Nanocomposite Materials Based on Electrochemically Synthesized Graphene Polymers: Molecular Architecture Strategies for Sensor Applications
Abstract
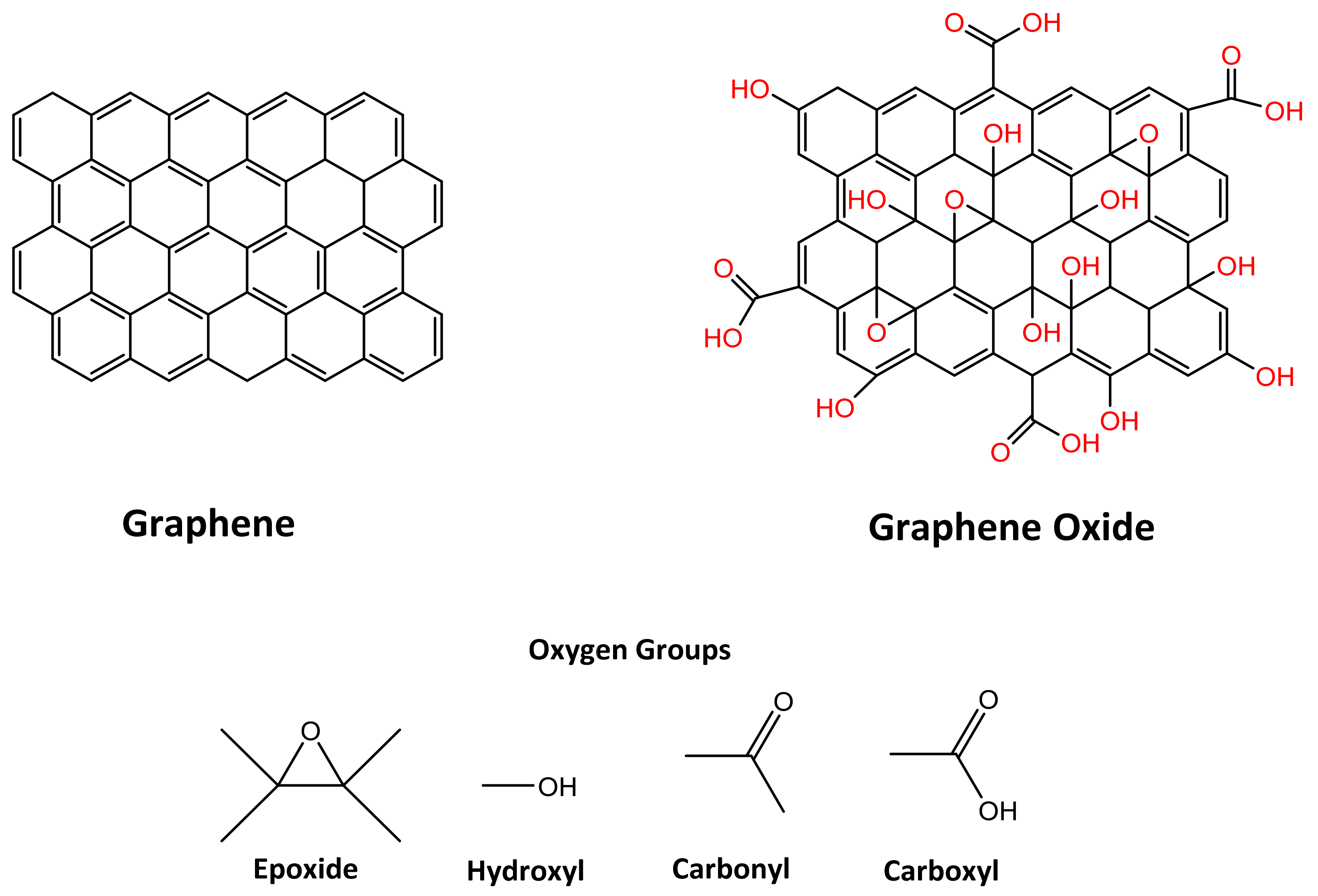
1. Introduction
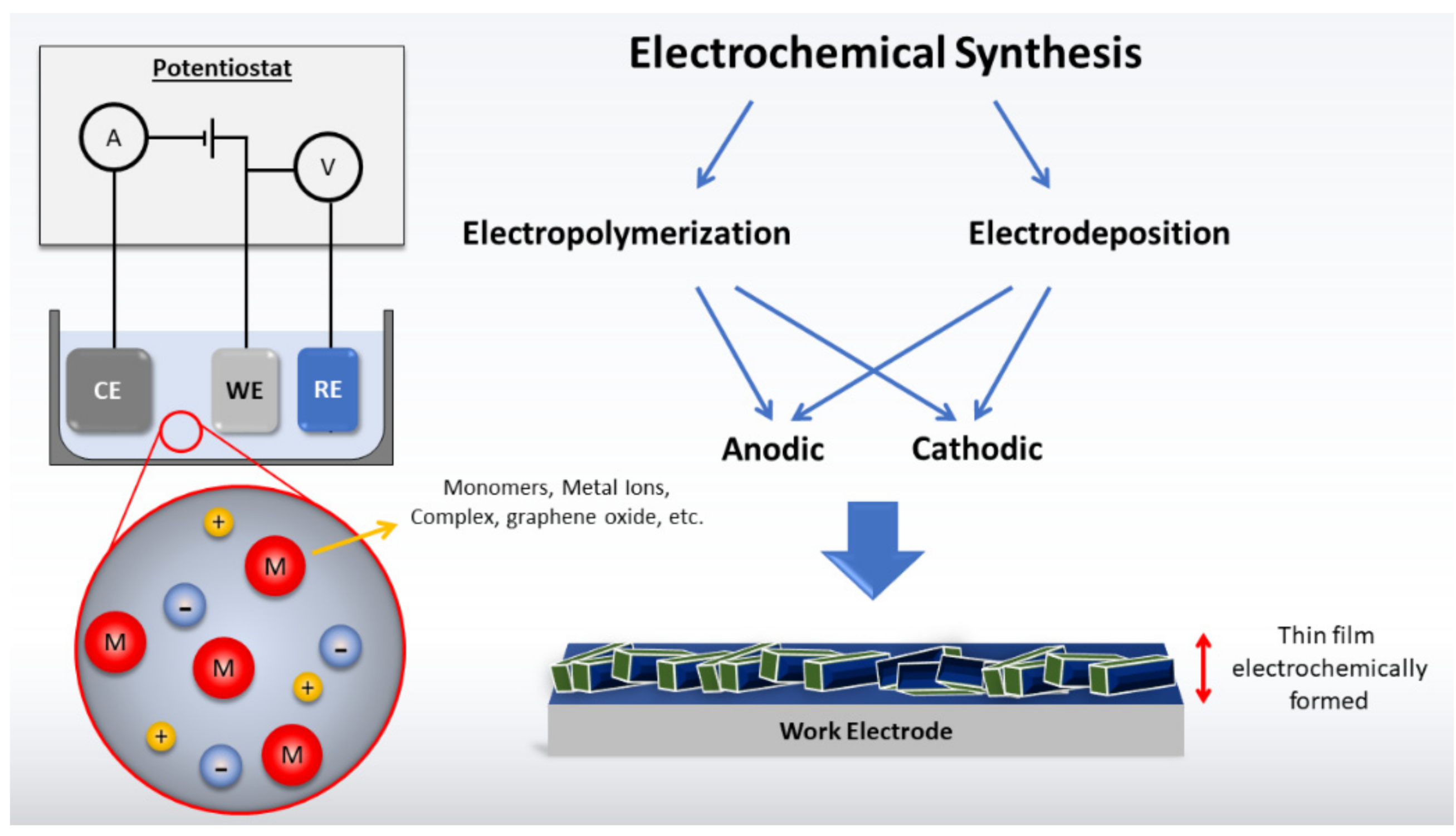
2. Electrochemical Synthesis
2.1. Main Aspects
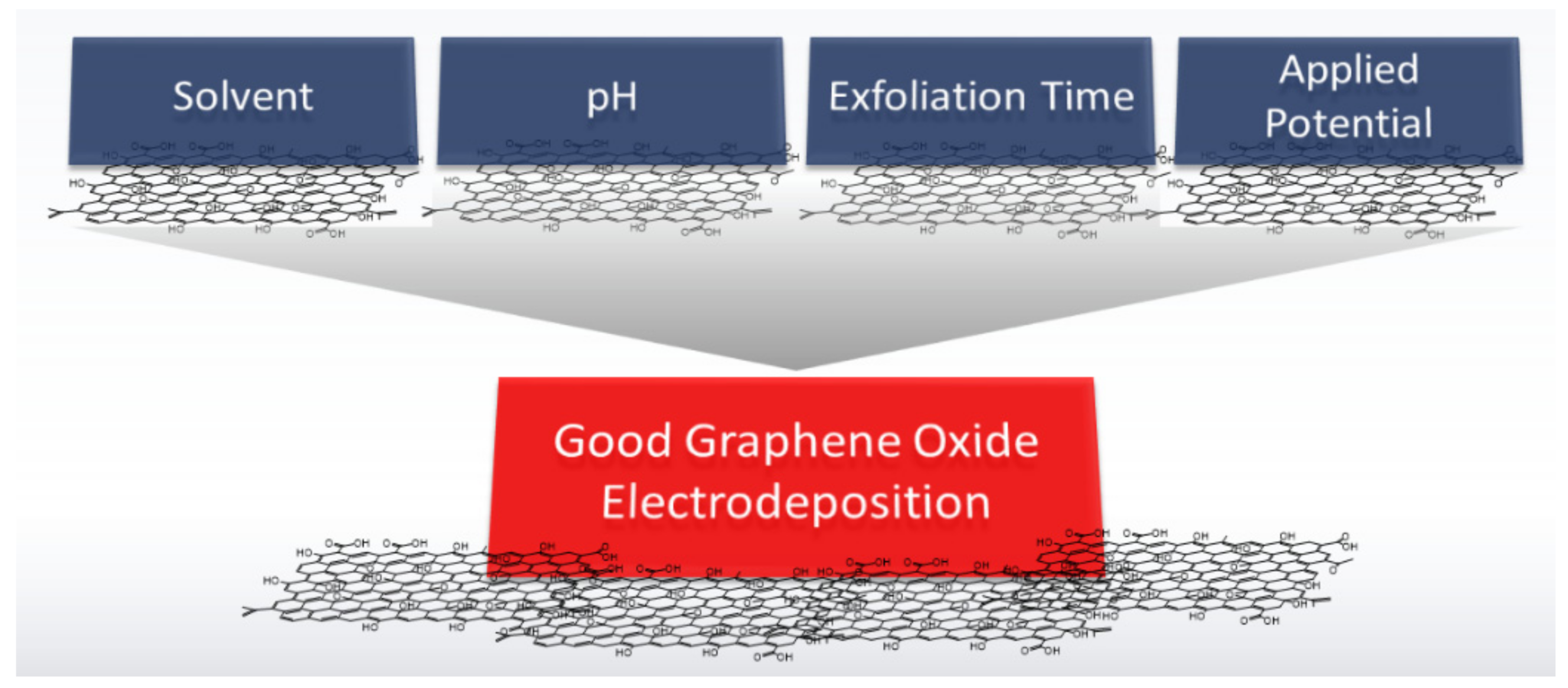
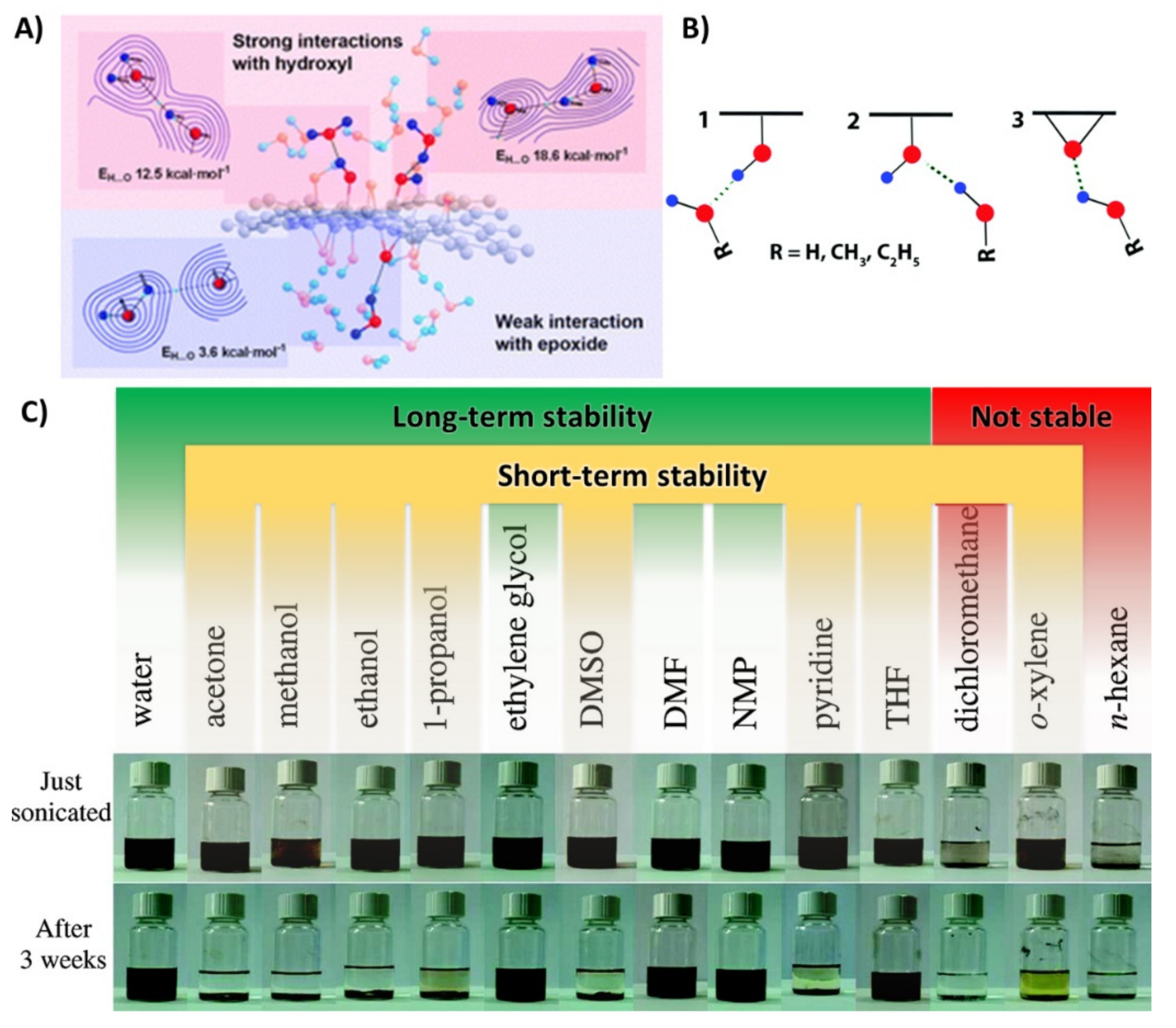
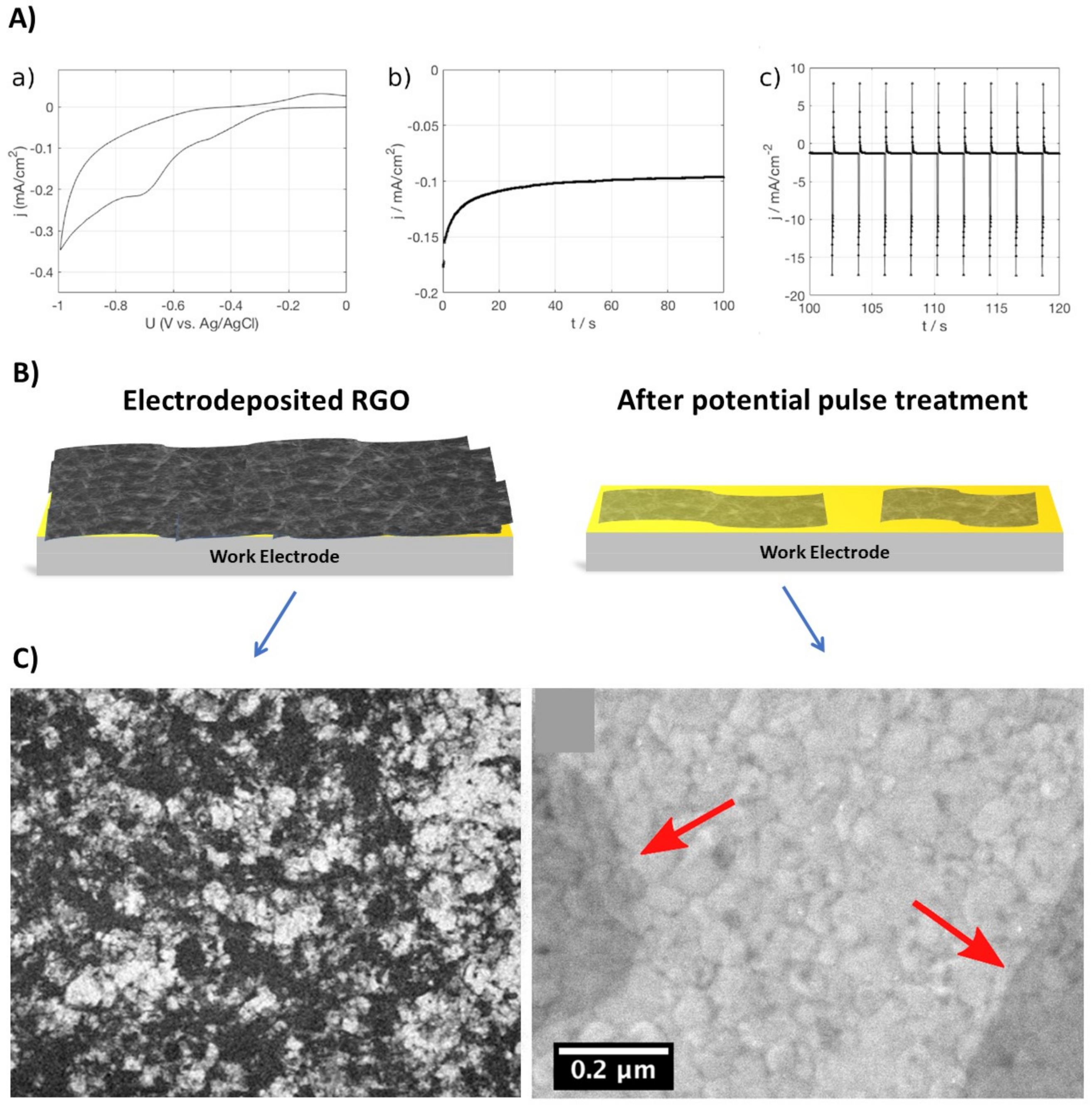
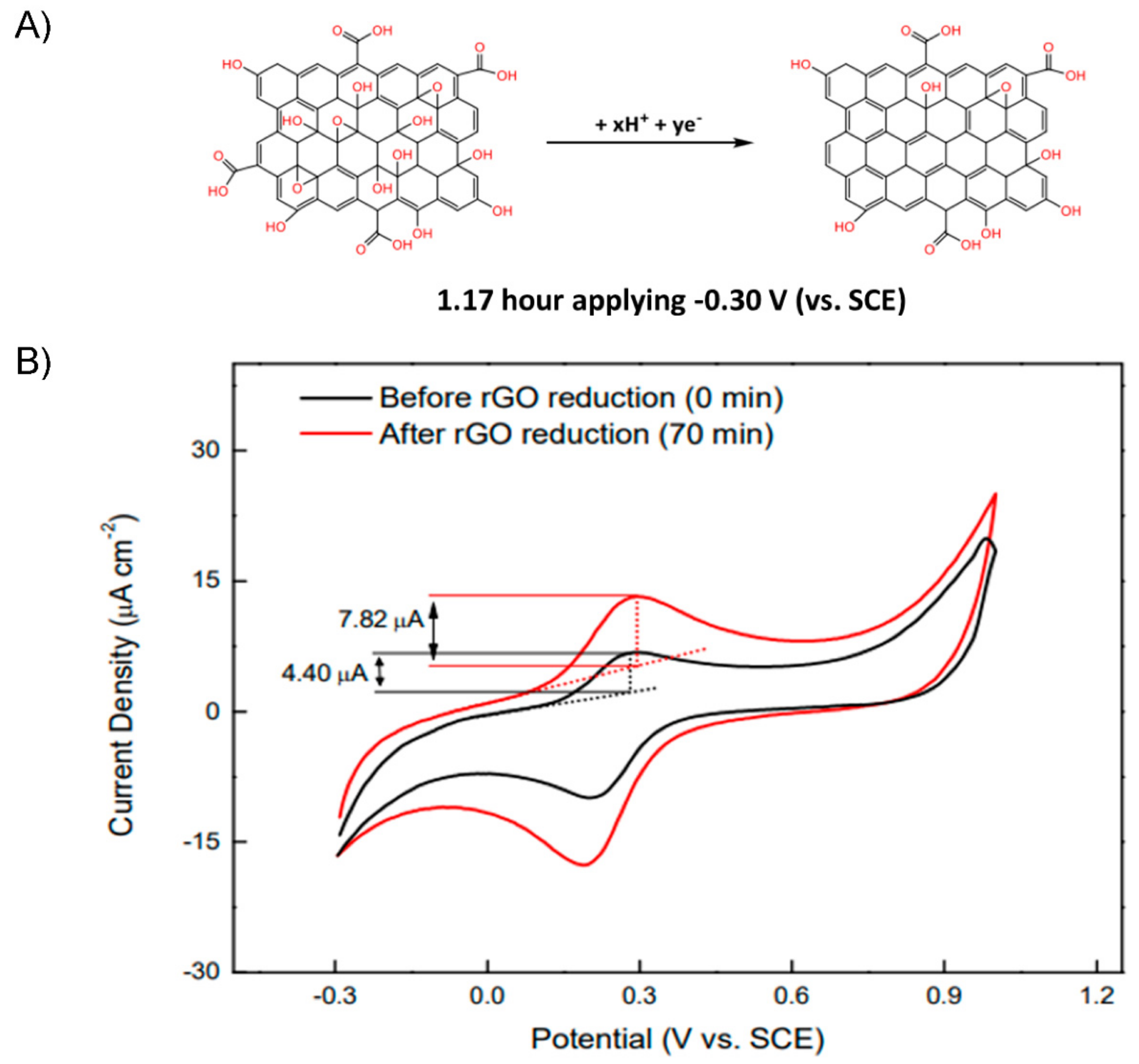
2.2. Electrodeposition of Graphene Oxide
3. Nanocomposite Architecture
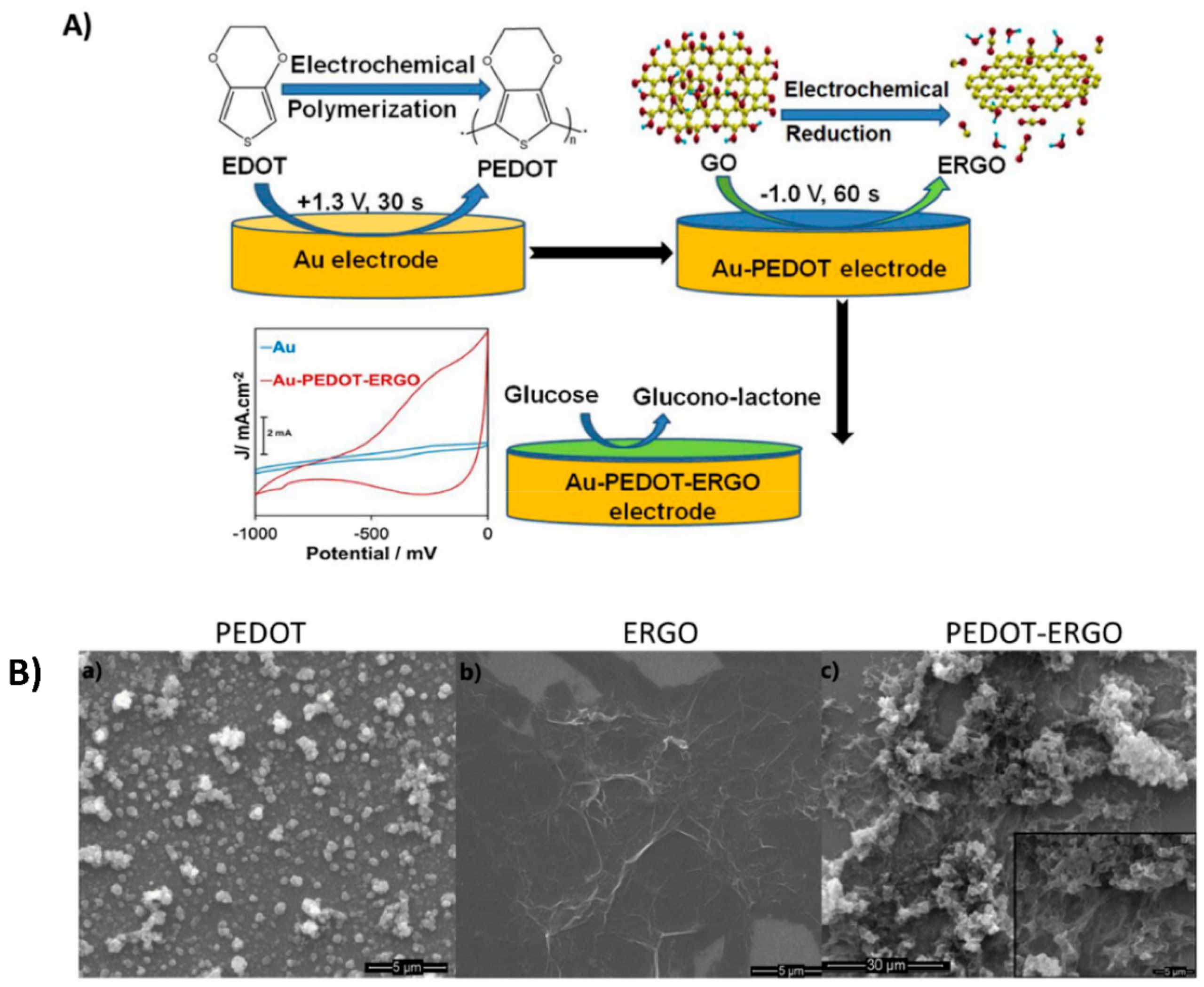
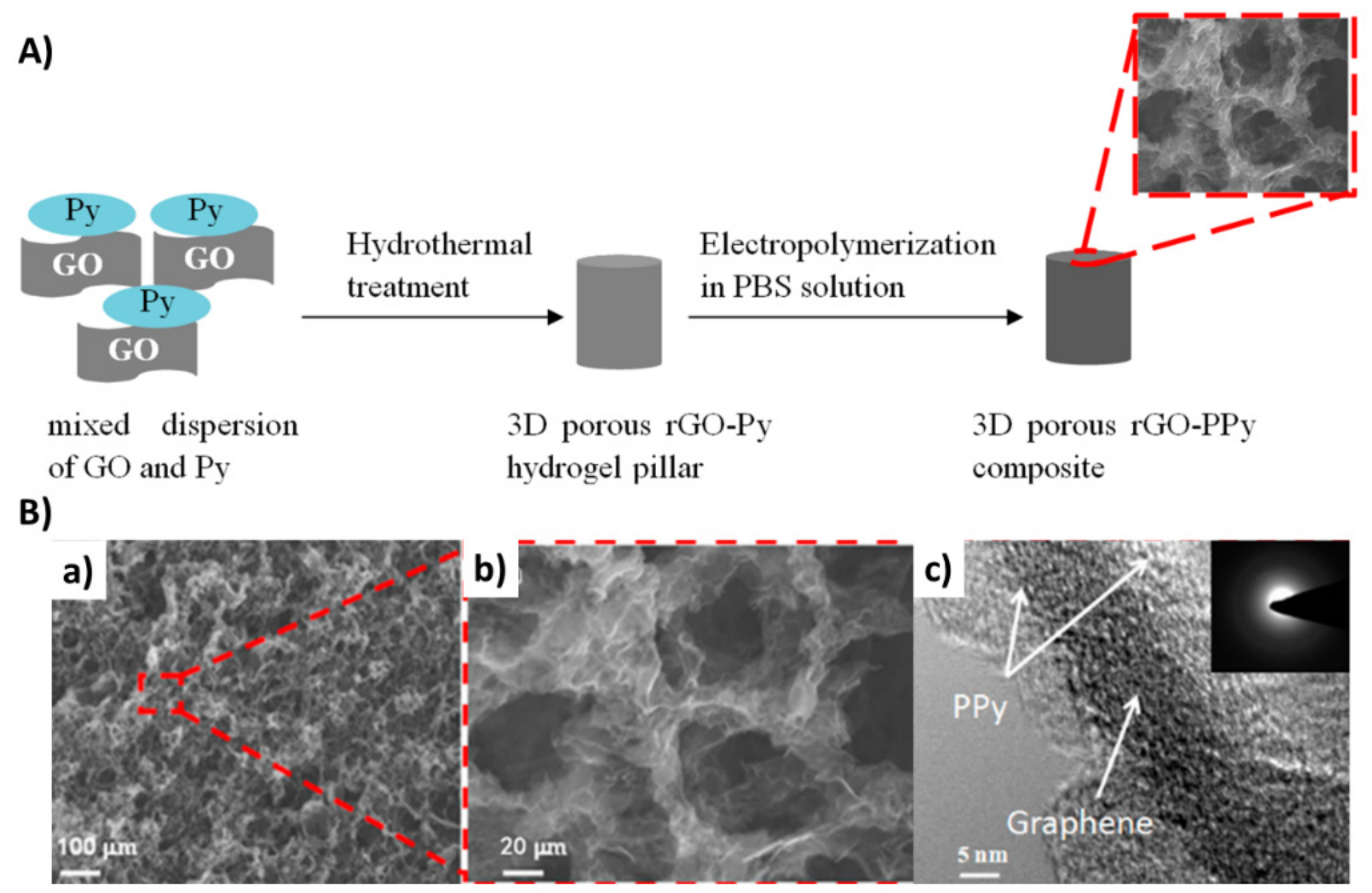
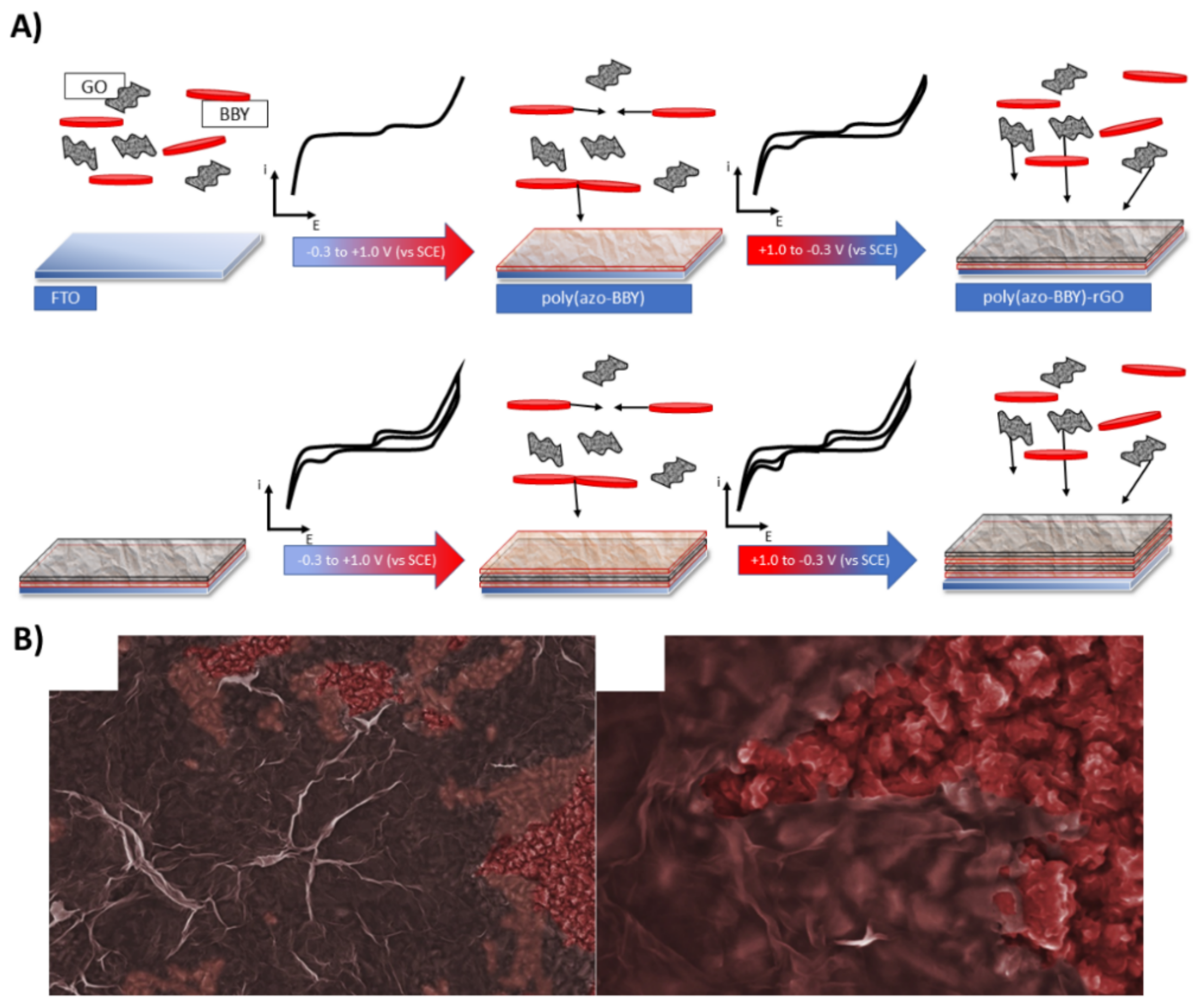
3.1. Multiple-Step Strategy
3.2. One-Step Approach
4. Sensing Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, W. The Chemistry of Graphene Oxide. Graphene Oxide 2015, 61–95. [Google Scholar] [CrossRef]
- Catania, F.; Marras, E.; Giorcelli, M.; Jagdale, P.; Lavagna, L.; Tagliaferro, A.; Bartoli, M. A Review on Recent Advancements of Graphene and Graphene-Related Materials in Biological Applications. Appl. Sci. 2021, 11, 614. [Google Scholar] [CrossRef]
- Olabi, A.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of graphene in energy storage device—A review. Renew. Sustain. Energy Rev. 2021, 135, 110026. [Google Scholar] [CrossRef]
- Hartmann, S.J.; Iurchenkova, A.A.; Kallio, T.; Fedorovskaya, E.O. Electrochemical Properties of Nitrogen and Oxygen Doped Reduced Graphene Oxide. Energies 2020, 13, 312. [Google Scholar] [CrossRef]
- Mouhat, F.; Coudert, F.-X.; Bocquet, M.-L. Structure and chemistry of graphene oxide in liquid water from first principles. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Olean-Oliveira, A.; Brito, G.A.O.; Teixeira, M.F.S. Mechanism of Nanocomposite Formation in the Layer-by-Layer Single-Step Electropolymerization of π-Conjugated Azopolymers and Reduced Graphene Oxide: An Electrochemical Impedance Spectroscopy Study. ACS Omega 2020, 5, 25954–25967. [Google Scholar] [CrossRef]
- Lawal, A.T. Graphene-based nano composites and their applications. A review. Biosens. Bioelectron. 2019, 141, 111384. [Google Scholar] [CrossRef]
- Karimzadeh, A.; Hasanzadeh, M.; Shadjou, N.; de la Guardia, M. Optical bio(sensing) using nitrogen doped graphene quantum dots: Recent advances and future challenges. TrAC Trends Anal. Chem. 2018, 108, 110–121. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Deshmukh, Y.S.; Abdala, A.A. Recent advances in chemical modifications of graphene. Nano Res. 2015, 8, 1039–1074. [Google Scholar] [CrossRef]
- Punetha, V.D.; Rana, S.; Yoo, H.J.; Chaurasia, A.; McLeskey, J.T.; Ramasamy, M.S.; Sahoo, N.G.; Cho, J.W. Functionalization of carbon nanomaterials for advanced polymer nanocomposites: A comparison study between CNT and graphene. Prog. Polym. Sci. 2017, 67, 1–47. [Google Scholar] [CrossRef]
- Xu, X.; Liu, C.; Sun, Z.; Cao, T.; Zhang, Z.; Wang, E.; Liu, Z.; Liu, K. Interfacial engineering in graphene bandgap. Chem. Soc. Rev. 2018, 47, 3059–3099. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Romero, U.A.; Pérez-García, S.A.; Xu, X.; Wang, E.; Licea-Jiménez, L. Functionalized reduced graphene oxide with tunable band gap and good solubility in organic solvents. Carbon 2019, 146, 491–502. [Google Scholar] [CrossRef]
- Hasan, T.; Senger, B.J.; Ryan, C.; Culp, M.; Gonzalez-Rodriguez, R.; Coffer, J.L.; Naumov, A.V. Optical Band Gap Alteration of Graphene Oxide via Ozone Treatment. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Abbaspour, S.; Sadatlu, M.A.A.; Mirkiani, S.; Ghasemi, A.; Hoseini-Ghahfarokhi, M.; Mozaffari, N.; Karimi, M.; Hamblin, M.R. Recent Developments in Graphene and Graphene Oxide: Properties, Synthesis, and Modifications: A Review. ChemistrySelect 2020, 5, 10200–10219. [Google Scholar] [CrossRef]
- Shende, P.; Augustine, S.; Prabhakar, B. A review on graphene nanoribbons for advanced biomedical applications. Carbon Lett. 2020, 30, 465–475. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2011, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M. Graphene-based nanomaterials for energy storage. Energy Environ. Sci. 2010, 4, 668–674. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-Based Supercapacitor with an Ultrahigh Energy Density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef]
- Zhou, X.; Qiao, J.; Yang, L.; Zhang, J. A Review of Graphene-Based Nanostructural Materials for Both Catalyst Supports and Metal-Free Catalysts in PEM Fuel Cell Oxygen Reduction Reactions. Adv. Energy Mater. 2014, 4, 1301523. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Q.; Huang, Y.; Ma, Y.; Yin, S.; Zhang, X.; Sun, W.; Chen, Y. Organic Photovoltaic Devices Based on a Novel Acceptor Material: Graphene. Adv. Mater. 2008, 20, 3924–3930. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-Based Materials: Synthesis, Characterization, Properties, and Applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Aliyev, E.; Filiz, V.; Khan, M.M.; Lee, Y.J.; Abetz, C.; Abetz, V. Structural Characterization of Graphene Oxide: Surface Functional Groups and Fractionated Oxidative Debris. Nanomaterials 2019, 9, 1180. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Kim, J.N.; Choi, H.J. Graphene Oxide and Its Inorganic Composites: Fabrication and Electrorheological Response. Materials 2019, 12, 2185. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, L.; Li, S.; Huang, S.; Sun, Y.; Chen, Y.; Wang, Z.; Liu, W.; Li, X. One-step electroreduction preparation of multilayered reduced graphene oxide/gold-palladium nanohybrid as a proficient electrocatalyst for development of sensitive hydrazine sensor. J. Colloid Interface Sci. 2020, 566, 473–484. [Google Scholar] [CrossRef]
- Zamiri, G.; Haseeb, A.S.M.A. Recent Trends and Developments in Graphene/Conducting Polymer Nanocomposites Chemiresistive Sensors. Materials 2020, 13, 3311. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Su, Z.; Wei, G. Recent advances in the synthesis and applications of graphene–polymer nanocomposites. Polym. Chem. 2015, 6, 6107–6124. [Google Scholar] [CrossRef]
- Sun, X.; Huang, C.; Wang, L.; Liang, L.; Cheng, Y.; Fei, W.; Li, Y. Recent Progress in Graphene/Polymer Nanocomposites. Adv. Mater. 2021, 33, 2001105. [Google Scholar] [CrossRef]
- Wang, M.; Duan, X.; Xu, Y.; Duan, X. Functional Three-Dimensional Graphene/Polymer Composites. ACS Nano 2016, 10, 7231–7247. [Google Scholar] [CrossRef]
- Wang, J.; Jin, X.; Li, C.; Wang, W.; Wu, H.; Guo, S. Graphene and graphene derivatives toughening polymers: Toward high toughness and strength. Chem. Eng. J. 2019, 370, 831–854. [Google Scholar] [CrossRef]
- Barakzehi, M.; Montazer, M.; Sharif, F.; Norby, T.; Chatzitakis, A. A textile-based wearable supercapacitor using reduced graphene oxide/polypyrrole composite. Electrochim. Acta 2019, 305, 187–196. [Google Scholar] [CrossRef]
- Aguilar, L.E. Nanotechnology in improving medical devices for smart drug delivery. In Biomimetic Nanoengineered Materials for Advanced Drug Delivery; Unnithan, A.R., Sasikala, A.R.K., Park, C.H., Kim, A.D.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 73–90. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, H.; Gu, C.; Ma, Y. Electrochemical polymerization: An emerging approach for fabricating high-quality luminescent films and super-resolution OLEDs. J. Mater. Chem. C 2020, 8, 5310–5320. [Google Scholar] [CrossRef]
- Cosnier, S. Biomolecule immobilization on electrode surfaces by entrapment or attachment to electrochemically polymerized films. A review. Biosens. Bioelectron. 1999, 14, 443–456. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2020, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Kaneto, K.; Yoshino, K. Dependences of electrical and mechanical properties of conducting polypyrrole films on conditions of electrochemical polymerization in an aqueous medium. Synth. Met. 1986, 14, 289–296. [Google Scholar] [CrossRef]
- Bidan, G. Electroconducting conjugated polymers: New sensitive matrices to build up chemical or electrochemical sensors. A review. Sens. Actuators B Chem. 1992, 6, 45–56. [Google Scholar] [CrossRef]
- Varsha, M.V.; Nageswaran, G. Review—Direct Electrochemical Synthesis of Metal Organic Frameworks. J. Electrochem. Soc. 2020, 167, 155527. [Google Scholar] [CrossRef]
- Yu, Y.; Zhong, J.; Xu, K.; Yuan, Y.; Ye, K. Recent Advances in the Electrochemical Synthesis and Functionalization of Indole Derivatives. Adv. Synth. Catal. 2020, 362, 2102–2119. [Google Scholar] [CrossRef]
- Chen, Z.; Villani, E.; Inagi, S. Recent progress in bipolar electropolymerization methods toward one-dimensional conducting polymer structures. Curr. Opin. Electrochem. 2021, 28, 100702. [Google Scholar] [CrossRef]
- Barroso, G.; Li, Q.; Bordia, R.K.; Motz, G. Polymeric and ceramic silicon-based coatings—A review. J. Mater. Chem. A 2018, 7, 1936–1963. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Cao, J.; Liu, Y.; Zhou, Y.; Ouyang, J.-H.; Jia, D. Facile Co-Electrodeposition Method for High-Performance Supercapacitor Based on Reduced Graphene Oxide/Polypyrrole Composite Film. ACS Appl. Mater. Interfaces 2016, 9, 19831–19842. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Ren, W.; Wang, D.-W.; Li, F.; Liu, B.; Cheng, H.-M. High-Energy MnO2Nanowire/Graphene and Graphene Asymmetric Electrochemical Capacitors. ACS Nano 2010, 4, 5835–5842. [Google Scholar] [CrossRef]
- Zhou, H.; Han, G.; Xiao, Y.; Chang, Y.; Zhai, H.-J. Facile preparation of polypyrrole/graphene oxide nanocomposites with large areal capacitance using electrochemical codeposition for supercapacitors. J. Power Sources 2014, 263, 259–267. [Google Scholar] [CrossRef]
- Hilder, M.; Winther-Jensen, B.; Li, D.; Forsyth, M.; Macfarlane, D.R. Direct electro-deposition of graphene from aqueous suspensions. Phys. Chem. Chem. Phys. 2011, 13, 9187–9193. [Google Scholar] [CrossRef]
- Chen, L.; Tang, Y.; Wang, K.; Liu, C.; Luo, S. Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem. Commun. 2011, 13, 133–137. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Xu, Y.; Wang, Y.; Wu, L.; Weng, X.; You, C.; Feng, J.-J. A one-step electrochemically reduced graphene oxide based sensor for sensitive voltammetric determination of furfural in milk products. Anal. Methods 2021, 13, 56–63. [Google Scholar] [CrossRef]
- Neklyudov, V.V.; Khafizov, N.R.; Sedov, I.A.; Dimiev, A.M. New insights into the solubility of graphene oxide in water and alcohols. Phys. Chem. Chem. Phys. 2017, 19, 17000–17008. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascon, J.M.D. Graphene Oxide Dispersions in Organic Solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef]
- Konkena, B.; Vasudevan, S. Understanding Aqueous Dispersibility of Graphene Oxide and Reduced Graphene Oxide through pKa Measurements. J. Phys. Chem. Lett. 2012, 3, 867–872. [Google Scholar] [CrossRef]
- Bakhshandeh, R.; Shafiekhani, A. Ultrasonic waves and temperature effects on graphene structure fabricated by electrochemical exfoliation method. Mater. Chem. Phys. 2018, 212, 95–102. [Google Scholar] [CrossRef]
- Mellado, C.; Figueroa, T.; Baez, R.; Meléndrez, M.; Fernández, K. Effects of probe and bath ultrasonic treatments on graphene oxide structure. Mater. Today Chem. 2019, 13, 1–7. [Google Scholar] [CrossRef]
- Shih, C.-J.; Lin, S.; Sharma, R.; Strano, M.S.; Blankschtein, D. Understanding the pH-Dependent Behavior of Graphene Oxide Aqueous Solutions: A Comparative Experimental and Molecular Dynamics Simulation Study. Langmuir 2012, 28, 235–241. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Y.; Hou, W.; Zhou, J.; Song, L. Effects of particle size and pH value on the hydrophilicity of graphene oxide. Appl. Surf. Sci. 2013, 273, 118–121. [Google Scholar] [CrossRef]
- Man, S.C.; Ly, D.; Whittaker, M.R.; Thickett, S.C.; Zetterlund, P.B. Nano-sized graphene oxide as sole surfactant in miniemulsion polymerization for nanocomposite synthesis: Effect of pH and ionic strength. Polymers 2014, 55, 3490–3497. [Google Scholar] [CrossRef]
- Ai, Y.; Liu, Y.; Lan, W.; Jin, J.; Xing, J.; Zou, Y.; Zhao, C.; Wang, X. The effect of pH on the U(VI) sorption on graphene oxide (GO): A theoretical study. Chem. Eng. J. 2018, 343, 460–466. [Google Scholar] [CrossRef]
- Toh, S.Y.; Loh, K.S.; Kamarudin, S.K.; Daud, W.R.W. Graphene production via electrochemical reduction of graphene oxide: Synthesis and characterisation. Chem. Eng. J. 2014, 251, 422–434. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Luo, S.; Tang, Y.; Chen, L. Direct Electrodeposition of Graphene Enabling the One-Step Synthesis of Graphene-Metal Nanocomposite Films. Small 2011, 7, 1203–1206. [Google Scholar] [CrossRef]
- Oliveira, Y.A.; Olean-Oliveira, A.; Teixeira, M.F. Short communication: Molecular architecture based on palladium-salen complex/graphene for low potential water oxidation. J. Electroanal. Chem. 2021, 880, 114928. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.M.; Tang, L.; Chen, J.; Zhu, Y.; He, X.X.; He, Y. Electrochemical Sensor Based on Electrodeposited Graphene-Au Modified Electrode and NanoAu Carrier Amplified Signal Strategy for Attomolar Mercury Detection. Anal. Chem. 2014, 87, 989–996. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, M.; He, H.; Cai, Z.; Gao, N.; He, C.; Chang, G.; Wang, X.; He, Y. Highly sensitive nitrite sensor based on AuNPs/RGO nanocomposites modified graphene electrochemical transistors. Biosens. Bioelectron. 2019, 146, 111751. [Google Scholar] [CrossRef]
- Nia, P.M.; Jenatabadi, H.S.; Woi, P.M.; Abouzari-Lotf, E.; Alias, Y. The optimization of effective parameters for electrodeposition of reduced graphene oxide through Taguchi method to evaluate the charge transfer. Measurement 2019, 137, 683–690. [Google Scholar] [CrossRef]
- Carrizo, R.; Ramírez, D.; Hernández, L.; Lobos, G.; Häberle, P.; Dalchiele, E.A.; Riveros, G. Electrodeposition and Characterization of a Tin Sulfide-Electrochemically Reduced Graphene Oxide Heterojunction. ChemElectroChem 2019, 6, 1047–1056. [Google Scholar] [CrossRef]
- Alonso, R.M.; San-Martín, M.I.; Sotres, A.; Escapa, A. Graphene oxide electrodeposited electrode enhances start-up and selective enrichment of exoelectrogens in bioelectrochemical systems. Sci. Rep. 2017, 7, 13726. [Google Scholar] [CrossRef]
- Shukla, R.P.; Cazelles, R.; Kelly, D.L.; Ben-Yoav, H. A reduced-graphene oxide-modified microelectrode for a repeatable detection of antipsychotic clozapine using microliters-volumes of whole blood. Talanta 2020, 209, 120560. [Google Scholar] [CrossRef]
- Pruna, A.I.; Rosas-Laverde, N.M.; Mataix, D.B. Effect of Deposition Parameters on Electrochemical Properties of Polypyrrole-Graphene Oxide Films. Materials 2020, 13, 624. [Google Scholar] [CrossRef]
- Della Noce, R.; Eugénio, S.; Siwek, K.; Silva, T.; Carmezim, M.; Sakita, A.; Lavall, R.; Montemor, M. Direct electrodeposition of hydrogenated reduced graphene oxide from unsonicated solution and its electrochemical response. Diam. Relat. Mater. 2020, 104, 107740. [Google Scholar] [CrossRef]
- Olean-Oliveira, A.; Olean-Oliveira, T.; Moreno, A.C.R.; Seraphim, P.M.; Teixeira, M.F.S. A Chemiresistor Sensor Based on Azo-Polymer and Graphene for Real-Time Monitoring of Mitochondrial Oxygen Consumption. ACS Sens. 2018, 4, 118–125. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, J.-G. Electroplating of reduced-graphene oxide on austenitic stainless steel to prevent hydrogen embrittlement. Int. J. Hydrog. Energy 2017, 42, 27428–27437. [Google Scholar] [CrossRef]
- Golkhatmi, S.Z.; Khalaj, M.; Izadpanahi, A.; Sedghi, A. One-step electrodeposition synthesis of high performance Graphene/Cu2O nanocomposite films on copper foils as binder-free supercapacitor electrodes. Solid State Sci. 2020, 106, 106336. [Google Scholar] [CrossRef]
- Li, Y.; Martens, I.; Cheung, K.C.; Bizzotto, D. Electrodeposition of reduced graphene oxide onto gold electrodes: Creating thin electrochemically active and optically transparent overlayers. Electrochim. Acta 2019, 319, 649–656. [Google Scholar] [CrossRef]
- Guex, L.G.; Sacchi, B.; Peuvot, K.F.; Andersson, R.L.; Pourrahimi, A.M.; Ström, V.; Farris, S.; Olsson, R.T. Experimental review: Chemical reduction of graphene oxide (GO) to reduced graphene oxide (rGO) by aqueous chemistry. Nanoscale 2017, 9, 9562–9571. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Highly conductive chemically converted graphene prepared from mildly oxidized graphene oxide. J. Mater. Chem. 2011, 21, 7376–7380. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene Oxide, Highly Reduced Graphene Oxide, and Graphene: Versatile Building Blocks for Carbon-Based Materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.L.; Timm, R.A.; Kubota, L.T.; Bonacin, J.A. Modulation of Electrochemical Properties of Graphene Oxide by Photochemical Reduction Using UV-Light Emitting Diodes. ChemistrySelect 2016, 1, 1168–1175. [Google Scholar] [CrossRef]
- Wang, J.; Hu, J.; Hu, S.; Gao, G.; Song, Y. A Novel Electrochemical Sensor Based on Electropolymerized Ion Imprinted PoPD/ERGO Composite for Trace Cd(II) Determination in Water. Sensors 2020, 20, 1004. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Chawla, S.; Pundir, C.; Jain, U. An electrochemical sensor for detection of neurotransmitter-acetylcholine using metal nanoparticles, 2D material and conducting polymer modified electrode. Biosens. Bioelectron. 2017, 89, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Liang, J.; Zhao, Z.; Liu, A.; Tian, Y. An electrochemical bisphenol A sensor based on one step electrochemical reduction of cuprous oxide wrapped graphene oxide nanoparticles modified electrode. Talanta 2017, 169, 37–43. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Feng, Y.; Feng, W. Electropolymerization of graphene oxide/polyaniline composite for high-performance supercapacitor. Electrochim. Acta 2013, 90, 95–100. [Google Scholar] [CrossRef]
- Chen, X.; Li, D.; Ma, W.; Yang, T.; Zhang, Y.; Zhang, D. Preparation of a glassy carbon electrode modified with reduced graphene oxide and overoxidized electropolymerized polypyrrole, and its application to the determination of dopamine in the presence of ascorbic acid and uric acid. Microchim. Acta 2019, 186, 407. [Google Scholar] [CrossRef]
- Eryiğit, M.; Çepni, E.; Urhan, B.K.; Doğan, H.Ö.; Özer, T. Öznülüer Nonenzymatic glucose sensor based on poly(3,4-ethylene dioxythiophene)/electroreduced graphene oxide modified gold electrode. Synth. Met. 2020, 268, 116488. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Ma, W.; Chen, X.; Zhang, Y. Electrodeposited reduced graphene oxide incorporating polymerization of l-lysine on electrode surface and its application in simultaneous electrochemical determination of ascorbic acid, dopamine and uric acid. Mater. Sci. Eng. C 2017, 70, 241–249. [Google Scholar] [CrossRef]
- Layek, R.K.; Nandi, A.K. A review on synthesis and properties of polymer functionalized graphene. Polymers 2013, 54, 5087–5103. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, W.; Yao, H.; Li, S.; Wu, Y.; Zhu, J.; Jiang, L. Solution Adsorption Formation of a π-Conjugated Polymer/Graphene Composite for High-Performance Field-Effect Transistors. Adv. Mater. 2017, 30, 1705377. [Google Scholar] [CrossRef]
- Ghosh, A.; Jana, B.; Maiti, S.; Bera, R.K.; Ghosh, H.N.; Patra, A. Light Harvesting and Photocurrent Generation in a Conjugated Polymer Nanoparticle-Reduced Graphene Oxide Composite. ChemPhysChem 2017, 18, 1308–1316. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Gao, G.; Xia, S. A Mediated BOD Biosensor Based on Immobilized B. Subtilis on Three-Dimensional Porous Graphene-Polypyrrole Composite. Sensors 2017, 17, 2594. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Q.; Lu, Y.; Ji, D.; Zhang, D.; Wu, J.; Chen, X.; Liu, Q. One step electrochemical deposition and reduction of graphene oxide on screen printed electrodes for impedance detection of glucose. Sens. Actuators B Chem. 2017, 244, 290–298. [Google Scholar] [CrossRef]
- Yang, T.; Kong, Q.; Li, Q.; Wang, X.; Chen, L.; Jiao, K. One-step electropolymerization of xanthurenic acid–graphene film prepared by a pulse potentiostatic method for simultaneous detection of guanine and adenine. Polym. Chem. 2014, 5, 2214–2218. [Google Scholar] [CrossRef]
- Gan, Z.; Song, N.; Zhang, H.; Ma, Z.; Wang, Y.; Chen, C. One-Step Electrofabrication of Reduced Graphene Oxide/Poly(N-methylthionine) Composite Film for High Performance Supercapacitors. J. Electrochem. Soc. 2020, 167, 085501. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, W.; Chang, Y.; Fu, D. Graphene oxide incorporated polypyrrole composite materials: Optimizing the electropolymerization conditions for improved supercapacitive properties. J. Mater. Sci. Mater. Electron. 2018, 30, 1109–1116. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Luan, C.; Wang, H.; Cheng, X.; Fang, L.; Wang, L.; Zhao, B.; Ma, C.; Zhang, H.; et al. Preparation and characterization of palladium/polypyrrole-reduced graphene oxide/foamed nickel composite electrode and its electrochemical dechlorination of triclosan. Arab. J. Chem. 2020, 13, 3963–3973. [Google Scholar] [CrossRef]
- Lv, Z.; Chen, Y.; Wei, H.; Li, F.; Hu, Y.; Wei, C.; Feng, C. One-step electrosynthesis of polypyrrole/graphene oxide composites for microbial fuel cell application. Electrochim. Acta 2013, 111, 366–373. [Google Scholar] [CrossRef]
- Kakaei, K.; Hamidi, M.; Kakaei, N. Simultaneous electro-synthesis of polyaniline graphene nanocomposite in dilute graphene oxide as dopant and aniline by electrochemical method and its high specific capacitance. Mater. Res. Express 2019, 6, 085623. [Google Scholar] [CrossRef]
- Imae, I.; Fujimoto, D.; Zhang, L.; Harima, Y. Electrosynthesis of a multilayer film stacked alternately by poly(3,4-ethylenedioxythiophene) and reduced graphene oxide from aqueous solution. Electrochem. Commun. 2017, 81, 65–69. [Google Scholar] [CrossRef]
- Ly, C.T.; Phan, C.T.; Vu, C.N.; Le, H.S.; Nguyen, T.T.; Tran, D.L.; Le, L.A.; Vu, T.T. Electrodeposition of PEDOT-rGO film in aqueous solution for detection of acetaminophen in traditional medicaments. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 015013. [Google Scholar] [CrossRef]
- Duan, Q.; Wang, L.; Wang, F.; Zhang, H.; Lu, K. Facile One-Step Electrodeposition Preparation of Cationic Pillar [6]arene-Modified Graphene Films on Glassy Carbon Electrodes for Enhanced Electrochemical Performance. Front. Chem. 2020, 8, 430. [Google Scholar] [CrossRef]
- Olean-Oliveira, A.; Pacheco, J.C.; Seraphim, P.M.; Teixeira, M.F. Synergistic effect of reduced graphene oxide/azo-polymer layers on electrochemical performance and application as nonenzymatic chemiresistor sensors for detecting superoxide anion radicals. J. Electroanal. Chem. 2019, 852. [Google Scholar] [CrossRef]
- Olean-Oliveira, A.; Teixeira, M.F. Development of a nanocomposite chemiresistor sensor based on π-conjugated azo polymer and graphene blend for detection of dissolved oxygen. Sens. Actuators B Chem. 2018, 271, 353–357. [Google Scholar] [CrossRef]
- Essousi, H.; Barhoumi, H.; Karastogianni, S.; Girousi, S.T. An Electrochemical Sensor Based on Reduced Graphene Oxide, Gold Nanoparticles and Molecular Imprinted Over-oxidized Polypyrrole for Amoxicillin Determination. Electroanalysis 2020, 32, 1546–1558. [Google Scholar] [CrossRef]
- Tığ, G.A.; Pekyardımcı, Ş. An electrochemical sandwich-type aptasensor for determination of lipocalin-2 based on graphene oxide/polymer composite and gold nanoparticles. Talanta 2020, 210, 120666. [Google Scholar] [CrossRef]
- Phetsang, S.; Jakmunee, J.; Mungkornasawakul, P.; Laocharoensuk, R.; Ounnunkad, K. Sensitive amperometric biosensors for detection of glucose and cholesterol using a platinum/reduced graphene oxide/poly(3-aminobenzoic acid) film-modified screen-printed carbon electrode. Bioelectrochemistry 2019, 127, 125–135. [Google Scholar] [CrossRef]
- Wen, Y.; Liao, X.; Deng, C.; Liu, G.; Yan, Q.; Li, L.; Wang, X. Imprinted voltammetric streptomycin sensor based on a glassy carbon electrode modified with electropolymerized poly(pyrrole-3-carboxy acid) and electrochemically reduced graphene oxide. Microchim. Acta 2017, 184, 935–941. [Google Scholar] [CrossRef]
- Ran, G.; Li, Y.; Xia, Y. Graphene oxide and electropolymerized p-aminobenzenesulfonic acid mixed film used as dopamine and serotonin electrochemical sensor. Mon. Chem. Chem. Mon. 2020, 151, 293–299. [Google Scholar] [CrossRef]
- Ji, Z. Electropolymerized Molecular Imprinting & Graphene Modified Electrode for Detection of Melamine. Int. J. Electrochem. Sci. 2017, 12, 11942–11954. [Google Scholar] [CrossRef]
- Tang, X.; Raskin, J.-P.; Kryvutsa, N.; Hermans, S.; Slobodian, O.; Nazarov, A.N.; Debliquy, M. An ammonia sensor composed of polypyrrole synthesized on reduced graphene oxide by electropolymerization. Sens. Actuators B Chem. 2020, 305, 127423. [Google Scholar] [CrossRef]
- Zaidi, S.A. Utilization of an environmentally-friendly monomer for an efficient and sustainable adrenaline imprinted electrochemical sensor using graphene. Electrochim. Acta 2018, 274, 370–377. [Google Scholar] [CrossRef]
- Nemakal, M.; Aralekallu, S.; Mohammed, I.; Swamy, S.; Sannegowda, L.K. Electropolymerized octabenzimidazole phthalocyanine as an amperometric sensor for hydrazine. J. Electroanal. Chem. 2019, 839, 238–246. [Google Scholar] [CrossRef]
- Pereira, T.C.; Stradiotto, N. Electrochemical sensing of lactate by using an electrode modified with molecularly imprinted polymers, reduced graphene oxide and gold nanoparticles. Microchim. Acta 2019, 186, 764. [Google Scholar] [CrossRef]
- Kumar, D.R.; Dhakala, G.; QuangNguyenab, V.; Shima, J.-J. Molecularly imprinted hornlike polymer@ electrochemically reduced graphene oxide electrode for the highly selective determination of an antiemetic drug. Anal. Chim. Acta 2021, 1141, 71–82. [Google Scholar] [CrossRef]
- Wu, B.; Hou, S.; Xue, Y.; Chen, Z. Electrodeposition–Assisted Assembled Multilayer Films of Gold Nanoparticles and Glucose Oxidase onto Polypyrrole-Reduced Graphene Oxide Matrix and Their Electrocatalytic Activity toward Glucose. Nanomaterials 2018, 8, 993. [Google Scholar] [CrossRef]
- Ghanbari, M.H.; Norouzi, Z. Using a nanocomposite consist of Boron-doped reduced graphene oxide and electropolymerized β-cyclodextrin for Flunitrazepam electrochemical sensor. Microchem. J. 2020, 156, 104994. [Google Scholar] [CrossRef]
- Shohibuddin, I.U.S.; Salim, W.W.A.W. Tailoring the Electrochemical and Morphological Properties of Electropolymerized and Dropcast Reduced Graphene Oxide-Poly(3,4-ethylene dioxythiophene):polystyrenesulfonate Transducers for Ion-Selective Sensors. Proceedings 2020, 60, 11. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, X.; Ma, W.; Yang, T.; Li, D.; Dai, B.; Zhang, Y. Direct electrochemistry of glucose oxidase based on one step electrodeposition of reduced graphene oxide incorporating polymerized l-lysine and its application in glucose sensing. Mater. Sci. Eng. C 2019, 104, 109880. [Google Scholar] [CrossRef] [PubMed]


| Electrochemical Approach | Platform | Analyte | Limit of Detection | Ref |
|---|---|---|---|---|
| One-step | P(ATT)-GO a | Lipocalin-2 | 0.3 nmol mL−1 | [98] |
| Pt/rGO/P3ABA b | Glucose/Cholesterol | 44.3/40.5 μmol L−1 | [99] | |
| Poly(BBY)-rGO c | Dissolved Oxygen | 0.36 μmol L−1 | [100] | |
| Two-steps | PPy3C/ERGO d | Streptomycin | 0.5 nmol L−1 | [97] |
| GO-poly(p-ABSA) e | Dopamine/Serotonin | 0.09/0.2 μmol L−1 | [101] | |
| MIPPy/GR/GCE f | Melamine | 10.2 nmol L−1 | [102] | |
| PPy/rGO | NH3 | 1 ppm | [103] | |
| Multiple steps | MIP-rGO/GCE | Adrenaline | 3 nmol L−1 | [104] |
| r-GO/poly(CoOBImPc) g | Hydrazine | 33 nmol L−1 | [105] | |
| MIP/AuNPs/RGO/GCE | Lactic acid | 8.9 × 10−11 mol L−1 | [106] | |
| MIP@ERGO/GC | Domperidone | 3.8 nmol L−1 | [107] | |
| PPy-RGO-(AuNPs-GOD)4/GCE | Glucose | 5.6 μmol L−1 | [108] | |
| Eβ-CD/B-RGO/GCE h | Flunitrazepam | 0.6 nmol L−1 | [109] | |
| rGO:PSS-PEDOT:PSS | Ion-sensor | - | [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olean-Oliveira, A.; Oliveira Brito, G.A.; Cardoso, C.X.; Teixeira, M.F.S. Nanocomposite Materials Based on Electrochemically Synthesized Graphene Polymers: Molecular Architecture Strategies for Sensor Applications. Chemosensors 2021, 9, 149. https://doi.org/10.3390/chemosensors9060149
Olean-Oliveira A, Oliveira Brito GA, Cardoso CX, Teixeira MFS. Nanocomposite Materials Based on Electrochemically Synthesized Graphene Polymers: Molecular Architecture Strategies for Sensor Applications. Chemosensors. 2021; 9(6):149. https://doi.org/10.3390/chemosensors9060149
Chicago/Turabian StyleOlean-Oliveira, André, Gilberto A. Oliveira Brito, Celso Xavier Cardoso, and Marcos F. S. Teixeira. 2021. "Nanocomposite Materials Based on Electrochemically Synthesized Graphene Polymers: Molecular Architecture Strategies for Sensor Applications" Chemosensors 9, no. 6: 149. https://doi.org/10.3390/chemosensors9060149
APA StyleOlean-Oliveira, A., Oliveira Brito, G. A., Cardoso, C. X., & Teixeira, M. F. S. (2021). Nanocomposite Materials Based on Electrochemically Synthesized Graphene Polymers: Molecular Architecture Strategies for Sensor Applications. Chemosensors, 9(6), 149. https://doi.org/10.3390/chemosensors9060149








