GABA Administration Ameliorates Sjogren’s Syndrome in Two Different Mouse Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Reagents
2.3. GABA Treatment
2.4. Measurements of Saliva Production
2.5. Measurement of Basal Tear Production
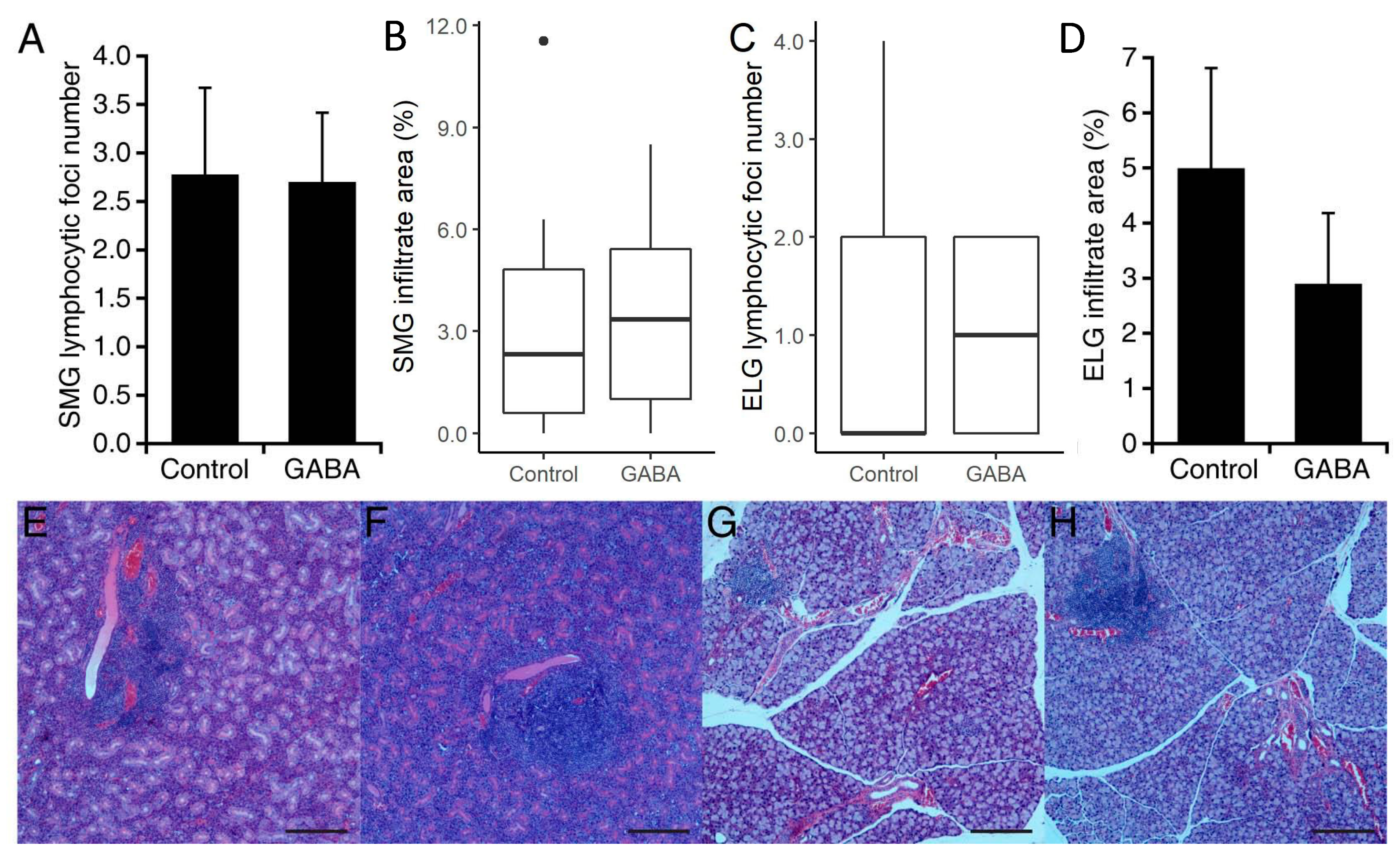
2.6. Histological Analysis of Salivary and Lacrimal Gland Tissues
2.7. Statistical Analysis
3. Results
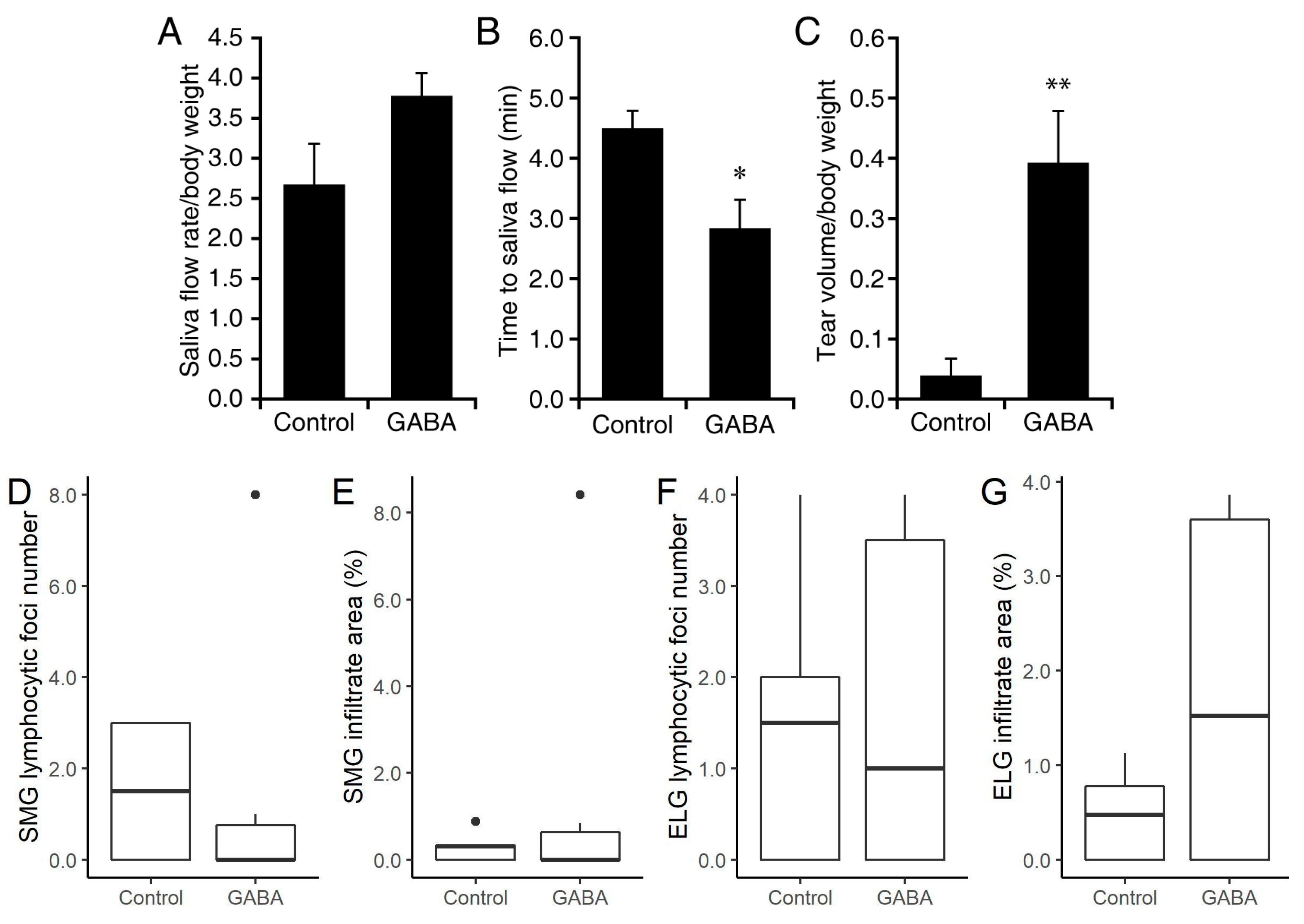
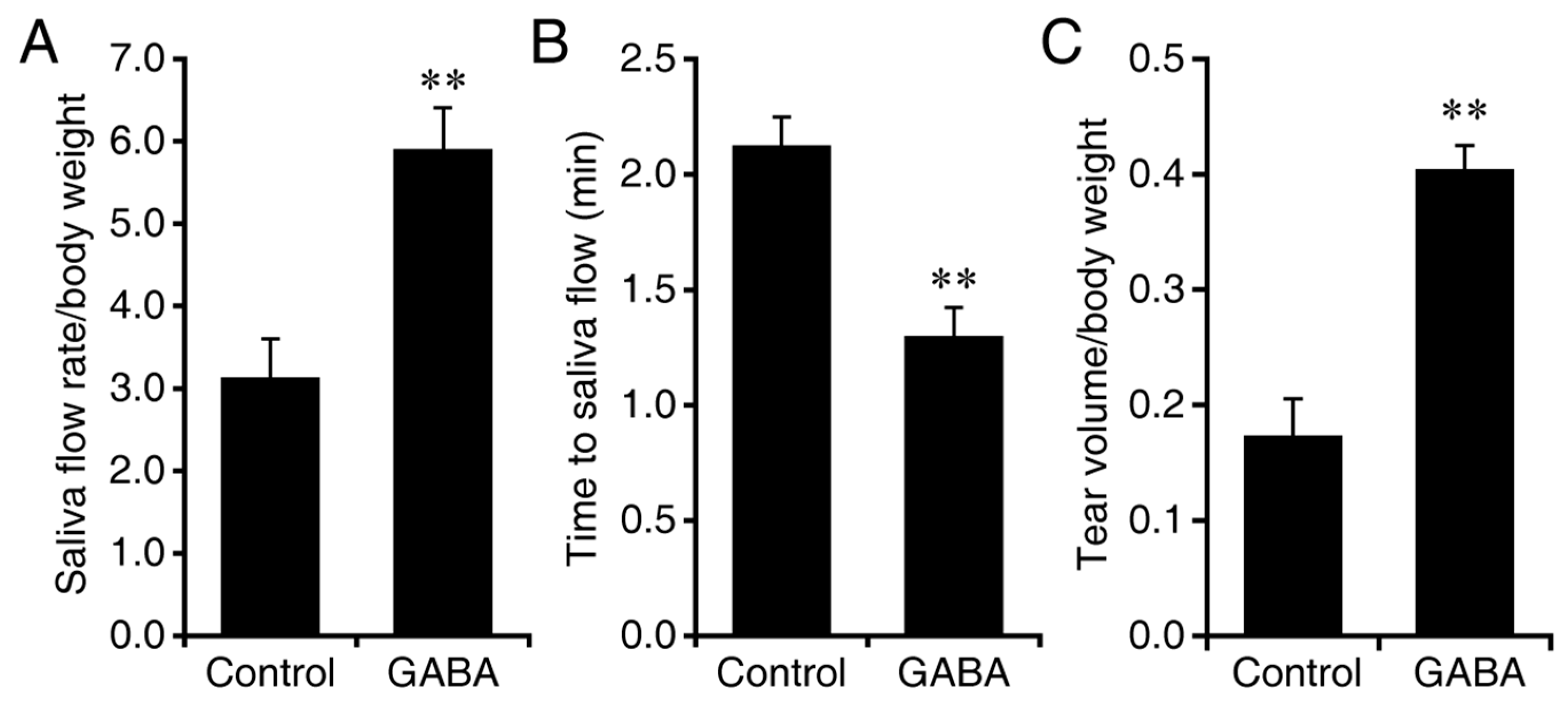
3.1. Prophylactic GABA Treatment in NOD.B10-H2b Mice
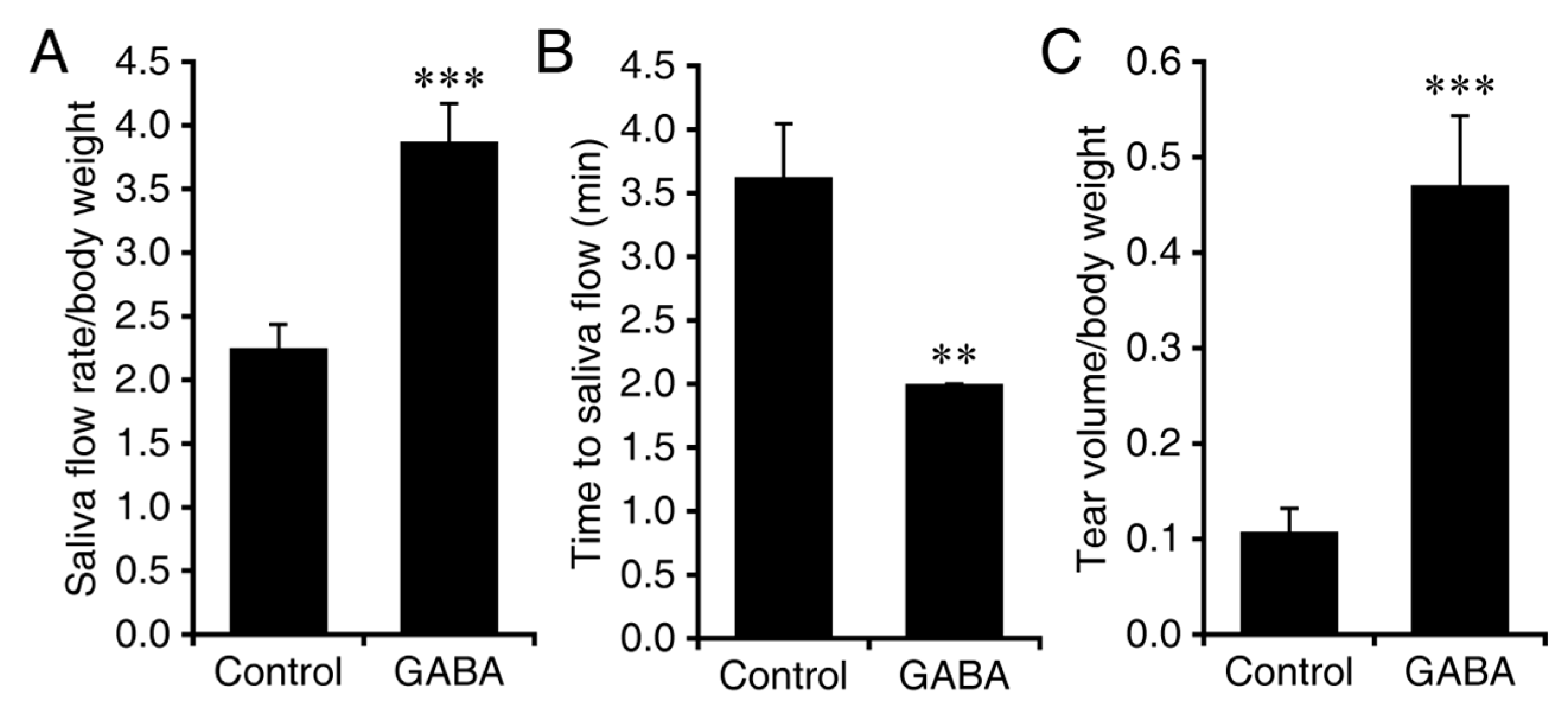
3.2. Interventive GABA Therapy after Disease Onset in NOD.B10-H2b Mice
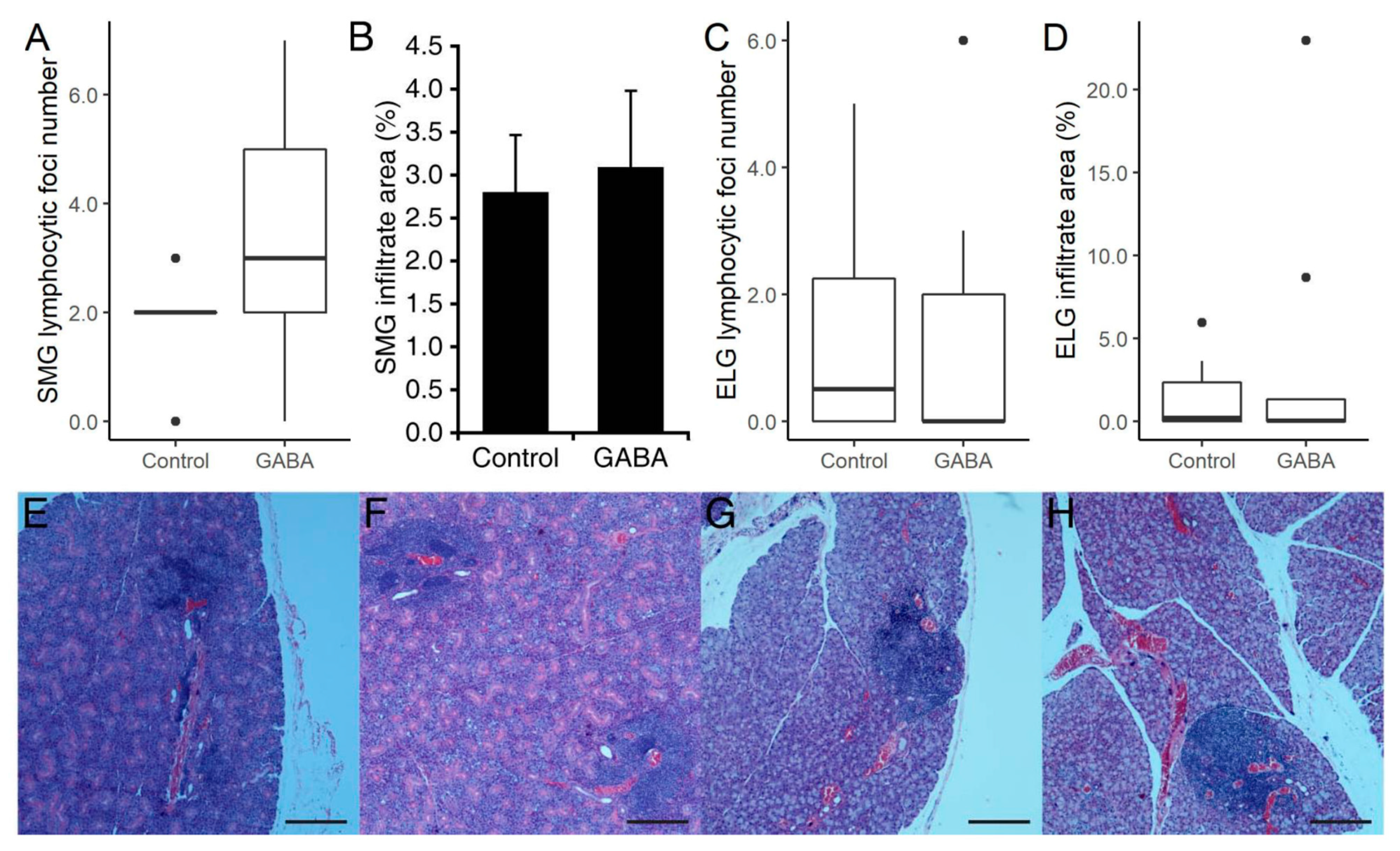
3.3. Prophylactic GABA Treatment in C57BL/6.NOD-Aec1Aec2 Mice
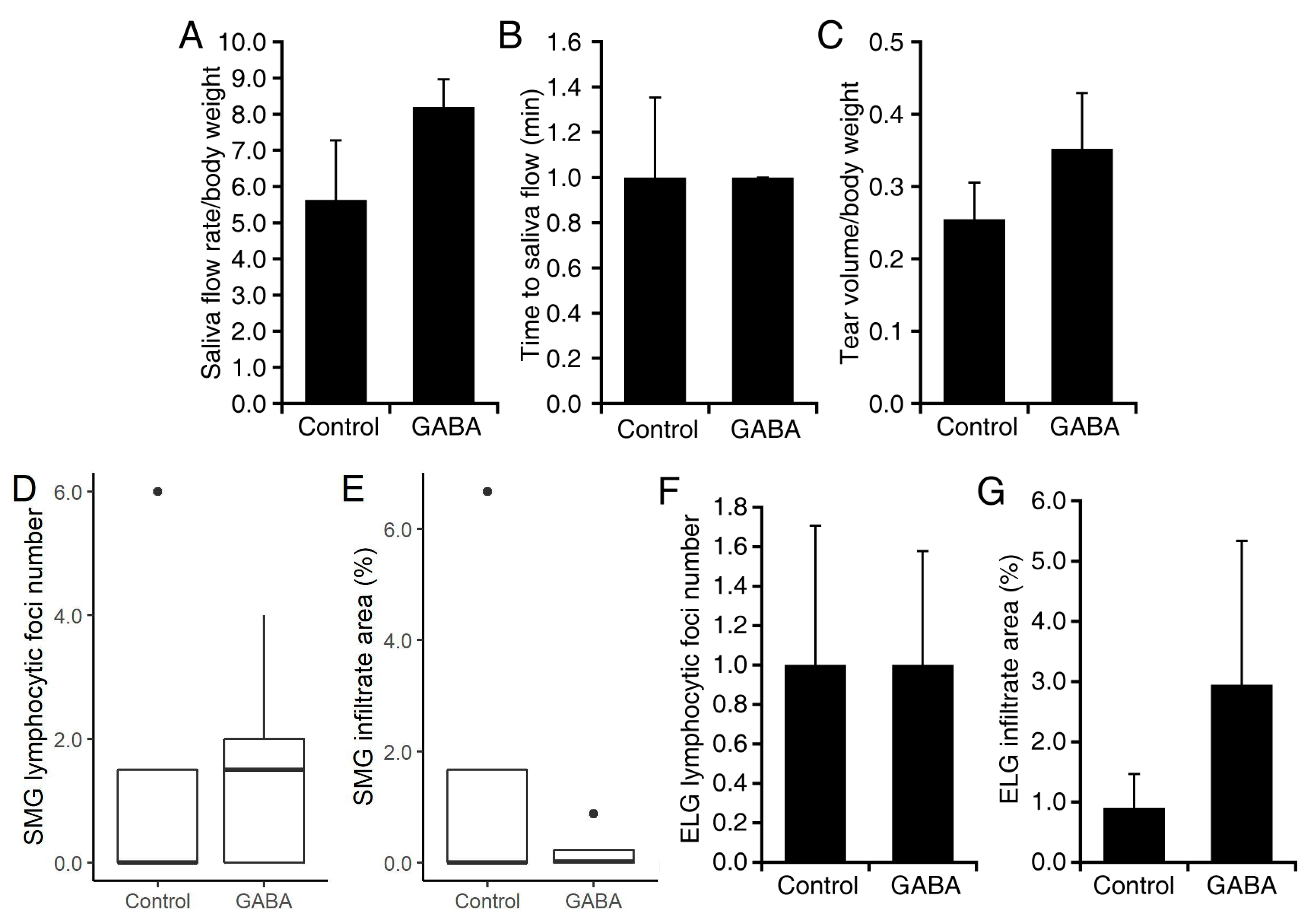
3.4. GABA Treatment after Disease Onset in C57BL/6.NOD-Aec1Aec2 Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fox, I.R. Sjögren’s syndrome. Lancet 2005, 366, 321–331. [Google Scholar] [CrossRef]
- Voulgarelis, M.; Tzioufas, A.G. Pathogenetic mechanisms in the initiation and perpetuation of Sjögren’s syndrome. Nat. Rev. Rheumatol. 2010, 6, 529–537. [Google Scholar] [CrossRef]
- Peri, Y.; Agmon-Levin, N.; Theodor, E.; Shoenfeld, Y. Sjögren’s syndrome, the old and the new. Best Pr. Res. Clin. Rheumatol. 2012, 26, 105–117. [Google Scholar] [CrossRef]
- Mavragani, C.P.; Moutsopoulos, H.M. Sjögren’s Syndrome. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, C.P.; Moutsopoulos, H.M. The geoepidemiology of Sjögren’s syndrome. Autoimmun. Rev. 2010, 9, A305–A310. [Google Scholar] [CrossRef] [PubMed]
- Skopouli, F.N.; Fox, P.C.; Galanopoulou, V.; Atkinson, J.C.; Jaffe, E.S.; Moutsopoulos, H.M. T cell subpopulations in the labial minor salivary gland histopathologic lesion of Sjogren’s syndrome. J. Rheumatol. 1991, 18, 210–214. [Google Scholar]
- Manoussakis, M.N.; Boiu, S.; Korkolopoulou, P.; Kapsogeorgou, E.K.; Kavantzas, N.; Ziakas, P.; Patsouris, E.; Moutsopoulos, H.M. Rates of infiltration by macrophages and dendritic cells and expression of interleukin-18 and interleukin-12 in the chronic inflammatory lesions of Sjögren’s syndrome: Correlation with certain features of immune hyperactivity and factors associated with high risk of lymphoma development. Arthritis Rheum. 2007, 56, 3977–3988. [Google Scholar] [CrossRef]
- Fox, R.I.; Carstens, S.A.; Fong, S.; Robinson, C.A.; Howell, F.; Vaughan, J.H. Use of monoclonal antibodies to analyze peripheral blood and salivary gland lymphocyte subsets in Sjogren’s syndrome. Arthritis Rheum. 1982, 25, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Adamson, T.C., 3rd; Fox, R.I.; Frisman, D.M.; Howell, F.V. Immunohistologic analysis of lymphoid infiltrates in primary Sjogren’s syndrome using monoclonal antibodies. J. Immunol. 1983, 130, 203–208. [Google Scholar]
- Talal, N.; Sylvester, R.A.; Daniels, T.E.; Greenspan, J.S.; Williams, R.C. T and B Lymphocytes in Peripheral Blood and Tissue Lesions in Sjögren’s Syndrome. J. Clin. Investig. 1974, 53, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.I.; Kapsogeorgou, E.K.; Moutsopoulos, H.M. Characteristics of the minor salivary gland infiltrates in Sjögren’s syndrome. J. Autoimmun. 2010, 34, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Witas, R.; Gupta, S.; Nguyen, C.Q. Contributions of Major Cell Populations to Sjögren’s Syndrome. J. Clin. Med. 2020, 9, 3057. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.Q.; Hu, M.H.; Li, Y.; Stewart, C.; Peck, A.B. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjögren’s syndrome: Findings in humans and mice. Arthritis Rheum. 2008, 58, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Sugawara, Y.; Kuroishi, T.; Sasano, T.; Sugawara, S. Identification of IL-18 and Th17 Cells in Salivary Glands of Patients with Sjögren’s Syndrome, and Amplification of IL-17-Mediated Secretion of Inflammatory Cytokines from Salivary Gland Cells by IL-18. J. Immunol. 2008, 181, 2898–2906. [Google Scholar] [CrossRef]
- Katsifis, G.E.; Rekka, S.; Moutsopoulos, N.M.; Pillemer, S.; Wahl, S.M. Systemic and Local Interleukin-17 and Linked Cytokines Associated with Sjögren’s Syndrome Immunopathogenesis. Am. J. Pathol. 2009, 175, 1167–1177. [Google Scholar] [CrossRef]
- Voigt, A.; Esfandiary, L.; Wanchoo, A.; Glenton, P.; Donate, A.; Craft, W.F.; Craft, S.L.M.; Nguyen, C.Q. Sexual dimorphic function of IL-17 in salivary gland dysfunction of the C57BL/6.NOD-Aec1Aec2 model of Sjögren’s syndrome. Sci. Rep. 2016, 6, 38717. [Google Scholar] [CrossRef]
- Voigt, A.; Bohn, K.; Sukumaran, S.; Stewart, C.M.; Bhattacharya, I.; Nguyen, C.Q. Unique glandular ex-vivo Th1 and Th17 receptor motifs in Sjögren’s syndrome patients using single-cell analysis. Clin. Immunol. 2018, 192, 58–67. [Google Scholar] [CrossRef]
- Luo, J.; Ming, B.; Zhang, C.; Deng, X.; Li, P.; Wei, Z.; Xia, Y.; Jiang, K.; Ye, H.; Ma, W.; et al. IL-2 Inhibition of Th17 Generation Rather Than Induction of Treg Cells Is Impaired in Primary Sjögren’s Syndrome Patients. Front. Immunol. 2018, 9, 1755. [Google Scholar] [CrossRef] [PubMed]
- Psianou, K.; Panagoulias, I.; Papanastasiou, A.D.; De Lastic, A.-L.; Rodi, M.; Spantidea, P.I.; Degn, S.E.; Georgiou, P.; Mouzaki, A. Clinical and immunological parameters of Sjögren’s syndrome. Autoimmun. Rev. 2018, 17, 1053–1064. [Google Scholar] [CrossRef]
- Lin, X.; Rui, K.; Deng, J.; Tian, J.; Wang, X.; Wang, S.; Ko, K.-H.; Jiao, Z.; Chan, V.S.-F.; Lau, W.C.S.; et al. Th17 cells play a critical role in the development of experimental Sjögren’s syndrome. Ann. Rheum. Dis. 2014, 74, 1302–1310. [Google Scholar] [CrossRef]
- Tian, J.; Chau, C.; Hales, T.G.; Kaufman, D.L. GABA(A) receptors mediate inhibition of T cell responses. J. Neuroimmunol. 1999, 96, 21–28. [Google Scholar] [CrossRef]
- Tian, J.; Yong, J.; Dang, H.; Kaufman, D.L. Oral GABA treatment downregulates inflammatory responses in a mouse model of rheumatoid arthritis. Autoimmunity 2011, 44, 465–470. [Google Scholar] [CrossRef]
- Bhat, R.; Axtell, R.; Mitra, A.; Miranda, M.; Lock, C.; Tsien, R.W.; Steinman, L. Inhibitory role for GABA in autoimmune inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 2580–2585. [Google Scholar] [CrossRef]
- Januzi, L.; Poirier, J.W.; Maksoud, M.J.; Xiang, Y.-Y.; Veldhuizen, R.A.; Gill, S.E.; Cregan, S.P.; Zhang, H.; Dekaban, G.A.; Lu, W.-Y. Autocrine GABA signaling distinctively regulates phenotypic activation of mouse pulmonary macrophages. Cell. Immunol. 2018, 332, 7–23. [Google Scholar] [CrossRef]
- Prud’Homme, G.J.; Glinka, Y.; Hasilo, C.; Paraskevas, S.; Li, X.; Wang, Q. GABA Protects Human Islet Cells Against the Deleterious Effects of Immunosuppressive Drugs and Exerts Immunoinhibitory Effects Alone. Transplantation 2013, 96, 616–623. [Google Scholar] [CrossRef]
- Mendu, S.K.; Åkesson, L.; Jin, Z.; Edlund, A.; Cilio, C.; Lernmark, Å.; Birnir, B. Increased GABAA channel subunits expression in CD8+ but not in CD4+ T cells in BB rats developing diabetes compared to their congenic littermates. Mol. Immunol. 2011, 48, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Bhandage, A.K.; Olivera, G.C.; Kanatani, S.; Thompson, E.; Loré, K.; Varas-Godoy, M.; Barragan, A. A motogenic GABAergic system of mononuclear phagocytes facilitates dissemination of coccidian parasites. eLife 2020, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Dang, H.; Wallner, M.; Olsen, R.; Kaufman, D.L. Homotaurine, a safe blood-brain barrier permeable GABAA-R-specific agonist, ameliorates disease in mouse models of multiple sclerosis. Sci. Rep. 2018, 8, 16555. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Dang, H.; O’Laco, K.A.; Song, M.; Tiu, B.-C.; Gilles, S.; Zakarian, C.; Kaufman, D.L. Homotaurine Treatment Enhances CD4+ and CD8+ Regulatory T Cell Responses and Synergizes with Low-Dose Anti-CD3 to Enhance Diabetes Remission in Type 1 Diabetic Mice. ImmunoHorizons 2019, 3, 498–510. [Google Scholar] [CrossRef]
- Huang, S.; Mao, J.; Wei, B.; Pei, G. The anti-spasticity drug baclofen alleviates collagen-induced arthritis and regulates dendritic cells. J. Cell. Physiol. 2015, 230, 1438–1447. [Google Scholar] [CrossRef]
- Duthey, B.; Hübner, A.; Diehl, S.; Boehncke, S.; Pfeffer, J.; Boehncke, W.-H. Anti-inflammatory effects of the GABAB receptor agonist baclofen in allergic contact dermatitis. Exp. Dermatol. 2010, 19, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Beales, P.E.; Hawa, M.; Williams, A.J.K.; Albertini, M.C.; Giorgini, A.; Pozzilli, P. Baclofen, a gamma-aminobutyric acid-b receptor agonist, delays diabetes onset in the non-obese diabetic mouse. Acta Diabetol. 1995, 32, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Crowley, T.; Fitzpatrick, J.-M.; Kuijper, T.; Cryan, J.F.; O’Toole, O.; O’Leary, O.F.; Downer, E.J. Modulation of TLR3/TLR4 inflammatory signaling by the GABAB receptor agonist baclofen in glia and immune cells: Relevance to therapeutic effects in multiple sclerosis. Front. Cell. Neurosci. 2015, 9, 284. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Middleton, B.; Lee, V.S.; Park, H.W.; Zhang, Z.; Kim, B.; Lowe, C.; Nguyen, N.; Liu, H.; Beyer, R.S.; et al. GABAB-Receptor Agonist-Based Immunotherapy for Type 1 Diabetes in NOD Mice. Biomedicines 2021, 9, 43. [Google Scholar] [CrossRef]
- Tian, J.; Song, M.; Kaufman, D.L. Homotaurine limits the spreading of T cell autoreactivity within the CNS and ameliorates disease in a model of multiple sclerosis. Sci. Rep. 2021, 11, 5402. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Middleton, B.; Kaufman, D. GABAA-Receptor Agonists Limit Pneumonitis and Death in Murine Coronavirus-Infected Mice. Viruses 2021, 13, 966. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Lu, Y.; Zhang, H.; Chau, C.H.; Dang, H.N.; Kaufman, D.L. Gamma-aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J. Immunol. 2004, 173, 5298–5304. [Google Scholar] [CrossRef]
- Bhandage, A.K.; Jin, Z.; Korol, S.V.; Shen, Q.; Pei, Y.; Deng, Q.; Espes, D.; Carlsson, P.-O.; Kamali-Moghaddam, M.; Birnir, B. GABA Regulates Release of Inflammatory Cytokines from Peripheral Blood Mononuclear Cells and CD4+ T Cells and Is Immunosuppressive in Type 1 Diabetes. EBioMedicine 2018, 30, 283–294. [Google Scholar] [CrossRef]
- Tian, J.; Dang, H.; Nguyen, A.V.; Chen, Z.; Kaufman, D.L. Combined Therapy with GABA and Proinsulin/Alum Acts Synergistically to Restore Long-term Normoglycemia by Modulating T-Cell Autoimmunity and Promoting β-Cell Replication in Newly Diabetic NOD Mice. Diabetes 2014, 63, 3128–3134. [Google Scholar] [CrossRef]
- Tian, J.; Dang, H.N.; Yong, J.; Chui, W.-S.; Dizon, M.P.G.; Yaw, C.K.Y.; Kaufman, D.L. Oral Treatment with γ-Aminobutyric Acid Improves Glucose Tolerance and Insulin Sensitivity by Inhibiting Inflammation in High Fat Diet-Fed Mice. PLoS ONE 2011, 6, e25338. [Google Scholar] [CrossRef]
- Alam, S.; Laughton, D.L.; Walding, A.; Wolstenholme, A.J. Human peripheral blood mononuclear cells express GABAA receptor subunits. Mol. Immunol. 2006, 43, 1432–1442. [Google Scholar] [CrossRef]
- Mendu, S.K.; Bhandage, A.K.; Jin, Z.; Birnir, B. Different Subtypes of GABA-A Receptors Are Expressed in Human, Mouse and Rat T Lymphocytes. PLoS ONE 2012, 7, e42959. [Google Scholar] [CrossRef]
- Robinson, C.P.; Yamachika, S.; Bounous, D.I.; Brayer, J.; Jonsson, R.; Holmdahl, R.; Peck, A.B.; Humphreys-Beher, M.G. A novel NOD-derived murine model of primary Sjogren’s syndrome. Arthritis Rheum. 1998, 41, 150–156. [Google Scholar] [CrossRef]
- Gao, J.; Killedar, S.; Cornelius, J.G.; Nguyen, C.; Cha, S.; Peck, A.B. Sjögren’s syndrome in the NOD mouse model is an interleukin-4 time-dependent, antibody isotype-specific autoimmune disease. J. Autoimmun. 2006, 26, 90–103. [Google Scholar] [CrossRef]
- Nguyen, C.Q.; Gao, J.-H.; Kim, H.; Saban, D.R.; Cornelius, J.G.; Peck, A.B. IL-4-STAT6 Signal Transduction-Dependent Induction of the Clinical Phase of Sjögren’s Syndrome-Like Disease of the Nonobese Diabetic Mouse. J. Immunol. 2007, 179, 382–390. [Google Scholar] [CrossRef]
- Kiripolsky, J.; Shen, L.; Liang, Y.; Li, A.; Suresh, L.; Lian, Y.; Li, Q.-Z.; Gaile, D.P.; Kramer, J.M. Systemic manifestations of primary Sjögren’s syndrome in the NOD.B10Sn- H2 b /J mouse model. Clin. Immunol. 2017, 183, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Allushi, B.; Bagavant, H.; Papinska, J.; Deshmukh, U.S. Hyperglycemia and Salivary Gland Dysfunction in the Non-obese Diabetic Mouse: Caveats for Preclinical Studies in Sjögren’s Syndrome. Sci. Rep. 2019, 9, 17969. [Google Scholar] [CrossRef]
- Cha, S.; Nagashima, H.; Brown, V.B.; Peck, A.B.; Humphreys-Beher, M.G. Two NODIdd-associated intervals contribute synergistically to the development of autoimmune exocrinopathy (Sjögren’s syndrome) on a healthy murine background. Arthritis Rheum. 2002, 46, 1390–1398. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.; Nagashima, H.; Peck, A.B.; Humphreys-Beher, M.G. IDD3 and IDD5 alleles from nod mice mediate Sjogren’s syndrome-like autoimmunity. Adv. Exp. Med. Biol. 2002, 506, 1035–1039. [Google Scholar] [PubMed]
- Horvath, S.; Nazmul-Hossain, A.N.; Pollard, R.P.; Kroese, F.G.; Vissink, A.; Kallenberg, C.G.; Spijkervet, F.K.; Bootsma, H.; A Michie, S.; Gorr, S.U.; et al. Systems analysis of primary Sjögren’s syndrome pathogenesis in salivary glands identifies shared pathways in human and a mouse model. Arthritis Res. Ther. 2012, 14, R238. [Google Scholar] [CrossRef]
- Peck, A.B.; Nguyen, C.Q. Transcriptome analysis of the interferon-signature defining the autoimmune process of Sjögren’s syndrome. Scand. J. Immunol. 2012, 76, 237–245. [Google Scholar] [CrossRef][Green Version]
- Nguyen, C.; Singson, E.; Kim, J.-Y.; Cornelius, J.G.; Attia, R.; Doyle, M.E.; Bulosan, M.; Cha, S.; Peck, A.B. Sjogren’s Syndrome-Like Disease of C57BL/6.NOD-Aec1 Aec2 Mice: Gender Differences in Keratoconjunctivitis Sicca Defined by a Cross-Over in the Chromosome 3 Aec1 Locus. Scand. J. Immunol. 2006, 64, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Carcamo, W.C.; Chiorini, J.A.; Peck, A.B.; Nguyen, C.Q. Gene therapy using IL-27 ameliorates Sjögren’s syndrome-like autoimmune exocrinopathy. Arthritis Res. Ther. 2012, 14, R172. [Google Scholar] [CrossRef]
- Bombardieri, M.; Barone, F.; Pittoni, V.; Alessandri, C.; Conigliaro, P.; Blades, M.C.; Priori, R.; McInnes, I.B.; Valesini, G.; Pitzalis, C. Increased circulating levels and salivary gland expression of interleukin-18 in patients with Sjögren’s syndrome: Relationship with autoantibody production and lymphoid organization of the periductal inflammatory infiltrate. Arthritis Res. Ther. 2004, 6, R447–R456. [Google Scholar] [CrossRef]
- Haga, H.J. Clinical and immunological factors associated with low lacrimal and salivary flow rate in patients with primary Sjogren’s syndrome. J. Rheumatol. 2002, 29, 305–308. [Google Scholar]
- Maehara, T.; Moriyama, M.; Hayashida, J.-N.; Tanaka, A.; Shinozaki, S.; Kubo, Y.; Matsumura, K.; Nakamura, S. Selective localization of T helper subsets in labial salivary glands from primary Sjögren’s syndrome patients. Clin. Exp. Immunol. 2012, 169, 89–99. [Google Scholar] [CrossRef]
- Doyle, M.E.; Boggs, L.; Attia, R.; Cooper, L.R.; Saban, D.R.; Nguyen, C.Q.; Peck, A.B. Autoimmune Dacryoadenitis of NOD/LtJ Mice and Its Subsequent Effects on Tear Protein Composition. Am. J. Pathol. 2007, 171, 1224–1236. [Google Scholar] [CrossRef]
- Mottram, P.L.; Murray-Segal, L.J.; Han, W.; Maguire, J.; Stein-Oakley, A.N. Remission and pancreas isograft survival in recent onset diabetic NOD mice after treatment with low-dose anti-CD3 monoclonal antibodies. Transpl. Immunol. 2002, 10, 63–72. [Google Scholar] [CrossRef]
- Ando, H.; Kurita, S.; Takamura, T. The specific p38 mitogen-activated protein kinase pathway inhibitor FR167653 keeps insulitis benign in nonobese diabetic mice. Life Sci. 2003, 74, 1817–1827. [Google Scholar] [CrossRef]
- Villalba, A.; Rodríguez-Fernández, S.; Ampudia, R.-M.; Cano-Sarabia, M.; Perna-Barrull, D.; Bertran-Cobo, C.; Ehrenberg, C.; Maspoch, D.; Vives-Pi, M. Preclinical evaluation of antigen-specific nanotherapy based on phosphatidylserine-liposomes for type 1 diabetes. Artif. Cells Nanomed. Biotechnol. 2019, 48, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Otomo, E.; Araki, G.; Mori, A.; Kurihara, M. Clinical evaluation of GABA in the treatment of cerebrovascular disorders. Multi-center double-blind study in comparison with pyrithioxine and placebo. Arzneimittelforschung 1981, 31, 1511–1523. [Google Scholar] [PubMed]
- Loeb, C.; Benassi, E.; Bo, G.-P.; Cocito, L.; Maffini, M.; Scotto, P. Preliminary evaluation of the effect of GABA and phosphatidylserine in epileptic patients. Epilepsy Res. 1987, 1, 209–212. [Google Scholar] [CrossRef]
- Tower, D.B.; Roberts, E. (Eds.) Inhibition in the Nervous System and GABA; Pergamon Press: New York, NY, USA, 1960. [Google Scholar]
- Li, J.; Zhang, Z.; Liu, X.; Wang, Y.; Mao, F.; Mao, J.; Lu, X.; Jiang, D.; Wan, Y.; Lv, J.-Y.; et al. Study of GABA in Healthy Volunteers: Pharmacokinetics and Pharmacodynamics. Front. Pharmacol. 2015, 6, 260. [Google Scholar] [CrossRef]
- Oketch-Rabah, H.; Madden, E.; Roe, A.; Betz, J. United States Pharmacopeia (USP) Safety Review of Gamma-Aminobutyric Acid (GABA). Nutrients 2021, 13, 2742. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.; Tian, J.; Middleton, B.; Nguyen, C.Q.; Kaufman, D.L. GABA Administration Ameliorates Sjogren’s Syndrome in Two Different Mouse Models. Biomedicines 2022, 10, 129. https://doi.org/10.3390/biomedicines10010129
Song M, Tian J, Middleton B, Nguyen CQ, Kaufman DL. GABA Administration Ameliorates Sjogren’s Syndrome in Two Different Mouse Models. Biomedicines. 2022; 10(1):129. https://doi.org/10.3390/biomedicines10010129
Chicago/Turabian StyleSong, Min, Jide Tian, Blake Middleton, Cuong Q. Nguyen, and Daniel L. Kaufman. 2022. "GABA Administration Ameliorates Sjogren’s Syndrome in Two Different Mouse Models" Biomedicines 10, no. 1: 129. https://doi.org/10.3390/biomedicines10010129
APA StyleSong, M., Tian, J., Middleton, B., Nguyen, C. Q., & Kaufman, D. L. (2022). GABA Administration Ameliorates Sjogren’s Syndrome in Two Different Mouse Models. Biomedicines, 10(1), 129. https://doi.org/10.3390/biomedicines10010129





