Abstract
Light-activated nanozymes possess several advantages, such as light-mediated activity regulation, utilization of molecular oxygen as a green oxidant, and highly enhanced activity; however, the types of light-activated nanozymes are still limited. In this study, we found that Mg aminoclay-based Fe3O4/TiO2 hybrids (MgAC-Fe3O4/TiO2) exhibited peroxidase-like catalytic activity to catalyze the oxidation of the peroxidase substrate 2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid) diammonium salt (ABTS) in the presence of H2O2, which was significantly enhanced under ultraviolet (UV)-light irradiation. Compared with MgAC-Fe3O4 and MgAC-TiO2, MgAC-Fe3O4/TiO2 showed around three-fold enhancement in the absorption intensity corresponding to the oxidized ABTS under UV-light irradiation, presumably due to the synergistic effect between Fe3O4 and TiO2, thereby facilitating photocatalytic electron transfer during the catalytic action. In addition, the MgAC-Fe3O4/TiO2 showed vivid stability enhancement in wide range of pH and temperature values compared with natural peroxidase. The UV-light-driven MgAC-Fe3O4/TiO2-based system was successfully applied for the colorimetric detection of phenolic compounds, including pyrocatechol and resorcinol, in a dynamic linear range of 0.15–1.30 mg/mL with a limit of detection as low as 0.1 mg/mL. Further, the system could successfully determine the phenolic compounds in spiked tap water, and thus, it can be used for practical applications. We believe that the UV-light-driven enhancement in the peroxidase-like catalytic performances highlights the potential of MgAC-Fe3O4/TiO2 for detecting phenolic compounds as well as other clinically and environmentally important substances.
1. Introduction
Phenolic compounds are considered as one of the most harmful pollutants due to their severe negative impacts on human health and ecosystems, in addition to the difficulty in their biodegradation. They are generally derived from industrial effluents that leach into water reservoirs and result in serious contamination [1]. Therefore, to effectively prevent and treat phenol contamination in water reservoirs, developing a simple, rapid, sensitive, and cost-effective strategy is crucial for the on-site detection of phenolic compounds. To achieve this, various analytical approaches have been proposed, which are based on chromatography [2], electrochemistry [3], fluorometry [4], and colorimetry [5,6]. Among them, colorimetric approach has attracted attention owing to its direct visual response, simple operation, and cost-effectiveness. Typically, colorimetric detection of phenolic compounds is performed through natural peroxidase-mediated oxidative coupling of the target phenols with 4-aminoantipyrine (4-AP) [5]. Although this assay method yields sensitive and accurate detection results, natural peroxidase should be further investigated, as its instability and high-cost significantly limit its practical applications [7].
To overcome the limitations associated with natural enzyme including peroxidase, several types of enzyme mimics have been developed to date. Among them, nanozymes, which are nanomaterials exhibiting enzyme-like activities, have been considered promising for replacing natural enzyme owing to their advantages, such as excellent stability and robustness during long-term operation/storage, low production cost resulting from their facile scale-up synthesis, and easy reutilization resulting from their heterogeneous nature [8,9]. After the first demonstration of intrinsic peroxidase-like activity of magnetic nanoparticles (MNPs), a huge number of nanozymes have been reported and extensively investigated to prove their potential applications in diverse fields [10,11]. Despite the great potentials of nanozymes, their real utilization has been limited majorly by their lower catalytic activity than that of natural enzyme. To resolve the limitation, several strategies to enhance the catalytic activity, such as surface modification with functional ligands, elemental composition engineering, morphology or crystal facet optimization, and development of unique composite, have been reported [12,13,14,15].
In this aspect, light-activated nanozymes have attracted attention due to their capability to catalyze unique photosensitized reactions [16], that can be utilized in diverse applications including chemical synthesis [17], pollutant removal [18], cancer therapy [19], antibacterial applications [20], and biosensing [21]. As light-activated nanozymes, TiO2 nanoparticles have been representatively investigated since they can efficiently produce hydroxyl or superoxide radicals from the reaction medium under ultraviolet (UV)/visible light irradiation during diverse catalytic reactions, which can be essentially used for oxidizing various organic substances [22]. However, when TiO2 nanoparticles are used alone, they suffer from low quantum efficiency and narrow light response range [23]. Thus, it is necessary to develop further nanozymes that are capable of efficiently enhancing the catalytic performances under light irradiation.
Herein, we have developed Mg aminoclay-based Fe3O4/TiO2 hybrids (MgAC-Fe3O4/TiO2) as new and efficient light-activated peroxidase-like nanozymes. We previously reported that Fe aminoclay showed intrinsic peroxidase-like activity that can be successfully applied to colorimetrically detect lung cancer cells [24]. As a continuing effort to develop nanozymes, particularly for light-activated ones, MgAC-Fe3O4/TiO2 was synthesized via a simple sol-gel reaction. The resulting nanohybrids showed a significant enhancement in peroxidase-like catalytic activity under UV-light irradiation, which is roughly three-times higher than those of control samples, including MgAC-Fe3O4 and MgAC-TiO2. The UV-light-driven MgAC-Fe3O4/TiO2-based system was further applied to colorimetrically determine phenolic compounds, and its analytical features were investigated.
2. Materials and Methods
2.1. Materials
Iron(III) chloride hexahydrate (FeCl3·H2O), titanium (IV) butoxide (TB, 97%), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), horseradish peroxidase (HRP), 4-AP, ethanol, sodium hydroxide, pyrocatechol, and resorcinol were purchased from Sigma-Aldrich (St Louis, MO, USA). Magnesium chloride hexahydrate (MgCl2.H2O) was purchased from Junsei Chemical Co. (Tokyo, Japan), and 3-aminopropyltrielthoxylane (APTES, ≥98%) was purchased from Tokyo Chemical Industry Co. (Tokyo, Japan). All solutions were prepared with distilled (DI) water purified using a Milli-Q Purification System (Millipore, MA, USA).
2.2. Synthesis of MgAC, MgAC-Fe3O4, MgAC-TiO2, and MgAC-Fe3O4/TiO2
MgAC-Fe3O4/TiO2 was synthesized via a facile sol–gel reaction. MgAC was synthesized by dissolving 1.68 g of MgCl2·H2O to 40 mL of ethanol, and subsequently, APTES (2.6 mL) was slowly added. The solution was incubated overnight and stirred, and the resulting solution was centrifuged and washed thrice with ethanol. The obtained MgAC was further dried at 40 °C and ground into powder [25]. For synthesizing MgAC-Fe3O4, as-prepared MgAC (0.7 g) was mixed with FeCl3·6H2O (3 g) in 40 mL of DI water, followed by the addition of NaOH solution (10 mL, 10 M). The solution was stirred for 12 h, centrifuged, and washed with DI water to remove residuals (mainly NaCl). The resulting brown solid products were then dried and calcined at 500 °C for 3 h under 4% H2/Ar condition (flow rate: 0.15 L/min) to obtain MgAC-Fe3O4 [26]. MgAC-TiO2 was synthesized by adding 1 mL of TB and 0.25 µL of DI water in 40 mL of ethanol containing MgAC (0.3 g). The solution was stirred for 12 h, followed by centrifuging, washing with ethanol, and drying. The resulting white precipitate was then collected and calcined at 350 °C for 3 h [27]. MgAC-Fe3O4/TiO2 was synthesized by mixing 0.05 g of MgAC-Fe3O4 and 1 mL of TB in 40 mL of ethanol. After stirring for 12 h, the mixture was centrifuged, washed with ethanol, and dried to obtain a gray-colored solid. After calcination at 350 °C for 3 h, the final MgAC-Fe3O4/TiO2 was obtained [28].
The synthesized MgAC-TiO2, MgAC-Fe3O4, and MgAC-Fe3O4/TiO2 were characterized via transmission electron microscopy (TEM; JEM-2100F, JEOL LTD, Peabody, MA, USA) and scanning electron microscopy (SEM; Hitachi S4700 at the Smart Materials Research Center for IoT at Gachon University for its instrumental support). X-ray diffraction (XRD) analysis (D/MAX-2500, Rigaku Corporation, Tokyo, Japan), equipped with a CuKα radiation generator powered at 40 kV and 30 mA, was used to characterize the crystallinity of the as-prepared samples. The chemical and electronic states of the elements on the surfaces of materials were investigated via X-ray photoelectron spectroscopy (XPS; ESCALAB250, VG scientific, East Grinstead, UK). The degree of crystallinity (, %) was calculated from the XRD pattern via the following equation [29]:
where Ac is the area of crystalline peaks and Aa is the area of amorphous peaks.
2.3. Determination of H2O2 Using MgAC-Fe3O4/TiO2 under UV-Light
Peroxidase-like activities of MgAC-Fe3O4/TiO2 were examined through the catalytic oxidations of ABTS in the presence of predetermined concentrations of H2O2. UV-light irradiation at around 312/365 nm was performed using a dual UV transilluminator (Core-Bio, Seoul, Korea). In a typical reaction, MgAC-Fe3O4/TiO2 (0.6 mg/mL), ABTS (0.6 mM), and H2O2 at diverse concentrations were added into sodium acetate buffer (0.1 M; pH 4.0), followed by UV-light exposure for 10 min. The control experiments were also carried out following the same assay conditions, but without UV-light exposure. After the reaction, the catalytic materials were separated by centrifugation (10,000 g; 2 min). The absorption intensities corresponding to the oxidized ABTS were measured in a scan mode from 380 to 500 nm or at 417 nm using a microplate reader (Synergy H1, BioTek, Winooski, VT, USA, at the Core-facility for Bionano Materials in Gachon University). The effects of UV irradiation time, reaction buffer pH, and nanohybrid concentration on the peroxidase activity were assessed following the above-mentioned procedures.
The steady-state kinetic analysis was conducted using MgAc-Fe3O4/TiO2 in 1 mL sodium acetate buffer solution (0.1 M, pH 4.0). For ABTS-based kinetic assay, 100 mM H2O2 was added in 1 mL reaction solution containing varying ABTS concentrations. For H2O2-based performance, 0.6 mM ABTS was added in 1 mL reaction buffer at varying H2O2 concentrations. The reactions were performed both in the presence and absence of UV-light irradiation and monitored by measuring the color changes of reaction solution using kinetic mode of spectrophotometer. The equation v = Vmax × [S]/(Km + [S]) was used to determine kinetic parameters including the maximal velocity Vmax and the Michalis constant Km.
2.4. Determination of Phenolic Compounds Using MgAC-Fe3O4/TiO2 under UV-Light
Phenolic compounds were colorimetrically determined by performing the chromogenic reaction for phenolic compounds with 4-AP in the presence of H2O2. Typically, MgAC-Fe3O4/TiO2 (0.6 mg/mL), H2O2 (1 mM), 4-AP (5 mM), and two representative phenolic compounds (pyrocatechol and resorcinol) at diverse concentrations were added into sodium acetate buffer (0.1 M, pH 4.0) under UV irradiation. After 10 min, the resulting solutions were centrifuged at 10,000 g for 2 min, and the supernatant was subjected to record the absorption intensity at 500 and 490 nm for pyrocatechol and resorcinol determination, respectively.
The reusability of MgAc-Fe3O4/TiO2 was explored through the consecutive runs involving the typical reaction with 1 mg/mL pyrocatechol under UV-light irradiation, centrifugation at 10,000 rpm for 3 min, and supernatant withdrawing with two washes using aqueous buffer (0.1 M sodium acetate, pH 4.0) to remove excess substrates. The hybrid was then reused for up to 9 iterative cycles and the residual detecting activity for pyrocatechol (%) at each round was calculated using the ratio of the residual activity to the initial one.
For determining phenolic compounds in real tap water solution, predetermined amounts of phenolic compounds were added into tap water to obtain spiked water samples. The concentration of pyrocatechol and resorcinol in each spiked sample was measured via the aforementioned procedures. The coefficient of variation [CV (%) = standard deviation (SD)/average × 100] and the recovery rate [recovery (%) = measured value/actual value × 100] were calculated to determine the reproducibility and precision of the assay.
3. Results and Discussion
For developing efficient light-activated peroxidase-like nanozymes, MgAC-Fe3O4/TiO2 was constructed via a simple sol–gel reaction. Fe3O4 composition can catalyze the peroxidase-like action, whereas TiO2 composition can act as a photocatalyst to facilitate hydroxyl radical generation under UV-light; therefore, we envisioned that the peroxidase activity of the resulting aminoclay-based nanohybrid (MgAC-Fe3O4/TiO2) will enhance significantly under UV-light irradiation, owing to the expected synergistic effects between Fe3O4 and TiO2 components (Figure 1a). The resulting UV-light-driven nanohybrid-based system was then used for the colorimetric determination of phenolic compounds, to prove the potential biosensing applicability (Figure 1b).
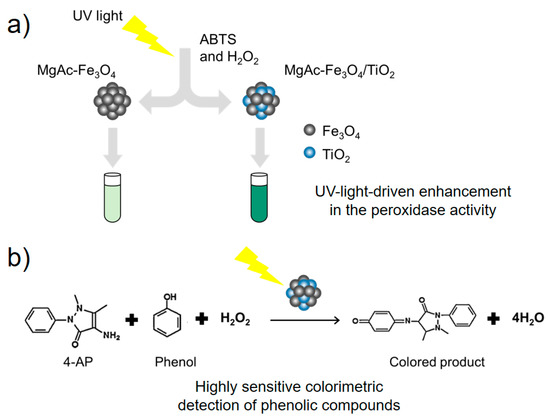
Figure 1.
Schematic illustrations for (a) UV-light-mediated enhancement in the peroxidase-like activity of MgAC-Fe3O4/TiO2, and (b) its utilization for colorimetric detection of phenolic compounds.
The morphologies of the as-prepared MgAC-Fe3O4/TiO2 were observed and compared with the control samples (MgAC-Fe3O4 and MgAC-TiO2), via TEM and SEM (Figure 2). The results indicated that MgAC-Fe3O4/TiO2 was successfully synthesized with an average diameter of ~135 ± 45 nm, which is marginally larger than those of control samples. Energy-dispersive X-ray mapping (EDX) analysis from our previous reports indicated that TiO2 was found to be uniformly distributed on the surface of MgAC-Fe3O4 particles (Supplementary Materials Figure S1) [28]. Further, we analyzed the crystallinity of the synthesized nanohybrid. The XRD pattern of MgAC-Fe3O4 showed peaks at 30.0°, 35.5°, 42.9°, 56.7°, and 62.0°, which corresponded to (200), (311), (400), (511), and (440) planes of Fe3O4 magnetite, respectively (JCPDS 00-021-1272) (Figure S2) [26]. In the case of MgAC-Fe3O4/TiO2, besides the peaks corresponding to Fe3O4, there were additional peaks at 25.5°, 37.8°, 48.3°, 54.2°, and 62.8°, which corresponded to (101), (004), (200), (105), and (204) planes of anatase TiO2 (JCPDS 00-064-0863), respectively, thereby confirming the presence of Fe3O4 and TiO2 crystals within the nanohybrid structure [25]. The calculated crystallinity degree of MgAC-Fe3O4 and MgAC-Fe3O4/TiO2 was ~58.60% and ~80.32%, respectively. XPS characterizations were then conducted to investigate the oxidation states of the elements within the nanohybrid. The full-scan spectra indicated Mg, Fe, Ti, C, Si, and O, which corresponded to Mg-based aminoclay, Fe3O4, and TiO2 within the nanohybrid (Figure S3). In the high-resolution XPS spectra, the peaks at 710.4 and 723.5 eV were attributed to Fe 2p3/2 and Fe 2p1/2, respectively, both of which revealed the presence Fe3O4 in both MgAC-Fe3O4/TiO2 and MgAC-Fe3O4 (Figure S4a,c) [30]. Furthermore, in both MgAC-Fe3O4/TiO2 and MgAC-TiO2, the peaks corresponding to Ti 2p3/2 and Ti 2p1/2 at 458.2 and 464.07 eV, respectively, were observed, confirming the presence of Ti4+ state for TiO2 (Figure S4b,d) [31]. All these characterizations demonstrate that the nanohybrid was synthesized by successfully combining MgAC, Fe3O4, and TiO2.
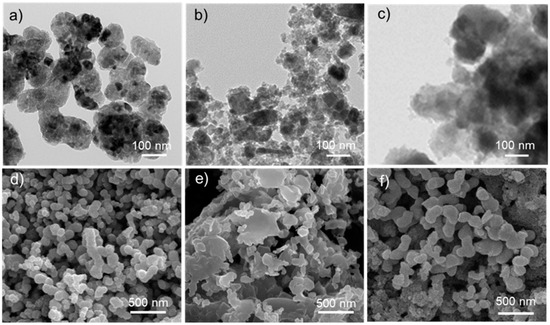
Figure 2.
TEM (a–c) and SEM (d–f) images of MgAC-Fe3O4/TiO2 (a,d), MgAC-Fe3O4 (b,e), and MgAC-TiO2 (c,f).
We performed typical ABTS oxidation reaction in the presence of H2O2, with or without UV-light irradiation, to examine the peroxidase activity of MgAC-Fe3O4/TiO2 and compared it with those of MgAC-Fe3O4 and MgAC-TiO2. The experimental results clearly suggest that MgAC-Fe3O4/TiO2 exhibited a significant enhancement in the peroxidase activity under UV-light irradiation (Figure 3). Compared with MgAC-Fe3O4 and MgAC-TiO2, the enhancement in the nanohybrid activity by UV-light irradiation was ~3-fold higher, presumably due to the expected synergistic effect of MgAC-Fe3O4/TiO2. TiO2 within the nanohybrid would catalyze hydroxyl radical generation by UV-light irradiation, which helps the peroxidase-mimicking Fe3O4-mediated oxidation of ABTS, thereby resulting in an enhanced peroxidase activity of the nanohybrid.
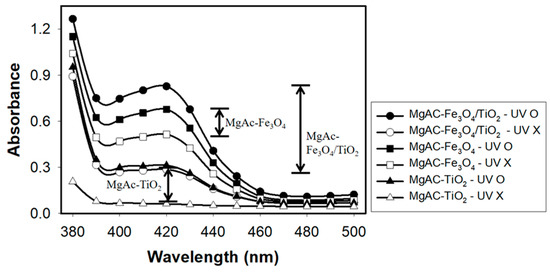
Figure 3.
Absorption spectra for the oxidation of ABTS in the presence of H2O2 by MgAC-Fe3O4/TiO2, MgAC-Fe3O4, and MgAC-TiO2, with or without UV-light irradiation.
To better understand the peroxidase-like activity, the steady-state kinetic assays of MgAc-Fe3O4/TiO2 were performed in the presence and absence of UV-light conditions using ABTS and H2O2 as substrate. MgAc-Fe3O4/TiO2 showed the typical Michalis–Menten kinetics toward H2O2 and ABTS in both cases (Figure S5). Its Michaelis–Menten constant (Km) and the maximal reaction velocity (Vmax) values were determined based on the double-reciprocal Lineweaver–Burk plots (Table S1). Under the UV-light irradiation, the Km values for both ABTS and H2O2 were lower than those without UV-light condition, proving the significant enhancement of the nanohybrid’s affinity toward both substrates. Furthermore, the Vmax values of MgAc-Fe3O4/TiO2 toward ABTS and H2O2 in the presence of UV-light irradiation were ~7.4 and ~3.4-fold higher than those observed in the absence of UV-light, respectively. These results provide the convincing evidence that UV-light acts as a superb inducer to impressively intensify the catalytic activity of MgAc-Fe3O4/TiO2.
The effects of duration of UV-light irradiation, pH of reaction buffer, and concentration of the employed MgAC-Fe3O4/TiO2 on the peroxidase activity were examined. As the duration of UV-light irradiation increased, the catalytic activity correspondingly increased. Considering the favorable detection speed and that the 10 min irradiation yielded sufficiently high ABTS oxidation (over 70%) compared to 20 min irradiation, UV-light exposure for 10 min was adopted for further studies (Figure 4a). MgAC-Fe3O4/TiO2 exhibited peroxidase activity at acidic pH conditions, and sodium acetate buffer at pH = 4 was adopted as the standard reaction buffer for our assay, since extreme acidic conditions could result in Fe ion leaching (Figure 4b) [8]. According to the peroxidase activity vs. the amount of MgAC-Fe3O4/TiO2, 0.6 mg/mL of MgAC-Fe3O4/TiO2 was the optimal amount and used for further studies (Figure 4c). Under the selected assay conditions, H2O2 was successfully determined from 2 to 25 μM with the limit of detection (LOD) as low as 1 μM (Figure 4d), which is among the best values reported so far for the colorimetric detection of H2O2 [32].
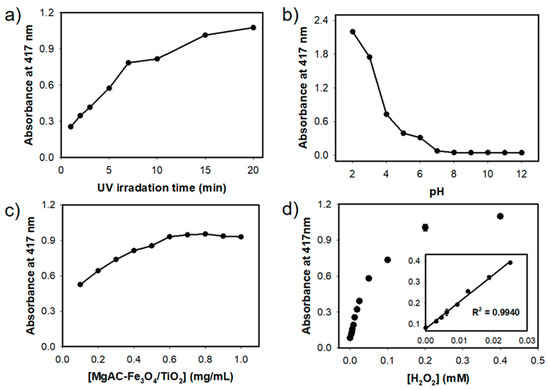
Figure 4.
Effects of (a) duration of UV-light irradiation, (b) buffer pH, and (c) employed concentration of MgAC-Fe3O4/TiO2 on the peroxidase activity. (d) Dose-response curve and calibration plot (inset) for H2O2 determination using UV-light-driven MgAC-Fe3O4/TiO2-based system.
In contrast with natural peroxidase, MgAC-Fe3O4/TiO2 are expected to be stable. To demonstrate this, the nanohybrid and HRP were incubated in a wide pH (3–11) and temperature (20–90 °C) range for 3 h, and the remaining activities were evaluated (Figure 5). According to the results, MgAC-Fe3O4/TiO2 efficiently maintained its activity under extreme pH and temperature conditions, while HRP faced a significant loss in its activity at both acidic and basic pH values or high temperatures (over 60 °C). Therefore, the excellent stability of MgAC-Fe3O4/TiO2 is considered beneficial for the practical applications.
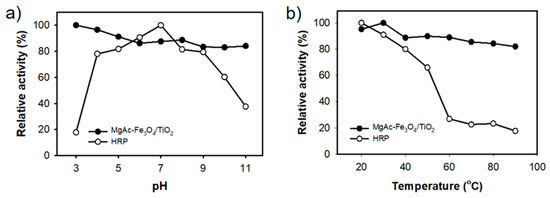
Figure 5.
Stabilities of MgAC-Fe3O4/TiO2 and HRP with varying (a) pH and (b) temperature.
Finally, we used our MgAC-Fe3O4/TiO2 to colorimetrically determine the selected phenolic compounds of pyrocatechol and resorcinol. Under the UV-light irradiation, our nanohybrid efficiently catalyzed the decomposition of H2O2 to produce hydroxyl radicals, which induced the oxidation of target phenolic compounds to generate quinoid radicals. The radicals further reacted with 4-AP to produce colored products, which can be observed by the naked eye and quantified by spectrophotometry [6]. The colors of the final products depended on the kind of phenol species, and thus, pyrocatechol and resorcinol induced slightly different colors and were quantitatively determined at different absorption intensities of 500 and 490 nm, respectively. We first performed experiments to verify the effects of UV-light on the detection of phenolic compounds. The results clearly showed that the absorption intensities toward phenolic compounds were significantly enhanced when irradiated with UV-light, indicating that UV-light-mediated activation of MgAC-Fe3O4/TiO2 would be crucial to sensitively determine phenolic compounds (Figure 6a,b). From the analysis of dose-response curves, both pyrocatechol and resorcinol were successfully determined with the same linear range of 0.15–1.30 mg/mL and LOD as low as 0.1 mg/mL, proving the high sensitivity for the determination of phenolic compounds (Figure 6c,d) [5]. In addition, the MgAc-Fe3O4/TiO2 were efficiently reused by simple centrifugation, by preserving over 90% of the original detecting activity for pyrocatechol even after nine consecutive cycles, proving the reusability of MgAc-Fe3O4/TiO2 (Figure S6).
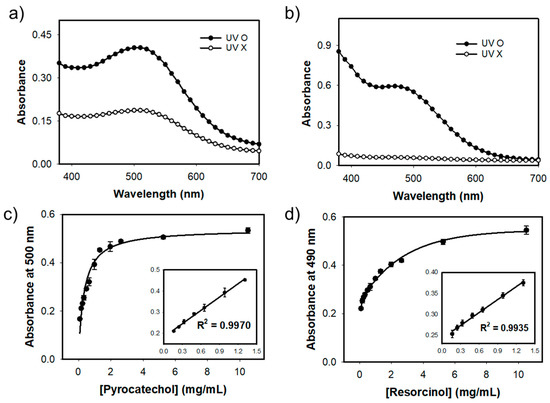
Figure 6.
Effects of UV-light irradiation for the detection of (a) pyrocatechol and (b) resorcinol, and dose-response curve and calibration plot (inset) for (c) pyrocatechol and (d) resorcinol using UV-light-driven MgAC-Fe3O4/TiO2-based system.
The practical utility of UV-light-driven MgAC-Fe3O4/TiO2-based system was demonstrated by determining several different levels of pyrocatechol and resorcinol in spiked tap water solutions. The system yielded acceptable CVs below 7% and recoveries of 97–109% (Table 1), validating the excellent reproducibility and reliability of this assay method. The CVs and recovery values of our system were comparable to those of previous nanomaterial-based strategies for phenolic compound detection (Table S2). These results demonstrated that the current method can be employed for the on-site practical determination of phenolic compounds.

Table 1.
Detection precision for the UV-light-driven MgAc-Fe3O4/TiO2-based system for the determination of phenolic compounds in spiked water samples.
4. Conclusions
Herein, we developed a novel UV-light-activated peroxidase-like nanozyme, MgAC-Fe3O4/TiO2, which was synthesized easily via sol–gel reaction. The activity of the developed nanozyme enhanced significantly in the presence of UV-light, owing to the synergistic effect between Fe3O4 and TiO2. With the enhanced activity, selected phenolic compounds of pyrocatechol and resorcinol were successfully determined with high sensitivity down to 0.1 mg/mL with excellent stability and reusability. The practical utility of UV-light-driven MgAC-Fe3O4/TiO2-based system was successfully demonstrated by precisely determining the concentrations of phenolic compounds in spiked tap water solution. The constructed nanohybrids could serve as new and efficient light-activated nanozymes, and we believe that they have great potential to be utilized in practical biosensing applications for detecting clinically and environmentally important substances.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/chemosensors9080219/s1, Figure S1: SEM (a), TEM (b), EDX (c) analysis, and (d) HRTEM of MgAC-Fe3O4/TiO2. Reprinted with permission from Bui et al. (2019) [28]. Copyright 2019, Springer Nature, Figure S2: XRD patterns for MgAC-Fe3O4/TiO2, MgAC-Fe3O4, and MgAC-TiO2, Figure S3: XPS full-survey spectra for (a) MgAC-Fe3O4/TiO2, (b) MgAC-Fe3O4, and (c) MgAC-TiO2, Figure S4: XPS spectra for (a) Fe 2p and (b) Ti 2p of MgAC-Fe3O4/TiO2; (c) Fe 2p of MgAC-Fe3O4; and (d) Ti 2p of MgAC-TiO2, Figure S5: Steady-state kinetic assays of MgAc-Fe3O4/TiO2 for H2O2 and ABTS in the (a) presence and (b) absence of UV-light irradiation, and their corresponding Lineweaver-Burk plots, Figure S6: Reusability of MgAc-Fe3O4/TiO2 hybrid for pyrocatechol determination, Table S1: Kinetic parameters of MgAc-Fe3O4/TiO2 with and without UV-light irradiation, Table S2: Comparison of CVs and recovery values of MgAc-Fe3O4/TiO2-based assay for phenolic compounds with those of previous nanomaterial-based strategies.
Author Contributions
Investigation, writing—original draft preparation, Y.J.J.; Validation, writing—review and editing, V.K.H.B.; Validation, P.T.N.; Conceptualization, supervision, writing—review and editing, Y.-C.L. and M.I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT (NRF-2019R1A2C1087459) and by the Korean National Police Agency (Project name: Development of visualization technology for biological evidence in crime scenes based on nano-bio technology/Project Number: PA-K000001-2019-401]. This research was also supported by Basic Science Research Program through NRF funded by the Ministry of Education (Grant No. 2021R1A6A1A03038996).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data of our study are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gul, I.; Ahmad, M.S.; Naqvi, S.S.; Hussain, A.; Wali, R.; Farooqi, A.A.; Ahmed, I. Polyphenol oxidase (PPO) based biosensors for detection of phenolic compounds: A review. J. Appl. Biol. Biotechnol. 2017, 5, 72–85. [Google Scholar]
- Hu, C.; Chen, B.; He, M.; Hu, B. Amino modified multi-walled carbon nanotubes/polydimethylsiloxane coated stir bar sorptive extraction coupled to high performance liquid chromatography-ultraviolet detection for the determination of phenols in environmental samples. J. Chromatogr. A 2013, 1300, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Moazampour, M.; Ensafi, A.A.; Mallakpour, S.; Hatami, M. An electrochemical nanocomposite modified carbon paste electrode as a sensor for simultaneous determination of hydrazine and phenol in water and wastewater samples. Environ. Sci. Pollut. Res. 2014, 21, 5879–5888. [Google Scholar] [CrossRef]
- Mitra, R.; Saha, A. Reduced graphene oxide based “turn-on” fluorescence sensor for highly reproducible and sensitive detection of small organic pollutants. ACS Sustain. Chem. Eng. 2017, 5, 604–615. [Google Scholar] [CrossRef]
- Tran, T.D.; Nguyen, P.T.; Le, T.N.; Kim, M.I. DNA-copper hybrid nanoflowers as efficient laccase mimics for colorimetric detection of phenolic compounds in paper microfluidic devices. Biosens. Bioelectron. 2021, 182, 113187. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Guo, D.; Xu, X.; Pan, J.; Niu, X. Colorimetric quantification and discrimination of phenolic pollutants based on peroxidase-like Fe3O4 nanoparticles. Sens. Actuators B Chem. 2020, 303, 127225. [Google Scholar] [CrossRef]
- Lin, Z.; Xiao, Y.; Yin, Y.; Hu, W.; Liu, W.; Yang, H. Facile synthesis of enzyme-inorganic hybrid nanoflowers and its application as a colorimetric platform for visual detection of hydrogen peroxide and phenol. ACS Appl. Mater. Interfaces 2014, 6, 10775–10782. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, W.; Cheng, J.; Qu, Y.; Dai, Y.; Liu, M.; Yu, J.; Wang, C.; Wang, H.; Wang, S.; et al. Stimuli-Responsive Manganese Single-Atom Nanozyme for Tumor Therapy via Integrated Cascade Reactions. Angew. Chem. 2021, 133, 9566–9574. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, J.; Kim, H.S.; Cho, A.; Shim, K.H.; Le, T.N.; An, S.S.A.; Han, J.W.; Kim, M.I.; Lee, J. Heme cofactor-resembling Fe–N single site embedded graphene as nanozymes to selectively detect H2O2 with high sensitivity. Adv. Funct. Mater. 2020, 30, 1905410. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface modification of magnetic iron oxide nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [Green Version]
- Badi, M.Y.; Azari, A.; Pasalari, H.; Esrafili, A.; Farzadkia, M. Modification of activated carbon with magnetic Fe3O4 nanoparticle composite for removal of ceftriaxone from aquatic solutions. J. Mol. Liq. 2018, 261, 146–154. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Zhang, P.; Xiong, J.; Mei, X.; Yu, Q.; Zhao, Z.; Liu, J. Boosting the removal of diesel soot particles by the optimal exposed crystal facet of CeO2 in Au/CeO2 catalysts. Environ. Sci. Technol. 2019, 54, 2002–2011. [Google Scholar] [CrossRef]
- Chen, L.; Yang, J.; Chen, W.; Sun, S.; Tang, H.; Li, Y. Perovskite mesoporous LaFeO3 with peroxidase-like activity for colorimetric detection of gallic acid. Sens. Actuators B Chem. 2020, 321, 128642. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, X.; Tang, D.; Wu, S.; Hou, X.; Liu, J.; Wu, P. Phosphorescent carbon dots for highly efficient oxygen photosensitization and as photo-oxidative nanozymes. ACS Appl. Mater. Interfaces 2018, 10, 40808–40814. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, S.; Chen, S.; Li, D.; Zhang, X.; Shao, W.; Sun, X.; Xie, J.; Zhao, Z.; Zhang, Q.; et al. Enhanced singlet oxygen generation in oxidized graphitic carbon nitride for organic synthesis. Adv. Mater. 2016, 28, 6940–6945. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Li, C.; Xu, S.; Hou, X.; Wu, P. Simultaneously broadened visible light absorption and boosted intersystem crossing in platinum-doped graphite carbon nitride for enhanced photosensitization. ACS Appl. Mater. Interfaces 2019, 11, 20770–20777. [Google Scholar] [CrossRef] [PubMed]
- Lismont, M.; Dreesen, L.; Wuttke, S. Metal-organic framework nanoparticles in photodynamic therapy: Current status and perspectives. Adv. Funct. Mater. 2017, 27, 1606314. [Google Scholar] [CrossRef]
- Meziani, M.J.; Dong, X.; Zhu, L.; Jones, L.P.; LeCroy, G.E.; Yang, F.; Wang, S.; Wang, P.; Zhao, Y.; Yang, L. Visible-light-activated bactericidal functions of carbon “Quantum” dots. ACS Appl. Mater. Interfaces 2016, 8, 10761–10766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, L.-Y.; Dong, Y.-M.; Wu, X.-M.; Cao, G.-X.; Wang, G.-L. Versatile and amplified biosensing through enzymatic cascade reaction by coupling alkaline phosphatase in situ generation of photoresponsive nanozyme. Anal. Chem. 2015, 87, 10429–10436. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding hydroxyl radical (•OH) generation processes in photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.; Lu, W.; Zhang, R.; Pan, F. Enhanced photocatalytic activity of TiO2-C hybrid aerogels for methylene blue degradation. Sci. Rep. 2013, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Kim, M.I.; Woo, M.A.; Park, H.G.; Han, J.I. Effective peroxidase-like activity of a water-solubilized Fe-aminoclay for use in immunoassay. Biosens. Bioelectron. 2013, 42, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Bui, V.K.H.; Park, D.; Lee, Y.-C. Aminoclays for biological and environmental applications: An updated review. Chem. Eng. J. 2018, 336, 757–772. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Pham, T.N.; Lee, Y.-C. One-Pot Synthesis of Magnesium Aminoclay–Iron Oxide Nanocomposites for Improved Photo-Fenton Catalytic Performance. J. Nanosci. Nanotechnol. 2019, 19, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Bui, V.K.H.; Park, D.; Tran, V.V.; Lee, G.-W.; Oh, S.Y.; Huh, Y.S.; Lee, Y.-C. One-pot synthesis of magnesium aminoclay-titanium dioxide nanocomposites for improved photocatalytic performance. J. Nanosci. Nanotechnol. 2018, 18, 6070–6074. [Google Scholar] [CrossRef] [PubMed]
- Bui, V.K.H.; Park, D.; Pham, T.N.; An, Y.; Choi, J.S.; Lee, H.U.; Kwon, O.-H.; Moon, J.-Y.; Kim, K.-T.; Lee, Y.-C. Synthesis of MgAC-Fe3O4/TiO2 hybrid nanocomposites via sol-gel chemistry for water treatment by photo-Fenton and photocatalytic reactions. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Doumeng, M.; Makhlouf, L.; Berthet, F.; Marsan, O.; Delbé, K.; Denape, J.; Chabert, F. A comparative study of the crystallinity of polyetheretherketone by using density, DSC, XRD, and Raman spectroscopy techniques. Polym. Test. 2021, 93, 106878. [Google Scholar] [CrossRef]
- Han, F.; Ma, L.; Sun, Q.; Lei, C.; Lu, A. Rationally designed carbon-coated Fe3O4 coaxial nanotubes with hierarchical porosity as high-rate anodes for lithium ion batteries. Nano Res. 2014, 7, 1706–1717. [Google Scholar] [CrossRef]
- Giorgi, L.; Dikonimos, T.; Giorgi, R.; Buonocore, F.; Faggio, G.; Messina, G.; Lisi, N. Electrochemical synthesis of self-organized TiO2 crystalline nanotubes without annealing. Nanotechnology 2018, 29, 095604. [Google Scholar] [CrossRef] [PubMed]
- Batule, B.S.; Park, K.S.; Gautam, S.; Cheon, H.J.; Kim, M.I.; Park, H.G. Intrinsic peroxidase-like activity of sonochemically synthesized protein copper nanoflowers and its application for the sensitive detection of glucose. Sens. Actuators B Chem. 2019, 283, 749–754. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).