Abstract
This work describes a novel L-lactate biosensor based on the immobilization of L-lactate dehydrogenase enzyme on the screen-printed electrode modified with a ternary composite based on gold nanoparticles, electrochemically-reduced graphene oxide, and poly (allylamine hydrochloride). The enzyme was stabilized by crosslinking with glutaraldehyde. Applied working potential, pH and NAD+ concentration were optimized. The biosensor reports a specific sensitivity of 1.08 µA/mM·cm2 in a range up to 3 mM L-lactic acid with a detection limit of 1 µM. The operational and long-term stability as well as good selectivity allowed the L-lactic acid measurement in dairy products and wine samples.
1. Introduction
L-lactate monitoring is widely used in different areas, from clinical diagnosis and sports medicine [1,2] to the wine industry [3] and fermentation processes [4]. L-lactic acid (2-hydroxy propionic acid) is the final product of the anaerobic phase of glycolysis, where pyruvate is converted to lactic acid by lactate dehydrogenase [5]. Under anaerobic conditions the lactate production occurs in all tissue, namely skeletal muscle, kidney, brain and red blood cells [6]. The lactate balance is related to acid–base homeostasis, the accumulation of lactic acid leading to lactic acidosis. In healthcare, L-lactate monitoring is crucial for the identification of metabolic disorders, heart failure and respiratory insufficiency. The monitoring of blood lactic levels is also a very important task in sport medicine, because it allows to evaluate the athlete’s performance following intensive exercise and endurance-based activities [7]. In the fermentation processes, the lactic acid level represents an indicator of the quality, stability and freshness of dairy products, such as creams, yogurt and milk, as well as of raw meat, fruits and vegetables. In wine industry, during the malolactic fermentation, the lactic acid presence leads to the lowering of the wine acidity and tartness.
Various techniques are used for lactic acid determination, including enzymatic and non-enzymatic methods like chemiluminescent [8], high-performance liquid chromatography—ion exclusion chromatography [9] and HPLC with fluorescence detection [10]. However, several drawbacks of these methods like time-consuming steps, invasive procedures and the need of specially trained personnel must be taken into account. Compared to spectrophotometric or chromatographic methods, electrochemical lactate biosensors possess the advantages of being simple, portable, highly selective and user-friendly. They do not require complex sample preparation or treatments and provide a fast response with, direct and real-time information about the L-lactate concentration.
There are mainly two types of L-lactate enzyme biosensors one based on L-lactate dehydrogenase (LDH) [11] and other on L-lactate oxidase (LOD) [12]. L-lactate dehydrogenase-based biosensors are better performing than L-lactate oxidase-based biosensors, in terms of cost, stability and substrate recycling and was extensively used in lactate detection. LDH enzyme has a high catalytic activity in the conversion of lactic acid to pyruvate and β-nicotinamide adenine dinucleotide (NAD+) to its reduced form (NADH), that is electrochemically detected by using an appropriate detector [13].
Several nanomaterials were reported in the development of the L-lactate dehydrogenase-based biosensors aiming the immobilization of L-lactate dehydrogenase through physical or chemical interactions as well as to improve the performances of the NADH detection. Carbon nanotubes [14], polymers [15], mediators [16] or gold nanoparticles [17] are reported in the literature as the immobilization support for LDH. Functionalized reduced graphene oxide (RGO) [18] or graphene oxide nanoparticles [19] were successfully used to covalently attach LDH on the surface of the electrode. Graphene-based nanocomposites have attracted attention recently, being in high demand now. The noble metal nanoparticles are of a great interest for electrochemical applications and have been used lately to decorate graphene-based materials [20]. Metal nanocomposites have an important role in preventing the graphene aggregation by dispersing among the graphene layers and display excellent properties for electrochemical detection of NADH [21]. A sensitive and selective detection of L-lactate was achieved by Azzouzi et al. by using a composite material based on RGO and gold nanoparticles (AuNPs), which improved the performances of the electrochemical detection of NADH [22].
Nevertheless, beside LDH immobilization it is also necessary to increase the performances of the electrochemical detectors mainly their sensitivity and selectivity. In the past two decades, the polyelectrolytes have gained increasing importance in the development of electrochemical biosensors, due to the fact that they are flexible materials that can be tailored for different purposes [23]. The literature reveals that polyelectrolytes are mainly used as coating-materials or as immobilization matrices for biomolecules [24]. The polyelectrolyte layer ensures a high active area, and the electrostatic interaction between NADH and polyelectrolyte could facilitate its accumulation at the electrode surface, resulting in the enhancement of the analytical signal [25].
Graphene based polymer nanocomposites have been recently reviewed and showed interesting properties in various fields including electrochemical sensors [26]. Our group have previously reported the advantages of using polyelectrolytes based nano-composites in the detection of NADH [21,27].
The present article describes the development of an LDH based biosensor for detection of L-lactic acid in real samples. The enzyme was immobilized on screen-printed electrodes modified with a ternary composite based on a polyelectrolyte, graphene and metal nanoparticles. Optimization and characterization of the biosensors was performed by using different electrochemical techniques.
2. Materials and Methods
2.1. Reagents
L-lactic dehydrogenase from rabbit muscle (L-LDH, 25 KU), L- (+)—lactic acid (L-LA), glutaraldehyde (GA, 25 wt% in H2O), nicotinamide adenine dinucleotide hydrate (NAD+), nicotinamide adenine dinucleotide reduced disodium salt (NADH), poly (allylamine hydrochloride) (PAH, MW 15 kDa), gold nanoparticles (AuNPs) (20 nm in diameter) suspension in PBS 0.1 mM were purchased from Sigma Aldrich and were used as received without further purification. Graphene oxide (GO) was purchased from DropSens (ref. GPHOX). All other chemicals were of analytical grade. Phosphate buffer 0.1 M, pH 7.5 was prepared from Na2HPO4 and NaH2PO4 and contains 0.1 M KCl. All solutions were prepared in ultrapure water (Millipore 18 MΩ∙cm).
2.2. Equipment and Materials
Autolab PGSTAT 101 (Metrohm-Autolab B.V., Utrecht, The Netherlands) electrochemical workstation was used for cyclic voltammetry and amperometric measurements. DRP C-110 screen-printed electrodes (SPE) from Dropsens (Metrohm Dropsens, Oviedo, Spain) based on carbon working electrode; silver pseudo-reference electrode and carbon counter electrode were used for the preparation of the LDH biosensor. All potentials are reported vs. pseudo-reference silver electrode. All measurements were performed at room temperature. A magnetic stirrer was used to provide a constant convective transport during the amperometric measurements. Prior to the immobilization of LDH, the enzyme activity was determined spectrometrically using a Shimadzu UV-1650PC UV–VIS spectrophotometer (Shimadzu, Kyoto, Japan) by monitoring the NADH at 340 nm [19]. The AuNPs-GO-PAH nanocomposite was prepared using an Elmasonic X-tra 50 H ultrasonic bath (Elmasonic, Singen, Germany) and Qualitron DW 41-230 centrifuge (Qualitron Inc., Gyeonggi-Do, South Korea), while pH measurements were performed with an InoLab WTW pH 730 pH meter (Inolab WTW, Weilheim, Germany).
2.3. Preparation of GA-LDH/AuNPs-ERGO-PAH/SPE Biosensor
The biosensor construction process is schematically presented in the Figure 1. The preparation of L-LDH biosensor was performed in three steps:
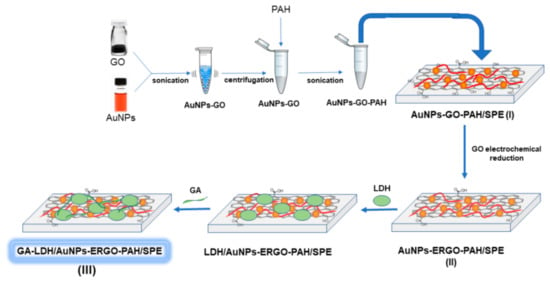
Figure 1.
Schematic representation of the GA-LDH/AuNPs-ERGO-PAH/SPE biosensor preparation.
- AuNPs-GO-PAH composite preparation and deposition on SPE electrode, labelled AuNPs-GO-PAH/SPE;
- Electrochemical reduction of GO on the AuNPs-GO-PAH/SPE modified sensor; and
- Immobilization of L-LDH on the surface of AuNPs-ERGO-PAH/SPE by cross-linking with glutaraldehyde (GA).
The first step was reported elsewhere in a previous publication of our group [27]. Shortly, The AuNPs-GO suspension was prepared by sonicating 1 mL AuNPs suspension with 25 µL aqueous GO suspension (1 mg/mL) for 1 h and followed by centrifugation of this mixture for 30 min. After removal of the supernatant, 80 µg of AuNPs-GO were mixed with 40 µL aqueous PAH solution (0.1 mg/mL) by sonication for 15 min to obtain a stable AuNPs-GO-PAH composite. A volume of 5 µL of this suspension was deposited on the working electrode surface of the SPE sensor and let to dry at the room temperature for 24 h.
The electrochemical reduction of GO (second step) was accomplished by scanning the potential in the range between −1000 and +500 mV in 0.1 M KCl solution, in the absence of O2, for 10 cycles, at a scan rate of 10 mV/s. The method is described elsewhere [21].
In the third step, LDH immobilization was performed by cross-linking with glutaraldehyde. A volume of 5 µL of L-LDH solution (6 IU/electrode) was deposited onto the AuNPs-GO-PAH/SPE and allowed to dry for 5 h at 4 °C. In order to stabilize the enzyme on the surface of the electrode, glutaraldehyde was added as a crosslinking agent. Thus, a volume of 5 µL of 0.1% GA solution (prepared in 0.1 M PBS, pH 7.5) was deposited on the enzyme layer and let to dry at 4 °C for 1 h [28]. The electrode surface was rinsed with ultrapure water to remove any unreacted GA.
3. Results and Discussion
3.1. Optimization of Immobilization Method
One of the most important aspects in the development of a biosensor is the immobilization of the enzyme on the surface of the electrode. In this sense, two immobilization methods were tested.
The first immobilization method consists in the embedding of the L-LDH into the ternary mixture AuNPs-GO-PAH. The L-LDH solution was mixed with PAH solution before the preparation of the AuNPs-GO-PAH composite. In this way the enzyme was incorporated in the composite used for the chemical modification of the SPE. A volume of 5 µL of LDH-AuNPs-GO-PAH (6 IU of LDH/electrode) was deposited onto the surface of the working electrode of SPE and let to dry at 4 °C for 24 h. After drying, the graphene oxide was electrochemically reduced at the surface of LDH-AuNPs-GO-PAH/SPE biosensor using the method described in Section 2.2.
The second method of immobilization, described in Section 2.3, implies the immobilization of the L-LDH by cross-linking with GA, as a separate biocatalytic layer.
Cyclic voltammograms were recorded for both types of biosensors in 5 mM NADH solution on the potential range between +100 and +1000 mV at a scan rate of 100 mV/s. Figure 2A shows variations in the intensity of the NADH oxidation peaks and potentials, depending on the immobilization method used. The LDH-AuNPs-ERGO-PAH/SPE biosensor exhibits an oxidation peak of NADH to a lower potential, at +400 mV, compared to the GA-LDH/AuNPs-ERGO-PAH/SPE biosensor, which shows a oxidation potential at +550 mV. The presence of the second LDH-GA layer, as the biocatalytical layer, can explain the higher over-potential required to oxidize the NADH.
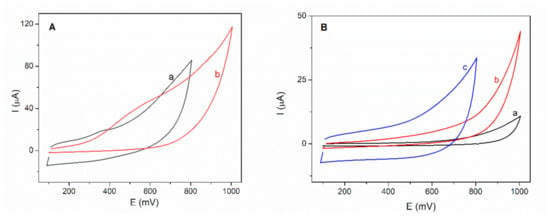
Figure 2.
(A) Cyclic voltammograms recorded in 5 mM NADH for (a) LDH-AuNPs-ERGO-PAH/SPE and (b) GA-LDH/AuNPs-ERGO-PAH/SPE (SR = 100 mV/s, PBS pH = 7.5). (B) Cyclic voltammograms recorded on L-lactic acid 2 mM for (a) AuNPs-GO-PAH/SPE, (b) AuNPs-ERGO-PAH/SPE and (c) GA-LDH/AuNPs-ERGO-PAH/SPE (SR = 100 mV/s, PBS pH = 7.5).
Despite the higher oxidation potential, the peak current is higher in the case of the biosensor based on the enzyme immobilized by crosslinking with GA (3.22 µA) compared to the peak current of the biosensor with the enzyme embedded in the ternary composite (1.22 µA).
Tests on LDH-AuNPs-ERGO-PAH/SPE biosensor for detection of 2 mM L-lactate in the presence of 8 mM NAD+ showed a low oxidation current and a constant decrease of the amperometric response for successive measurements. It was observed a decrease of the initial current (70 nA) with approximately 50% after seven measurements. This behavior can be attributed to the leaking out of the enzyme from the electrode surface.
The immobilization of L-LDH by crosslinking with GA was considered optimal and used for subsequent experiments. Therefore, cyclic voltammograms were recorded after each preparation step of the GA-LDH/AuNPs-ERGO-PAH/SPE biosensor in order to test the response to L-lactic acid. As it is shown in the Figure 2B there is no response to L-lactic acid for the modified SPE electrodes as well as for the LDH based biosensor in the absence of NAD+.
3.2. Optimization of the Working pH
The L-LDH enzyme activity is strongly dependent on the working pH and the chemical equilibrium is shifted to the formation of L-lactate or pyruvate. The activity of the L-LDH was determined spectrometrically by monitoring the formation of NADH at the wavelength of 340 nm in the pH range from 2 to 8 [19]. As it is depicted in the Figure 3, an increase of the enzyme activity was observed in the pH range from 2 to 7.5 reaching a maximum value at pH of 7.5. Therefore, all subsequent studies were realized at pH = 7.5. This value is in the optimal pH range reported in the literature for this enzyme, which is between 6 and 7.8 [29].
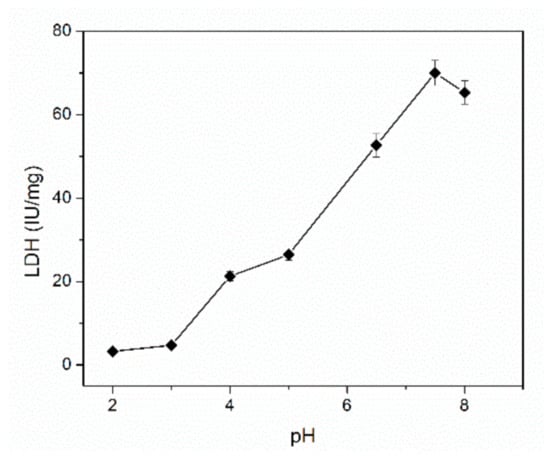
Figure 3.
Influence of pH on the enzymatic activity of L-LDH (n = 3).
3.3. Optimization of the Working Potential
The optimization of working potential was achieved by recording the chronoamperometric response of the biosensor GA-LDH/AuNPs-ERGO-PAH/SPE for 2 mM L-lactic acid solution in the presence of 8 mM NAD+ at different working potentials in the range from +100 mV to +600 mV. The Figure 4 shows a very low response in the range from +100 to +300 mV with a significant increase from +300 to +500 mV. The maximum current was achieved at +500 mV and all further amperometric measurements were realized at this potential.
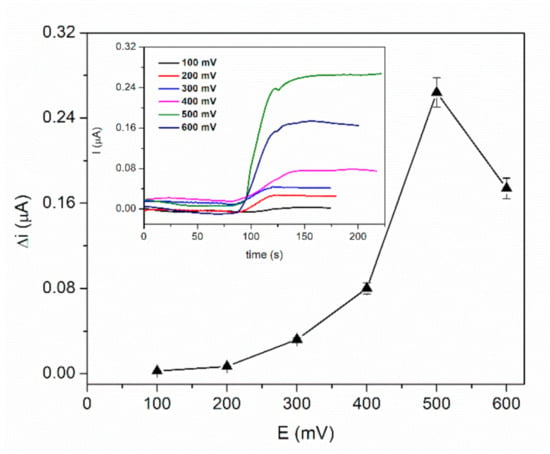
Figure 4.
Influence of working potential on the biosensor response (PBS 0.1 M pH = 7.5, 8 mM NAD+, 2 mM L-lactic acid, n = 3). (inset: chronoamperometric response curves at an applied potential between 100 and 600 mV).
3.4. Optimization of Coenzyme Concentration
L-LDH is a dehydrogenase that catalyses a reversible reaction that imply L-lactate and pyruvate. The oxidation of L-lactate by can be realized in the presence of the coenzyme NAD+, which acts as electrons and H+ ions acceptor. Usually, it is necessary a higher amount of the coenzyme in order to reach the maximal reaction rate of the enzyme reaction and to shift the chemical equilibrium in the direction of L-lactate oxidation. The influence of the NAD+ concentration on the biosensor response was investigated by chronoamperometric measurements. The biosensor response was recorded at an applied potential of +500 mV, in PBS buffer pH = 7.5 containing different concentrations of NAD from 1 to 12 mM after addition of 2 mM L-lactic acid. The response of the biosensor presented in the Figure 5 shows an increase of the signal from 1 to 8 mM NAD+ followed by a decrease of the current at higher concentrations of NAD+. Diffusion issues can explain this behaviour at higher concentrations of NAD+. Consequently, 8 mM NAD+ that ensures the highest sensitivity was the concentration used for biosensor calibration and real samples determinations.
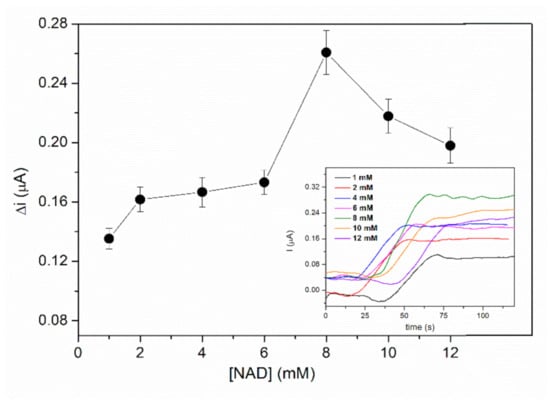
Figure 5.
Influence of NAD+ concentration on the biosensor response (E = +500 mV, PBS 0.1 M pH = 7.5, 2 mM L-lactic acid, n = 3) (inset: chronoamperometric response curves recorded for NAD+ concentrations range 1–12 mM).
3.5. Biosensor Calibration for L-Lactic Acid
Calibration of the GA-LDH/AuNPs-ERGO-PAH/SPE biosensor was performed in a 5 mL cell under continuous stirring, at an applied potential of +500 mV in PBS buffer containing 8 mM NAD+. Successive volumes of lactic acid were added at regular intervals. Figure 6 shows the dynamic response range of the biosensor corresponding to the anodic current measured as a function of the L-lactic acid concentration, where each point represents the mean value for three measurements. Experimental data allowed us to split the dynamic range into two linear domains. The specific sensitivities for two linear domains were calculated. A specific sensitivity of 1.08 ± 0.05 µA/mM·cm2 was calculated for the first linear range between 0.5–3 mM, while for the second linear range assigned to the 4–16 mM domain the specific sensitivity was 0.28 ± 0.03 µA/mM·cm2. The detection limit of the biosensor was estimated to 1 µM (based on signal to noise ratio of 3). Biosensor presents a fast response of about 20 s for the entire L-lactate concentration range.
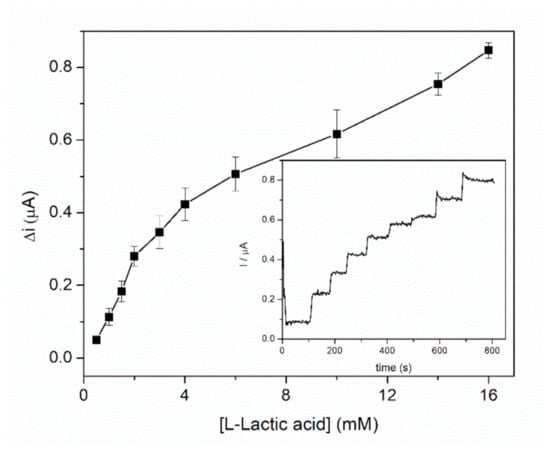
Figure 6.
Calibration graph of GA-LDH/AuNPs-ERGO-PAH/SPE biosensor (E = +500 mV, [NAD+] = 8 mM, PBS 0.1 M pH = 7.5, n = 3) (inset: biosensor response to successive L-lactic acid additions).
The performances of the GA-LDH/AuNPs-ERGO-PAH/SPE biosensor were compared with data from the literature and the results are summarized in the Table 1. The working pH varies between 6.5 and 7.5 for all reported works. As it can be seen, the biosensor presented in this work exhibits one of the largest response ranges, with a low detection limit of 1 µM L-lactate. Specific sensitivities are comparable for references [14,30] and biosensor reported in this paper with a clear advantage related to the response range of our biosensor.

Table 1.
Comparison of the performances of some L-lactate biosensors based on mediators, polymers or nanomaterials.
3.6. Stability of the GA-LDH/AuNPs-ERGO-PAH/SPE Biosensor
Operational stability of the biosensor proposed in this work was studied by performing successive chronoamperometric measurements, every 2 min for 2 mM lactic acid in a buffer solution that contains 8 mM NAD+. Between measurements, the biosensor was rinsed with phosphate buffer. The currents recorded for 10 successive measurements is presented in the Figure 7A. The relative standard deviation (RSD) was calculated to estimate the stability of the biosensor response. The biosensor presents a very good operational stability with RSD = 4.2% for 10 successive measurements.
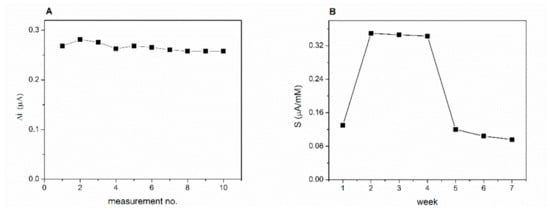
Figure 7.
(A) Operational stability of the GA-LDH/AuNPs-ERGO-PAH/SPE biosensor. (B) Long term stability of the GA-LDH/AuNPs-ERGO-PAH/SPE biosensor (E = +500 mV, [NAD+] = 8 mM, PBS 0.1 M pH = 7.5).
The long-term stability of the biosensor was studied by performing three calibrations curves of the biosensor every week for seven weeks. The biosensor was kept in the desiccator at 4 °C when not in use. A significant 2.7-fold increase in sensitivity was observed in the second week of testing compared to the first week (Figure 7B). The response of the biosensor keeps stable for the next three weeks. Finally, at the end of the seven week the sensitivity reached 75% of the initial value. The same behaviour of the LDH biosensor was reported by Azzouzi et al. [22] where the biosensor showed an increase of the response after one week of storage.
3.7. Selectivity of the GA-L-LDH/AuNPs-ERGO-PAH/SPE Biosensor
One critical aspect to be considered for the analytical application of the biosensor is the potential interfering effect of other compounds present in real samples. Therefore, to test the usefulness of the proposed biosensor in detection of L-lactic acid in real samples (such as yoghurt, wine) a study of the influence of the most common compounds that may be present in these real samples was realized.
GA-LDH/AuNPs-ERGO-PAH/SPE biosensor was tested, in the experimental conditions previously optimized, for detection of 1 mM L-lactic acid in the absence and in the presence of glutamic acid, acetic acid, ascorbic acid, ethanol and glucose. The biosensor response before and after addition of the potential interfering compound is presented in the Figure 8. The potential interfering compounds (I) were tested at the same level of concentration as L-lactic acid (1:1 ratio) and at a concentration 10 times lower than L-lactic acid (1:0.1 ratio). The current ratio expressed as the ratio between the current recorded for 1 mM L-lactic acid in the presence of the interfering compound and the current recorded for 1 mM L-lactic acid are presented in the Table 2.
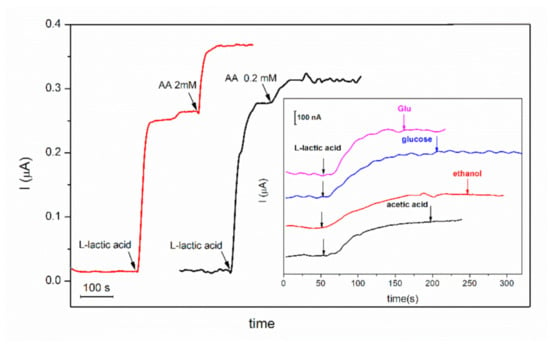
Figure 8.
Biosensors response to successive addition of L-lactic acid 2 mM and ascorbic acid (AA) 2 mM (red line) and 0.2 mM (black line), respectively (inset: biosensor response to successive addition of L-lactic acid 2 mM and acetic acid, ethanol and glucose and glutamic acid (Glu) 0.2 mM; E = +500 mV, [NAD+] = 8 mM, PBS 0.1 M pH = 7.5).

Table 2.
Influence of interfering compounds on the detection of lactic acid.
The experimental results showed that only ascorbic acid significantly interferes in the detection of L-lactic acid when it is at the same level of concentration as L-lactic acid. The results of this study highlight that GA-LDH/AuNPs-ERGO-PAH/SPE biosensor is suitable for measurements of L-lactic acid concentrations in the food products with a low concentration of ascorbic acid such as dairy products and wines.
3.8. Detection of L-Lactic Acid in Food Sample
The GA-LDH/AuNPs-ERGO-PAH/SPE biosensor was tested to determine the concentration of lactic acid in the real samples of yogurt and wine. Prior to chronoamperometric measurements the real sample of yogurt was diluted by factor of 50, while the wine sample was diluted by factor of 25 with PBS 0.1 M pH = 7.5. The yogurt sample was filtered before performing the electrochemical measurements. Amperometric measurements were performed in a 5 mL cell, where volumes of samples from 0.5 to 4 mL were added. Addition of 2.5 mL of diluted yogurt samples and 4 mL of wine sample respectively, provided an analytical signal, that allowed us to determine the concentration of L-lactic acid in the real samples from the first linear calibration graph.
The lactic acid concentrations in yogurt found in trade yogurt and home-made red wine are shown in the Table 3. The R-Biopharm kit, based on the UV detection of NADH in the enzyme oxidation of L-lactate, was used as reference method for determination of L-lactic acid in the same samples. A good correlation between the biosensor and spectrometric method was achieved. This proves that the new developed amperometric biosensor can be successfully used in food quality control.

Table 3.
L-lactic acid determination in yogurt and red wine samples.
4. Conclusions
In this study a new amperometric biosensor for the detection of L-lactic acid based on L-lactate dehydrogenase was developed. The surface of carbon screen-printed electrode was modified with a ternary composite based on gold nanoparticles, electrochemically reduced graphene oxide and poly(allylamine) hydrochloride, that as it has been proved in a previous work, showed good performances for detection of NADH. The enzyme was immobilized on the top by crosslinking with glutaraldehyde. In this way the LDH activity was stabilized and allowed us to use the biosensor up to seven weeks. The GA-LDH/AuNPs-ERGO-PAH/SPE biosensor exhibits a large dynamic response range that includes two linear ranges. The simple manufacturing method, low costs and good analytical performances (high sensibility, low detection limit, good stability and selectivity) recommend this biosensor for applications in the field of quality control of foodstuffs.
Author Contributions
Conceptualization: O.-M.I., L.R. and C.B.; methodology: O.-M.I. and L.R.; validation: C.B. and L.R.; experimental data analysis: O.-M.I. and L.R.; writing—original draft, O.-M.I. and L.R.; writing—review and editing: L.R. and C.B.; project administration: C.B.; funding acquisition: C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Romanian Ministry of Education and Research, CNCS—UEFISCDI, project number PN-III-P4-ID-PCE-2020-0998, within PNCDI III.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Oana-Maria Istrate gratefully acknowledges the ICUB Fellowship no. 15260/2020.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Kuşbaz, A.; Göcek, İ.; Baysal, G.; Kök, F.N.; Trabzon, L.; Kizil, H.; Karagüzel Kayaoğlu, B. Lactate detection by colorimetric measurement in real human sweat by microfluidic-based biosensor on flexible substrate. J. Text. Inst. 2019, 110, 1725–1732. [Google Scholar] [CrossRef]
- Bravo, I.; Revenga-Parra, M.; Pariente, F.; Lorenzo, E. Reagent-Less and Robust Biosensor for Direct Determination of Lactate in Food Samples. Sensors 2017, 17, 144. [Google Scholar] [CrossRef]
- Shkotova, L.; Bohush, A.; Voloshina, I.; Smutok, O.; Dzyadevych, S. Amperometric biosensor modified with platinum and palladium nanoparticles for detection of lactate concentrations in wine. Sn Appl. Sci. 2019, 1, 8. [Google Scholar] [CrossRef]
- Farina, D.; Zinellu, M.; Fanari, M.; Porcu, M.C.; Scognamillo, S.; Puggioni, G.M.G.; Rocchitta, G.; Serra, P.A.; Pretti, L. Development of a biosensor telemetry system for monitoring fermentation in craft breweries. Food Chem. 2017, 218, 479–486. [Google Scholar] [CrossRef]
- Seheult, J.; Fitzpatrick, G.; Boran, G. Lactic acidosis: an update. Clin. Chem. Lab. Med. 2017, 55, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Komesu, A.; Oliveira, J.A.R.d.; Martins, L.H.d.S.; Wolf Maciel, M.R.; Maciel Filho, R. Lactic Acid Production to Purification: A Review. BioResources 2017, 12, 20. [Google Scholar] [CrossRef]
- Fernandes Nascimento, E.M.; Augusta Pedutti Dal Molin Kiss, M.; Meireles Santos, T.; Lambert, M.; Pires, F.O. Determination of Lactate Thresholds in Maximal Running Test by Heart Rate Variability Data Set. Asian J. Sports Med. 2017, 8, e58480. [Google Scholar] [CrossRef]
- Rishi, L.; Yaqoob, M.; Asghar, M.; Nabi, A.; Munawar, N. Flow Injection Determination of Lactate Using Immobilized Lactate Dehydrogenase Enzyme with Tris(2,2 ’-Bipyridyl)Ruthenium(III) Chemiluminescence Detection. Anal. Lett. 2016, 49, 654–664. [Google Scholar] [CrossRef]
- Milagres, M.P.; Brandão, S.C.C.; Magalhães, M.A.; Minim, V.P.R.; Minim, L.A. Development and validation of the high performance liquid chromatography–ion exclusion method for detection of lactic acid in milk. Food Chem. 2012, 135, 1078–1082. [Google Scholar] [CrossRef]
- Hasegawa, H.; Fukushima, T.; Lee, J.-A.; Tsukamoto, K.; Moriya, K.; Ono, Y.; Imai, K. Determination of serum d-lactic and l-lactic acids in normal subjects and diabetic patients by column-switching HPLC with pre-column fluorescence derivatization. Anal. Bioanal. Chem. 2003, 377, 886–891. [Google Scholar] [CrossRef]
- Chan, D.; Barsan, M.M.; Korpan, Y.; Brett, C.M.A. L-lactate selective impedimetric bienzymatic biosensor based on lactate dehydrogenase and pyruvate oxidase. Electrochim. Acta 2017, 231, 209–215. [Google Scholar] [CrossRef]
- Dagar, K.; Pundir, C.S. An improved amperometric L-lactate biosensor based on covalent immobilization of microbial lactate oxidase onto carboxylated multiwalled carbon nanotubes/copper nanoparticles/polyaniline modified pencil graphite electrode. Enzym. Microb. Technol. 2017, 96, 177–186. [Google Scholar] [CrossRef]
- Kucherenko, I.S.; Topolnikova, Y.V.; Soldatkin, O.O. Advances in the biosensors for lactate and pyruvate detection for medical applications: A review. Trac-Trends Anal. Chem. 2019, 110, 160–172. [Google Scholar] [CrossRef]
- Pereira, A.C.; Aguiar, M.R.; Kisner, A.; Macedo, D.V.; Kubota, L.T. Amperometric biosensor for lactate based on lactate dehydrogenase and Meldola Blue coimmobilized on multi-wall carbon-nanotube. Sens. Actuators B Chem. 2007, 124, 269–276. [Google Scholar] [CrossRef]
- Rahman, M.M.; Shiddiky, M.J.A.; Rahman, M.A.; Shim, Y.-B. A lactate biosensor based on lactate dehydrogenase/nictotinamide adenine dinucleotide (oxidized form) immobilized on a conducting polymer/multiwall carbon nanotube composite film. Anal. Biochem. 2009, 384, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Piano, M.; Serban, S.; Pittson, R.; Drago, G.A.; Hart, J.P. Amperometric lactate biosensor for flow injection analysis based on a screen-printed carbon electrode containing Meldola’s Blue-Reinecke salt, coated with lactate dehydrogenase and NAD(+). Talanta 2010, 82, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, J.S.; Slaughter, G. Lactic Acid Biosensor Based on Lactate Dehydrogenase Immobilized on Au Nanoparticle Modified Microwire Electrode. IEEE Sens. J. 2020, 20, 4034–4040. [Google Scholar] [CrossRef]
- Manna, B.; Raj, C.R. Covalent functionalization and electrochemical tuning of reduced graphene oxide for the bioelectrocatalytic sensing of serum lactate. J. Mater. Chem. B 2016, 4, 4585–4593. [Google Scholar] [CrossRef]
- Batra, B.; Narwal, V.; Pundir, C.S. An amperometric lactate biosensor based on lactate dehydrogenase immobilized onto graphene oxide nanoparticles-modified pencil graphite electrode. Eng. Life Sci. 2016, 16, 786–794. [Google Scholar] [CrossRef]
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-warthan, A.A.; Tremel, W. Graphene based metal and metal oxide nanocomposites: synthesis, properties and their applications. J. Mater. Chem. A 2015, 3, 18753–18808. [Google Scholar] [CrossRef]
- Istrate, O.M.; Rotariu, L.; Marinescu, V.E.; Bala, C. NADH sensing platform based on electrochemically generated reduced graphene oxide-gold nanoparticles composite stabilized with poly(allylamine hydrochloride). Sens. Actuators B Chem. 2016, 223, 697–704. [Google Scholar] [CrossRef]
- Azzouzi, S.; Rotariu, L.; Benito, A.M.; Maser, W.K.; Ben Ali, M.; Bala, C. A novel amperometric biosensor based on gold nanoparticles anchored on reduced graphene oxide for sensitive detection of l-lactate tumor biomarker. Biosens. Bioelectron. 2015, 69, 280–286. [Google Scholar] [CrossRef]
- Barsan, M.M.; Ghica, M.E.; Brett, C.M.A. Electrochemical sensors and biosensors based on redox polymer/carbon nanotube modified electrodes: A review. Anal. Chim. Acta 2015, 881, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.Y.; Wang, Y.Y.; Duan, X.X. Biofunctional polyelectrolytes assembling on biosensors—A versatile surface coating method for protein detections. Anal. Chim. Acta 2017, 964, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Rotariu, L.; Istrate, O.-M.; Bala, C. Poly(allylamine hydrochloride) modified screen-printed carbon electrode for sensitive and selective detection of NADH. Sens. Actuators B Chem. 2014, 191, 491–497. [Google Scholar] [CrossRef]
- Lawal, A.T. Recent progress in graphene based polymer nanocomposites. Cogent Chem. 2020, 6, 1833476. [Google Scholar] [CrossRef]
- Istrate, O.M.; Rotariu, L.; Bala, C. Electrochemical determination of NADH using screen printed carbon electrodes modified with reduced graphene oxide and poly(allylamine hydrochloride). Microchim. Acta 2016, 183, 57–65. [Google Scholar] [CrossRef]
- Bilgi, M.; Ayranci, E. Biosensor application of screen-printed carbon electrodes modified with nanomaterials and a conducting polymer: Ethanol biosensors based on alcohol dehydrogenase. Sens. Actuators B Chem. 2016, 237, 849–855. [Google Scholar] [CrossRef]
- Fritz, P.J. Rabbit Lactate Dehydrogenase Isozymes: Effect of pH on Activity. Science 1967, 156, 82–83. [Google Scholar] [CrossRef]
- Teymourian, H.; Salimi, A.; Hallaj, R. Low potential detection of NADH based on Fe3O4 nanoparticles/multiwalled carbon nanotubes composite: Fabrication of integrated dehydrogenase-based lactate biosensor. Biosens. Bioelectron. 2012, 33, 60–68. [Google Scholar] [CrossRef]
- Nesakumar, N.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. Fabrication of lactate biosensor based on lactate dehydrogenase immobilized on cerium oxide nanoparticles. J. Colloid Interface Sci. 2013, 410, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Vargas, E.; Ruiz, M.A.; Campuzano, S.; de Rivera, G.G.; López-Colino, F.; Reviejo, A.J.; Pingarrón, J.M. Implementation of a new integrated d-lactic acid biosensor in a semiautomatic FIA system for the simultaneous determination of lactic acid enantiomers. Application to the analysis of beer samples. Talanta 2016, 152, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ding, S.; Cao, S.; Zhu, A.; Shi, G. Functional surface engineering of quantum dot hydrogels for selective fluorescence imaging of extracellular lactate release. Biosens. Bioelectron. 2016, 80, 315–322. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).