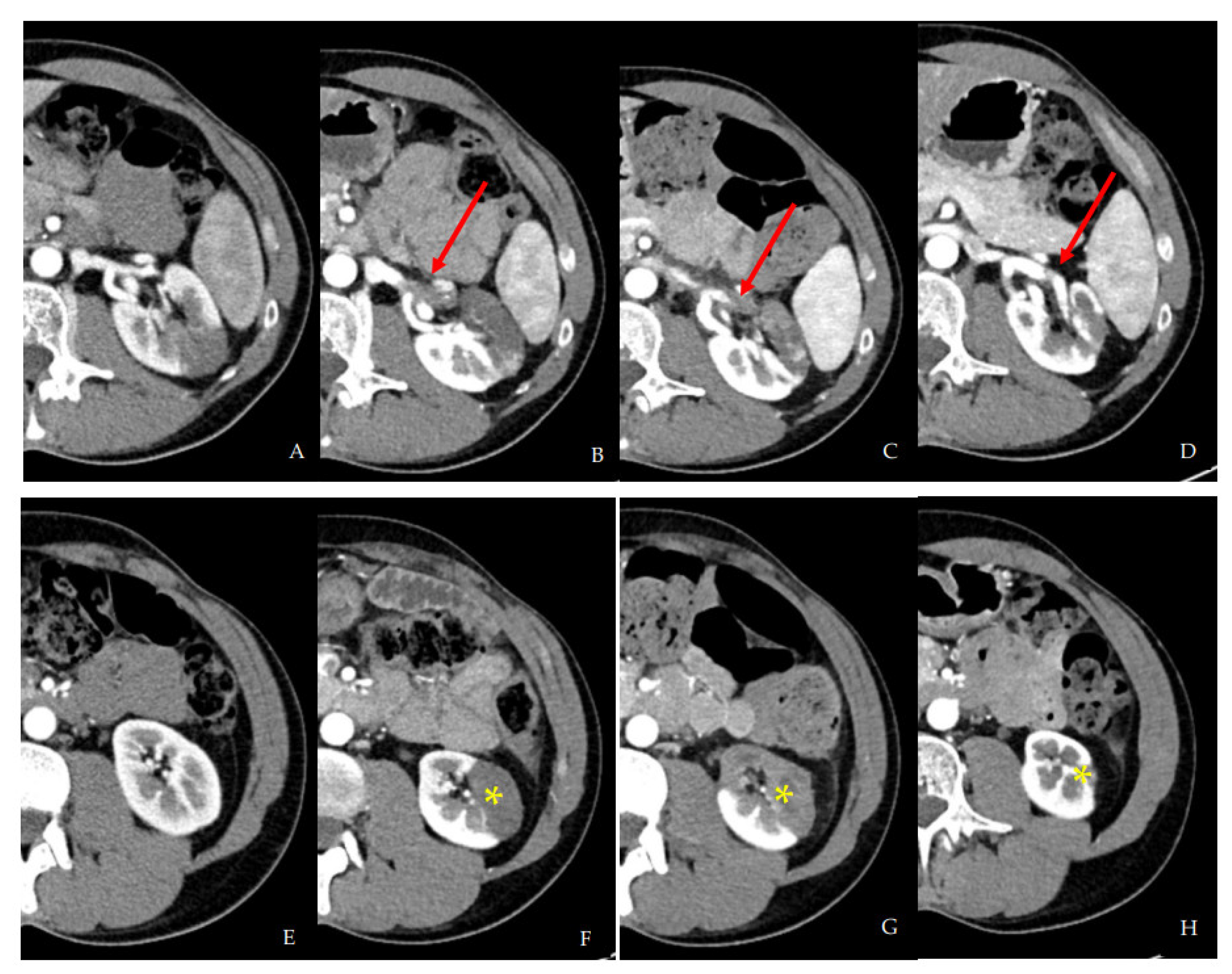
2.1. First Hospital Admission
2.2. Second Hospital Admission
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourgault, M.; Grimbert, P.; Verret, C.; Pourrat, J.; Herody, M.; Halimi, J.M.; Karras, A.; Amoura, Z.; Jourde-Chiche, N.; Izzedine, H.; et al. Acute Renal Infarction: A Case Series. Clin. J. Am. Soc. Nephrol. 2013, 8, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Piazza, G.; Morrow, D.A. Diagnosis, management, and pathophysiology of arterial and venous thrombosis in covid-19. JAMA-J. Am. Med. Assoc. 2020, 324, 2548–2549. [Google Scholar] [CrossRef] [PubMed]
- Piazza, G.; Campia, U.; Hurwitz, S.; Snyder, J.E.; Rizzo, S.M.; Pfeferman, M.B.; Morrison, R.B.; Leiva, O.; Fanikos, J.; Nauffal, V.; et al. Registry of Arterial and Venous Thromboembolic Complications in Patients with COVID-19. J. Am. Coll. Cardiol. 2020, 76, 2060–2072. [Google Scholar] [CrossRef] [PubMed]
- Cheruiyot, I.; Kipkorir, V.; Ngure, B.; Misiani, M.; Munguti, J.; Ogeng’o, J. Arterial Thrombosis in Coronavirus Disease 2019 Patients: A Rapid Systematic Review. Ann. Vasc. Surg. 2021, 70, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Zaid, Y.; Puhm, F.; Allaeys, I.; Naya, A.; Oudghiri, M.; Khalki, L.; Limami, Y.; Zaid, N.; Sadki, K.; Ben El Haj, R.; et al. Platelets Can Associate with SARS-CoV-2 RNA and Are Hyperactivated in COVID-19. Circ. Res. 2020, 127, 1404–1418. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Oomen, L.; Leijte, E.; Shilhan, D.E.; Battye, M.; Waltregny, D.; Van der Aa, F.; Spinoit, A.F.; Rösch, W.H.; Schmiedeke, E.; Fisch, M.; et al. Rare and Complex Urology: Clinical Overview of ERN eUROGEN. Eur. Urol. 2022, 81, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Zazzara, M.; Zattoni, F. Stem cells, biomarkers and genetic profiling: Approaching future challenges in Urology. Urologia 2016, 83, 4–13. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 23 August 2022).
- Schulman, S.; Sholzberg, M.; Spyropoulos, A.C.; Zarychanski, R.; Resnick, H.E.; Bradbury, C.A.; Broxmeyer, L.; Connors, J.M.; Falanga, A.; Iba, T.; et al. ISTH guidelines for antithrombotic treatment in COVID-19. J. Thromb. Haemost. 2022, 20, 2214–2225. [Google Scholar] [CrossRef] [PubMed]
- Katsoularis, I.; Fonseca-Rodríguez, O.; Farrington, P.; Jerndal, H.; Lundevaller, E.H.; Sund, M.; Lindmark, K.; Connolly, A.M.F. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: Nationwide self-controlled cases series and matched cohort study. BMJ 2022, 377, e069590. [Google Scholar] [CrossRef] [PubMed]


| Value | Reference Range | |
| Inflammatory markers | ||
| Leukocytes, ×109/L | 12.3 | 4.4–11 |
| CRP, mg/dL | 127.6 | 0–5 |
| Procalcitonin, µg/L | 0.08 | <0.5 |
| Creatinine, µmol/L | 75 | 59–104 |
| ESR, mm/h | 46 | 2–28 |
| LDH, U/L | 462 | 135–225 |
| Immunological markers | ||
| S-ICC-C1q, µgEq/mL | 1.2 | <16 |
| S-ICC-C3d, µgEq/mL | 1.7 | <16 |
| C3 factor, g/L | 1.6 | 0.9–1.8 |
| C4 factor, g/L | 0.32 | 0.09–0.36 |
| Plasmatic crioglobulins | Negative | |
| Rheumatoid factor, kU/L | <10 | 0–30 |
| Anti-ENA ab | Negative | |
| Anti-ANCA ab | Negative | |
| Anti-proteinase III ab | Negative | |
| Anti-MPO ab | Negative | |
| Anti-native DNA ab | Negative | |
| Coagulation panel | ||
| INR | 1.12 | |
| aPTT, s | 23 | 22–32 |
| D-dimer, µg/L | <150 | 0–250 |
| Factor II activity, % | 134.8 | 80–120 |
| Factor VIII activity, % | 265.3 | 60–160 |
| Factor IX activity, % | 175.4 | 80–120 |
| Factor X activity, % | 117.6 | 80–120 |
| Factor XI activity, % | 153.4 | 80–120 |
| Fibrinogen (Clauss assay), mg/dL | 681.2 | 150–450 |
| Antitrombin activity, % | 101.2 | 80–120 |
| Protein C, % | 109 | 80–120 |
| Protein S, % | 118 | 80–120 |
| Factor V Leiden mutation | Negative | |
| Protrombin variant G20210A | Negative | |
| Plasminogen activity, % | 120.4 | 75.0–140 |
| Anti beta-2 GPI IgG, U/mL | 8.6 | <8 |
| Anti beta-2 GPI IgM, U/mL | 1.7 | <8 |
| Anti cardiolipin IgG, U/mL | 8.2 | <10 |
| Anti cardiolipin IgM, U/mL | 2.1 | <10 |
| Value | Reference | |
| Oncological Markers | ||
| AFP, μg/L | 3.0 | 0.0–7.4 |
| CA-19-9, kU/L | 12.5 | 0.0–30.0 |
| CEA, μg/L | 1.1 | 0.0–4.0 |
| 24-h urine metanephrines μmol/L | 0.48 | 0.01–1.62 |
| beta-HCG, IU/L | <0.05 | 0.0–1.5 |
| PSA, μg/L | 0.41 | 0.02–4.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancini, M.; Randazzo, G.; Piazza, G.; Dal Moro, F. Arterial Thrombotic Complications in COVID-19: A Case of Renal Infarction. Biomedicines 2022, 10, 2354. https://doi.org/10.3390/biomedicines10102354
Mancini M, Randazzo G, Piazza G, Dal Moro F. Arterial Thrombotic Complications in COVID-19: A Case of Renal Infarction. Biomedicines. 2022; 10(10):2354. https://doi.org/10.3390/biomedicines10102354
Chicago/Turabian StyleMancini, Mariangela, Gianmarco Randazzo, Gregory Piazza, and Fabrizio Dal Moro. 2022. "Arterial Thrombotic Complications in COVID-19: A Case of Renal Infarction" Biomedicines 10, no. 10: 2354. https://doi.org/10.3390/biomedicines10102354
APA StyleMancini, M., Randazzo, G., Piazza, G., & Dal Moro, F. (2022). Arterial Thrombotic Complications in COVID-19: A Case of Renal Infarction. Biomedicines, 10(10), 2354. https://doi.org/10.3390/biomedicines10102354








