Cancer Studies under Space Conditions: Finding Answers Abroad
Abstract
1. Introduction
2. Cancer
2.1. Definition of Cancer
2.2. Epidemiology for Cancer in Astronauts/Cosmonauts
3. General Effects of Microgravity
3.1. In Vivo Animal Models
3.2. Important Considerations for Analysing Cancer Cells in Real or Simulated Microgravity
3.3. At the Cell Level
3.4. Multicellular Spheroid Formation
3.5. Ground and Space Facilities to Study Microgravity Changes
3.6. Brief Description of the Biophysics of Cancer in Space
3.7. Graviperception System in Non-specialised Mammalian Cells
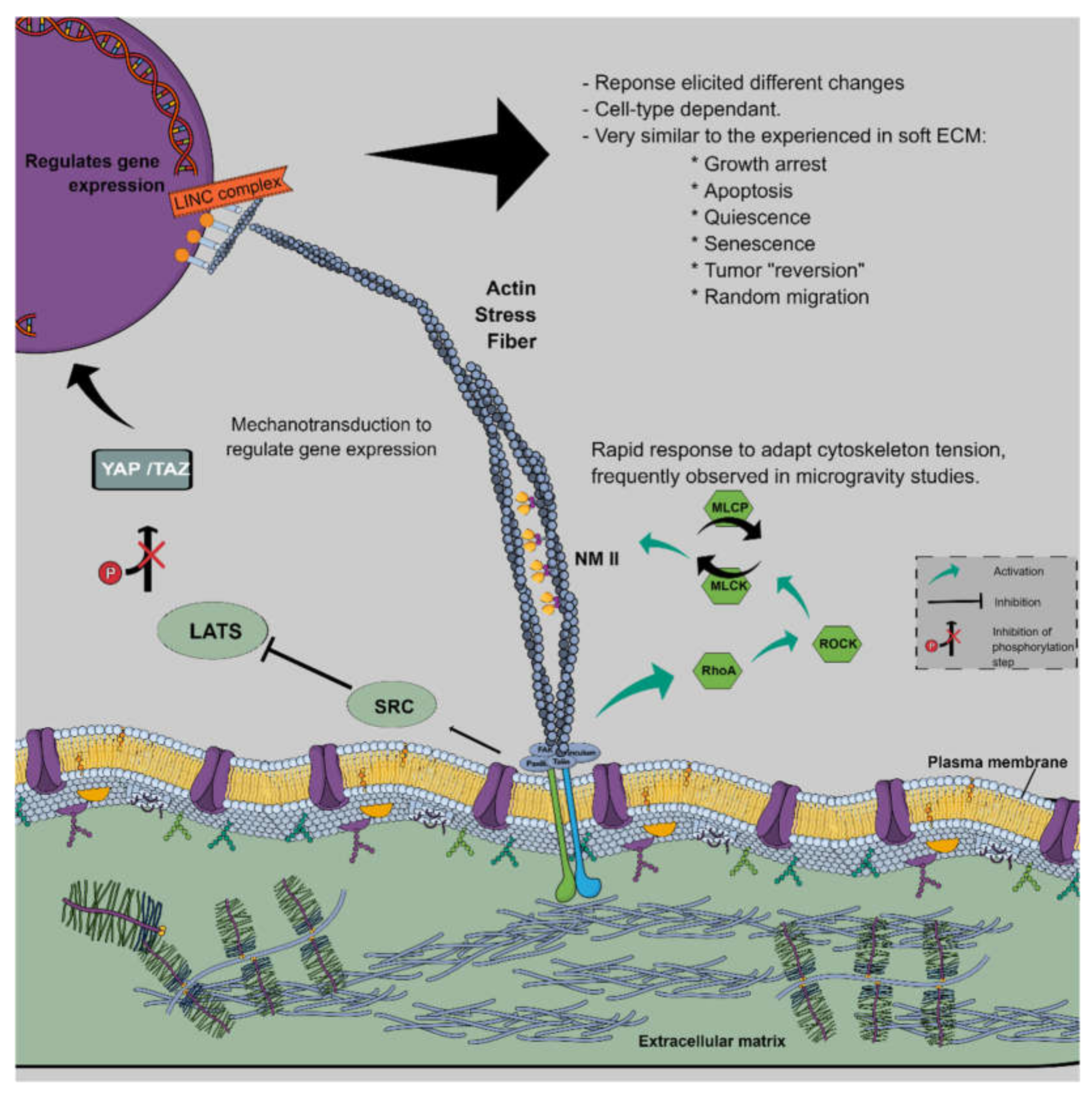
3.7.1. The Cytoskeleton Interaction with Microgravity
3.7.2. YAP/TAZ: Mechanosensor Hub and Mechano-Effector
- signals from the ECM, mediated by focal adhesions, activate different kinases, like RhoA and Src, depending on ECM stiffness and available area;
- signals from neighbouring cells, by way of tight and adherens junctions, generally downregulate YAP/TAZ nuclear entry by Hippo-dependent and independent mechanisms, which mediate the contact-inhibition process [50];
- polarity in the epithelial cells by the Hippo pathway [51], its primary inhibitor, mainly by phosphorylation and proteasomal degradation, preventing nuclear entry.
3.7.3. Coherent Model: Mechanobiology and Cancer in Microgravity
4. General Effects of Radiation on DNA/Cancer Cells
4.1. High Versus Low Linear Energy Transfer
4.2. Mixed Beam Radiation and Sequential Exposure
4.3. Indirect Damage, Non-Targeted Effects, and Bystander Effects
4.4. Accurate Space Radiation Simulation
4.5. Low-Dose Radiation
4.6. DNA Repair Pathways and Markers under Space Conditions
5. Combination of Radiation and Microgravity
6. Updated Knowledge on Microgravity Research
6.1. Breast Cancer
6.1.1. Real Microgravity Studies
6.1.2. Simulated Microgravity Studies
6.2. Thyroid Cancer
6.2.1. Real Microgravity Studies
6.2.2. Simulated Microgravity Studies
6.3. Melanoma
6.4. Haematological Disorders
6.5. Gastrointestinal Tract and Liver
6.6. Prostate Cancer
6.7. Lung Cancer
6.8. Brain Tumours
6.9. Bone Tumours
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akiyama, T.; Horie, K.; Hinoi, E.; Hiraiwa, M.; Kato, A.; Maekawa, Y.; Takahashi, A.; Furukawa, S. How does spaceflight affect the acquired immune system? NPJ Microgravity 2020, 6, 14. [Google Scholar] [CrossRef]
- Tackling the cancer epidemic. Lancet Oncol. 2015, 16, 349. [CrossRef]
- Hassanpour, S.H.; Dehghani, M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017, 4, 127–129. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Grimm, D.; Bauer, J.; Wise, P.; Krüger, M.; Simonsen, U.; Wehland, M.; Infanger, M.; Corydon, T.J. The role of SOX family members in solid tumours and metastasis. Semin. Cancer Biol. 2020, 67, 122–153. [Google Scholar] [CrossRef] [PubMed]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, 144. [Google Scholar] [CrossRef]
- Elgart, S.R.; Little, M.P.; Chappell, L.J.; Milder, C.M.; Shavers, M.R.; Huff, J.L.; Patel, Z.S. Radiation Exposure and Mortality from Cardiovascular Disease and Cancer in Early NASA Astronauts. Sci. Rep. 2018, 8, 8480. [Google Scholar] [CrossRef]
- Reynolds, R.J.; Day, S.M. Mortality of US astronauts: Comparisons with professional athletes. Occup. Environ. Med. 2019, 76, 114–117. [Google Scholar] [CrossRef]
- Ushakov, I.B.; Voronkov, Y.I.; Bukhtiyarov, I.V.; Tikhonova, G.I.; Gorchakova, T.Y.; Bryleva, M.S. A Cohort Mortality Study Among Soviet and Russian Cosmonauts, 1961–2014. Aerosp. Med. Hum. Perform. 2017, 88, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.J.; Bukhtiyarov, I.V.; Tikhonova, G.I.; Day, S.M.; Ushakov, I.B.; Gorchakova, T.Y.U. Contrapositive logic suggests space radiation not having a strong impact on mortality of US astronauts and Soviet and Russian cosmonauts. Sci. Rep. 2019, 9, 8583. [Google Scholar] [CrossRef]
- Gridley, D.S.; Mao, X.W.; Stodieck, L.S.; Ferguson, V.L.; Bateman, T.A.; Moldovan, M.; Cunningham, C.E.; Jones, T.A.; Slater, J.M.; Pecaut, M.J. Changes in mouse thymus and spleen after return from the STS-135 mission in space. PLoS ONE 2013, 8, e75097. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.G.; Allen, P.L.; Birdsall, H.H. Effects of Space Flight on Mouse Liver versus Kidney: Gene Pathway Analyses. Int. J. Mol. Sci. 2018, 19, 4106. [Google Scholar] [CrossRef] [PubMed]
- Eyckmans, J.; Boudou, T.; Yu, X.; Chen, C.S. A hitchhiker’s guide to mechanobiology. Dev. Cell 2011, 21, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Dietz, C.; Infanger, M.; Romswinkel, A.; Strube, F.; Kraus, A. Apoptosis Induction and Alteration of Cell Adherence in Human Lung Cancer Cells under Simulated Microgravity. Int. J. Mol. Sci. 2019, 20, 3601. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.; Grimm, D.; Strauch, S.M.; Erzinger, G.S.; Corydon, T.J.; Lebert, M.; Magnusson, N.E.; Infanger, M.; Richter, P.; Krüger, M. Influence of Microgravity on Apoptosis in Cells, Tissues, and Other Systems In Vivo and In Vitro. Int. J. Mol. Sci. 2020, 21, 9373. [Google Scholar] [CrossRef]
- Arun, R.P.; Sivanesan, D.; Patra, B.; Varadaraj, S.; Verma, R.S. Simulated microgravity increases polyploid giant cancer cells and nuclear localization of YAP. Sci. Rep. 2019, 9, 10684. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Guo, S.; Li, B.-B.; Jiang, N.; Li, A.; Yan, H.-F.; Yang, H.-M.; Zhou, J.-L.; Li, C.-L.; Cui, Y. Effect of Weightlessness on the 3D Structure Formation and Physiologic Function of Human Cancer Cells. Biomed. Res. Int. 2019, 2019, 4894083. [Google Scholar] [CrossRef]
- Krüger, M.; Bauer, J.; Grimm, D. Cancer Research in Space. In Biotechnology in Space; Ruyters, G., Betzel, C., Grimm, D., Eds.; Springer: Cham, Switzerland, 2017; pp. 87–106. [Google Scholar]
- Nassef, M.Z.; Melnik, D.; Kopp, S.; Sahana, J.; Infanger, M.; Lützenberg, R.; Relja, B.; Wehland, M.; Grimm, D.; Krüger, M. Breast Cancer Cells in Microgravity: New Aspects for Cancer Research. Int. J. Mol. Sci. 2020, 21, 7345. [Google Scholar] [CrossRef]
- Krüger, M.; Melnik, D.; Kopp, S.; Buken, C.; Sahana, J.; Bauer, J.; Wehland, M.; Hemmersbach, R.; Corydon, T.J.; Infanger, M.; et al. Fighting thyroid cancer with microgravity research. Int. J. Mol. Sci. 2019, 20, 2553. [Google Scholar] [CrossRef]
- Kopp, S.; Krüger, M.; Bauer, J.; Wehland, M.; Corydon, T.J.; Sahana, J.; Nassef, M.Z.; Melnik, D.; Bauer, T.J.; Schulz, H.; et al. Microgravity Affects Thyroid Cancer Cells during the TEXUS-53 Mission Stronger than Hypergravity. Int. J. Mol. Sci. 2018, 19, 4001. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Gilmour, D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2009, 10, 445–457. [Google Scholar] [CrossRef]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Melnik, D.; Sahana, J.; Corydon, T.J.; Kopp, S.; Nassef, M.Z.; Wehland, M.; Infanger, M.; Grimm, D.; Krüger, M. Dexamethasone Inhibits Spheroid Formation of Thyroid Cancer Cells Exposed to Simulated Microgravity. Cells 2020, 9, 367. [Google Scholar] [CrossRef]
- Sahana, J.; Nassef, M.Z.; Wehland, M.; Kopp, S.; Krüger, M.; Corydon, T.J.; Infanger, M.; Bauer, J.; Grimm, D. Decreased E-Cadherin in MCF7 Human Breast Cancer Cells Forming Multicellular Spheroids Exposed to Simulated Microgravity. Proteomics 2018, 18, e1800015. [Google Scholar] [CrossRef] [PubMed]
- Weaver, V.M.; Petersen, O.W.; Wang, F.; Larabell, C.A.; Briand, P.; Damsky, C.; Bissell, M.J. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 1997, 137, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Reddig, P.J.; Juliano, R.L. Clinging to life: Cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005, 24, 425–439. [Google Scholar] [CrossRef]
- Morozevich, G.E.; Kozlova, N.I.; Susova, O.Y.; Karalkin, P.A.; Berman, A.E. Implication of α2β1 integrin in anoikis of MCF-7 human breast carcinoma cells. Biochemistry 2015, 80, 97–103. [Google Scholar] [CrossRef]
- Akekawatchai, C.; Roytrakul, S.; Kittisenachai, S.; Isarankura-Na-Ayudhya, P.; Jitrapakdee, S. Protein profiles associated with anoikis resistance of metastatic MDA-MB-231 breast cancer cells. Asian Pac. J. Cancer Prev. 2016, 17, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-J.; Hsu, Y.-C.; Li, Y.-S.; Cheng, S.-P. Galectin-3 inhibitors suppress anoikis resistance and invasive capacity in thyroid cancer cells. Int. J. Endocrinol. 2021, 2021, 5583491. [Google Scholar] [CrossRef]
- Hargens, A.R.; Vico, L. Long-duration bed rest as an analog to microgravity. J. Appl. Physiol. 2016, 120, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Poon, C. Factors implicating the validity and interpretation of mechanobiology studies in simulated microgravity environments. Eng. Rep. 2020, 2, e12242. [Google Scholar] [CrossRef]
- Montagner, M.; Dupont, S. Mechanical Forces as Determinants of Disseminated Metastatic Cell Fate. Cells 2020, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Meng, X.; Dong, D.; Xue, Y.; Chen, X.; Wang, S.; Shen, Y.; Zhang, G.; Shang, P. Iron overload involved in the enhancement of unloading-induced bone loss by hypomagnetic field. Bone 2018, 114, 235–245. [Google Scholar] [CrossRef]
- Xue, Y.; Yang, J.; Luo, J.; Ren, L.; Shen, Y.; Dong, D.; Fang, Y.; Hu, L.; Liu, M.; Liao, Z.; et al. Disorder of iron metabolism inhibits the recovery of unloading-induced bone loss in hypomagnetic field. J. Bone Miner. Res. 2020, 35, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D. How cells (might) sense microgravity. FASEB J. 1999, 13, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Sieber, M.; Hanke, W.; Kohn, F.P.M. Modification of membrane fluidity by gravity. Open J. Biophys. 2014, 04, 105–111. [Google Scholar] [CrossRef][Green Version]
- Corydon, T.J.; Kopp, S.; Wehland, M.; Braun, M.; Schütte, A.; Mayer, T.; Hülsing, T.; Oltmann, H.; Schmitz, B.; Hemmersbach, R.; et al. Alterations of the cytoskeleton in human cells in space proved by life-cell imaging. Sci. Rep. 2016, 6, 20043. [Google Scholar] [CrossRef]
- Ishihara, E.; Nishina, H. The hippo-YAP pathway regulates 3D organ formation and homeostasis. Cancers 2018, 10, 122. [Google Scholar] [CrossRef]
- Moes, M.J.A.; Gielen, J.C.; Bleichrodt, R.-J.; van Loon, J.J.W.A.; Christianen, P.C.M.; Boonstra, J. Simulation of Microgravity by Magnetic Levitation and Random Positioning: Effect on Human A431 Cell Morphology. Microgravity Sci. Technol. 2011, 23, 249–261. [Google Scholar] [CrossRef]
- Gershovich, P.M.; Gershovich, J.G.; Buravkova, L.B. Cytoskeleton structure and adhesion properties of human stromal precursors under conditions of simulated microgravity. Cell Tissue Biol. 2009, 3, 423–430. [Google Scholar] [CrossRef]
- Zayzafoon, M.; Meyers, V.E.; McDonald, J.M. Microgravity: The immune response and bone. Immunol. Rev. 2005, 208, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Croute, F.; Gaubin, Y.; Pianezzi, B.; Soleilhavoup, J.P. Effects of hypergravity on the cell shape and on the organization of cytoskeleton and extracelluar matrix molecules of in vitro human dermal fibroblasts. Microgravity Sci. Technol. 1995, 8, 118–124. [Google Scholar] [PubMed]
- Vorselen, D.; Roos, W.H.; MacKintosh, F.C.; Wuite, G.J.L.; van Loon, J.J.W.A. The role of the cytoskeleton in sensing changes in gravity by nonspecialized cells. FASEB J. 2014, 28, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Xu, A.; Zhao, T.; Zhao, Q.; Zhang, J.; Fan, C.; Deng, Y.; Freywald, A.; Genth, H.; Xiang, J. Simulated microgravity inhibits cell focal adhesions leading to reduced melanoma cell proliferation and metastasis via FAK/RhoA-regulated mTORC1 and AMPK pathways. Sci. Rep. 2018, 8, 3769. [Google Scholar] [CrossRef]
- Deng, B.; Liu, R.; Tian, X.; Han, Z.; Chen, J. Simulated microgravity inhibits the viability and migration of glioma via FAK/RhoA/Rock and FAK/Nek2 signaling. In Vitro Cell. Dev. Biol. Anim. 2019, 55, 260–271. [Google Scholar] [CrossRef]
- Finch-Edmondson, M.; Sudol, M. Framework to function: Mechanosensitive regulators of gene transcription. Cell. Mol. Biol. Lett. 2016, 21, 28. [Google Scholar] [CrossRef]
- Pocaterra, A.; Romani, P.; Dupont, S. YAP/TAZ functions and their regulation at a glance. J. Cell Sci. 2020, 133, jcs230425. [Google Scholar] [CrossRef]
- Charras, G.; Yap, A.S. Tensile Forces and Mechanotransduction at Cell-Cell Junctions. Curr. Biol. 2018, 28, R445–R457. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, D. The Hippo signaling pathway in development and disease. Dev. Cell 2019, 50, 264–282. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ye, X.; Yu, J.; Li, L.; Li, W.; Li, S.; Yu, J.; Lin, J.D.; Wang, C.-Y.; Chinnaiyan, A.M.; et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008, 22, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Shreberk-Shaked, M.; Oren, M. New insights into YAP/TAZ nucleo-cytoplasmic shuttling: New cancer therapeutic opportunities? Mol. Oncol. 2019, 13, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Elosegui-Artola, A.; Andreu, I.; Beedle, A.E.M.; Lezamiz, A.; Uroz, M.; Kosmalska, A.J.; Oria, R.; Kechagia, J.Z.; Rico-Lastres, P.; Le Roux, A.-L.; et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 2017, 171, 1397–1410.e14. [Google Scholar] [CrossRef] [PubMed]
- Nardone, G.; Oliver-De La Cruz, J.; Vrbsky, J.; Martini, C.; Pribyl, J.; Skládal, P.; Pešl, M.; Caluori, G.; Pagliari, S.; Martino, F.; et al. YAP regulates cell mechanics by controlling focal adhesion assembly. Nat. Commun. 2017, 8, 15321. [Google Scholar] [CrossRef] [PubMed]
- Neelam, S.; Richardson, B.; Barker, R.; Udave, C.; Gilroy, S.; Cameron, M.J.; Levine, H.G.; Zhang, Y. Changes in Nuclear Shape and Gene Expression in Response to Simulated Microgravity Are LINC Complex-Dependent. Int. J. Mol. Sci. 2020, 21, 6762. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, Y.; Nishina, H.; Furutani-Seiki, M. YAP is essential for 3D organogenesis withstanding gravity. Dev. Growth Differ. 2017, 59, 52–58. [Google Scholar] [CrossRef]
- Thompson, M.; Woods, K.; Newberg, J.; Oxford, J.T.; Uzer, G. Low-intensity vibration restores nuclear YAP levels and acute YAP nuclear shuttling in mesenchymal stem cells subjected to simulated microgravity. NPJ Microgravity 2020, 6, 35. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, Q.; Lin, C.; Kuang, D.; Song, G. Simulated microgravity inhibits osteogenic differentiation of mesenchymal stem cells via depolymerizing F-actin to impede TAZ nuclear translocation. Sci. Rep. 2016, 6, 30322. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Kopp, S.; Schlagberger, E.M.; Grosse, J.; Sahana, J.; Riwaldt, S.; Wehland, M.; Luetzenberg, R.; Infanger, M.; Grimm, D. Proteome Analysis of Human Follicular Thyroid Cancer Cells Exposed to the Random Positioning Machine. Int. J. Mol. Sci. 2017, 18, 546. [Google Scholar] [CrossRef]
- Romani, P.; Valcarcel-Jimenez, L.; Frezza, C.; Dupont, S. Crosstalk between mechanotransduction and metabolism. Nat. Rev. Mol. Cell Biol. 2021, 22, 22–38. [Google Scholar] [CrossRef]
- Callahan, R.; Webster, E.; Mahmood, U. Imaging Physics. In Primer of Diagnostic Imaging; Elsevier: Amsterdam, The Netherlands, 2011; pp. 690–746. ISBN 9780323065382. [Google Scholar]
- Sage, E.; Harrison, L. Clustered DNA lesion repair in eukaryotes: Relevance to mutagenesis and cell survival. Mutat. Res. 2011, 711, 123–133. [Google Scholar] [CrossRef]
- Lorat, Y.; Brunner, C.U.; Schanz, S.; Jakob, B.; Taucher-Scholz, G.; Rübe, C.E. Nanoscale analysis of clustered DNA damage after high-LET irradiation by quantitative electron microscopy--the heavy burden to repair. DNA Repair 2015, 28, 93–106. [Google Scholar] [CrossRef]
- Asaithamby, A.; Chen, D.J. Mechanism of cluster DNA damage repair in response to high-atomic number and energy particles radiation. Mutat. Res. 2011, 711, 87–99. [Google Scholar] [CrossRef] [PubMed]
- George, K.A.; Hada, M.; Cucinotta, F.A. Biological effectiveness of accelerated protons for chromosome exchanges. Front. Oncol. 2015, 5, 226. [Google Scholar] [CrossRef] [PubMed]
- Michalettou, T.-D.; Michalopoulos, I.; Costes, S.V.; Hellweg, C.E.; Hada, M.; Georgakilas, A.G. A meta-analysis of the effects of high-LET ionizing radiations in human gene expression. Life 2021, 11, 115. [Google Scholar] [CrossRef]
- Hada, M.; Meador, J.A.; Cucinotta, F.A.; Gonda, S.R.; Wu, H. Chromosome aberrations induced by dual exposure of protons and iron ions. Radiat. Environ. Biophys. 2007, 46, 125–129. [Google Scholar] [CrossRef]
- Hada, M.; Georgakilas, A.G. Formation of clustered DNA damage after high-LET irradiation: A review. J. Radiat. Res. 2008, 49, 203–210. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Oike, T.; Niimi, A.; Yamauchi, M.; Sato, H.; Limsirichaikul, S.; Held, K.D.; Nakano, T.; Shibata, A. Clustered DNA double-strand break formation and the repair pathway following heavy-ion irradiation. J. Radiat. Res. 2019, 60, 69–79. [Google Scholar] [CrossRef]
- Turker, M.S.; Grygoryev, D.; Lasarev, M.; Ohlrich, A.; Rwatambuga, F.A.; Johnson, S.; Dan, C.; Eckelmann, B.; Hryciw, G.; Mao, J.-H.; et al. Simulated space radiation-induced mutants in the mouse kidney display widespread genomic change. PLoS ONE 2017, 12, e0180412. [Google Scholar] [CrossRef]
- Datta, K.; Suman, S.; Kallakury, B.V.S.; Fornace, A.J., Jr. Heavy ion radiation exposure triggered higher intestinal tumor frequency and greater β-catenin activation than γ radiation in APC(Min/+) mice. PLoS ONE 2013, 8, e59295. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Ogiu, T.; Nishimura, M.; Masaoka, Y.; Kurosumi, M.; Takahashi, T.; Oguri, T.; Shoji, S.; Katoh, O. Comparison of tumorigenesis between accelerated heavy ion and X-ray in B6C3F1 mice. J. Radiat. Res. 1998, 39, 93–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bielefeldt-Ohmann, H.; Genik, P.C.; Fallgren, C.M.; Ullrich, R.L.; Weil, M.M. Animal studies of charged particle-induced carcinogenesis. Health Phys. 2012, 103, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Weil, M.M.; Ray, F.A.; Genik, P.C.; Yu, Y.; McCarthy, M.; Fallgren, C.M.; Ullrich, R.L. Effects of 28Si ions, 56Fe ions, and protons on the induction of murine acute myeloid leukemia and hepatocellular carcinoma. PLoS ONE 2014, 9, e104819. [Google Scholar] [CrossRef] [PubMed]
- Hada, M.; Cucinotta, F.A.; Gonda, S.R.; Wu, H. mBAND analysis of chromosomal aberrations in human epithelial cells exposed to low- and high-LET radiation. Radiat. Res. 2007, 168, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Aoto, T.; Mohri, Y.; Yokozeki, H.; Nishimura, E.K. Coupling of the radiosensitivity of melanocyte stem cells to their dormancy during the hair cycle. J. Dermatol. Sci. 2016, 84, e80. [Google Scholar] [CrossRef]
- Bergonie, J.; Tribondeau, L. Interpretation of some results of radiotherapy and an attempt at determining a logical technique of treatment. Radiat. Res. 1959, 11, 587–588. [Google Scholar] [CrossRef]
- Zhou, G.; Bennett, P.V.; Cutter, N.C.; Sutherland, B.M. Proton-HZE-particle sequential dual-beam exposures increase anchorage-independent growth frequencies in primary human fibroblasts. Radiat. Res. 2006, 166, 488–494. [Google Scholar] [CrossRef]
- Staaf, E.; Brehwens, K.; Haghdoost, S.; Nievaart, S.; Pachnerova-Brabcova, K.; Czub, J.; Braziewicz, J.; Wojcik, A. Micronuclei in human peripheral blood lymphocytes exposed to mixed beams of X-rays and alpha particles. Radiat. Environ. Biophys. 2012, 51, 283–293. [Google Scholar] [CrossRef]
- Staaf, E.; Deperas-Kaminska, M.; Brehwens, K.; Haghdoost, S.; Czub, J.; Wojcik, A. Complex aberrations in lymphocytes exposed to mixed beams of (241)Am alpha particles and X-rays. Mutat. Res. 2013, 756, 95–100. [Google Scholar] [CrossRef]
- Staaf, E.; Brehwens, K.; Haghdoost, S.; Czub, J.; Wojcik, A. Gamma-H2AX foci in cells exposed to a mixed beam of X-rays and alpha particles. Genome Integr. 2012, 3, 8. [Google Scholar] [CrossRef]
- Cheng, L.; Brzozowska, B.; Sollazzo, A.; Lundholm, L.; Lisowska, H.; Haghdoost, S.; Wojcik, A. Simultaneous induction of dispersed and clustered DNA lesions compromises DNA damage response in human peripheral blood lymphocytes. PLoS ONE 2018, 13, e0204068. [Google Scholar] [CrossRef]
- Brzozowska, B.; Tartas, A.; Wojcik, A. Monte Carlo modeling of DNA lesions and chromosomal aberrations induced by mixed beams of alpha particles and X-rays. Front. Phys. 2020, 8, 512. [Google Scholar] [CrossRef]
- Wojcik, A.; Obe, G.; Lisowska, H.; Czub, J.; Nievaart, V.; Moss, R.; Huiskamp, R.; Sauerwein, W. Chromosomal aberrations in peripheral blood lymphocytes exposed to a mixed beam of low energy neutrons and gamma radiation. J. Radiol. Prot. 2012, 32, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.V.; Cutter, N.C.; Sutherland, B.M. Split-dose exposures versus dual ion exposure in human cell neoplastic transformation. Radiat. Environ. Biophys. 2007, 46, 119–123. [Google Scholar] [CrossRef]
- Buonanno, M.; De Toledo, S.M.; Howell, R.W.; Azzam, E.I. Low-dose energetic protons induce adaptive and bystander effects that protect human cells against DNA damage caused by a subsequent exposure to energetic iron ions. J. Radiat. Res. 2015, 56, 502–508. [Google Scholar] [CrossRef]
- Stoilov, L.M.; Mullenders, L.H.F.; Darroudi, F.; Natarajan, A.T. Adaptive response to DNA and chromosomal damage induced by X-rays in human blood lymphocytes. Mutagenesis 2007, 22, 117–122. [Google Scholar] [CrossRef]
- Hada, M.; Saganti, P.B.; Cucinotta, F.A. Nitric oxide is involved in heavy ion-induced non-targeted effects in human fibroblasts. Int. J. Mol. Sci. 2019, 20, 4327. [Google Scholar] [CrossRef]
- Barcellos-Hoff, M.H.; Adams, C.; Balmain, A.; Costes, S.V.; Demaria, S.; Illa-Bochaca, I.; Mao, J.H.; Ouyang, H.; Sebastiano, C.; Tang, J. Systems biology perspectives on the carcinogenic potential of radiation. J. Radiat. Res. 2014, 55, i145–i154. [Google Scholar] [CrossRef][Green Version]
- Mavragani, I.V.; Nikitaki, Z.; Souli, M.P.; Aziz, A.; Nowsheen, S.; Aziz, K.; Rogakou, E.; Georgakilas, A.G. Complex DNA damage: A route to radiation-induced genomic instability and carcinogenesis. Cancers 2017, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, A.; McDonald, J.T.; Miller, J.; Grabham, P.; Costes, S.V. GeneLab database analyses suggest long-term impact of space radiation on the cardiovascular system by the activation of FYN through reactive oxygen species. Int. J. Mol. Sci. 2019, 20, 661. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-T.; Reiter, R.J. Molecular mechanisms of melatonin’s protection against high-LET radiation: Implications for space travel. Melatonin Res. 2020, 3, 503–514. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; To, K.; Cacao, E. Predictions of space radiation fatality risk for exploration missions. Life Sci. Space Res. 2017, 13, 1–11. [Google Scholar] [CrossRef]
- Moreno-Villanueva, M.; Wu, H. Radiation and microgravity—Associated stress factors and carcinogensis. Reach. Out 2019, 13, 100027. [Google Scholar] [CrossRef]
- Norbury, J.W.; Slaba, T.C.; Aghara, S.; Badavi, F.F.; Blattnig, S.R.; Clowdsley, M.S.; Heilbronn, L.H.; Lee, K.; Maung, K.M.; Mertens, C.J.; et al. Advances in space radiation physics and transport at NASA. Life Sci. Space Res. 2019, 22, 98–124. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yamashita, S. Low-dose radiation exposure and carcinogenesis. Jpn. J. Clin. Oncol. 2012, 42, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.F.; Cucinotta, F.A.; Ning-Ang, L.; Zhou, G. Cancer risk of low dose ionizing radiation. Front. Phys. 2020, 8, 234. [Google Scholar] [CrossRef]
- Mewaldt, R.; Davis, A.; Binns, W.; Nolfo, G.; George, J.; Israel, M.; Leske, R.; Stone, E.; Wiedenbeck, M.; Rosenvinge, T. The Cosmic Ray Radiation Dose in Interplanetary Space–Present Day and Worst-Case Evaluations. In Proceedings of the 29th International Cosmic Ray Conference, Pune, India, 3–10 August 2005; Volume 00, pp. 101–104. [Google Scholar]
- Moreno-Villanueva, M.; Wong, M.; Lu, T.; Zhang, Y.; Wu, H. Interplay of space radiation and microgravity in DNA damage and DNA damage response. NPJ Microgravity 2017, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Muratani, M.; Hidema, J.; Hada, M.; Fujiwara, K.; Souda, H.; Yoshida, Y.; Takahashi, A. Expression Profile of Cell Cycle-Related Genes in Human Fibroblasts Exposed Simultaneously to Radiation and Simulated Microgravity. Int. J. Mol. Sci. 2019, 20, 4791. [Google Scholar] [CrossRef]
- Mognato, M.; Girardi, C.; Fabris, S.; Celotti, L. DNA repair in modeled microgravity: Double strand break rejoining activity in human lymphocytes irradiated with γ-rays. Mutat. Res. 2009, 663, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Mognato, M.; Celotti, L. Modeled microgravity affects cell survival and HPRT mutant frequency, but not the expression of DNA repair genes in human lymphocytes irradiated with ionising radiation. Mutat. Res. 2005, 578, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tang, X.; Umeshappa, C.S.; Ma, H.; Gao, H.; Deng, Y.; Freywald, A.; Xiang, J. Simulated microgravity promotes cell apoptosis through suppressing Uev1A/TICAM/TRAF/NF-κB-regulated anti-apoptosis and p53/PCNA- and ATM/ATR-Chk1/2-controlled DNA-damage response pathways. J. Cell. Biochem. 2016, 117, 2138–2148. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Rajput, M.; Singh, R.P. Simulated microgravity triggers DNA damage and mitochondria-mediated apoptosis through ROS generation in human promyelocytic leukemic cells. Mitochondrion 2021, 61, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Pei, W.; Huang, H.; Zhou, G.; Hu, W. Additive effects of simulated microgravity and ionizing radiation in cell death, induction of ROS and expression of RAC2 in human bronchial epithelial cells. NPJ Microgravity 2020, 6, 34. [Google Scholar] [CrossRef]
- Yamanouchi, S.; Rhone, J.; Mao, J.-H.; Fujiwara, K.; Saganti, P.B.; Takahashi, A.; Hada, M. Simultaneous Exposure of Cultured Human Lymphoblastic Cells to Simulated Microgravity and Radiation Increases Chromosome Aberrations. Life 2020, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Canova, S.; Fiorasi, F.; Mognato, M.; Grifalconi, M.; Reddi, E.; Russo, A.; Celotti, L. “Modeled microgravity” affects cell response to ionizing radiation and increases genomic damage. Radiat. Res. 2005, 163, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Nagamatsu, A.; Nenoi, M.; Fujimori, A.; Kakinuma, S.; Katsube, T.; Wang, B.; Tsuruoka, C.; Shirai, T.; Nakamura, A.J.; et al. Space Radiation Biology for “Living in Space”. Biomed Res. Int. 2020, 2020, 4703286. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, A.; McDonald, J.T.; Hada, M.; Takahashi, A.; Mason, C.E.; Mognato, M. Genomic Changes Driven by Radiation-Induced DNA Damage and Microgravity in Human Cells. Int. J. Mol. Sci. 2021, 22, 10507. [Google Scholar] [CrossRef] [PubMed]
- Risin, D.; Pellis, N.R. Modeled microgravity inhibits apoptosis in peripheral blood lymphocytes. In Vitro Cell. Dev. Biol. Anim. 2001, 37, 66–72. [Google Scholar] [CrossRef]
- Ishizaki, K.; Nishizawa, K.; Kato, T.; Kitao, H.; Han, Z.B.; Hirayama, J.; Suzuki, F.; Cannon, T.F.; Kamigaichi, S.; Tawarayama, Y.; et al. Genetic changes induced in human cells in Space Shuttle experiment (STS-95). Aviat. Space Environ. Med. 2001, 72, 794–798. [Google Scholar]
- Wu, H.; George, K.; Willingham, V.; Cucinotta, F.A. Comparison of chromosome aberration frequencies in pre- and post-flight astronaut lymphocytes irradiated in vitro with gamma rays. Phys. Med. 2001, 17 (Suppl. 1), 229–231. [Google Scholar]
- Girardi, C.; De Pittà, C.; Casara, S.; Sales, G.; Lanfranchi, G.; Celotti, L.; Mognato, M. Analysis of miRNA and mRNA expression profiles highlights alterations in ionizing radiation response of human lymphocytes under modeled microgravity. PLoS ONE 2012, 7, e31293. [Google Scholar] [CrossRef]
- Dang, B.; Yang, Y.; Zhang, E.; Li, W.; Mi, X.; Meng, Y.; Yan, S.; Wang, Z.; Wei, W.; Shao, C.; et al. Simulated microgravity increases heavy ion radiation-induced apoptosis in human B lymphoblasts. Life Sci. 2014, 97, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.; Moreels, M.; Quintens, R.; Abou-El-Ardat, K.; El-Saghire, H.; Tabury, K.; Michaux, A.; Janssen, A.; Neefs, M.; Van Oostveldt, P.; et al. Chronic exposure to simulated space conditions predominantly affects cytoskeleton remodeling and oxidative stress response in mouse fetal fibroblasts. Int. J. Mol. Med. 2014, 34, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Pani, G.; Verslegers, M.; Quintens, R.; Samari, N.; de Saint-Georges, L.; van Oostveldt, P.; Baatout, S.; Benotmane, M.A. Combined Exposure to Simulated Microgravity and Acute or Chronic Radiation Reduces Neuronal Network Integrity and Survival. PLoS ONE 2016, 11, e0155260. [Google Scholar]
- Moreno-Villanueva, M.; Feiveson, A.H.; Krieger, S.; Kay Brinda, A.; von Scheven, G.; Bürkle, A.; Crucian, B.; Wu, H. Synergistic Effects of Weightlessness, Isoproterenol, and Radiation on DNA Damage Response and Cytokine Production in Immune Cells. Int. J. Mol. Sci. 2018, 19, 3689. [Google Scholar] [CrossRef] [PubMed]
- Hada, M.; Ikeda, H.; Rhone, J.R.; Beitman, A.J.; Plante, I.; Souda, H.; Yoshida, Y.; Held, K.D.; Fujiwara, K.; Saganti, P.B.; et al. Increased Chromosome Aberrations in Cells Exposed Simultaneously to Simulated Microgravity and Radiation. Int. J. Mol. Sci. 2018, 20, 43. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhang, H.; Miao, G.Y.; Xie, Y.; Sun, C.; Di, C.X.; Liu, Y.; Liu, Y.Y.; Zhang, X.; Ma, X.F.; et al. Simulated microgravity conditions and carbon ion irradiation induce spermatogenic cell apoptosis and sperm DNA damage. Biomed. Environ. Sci. 2013, 26, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.W.; Nishiyama, N.C.; Pecaut, M.J.; Campbell-Beachler, M.; Gifford, P.; Haynes, K.E.; Becronis, C.; Gridley, D.S. Simulated Microgravity and Low-Dose/Low-Dose-Rate Radiation Induces Oxidative Damage in the Mouse Brain. Radiat. Res. 2016, 185, 647–657. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, Y.; Kidane, Y.; Feiveson, A.; Stodieck, L.; Karouia, F.; Ramesh, G.; Rohde, L.; Wu, H. Cellular responses and gene expression profile changes due to bleomycin-induced DNA damage in human fibroblasts in space. PLoS ONE 2017, 12, e0170358. [Google Scholar]
- Mao, X.W.; Boerma, M.; Rodriguez, D.; Campbell-Beachler, M.; Jones, T.; Stanbouly, S.; Sridharan, V.; Nishiyama, N.C.; Wroe, A.; Nelson, G.A. Combined Effects of Low-Dose Proton Radiation and Simulated Microgravity on the Mouse Retina and the Hematopoietic System. Radiat. Res. 2019, 192, 241–250. [Google Scholar] [CrossRef]
- Nassef, M.Z.; Kopp, S.; Wehland, M.; Melnik, D.; Sahana, J.; Krüger, M.; Corydon, T.J.; Oltmann, H.; Schmitz, B.; Schütte, A.; et al. Real Microgravity Influences the Cytoskeleton and Focal Adhesions in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 3156. [Google Scholar] [CrossRef]
- Nassef, M.Z.; Kopp, S.; Melnik, D.; Corydon, T.J.; Sahana, J.; Krüger, M.; Wehland, M.; Bauer, T.J.; Liemersdorf, C.; Hemmersbach, R.; et al. Short-Term Microgravity Influences Cell Adhesion in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 5730. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Paoli, P.; Giannoni, E.; Chiarugi, P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta 2013, 1833, 3481–3498. [Google Scholar] [CrossRef]
- Kopp, S.; Sahana, J.; Islam, T.; Petersen, A.G.; Bauer, J.; Corydon, T.J.; Schulz, H.; Saar, K.; Huebner, N.; Slumstrup, L.; et al. The role of NFκB in spheroid formation of human breast cancer cells cultured on the Random Positioning Machine. Sci. Rep. 2018, 8, 921. [Google Scholar] [CrossRef]
- Jiang, N.; Chen, Z.; Li, B.; Guo, S.; Li, A.; Zhang, T.; Fu, X.; Si, S.; Cui, Y. Effects of rotary cell culture system-simulated microgravity on the ultrastructure and biological behavior of human MDA-MB-231 breast cancer cells. Precis. Radiat. Oncol. 2019, 3, 87–93. [Google Scholar] [CrossRef]
- Strube, F.; Infanger, M.; Dietz, C.; Romswinkel, A.; Kraus, A. Short-term effects of simulated microgravity on morphology and gene expression in human breast cancer cells. Physiol. Int. 2019, 106, 311–322. [Google Scholar] [CrossRef]
- Strube, F.; Infanger, M.; Wehland, M.; Delvinioti, X.; Romswinkel, A.; Dietz, C.; Kraus, A. Alteration of Cytoskeleton Morphology and Gene Expression in Human Breast Cancer Cells under Simulated Microgravity. Cell J. 2020, 22, 106–114. [Google Scholar] [CrossRef]
- Bauer, T.J.; Gombocz, E.; Wehland, M.; Bauer, J.; Infanger, M.; Grimm, D. Insight in Adhesion Protein Sialylation and Microgravity Dependent Cell Adhesion-An Omics Network Approach. Int. J. Mol. Sci. 2020, 21, 1749. [Google Scholar] [CrossRef]
- Monti, N.; Masiello, M.G.; Proietti, S.; Catizone, A.; Ricci, G.; Harrath, A.H.; Alwasel, S.H.; Cucina, A.; Bizzarri, M. Survival Pathways Are Differently Affected by Microgravity in Normal and Cancerous Breast Cells. Int. J. Mol. Sci. 2021, 22, 862. [Google Scholar] [CrossRef]
- Sahana, J.; Corydon, T.J.; Wehland, M.; Krüger, M.; Kopp, S.; Melnik, D.; Kahlert, S.; Relja, B.; Infanger, M.; Grimm, D. Alterations of Growth and Focal Adhesion Molecules in Human Breast Cancer Cells Exposed to the Random Positioning Machine. Front. Cell Dev. Biol. 2021, 9, 672098. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xue, F.; Russo, A.; Wan, Y. Proteomic Analysis of Extracellular Vesicles Derived from MDA-MB-231 Cells in Microgravity. Protein J. 2021, 40, 108–118. [Google Scholar] [CrossRef]
- Elia, I.; Broekaert, D.; Christen, S.; Boon, R.; Radaelli, E.; Orth, M.F.; Verfaillie, C.; Grünewald, T.G.P.; Fendt, S.-M. Proline metabolism supports metastasis formation and could be inhibited to selectively target metastasizing cancer cells. Nat. Commun. 2017, 8, 15267. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lee, Y.; Johansson, H.J.; Mäger, I.; Vader, P.; Nordin, J.Z.; Wiklander, O.P.B.; Lehtiö, J.; Wood, M.J.A.; Andaloussi, S.E. Serum-free culture alters the quantity and protein composition of neuroblastoma-derived extracellular vesicles. J. Extracell Vesicles 2015, 4, 26883. [Google Scholar] [CrossRef]
- Melnik, D.; Krüger, M.; Kopp, S.; Wehland, M.; Bauer, J.; Infanger, M.; Grimm, D. Microgravity-based modulation of VEGF expression in human thyroid carcinoma cells. In Proceedings of the 39th ISGP Meeting & ESA Life Sciences Meeting, Noordwijk, The Ntherlands, 18–22 June 2018. [Google Scholar]
- Wise, P.M.; Neviani, P.; Riwaldt, S.; Corydon, T.J.; Wehland, M.; Braun, M.; Krüger, M.; Infanger, M.; Grimm, D. Changes in Exosome Release in Thyroid Cancer Cells after Prolonged Exposure to Real Microgravity in Space. Int. J. Mol. Sci. 2021, 22, 2132. [Google Scholar] [CrossRef] [PubMed]
- Wise, P.M.; Neviani, P.; Riwaldt, S.; Corydon, T.J.; Wehland, M.; Braun, M.; Krüger, M.; Infanger, M.; Grimm, D. Changes in exosomal miRNA composition in thyroid cancer cells after prolonged exposure to real microgravity in space. Int. J. Mol. Sci. 2021, 22, 12841. [Google Scholar] [CrossRef] [PubMed]
- Riwaldt, S.; Pietsch, J.; Sickmann, A.; Bauer, J.; Braun, M.; Segerer, J.; Schwarzwälder, A.; Aleshcheva, G.; Corydon, T.J.; Infanger, M.; et al. Identification of proteins involved in inhibition of spheroid formation under microgravity. Proteomics 2015, 15, 2945–2952. [Google Scholar] [CrossRef]
- Kopp, S.; Krüger, M.; Feldmann, S.; Oltmann, H.; Schütte, A.; Schmitz, B.; Bauer, J.; Schulz, H.; Saar, K.; Huebner, N.; et al. Thyroid cancer cells in space during the TEXUS-53 sounding rocket mission - The THYROID Project. Sci. Rep. 2018, 8, 10355. [Google Scholar] [CrossRef]
- Bauer, J.; Wehland, M.; Infanger, M.; Grimm, D.; Gombocz, E. Semantic Analysis of Posttranslational Modification of Proteins Accumulated in Thyroid Cancer Cells Exposed to Simulated Microgravity. Int. J. Mol. Sci. 2018, 19, 2257. [Google Scholar] [CrossRef]
- Zhao, T.; Li, R.; Tan, X.; Zhang, J.; Fan, C.; Zhao, Q.; Deng, Y.; Xu, A.; Lukong, K.E.; Genth, H.; et al. Simulated Microgravity Reduces Focal Adhesions and Alters Cytoskeleton and Nuclear Positioning Leading to Enhanced Apoptosis via Suppressing FAK/RhoA-Mediated mTORC1/NF-κB and ERK1/2 Pathways. Int. J. Mol. Sci. 2018, 19, 1994. [Google Scholar] [CrossRef]
- Jeong, A.J.; Kim, Y.J.; Lim, M.H.; Lee, H.; Noh, K.; Kim, B.-H.; Chung, J.W.; Cho, C.-H.; Kim, S.; Ye, S.-K. Microgravity induces autophagy via mitochondrial dysfunction in human Hodgkin’s lymphoma cells. Sci. Rep. 2018, 8, 14646. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, D.; Suresh, S.; Prathivadhi-Bhayankaram, S.; Mimlitz, M.; Zetocha, N.; Lee, B.; Ekpenyong, A. Microgravity Modulates Effects of Chemotherapeutic Drugs on Cancer Cell Migration. Life 2020, 10, 162. [Google Scholar] [CrossRef]
- Arun, R.P.; Sivanesan, D.; Vidyasekar, P.; Verma, R.S. PTEN/FOXO3/AKT pathway regulates cell death and mediates morphogenetic differentiation of Colorectal Cancer Cells under Simulated Microgravity. Sci. Rep. 2017, 7, 5952. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Jiang, N.; Guo, S.; Li, B.-B.; Yang, J.-Q.; Chai, S.-B.; Yan, H.-F.; Sun, P.-M.; Zhang, T.; Sun, H.-W.; et al. Effect of simulated microgravity on metabolism of HGC-27 gastric cancer cells. Oncol. Lett. 2020, 19, 3439–3450. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, T.; Tanimoto, K.; Shrestha, L.; Imura, T.; Takahashi, S.; Sueda, T.; Hirohashi, N.; Hiyama, E.; Yuge, L. Simulated microgravity enhances CDDP-induced apoptosis signal via p53-independent mechanisms in cancer cells. PLoS ONE 2019, 14, e0219363. [Google Scholar] [CrossRef]
- Hybel, T.E.; Dietrichs, D.; Sahana, J.; Corydon, T.J.; Nassef, M.Z.; Wehland, M.; Krüger, M.; Magnusson, N.E.; Bauer, J.; Utpatel, K.; et al. Simulated Microgravity Influences VEGF, MAPK, and PAM Signaling in Prostate Cancer Cells. Int. J. Mol. Sci. 2020, 21, 1263. [Google Scholar] [CrossRef]
- Chung, J.H.; Ahn, C.B.; Son, K.H.; Yi, E.; Son, H.S.; Kim, H.-S.; Lee, S.H. Simulated Microgravity Effects on Nonsmall Cell Lung Cancer Cell Proliferation and Migration. Aerosp. Med. Hum. Perform. 2017, 88, 82–89. [Google Scholar] [CrossRef]
- Ahn, C.B.; Lee, J.-H.; Han, D.G.; Kang, H.-W.; Lee, S.-H.; Lee, J.-I.; Son, K.H.; Lee, J.W. Simulated microgravity with floating environment promotes migration of non-small cell lung cancers. Sci. Rep. 2019, 9, 14553. [Google Scholar] [CrossRef] [PubMed]
- Degan, P.; Cortese, K.; Pulliero, A.; Bruno, S.; Gagliani, M.C.; Congiu, M.; Izzotti, A. Simulated Microgravity Effects on Human Adenocarcinoma Alveolar Epithelial Cells: Characterization of Morphological, Functional, and Epigenetic Parameters. Int. J. Mol. Sci. 2021, 22, 6951. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ma, H.; Wu, L.; Cao, L.; Yang, Q.; Dong, H.; Wang, Z.; Ma, J.; Li, Z. The influence of simulated microgravity on proliferation and apoptosis in U251 glioma cells. In Vitro Cell. Dev. Biol. Anim. 2017, 53, 744–751. [Google Scholar] [CrossRef]
- Romswinkel, A.; Infanger, M.; Dietz, C.; Strube, F.; Kraus, A. The Role of C-X-C Chemokine Receptor Type 4 (CXCR4) in Cell Adherence and Spheroid Formation of Human Ewing’s Sarcoma Cells under Simulated Microgravity. Int. J. Mol. Sci. 2019, 20, 6073. [Google Scholar] [CrossRef] [PubMed]
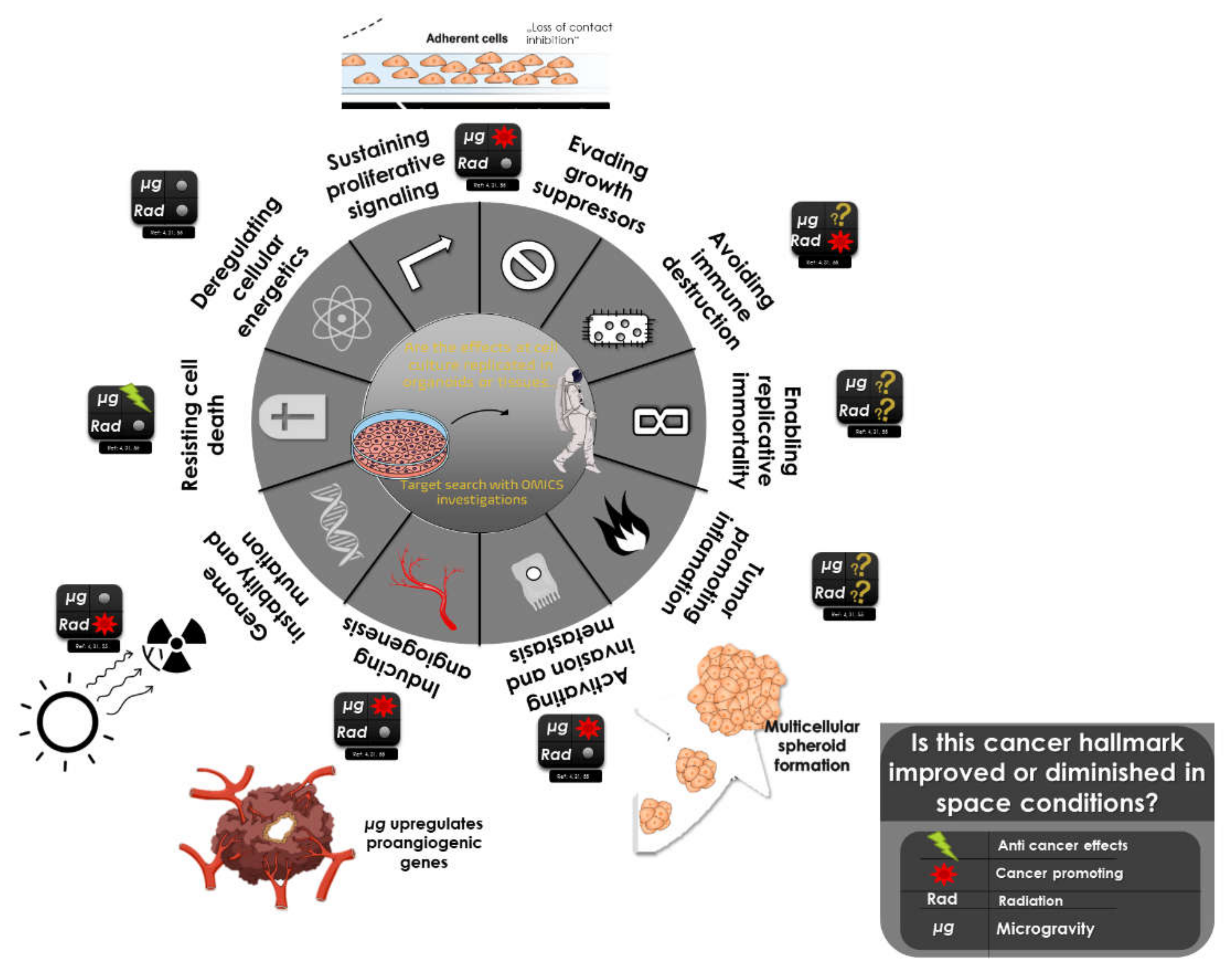
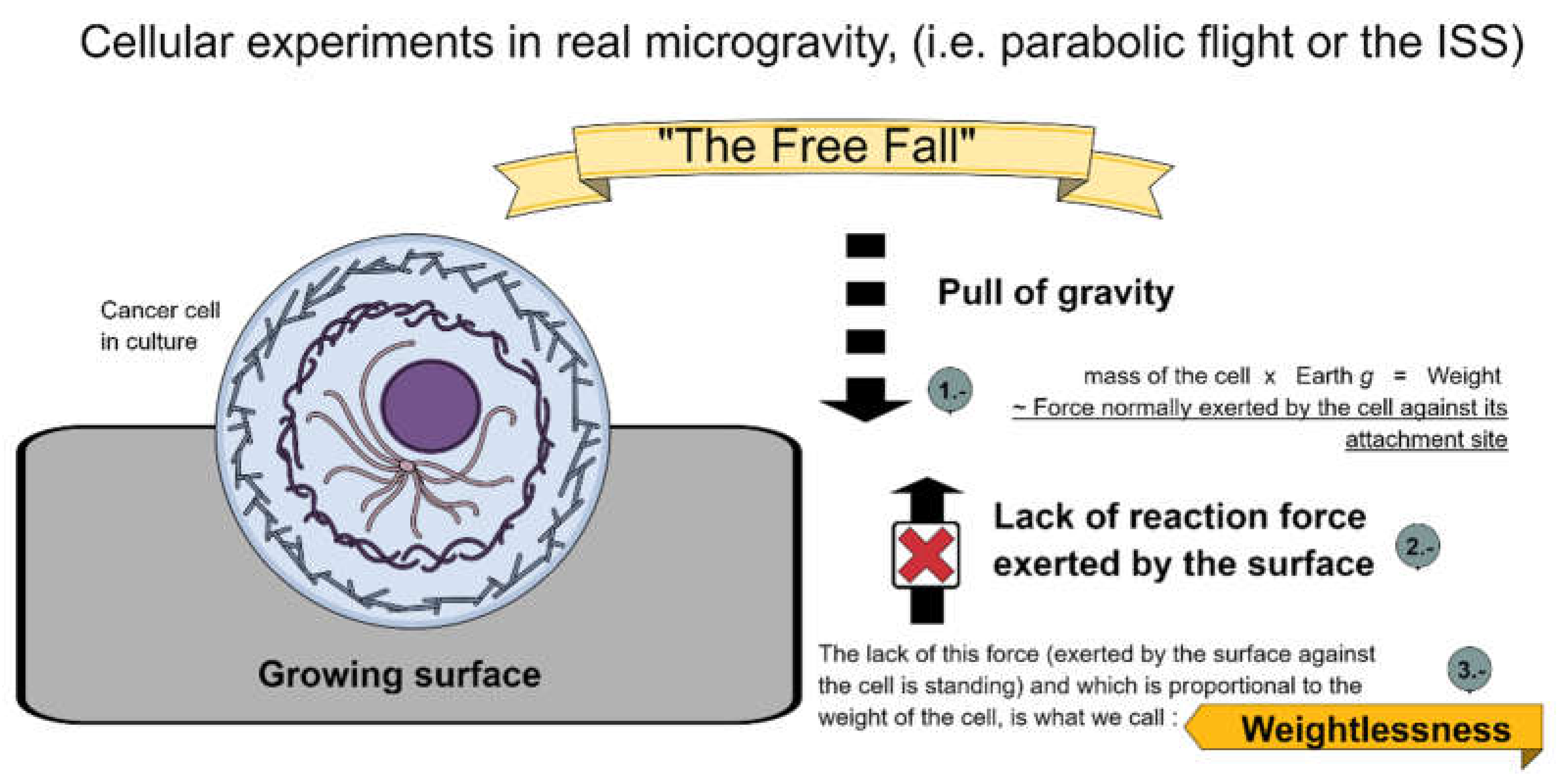

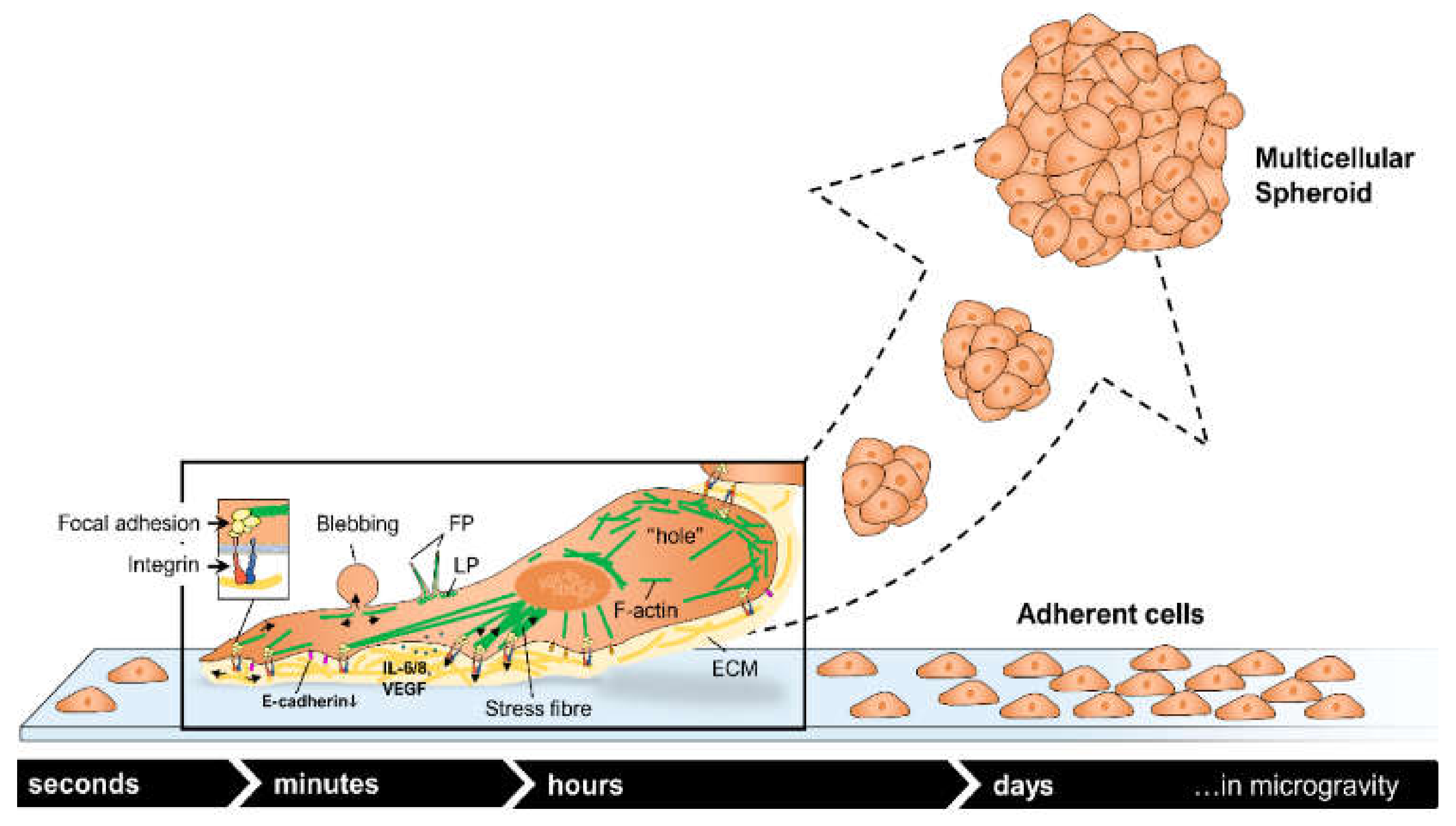
| Cancer Type | Microgravity Effects | s-µg | r-µg |
|---|---|---|---|
| Breast | NF-κB p65 plays a crucial role in MCS formation [128] | RPM | |
| Decreased E-cadherin in MCS; the balance of proteins that up- or downregulate E-cadherin mediates the tendency to form MCS [26] | RPM | ||
| Upregulation of KRT8, RDX, TIMP1, CXCL8 mRNAs and downregulation of VCL. E-cadherin protein was significantly reduced [124]; rearrangement of F-actin and tubulin, with the formation of holes | SR & PF | ||
| MCS have an altered cytoskeleton and appreciable apoptosis after 72 h; survival strategies cannot provide sufficient protection [133] | RPM | ||
| The process of linking cells to each other or the ECM under µg includes sialylation of extracellular domains of adhesion proteins [132] | RPM | ISS | |
| Vinculin and β-catenin are critical to form MCS during incubation in an RPM for 24 h [134] | RPM | ||
| MCS formation; BRCA1 increased, KRAS decreased in AD cells; VCAM1 upregulated, VIM downregulated in µg [130] | RPM | ||
| Increased metastatic ability; considerable changes in morphology, cytoskeletal shape, and gene expression [131] | RPM | ||
| Induces gene expression of cell adhesion molecules [125] | RPM | PF | |
| EV release rate decreases while average EV size increases; significant correlation with GTPases and proliferation [135] | Gravite | ||
| Lysosomal vesicles, cyclin D3, and apoptosis increase; migration ability and the expression of BCL-2 and MMP9 proteins decrease [129] | RWV | ||
| Thyroid | Altered integrin signalling, facilitating cytoskeletal changes, and weakening focal adhesion complexes, promoting MCS formation [60] | RPM | |
| Moderate gene expression changes indicate orbital survival [142] | hyper-g | SR | |
| µg is a more potent regulator of gene expression than hyper-g [22] | RPM | SR | |
| Proteins undergo extensive posttranslational modification [143] | s-µg | ||
| Spheroids formed in all hardware units; enhanced release of VEGF versus RPM samples [138] | RPM | ISS | |
| Alters expression of adhesion proteins and enzymes for their posttranslational modifications [132] | RPM | ||
| Dexamethasone inhibits the formation of MCS in a dose-dependent manner through the E-cadherin/β-catenin pathway [25] | RPM | ||
| Differences in the number of secreted exosomes, alteration of their population regarding the tetraspanin surface expression [139] | ISS | ||
| Skin (melanoma) | Inhibits focal adhesions, leading to reduced proliferation and metastasis via FAK/RhoA-regulated mTORC1 and AMPK pathways [46] | Clinostat | |
| Fewer focal adhesions; enhanced apoptosis via FAK/RhoA-mediated mTORC1/NF-κB and ERK1/2 pathways suppression [144] | Clinostat | ||
| Haematological | Induced autophagy via mitochondrial dysfunction [145] | 3D-C | |
| Modulated chemotherapeutics effects on cancer cell migration [146] | RWV | ||
| Gastrointestinal | PTEN/FOXO3/AKT pathway regulates cell death and mediates morphogenetic differentiation [147] | RCCS-H | |
| More polyploid giant cancer cells and YAP nuclear localisation [17] | RCCS | ||
| Effects on lipid metabolism [148] | RCCS | ||
| Enhances CDDP-induced apoptosis via independent of p53 [149] | RPM | ||
| Prostate | Influenced VEGF, MAPK, and PAM signalling [150]. | RPM | |
| Lung | Cell type–dependent effects on proliferation and migration [151] | 3D-C | |
| Promotes migration of non-small cell lung cancer [152] | RPM | ||
| Apoptosis induction and alteration of cell adherence [15] | RPM | ||
| Mitochondria are susceptible to μg; global miRNA analysis defined a pool of miRNAs associated with μg exposure [153] | RPM | ||
| Brain | Influence on proliferation and apoptosis in glioma cells [154] | 2D-C | |
| Inhibits viability and migration via FAK/RhoA/Rock and FAK/Nek2 [47] | SM-31 | ||
| Bone | Increased EWS/FLI1 expression; CXCR4 does not affect MCS formation [155] | RPM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Sánchez, J.L.; Callant, J.; Krüger, M.; Sahana, J.; Kraus, A.; Baselet, B.; Infanger, M.; Baatout, S.; Grimm, D. Cancer Studies under Space Conditions: Finding Answers Abroad. Biomedicines 2022, 10, 25. https://doi.org/10.3390/biomedicines10010025
Cortés-Sánchez JL, Callant J, Krüger M, Sahana J, Kraus A, Baselet B, Infanger M, Baatout S, Grimm D. Cancer Studies under Space Conditions: Finding Answers Abroad. Biomedicines. 2022; 10(1):25. https://doi.org/10.3390/biomedicines10010025
Chicago/Turabian StyleCortés-Sánchez, José Luis, Jonas Callant, Marcus Krüger, Jayashree Sahana, Armin Kraus, Bjorn Baselet, Manfred Infanger, Sarah Baatout, and Daniela Grimm. 2022. "Cancer Studies under Space Conditions: Finding Answers Abroad" Biomedicines 10, no. 1: 25. https://doi.org/10.3390/biomedicines10010025
APA StyleCortés-Sánchez, J. L., Callant, J., Krüger, M., Sahana, J., Kraus, A., Baselet, B., Infanger, M., Baatout, S., & Grimm, D. (2022). Cancer Studies under Space Conditions: Finding Answers Abroad. Biomedicines, 10(1), 25. https://doi.org/10.3390/biomedicines10010025










