Serpinin in the Skin
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Western Blot
2.3. RP-HPLC with EIA
2.4. Double Immunofluorescence for Serpinin/Substance P (SP) in Rat DRG
2.5. Double Immunofluorescence for Serpinin/Protein Gene Product (PGP) 9.5 and Serpinin/SP in the Human Skin
3. Results
3.1. Western Blot Analysis of Immunoreactive Forms of Serpinin in Trigeminal Ganglia (TG), DRG and Skin
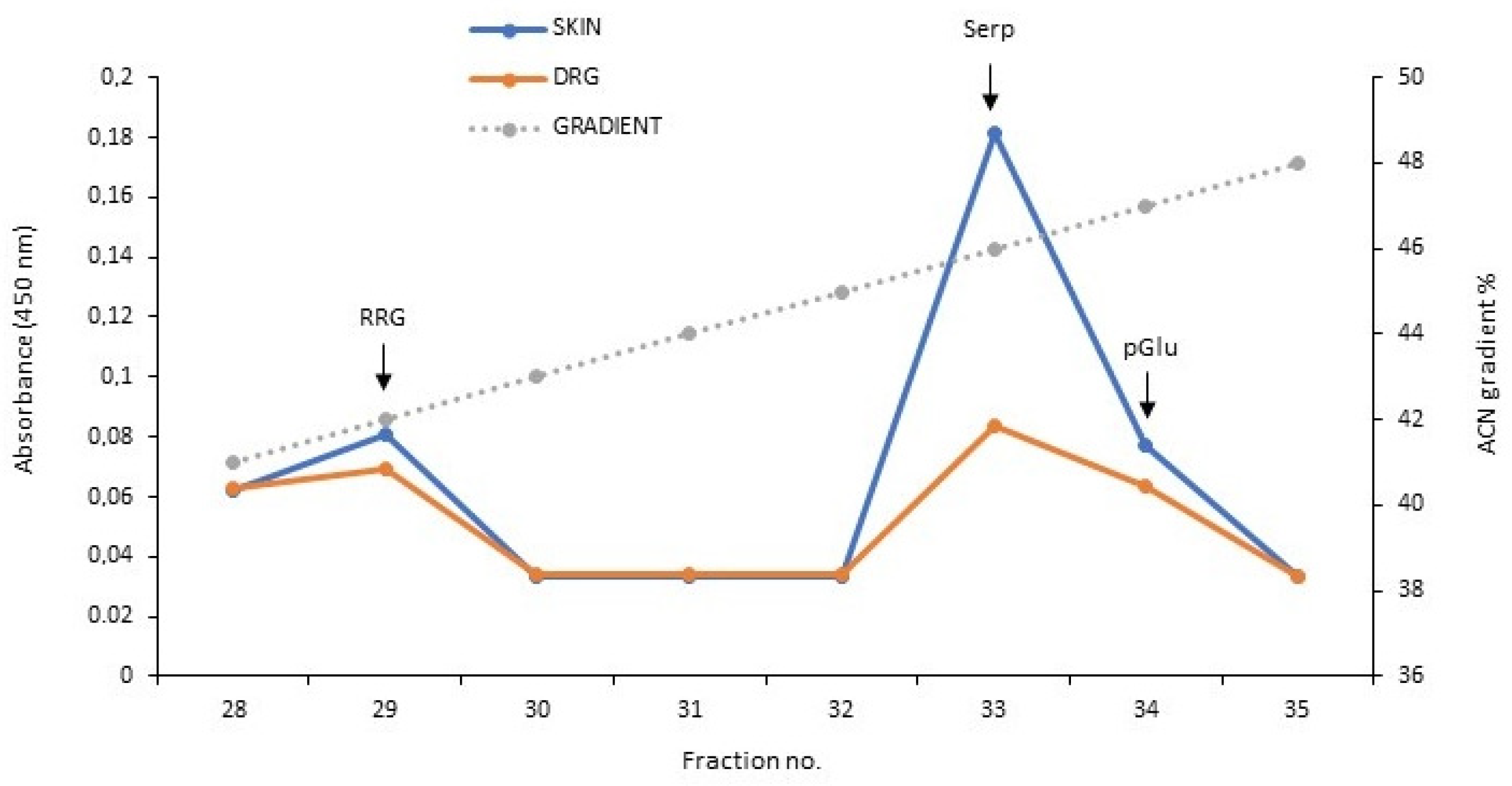
3.2. Analysis of Serpinin Peptides in Rat Thoracic DRG and Skin
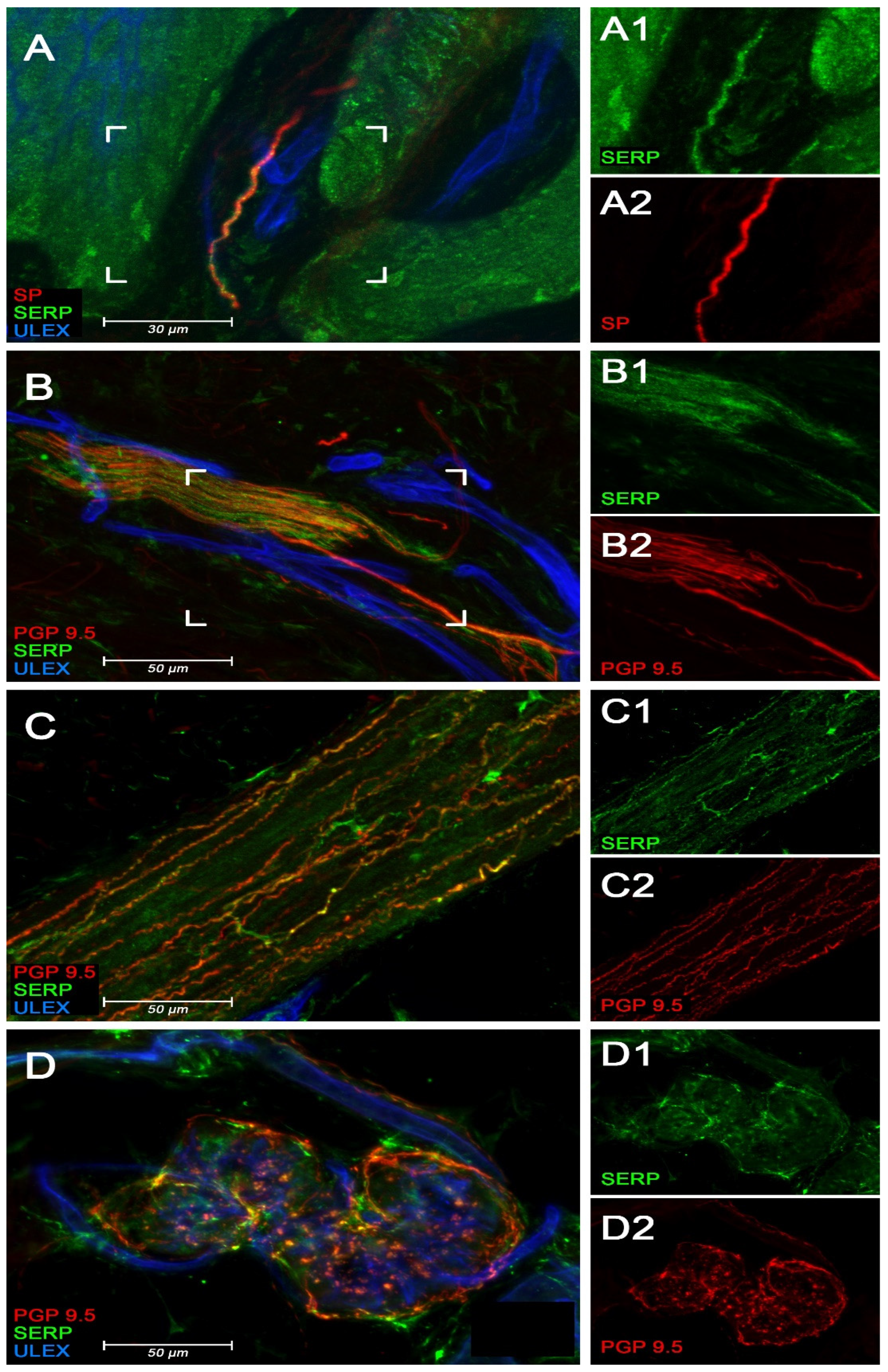
3.3. Immunofluorescence Studies of Serpinin and SP in Rat Thoracic DRG
3.4. Immunofluorescence Studies of Serpinin in Human Skin
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Koshimizu, H.; Cawley, N.X.; Kim, T.; Yergey, A.L.; Loh, Y.P. Serpinin: A Novel Chromogranin A-Derived, Secreted Peptide Up-Regulates Protease Nexin-1 Expression and Granule Biogenesis in Endocrine Cells. Mol. Endocrinol. 2011, 25, 732–744. [Google Scholar] [CrossRef]
- Koshimizu, H.; Cawley, N.X.; Yergey, A.L.; Loh, Y.P. Role of pGlu-Serpinin, a Novel Chromogranin A-Derived Peptide in Inhibition of Cell Death. J. Mol. Neurosci. 2011, 45, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Winkler, H.; Fischer-Colbrie, R. The Chromogranins A and B: The First 25 Years and Future Perspectives. Neuroscience 1992, 49, 497–528. [Google Scholar] [CrossRef]
- Fischer-Colbrie, R.; Laslop, A.; Kirchmair, R. Secretogranin II: Molecular Properties, Regulation of Biosynthesis and Processing to the Neuropeptide Secretoneurin. Prog. Neurobiol. 1995, 46, 49–70. [Google Scholar] [CrossRef]
- Laslop, A.; Doblinger, A.; Weiss, U. Proteolytic Processing of Chromogranins. Adv. Exp. Med. Biol. 2000, 482, 155–166. [Google Scholar] [PubMed]
- Troger, J.; Theurl, M.; Kirchmair, R.; Pasqua, T.; Tota, B.; Angelone, T.; Cerra, M.C.; Nowosielski, Y.; Mätzler, R.; Troger, J.; et al. Granin-Derived Peptides. Prog. Neurobiol. 2017, 154, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Koshimizu, H.; Kim, T.; Cawley, N.X.; Loh, Y.P. Chromogranin A: A New Proposal for Trafficking, Processing and Induction of Granule Biogenesis. Regul. Pept. 2010, 160, 153–159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loh, Y.P.; Koshimizu, H.; Cawley, N.X.; Tota, B. Serpinins: Role in Granule Biogenesis, Inhibition of Cell Death and Cardiac Function. Curr. Med. Chem. 2012, 19, 4086–4092. [Google Scholar] [CrossRef][Green Version]
- Tota, B.; Gentile, S.; Pasqua, T.; Bassino, E.; Koshimizu, H.; Cawley, N.X.; Cerra, M.C.; Loh, Y.P.; Angelone, T. The Novel Chromogranin A-Derived Serpinin and Pyroglutamated Serpinin Peptides Are Positive Cardiac ß-Adrenergic-Like Inotropes. FASEB J. 2012, 26, 2888–2898. [Google Scholar] [CrossRef]
- Pasqua, T.; Tota, B.; Penna, C.; Corti, A.; Cerra, M.C.; Loh, Y.P.; Angelone, T. pGlu-Serpinin Protects the Normotensive and Hypertensive Heart from Ischemic Injury. J. Endocrinol. 2015, 227, 167–178. [Google Scholar] [CrossRef][Green Version]
- Imbrogno, S.; Mazza, R.; Pugliese, C.; Filice, M.; Angelone, T.; Loh, Y.P.; Tota, B.; Cerra, M.C. The Chromogranin A-derived sympathomimetic serpinin depresses myocardial performance in teleost and amphibian hearts. Gen. Comp. Endocrinol. 2017, 240, 1–9. [Google Scholar] [CrossRef]
- Mätzler, R.; Humpel, C.; Leierer, J.; Staudinger, P.; Gramlich, O.; Cawley, N.; Loh, P.; Nowosielski, Y.; Teuchner, B.; Troger, J.; et al. Serpinin Immunoreactivity in the Rat Eye. Exp. Eye Res. 2022, in press. [Google Scholar]
- Steiner, R.; Humpel, C.; Bletsa, A.; Laimer, J.; Rauchegger, T.; Psomiadi, A.; Troger, J. Serpinin Immunoreactivity in the Dental Pulp. J. Dent. Res. 2022, in press. [Google Scholar]
- Gramlich, O.W.; Lorenz, K.; Grus, F.H.; Kriechbaum, M.; Ehrlich, D.; Humpel, C.; Fischer-Colbrie, R.; Bechrakis, N.E.; Troger, J. Catestatin-Like Immunoreactivity in the Rat Eye. Neuropeptides 2014, 48, 7–13. [Google Scholar] [CrossRef]
- Kennedy, W.R.; Wendelschafer-Crabb, G. The Innervation of Human Epidermis. J. Neurol. Sci. 1993, 115, 184–190. [Google Scholar] [CrossRef]
- Troger, J.; Humpel, C.; Fischer-Colbrie, R.; Nolano, M.; Provitera, V.; Sharma, V.K.; Loh, Y.P.; Pidsudko, Z.; Steiner, R.; Rauchegger, T.; et al. Chromogranin B-Derived Peptides in the Skin. J. Dermatol. Sci. 2022; in press. [Google Scholar]
- Troger, J.; Doblinger, A.; Leierer, J.; Laslop, A.; Schmid, E.; Teuchner, B.; Opatril, M.; Philipp, W.; Klimaschewski, L.; Pfaller, K.; et al. Secretoneurin in the Peripheral Ocular Innervation. Investig. Ophthalmol. Vis. Sci. 2005, 46, 647–654. [Google Scholar] [CrossRef]
- Lorenz, K.; Troger, J.; Gramlich, O.; Grus, F.; Hattmannstorfer, R.; Fischer-Colbrie, R.; Joachim, S.; Schmid, E.; Teuchner, B.; Haas, G.; et al. PE-11, a Peptide Derived from Chromogranin B, in the Rat Eye. Peptides 2011, 32, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.; Gramlich, O.; Grus, F.; Ehrlich, D.; Humpel, C.; Nogalo, M.; Fischer-Colbrie, R.; Bechrakis, N.; Hattmannstorfer, R.; Troger, J. GE-25-Like Immunoreactivity in the Rat Eye. Peptides 2012, 36, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Steiner, R.; Fischer-Colbrie, R.; Bletsa, A.; Laimer, J.; Troger, J. Secretoneurin and PE-11 Immunoreactivity in the Human Dental Pulp. Arch. Oral. Biol. 2018, 86, 13–17. [Google Scholar] [CrossRef]
- Holzer, P. Local Effector Functions of Capsaicin-Sensitive Sensory Nerve Endings: Involvement of Tachykinins, Calcitonin Gene-Related Peptide and Other Neuropeptides. Neuroscience 1988, 24, 739–768. [Google Scholar] [CrossRef]
- Ding, W.; Manni, M.; Stohl, L.L.; Zhou, X.K.; Wagner, J.A.; Granstein, R.D. Pituitary Adenylate Cyclase-Activating Peptide and Vasoactive Intestinal Polypeptide Bias Langerhans Cell Ag Presentation toward Th17 Cells. Eur. J. Immunol. 2012, 42, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Toth, D.; Szabo, E.; Tamas, A.; Juhasz, T.; Horvath, G.; Fabian, E.; Opper, B.; Szabo, D.; Maugeri, G.; D´Amico, A.G.; et al. Protective Effects of PACAP in Peripheral Organs. Front. Endocrinol. 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Lands, A.M.; Arnold, A.; McAnliff, J.P.; Induena, F.P.; Brown, R.J.J. Differentiation of Receptor Systems Activated by Sympathomimetic Amines. Nature 1967, 214, 597–598. [Google Scholar] [CrossRef]
- Ahlquist, R.P. Present State of α- and ß-Adrenergic Drugs I. The Adrenergic Receptor. Am. Heart J. 1976, 92, 661–664. [Google Scholar]
- Chruscinski, A.J.; Rohrer, D.K.; Schauble, E.; Desai, K.H.; Bernstein, D.; Kobilka, B.K. Targeted Disruption of the ß2 Adrenergic Receptor Gene. J. Biol. Chem. 1999, 274, 16694–16700. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraquelli, C.; Hauzinger, J.; Humpel, C.; Nolano, M.; Provitera, V.; Sharma, V.K.; Loh, P.; Pidsudko, Z.; Blatsios, G.; Troger, J. Serpinin in the Skin. Biomedicines 2022, 10, 183. https://doi.org/10.3390/biomedicines10010183
Fraquelli C, Hauzinger J, Humpel C, Nolano M, Provitera V, Sharma VK, Loh P, Pidsudko Z, Blatsios G, Troger J. Serpinin in the Skin. Biomedicines. 2022; 10(1):183. https://doi.org/10.3390/biomedicines10010183
Chicago/Turabian StyleFraquelli, Cristina, Jasmine Hauzinger, Christian Humpel, Maria Nolano, Vincenzo Provitera, Vinay Kumar Sharma, Peng Loh, Zenon Pidsudko, Georgios Blatsios, and Josef Troger. 2022. "Serpinin in the Skin" Biomedicines 10, no. 1: 183. https://doi.org/10.3390/biomedicines10010183
APA StyleFraquelli, C., Hauzinger, J., Humpel, C., Nolano, M., Provitera, V., Sharma, V. K., Loh, P., Pidsudko, Z., Blatsios, G., & Troger, J. (2022). Serpinin in the Skin. Biomedicines, 10(1), 183. https://doi.org/10.3390/biomedicines10010183







