Dopamine D2L Receptor Deficiency Alters Neuronal Excitability and Spine Formation in Mouse Striatum
Abstract
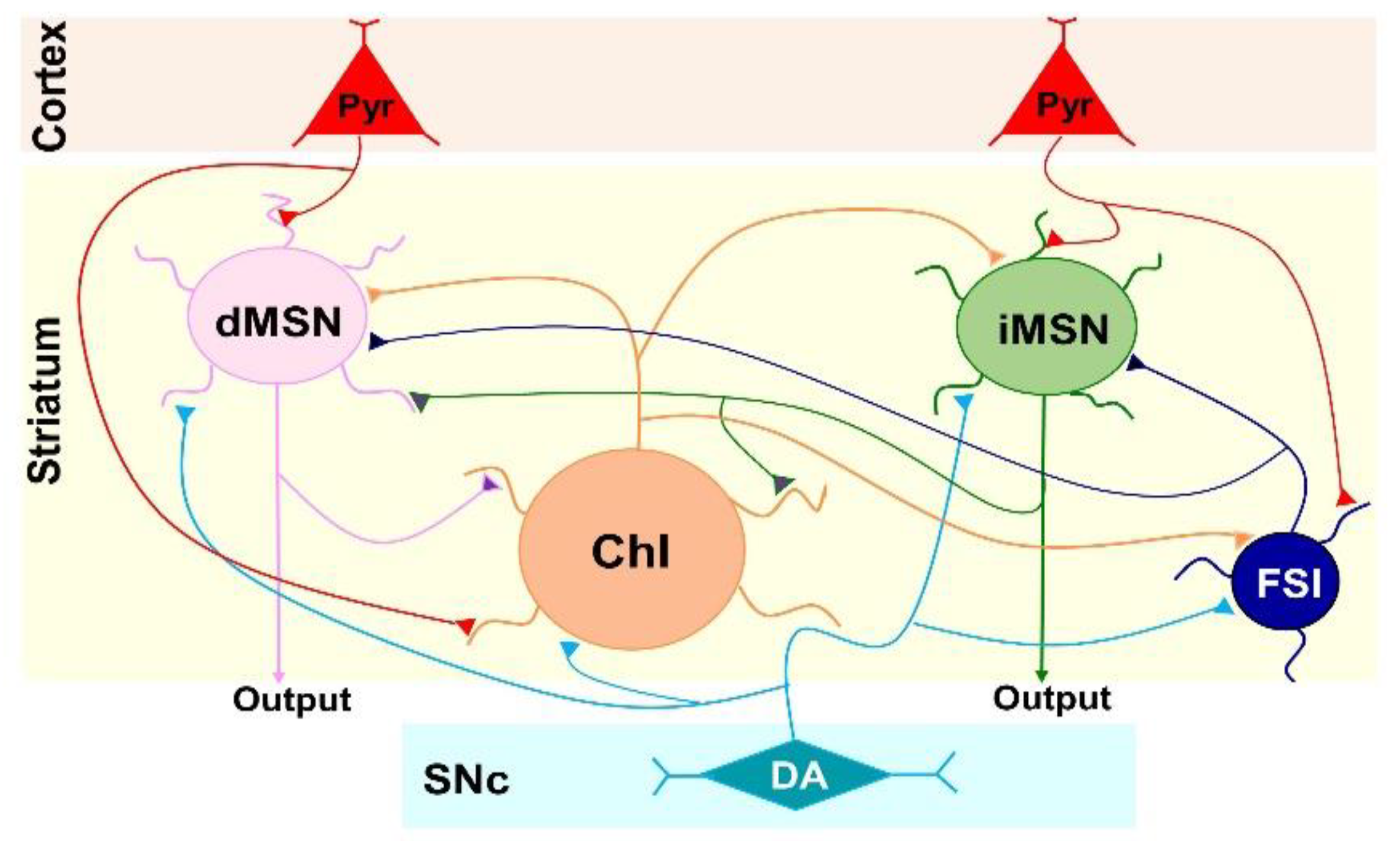
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Brain Slice Preparation
2.3. Whole-Cell Recordings and Data Analysis
2.4. Imaging and Data Analysis
2.5. Drugs
2.6. Analysis and Statistics
3. Results
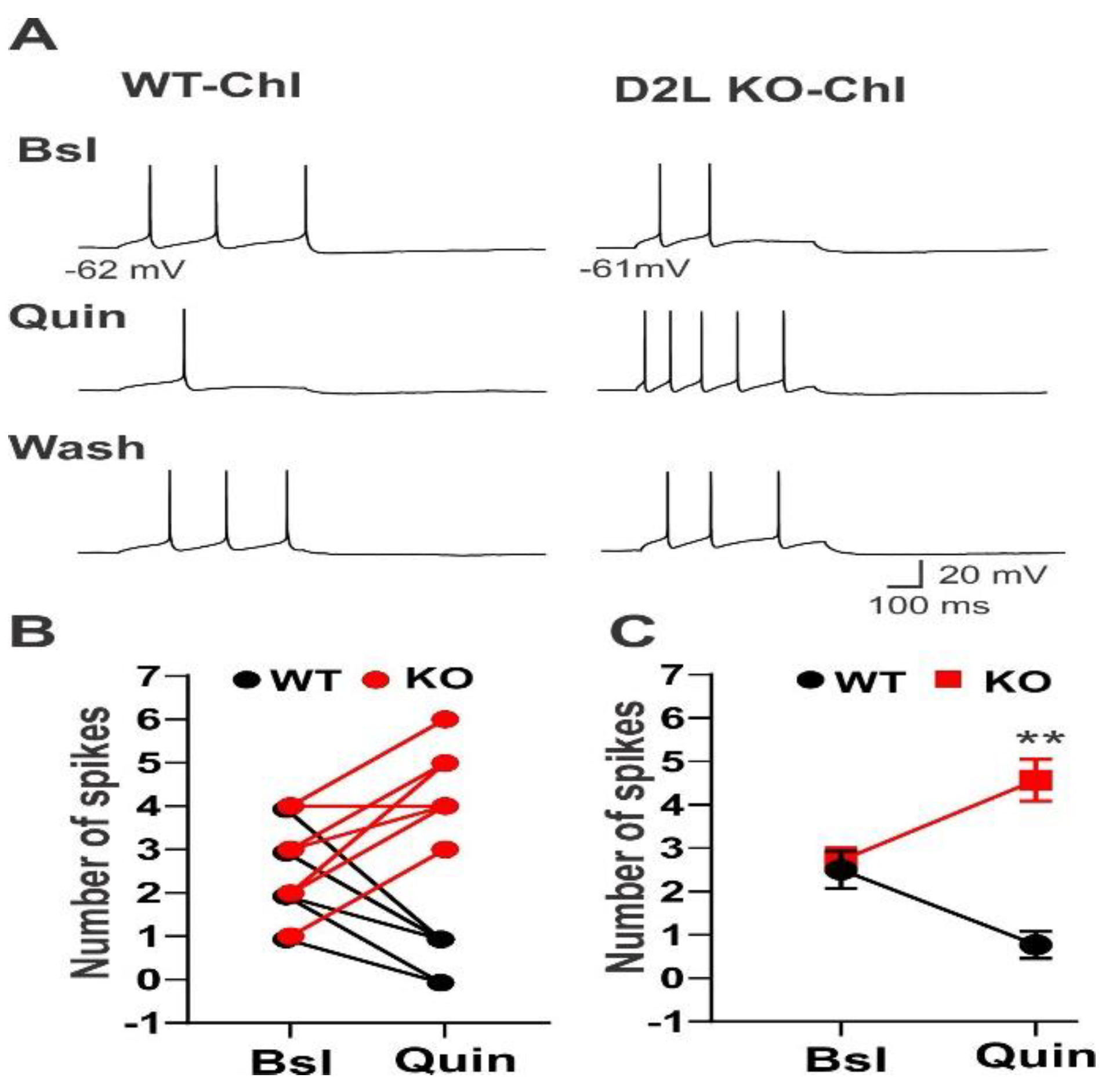
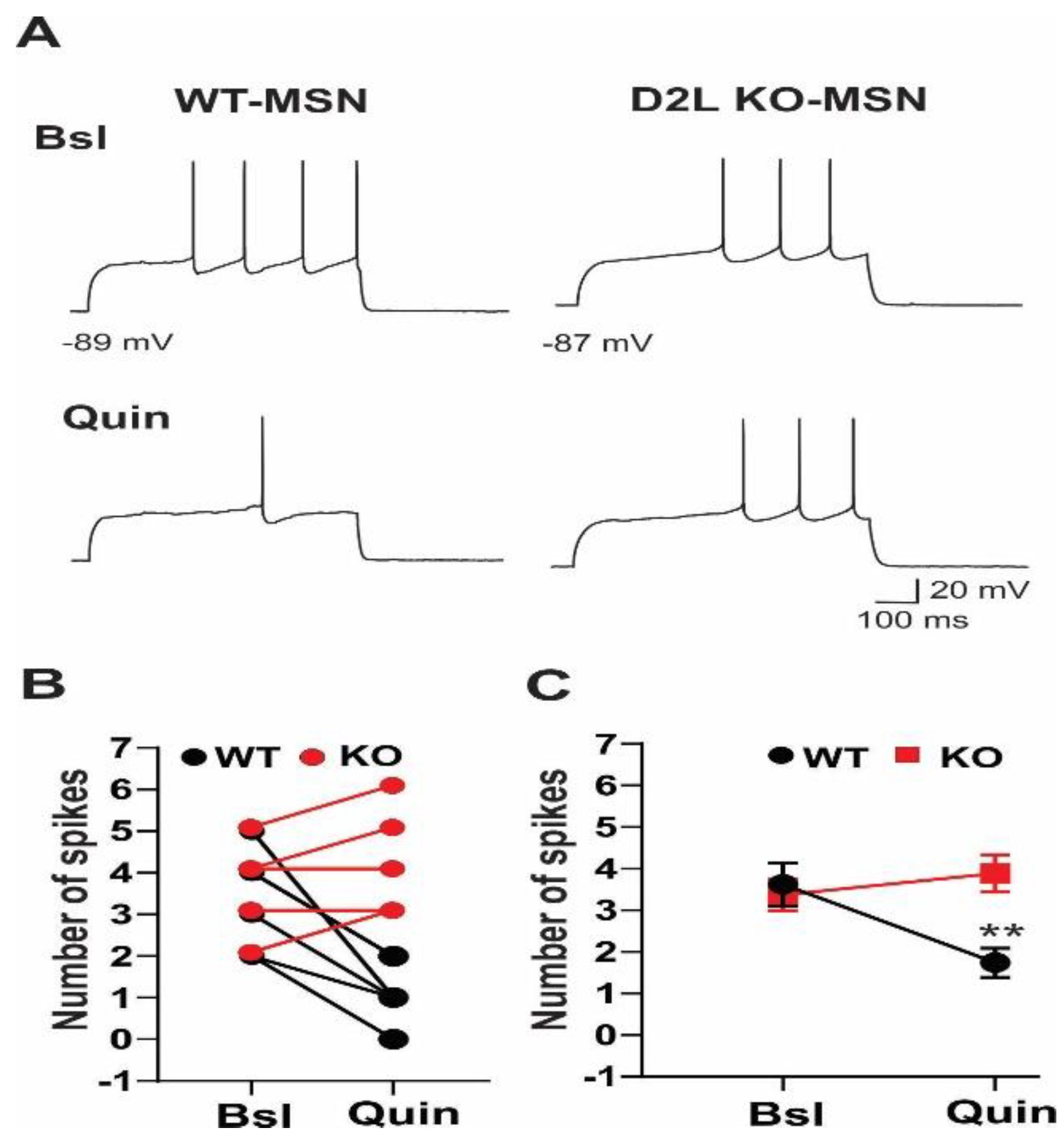
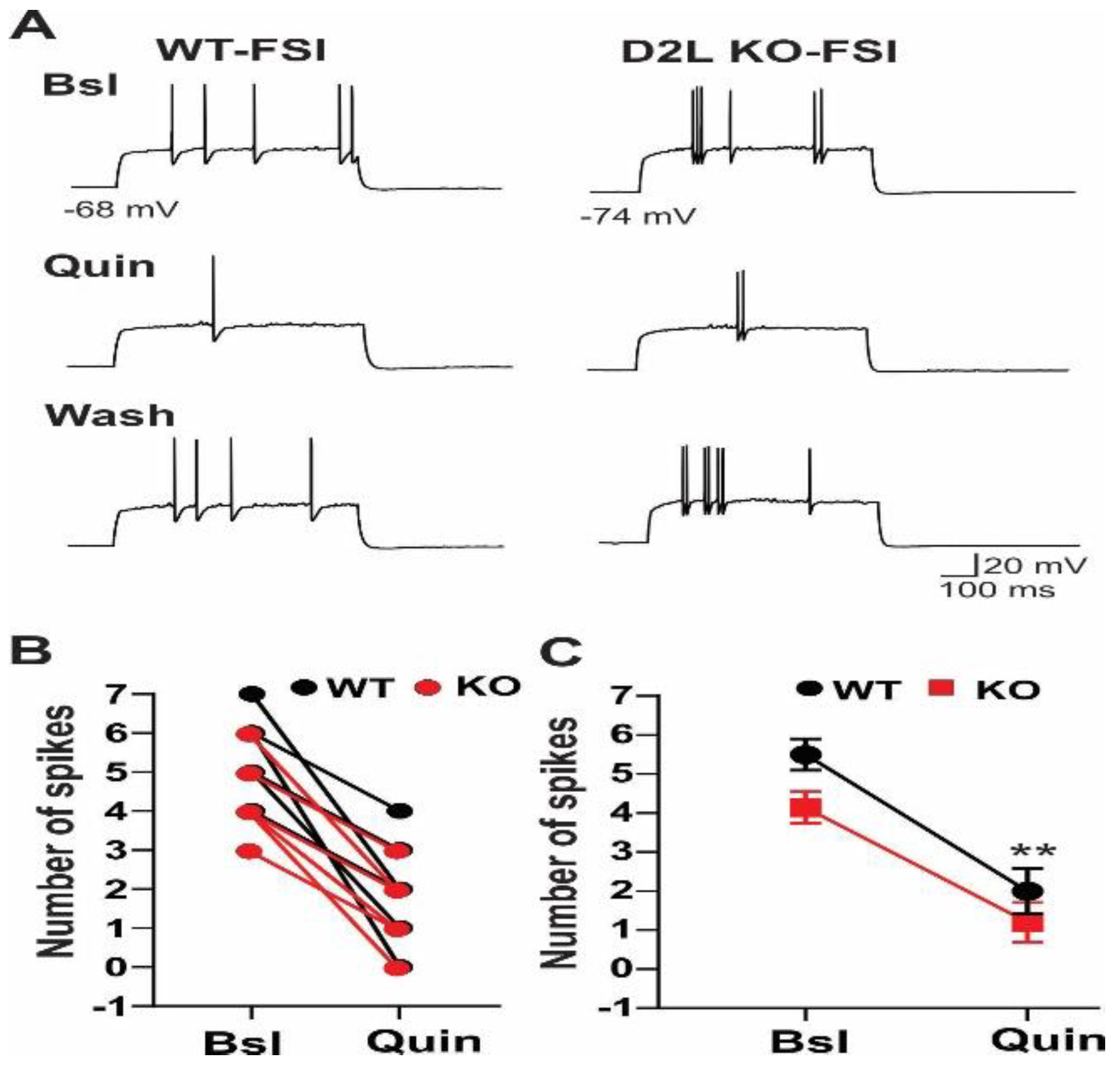
3.1. D2L Deficiency Produced Differential Effects on Neuronal Excitability in Three Types of Striatal Neurons
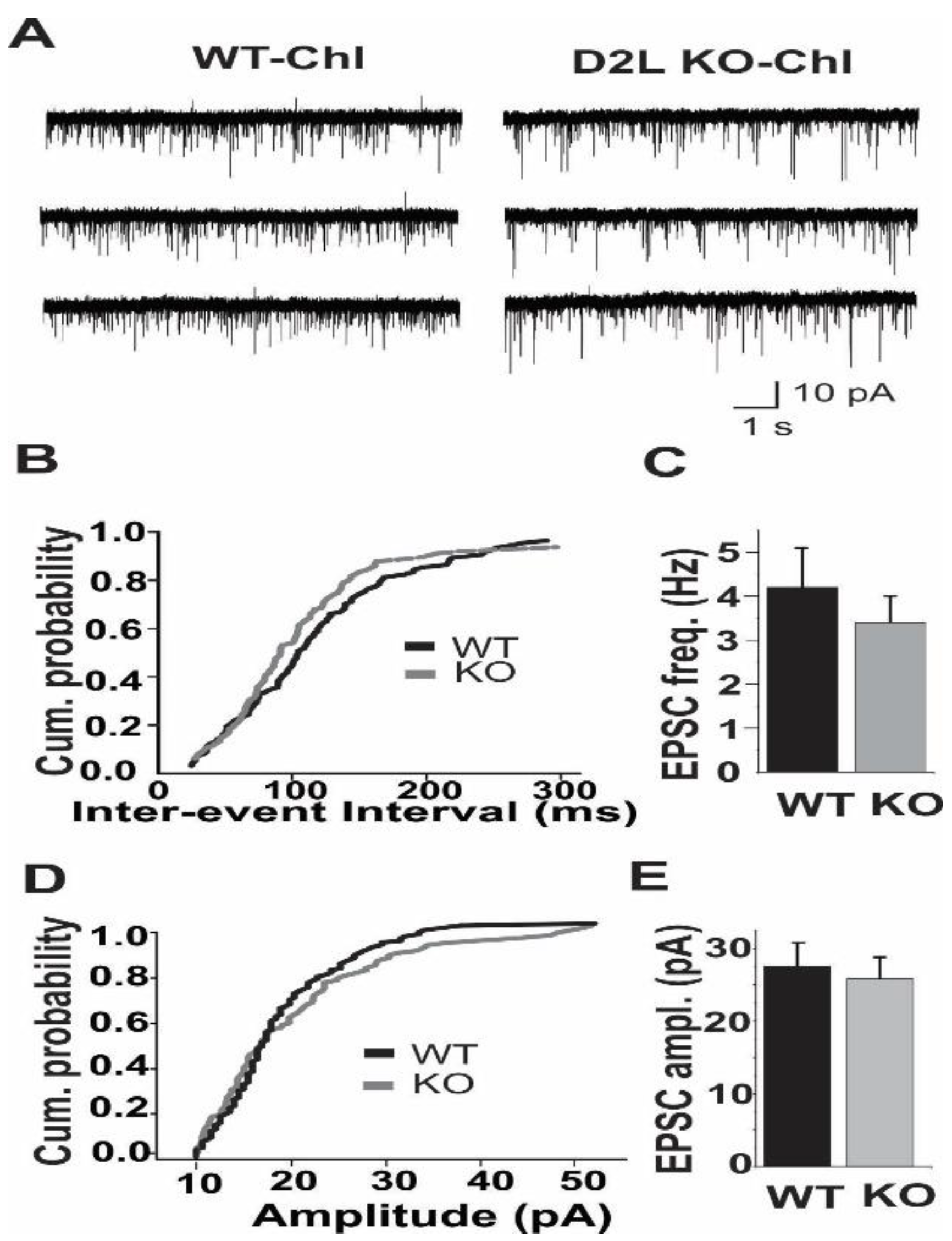
3.2. D2L Deficiency Resulted in a Decrease in mIPSC Frequency in Cholinergic Interneurons
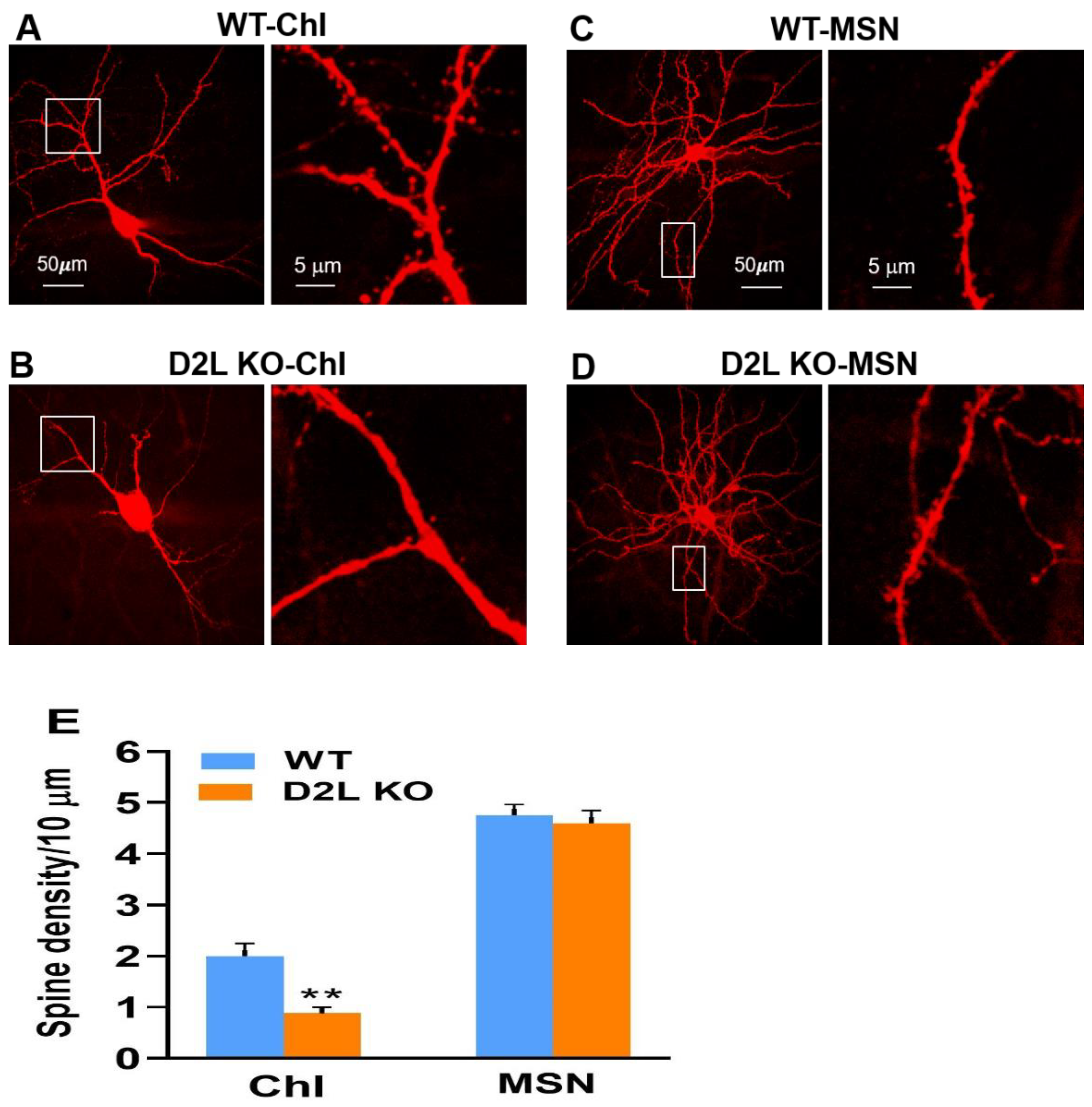
3.3. D2L Deficiency Caused a Reduction in Dendritic Spine Density in Cholinergic Interneurons
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerdeman, G.L.; Partridge, J.G.; Lupica, C.R.; Lovinger, D.M. It could be habit forming: Drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003, 26, 184–192. [Google Scholar] [CrossRef]
- Smith, D.V.; Rigney, A.E.; Delgado, M.R. Distinct reward properties are encoded via corticostriatal interactions. Sci. Rep. 2016, 6, 20093. [Google Scholar] [CrossRef] [Green Version]
- Graybiel, A.M.; Canales, J.J.; Capper-Loup, C. Levodopa-induced dyskinesias and dopamine-dependent stereotypies: A new hypothesis. Trends Neurosci. 2000, 23, S71–S77. [Google Scholar] [CrossRef]
- Cenci, M.A.; Crossman, A.R. Animal models of l-dopa-induced dyskinesia in Parkinson’s disease. Mov. Disord. 2018, 33, 889–899. [Google Scholar] [CrossRef]
- Graveland, G.A.; Difiglia, M. The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Res. 1985, 327, 307–311. [Google Scholar] [CrossRef]
- David, H.N. Towards a reconceptualization of striatal interactions between glutamatergic and dopaminergic neurotransmission and their contribution to the production of movements. Curr. Neuropharmacol. 2009, 7, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Chiken, S.; Sato, A.; Ohta, C.; Kurokawa, M.; Arai, S.; Maeshima, J.; Sunayama-Morita, T.; Sasaoka, T.; Nambu, A. Dopamine D1 receptor-mediated transmission maintains information flow through the cortico-striato-entopeduncular direct pathway to release movements. Cereb. Cortex 2015, 25, 4885–4897. [Google Scholar] [CrossRef] [Green Version]
- Tepper, J.M.; Bolam, J.P. Functional diversity and specificity of neostriatal interneurons. Curr. Opin. Neurobiol. 2004, 14, 685–692. [Google Scholar] [CrossRef]
- Wilson, C.J. GABAergic inhibition in the neostriatum. Prog. Brain Res. 2007, 160, 91–110. [Google Scholar]
- Kawaguchi, Y.; Wilson, C.J.; Augood, S.J.; Emson, P.C. Striatal Interneurons—Chemical, Physiological and Morphological Characterization. Trends Neurosci. 1995, 18, 527–535. [Google Scholar] [CrossRef]
- Pakhotin, P.; Bracci, E. Cholinergic interneurons control the excitatory input to the straitum. J. Neurosci. 2007, 27, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Licheri, V.; Lagstrom, O.; Lotfi, A.; Patton, M.H.; Wigstrom, H.; Mathur, B.; Adermark, L. Complex control of striatal neurotransmission by nicotinic acetylcholine receptors via excitatory inputs onto medium spiny neurons. J. Neurosci. 2018, 38, 6597–6607. [Google Scholar] [CrossRef]
- Kita, H.; Kosaka, T.; Heizmann, C.W. Parvalbumin-immunoreactive neurons in the rat neostriatum: A light and electron microscopic study. Brain Res. 1990, 536, 1–15. [Google Scholar] [CrossRef]
- Szydlowski, S.N.; Dorocic, I.P.; Planert, H.; Carlen, M.; Meletis, K.; Silberberg, G. Target selectivity of feedforward inhibition by striatal fast-spiking interneurons. J. Neurosci. 2013, 33, 1678–1683. [Google Scholar] [CrossRef] [Green Version]
- Govindaiah, G.; Wang, Y.; Cox, C.L. Substance P selectively modulates GABAA receptor-mediated synaptic transmission in striatal cholinergic interneurons. Neuropharmacology 2010, 58, 413–422. [Google Scholar] [CrossRef]
- Paladini, C.A.; Robinson, S.; Morikawa, H.; Williams, J.T.; Palmiter, R.D. Dopamine controls the firing pattern of dopamine neurons via a network feedback mechanism. Proc. Natl. Acad. Sci. USA 2003, 100, 2866–2871. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.A.O.; Kang, U.J.; McGehee, D.S. Striatal cholinergic interneuron regulation and circuit effects. Front. Synaptic. Neurosci. 2014, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Song, W.J.; Surmeier, J. D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited protein-kinase-C-insensitive pathway. J. Neurophysiol. 1997, 77, 1003–1015. [Google Scholar] [CrossRef] [Green Version]
- Aizman, O.; Brismar, H.; Uhlen, P.; Zettergren, E.; Levey, A.I.; Forssberg, H.; Greengard, P.; Aperia, A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat. Neurosci. 2000, 3, 226–230. [Google Scholar] [CrossRef]
- Alcantara, A.A.; Chen, V.; Herring, B.E.; Mendenhall, J.M.; Berlanga, M.L. Localization of dopamine D2 receptors on cholinergic interneurons of the dorsal striatum and nucleus accumbens of the rat. Brain Res. 2003, 986, 22–29. [Google Scholar] [CrossRef]
- Wu, J.; Dougherty, J.J.; Nichols, R.A. Dopamine receptor regulation of Ca2+ levels in individual-isolated nerve terminals from rat striatum: Comparison of presynaptic D1-like and D2-like receptors. J. Neurochem. 2006, 98, 481–494. [Google Scholar] [CrossRef]
- Delgado, A.; Sierra, A.; Querejeta, E.; Valdiosera, R.F.; Aceves, J. Inhibitory control of the GABAergic transmission in the rat neostriatum by D2 dopamine receptors. Neuroscience 2000, 95, 1043–1048. [Google Scholar] [CrossRef]
- Momiyama, T.; Koga, E. Dopamine D2-like receptors selectively block N-type Ca2+ channels to reduce GABA release onto rat striatal cholinergic interneurons. J. Physiol. 2001, 533, 479–492. [Google Scholar] [CrossRef]
- Centonze, D.; Grande, C.; Usiello, A.; Gubellini, P.; Erbs, E.; Martin, A.B.; Pisani, A.; Tognazzi, N.; Bernardi, G.; Moratalla, R.; et al. Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. J. Neurosci. 2003, 23, 6245–6254. [Google Scholar] [CrossRef] [Green Version]
- Sato, A.; Sasaoka, T.; Nishijo, T.; Momiyama, T. GABAergic synaptic transmission onto striatal cholinergic interneurons in dopaminergic D2 receptor knock-out mice. Neuroscience 2014, 263, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Dal Toso, R.; Sommer, B.; Ewert, M.; Herb, A.; Pritchett, D.B.; Bach, A.; Shivers, B.D.; Seeburg, P.H. The dopamine D2 receptor: Two molecular forms generated by alternative splicing. EMBO J. 1989, 8, 4025–4034. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, R.; Sasaoka, T.; Tonegawa, S.; Kung, M.-P.; Sankoorikal, E.-B. Dopamine D2 long receptor-deficient mice display alterations in striatum-dependent functions. J. Neurosci. 2000, 20, 8305–8314. [Google Scholar] [CrossRef] [Green Version]
- Fetsko, L.A.; Xu, R.; Wang, Y. Alterations in D1/D2 synergism may account for enhanced stereotypy and reduced climbing in mice lacking dopamine D2L receptor. Brain Res. 2003, 967, 191–200. [Google Scholar] [CrossRef]
- Smith, J.W.; Fetsko, L.A.; Xu, R.; Wang, Y. Dopamine D2L receptor knockout mice display deficits in positive and negative reinforcing properties of morphine and in avoidance learning. Neuroscience 2002, 113, 755–765. [Google Scholar] [CrossRef]
- Morita, M.; Wang, Y.; Sasaoka, T.; Okada, K.; Niwa, M.; Sawa, A.; Hikida, T. Dopamine D2L receptor is required for visual discrimination and reversal learning. Mol. Neuropsychiatry 2016, 2, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Hranilovic, D.; Fetsko, L.A.; Bucan, M.; Wang, Y. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol. Psychiatry 2002, 7, 1075–1082. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Cheng, L.; Wang, Y. Differential roles of two isoforms of dopamine D2 receptors in L-dopa-induced abnormal involuntary movements in mice. NeuroReport 2021, 32, 555–561. [Google Scholar] [CrossRef]
- Kawahata, I.; Sekimori, T.; Wang, H.; Wang, Y.; Sasaoka, T.; Bousset, L.; Melki, R.; Mizobata, T.; Kawata, Y.; Fukunaga, K. Dopamine D2L long receptors are crtitical for caveolae-mediated a-synuclein uptake in cultured dopaminergic neurons. Biomedicines 2021, 9, 49. [Google Scholar] [CrossRef]
- Wang, Y. Differential effect of aging on synaptic plasticity in the ventral and dorsal striatum. Neurobiol. Learn. Mem. 2008, 89, 70–75. [Google Scholar] [CrossRef]
- Stevens, C.F.; Wang, Y. Changes in reliability of synaptic function as a mechanism for plasticity. Nature 1994, 371, 704–707. [Google Scholar] [CrossRef]
- Govindaiah, G.; Venkitaramani, D.V.; Chaki, S.; Cox, C.L. Spatially distinct actions of metabotropic glutamate receptor activation in dorsal lateral geniculate nucleus. J. Neurophysiol. 2012, 107, 1157–1163. [Google Scholar] [CrossRef]
- Sears, R.M.; Liu, R.-J.; Narayanan, N.S.; Sharf, R.; Yeckel, M.F.; Laubach, M.; Aghajanian, G.K.; DiLeone, R.J. Regulation of nucleus accumbens activity by the hypothalamic neuropeptide melanin-concentrating hormone. J. Neurosci. 2010, 30, 8263–8273. [Google Scholar] [CrossRef]
- Liu, R.-J.; Aghajanian, G.K. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: Role of corticosterone-mediated apical dendritic atrophy. Proc. Nat. Acad. Sci. USA 2008, 105, 359–364. [Google Scholar] [CrossRef] [Green Version]
- Horvath, P.M.; Piazza, M.K.; Monteggia, L.M.; Kavalali, E.T. Spontaneous and evoked neurotransmission are partially segregated at inhibitory synapses. eLife 2020, 9, e52852. [Google Scholar] [CrossRef]
- Banerjee, A.; Larsen, R.S.; Philpot, B.D.; Paulsen, O. Roles of presynaptic NMDA receptors in neurotransmission and plasticity. Trends Neurosci. 2016, 39, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Martone, M.E.; Armstrong, D.M.; Young, S.J.; Groves, P.M. Ultrastructural examination of enkephalin and substance P input to cholinergic neurons within the rat neostriatum. Brain Res. 1992, 594, 253–262. [Google Scholar] [CrossRef]
- Hersch, S.M.; Gutekunst, C.A.; Rees, H.D.; Heilman, C.J.; Levey, A.I. Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: Light and electron microscopic immunocytochemistry using subtype-specific antibodies. J. Neurosci. 1994, 14, 3351–3363. [Google Scholar] [CrossRef] [Green Version]
- Mamaligas, A.A.; Cai, Y.; Ford, C.P. Nicotinic and opioid receptor regulation of striatal dopamine D2-receptor mediated transmission. Sci. Rep. 2016, 6, 37834. [Google Scholar] [CrossRef] [Green Version]
- Conti, M.M.; Chambers, N.; Bishop, C. A new outlook on cholinergic interneurons in Parkinson’s disease and L-DOPA-induced dyskinesia. Neurosci. Biobehav. Rev. 2018, 92, 67–82. [Google Scholar] [CrossRef]
- Hisahara, S.; Shimohama, S. Dopamine receptors and Parkinson’s disease. Int. J. Med. Chem. 2011, 2011, 403039. [Google Scholar] [CrossRef]
- Ding, Y.; Won, L.; Britt, J.P.; Lim, S.A.O.; McGehee, D.S.; Kang, U.J. Enhanced striatal cholinergic neuronal activity mediates L-DOPA-induced dyskinesia in parkinsonian mice. Proc. Natl. Acad. Sci. USA 2011, 108, 840–845. [Google Scholar] [CrossRef] [Green Version]
- Bordia, T.; Perez, X.A.; Heiss, J.; Zhang, D.; Quik, M. Optogenetic activation of striatal cholinergic interneurons regulates L-dopa-induced dyskinesias. Neurobiol. Dis. 2016, 91, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Smith, Y.; Villalba, R. Striatal and extrastriatal dopamine in the basal ganglia: An overview of its anatomical organization in normal and parkinsonian brains. Mov. Disord. 2008, 23 (Suppl. 3), S534–S547. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govindaiah, G.; Liu, R.-J.; Wang, Y. Dopamine D2L Receptor Deficiency Alters Neuronal Excitability and Spine Formation in Mouse Striatum. Biomedicines 2022, 10, 101. https://doi.org/10.3390/biomedicines10010101
Govindaiah G, Liu R-J, Wang Y. Dopamine D2L Receptor Deficiency Alters Neuronal Excitability and Spine Formation in Mouse Striatum. Biomedicines. 2022; 10(1):101. https://doi.org/10.3390/biomedicines10010101
Chicago/Turabian StyleGovindaiah, Gubbi, Rong-Jian Liu, and Yanyan Wang. 2022. "Dopamine D2L Receptor Deficiency Alters Neuronal Excitability and Spine Formation in Mouse Striatum" Biomedicines 10, no. 1: 101. https://doi.org/10.3390/biomedicines10010101
APA StyleGovindaiah, G., Liu, R.-J., & Wang, Y. (2022). Dopamine D2L Receptor Deficiency Alters Neuronal Excitability and Spine Formation in Mouse Striatum. Biomedicines, 10(1), 101. https://doi.org/10.3390/biomedicines10010101





