Sensing Methods for Hazardous Phenolic Compounds Based on Graphene and Conducting Polymers-Based Materials
Abstract
:1. Introduction
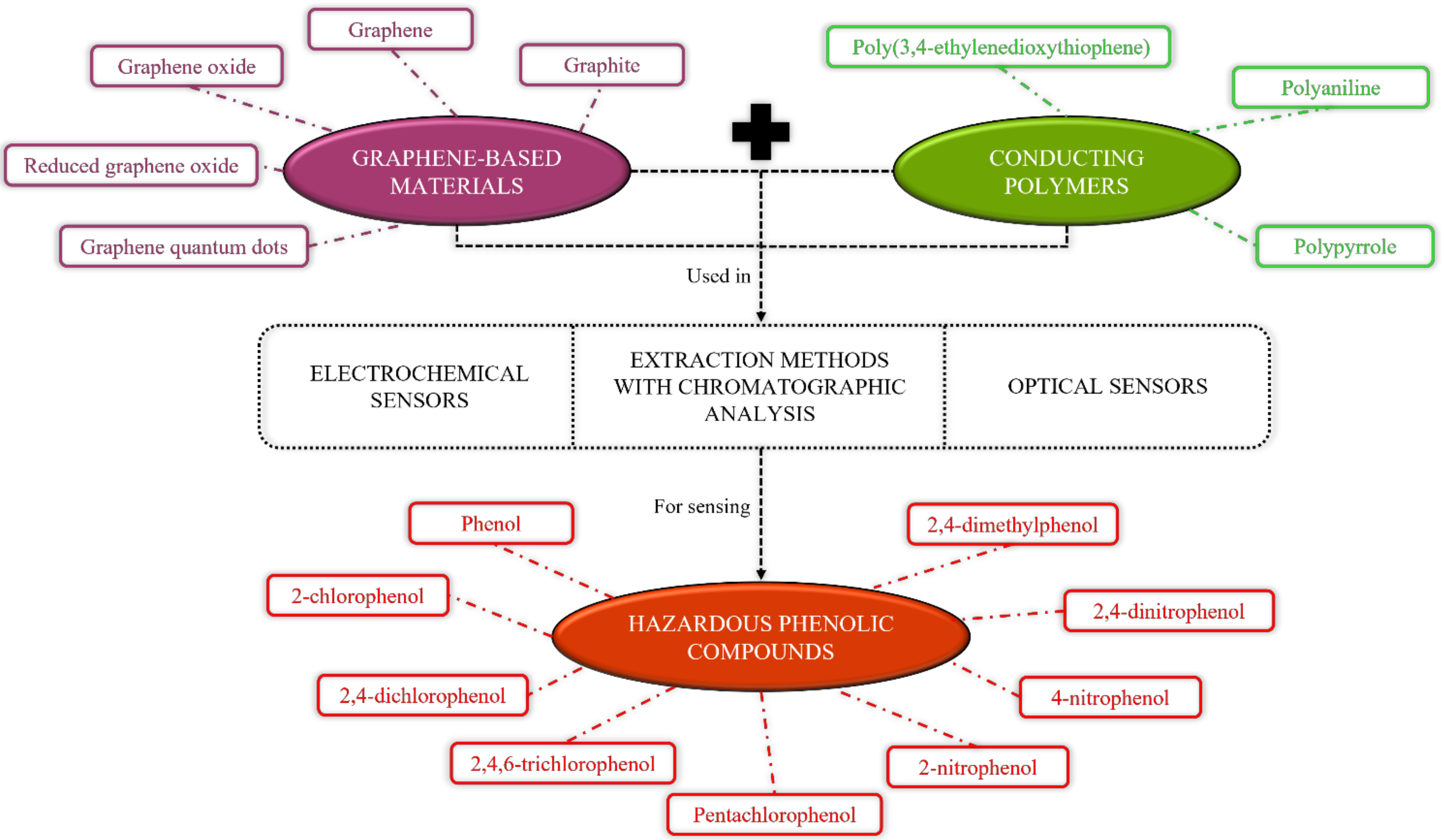
2. Graphene-Based Materials Incorporated with Various Sensors for the Detection of Hazardous Phenolic Compounds
2.1. Electrochemical Sensors
2.1.1. Graphite
2.1.2. Graphene
2.1.3. Graphene Oxide
2.1.4. Reduced Graphene Oxide
2.1.5. Graphene Quantum Dots
2.2. Extraction Methods with Chromatographic Analysis
2.2.1. Graphene
2.2.2. Graphene Oxide
2.2.3. Reduced Graphene Oxide
2.3. Optical Sensors
2.3.1. Graphene
2.3.2. Graphene Oxide
2.3.3. Reduced Graphene Oxide
2.3.4. Graphene Quantum Dots
3. Conducting Polymers-Based Materials Incorporated with Various Sensors for the Detection of Hazardous Phenolic Compounds
3.1. Electrochemical Sensors
3.1.1. Polypyrrole
3.1.2. Polyaniline and Its Derivative
3.1.3. Poly(3,4-ethylenedioxythiophene)
3.2. Extraction Methods with Chromatographic Analysis
3.2.1. Polypyrrole
3.2.2. Polyaniline and Its Derivatives
4. Graphene and Conducting Polymers-Based Composite Materials Incorporated with Various Sensors for Detection of Hazardous Phenolic Compounds
4.1. Electrochemical Sensors
4.2. Extraction Methods with Chromatographic Analysis
4.3. Optical Sensors
5. Future Perspectives of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gami, A.A.; Shukor, M.Y.; Khalil, K.A.; Dahalan, F.A.; Khalid, A.; Ahmad, S.A. Phenol and its toxicity. J. Environ. Microbiol. Toxicol. 2014, 2, 11–24. [Google Scholar]
- Bohdziewicz, J.; Kamińska, G.; Tytła, M. The removal of phenols from wastewater through sorption on the removal of phenols from wastewater through sorption on activated carbon. Archit. Civ. Eng. Environ. 2012, 2, 89–94. [Google Scholar]
- Kafi, A.K.M.; Chen, A. A novel amperometric biosensor for the detection of nitrophenol. Talanta 2009, 79, 97–102. [Google Scholar] [CrossRef]
- Belekbir, S.; El Azzouzi, M.; El Hamidi, A.; Rodríguez-Lorenzo, J.A.; Santaballa, L.; Canle, M. Improved photocatalyzed degradation of phenol, as a model pollutant, over metal-impregnated nanosized TiO2. Nanomaterials 2020, 10, 996. [Google Scholar] [CrossRef]
- Guan, H.; Liu, X.; Wang, W. Encapsulation of tyrosinase within liposome bioreactors for developing an amperometric phenolic compounds biosensor. J. Solid State Electrochem. 2013, 17, 2887–2893. [Google Scholar] [CrossRef]
- Abdullah, J.; Ahmad, M.; Karuppiah, N.; Heng, L.Y.; Sidek, H. Immobilization of tyrosinase in chitosan film for an optical detection of phenol. Sens. Actuators B 2006, 114, 604–609. [Google Scholar] [CrossRef]
- Wen, Y.; Li, R.; Liu, J.; Zhang, X.; Wang, P.; Zhang, X.; Zhou, B.; Li, H.; Wang, J.; Li, Z.; et al. Promotion effect of Zn on 2D bimetallic NiZn metal organic framework nanosheets for tyrosinase immobilization and ultrasensitive detection of phenol. Anal. Chim. Acta 2020, 1127, 131–139. [Google Scholar] [CrossRef]
- Jiang, L.; Santiago, I.; Foord, J. Nanocarbon and nanodiamond for high performance phenolics sensing. Commun. Chem. 2018, 1, 43. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Health and Human Services. Toxicological Profile for Asbestos; Update; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 1999.
- Mu’azu, N.D.; Jarrah, N.; Zubair, M.; Alagha, O. Removal of phenolic compounds from water using sewage sludge-based activated carbon adsorption: A review. Int. J. Environ. Res. Public Health 2017, 14, 1094. [Google Scholar] [CrossRef] [Green Version]
- Raza, W.; Lee, J.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J. Removal of phenolic compounds from industrial waste water based on membrane-based technologies. J. Ind. Eng. Chem. 2019, 71, 1–18. [Google Scholar] [CrossRef]
- Boruah, P.K.; Sharma, B.; Karbhal, I.; Shelke, M.V.; Das, M.R. Ammonia-modified graphene sheets decorated with magnetic Fe3O4 nanoparticles for the photocatalytic and photo-Fenton degradation of phenolic compounds under sunlight irradiation. J. Hazard. Mater. 2017, 325, 90–100. [Google Scholar] [CrossRef]
- Shandilya, P.; Mittal, D.; Soni, M.; Raizada, P.; Lim, J.-H.; Jeong, D.Y.; Dewedi, R.P.; Saini, A.K.; Singh, P. Islanding of EuVO4 on high-dispersed fluorine doped few layered graphene sheets for efficient photocatalytic mineralization of phenolic compounds and bacterial disinfection. J. Taiwan Inst. Chem. Eng. 2018, 93, 528–542. [Google Scholar] [CrossRef]
- MeiJiao, L.; Jing, L.; XuYu, Y.; ChangAn, Z.; Jia, Y.; Hao, H.; XianBao, W. Applications of graphene-based materials in environmental protection and detection. Mol. Mater. Devices 2013, 58, 2698–2710. [Google Scholar]
- Hashim, H.S.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Saleviter, S.; Abdullah, J. Structural, optical and potential sensing properties of tyrosinase immobilized graphene oxide thin film on gold surface. Optik 2020, 212, 164786. [Google Scholar] [CrossRef]
- Nurrohman, D.T.; Chiu, N.-F. A review of graphene-based surface plasmon resonance and surface-enhanced raman scattering biosensors: Current status and future prospects. Nanomaterials 2021, 11, 216. [Google Scholar] [CrossRef]
- Popov, A.; Aukstakojyte, R.; Gaidukevic, J.; Lisyte, V.; Kausaite-Minkstimiene, A.; Barkauskas, J.; Ramanaviciene, A. Reduced graphene oxide and polyaniline nanofibers nanocomposite for the development of an amperometric glucose biosensor. Sensors 2021, 21, 948. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Baig, N.; Kawde, A.N. A novel, fast and cost effective graphene-modified graphite pencil electrode for trace quantification of l-tyrosine. Anal. Methods 2015, 7, 9535–9541. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Kamil, Y.M.; Daniyal, W.M.E.M.M.; Sadrolhosseini, A.R.; Mahdi, M.A. Sensitive detection of dengue virus type 2 E-proteins signals using self-assembled monolayers/reduced graphene oxide-PAMAM dendrimer thin film-SPR optical sensor. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Baig, N.; Saleh, T.A. Electrodes modified with 3D graphene composites: A review on methods for preparation, properties and sensing applications. Microchim. Acta 2018, 2, 1–21. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent advances in graphene quantum dots for sensing. Mater. Today 2013, 16, 433–442. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, R.; Sun, D.; Li, Y.; Wu, T. Sustainable fabrication of graphene oxide/manganese oxide composites for removing phenolic compounds by adsorption-oxidation process. J. Clean. Prod. 2019, 215, 165–174. [Google Scholar] [CrossRef]
- Abdolmaleki, A.; Mahmoudian, M. Use of biomass sericin as matrices in functionalized graphene/sericin nanocomposites for the removal of phenolic compounds. Heliyon 2020, 6, e04955. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Kim, C.M.; Jhung, S.H. Melamine/polyaniline-derived carbons with record-high adsorption capacities for effective removal of phenolic compounds from water. Chem. Eng. J. 2020, 127627. [Google Scholar] [CrossRef]
- Majumdar, S.; Nath, J.; Mahanta, D. Surface modified polypyrrole for the efficient removal of phenolic compounds from aqueous medium. J. Environ. Chem. Eng. 2018, 6, 2588–2596. [Google Scholar] [CrossRef]
- Tuncagil, S.; Varis, S.; Toppare, L. Design of a biosensor based on 1-(4-nitrophenyl)-2,5-di(2-thienyl)-1H pyrrole. J. Mol. Catal. B Enzym. 2010, 64, 195–199. [Google Scholar] [CrossRef]
- Abdi, M.M.; Abdullah, L.C.; Sadrolhosseini, A.R.; Yunus, W.M.M.; Moksin, M.M.; Tahir, P.M. Surface plasmon resonance sensing detection of mercury and lead ions based on conducting polymer composite. PLoS ONE 2011, 6, e24578. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Charge transfer and biocompatibility aspects in conducting polymer-based enzymatic biosensors and biofuel cells. Nanomaterials 2021, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.-B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Sulak, M.T.; Erhan, E.; Keskinler, B.; Yılmaz, F.; Celik, A. Development of amperometric biosensor for phenolic compounds using a modified electrode with poly(GMA-co-MTM) and laccase. Sens. Lett. 2010, 8, 1–6. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, L.; Li, L.; Cheng, W.; Xu, L.; Ping, X.; Pan, L.; Shi, Y. Conducting polymers and their applications in diabetes management. Sensors 2016, 16, 1787. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, K.; Amalnerkar, D.P.; Trivedi, D.C. Synthesis and characterization of conducting polymer composite (PAn/TiO2) for cathode material in rechargeable battery. Mater. Lett. 2003, 57, 1642–1648. [Google Scholar] [CrossRef]
- Coskun, Y.; Cirpan, A.; Toppare, L. Conducting polymers of terepthalic acid bis-(2-thiophen-3-yl-ethyl) ester and their electrochromic properties. Polymer 2004, 45, 4989–4995. [Google Scholar] [CrossRef]
- Lee, A.S.; Peteu, S.F.; Ly, J.V.; Requicha, A.A.G.; Thompson, M.E.; Zhou, C. Actuation of polypyrrole nanowires. Nanotechnology 2008, 19, 165501. [Google Scholar] [CrossRef] [PubMed]
- Otero, T.F.; Sanchez, J.J.; Martinez, J.G. Biomimetic dual sensing-actuators based on conducting polymers. Galvanostatic theoretical model for actuators sensing temperature. J. Phys. Chem. B 2012, 116, 5279–5290. [Google Scholar] [CrossRef]
- Mi, H.; Zhang, X.; Ye, X.; Yang, S. Preparation and enhanced capacitance of core-shell polypyrrole/polyaniline composite electrode for supercapacitors. J. Power Sources 2008, 176, 403–409. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Saleviter, S.; Daniyal, W.M.E.M.M.; Anas, N.A.A.; Ramdzan, N.S.M.; Roshidi, M.D.A. Development of a graphene-based surface plasmon resonance optical sensor chip for potential biomedical application. Materials 2019, 12, 1928. [Google Scholar] [CrossRef] [Green Version]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Saleviter, S.; Omar, N.A.S. Label-free optical spectroscopy for characterizing binding properties of highly sensitive nanocrystalline cellulose-graphene oxide based nanocomposite towards nickel ion. Spectrochim. Acta Part A 2019, 212, 25–31. [Google Scholar] [CrossRef]
- Ramdzan, N.S.M.; Fen, Y.W.; Omar, N.A.S.; Anas, N.A.A.; Liew, J.Y.C.; Daniyal, W.M.E.M.M.; Hashim, H.S. Detection of mercury ion using surface plasmon resonance spectroscopy based on nanocrystalline cellulose/poly(3,4-ethylenedioxythiophene) thin film. Measurement 2021, 182, 109728. [Google Scholar] [CrossRef]
- Coros, M.; Pruneanu, S.; Staden, R.-I.S. Review—Recent progress in the graphene-based electrochemical sensors and biosensors. J. Electrochem. Soc. 2020, 167, 037528. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Pumera, M.; Ambrosi, A.; Bonanni, A.; Chng, E.L.K.; Poh, H.L. Graphene for electrochemical sensing and biosensing. TrAC-Trends Anal. Chem. 2010, 29, 954–965. [Google Scholar] [CrossRef]
- Ma, H.; Wu, D.; Cui, Z.; Li, Y.; Zhang, Y.; Du, B.; Wei, Q. Graphene-based optical and electrochemical biosensors: A review. Anal. Lett. 2013, 46, 1–17. [Google Scholar] [CrossRef]
- Brownson, D.A.C.; Banks, C.E. Graphene electrochemistry: An overview of potential applications. Analyst 2010, 135, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Kumar, P.; Park, D.-S.; Shim, Y.-B. Electrochemical sensors based on organic conjugated polymers. Sensors 2008, 8, 118–141. [Google Scholar] [CrossRef]
- Das, T.K.; Prusty, S. Review on conducting polymers and their applications. Polym.-Plast. Technol. Eng. 2012, 51, 1487–1500. [Google Scholar] [CrossRef]
- Gupta, N.; Sharma, S.; Mir, I.A.; Kumar, D. Advances in sensors based on conducting polymers. J. Sci. Ind. Res. 2006, 65, 549–557. [Google Scholar]
- Ates, M. A review study of (bio)sensor systems based on conducting polymers. Mater. Sci. Eng. C 2013, 33, 1853–1859. [Google Scholar] [CrossRef]
- Krampa, F.D.; Aniweh, Y.; Awandare, G.A.; Kanyong, P. A disposable amperometric sensor based on high-performance PEDOT:PSS/ionic liquid nanocomposite thin film-modified screen-printed electrode for the analysis of catechol in natural water samples. Sensors 2017, 17, 1716. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Fu, B.; Chen, J.; Yan, Z.; Li, K. A non-enzymatic electrochemical sensor for detection of sialic acid based on a porphine/graphene oxide modified electrode via indicator displacement assay. Electrochim. Acta 2018, 269, 136–143. [Google Scholar] [CrossRef]
- Capannesi, C.; Palchetti, I.; Mascini, M.; Parenti, A. Electrochemical sensor and biosensor for polyphenols detection in olive oils. Food Chem. 2000, 71, 553–562. [Google Scholar] [CrossRef]
- Fang, Y.; Bullock, H.; Lee, S.A.; Sekar, N.; Eiteman, M.A.; Whitman, W.B.; Ramasamy, R.P. Detection of methyl salicylate using bi-enzyme electrochemical sensor consisting salicylate hydroxylase and tyrosinase. Biosens. Bioelectron. 2016, 85, 603–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terán-Alcocer, Á.; Bravo-Plascencia, F.; Cevallos-Morillo, C.; Palma-Cando, A. electrochemical sensors based on conducting polymers for the aqueous detection of biologically relevant molecules. Nanomaterials 2021, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Bounegru, A.V.; Apetrei, C. Development of a novel electrochemical biosensor based on carbon nanofibers-gold nanoparticles- tyrosinase for the detection of ferulic acid in cosmetics. Sensors 2020, 20, 6724. [Google Scholar] [CrossRef] [PubMed]
- Dăscălescu, D.; Apetrei, C. Nanomaterials based electrochemical sensors for serotonin detection: A review. Chemosensors 2021, 9, 14. [Google Scholar] [CrossRef]
- Coelho, M.K.L.; da Silva, D.N.; Pereira, A.C. Development of electrochemical sensor based on carbonaceal and metal phthalocyanines materials for determination of ethinyl estradiol. Chemosensors 2019, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Poole, C.F. New trends in solid-phase extraction. Trends Anal. Chem. 2003, 22, 362–373. [Google Scholar] [CrossRef]
- Ramdzan, N.S.M.; Fen, Y.W.; Anas, N.A.A.; Omar, N.A.S.; Saleviter, S. Development of biopolymer and conducting polymer-based optical sensors for heavy metal ion detection. Molecules 2020, 25, 2548. [Google Scholar] [CrossRef] [PubMed]
- Eddin, F.B.K.; Fen, Y.W. Recent advances in electrochemical and optical sensing of dopamine. Sensors 2020, 20, 1039. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Liu, L.; Chen, C.; Kang, X.; Xie, Q. Effective immobilization of tyrosinase via enzyme catalytic polymerization of L-DOPA for highly sensitive phenol and atrazine sensing. Talanta 2016, 160, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Delgado, M.M.; Alemán-Nava, G.S.; Rodríguez-Delgado, J.M.; Dieck-Assad, G.; Martínez-Chapa, S.O.; Barceló, D.; Parra, R. Laccase-based biosensors for detection of phenolic compounds. Trends Anal. Chem. 2015, 74, 21–45. [Google Scholar] [CrossRef] [Green Version]
- Bellido-Milla, D.; Cubillana-Aguilera, L.M.; Kaoutit, M.E.; Hernández-Artiga, M.P.; de Cisneros, J.L.H.-H.; Naranjo-Rodríguez, I.; Palacios-Santander, J.M. Recent advances in graphite powder-based electrodes. Anal. Bioanal. Chem. 2013, 405, 3525–3539. [Google Scholar] [CrossRef]
- Ortega, F.; Dominguez, E.; Jonsson-Pettersson, G.; Gorton, L. Amperometric biosensor for the determination of phenolic compounds using a tyrosinase graphite electrode in a flow injection system. J. Biotechnol. 1993, 31, 289–300. [Google Scholar] [CrossRef]
- Liu, F.; Reviejo, A.J.; Pingarron, J.M.; Wang, J. Development of an amperometric for the determination of phenolic in reversed micelles. Talanta 1994, 41, 455–459. [Google Scholar] [CrossRef]
- Stoytcheva, M.; Zlatev, R.; Gochev, V.; Velkova, Z.; Montero, G.; Beleno, M.T. Amperometric biosensors precision improvement. Application to phenolic pollutants determination. Electrochim. Acta 2014, 147, 25–30. [Google Scholar] [CrossRef]
- Wang, J.; Reviejo, A.J.; Mannino, S. Organic-phase enzyme electrode for the determination of phenols in olive oils. Anal. Lett. 1992, 25, 1399–1409. [Google Scholar] [CrossRef]
- Haghighi, B.; Gorton, L.; Ruzgas, T.; Jonsson, L.J. Characterization of graphite electrodes modified with laccase from Trametes versicolor and their use for bioelectrochemical monitoring of phenolic compounds in flow injection analysis. Anal. Chim. Acta 2003, 487, 3–14. [Google Scholar] [CrossRef]
- Jarosz-Wilkołazka, A.; Ruzgas, T.; Gorton, L. Amperometric detection of mono- and diphenols at Cerrena unicolor laccase-modified graphite electrode: Correlation between sensitivity and substrate structure. Talanta 2005, 66, 1219–1224. [Google Scholar] [CrossRef]
- Parellada, J.; Narvaez, A.; Lopez, M.A.; Dominguez, E.; Fernandez, J.J.; Pavlov, V.; Katakis, I. Amperometric immunosensors and enzyme electrodes for environmental applications. Anal. Chim. Acta 1998, 362, 47–57. [Google Scholar] [CrossRef]
- Kulys, J.; Schmid, R.D. A sensitive enzyme electrode for phenol monitoring. Anal. Lett. 1990, 23, 589–597. [Google Scholar] [CrossRef]
- Svitel, J.; Miertus, S. Development of tyrosinase-based biosensor and its application for monitoring of bioremediation of phenol and phenolic compounds. Environ. Sci. Technol. 1998, 32, 828–832. [Google Scholar] [CrossRef]
- Wang, J.; Fang, L.; Lopez, D. Amperometric biosensor for phenols based on a tyrosinase-graphite-epoxy biocomposite. Analyst 1994, 119, 455–458. [Google Scholar] [CrossRef]
- Onnerfjord, P.; Emneus, J.; Marko-Varga, G.; Gorton, L. Tyrosinase graphite-epoxy based composite electrodes for detection of phenols. Biosens. Bioelectron. 1995, 10, 607–619. [Google Scholar] [CrossRef]
- Hernandez, S.R.; Kergaravat, S.V.; Pividori, M.I. Enzymatic electrochemical detection coupled to multivariate calibration for the determination of phenolic compounds in environmental samples. Talanta 2013, 106, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, F.-D.; Lindgren, A.; Emneus, J.; Gorton, L.; Ruzgas, T.; Csoregi, E.; Ciucu, A.; van Huystee, R.B.; Gazaryan, I.G.; Lagrimini, L.M. Bioelectrochemical monitoring of phenols and aromatic amines in flow injection using novel plant peroxidases. Anal. Chem. 1998, 70, 2596–2600. [Google Scholar] [CrossRef] [PubMed]
- Serra, B.; Mateo, E.; Pedrero, M.; Reviejo, A.J.; Pingarron, J.M. Graphite-teflon-tyrosinase composite electrodes for the monitoring of phenolic compounds in predominantly non-aqueous media. Analusis 1999, 27, 592–599. [Google Scholar] [CrossRef]
- Serra, B.; Benito, B.; Agui, L.; Reviejo, A.J.; Pingarron, J.M. Graphite-teflon-peroxidase composite electrochemical biosensors. a tool for the wide detection of phenolic compounds. Electroanalysis 2001, 13, 693–700. [Google Scholar] [CrossRef]
- Serra, B.; Reviejo, A.J.; Pingarron, J.M. Flow injection amperometric detection of phenolic compounds at enzyme composite biosensors application to their monitoring during industrial waste waters purification processes. Anal. Lett. 2003, 36, 1965–1986. [Google Scholar] [CrossRef]
- Serra, B.; Reviejo, A.J.; Pingarron, J.M. Composite multienzyme amperometric biosensors for an improved detection of phenolic compounds. Electroanalysis 2003, 15, 1737–1744. [Google Scholar] [CrossRef]
- Serra, B.; Jimenez, S.; Mena, M.L.; Reviejo, A.J.; Pingarron, J.M. Composite electrochemical biosensors: A comparison of three different electrode matrices for the construction of amperometric tyrosinase biosensors. Biosens. Bioelectron. 2002, 17, 217–226. [Google Scholar] [CrossRef]
- Carralero-Sanz, V.; Mena, M.L.; Gonzalez-Cortes, A.; Yanez-Sedeno, P.; Pingarron, J.M. Development of a high analytical performance-tyrosinase biosensor based on a composite graphite-Teflon electrode modified with gold nanoparticles. Biosens. Bioelectron. 2006, 22, 730–736. [Google Scholar] [CrossRef]
- Sapelnikova, S.; Dock, E.; Solna, R.; Skladal, P.; Ruzgas, T.; Emneus, J. Screen-printed multienzyme arrays for use in amperometric batch and flow systems. Anal. Bioanal. Chem. 2003, 376, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Solna, R.; Dock, E.; Christenson, A.; Winther-Nielsen, M.; Carlsson, C.; Emneus, J.; Ruzgas, T.; Skladal, P. Amperometric screen-printed biosensor arrays with co-immobilised oxidoreductases and cholinesterases. Anal. Chim. Acta 2005, 528, 9–19. [Google Scholar] [CrossRef]
- Kawde, A.-N.; Aziz, M.A. Porous copper-modified graphite pencil electrode for the amperometric detection of 4-nitrophenol. Electroanalysis 2014, 26, 1–8. [Google Scholar] [CrossRef]
- Rana, A.; Kawde, A.N. Open-circuit electrochemical polymerization for the sensitive detection of phenols. Electroanalysis 2016, 28, 898–902. [Google Scholar] [CrossRef]
- Rana, A.; Kawde, A.-N.; Ibrahim, M. Simple and sensitive detection of 4-nitrophenol in real water samples using gold nanoparticles modified pretreated graphite pencil electrode. J. Electroanal. Chem. 2018, 820, 24–31. [Google Scholar] [CrossRef]
- Shahbakhsh, M.; Noroozifar, M. Poly (dopamine quinone-chromium (III) complex) microspheres as new modifier for simultaneous determination of phenolic compounds. Biosens. Bioelectron. 2018, 102, 439–448. [Google Scholar] [CrossRef]
- Nissim, R.; Compton, R.G. Introducing absorptive stripping voltammetry: Wide concentration range voltammetric phenol detection. Analyst 2014, 139, 5911–5918. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, X.; Wu, L.; Chen, J. Direct electrochemical tyrosinase biosensor based on mesoporous carbon and co3o4 nanorods for the rapid detection of phenolic pollutants. ChemElectroChem 2014, 1, 808–816. [Google Scholar] [CrossRef]
- Wang, P.; Xiao, J.; Guo, M.; Xia, Y.; Li, Z.; Jiang, X.; Huang, W. Voltammetric determination of 4-nitrophenol at graphite nanoflakes modified glassy carbon electrode. J. Electrochem. Soc. 2015, 162, H72–H78. [Google Scholar] [CrossRef]
- Yin, H.Y.; Zheng, Y.F.; Wang, L. Au/CeO2/g-C3N4 nanocomposite modified electrode as electrochemical sensor for the determination of phenol. J. Nanosci. Nanotechnol. 2020, 20, 5539–5545. [Google Scholar] [CrossRef] [PubMed]
- Arvinte, A.; Mahosenaho, M.; Pinteala, M.; Sesay, A.-M.; Virtanen, V. Electrochemical oxidation of p-nitrophenol using graphene-modified electrodes, and a comparison to the performance of MWNT-based electrodes. Microchem. J. 2011, 174, 337–343. [Google Scholar] [CrossRef]
- Shi, J.-J.; Zhu, J.-J. Sonoelectrochemical fabrication of Pd-graphene nanocomposite and its application in the determination of chlorophenols. Electrochim. Acta 2011, 56, 6008–6013. [Google Scholar] [CrossRef]
- Wu, C.; Cheng, Q.; Wu, K. Electrochemical functionalization of N-methyl-2-pyrrolidone-exfoliated graphene nanosheets as highly sensitive analytical platform for phenols. Anal. Chem. 2015, 87, 3294–3299. [Google Scholar] [CrossRef]
- Hu, F.-T.; Liu, S.-Q. Enhanced effects of surfactant on sensing of phenol on a graphene nano-sheet paste electrode. Int. J. Electrochem. Sci. 2012, 7, 11338–11350. [Google Scholar]
- Hashemnia, S.; Khayatzadeh, S.; Hashemnia, M. Electrochemical detection of phenolic compounds using composite film of multiwall carbon nanotube/surfactant/tyrosinase on a carbon paste electrode. J. Solid State Electrochem. 2012, 16, 473–479. [Google Scholar] [CrossRef]
- Li, X.; Shen, J.; Wu, C.; Wu, K. Ball-mill-exfoliated graphene: Tunable electrochemistry and phenol sensing. Small 2019, 15, 1805567. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, D.; Ju, T.; Wang, L.; Lin, S.; Zhao, Y.; Wang, C.; He, H.; Du, Y. Biodegradation of phenol using Bacillus cereus WJ1 and evaluation of degradation efficiency based on a graphene-modified electrode. Int. J. Electrochem. Sci. 2013, 8, 504–519. [Google Scholar]
- Li, J.; Miao, D.; Yang, R.; Qu, L.; Harrington, P. de B. Synthesis of poly(sodium 4-styrenesulfonate) functionalized graphene/cetyltrimethylammonium bromide (CTAB) nanocomposite and its application in electrochemical oxidation of 2,4-dichlorophenol. Electrochim. Acta 2014, 125, 1–8. [Google Scholar] [CrossRef]
- Devasenathipathy, R.; Mani, V.; Chen, S.-M.; Manibalan, K.; Huang, S.-T. Determination of 4-nitrophenol at iron phthalocyanine decorated graphene nanosheets film modified electrode. Int. J. Electrochem. Sci. 2015, 10, 1384–1392. [Google Scholar]
- Zhang, W.; Chang, J.; Chen, J.; Xu, F.; Wang, F.; Jiang, K.; Gao, Z. Graphene-Au composite sensor for electrochemical detection of para-nitrophenol. Res. Chem. Intermed. 2012, 38, 2443–2455. [Google Scholar] [CrossRef]
- Jiao, X.X.; Luo, H.Q.; Li, N.B. Fabrication of graphene–gold nanocomposites by electrochemical co-reduction and their electrocatalytic activity toward 4-nitrophenol oxidation. J. Electroanal. Chem. 2013, 691, 83–89. [Google Scholar] [CrossRef]
- Deng, P.; Xu, Z.; Li, J. Simultaneous voltammetric determination of 2-nitrophenol and 4-nitrophenol based on an acetylene black paste electrode modified with a graphene-chitosan composite. Microchim. Acta 2014, 181, 1077–1084. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, L.; Han, G.; Liu, Y.; Tang, W. Graphene-chitosan composite modified electrode for simultaneous detection of nitrophenol isomers. J. Electrochem. Soc. 2015, 162, 269–274. [Google Scholar] [CrossRef]
- Liu, F.; Piao, Y.; Choi, J.S.; Seo, T.S. Three-dimensional graphene micropillar based electrochemical sensor for phenol detection. Biosens. Bioelectron. 2013, 50, 387–392. [Google Scholar] [CrossRef]
- Xu, Q.; Li, X.; Zhou, Y.; Wei, H.; Hu, X.-Y.; Wang, Y.; Yang, Z. An enzymatic amplified system for the detection of 2,4-dichlorophenol based on graphene membrane modified electrode. Anal. Methods 2012, 4, 3429–3435. [Google Scholar] [CrossRef]
- Fartas, F.M.; Abdullah, J.; Yusof, N.A.; Sulaiman, Y.; Saiman, M.I. Biosensor based on tyrosinase immobilized on graphene-decorated gold nanoparticle/chitosan for phenolic detection in aqueous. Sensors 2017, 17, 1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Y.; Ma, M.; Wang, Z.; Zhan, G.; Li, B.; Wang, X.; Fang, H.; Zhang, H.; Li, C. Sensitive amperometric biosensor for phenolic compounds based on graphene-silk peptide/tyrosinase composite nanointerface. Biosens. Bioelectron. 2013, 44, 85–88. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Guo, Y.; Yang, F.; Cheng, F. Electrochemical sensor for o-nitrophenol based on β-cyclodextrin functionalized graphene nanosheets. J. Nanomater. 2013, 2013, 632809. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.; Qian, J.; Sun, H.; Wu, X.; Wang, K.; Yi, Y. Voltammetric determination of o-chlorophenol using β-cyclodextrin/graphene nanoribbon hybrids modified electrode. J. Electroanal. Chem. 2017, 794, 126–131. [Google Scholar] [CrossRef]
- Wei, M.; Tian, D.; Liu, S.; Zheng, X.; Duan, S.; Zhou, C. β-cyclodextrin functionalized graphene material: A novel electrochemical sensor for simultaneous determination of 2-chlorophenol and 3-chlorophenol. Sens. Actuators B 2014, 195, 452–458. [Google Scholar] [CrossRef]
- Gao, J.; Liu, M.; Song, H.; Zhang, S.; Qian, Y.; Li, A. Highly-sensitive electrocatalytic determination for toxic phenols based on coupled cMWCNT/cyclodextrin edge-functionalized graphene composite. J. Hazard. Mater. 2016, 318, 99–108. [Google Scholar] [CrossRef]
- Liu, W.; Li, C.; Gu, Y.; Tang, L.; Zhang, Z.; Yang, M. One-step synthesis of β-cyclodextrin functionalized graphene/Ag nanocomposite and its application in sensitive determination of 4-nitrophenol. Electroanalysis 2013, 25, 2367–2376. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, H.; Li, Y.; Li, C.-P. Electrochemical simultaneous determination of hydroquinone and p-nitrophenol based on host-guest molecular recognition capability of dual β-cyclodextrin functionalized Au@graphene nanohybrids. Sens. Actuators B 2015, 207, 1–8. [Google Scholar] [CrossRef]
- He, Q.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; Deng, P.; Chen, D. Facile and ultrasensitive determination of 4-nitrophenol based on acetylene black paste and graphene hybrid electrode. Nanomaterials 2019, 9, 429. [Google Scholar] [CrossRef] [Green Version]
- Bharath, G.; Veeramani, V.; Chen, S.-M.; Madhu, R.; Raja, M.M.; Balamurugan, A.; Mangalaraj, D.; Viswanathana, C.; Ponpandian, N. Edge-carboxylated graphene anchoring magnetite-hydroxyapatite nanocomposite for an efficient 4-nitrophenol sensor. RSC Adv. 2015, 5, 13392–13401. [Google Scholar] [CrossRef]
- Meng, Z.; Li, M.; Li, C.; Liu, X.; Lei, Z. A sensitive phenol electrochemical sensor based on magnetic oxide/amino-functional graphene nanocomposite. Int. J. Electrochem. Sci. 2019, 14, 3126–3137. [Google Scholar] [CrossRef]
- Nurdin, M.; Agusu, L.; Putra, A.A.M.; Maulidiyah, M.; Arham, Z.; Wibowo, D.; Muzakkar, M.Z.; Umar, A.A. Synthesis and electrochemical performance of graphene-TiO2-carbon paste nanocomposites electrode in phenol detection. J. Phys. Chem. Solids 2019, 131, 104–110. [Google Scholar] [CrossRef]
- Wibowo, D.; Sufandy, Y.; Irwan, I.; Azis, T.; Maulidiyah, M.; Nurdin, M. Investigation of nickel slag waste as a modifier on graphene-TiO2 microstructure for sensing phenolic compound. J. Mater. Sci. Mater. Electron. 2020, 31, 14375–14383. [Google Scholar] [CrossRef]
- Yin, H.Y.; Zheng, Y.F.; Wang, L. Electrochemical sensor based on Na+-doped g-C3N4 for detection of phenol. Bull. Mater. Sci. 2020, 43, 110. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Saleviter, S.; Omar, N.A.S. Preparation and characterization of hexadecyltrimethylammonium bromide modified nanocrystalline cellulose/graphene oxide composite thin film and its potential in sensing copper ion using surface plasmon resonance technique. Optik 2018, 173, 71–77. [Google Scholar] [CrossRef]
- Li, J.; Kuang, D.; Feng, Y.; Zhang, F.; Xu, Z.; Liu, M. A graphene oxide-based electrochemical sensor for sensitive determination of 4-nitrophenol. J. Hazard. Mater. 2012, 201–202, 250–259. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, L.; Zhang, Y.; Tang, H. Electrochemical sensoring of 2,4-dinitrophenol by using composites of graphene oxide with surface molecular imprinted polymer. Sens. Actuators B 2012, 171–172, 1151–1158. [Google Scholar] [CrossRef]
- Liang, Y.; Yu, L.; Yang, R.; Li, X.; Qu, L.; Li, J. High sensitive and selective graphene oxide/molecularly imprinted polymer electrochemical sensor for 2,4-dichlorophenol in water. Sens. Actuators B 2017, 240, 1330–1335. [Google Scholar] [CrossRef]
- Arfin, T.; Bushra, R.; Mohammad, F. Electrochemical sensor for the sensitive detection of o-nitrophenol using graphene oxide-poly(ethyleneimine) dendrimer-modified glassy carbon electrode. Graphene Technol. 2016, 1, 1–15. [Google Scholar] [CrossRef]
- Ragu, S.; Chen, S.-M.; Ranganathan, P.; Rwei, S.-P. Fabrication of a novel nickel-curcumin/graphene oxide nanocomposites for superior electrocatalytic activity toward the detection of toxic p-nitrophenol. Int. J. Electrochem. Sci. 2016, 11, 9133–9144. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, K.; Lu, N.; Yuan, X. Simultaneous determination of 2,4,6-trichlorophenol and pentachlorophenol based on poly(Rhodamine B)/graphene oxide/multiwalled carbon nanotubes composite film modified electrode. Appl. Surf. Sci. 2016, 361, 72–79. [Google Scholar] [CrossRef]
- Gan, T.; Lv, Z.; Sun, J.; Shi, Z.; Liu, Y. Preparation of graphene oxide-wrapped carbon sphere@silver spheres for high performance chlorinated phenols sensor. J. Hazard. Mater. 2016, 302, 188–197. [Google Scholar] [CrossRef]
- Zhan, T.; Tan, Z.; Tian, X.; Hou, W. Ionic liquid functionalized graphene oxide-Au nanoparticles assembly for fabrication of electrochemical 2,4-dichlorophenol sensor. Sens. Actuators B 2017, 246, 638–646. [Google Scholar] [CrossRef]
- Arfin, T.; Rangari, S.N. Graphene oxide–ZnO nanocomposite modified electrode for the detection of phenol. Anal. Methods 2018, 10, 347–358. [Google Scholar] [CrossRef]
- Gan, T.; Wang, Z.; Gao, J.; Sun, J.; Wu, K.; Wang, H.; Liu, Y. Morphology-dependent electrochemical activity of Cu2O polyhedrons and construction of sensor for simultaneous determination of phenolic compounds with graphene oxide as reinforcement. Sens. Actuators B 2019, 282, 549–558. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, N.; Meena, V.K.; Kranz, C.; Mishra, S. Impedometric phenol sensing using graphenated electrochip. Sens. Actuators B 2016, 237, 318–328. [Google Scholar] [CrossRef]
- Wiench, P.; Grzyb, B.; González, Z.; Menéndez, R.; Handke, B.; Gryglewicz, G. pH robust electrochemical detection of 4-nitrophenol on a reduced graphene oxide modified glassy carbon electrode. J. Electroanal. Chem. 2017, 787, 80–87. [Google Scholar] [CrossRef]
- Rao, H.; Guo, W.; Hou, H.; Wang, H.; Yin, B.; Xue, Z.; Zhao, G. Electroanalytical investigation of p-nitrophenol with dual electroactive groups on a reduced graphene oxide modified glassy carbon electrode. Int. J. Electrochem. Sci. 2017, 12, 1052–1063. [Google Scholar] [CrossRef]
- Xu, C.; Wang, J.; Wan, L.; Lin, J.; Wang, X. Microwave-assisted covalent modification of graphene nanosheets with hydroxypropyl-β-cyclodextrin and its electrochemical detection of phenolic organic pollutants. J. Mater. Chem. 2011, 21, 10463–10471. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, X.; Zhang, H.; Lu, W.; Ma, H.; Hou, S. Simultaneous Determination of nitrophenol isomers based on β-cyclodextrin functionalized reduced graphene oxide. Electroanalysis 2012, 24, 1178–1185. [Google Scholar] [CrossRef]
- Li, C.; Wu, Z.; Yang, H.; Deng, L.; Chen, X. Reduced graphene oxide-cyclodextrin-chitosan electrochemical sensor: Effective and simultaneous determination of o- and p-nitrophenols. Sens. Actuators B 2017, 251, 446–454. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Q.; Gu, Y.; Tang, L.; Li, C.; Zhang, Z. One-pot green synthesis of Prussian blue nanocubes decorated reduced graphene oxide using mushroom extract for efficient 4-nitrophenol reduction. Anal. Chim. Acta 2014, 853, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Ikhsan, N.I.; Rameshkumar, P.; Huang, N.M. Controlled synthesis of reduced graphene oxide supported silver nanoparticles for selective and sensitive electrochemical detection of 4-nitrophenol. Electrochim. Acta 2016, 192, 392–399. [Google Scholar] [CrossRef]
- Noor, A.M.; Rameshkumar, P.; Yusoff, N.; Ming, H.N.; Sajab, M.S. Microwave synthesis of reduced graphene oxide decorated with silver nanoparticles for electrochemical determination of 4-nitrophenol. Ceram. Int. 2016, 42, 18813–18820. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Yang, R.; Li, J.-J.; Qu, L.-B. A highly sensitive and selective electrochemical sensor for pentachlorophenol based on reduced graphite oxide-silver nanocomposites. Food Anal. Methods 2020, 13, 2050–2058. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, R.; Liu, C.; Yang, S.; Lu, Z.; Luo, S. Electrochemical detection of 4-nitrophenol based on a glassy carbon electrode modified with a reduced graphene oxide/Au nanoparticle composite. Anal. Methods 2013, 5, 5508–5514. [Google Scholar] [CrossRef]
- Liu, F.; Piao, Y.; Choi, K.S.; Seo, T.S. Fabrication of free-standing graphene composite films as electrochemical biosensors. Carbon N. Y. 2012, 50, 123–133. [Google Scholar] [CrossRef]
- Penu, R.; Obreja, A.C.; Patroi, D.; Diaconu, M.; Radu, G.L. Graphene and gold nanoparticles based reagentless biodevice for phenolic endocrine disruptors monitoring. Microchem. J. 2015, 121, 130–135. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Wu, H.; Guo, S.; Zhang, J. Glass carbon electrode modified with horseradish peroxidase immobilized on partially reduced graphene oxide for detecting phenolic compounds. J. Electroanal. Chem. 2012, 681, 49–55. [Google Scholar] [CrossRef]
- Boujakhrout, A.; Jimenez-Falcao, S.; Martínez-Ruiz, P.; Sánchez, A.; Díez, P.; Pingarrón, J.M.; Villalonga, R. Novel reduced graphene oxide-glycol chitosan nanohybrid for the assembly of amperometric enzyme biosensor for phenols. Analyst 2016, 141, 4162–4169. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Choe, S.R.; Giribabu, K.; Jang, S.-C.; Roh, C.; Huh, Y.S.; Han, Y.-K. Pd nanospheres decorated reduced graphene oxide with multi-functions: Highly efficient catalytic reduction and ultrasensitive sensing of hazardous 4-nitrophenol pollutant. J. Hazard. Mater. 2017, 333, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.A.; Shin, J.H. A novel and highly sensitive electrochemical monitoring platform for 4-nitrophenol on MnO2 nanoparticles modified graphene surface. RSC Adv. 2015, 5, 88996–89002. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Y.; Li, D.; Zhang, B.; Hao, R.; Sang, S. A sensor for detection of 4-nitrophenol based on a glassy carbon electrode modified with a reduced graphene oxide/Fe3O4 nanoparticle composite. Int. J. Electrochem. Sci. 2017, 12, 7754–7764. [Google Scholar] [CrossRef]
- Sha, R.; Puttapati, S.K.; Srikanth, V.V.; Badhulika, S. Ultra-sensitive phenol sensor based on overcoming surface fouling of reduced graphene oxide-zinc oxide composite electrode. J. Electroanal. Chem. 2017, 785, 26–32. [Google Scholar] [CrossRef]
- Mohammad, A.; Ahmad, K.; Rajak, R.; Mobin, S.M. Binder free modification of glassy carbon electrode by employing reduced graphene oxide/ZnO composite for voltammetric determination of certain nitroaromatics. Electroanalysis 2017, 29, 1–10. [Google Scholar] [CrossRef]
- Alam, M.K.; Rahman, M.M.; Abbas, M.; Torati, S.R.; Asiri, A.M.; Kim, D.; Kim, C. Ultra-sensitive 2-nitrophenol detection based on reduced graphene oxide/ZnO nanocomposites. J. Electroanal. Chem. 2017, 788, 66–73. [Google Scholar] [CrossRef]
- Dutta, S.; Biswas, S.; Maji, R.C.; Saha, R. Environmentally sustainable fabrication of Cu1.94S-rGO composite for dual environmental application: Visible-light-active photocatalyst and room-temperature phenol sensor. ACS Sustain. Chem. Eng. 2018, 6, 835–845. [Google Scholar] [CrossRef]
- Giribabu, K.; Suresh, R.; Manigandan, R.; Kumar, S.P.; Muthamizh, S.; Munusamy, S.; Narayanan, V. Preparation of nitrogen-doped reduced graphene oxide and its use in a glassy carbon electrode for sensing 4-nitrophenol at nanomolar levels. Microchim. Acta 2014, 181, 1863–1870. [Google Scholar] [CrossRef]
- Kumar, D.R.; Kesavan, S.; Baynosa, M.L.; Shim, J.J. 3,5-Diamino-1,2,4-triazole@electrochemically reduced graphene oxide film modified electrode for the electrochemical determination of 4-nitrophenol. Electrochim. Acta 2017, 246, 1131–1140. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Yang, R.; Li, J.; Qu, L. A highly sensitive and selective electrochemical sensor based on polydopamine functionalized graphene and molecularly imprinted polymer for the 2,4-dichlorophenol recognition and detection. Talanta 2019, 195, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.H.; Zhu, J.; Liu, X. Reduced graphene oxide-conjugated urchin-like NiCo2O4 nanostructures for individual detection of o-nitro and p-amino phenol. ACS Omega 2019, 4, 11433–11439. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, L.; Zhang, J.; Guo, S. Gold electrode fused with AuNPs/GQDs showing enhanced electrochemical performance for detection of phenolic compounds. J. Electrochem. Soc. 2019, 166, 1707–1711. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Zeng, L.; Wang, T.; Cai, Y.; Jiang, G. Evaluation of graphene as an advantageous adsorbent for solid-phase extraction with chlorophenols as model analytes. J. Chromatogr. A 2011, 1218, 197–204. [Google Scholar] [CrossRef]
- Pan, S.-D.; Zhou, L.-X.; Zhao, Y.-G.; Chen, X.-H.; Shen, H.-Y.; Cai, M.-Q.; Jin, M.-C. Amine-functional magnetic polymer modified graphene oxide as magnetic solid-phase extraction materials combined with liquid chromatography-tandem mass spectrometry for chlorophenols analysis in environmental water. J. Chromatogr. A 2014, 1362, 34–42. [Google Scholar] [CrossRef]
- Cai, M.-Q.; Su, J.; Hu, J.-Q.; Wang, Q.; Dong, C.-Y.; Pan, S.-D.; Jin, M.-C. Planar graphene oxide-based magnetic ionic liquid nanomaterial for extraction of chlorophenols from environmental water samples coupled with liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2016, 1459, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-H.; Pan, S.-D.; Ye, M.-J.; Li, X.-P.; Zhao, Y.-G.; Jin, M.-C. Magnetic solid-phase extraction based on a triethylenetetramine-functionalized magnetic graphene oxide composite for the detection of ten trace phenolic environmental estrogens in environmental water. J. Sep. Sci. 2016, 39, 762–768. [Google Scholar] [CrossRef]
- Zhang, R.; Su, P.; Yang, Y. Microwave-assisted preparation of magnetic nanoparticles modified with graphene oxide for the extraction and analysis of phenolic compounds. J. Sep. Sci. 2014, 37, 3339–3346. [Google Scholar] [CrossRef]
- Abdolmohammad-Zadeh, H.; Zamani, A.; Shamsi, Z. Extraction of four endocrine-disrupting chemicals using a Fe3O4/graphene oxide/di-(2-ethylhexyl) phosphoric acid nano-composite, and their quantification by HPLC-UV. Microchem. J. 2020, 157, 104964. [Google Scholar] [CrossRef]
- Li, F.; Cai, C.; Cheng, J.; Zhou, H.; Ding, K.; Zhang, L. Extraction of endocrine disrupting phenols with iron-ferric oxide core-shell nanowires on graphene oxide nanosheets, followed by their determination by HPLC. Microchim. Acta 2015, 182, 2503–2511. [Google Scholar] [CrossRef]
- Hou, X.; Yu, H.; Guo, Y.; Liang, X.; Wang, S.; Wang, L.; Liu, X. Polyethylene glycol/graphene oxide coated solid-phase microextraction fiber for analysis of phenols and phthalate esters coupled with gas chromatography. J. Sep. Sci. 2015, 38, 2700–2707. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Chen, G.; Huang, J.; Zheng, J.; Xu, J.; Liu, S.; Qiu, J.; Yin, L.; Ruan, W.; et al. A graphene oxide-based polymer composite coating for highly-efficient solid phase microextraction of phenols. Anal. Chim. Acta 2018, 1015, 20–26. [Google Scholar] [CrossRef]
- Sun, M.; Bu, Y.; Feng, J.; Luo, C. Graphene oxide reinforced polymeric ionic liquid monolith solid-phase microextraction sorbent for high-performance liquid chromatography analysis of phenolic compounds in aqueous environmental samples. J. Sep. Sci. 2016, 39, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-B.; Zhu, G.-T.; Li, X.-S.; Yuan, B.-F.; Feng, Y.-Q. Facile fabrication of reduced graphene oxide-encapsulated silica: A sorbent for solid-phase extraction. J. Chromatogr. A 2013, 1299, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liang, J.; Luo, S.; Liu, C.; Tang, Y. Supersensitive detection of chlorinated phenols by multiple amplification electrochemiluminescence sensing based on carbon quantum dots/graphene. Anal. Chem. 2013, 85, 7720–7725. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Xiao, H.; Yang, S.; Liu, C.; Liang, J.; Tang, Y. Ultrasensitive detection of pentachlorophenol based on enhanced electrochemiluminescence of Au nanoclusters/graphene hybrids. Sens. Actuators B 2014, 194, 325–331. [Google Scholar] [CrossRef]
- Jiang, D.; Du, X.; Liu, Q.; Zhou, L.; Qian, J.; Wang, K. One-step thermal-treatment route to fabricate well-dispersed ZnO nanocrystals on nitrogen-doped graphene for enhanced electrochemiluminescence and ultrasensitive detection of pentachlorophenol. ACS Appl. Mater. Interfaces 2015, 7, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Qiu, N.; Liu, Y.; Xiang, M.; Lu, X.; Yang, Q.; Guo, R. A facile and stable colorimetric sensor based on three-dimensional graphene/mesoporous Fe3O4 nanohybrid for highly sensitive and selective detection of p-nitrophenol. Sens. Actuators B 2018, 266, 86–94. [Google Scholar] [CrossRef]
- Hashim, H.S.; Fen, Y.W.; Omar, N.A.S.; Fauzi, N.I.M.; Daniyal, W.M.E.M.M. Recent advances of priority phenolic compounds detection using phenol oxidases-based electrochemical and optical sensors. Measurement 2021, 184, 109855. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Fauzi, N.I.M.; Hashim, H.S.; Ramdzan, N.S.M.; Omar, N.A.S. Recent advances in surface plasmon resonance optical sensors for potential application in environmental monitoring. Sensors Mater. 2020, 32, 4191. [Google Scholar] [CrossRef]
- Ramdzan, N.S.M.; Fen, Y.W.; Omar, N.A.S.; Anas, N.A.A.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainudin, A.A. Optical and surface plasmon resonance sensing properties for chitosan/carboxyl-functionalized graphene quantum dots thin film. Optik 2019, 178, 802–812. [Google Scholar] [CrossRef]
- Hashim, H.S.; Fen, Y.W.; Omar, N.A.S.; Abdullah, J.; Daniyal, W.M.E.M.M.; Saleviter, S. Detection of phenol by incorporation of gold modified-enzyme based graphene oxide thin film with surface plasmon resonance technique. Opt. Express 2020, 28, 9738–9752. [Google Scholar] [CrossRef]
- Deng, Y.; Chang, Q.; Yin, K.; Liu, C.; Wang, Y. A highly stable electrochemiluminescence sensing system of cadmium sulfide nanowires/graphene hybrid for supersensitive detection of pentachlorophenol. Chem. Phys. Lett. 2017, 685, 157–164. [Google Scholar] [CrossRef]
- Mitra, R.; Saha, A. Reduced graphene oxide based ‘turn-on’ fluorescence sensor for highly reproducible and sensitive detection of small organic pollutants. ACS Sustain. Chem. Eng. 2017, 5, 604–615. [Google Scholar] [CrossRef]
- Darabdhara, G.; Das, M.R. Dual responsive magnetic Au@Ni nanostructures loaded reduced graphene oxide sheets for colorimetric detection and photocatalytic degradation of toxic phenolic compounds. J. Hazard. Mater. 2019, 368, 365–377. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Hou, C.; Yang, X.; Li, Z.; Meng, Q.; Liang, C. Graphene-based magnetic metal organic framework nanocomposite for sensitive colorimetric detection and facile degradation of phenol. J. Taiwan Inst. Chem. Eng. 2019, 102, 312–320. [Google Scholar] [CrossRef]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Ramdzan, N.S.M.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainudin, A.A. Optical properties of chitosan/hydroxyl-functionalized graphene quantum dots thin film for potential optical detection of ferric (III) ion. Opt. Laser Technol. 2019, 120, 105724. [Google Scholar] [CrossRef]
- Sun, R.; Wang, Y.; Ni, Y.; Kokot, S. Graphene quantum dots and the resonance light scattering technique for trace analysis of phenol in different water samples. Talanta 2014, 125, 341–346. [Google Scholar] [CrossRef]
- Anh, N.T.N.; Doong, R.-A. One-step synthesis of size-tunable gold@sulfur-doped graphene quantum dot nanocomposites for highly selective and sensitive detection of nanomolar 4-nitrophenol in aqueous solutions with complex matrix. ACS Appl. Nano Mater. 2018, 1, 2153–2163. [Google Scholar] [CrossRef]
- Zhou, Y.; Qu, Z.-B.; Zeng, Y.; Zhou, T.; Shi, G. A novel composite of graphene quantum dots and molecularly imprinted polymer for fluorescent detection of paranitrophenol. Biosens. Bioelectron. 2014, 52, 317–323. [Google Scholar] [CrossRef]
- Anh, N.T.N.; Chang, P.-Y.; Doong, R.-A. Sulfur-doped graphene quantum dot-based paper sensor for highly sensitive and selective detection of 4-nitrophenol in contaminated water and wastewater. RSC Adv. 2019, 9, 26588–26597. [Google Scholar]
- Wang, Y.; Liu, A.; Han, Y.; Li, T. Sensors based on conductive polymers and their composites: A review. Polym. Int. 2020, 69, 7–17. [Google Scholar] [CrossRef]
- Hathoot, A.A.; Yousef, U.S.; Shatla, A.S.; Abdel-Azzem, M. Voltammetric simultaneous determination of glucose, ascorbic acid and dopamine on glassy carbon electrode modified by NiNPs@poly 1,5-diaminonaphthalene. Electrochim. Acta 2012, 85, 531–537. [Google Scholar] [CrossRef]
- Abdelwahab, A.A.; Lee, H.-M.; Shim, Y.-B. Selective determination of dopamine with a cibacron blue/poly-1,5-diaminonaphthalene composite film. Anal. Chim. Acta 2009, 650, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Son, N.X.; Noh, H.-B.; Goyal, R.N.; Shim, Y.-B. Investigation on the downregulation of dopamine by acetaminophen administration based on their simultaneous determination in urine. Biosens. Bioelectron. 2013, 39, 139–144. [Google Scholar] [CrossRef]
- Kim, D.-M.; Cho, S.J.; Cho, C.-H.; Kim, K.B.; Kim, M.-Y.; Shim, Y.-B. Disposable all-solid-state pH and glucose sensors based on conductive polymer covered hierarchical AuZn oxide. Biosens. Bioelectron. 2016, 79, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, A.A.; Shim, Y.-B. Nonenzymatic H2O2 sensing based on silver nanoparticles capped polyterthiophene/MWCNT nanocomposite. Sens. Actuators B 2014, 201, 51–58. [Google Scholar] [CrossRef]
- Won, M.-S.; Yoon, J.-H.; Shim, Y.-B. Determination of selenium with a poly(1,8-diamino-naphthalene)-modified electrode. Electroanalysis 2005, 17, 1952–1958. [Google Scholar] [CrossRef]
- Pallela, R.; Chandra, P.; Noh, H.-B.; Shim, Y.-B. An amperometric nanobiosensor using a biocompatible conjugate for early detection of metastatic cancer cells in biological fluid. Biosens. Bioelectron. 2016, 85, 883–890. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Habibiasr, M.; Soleimani, H.; Hamidon, M.N.; Fen, Y.W.; Lim, H.N. Surface plasmon resonance sensor to detect n-hexane in palm kernel oil using polypyrrole nanoparticles reduced graphene oxide layer. J. Sensors 2021, 2021, 1–13. [Google Scholar] [CrossRef]
- Ozdokur, K.V.; Pelit, L.; Ertas, H.; Timur, S.; Ertas, F.N. Head space voltammetry: A novel voltammetric method for volatile organics and a case study for phenol. Talanta 2012, 98, 34–39. [Google Scholar] [CrossRef]
- Besombes, J.L.; Cosnier, S.; Labbé, P.; Reverdy, G. Determination of phenol and chlorinated phenolic compounds based on a PPO-bioelectrode and its inhibition. Anal. Lett. 1995, 28, 405–424. [Google Scholar] [CrossRef]
- Cosnier, S.; Popescu, I.C. Poly(amphiphilic pyrrole)-tyrosinase-peroxidase electrode for amplified flow injection-amperometric detection of phenol. Anal. Chim. Acta 1996, 319, 145–151. [Google Scholar] [CrossRef]
- Cosnier, S.; Fombon, J.-J.; Labbe, P.; Limosin, D. Development of a PPO-poly(amphiphilic pyrrole) electrode for on site monitoring of phenol in aqueous effluents. Sens. Actuators B 1999, 59, 134–139. [Google Scholar] [CrossRef]
- Mailley, P.; Cummings, E.A.; Mailley, S.C.; Eggins, B.R.; McAdams, E.; Cosnier, S. Composite carbon paste biosensor for phenolic derivatives based on in situ electrogenerated. Anal. Chem. 2003, 75, 5422–5428. [Google Scholar] [CrossRef]
- Cosnier, S.; Innocent, C. A new strategy for the construction of a tyrosinase-based amperometric phenol and o-diphenol sensor. Bioelectrochem. Bioenerg. 1993, 31, 147–160. [Google Scholar] [CrossRef]
- Tingry, S.; Innocent, C.; Touil, S.; Deratani, A.; Seta, P. Carbon paste biosensor for phenol detection of impregnated tissue: Modification of selectivity by using β-cyclodextrin-containing PVA membrane. Mater. Sci. Eng. C 2006, 26, 222–226. [Google Scholar] [CrossRef]
- Li, H.; Hu, X.; Zhu, H.; Zang, Y.; Xue, H. Amperometric phenol biosensor based on a new immobilization matrix: Polypyrrole nanotubes derived from methyl orange as dopant. Int. J. Electrochem. Sci. 2017, 12, 6714–6728. [Google Scholar] [CrossRef]
- Lu, W.; Wallace, G.G.; Imisides, M.D. Development of conducting polymer modified electrodes for the detection of phenol. Electroanalysis 2002, 14, 325–332. [Google Scholar] [CrossRef]
- Stanca, S.E.; Popescu, I.C. Phenols monitoring and Hill coefficient evaluation using tyrosinase-based amperometric biosensors. Bioelectrochemistry 2004, 64, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Ozoner, S.K.; Kilic, M.S.; Erhan, E. Modified poly(pyrrole) film based biosensors for phenol detection. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2015, 13, 1173–1176. [Google Scholar]
- Rajesh; Takashima, W.; Kaneto, K. Amperometric tyrosinase based biosensor using an electropolymerized PTS-doped polypyrrole film as an entrapment support. React. Funct. Polym. 2004, 59, 163–169. [Google Scholar] [CrossRef]
- Rajesh; Takashima, W.; Kaneto, K. Amperometric phenol biosensor based on covalent immobilization of tyrosinase onto an electrochemically prepared novel copolymer poly(N-3-aminopropyl pyrrole-co-pyrrole) film. Sens. Actuators B 2004, 102, 271–277. [Google Scholar] [CrossRef]
- Rajesh; Kaneto, K. A new tyrosinase biosensor based on covalent immobilization of enzyme on N-(3-aminopropyl) pyrrole polymer film. Curr. Appl. Phys. 2005, 5, 178–183. [Google Scholar] [CrossRef]
- Rajesh; Pandey, S.S.; Takashima, W.; Kaneto, K. Development of an amperometric biosensor based on a redox-mediator-doped polypyrrole film. J. Appl. Polym. Sci. 2004, 93, 927–933. [Google Scholar] [CrossRef]
- Rajesh; Pandey, S.S.; Takashima, W.; Kaneto, K. Simultaneous co-immobilization of enzyme and a redox mediator in polypyrrole film for the fabrication of an amperometric phenol biosensor. Curr. Appl. Phys. 2005, 5, 184–188. [Google Scholar] [CrossRef]
- Njagi, J.; Andreescu, S. Stable enzyme biosensors based on chemically synthesized Au-polypyrrole nanocomposites. Biosens. Bioelectron. 2007, 23, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Daoa, Y.; Thanachayanont, C.; Prichanont, S. Glassy carbon electrode with decorated gold nanoparticles for horseradish peroxidase/polypyrrole nanorod biosensor. Sensors Mater. 2016, 28, 1–11. [Google Scholar]
- Sulak, M.T.; Erhan, E.; Keskinler, B. Electrochemical phenol biosensor configurations based on nanobiocomposites. Sensors Mater. 2012, 24, 141–152. [Google Scholar]
- Ozoner, S.K.; Keskinler, B.; Erhan, E. An amperometric biosensor based on multiwalled carbon nanotube-poly(pyrrole)-horseradish peroxidase nanobiocomposite film for determination of phenol derivatives. Talanta 2008, 76, 1147–1152. [Google Scholar]
- Ozoner, S.K.; Yilmaz, F.; Celik, A.; Keskinler, B.; Erhan, E. A novel poly(glycine methacrylate-co-3-thienylmethyl methacrylate )-polypyrrole-carbon nanotube-horseradish peroxidase composite film electrode for the detection of phenolic compounds. Curr. Appl. Phys. 2011, 11, 402–408. [Google Scholar] [CrossRef]
- Sulak, M.T.; Erhan, E.; Keskinler, B. Amperometric phenol biosensor based on horseradish peroxidase entrapped PVF and PPy composite film coated GC electrode. Appl. Biochem. Biotechnol. 2010, 160, 856–867. [Google Scholar] [CrossRef]
- Dursun, F.; Ozoner, S.K.; Demirci, A.; Gorur, M.; Yilmaz, F.; Erhan, E. Vinylferrocene copolymers based biosensors for phenol derivatives. J. Chem. Tech. Biotechnol. 2012, 87, 95–104. [Google Scholar] [CrossRef]
- Arslan, H.; Arslan, F. Preparation of a polypyrrole-polyvinylsulphonate composite film biosensor for determination of phenol based on entrapment of polyphenol oxidase. Artif. Cells, Blood Substitutes, Biotechnol. 2011, 39, 341–345. [Google Scholar] [CrossRef]
- Arulraj, A.D.; Vijayan, M.; Vasantha, V.S. Highly selective and sensitive simple sensor based on electrochemically treated nano polypyrrole-sodium dodecyl sulphate film for the detection of para-nitrophenol. Anal. Chim. Acta 2015, 899, 66–74. [Google Scholar] [CrossRef]
- Seo, H.-K.; Ameen, S.; Akhtar, M.S.; Shin, H.S. Structural, morphological and sensing properties of layered polyaniline nanosheets towards hazardous phenol chemical. Talanta 2013, 104, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Timur, S.; Pazarlioglu, N.; Pilloton, R.; Telefoncu, A. Thick film sensors based on laccases from different sources immobilized in polyaniline matrix. Sens. Actuators B 2004, 97, 132–136. [Google Scholar] [CrossRef]
- Kushwah, B.S.; Upadhyaya, S.C.; Shukla, S.; Sikarwar, A.S.; Sengar, R.M.S.; Bhadauria, S. Performance of nanopolyaniline-fungal enzyme based biosensor for water pollution. Adv. Mat. Lett. 2011, 2, 43–51. [Google Scholar] [CrossRef]
- Li, X.; Sun, C. Bioelectrochemical response of the polyaniline tyrosinase electrode to phenol. J. Anal. Chem. 2005, 60, 1073–1077. [Google Scholar] [CrossRef]
- Xue, H.; Shen, Z. A highly stable biosensor for phenols prepared by immobilizing polyphenol oxidase into polyaniline-polyacrylonitrile composite matrix. Talanta 2002, 57, 289–295. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, J.; Liu, Y.; Zhao, J.; Ju, H. Highly sensitive amperometric biosensors for phenols based on polyaniline-ionic liquid-carbon nanofiber composite. Biosens. Bioelectron. 2009, 24, 1858–1863. [Google Scholar] [CrossRef]
- Arslan, H.; Senarslan, D.; Cevrimli, B.S.; Zengin, H.; Uzun, D.; Arslan, F. Preparation of carbon paste electrode containing polyaniline-activated carbon composite for amperometric detection of phenol. Bulg. Chem. Commun. 2018, 50, 16–20. [Google Scholar]
- Guo, L.; Du, H.; Zhao, H.; Li, J. Amplified electrochemical response of phenol by oxygenation of tyrosinase coupling with electrochemical-chemical-chemical redox cycle. Electroanalysis 2019, 31, 1728–1735. [Google Scholar] [CrossRef]
- Roy, A.C.; Nisha, V.S.; Dhand, C.; Ali, M.A.; Malhotra, B.D. Molecularly imprinted polyaniline-polyvinyl sulphonic acid composite based sensor for para-nitrophenol detection. Anal. Chim. Acta 2013, 777, 63–71. [Google Scholar] [CrossRef]
- Jovic, A.; Dordevic, A.; Cebela, M.; Simatovic, I.S.; Hercigonja, R.; Sljukic, B. Composite zeolite/carbonized polyaniline electrodes for p-nitrophenol sensing. J. Electroanal. Chem. 2016, 778, 137–147. [Google Scholar] [CrossRef]
- Klink, M.J.; Iwuoha, E.I.; Ebenso, E.E. The electro-catalytic and redox-mediator effects of nanostructured PDMA-PSA modified-electrodes as phenol derivative sensors. Int. J. Electrochem. Sci. 2011, 6, 2429–2442. [Google Scholar]
- Vedrine, C.; Fabiano, S.; Tran-Minh, C. Amperometric tyrosinase based biosensor using an electrogenerated polythiophene film as an entrapment support. Talanta 2003, 59, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Heras, M.A.; Lupu, S.; Pigani, L.; Pirvu, C.; Seeber, R.; Terzi, F.; Zanardi, C. A poly(3,4-ethylenedioxythiophene)-poly(styrene sulphonate) composite electrode coating in the electrooxidation of phenol. Electrochim. Acta 2005, 50, 1685–1691. [Google Scholar] [CrossRef]
- Hryniewicz, B.M.; Orth, E.S.; Vidotti, M. Enzymeless PEDOT-based electrochemical sensor for the detection of nitrophenols and organophosphates. Sens. Actuators B 2017, 257, 570–578. [Google Scholar] [CrossRef]
- Negash, N.; Alemu, H.; Tessema, M. Determination of phenol and chlorophenols at single-wall carbon nanotubes/poly(3,4-ethylenedioxythiophene) modified glassy carbon electrode using flow injection amperometry. Anal. Chem. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Negash, N.; Alemu, H.; Tessema, M. Electrochemical characterization and determination of phenol and chlorophenols by voltammetry at single wall carbon nanotube/poly(3,4-ethylenedioxythiophene) modified screen printed carbon electrode. Int. Sch. Res. Not. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ann Maria, C.G.; Akshaya, K.B.; Rison, S.; Varghese, A.; George, L. Molecularly imprinted PEDOT on carbon fiber paper electrode for the electrochemical determination of 2,4-dichlorophenol. Synth. Met. 2020, 261, 116309. [Google Scholar] [CrossRef]
- Bagheri, H.; Mohammadi, A. Pyrrole-based conductive polymer as the solid-phase extraction medium for the preconcentration of environmental pollutants in water samples followed by gas chromatography with flame ionization and mass spectrometry detection. J. Chromatogr. A 2003, 1015, 23–30. [Google Scholar] [CrossRef]
- Bagheri, H.; Mohammadi, A.; Salemi, A. On-line trace enrichment of phenolic compounds from water using a pyrrole-based polymer as the solid-phase extraction sorbent coupled with high-performance liquid chromatography. Anal. Chim. Acta 2004, 513, 445–449. [Google Scholar] [CrossRef]
- Tahmasebi, E.; Yamini, Y.; Seidi, S.; Rezazadeh, M. Extraction of three nitrophenols using polypyrrole-coated magnetic nanoparticles based on anion exchange process. J. Chromatogr. A 2013, 1314, 15–23. [Google Scholar] [CrossRef]
- Abolghasemi, M.M.; Parastari, S.; Yousefi, V. Polypyrrole-montmorillonite nanocomposite as sorbent for solid-phase microextraction of phenolic compounds in water. J. Sep. Sci. 2014, 37, 3526–3532. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Saraji, M. New polymeric sorbent for the solid-phase extraction of chlorophenols from water samples followed by gas chromatography-electron-capture detection. J. Chromatogr. A 2001, 910, 87–93. [Google Scholar] [CrossRef]
- Bagheri, H.; Saraji, M. Conductive polymers as new media for solid-phase extraction: Isolation of chlorophenols from water sample. J. Chromatogr. A 2003, 986, 111–119. [Google Scholar] [CrossRef]
- Bagheri, H.; Mir, A.; Babanezhad, E. An electropolymerized aniline-based fiber coating for solid phase microextraction of phenols from water. Anal. Chim. Acta 2005, 532, 89–95. [Google Scholar] [CrossRef]
- Mousavi, M.; Noroozian, E.; Jalali-Heravi, M.; Mollahosseini, A. Optimization of solid-phase microextraction of volatile phenols in water by a polyaniline-coated Pt-fiber using experimental design. Anal. Chim. Acta 2007, 581, 71–77. [Google Scholar] [CrossRef]
- Du, W.; Zhao, F.; Zeng, B. Novel multiwalled carbon nanotubes–polyaniline composite film coated platinum wire for headspace solid-phase microextraction and gas chromatographic determination of phenolic compounds. J. Chromatogr. A 2009, 1216, 3751–3757. [Google Scholar] [CrossRef]
- Meng, J.; Shi, C.; Wei, B.; Yu, W.; Deng, C.; Zhang, X. Preparation of Fe3O4@C@PANI magnetic microspheres for the extraction and analysis of phenolic compounds in water samples by gas chromatography-mass spectrometry. J. Chromatogr. A 2011, 1218, 2841–2847. [Google Scholar] [CrossRef] [PubMed]
- Tafazoli, Z.; Azar, P.A.; Tehrani, M.S.; Husain, S.W. Facile preparation of multifunctional carbon nanotube/magnetite/polyaniline nanocomposite offering a strong option for efficient solid-phase microextraction coupled with GC-MS for the analysis of phenolic compounds. J. Sep. Sci. 2018, 41, 2736–2742. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Lei, W.; Xia, X.; Xia, M.; Hao, Q. Electrochemical determination of 4-nitrophenol at polycarbazole/N-doped graphene modified glassy carbon electrode. Electrochim. Acta 2014, 146, 568–576. [Google Scholar] [CrossRef]
- Saadati, F.; Ghahramani, F.; Shayani-jam, H.; Piri, F.; Yaftian, M.R. Synthesis and characterization of nanostructure molecularly imprinted polyaniline/graphene oxide composite as highly selective electrochemical sensor for detection of p-nitrophenol. J. Taiwan Inst. Chem. Eng. 2018, 86, 213–221. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Mousavi, S.M.; Bahrani, S.; Ramakrishna, S. Integrated polyaniline with graphene oxide-iron tungsten nitride nanoflakes as ultrasensitive electrochemical sensor for precise detection of 4-nitrophenol within aquatic media. J. Electroanal. Chem. 2020, 873, 114406. [Google Scholar] [CrossRef]
- Zhu, G.; Tang, Q.; Dou, J.; Li, X.; Yang, J.; Xu, R.; Liu, J. Partially reduced graphene oxide sheet-covered polyaniline nanotubes for the simultaneous determination of bisphenol A and phenol. J. Electrochem. Soc. 2019, 166, 1661–1668. [Google Scholar] [CrossRef]
- Peleyeju, M.G.; Idris, A.O.; Umukoro, E.H.; Babalola, J.O.; Arotiba, O.A. Electrochemical detection of 2,4-dichlorophenol on a ternary composite electrode of diamond, graphene, and polyaniline. ChemElectroChem 2017, 3, 1074–1080. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.A.P.; Rahman, M.M.; Asiri, A.M.; Inamuddin; Alamry, K.A.; Hameed, S.A. Preparation and characterization of PANI@G/CWO nanocomposite for enhanced 2-nitrophenol sensing. Appl. Surf. Sci. 2018, 433, 696–704. [Google Scholar] [CrossRef]
- Zou, J.; Song, X.; Ji, J.; Xu, W.; Chen, J.; Jiang, Y.; Wang, Y.; Chen, X. Polypyrrole/graphene composite-coated fiber for the solid-phase microextraction of phenols. J. Sep. Sci. 2011, 34, 2765–2772. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gu, Y.; Nie, G.; Chi, M.; Yang, Z.; Wang, C.; Wei, Y.; Lu, X. Synthesis of RGO/Cu8S5/PPy composite nanosheets with enhanced peroxidase-like activity for sensitive colorimetric detection of H2O2 and phenol. Part. Part. Syst. Charact. 2017, 34, 1600233. [Google Scholar] [CrossRef]
- Talarico, D.; Arduini, F.; Constantino, A.; Del Carlo, M.; Compagnone, D.; Moscone, D.; Palleschi, G. Carbon black as successful screen-printed electrode modifier for phenolic compound detection. Electrochem. Commun. 2015, 60, 78–82. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, Z.-L.; Liang, Y.-M.; Liu, W. A graphene-based electrochemical sensor for rapid determination of phenols in water. Sensors 2013, 13, 6204–6216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Ramdzan, N.S.M.; Saleviter, S. Development of graphene quantum dots-based optical sensor for toxic metal ion detection. Sensors 2019, 19, 3850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Sensing Materials | Sensor | Hazardous Phenolic Compounds | Linear Range | LOD 1 | Ref. |
|---|---|---|---|---|---|
| Tyr–graphite electrode | Amperometry | Ph | 0.02–0.14 mM | 6 µM | [65] |
| 2,4-DMP | 0.08–0.64 mM | 29 µM | |||
| Tyr–graphite electrode | Pulsed amperometry | Ph | Up to 2 mM | 5.2 µM | [66] |
| Tyr–graphite electrode | Amperometry | Ph | Up to 600 µM | 0.4 µM | [67] |
| Trametes versicolor laccase–graphite electrode | Amperometry | Ph | 1000–10,000 µM | 557 µM | [68] |
| Cerrena unicolor laccase–graphite electrode | Amperometry | Ph | 1000–10,000 µM | 296 ± 10 µM | [69] |
| Tyr-mediated graphite electrode | Amperometry | Ph | - | 0.006 µM | [70] |
| Entrapped Tyr–tetracyanoquinodimethane–graphite electrode | Amperometry | Ph | Up to 65 µM | - | [71] |
| Immobilized Tyr–tetracyanoquinodimethane–graphite electrode | Up to 25 µM | 0.23 µM | |||
| Tyr/graphite/2–hexadecanol | Chronoamperometry | Ph | Up to 2.5 µM | 0.2 µM | [72] |
| Tyr–glutaraldehyde–carbodiimide-activated graphite electrode | Amperometry | Ph | 0.01–5 µM | 0.003 µM | [64] |
| Tyr–graphite–epoxy electrode | Amperometry | Ph | - | 0.5 µM | [73] |
| Tyr–graphite–epoxy electrode | Amperometry | Ph | Up to 300 µM | 1 µM | [74] |
| HRP–graphite–epoxy composite electrode | Square wave voltammetry/weighted least-squares | Ph | 1.95–5.5 µM | 0.65 µM | [75] |
| Square wave voltammetry/partial least-squares 1 | 1–62 µM | 1.1 µM | |||
| HRP–GOD–mutarotase reactor–graphite electrode | Amperometry | Ph | - | 3.6 ± 0.5 µM | [76] |
| Tobacco peroxidase–GOD–mutarotase reactor–graphite electrode | 10.1 ± 0.8 µM | ||||
| Peroxidase from peanut cell culture–GOD–mutarotase reactor–graphite electrode | 7 ± 0.6 µM | ||||
| Graphite–Teflon–Tyr composite electrodes | Amperometry | Ph | 4–80 µM | 1.1 µM | [77] |
| 2,4-DMP | 0.05–3 mM | 33.8 µM | |||
| Graphite–Teflon–HRP composite electrodes | Amperometry | 2-CP | 0.2–20 µM | 0.16 µM | [78] |
| 2,4-DCP | 0.4–20 µM | 0.45 µM | |||
| 2,4-DMP | 4.5–10 µM | 0.45 µM | |||
| Graphite–Teflon–Tyr composite electrode | Flow injection with amperometry | Ph | 0.01–40 µM | 0.01 µM | [79] |
| 2-CP | 10–750 µM | 7.3 µM | |||
| Flow injection with dual amperometry | Ph | 0.25–50 µM | 0.12 µM | ||
| Graphite–Teflon–GOD–HRP composite electrode | Flow injection with amperometry | Ph | 1–100 µM | 1.5 µM | |
| 2-CP | 1–50 µM | 2.2 µM | |||
| 2,4,6-TCP | 1–50 µM | 1.3 µM | |||
| Flow injection with dual amperometry | Ph | 1–100 µM | 2.3 µM | ||
| 2,4,6-TCP | 5–60 µM | 3 µM | |||
| Graphite–Teflon–GOD–HRP–Tyr composite electrodes | Amperometry | Ph | 0.1–20 µM | 0.08 µM | [80] |
| 2,4,6-TCP | 0.1–40 µM | 0.14 µM | |||
| PCP | 0.02–1000 µM | 7.6 µM | |||
| Graphite–Teflon–GOD–HRP composite electrodes | Ph | 0.5–60 µM | 0.43 µM | ||
| 2,4,6-TCP | 0.1–60 µM | 0.11 µM | |||
| PCP | 0.01–1500 µM | 4.7 µM | |||
| Graphite–ethylene–propylene–diene–Tyr composite electrode | Amperometry | Ph | 0.05–6 µM | 26.3 nM | [81] |
| 2,4-DMP | 1–50 µM | 0.67 µM | |||
| Graphite–Teflon–Tyr composite electrode | Ph | 0.1–25 µM | 99 nM | ||
| 2,4-DMP | 0.7–100 µM | 0.71 µM | |||
| Tyr–colloidal gold nanoparticles–graphite–Teflon electrode | Amperometry | Ph | 0.025–4 µM | 0.02 µM | [82] |
| HRP–graphite-coated screen-printed four-channel gold-array | Amperometry | Ph | 2–300 µM | - | [83] |
| Tyr–graphite-coated screen-printed four-channel gold-array | 2–40 µM | ||||
| HRP–graphite-coated screen-printed four-channel gold-array | 2,4,6-TCP | 2–140 µM | |||
| Tyr–screen-printed graphite electrode | Amperometry | Ph | - | 0.41 µM | [84] |
| HRP/GOD–screen-printed graphite electrode | 1.8 µM | ||||
| Copper-modified graphite pencil electrode | Amperometry | 4-NP | 50–850 µM | 1.9 µM | [85] |
| Bare graphite pencil electrode | - | 1 mM | |||
| Pre-charged disposable graphite pencil electrode | Square wave voltammetry | Ph | 0.05–1 µM | 4.17 nM | [86] |
| Gold nanoparticles–pre-treated graphite pencil electrode | Square wave voltammetry | 4-NP | 0.5–100 µM | - | [87] |
| Pre-treated graphite pencil electrode | 0.01–0.8 µM | 0.002 µM | |||
| Poly(dopamine–quinone chromium (III))–microspheres/graphite paste electrode | Differential pulse voltammetry | Ph | 2.5–107.5 µM | 0.6 µM | [88] |
| 4-NP | 2.5–130 µM | 0.8 µM | |||
| Graphite–dioctyl phthalate–CPE | Voltammetry | Ph | 2.5 µM–60 mM | 2.5 µM | [89] |
| Graphitized-ordered mesoporous carbon–Tyr–cobaltosic oxide nanorod–chitosan–GCE | Amperometry | Ph | 0.05–11 µM | 0.025 µM | [90] |
| Graphite nanoflakes–GCE | Cyclic voltammetry at peak c1 | 4-NP | 0.5–6000 µM | 0.18 µM | [91] |
| Cyclic voltammetry at peak a2 | 1–6000 µM | 0.7 µM | |||
| Gold–cerium oxide–graphite–carbon nitride modified carbon paper | Amperometry | Ph | 10–90 µM | 2.33 µM | [92] |
| Sensing Materials | Sensor | Hazardous Phenolic Compounds | Linear Range | LOD 1 | Ref. |
|---|---|---|---|---|---|
| Gr–Nafion–screen-printed electrode | Differential pulse voltammetry | 4-NP | 10 µM–0.62 mM | 0.6 µM | [93] |
| Ion liquid–palladium–Gr–Nafion–GCE | Differential pulse voltammetry | 2-CP | 4–800 µM | 1.5 µM | [94] |
| N-methyl-2-pyrrolidone exfoliated Gr nanosheets–GCE | Differential pulse voltammetry | 4-NP | - | 0.04 µM | [95] |
| Gr nanosheet paste electrode | Differential pulse voltammetry | Ph | 0.08–80 µM | 0.05 µM | [96] |
| Gr nanosheets–CTAB | Differential pulse voltammetry | 4-NP | 7.2–107.8 µM | 3 µM | [98] |
| Gr–CTAB–GCE | Differential pulse voltammetry | Ph | 0.53–1063 µM | 0.21 µM | [99] |
| Nafion–poly(sodium 4–styrenesulfonate)–Gr–CTAB–GCE | Linear sweep voltammetry | 2,4-DCP | 0.01–2 µM | 2 nM | [100] |
| Gr nanosheets–iron phthalocyanine–GCE | Cyclic voltammetry | 4-NP | 0.1–0.7 mM | 10 µM | [101] |
| Gr–gold nanoparticles–GCE | Amperometry | 4-NP | 0.47–10,750 µM | 0.47 µM | [102] |
| Gr–gold nanoparticles–GCE | Linear sweep voltammetry | 4-NP | 0.036–90 µM | 0.01 µM | [103] |
| Gr–chitosan–acetylene black paste electrode | Linear sweep voltammetry | 2-NP | 0.4–80 µM | 0.2 µM | [104] |
| 4-NP | 0.1–20 µM | 0.08 µM | |||
| 20–80 µM | |||||
| Gr–chitosan–GCE | Linear sweep voltammetry | 2-NP | 1–240 µM | 0.1 µM | [105] |
| 4-NP | 0.1–140 µM | 0.09 µM | |||
| Three-dimensional Gr–tyrosinase | Amperometry | Ph | 0.05–2 µM | 0.05 µM | [106] |
| Horseradish peroxidase–Gr–chitosan–GCE | Amperometry | 2,4-DCP | 0.01–13 µM | 5 nM | [107] |
| Gr–gold nanoparticles–chitosan–tyrosinase–screen-printed carbon electrode | Differential pulse voltammetry | Ph | 0.05–15 µM | 0.016 µM | [108] |
| 2,4-DCP | 0.05–15 µM | 0.041 µM | |||
| 4-NP | 0.05–19 µM | 0.031 µM | |||
| Tyrosinase–Gr–silk peptide–GCE | Amperometry | Ph | 0.0015–21.12 µM | 0.35 nM | [109] |
| β-cyclodextrin-functionalized Gr nanosheets–GCE | Differential pulse voltammetry | 2-NP | 5–400 µM | 0.3 µM | [110] |
| 2-hydroxypropyl–β-cyclodextrin–Gr nanoribbon–GCE | Differential pulse voltammetry | 2-CP | 0.01–16 µM | 0.004 µM | [111] |
| β-cyclodextrin–Gr–carbon paste electrode | Differential pulse voltammetry | 2-CP | 0.5–40 µM | 0.2 µM | [112] |
| Carboxyl–multiwalled carbon nanotubes–β-cyclodextrin edge-functionalized Gr–GCE | Differential pulse voltammetry | 4-NP | 0.3–10 µM | 0.027 µM | [113] |
| 10–50 µM | |||||
| β-cyclodextrin functionalized Gr–silver–GCE | Amperometry | 4-NP | 0.01–0.1 µM | 0.89 nM | [114] |
| 0.1–1500 µM | |||||
| β-cyclodextrin–gold@carboxylic Gr nanosheets–GCE | Differential pulse voltammetry | 4-NP | 0.01–5 µM | 3.8 nM | [115] |
| 5–200 µM | |||||
| Gr–acetylene black paste hybridized electrode | Linear sweep voltammetry | 4-NP | 0.02–8 µM | 0.008 µM | [116] |
| 8–100 µM | |||||
| Magnetite–hydroxyapatite dispersed edge–carboxylated Gr–GCE | Cyclic voltammetry | 4-NP | 30–1455 µM | - | [117] |
| Differential pulse voltammetry | 0.2–994 µM | 0.27 µM | |||
| Fe3O4–amino functionalized Gr–GCE | Linear sweep voltammetry | Ph | 0.45–56 µM | 0.4 µM | [118] |
| 156–456 µM | |||||
| Gr–TiO2 anatase–carbon paste electrode | Cyclic voltammetry | Ph | 0.01–1 nM | 36.6 µM | [119] |
| 1–1000 nM | |||||
| Gr–nickel slag waste–TiO2 electrode | Voltammetry | Ph | 1.06–10.63 µM | 0.39 µM | [120] |
| Na+-doped Gr-like carbon nitride electrode | Amperometry | Ph | 10–110 µM | 0.23 µM | [121] |
| Sensing Materials | Sensor | Hazardous Phenolic Compounds | Linear Range | LOD 1 | Ref. |
|---|---|---|---|---|---|
| GO-GCE | Linear sweep voltammetry | 4-NP | 0.1–120 µM | 0.02 µM | [123] |
| GO–molecularly imprinted polymer–GCE | Differential pulse voltammetry | 2,4-DNP | 1–150 µM | 0.4 µM | [124] |
| Molecularly imprinted polymer–GO–GCE | Differential pulse voltammetry | 2,4-DCP | 0.004–10 µM | 0.5 nM | [125] |
| GO–poly(ethyleneimine)–GCE | Square wave voltammetry | 2-NP | 5–155 µM | 0.1 µM | [126] |
| GO/nickel–curcumin/GCE | Linear sweep voltammetry | 4-NP | 0.49–760 µM | 0.016 µM | [127] |
| Poly(Rhodamine B)–GO–multiwalled carbon nanotubes–GCE | Differential pulse voltammetry | 2,4,6-TCP | 0.004–0.1 µM | 0.8 nM | [128] |
| 0.1–100 µM | |||||
| PCP | 0.002–0.1 µM | 0.5 nM | |||
| 0.1–90 µM | |||||
| Core/shell structured carbon sphere–silver–GO–GCE | Differential pulse voltammetry | 2-CP | 0.05–25 µM | 13.9 nM | [129] |
| 2,4-DCP | 0.05–35 µM | 7.52 nM | |||
| 2,4,6-TCP | 0.03–35 µM | 9.71 nM | |||
| Ionic liquid–GO–gold nanoparticles–GCE | Differential pulse voltammetry | 2,4-DCP | 0.01–5 µM | 3 nM | [130] |
| GO–zinc oxide–GCE | Square wave voltammetry | Ph | 5–155 µM | 2.2 nM | [131] |
| Octahedral cuprous oxide–GO–GCE | Differential pulse voltammetry | 4-NP | 0.08–30 µM | 8.5 nM | [132] |
| Sensing Materials | Sensor | Hazardous Phenolic Compounds | Linear Range | LOD 1 | Ref. |
|---|---|---|---|---|---|
| RGO–screen-printed electrode | Electrochemical impedance spectroscopy | Ph | 1–40 µM | 0.2 µM | [133] |
| RGO–GCE | Differential pulse voltammetry | 4-NP | 50–800 µM | 42 µM | [134] |
| RGO–GCE | Differential pulse voltammetry by oxidation reaction | 4-NP | 8.3–79.8 µM | 2.13 µM | [135] |
| Differential pulse voltammetry by reduction reaction | 3.3–34.4 µM | 0.55 µM | |||
| Hydroxypropyl–β-cyclodextrin–RGO–GCE | Cyclic voltammetry | 2-NP | 0.05–100 µM | 0.01 µM | [136] |
| β-cyclodextrin–RGO–GCE | Differential pulse voltammetry | 2-NP | 7.2–64.7 µM | 0.14 µM | [137] |
| 4-NP | 7.2–73 µM | 0.36 µM | |||
| RGO/β-cyclodextrin/chitosan/GCE | Differential pulse voltammetry | 2-NP | 0.16–0.28 µM | 0.018 µM | [138] |
| 5–40 µM | |||||
| 4-NP | 0.06–0.16 µM | 0.016 µM | |||
| 5–40 µM | |||||
| β-cyclodextrin–Prussian blue nanocubes–RGO–GCE | Linear sweep voltammetry | 4-NP | 0.01–700 µM | 2.34 nM | [139] |
| RGO–silver nanoparticles–GCE | Square wave voltammetry | 4-NP | 0.01–0.1 µM | 1.2 nM | [140] |
| RGO–silver nanoparticles–GCE | Amperometry | 4-NP | 1–10 µM | 0.32 µM | [141] |
| 10–110 µM | |||||
| 110–1110 µM | |||||
| Silver nanoparticles–RGO–GCE | Differential pulse voltammetry | PCP | 0.008–10 µM | 0.001 µM | [142] |
| Au nanoparticles–RGO–GCE | Differential pulse voltammetry | 4-NP | 0.05–2 µM | 0.01 µM | [143] |
| 4–100 µM | |||||
| Square wave voltammetry | 0.05–2 µM | 0.02 µM | |||
| Tyrosinase–RGO–Au nanoparticles–Au/chromium electrode | Amperometry | Ph | - | 0.1 µM | [144] |
| Au nanoparticles–RGO–tyrosinase–indium tin oxide electrode | Chronoamperometry | Ph | Up to 19.5 µM | 0.072 µM | [145] |
| Horseradish peroxidase–partially RGO–GCE | Amperometry | Ph | 0.05–0.1 mM | 4.4 µM | [146] |
| 0.2–1 mM | |||||
| 2–10 mM | |||||
| RGO–chitosan–laccase–GCE | Amperometry | Ph | 5–30 µM | 4.9 µM | [147] |
| Palladium nanoparticles–RGO–gum arabic–GCE | Linear sweep voltammetry | 4-NP | 20–400 pM | - | [148] |
| Electrochemical impedance spectroscopy | 5–300 pM | 2 pM | |||
| Square wave voltammetry | 2–80 pM | 9 fM | |||
| Manganese dioxide nanoparticles–RGO–GCE | Linear sweep voltammetry | 4-NP | 0.02–0.5 µM | 0.01 µM | [149] |
| 2–180 µM | |||||
| RGO–Fe3O4 nanoparticles–GCE | Differential pulse voltammetry | 4-NP | 0.2–10 µM | 0.26 µM | [150] |
| 20–100 µM | |||||
| Square wave voltammetry | 0.2–10 µM | 0.012 µM | |||
| RGO–zinc oxide–GCE | Differential pulse voltammetry | Ph | 2–15 µM | 1.94 µM | [151] |
| 15–40 µM | |||||
| RGO–zinc oxide nanoflowers–GCE | Linear sweep voltammetry | 4-NP | 0.2–0.9 mM | 0.93 µM | [152] |
| 2,4-DNP | 0.1–0.9 mM | 6.2 µM | |||
| RGO–zinc oxide–GCE | I-V method | 2-NP | 0.01–10,000 µM | 0.27 nM | [153] |
| Copper sulfide djurleite–RGO–GCE | Cyclic voltammetry | Ph | 0.2–1.4 µM | - | [154] |
| Nitrogen-doped–RGO–GCE | Linear sweep voltammetry | 4-NP | 0.02–0.5 µM | 0.007 µM | [155] |
| Polymerized 3,5-diamino-1,2,4-triazole/RGO/GCE | Differential pulse voltammetry | 4-NP | 2–16 µM | 0.037 µM | [156] |
| 5–200 µM | |||||
| 300–1500 µM | |||||
| Molecularly imprinted polymer–polydopamine–RGO–GCE | Differential pulse voltammetry | 2,4-DCP | 2–10 nM | 0.8 nM | [157] |
| 10–100 nM | |||||
| RGO/NiCo2O4/3-aminopropyltriethoxysilane/GCE | Differential pulse voltammetry | 2-NP | 0.005–0.5 µM | 5 nM | [158] |
| 1–25 µM |
| Sensing Materials | Sensor | Hazardous Phenolic Compounds | Linear Range | LOD 1 | Ref. |
|---|---|---|---|---|---|
| PPy–GCE | Headspace-voltammetry | Ph | 0.25–1.25 µM | 0.07 µM | [197] |
| PPy–PPO–GCE | Amperometry | Ph | 0.01–70 µM | 0.1 µM | [198] |
| 2-CP | - | 0.2 µM | |||
| 2,4-DCP | 0.4 µM | ||||
| 2,4,6-TCP | 0.2 µM | ||||
| PCP | 0.2 µM | ||||
| PPy–Tyr–GCE | Amperometry | Ph | 0.06–125 µM | - | [199] |
| PPy–Tyr–HRP–GCE | 0.24–125 µM | ||||
| PPy–Tyr–GCE | Amperometry | Ph | Up to 10 µM | 10 nM | [200] |
| PPy–Tyr–GCE | Amperometry | Ph | - | 5 nM | [202] |
| PPy–Tyr–CPE | Amperometry | Ph | Up to 7 µM | - | [203] |
| PPy nanotubes–PPO–GCE | Amperometry | Ph | 0.5–40 µM | 14.4 nM | [204] |
| PPy–o-amino-benzenesulfonic acid–Pt electrode | Amperometry | Ph | Up to 100 µM | 0.5 µM | [205] |
| Pulsed amperometry | 0.1 µM | ||||
| PPy–Tyr–Pt electrode in aqueous | Amperometry | Ph | Up to 7 mM | 0.8 nM | [206] |
| PPy–Tyr–GA–Pt electrode in aqueous | - | ||||
| PPy–Tyr–Pt electrode in chloroform | Up to 2.5 mM | 50 nM | |||
| PPy–Tyr–GA–Pt electrode in chloroform | - | ||||
| Polyglutaraldehyde–PPy–HRP–gold electrode | Amperometry | Ph | 16–112 µM | 0.087 µM | [207] |
| 2-CP | 4–128 µM | 0.114 µM | |||
| Tyr/p-toluene sulfonate ion–PPy/GCE | Amperometry | Ph | 3.3–220.3 µM | 0.8 µM | [208] |
| Tyr–poly(N-3-aminopropyl pyrrole–co-pyrrole)–ITO electrode | Amperometry | Ph | 1.35–222.3 µM | 0.7 µM | [209] |
| Tyr–N-amino substituted PPy electrode | Amperometry | Ph | 1.8–170.2 µM | 0.9 µM | [210] |
| Tyr/Fe(CN)64−–PPy/ITO electrode | Amperometry | Ph | 9.9–84.7 µM | 2.9 µM | [211] |
| Tyr/Fe(CN)64−–PPy/ITO electrode | Amperometry | Ph | 4.5–107.4 µM | 0.7 µM | [212] |
| PPy–Tyr–gold nanoparticles–GCE | Amperometry | Ph | 0.25–70 µM | 0.03 µM | [213] |
| PPy–HRP–gold nanoparticles–GCE | Amperometry | Ph | 0.01–0.2 mM | 15 µM | [214] |
| Lac–PPy–GCE | Amperometry | Ph | 0.2–1.4 µM | 0.04 µM | [215] |
| Lac–PPy–MWCNTs–GCE | 0.39–1.4 µM | 0.03 µM | |||
| Lac–PPy–MWCNTs–Prussian blue–GCE | 0.2–2.56 µM | 0.03 µM | |||
| MWCNTs–PPy–HRP–gold electrode | Amperometry | Ph | 16–44 µM | 3.52 µM | [216] |
| 2-CP | 1.6–8 µM | 0.26 µM | |||
| 2,4-DMP | 64–240 µM | 27.9 µM | |||
| Poly(glycidyl methacrylate–co-3-methylthienyl methacrylate)–MWCNTs–PPy–HRP–gold electrode | Amperometry | Ph | 1.6–72 µM | 0.732 µM | [217] |
| 2-CP | 1.6–68.8 µM | 0.249 µM | |||
| 2,4-DMP | 1.6–40 µM | 0.382 µM | |||
| HRP–PPy–polyvinylferrocene–GCE | Amperometry | Ph | 0.5–10 µM | 0.23 µM | [218] |
| Tyr–poly(glycidyl methacrylate85–co-vinylferrocene15)–polyglutaraldehyde–PPy–GCE | Amperometry | Ph | 20–70 µM | 0.781 µM | [219] |
| PPy–polyvinylsulfonate–Tyr–Pt electrode | Amperometry | Ph | 0.1–5 µM | 10 nM | [220] |
| Nano PPy–sodium dodecyl sulfate film modified GCE | Square wave voltammetry | 4-NP | 0.1 nM–100 µM | 0.1 nM | [221] |
| Sensing Materials | Sensor | Hazardous Phenolic Compounds | Linear Range | LOD 1 | Ref. |
|---|---|---|---|---|---|
| PANI nanosheets electrode | Cyclovoltammetry | Ph | 20–80 µM | 4.43 µM | [222] |
| Trametes versicolor–PANI thick film electrode | Amperometry | Ph | 0.4–6 µM | - | [223] |
| Aspergillus niger–PANI thick film electrode | 0.4–4 µM | ||||
| Agaricus bisporus–PANI thick film electrode | 1–10 µM | ||||
| PANI–laccase–indium tin oxide electrode | Amperometry | Ph | 0.5–4.5 µM | - | [224] |
| PANI–tyrosinase–platinum electrode | Amperometry | Ph | 0.04–25 µM | 10 nM | [225] |
| PANI–polyacrylonitrile–tyrosinase–platinum electrode | Amperometry | Ph | 0.1–75 µM | - | [226] |
| PANI–ionic liquid–carbon nanofiber–tyrosinase–glassy carbon electrode | Amperometry | Ph | 0.4 nM–1.9 µM | 0.1 nM | [227] |
| PANI-activated carbon–tyrosinase–carbon paste electrode | Amperometry | Ph | 1–50 µM | 0.5 µM | [228] |
| Sulfonated PANI–chitosan–tyrosinase–glassy carbon electrode | Voltammetry | Ph | 3.5–200 nM | 0.8 nM | [229] |
| 200–2000 nM | |||||
| 4-NP imprinted–PANI–polyvinyl sulfonic acid–indium tin oxide electrode | Cyclic voltammetry | 4-NP | - | 1 µM | [230] |
| NaX/carbonized–PANI | Cyclic voltammetry | 4-NP | 100 µM–1 mM | 1.27 µM | [231] |
| Poly(2,5-dimethoxyaniline)–phenanthrene sulfonic acid–platinum electrode | Amperometry | Ph | - | 2.09 mM | [232] |
| 2,4-DCP | 2.53 mM | ||||
| 2,4,6-TCP | 0.07 mM | ||||
| PCP | 0.61 mM | ||||
| 2,4-NP | 0.63 mM | ||||
| 2,4-DMP | 3.71 mM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashim, H.S.; Fen, Y.W.; Omar, N.A.S.; Fauzi, N.I.M. Sensing Methods for Hazardous Phenolic Compounds Based on Graphene and Conducting Polymers-Based Materials. Chemosensors 2021, 9, 291. https://doi.org/10.3390/chemosensors9100291
Hashim HS, Fen YW, Omar NAS, Fauzi NIM. Sensing Methods for Hazardous Phenolic Compounds Based on Graphene and Conducting Polymers-Based Materials. Chemosensors. 2021; 9(10):291. https://doi.org/10.3390/chemosensors9100291
Chicago/Turabian StyleHashim, Hazwani Suhaila, Yap Wing Fen, Nur Alia Sheh Omar, and Nurul Illya Muhamad Fauzi. 2021. "Sensing Methods for Hazardous Phenolic Compounds Based on Graphene and Conducting Polymers-Based Materials" Chemosensors 9, no. 10: 291. https://doi.org/10.3390/chemosensors9100291
APA StyleHashim, H. S., Fen, Y. W., Omar, N. A. S., & Fauzi, N. I. M. (2021). Sensing Methods for Hazardous Phenolic Compounds Based on Graphene and Conducting Polymers-Based Materials. Chemosensors, 9(10), 291. https://doi.org/10.3390/chemosensors9100291






