Potential Assessment of Dehydration during High-Intensity Training Using a Capacitance Sensor for Oral Mucosal Moisture: Evaluation of Elite Athletes in a Field-Based Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
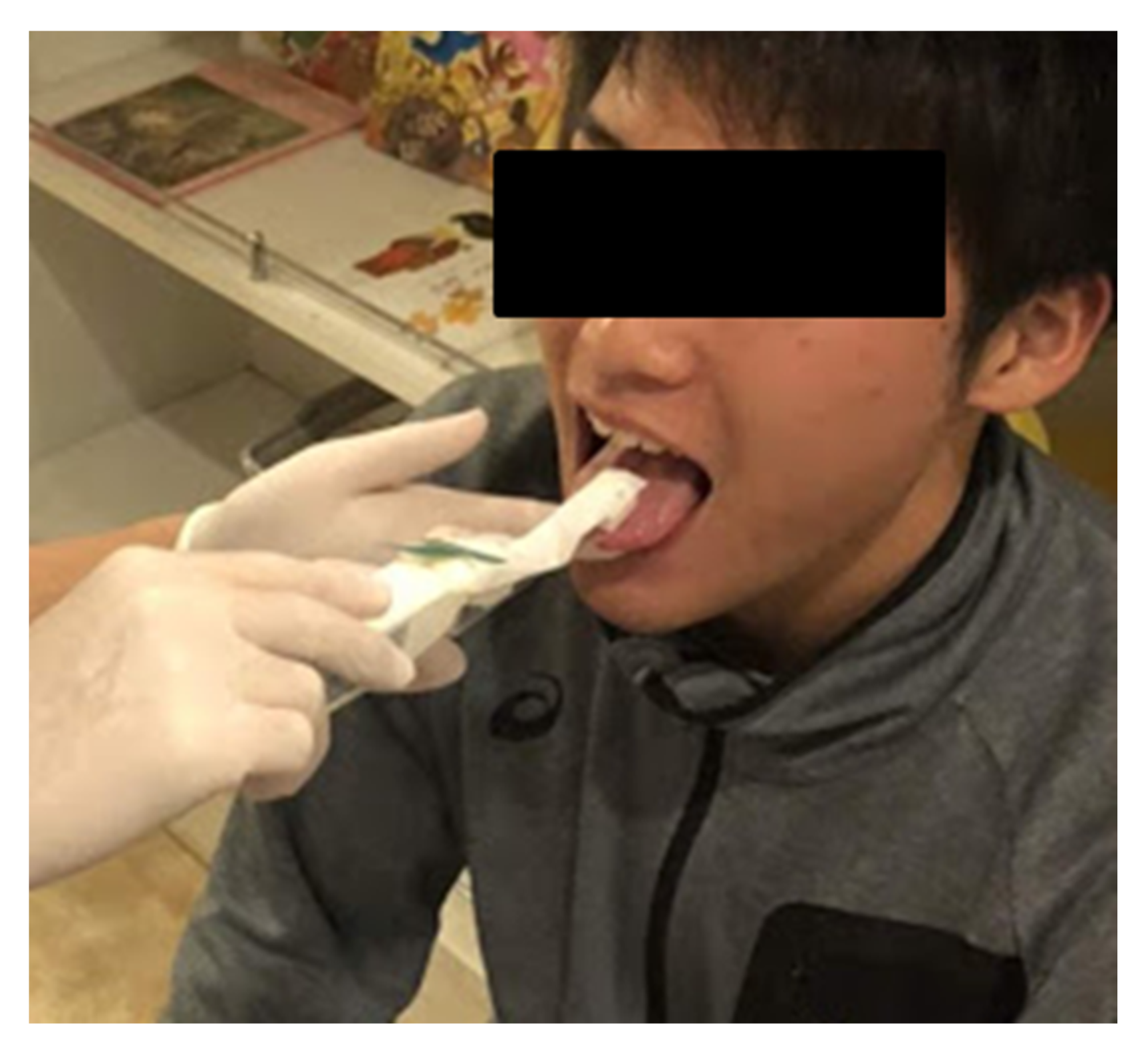
2.2. Data Collection
2.3. Data Analysis
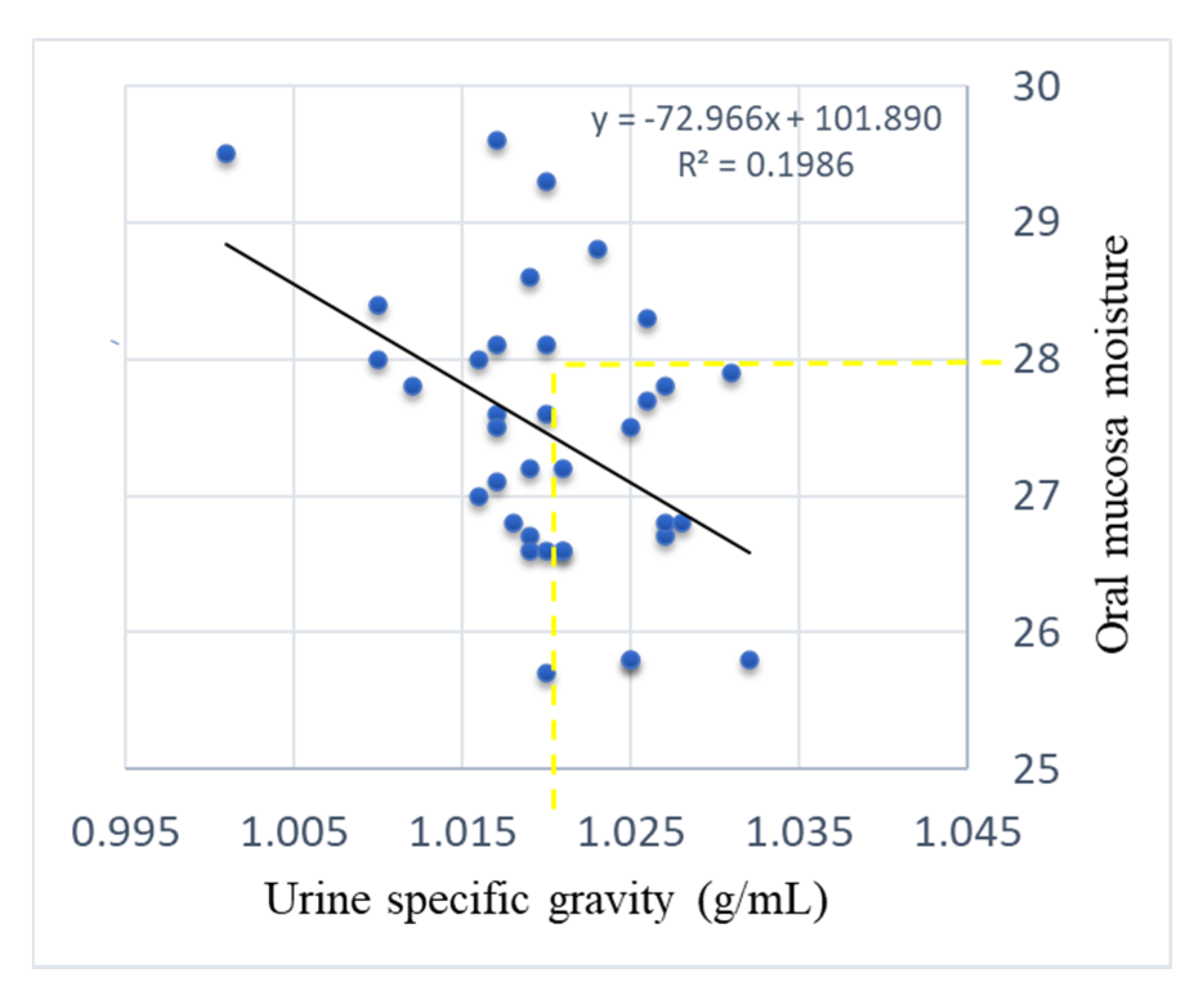
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sawka, M.N.; Young, A.J. Physiological Systems and Their Responses to Conditions of Heat and Cold. In ACSM’s Advanced Exercise Physiology; Tipton, C.M., Sawka, M.N., Tate, C.A., Terjung, R.L., Baltimore, M.D., Eds.; Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 535–563. [Google Scholar]
- Sawka, M.N.; Burke, L.M.; Eichner, E.R.; Maughan, R.J.; Montain, S.J.; Stachenfeld, N.S. American College of Sports Medicine position stand: Exercise and fluid replacement. Med. Sci. Sports Exerc. 2007, 39, 377–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheuvront, S.N.; Carter, R., 3rd; Sawka, M.N. Fluid balance and endurance exercise performance. Curr. Sports Med. Rep. 2003, 2, 202–208. [Google Scholar] [CrossRef]
- Hancock, P.A.; Vasmatzidis, I. Effects of heat stress on cognitive performance: The current state of knowledge. Int. J. Hyperth. 2003, 19, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Greiwe, J.S.; Staffey, K.S.; Melrose, D.R.; Narve, M.D.; Knowlton, R.G. Effects of dehydration on isometric muscular strength and endurance. Med. Sci. Sports Exerc. 1998, 30, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Sawka, M.N.; Young, A.J.; Latzka, W.A.; Neufer, P.D.; Quigley, M.D.; Pandolf, K.B. Human tolerance to heat strain during exercise: Influence of hydration. J. Appl. Physiol. 1992, 73, 368–375. [Google Scholar] [CrossRef]
- Carter, R., 3rd; Cheuvront, S.N.; Williams, J.O.; Kolka, M.A.; Stephenson, L.A.; Sawka, M.N.; Amoroso, P.J. Epidemiology of hospitalizations and deaths from heat illness in soldiers. Med. Sci. Sports Exerc. 2005, 37, 1338–1344. [Google Scholar] [CrossRef]
- Sayers, S.P.; Clarkson, P.M. Exercise-induced rhabdomyolysis. Curr. Sports Med. Rep. 2002, 1, 59–60. [Google Scholar] [CrossRef]
- Dancaster, C.P.; Whereat, S.J. Fluid and electrolyte balance during the comrades marathon. S. Afr. Med. J. 1971, 45, 147–150. [Google Scholar]
- Armstrong, L.E.; Maresh, C.M.; Castellani, J.W.; Bergeron, M.F.; Kenefick, R.W.; LaGasse, K.E.; Riebe, D. Urinary indices of hydration status. Int. J. Sport Nutr. 1994, 4, 265–279. [Google Scholar] [CrossRef]
- Armstrong, L.E. Hydration assessment techniques. Nutr. Rev. 2005, 63 (Suppl. 1), S40–S54. [Google Scholar] [CrossRef]
- Yoshihara, A.; Hirotomi, T.; Takano, N.; Kondo, T.; Hanada, N.; Miyazaki, H. Serum markers of chronic dehydration are associated with saliva spinability. J. Oral Rehabil. 2007, 34, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Fortes, M.B.; Owen, J.A.; Raymond-Barker, P.; Bishop, C.; Elghenzai, S.; Oliver, S.J.; Walsh, N.P. Is this elderly patient dehydrated? Diagnostic accuracy of hydration assessment using physical signs, urine, and saliva markers. J. Am. Med. Dir. Assoc. 2015, 16, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, M.; Kinoshita, K.; Hattori, K.; Ota, Y.; Kanai, T.; Kobayashi, H.; Tokuda, Y. Physical signs of dehydration in the elderly. Intern. Med. 2012, 51, 1207–1210. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, Y.; Sano, Y.; Isozaki, Y.; Endo, M.; Tomoda, T.; Kitamura, T.; Sato, T.; Kamijo, Y.; Haga, Y.; Yoda, T. A pilot clinical evaluation of oral mucosal dryness in dehydrated patients using a moisture-checking device. Clin. Exp. Dent. Res. 2019, 5, 116–120. [Google Scholar] [CrossRef]
- McGee, S.; Abernethy, W.B., 3rd; Simel, D.L. The rational clinical examination. Is this patient hypovolemic? JAMA 1999, 281, 1022–1029. [Google Scholar] [CrossRef]
- Saito, M.; Ono, Y.; Kitamura, N.; Yamaguchi, M.; Saito, C. A study of moisture content in the oral mucosa in the elderly, Part II. Investigating moisture content in the oral mucosa in the elderly population residing at a nursing home. Ann. Jpn. Gerodont. Soc. 2008, 23, 97–105. [Google Scholar]
- Murakami, M.; Nishi, Y.; Kamashita, Y.; Nagaoka, E. Comparison of a saliva wetness tester and a moisture-checking device in patients with maxillary obturator prostheses. Gerodontology 2014, 31, 83–88. [Google Scholar] [CrossRef]
- Kanazaki, A.; Hu, J.; Otomaru, T.; Sumita, Y.I. Evaluation of xerostomia and effect of maxillofacial prosthesis use on mouth dryness in head and neck tumor patients. Int. J. Maxillofac. Prosthet. 2021, 4, 9–17. [Google Scholar] [CrossRef]
- Nishii, M.; Akashi, M.; Kakei, Y.; Hasegawa, T.; Minamikawa, T.; Furudoi, S.; Shibuya, Y.; Takahashi, M.; Otsuki, N.; Nibu, K.; et al. Sequential changes in oral dryness evaluated by a moisture-checking device in patients with oropharyngeal cancer during chemoradiotherapy: A pilot study. Oral Health Dent. Manag. 2014, 13, 507–511. [Google Scholar]
- Takahashi, F.; Takahashi, M.; Toya, S.; Morita, O. Relationship between medicine and stimulated saliva and oral moisture. Nihon Hotetsu Shika Gakkai Zasshi 2008, 52, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Koji, T.; Morita, O. The usefulness of an oral moisture checking device (Moisture Checker for Mucus). Nihon Hotetsu Shika Gakkai Zasshi 2005, 49, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, Y.; Yoda, T.; Araki, R.; Sakai, T.; Toya, S.; Ito, K.; Sato, T. Evaluation of oral wetness using an improved moisture-checking device for the diagnosis of dry mouth. Oral Sci. Int. 2017, 14, 33–36. [Google Scholar] [CrossRef]
- Takano, T.; Kugimiya, Y.; Morita, K.; Tazawa, S.; Ueda, T.; Sakurai, K. Intra- and inter-investigator reliabilities of oral moisture measured using an oral moisture-checking device. J. Oral Rehabil. 2020, 47, 480–484. [Google Scholar] [CrossRef]
- Foster, C.; Florhaug, J.A.; Franklin, J.; Gottschall, L.; Hrovatin, L.A.; Parker, S.; Doleshal, P.; Dodge, C. A new approach to monitoring exercise training. J. Strength Cond. Res. 2001, 15, 109–115. [Google Scholar]
- Yoshida, G.; Iwama, A.; Sakata, T.M.; Nakamura, N.; Eiraku, H.; Higashionna, A.; Akamine, T.; Yoshitake, Y. Fluid Balance during Training for Top University Canoe Sprint Athletes: A Preliminary Study Investigating the Differences in Controlling Energy Expenditure by Season. Jpn. J. Nutr. Diet. 2021, 79, 103–111. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Kenefick, R.W. Am I Drinking Enough? Yes, No, and Maybe. J. Am. Coll. Nutr. 2016, 35, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, E.; Kashimura, O. Relationship between physiological responses and thirst sensation at exercise. Jpn. J. Biometeor. 2015, 52, 165–174. [Google Scholar] [CrossRef]


| Measure | Euhydration Cutoff | |
|---|---|---|
| Blood | Plasma osmolality | <290 mOsmol |
| Urine | Urine specific gravity | <1.020 g/mL |
| Urine osmolality | <700 mOsmol | |
| Urine color | ||
| Weight | Body weight | <1% |
| Total body water | <2% | |
| Oral | Thirst | |
| Mouth dryness |
| Number of subjects | 19 (Male 10; Female 9) |
| Mean age | 20.8 ± 0.8 |
| Weight (kg) | 58.1 ± 8.7 |
| Summer | Day1 | Day2 | Day3 | Day4 | Day5 | Day6 | Day7 |
|---|---|---|---|---|---|---|---|
| Weather | Sunny | Sunny | Sunny | Sunny | Sunny | Sunny | Sunny |
| Maximum temperature (°C) | 30.1 | 30.3 | 30.5 | 33.5 | 32.6 | 31.1 | 32.0 |
| s-RPE 1 | 6.6 ± 2.7 * | 34.0 ± 10.5 | 25.5 ± 7.4 | 13.3 ± 4.5 | 33.0 ± 10.5 | 39.4 ± 8.8 | 10.3 ± 2.9 |
| Winter | Day1 | Day2 | Day3 | Day4 | Day5 | Day6 | Day7 |
| Weather | Rain | Rain | Sunny | Cloudy | Rain | Rain | Sunny |
| Maximum temperature (°C) | 13.2 | 10.2 | 17.1 | 15.8 | 16.5 | 16.4 | 12.7 |
| s-RPE | 2.3 ± 3.60 | 9.9 ± 3.7 | 32.8 ± 12.3 | 17.6 ± 4.2 | 24.5 ± 11.3 | 20.7 ± 6.6 | 24.6 ± 6.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanabe, G.; Hasunuma, T.; Inai, Y.; Takeuchi, Y.; Kobayashi, H.; Hayashi, K.; Shimizu, S.; Kamiya, N.S.; Churei, H.; Sumita, Y.I.; et al. Potential Assessment of Dehydration during High-Intensity Training Using a Capacitance Sensor for Oral Mucosal Moisture: Evaluation of Elite Athletes in a Field-Based Survey. Chemosensors 2021, 9, 196. https://doi.org/10.3390/chemosensors9080196
Tanabe G, Hasunuma T, Inai Y, Takeuchi Y, Kobayashi H, Hayashi K, Shimizu S, Kamiya NS, Churei H, Sumita YI, et al. Potential Assessment of Dehydration during High-Intensity Training Using a Capacitance Sensor for Oral Mucosal Moisture: Evaluation of Elite Athletes in a Field-Based Survey. Chemosensors. 2021; 9(8):196. https://doi.org/10.3390/chemosensors9080196
Chicago/Turabian StyleTanabe, Gen, Tetsuya Hasunuma, Yuto Inai, Yasuo Takeuchi, Hiroaki Kobayashi, Kairi Hayashi, Shintaro Shimizu, Nana S Kamiya, Hiroshi Churei, Yuka I Sumita, and et al. 2021. "Potential Assessment of Dehydration during High-Intensity Training Using a Capacitance Sensor for Oral Mucosal Moisture: Evaluation of Elite Athletes in a Field-Based Survey" Chemosensors 9, no. 8: 196. https://doi.org/10.3390/chemosensors9080196
APA StyleTanabe, G., Hasunuma, T., Inai, Y., Takeuchi, Y., Kobayashi, H., Hayashi, K., Shimizu, S., Kamiya, N. S., Churei, H., Sumita, Y. I., Suzuki, K., Moriya, N., & Ueno, T. (2021). Potential Assessment of Dehydration during High-Intensity Training Using a Capacitance Sensor for Oral Mucosal Moisture: Evaluation of Elite Athletes in a Field-Based Survey. Chemosensors, 9(8), 196. https://doi.org/10.3390/chemosensors9080196








