Abstract
Technologies for quantifying bitterness are essential for classifying medicines. As previously reported, taste sensors with lipid polymer membranes can respond to bitter hydrochloride substances in pharmaceuticals. However, the acid hydrolysis reaction between the lipid phosphoric acid di-n-decyl ester (PADE) and the plasticizer tributyl o-acetylcitrate (TDAB) led to a deterioration in sensor responses during storage. Given the cost of transportation and preservation for commercialization, membrane components that maintain physical and chemical stability during long-term storage are needed. Here we present a membrane electrode based on hydrophobic tetrakis [3,5-bis (trifluoromethyl) phenyl] borate (TFPB) and a plasticizer 2-nitrophenyl octyl ether (NPOE) for the quantification of pharmaceutical bitterness; they maintain a stable response before and after accelerated deterioration, as well as high selectivity and sensitivity. It is a first attempt to use a completely dissociative substance to replace non-completely dissociative lipids. Our work offsets the long-term stability issue of a bitterness sensor with a negatively charged hydrophobic membrane. Meanwhile, we provide the opportunity to select surface charge modifiers for a membrane surface using ester plasticizers containing oppositely charged impurities.
1. Introduction
Taste plays a vital role in the acceptance of a pharmaceutical formulation. Many active pharmaceutical ingredients (APIs) have a bitter taste, and therefore are aversive not only for children, but also for many adults [1]. To deal with this problem, the evaluation of bitterness has become an important step during the process of pharmaceutical development [2]. Traditional sensory testing relies on the subjective feelings of panelists, with the limitations of low reproducibility, low objectivity, and possible side effects. In the past few decades, several studies on electronic tongues have been drawing intense research interest because of their potential application in taste assessment [3,4,5,6,7,8,9]. The use of an electronic tongue, called a “taste sensor,” provides an objective solution for taste evaluation. The taste sensor is an analytical sensor array system with different artificial lipid polymer membranes. Unlike other electronic tongues with cross-selectivity, each sensor electrode of the taste sensor can detect substances with specific physiochemical properties (or taste qualities). We call global selectivity. The two-electrode method is used as the measurement system, with a sensor electrode and an Ag/AgCl reference electrode. The membrane potential between the two electrodes is measured by a potentiometer. When a sensor membrane is immersed in a taste sample, hydrophobic and electrical interactions occur between the taste substances and the membrane, which causes a change in the membrane potential. The changes in membrane potential are used as the outputs of the taste sensor [10,11,12,13,14,15].
A bitterness sensor called BT0 has been reported as one of the sensor electrodes of the taste sensor [12,16]. It shows global selectivity to hydrochloride drugs and has been used to quantify the bitterness of commercial hydrochloride medicines. The BT0 membrane contains phosphoric acid di-n-decyl ester (PADE), bis(1-butylpentyl) adipate (BBPA), and tributyl o-acetylcitrate (TBAC), with polyvinyl chloride (PVC) as the supporting material. PADE is a phospholipid, which adjusts the surface charge density of the membrane. BBPA and TBAC are plasticizers, which change the hydrophobicity of the membrane. Earlier studies have suggested that the BT0 sensor possesses high sensory correlation as well as a good reaction to the bitterness suppression effect of sucrose and other bitter-masking materials [16]. However, long-term preservation at room temperature is still a challenge, and humidity is a drawback in the laboratory. A previous study has suggested that the sensor response gradually decreases by half after one year and keeps decreasing to almost zero after two years. We revealed that the reason behind the deterioration is that the phospholipid PADE creates hydrogen ions, which promote TBAC hydrolysis [17].
Tetrakis [3,5-bis (trifluoromethyl) phenyl] borate (TFPB) is a highly hydrophobic fluoroaromatic borate ion with little nucleophilicity and high chemical durability [18,19,20]. Because of the trifluoromethyl groups, the ipso-carbons of TFPB have low electron density. Thus, TFPB is stable under acidic conditions [19]. It has been used as an anion phase-transfer catalyst in Friedel‒Craft alkylation or diazo coupling reactions [21], and as an ion exchanger in ion-selective electrodes (ISEs) [22,23,24]. As an anionic lipophilic additive for potentiometer sensors, appropriate TFPB content is important for cation sensing to have better sensitivity and a shorter response time [25]. To sum up, TFPB can be considered an ideal anion amphiphile for sensing bitter hydrochloride substances in pharmaceuticals, which are usually hydrophobic and positively charged. Also, as a completely dissociative substance, TFPB does not create an acidic environment in the sensing element and the degree of dissociation is not affected by pH conditions.
The purpose of this paper is to develop a bitterness sensor for quantifying pharmaceutical bitterness with high stability in long-term storage. In this work, a completely dissociative TFPB was first used as an anionic amphiphile in the sensor membrane instead of the non-completely dissociative lipid PADE. To obtain good sensitivity, we determined the optimal concentration of TFPB and adopted NPOE as the plasticizer, which contains positively charged impurities to neutralize the excessive surface charge density of TFPB. The results of this paper proved that the surface charge density of the sensor membrane determines the sensitivity of sensor responses to the adsorption of bitterness substances into the membrane. Finally, the sensor withstood the long-term stability test and showed high selectivity and sensitivity for pharmaceutical bitterness.
2. Materials and Methods
2.1. Materials
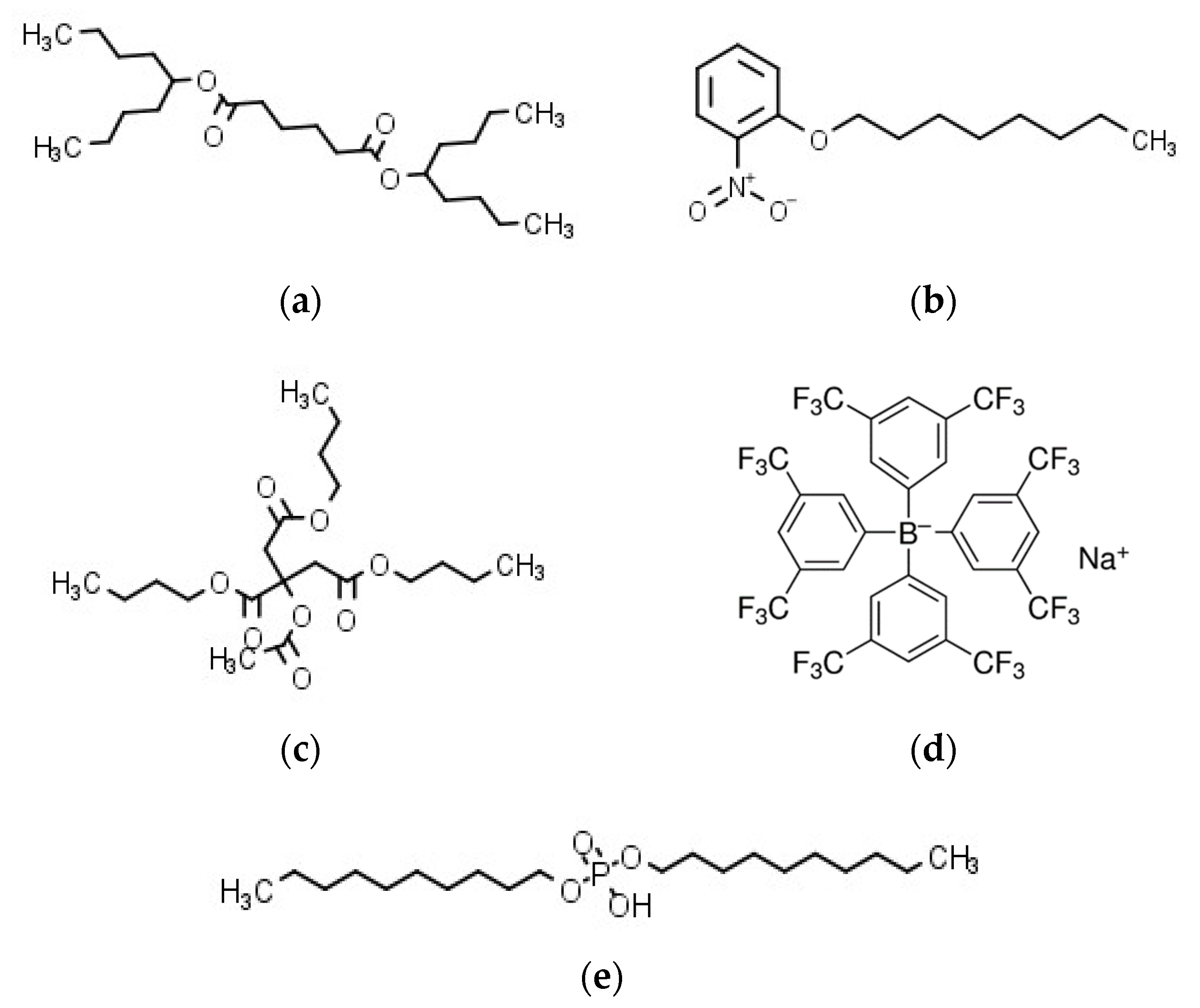
Sodium tetrakis [3,5-bis (trifluoromethyl) phenyl] borate (Na-TFPB) was supplied by Dojindo Laboratories Co., Ltd. (Kumamoto, Japan). Phosphoric acid di-n-decyl ester (PADE) were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). The plasticizers, bis(1-butylpentyl) adipate (BBPA), 2-nitrophenyl octyl ether (NPOE), and tributyl O-acetylcitrate (TBAC) were purchased from Sigma-Aldrich Japan K.K. (Tokyo, Japan) and Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Polyvinyl chloride (PVC) purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan) and tetrahydrofuran (THF) purchased form Sigma-Aldrich Japan K.K. (Tokyo, Japan) were used as the supporting material and the organic solvent, respectively. The structures of membrane components are summarized in Figure 1. Potassium chloride (KCl), tannic acid, monosodium glutamate (MSG), tartaric acid, quinine hydrochloride, iso-alpha acid, and sucrose were obtained from Kanto Chemical Co., Inc. (Tokyo, Japan). Azelastine hydrochloride was obtained from LKT Laboratories, Inc. (St. Paul, MN, USA). Cetirizine hydrochloride and eperisone hydrochloride were obtained from Combi-Blocks, Inc. (San Diego, CA, USA). Loperamide hydrochloride was obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Hydroxyzine hydrochloride was obtained from Sigma-Aldrich Japan K.K. (Tokyo, Japan).
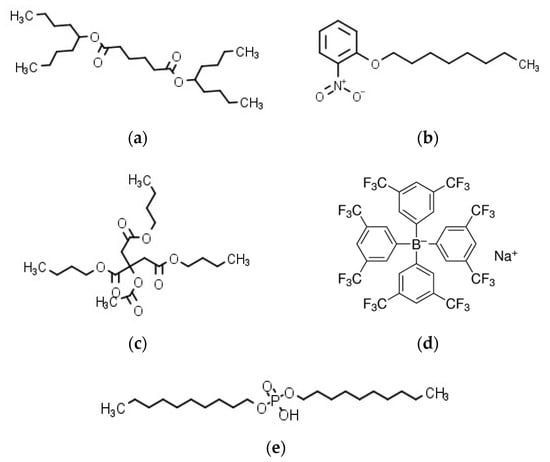
Figure 1.
Structures of membrane components: (a) BBPA; (b) NPOE; (c) TBAC; (d) Na–TFPB; (e) PADE.
A reference solution composed of 30 mM KCl and 0.3 mM tartaric acid was used as a buffer solution to prepare all taste samples. A cleaning solution composed of 30 vol% ethanol and 100 mM HCl was used to clean the membrane for the next measurement.
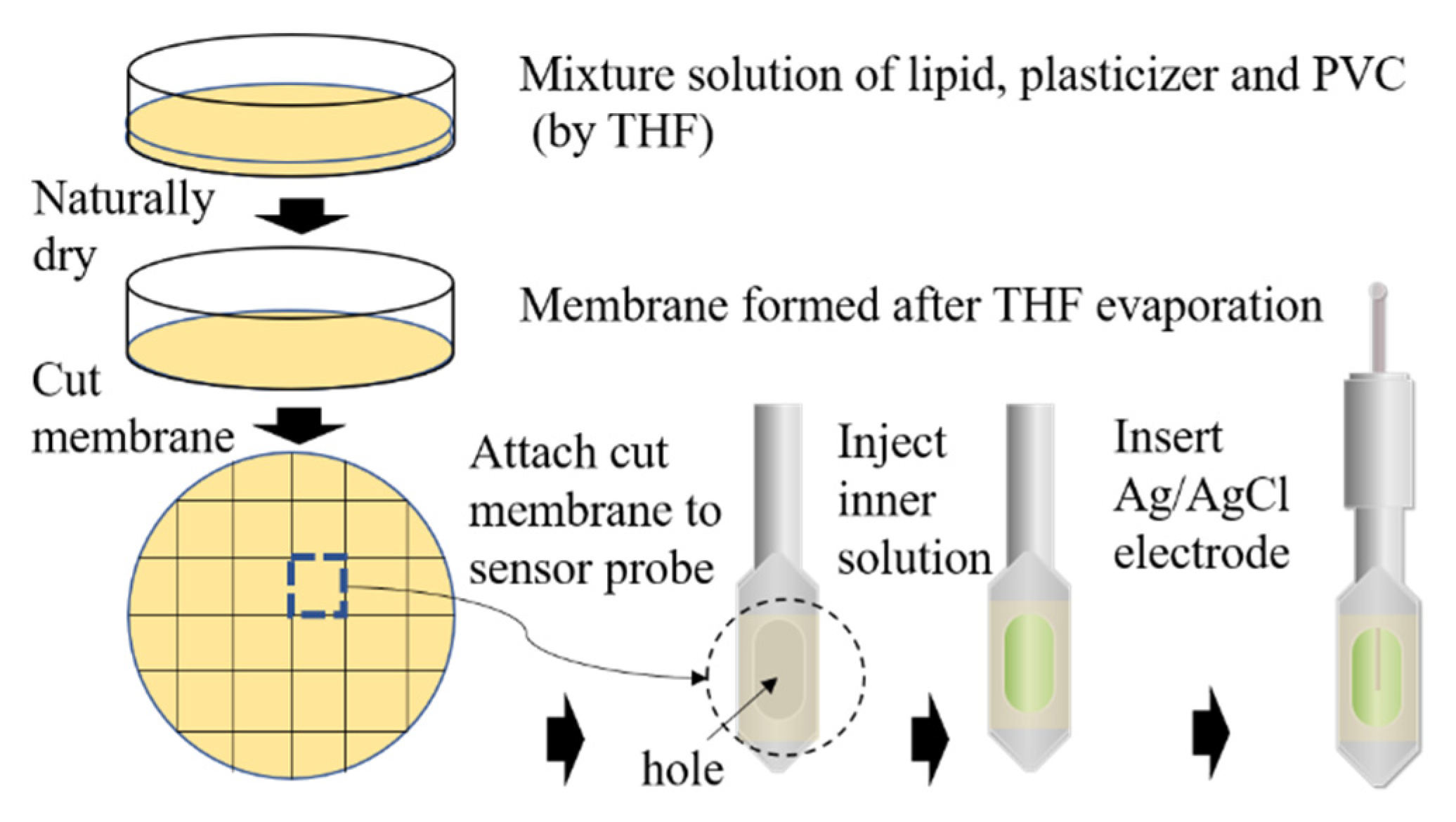
2.2. Fabrication Process of the Sensor Electrode
The membrane electrode based on TFPB has been fabricated in a multistep sequence (Figure 2). First, Na–TFPB and plasticizers BBPA, TBAC (or NPOE), and PVC were mixed by THF for 1 h. The mixture solution was then poured into a glass Petri plate to dry naturally. After three days, a transparent membrane had formed on the bottom of the Petri plate. Then the membrane was divided to fit the size of the sensor probe. Finally, an Ag/AgCl electrode was fixed to the sensor probe after injecting an inner solution (3.3 M KCl and saturated AgCl). To obtain the best performance for over multiple measurements, the sensor electrode was covered with a reference solution comprised of 30 mM KCl and 0.3 mM tartaric acid for 24 h before measurements. This conditioning process aimed to optimize the structure of the membrane surface to reach a stable initial potential [26].
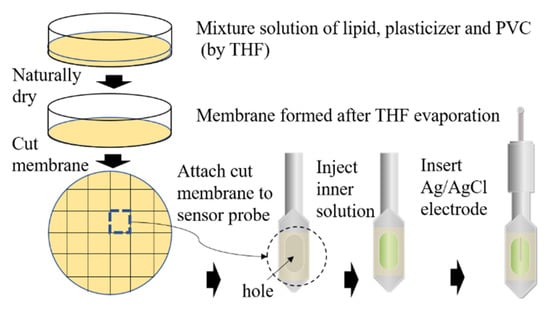
Figure 2.
Fabrication process of sensor electrode.
2.3. Measurement Procedure
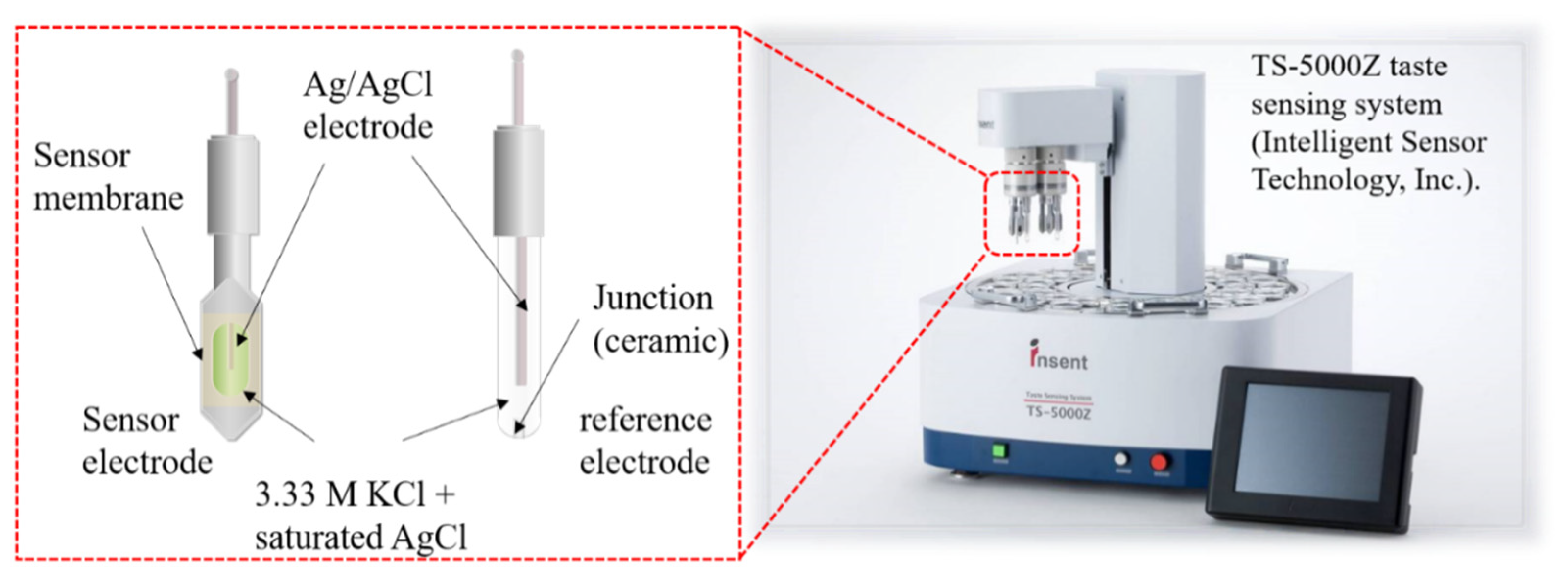
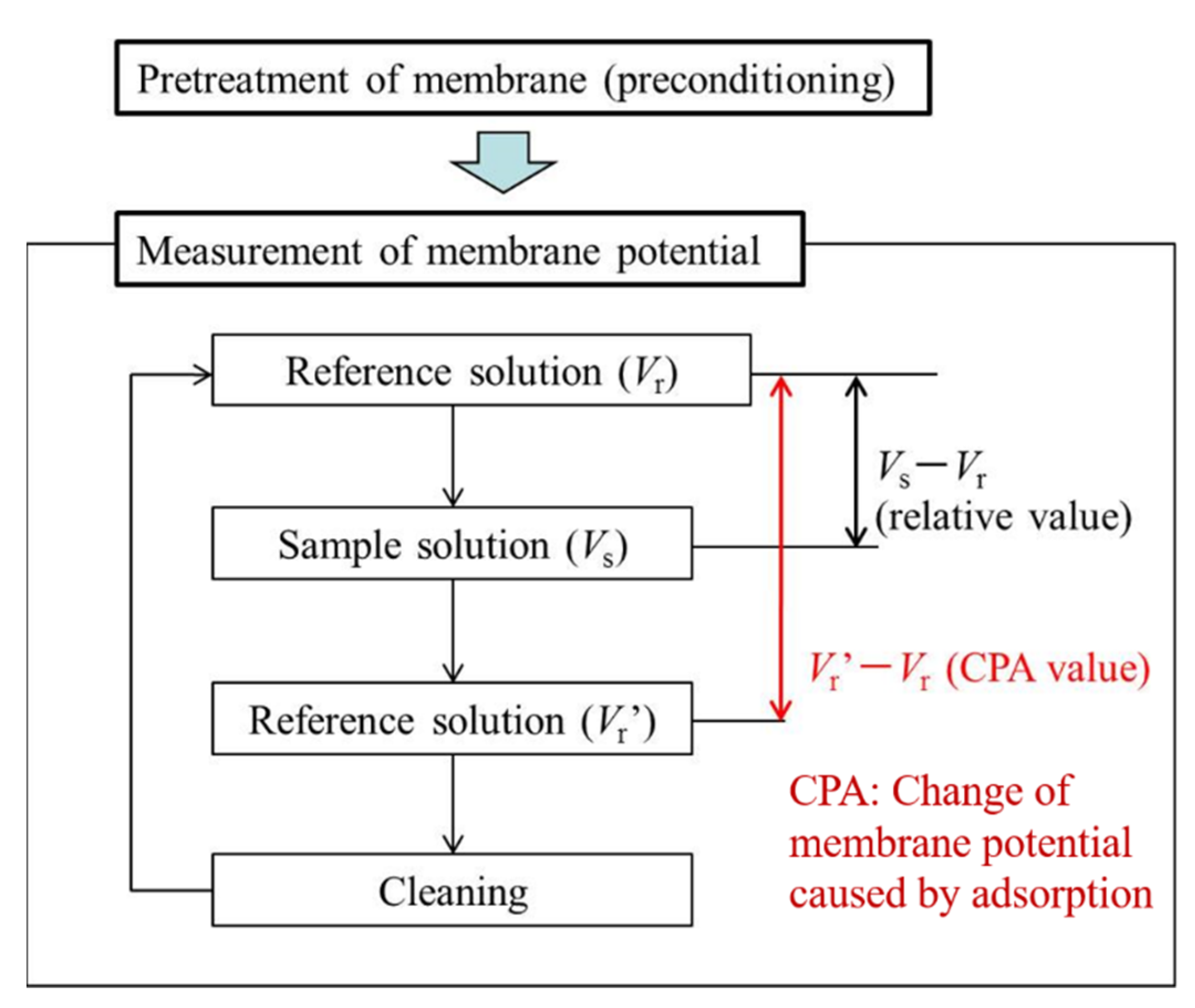
As shown in Figure 3, the two-electrode method was used to measure membrane potentials between the sensor electrode and the reference electrode. Before the measurement, the sensor membrane was immersed in a reference solution for one day to obtain a relatively stable initial membrane potential. The measurement procedure is shown in Figure 4. First, the membrane potential was obtained in a reference solution. Second, was obtained in a sample solution. All samples were made with a reference solution as a buffer. Third, membrane potential was obtained after lightly rinsing the sensor electrodes in a reference solution. The values of and are often different because some hydrophobic substances from the samples remain on the membrane after a light rinse, causing changes in the membrane potential. The change in membrane potential caused by the adsorption is called the CPA value. Finally, the membrane potential was restored to the original value after sufficiently rinsing the sensor electrodes with a cleaning solution comprised of 30 vol % ethanol and 100 mM HCl. The relative value and the CPA value, determined using the following equations, were used as the sensor outputs [27,28]:
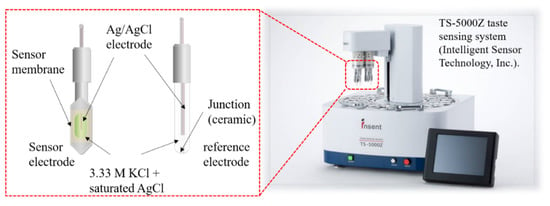
Figure 3.
Experimental setup of taste sensor system.
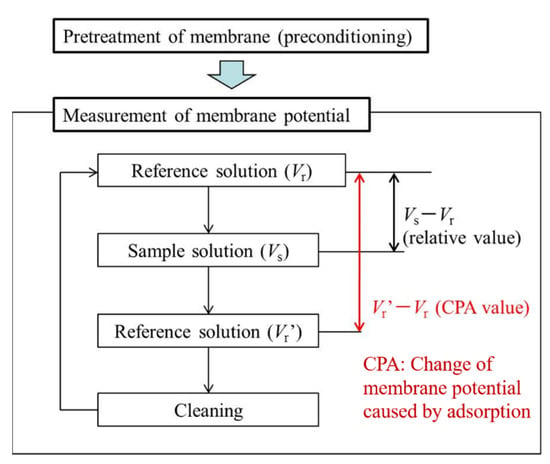
Figure 4.
Measurement procedure using taste sensor.
2.4. Investigation of TFPB Contents
As shown in Figure 1, TFPB is fully dissociated with a sodium ion as the counter ion in the solution. There are four benzene rings around the boron ion, which makes TFPB have high hydrophobicity. On the other hand, PADE becomes negatively charged when it dissociates from hydrogen ion. The degree of the dissociation is not complete, and is influenced by the pH of the environment. Moreover, previous research has suggested that PADE generates an acidic environment in the BT0 membrane and promotes the hydrolysis of the ester plasticizer TBAC, which causes a decrease in membrane sensitivity [17]. The carbon chains (C10) on both sides make PADE have high hydrophobicity, but the hydrophobicity of PADE (logD: 4.307) is weaker than that of TFPB (logD: 9.735). In the first experiment, a sensor electrode was fabricated with different contents of TFPB, while TBAC and BBPA were used as plasticizers in certain amounts. Considering the content of PADE used in the BT0 sensor as well as the difference in the dissociation of TFPB and PADE, the minimum concentration of TFPB (0.1 wt %) was set to be smaller than that of PADE. To test the bitterness sensitivity of sensor membranes containing TFPB, 0.1 mM quinine hydrochloride was measured for each membrane.
2.5. Contact Angle
The contact angle of the surface of each membrane containing TFPB was measured using a contact angle meter (DM 500, Kyowa Interface Science Co., Ltd., Saitama, Japan). The contact angles were measured with a 2 µL water droplet.
2.6. Adsorption Amount
The adsorption amount of quinine hydrochloride was measured using an UV-visible spectrometer (UV-1800, Shimadzu Corp., Kyoto, Japan). First, a calibration curve was calculated by measuring the absorbance of quinine hydrochloride solutions with known concentrations. Then 5 mL of quinine hydrochloride solution were poured onto the Petri plate. The absorbance of the solution extracted from the Petri plate was measured after 30 s of soaking. Finally, the sample concentration can be estimated from the obtained absorbance using the calibration curve. After the measurement, the cleaning solution was poured into the Petri plate for 1 min. to wash the membrane for reuse.
2.7. Evaluation of TFPB-NPOE Membranes
The plasticizers BBPA and TBAC were replaced with NPOE. The TFPB content in TFPB-NPOE membranes was set to 0.0025–2.5 wt %. The initial membrane potential and CPA values were measured for quinine hydrochloride using each TFPB-NPOE membrane. To confirm the selectivity to the different taste qualities, the responses to saltiness, sourness, umami, astringency, bitterness, and sweetness were measured by the TFPB-NPOE sensor. Two representative bitter substances were chosen for the bitterness sensing: 0.01 vol % iso-alpha acid (called “bitterness (−)”) was used as the representative of the acidic materials included in food or beverages, and 0.1 mM quinine hydrochlorde, azelastine hydrochlorde, cetirizine hydrochlorde, loperamide hydrochlorde, eperisone hydrochlorde, hydroxyzine hydrochlorde (called “bitterness (+)”) were used as representatives of the hydrochloride salts included in medicines. In this taste sensor system, the two types of bitterness were evaluated by two different types of bitterness sensors with opposite membrane potentials. In this paper, we aim to design a bitterness sensor that responds to the bitterness of pharmaceuticals. Therefore, the selectivity of bitterness (+) is expected.
2.8. Long-Term Stability Test
Since it is not efficient to wait for the natural deterioration of the membrane, we accelerated the deterioration process to test the long-term stability of the sensor electrode. The sensor membranes were put in a chamber at 45 °C and 95% relative humidity (RH) for 28 days. The accelerated deterioration process was initiated according to our previous report [17]. The sensor responses (CPA values) to a 0.1 mM quinine hydrochloride solution before and after the accelerated deterioration process were defined as and , respectively.
The residual voltage ratio D was defined by the following equation to evaluate the degree of deterioration:
3. Results and Discussion
3.1. Bitterness Responses with TFPB-BBPA-TBAC Membranes
3.1.1. Sensor Responses
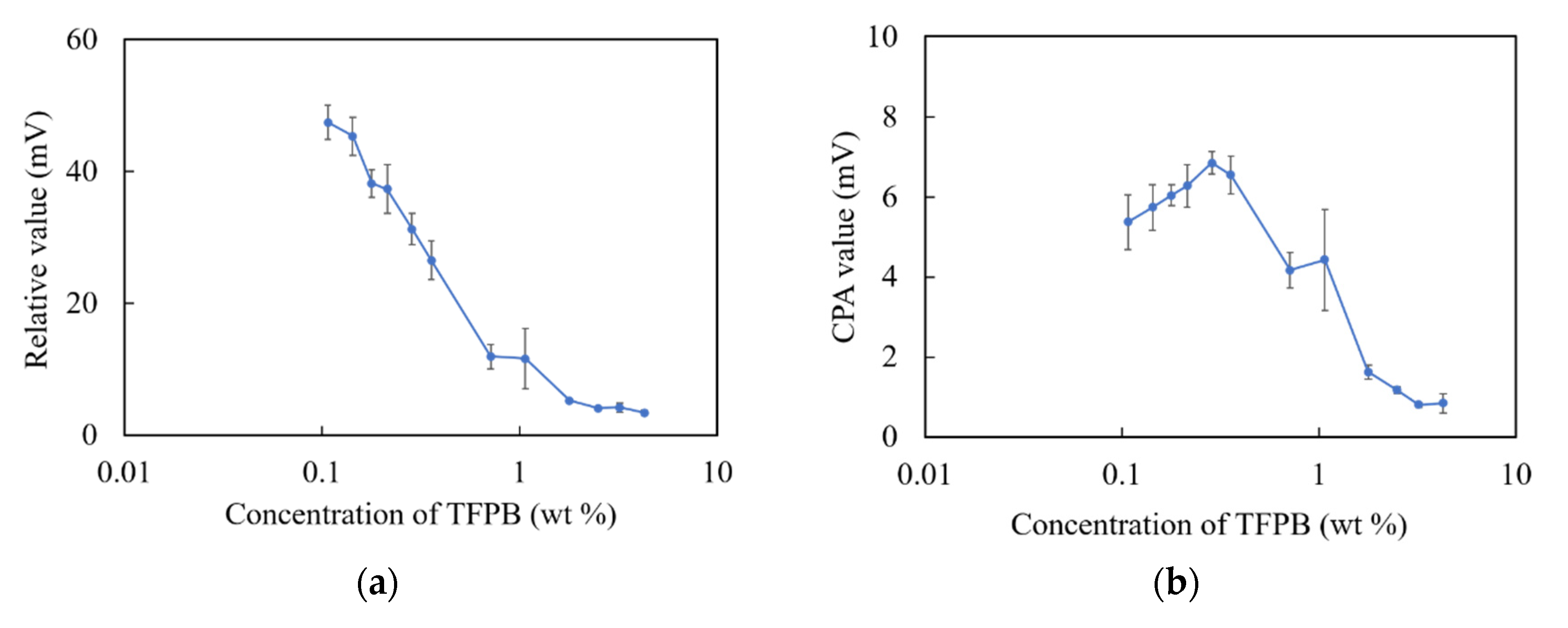
The relative values and CPA values are shown in Figure 2. As shown in Figure 5a, there was a consistent decrease in the relative values with respect to the increase in the concentration of TFPB until 1.8 wt %. The relative value remained unchanged under 5 mV during 1.8–4.3 wt % TFPB. As shown in Figure 5b, the CPA values were found to be insignificant with a maximum of 6–7 mV, while the CPA value of the BT0 sensor was about 36 mV for 0.1 mM quinine hydrochloride [17]. It can be seen that the TFPB-BBPA-TBAC membrane showed poorer performance than the conventional BT0. The hydrophobic interaction between the membrane and quinine hydrochloride contributed little to the sensor output, and a low response is likely to be obtained around the high concentration of TFPB.
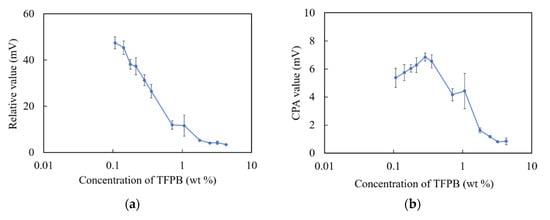
Figure 5.
Responses of sensor membranes with different TFPB contents in TFPB-BBPA-TBAC membranes to quinine hydrochloride (0.1 mM): (a) Relative values; (b) CPA values. The results are expressed as means ± SD (n = 16, 4 membranes × 4 times).
3.1.2. Characteristics of Adsorption
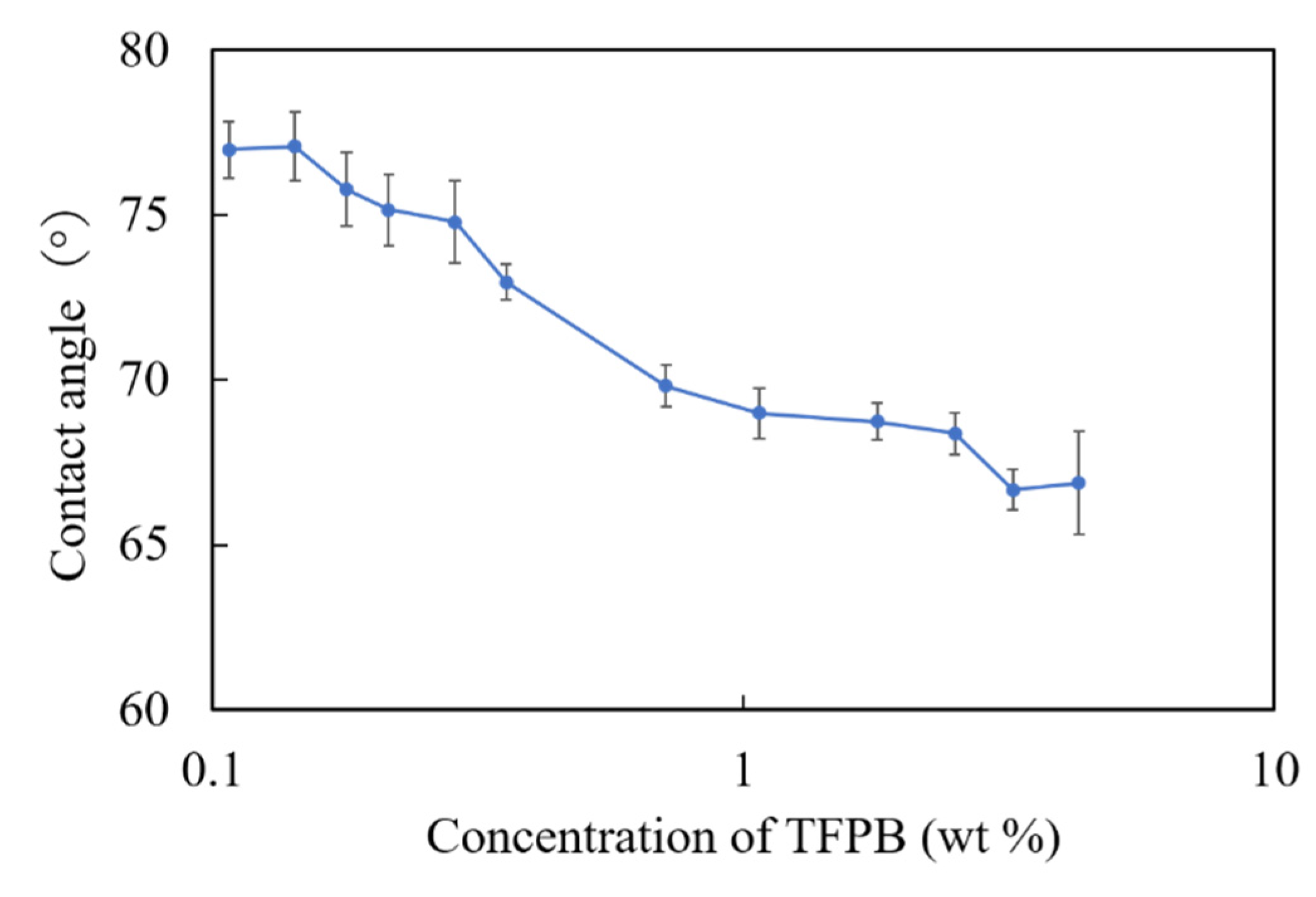
According to Figure 6, there was a consistent decrease in the contact angles with respect to the increase in the concentration of TFPB. From the results, we found that the hydrophobicity of the membrane surface decreased with the increased concentrations of TFPB. As the contact angle of the conventional BT0 sensor was about 75°, the membranes with 0.1–0.3 wt % TFPB showed the same surface hydrophobicity as the BT0.
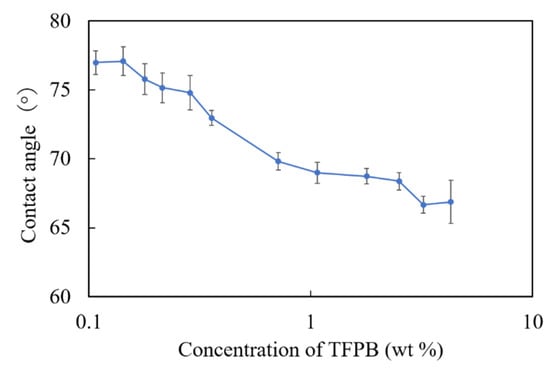
Figure 6.
The surface contact angles of TFPB-BBPA-TBAC membranes in different TFPB concentrations. The results are expressed as means ± SD (n = 9, 3 membranes × 3 times).
In terms of the measurement procedure of the taste sensor, bitter substances are attracted to the sensor membrane mainly by electrostatic interaction (reflected by relative values) and then adsorbed in the membrane by hydrophobic interaction (reflected by CPA values) after being lightly washed. In this experiment, relative values were obviously observed in the low-concentration region of TFPB, which indicated electrostatic interactions. On the other hand, although the membrane with 0.1–0.3 wt % TFPB showed the same surface hydrophobicity as the BT0, the CPA values (maximum: 6–7 mV) were much smaller than that of the BT0 (about 36 mV).
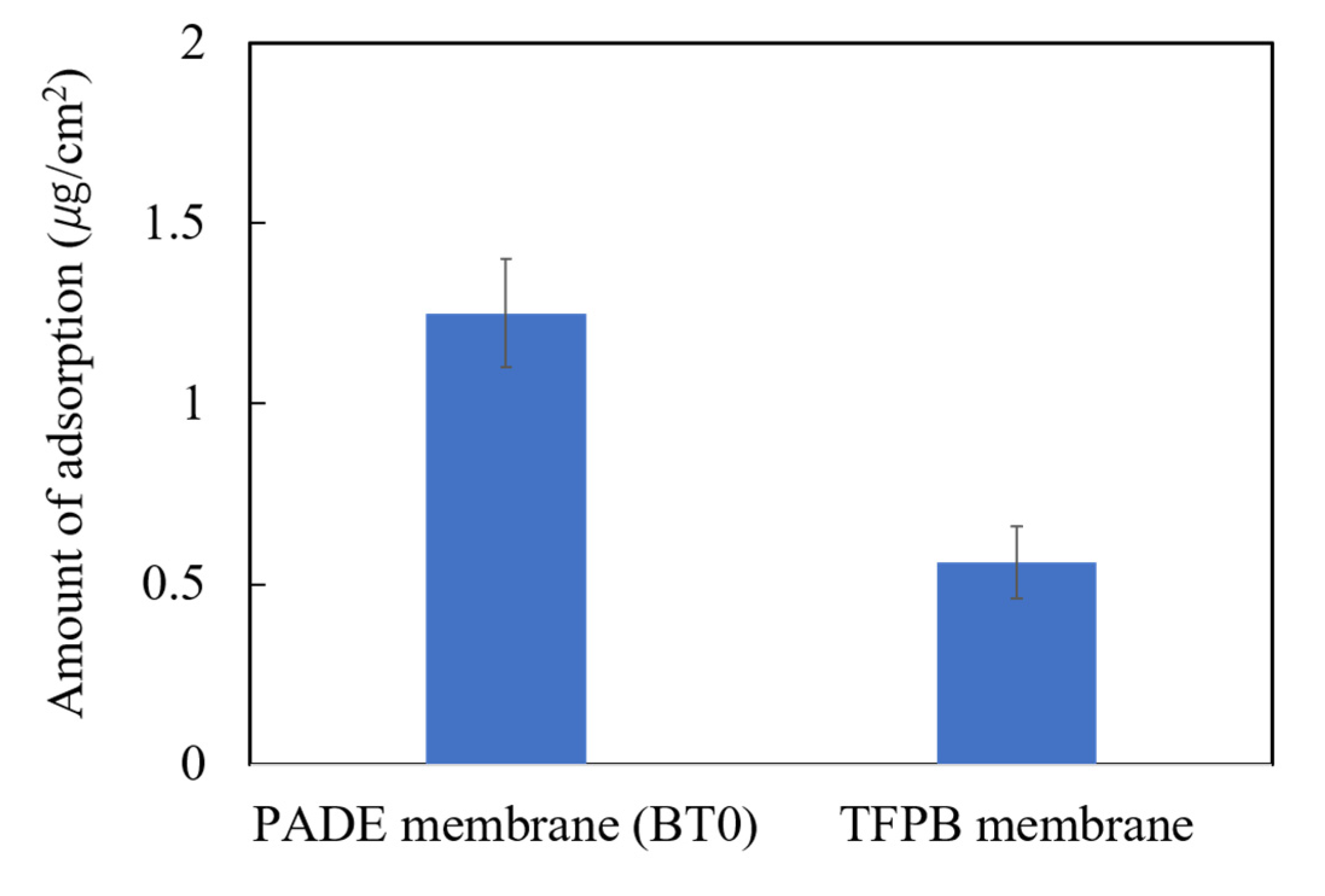
The wavelength we used was 249 nm. The adsorption amounts of 0.1 mM quinine hydrochloride on the membranes of conventional BT0 and the sensor containing 0.14 wt % TFPB were investigated. As shown in Figure 7, the adsorption amount on the BT0 and TFPB membrane was 1.25 and 0.56 µg/cm2, respectively.
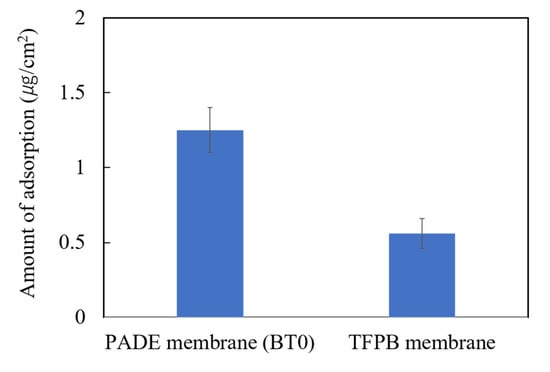
Figure 7.
Amount of adsorbed quinine hydrochloride (0.1 mM) on BT0 (PADE-BBPA-TBAC) and TFPB-BBPA-TBAC membrane. The results are expressed as the means ± SD (n = 6, 3 membranes × 2 times).
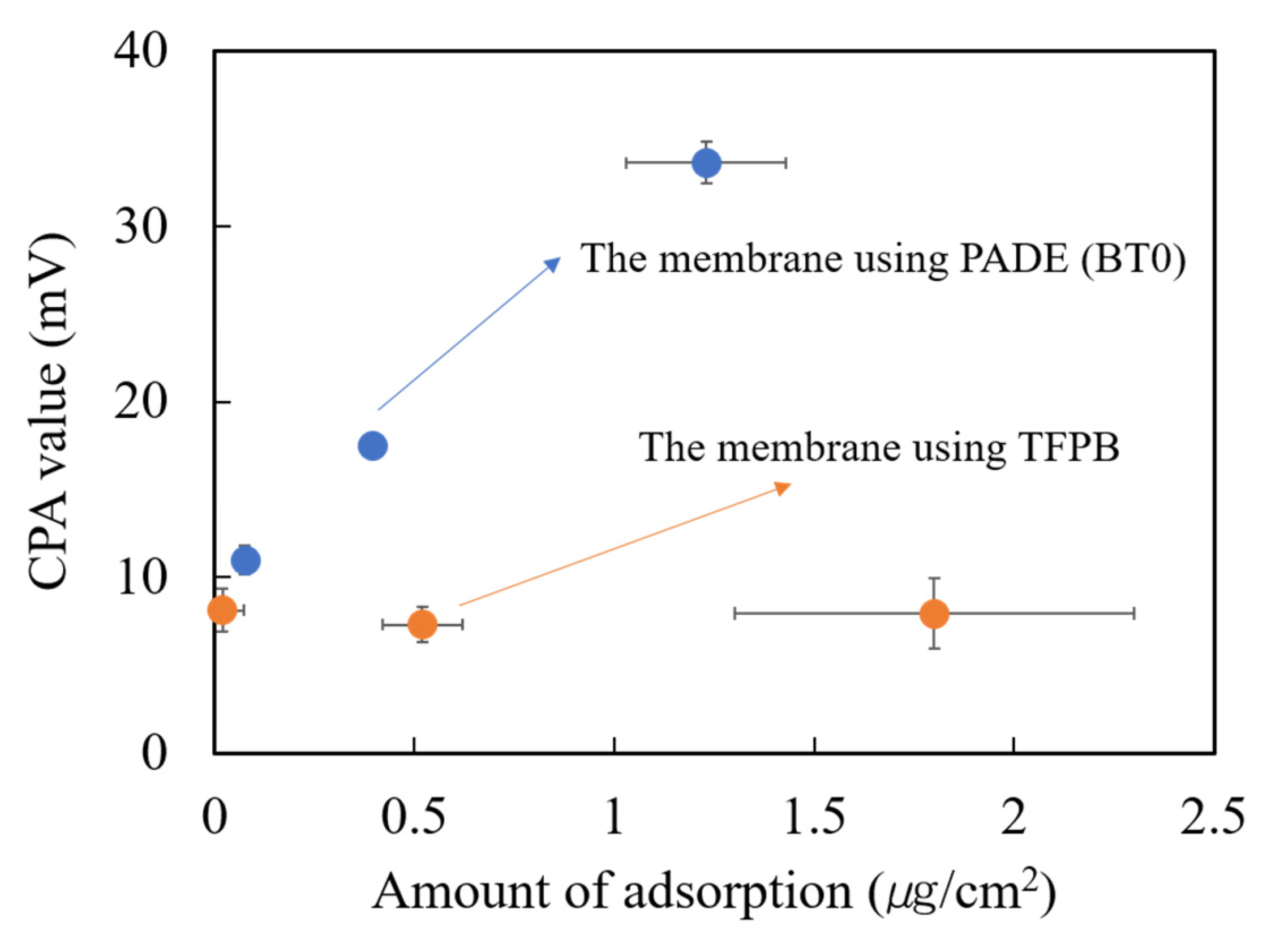
As shown in Figure 8, the CPA value of the BT0 sensor membrane increased with the increasing adsorption amount of quinine hydrochloride, but the CPA value of TFPB membrane showed no significant change. The results also showed that the BT0 membrane has larger CPA values than the TFPB membrane even if the adsorption amount of quinine hydrochloride was the same. These results suggested that the TFPB-BBPA-TABC membrane had low sensitivity for quinine hydrochloride.
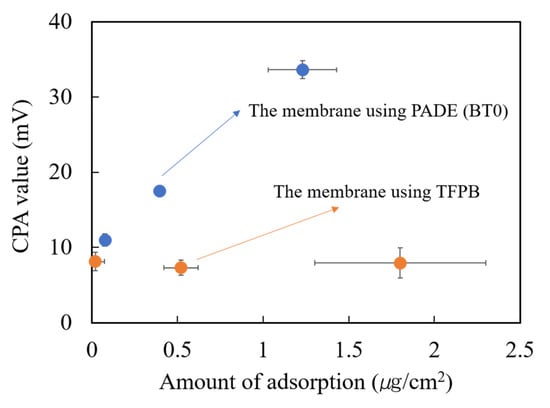
Figure 8.
The change of CPA value with the amount of adsorption of quinine hydrochloride (0.1 mM) onto the membrane. The blue dots indicate the BT0 sensor and the orange dots indicate the sensor with the TFPB-BBPA-TBAC membrane. The results are expressed as means ± SD (n = 6, 3 membranes 2 times).
Previous research has suggested that the responses of the taste sensor were determined by both the charge density and the adsorption amount on the membrane surface [29,30]. In this experiment, the low CPA values of the TFPB membrane may be caused by the excessive surface charge density caused by negatively charged TFPB because the amount of adsorption indeed rose.
3.2. Evaluation of Bitterness Sensining with TFPB-NPOE Membranes
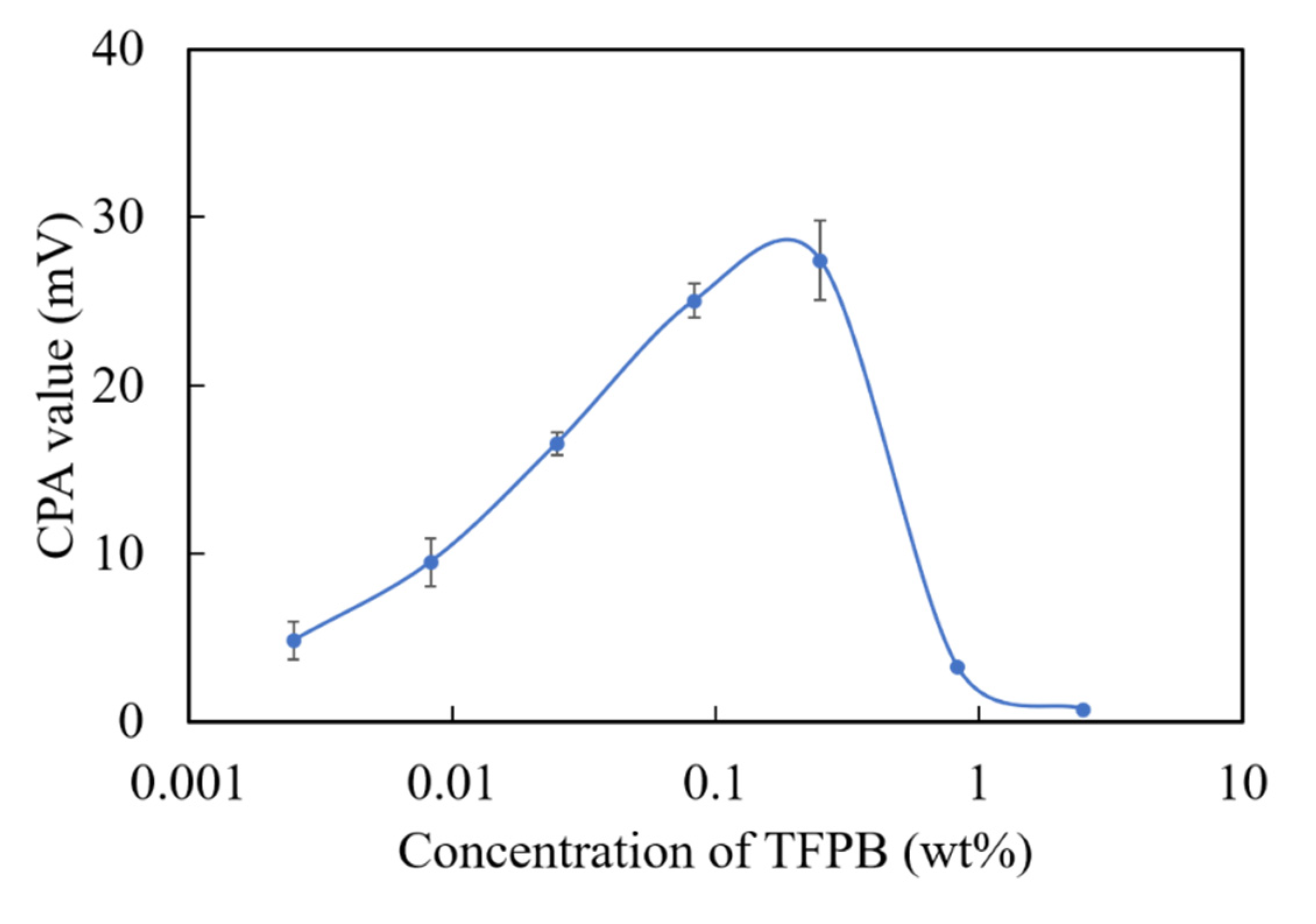
Since the TFPB-BBPA-TBAC membrane showed much lower output than the conventional BT0, and the further reduction of TFPB caused the instability of the membrane potential (data not shown), we chose to change the plasticizers to adjust the surface charge density. As we know, the plasticizers are ester compounds mainly synthesized from acids and alcohols [31]. For example, adipic esters, such as BBPA, are synthesized by alkylating the adipic acids. Citrates such as TBAC are synthesized by alkylating citric acids. However, it is conceivable that acids such as adipic acid and citric acid that were not totally alkylated during the synthesis exist as impurities in the reagent. All these acids released protons and showed negative charge, which was thought to contribute to increasing the negative charge density on the sensor membrane surface. In a previous study, a NPOE-PVC membrane (without TFPB) showed a positive membrane potential in a reference solution, which indicants the existence of positively charged impurities in NPOE [32]. Therefore, we chose NPOE as a new potential plasticizer of the TFPB membrane. NPOE has also been used as the plasticizer in other taste sensors [10]. Figure 9 shows the CPA value for each TFPB-NPOE membrane with different TFPB contents. A response peak for quinine hydrochloride appeared around 0.08–0.25 wt % of TFPB, and the CPA value was about 27 mV. Although the response value was about three-quarters of that of the BT0 (36 mV), it was much higher than that of TFPB-BBPA-TBAC (6–7 mV). The results revealed that the positively charged impurities in NPOE were helpful in neutralizing the excess negative charges of TFPB and provided an appropriate surface charge density.
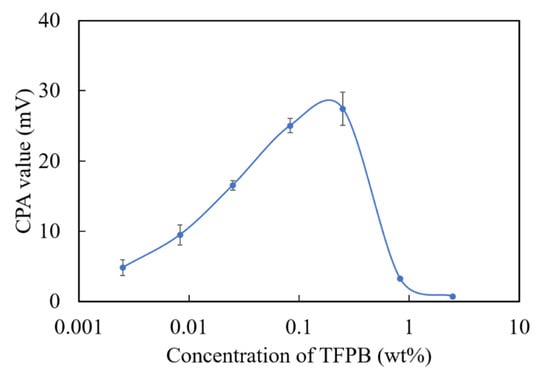
Figure 9.
The relationship of CPA values and TFPB contents in TFPB-NPOE membranes. The results are expressed as means ± SD (n = 16, 4 membranes × 4 times).
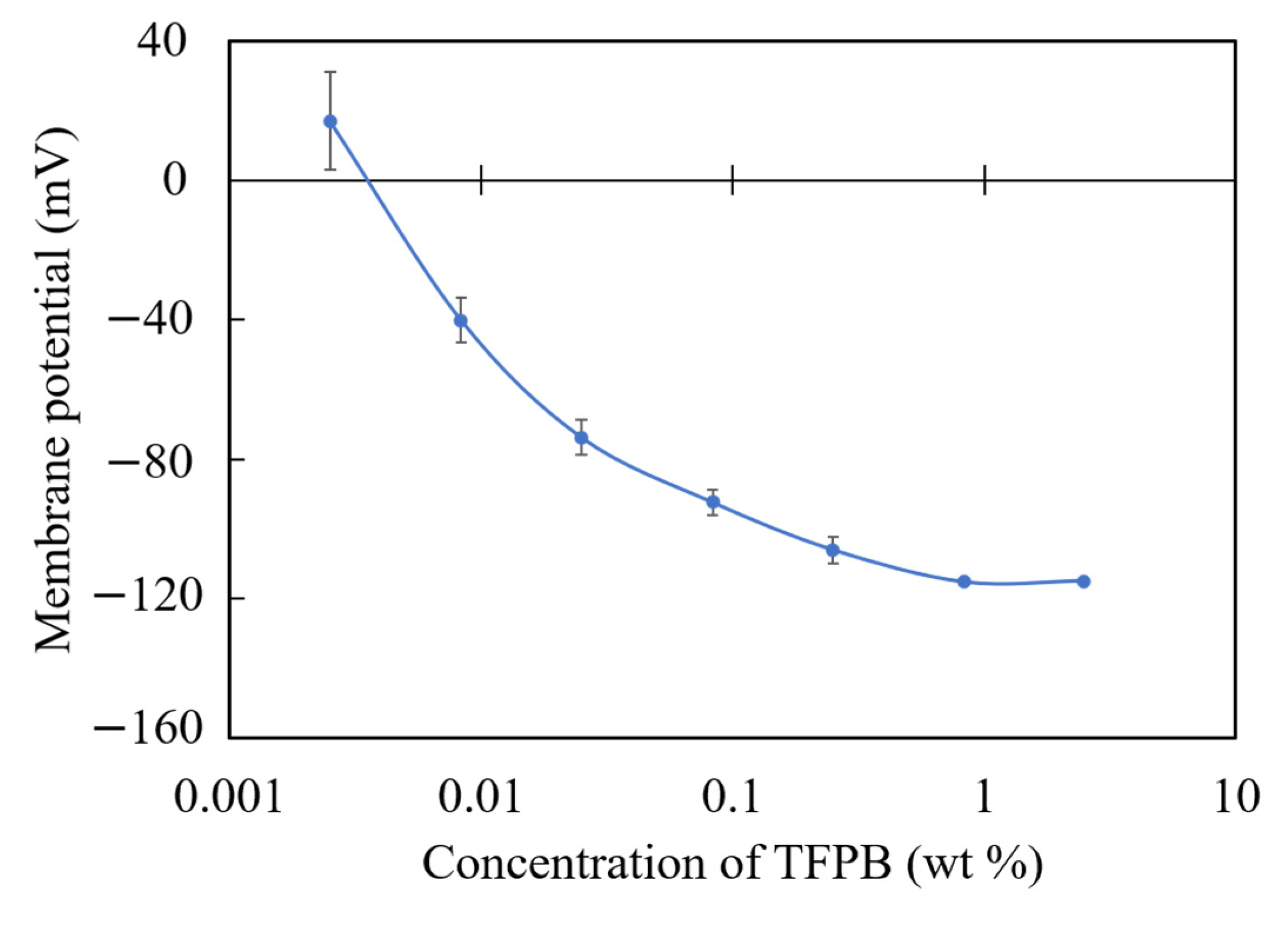
Figure 10 shows the membrane potentials of each TFPB-NPOE membrane in the reference solution. It can be seen that the membrane potential gradually becomes saturated, and a high response is likely to be obtained around the low concentration of TFPB, where a small change in TFPB concentration can make a large change in the membrane potential.
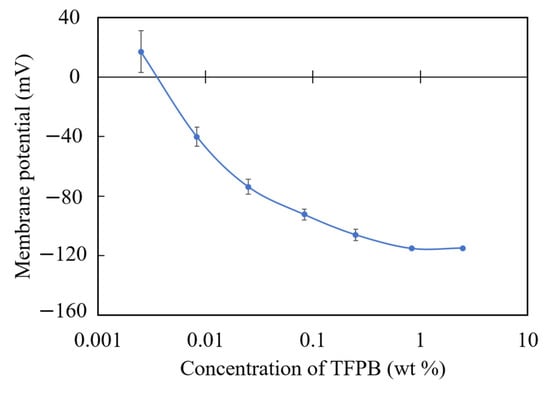
Figure 10.
The membrane potential of TFPB-NPOE membranes with different TFPB contents. The results are expressed as means ± SD (n = 16, 4 membranes × 4 times).
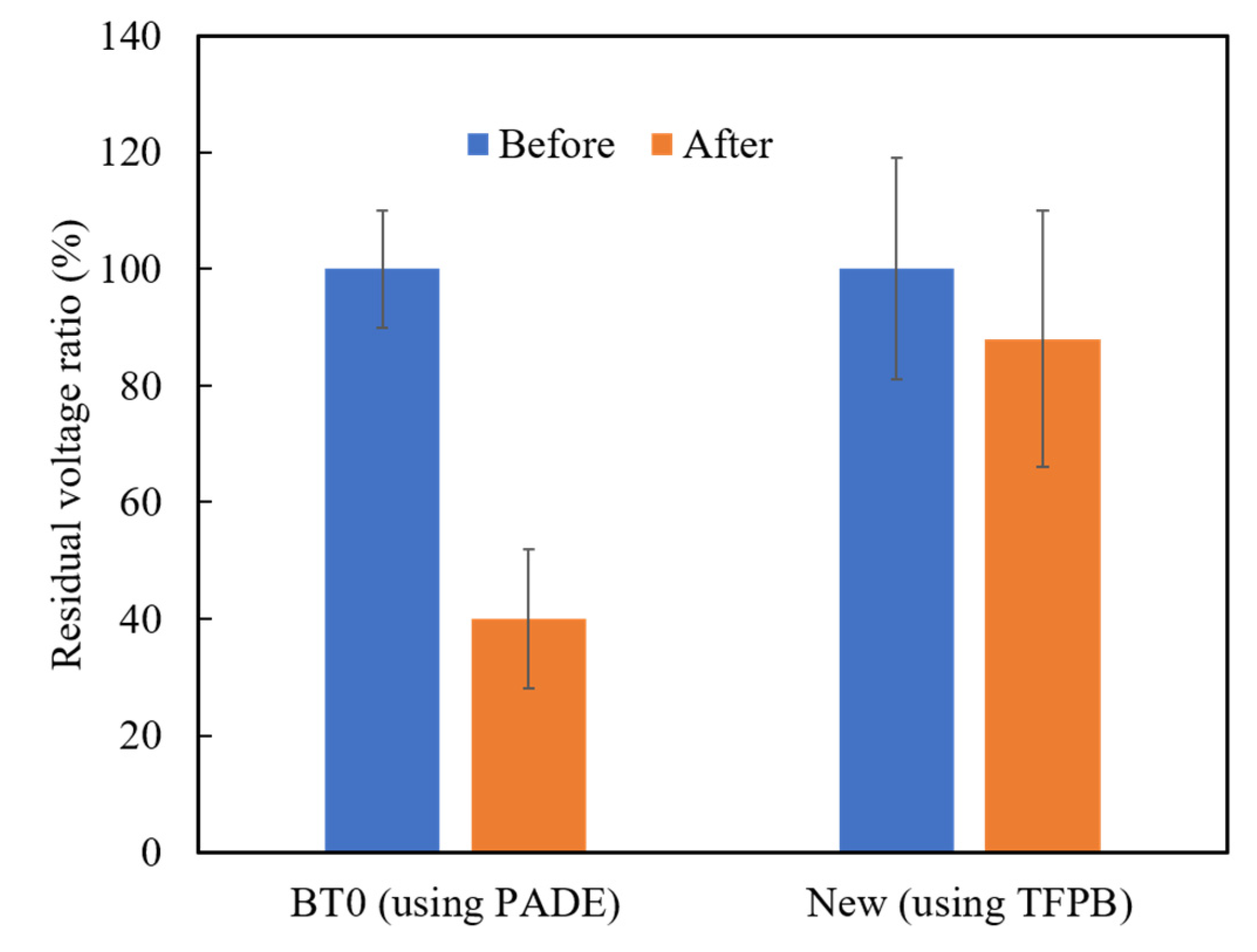
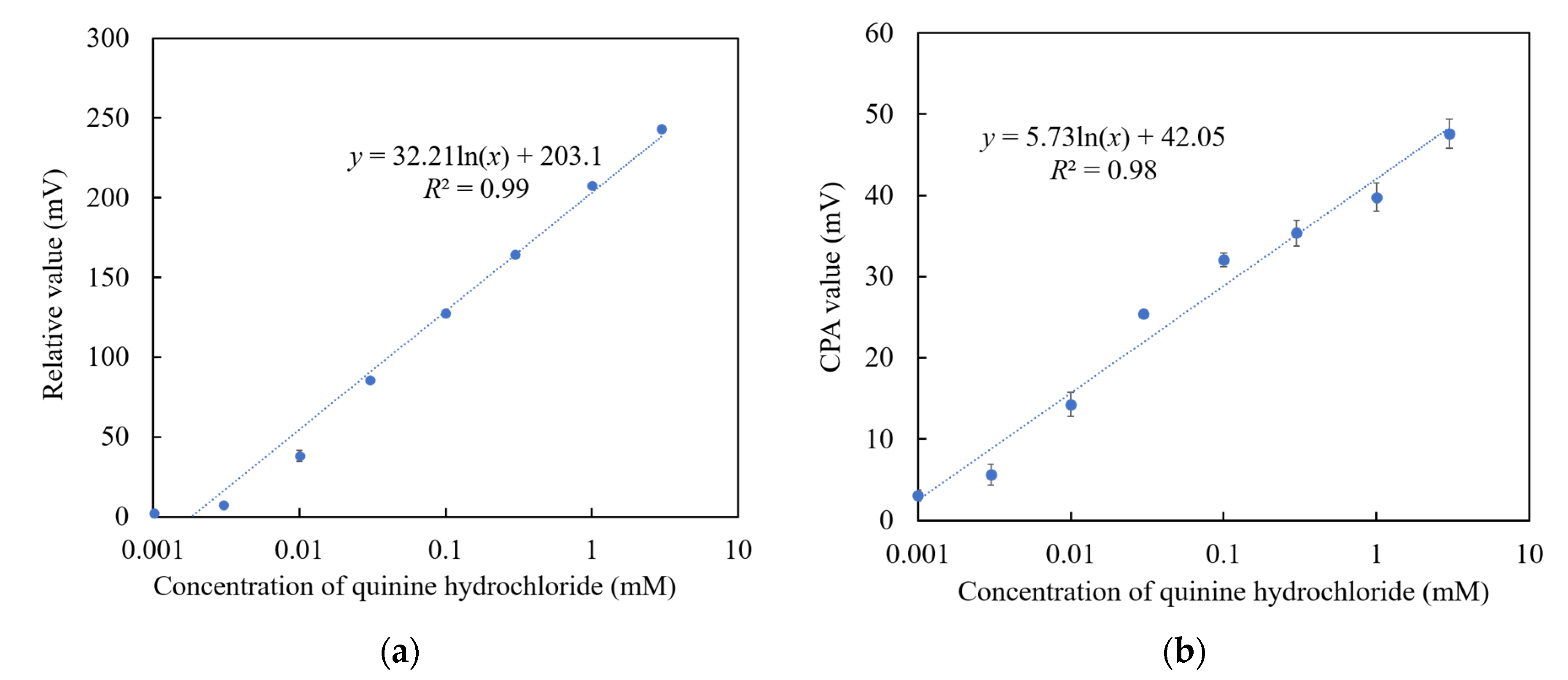
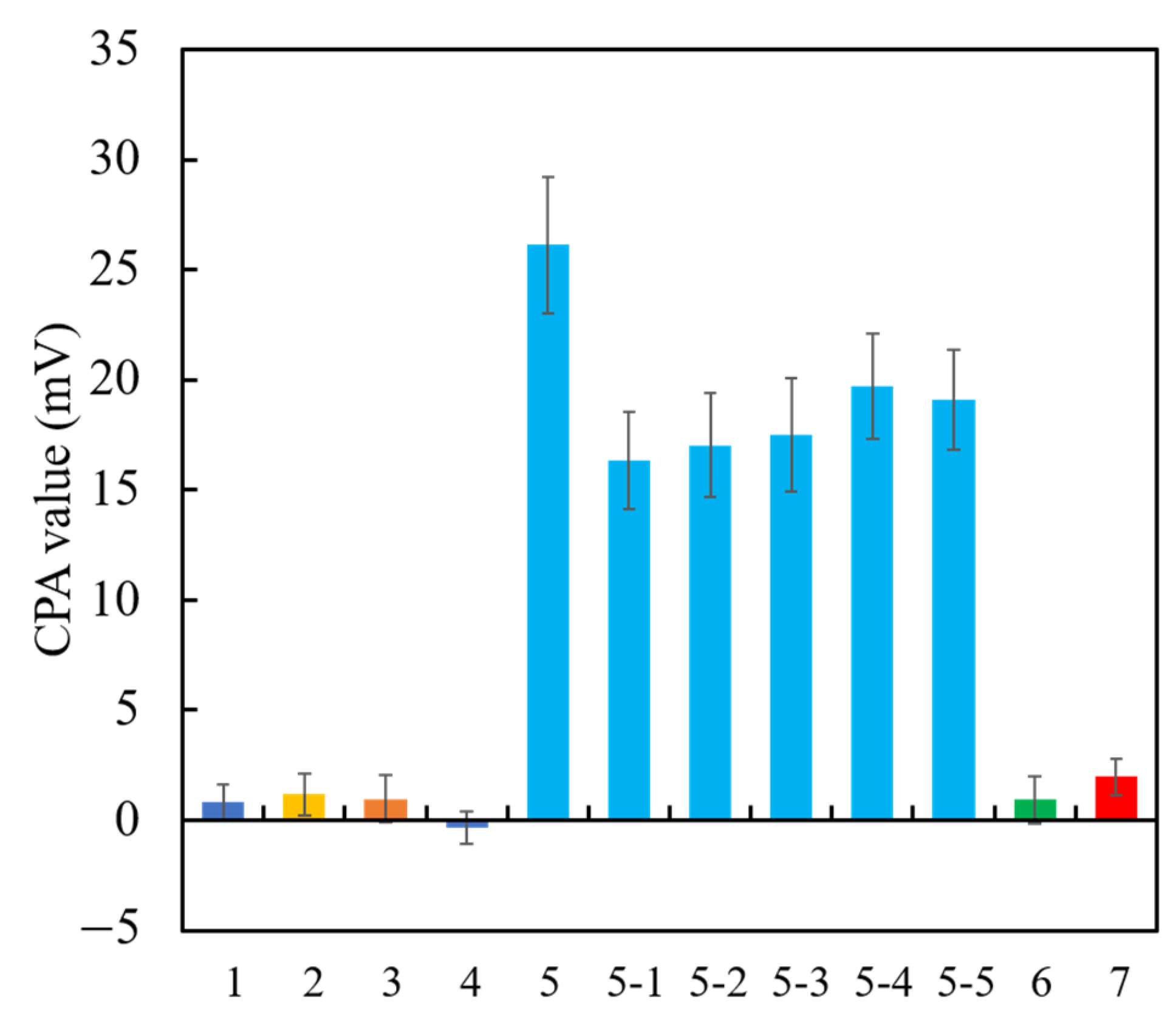
Among the above TFPB-NPOE membranes, the sensor membrane with 0.25 wt % TFPB, which had the highest CPA value response to the quinine hydrochloride, was subjected to the long-term stability test. Figure 11 shows the residual voltage ratios (D), expressed by Equation (2), of the TFPB-NPOE membrane and BT0 sensor. From the results, it can be said that, although the response was somewhat reduced, the reduction was not as large as for the BT0 sensor. Therefore, the response of the TFPB-NPOE membrane is less likely to deteriorate at room temperature and in humid conditions during long-term storage. We investigated the concentration dependence of the TFPB-NPOE sensor for quinine hydrochloride (Figure 12). The results indicated that this sensor response was proportional to the logarithm of the concentration of quinine hydrochloride from 0.001 to 3 mM. In addition, as shown in Figure 13, the TFPB-NPOE sensor membrane responds selectively to the bitter pharmaceutical drugs hydrochloride, but not to any other taste qualities, indicating this sensor has global selectivity to bitterness.
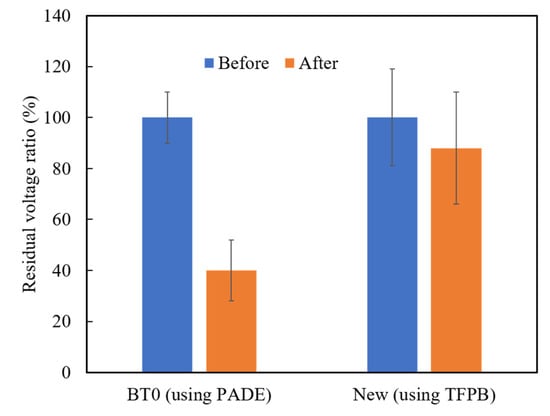
Figure 11.
Residual voltage ratios of the BT0 and new TFPB-NPOE sensor. The blue columns represent the residual voltage ratio before the accelerated deterioration process. The orange columns represent the residual voltage ratio after the accelerated deterioration process. The results are expressed as means ± SD (n = 20, 4 membranes × 5 times).
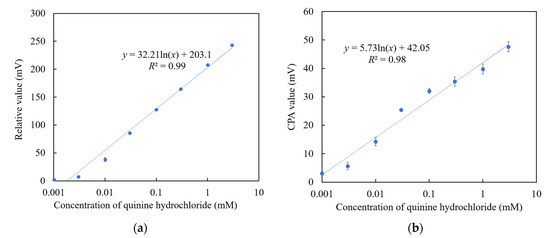
Figure 12.
Concentration dependence of TFPB-NPOE sensor on quinine hydrochloride: (a) Relative values; (b) CPA values. The results are expressed as means ± SD (n = 20, 4 membranes × 5 times).
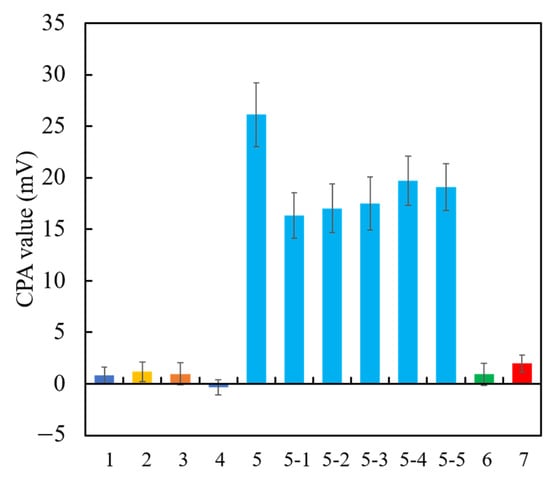
Figure 13.
The CPA values for different taste qualities. (1) Saltiness sample (300 mM KCl); (2) sourness sample (3 mM tartaric acid); (3) umami sample (10 mM sodium glutamate); (4) astringency sample (0.05% tannic acid); (5) bitterness (+) sample (0.1 mM of hydrochloride salts of (5) quinine, (5-1) azelastine, (5-2) cetirizine, (5-3) loperamide, (5-4) eperisone, (5-5) hydroxyzine; (6) bitterness (−) (0.01 vol% iso-alpha acid); (7) sweetness sample (1 M sucrose). All taste samples were prepared with the reference solution.
4. Conclusions
Taste sensors using non-completely dissociative lipids have been used for the taste evaluation of foods and pharmaceuticals. Since the non-completely dissociative lipids are prone to produce an acidic environment in the sensor membrane and affect the degradation rate of the membrane, we attempted to construct a bitterness sensor for the quantification of pharmaceutical bitterness based on TFPB as a completely dissociative anionic amphiphile. With the use of highly hydrophobic TFPB, only a small amount of TFPB can realize a highly hydrophobic membrane surface, which is suitable for the detection of bitter substances. The replacement of the conventional plasticizers BBPA and TBAC with NPOE, which contains impurities with charges opposite to TFPB, avoided the excessive surface charge density and improved the response sensitivity of TFPB membranes to quinine hydrochloride. It necessarily follows that the response sensitivity is determined not only by the amount of adsorption of bitter substances, but also by the surface charge density. Finally, by using the fully dissociated TFPB instead of the conventional PADE, which is a partially dissociated phosphoric acid, response deterioration was significantly prevented. The TFPB-NPOE sensor withstood the long-term stability test and showed high selectivity and sensitivity for pharmaceutical bitterness. This sensor also provided a route for the design of the surface charge density of membrane surfaces using ester plasticizers that contain oppositely charged impurities. In the future, the sensory correlation and the reaction to bitterness suppression effect should be discussed in more detail using commercially available medicines.
As the novelty of our research, taste sensor using a completely dissociative substance is the first attempt ever made. The new attempt helps to improve the long-term stability of bitterness sensor and is also expected to be applied to the membranes of taste sensors using non-completely dissociative lipids other than the bitterness sensor.
Author Contributions
Conceptualization, K.T. and H.I.; methodology, T.S., X.W., Y.T., H.I., and K.T.; experiments, T.S. and X.W.; data analysis, T.S., X.W., Y.T., H.I., and K.T.; writing—original draft preparation, X.W.; writing—review and editing, Y.T. and K.T.; funding acquisition, K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This paper is based on results obtained from a project commissioned by the New Energy and Industrial Development Organization (NEDO).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Hidekazu Ikezaki is the president of Intelligent Sensor Technology, Inc., the maker of the taste sensing system TS–5000Z. Kiyoshi Toko holds stock in Intelligent Sensor Technology, Inc. The other authors declare no conflicts of interest.
References
- Mennella, J.; Spector, A.; Reed, D.C. Bad taste of medicines. Clin. Ther. 2013, 35, 1225–1246. [Google Scholar] [CrossRef] [PubMed]
- Anand, V.; Kataria, M.; Kukkar, V.; Saharan, V.; Choudhury, P.K. The latest trends in the taste assessment of pharmaceuticals. Drug Discov. Today 2007, 12, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Winquist, F. Voltammetric electronic tongues–Basic principles and applications. Microchim. Acta 2008, 163, 3–10. [Google Scholar] [CrossRef]
- Facure, M.H.M.; Mercante, L.A.; Mattoso, L.H.C.; Correa, D.S. Detection of trace levels of organophosphate pesticides using an electronic tongue based on graphene hybrid nanocomposites. Talanta 2017, 167, 59–66. [Google Scholar] [CrossRef]
- Ciosek, P.; Wróblewski, W. Sensor arrays for liquid sensing—Electronic tongue systems. Analyst 2007, 132, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Marx, Í.; Rodrigues, N.; Dias, L.G.; Veloso, A.C.A.; Pereira, J.A.; Drunkler, D.A.; Peres, A.M. Sensory classification of table olives using an electronic tongue: Analysis of aqueous pastes and brines. Talanta 2017, 162, 98–106. [Google Scholar] [CrossRef]
- Citterio, D.; Suzuki, K. Smart taste sensors. Anal. Chem. 2008, 80, 3965–3972. [Google Scholar]
- Nuñez, L.; Cetó, X.; Pividori, M.I.; Zanoni, M.V.B.; del Valle, M. Development and application of an electronic tongue for detection and monitoring of nitrate, nitrite and ammonium levels in waters. Microchem. J. 2013, 110, 273–279. [Google Scholar] [CrossRef]
- Daikuzono, C.M.; Dantas, C.A.R.; Volpati, D.; Constantino, C.J.L.; Piazzetta, M.H.O.; Gobbi, A.L.; Taylor, D.M.; Oliveira, O.N.; Riul, A. Microfluidic electronic tongue. Sens. Actuators B Chem. 2015, 207, 1129–1135. [Google Scholar] [CrossRef]
- Wu, X.; Tahara, Y.; Yatabe, R.; Toko, K. Taste sensor: Electronic tongue with lipid membranes. Anal. Sci. 2020, 36, 147–159. [Google Scholar] [CrossRef]
- Hayashi, N.; Ujihara, T.; Chen, R.; Irie, K.; Ikezaki, H. Objective evaluation methods for the bitter and astringent taste intensities of black and oolong teas by a taste sensor. Food Res. Int. 2013, 53, 816–821. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Habara, M.; Ikezazki, H.; Chen, R.; Naito, Y.; Toko, K. Advanced taste sensors based on artificial lipids with global selectivity to basic taste qualities and high correlation to sensory scores. Sensors 2010, 10, 3411–3443. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Toko, K. Electronic tongues-a review. IEEE Sens. J. 2013, 13, 3001–3011. [Google Scholar] [CrossRef]
- Ito, M.; Wada, K.; Yoshida, M.; Hazekawa, M. Quantitative evaluation of bitterness of H-1-receptor antagonists and masking effect of acesulfame potassium, an artificial sweetener, using a taste sensor. Sens. Mater. 2013, 25, 17–30. [Google Scholar]
- Tahara, Y.; Hattori, T.; Wu, X.; Yatabe, R.; Ikezaki, H.; Habara, M.; Toko, K. Development of sweetness sensor for high-potency sweeteners using lipid polymer membrane. In Proceedings of the 2017 ISOCS/IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Montreal, QC, Canada, 28–31 May 2017; pp. 265–266. [Google Scholar]
- Kobayashi, Y.; Hamada, H.; Yamaguchi, Y.; Ikezaki, H.; Toko, K. Development of an artificial lipid-based membrane sensor with high selectivity and sensitivity to the bitterness of drugs and with high correlation with sensory score. IEEJ Trans. Electr. Electron. Eng. 2009, 4, 710–719. [Google Scholar] [CrossRef]
- Wu, X.; Onitake, H.; Huang, Z.; Shiino, T.; Tahara, Y.; Yatabe, R.; Ikezaki, H.; Toko, K. Improved durability and sensitivity of bitterness-sensing membrane for medicines. Sensors 2017, 17, 2541. [Google Scholar] [CrossRef]
- Kobayashi, H.; Sonoda, T.; Iwamoto, H.; Yoshimura, M. Tetrakis [3,5-di( F -Methyl) phenyl] borate as the first Efficient negatively charged phase transfer catalyst. Kinetic evidences. Chem. Lett. 1981, 10, 579–580. [Google Scholar] [CrossRef]
- Nishida, H.; Takada, N.; Yoshimura, M.; Sonoda, T.; Kobayashi, H. Tetrakis [3,5-bis(trifluoromethyl) phenyl] borate. Highly lipophilic stable anionic agent for solvent-extraction of cations. Bull. Chem. Soc. Jpn. 1984, 57, 2600–2604. [Google Scholar] [CrossRef]
- Nishi, N.; Imakura, S.; Kakiuchi, T. Wide electrochemical window at the interface between water and a hydrophobic room-temperature ionic liquid of tetrakis [3,5-bis(trifluoromethyl) phenyl] borate. Anal. Chem. 2006, 78, 2726–2731. [Google Scholar] [CrossRef]
- Ichikawa, J.; Kobayashi, H.; Sonoda, T. Anionic phase-transfer catalysis with TFPB ion. J. Synth. Org. Chem. Jpn. 1988, 46, 943–954. [Google Scholar] [CrossRef][Green Version]
- Peper, S.; Gonczy, C. Potentiometric response characteristics of membrane-based Cs+-selective electrodes containing ionophore-functionalized polymeric microspheres. Int. J. Electrochem. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Wakida, S.; Masadome, T.; Imato, T. Response mechanism of additive salt effects of potassium-selective neutral carrier based electrode using their liquid membrane based ion-sensitive field. Anal. Sci. 2001, 17, 15–18. [Google Scholar]
- Faozia, H.; Itadani, N.; Nomura, M.; Suzuki, K.; Yasui, T.; Takada, K.; Yuchi, A. Potential changes during in situ formation of carriers for cationic surfactant ion-selective electrodes by conditioning. J. Electroanal. Chem. 2013, 696, 20–23. [Google Scholar] [CrossRef]
- Ying, K.S.; Lee, Y.H.; Hassan, N.I.; Hasbullah, S.A. A new copper ionophore N1, N3-Bis [[3,5-bis(trifluoromethyl)phenyl] carbamothioyl] isophtalamide for potentiometric sensor. Sains Malays. 2018, 47, 2657–2666. [Google Scholar]
- Yatabe, R.; Noda, J.; Tahara, Y.; Naito, Y.; Ikezaki, H.; Toko, K. Analysis of a lipid/polymer membrane for bitterness sensing with a preconditioning process. Sensors 2015, 15, 22439–22450. [Google Scholar] [CrossRef]
- Harada, Y.; Tahara, Y.; Toko, K. Study of the relationship between taste sensor response and the amount of epigallocatechin gallate adsorbed onto a lipid-polymer membrane. Sensors 2015, 15, 6241–6249. [Google Scholar] [CrossRef]
- Fukagawa, T.; Tahara, Y.; Yasuura, M.; Habara, M.; Ikezaki, H.; Toko, K. Relationship between taste sensor response and amount of quinine adsorbed on lipid/polymer membrane. J. Innov. Electron. Commun. 2012, 2, 1–6. [Google Scholar]
- Toko, K.; Hara, D.; Tahara, Y.; Yasuura, M.; Ikezaki, H. Relationship between the amount of bitter substances adsorbed onto lipid/polymer membrane and the electric response of taste sensors. Sensors 2014, 14, 16274–16286. [Google Scholar] [CrossRef]
- Harada, Y.; Noda, J.; Yatabe, R.; Ikezaki, H.; Toko, K. Research on the changes to the lipid/polymer membrane used in the acidic bitterness sensor caused by preconditioning. Sensors 2016, 16, 230. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ghosh, M. Studies on performance evaluation of a green plasticizer made by enzymatic esterification of furfuryl alcohol and castor oil fatty acid. Carbohydr. Polym. 2017, 157, 1076–1084. [Google Scholar] [CrossRef]
- Watanabe, M.; Toko, K.; Sato, K.; Kina, K.; Takahashi, Y.; Iiyama, S. Charged impurities of plassticizer used for ion-selective electrode and taste sensor. Sens. Mater. 1998, 10, 103–112. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).