NOG-Derived Peptides Can Restore Neuritogenesis on a CRASH Syndrome Cell Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Structural Modeling and Molecular Dynamics
2.2. Peptide Synthesis, Purification and Characterization
2.3. Cell Culture and Peptide Treatment
2.4. L1CAM Clones and SH-SY5Y Transfection
2.5. Neuritogenesis Assays
2.6. Immunofluorescence
2.7. Statistical Analysis
3. Results and Discussion
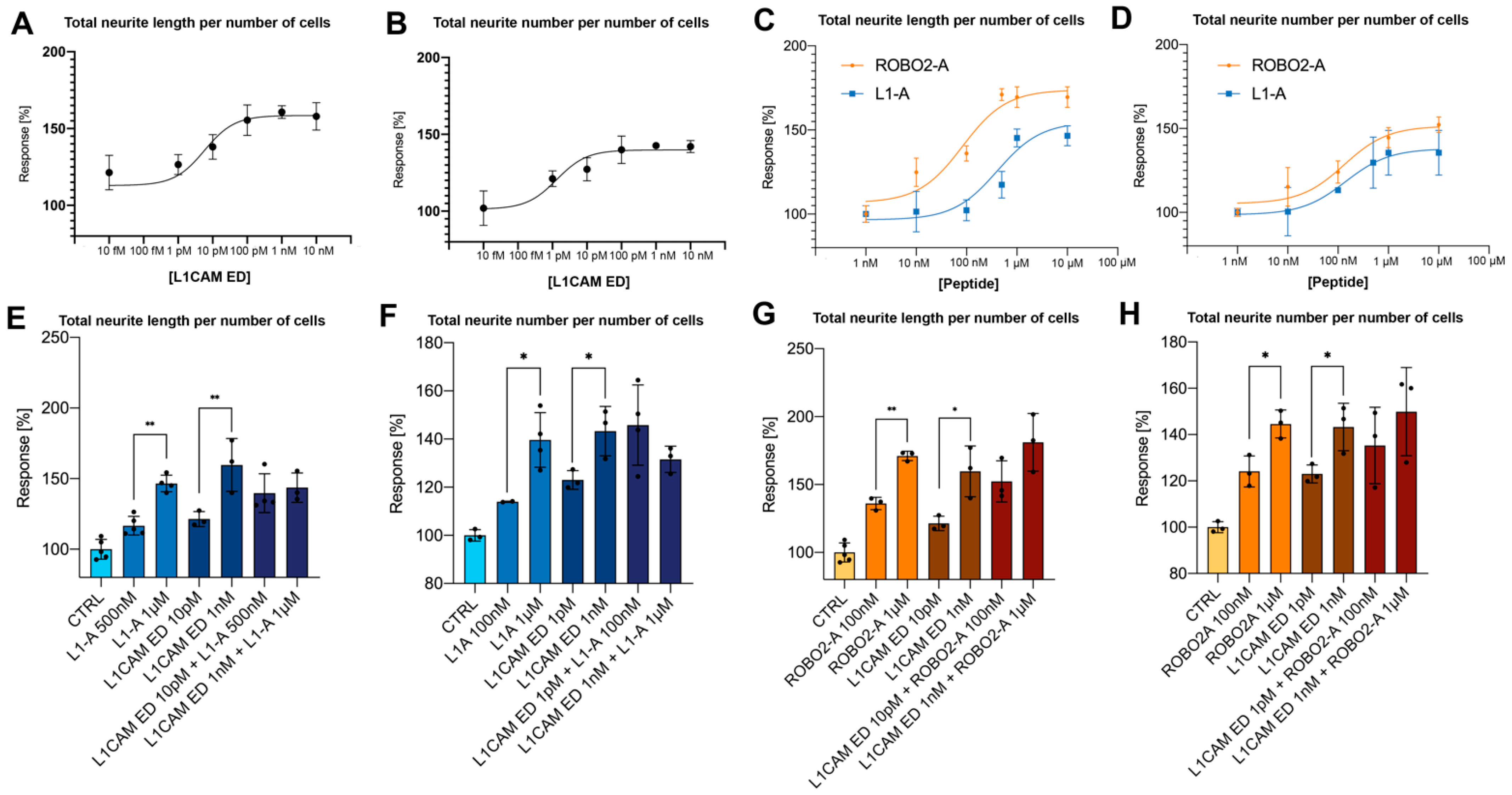
3.1. NOG-Derived Peptides and L1CAM ED Show Comparable Proneuritogenic Capacity
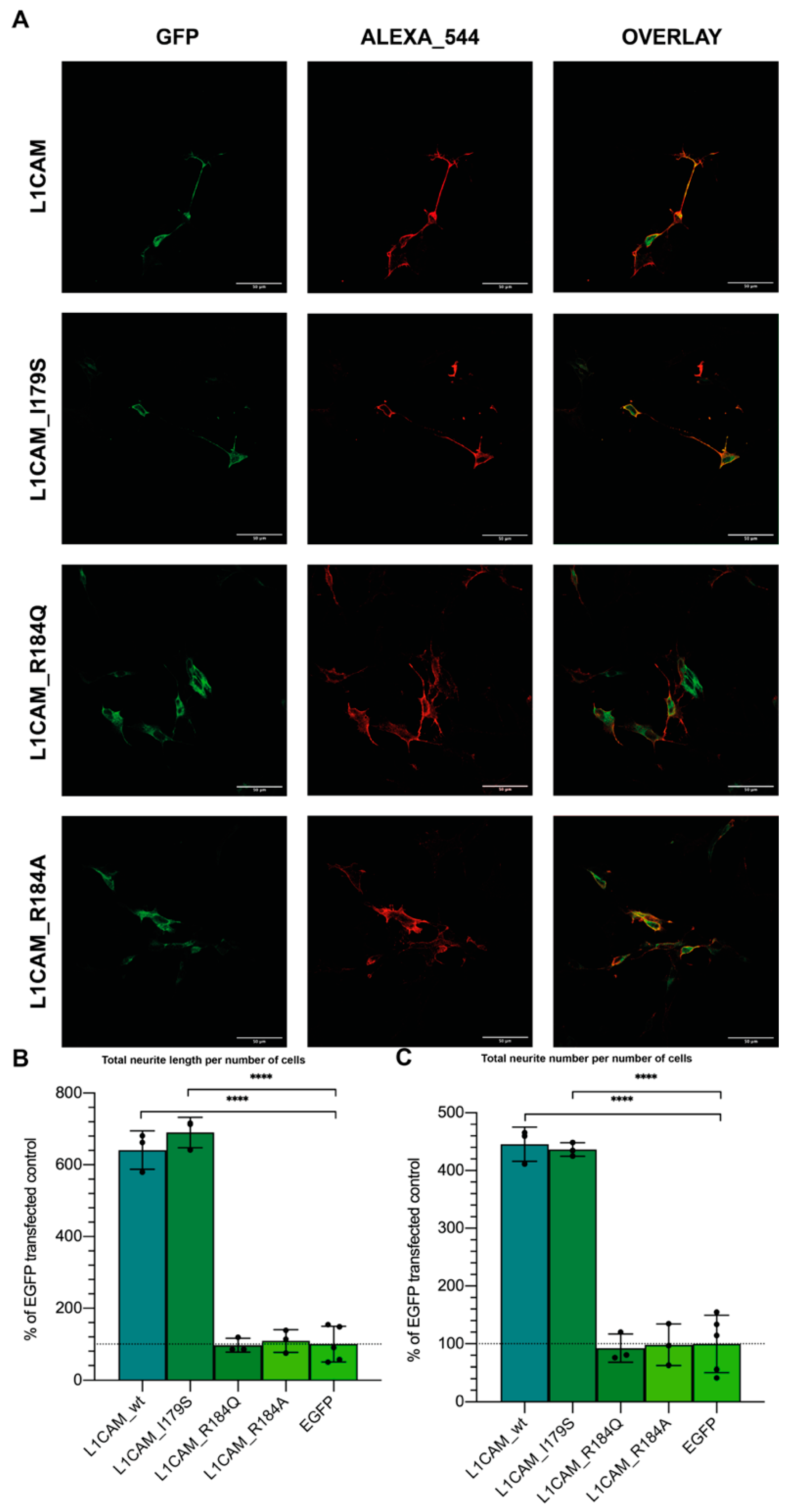
3.2. L1-A Peptides with Known L1CAM Mutations Show Impaired Neuritogenic Capacity
3.3. CRASH Mutations Differentially Impact on L1CAM Proneuritogenic Activity
3.4. In Silico Simulations Suggest a Rationale for CRASH Differential Severity and Translational Perspectives
3.5. L1-A Can Restore Neuritogenesis on a CRASH Syndrome Cell Model, While L1CAM ED Is Unable
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- McFarlane, S. Attraction vs. repulsion: The growth cone decides. Biochem. Cell Biol. 2000, 78, 563–568. [Google Scholar] [CrossRef]
- Zhang, Y.; Yeh, J.; Richardson, P.M.; Bo, X. Cell adhesion molecules of the immunoglobulin superfamily in axonal regeneration and neural repair. Restor. Neurol. Neurosci. 2008, 26, 81–96. [Google Scholar]
- Hortsch, M.; Nagaraj, K.; Mualla, R. The L1 family of cell adhesion molecules: A sickening number of mutations and protein functions. Adv. Neurobiol. 2014, 8, 195–229. [Google Scholar] [CrossRef]
- Abe, K.; Katsuno, H.; Toriyama, M.; Baba, K.; Mori, T.; Hakoshima, T.; Kanemura, Y.; Watanabe, R.; Inagaki, N. Grip and slip of L1-CAM on adhesive substrates direct growth cone haptotaxis. Proc. Natl. Acad. Sci. USA 2018, 115, 2764–2769. [Google Scholar] [CrossRef] [Green Version]
- Duncan, B.W.; Murphy, K.E.; Maness, P.F. Molecular Mechanisms of L1 and NCAM Adhesion Molecules in Synaptic Pruning, Plasticity, and Stabilization. Front. Cell Dev. Biol. 2021, 9, 625340. [Google Scholar] [CrossRef]
- Liu, H.; Focia, P.J.; He, X. Homophilic adhesion mechanism of neurofascin, a member of the L1 family of neural cell adhesion molecules. J. Biol. Chem. 2011, 286, 797–805. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Jensen, G.J.; Bjorkman, P.J. Cryo-electron tomography of homophilic adhesion mediated by the neural cell adhesion molecule L1. Structure 2009, 17, 460–471. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, R.M.; Gomes, C.M.; Sousa, M.; Alves, P.M.; Costa, J. Kinetic analysis of L1 homophilic interaction: Role of the first four immunoglobulin domains and implications on binding mechanism. J. Biol. Chem. 2008, 283, 28038–28047. [Google Scholar] [CrossRef] [Green Version]
- Itoh, K.; Fushiki, S. The role of L1cam in murine corticogenesis, and the pathogenesis of hydrocephalus. Pathol. Int. 2015, 65, 58–66. [Google Scholar] [CrossRef]
- Zhang, L. CRASH syndrome: Does it teach us about neurotrophic functions of cell adhesion molecules? Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2010, 16, 470–474. [Google Scholar] [CrossRef]
- Vos, Y.J.; Hofstra, R.M.W. An updated and upgraded L1CAM mutation database. Hum. Mutat. 2010, 31, E1102–E1109. [Google Scholar] [CrossRef]
- Christaller, W.A.A.; Vos, Y.; Gebre-Medhin, S.; Hofstra, R.M.W.; Schäfer, M.K.E. L1 syndrome diagnosis complemented with functional analysis of L1CAM variants located to the two N-terminal Ig-like domains. Clin. Genet. 2017, 91, 115–120. [Google Scholar] [CrossRef]
- Vos, Y.J.; de Walle, H.E.K.; Bos, K.K.; Stegeman, J.A.; Ten Berge, A.M.; Bruining, M.; van Maarle, M.C.; Elting, M.W.; den Hollander, N.S.; Hamel, B.; et al. Genotype-phenotype correlations in L1 syndrome: A guide for genetic counselling and mutation analysis. J. Med. Genet. 2010, 47, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.M.; Orth, U.; Veske, A.; Enders, H.; Klunder, K.; Schlosser, M.; Engel, W.; Schwinger, E.; Gal, A. Five novel mutations in the L1CAM gene in families with X linked hydrocephalus. J. Med. Genet. 1996, 33, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Jouet, M.; Rosenthal, A.; Armstrong, G.; MacFarlane, J.; Stevenson, R.; Paterson, J.; Metzenberg, A.; Ionasescu, V.; Temple, K.; Kenwrick, S. X-linked spastic paraplegia (SPG1), MASA syndrome and X-linked hydrocephalus result from mutations in the L1 gene. Nat. Genet. 1994, 7, 402–407. [Google Scholar] [CrossRef]
- De Angelis, E.; MacFarlane, J.; Du, J.-S.; Yeo, G.; Hicks, R.; Rathjen, F.G.; Kenwrick, S.; Brümmendorf, T. Pathological missense mutations of neural cell adhesion molecule L1 affect homophilic and heterophilic binding activities. EMBO J. 1999, 18, 4744–4753. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Yip, P.M.; Siu, C.H. Identification of a homophilic binding site in immunoglobulin-like domain 2 of the cell adhesion molecule L1. J. Neurochem. 1998, 71, 960–971. [Google Scholar] [CrossRef]
- Fryns, J.P.; Spaepen, A.; Cassiman, J.J.; van den Berghe, H. X linked complicated spastic paraplegia, MASA syndrome, and X linked hydrocephalus owing to congenital stenosis of the aqueduct of Sylvius: Variable expression of the same mutation at Xq28. J. Med. Genet. 1991, 28, 429–431. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.C.; Cuppens, H.; Legius, E.; Fryns, J.P.; Glover, T.; Marynen, P.; Cassiman, J.J. Mutations in L1-CAM in two families with X linked complicated spastic paraplegia, MASA syndrome, and HSAS. J. Med. Genet. 1995, 32, 549–552. [Google Scholar] [CrossRef] [Green Version]
- Michelson, P.; Hartwig, C.; Schachner, M.; Gal, A.; Veske, A.; Finckh, U. Missense mutations in the extracellular domain of the human neural cell adhesion molecule L1 reduce neurite outgrowth of murine cerebellar neurons. Hum. Mutat. 2002, 20, 481–482. [Google Scholar] [CrossRef]
- Scapin, G.; Salice, P.; Tescari, S.; Menna, E.; De Filippis, V.; Filippini, F. Enhanced neuronal cell differentiation combining biomimetic peptides and a carbon nanotube-polymer scaffold. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 621–632. [Google Scholar] [CrossRef]
- Scapin, G.; Gasparotto, M.; Peterle, D.; Tescari, S.; Porcellato, E.; Piovesan, A.; Righetto, I.; Acquasaliente, L.; De Filippis, V.; Filippini, F. A conserved Neurite Outgrowth and Guidance motif with biomimetic potential in neuronal Cell Adhesion Molecules. Comput. Struct. Biotechnol. J. 2021, 19, 5622–5636. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics 2005, 22, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Kiefer, F.; Arnold, K.; Künzli, M.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2008, 37, D387–D392. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Canutescu, A.A.; Dunbrack, R.L. SCWRL and MolIDE: Computer programs for side-chain conformation prediction and homology modeling. Nat. Protoc. 2008, 3, 1832–1847. [Google Scholar] [CrossRef] [Green Version]
- Canutescu, A.A.; Shelenkov, A.A.; Dunbrack, R.L. A graph-theory algorithm for rapid protein side-chain prediction. Protein Sci. 2003, 12, 2001–2014. [Google Scholar] [CrossRef]
- Benkert, P.; Biasini, M.; Schwede, T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 2010, 27, 343–350. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindah, E. Gromacs: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Wu, C.; Chowdhury, S.; Lee, M.C.; Xiong, G.; Zhang, W.; Yang, R.; Cieplak, P.; Luo, R.; Lee, T.; et al. A Point-Charge Force Field for Molecular Mechanics Simulations of Proteins Based on Condensed-Phase Quantum Mechanical Calculations. J. Comput. Chem. 2003, 24, 1999–2012. [Google Scholar] [CrossRef]
- Berendsen, H.; Postma, J.P.M.; van Gunsteren, W.; DiNola, A.D.; Haak, J.R. Molecular-Dynamics with Coupling to An External Bath. J. Chem. Phys. 1984, 81, 3684. [Google Scholar] [CrossRef] [Green Version]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.C.; Meng, E.C.; Morris, J.H.; Pettersen, E.F.; Ferrin, T.E. Enhancing UCSF Chimera through web services. Nucleic Acids Res. 2014, 42, W478–W484. [Google Scholar] [CrossRef] [Green Version]
- Lavi, A.; Ngan, C.H.; Movshovitz-Attias, D.; Bohnuud, T.; Yueh, C.; Beglov, D.; Schueler-Furman, O.; Kozakov, D. Detection of peptide-binding sites on protein surfaces: The first step toward the modeling and targeting of peptide-mediated interactions. Proteins Struct. Funct. Bioinform. 2013, 81, 2096–2105. [Google Scholar] [CrossRef] [Green Version]
- Brenke, R.; Kozakov, D.; Chuang, G.-Y.; Beglov, D.; Hall, D.; Landon, M.R.; Mattos, C.; Vajda, S. Fragment-based identification of druggable “hot spots” of proteins using Fourier domain correlation techniques. Bioinformatics 2009, 25, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Schrödinger, LLC. The {PyMOL} Molecular Graphics System, Version 1.8; Schrödinger, LLC: New York, NY, USA, 2015. [Google Scholar]
- Atherton, E. Solid Phase Peptide Synthesis: A Practical Approach; IRL Press: Oxford, UK, 1989. [Google Scholar]
- De Filippis, V.; Quarzago, D.; Vindigni, A.; Di Cera, E.; Fontana, A. Synthesis and characterization of more potent analogues of hirudin fragment 1-47 containing non-natural amino acids. Biochemistry 1998, 37, 13507–13515. [Google Scholar] [CrossRef]
- Ross, R.A.; Spengler, B.A.; Biedler, J.L. Coordinate Morphological and Biochemical Interconversion of Human Neuroblastoma Cells2. JNCI J. Natl. Cancer Inst. 1983, 71, 741–747. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Ni, Y.; Montana, V.; Haddon, R.C.; Parpura, V. Chemically Functionalized Carbon Nanotubes as Substrates for Neuronal Growth. Nano Lett. 2004, 4, 507–511. [Google Scholar] [CrossRef] [Green Version]
- Munnamalai, V.; Weaver, C.J.; Weisheit, C.E.; Venkatraman, P.; Agim, Z.S.; Quinn, M.T.; Suter, D.M. Bidirectional interactions between NOX2-type NADPH oxidase and the F-actin cytoskeleton in neuronal growth cones. J. Neurochem. 2014, 130, 526–540. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, M.; Thompson, P.; Lemmon, V. CRASH Syndrome: Mutations in L1CAM Correlate with Severity of the Disease. Neuropediatrics 1997, 28, 175–178. [Google Scholar] [CrossRef]
- Romano, N.H.; Madl, C.M.; Heilshorn, S.C. Matrix RGD ligand density and L1CAM-mediated Schwann cell interactions synergistically enhance neurite outgrowth. Acta Biomater. 2015, 11, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Bellet, P.; Gasparotto, M.; Pressi, S.; Fortunato, A.; Scapin, G.; Mba, M.; Menna, E.; Filippini, F. Graphene-based scaffolds for regenerative medicine. Nanomaterials 2021, 11, 404. [Google Scholar] [CrossRef]
- De Angelis, E.; Watkins, A.; Schäfer, M.; Brümmendorf, T.; Kenwrick, S. Disease-associated mutations in L1 CAM interfere with ligand interactions and cell-surface expression. Hum. Mol. Genet. 2002, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasparotto, M.; Hernandez Gomez, Y.S.; Peterle, D.; Grinzato, A.; Zen, F.; Pontarollo, G.; Acquasaliente, L.; Scapin, G.; Bergantino, E.; De Filippis, V.; et al. NOG-Derived Peptides Can Restore Neuritogenesis on a CRASH Syndrome Cell Model. Biomedicines 2022, 10, 102. https://doi.org/10.3390/biomedicines10010102
Gasparotto M, Hernandez Gomez YS, Peterle D, Grinzato A, Zen F, Pontarollo G, Acquasaliente L, Scapin G, Bergantino E, De Filippis V, et al. NOG-Derived Peptides Can Restore Neuritogenesis on a CRASH Syndrome Cell Model. Biomedicines. 2022; 10(1):102. https://doi.org/10.3390/biomedicines10010102
Chicago/Turabian StyleGasparotto, Matteo, Yuriko Suemi Hernandez Gomez, Daniele Peterle, Alessandro Grinzato, Federica Zen, Giulia Pontarollo, Laura Acquasaliente, Giorgia Scapin, Elisabetta Bergantino, Vincenzo De Filippis, and et al. 2022. "NOG-Derived Peptides Can Restore Neuritogenesis on a CRASH Syndrome Cell Model" Biomedicines 10, no. 1: 102. https://doi.org/10.3390/biomedicines10010102
APA StyleGasparotto, M., Hernandez Gomez, Y. S., Peterle, D., Grinzato, A., Zen, F., Pontarollo, G., Acquasaliente, L., Scapin, G., Bergantino, E., De Filippis, V., & Filippini, F. (2022). NOG-Derived Peptides Can Restore Neuritogenesis on a CRASH Syndrome Cell Model. Biomedicines, 10(1), 102. https://doi.org/10.3390/biomedicines10010102







