Collagenase-Induced Patellar Tendinopathy with Neovascularization: First Results towards a Piglet Model of Musculoskeletal Embolization
Abstract
:1. Introduction
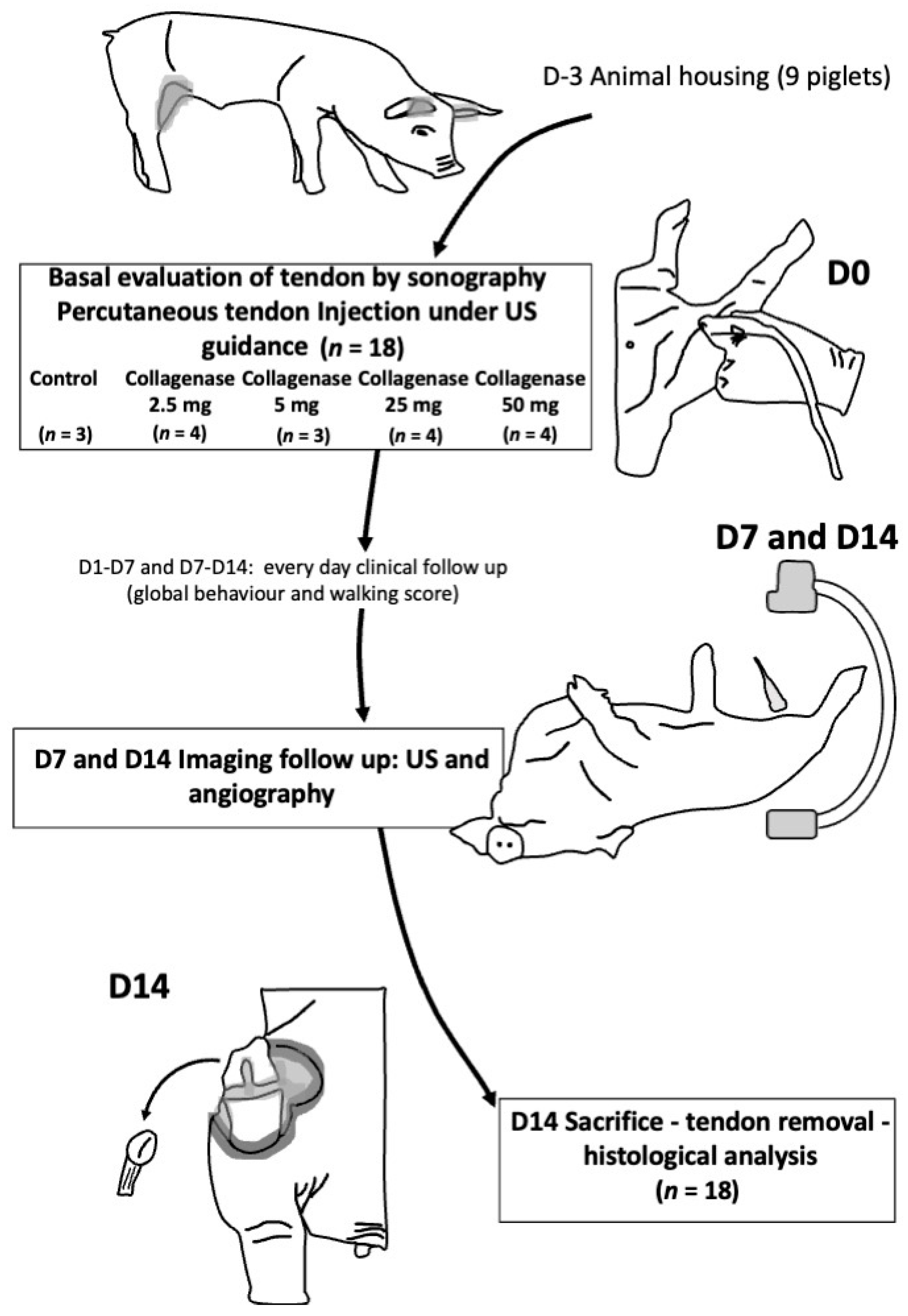
2. Materials and Methods
2.1. Animal Model
2.2. Induction of Patellar Tendinopathy
2.3. Clinical Assessment
2.4. Ultrasound Exploration
2.5. Angiography Exploration
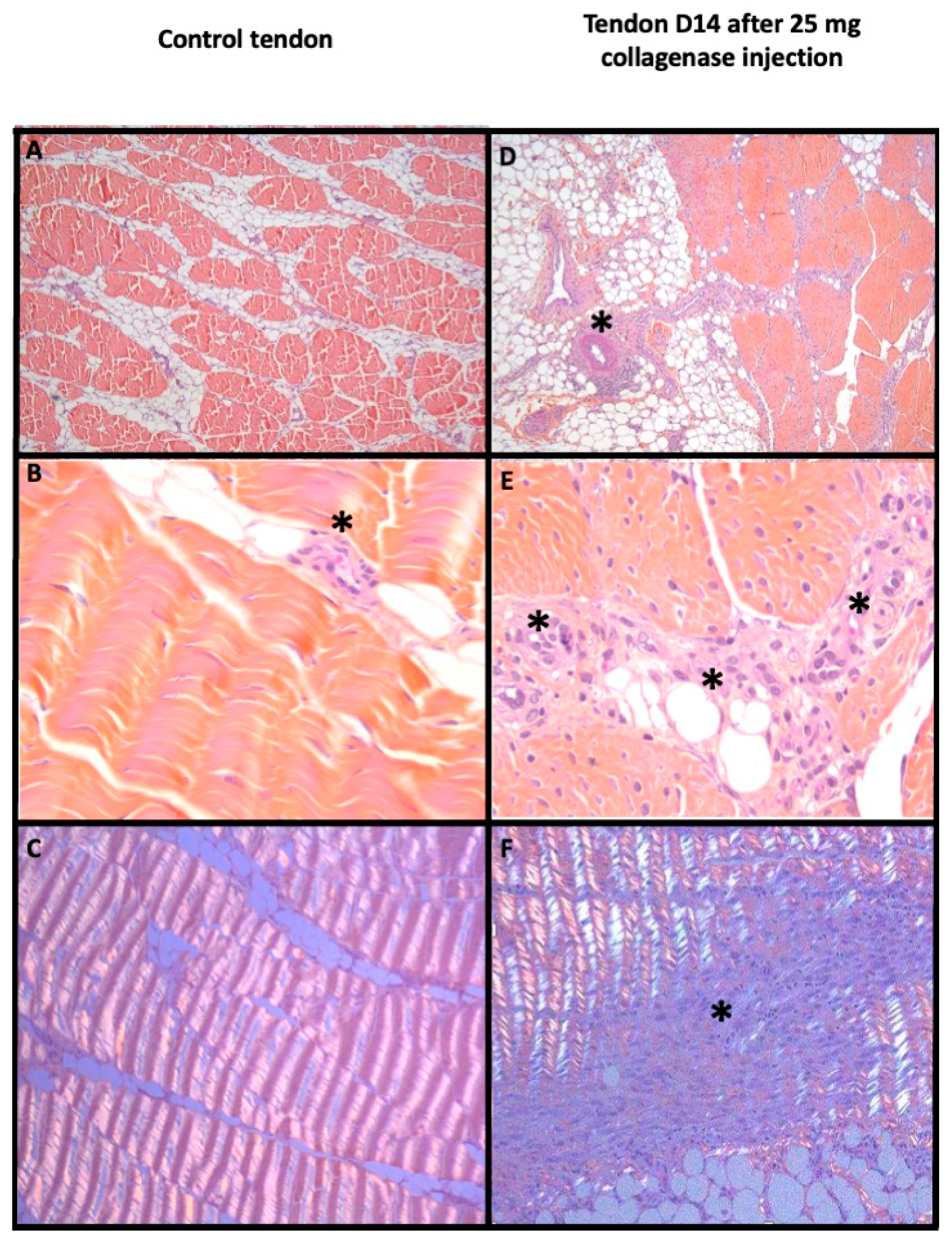
2.6. Histological Analyses
2.7. Statistical Analysis
3. Results
3.1. Development of Tendinopathy Model
3.2. Neovascularization Evaluation
3.3. Efficiency of the Model Obtained
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dominick, K.L.; Ahern, F.M.; Gold, C.H.; Heller, D.A. Health-Related Quality of Life and Health Service Use among Older Adults with Osteoarthritis. Arthritis Care Res. 2004, 51, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T. The Epidemiology and Impact of Pain in Osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brockmeyer, M.; Diehl, N.; Schmitt, C.; Kohn, D.M.; Lorbach, O. Results of Surgical Treatment of Chronic Patellar Tendinosis (Jumper’s Knee): A Systematic Review of the Literature. Arthroscopy 2015, 31, 2424–2429.e3. [Google Scholar] [CrossRef] [PubMed]
- Kettunen, J.A.; Kvist, M.; Alanen, E.; Kujala, U.M. Long-Term Prognosis for Jumper’s Knee in Male Athletes. A Prospective Follow-up Study. Am. J. Sports Med. 2002, 30, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Silbernagel, K.G.; Thorborg, K.; Kirwan, P.D.; Galatz, L.M.; Abrams, G.D.; Murrell, G.A.C.; McInnes, I.B.; Rodeo, S.A. Tendinopathy. Nat. Rev. Dis. Primers 2021, 7, 1. [Google Scholar] [CrossRef]
- Chen, P.-C.; Wu, K.-T.; Chou, W.-Y.; Huang, Y.-C.; Wang, L.-Y.; Yang, T.-H.; Siu, K.-K.; Tu, Y.-K. Comparative Effectiveness of Different Nonsurgical Treatments for Patellar Tendinopathy: A Systematic Review and Network Meta-Analysis. Arthroscopy 2019, 35, 3117–3131.e2. [Google Scholar] [CrossRef]
- Coombes, B.K.; Bisset, L.; Vicenzino, B. Efficacy and Safety of Corticosteroid Injections and Other Injections for Management of Tendinopathy: A Systematic Review of Randomised Controlled Trials. Lancet 2010, 376, 1751–1767. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Bonar, F.; Murrell, G.A.C. Neoinnervation in Rotator Cuff Tendinopathy. Sports Med. Arthrosc. Rev. 2011, 19, 354–359. [Google Scholar] [CrossRef]
- Zayni, R.; Thaunat, M.; Fayard, J.-M.; Hager, J.-P.; Carrillon, Y.; Clechet, J.; Gadea, F.; Archbold, P.; Sonnery Cottet, B. Platelet-Rich Plasma as a Treatment for Chronic Patellar Tendinopathy: Comparison of a Single versus Two Consecutive Injections. Muscles Ligaments Tendons J. 2015, 5, 92–98. [Google Scholar] [CrossRef]
- Mapp, P.I.; Walsh, D.A. Mechanisms and Targets of Angiogenesis and Nerve Growth in Osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 390–398. [Google Scholar] [CrossRef]
- Alfredson, H.; Ohberg, L. Neovascularisation in Chronic Painful Patellar Tendinosis—Promising Results after Sclerosing Neovessels Outside the Tendon Challenge the Need for Surgery. Knee Surg. Sports Traumatol. Arthrosc. 2005, 13, 74–80. [Google Scholar] [CrossRef]
- Ashraf, S.; Wibberley, H.; Mapp, P.I.; Hill, R.; Wilson, D.; Walsh, D.A. Increased Vascular Penetration and Nerve Growth in the Meniscus: A Potential Source of Pain in Osteoarthritis. Ann. Rheum. Dis. 2011, 70, 523–529. [Google Scholar] [CrossRef]
- Alfredson, H.; Lorentzon, R. Sclerosing Polidocanol Injections of Small Vessels to Treat the Chronic Painful Tendon. Cardiovasc. Hematol. Agents Med. Chem. (Former. Curr. Med. Chem. -Cardiovasc. Hematol. Agents) 2007, 5, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Hoksrud, A.; Torgalsen, T.; Harstad, H.; Haugen, S.; Andersen, T.E.; Risberg, M.A.; Bahr, R. Ultrasound-Guided Sclerosis of Neovessels in Patellar Tendinopathy: A Prospective Study of 101 Patients. Am. J. Sports Med. 2012, 40, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.L.; Abreu, F.G.; Queirós, C.M.; Pisanu, G.; Clechet, J.; Vieira, T.D.; Sonnery-Cottet, B. Ultrasound-Guided Electrocoagulation of Neovessels for Chronic Patellar Tendinopathy. Arthrosc. Tech. 2020, 9, e803–e807. [Google Scholar] [CrossRef] [PubMed]
- Okuno, Y.; Iwamoto, W.; Matsumura, N.; Oguro, S.; Yasumoto, T.; Kaneko, T.; Ikegami, H. Clinical Outcomes of Transcatheter Arterial Embolization for Adhesive Capsulitis Resistant to Conservative Treatment. J. Vasc. Interv. Radiol. 2017, 28, 161–167.e1. [Google Scholar] [CrossRef] [PubMed]
- Okuno, Y.; Korchi, A.M.; Shinjo, T.; Kato, S.; Kaneko, T. Midterm Clinical Outcomes and MR Imaging Changes after Transcatheter Arterial Embolization as a Treatment for Mild to Moderate Radiographic Knee Osteoarthritis Resistant to Conservative Treatment. J. Vasc. Interv. Radiol. 2017, 28, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Okuno, Y.; Matsumura, N.; Oguro, S. Transcatheter Arterial Embolization Using Imipenem/Cilastatin Sodium for Tendinopathy and Enthesopathy Refractory to Nonsurgical Management. J. Vasc. Interv. Radiol. 2013, 24, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, H.; Tanaka, T.; Nishiofuku, H.; Fukuoka, Y.; Minamiguchi, K.; Taiji, R.; Takayama, K.; Takeda, M.; Hatakeyama, K.; Inoue, T.; et al. A Rat Model of Frozen Shoulder Demonstrating the Effect of Transcatheter Arterial Embolization on Angiography, Histopathology, and Physical Activity. J. Vasc. Interv. Radiol. 2021, 32, 376–383. [Google Scholar] [CrossRef]
- Warden, S.J. Animal Models for the Study of Tendinopathy. Br. J. Sports Med. 2007, 41, 232–240. [Google Scholar] [CrossRef]
- de Cesar Netto, C.; Godoy-Santos, A.L.; Augusto Pontin, P.; Natalino, R.J.; Pereira, C.A.; Lima, F.D.; da Fonseca, L.F.; Staggers, J.R.; Cavinatto, L.M.; Schon, L.C.; et al. Novel Animal Model for Achilles Tendinopathy: Controlled Experimental Study of Serial Injections of Collagenase in Rabbits. PLoS ONE 2018, 13, e0192769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanno, A.; Sano, H.; Itoi, E. Development of a Shoulder Contracture Model in Rats. J. Shoulder Elb. Surg. 2010, 19, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Oki, S.; Shirasawa, H.; Yoda, M.; Matsumura, N.; Tohmonda, T.; Yuasa, K.; Nakamura, M.; Matsumoto, M.; Horiuchi, K. Generation and Characterization of a Novel Shoulder Contracture Mouse Model. J. Orthop. Res. 2015, 33, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Perucca Orfei, C.; Lovati, A.B.; Viganò, M.; Stanco, D.; Bottagisio, M.; Di Giancamillo, A.; Setti, S.; de Girolamo, L. Dose-Related and Time-Dependent Development of Collagenase-Induced Tendinopathy in Rats. PLoS ONE 2016, 11, e0161590. [Google Scholar] [CrossRef] [PubMed]
- Boesen, M.I.; Nanni, S.; Langberg, H.; Boesen, M.; Falk-Ronne, J.; Bliddal, H.; Torp-Pedersen, S. Colour Doppler Ultrasonography and Sclerosing Therapy in Diagnosis and Treatment of Tendinopathy in Horses-a Research Model for Human Medicine. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Fearon, A.; Dahlstrom, J.E.; Twin, J.; Cook, J.; Scott, A. The Bonar Score Revisited: Region of Evaluation Significantly Influences the Standardized Assessment of Tendon Degeneration. J. Sci. Med. Sport 2014, 17, 346–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, K.M.; Cook, J.L.; Bonar, F.; Harcourt, P.; Astrom, M. Histopathology of Common Tendinopathies. Update and Implications for Clinical Management. Sports Med. 1999, 27, 393–408. [Google Scholar] [CrossRef]
- Maffulli, N.; Longo, U.G.; Franceschi, F.; Rabitti, C.; Denaro, V. Movin and Bonar Scores Assess the Same Characteristics of Tendon Histology. Clin. Orthop. Relat. Res. 2008, 466, 1605–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal Models of Osteoarthritis: Classification, Update, and Measurement of Outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef] [Green Version]
- Nuelle, C.W.; Stokes, D.C.; Kuroki, K.; Crim, J.R.; Sherman, S.L. Radiologic and Histologic Evaluation of Proximal Bicep Pathology in Patients with Chronic Biceps Tendinopathy Undergoing Open Subpectoral Biceps Tenodesis. Arthroscopy 2018, 34, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Stange, R.; Sahin, H.; Wieskötter, B.; Persigehl, T.; Ring, J.; Bremer, C.; Raschke, M.J.; Vieth, V. In Vivo Monitoring of Angiogenesis during Tendon Repair: A Novel MRI-Based Technique in a Rat Patellar Tendon Model. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2433–2439. [Google Scholar] [CrossRef] [PubMed]
- Zabrzyński, J.; Gagat, M.; Łapaj, Ł.; Paczesny, Ł.; Yataganbaba, A.; Szwedowski, D.; Huri, G. Relationship between Long Head of the Biceps Tendon Histopathology and Long-Term Functional Results in Smokers. A Time to Reevaluate the Bonar Score? Adv. Chronic. Dis. 2021, 12, 2040622321990262. [Google Scholar] [CrossRef] [PubMed]
- Szwedowski, D.; Jaworski, Ł.; Szwedowska, W.; Pękala, P.; Gagat, M. Neovascularization in Meniscus and Tendon Pathology as a Potential Mechanism in Regenerative Therapies: Special Reference to Platelet-Rich Plasma Treatment. Appl. Sci. 2021, 11, 8310. [Google Scholar] [CrossRef]
- Di Meglio, F.; Sacco, A.M.; Belviso, I.; Romano, V.; Sirico, F.; Loiacono, C.; Palermi, S.; Pempinello, C.; Montagnani, S.; Nurzynska, D.; et al. Influence of Supplements and Drugs used for the Treatment of Musculoskeletal Disorders on Adult Human Tendon-Derived Stem Cells. Muscles Ligaments Tendons J. 2020, 10, 376–384. [Google Scholar] [CrossRef]
- Zabrzyński, J.; Gagat, M.; Huri, G.; Łapaj, Ł.; Paczesny, Ł.; Zielińska, W.; Zabrzyńska, M.; Szwedowski, D.; Kruczyński, J. Therapeutic Advances in Tendinopathy Quantified Microscopically Using Bonar Score, with a Special Reference to PRP Therapy—A Systematic Review of Experimental Studies. Appl. Sci. 2021, 11, 4973. [Google Scholar] [CrossRef]
- Korchi, A.M.; Cengarle-Samak, A.; Okuno, Y.; Martel-Pelletier, J.; Pelletier, J.P.; Boesen, M.; Doyon, J.; Bodson-Clermont, P.; Lussier, B.; Héon, H.; et al. Inflammation and Hypervascularization in a Large Animal Model of Knee Osteoarthritis: Imaging with Pathohistologic Correlation. J. Vasc. Interv. Radiol. 2019, 30, 1116–1127. [Google Scholar] [CrossRef] [PubMed]

| Tendon Surface at Baseline (cm2) | Increased Tendon Surface Compared to Surface at Baseline, % [IQR range] | Tendon Lesions (n) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median [Range] | p-Value | D7 | p-Value | D14 | p-Value | None | Hypoechoic Change | Neovas-Cularization | Tendon Rupture | p-Value | |
| Control\(n = 3) | 0.19 (0.19–0.19) | 0.244 | 32 (29–31) | 0.167 | 53 (50–56) | 0.065 | 3 | 0 | 0 | 0 | 0.012 |
| 2.5 mg Collagenase (n = 4) | 0.18 (0.18–0.19) | 37 (16–67) | 40 (34–47) | 4 | 0 | 0 | 0 | ||||
| 5 mg Collagenase (n = 3) | 0.27 (0.24–0.27) | 25 (16–46) | 89 (77–96) | 1 | 2 | 0 | 0 | ||||
| 25 mg Collagenase (n = 4) | 0.30 (0.26–0.33) | 37 (20–44) | 54 (45–60) | 0 | 3 | 1 | 1 | ||||
| 50 mg Collagenase (n = 4) | 0.18 (0.15–0.21) | 29 (26–37) | 104 (92–124) | 1 | 3 | 0 | 0 | ||||
| Angiographic Neovascularisation (Visual Evaluation) | BONAR Score at Day 14 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 7 | Day 14 | |||||||||||||||
| None (N) | Mild (N) | Important (N) | p-Value | Rs p | None (N) | Mild (N) | Important (N) | p-Value | Rs p | Global Score Median [IQR] | p-Value | Rs p | Vascularity Subclass Median [IQR] | p-Value | Rs p | |
| Control (n = 3) | 3 | 0 | 0 | 0.009 | 0.82 <0.001 | 3 | 0 | 0 | 0.008 | 0.791 <0.001 | 2.0 (2.0–2.0) | 0.024 | 0.666 0.003 | 1.0 (1.0–1.0) | 0.054 | 0.373 0.127 |
| 2.5 mg Collagenase (n = 4) | 2 | 2 | 0 | 2 | 2 | 0 | 2.5 (2.0–4.5) | 1.0 (1.0–1.5) | ||||||||
| 5 mg Collagenase (n = 3) | 0 | 2 | 1 | 0 | 1 | 2 | 6.0 (4.0–8.5) | 2.0 (1.5–2.5) | ||||||||
| 25 mg Collagenase (n = 4) | 0 | 0 | 4 | 0 | 0 | 4 | 7.5 (5.8–10.0) | 1.5 (1.0–2.3) | ||||||||
| 50 mg Collagenase (n = 4) | 0 | 1 | 3 | 0 | 1 | 3 | 9.0 (6.8–10.8) | 2.0 (1.8–2.0) | ||||||||
| Piglets (Amount of Collagenase Injected; R and L, in mg) | Global Behavior | Walking Score | Weight (kg) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | D1 | D7 | D14 | p-Value | Baseline | D1 | D7 | D14 | p-Value | Baseline | D7 Weight Gain | D14 Weight Gain | p-Value | |
| P1 (R:0, L:0) | 11 | 11 | 11 | 11 | 1 | 3 | 3 | 3 | 3 | 1 | 33.7 | 6% | 17% | 0.956 |
| P2 (R:0, L:2.5) | 11 | 9 | 11 | 11 | 3 | 1 | 3 | 3 | 30.2 | 2% | 19% | |||
| P3 (R:2.5, L:2.5) | 11 | 9 | 11 | 10 | 3 | 1 | 3 | 2 | 34.4 | 2% | 6% | |||
| P4 (R:2.5, L:5) | 11 | 11 | 11 | 11 | 3 | 3 | 3 | 3 | 33.1 | 2% | 9% | |||
| P5 (R:5, L:5) | 11 | 11 | 11 | 11 | 3 | 3 | 3 | 3 | 29.3 | 5% | 17% | |||
| P6 (R:25, L:25) | 11 | 11 | 11 | 11 | 3 | 3 | 3 | 3 | 20.2 | 5% | 14% | |||
| P7 (R:25, L:25) | 11 | 11 | 11 | 11 | 3 | 3 | 3 | 3 | 30.7 | 3% | 10% | |||
| P8 (R:50, L:50) | 11 | 11 | 11 | 11 | 3 | 3 | 3 | 3 | 22.5 | 4% | 12% | |||
| P9 (R:50, L:50) | 11 | 11 | 11 | 10 | 3 | 3 | 3 | 2 | 20.9 | 4% | 12% | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghelfi, J.; Bacle, M.; Stephanov, O.; de Forges, H.; Soulairol, I.; Roger, P.; Ferretti, G.R.; Beregi, J.-P.; Frandon, J. Collagenase-Induced Patellar Tendinopathy with Neovascularization: First Results towards a Piglet Model of Musculoskeletal Embolization. Biomedicines 2022, 10, 2. https://doi.org/10.3390/biomedicines10010002
Ghelfi J, Bacle M, Stephanov O, de Forges H, Soulairol I, Roger P, Ferretti GR, Beregi J-P, Frandon J. Collagenase-Induced Patellar Tendinopathy with Neovascularization: First Results towards a Piglet Model of Musculoskeletal Embolization. Biomedicines. 2022; 10(1):2. https://doi.org/10.3390/biomedicines10010002
Chicago/Turabian StyleGhelfi, Julien, Marylène Bacle, Olivier Stephanov, Hélène de Forges, Ian Soulairol, Pascal Roger, Gilbert R. Ferretti, Jean-Paul Beregi, and Julien Frandon. 2022. "Collagenase-Induced Patellar Tendinopathy with Neovascularization: First Results towards a Piglet Model of Musculoskeletal Embolization" Biomedicines 10, no. 1: 2. https://doi.org/10.3390/biomedicines10010002
APA StyleGhelfi, J., Bacle, M., Stephanov, O., de Forges, H., Soulairol, I., Roger, P., Ferretti, G. R., Beregi, J.-P., & Frandon, J. (2022). Collagenase-Induced Patellar Tendinopathy with Neovascularization: First Results towards a Piglet Model of Musculoskeletal Embolization. Biomedicines, 10(1), 2. https://doi.org/10.3390/biomedicines10010002







