1. Introduction
The molecular imprinting technique is extensively used for various applications such as sorbents and sensors. Molecularly imprinted polymers (MIP) can be formed using this technique by undergoing a thermal/photopolymerization process between the monomers and the crosslinkers in the presence of target molecules to make mimicked cavities in the polymer matrix after template removal [
1,
2]. As a favorable sensing approach, molecular imprinting is widely employed as optical sensors [
3], electrochemical biosensors [
4], and biomimetic sensors [
5,
6] as well as is used for drug extraction applications [
7].
Enormous effort has been invested into the development of MIP-based sensors, for example, for core–shell nanoparticles [
8], lithographical patterns [
9,
10], and organic–inorganic hybrids [
11]. Among them, the lithographic approach is an efficient tool for MIP structuring on sensing surfaces associated with quartz crystal microbalance (QCM) and could be combined with electro-/photochemical polymerization to fabricate specifically designed imprinted films. Various specific templates, from small molecules to large proteins, could be successfully imprinted on sensor platforms [
12,
13,
14,
15,
16]. Recently, our group focused on increasing the sensing response of QCM-based MIP sensors by controlling the surface area of MIP films, which were fabricated using colloidal/soft lithography and photo-/electropolymerization [
17]. Compared to their planar MIP film counterparts, the structured MIP films exhibited enhanced sensing signal response during a limited period owing to the increased surface area into which the specific template diffused in solution. The use of a poly(dimethylsiloxane) (PDMS) replica mold is a ubiquitous lithographic tool for generating MIP films during photopolymerization. However, pre-curing of the MIP precursor solution is required to prevent functional monomers, templates, and crosslinkers from being absorbed into the molds. Moreover, polystyrene (PS) or silica colloids can be used as a master mold to fabricate convex or concave hemispherical patterns [
18]. Despite these lithographic approaches, the occurrence of undesirable nonspecific adsorption on non-imprinted polymer (NIP) thin films seriously affects the sensing parameters (i.e., the imprinting factor (IF) and the selectivity coefficient). In the past, we reported the adsorption of 2,4-dichlorophenoxyacetic (2,4-D) molecules on a template-extracted porous MIP films, which were lithographically fabricated using the silica colloidal array (
d = 500 nm) as a master mold [
19]. In that study, it was revealed that the hydroxyl groups on the surfaces of the silica colloids were involved in monomer–template complex formation during the polymerization process and increased nonspecific adsorption on the surface of the MIP/NIP films, consequently resulting in lowering the imprinting effect.
Thus, solving this problem in the lithographic process necessitates a new experimental strategy to control structural conformation on the surface of the MIP film formed in contact with patterned molds under photoirradiation.
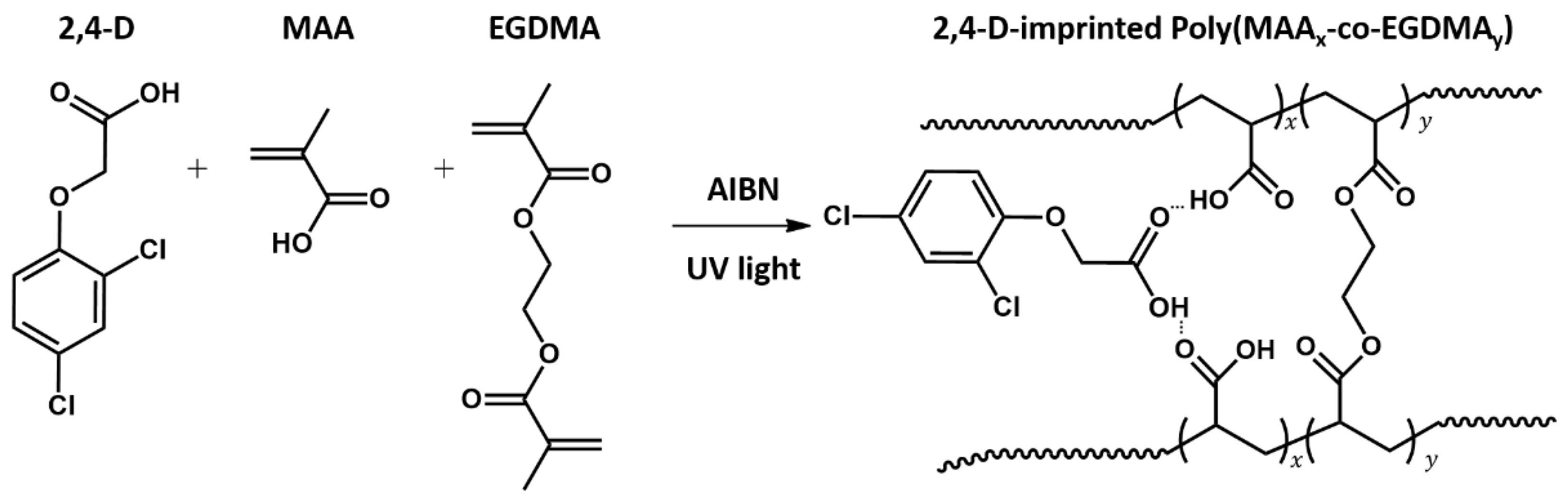
Here, we report a simple strategy to decrease nonspecific binding on the surface of MIP/NIP films, leading to improved sensing properties. To this end, the surface of the structured silica particle arrays as a mold was modified with methylsilane and 2,4-D, which was used as a standard template to manipulate porous MIP thin films during the lithographic process. Fabrication of the porous MIP systems was conducted using methacrylic acid (MAA) as the functional monomer and ethylene glycol dimethacrylate (EGDMA) as the crosslinker via polymerization during microcontact lithography under ultraviolet (UV) exposure. Using a QCM, the sensing properties of the nanopatterned MIP films obtained from the silanized silica molds were measured in situ and compared to those of the MIP films that had been prepared with a non-surface-treated silica mold. Furthermore, the two corresponding NIP films were evaluated to identify differences resulting from the use of the two molds. Finally, other analogous herbicides were employed to investigate a change in selectivity of the two MIP films.
2. Materials and Methods
2.1. Materials
PS latex microspheres (d = 1 μm, 2.5 wt% dispersion in water) with a slight anionic charge were bought from Alfa Aesar Co., Ward Hill, MA, USA. Sodium dodecyl sulfate (SDS, Sigma–Aldrich Co., St. Louis, MO, USA) was used as an anionic surfactant to generate a robust PS colloidal monolayer. The SiO2 precursor was prepared using tetraethyl orthosilicate (TEOS, 98%, reagent grade) and absolute ethanol (99.5%, analytical reagent), which were purchased from Sigma–Aldrich Co. (St. Louis, MO, USA). Trimethoxy(methyl)silane (TMMS, Tokyo Chemical Industry Co., Tokyo, Japan) was used for methylation on the surface of SiO2. MAA, 2,4-D, and EGDMA were purchased from Tokyo Chemical Industry Co. (Tokyo, Japan) to be utilized as the functional monomer, template, and crosslinker, respectively. Dimethylformamide (DMF, ≥99%, Sigma–Aldrich Co., St. Louis, MO, USA) and 2,2′-azobisisobutyronitrile (AIBN, Daejung Chemicals & Metals Co. Ltd., Siheung, South Korea) were used as the solvent and photoinitiator, respectively. Other analogous chemicals such as atrazine (Sigma–Aldrich Co., St. Louis, MO, USA), ametryn (Sigma–Aldrich Co., St. Louis, MO, USA), and (4-chloro-2-methylphenoxy) acetic acid (MCPA, Tokyo Chemical Industry Co., Tokyo, Japan) were utilized for the selectivity test. Acetic acid and methanol were obtained from Duksan Co. Ltd., Ansan, South Korea. All other chemicals were used as received.
2.2. Close-Packed Arrangement of PS Microspheres
Close-packed PS microspheres were prepared on a glass substrate as per a previously reported technique [
17]. Glass substrates were ultrasonicated for 5 min in acetone, ethanol, and DI water, sequentially, and dried under nitrogen (N
2) gas. Next, these substrates were immersed in a piranha mixture (H
2SO
4:H
2O
2, 3:1 v/v) for 40 min to remove all impurities. A small quantity of the PS dispersion (80 μL) was diffused on the cleaned substrate using a micropipette and spin-coated with 300 rpm for 5 min via a spin-coater (BGK, NSF-100DP). The randomly arranged PS microspheres on the substrate were floated carefully onto SDS assembled on an air/water interface in a Petri-dish. In this process, the PS microspheres were closely packed using an SDS aqueous solution of 200 μL (2 wt%). Finally, the monolayer was transferred onto the glass substrate (≈2.5 × 2.5 cm
2) and allowed to dry completely for a day.
2.3. Porous Hexagonal-Patterned Polydimethylsiloxane (PDMS) Mold
A porous hexagonal-patterned PDMS mold was prepared using the PS colloidal monolayer as the sacrificial mask. First, the base elastomer (30 g) and 3 g of the Sylgard 184 curing agent (10:1 wt%, Dow Corning Co., Midland, MI, USA) were added to a glass bottle. After mixing for about ten mins, the air bubbles were removed using a DOA-P704-AC vacuum pump (Gast Manufacturing Inc., Benton Harbor, MI, USA). Next, the mixture was poured onto the colloidal mask in the plastic Petri-dish, and thermal curing was then performed at 60 °C in an oven for 2.5 h. After removing the colloidal mask, the obtained PDMS mold was cut into pieces (2.0 cm × 1.5 cm) and rinsed with toluene for 5 min to remove the remaining microspheres.
2.4. Hemispherical SiO2 Film
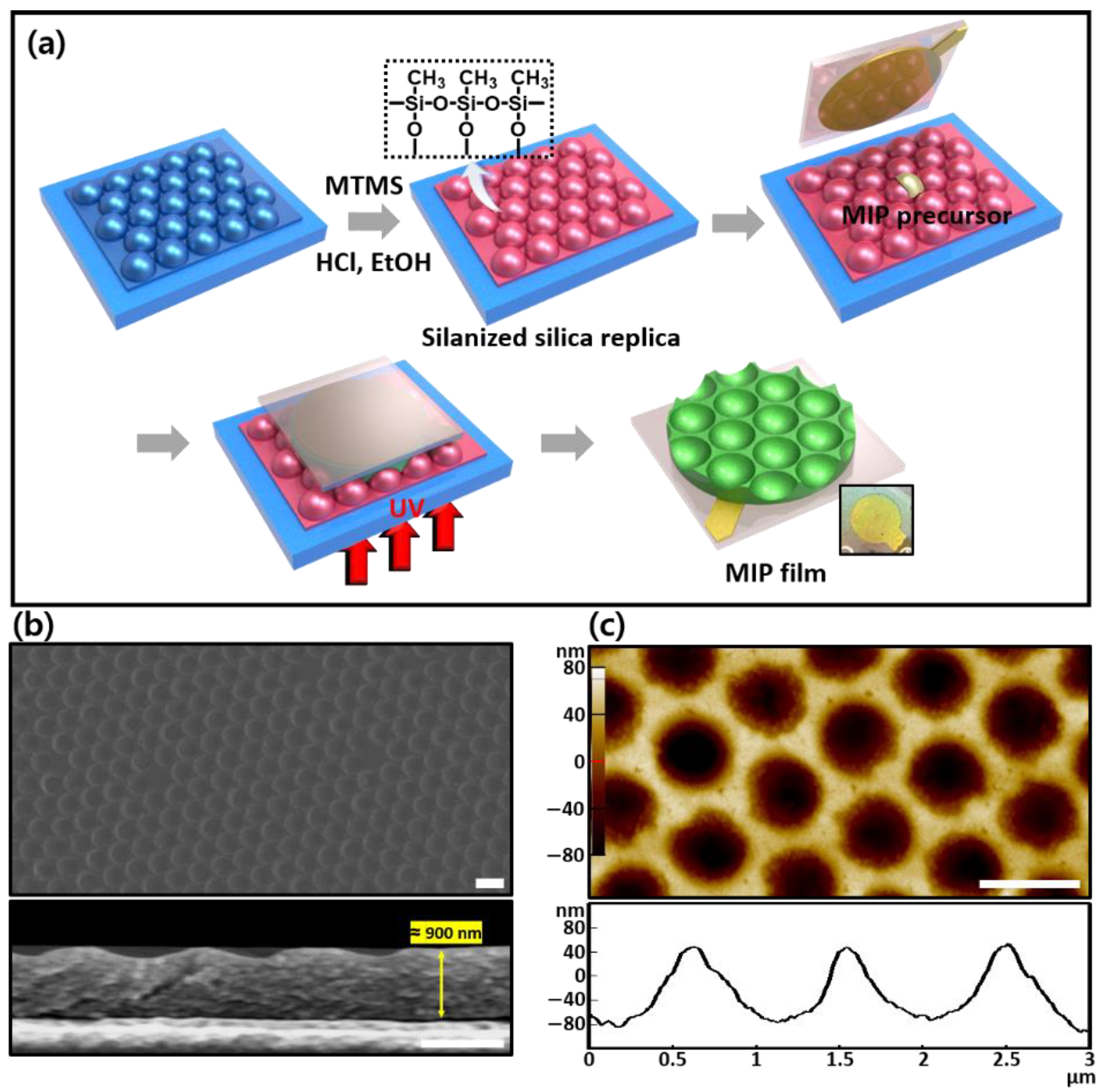
The hemispherical SiO2 film was replicated in the reverse structure of the patterned PDMS mold. The SiO2 precursor solution was prepared by sequentially placing TEOS (5.32 mL), absolute ethanol (9.5 mL), and HCl aqueous solution (0.1 N, 5 mL) in an N2-filled glass bottle and stirring slightly for 1 h. After adding absolute ethanol (17.83 mL), the pre-hydrolyzed mixture was stirred again for 1 h before being stored at 5 °C. The SiO2 precursor (100 μL) was added dropwise onto the glass slide (2.5 cm × 2.5 cm). After covering the PDMS elastomer on the surface, thermal curing was conducted at 100 °C on a hotplate for 1 h at 11.10 kPa. After peeling off the PDMS mold, the SiO2 film was immersed in a mixture of TMMS (28.8 mL), absolute ethanol (25 mL), and HCl aqueous solution (0.1 N) for 4 h at room temperature to facilitate methylation on the surface for silane-treated replica molds (st-replica mold). The silanization step was omitted in non-treated replica molds (n-replica mold).
2.5. Porous Patterned MIP Film
MAA (0.4 mmol), 2,4-D (0.1 mmol), and EGDMA (2.0 mmol) were injected sequentially into a 5-mL vial. AIBN (0.05 mmol) was dissolved in DMF (50 μL) and added in the mixture solution. After purging with N
2 gas for 10 min, the MIP precursor (0.1 μL) was added dropwise to the SiO
2 film using a micropipette. Next, 9 MHz gold-coated AT-cut quartz crystal substrates (QCs, QA-A9M AU[M], 5 mm in diameter, Seiko EG&G, Seiko instruments Inc., Chiba, Japan) with an active gold area of 0.196 cm
2 was placed in contact with the SiO
2 film before being subjected to UV light (370 nm, 36 W) for 5 min. After photopolymerization (
Figure 1), the QCs were gently peeled off the SiO
2 film. Finally, the MIP film was immersed in a mixture consisting of methanol and acetic acid (9:1 v/v) for 30 min to extract the 2.4-D molecules in the polymer matrix. The NIP film was fabricated under the same procedures without the presence of 2,4-D.
Each MIP/NIP film was installed in a dip cell, and after the stabilization of resonant frequency in a few min, the frequency shift was measured in various 2,4-D solutions (from 40 to 100 nM) for sensitivity determination and in 100 nM of each herbicide solution (2,4-D, atrazine, ametryn, or MCPA) for selectivity analysis for a 2 h rebinding process using a QCA 922 quartz crystal analyzer (Seiko EG&G, Seiko instruments Inc., Chiba, Japan).
For the 9 MHz AT-cut gold-coated quartz crystals, the sensitivity factor was approximately 0.1834 Hz cm
2 ng
−1 [
20]. A decrease of 1 Hz in the resonant frequency was almost identical to 1.07 ng in the mass loaded on the defined gold area. Thus, the mass of each imprinted polymer film (10–12 μg) was calculated from the resonant frequency differentiation of the bare and the 2,4-D-extracted imprinted quartz crystals oscillating in air. In addition, Q values (equilibrium binding capacities), denoted as the mass of the adsorbed 2,4-D molecules over the template-extracted poly(MAA–co–TFMAA–co–EGDMA) film were obtained from the Δ
f of each film in the sensing response.
2.6. Characteristics
The surface topographies of the MIP and NIP films were analyzed using field emission scanning electron microscopy (FE–SEM, Hitachi SU8220, Tokyo, Japan) and scanning probe microscopy (SPM, NX20, Park Systems, Suwon, South Korea).
3. Results and Discussion
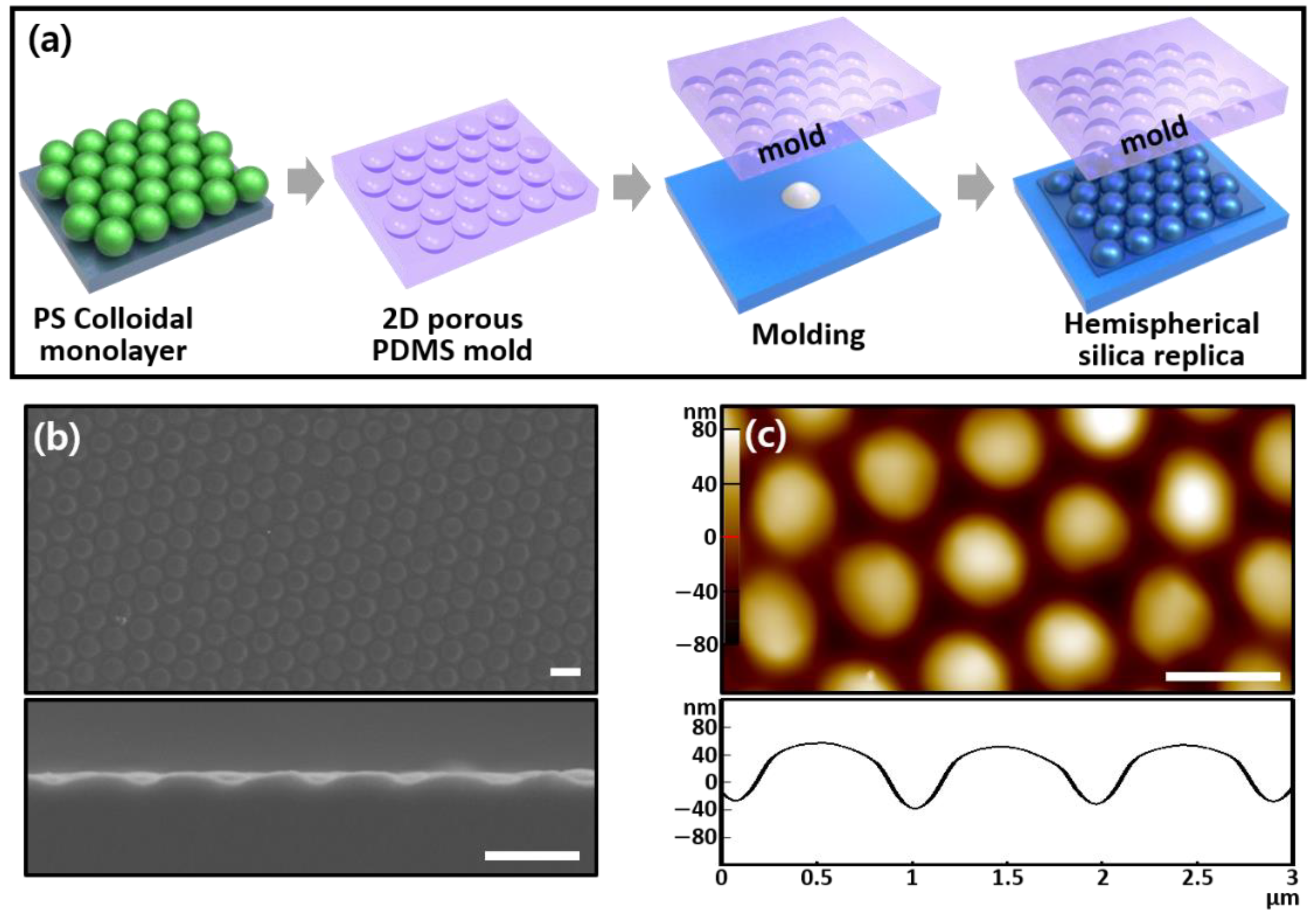
As shown in
Figure 2a, a convex hemispherical silica replica mold was manipulated using a pore-arrayed PDMS mold as a master mold, which was obtained from the PS colloidal monolayer, to form the porous patterned MIP/NIP films on gold-coated QC substrates. The well-ordered close-packed pattern in the silica observed in the SEM image had a small curvature of the hemispheres with a pore diameter of 830 nm and a pore height of 130 nm due to repeated executions of the replication process (
Figure 2b). The atomic force microscopy (AFM) image was also obtained to determine a more accurate dimensional structure. The height (i.e.,
h at ≈90 nm) from the valley of the hemispheres was not precisely measured due to the AFM tip effect [
21], but the hexagonal array of the silica was clearly observed (
Figure 2c). For the porous MIP/NIP films, two silica replica molds (i.e., non-treated and silanized molds) were prepared to examine the differences in the nonspecific binding properties in the MIP/NIP films formed during the μ-contact printing process.
As shown in
Figure 3a, the porous MIP/NIP film surface, which was in contact with the mold’s surface during photopolymerization, could be used as a sensitive interface for template detection; thus, the conformational control of this surface played an important role in determining the sensitivity of the QCM sensors. The MIP films (
hfilm at ≈900 nm) formed using the silanized silica molds (
st-MIP) appeared to be thicker than those obtained from the silica colloidal arrayed molds previously reported [
19], with an 800-nm pore diameter and a pore height of 125 nm, as determined via SEM and AFM surface analysis (
Figure 3b). In addition, the surface-to-volume (S/N) ratio was estimated using surface statistics in the AFM analytical software to obtain an A/A
0 (i.e., surface area/geometric area) value of 1.096 (see
Figure S1). In general, an increase in the surface area magnified the sensing signal relative to the planar MIP film’s signal due to a larger diffusion area during the limited sensing period [
19]. In the absence of 2,4-D molecules, a non-imprinted polymer (NIP) film (
st-NIP) was also prepared using the same procedure with a precursor solution to compare the sensing results with those obtained from the
st-MIP film. As control samples, the MIP and NIP films were manipulated using non-silane treated silica molds to determine the effects exerted by the conformation structures of the imprinted films in the encapsulating process with two different molds on sensing properties. The surface morphology and geometrical dimensions of the
st-NIP and MIP/NIP films were identical to those observed for the
st-MIP film (see
Figure S2).
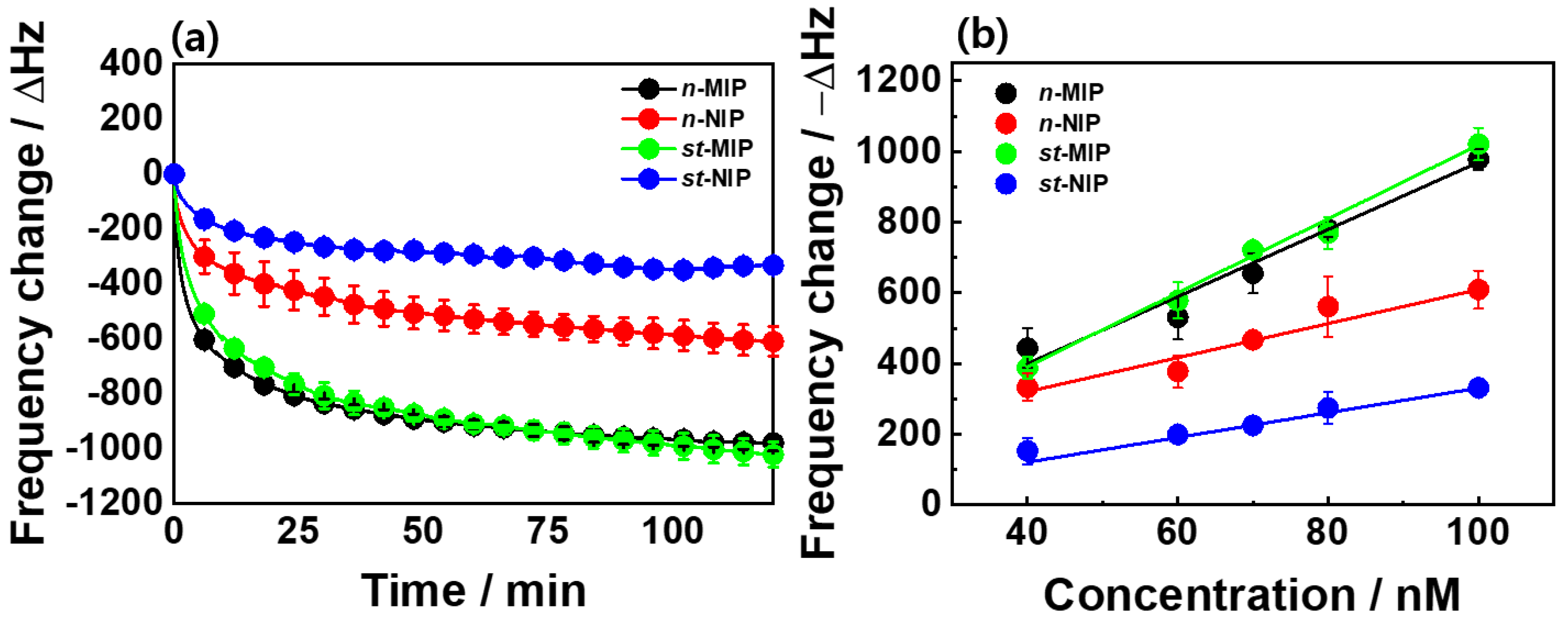
The sensing behaviors of the 2,4-D-imprinted
st-MIP and
st-NIP films on gold-coated QCs were investigated by measuring the resonant frequency changes (Δ
f) of QCM sensors in a 100-nM 2,4-D aqueous solution for 2 h (
Figure 4a). The MIP and NIP films, which were lithographically made using non-treated silica molds, were used as control samples and tested in the same solution. The functional MAA monomer served as a robust H-bond acceptor during the rebinding process and was linked noncovalently to the carboxylic group of the 2,4-D molecule via hydrogen bonding, thereby forming complexes in the template-extracted cavities of the MIP films. As a control sample, the
n-MIP film showed a Δ
f value of −978 Hz, which corresponded to the adsorbed mass of 1046 ng on the defined gold surface of the QCs (
Figure 4a). The
st-MIP film (Δ
f = −1021 Hz) also had a similar sensing response due to its almost identical porous structure, regardless of the surface modifications that were conducted on the silica molds. The recovery percentage values of the two MIP films were 87% (
n-MIP) and 92% (
st-MIP) during the limited sensing period, as calculated from the maximum capacity (Δ
fmax = −1119 Hz and −1111 Hz, respectively) of the MIP films. However, the sensing response between the
n-NIP and the
st-NIP films exhibited significant differences in their respective Δ
f values (Δ
fn-NIP = −610 Hz and Δ
fst-NIP = −332 Hz). In the
st-NIP film, the silica mold’s hydrophobic surface minimized conformation of the functional groups linked to the 2,4-D molecules in the imprinting process. The Δ
f value decreased as a result, even though nonspecific adsorption still occurred in an inner region near the NIP film’s surface. From these results, the imprinting factor (IF) was calculated based on the adsorbed 2,4-D mass per poly(MAA–co–EGDMA) unit weight (i.e., Q
MIP/Q
NIP) [
22]. The
st-MIP and
n-MIP films had IF values of 3.38 and 1.86, respectively. This difference indicated that surface modification of the patterned molds used in the lithographically patterned MIP systems could inhibit nonspecific adsorption on the NIP films’ surface, thereby improving sensing properties. The molecular imprinting recognition on the porous MIP films, formed using
st- and
n-replica molds, is schematically represented in
Figure 5.
As shown in
Figure 4b and
Figure S3, the Δ
f values were monitored in 2,4-D aqueous solutions with a concentration range between 40 and 100 nM to determine the sensitivity of the 2,4-D-detectable MIP films. Both the
n-MIP and
st-MIP films exhibited an increase in the Δ
f value with a corresponding increase in the concentration of 2,4-D. Additionally, the calibration curves were linear with coefficients of determination (R
2) of 0.982 (
n-MIP) and 0.949 (
st-MIP), and the sensitivities obtained from the slope of linear fitting lines were ≈9.5 and 10.5 Hz/nM, respectively. Compared with the imprinted films, the
n-NIP (4.8 Hz/nM) and
st-NIP (3.5 Hz/nM) films showed significantly lower sensitivity. In particular, the
st-NIP film retained the relatively low Δ
f value within the examined concentration range due to the small amount of nonspecifically bound 2,4-D molecules on the surface of the NIP film. Moreover, the Δ
f value of the
n-NIP film was almost identical to that of the MIP film at the lowest concentration (40 nM), thereby confirming that this MIP system (IF ≈ 1.33) was not validated by the imprinting effect. However, the
st-MIP film still had a high IF value of 2.40. Thus, surface modification protocols are required to enhance the sensing properties once a lithographic approach for fabricating the MIP system is applied. In addition, the MIP films were long-term stable and showed high reproducibility in sensing responses via several repeated measurements (
n < 10).
As shown in
Figure 6a, four herbicide molecules, including 2,4-D, were used for determining the specific selectivity, thereby verifying the efficiency of the 2,4-D-imprinted sensor. The Δ
f values of the
n-MIP/NIP and
st-MIP/NIP films were measured in herbicide solutions of 100 nM each for 2 h (
Figure 6b and
Figure S4). Both MIP films showed a similar sensing response to the 2,4-D molecule, but in the case of the
st-MIP films, the sensing response was 45–100 Hz lower than that of the
n-MIP film for the other herbicides. This was due to silanization of the used molds, which exerted influence on the surface conformation, regardless of the type of herbicide present. From the Q values described in
Tables S1 and S2, we found that the sensing responses of the three similar herbicides on the
st-MIP film ranged from 10.2 to 17.4 ng/μg. However, the
n-MIP films exhibited a higher sensing response between 17.4 and 31.1 ng/μg due to increased nonspecific binding of the analogous herbicide molecules on the surface of the MIP film. From the Q values, we determined that the selectivity values (α = Q
MIP,2,4-D/Q
MIP, other herbicides) of the
st-MIP and
n-MIP films were within the ranges of 5.82–9.93 and 3.24–5.80, respectively. These results revealed that controlling the MIP films using silanized molds also contributed to the film’s selectivity. Similarly, in the case of the NIP films, the Δ
f value of the
st-NIP film (250–335 Hz) was almost half-fold lower than that of the
n-NIP film (480–570 Hz), regardless of the type of herbicide present.