Movement Disorders in Oncology: From Clinical Features to Biomarkers
Abstract
:1. Introduction
2. Movement Disorders in the Context of Brain Malignancies
3. Paraneoplastic Movement Disorders
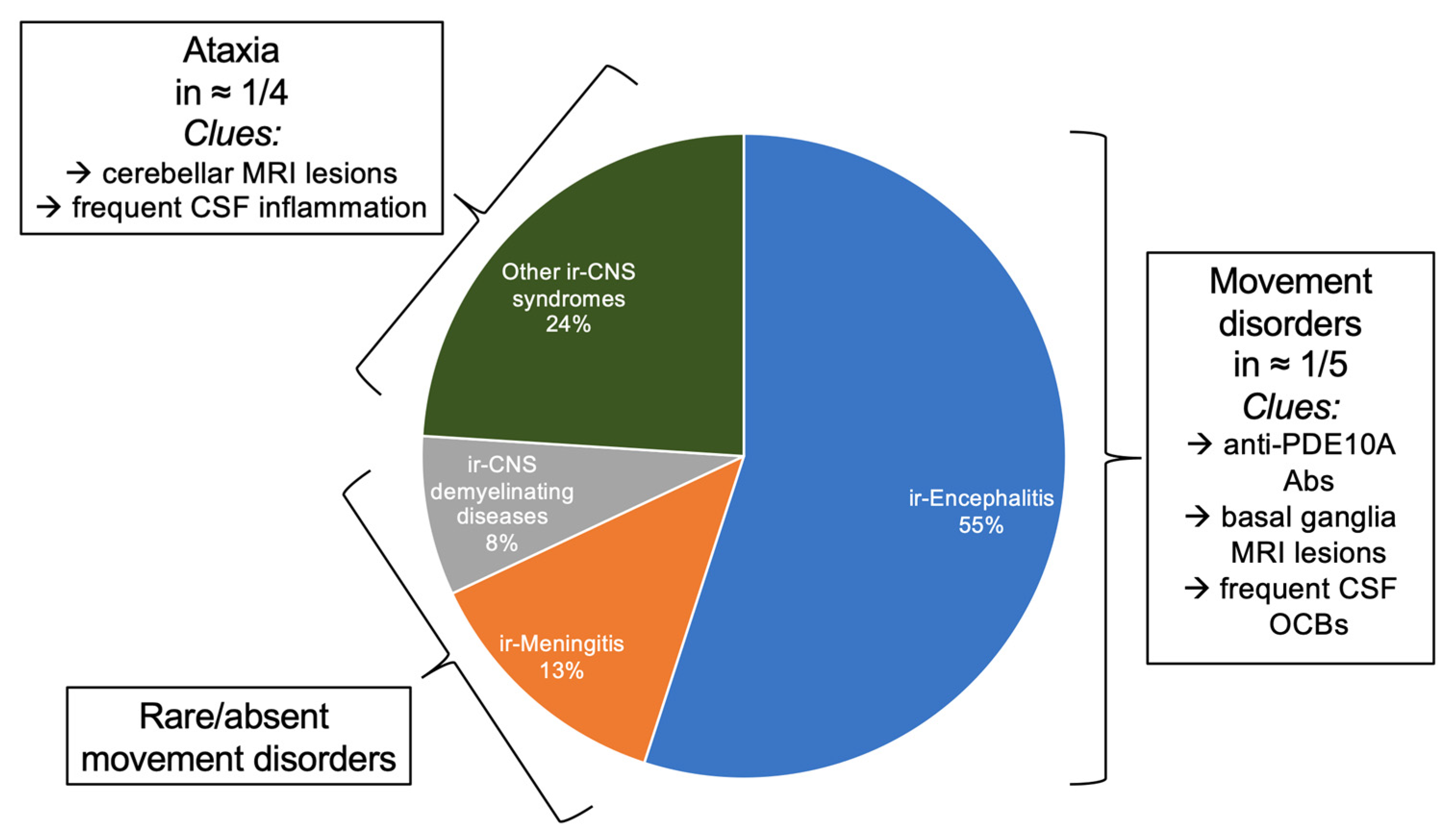
4. Immune-Checkpoint Inhibitors Associated Movement Disorders
5. Diagnostic Algorithm and Testing
6. Therapeutic Approach
7. Challenges and Future Directions
8. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahajan, A.; Chirra, M.; Dwivedi, A.K.; Sturchio, A.; Keeling, E.G.; Marsili, L.; Espay, A.J. Skin Cancer May Delay Onset but Not Progression of Parkinson’s Disease: A Nested Case-Control Study. Front. Neurol. 2020, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Sturchio, A.; Dwivedi, A.K.; Vizcarra, J.A.; Chirra, M.; Keeling, E.G.; Mata, I.F.; Kauffman, M.A.; Pandey, M.K.; Roviello, G.; Comi, C.; et al. Genetic parkinsonisms and cancer: A systematic review and meta-analysis. Rev. Neurosci. 2021, 32, 159–167. [Google Scholar] [CrossRef]
- Magnusen, A.F.; Hatton, S.L.; Rani, R.; Pandey, M.K. Genetic Defects and Pro-inflammatory Cytokines in Parkinson’s Disease. Front. Neurol. 2021, 12, 636139. [Google Scholar] [CrossRef]
- Hatano, T.K.S.; Hattori, N.; Mizuno, Y. Movement disorders in neoplastic brain disease. In Movement Disorders in Neurologic and Systemic Disease; W. Poewe, J.J., Ed.; Cambridge University Press: Cambridge, UK, 2014; pp. 279–292. [Google Scholar]
- Hoos, A. Development of immuno-oncology drugs-from CTLA4 to PD1 to the next generations. Nat. Rev. Drug Discov. 2016, 15, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Duker, A.P.; Espay, A.J. Images in clinical medicine. Hemichorea–hemiballism after diabetic ketoacidosis. N. Engl. J. Med. 2010, 363, e27. [Google Scholar] [CrossRef] [PubMed]
- Marsili, L.; Gallerini, S.; Bartalucci, M.; Marotti, C.; Marconi, R. Paroxysmal painful spasms associated with central pontine myelinolisis in the context of nonketotic hyperglycemia. J. Neurol. Sci. 2018, 388, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Moore, F.G. Bilateral hemichorea-hemiballism caused by metastatic lung cancer. Mov. Disord. 2009, 24, 1405–1406. [Google Scholar] [CrossRef] [PubMed]
- Patankar, A.P. Hemi-chorea: An unusual presentation of brainstem glioma. Br. J. Neurosurg. 2013, 27, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Ząbek, M.; Sobstyl, M.; Dzierzęcki, S.; Górecki, W.; Jakuciński, M. Right hemichorea treated successfully by surgical removal of a left putaminal cavernous angioma. Clin. Neurol. Neurosurg. 2013, 115, 844–846. [Google Scholar] [CrossRef] [PubMed]
- Zagrajek, M.; Chojdak-Łukasiewicz, J. Ballism as a rare form of hyperkinetic movement disorder. Wiad. Lek. 2013, 66, 171–174. [Google Scholar]
- Chuang, C.; Fahn, S.; Frucht, S.J. The natural history and treatment of acquired hemidystonia: Report of 33 cases and review of the literature. J. Neurol. Neurosurg. Psychiatry 2002, 72, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Marsden, C.D. Movement disorders following lesions of the thalamus or subthalamic region. Mov. Disord. 1994, 9, 493–507. [Google Scholar] [CrossRef]
- Colosimo, C.; Bologna, M.; Lamberti, S.; Avanzino, L.; Marinelli, L.; Fabbrini, G.; Abbruzzese, G.; Defazio, G.; Berardelli, A. A comparative study of primary and secondary hemifacial spasm. Arch. Neurol. 2006, 63, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P. New insights into the pathophysiology of primary hemifacial spasm. Neurochirurgie 2018, 64, 87–93. [Google Scholar] [CrossRef]
- Marsili, L.; Rizzo, G.; Colosimo, C. Diagnostic Criteria for Parkinson’s Disease: From James Parkinson to the Concept of Prodromal Disease. Front. Neurol. 2018, 9, 156. [Google Scholar] [CrossRef]
- Krauss, J.K.; Paduch, T.; Mundinger, F.; Seeger, W. Parkinsonism and rest tremor secondary to supratentorial tumours sparing the basal ganglia. Acta Neurochir. 1995, 133, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, D.; Romano, A.; Mastronardi, L.; Pellicano, C.; Bozzao, A.; Pontieri, F.E. Hemiparkinsonism due to frontal meningioma. Acta Neurol. Belg. 2008, 108, 29–32. [Google Scholar] [PubMed]
- Yoshimura, M.; Yamamoto, T.; Iso-o, N.; Imafuku, I.; Momose, T.; Shirouzu, I.; Kwak, S.; Kanazawa, I. Hemiparkinsonism associated with a mesencephalic tumor. J. Neurol. Sci. 2002, 197, 89–92. [Google Scholar] [CrossRef]
- Fabbrini, G.; Baronti, F.; Ruggieri, S.; Lenzi, G.L. Meningioma-induced loss of antiparkinsonian response to levodopa. Mov. Disord. 1995, 10, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W.; Rademakers, R.; Hutton, M.L. Progressive supranuclear palsy: Pathology and genetics. Brain Pathol. 2007, 17, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Siderowf, A.D.; Galetta, S.L.; Hurtig, H.I.; Liu, G.T. Posey and Spiller and progressive supranuclear palsy: An incorrect attribution. Mov. Disord. 1998, 13, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, J.; Rosenfeld, M.R. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008, 7, 327–340. [Google Scholar] [CrossRef] [Green Version]
- Chirra, M.; Marsili, L.; Gallerini, S.; Keeling, E.G.; Marconi, R.; Colosimo, C. Paraneoplastic movement disorders: Phenomenology, diagnosis, and treatment. Eur. J. Intern. Med. 2019, 67, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, G.B.; Klein, R.; Simmonds, M.A.; Kornguth, S.E. Autoimmune basis for visual paraneoplastic syndrome in patients with small-cell lung carcinoma. Lancet 1985, 1, 658–661. [Google Scholar] [CrossRef]
- Vogrig, A.; Muñiz-Castrillo, S.; Desestret, V.; Joubert, B.; Honnorat, J. Pathophysiology of paraneoplastic and autoimmune encephalitis: Genes, infections, and checkpoint inhibitors. Adv. Neurol. Disord. 2020, 13, 1756286420932797. [Google Scholar] [CrossRef] [PubMed]
- Honorat, J.A.; Komorowski, L.; Josephs, K.A.; Fechner, K.; St Louis, E.K.; Hinson, S.R.; Lederer, S.; Kumar, N.; Gadoth, A.; Lennon, V.A.; et al. IgLON5 antibody: Neurological accompaniments and outcomes in 20 patients. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graus, F.; Vogrig, A.; Muñiz-Castrillo, S.; Antoine, J.G.; Desestret, V.; Dubey, D.; Giometto, B.; Irani, S.R.; Joubert, B.; Leypoldt, F.; et al. Updated Diagnostic Criteria for Paraneoplastic Neurologic Syndromes. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Graus, F.; Titulaer, M.J.; Balu, R.; Benseler, S.; Bien, C.G.; Cellucci, T.; Cortese, I.; Dale, R.C.; Gelfand, J.M.; Geschwind, M.; et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016, 15, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Gövert, F.; Leypoldt, F.; Junker, R.; Wandinger, K.P.; Deuschl, G.; Bhatia, K.P.; Balint, B. Antibody-related movement disorders—A comprehensive review of phenotype-autoantibody correlations and a guide to testing. Neurol. Res. Pract. 2020, 2, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogrig, A.; Péricart, S.; Pinto, A.L.; Rogemond, V.; Muñiz-Castrillo, S.; Picard, G.; Selton, M.; Mittelbronn, M.; Lanoiselée, H.M.; Michenet, P.; et al. Immunopathogenesis and proposed clinical score for identifying Kelch-like protein-11 encephalitis. Brain Commun. 2021, 3, fcab185. [Google Scholar] [CrossRef]
- Peterson, K.; Rosenblum, M.K.; Kotanides, H.; Posner, J.B. Paraneoplastic cerebellar degeneration. I. A clinical analysis of 55 anti-Yo antibody-positive patients. Neurology 1992, 42, 1931–1937. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Kryzer, T.J.; Griesmann, G.E.; Kim, K.; Benarroch, E.E.; Lennon, V.A. CRMP-5 neuronal autoantibody: Marker of lung cancer and thymoma-related autoimmunity. Ann. Neurol. 2001, 49, 146–154. [Google Scholar] [CrossRef]
- Balint, B.; Vincent, A.; Meinck, H.M.; Irani, S.R.; Bhatia, K.P. Movement disorders with neuronal antibodies: Syndromic approach, genetic parallels and pathophysiology. Brain 2018, 141, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Darnell, R.B.; Posner, J.B. Paraneoplastic syndromes involving the nervous system. N. Engl. J. Med. 2003, 349, 1543–1554. [Google Scholar] [CrossRef]
- Dalmau, J.; Graus, F.; Villarejo, A.; Posner, J.B.; Blumenthal, D.; Thiessen, B.; Saiz, A.; Meneses, P.; Rosenfeld, M.R. Clinical analysis of anti-Ma2-associated encephalitis. Brain 2004, 127, 1831–1844. [Google Scholar] [CrossRef] [Green Version]
- Xing, F.; Marsili, L.; Truong, D.D. Parkinsonism in Viral, Paraneoplastic, and Autoimmune Diseases. J. Neurol. Sci. 2021, 120014, in press. [Google Scholar] [CrossRef]
- Gaig, C.; Graus, F.; Compta, Y.; Högl, B.; Bataller, L.; Brüggemann, N.; Giordana, C.; Heidbreder, A.; Kotschet, K.; Lewerenz, J.; et al. Clinical manifestations of the anti-IgLON5 disease. Neurology 2017, 88, 1736–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, D.M.; Tai, P.; Dalmau, J.; Wennberg, R. Tonic seizures: A diagnostic clue of anti-LGI1 encephalitis? Neurology 2011, 76, 1355–1357. [Google Scholar] [CrossRef] [Green Version]
- Meinck, H.M.; Thompson, P.D. Stiff man syndrome and related conditions. Mov. Disord. 2002, 17, 853–866. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Guidon, A.C.; Burton, L.B.; Chwalisz, B.K.; Hillis, J.; Schaller, T.H.; Amato, A.A.; Betof Warner, A.; Brastianos, P.K.; Cho, T.A.; Clardy, S.L.; et al. Consensus disease definitions for Neurologic immune-related adverse events of immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e002890. [Google Scholar] [CrossRef]
- Marini, A.; Bernardini, A.; Gigli, G.L.; Valente, M.; Muñiz-Castrillo, S.; Honnorat, J.; Vogrig, A. Neurologic Adverse Events of Immune Checkpoint Inhibitors: A Systematic Review. Neurology 2021, 96, 754–766. [Google Scholar] [CrossRef]
- Graus, F.; Dalmau, J. Paraneoplastic Neurological syndromes in the era of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2019, 16, 535–548. [Google Scholar] [CrossRef]
- Yshii, L.M.; Gebauer, C.M.; Pignolet, B.; Mauré, E.; Quériault, C.; Pierau, M.; Saito, H.; Suzuki, N.; Brunner-Weinzierl, M.; Bauer, J.; et al. CTLA4 blockade elicits paraneoplastic Neurological disease in a mouse model. Brain 2016, 139, 2923–2934. [Google Scholar] [CrossRef] [Green Version]
- Vogrig, A.; Fouret, M.; Joubert, B.; Picard, G.; Rogemond, V.; Pinto, A.L.; Muñiz-Castrillo, S.; Roger, M.; Raimbourg, J.; Dayen, C.; et al. Increased frequency of anti-Ma2 encephalitis associated with immune checkpoint inhibitors. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e604. [Google Scholar] [CrossRef] [Green Version]
- Zekeridou, A.; Kryzer, T.; Guo, Y.; Hassan, A.; Lennon, V.; Lucchinetti, C.F.; Pittock, S.; McKeon, A. Phosphodiesterase 10A IgG: A novel biomarker of paraneoplastic Neurologic autoimmunity. Neurology 2019, 93, e815–e822. [Google Scholar] [CrossRef] [Green Version]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, B.W.; Priesterbach-Ackley, L.P.; Petersen, J.K.; Wesseling, P. Molecular pathology of tumors of the central nervous system. Ann. Oncol. 2019, 30, 1265–1278. [Google Scholar] [CrossRef]
- Jelski, W.; Mroczko, B. Molecular and Circulating Biomarkers of Brain Tumors. Int. J. Mol. Sci. 2021, 22, 7039. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, K.; O’Mahoney, K.; Dowling, P.; O’Gorman, P.; Bazou, D. Clinical Proteomics of Biofluids in Haematological Malignancies. Int. J. Mol. Sci. 2021, 22, 8021. [Google Scholar] [CrossRef]
- Mayo Foundation for Medical Education and Research. Paraneoplastic, Autoantibody Evaluation, Serum. Available online: https://Neurology.testcatalog.org/show/PAVAL (accessed on 28 September 2021).
- Déchelotte, B.; Muñiz-Castrillo, S.; Joubert, B.; Vogrig, A.; Picard, G.; Rogemond, V.; Pinto, A.L.; Lombard, C.; Desestret, V.; Fabien, N.; et al. Diagnostic yield of commercial immunodots to diagnose paraneoplastic Neurologic syndromes. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e701. [Google Scholar] [CrossRef] [Green Version]
- Alruwaili, A.A.; De Jesus, O. Meningioma. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ko, C.C.; Yeh, L.R.; Kuo, Y.T.; Chen, J.H. Imaging biomarkers for evaluating tumor response: RECIST and beyond. Biomark Res. 2021, 9, 52. [Google Scholar] [CrossRef]
- Zoccarato, M.; Gastaldi, M.; Zuliani, L.; Biagioli, T.; Brogi, M.; Bernardi, G.; Corsini, E.; Bazzigaluppi, E.; Fazio, R.; Giannotta, C.; et al. Diagnostics of paraneoplastic Neurological syndromes. Neurol. Sci. 2017, 38, 237–242. [Google Scholar] [CrossRef]
- Dalakas, M.C.; Fujii, M.; Li, M.; Lutfi, B.; Kyhos, J.; McElroy, B. High-dose intravenous immune globulin for stiff-person syndrome. N. Engl. J. Med. 2001, 345, 1870–1876. [Google Scholar] [CrossRef]
- Dubey, D.; Britton, J.; McKeon, A.; Gadoth, A.; Zekeridou, A.; Lopez Chiriboga, S.A.; Devine, M.; Cerhan, J.H.; Dunlay, K.; Sagen, J.; et al. Randomized Placebo-Controlled Trial of Intravenous Immunoglobulin in Autoimmune LGI1/CASPR2 Epilepsy. Ann. Neurol. 2020, 87, 313–323. [Google Scholar] [CrossRef] [Green Version]
- National Comprehensive Cancer Network. Available online: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf (accessed on 29 September 2021).
- Cardoso, F. Autoimmune choreas. J. Neurol. Neurosurg. Psychiatry 2017, 88, 412–417. [Google Scholar] [CrossRef]
- McKeon, A.; Marnane, M.; O’Connell, M.; Stack, J.P.; Kelly, P.J.; Lynch, T. Potassium channel antibody associated encephalopathy presenting with a frontotemporal dementia like syndrome. Arch. Neurol. 2007, 64, 1528–1530. [Google Scholar] [CrossRef] [Green Version]
- Sabater, L.; Gaig, C.; Gelpi, E.; Bataller, L.; Lewerenz, J.; Torres-Vega, E.; Contreras, A.; Giometto, B.; Compta, Y.; Embid, C.; et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: A case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014, 13, 575–586. [Google Scholar] [CrossRef] [Green Version]
- Giannoccaro, M.P.; Gastaldi, M.; Rizzo, G.; Jacobson, L.; Vacchiano, V.; Perini, G.; Capellari, S.; Franciotta, D.; Costa, A.; Liguori, R.; et al. Antibodies to neuronal surface antigens in patients with a clinical diagnosis of neurodegenerative disorder. Brain Behav. Immun. 2021, 96, 106–112. [Google Scholar] [CrossRef]
- Çoban, A.; Ismail Küçükali, C.; Bilgiç, B.; Yalçınkaya, N.; Haytural, H.; Ulusoy, C.; Turan, S.; Çakır, S.; Uçok, A.; Ünübol, H.; et al. Evaluation of incidence and clinical features of antibody-associated autoimmune encephalitis mimicking dementia. Behav. Neurol. 2014, 2014, 935379. [Google Scholar] [CrossRef]
- Flanagan, E.P.; McKeon, A.; Lennon, V.A.; Boeve, B.F.; Trenerry, M.R.; Tan, K.M.; Drubach, D.A.; Josephs, K.A.; Britton, J.W.; Mandrekar, J.N.; et al. Autoimmune dementia: Clinical course and predictors of immunotherapy response. Mayo Clin. Proc. 2010, 85, 881–897. [Google Scholar] [CrossRef] [PubMed]
- Doss, S.; Wandinger, K.P.; Hyman, B.T.; Panzer, J.A.; Synofzik, M.; Dickerson, B.; Mollenhauer, B.; Scherzer, C.R.; Ivinson, A.J.; Finke, C.; et al. High prevalence of NMDA receptor IgA/IgM antibodies in different dementia types. Ann. Clin. Transl. Neurol. 2014, 1, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; McGuirk, J.P. CAR T cells: Continuation in a revolution of immunotherapy. Lancet Oncol. 2020, 21, e168–e178. [Google Scholar] [CrossRef]
- Perrinjaquet, C.; Desbaillets, N.; Hottinger, A.F. Neurotoxicity associated with cancer immunotherapy: Immune checkpoint inhibitors and chimeric antigen receptor T-cell therapy. Curr. Opin. Neurol. 2019, 32, 500–510. [Google Scholar] [CrossRef] [PubMed]

| Antibody | Antigen Location | Cancer Risk | Cancer Type | Associated Movement Disorders | Other Clinical Features | |
|---|---|---|---|---|---|---|
| Anti-Yo | I | High | Breast cancer, ovary cancer | Ataxia | Uncommon | |
| Anti-Hu/ANNA1 | I | High | SCLC, NSCLC | Ataxia, chorea, OMS | SN, EM, LE | |
| Anti-Ri/ANNA2 | I | High | Breast cancer in women, lung cancer in men | Ataxia, OMS, parkinsonism, jaw dystonia, laryngospasm | BE | |
| Anti-Tr/DNER | I | High | Hodgkin lymphoma | Ataxia | Uncommon | |
| * Anti- KLHL11 | I | High | Testicular germ cell cancer (Typically, “burned-out”) | Ataxia (including paroxysmal) | BE, myelitis, LE | |
| Anti-PCA2 | I | High | SCLC, NSCLC, breast cancer | Ataxia | Neuropathy, EM | |
| Anti-Ma2 | I | High | Testicular cancer and NSCLC | Parkinsonism | LE, BE, diencephalitis | |
| Anti-CV2/CRMP5 | I | High | SCLC and thymoma | Ataxia, chorea | SN and EM | |
| Anti-Amphiphysin | I | High | SCLC and breast cancer | SPS | EM, SN, polyradiculoneuropathy | |
| Anti-GABAB-R | E | Intermediate | SCLC | Ataxia, OMS | LE | |
| Anti-CASPR2 | E | Intermediate | Thymoma | Ataxia (including paroxysmal), chorea | LE, Morvan syndrome, neuromyotonia | |
| Anti-NMDA-R | E | Intermediate | Ovarian or extra-ovarian teratoma | Dyskinesia (orofacial and limb), chorea, dystonia, stereotypies, myoclonus, ataxia, parkinsonism | Encephalitis | |
| Anti-AMPA-R | E | Intermediate | SCLC and thymoma | Tremor | LE | |
| Anti-LGI1 | E | Low | Thymoma | Facio-brachial dystonic seizures, chorea, myoclonus, tremor | LE | |
| Anti-GAD | S/I | Low | Rare (SCLC and thymoma) | Ataxia (including paroxysmal), SPS | LE | |
| Anti-DPPX | E | Low | Lymphoma | Tremor, myoclonus, startle, ataxia, parkinsonism, PERM, SPS | Encephalitis | |
| Anti-GFAP | I | Low | Ovarian teratoma and adenocarcinoma | Ataxia, tremor | Meningoencephalitis | |
| Anti-Glycine-R | E | Low | Lymphoma, thymoma and lung cancer | PERM and SPS | LE | |
| Anti-mGLUR-1 | E | Low | Lymphoma | Ataxia, myoclonus, dystonia, tremor | Behavioral changes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marsili, L.; Vogrig, A.; Colosimo, C. Movement Disorders in Oncology: From Clinical Features to Biomarkers. Biomedicines 2022, 10, 26. https://doi.org/10.3390/biomedicines10010026
Marsili L, Vogrig A, Colosimo C. Movement Disorders in Oncology: From Clinical Features to Biomarkers. Biomedicines. 2022; 10(1):26. https://doi.org/10.3390/biomedicines10010026
Chicago/Turabian StyleMarsili, Luca, Alberto Vogrig, and Carlo Colosimo. 2022. "Movement Disorders in Oncology: From Clinical Features to Biomarkers" Biomedicines 10, no. 1: 26. https://doi.org/10.3390/biomedicines10010026
APA StyleMarsili, L., Vogrig, A., & Colosimo, C. (2022). Movement Disorders in Oncology: From Clinical Features to Biomarkers. Biomedicines, 10(1), 26. https://doi.org/10.3390/biomedicines10010026







