Telomeres and Telomerase in the Control of Stem Cells
Abstract
:1. Introduction
2. What Are Stem Cells?
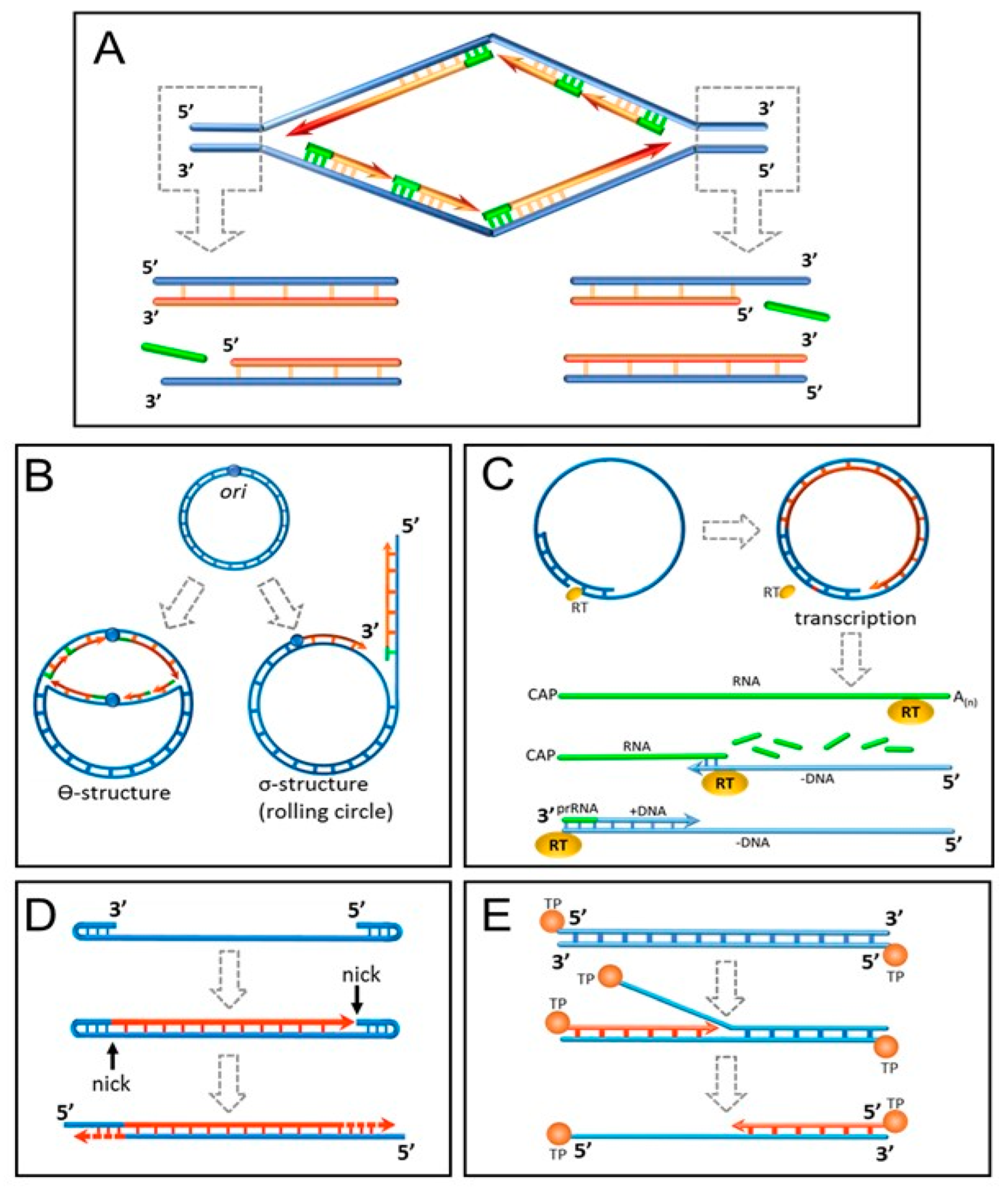
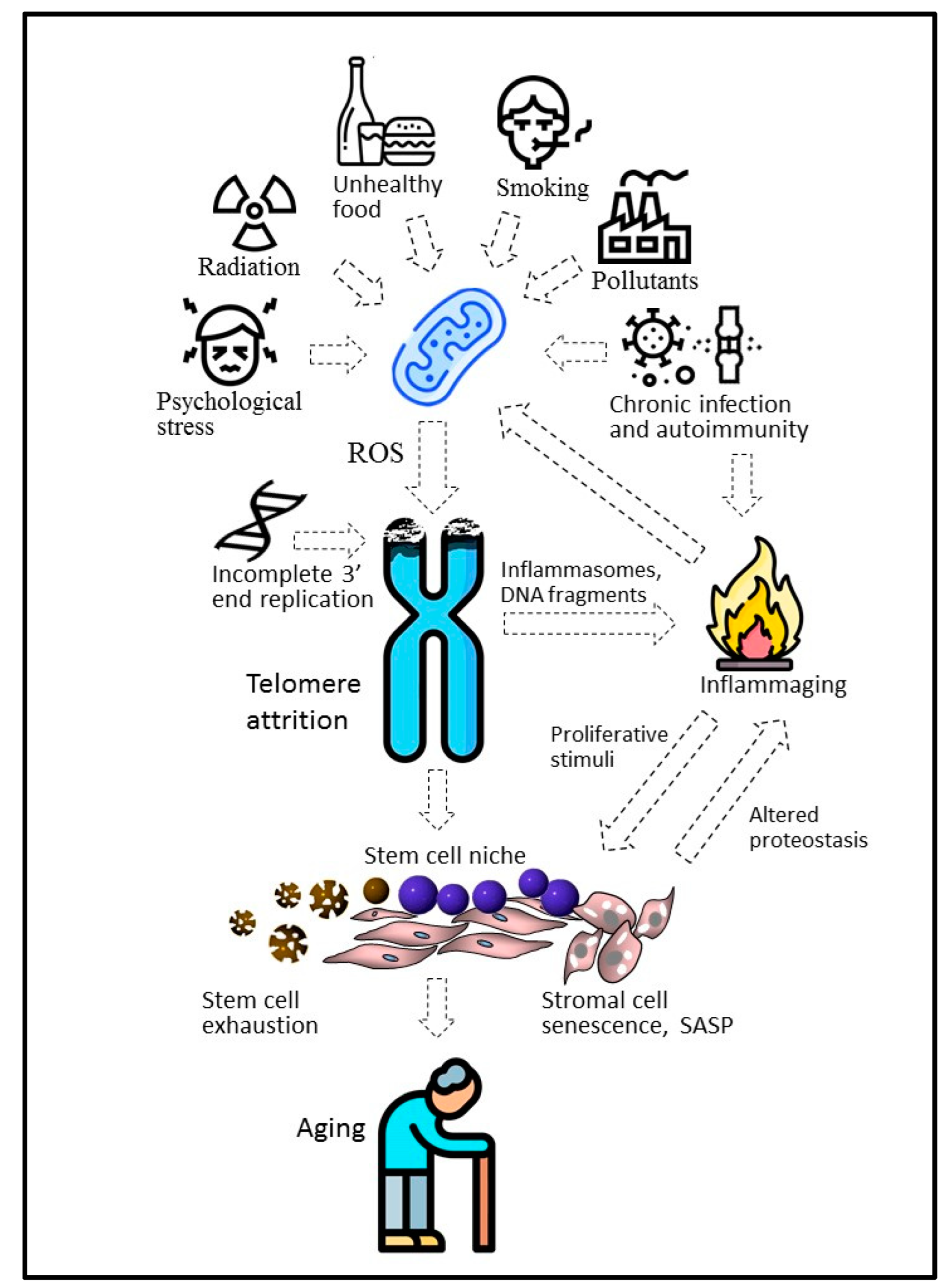
3. Proliferative Senescence and Telomere Shortening: From Mitotic Clock to Molecular Biosensor
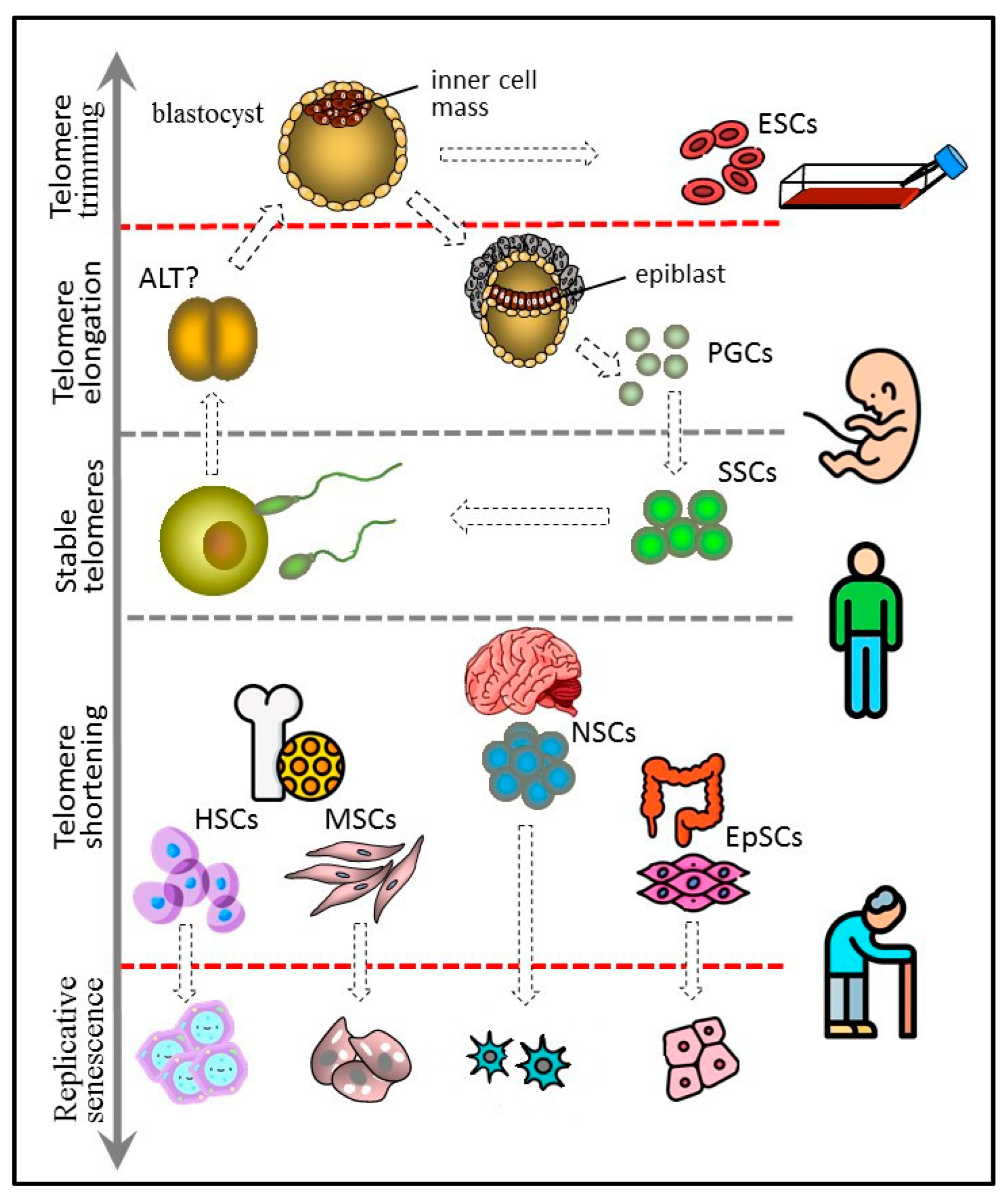
4. Replicative Senescence in Different Types of Stem Cells
4.1. Embryonic Stem Cells
4.2. Germline Stem Cells
4.3. Hematopoietic Stem Cells
4.4. Mesenchymal Stem Cells
4.5. Neural Stem Cells
4.6. Tissue-Specific Epithelial Stem Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiley, L. Transgenic animals resistant to infectious diseases. Rev. Sci. Technol. 2016, 35, 121–132. [Google Scholar] [CrossRef]
- Brüggemann, M.; Osborn, M.J.; Ma, B.; Hayre, J.; Avis, S.; Lundstrom, B.; Buelow, R. Human antibody production in transgenic animals. Arch. Immunol. Ther. Exp. 2015, 63, 101–108. [Google Scholar] [CrossRef]
- Gouveia, C.; Huyser, C.; Egli, D.; Pepper, M.S. Lessons learned from somatic cell nuclear transfer. Int. J. Mol. Sci. 2020, 21, 2314. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, Y.; Zou, K. Germline stem cells: A useful tool for therapeutic cloning. Curr. Stem. Cell Res. Ther. 2018, 13, 236–242. [Google Scholar] [CrossRef]
- Alcalde, I.; Sánchez-Fernández, C.; Martín, C.; De Pablo, N.; Jemni-Damer, N.; Guinea, G.V.; Merayo-Lloves, J.; Del Olmo-Aguado, S. Human stem cell transplantation for retinal degenerative diseases: Where Are We Now? Medicina 2022, 58, 102. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Islam, M.T.; Harun-Or-Rashid, M.; Islam, M.; Abdullah, S.; Uddin, M.B.; Das, S.; Rahaman, M.S.; Ahmed, M.; et al. Stem cell transplantation therapy and neurological disorders: Current status and future perspectives. Biology 2022, 11, 147. [Google Scholar] [CrossRef]
- Yarygin, K.N.; Lupatov, A.Y.; Kholodenko, I.V. Cell-based therapies of liver diseases: Age-related challenges. Clin. Interv. Aging 2015, 10, 1909–1924. [Google Scholar] [CrossRef]
- Greil, C.; Engelhardt, M.; Finke, J.; Wäsch, R. Allogeneic stem cell transplantation in multiple myeloma. Cancers 2021, 14, 55. [Google Scholar] [CrossRef]
- Lee, J.K.; Link, J.M.; Hu, J.C.Y.; Athanasiou, K.A. The Self-Assembling Process and Applications in Tissue Engineering. Cold Spring Harb. Perspect Med. 2017, 7, a025668. [Google Scholar] [CrossRef]
- Crippa, S.; Santi, L.; Berti, M.; De Ponti, G.; Bernardo, M.E. Role of ex vivo Expanded Mesenchymal Stromal Cells in Determining Hematopoietic Stem Cell Transplantation Outcome. Front. Cell Dev. Biol. 2021, 9, 663316. [Google Scholar] [CrossRef]
- Leyendecker, A., Jr.; Pinheiro, C.C.G.; Amano, M.T.; Bueno, D.F. The Use of Human Mesenchymal Stem Cells as Therapeutic Agents for the in vivo Treatment of Immune-Related Diseases: A Systematic Review. Front Immunol. 2018, 9, 2056. [Google Scholar] [CrossRef]
- Yegorov, Y.E.; Poznyak, A.V.; Nikiforov, N.G.; Sobenin, I.A.; Orekhov, A.N. The link between chronic stress and accelerated aging. Biomedicines 2020, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Maurya, P.K. Correlation between telomere length and biomarkers of oxidative stress in human aging. Rejuvenation Res. 2022, 25, 25–29. [Google Scholar] [CrossRef]
- Dahiya, R.; Mohammad, T.; Alajmi, M.F.; Rehman, M.T.; Hasan, G.M.; Hussain, A.; Hassan, M.I. Insights into the conserved regulatory mechanisms of human and yeast aging. Biomolecules 2020, 10, 882. [Google Scholar] [CrossRef] [PubMed]
- Koltzenburg, C.; Chukhlovin, A.; Anagnostou, A.; Stocking, C. The lymphocyte as a stem cell, common to different blood elements in embryonic development and during the post-fetal life of mammals. Cell Ther. Transpl. 2009, 1, 14–18. [Google Scholar] [CrossRef]
- Martin, G.R. Isolation of a pluripotent cell-line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem-cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7634–7638. [Google Scholar] [CrossRef]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef]
- Pirity, M.; Hadjantonakis, A.K.; Nagy, A. Embryonic stem cells, creating transgenic animals. Methods Cell Biol. 1998, 57, 279–293. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, S.; Liu, S. Aging of Human Adult Stem Cells. Adv. Exp. Med. Biol. 2018, 1086, 105–115. [Google Scholar] [CrossRef]
- Slack, J.M.W. What is a stem cell? Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e323. [Google Scholar] [CrossRef]
- Yasui, R.; Sekine, K.; Taniguchi, H. Clever experimental designs: Shortcuts for better iPSC differentiation. Cells 2021, 10, 3540. [Google Scholar] [CrossRef]
- Bhartiya, D. Pluripotent stem cells in adult tissues: Struggling to be acknowledged over two decades. Stem. Cell Rev. Rep. 2017, 13, 713–724. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; Lui, M.; Chu, P.J.; Yoo, J.; Chang, M.; Yen, Y. Identification of a distinct small cell population from human bone marrow reveals its multipotency in vivo and in vitro. PLoS ONE 2014, 9, e85112. [Google Scholar] [CrossRef]
- Dulak, J.; Szade, K.; Szade, A.; Nowak, W.; Józkowicz, A. Adult stem cells: Hopes and hypes of regenerative medicine. Acta Biochim. Pol. 2015, 62, 329–337. [Google Scholar] [CrossRef]
- Schofield, R. The relationship between the spleen colony forming cell and the haemopoietic stem cell. Blood Cells. 1978, 4, 7–25. [Google Scholar] [PubMed]
- Xin, T.; Greco, V.; Myung, P. Hardwiring stem cell communication through tissue structure. Cell 2016, 164, 1212–1225. [Google Scholar] [CrossRef]
- Mohammad, K.; Dakik, P.; Medkour, Y.; Mitrofanova, D.; Titorenko, V.I. Quiescence entry, maintenance, and exit in adult stem cells. Int. J. Mol. Sci. 2019, 20, 2158. [Google Scholar] [CrossRef]
- Knoblich, J.A. Mechanisms of asymmetric stem cell division. Cell 2008, 132, 583–597. [Google Scholar] [CrossRef]
- Morrison, S.J.; Kimble, J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 2006, 441, 1068–1074. [Google Scholar] [CrossRef]
- Cao, H.; Cao, B.; Heazlewood, C.K.; Domingues, M.; Sun, X.; Debele, E.; McGregor, N.E.; Sims, N.A.; Heazlewood, S.Y.; Nilsson, S.K. Osteopontin is an important regulative component of the fetal bone marrow hematopoietic stem cell niche. Cells 2019, 8, 985. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Al, M.; Kyeremeh, G.K.; Saeinasab, M.; Heidari Keshel, S.; Sefat, F. Stem cell niche microenvironment: Review. Bioengineering 2021, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Yadav, C.B.; Tabassum, N.; Bajpeyee, A.K.; Verma, V. Stem cell niche: Dynamic neighbor of stem cells. Eur. J. Cell Biol. 2019, 98, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Püschel, J.; Dubrovska, A.; Gorodetska, I. The multifaceted role of aldehyde dehydrogenases in prostate cancer stem cells. Cancers 2021, 13, 4703. [Google Scholar] [CrossRef] [PubMed]
- Gisina, A.M.; Kim, Y.S.; Yarygin, K.N.; Lupatov, A.Y. Identification of the side population associated with ATP-binding cassette transporters activity using imaging flow cytometry. Biochem. Mosc. Suppl. B 2021, 15, 248–254. [Google Scholar] [CrossRef]
- Goodell, M.A.; Brose, K.; Paradis, G.; Conner, A.S.; Mulligan, R.C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 1996, 183, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Challen, G.A.; Little, M.H. A side order of stem cells: The SP phenotype. Stem Cells 2006, 24, 3–12. [Google Scholar] [CrossRef]
- Zeng, H.; Park, J.W.; Guo, M.; Lin, G.; Crandall, L.; Compton, T.; Wang, X.; Li, X.J.; Chen, F.P.; Xu, R.H. Lack of ABCG2 expression and side population properties in human pluripotent stem cells. Stem cells 2009, 27, 2435–2445. [Google Scholar] [CrossRef]
- Gisina, A.M.; Lupatov, A.Y.; Karalkin, P.A.; Mainovskaya, O.A.; Petrov, L.O.; Sidorov, D.V.; Frank, G.A.; Yarygin, K.N. CD133+ human colorectal adenocarcinoma cells are resistant to staining with fluorescent dyes used for analysis of ABC- Transporter activities. Bull. Exp. Biol. Med. 2014, 158, 80–83. [Google Scholar] [CrossRef]
- Carrel, A. On the permanent life of tissues outside of the organism. J. Exp. Med. 1912, 15, 516–528. [Google Scholar] [CrossRef]
- Swim, H.E.; Parker, R.F. Culture characteristics of human fibroblasts propagated serially. Am. J. Hyg. 1957, 66, 235–243. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Olovnikov, A.M. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J. Theor. Biol. 1973, 41, 181–190. [Google Scholar] [CrossRef]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef]
- Pellegrini, L. The Pol α-primase complex. Subcell. Biochem. 2012, 62, 157–169. [Google Scholar] [CrossRef]
- Chow, T.T.; Zhao, Y.; Mak, S.S.; Shay, J.W.; Wright, W.E. Early and late steps in telomere overhang processing in normal human cells: The position of the final RNA primer drives telomere shortening. Genes Dev. 2012, 26, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Doksani, Y.; Wu, J.Y.; de Lange, T.; Zhuang, X. Super-resolution fluorescence imaging of telomeres reveals TRF2-dependent T-loop formation. Cell 2013, 155, 345–356. [Google Scholar] [CrossRef]
- Sahu, S.; Dattani, A.; Aboobaker, A.A. Secrets from immortal worms: What can we learn about biological ageing from the planarian model system? Semin. Cell Dev. Biol. 2017, 70, 108–121. [Google Scholar] [CrossRef]
- de Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef]
- Azzalin, C.M.; Lingner, J. Telomere functions grounding on TERRA firma. Trends Cell Biol. 2015, 25, 29–36. [Google Scholar] [CrossRef]
- Chan, S.W.; Blackburn, E.H. New ways not to make ends meet: Telomerase, DNA damage proteins and heterochromatin. Oncogene 2002, 21, 553–563. [Google Scholar] [CrossRef]
- Collins, K.; Mitchell, J.R. Telomerase in the human organism. Oncogene 2002, 21, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Shmookler Reis, R.J. Model systems for aging research: Syncretic concepts and diversity of mechanisms. Genome 1989, 31, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.; Blasco, M.A.; Serrano, M. Cellular senescence in cancer and aging. Cell 2007, 130, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E.; Brasiskyte, D.; Van der Haegen, B.A. E6 of human papillomavirus type 16 can overcome the M1 stage of immortalization in human mammary epithelial cells but not in human fibroblasts. Oncogene 1993, 8, 1407–1413. [Google Scholar]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The essence of senescence. Genes Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef]
- Lazzerini-Denchi, E.; Sfeir, A. Stop pulling my strings—What telomeres taught us about the DNA damage response. Nat. Rev. Mol. Cell Biol. 2016, 17, 364–378. [Google Scholar] [CrossRef]
- Gomes, N.M.; Ryder, O.A.; Houck, M.L.; Charter, S.J.; Walker, W.; Forsyth, N.R.; Austad, S.N.; Venditti, C.; Pagel, M.; Shay, J.W.; et al. Comparative biology of mammalian telomeres: Hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell. 2011, 10, 761–768. [Google Scholar] [CrossRef]
- Vera, E.; Bernardes de Jesus, B.; Foronda, M.; Flores, J.M.; Blasco, M.A. The rate of increase of short telomeres predicts longevity in mammals. Cell Rep. 2012, 2, 732–737. [Google Scholar] [CrossRef]
- Sahin, E.; DePinho, R.A. Axis of ageing: Telomeres, p53 and mitochondria. Nat. Rev. Mol. Cell Biol. 2012, 13, 397–404. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Telomeres and telomerase: Three decades of progress. Nat. Rev. Genet. 2019, 20, 299–309. [Google Scholar] [CrossRef]
- Celtikci, B.; Erkmen, G.K.; Dikmen, Z.G. Regulation and effect of telomerase and telomeric length in stem cells. Curr. Stem Cell Res. Ther. 2021, 16, 809–823. [Google Scholar] [CrossRef]
- Gavia-García, G.; Rosado-Pérez, J.; Arista-Ugalde, T.L.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Telomere length and oxidative stress and its relation with metabolic syndrome components in the aging. Biology 2021, 10, 253. [Google Scholar] [CrossRef]
- Reichert, S.; Stier, A. Does oxidative stress shorten telomeres in vivo? A review. Biol. Lett. 2017, 13, 20170463. [Google Scholar] [CrossRef]
- Sen, A.; Marsche, G.; Freudenberger, P.; Schallert, M.; Toeglhofer, A.M.; Nagl, C.; Schmidt, R.; Launer, L.J.; Schmidt, H. Association between higher plasma lutein, zeaxanthin, and vitamin C concentrations and longer telomere length: Results of the Austrian Stroke Prevention Study. J. Am. Geriatr. Soc. 2014, 62, 222–229. [Google Scholar] [CrossRef]
- Shen, J.; Gammon, M.D.; Terry, M.B.; Wang, Q.; Bradshaw, P.; Teitelbaum, S.L.; Neugut, A.I.; Santella, R.M. Telomere length, oxidative damage, antioxidants and breast cancer risk. Int. J. Cancer 2009, 124, 1637–1643. [Google Scholar] [CrossRef]
- Koju, N.; Taleb, A.; Zhou, J.; Lv, G.; Yang, J.; Cao, X.; Lei, H.; Ding, Q. Pharmacological strategies to lower crosstalk between nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and mitochondria. Biomed. Pharmacother 2019, 111, 1478–1498. [Google Scholar] [CrossRef]
- Brunk, U.T.; Terman, A. The mitochondrial-lysosomal axis theory of aging: Accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur. J. Biochem. 2002, 269, 1996–2002. [Google Scholar] [CrossRef]
- Victorelli, S.; Passos, J.F. Telomeres and cell senescence—Size matters not. EBioMedicine 2017, 21, 14–20. [Google Scholar] [CrossRef]
- de Lange, T. Shelterin-mediated telomere protection. Annu. Rev. Genet. 2018, 52, 223–247. [Google Scholar] [CrossRef]
- Martínez, P.; Flores, J.M.; Blasco, M.A. 53BP1 deficiency combined with telomere dysfunction activates ATR-dependent DNA damage response. J. Cell Biol. 2012, 197, 283–300. [Google Scholar] [CrossRef]
- Petersen, S.; Saretzki, G.; von Zglinicki, T. Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Exp. Cell Res. 1998, 239, 152–160. [Google Scholar] [CrossRef]
- Storci, G.; De Carolis, S.; Olivieri, F.; Bonafe, M. Changes in the biochemical taste of cytoplasmic and cell-free DNA are major fuels for inflamm-aging. Semin. Immunol. 2018, 40, 6–16. [Google Scholar] [CrossRef]
- Fulop, T.; Witkowski, J.M.; Olivieri, F.; Larbi, A. The integration of inflammaging in agerelated diseases. Semin. Immunol. 2018, 40, 17–35. [Google Scholar] [CrossRef]
- Mender, I.; Zhang, A.; Ren, Z.; Han, C.; Deng, Y.; Siteni, S.; Li, H.; Zhu, J.; Vemula, A.; Shay, J.W.; et al. Telomere Stress Potentiates STING Dependent Anti-tumor Immunity. Cancer Cell. 2020, 38, 400–411.e6. [Google Scholar] [CrossRef]
- Chakravarti, D.; Hu, B.; Mao, X.; Rashid, A.; Li, J.; Li, J.; Liao, W.T.; Whitley, E.M.; Dey, P.; Hou, P.l.; et al. Telomere dysfunction activates YAP1 to drive tissue inflammation. Nat. Commun. 2020, 11, 4766. [Google Scholar] [CrossRef]
- Fernandes, S.G.; Dsouza, R.; Khattar, E. External environmental agents influence telomere length and telomerase activity by modulating internal cellular processes: Implications in human aging. Environ. Toxicol. Pharmacol. 2021, 85, 103633. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Yegorov, Y.E. Smoking and health: Association between telomere length and factors impacting on human disease, quality of life and life span in a large population-based cohort under the effect of smoking duration. Fundam. Clin. Pharmacol. 2011, 25, 425–442. [Google Scholar] [CrossRef]
- Lin, J.; Epel, E. Stress and telomere shortening: Insights from cellular mechanisms. Ageing Res. Rev. 2022, 73, 101507. [Google Scholar] [CrossRef]
- Turkiewicz, S.; Ditmer, M.; Sochal, M.; Białasiewicz, P.; Strzelecki, D.; Gabryelska, A. Obstructive sleep apnea as an acceleration trigger of cellular senescence processes through telomere shortening. Int. J. Mol. Sci. 2021, 22, 12536. [Google Scholar] [CrossRef]
- Rotar, O.; Moguchaia, E.; Boyarinova, M.; Kolesova, E.; Khromova, N.; Freylikhman, O.; Smolina, N.; Solntsev, V.; Kostareva, A.; Konradi, A.; et al. Seventy years after the siege of Leningrad: Does early life famine still affect cardiovascular risk and aging? J. Hypertens 2015, 33, 1772–1779. [Google Scholar] [CrossRef]
- Garatachea, N.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Santos-Lozano, A.; Fiuza-Luces, C.; Morán, M.; Emanuele, E.; Joyner, M.J.; Lucia, A. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 2015, 18, 57–89. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Ibarra, M.J.; Hernández, J.; Caire-Juvera, G. Diet, physical activity and telomere length in adults. Nutr. Hosp. 2019, 36, 1403–1417. [Google Scholar] [CrossRef]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA twins study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, eaau8650. [Google Scholar] [CrossRef]
- Fouquerel, E.; Lormand, J.; Bose, A.; Lee, H.T.; Kim, G.S.; Li, J.; Sobol, R.W.; Freudenthal, B.D.; Myong, S.; Opresko, P.L. Oxidative guanine base damage regulates human telomerase activity. Nat. Struct. Mol. Biol. 2016, 23, 1092–1100. [Google Scholar] [CrossRef]
- Ahmed, S.; Passos, J.F.; Birket, M.J.; Beckmann, T.; Brings, S.; Peters, H.; Birch-Machin, M.A.; von Zglinicki, T.; Saretzki, G. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J. Cell Sci. 2008, 121 Pt 7, 1046–1053. [Google Scholar] [CrossRef]
- Kaul, Z.; Cesare, A.J.; Huschtscha, L.I.; Neumann, A.A.; Reddel, R.R. Five dysfunctional telomeres predit onset of senescence in human cells. EMBO Rep. 2012, 13, 52–59. [Google Scholar] [CrossRef]
- Leem, S.H.; Londoño-Vallejo, J.A.; Kim, J.H.; Bui, H.; Tubacher, E.; Solomon, G.; Park, J.E.; Horikawa, I.; Kouprina, N.; Barrett, J.C.; et al. The human telomerase gene: Complete genomic sequence and analysis of tandem repeat polymorphisms in intronic regions. Oncogene 2002, 21, 769–777. [Google Scholar] [CrossRef]
- Kim, W.; Shay, J.W. Long-range telomere regulation of gene expression: Telomere looping and telomere position effect over long distances (TPE-OLD). Differentiation 2018, 99, 1–9. [Google Scholar] [CrossRef]
- Dogan, F.; Forsyth, N.R. Telomerase regulation: A role for epigenetics. Cancers 2021, 13, 1213. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Wang, Z.; Liu, J.P. Roles of telomere biology in cell senescence, replicative and chronological ageing. Cells 2019, 8, 54. [Google Scholar] [CrossRef]
- Sharpless, N.E.; DePinho, R.A. Telomeres, stem cells, senescence, and cancer. J. Clin. Investig. 2004, 113, 160–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, D.; Wang, H.; Li, T.; Shi, Z.; Jin, X.; Sperka, T.; Zhu, X.; Zhang, M.; Yang, F.; Cong, Y.; et al. Telomeric epigenetic response mediated by Gadd45a regulates stem cell aging and lifespan. EMBO Rep. 2018, 19, e45494. [Google Scholar] [CrossRef]
- Böcker, W.; Yin, Z.; Drosse, I.; Haasters, F.; Rossmann, O.; Wierer, M.; Popov, C.; Locher, M.; Mutschler, W.; Docheva, D.; et al. Introducing a single-cell-derived human mesenchymal stem cell line expressing hTERT after lentiviral gene transfer. J. Cell Mol. Med. 2008, 12, 1347–1359. [Google Scholar] [CrossRef]
- Dai, F.; Yang, S.; Zhang, F.; Shi, D.; Zhang, Z.; Wu, J.; Xu, J. hTERT- and hCTLA4Ig-expressing human bone marrow-derived mesenchymal stem cells: In vitro and in vivo characterization and osteogenic differentiation. J. Tissue Eng. Regen. Med. 2017, 11, 400–411. [Google Scholar] [CrossRef]
- Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weissman, I.L.; Rando, T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005, 433, 760–764. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Allsopp, R.C. Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood 2003, 102, 517–520. [Google Scholar] [CrossRef]
- Kumar, M.; Lechel, A.; Güneş, Ç. Telomerase: The devil inside. Genes 2016, 7, 43. [Google Scholar] [CrossRef]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Wu, J.; Runnels, J.M.; Zaher, W.; Mortensen, L.J.; Alt, C.; Turcotte, R.; et al. Direct measurement of local oxygen concentration R in the bone marrow of live animals. Nature 2014, 508, 269–273. [Google Scholar] [CrossRef]
- Kunisaki, Y.; Bruns, I.; Scheiermann, C.; Ahmed, J.; Pinho, S.; Zhang, D.; Mizoguchi, T.; Wei, Q.; Lucas, D.; Ito, K.; et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013, 502, 637–643. [Google Scholar] [CrossRef]
- Culver, J.C.; Vadakkan, T.J.; Dickinson, M.E. A specialized microvascular domain in the mouse neural stem cell niche. PLoS ONE 2013, 8, e53546. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef]
- Suda, T.; Takubo, K.; Semenza, G.L. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 2011, 9, 298–310. [Google Scholar] [CrossRef]
- Singh, R.P.; Franke, K.; Wielockx, B. Hypoxia-mediated regulation of stem cell fate. High Alt. Med. Biol. 2012, 13, 162–168. [Google Scholar] [CrossRef]
- Prigione, A.; Rohwer, N.; Hoffmann, S.; Mlody, B.; Drews, K.; Bukowiecki, R.; Blümlein, K.; Wanker, E.E.; Ralser, M.; Cramer, T.; et al. HIF1α modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK1-3 and PKM2. Stem Cells 2014, 32, 364–376. [Google Scholar] [CrossRef]
- Yatabe, N.; Kyo, S.; Maida, Y.; Nishi, H.; Nakamura, M.; Kanaya, T.; Tanaka, M.; Isaka, K.; Ogawa, S.; Inoue, M. HIF-1-mediated activation of telomerase in cervical cancer cells. Oncogene 2004, 23, 3708–3715. [Google Scholar] [CrossRef]
- Ramlee, M.K.; Wang, J.; Toh, W.X.; Li, S. Transcription regulation of the human telomerase reverse transcriptase (hTERT) gene. Genes 2016, 7, 50. [Google Scholar] [CrossRef]
- Hiyama, E.; Hiyama, K. Telomere and telomerase in stem cells. Br. J. Cancer 2007, 96, 1020–1024. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, D. Telomerase reverse transcriptase (TERT) in action: Cross-talking with epigenetics. Int. J. Mol. Sci. 2019, 20, 3338. [Google Scholar] [CrossRef]
- Park, J.I.; Venteicher, A.S.; Hong, J.Y.; Choi, J.; Jun, S.; Shkreli, M.; Chang, W.; Meng, Z.; Cheung, P.; Ji, H.; et al. Telomerase modulates Wnt signaling by association with target gene chromatin. Nature 2009, 460, 66–72. [Google Scholar] [CrossRef]
- Radan, L.; Hughes, C.S.; Teichroeb, J.H.; Vieira Zamora, F.M.; Jewer, M.; Postovit, L.M.; Betts, D.H. Microenvironmental regulation of telomerase isoforms in human embryonic stem cells. Stem Cells Dev. 2014, 23, 2046–2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolte, J. Lrrc34 Interacts with Oct4 and Has an Impact on Telomere Length in Mouse Embryonic Stem Cells. Stem Cells Dev. 2021, 30, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Meerdo, L.N.; Reed, W.A.; White, K.L. Telomere-to-centromere ratio of bovine clones, embryos, gametes, fetal cells, and adult cells. Cloning Stem Cells 2005, 7, 62–73. [Google Scholar] [CrossRef]
- Marión, R.M.; López de Silanes, I.; Mosteiro, L.; Gamache, B.; Abad, M.; Guerra, C.; Megías, D.; Serrano, M.; Blasco, M.A. Common telomere changes during in vivo reprogramming and early stages of tumorigenesis. Stem Cell Rep. 2017, 8, 460–475. [Google Scholar] [CrossRef]
- Agarwal, S.; Loh, Y.H.; McLoughlin, E.M.; Huang, J.; Park, I.H.; Miller, J.D.; Huo, H.; Okuka, M.; dos Reis, R.M.; Loewer, S.; et al. Telomere elongation in induced pluripotent stem cells from dyskeratosis congenita patients. Nature 2010, 464, 292–296. [Google Scholar] [CrossRef]
- Kamada, M.; Mitsui, Y.; Matsuo, T.; Takahashi, T. Reversible transformation and de-differentiation of human cells derived from induced pluripotent stem cell teratomas. Hum. Cell 2016, 29, 1–9. [Google Scholar] [CrossRef]
- Liu, L.; Bailey, S.M.; Okuka, M.; Munoz, P.; Li, C.; Zhou, L.; Wu, C.; Czerwiec, E.; Sandler, L.; Seyfang, A.; et al. Telomere lengthening early in development. Nat. Cell Biol. 2007, 9, 1436–1441. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, L.; Sun, Y.; Xie, P.; Hu, L.; Yuan, D.; Chen, D.; Ouyang, Q.; Lin, G.; Lu, G. Telomerase-mediated telomere elongation from human blastocysts to embryonic stem cells. J. Cell Sci. 2014, 127, 752–762. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, F.; Liu, L. Alternative lengthening of telomeres (ALT) in tumors and pluripotent stem cells. Genes 2019, 10, 1030. [Google Scholar] [CrossRef]
- Zalzman, M.; Falco, G.; Sharova, L.V.; Nishiyama, A.; Thomas, M.; Lee, S.L.; Stagg, C.A.; Hoang, H.G.; Yang, H.T.; Indig, F.E.; et al. Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature 2010, 464, 858–863. [Google Scholar] [CrossRef]
- Dan, J.; Zhou, Z.; Wang, F.; Wang, H.; Guo, R.; Keefe, D.L.; Liu, L. Zscan4 Contributes to Telomere Maintenance in Telomerase-Deficient Late Generation Mouse ESCs and Human ALT Cancer Cells. Cells 2022, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.; Liu, Y.; Liu, N.; Chiourea, M.; Okuka, M.; Wu, T.; Ye, X.; Mou, C.; Wang, L.; Wang, L.; et al. Rif1 maintains telomere length homeostasis of ESCs by mediating heterochromatin silencing. Dev. Cell 2014, 29, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Le, R.; Huang, Y.; Zhang, Y.; Wang, H.; Lin, J.; Dong, Y.; Li, Z.; Guo, M.; Kou, X.; Zhao, Y.; et al. Dcaf11 activates Zscan4-mediated alternative telomere lengthening in early embryos and embryonic stem cells. Cell Stem Cell 2021, 28, 732–747.e9. [Google Scholar] [CrossRef]
- Markiewicz-Potoczny, M.; Lobanova, A.; Loeb, A.M.; Kirak, O.; Olbrich, T.; Ruiz, S.; Lazzerini Denchi, E. TRF2-mediated telomere protection is dispensable in pluripotent stem cells. Nature 2021, 589, 110–115. [Google Scholar] [CrossRef]
- Rivera, T.; Haggblom, C.; Cosconati, S.; Karlseder, J. A balance between elongation and trimming regulates telomere stability in stem cells. Nat. Struct. Mol. Biol. 2017, 24, 30–39. [Google Scholar] [CrossRef]
- Varela, E.; Munoz-Lorente, M.A.; Tejera, A.M.; Ortega, S.; Blasco, M.A. Generation of mice with longer and better preserved telomeres in the absence of genetic manipulations. Nat. Commun. 2016, 7, 11739. [Google Scholar] [CrossRef]
- Li, J.S.; Miralles Fuste, J.; Simavorian, T.; Bartocci, C.; Tsai, J.; Karlseder, J.; Lazzerini Denchi, E. TZAP: A telomere-associated protein involved in telomere length control. Science 2017, 355, 638–641. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, G.; He, C.; Mei, Y.; Shi, Y.; Li, F. The 11th C2H2 zinc finger and an adjacent C-terminal arm are responsible for TZAP recognition of telomeric DNA. Cell Res. 2018, 28, 130–134. [Google Scholar] [CrossRef]
- Nikolic, A.; Volarevic, V.; Armstrong, L.; Lako, M.; Stojkovic, M. Primordial Germ Cells: Current Knowledge and Perspectives. Stem Cells Int. 2016, 2016, 1741072. [Google Scholar] [CrossRef]
- Guo, J.; Grow, E.J.; Yi, C.; Mlcochova, H.; Maher, G.J.; Lindskog, C.; Murphy, P.J.; Wike, C.L.; Carrel, D.T.; Goriely, A.; et al. Chromatin and single-cell RNA-seq profiling reveal dynamic signaling and metabolic transitions during human spermatogonial stem cell development. Cell Stem Cell 2017, 21, 533–546.e6. [Google Scholar] [CrossRef]
- Kerr, C.L.; Shamblott, M.J.; Gearhart, J.D. Pluripotent stem cells from germ cells. Methods Enzymol. 2006, 419, 400–426. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.C.; Kobayash, T.; Irie, N.; Dietmann, S.; Surani, M.A. Specification and epigenetic programming of the human germ line. Nat. Rev. Genet. 2016, 17, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Wistuba, J.; Pock, T.; Schlatt, S.; Neuhaus, N. Spermatogonial stem cells: Updates from specification to clinical relevance. Hum. Reprod. Updat. 2019, 25, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Pech, M.F.; Garbuzov, A.; Hasegawa, K.; Sukhwani, M.; Zhang, R.J.; Benayoun, B.A.; Brockman, S.A.; Lin, S.; Brunet, A.; Orwig, K.E.; et al. High telomerase is a hallmark of undifferentiated spermatogonia and is required for maintenance of male germline stem cells. Genes Dev. 2015, 29, 2420–2434. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Ogonuki, N.; Iwano, T.; Lee, J.; Kazuki, Y.; Inoue, K.; Miki, H.; Takehashi, M.; Toyokuni, S.; Shinkai, Y.; et al. Genetic and epigenetic properties of mouse male germline stem cells during long-term culture. Development 2005, 132, 4155–4163. [Google Scholar] [CrossRef]
- Ferlin, A.; Rampazzo, E.; Rocca, M.S.; Keppel, S.; Frigo, A.C.; De Rossi, A.; Foresta, C. In young men sperm telomere length is related to sperm number and parental age. Hum. Reprod. 2017, 28, 3370–3376. [Google Scholar] [CrossRef]
- Dunlop, C.E.; Telfer, E.E.; Anderson, R.A. Ovarian germline stem cells. Stem Cell Res. Ther. 2014, 5, 98. [Google Scholar] [CrossRef]
- Zhang, H.; Panula, S.; Petropoulos, S.; Edsgärd, D.; Busayavalasa, K.; Liu, L.; Li, X.; Risal, S.; Shen, Y.; Shao, J.; et al. Adult human and mouse ovaries lack DDX4-expressing functional oogonial stem cells. Nat. Med. 2015, 21, 1116–1118. [Google Scholar] [CrossRef]
- Turner, S.; Hartshorne, G.M. Telomere lengths in human pronuclei, oocytes and spermatozoa. Mol. Hum. Reprod. 2013, 19, 510–518. [Google Scholar] [CrossRef]
- Wright, W.E.; Piatyszek, M.A.; Rainey, W.E.; Byrd, W.; Shay, J.W. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 1996, 18, 173–179. [Google Scholar] [CrossRef]
- Brummendorf, T.H.; Balabanov, S. Telomere length dynamics in normal hematopoiesis and in disease states characterized by increased stem cell turnover. Leukemia 2006, 29, 273–275. [Google Scholar] [CrossRef]
- Brazvan, B.; Ebrahimi-Kalan, A.; Velaei, K.; Mehdipour, A.; Aliyari Serej, Z.; Ebrahimi, A.; Ghorbani, M.; Cheraghi, O.; Nozad Charoudeh, H. Telomerase activity and telomere on stem progeny senescence. Biomed. Pharmacother. 2018, 102, 9–17. [Google Scholar] [CrossRef]
- Patrick, M.; Weng, N.P. Expression and regulation of telomerase in human T cell differentiation, activation, aging and diseases. Cell Immunol. 2019, 345, 103989. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; de Haan, G. Inflammation and aging of hematopoietic stem cells in their niche. Cells 2021, 10, 1849. [Google Scholar] [CrossRef]
- De Haan, G.; Lazare, S.S. Aging of hematopoietic stem cells. Blood 2018, 131, 479–487. [Google Scholar] [CrossRef]
- Brzeźniakiewicz-Janus, K.; Rupa-Matysek, J.; Gil, L. Acquired aplastic anemia as a clonal disorder of hematopoietic stem cells. Stem Cell Rev. Rep. 2020, 16, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, E.; Santoni, A.; Colla, S. Dysfunctional telomeres and hematological disorders. Differentiation 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Yarygin, K.N.; Lupatov, A.Y.; Sukhikh, G.T. Modulation of immune responses by mesenchymal stromal cells. Bull. Exp. Biol. Med. 2016, 161, 561–565. [Google Scholar] [CrossRef]
- Choumerianou, D.M.; Martimianaki, G.; Stiakaki, E.; Kalmanti, L.; Kalmanti, M.; Dimitriou, H. Comparative study of stemness characteristics of mesenchymal cells from bone marrow of children and adults. Cytotherapy 2010, 12, 881–887. [Google Scholar] [CrossRef]
- Yang, Y.K.; Ogando, C.R.; Wang See, C.; Chang, T.Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, E.N.; Vega, S.; Fu, C.; De León, R.; Beltran, D.; Solis, M.A. Increased proliferation and differentiation capacity of placenta-derived mesenchymal stem cells from women of median maternal age correlates with telomere shortening. Aging 2021, 13, 24542–24559. [Google Scholar] [CrossRef]
- Ryu, E.; Hong, S.; Kang, J.; Woo, J.; Park, J.; Lee, J.; Seo, J.S. Identification of senescence-associated genes in human bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2008, 371, 431–436. [Google Scholar] [CrossRef]
- Fehrer, C.; Lepperdinger, G. Mesenchymal stem cell aging. Exp. Gerontol. 2005, 40, 926–930. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Wang, S.; Deng, Q.; Wang, K.; Dai, X.; An, Y.; Dong, G.; Ke, W.; Chen, F.; et al. Single-cell transcriptome profiling reveals molecular heterogeneity in human umbilical cord tissue and culture-expanded mesenchymal stem cells. FEBS J. 2021, 288, 5311–5330. [Google Scholar] [CrossRef]
- Hwang, E.S.; Ok, J.S.; Song, S. Chemical and physical approaches to extend the replicative and differentiation potential of stem cells. Stem Cell Rev. Rep. 2016, 12, 315–326. [Google Scholar] [CrossRef]
- Wolbank, S.; Stadler, G.; Peterbauer, A.; Gillich, A.; Karbiener, M.; Streubel, B.; Wieser, M.; Katinger, H.; van Griensven, M.; Redl, H.; et al. Telomerase immortalized human amnion- and adipose-derived mesenchymal stem cells: Maintenance of differentiation and immunomodulatory characteristics. Tissue Eng. Part A 2009, 15, 1843–1854. [Google Scholar] [CrossRef]
- Trachana, V.; Petrakis, S.; Fotiadis, Z.; Siska, E.K.; Balis, V.; Gonos, E.S.; Kaloyianni, M.; Koliakos, G. Human mesenchymal stem cells with enhanced telomerase activity acquire resistance against oxidative stress-induced genomic damage. Cytotherapy 2017, 19, 808–820. [Google Scholar] [CrossRef]
- Poltavtseva, R.A.; Silachev, D.N.; Pavlovich, S.V.; Kesova, M.I.; Yarygin, K.N.; Lupatov, A.Y.; Van’ko, L.V.; Shuvalova, M.P.; Sukhikh, G.T. Neuroprotective effect of mesenchymal and neural stem and progenitor cells on sensorimotor recovery after brain injury. Bull Exp. Biol. Med. 2012, 153, 586–590. [Google Scholar] [CrossRef]
- Ferrón, S.R.; Marqués-Torrejón, M.A.; Mira, H.; Flores, I.; Taylor, K.; Blasco, M.A.; Fariñas, I. Telomere shortening in neural stem cells disrupts neuronal differentiation and neuritogenesis. J. Neurosci. 2009, 29, 14394–14407. [Google Scholar] [CrossRef]
- Grammatikakis, I.; Zhang, P.; Mattson, M.P.; Gorospe, M. The long and the short of TRF2 in neurogenesis. Cell Cycle 2016, 15, 3026–3032. [Google Scholar] [CrossRef] [Green Version]
- Lobanova, A.; She, R.; Pieraut, S.; Clapp, C.; Maximov, A.; Denchi, E.L. Different requirements of functional telomeres in neural stem cells and terminally differentiated neurons. Genes Dev. 2017, 31, 639–647. [Google Scholar] [CrossRef]
- Martín-Rivera, L.; Herrera, E.; Albar, J.P.; Blasco, M.A. Expression of mouse telomerase catalytic subunit in embryos and adult tissues. Proc. Natl. Acad. Sci. USA 1998, 95, 10471–10476. [Google Scholar] [CrossRef]
- Schwob, A.E.; Nguyen, L.J.; Meiri, K.F. Immortalization of neural precursors when telomerase is overexpressed in embryonal carcinomas and stem cells. Mol. Biol. Cell 2008, 19, 1548–1560. [Google Scholar] [CrossRef]
- Zhou, Q.G.; Liu, M.Y.; Lee, H.W.; Ishikawa, F.; Devkota, S.; Shen, X.R.; Jin, X.; Wu, H.Y.; Liu, Z.; Liu, X.; et al. Hippocampal TERT regulates spatial memory formation through modulation of neural development. Stem Cell Rep. 2017, 9, 543–556. [Google Scholar] [CrossRef]
- Richardson, R.M.; Nguyen, B.; Holt, S.E.; Broaddus, W.C.; Fillmore, H.L. Ectopic telomerase expression inhibits neuronal differentiation of NT2 neural progenitor cells. Neurosci. Lett. 2007, 421, 168–172. [Google Scholar] [CrossRef]
- Spilsbury, A.; Miwa, S.; Attems, J.; Saretzki, G. The role of telomerase protein TERT in Alzheimer’s disease and in tau-related pathology in vitro. J. Neurosci. 2015, 35, 1659–1674. [Google Scholar] [CrossRef]
- Liu, M.Y.; Nemes, A.; Zhou, Q.G. The emerging roles for telomerase in the central nervous system. Front. Mol. Neurosci. 2018, 11, 160. [Google Scholar] [CrossRef]
- Caporaso, G.L.; Lim, D.A.; Alvarez-Buylla, A.; Chao, M.V. Telomerase activity in the subventricular zone of adult mice. Mol. Cell Neurosci. 2003, 23, 693–702. [Google Scholar] [CrossRef]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Cameron, H.A.; McKay, R.D. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001, 435, 406–417. [Google Scholar] [CrossRef]
- Augusto-Oliveira, M.; Arrifano, G.P.F.; Malva, J.O.; Crespo-Lopez, M.E. Adult hippocampal neurogenesis in different taxonomic groups: Possible functional similarities and striking controversies. Cells 2019, 8, 125. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Takubo, K.; Izumiyama-Shimomura, N.; Honma, N.; Sawabe, M.; Arai, T.; Kato, M.; Oshimura, M.; Nakamura, K. Telomere lengths are characteristic in each human individual. Exp. Gerontol. 2002, 37, 523–531. [Google Scholar] [CrossRef]
- Anitha, A.; Thanseem, I.; Vasu, M.M.; Viswambharan, V.; Poovathinal, S.A. Telomeres in neurological disorders. Adv. Clin. Chem. 2019, 90, 81–132. [Google Scholar] [CrossRef] [PubMed]
- Flores, I.; Canela, A.; Vera, E.; Tejera, A.; Cotsarelis, G.; Blasco, M.A. The longest telomeres: A general signature of adult stem cell compartments. Genes Dev. 2008, 22, 654–667. [Google Scholar] [CrossRef]
- Buckingham, E.M.; Klingelhutz, A.J. The role of telomeres in the ageing of human skin. Exp. Dermatol. 2011, 20, 297–302. [Google Scholar] [CrossRef]
- Orioli, D. Dellambra, E. Epigenetic regulation of skin cells in natural aging and premature aging diseases. Cells 2018, 7, 268. [Google Scholar] [CrossRef]
- Enzo, E.; Secone Seconetti, A.; Forcato, M.; Tenedini, E.; Polito, M.P.; Sala, I.; Carulli, S.; Contin, R.; Peano, C.; Tagliafico, E.; et al. Single-keratinocyte transcriptomic analyses identify different clonal types and proliferative potential mediated by FOXM1 in human epidermal stem cells. Nat. Commun. 2021, 12, 2505. [Google Scholar] [CrossRef]
- Sarin, K.Y.; Cheung, P.; Gilison, D.; Lee, E.; Tennen, R.I.; Wang, E.; Artandi, M.K.; Oro, A.E.; Artandi, S.E. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature 2005, 436, 1048–1052. [Google Scholar] [CrossRef]
- Vulliamy, T.; Marrone, A.; Szydlo, R.; Walne, A.; Mason, P.J.; Dokal, I. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nat. Genet. 2004, 36, 447–449. [Google Scholar] [CrossRef] [Green Version]
- Gourronc, F.A.; Robertson, M.M.; Herrig, A.K.; Lansdorp, P.M.; Goldman, F.D.; Klingelhutz, A.J. Proliferative defects in dyskeratosis congenita skin keratinocytes are corrected by expression of the telomerase reverse transcriptase, TERT, or by activation of endogenous telomerase through expression of papillomavirus E6/E7 or the telomerase RNA component, TERC. Exp. Dermatol. 2010, 19, 279–288. [Google Scholar] [CrossRef]
- Ibrahim, B.; Sheerin, A.N.; Jennert-Burston, K.; Bird, J.L.; Massala, M.V.; Illsley, M.; James, S.E.; Faragher, R.G. Absence of premature senescence in Werner’s syndrome keratinocytes. Exp. Gerontol. 2016, 83, 139–147. [Google Scholar] [CrossRef]
- Buckingham, E.M.; Goldman, F.D.; Klingelhutz, A.J. Dyskeratosis congenita dermal fibroblasts are defective in supporting the clonogenic growth of epidermal keratinocytes. Aging Dis. 2012, 3, 427–437. [Google Scholar]
- Liu, N.; Yin, Y.; Wang, H.; Zhou, Z.; Sheng, X.; Fu, H.; Guo, R.; Wang, H.; Yang, J.; Gong, P.; et al. Telomere dysfunction impairs epidermal stem cell specification and differentiation by disrupting BMP/pSmad/P63 signaling. PLoS Genet. 2019, 15, e1008368. [Google Scholar] [CrossRef]
- Montgomery, R.K.; Carlone, D.L.; Richmond, C.A.; Farilla, L.; Kranendonk, M.E.; Henderson, D.E.; Baffour-Awuah, N.Y.; Ambruzs, D.M.; Fogli, L.K.; Algra, S.; et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc. Natl. Acad. Sci. USA 2011, 108, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Carlone, D.L.; Breault, D.T. Slowly cycling versus rapidly cycling intestinal stem cells: Distinct roles or redundancy. Cell Cycle 2011, 10, 723–724. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The intestinal epithelium: Central coordinator of mucosal immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef]
- Clevers, H. The intestinal crypt, a prototype stem cell compartment. Cell 2013, 154, 274–284. [Google Scholar] [CrossRef]
- Hiyama, E.; Tatsumoto, N.; Kodama, T.; Hiyama, K.; Shay, J.; Yokoyama, T. Telomerase activity in human intestine. Int. J. Oncol. 1996, 9, 453–458. [Google Scholar] [CrossRef]
- Schepers, A.G.; Vries, R.; van den Born, M.; van de Wetering, M.; Clevers, H. Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO J. 2011, 30, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Pasztoi, M.; Ohnmacht, C. Tissue niches formed by intestinal mesenchymal stromal cells in mucosal homeostasis and immunity. Int. J. Mol. Sci. 2022, 23, 5181. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Chakrabarti, P.; Sen, S.; Barui, A. Reactive oxygen species in modulating intestinal stem cell dynamics and function. Stem Cell Rev. Rep. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Flores-Gonzalez, J.; Buendia-Roldan, I.; Chavez-Galan, L. Telomere shortening and its association with cell dysfunction in lung diseases. Int. J. Mol. Sci. 2021, 23, 425. [Google Scholar] [CrossRef]
- Kathiriya, J.J.; Brumwell, A.N.; Jackson, J.R.; Tang, X.; Chapman, H.A. Distinct airway epithelial stem cells hide among club cells but mobilize to promote alveolar regeneration. Cell Stem Cell 2020, 26, 346–358.e4. [Google Scholar] [CrossRef]
- Rao, W.; Wang, S.; Duleba, M.; Niroula, S.; Goller, K.; Xie, J.; Mahalingam, R.; Neupane, R.; Liew, A.A.; Vincent, M.; et al. Regenerative metaplastic clones in COPD lung drive inflammation and fibrosis. Cell 2020, 181, 848–864.e18. [Google Scholar] [CrossRef]
- Hong, X.; Wang, L.; Zhang, K.; Liu, J.; Liu, J.P. Molecular mechanisms of alveolar epithelial stem cell senescence and senescence-associated differentiation disorders in pulmonary fibrosis. Cells 2022, 11, 877. [Google Scholar] [CrossRef]
- Wang, L.; Chen, R.; Li, G.; Wang, Z.; Liu, J.; Liang, Y.; Liu, J.P. FBW7 mediates senescence and pulmonary fibrosis through telomere uncapping. Cell Metab. 2020, 32, 860–877.e9. [Google Scholar] [CrossRef]
- Lee, S.; Yu, Y.; Trimpert, J.; Benthani, F.; Mairhofer, M.; Richter-Pechanska, P.; Wyler, E.; Belenki, D.; Kaltenbrunner, S.; Pammer, M.; et al. Virus-induced senescence is driver and therapeutic target in COVID-19. Nature 2021, 599, 283–289. [Google Scholar] [CrossRef]
- Saito, S.; Ku, C.C.; Wuputra, K.; Pan, J.B.; Lin, C.S.; Lin, Y.C.; Wu, D.C.; Yokoyama, K.K. Biomarkers of Cancer Stem Cells for Experimental Research and Clinical Application. J. Pers. Med. 2022, 12, 715. [Google Scholar] [CrossRef]
- Harman, D. Free radical theory of aging. Mutat. Res. 1992, 275, 257–266. [Google Scholar] [CrossRef]
- Vaziri, H.; Dragowska, W.; Allsopp, R.C.; Thomas, T.E.; Harley, C.B.; Lansdorp, P.M. Evidence for a mitotic clock in human hematopoietic stem cells: Loss of telomeric DNA with age. Proc. Natl. Acad. Sci. USA 1994, 91, 9857–9860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada, J.C.; Albo, C.; Benguría, A.; Dopazo, A.; López-Romero, P.; Carrera-Quintanar, L.; Roche, E.; Clemente, E.P.; Enríquez, J.A.; Bernad, A.; et al. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death Differ. 2012, 19, 743–755. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Yoo, S.Y.; Hur, J.; Kim, H.S.; Kwon, S.M. Hypoxia inhibits cellular senescence to restore the therapeutic potential of old human endothelial progenitor cells via the hypoxia-inducible factor-1α-TWIST-p21 axis. Arter. Thromb. Vasc. Biol. 2013, 33, 2407–2414. [Google Scholar] [CrossRef]
- Kawasaki, H.; Guan, J.; Tamama, K. Hydrogen gas treatment prolongs replicative lifespan of bone marrow multipotential stromal cells in vitro while preserving differentiation and paracrine potentials. Biochem. Biophys. Res. Commun. 2010, 397, 608–613. [Google Scholar] [CrossRef]
- Li, J.; Hansen, K.C.; Zhang, Y.; Dong, C.; Dinu, C.Z.; Dzieciatkowska, M.; Pei, M. Rejuvenation of chondrogenic potential in a young stem cell microenvironment. Biomaterials 2014, 35, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Yanada, S.; Ochi, M.; Kojima, K.; Sharman, P.; Yasunaga, Y.; Hiyama, E. Possibility of selection of chondrogenic progenitor cells by telomere length in FGF-2-expanded mesenchymal stromal cells. Cell Prolif. 2006, 39, 575–584. [Google Scholar] [CrossRef]
- Pirmoradi, S.; Fathi, E.; Farahzadi, R.; Pilehvar-Soltanahmadi, Y.; Zarghami, N. Curcumin affects adipose tissue-derived mesenchymal stem cell aging through tert gene expression. Drug Res. 2018, 68, 213–221. [Google Scholar] [CrossRef]
- Bodnar, A.G.; Ouellette, M.; Frolkis, M.; Holt, S.E.; Chiu, C.P.; Morin, G.B.; Harley, C.B.; Shay, J.W.; Lichtsteiner, S.; Wright, W.E. Extension of life-span by introduction of telomerase into normal human cells. Science 1998, 279, 349–352. [Google Scholar] [CrossRef]
- Yegorov, Y.E.; Moldaver, M.V.; Vishnyakova, K.S.; Chumakov, P.M.; Zelenin, A.V.; Terekhov, S.M.; Dashinimaev, E.B.; Burnaevskii, N.S.; Cheglakov, I.B.; Toropygin, I.Y.; et al. Enhanced control of proliferation in telomerized cells. Russ. J. Dev. Biol. 2007, 38, 76–89. [Google Scholar] [CrossRef]
- Gong, M.; Bi, Y.; Jiang, W.; Zhang, Y.; Chen, L.; Hou, N.; Liu, Y.; Wei, X.; Chen, J.; Li, T. Immortalized mesenchymal stem cells: An alternative to primary mesenchymal stem cells in neuronal differentiation and neuroregeneration associated studies. J. Biomed. Sci. 2011, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Tolmachov, O.E. Transgenic DNA modules with pre-programmed self-destruction: Universal molecular devices to escape ‘genetic litter’ in gene and cell therapy. Med. Hypotheses 2015, 85, 686–689. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupatov, A.Y.; Yarygin, K.N. Telomeres and Telomerase in the Control of Stem Cells. Biomedicines 2022, 10, 2335. https://doi.org/10.3390/biomedicines10102335
Lupatov AY, Yarygin KN. Telomeres and Telomerase in the Control of Stem Cells. Biomedicines. 2022; 10(10):2335. https://doi.org/10.3390/biomedicines10102335
Chicago/Turabian StyleLupatov, Alexey Yu., and Konstantin N. Yarygin. 2022. "Telomeres and Telomerase in the Control of Stem Cells" Biomedicines 10, no. 10: 2335. https://doi.org/10.3390/biomedicines10102335
APA StyleLupatov, A. Y., & Yarygin, K. N. (2022). Telomeres and Telomerase in the Control of Stem Cells. Biomedicines, 10(10), 2335. https://doi.org/10.3390/biomedicines10102335





