Structural and Electrochemical Studies of Cobalt(II) and Nickel(II) Coordination Polymers with 6-Oxonicotinate and 4,4′-Bipyridine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Physical Measurements
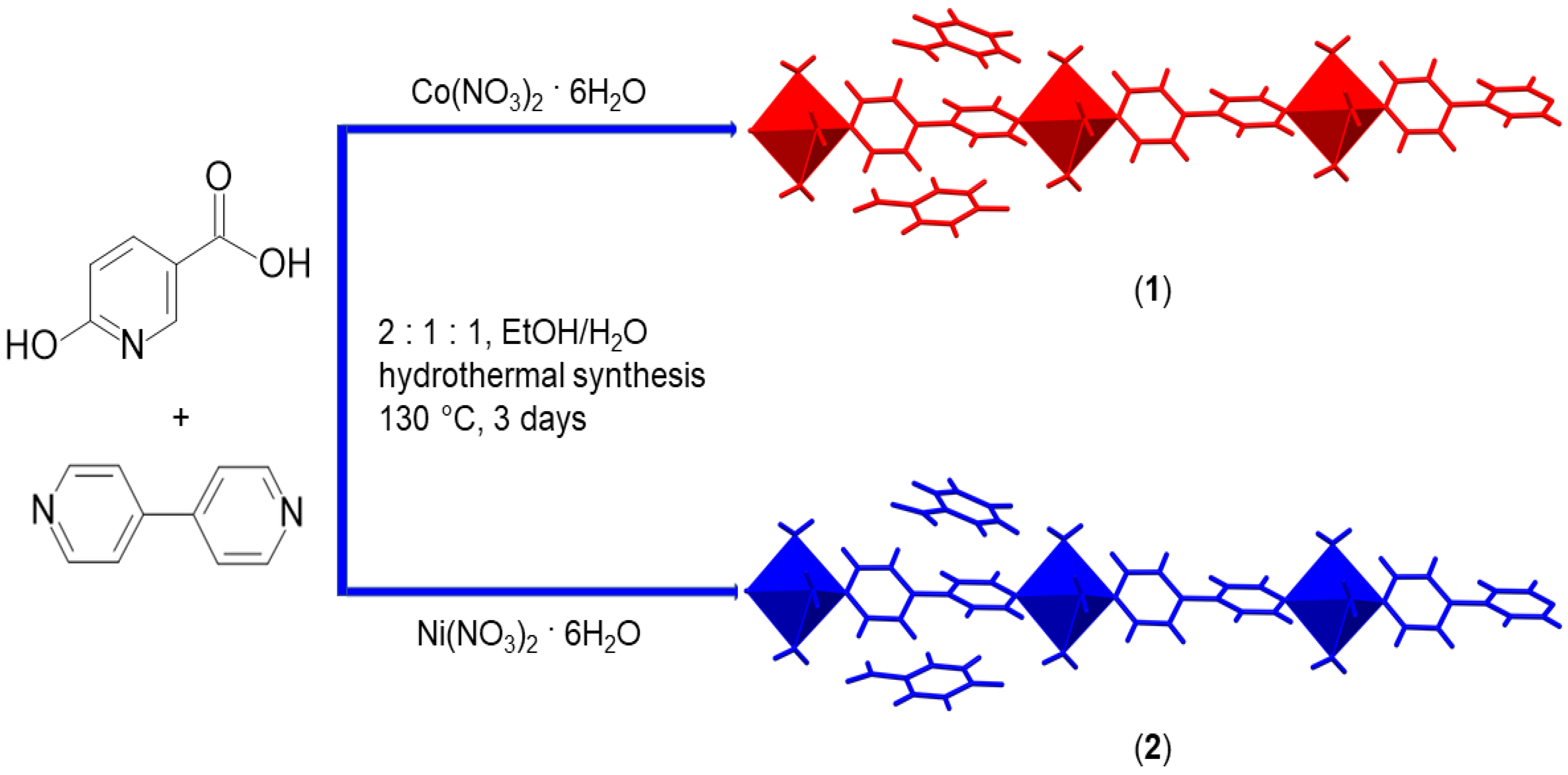
2.2. Preparation of Coordination Polymers 1 and 2
2.3. Electrochemical Measurements
2.4. X-ray Crystallographic Analysis
3. Results and Discussion
3.1. Syntheses
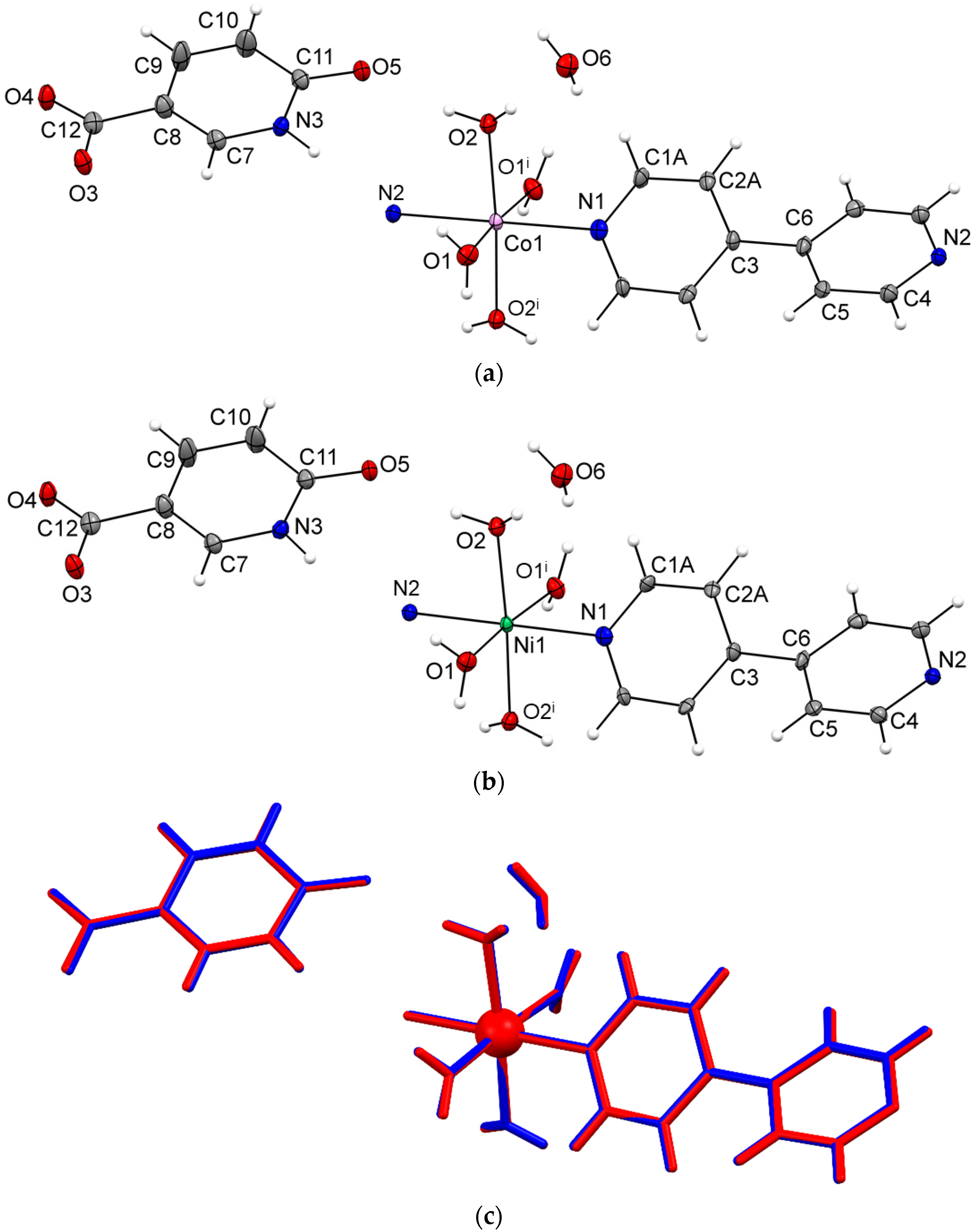
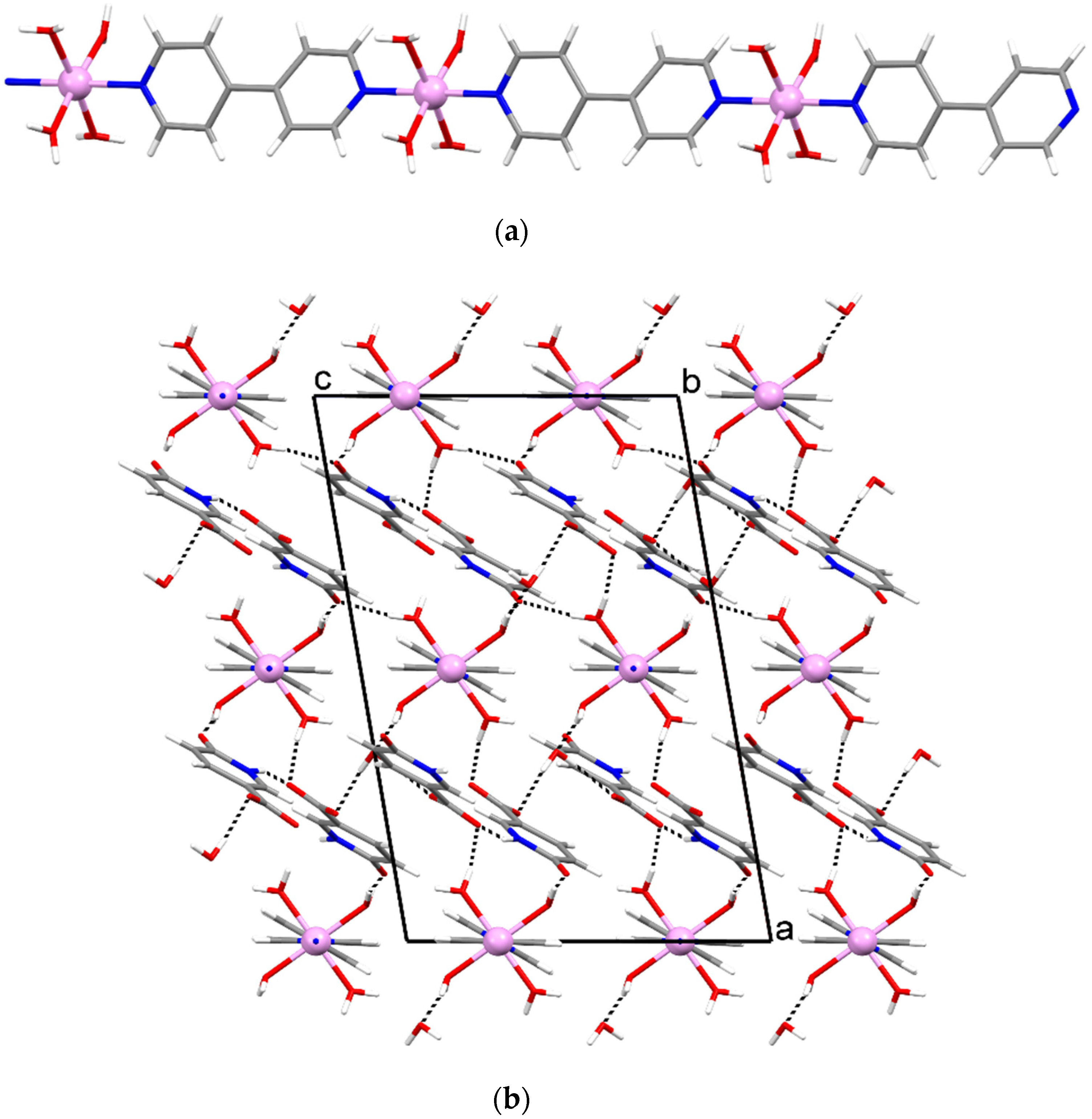
3.2. Crystal Structures
3.3. Thermal Analysis
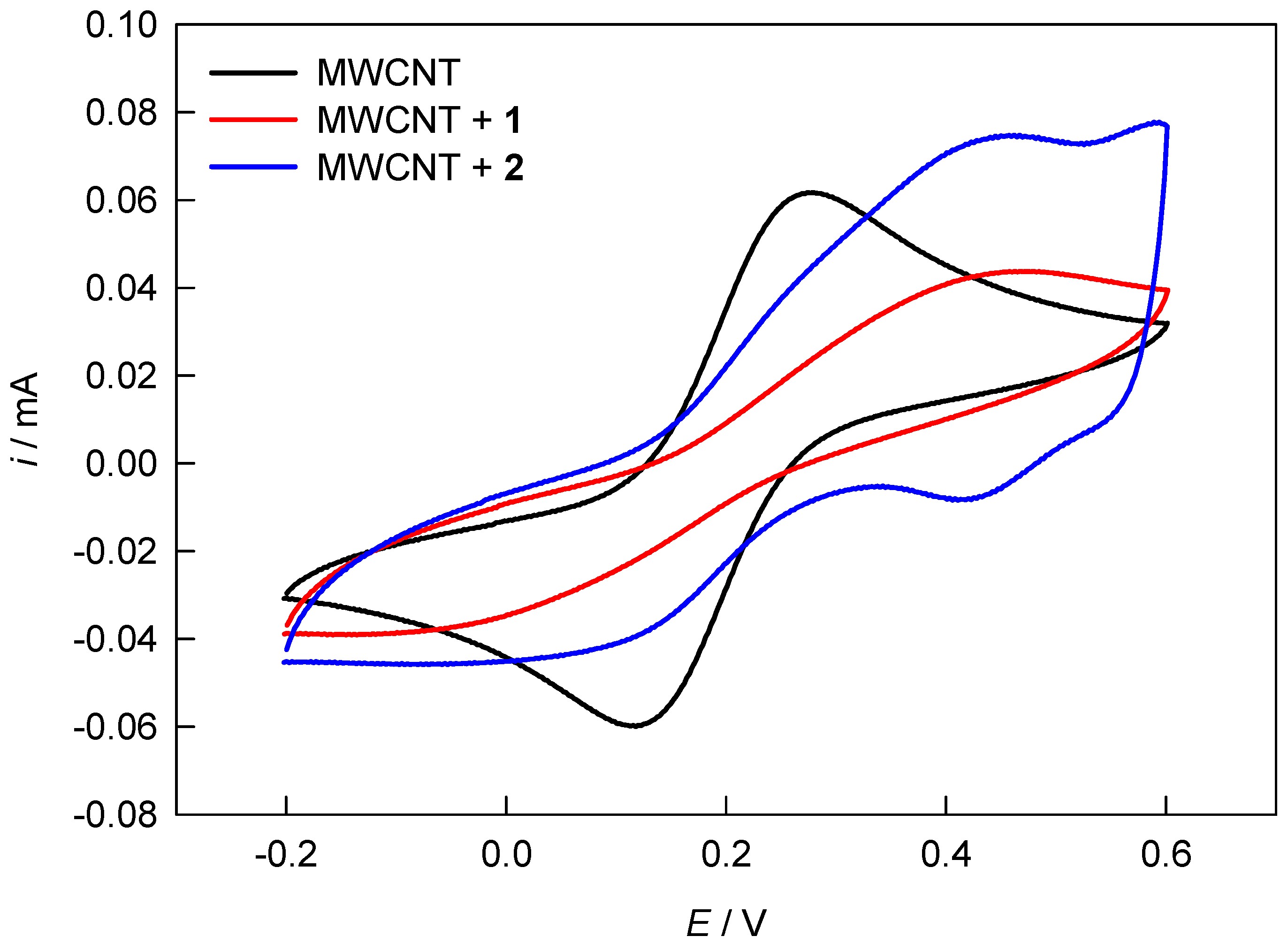
3.4. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.-X.; Du, Z.-X.; Xiong, L.-Y.; Fu, L.-L.; Bo, W.-B. Supramolecular isomerism in two nickel(II) coordination polymers constructed with the flexible 2-carboxyphenoxyacetate linker: Syntheses, structure analyses and magnetic properties. J. Solid State Chem. 2021, 293, 121799. [Google Scholar] [CrossRef]
- Bosch, M.; Yuan, S.; Rutledge, W.; Zhou, H.-C. Stepwise synthesis of metal–organic frameworks. Acc. Chem. Res. 2017, 50, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-X.; Liao, P.-Q.; Lin, R.-B.; Wei, Y.-S.; Zeng, M.-H.; Chen, X.-M. Metal cluster-based functional porous coordination polymers. Coord. Chem. Rev. 2015, 293–294, 263–278. [Google Scholar] [CrossRef]
- Zeng, M.-H.; Yin, Z.; Liu, Z.-H.; Xu, H.-B.; Feng, Y.-C.; Hu, Y.-Q.; Chang, L.-X.; Zhang, Y.-X.; Huang, J.; Kurmoo, M. Assembly of a highly stable luminescent Zn5 cluster and application to bio-imaging. Angew. Chem. Int. Ed. 2016, 55, 11407–11411. [Google Scholar] [CrossRef]
- Zeng, M.-H.; Yin, Z.; Tan, Y.-X.; Zhang, W.-X.; He, Y.-P.; Kurmoo, M. Nanoporous cobalt(II) MOF exhibiting four magnetic ground states and changes in gas sorption upon post-synthetic modification. J. Am. Chem. Soc. 2014, 136, 4680–4688. [Google Scholar] [CrossRef]
- Douvali, A.; Tsipis, A.C.; Eliseeva, S.V.; Petoud, S.; Papaefstathiou, G.S.; Malliakas, C.D.; Papadas, I.; Armatas, G.S.; Margiolaki, I.; Kanatzidis, M.G.; et al. Turn-on luminescence sensing and real-time detection of traces of water in organic solvents by a flexible metal–organic framework. Angew. Chem. Int. Ed. 2015, 54, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yuan, S.; Chen, X.-Y.; Chang, Y.-J.; Day, G.; Gu, Z.-Y.; Zhou, H.-C. Two-dimensional metal–organic framework nanosheets as an enzyme inhibitor: Modulation of the α-chymotrypsin activity. J. Am. Chem. Soc. 2017, 139, 8312–8319. [Google Scholar] [CrossRef]
- Zhou, Z.; He, C.; Yang, L.; Wang, Y.; Liu, T.; Duan, C. Alkyne activation by a porous silver coordination polymer for heterogeneous catalysis of carbon dioxide cycloaddition. ACS Catal. 2017, 7, 2248–2256. [Google Scholar] [CrossRef]
- Suh, M.P.; Cheon, Y.E.; Lee, E.Y. Syntheses and functions of porous metallosupramolecular networks. Coord. Chem. Rev. 2008, 252, 1007–1026. [Google Scholar] [CrossRef]
- Swiegers, G.F.; Malefetse, T.J. New self-assembled structural motifs in coordination chemistry. Chem. Rev. 2000, 100, 3483–3538. [Google Scholar] [CrossRef]
- Wen, L.; Lu, Z.; Lin, L.G.; Tian, Z.; Zhu, H.; Meng, Q. Syntheses, structures, and physical properties of three novel metal−organic frameworks constructed from aromatic polycarboxylate acids and flexible imidazole-based synthons. Cryst. Growth Des. 2007, 7, 93–99. [Google Scholar] [CrossRef]
- Wang, Y.; Englert, U. The first linear coordination oligomer linked by 4,4′-bipyridine. Eur. J. Inorg. Chem. 2007, 5623–5625. [Google Scholar] [CrossRef]
- Schönfeld, S.; Dankhoff, K.; Baabe, D.; Zaretzke, M.-K.; Bröring, M.; Schötz, K.; Köhler, A.; Hörner, G.; Weber, B. Iron(II) spin crossover complexes based on a redox active equatorial Schiff-base-like ligand. Inorg. Chem. 2020, 59, 8320–8333. [Google Scholar] [CrossRef] [PubMed]
- Karushev, M.A. Novel cobalt metallopolymer with redox-matched conjugated organic backbone via electropolymerization of a readily available N4 cobalt complex. Polymers 2021, 13, 1667. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ding, D.; Jiang, X.; Zhou, B. Electrochemical sensors based on copper–cadmium bimetallic porphyrin coordination polymers with various Cu/Cd ratios. J. Analyt. Chem. 2021, 76, 772–778. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.-X.; Zhang, Y.; Xu, N.; Wang, X.-L. A series of cobalt-based coordination polymer crystalline materials as highly sensitive electrochemical sensors for detecting trace Cr(VI), Fe(III) ions, and ascorbic acid. Cryst. Growth Des. 2021, 21, 4390–4397. [Google Scholar] [CrossRef]
- Huang, Y.; Luo, M.; Li, S.; Xia, D.; Tang, Z.; Hu, S.; Ye, S.; Sun, M.; He, C.; Shu, D. Efficient catalytic activity and bromate minimization over lattice oxygen-rich MnOOH nanorods in catalytic ozonation of Bromide-containing organic pollutants: Lattice oxygen-directed redox cycle and bromate reduction. J. Hazard. Mater. 2021, 410, 124545. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, J.; Zhang, R.; Shi, H.; Wang, N.; Li, J.; Ma, F.; Zhang, D. Cu-based metal−organic framework as a novel sensing platform for the enhanced electro-oxidation of nitrite. Sens. Actuators B 2016, 222, 632–637. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Xu, N.; Chang, Z.; Liu, G.; Lin, H.; Wang, X. Four octamolybdate complexes constructed from a quinoline−imidazole−monoamide ligand: Structures and electrochemical, photocatalytic and magnetic properties. CrystEngComm 2020, 22, 8322–8329. [Google Scholar] [CrossRef]
- Cui, Z.-W.; Wang, X.-L.; Lin, H.-Y.; Xu, N.; Wang, X.; Liu, G.-C.; Chang, Z.-H. Two Anderson-type polyoxometalate-based metal−organic complexes with a flexible bis(pyrazine)-bis(amide) ligand for rapid adsorption and selective separation of cationic dyes. Inorg. Chim. Acta 2020, 513, 119937. [Google Scholar] [CrossRef]
- Zhou, S.F.; Hao, B.B.; Lin, T.; Zhang, C.X. A dual-functional MOF for high proton conduction and sensitive detection of ascorbic acid. Dalton Trans. 2020, 49, 14490–14496. [Google Scholar] [CrossRef]
- Li, Y.; Xie, M.; Zhang, X.; Liu, Q.; Lin, D.; Xu, C.; Xie, F.; Sun, X. Co-MOF nanosheet array: A high-performance electrochemical sensor for non-enzymatic glucose detection. Sens. Actuators B 2019, 278, 126–132. [Google Scholar] [CrossRef]
- Pang, L.Y.; Wang, P.; Gao, J.J.; Wen, Y.; Liu, H. An active metal-organic anion framework with highly exposed SO42− on {001} facets for the enhanced electrochemical detection of trace Fe3+. J. Electroanal. Chem. 2019, 836, 85–93. [Google Scholar] [CrossRef]
- Hok, L.; Lluch Sanchez, E.; Vianello, R.; Kukovec, B.-M.; Popović, Z. Self-assembly of cobalt(II) coordination polymers with differently halosubstituted nicotinate ligands and 4,4’-bipyridine—The effect of the halosubstituent positions on polymer types. Eur. J. Inorg. Chem. 2021, 1470–1480. [Google Scholar] [CrossRef]
- Politeo, N.; Pisačić, M.; Đaković, M.; Sokol, V.; Kukovec, B.-M. The first coordination compound of 6-fluoronicotinate: The crystal structure of a one-dimensional nickel(II) coordination polymer containing the mixed ligands 6-fluoronicotinate and 4,4′-bipyridine. Acta Crystallogr. 2020, E76, 500–505. [Google Scholar] [CrossRef]
- Politeo, N.; Pisačić, M.; Đaković, M.; Sokol, V.; Kukovec, B.-M. Synthesis and crystal structure of a 6-chloronicotinate salt of a one-dimensional cationic nickel(II) coordination polymer with 4,4′-bipyridine. Acta Crystallogr. 2020, E76, 599–604. [Google Scholar] [CrossRef] [Green Version]
- SAINT (including XPREP); Bruker AXS Inc.: Madison, WI, USA, 2012.
- Sheldrick, G.M. SADABS: Area-Detector Absorption Correction; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Q.; Kong, Z.-P.; Lin, J.-L. Crystal structure of tetraaqua(4,4’-bipyridine-N,N’)nickel(II) fumarate tetrahydrate, Ni(H2O)4(C10H8N2)(C4H2O4) · 4H2O. Z. Kristallogr. New Cryst. Struct. 2002, 217, 195–196. [Google Scholar] [CrossRef]
- Gong, Y.-N.; Liu, C.-B.; Huang, D.-H.; Xiong, Z.-Q. Crystal structure of tetraaqua-(4,4’-bipyridyl)zinc(II) 3-(4-(carboxymethoxy)phenyl)propanoate, [Ni(H2O)4(C10H8N2)][C11H10O5]. Z. Kristallogr. New Cryst. Struct. 2009, 224, 421–422. [Google Scholar] [CrossRef]
- Li, N.-Y. catena-Poly[[[tetraaquanickel(II)]-μ-4,4’-bipyridyl-κ2N:N’] 3,3’-(p-phenylene)diacrylate]. Acta Crystallogr. 2011, E67, m1397. [Google Scholar] [CrossRef] [Green Version]
- Gao, P.; Bai, H.; Bing, Y.-Y.; Hu, M. Syntheses, structures, and properties of transition metal coordination polymers based on a long semirigid tetracarboxylic acid and multidentate N-donor ligands. Solid State Sci. 2016, 52, 118–125. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Li, W.-J.; Che, P. Impact of reaction conditions on the structures of nickel(II) complexes based on 3-(4-Carboxyphenyl)propionic acid. Z. Anorg. Allg. Chem. 2013, 639, 129–133. [Google Scholar] [CrossRef]
- Wang, X.-L.; Qin, C.; Wang, E.-B. Polythreading of infinite 1D chains into different structural motifs: Two poly(pseudo-rotaxane) architectures constructed by concomitant coordinative and hydrogen bonds. Cryst. Growth Des. 2006, 6, 439–443. [Google Scholar] [CrossRef]
- Sanram, S.; Boonmak, J.; Youngme, S. Ni(II)-metal–organic frameworks based on 1,4-phenylenedipropionic acid: Solvothermal syntheses, structures, and photocatalytic properties. Polyhedron 2016, 119, 151–159. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, Q. Crystal structure of tetraaqua-(4,4’-bipyridine-κ2N,N’)nickel(II) 2,3-naphthalenedicarboxylate, [Ni(H2O)4(C10H8N2)][C12H6O4]. Z. Kristallogr. New Cryst. Struct. 2010, 225, 155–156. [Google Scholar] [CrossRef]
- Verma, S.K.; Singh, V.K. [M{κ2S,S-S2C-piperazine-C2H4N=C(R)}n] {Co(III), Ni(II), Cu(II) or Zn(II)} complexes bearing pendant Schiff base moieties: Spectral characterization, fluorescence, cyclic voltammetric and TGA/DTA study. J. Coord. Chem. 2015, 68, 1072–1087. [Google Scholar] [CrossRef]

| Compound | 1 | 2 |
|---|---|---|
| Formula | {[C10H16CoN2O4](C6H4NO3)2·2H2O}n | {[C10H16NiN2O4](C6H4NO3)2·2H2O}n |
| Mr | 599.41 | 599.19 |
| Crystal system, space group | monoclinic, C2/c (No. 15) | monoclinic, C2/c (No. 15) |
| a (Å) | 19.0114(13) | 18.9891(9) |
| b (Å) | 11.4133(7) | 11.3111(5) |
| c (Å) | 12.0374(7) | 12.0639(6) |
| β (°) | 99.333(2) | 99.169(2) |
| V (Å3) | 2577.3(3) | 2558.1(2) |
| Z | 4 | 4 |
| Dcalc (g cm−3) | 1.545 | 1.556 |
| μ (mm−1) | 0.737 | 0.830 |
| R [I ≥ 2σ(I)] | 0.0480 | 0.0473 |
| wR [all data] | 0.1260 | 0.1228 |
| D–H···A | d(D–H)/Å | d(H···A)/Å | d(D···A)/Å | ∠(D–H···A)/° | Symmetry Code on A |
|---|---|---|---|---|---|
| 1 | |||||
| N3–H31···O3 | 0.88(1) | 1.88(2) | 2.732(4) | 163(4) | −x + 1/2, y + 1/2, −z + 3/2 |
| O1–H11···O5 | 0.84(1) | 1.85(1) | 2.680(4) | 172(4) | x, −y + 1, z + 1/2 |
| O1–H12···O3 | 0.84(1) | 1.87(1) | 2.699(4) | 169(4) | −x + 1/2, y + 1/2, −z + 3/2 |
| O2–H21···O6 | 0.84(1) | 1.87(1) | 2.704(4) | 170(5) | x, y, z |
| O2–H22···O5 | 0.84(1) | 1.90(2) | 2.729(4) | 167(5) | x, y, z |
| O6–H61···O4 | 0.84(1) | 1.99(1) | 2.823(4) | 175(5) | −x + 1/2, −y + 1/2, −z + 1 |
| O6–H62···O4 | 0.84(1) | 1.92(1) | 2.755(4) | 175(4) | x, y + 1, z |
| C1B–H1B···O1 | 0.95 | 2.45 | 2.989(9) | 116 | −x + 1, y, −z + 3/2 |
| C7–H7···O4 | 0.95 | 2.60 | 3.287(5) | 130 | −x + 1/2, y + 1/2, −z + 3/2 |
| 2 | |||||
| N3–H31···O3 | 0.88(1) | 1.88(2) | 2.721(4) | 161(4) | −x + 1/2, y + 1/2, −z + 3/2 |
| O1–H11···O5 | 0.84(1) | 1.86(2) | 2.687(4) | 166(5) | x, −y + 1, z + 1/2 |
| O1–H12···O3 | 0.84(1) | 1.86(1) | 2.696(4) | 171(4) | −x + 1/2, y + 1/2, −z + 3/2 |
| O2–H21···O6 | 0.84(1) | 1.87(1) | 2.702(4) | 172(5) | x, y, z |
| O2–H22···O5 | 0.84(1) | 1.90(2) | 2.724(4) | 165(5) | x, y, z |
| O6–H61···O4 | 0.84(1) | 1.99(1) | 2.823(4) | 175(4) | −x + 1/2, −y + 1/2, −z + 1 |
| O6–H62···O4 | 0.84(1) | 1.91(1) | 2.748(4) | 174(5) | x, y + 1, z |
| C1B–H1B···O1 | 0.95 | 2.40 | 2.923(9) | 115 | −x + 1, y, −z + 3/2 |
| C7–H7···O4 | 0.95 | 2.57 | 3.270(5) | 131 | −x + 1/2, y + 1/2, −z + 3/2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škugor Rončević, I.; Vladislavić, N.; Chatterjee, N.; Sokol, V.; Oliver, C.L.; Kukovec, B.-M. Structural and Electrochemical Studies of Cobalt(II) and Nickel(II) Coordination Polymers with 6-Oxonicotinate and 4,4′-Bipyridine. Chemosensors 2021, 9, 352. https://doi.org/10.3390/chemosensors9120352
Škugor Rončević I, Vladislavić N, Chatterjee N, Sokol V, Oliver CL, Kukovec B-M. Structural and Electrochemical Studies of Cobalt(II) and Nickel(II) Coordination Polymers with 6-Oxonicotinate and 4,4′-Bipyridine. Chemosensors. 2021; 9(12):352. https://doi.org/10.3390/chemosensors9120352
Chicago/Turabian StyleŠkugor Rončević, Ivana, Nives Vladislavić, Nabanita Chatterjee, Vesna Sokol, Clive L. Oliver, and Boris-Marko Kukovec. 2021. "Structural and Electrochemical Studies of Cobalt(II) and Nickel(II) Coordination Polymers with 6-Oxonicotinate and 4,4′-Bipyridine" Chemosensors 9, no. 12: 352. https://doi.org/10.3390/chemosensors9120352
APA StyleŠkugor Rončević, I., Vladislavić, N., Chatterjee, N., Sokol, V., Oliver, C. L., & Kukovec, B.-M. (2021). Structural and Electrochemical Studies of Cobalt(II) and Nickel(II) Coordination Polymers with 6-Oxonicotinate and 4,4′-Bipyridine. Chemosensors, 9(12), 352. https://doi.org/10.3390/chemosensors9120352







