Fluorescence Enhancement via Dual Coupling of Dye Molecules with Silver Nanostructures
Abstract
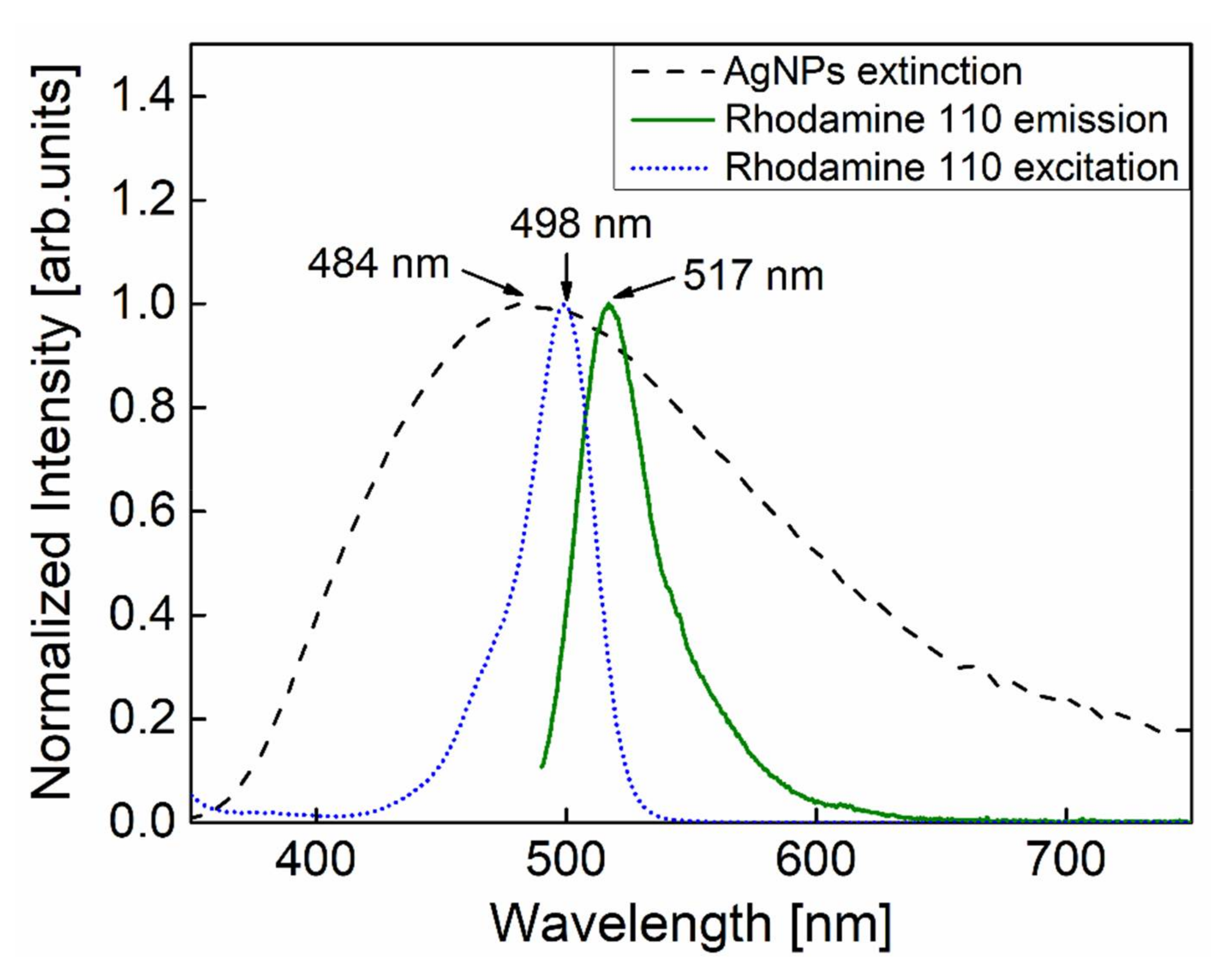
:1. Introduction
2. Experimental Section
2.1. Fabrication of the Plasmonic Chip for Fluorescence Enhancement
2.2. Fluorescence Detection System
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horton, M.J.; Ojambati, O.S.; Chikkaraddy, R.; Deacon, W.M.; Kongsuwan, N.; Demetriadou, A.; Hess, O.; Baumberg, J.J. Nanoscopy through a plasmonic nanolens. Proc. Natl. Acad. Sci. USA 2020, 117, 2275–2281. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Liu, X.; Jarrett, J.W.; Brown, D.; Pustovit, V.; Urbas, A.; Knappenberger, K.L., Jr.; Nealey, P.F.; Vaia, R.A. Nonlinear Chiro-Optical Amplification by Plasmonic Nanolens Arrays Formed via Directed Assembly of Gold Nanoparticles. Nano Lett. 2015, 15, 1836–1842. [Google Scholar] [CrossRef]
- Yuan, H.; Fales, A.M.; Vo-Dinh, T. TAT Peptide-Functionalized Gold Nanostars: Enhanced Intracellular Delivery and Efficient NIR Photothermal Therapy Using Ultralow Irradiance. J. Am. Chem. Soc. 2012, 134, 11358–11361. [Google Scholar] [CrossRef] [Green Version]
- Aioub, M.; Panikkanvalappil, S.R.; El-Sayed, M.A. Platinum-Coated Gold Nanorods: Efficient Reactive Oxygen Scavengers That Prevent Oxidative Damage toward Healthy, Untreated Cells during Plasmonic Photothermal Therapy. ACS Nano 2017, 11, 579–586. [Google Scholar] [CrossRef]
- Sivis, M.; Duwe, M.; Abel, B.; Ropers, C. Extreme-ultraviolet light generation in plasmonic nanostructures. Nat. Phys. 2013, 9, 304–309. [Google Scholar] [CrossRef]
- Krasavin, A.V.; Ginzburg, P.; Wurtz, G.A.; Zayats, A.V. Nonlocality-driven supercontinuum white light generation in plasmonic nanostructures. Nat. Commun. 2016, 7, 11497. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.; Thomas, M.R.; Bergholt, M.S.; Pence, I.J.; Seong, H.; Charchar, P.; Todorova, N.; Nagelkerke, A.; Belessiotis-Richards, A.; Payne, D.J.; et al. Surface enhanced Raman scattering artificial nose for high dimensionality fingerprinting. Nat. Commun. 2020, 11, 207. [Google Scholar] [CrossRef]
- Shiota, M.; Naya, M.; Yamamoto, T.; Hishiki, T.; Tani, T.; Takahashi, H.; Kubo, A.; Koike, D.; Itoh, M.; Ohmura, M.; et al. Gold-nanofève surface-enhanced Raman spectroscopy visualizes hypotaurine as a robust anti-oxidant consumed in cancer survival. Nat. Commun. 2018, 9, 1561. [Google Scholar] [CrossRef]
- Carretero-Palacios, S.; Jimenez-Solano, A.; Míguez, H. Plasmonic Nanoparticles as Light-Harvesting Enhancers in Perovskite Solar Cells: A User’s Guide. ACS Energy Lett. 2016, 1, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Li, Y.; Liu, L.; Chen, L.; Xu, J.; Ma, J.; Fang, G.; Zhu, E.; Wu, H.; Zhao, L.; et al. Near-Infrared Plasmonic-Enhanced Solar Energy Harvest for Highly Efficient Photocatalytic Reactions. Nano Lett. 2015, 15, 6295–6301. [Google Scholar] [CrossRef]
- Masuda, S.; Yanase, Y.; Usukura, E.; Ryuzaki, S.; Wang, P.; Okamoto, K.; Kuboki, T.; Kidoaki, S.; Tamada, K. High-resolution imaging of a cellattached nanointerface using a gold-nanoparticle two-dimensional sheet. Sci. Rep. 2017, 7, 3720. [Google Scholar] [CrossRef]
- Williams, C.; Rughoobur, G.; Flewitt, A.J.; Wilkinson, T.D. Nanostructured plasmonic metapixels. Sci. Rep. 2017, 7, 7745. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, S.; Nguyen, T.T.; Lee, R.; Li, T.; Yun, C.; Ham, Y.; An, S.S.A.; Ju, H. Label-free quantitative immunoassay of fibrinogen in Alzheimer disease patient plasma using fiber optical surface plasmon resonance. J. Electron. Mater. 2016, 45, 2354–2360. [Google Scholar] [CrossRef]
- Tran, V.T.; Yoon, W.J.; Lee, J.-H.; Ju, H. DNA sequence-induced modulation of bimetallic surface plasmons in optical fibers for sub-ppq (parts-per-quadrillion) detection of mercury ions in water. J. Mater. Chem. A 2018, 6, 23894–23902. [Google Scholar] [CrossRef]
- Kim, J.; Son, C.; Choi, S.; Yoon, W.J.; Ju, H. A plasmonic fiber-based glucometer and its temperature dependence. Micromachines 2018, 9, 506. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-H.; Choi, J.-W. Metal-Enhanced Fluorescence by Bifunctional Au Nanoparticles for Highly Sensitive and Simple Detection of Proteolytic Enzyme. Nano Lett. 2020, 20, 7100–7107. [Google Scholar] [CrossRef]
- Fothergill, S.M.; Joyce, C.; Xie, F. Metal enhanced fluorescence biosensing: From ultra-violet towards second near-infrared window. Nanoscale 2018, 10, 20914–20929. [Google Scholar] [CrossRef] [Green Version]
- Tran, N.H.T.; Trinh, K.T.L.; Lee, J.-H.; Yoon, W.J.; Ju, H. Reproducible Enhancement of Fluorescence by Bimetal Mediated Surface Plasmon Coupled Emission for Highly Sensitive Quantitative Diagnosis of Double-Stranded DNA. Small 2018, 14, 1801385. [Google Scholar] [CrossRef]
- Tran, V.T.; Ju, H. Fluorescence Based on Surface Plasmon Coupled Emission for Ultrahigh Sensitivity Immunoassay of Cardiac Troponin I. Biomedicines 2021, 9, 448. [Google Scholar] [CrossRef]
- Gryczynski, I.; Malicka, J.; Gryczynski, Z.; Lakowicz, J.R. Surface Plasmon-Coupled Emission with Gold Films. J. Phys. Chem. B 2004, 108, 12568–12574. [Google Scholar] [CrossRef] [Green Version]
- Badugu, R.; Szmacinski, H.; Ray, K.; Descrovi, E.; Ricciardi, S.; Zhang, D.; Chen, J.; Huo, Y.; Lakowicz, J.R. Metal−Dielectric Waveguides for High-Efficiency Coupled Emission. ACS Photonics 2015, 2, 810–815. [Google Scholar] [CrossRef] [Green Version]
- Geddes, C.D.; Gryczynski, I.; Malicka, J.; Gryczynski, Z.; Lakowicz, J.R. Directional Surface Plasmon Coupled Emission. J. Fluoresc. 2004, 14, 119–123. [Google Scholar] [CrossRef]
- Meng, L.; Yang, Z. Directional surface plasmon-coupled emission of tilted-tip enhanced spectroscopy. Nanophotonics 2018, 7, 1325–1332. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Malicka, J.; Gryczynski, I.; Gryczynski, Z. Directional surface plasmon-coupled emission: A new method for high sensitivity detection. Biochem. Biophys. Res. Commun. 2003, 307, 435–439. [Google Scholar] [CrossRef] [Green Version]
- Gryczynski, I.; Malicka, J.; Nowaczyk, K.; Gryczynski, Z.; Lakowicz, J.R. Effects of Sample Thickness on the Optical Properties of Surface Plasmon-Coupled Emission. J. Phys. Chem. B 2004, 108, 12073–12083. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, G.J.; Scott, G.D. Optical excitation of surface plasma waves in layered media. Phys. Rev. B 1977, 16, 1297. [Google Scholar] [CrossRef]
- Kovacs, G.J.; Scott, G.D. Attenuated total reflection angular spectra and associated resonant electromagnetic oscillations of a dielectric slab bounded by Ag films. Appl. Opt. 1978, 17, 3314–3322. [Google Scholar] [CrossRef] [PubMed]
- Chyou, J.-J.; Chu, C.-S.; Shih, C.-H.; Lin, C.-Y.; Huang, K.-T.; Chen, S.-J.; Shu, S.-F. High-efficiency electro-optic polymer light modulator based on waveguide-coupled surface plasmon resonance. In Plasmonics: Metallic Nanostructures and Their Optical Properties; International Society for Optics and Photonics: Bellingham, DC, USA, 2003; Volume 5211, pp. 197–206. [Google Scholar]
- Gryczynski, I.; Malicka, J.; Nowaczyk, K.; Gryczynski, Z.; Lakowicz, J.R. Waveguide-modulated surface plasmon-coupled emission of Nile blue in poly(vinyl alcohol) thin films. Thin Solid Films 2006, 510, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calander, N. Surface Plasmon-Coupled Emission and Fabry-Perot Resonance in the Sample Layer: A Theoretical Approach. J. Phys. Chem. B 2005, 109, 13957–13963. [Google Scholar] [CrossRef] [PubMed]
- Salamon, Z.; Macleod, H.A.; Tollin, G. Coupled Plasmon-Waveguide Resonators: A New Spectroscopic Tool for Probing Proteolipid Film Structure and Properties. Biophys. J. 1997, 73, 2791–2797. [Google Scholar] [CrossRef] [Green Version]
- Salamon, Z.; Tollin, G. Optical Anisotropy in Lipid Bilayer Membranes: Coupled Plasmon-Waveguide Resonance Measurements of Molecular Orientation, Polarizability, and Shape. Biophys. J. 2001, 80, 1557–1567. [Google Scholar] [CrossRef] [Green Version]
- Toyama, S.; Doumae, N.; Shoji, A.; Ikariyama, Y. Design and fabrication of a waveguide-coupled prism device for surface plasmon resonance sensor. Sens. Actuat. B Chem. 2000, 65, 32–34. [Google Scholar] [CrossRef]
- Song, B.; Jiang, Z.; Liu, Z.; Wang, Y.; Liu, F.; Cronin, S.B.; Yang, H.; Meng, D.; Chen, B.; Hu, P.; et al. Probing the Mechanisms of Strong Fluorescence Enhancement in Plasmonic Nanogaps with Sub-nanometer Precision. ACS Nano 2020, 14, 14769–14778. [Google Scholar] [CrossRef]
- Cao, S.-H.; Cai, W.-P.; Liu, Q.; Xie, K.-X.; Weng, Y.-H.; Huo, S.-X.; Tian, Z.-Q.; Li, Y.-Q. Label-Free Aptasensor Based on Ultrathin-Linker-Mediated Hot-Spot Assembly to Induce Strong Directional Fluorescence. J. Am. Chem. Soc. 2014, 136, 6802–6805. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ju, H. Label-free optical biosensors based on a planar optical waveguide. Biochip J. 2013, 7, 295–318. [Google Scholar] [CrossRef]
- Tran, N.H.T.; Phan, B.T.; Yoon, W.J.; Khym, S.; Ju, H. Dielectric metal-based multilayers for surface plasmon resonance with enhanced quality factor of the plasmonic waves. J. Electron. Mater. 2017, 46, 3654–3689. [Google Scholar] [CrossRef]
- Michal, T.; Joerg, E.; Thomas, R.; Colette, M.; Brian, D.M. Experimental and theoretical evaluation of surface plasmon-coupled emission for sensitive fluorescence detection. J. Biomed. Opt. 2008, 13, 054021. [Google Scholar]
- Venkatesh, S.; Sai Sathish, R. Purcell Factor: A Tunable Metric for Plasmon-Coupled Fluorescence Emission Enhancements in Cermet Nanocavities. J. Phys. Chem. C 2016, 120, 2908–2913. [Google Scholar]
- Kaushal, S.; Nanda, S.S.; Yi, D.K.; Ju, H. Effects of Aspect Ratio Heterogeneity of an Assembly of Gold Nanorod on Localized Surface Plasmon Resonance. J. Phys. Chem. Lett. 2020, 11, 5972–5979. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Effects of Solvents on Fluorescence Emission Spectra. In Principles of Fluorescence Spectroscopy; Plenum Press: New York, NY, USA, 1983; pp. 187–215. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, V.T.; Ju, H. Fluorescence Enhancement via Dual Coupling of Dye Molecules with Silver Nanostructures. Chemosensors 2021, 9, 217. https://doi.org/10.3390/chemosensors9080217
Tran VT, Ju H. Fluorescence Enhancement via Dual Coupling of Dye Molecules with Silver Nanostructures. Chemosensors. 2021; 9(8):217. https://doi.org/10.3390/chemosensors9080217
Chicago/Turabian StyleTran, Vien Thi, and Heongkyu Ju. 2021. "Fluorescence Enhancement via Dual Coupling of Dye Molecules with Silver Nanostructures" Chemosensors 9, no. 8: 217. https://doi.org/10.3390/chemosensors9080217
APA StyleTran, V. T., & Ju, H. (2021). Fluorescence Enhancement via Dual Coupling of Dye Molecules with Silver Nanostructures. Chemosensors, 9(8), 217. https://doi.org/10.3390/chemosensors9080217





