Antioxidants as Therapeutic Agents in Acute Respiratory Distress Syndrome (ARDS) Treatment—From Mice to Men
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Models of ARDS/ALI
| Direct Lung Damage [17,22,23] | Route of Application | ARDS-Like Affects | Antioxidant Approaches Already Used | Ref. |
|---|---|---|---|---|
| LPS [24,25,26] | intranasal/intratracheal instillation | lung accumulation of neutrophils, induction of proinflammatory cytokines | NAC, SAMC | [27,28,29] |
| Bacteria [30,31,32] | intratracheal instillation | lung accumulation of neutrophils, induction of proinflammatory cytokines | CDC | [33] |
| HCl [34,35,36] | intratracheal instillation | neutrophil infiltration, damage of alveolar/ vascular barrier | apocynin, MitoTempo | [37,38] |
| Hyperoxia (HALI) [39,40,41] | intratracheal | damage of epithelial cells, neutrophil infiltration | AA, BNF, SFN, MnSOD | [42,43,44] |
| MV (VILI) [45,46,47,48] | intratracheal | inflammasome-mediated proinflammatory cytokine expression | NAC, Nrf2+/+, Nrf2−/−, PIP-2; PC-SOD | [49,50,51,52] |
| Bleomycin [53,54,55] | intratracheal instillation | invertible fibrosis | BRNPs, adelmidrol, EC-SOD | [56,57,58] |
| Pulmonary ischemia/reperfusion [59,60,61] | surgery; mesenteric artery clamping or hilar ligation and reperfusion | neutrophil infiltration, damage of alveolar/ vascular barrier | irisin | [60] |
| Indirect lung damage [17,22,23] | ||||
| Sepsis (live bacteria, CASP, CLP, CSI) [62,63,64,65,66,67] | i.p.¸ peritonitis | damage of alveolar/ vascular barrier | PC-SOD, SOD mimetic, Prdx6−/− | [51,68,69] |
| Endotoxemia [70,71,72] | i.v. or i.p. | damage of alveolar/ vascular barrier | NAC, EUK-8, CypD | [73,74,75] |
| Oleic acid [70,76,77] | i.v. | mimics fat embolism | BAY 60-6583, leptin | [76,78] |
| Multiple transfusions (TRALI) [79,80,81,82] | i.v.; syngeneic or allogenic | acute onset; underlying a 2-hit onset, pulmonary neutrophil sequestration, involvement of MΦ | MΦ depletion, C3−/−, C5−/−, C5aR−/− | [83,84,85] |
| Multiple trauma [86,87,88] | externally received | neutrophil infiltration, complement activation | p47phox−/− | [89] |
| H2O2 [90,91,92] | i.v. | increased vascular permeability and fluid retention, edema formation | AA, TP | [93] |
| Nonpulmonary ischemia/reperfusion [94,95,96] | surgery; liver, gut, kidney | neutrophil sequestration, acceleration of microvascular permeability | CypDPlt−/−, SB239063, FK866, LY333531 | [97,98] |
| Two-hit models | ||||
| LPS + MV [99,100,101] | intratracheal, i.v., i.p. | inflammasome-dependent | ATF3 OE/KD; HIF1α−/−, enoxaparin, DJ-1, paracoxib | [102,103,104,105] |
| Sepsis + MV [106,107,108] | i.p., peritonitis, intratracheal | augment sepsis-mediated organ damage | AM | [109] |
| HCl + MV [100,110,111,112] | intratracheal | enhanced HCl impact | IL-6−/− | [113] |
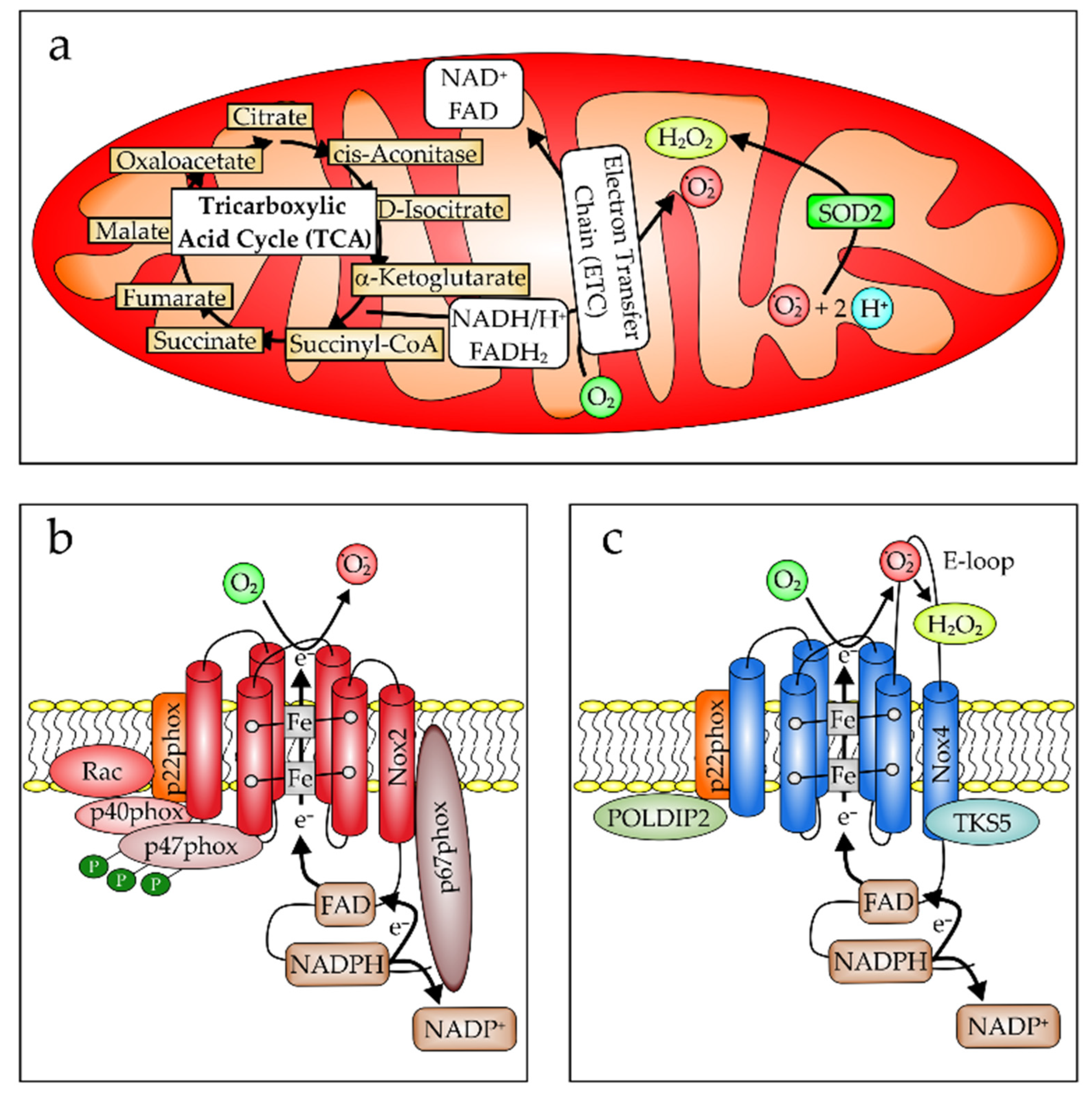
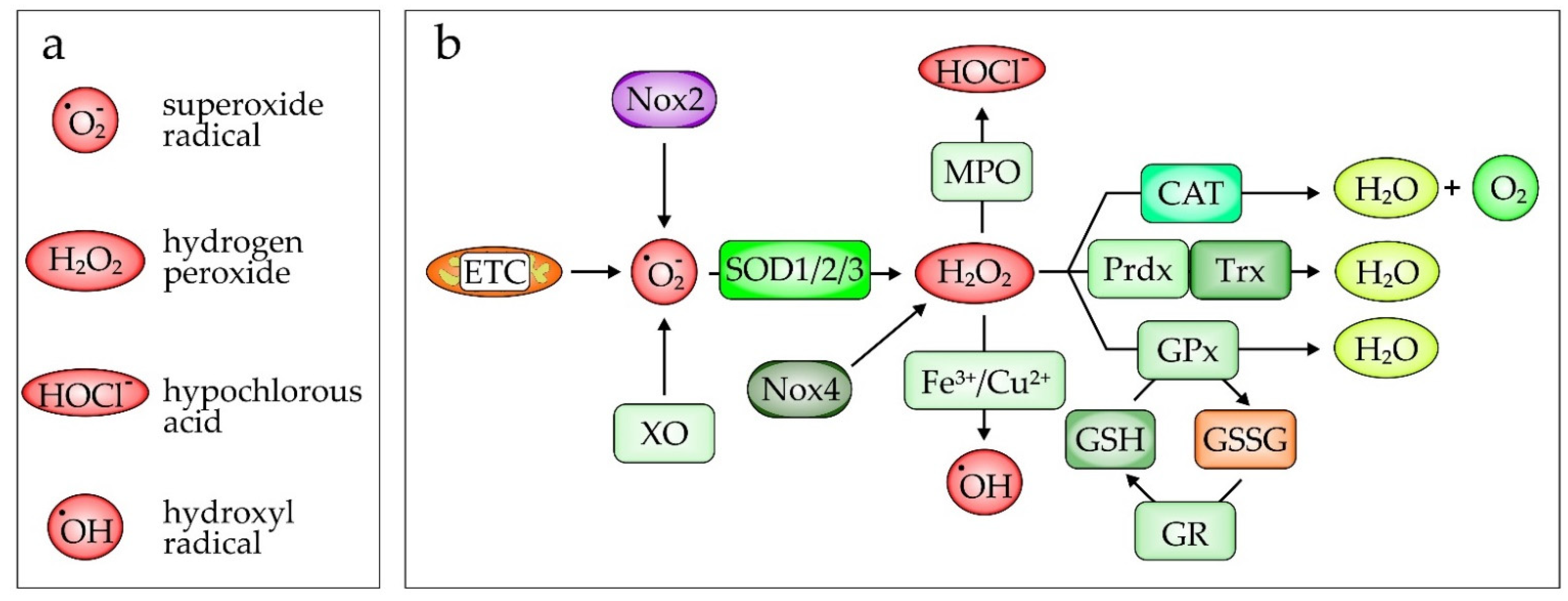
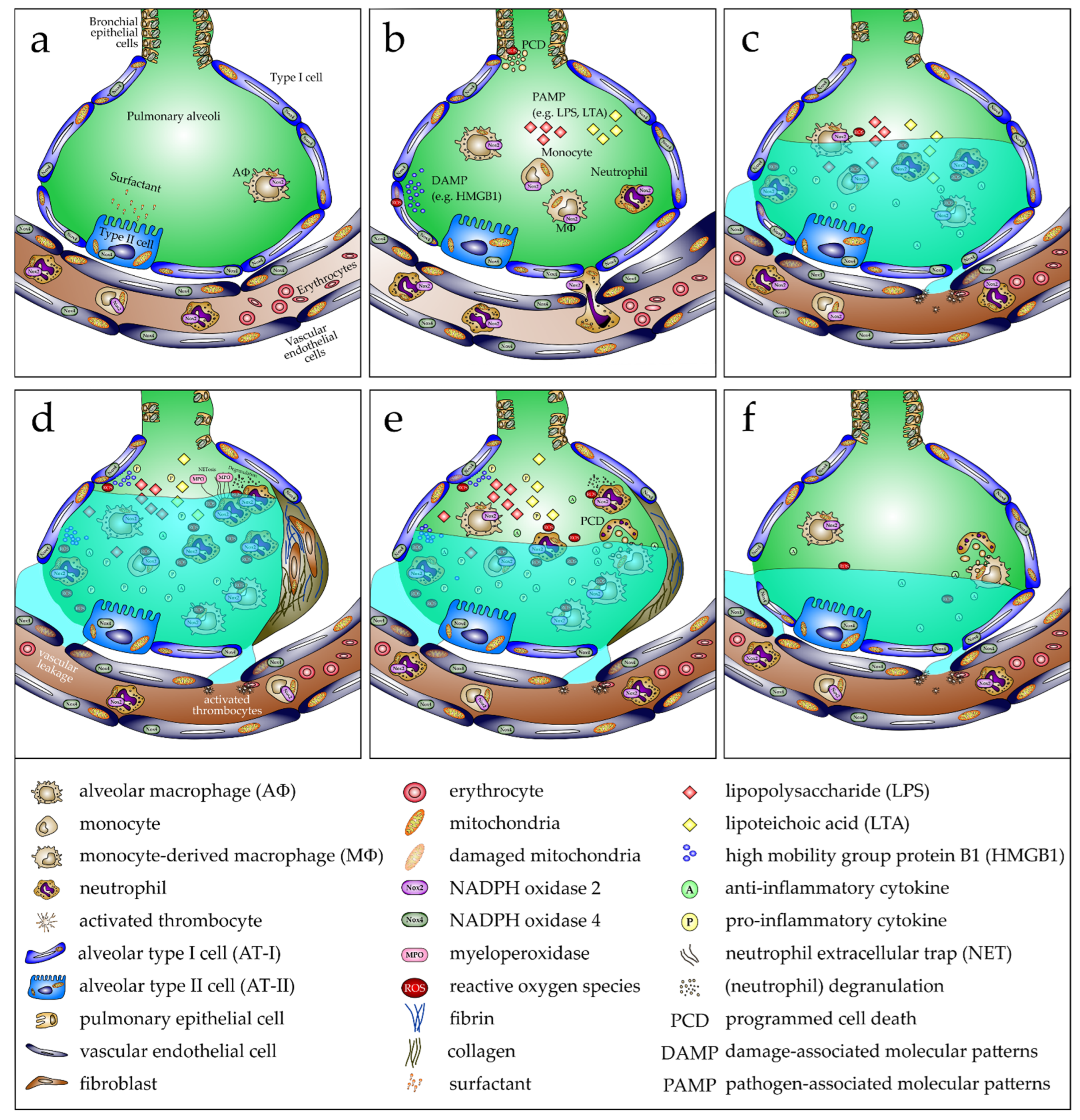
2.2. Oxidative Stress in Pathogenesis of ARDS/ALI
2.3. Antioxidative Treatments
2.3.1. Pharmacological Antioxidants
2.3.2. Nrf2
2.3.3. PPARγ
2.4. Mouse Data Translated to the ARDS Patients’ Situation
3. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashbaugh, D.G.; Bigelow, D.B.; Petty, T.L.; Levine, B.E. Acute respiratory distress syndrome in adults. Lancet 1967, 61, 319–323. [Google Scholar] [CrossRef]
- Bernard, G.R.; Artigas, A.; Brigham, K.L.; Carlet, J.; Falke, K.; Hudson, L.; Lamy, M.; LeGall, J.R.; Morris, A.; Spragg, R.; et al. Report of the American-European Consensus Conference on acute respiratory distress syndrome: Definitions, mechanisms, relevant outcomes, and clinical trial coordination. J. Crit. Care 1994, 9, 72–81. [Google Scholar] [CrossRef]
- Luterman, A.; Horovitz, J.H.; Carrico, C.J.; Canizaro, P.C.; Heimbach, D.; Colocousis, J. Withdrawal from positive end-expiratory pressure. Surgery 1978, 83, 328–332. [Google Scholar] [CrossRef]
- Esteban, A.; Fernández-Segoviano, P.; Frutos-Vivar, F.; Aramburu, J.A.; Nájera, L.; Ferguson, N.D.; Alía, I.; Gordo, F.; Ríos, F. Comparison of Clinical Criteria for the Acute Respiratory Distress Syndrome With Autopsy Findings. Ann. Intern. Med. 2004, 141, 440–445. [Google Scholar] [CrossRef]
- Ferguson, N.D.; Davis, A.M.; Slutsky, A.S.; Stewart, T.E. Development of a clinical definition for acute respiratory distress syndrome using the Delphi technique. J. Crit. Care 2005, 20, 147–154. [Google Scholar] [CrossRef]
- Raghavendran, K.; Napolitano, L.M. Definition of ALI/ARDS. Crit. Care Clin. 2011, 27, 429–437. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Riviello, E.D.; Kiviri, W.; Twagirumugabe, T.; Mueller, A.; Banner-Goodspeed, V.M.; Officer, L.; Novack, V.; Mutumwinka, M.; Talmor, D.S.; Fowler, R.A. Hospital Incidence and Outcomes of the Acute Respiratory Distress Syndrome Using the Kigali Modification of the Berlin Definition. Am. J. Respir. Crit. Care Med. 2016, 193, 52–59. [Google Scholar] [CrossRef]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef]
- Ficial, B.; Vasques, F.; Zhang, J.; Whebell, S.; Slattery, M.; Lamas, T.; Daly, K.; Agnew, N.; Camporota, L. Physiological Basis of Extracorporeal Membrane Oxygenation and Extracorporeal Carbon Dioxide Removal in Respiratory Failure. Membranes 2021, 11, 225. [Google Scholar] [CrossRef]
- Guérin, C.; Reignier, J.; Richard, J.-C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef]
- Cavalcanti, A.B.; Suzumura, É.A.; Laranjeira, L.N.; de Moraes Paisani, D.; Damiani, L.P.; Guimarães, H.P.; Romano, E.R.; de Moraes Regenga, M.; Taniguchi, L.N.T.; Teixeira, C.; et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2017, 318, 1335–1345. [Google Scholar] [CrossRef]
- Suzumura, E.A.; Amato, M.B.P.; Cavalcanti, A.B. Understanding recruitment maneuvers. Intensive Care Med. 2016, 42, 908–911. [Google Scholar] [CrossRef]
- Wei, X.-B.; Wang, Z.-H.; Liao, X.-L.; Guo, W.-X.; Qin, T.-H.; Wang, S.-H. Role of Neuromuscular Blocking Agents in Acute Respiratory Distress Syndrome: An Updated Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2019, 10, 1637. [Google Scholar] [CrossRef]
- Cui, Y.-Q.; Ding, X.-F.; Liang, H.-Y.; Wang, D.; Zhang, X.-J.; Li, L.-F.; Kan, Q.-C.; Wang, L.-X.; Sun, T.-W. Efficacy and safety of low-dose corticosteroids for acute respiratory distress syndrome: A systematic review and meta-analysis. World J. Emerg. Med. 2021, 12, 207–213. [Google Scholar] [CrossRef]
- Aeffner, F.; Bolon, B.; Davis, I.C. Mouse Models of Acute Respiratory Distress Syndrome: A Review of Analytical Approaches, Pathologic Features, and Common Measurements. Toxicol. Pathol. 2015, 43, 1074–1092. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef]
- Joelsson, J.P.; Ingthorsson, S.; Kricker, J.; Gudjonsson, T.; Karason, S. Ventilator-induced lung-injury in mouse models: Is there a trap? Lab. Anim. Res. 2021, 37, 30. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Q.; Liu, J.; Yang, X.; Zhang, Y.; Huang, F. Sinomenine protects against E. coli-induced acute lung injury in mice through Nrf2-NF-κB pathway. Biomed. Pharmacother. 2018, 107, 696–702. [Google Scholar] [CrossRef]
- El Kebir, D.; Gjorstrup, P.; Filep, J.G. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc. Natl. Acad. Sci. USA 2012, 109, 14983–14988. [Google Scholar] [CrossRef]
- Semple, J.W.; Rebetz, J.; Kapur, R. Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood 2019, 133, 1840–1853. [Google Scholar] [CrossRef]
- Chimenti, L.; Morales-Quinteros, L.; Puig, F.; Camprubi-Rimblas, M.; Guillamat-Prats, R.; Gómez, M.N.; Tijero, J.; Blanch, L.; Matute-Bello, G.; Artigas, A. Comparison of direct and indirect models of early induced acute lung injury. Intensive Care Med. Exp. 2020, 8, 62. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Frevert, C.W.; Martin, T.R. Animal models of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L379–L399. [Google Scholar] [CrossRef]
- Ribeiro, A.P.; Mayer, C.A.; Wilson, C.G.; Martin, R.J.; MacFarlane, P.M. Intratracheal LPS administration attenuates the acute hypoxic ventilatory response: Role of brainstem IL-1β receptors. Respir. Physiol. Neurobiol. 2017, 242, 45–51. [Google Scholar] [CrossRef]
- Peritore, A.F.; D’amico, R.; Siracusa, R.; Cordaro, M.; Fusco, R.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Di Paola, R.; Cuzzocrea, S.; et al. Management of Acute Lung Injury: Palmitoylethanolamide as a New Approach. Int. J. Mol. Sci. 2021, 22, 5533. [Google Scholar] [CrossRef]
- Khadangi, F.; Forgues, A.-S.; Tremblay-Pitre, S.; Dufour-Mailhot, A.; Henry, C.; Boucher, M.; Beaulieu, M.-J.; Morissette, M.; Fereydoonzad, L.; Brunet, D.; et al. Intranasal versus intratracheal exposure to lipopolysaccharides in a murine model of acute respiratory distress syndrome. Sci. Rep. 2021, 11, 7777. [Google Scholar] [CrossRef]
- Song, Q.; Lin, L.; Chen, L.; Cheng, L.; Zhong, W. Co-administration of N-acetylcysteine and dexmedetomidine plays a synergistic effect on protection of LPS-induced acute lung injury via correcting Th1/Th2/Th17 cytokines imbalance. Clin. Exp. Pharmacol. Physiol. 2020, 47, 294–301. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Liu, R.; Qian, T.; Liu, J.; Wang, L.; Chu, Y. Reactive oxygen species stimulated pulmonary epithelial cells mediate the alveolar recruitment of FasL+ killer B cells in LPS-induced acute lung injuries. J. Leukoc. Biol. 2018, 104, 1187–1198. [Google Scholar] [CrossRef]
- Mo, M.; Li, S.; Dong, Z.; Li, C.; Sun, Y.; Li, A.; Zhao, Z. S-allylmercaptocysteine ameliorates lipopolysaccharide-induced acute lung injury in mice by inhibiting inflammation and oxidative stress via nuclear factor kappa B and Keap1/Nrf2 pathways. Int. Immunopharmacol. 2020, 81, 106273. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Fusco, R.; Ginestra, G.; D’amico, R.; Bisignano, C.; Mandalari, G.; Cuzzocrea, S.; Di Paola, R. Involvement of TLR4 and PPAR-α Receptors in Host Response and NLRP3 Inflammasome Activation, Against Pulmonary Infection With Pseudomonas Aeruginosa. Shock 2019, 51, 221–227. [Google Scholar] [CrossRef]
- Aoyagi, T.; Newstead, M.W.; Zeng, X.; Nanjo, Y.; Peters-Golden, M.; Kaku, M.; Standiford, T.J. Interleukin-36γ and IL-36 receptor signaling mediate impaired host immunity and lung injury in cytotoxic Pseudomonas aeruginosa pulmonary infection: Role of prostaglandin E2. PLoS Pathog. 2017, 13, e1006737. [Google Scholar] [CrossRef]
- McHugh, W.M.; Russell, W.W.; Fleszar, A.J.; Rodenhouse, P.E.; Rietberg, S.P.; Sun, L.; Shanley, T.P.; Cornell, T.T. Protein phosphatase 2A activation attenuates inflammation in murine models of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L903–L912. [Google Scholar] [CrossRef]
- Zhang, B.; Swamy, S.; Balijepalli, S.; Panicker, S.; Mooliyil, J.; Sherman, M.A.; Parkkinen, J.; Raghavendran, K.; Suresh, M.V. Direct pulmonary delivery of solubilized curcumin reduces severity of lethal pneumonia. FASEB J. 2019, 33, 13294–13309. [Google Scholar] [CrossRef]
- Hashimoto, S.; Amaya, F.; Oh-Hashi, K.; Kiuchi, K.; Hashimoto, S. Expression of neutral endopeptidase activity during clinical and experimental acute lung injury. Respir. Res. 2010, 11, 164. [Google Scholar] [CrossRef]
- Aoki-Nagase, T.; Nagase, T.; Oh-Hashi, Y.; Kurihara, Y.; Yamaguchi, Y.; Yamamoto, H.; Nagata, T.; Kurihara, H.; Ouchi, Y. Calcitonin gene-related peptide mediates acid-induced lung injury in mice. Respirology 2007, 12, 807–813. [Google Scholar] [CrossRef]
- Zarbock, A.; Schmolke, M.; Spieker, T.; Jurk, K.; van Aken, H.; Singbartl, K. Acute uremia but not renal inflammation attenuates aseptic acute lung injury: A critical role for uremic neutrophils. J. Am. Soc. Nephrol. 2006, 17, 3124–3131. [Google Scholar] [CrossRef]
- Puri, G.; Naura, A.S. Critical role of mitochondrial oxidative stress in acid aspiration induced ALI in mice. Toxicol. Mech. Methods 2020, 30, 266–274. [Google Scholar] [CrossRef]
- Yuan, Q.; Basit, A.; Liang, W.; Qu, R.; Luan, Y.; Ren, C.; Li, A.; Xu, X.; Liu, X.; Yang, C.; et al. Pazopanib ameliorates acute lung injuries via inhibition of MAP3K2 and MAP3K3. Sci. Transl. Med. 2021, 13, eabc2499. [Google Scholar] [CrossRef]
- Bailey, T.C.; Martin, E.L.; Zhao, L.; Veldhuizen, R.A.W. High oxygen concentrations predispose mouse lungs to the deleterious effects of high stretch ventilation. J. Appl. Physiol. (1985) 2003, 94, 975–982. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Yanagihara, T.; Yokoyama, T.; Suetsugu-Ogata, S.; Hamada, N.; Harada-Ikeda, C.; Suzuki, K.; Maeyama, T.; Kuwano, K.; Nakanishi, Y. Probucol attenuates hyperoxia-induced lung injury in mice. PLoS ONE 2017, 12, e0175129. [Google Scholar] [CrossRef]
- Shah, D.; Das, P.; Acharya, S.; Agarwal, B.; Christensen, D.J.; Robertson, S.M.; Bhandari, V. Small Immunomodulatory Molecules as Potential Therapeutics in Experimental Murine Models of Acute Lung Injury (ALI)/Acute Respiratory Distress Syndrome (ARDS). Int. J. Mol. Sci. 2021, 22, 2573. [Google Scholar] [CrossRef] [PubMed]
- Callaway, D.A.; Jiang, W.; Wang, L.; Lingappan, K.; Moorthy, B. Oxygen-mediated lung injury in mice lacking the gene for NRF2: Rescue with the cytochrome P4501A-inducer, beta-naphthoflavone (BNF), and differential sex-specific effects. Free Radic. Biol. Med. 2020, 160, 208–218. [Google Scholar] [CrossRef]
- Patel, V.; Dial, K.; Wu, J.; Gauthier, A.G.; Wu, W.; Lin, M.; Espey, M.G.; Thomas, D.D.; Ashby, C.R.; Mantell, L.L. Dietary Antioxidants Significantly Attenuate Hyperoxia-Induced Acute Inflammatory Lung Injury by Enhancing Macrophage Function via Reducing the Accumulation of Airway HMGB1. Int. J. Mol. Sci. 2020, 21, 977. [Google Scholar] [CrossRef]
- Tian, Y.G.; Zhang, J. Protective effect of SIRT3 on acute lung injury by increasing manganese superoxide dismutase-mediated antioxidation. Mol. Med. Rep. 2018, 17, 5557–5565. [Google Scholar] [CrossRef] [PubMed]
- Szabari, M.V.; Takahashi, K.; Feng, Y.; Locascio, J.J.; Chao, W.; Carter, E.A.; Vidal Melo, M.F.; Musch, G. Relation between Respiratory Mechanics, Inflammation, and Survival in Experimental Mechanical Ventilation. Am. J. Respir. Cell Mol. Biol. 2019, 60, 179–188. [Google Scholar] [CrossRef]
- Wilson, M.R.; Patel, B.V.; Takata, M. Ventilation with “clinically relevant” high tidal volumes does not promote stretch-induced injury in the lungs of healthy mice. Crit. Care Med. 2012, 40, 2850–2857. [Google Scholar] [CrossRef] [PubMed]
- Lex, D.; Uhlig, S. One-hit models of ventilator-induced lung injury-benign inflammation versus inflammation as a by-product. Anesthesiology 2017, 126, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Dolinay, T.; Kim, Y.S.; Howrylak, J.; Hunninghake, G.M.; An, C.H.; Fredenburgh, L.; Massaro, A.F.; Rogers, A.; Gazourian, L.; Nakahira, K.; et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am. J. Respir. Crit. Care Med. 2012, 185, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Papaiahgari, S.; Yerrapureddy, A.; Reddy, S.R.; Reddy, N.M.; Dodd-O, J.M.; Crow, M.T.; Grigoryev, D.N.; Barnes, K.; Tuder, R.M.; Yamamoto, M.; et al. Genetic and pharmacologic evidence links oxidative stress to ventilator-induced lung injury in mice. Am. J. Respir. Crit. Care Med. 2007, 176, 1222–1235. [Google Scholar] [CrossRef]
- Fisher, A.B.; Dodia, C.; Chatterjee, S. A Peptide Inhibitor of Peroxiredoxin 6 Phospholipase A2 Activity Significantly Protects against Lung Injury in a Mouse Model of Ventilator Induced Lung Injury (VILI). Antioxidants 2021, 10, 925. [Google Scholar] [CrossRef]
- Tanaka, K.-I.; Tamura, F.; Sugizaki, T.; Kawahara, M.; Kuba, K.; Imai, Y.; Mizushima, T. Evaluation of Lecithinized Superoxide Dismutase for the Prevention of Acute Respiratory Distress Syndrome in Animal Models. Am. J. Respir. Cell Mol. Biol. 2017, 56, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Veskemaa, L.; Graw, J.A.; Pickerodt, P.A.; Taher, M.; Boemke, W.; González-López, A.; Francis, R.C.E. Tert-butylhydroquinone augments Nrf2-dependent resilience against oxidative stress and improves survival of ventilator-induced lung injury in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L17–L28. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hult, E.M.; Cornell, T.T.; Kim, K.K.; Shanley, T.P.; Wilke, C.A.; Agarwal, M.; Gurczynski, S.J.; Moore, B.B.; Dahmer, M.K. Loss of myeloid-specific protein phosphatase 2A enhances lung injury and fibrosis and results in IL-10-dependent sensitization of epithelial cell apoptosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L1035–L1048. [Google Scholar] [CrossRef]
- Shaver, C.M.; Grove, B.S.; Clune, J.K.; Mackman, N.; Ware, L.B.; Bastarache, J.A. Myeloid tissue factor does not modulate lung inflammation or permeability during experimental acute lung injury. Sci. Rep. 2016, 6, 22249. [Google Scholar] [CrossRef] [PubMed]
- LaRivière, W.B.; Liao, S.; McMurtry, S.A.; Oshima, K.; Han, X.; Zhang, F.; Yan, S.; Haeger, S.M.; Ransom, M.; Bastarache, J.A.; et al. Alveolar heparan sulfate shedding impedes recovery from bleomycin-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L1198–L1210. [Google Scholar] [CrossRef]
- Keum, H.; Kim, D.; Kim, J.; Kim, T.W.; Whang, C.-H.; Jung, W.; Jon, S. A bilirubin-derived nanomedicine attenuates the pathological cascade of pulmonary fibrosis. Biomaterials 2021, 275, 120986. [Google Scholar] [CrossRef]
- Fusco, R.; Cordaro, M.; Genovese, T.; Impellizzeri, D.; Siracusa, R.; Gugliandolo, E.; Peritore, A.F.; D’amico, R.; Crupi, R.; Cuzzocrea, S.; et al. Adelmidrol: A New Promising Antioxidant and Anti-Inflammatory Therapeutic Tool in Pulmonary Fibrosis. Antioxidants 2020, 9, 601. [Google Scholar] [CrossRef]
- Allawzi, A.; McDermott, I.; Delaney, C.; Nguyen, K.; Banimostafa, L.; Trumpie, A.; Hernandez-Lagunas, L.; Riemondy, K.; Gillen, A.; Hesselberth, J.; et al. Redistribution of EC-SOD resolves bleomycin-induced inflammation via increased apoptosis of recruited alveolar macrophages. FASEB J. 2019, 33, 13465–13475. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Huang, W.; Wu, F.; Shang, J.; Ping, F.; Wang, W.; Li, Y.; Zhao, X.; Zhang, X. ZFP36 protects lungs from intestinal I/R-induced injury and fibrosis through the CREBBP/p53/p21/Bax pathway. Cell Death Dis. 2021, 12, 685. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xu, Z.; Liu, Y.; Wang, Z.; Li, Y.; Xu, X.; Chen, C.; Xia, T.; Liao, Q.; Yao, Y.; et al. Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci. Transl. Med. 2017, 9, eaao6298. [Google Scholar] [CrossRef]
- Dodd-o, J.M.; Hristopoulos, M.L.; Kibler, K.; Gutkowska, J.; Mukaddam-Daher, S.; Gonzalez, A.; Welsh-Servinsky, L.E.; Pearse, D.B. The role of natriuretic peptide receptor-A signaling in unilateral lung ischemia-reperfusion injury in the intact mouse. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L714–L723. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Hong, Z.; Huang, L.S.; Tsukasaki, Y.; Nepal, S.; Di, A.; Zhong, M.; Wu, W.; Ye, Z.; Gao, X.; et al. IL-1β suppression of VE-cadherin transcription underlies sepsis-induced inflammatory lung injury. J. Clin. Investig. 2020, 130, 3684–3698. [Google Scholar] [CrossRef]
- Zou, Y.; Bao, S.; Wang, F.; Guo, L.; Zhu, J.; Wang, J.; Deng, X.; Li, J. FN14 Blockade on Pulmonary Microvascular Endothelial Cells Improves the Outcome of Sepsis-Induced Acute Lung Injury. Shock 2018, 49, 213–220. [Google Scholar] [CrossRef]
- Murando, F.; Peloso, A.; Cobianchi, L. Experimental Abdominal Sepsis: Sticking to an Awkward but Still Useful Translational Model. Mediat. Inflamm. 2019, 2019, 8971036. [Google Scholar] [CrossRef]
- Fallon, E.A.; Chung, C.-S.; Heffernan, D.S.; Chen, Y.; de Paepe, M.E.; Ayala, A. Survival and Pulmonary Injury After Neonatal Sepsis: PD1/PDL1’s Contributions to Mouse and Human Immunopathology. Front. Immunol. 2021, 12, 634529. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, S.M.; Dumasius, A.; Easington, C.; Colilla, S.A.; Neumann, A.; Parrillo, J.E. Characterization of a Hyperdynamic Murine Model of Resuscitated Sepsis Using Echocardiography. Am. J. Respir. Crit. Care Med. 2001, 164, 891–895. [Google Scholar] [CrossRef]
- Neumann, B.; Zantl, N.; Veihelmann, A.; Emmanuilidis, K.; Pfeffer, K.; Heidecke, C.D.; Holzmann, B. Mechanisms of acute inflammatory lung injury induced by abdominal sepsis. Int. Immunol. 1999, 11, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Constantino, L.; Gonçalves, R.C.; Giombelli, V.R.; Tomasi, C.D.; Vuolo, F.; Kist, L.W.; de Oliveira, G.M.T.; de Bittencourt Pasquali, M.A.; Bogo, M.R.; Mauad, T.; et al. Regulation of lung oxidative damage by endogenous superoxide dismutase in sepsis. Intensive Care Med. Exp. 2014, 2, 17. [Google Scholar] [CrossRef]
- Wang, X.; An, X.; Wang, X.; Hu, X.; Bi, J.; Tong, L.; Yang, D.; Song, Y.; Bai, C. Peroxiredoxin 6 knockout aggravates cecal ligation and puncture-induced acute lung injury. Int. Immunopharmacol. 2019, 68, 252–258. [Google Scholar] [CrossRef]
- Zhou, Z.; Kozlowski, J.; Schuster, D.P. Physiologic, biochemical, and imaging characterization of acute lung injury in mice. Am. J. Respir. Crit. Care Med. 2005, 172, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Tesse, A.; Gena, P.; Rützler, M.; Calamita, G. Ablation of Aquaporin-9 Ameliorates the Systemic Inflammatory Response of LPS-Induced Endotoxic Shock in Mouse. Cells 2021, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- Colón, D.F.; Wanderley, C.W.; Franchin, M.; Silva, C.M.; Hiroki, C.H.; Castanheira, F.V.S.; Donate, P.B.; Lopes, A.H.; Volpon, L.C.; Kavaguti, S.K.; et al. Neutrophil extracellular traps (NETs) exacerbate severity of infant sepsis. Crit. Care 2019, 23, 113. [Google Scholar] [CrossRef]
- Baboolal, H.A.; Ichinose, F.; Ullrich, R.; Kawai, N.; Bloch, K.D.; Zapol, W.M. Reactive oxygen species scavengers attenuate endotoxin-induced impairment of hypoxic pulmonary vasoconstriction in mice. Anesthesiology 2002, 97, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Jones, D.P.; Brigham, K.L.; Rojas, M. Oxidation of plasma cysteine/cystine redox state in endotoxin-induced lung injury. Am. J. Respir. Cell Mol. Biol. 2009, 40, 90–98. [Google Scholar] [CrossRef]
- Fonai, F.; Priber, J.K.; Jakus, P.B.; Kalman, N.; Antus, C.; Pollak, E.; Karsai, G.; Tretter, L.; Sumegi, B.; Veres, B. Lack of cyclophilin D protects against the development of acute lung injury in endotoxemia. Biochim. Biophys. Acta 2015, 1852, 2563–2573. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhu, Q.; Niu, F.; Zhang, R.; Wang, Y.; Wang, W.; Sun, D.; Wang, X.; Wang, A. A2BAR activation attenuates acute lung injury by inhibiting alveolar epithelial cell apoptosis both in vivo and in vitro. Am. J. Physiol. Cell Physiol. 2018, 315, C558–C570. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, D.; Li, S.; He, B.; Huang, Y.; Xu, M.; Ren, S.; Li, S.; Wang, H.; Xie, W. Activation of Liver X Receptor Attenuates Oleic Acid-Induced Acute Respiratory Distress Syndrome. Am. J. Pathol. 2016, 186, 2614–2622. [Google Scholar] [CrossRef]
- Dong, H.-Y.; Xu, M.; Ji, Z.-Y.; Wang, Y.-X.; Dong, M.-Q.; Liu, M.-L.; Xu, D.-Q.; Zhao, P.-T.; Liu, Y.; Luo, Y.; et al. Leptin attenuates lipopolysaccharide or oleic acid-induced acute lung injury in mice. Am. J. Respir. Cell Mol. Biol. 2013, 49, 1057–1063. [Google Scholar] [CrossRef]
- Kapur, R.; Kim, M.; Rebetz, J.; Hallström, B.; Björkman, J.T.; Takabe-French, A.; Kim, N.; Liu, J.; Shanmugabhavananthan, S.; Milosevic, S.; et al. Gastrointestinal microbiota contributes to the development of murine transfusion-related acute lung injury. Blood Adv. 2018, 2, 1651–1663. [Google Scholar] [CrossRef]
- Hechler, B.; Maître, B.; Magnenat, S.; Heim, V.; El Mdawar, M.B.; Gachet, C.; de la Salle, H. Platelets are dispensable for antibody-mediated transfusion-related acute lung injury in the mouse. J. Thromb. Haemost. 2016, 14, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Kapur, R.; Kim, M.; Shanmugabhavananthan, S.; Liu, J.; Li, Y.; Semple, J.W. C-reactive protein enhances murine antibody-mediated transfusion-related acute lung injury. Blood 2015, 126, 2747–2751. [Google Scholar] [CrossRef]
- Fung, Y.L.; Tung, J.P. How different animal models help us understand TRALI. ISBT Sci. Ser. 2018, 13, 197–205. [Google Scholar] [CrossRef]
- Semple, J.W.; Kim, M.; Hou, J.; McVey, M.; Lee, Y.J.; Tabuchi, A.; Kuebler, W.M.; Chai, Z.-W.; Lazarus, A.H. Intravenous immunoglobulin prevents murine antibody-mediated acute lung injury at the level of neutrophil reactive oxygen species (ROS) production. PLoS ONE 2012, 7, e31357. [Google Scholar] [CrossRef] [PubMed]
- Van der Zeeuw Laan, E.A.N.; van der Velden, S.; Porcelijn, L.; Semple, J.W.; van der Schoot, C.E.; Kapur, R. Update on the pathophysiology of transfusion-related acute lung injury. Curr. Opin. Hematol. 2020, 27, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Strait, R.T.; Hicks, W.; Barasa, N.; Mahler, A.; Khodoun, M.; Köhl, J.; Stringer, K.; Witte, D.; van Rooijen, N.; Susskind, B.M.; et al. MHC class I-specific antibody binding to nonhematopoietic cells drives complement activation to induce transfusion-related acute lung injury in mice. J. Exp. Med. 2011, 208, 2525–2544. [Google Scholar] [CrossRef]
- Langgartner, D.; Palmer, A.; Rittlinger, A.; Reber, S.O.; Huber-Lang, M. Effects of Prior Psychosocial Trauma on Subsequent Immune Response After Experimental Thorax Trauma. Shock 2018, 49, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Kalbitz, M.; Karbach, M.; Braumueller, S.; Kellermann, P.; Gebhard, F.; Huber-Lang, M.; Perl, M. Role of Complement C5 in Experimental Blunt Chest Trauma-Induced Septic Acute Lung Injury (ALI). PLoS ONE 2016, 11, e0159417. [Google Scholar] [CrossRef]
- Wen, Z.; Fan, L.; Li, Y.; Zou, Z.; Scott, M.J.; Xiao, G.; Li, S.; Billiar, T.R.; Wilson, M.A.; Shi, X.; et al. Neutrophils counteract autophagy-mediated anti-inflammatory mechanisms in alveolar macrophage: Role in posthemorrhagic shock acute lung inflammation. J. Immunol. 2014, 193, 4623–4633. [Google Scholar] [CrossRef]
- Barrett, C.D.; Hsu, A.T.; Ellson, C.D.; Miyazawa, B.Y.; Kong, Y.-W.; Greenwood, J.D.; Dhara, S.; Neal, M.D.; Sperry, J.L.; Park, M.S.; et al. Blood clotting and traumatic injury with shock mediates complement-dependent neutrophil priming for extracellular ROS, ROS-dependent organ injury and coagulopathy. Clin. Exp. Immunol. 2018, 194, 103–117. [Google Scholar] [CrossRef]
- Christofidou-Solomidou, M.; Scherpereel, A.; Wiewrodt, R.; Ng, K.; Sweitzer, T.; Arguiri, E.; Shuvaev, V.; Solomides, C.C.; Albelda, S.M.; Muzykantov, V.R. PECAM-directed delivery of catalase to endothelium protects against pulmonary vascular oxidative stress. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L283–L292. [Google Scholar] [CrossRef]
- Hammerschmidt, S.; Wahn, H. The oxidants hypochlorite and hydrogen peroxide induce distinct patterns of acute lung injury. Biochim. Biophys. Acta 2004, 1690, 258–264. [Google Scholar] [CrossRef][Green Version]
- Kim, S.R.; Lee, K.S.; Park, S.J.; Min, K.H.; Lee, K.Y.; Choe, Y.H.; Hong, S.H.; Koh, G.Y.; Lee, Y.C. Angiopoietin-1 variant, COMP-Ang1 attenuates hydrogen peroxide-induced acute lung injury. Exp. Mol. Med. 2008, 40, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Mraheil, M.A.; Toque, H.A.; La Pietra, L.; Hamacher, J.; Phanthok, T.; Verin, A.; Gonzales, J.; Su, Y.; Fulton, D.; Eaton, D.C.; et al. Dual Role of Hydrogen Peroxide as an Oxidant in Pneumococcal Pneumonia. Antioxid. Redox Signal. 2021, 34, 962–978. [Google Scholar] [CrossRef]
- Hepokoski, M.; Wang, J.; Li, K.; Li, Y.; Gupta, P.; Mai, T.; Moshensky, A.; Alotaibi, M.; Crotty Alexander, L.E.; Malhotra, A.; et al. Altered lung metabolism and mitochondrial DAMPs in lung injury due to acute kidney injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L821–L831. [Google Scholar] [CrossRef]
- Imtiazul, I.M.; Asma, R.; Lee, J.-H.; Cho, N.-J.; Park, S.; Song, H.-Y.; Gil, H.-W. Change of surfactant protein D and A after renal ischemia reperfusion injury. PLoS ONE 2019, 14, e0227097. [Google Scholar] [CrossRef]
- Gray, K.D.; MacMillan-Crow, L.-A.; Simovic, M.O.; Stain, S.C.; May, A.K. Pulmonary MnSOD is nitrated following hepatic ischemia-reperfusion. Surg. Infect. (Larchmt) 2004, 5, 166–173. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Fan, Z. Cellular Signal Transduction Pathways Involved in Acute Lung Injury Induced by Intestinal Ischemia-Reperfusion. Oxid. Med. Cell. Longev. 2021, 2021, 9985701. [Google Scholar] [CrossRef]
- Yuan, Y.; Alwis, I.; Wu, M.C.L.; Kaplan, Z.; Ashworth, K.; Bark, D.; Pham, A.; Mcfadyen, J.; Schoenwaelder, S.M.; Josefsson, E.C.; et al. Neutrophil macroaggregates promote widespread pulmonary thrombosis after gut ischemia. Sci. Transl. Med. 2017, 9, eaam5861. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, T.-T.; Xu, D.-F.; Zhu, X.-Y.; Dong, W.-W.; Lv, Z.; Liu, Y.-J.; Jiang, L. Upregulation of sphingosine kinase 1 contributes to ventilator-associated lung injury in a two-hit model. Int. J. Mol. Med. 2019, 44, 2077–2090. [Google Scholar] [CrossRef] [PubMed]
- Hoegl, S.; Burns, N.; Angulo, M.; Francis, D.; Osborne, C.M.; Mills, T.W.; Blackburn, M.R.; Eltzschig, H.K.; Vohwinkel, C.U. Capturing the multifactorial nature of ARDS—“Two-hit” approach to model murine acute lung injury. Physiol. Rep. 2018, 6, e13648. [Google Scholar] [CrossRef]
- Jones, H.D.; Crother, T.R.; Gonzalez-Villalobos, R.A.; Jupelli, M.; Chen, S.; Dagvadorj, J.; Arditi, M.; Shimada, K. The NLRP3 inflammasome is required for the development of hypoxemia in LPS/mechanical ventilation acute lung injury. Am. J. Respir. Cell Mol. Biol. 2014, 50, 270–280. [Google Scholar] [CrossRef]
- Shan, Y.; Akram, A.; Amatullah, H.; Zhou, D.Y.; Gali, P.L.; Maron-Gutierrez, T.; González-López, A.; Zhou, L.; Rocco, P.R.M.; Hwang, D.; et al. ATF3 protects pulmonary resident cells from acute and ventilator-induced lung injury by preventing Nrf2 degradation. Antioxid. Redox Signal. 2015, 22, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-F.; Liu, Y.-Y.; Lin, S.-W.; Chang, C.-H.; Chen, N.-H.; Hung, C.-Y.; Lee, C.-S. Low-Molecular-Weight Heparin Reduces Ventilation-Induced Lung Injury through Hypoxia Inducible Factor-1α in a Murine Endotoxemia Model. Int. J. Mol. Sci. 2020, 21, 3097. [Google Scholar] [CrossRef] [PubMed]
- Amatullah, H.; Maron-Gutierrez, T.; Shan, Y.; Gupta, S.; Tsoporis, J.N.; Varkouhi, A.K.; Teixeira Monteiro, A.P.; He, X.; Yin, J.; Marshall, J.C.; et al. Protective function of DJ-1/PARK7 in lipopolysaccharide and ventilator-induced acute lung injury. Redox Biol. 2021, 38, 101796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, S.; Zosky, G.R.; Wei, X.; Shu, S.; Wang, D.; Chai, X. Paracoxib Alleviates Ventilator-Induced Lung Injury Through Functional Modulation of Lung-Recruited CD11bloLy6Chi Monocytes. Shock 2021, 55, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Tong, Y.; Jin, S.; Chen, Z.; Li, T.; Billiar, T.R.; Pitt, B.R.; Li, Q.; Zhang, L.-M. Mechanical ventilation enhances extrapulmonary sepsis-induced lung injury: Role of WISP1-αvβ5 integrin pathway in TLR4-mediated inflammation and injury. Crit. Care 2018, 22, 302. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Huang, Y.; Islam, D.; Wen, X.-Y.; Wu, S.; Streutker, C.; Luo, A.; Li, M.; Khang, J.; Han, B.; et al. Dual effects of human neutrophil peptides in a mouse model of pneumonia and ventilator-induced lung injury. Respir. Res. 2018, 19, 190. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Deng, M.; Pan, P.; Turnquist, H.R.; Pitt, B.R.; Billiar, T.R.; Zhang, L.-M. Mechanical Ventilation With Moderate Tidal Volume Exacerbates Extrapulmonary Sepsis-Induced Lung Injury via IL33-WISP1 Signaling Pathway. Shock 2021, 56, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Müller-Redetzky, H.C.; Will, D.; Hellwig, K.; Kummer, W.; Tschernig, T.; Pfeil, U.; Paddenberg, R.; Menger, M.D.; Kershaw, O.; Gruber, A.D.; et al. Mechanical ventilation drives pneumococcal pneumonia into lung injury and sepsis in mice: Protection by adrenomedullin. Crit. Care 2014, 18, R73. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Han, B.; Fanelli, V.; Wen, X.; Huang, Y.; Luo, A.; Ghazarian, M.; Wang, D.; Khang, J.; Morriello, F.; et al. Distinctive Roles and Mechanisms of Human Neutrophil Peptides in Experimental Sepsis and Acute Respiratory Distress Syndrome. Crit. Care Med. 2018, 46, e921–e927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Pan, Y.; Fanelli, V.; Wu, S.; Luo, A.A.; Islam, D.; Han, B.; Mao, P.; Ghazarian, M.; Zeng, W.; et al. Mechanical Stress and the Induction of Lung Fibrosis via the Midkine Signaling Pathway. Am. J. Respir. Crit. Care Med. 2015, 192, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.G.; Yao, L.-J.; Patterson, E.K.; Joseph, M.G.; Cepinskas, G.; Veldhuizen, R.A.W.; Lewis, J.F.; Yamashita, C.M. The effect of tidal volume on systemic inflammation in Acid-induced lung injury. Respiration 2011, 81, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Gurkan, O.U.; He, C.; Zielinski, R.; Rabb, H.; King, L.S.; Dodd-o, J.M.; D’Alessio, F.R.; Aggarwal, N.; Pearse, D.; Becker, P.M. Interleukin-6 mediates pulmonary vascular permeability in a two-hit model of ventilator-associated lung injury. Exp. Lung Res. 2011, 37, 575–584. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Airway Redox Homeostasis and Inflammation Gone Awry: From Molecular Pathogenesis to Emerging Therapeutics in Respiratory Pathology. Int. J. Mol. Sci. 2020, 21, 9317. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Kellner, M.; Noonepalle, S.; Lu, Q.; Srivastava, A.; Zemskov, E.; Black, S.M. ROS Signaling in the Pathogenesis of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS). Adv. Exp. Med. Biol. 2017, 967, 105–137. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Coelho-Junior, H.J.; Marzetti, E. Cell Death and Inflammation: The Role of Mitochondria in Health and Disease. Cells 2021, 10, 537. [Google Scholar] [CrossRef] [PubMed]
- Hernansanz-Agustín, P.; Enríquez, J.A. Generation of Reactive Oxygen Species by Mitochondria. Antioxidants 2021, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-C.; Fessler, M.B. Regulatory mechanisms of neutrophil migration from the circulation to the airspace. Cell. Mol. Life Sci. 2021, 78, 4095–4124. [Google Scholar] [CrossRef]
- Peters, D.M.; Vadász, I.; Wujak, L.; Wygrecka, M.; Olschewski, A.; Becker, C.; Herold, S.; Papp, R.; Mayer, K.; Rummel, S.; et al. TGF-β directs trafficking of the epithelial sodium channel ENaC which has implications for ion and fluid transport in acute lung injury. Proc. Natl. Acad. Sci. USA 2014, 111, E374–E383. [Google Scholar] [CrossRef] [PubMed]
- Witten, M.L.; Chau, B.; Sáez, E.; Boitano, S.; Clark Lantz, R. Early life inhalation exposure to mine tailings dust affects lung development. Toxicol. Appl. Pharmacol. 2019, 365, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-M.; Xu, S.-Y.; Feng, Y.-Z.; Cheng, Y.-R.; Xiong, J.-B.; Zhou, Y.; Guan, C.-X. The role of NOX4 in pulmonary diseases. J. Cell. Physiol. 2021, 236, 1628–1637. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- O’Malley, Y.; Fink, B.D.; Ross, N.C.; Prisinzano, T.E.; Sivitz, W.I. Reactive oxygen and targeted antioxidant administration in endothelial cell mitochondria. J. Biol. Chem. 2006, 281, 39766–39775. [Google Scholar] [CrossRef]
- Schumacker, P.T.; Gillespie, M.N.; Nakahira, K.; Choi, A.M.K.; Crouser, E.D.; Piantadosi, C.A.; Bhattacharya, J. Mitochondria in lung biology and pathology: More than just a powerhouse. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L962–L974. [Google Scholar] [CrossRef]
- Takac, I.; Schröder, K.; Zhang, L.; Lardy, B.; Anilkumar, N.; Lambeth, J.D.; Shah, A.M.; Morel, F.; Brandes, R.P. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 2011, 286, 13304–13313. [Google Scholar] [CrossRef]
- Diaz, B.; Shani, G.; Pass, I.; Anderson, D.; Quintavalle, M.; Courtneidge, S.A. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci. Signal. 2009, 2, ra53. [Google Scholar] [CrossRef]
- Lyle, A.N.; Deshpande, N.N.; Taniyama, Y.; Seidel-Rogol, B.; Pounkova, L.; Du, P.; Papaharalambus, C.; Lassègue, B.; Griendling, K.K. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ. Res. 2009, 105, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Faraci, F.M.; Didion, S.P. Vascular protection: Superoxide dismutase isoforms in the vessel wall. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide anion radical (O2−.), superoxide dismutases, and related matters. J. Biol. Chem. 1997, 272, 18515–18517. [Google Scholar] [CrossRef] [PubMed]
- Budinger, G.R.S.; Mutlu, G.M.; Urich, D.; Soberanes, S.; Buccellato, L.J.; Hawkins, K.; Chiarella, S.E.; Radigan, K.A.; Eisenbart, J.; Agrawal, H.; et al. Epithelial cell death is an important contributor to oxidant-mediated acute lung injury. Am. J. Respir. Crit. Care Med. 2011, 183, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Gongora, M.C.; Lob, H.E.; Landmesser, U.; Guzik, T.J.; Martin, W.D.; Ozumi, K.; Wall, S.M.; Wilson, D.S.; Murthy, N.; Gravanis, M.; et al. Loss of extracellular superoxide dismutase leads to acute lung damage in the presence of ambient air: A potential mechanism underlying adult respiratory distress syndrome. Am. J. Pathol. 2008, 173, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Kinnula, V.L.; Crapo, J.D. Superoxide dismutases in the lung and human lung diseases. Am. J. Respir. Crit. Care Med. 2003, 167, 1600–1619. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C.; Kettle, A.J. Redox reactions and microbial killing in the neutrophil phagosome. Antioxid. Redox Signal. 2013, 18, 642–660. [Google Scholar] [CrossRef]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef]
- Davies, M.J.; Hawkins, C.L. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxid. Redox Signal. 2020, 32, 957–981. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Rahman, I.; MacNee, W. Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 2000, 16, 534–554. [Google Scholar] [CrossRef]
- Zuo, L.; Wijegunawardana, D. Redox Role of ROS and Inflammation in Pulmonary Diseases. Adv. Exp. Med. Biol. 2021, 1304, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.S.; Fuloria, N.K.; Fuloria, S.; Rahman, S.B.; Al-Malki, W.H.; Javed Shaikh, M.A.; Thangavelu, L.; Singh, S.K.; Rama Raju Allam, V.S.; Jha, N.K.; et al. Nuclear factor-kappa B and its role in inflammatory lung disease. Chem. Biol. Interact. 2021, 345, 109568. [Google Scholar] [CrossRef] [PubMed]
- Herrero, R.; Sanchez, G.; Lorente, J.A. New insights into the mechanisms of pulmonary edema in acute lung injury. Ann. Transl. Med. 2018, 6, 32. [Google Scholar] [CrossRef]
- Barabutis, N. Unfolded Protein Response: A Regulator of the Endothelial Barrier. Endocr. Metab. Sci. 2021, 3, 100092. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gauthier, A.; Daley, L.; Dial, K.; Wu, J.; Woo, J.; Lin, M.; Ashby, C.; Mantell, L.L. The Role of HMGB1, a Nuclear Damage-Associated Molecular Pattern Molecule, in the Pathogenesis of Lung Diseases. Antioxid. Redox Signal. 2019, 31, 954–993. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury. Front. Immunol. 2020, 11, 1722. [Google Scholar] [CrossRef] [PubMed]
- Dessing, M.C.; Schouten, M.; Draing, C.; Levi, M.; Von Aulock, S.; van der Poll, T. Role played by Toll-like receptors 2 and 4 in lipoteichoic acid-induced lung inflammation and coagulation. J. Infect. Dis. 2008, 197, 245–252. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef] [PubMed]
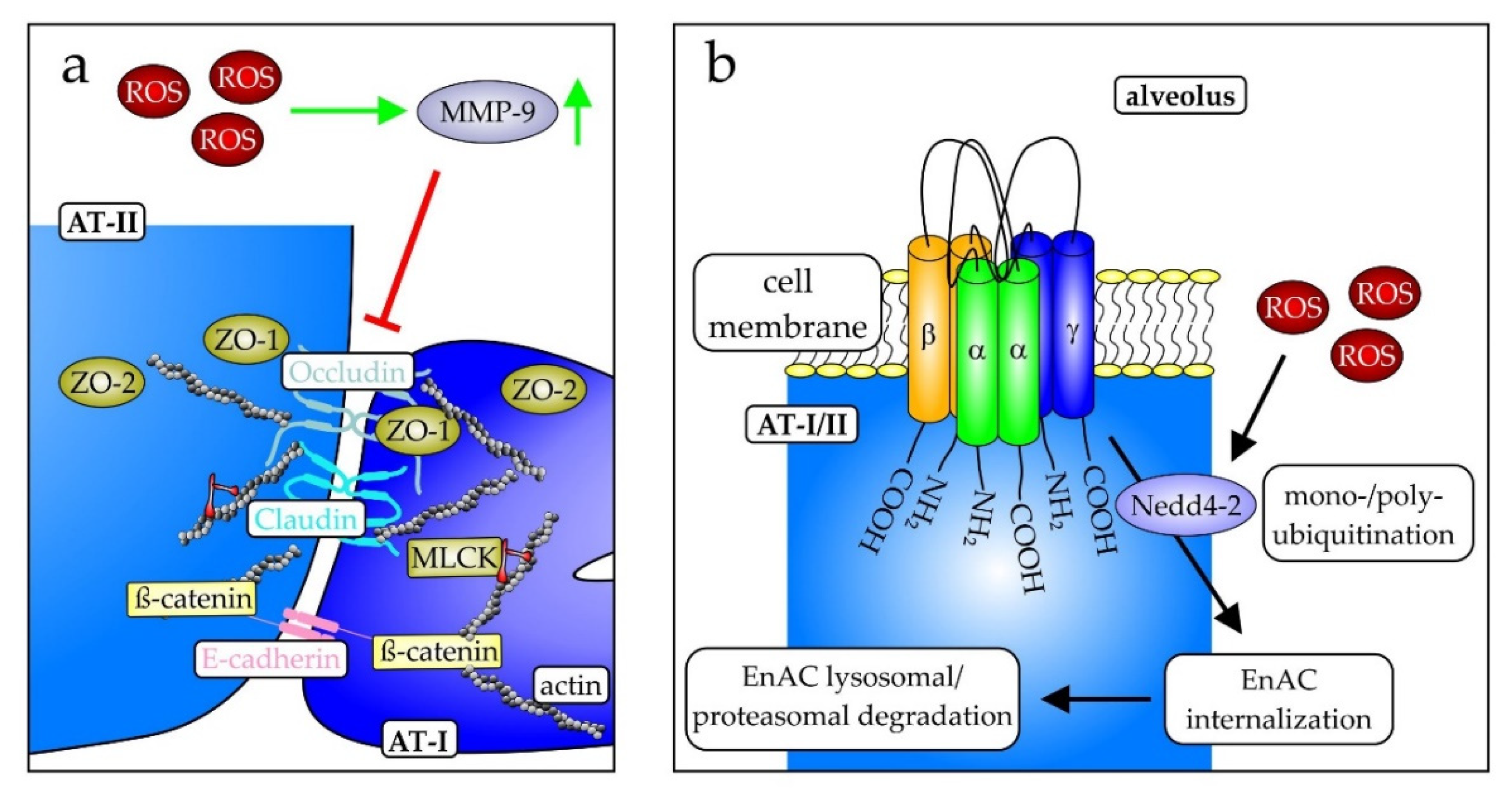
- Wittekindt, O.H. Tight junctions in pulmonary epithelia during lung inflammation. Pflugers Arch. 2017, 469, 135–147. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, Y.-F.; Zhao, Y.; Xu, D.-F.; Wang, Y.; Xu, C.-F.; Dong, W.-W.; Zhu, X.-Y.; Ding, N.; Jiang, L.; et al. Upregulation of Matrix Metalloproteinase-9 Protects against Sepsis-Induced Acute Lung Injury via Promoting the Release of Soluble Receptor for Advanced Glycation End Products. Oxid. Med. Cell. Longev. 2021, 2021, 8889313. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-T.; Yang, C.-M. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem. Pharmacol. 2012, 84, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qi, Z.; Li, D.; Huang, X.; Qi, B.; Feng, J.; Qu, J.; Wang, X. Alveolar epithelial glycocalyx shedding aggravates the epithelial barrier and disrupts epithelial tight junctions in acute respiratory distress syndrome. Biomed. Pharmacother. 2021, 133, 111026. [Google Scholar] [CrossRef]
- He, H.; Huang, C.; Chen, Z.; Huang, H.; Wang, X.; Chen, J. An outlined review for the role of Nedd4-1 and Nedd4-2 in lung disorders. Biomed. Pharmacother. 2020, 125, 109983. [Google Scholar] [CrossRef]
- Duerr, J.; Leitz, D.H.W.; Szczygiel, M.; Dvornikov, D.; Fraumann, S.G.; Kreutz, C.; Zadora, P.K.; Seyhan Agircan, A.; Konietzke, P.; Engelmann, T.A.; et al. Conditional deletion of Nedd4-2 in lung epithelial cells causes progressive pulmonary fibrosis in adult mice. Nat. Commun. 2020, 11, 2012. [Google Scholar] [CrossRef] [PubMed]
- Eaton, A.F.; Yue, Q.; Eaton, D.C.; Bao, H.-F. ENaC activity and expression is decreased in the lungs of protein kinase C-α knockout mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L374–L385. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.C.; Helms, M.N.; Koval, M.; Bao, H.F.; Jain, L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annu. Rev. Physiol. 2009, 71, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Wynne, B.M.; Zou, L.; Linck, V.; Hoover, R.S.; Ma, H.-P.; Eaton, D.C. Regulation of Lung Epithelial Sodium Channels by Cytokines and Chemokines. Front. Immunol. 2017, 8, 766. [Google Scholar] [CrossRef]
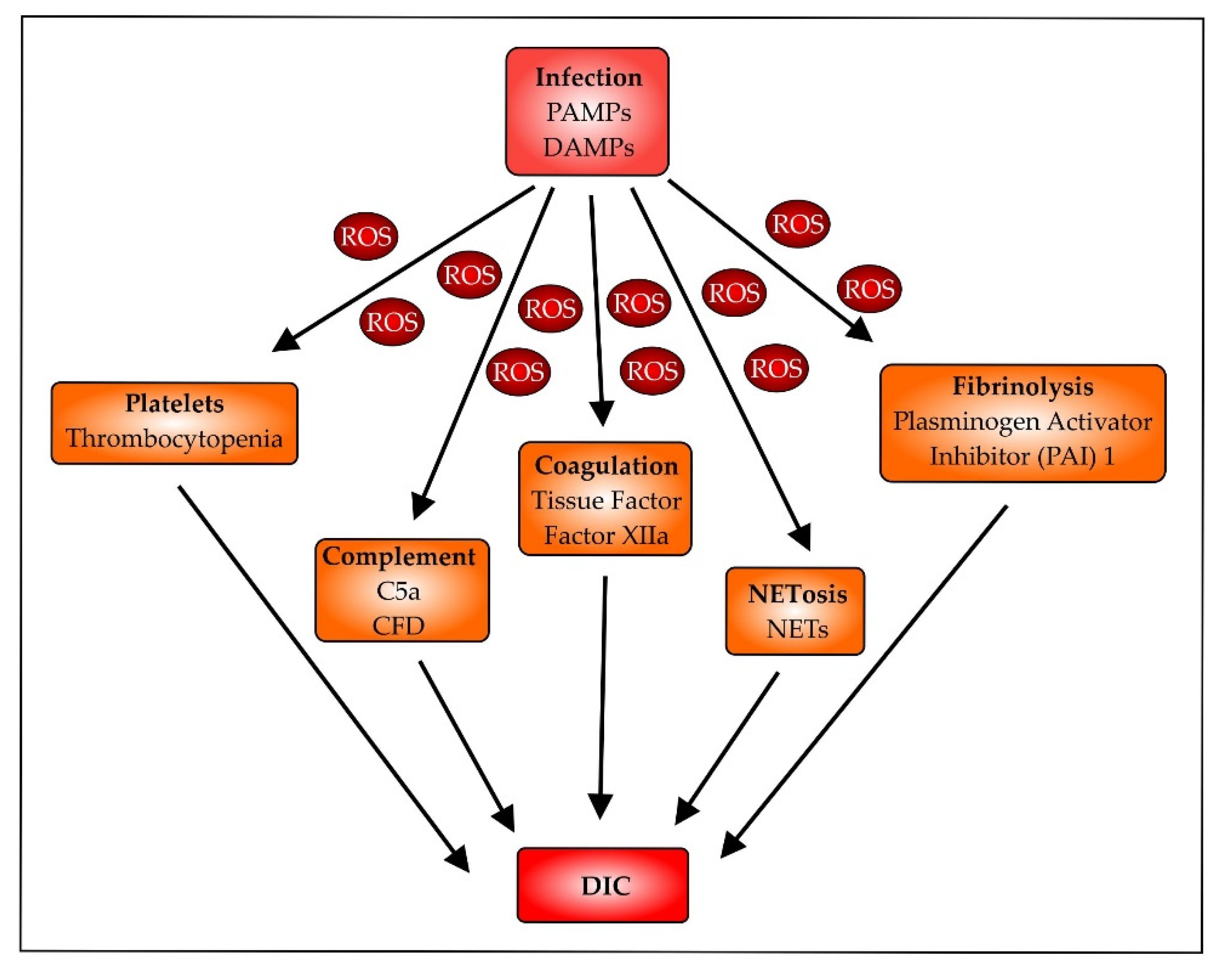
- Yadav, H.; Kor, D.J. Platelets in the pathogenesis of acute respiratory distress syndrome. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L915–L923. [Google Scholar] [CrossRef] [PubMed]
- Hirata, N.; Ngo, D.T.; Phan, P.H.; Ainai, A.; Phung, T.T.B.; Ta, T.A.; Takasaki, J.; Kawachi, S.; Nunoi, H.; Nakajima, N.; et al. Recombinant human thrombomodulin for pneumonia-induced severe ARDS complicated by DIC in children: A preliminary study. J. Anesth. 2021, 35, 638–645. [Google Scholar] [CrossRef]
- Wake, H.; Mori, S.; Liu, K.; Morioka, Y.; Teshigawara, K.; Sakaguchi, M.; Kuroda, K.; Gao, Y.; Takahashi, H.; Ohtsuka, A.; et al. Histidine-Rich Glycoprotein Prevents Septic Lethality through Regulation of Immunothrombosis and Inflammation. EBioMedicine 2016, 9, 180–194. [Google Scholar] [CrossRef]
- Middleton, E.A.; Rondina, M.T.; Schwertz, H.; Zimmerman, G.A. Amicus or Adversary Revisited: Platelets in Acute Lung Injury and Acute Respiratory Distress Syndrome. Am. J. Respir. Cell Mol. Biol. 2018, 59, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.; Perdomo, J.; Ahmadi, Z.; Yan, F.; McKenzie, S.E.; Chong, B.H. Inhibition of NADPH oxidase blocks NETosis and reduces thrombosis in heparin-induced thrombocytopenia. Blood Adv. 2021, 5, 5439–5451. [Google Scholar] [CrossRef]
- Delaney, M.K.; Kim, K.; Estevez, B.; Xu, Z.; Stojanovic-Terpo, A.; Shen, B.; Ushio-Fukai, M.; Cho, J.; Du, X. Differential Roles of the NADPH-Oxidase 1 and 2 in Platelet Activation and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Gibbins, J.M.; Holbrook, L.M.; Palomo, I. NADPH oxidase 2 (NOX2): A key target of oxidative stress-mediated platelet activation and thrombosis. Trends Cardiovasc. Med. 2018, 28, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Renné, T.; Pozgajová, M.; Grüner, S.; Schuh, K.; Pauer, H.-U.; Burfeind, P.; Gailani, D.; Nieswandt, B. Defective thrombus formation in mice lacking coagulation factor XII. J. Exp. Med. 2005, 202, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Mutch, N.J.; Baskar, D.; Rohloff, P.; Docampo, R.; Morrissey, J.H. Polyphosphate modulates blood coagulation and fibrinolysis. Proc. Natl. Acad. Sci. USA 2006, 103, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, Y.; Qu, M.; Yu, Y.; Chen, Z.; Zhu, S.; Guo, K.; Chen, W.; Miao, C. Tissue Factor-Enriched Neutrophil Extracellular Traps Promote Immunothrombosis and Disease Progression in Sepsis-Induced Lung Injury. Front. Cell. Infect. Microbiol. 2021, 11, 677902. [Google Scholar] [CrossRef]
- Mannes, M.; Schmidt, C.Q.; Nilsson, B.; Ekdahl, K.N.; Huber-Lang, M. Complement as driver of systemic inflammation and organ failure in trauma, burn, and sepsis. Semin. Immunopathol. 2021, 43, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front. Cell. Infect. Microbiol. 2017, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.-J.; Lin, W.-C.; Yang, Y.-H.; Tseng, Y.-L.; Lin, Y.-H.; Chou, C.-H.; Tsau, Y.-K. High Concentration of C5a-Induced Mitochondria-Dependent Apoptosis in Murine Kidney Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 4465. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Schultz, M.J.; Rijneveld, A.W.; van der Poll, T. Bronchoalveolar coagulation and fibrinolysis in endotoxemia and pneumonia. Crit. Care Med. 2003, 31, S238–S242. [Google Scholar] [CrossRef]
- Akinosoglou, K.; Alexopoulos, D. Use of antiplatelet agents in sepsis: A glimpse into the future. Thromb. Res. 2014, 133, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Naime, A.C.A.; Ganaes, J.O.F.; Lopes-Pires, M.E. Sepsis: The Involvement of Platelets and the Current Treatments. Curr. Mol. Pharmacol. 2018, 11, 261–269. [Google Scholar] [CrossRef]
- Gill, S.E.; Rohan, M.; Mehta, S. Role of pulmonary microvascular endothelial cell apoptosis in murine sepsis-induced lung injury In Vivo. Respir. Res. 2015, 16, 109. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Li, G.; Ma, W.; Zhou, X.-S.; Wang, J.; Liu, B. The Nox1/Nox4 inhibitor attenuates acute lung injury induced by ischemia-reperfusion in mice. PLoS ONE 2018, 13, e0209444. [Google Scholar] [CrossRef]
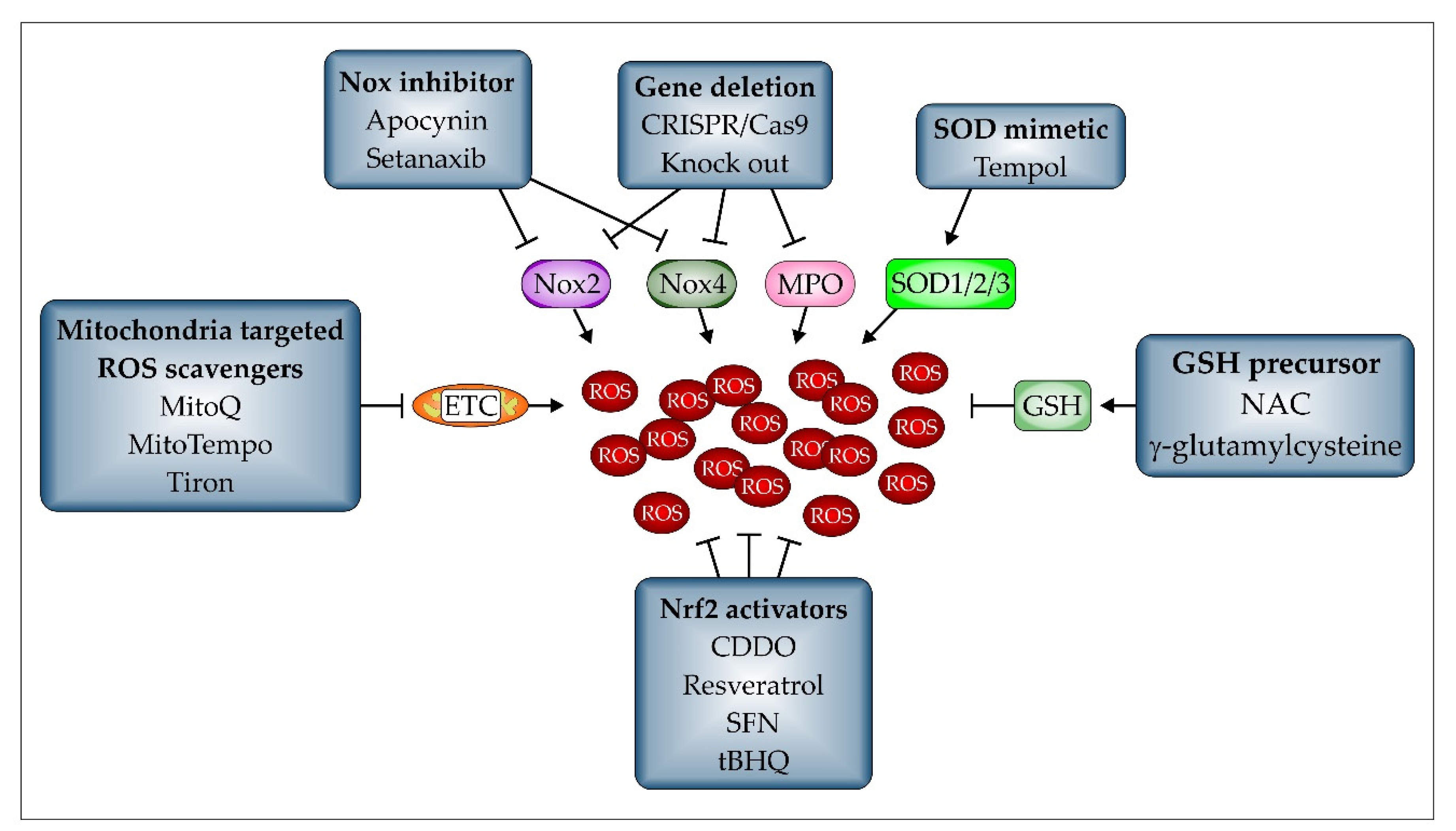
- Park, W.H. Tempol differently affects cellular redox changes and antioxidant enzymes in various lung-related cells. Sci. Rep. 2021, 11, 14869. [Google Scholar] [CrossRef]
- Wu, N.-C.; Liao, F.-T.; Cheng, H.-M.; Sung, S.-H.; Yang, Y.-C.; Wang, J.-J. Intravenous superoxide dismutase as a protective agent to prevent impairment of lung function induced by high tidal volume ventilation. BMC Pulm. Med. 2017, 17, 105. [Google Scholar] [CrossRef] [PubMed]
- Oyewole, A.O.; Birch-Machin, M.A. Mitochondria-targeted antioxidants. FASEB J. 2015, 29, 4766–4771. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Gu, C.; Liu, M.; Liu, H.; Wang, D.; Liu, X.; Wang, Y. Protective role of p120-catenin on mitochondria by inhibiting NLRP3 in ventilator-induced lung injury. J. Cell. Mol. Med. 2019, 23, 7360–7371. [Google Scholar] [CrossRef] [PubMed]
- Matuz-Mares, D.; Riveros-Rosas, H.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Glutathione Participation in the Prevention of Cardiovascular Diseases. Antioxidants 2021, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Rahman, I. Environmental toxicity, redox signaling and lung inflammation: The role of glutathione. Mol. Aspects Med. 2009, 30, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Lou, Y.; Wang, Y.; Zhang, M.; Jiang, Q.; Mo, Y.; Han, K.; Jin, S.; Dai, Q.; Yu, Y.; et al. PICK1 deficiency exacerbates sepsis-associated acute lung injury and impairs glutathione synthesis via reduction of xCT. Free Radic. Biol. Med. 2018, 118, 23–34. [Google Scholar] [CrossRef]
- Bao, X.; Liu, X.; Liu, N.; Zhuang, S.; Yang, Q.; Ren, H.; Zhao, D.; Bai, J.; Zhou, X.; Tang, L. Inhibition of EZH2 prevents acute respiratory distress syndrome (ARDS)-associated pulmonary fibrosis by regulating the macrophage polarization phenotype. Respir. Res. 2021, 22, 194. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Hang, Q.; Fang, Y.; Dong, X.; Cao, P.; Yin, Z.; Luo, L. γ-glutamylcysteine exhibits anti-inflammatory effects by increasing cellular glutathione level. Redox Biol. 2019, 20, 157–166. [Google Scholar] [CrossRef]
- Ghezzi, P. Redox regulation of immunity and the role of small molecular weight thiols. Redox Biol. 2021, 44, 102001. [Google Scholar] [CrossRef]
- Parnham, M.J.; Sies, H. The early research and development of ebselen. Biochem. Pharmacol. 2013, 86, 1248–1253. [Google Scholar] [CrossRef]
- Sies, H. Ebselen, a selenoorganic compound as glutathione peroxidase mimic. Free Radic. Biol. Med. 1993, 14, 313–323. [Google Scholar] [CrossRef]
- Azad, G.K.; Tomar, R.S. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014, 41, 4865–4879. [Google Scholar] [CrossRef]
- Ishii, Y.; Hashimoto, K.; Hirano, K.; Morishima, Y.; Mochizuki, M.; Masuyama, K.; Nomura, A.; Sakamoto, T.; Uchida, Y.; Sagai, M.; et al. Ebselen decreases ozone-induced pulmonary inflammation in rats. Lung 2000, 178, 225–234. [Google Scholar] [CrossRef]
- Sies, H.; Parnham, M.J. Potential therapeutic use of ebselen for COVID-19 and other respiratory viral infections. Free Radic. Biol. Med. 2020, 156, 107–112. [Google Scholar] [CrossRef]
- Tyml, K.; Li, F.; Wilson, J.X. Septic impairment of capillary blood flow requires nicotinamide adenine dinucleotide phosphate oxidase but not nitric oxide synthase and is rapidly reversed by ascorbate through an endothelial nitric oxide synthase-dependent mechanism. Crit. Care Med. 2008, 36, 2355–2362. [Google Scholar] [CrossRef]
- Fisher, B.J.; Kraskauskas, D.; Martin, E.J.; Farkas, D.; Wegelin, J.A.; Brophy, D.; Ward, K.R.; Voelkel, N.F.; Fowler, A.A.; Natarajan, R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L20–L32. [Google Scholar] [CrossRef]
- Audousset, C.; McGovern, T.; Martin, J.G. Role of Nrf2 in Disease: Novel Molecular Mechanisms and Therapeutic Approaches—Pulmonary Disease/Asthma. Front. Physiol. 2021, 12, 727806. [Google Scholar] [CrossRef]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; Von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jang, J.; Park, S.-M.; Yang, S.-R. An Update on the Role of Nrf2 in Respiratory Disease: Molecular Mechanisms and Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 8406. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Xia, Y.; Jin, S.; Xue, C.; Wang, Y.; Hu, R.; Jiang, H. Nrf2 attenuates ferroptosis-mediated IIR-ALI by modulating TERT and SLC7A11. Cell Death Dis. 2021, 12, 1027. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Greven, J.; Fragoulis, A.; Horst, K.; Bläsius, F.; Wruck, C.; Pufe, T.; Kobbe, P.; Hildebrand, F.; Lichte, P. Sulforaphane-Dependent Up-Regulation of NRF2 Activity Alleviates Both Systemic Inflammatory Response and Lung Injury After Hemorrhagic Shock/Resuscitation In Mice. Shock 2021. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, G.; Shi, X.; Zhou, H.; Liu, M.; Chen, Y.; Feng, D.; Zhang, P.; Wu, L.; Lv, X. Nrf2 activation protects against intratracheal LPS induced mouse/murine acute respiratory distress syndrome by regulating macrophage polarization. Biochem. Biophys. Res. Commun. 2018, 500, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Zhang, X.-J.; Chen, H.-M. Bardoxolone treatment alleviates lipopolysaccharide (LPS)-induced acute lung injury through suppressing inflammation and oxidative stress regulated by Nrf2 signaling. Biochem. Biophys. Res. Commun. 2019, 516, 270–277. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.T.P.; Coutinho, D.D.S.; Guterres, S.S.; Pohlmann, A.R.; Martins, M.A.; Bernardi, A. Resveratrol-Loaded Lipid-Core Nanocapsules Modulate Acute Lung Inflammation and Oxidative Imbalance Induced by LPS in Mice. Pharmaceutics 2021, 13, 683. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, S.; Zhang, X.; Liu, F.; Yang, K.; Du, G.; Rui, X. Dasatinib protects against acute respiratory distress syndrome via Nrf2-regulated M2 macrophages polarization. Drug Dev. Res. 2021, 82, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Cen, M.; Ouyang, W.; Zhang, W.; Yang, L.; Lin, X.; Dai, M.; Hu, H.; Tang, H.; Liu, H.; Xia, J.; et al. MitoQ protects against hyperpermeability of endothelium barrier in acute lung injury via a Nrf2-dependent mechanism. Redox Biol. 2021, 41, 101936. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, J.; Liu, Y.; Fang, H.; Liao, L.; Wang, Z.; Yuan, J.; Wang, X.; Sun, J.; Tang, B.; et al. MitoQ alleviates LPS-mediated acute lung injury through regulating Nrf2/Drp1 pathway. Free Radic. Biol. Med. 2021, 165, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Buelna-Chontal, M.; Zazueta, C. Redox activation of Nrf2 & NF-kB: A double end sword? Cell. Signal. 2013, 25, 2548–2557. [Google Scholar]
- Fratantonio, D.; Virgili, F.; Zucchi, A.; Lambrechts, K.; Latronico, T.; Lafère, P.; Germonpré, P.; Balestra, C. Increasing oxygen partial pressures induce a distinct transcriptional response in human PBMC: A pilot study on the “normobaric oxygen paradox”. Int. J. Mol. Sci. 2021, 22, 458. [Google Scholar] [CrossRef]
- von Knethen, A.; Tzieply, N.; Jennewein, C.; Brüne, B. Casein-kinase-II-dependent phosphorylation of PPARgamma provokes CRM1-mediated shuttling of PPARgamma from the nucleus to the cytosol. J. Cell Sci. 2010, 123, 192–201. [Google Scholar] [CrossRef]
- Odegaard, J.I.; Ricardo-Gonzalez, R.R.; Goforth, M.H.; Morel, C.R.; Subramanian, V.; Mukundan, L.; Red Eagle, A.; Vats, D.; Brombacher, F.; Ferrante, A.W.; et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 2007, 447, 1116–1120. [Google Scholar] [CrossRef]
- Bouhlel, M.A.; Derudas, B.; Rigamonti, E.; Dièvart, R.; Brozek, J.; Haulon, S.; Zawadzki, C.; Jude, B.; Torpier, G.; Marx, N.; et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007, 6, 137–143. [Google Scholar] [CrossRef]
- Trümper, V.; Wittig, I.; Heidler, J.; Richter, F.; Brüne, B.; von Knethen, A. Redox Regulation of PPARγ in Polarized Macrophages. PPAR Res. 2020, 2020, 8253831. [Google Scholar] [CrossRef]
- He, J.; Qi, D.; Tang, X.-M.; Deng, W.; Deng, X.-Y.; Zhao, Y.; Wang, D.-X. Rosiglitazone promotes ENaC-mediated alveolar fluid clearance in acute lung injury through the PPARγ/SGK1 signaling pathway. Cell. Mol. Biol. Lett. 2019, 24, 35. [Google Scholar] [CrossRef]
- Wang, G.; Liu, L.; Zhang, Y.; Han, D.; Liu, J.; Xu, J.; Xie, X.; Wu, Y.; Zhang, D.; Ke, R.; et al. Activation of PPARγ attenuates LPS-induced acute lung injury by inhibition of HMGB1-RAGE levels. Eur. J. Pharmacol. 2014, 726, 27–32. [Google Scholar] [CrossRef]
- Xie, K.; Chen, Y.-Q.; Chai, Y.-S.; Lin, S.-H.; Wang, C.-J.; Xu, F. HMGB1 suppress the expression of IL-35 by regulating Naïve CD4+ T cell differentiation and aggravating Caspase-11-dependent pyroptosis in acute lung injury. Int. Immunopharmacol. 2021, 91, 107295. [Google Scholar] [CrossRef] [PubMed]
- Ricote, M.; Li, A.C.; Willson, T.M.; Kelly, C.J.; Glass, C.K. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 1998, 391, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-S.; Wang, H.; Li, X.; Chao, T.; Teav, T.; Christen, S.; Di Conza, G.; Cheng, W.-C.; Chou, C.-H.; Vavakova, M.; et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 2017, 18, 985–994. [Google Scholar] [CrossRef]
- Pearce, E.L.; Pearce, E.J. Metabolic pathways in immune cell activation and quiescence. Immunity 2013, 38, 633–643. [Google Scholar] [CrossRef]
- Arora, S.; Dev, K.; Agarwal, B.; Das, P.; Syed, M.A. Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology 2018, 223, 383–396. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Y.; Wang, S.; Zhou, H.; Feng, D.; Wei, J.; Shi, X.; Wu, L.; Zhang, P.; Yang, H.; et al. α-Ketoglutarate Modulates Macrophage Polarization Through Regulation of PPARγ Transcription and mTORC1/p70S6K Pathway to Ameliorate ALI/ARDS. Shock 2020, 53, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Budinger, G.R.S.; Lo, A.; Urich, D.; Rivera, S.E.; Ghosh, A.K.; Gonzalez, A.; Chiarella, S.E.; Marks, K.; Donnelly, H.K.; et al. Leptin promotes fibroproliferative acute respiratory distress syndrome by inhibiting peroxisome proliferator-activated receptor-γ. Am. J. Respir. Crit. Care Med. 2011, 183, 1490–1498. [Google Scholar] [CrossRef]
- Schmidt, M.V.; Paulus, P.; Kuhn, A.M.; Weigert, A.; Morbitzer, V.; Zacharowski, K.; Kempf, V.A.; Brüne, B.; von Knethen, A. Peroxisome proliferator-activated receptor γ-induced T cell apoptosis reduces survival during polymicrobial sepsis. Am. J. Respir. Crit. Care Med. 2011, 184, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Menezes, S.L.S.; Bozza, P.T.; Neto, H.C.C.F.; Laranjeira, A.P.; Negri, E.M.; Capelozzi, V.L.; Zin, W.A.; Rocco, P.R.M. Pulmonary and extrapulmonary acute lung injury: Inflammatory and ultrastructural analyses. J. Appl. Physiol. (1985) 2005, 98, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Capelozzi, V.L.; Allen, T.C.; Beasley, M.B.; Cagle, P.T.; Guinee, D.; Hariri, L.P.; Husain, A.N.; Jain, D.; Lantuejoul, S.; Larsen, B.T.; et al. Molecular and Immune Biomarkers in Acute Respiratory Distress Syndrome: A Perspective From Members of the Pulmonary Pathology Society. Arch. Pathol. Lab. Med. 2017, 141, 1719–1727. [Google Scholar] [CrossRef]
- Radermacher, P.; Haouzi, P. A mouse is not a rat is not a man: Species-specific metabolic respsonses to sepsis—A nail in the coffin of murine models for critical care research? Intensive Care Med. Exp. 2013, 1, 7. [Google Scholar] [CrossRef]
- Zolfaghari, P.S.; Pinto, B.B.; Dyson, A.; Singer, M. The metabolic phenotype of rodent sepsis: Cause for concern? Intensive Care Med. Exp. 2013, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Drohan, C.M.; Nouraie, S.M.; Bain, W.; Shah, F.A.; Evankovich, J.; Zhang, Y.; Morris, A.; McVerry, B.J.; Kitsios, G.D. Biomarker-Based Classification of Patients With Acute Respiratory Failure Into Inflammatory Subphenotypes: A Single-Center Exploratory Study. Crit. Care Explor. 2021, 3, e0518. [Google Scholar] [CrossRef]
- Spadaro, S.; Park, M.; Turrini, C.; Tunstall, T.; Thwaites, R.; Mauri, T.; Ragazzi, R.; Ruggeri, P.; Hansel, T.T.; Caramori, G.; et al. Biomarkers for Acute Respiratory Distress syndrome and prospects for personalised medicine. J. Inflamm. 2019, 16, 1. [Google Scholar] [CrossRef]
- Wick, K.D.; McAuley, D.F.; Levitt, J.E.; Beitler, J.R.; Annane, D.; Riviello, E.D.; Calfee, C.S.; Matthay, M.A. Promises and challenges of personalized medicine to guide ARDS therapy. Crit. Care 2021, 25, 404. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Arabi, Y.M.; Siegel, E.R.; Ware, L.B.; Bos, L.D.J.; Sinha, P.; Beitler, J.R.; Wick, K.D.; Curley, M.A.Q.; Constantin, J.-M.; et al. Phenotypes and personalized medicine in the acute respiratory distress syndrome. Intensive Care Med. 2020, 46, 2136–2152. [Google Scholar] [CrossRef]
- Sweeney, T.E.; Thomas, N.J.; Howrylak, J.A.; Wong, H.R.; Rogers, A.J.; Khatri, P. Multicohort Analysis of Whole-Blood Gene Expression Data Does Not Form a Robust Diagnostic for Acute Respiratory Distress Syndrome. Crit. Care Med. 2018, 46, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Qadir, N.; Chang, S.Y. Pharmacologic Treatments for Acute Respiratory Distress Syndrome. Crit. Care Clin. 2021, 37, 877–893. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Horie, S.; Laffey, J.G. Emerging cellular and pharmacologic therapies for acute respiratory distress syndrome. Curr. Opin. Crit. Care 2021, 27, 20–28. [Google Scholar] [CrossRef]
- Jaiswal, N.; Bhatnagar, M.; Shah, H. N-acetycysteine: A potential therapeutic agent in COVID-19 infection. Med. Hypotheses 2020, 144, 110133. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Rogliani, P.; Salvi, S.S.; Ora, J.; Matera, M.G. Use of Thiols in the Treatment of COVID-19: Current Evidence. Lung 2021, 199, 335–343. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Knethen, A.; Heinicke, U.; Laux, V.; Parnham, M.J.; Steinbicker, A.U.; Zacharowski, K. Antioxidants as Therapeutic Agents in Acute Respiratory Distress Syndrome (ARDS) Treatment—From Mice to Men. Biomedicines 2022, 10, 98. https://doi.org/10.3390/biomedicines10010098
von Knethen A, Heinicke U, Laux V, Parnham MJ, Steinbicker AU, Zacharowski K. Antioxidants as Therapeutic Agents in Acute Respiratory Distress Syndrome (ARDS) Treatment—From Mice to Men. Biomedicines. 2022; 10(1):98. https://doi.org/10.3390/biomedicines10010098
Chicago/Turabian Stylevon Knethen, Andreas, Ulrike Heinicke, Volker Laux, Michael J. Parnham, Andrea U. Steinbicker, and Kai Zacharowski. 2022. "Antioxidants as Therapeutic Agents in Acute Respiratory Distress Syndrome (ARDS) Treatment—From Mice to Men" Biomedicines 10, no. 1: 98. https://doi.org/10.3390/biomedicines10010098
APA Stylevon Knethen, A., Heinicke, U., Laux, V., Parnham, M. J., Steinbicker, A. U., & Zacharowski, K. (2022). Antioxidants as Therapeutic Agents in Acute Respiratory Distress Syndrome (ARDS) Treatment—From Mice to Men. Biomedicines, 10(1), 98. https://doi.org/10.3390/biomedicines10010098







