Galectin-9 Triggers Neutrophil-Mediated Anticancer Immunity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Galectin-9 and Inhibitors
2.2. Cell Lines
2.3. Isolation of Immune Cells
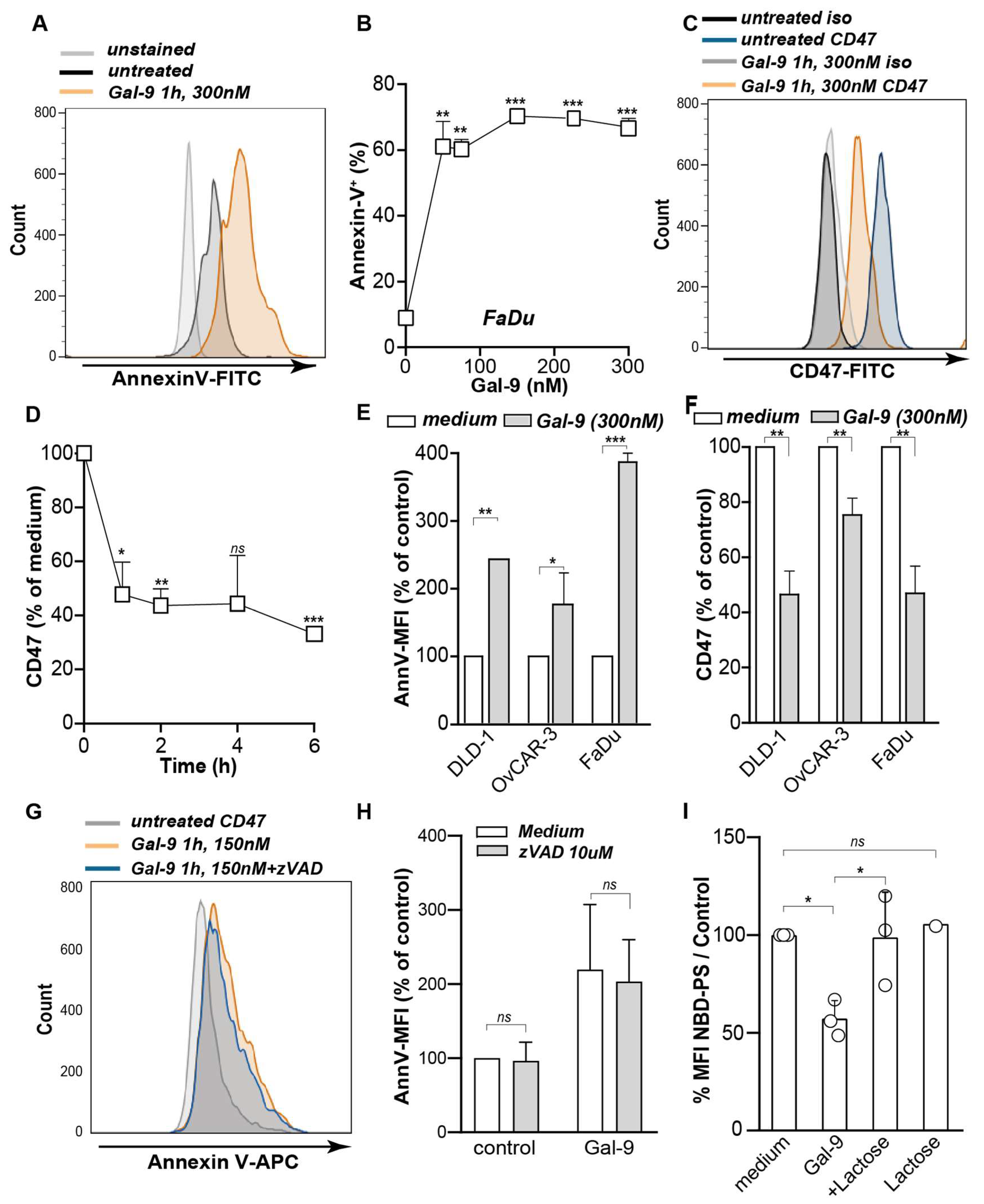
2.4. Expression of PS and CD47 on FaDu
2.5. Detection of PS Flip-Flop Using NBD-Labeled PS
2.6. Flow Cytometry-Based Trogocytosis Assay
2.7. Antibody-Dependent Cellular Phagocytosis (ADCP)
2.8. Neutrophil Activation Assays
2.9. Cell Killing Assay
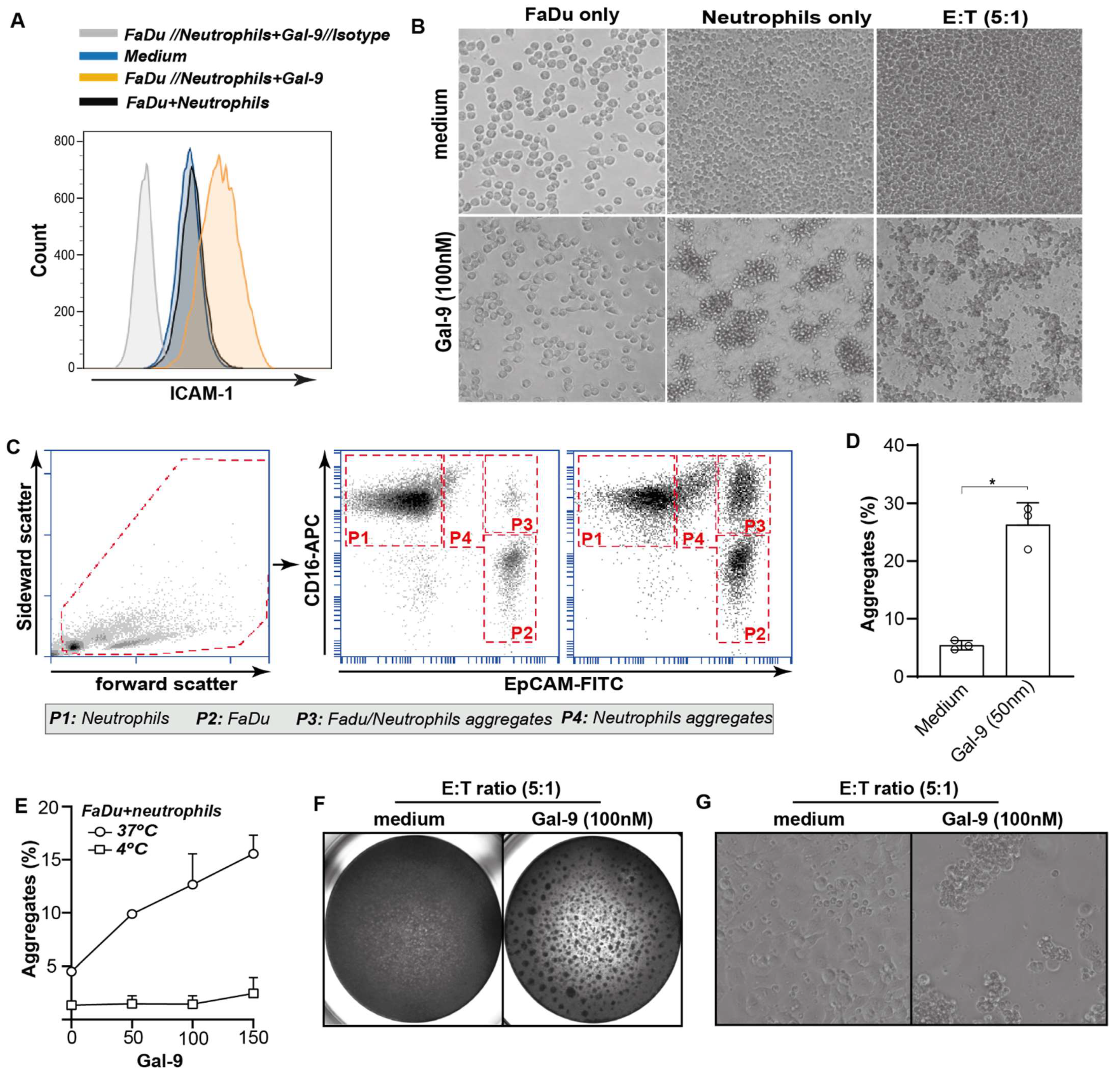
2.10. MTS Assays to Determine Cell Adhesion
2.11. Statistical Analysis
3. Results
3.1. Galectin-9 Shifted the Phagocytic Balance in Cancer Cells
3.2. Galectin-9 Triggered Neutrophil but Not Macrophage-Mediated Cancer Cell Uptake
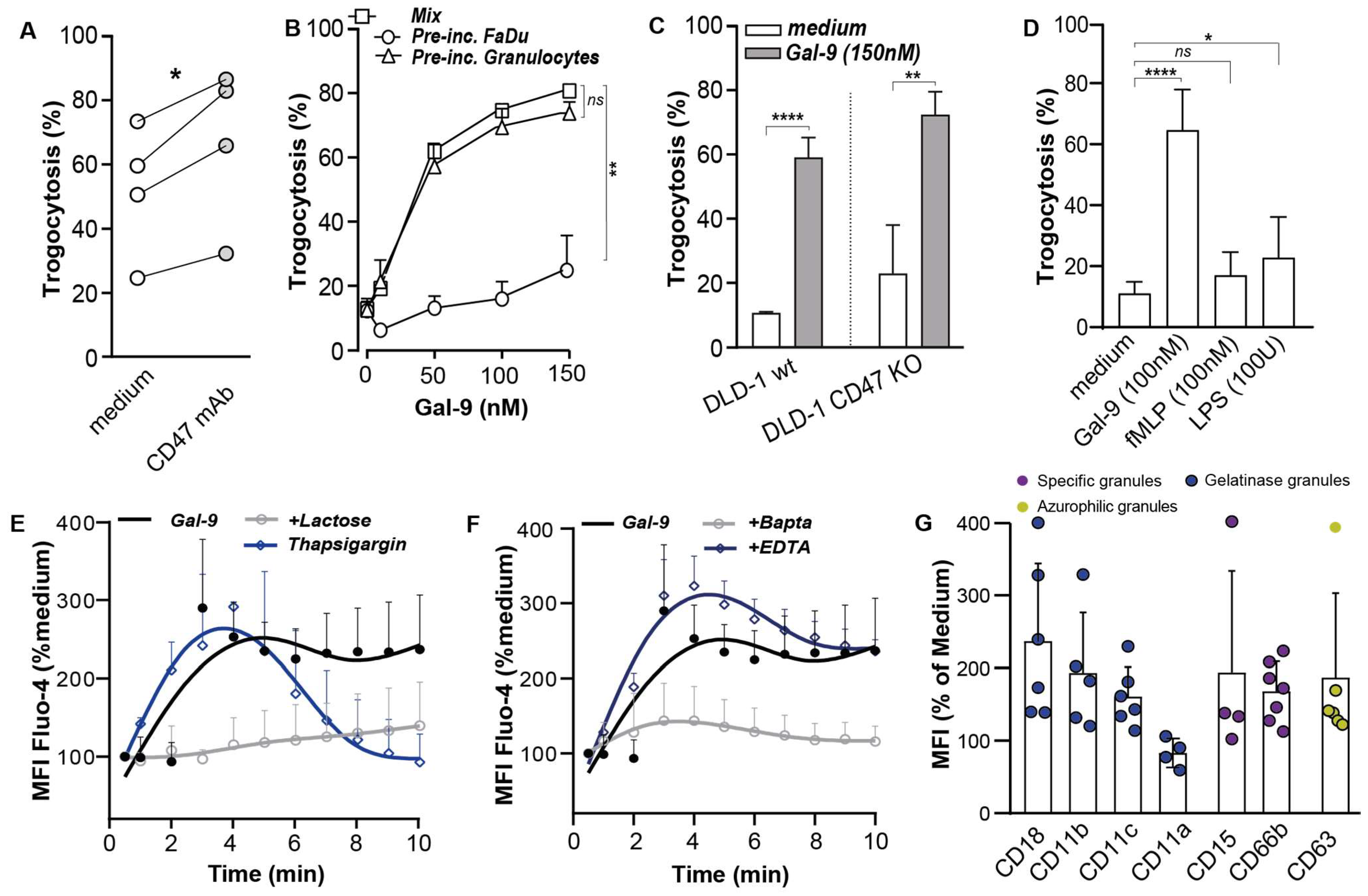
3.3. Galectin-9 Potentiated Antibody-Dependent Cellular Trogocytosis (ADCP)
3.4. Gal-9-Mediated Trogocytosis Can Largely Be Attributed to Neutrophil Activation
3.5. Galectin-9 Had Multi-Fold Stimulatory Activity on Neutrophils
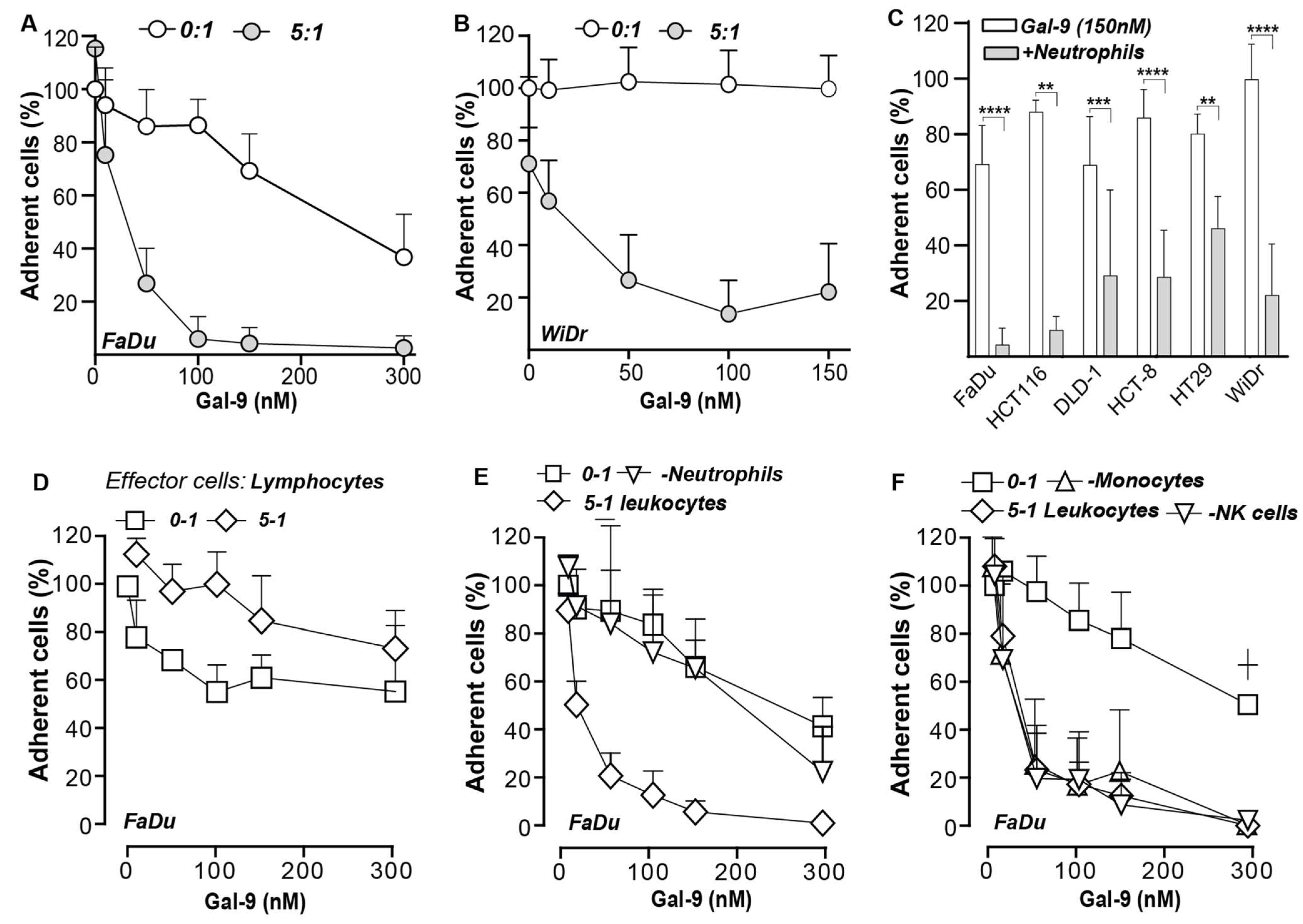
3.6. Galectin-9-Mediated Activation of Neutrophils Abrogates Cancer Cell Adhesion
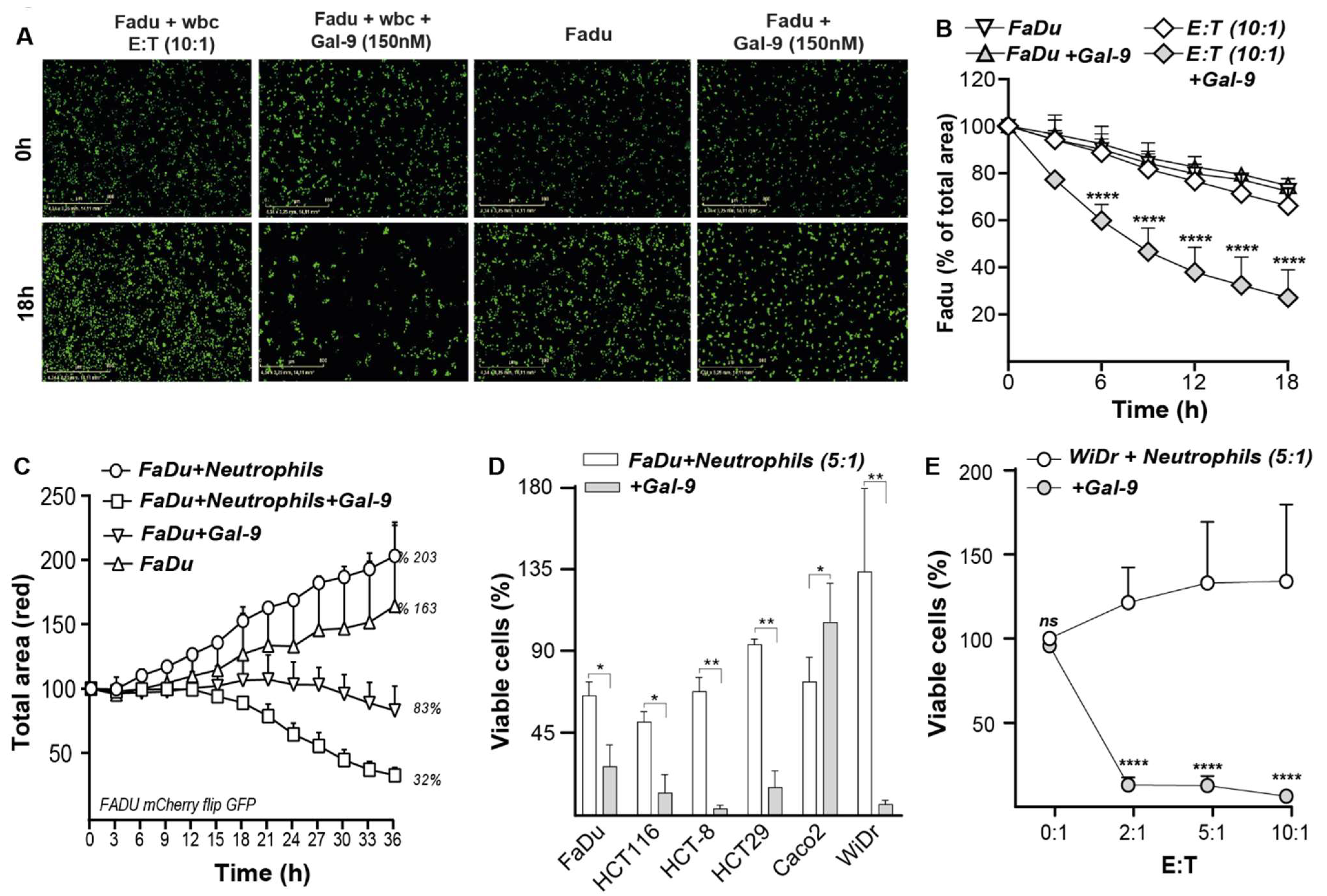
3.7. Galectin-9-Treated Neutrophils Trigger Cytotoxic Elimination of Cancer Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wiersma, V.R.; de Bruyn, M.; Helfrich, W.; Bremer, E. Therapeutic potential of Galectin-9 in human disease. Med. Res. Rev. 2013, 33, E102–E126. [Google Scholar] [CrossRef] [PubMed]
- Hirashima, M.; Kashio, Y.; Nishi, N.; Yamauchi, A.; Imaizumi, T.-A.; Kageshita, T.; Saita, N.; Nakamura, T. Galectin-9 in physiological and pathological conditions. Glycoconj. J. 2002, 19, 593–600. [Google Scholar] [CrossRef]
- Irie, A.; Yamauchi, A.; Kontani, K.; Kihara, M.; Liu, D.; Shirato, Y.; Seki, M.; Nishi, N.; Nakamura, T.; Yokomise, H. Galectin-9 as a prognostic factor with antimetastatic potential in breast cancer. Clin. Cancer Res. 2005, 11, 2962–2968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Chen, Z.; Wu, R.; Yin, J.; Fan, M.; Xu, X. Prognostic role of high gal-9 expression in solid tumours: A meta-analysis. Cell. Physiol. Biochem. 2018, 45, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Wiersma, V.R.; de Bruyn, M.; van Ginkel, R.J.; Sigar, E.; Hirashima, M.; Niki, T.; Nishi, N.; Samplonius, D.F.; Helfrich, W.; Bremer, E. The glycan-binding protein galectin-9 has direct apoptotic activity toward melanoma cells. J. Investig. Dermatol. 2012, 132, 2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.-Y.; Dong, J.-H.; Chen, Y.-W.; Wang, X.-Q.; Li, C.-H.; Wang, J.; Wang, G.-Q.; Li, H.-L.; Wang, X.-D. Galectin-9 acts as a prognostic factor with antimetastatic potential in hepatocellular carcinoma. Asian Pac. J. Cancer Prev. 2012, 13, 2503–2509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, K.; Morishita, A.; Iwama, H.; Fujita, K.; Okura, R.; Fujihara, S.; Yamashita, T.; Fujimori, T.; Kato, K.; Kamada, H. Galectin-9 suppresses cholangiocarcinoma cell proliferation by inducing apoptosis but not cell cycle arrest. Oncol. Rep. 2015, 34, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Morishita, A.; Nomura, K.; Tani, J.; Fujita, K.; Iwama, H.; Takuma, K.; Nakahara, M.; Tadokoro, T.; Oura, K.; Chiyo, T. Galectin-9 suppresses the tumor growth of colon cancer in vitro and in vivo. Oncol. Rep. 2021, 45, 105. [Google Scholar] [CrossRef] [PubMed]
- Wiersma, V.R.; de Bruyn, M.; Wei, Y.; van Ginkel, R.J.; Hirashima, M.; Niki, T.; Nishi, N.; Zhou, J.; Pouwels, S.D.; Samplonius, D.F. The epithelial polarity regulator LGALS9/galectin-9 induces fatal frustrated autophagy in KRAS mutant colon carcinoma that depends on elevated basal autophagic flux. Autophagy 2015, 11, 1373–1388. [Google Scholar] [CrossRef] [Green Version]
- Nobumoto, A.; Nagahara, K.; Oomizu, S.; Katoh, S.; Nishi, N.; Takeshita, K.; Niki, T.; Tominaga, A.; Yamauchi, A.; Hirashima, M. Galectin-9 suppresses tumor metastasis by blocking adhesion to endothelium and extracellular matrices. Glycobiology 2008, 18, 735–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, A.; Kontani, K.; Kihara, M.; Nishi, N.; Yokomise, H.; Hirashima, M. Galectin-9, a novel prognostic factor with antimetastatic potential in breast cancer. Breast J. 2006, 12, S196–S200. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, N.; Nishi, N.; Seki, M.; Matsumoto, R.; Kuwabara, I.; Liu, F.-T.; Hata, Y.; Nakamura, T.; Hirashima, M. Requirement of divalent galactoside-binding activity of ecalectin/galectin-9 for eosinophil chemoattraction. J. Biol. Chem. 2000, 275, 8355–8360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.-C.; Li, C.-S.; Weng, I.-C.; Chen, H.-Y.; Lu, H.-H.; Huang, C.-C.; Liu, F.-T. Galectin-9 Is Critical for Mucosal Adaptive Immunity through the T Helper 17–IgA Axis. Am. J. Pathol. 2018, 188, 1225–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steichen, A.L.; Simonson, T.J.; Salmon, S.L.; Metzger, D.W.; Mishra, B.B.; Sharma, J. Alarmin function of galectin-9 in murine respiratory tularemia. PLoS ONE 2015, 10, e0123573. [Google Scholar]
- Wiersma, V.R.; Clarke, A.; Pouwels, S.D.; Perry, E.; Abdullah, T.M.; Kelly, C.; Soyza, A.D.; Hutchinson, D.; Eggleton, P.; Bremer, E. Galectin-9 is a possible promoter of immunopathology in rheumatoid arthritis by activation of peptidyl arginine deiminase 4 (PAD-4) in granulocytes. Int. J. Mol. Sci. 2019, 20, 4046. [Google Scholar] [CrossRef] [Green Version]
- Kurose, Y.; Wada, J.; Kanzaki, M.; Teshigawara, S.; Nakatsuka, A.; Murakami, K.; Inoue, K.; Terami, T.; Katayama, A.; Watanabe, M. Serum galectin-9 levels are elevated in the patients with type 2 diabetes and chronic kidney disease. BMC Nephrol. 2013, 14, 23. [Google Scholar] [CrossRef] [Green Version]
- Nagahara, K.; Arikawa, T.; Oomizu, S.; Kontani, K.; Nobumoto, A.; Tateno, H.; Watanabe, K.; Niki, T.; Katoh, S.; Miyake, M. Galectin-9 increases Tim-3+ dendritic cells and CD8+ T cells and enhances antitumor immunity via galectin-9-Tim-3 interactions. J. Immunol. 2008, 181, 7660–7669. [Google Scholar] [CrossRef] [Green Version]
- Oomizu, S.; Arikawa, T.; Niki, T.; Kadowaki, T.; Ueno, M.; Nishi, N.; Yamauchi, A.; Hattori, T.; Masaki, T.; Hirashima, M. Cell surface galectin-9 expressing Th cells regulate Th17 and Foxp3+ Treg development by galectin-9 secretion. PLoS ONE 2012, 7, e48574. [Google Scholar] [CrossRef] [Green Version]
- Gooden, M.J.; Wiersma, V.R.; Samplonius, D.F.; Gerssen, J.; van Ginkel, R.J.; Nijman, H.W.; Hirashima, M.; Niki, T.; Eggleton, P.; Helfrich, W. Galectin-9 activates and expands human T-helper 1 cells. PLoS ONE 2013, 8, e65616. [Google Scholar] [CrossRef] [Green Version]
- Lhuillier, C.; Barjon, C.; Niki, T.; Gelin, A.; Praz, F.; Morales, O.; Souquere, S.; Hirashima, M.; Wei, M.; Dellis, O. Impact of exogenous galectin-9 on human T cells: Contribution of the T cell receptor complex to antigen-independent activation but not to apoptosis induction. J. Biol. Chem. 2015, 290, 16797–16811. [Google Scholar] [CrossRef] [Green Version]
- Matsuura, A.; Tsukada, J.; Mizobe, T.; Higashi, T.; Mouri, F.; Tanikawa, R.; Yamauchi, A.; Hirashima, M.; Tanaka, Y. Intracellular galectin-9 activates inflammatory cytokines in monocytes. Genes Cells 2009, 14, 511–521. [Google Scholar] [CrossRef]
- Seifert, A.M.; Reiche, C.; Heiduk, M.; Tannert, A.; Meinecke, A.-C.; Baier, S.; von Renesse, J.; Kahlert, C.; Distler, M.; Welsch, T. Detection of pancreatic ductal adenocarcinoma with galectin-9 serum levels. Oncogene 2020, 39, 3102–3113. [Google Scholar] [CrossRef] [Green Version]
- Enninga, E.A.L.; Nevala, W.K.; Holtan, S.G.; Leontovich, A.A.; Markovic, S.N. Galectin-9 modulates immunity by promoting Th2/M2 differentiation and impacts survival in patients with metastatic melanoma. Melanoma Res. 2016, 26, 429. [Google Scholar] [CrossRef] [PubMed]
- Krautter, F.; Recio, C.; Hussain, M.T.; Lezama, D.R.; Maione, F.; Chimen, M.; Iqbal, A.J. Characterisation of endogenous Galectin-1 and-9 expression in monocyte and macrophage subsets under resting and inflammatory conditions. Biomed. Pharmacother. 2020, 130, 110595. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Arikawa, T.; Shinonaga, R.; Oomizu, S.; Inagawa, H.; Soma, G.; Niki, T.; Hirashima, M. Galectin-9 signaling prolongs survival in murine lung-cancer by inducing macrophages to differentiate into plasmacytoid dendritic cell-like macrophages. Clin. Immunol. 2012, 142, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Vega-Carrascal, I.; Bergin, D.A.; McElvaney, O.J.; McCarthy, C.; Banville, N.; Pohl, K.; Hirashima, M.; Kuchroo, V.K.; Reeves, E.P.; McElvaney, N.G. Galectin-9 signaling through TIM-3 is involved in neutrophil-mediated Gram-negative bacterial killing: An effect abrogated within the cystic fibrosis lung. J. Immunol. 2014, 192, 2418–2431. [Google Scholar] [CrossRef] [PubMed]
- Andzinski, L.; Kasnitz, N.; Stahnke, S.; Wu, C.F.; Gereke, M.; von Köckritz-Blickwede, M.; Schilling, B.; Brandau, S.; Weiss, S.; Jablonska, J. Type I IFN s induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int. J. Cancer 2016, 138, 1982–1993. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V.; Assi, S.; Gershkovitz, M.; Sagiv, J.Y.; Polyansky, L.; Mishalian, I.; Fridlender, Z.G.; Granot, Z. Isolation and characterization of neutrophils with anti-tumor properties. J. Vis. Exp. JoVE 2015, Nr100, 52933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadok, V.A.; de Cathelineau, A.; Daleke, D.L.; Henson, P.M.; Bratton, D.L. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol. Chem. 2001, 276, 1071–1077. [Google Scholar] [CrossRef] [Green Version]
- Nishi, N.; Itoh, A.; Fujiyama, A.; Yoshida, N.; Araya, S.I.; Hirashima, M.; Shoji, H.; Nakamura, T. Development of highly stable galectins: Truncation of the linker peptide confers protease-resistance on tandem-repeat type galectins. FEBS Lett. 2005, 579, 2058–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bratton, D.L.; Fadok, V.A.; Richter, D.A.; Kailey, J.M.; Frasch, S.C.; Nakamura, T.; Henson, P.M. Polyamine regulation of plasma membrane phospholipid flip-flop during apoptosis. J. Biol. Chem. 1999, 274, 28113–28120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlegel, R.A.; Callahan, M.K.; Williamson, P. The central role of phosphatidylserine in the phagocytosis of apoptotic thymocytes. Ann. N. Y. Acad. Sci. 2000, 926, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Suzuki, J.; Segawa, K.; Fujii, T. Exposure of phosphatidylserine on the cell surface. Cell Death Differ. 2016, 23, 952–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segawa, K.; Kurata, S.; Yanagihashi, Y.; Brummelkamp, T.R.; Matsuda, F.; Nagata, S. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 2014, 344, 1164–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valgardsdottir, R.; Cattaneo, I.; Klein, C.; Introna, M.; Figliuzzi, M.; Golay, J. Human neutrophils mediate trogocytosis rather than phagocytosis of CLL B cells opsonized with anti-CD20 antibodies. Blood J. Am. Soc. Hematol. 2017, 129, 2636–2644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollinedo, F.; Calafat, J.; Janssen, H.; Martín-Martín, B.; Canchado, J.; Nabokina, S.M.; Gajate, C. Combinatorial SNARE complexes modulate the secretion of cytoplasmic granules in human neutrophils. J. Immunol. 2006, 177, 2831–2841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witting, A.; Müller, P.; Herrmann, A.; Kettenmann, H.; Nolte, C. Phagocytic clearance of apoptotic neurons by microglia/brain macrophages in vitro: Involvement of lectin-, integrin-, and phosphatidylserine-mediated recognition. J. Neurochem. 2000, 75, 1060–1070. [Google Scholar] [CrossRef]
- Maugeri, N.; Rovere-Querini, P.; Evangelista, V.; Covino, C.; Capobianco, A.; Bertilaccio, M.T.; Piccoli, A.; Totani, L.; Cianflone, D.; Maseri, A. Neutrophils phagocytose activated platelets in vivo: A phosphatidylserine, P-selectin, and β2 integrin–dependent cell clearance program. Blood J. Am. Soc. Hematol. 2009, 113, 5254–5265. [Google Scholar] [CrossRef]
- Ring, N.G.; Herndler-Brandstetter, D.; Weiskopf, K.; Shan, L.; Volkmer, J.-P.; George, B.M.; Lietzenmayer, M.; McKenna, K.M.; Naik, T.J.; McCarty, A. Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc. Natl. Acad. Sci. USA 2017, 114, E10578–E10585. [Google Scholar] [CrossRef] [Green Version]
- Voets, E.; Paradé, M.; Hulsik, D.L.; Spijkers, S.; Janssen, W.; Rens, J.; Reinieren-Beeren, I.; van den Tillaart, G.; van Duijnhoven, S.; Driessen, L. Functional characterization of the selective pan-allele anti-SIRPα antibody ADU-1805 that blocks the SIRPα–CD47 innate immune checkpoint. J. Immunother. Cancer 2019, 7, 340. [Google Scholar] [CrossRef]
- Avtenyuk, N.U.; Visser, N.; Bremer, E.; Wiersma, V.R. The neutrophil: The underdog that packs a punch in the fight against cancer. Int. J. Mol. Sci. 2020, 21, 7820. [Google Scholar] [CrossRef] [PubMed]
- Treffers, L.W.; Broeke, T.T.; Rösner, T.; Jansen, J.M.; van Houdt, M.; Kahle, S.; Schornagel, K.; Verkuijlen, P.J.; Prins, J.M.; Franke, K. IgA mediated killing of tumor cells by neutrophils is enhanced by CD47-SIRPα checkpoint inhibition. Cancer Immunol. Res. 2020, 8, 120–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.W.; van Beek, E.M.; Schornagel, K.; van der Maaden, H.; van Houdt, M.; Otten, M.A.; Finetti, P.; van Egmond, M.; Matozaki, T.; Kraal, G. CD47–signal regulatory protein-α (SIRPα) interactions form a barrier for antibody-mediated tumor cell destruction. Proc. Natl. Acad. Sci. USA 2011, 108, 18342–18347. [Google Scholar] [CrossRef] [Green Version]
- Matlung, H.L.; Babes, L.; Zhao, X.W.; van Houdt, M.; Treffers, L.W.; van Rees, D.J.; Franke, K.; Schornagel, K.; Verkuijlen, P.; Janssen, H. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. 2018, 23, 3946–3959.e6. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Jin, M.-S.; Kong, F.; Cao, D.; Ma, H.-X.; Jia, Z.; Wang, Y.-P.; Suo, J.; Cao, X. Decreased galectin-9 and increased Tim-3 expression are related to poor prognosis in gastric cancer. PLoS ONE 2013, 8, e81799. [Google Scholar] [CrossRef]
- Simons, M.P.; Nauseef, W.M.; Griffith, T.S. Neutrophils and TRAIL: Neutrophils and TRAIL: Insights into BCG immunotherapy for bladder cancer. Immunol. Res. 2007, 39, 79–93. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ustyanovska Avtenyuk, N.; Choukrani, G.; Ammatuna, E.; Niki, T.; Cendrowicz, E.; Lourens, H.J.; Huls, G.; Wiersma, V.R.; Bremer, E. Galectin-9 Triggers Neutrophil-Mediated Anticancer Immunity. Biomedicines 2022, 10, 66. https://doi.org/10.3390/biomedicines10010066
Ustyanovska Avtenyuk N, Choukrani G, Ammatuna E, Niki T, Cendrowicz E, Lourens HJ, Huls G, Wiersma VR, Bremer E. Galectin-9 Triggers Neutrophil-Mediated Anticancer Immunity. Biomedicines. 2022; 10(1):66. https://doi.org/10.3390/biomedicines10010066
Chicago/Turabian StyleUstyanovska Avtenyuk, Natasha, Ghizlane Choukrani, Emanuele Ammatuna, Toshiro Niki, Ewa Cendrowicz, Harm Jan Lourens, Gerwin Huls, Valerie R. Wiersma, and Edwin Bremer. 2022. "Galectin-9 Triggers Neutrophil-Mediated Anticancer Immunity" Biomedicines 10, no. 1: 66. https://doi.org/10.3390/biomedicines10010066
APA StyleUstyanovska Avtenyuk, N., Choukrani, G., Ammatuna, E., Niki, T., Cendrowicz, E., Lourens, H. J., Huls, G., Wiersma, V. R., & Bremer, E. (2022). Galectin-9 Triggers Neutrophil-Mediated Anticancer Immunity. Biomedicines, 10(1), 66. https://doi.org/10.3390/biomedicines10010066







