Core-Shell Iron-Nickel Hexacyanoferrate Nanoparticle-Based Sensors for Hydrogen Peroxide Scavenging Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Objects of Analysis
2.2. Sensor Preparation
2.3. Amperometric Measurements
3. Results
3.1. Analytical Performances of PB-NiHCF Nanoparticle-Based Sensors for Hydrogen Peroxide
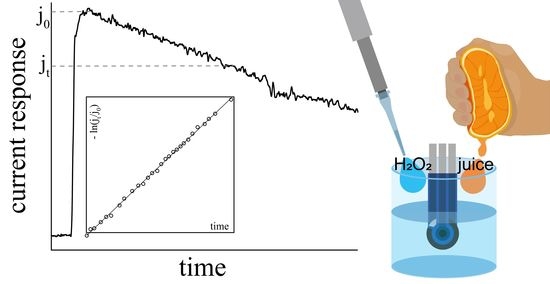
3.2. Hydrogen Peroxide Scavenging Assay as a Tool for Total Antioxidant Activity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khurana, S.; Piche, M.; Hollingsworth, A.; Venkataraman, K.; Tai, T.C. Oxidative stress and cardiovascular health: Therapeutic potentialof polyphenols. Can. J. Physiol. Pharmacol. 2013, 91, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.N.; Firuzi, O.; Gama, M.J.; van Horssen, J.; Saso, L. Oxidative Stress and Antioxidants in Neurological Diseases: Is There Still Hope? Curr. Drug Targets 2017, 18, 705–718. [Google Scholar] [CrossRef]
- Castelli, V.; Paladini, A.; d’Angelo, M.; Allegretti, M.; Mantelli, F.; Brandolini, L.; Cocchiaro, P.; Cimini, A.; Varrass, G. Taurine and oxidative stress in retinal health and disease. CNS Neurosci. Ther. 2021, 27, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Kitada, M.; Koya, D. Sirtuins and Renal Oxidative Stress. Antioxidants 2021, 10, 1198. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Perez-Matute, P.; Zulet, M.A.; Martinez, J.A. Reactive species and diabetes: Counteracting oxidative stress to improve health. Curr. Opin. Pharmacol. 2009, 9, 771–779. [Google Scholar] [CrossRef]
- Reddy, V.P.; Zhu, X.; Perry, G.; Smith, M.A. Oxidative Stress in Diabetes and Alzheimer’s Disease. J. Alzheimers Dis. 2009, 16, 763–774. [Google Scholar] [CrossRef]
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef]
- Salmon, A.B.; Richardson, A.; Pérez, V.I. Update on the oxidative stress theory of aging: Does oxidative stress play a role in aging or healthy aging? Free Radic. Biol. Med. 2010, 48, 642–655. [Google Scholar]
- Hughes, M.; Williams, G.; Pageon, H.; Fourtanier, A.; Green, A. Dietary Antioxidant Capacity and Skin Photoaging: A 15-Year Longitudinal Study. J. Investig. Dermatol. 2021, 141, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Comert, E.D.; Gokmen, V. Evolution of food antioxidants as a core topic of food science for a century. Food Res. Int. 2018, 105, 76–93. [Google Scholar] [CrossRef]
- Ohkatsu, Y.; Suzuki, F. Synergism between Phenolic Antioxidants in Autoxidation. J. Jpn. Petrol. Inst. 2011, 54, 22–29. [Google Scholar] [CrossRef][Green Version]
- Pereira, R.B.; Sousa, C.; Costa, A.; Andrade, P.B.; Valentao, P. Glutathione and the Antioxidant Potential of Binary Mixtures with Flavonoids: Synergisms and Antagonisms. Molecules 2013, 18, 8858–8872. [Google Scholar] [CrossRef]
- Celik, E.E.; Gokmen, V.; Skibsted, L.H. Synergism between Soluble and Dietary Fiber Bound Antioxidants. J. Agric. Food Chem. 2015, 63, 2338–2343. [Google Scholar] [CrossRef] [PubMed]
- Sazhina, N.; Plotnikov, E.; Korotkova, E.; Dorozhko, E.; Voronova, O. Electrochemical Oxidability of Antioxidants: Synergism and Antagonism in Mixes. J. Pharm. Bioallied Sci. 2018, 10, 60–65. [Google Scholar]
- Ghani, M.A.; Barril, C.; Bedgood, D.R., Jr.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef]
- Ghani, M.A.; Barril, C.; Bedgood, D.R., Jr.; Prenzler, P.D. Substrate and TBARS variability in a multi-phase oxidation system. Eur. J. Lipid Sci. Technol. 2017, 119, 1500500. [Google Scholar] [CrossRef]
- Juárez-Gómez, J.; Ramírez-Silva, M.T.; Guzmán-Hernández, D.S.; Romero-Romo, M.; Palomar-Pardavé, M. Novel electrochemical method to evaluate the antioxidant capacity of infusions and beverages, based on in situ formation of free superoxide radicals. Food Chem. 2020, 332, 127409. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar]
- Ilyasov, I.R.; Beloborodov, B.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Salikhova, I.; Budnikov, H. Coulometric titration with electrogenerated oxidants as a tool for evaluation of cognac and brandy antioxidant properties. Food Chem. 2014, 150, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Brainina, K.Z.; Varzakova, D.P.; Gerasimova, E.L. A chronoamperometric method for determining total antioxidant activity. J. Anal. Chem. 2012, 67, 364–369. [Google Scholar] [CrossRef]
- Papadopoulos, K.; Triantis, T.; Yannakopoulou, E.; Nikokavoura, A.; Dimotikali, D. Comparative studies on the antioxidant activity of aqueous extracts of olive oils and seed oils using chemiluminescence. Anal. Chim. Acta 2003, 494, 41–47. [Google Scholar] [CrossRef]
- Karyakin, A.A. Advances of Prussian blue and its analogues in (bio)sensors. Curr. Opin. Electrochem. 2017, 5, 92–98. [Google Scholar] [CrossRef]
- Lukachova, L.V.; Kotel’nikova, E.A.; D’Ottavi, D.; Shkerin, E.A.; Karyakina, E.E.; Moscone, D.; Palleschi, G.; Curulli, A.; Karyakin, A.A. Nonconducting Polymers on Prussian Blue Modified Electrodes: Improvement of Selectivity and Stability of the Advanced H2O2 Transducer. IEEE Sens. J. 2003, 3, 326–332. [Google Scholar] [CrossRef]
- Karyakina, E.E.; Vokhmyanina, D.V.; Sizova, N.V.; Sabitov, A.N.; Borisova, A.V.; Sazontova, T.G.; Arkhipenko, Y.V.; Tkachuk, V.A.; Zolotov, Y.A.; Karyakin, A.A. Kinetic approach for evaluation of total antioxidant activity. Talanta 2009, 80, 749–753. [Google Scholar] [CrossRef]
- Sitnikova, N.A.; Borisova, A.V.; Komkova, M.A.; Karyakin, A.A. Superstable Advanced Hydrogen Peroxide Transducer Based on Transition Metal Hexacyanoferrates. Anal. Chem. 2011, 83, 2359–2363. [Google Scholar] [CrossRef]
- Sitnikova, N.A.; Komkova, M.A.; Khomyakova, I.V.; Karyakina, E.E.; Karyakin, A.A. Transition Metal Hexacyanoferrates in Electrocatalysis of H2O2 Reduction: An Exclusive Property of Prussian Blue. Anal. Chem. 2014, 86, 4131–4134. [Google Scholar] [CrossRef]
- Karpova, E.V.; Karyakina, E.E.; Karyakin, A.A. Iron-nickel hexacyanoferrate bilayer as an advanced electrocatalyst for H2O2 reduction. RSC Adv. 2016, 6, 103328–103331. [Google Scholar] [CrossRef]
- Salimi, A.; Abdi, K. Enhancement of the analytical properties and catalytic activity of a nickel hexacyanoferrate modified carbon ceramic electrode prepared by two-step sol-gel technique: Application to amperometric detection of hydrazine and hydroxyl amine. Talanta 2004, 63, 475–483. [Google Scholar] [CrossRef]
- Borisova, A.V.; Karyakina, E.E.; Cosnier, S.; Karyakin, A.A. Current-Free Deposition of Prussian Blue with Organic Polymers: Towards Improved Stability and Mass Production of the Advanced Hydrogen Peroxide Transducer. Electroanalysis 2009, 21, 409–414. [Google Scholar] [CrossRef]
- Komkova, M.A.; Karyakina, E.E.; Karyakin, A.A. Catalytically synthesized Prussian Blue nanoparticles defeating natural enzyme peroxidase. J. Am. Chem. Soc. 2018, 140, 11302–11307. [Google Scholar] [CrossRef]
- Komkova, M.A.; Vetoshev, K.R.; Andreev, E.A.; Karyakin, A.A. Flow-electrochemical synthesis of Prussian Blue based nanozyme ‘artificial peroxidase’. Dalton Trans. 2021, 50, 11385–11389. [Google Scholar] [CrossRef]
- Komkova, M.A.; Zarochintsev, A.A.; Karyakina, E.E.; Karyakin, A.A. Electrochemical and sensing properties of Prussian Blue based nanozymes “artificial peroxidase”. J. Electroanal. Chem. 2020, 872, 114048. [Google Scholar] [CrossRef]
- Vokhmyanina, D.V.; Andreeva, K.D.; Komkova, M.A.; Karyakina, E.E.; Karyakin, A.A. ‘Artificial peroxidase’ nanozyme—Enzyme based lactate biosensor. Talanta 2020, 208, 120393. [Google Scholar] [CrossRef] [PubMed]
- Komkova, M.A.; Andreeva, K.D.; Zarochintsev, A.A.; Karyakin, A.A. Nanozymes ‘Artificial Peroxidase’—Enzymes Oxidases Mixtures for Single-Step Fabrication of Advanced Electrochemical Biosensors. ChemElectroChem 2021, 8, 1117–1122. [Google Scholar] [CrossRef]
- Karpova, E.V.; Shcherbacheva, E.V.; Komkova, M.A.; Eliseev, A.A.; Karyakin, A.A. Core–Shell Nanozymes “Artificial Peroxidase”: Stability with Superior Catalytic Properties. J. Phys. Chem. Lett. 2021, 12, 5547–5551. [Google Scholar] [CrossRef]



| Sample | ctrolox, mM |
|---|---|
| grapefruit, fresh (PB-NiHCF film) | 13 ± 1 |
| grapefruit, fresh (PB-NiHCF nanoparticles) | 12 ± 1 |
| orange, fresh (PB-NiHCF film) | 13 ± 2 |
| orange, fresh (PB-NiHCF nanoparticles) | 14 ± 2 |
| orange, “Ya” (PB-NiHCF film) | 2.9 ± 0.3 |
| orange, “Ya” (PB-NiHCF nanoparticles) | 3.2 ± 0.3 |
| orange, “J7” | 2.5 ± 0.5 |
| orange, “Bioitalia” | 4.7 ± 0.3 |
| orange, “Aushan” | 4.33 ± 0.06 |
| orange, “Dobryi” | <0.1 |
| birch sap, “Vkusvill” | 0.52 ± 0.08 |
| sea buckthorn, “Vkusvill” | 0.9 ± 0.5 |
| carrot, “Bioitalia” | 0.12 ± 0.01 |
| apple, “Aushan” | 0.9 ± 0.3 |
| black currant, “4 seasons” | 2.4 ± 0.8 |
| cranberry, “4 seasons” | <0.1 |
| cranberry, “Absolute nature” | <0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vokhmyanina, D.V.; Shcherbacheva, E.V.; Daboss, E.V.; Karyakina, E.E.; Karyakin, A.A. Core-Shell Iron-Nickel Hexacyanoferrate Nanoparticle-Based Sensors for Hydrogen Peroxide Scavenging Activity. Chemosensors 2021, 9, 344. https://doi.org/10.3390/chemosensors9120344
Vokhmyanina DV, Shcherbacheva EV, Daboss EV, Karyakina EE, Karyakin AA. Core-Shell Iron-Nickel Hexacyanoferrate Nanoparticle-Based Sensors for Hydrogen Peroxide Scavenging Activity. Chemosensors. 2021; 9(12):344. https://doi.org/10.3390/chemosensors9120344
Chicago/Turabian StyleVokhmyanina, Darya V., Elizaveta V. Shcherbacheva, Elena V. Daboss, Elena E. Karyakina, and Arkady A. Karyakin. 2021. "Core-Shell Iron-Nickel Hexacyanoferrate Nanoparticle-Based Sensors for Hydrogen Peroxide Scavenging Activity" Chemosensors 9, no. 12: 344. https://doi.org/10.3390/chemosensors9120344
APA StyleVokhmyanina, D. V., Shcherbacheva, E. V., Daboss, E. V., Karyakina, E. E., & Karyakin, A. A. (2021). Core-Shell Iron-Nickel Hexacyanoferrate Nanoparticle-Based Sensors for Hydrogen Peroxide Scavenging Activity. Chemosensors, 9(12), 344. https://doi.org/10.3390/chemosensors9120344