Conductive Hydrogel-Based Electrochemical Sensor: A Soft Platform for Capturing Analyte
Abstract
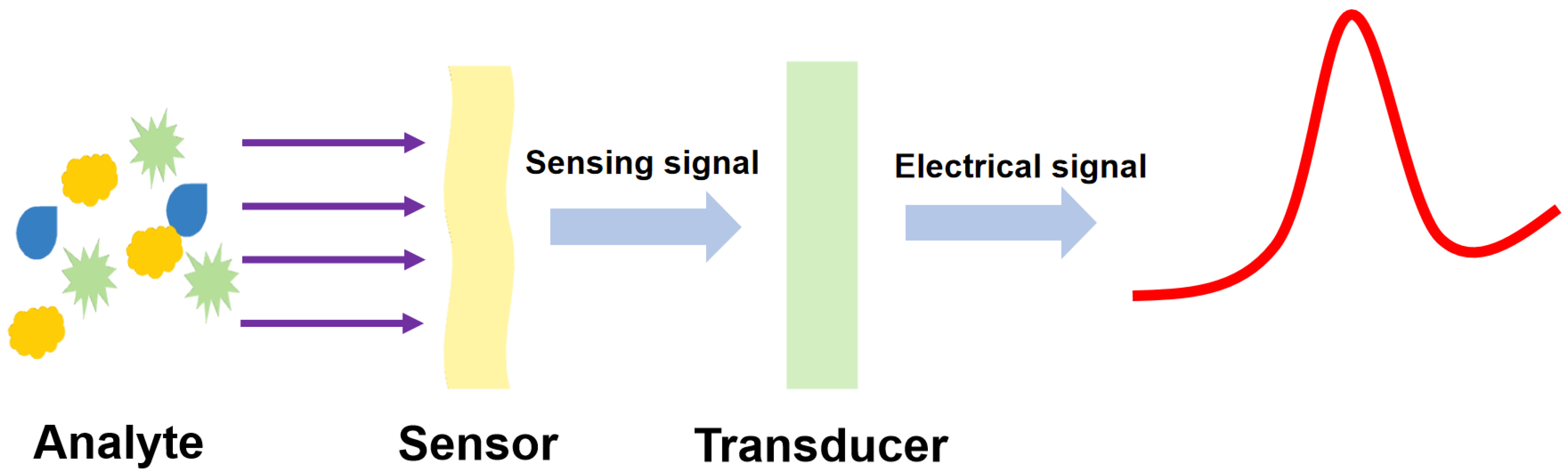
:1. Introduction
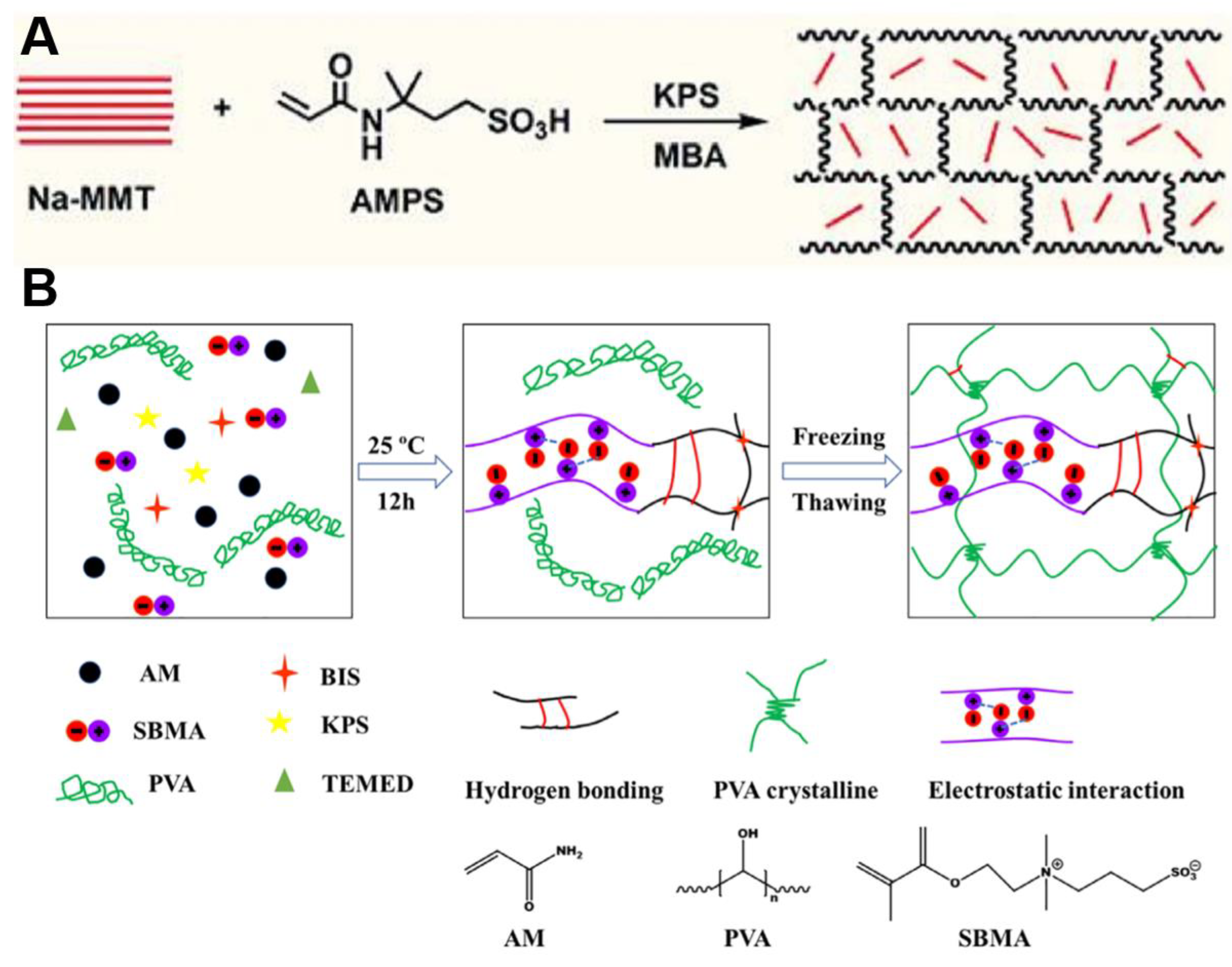
2. Polyelectrolyte Conductive Hydrogel
3. Acid-Doped Conductive Hydrogel
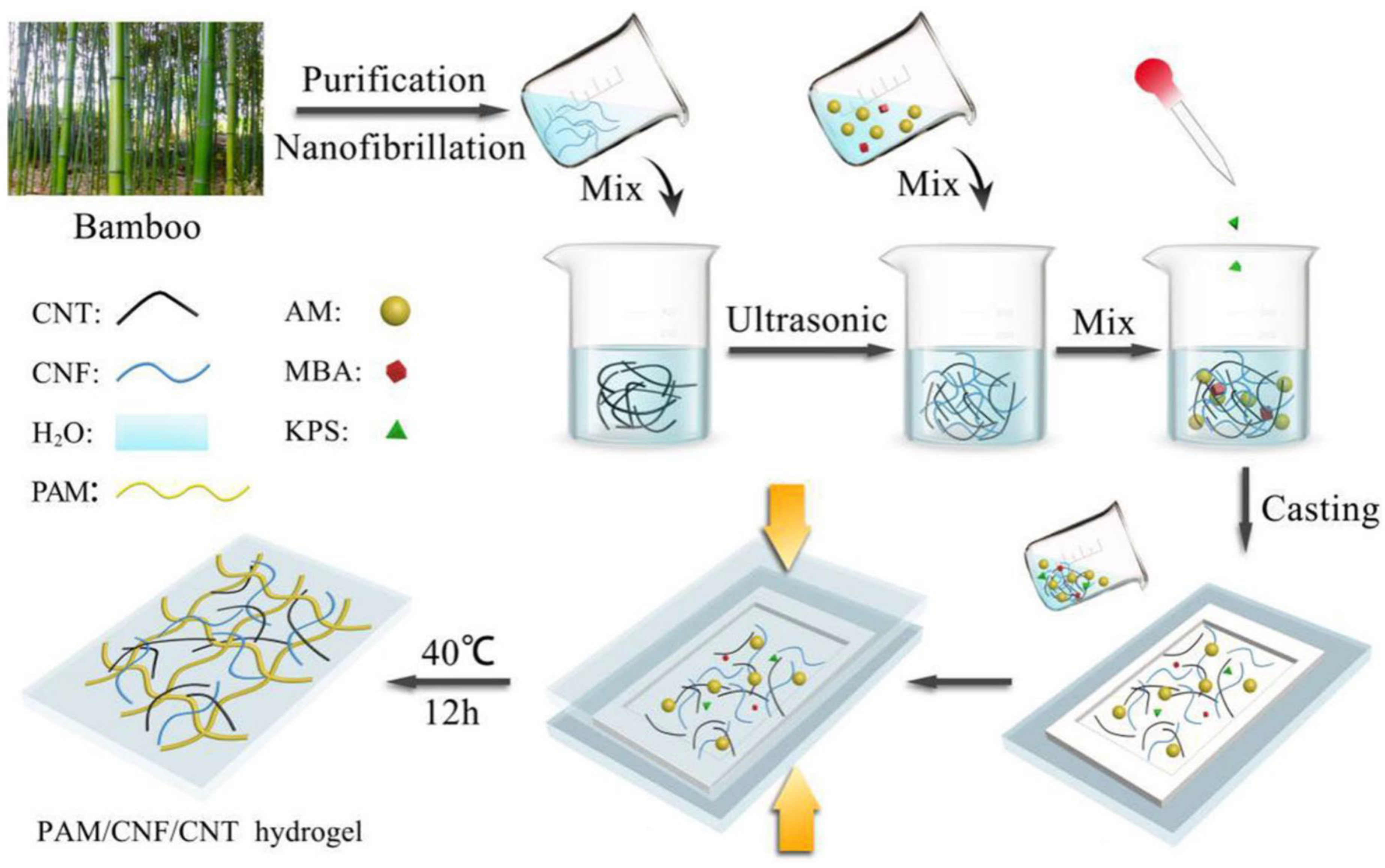
4. Inorganic Material Filled Conductive Hydrogel
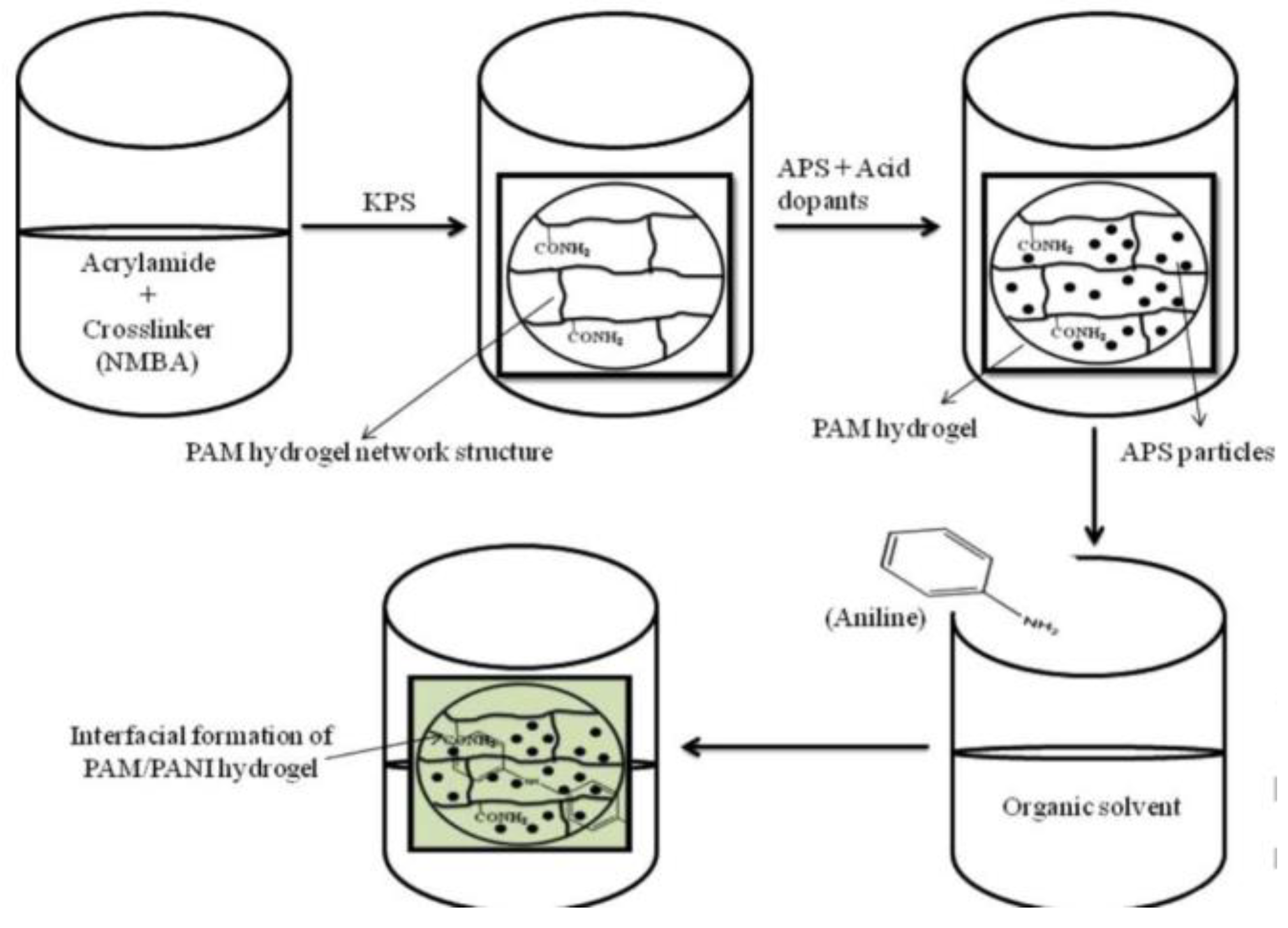
5. Conductive Polymer-Based Conductive Hydrogel
6. Other Hydrogels
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A Review on Polymeric Hydrogel Membranes for Wound Dressing Applications: PVA-Based Hydrogel Dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Chimene, D.; Kaunas, R.; Gaharwar, A.K. Hydrogel Bioink Reinforcement for Additive Manufacturing: A Focused Review of Emerging Strategies. Adv. Mater. 2020, 32, 1902026. [Google Scholar] [CrossRef]
- Satarkar, N.S.; Biswal, D.; Hilt, J.Z. Hydrogel Nanocomposites: A Review of Applications as Remote Controlled Biomaterials. Soft Matter 2010, 6, 2364–2371. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Shewan, H.M.; Stokes, J.R. Review of Techniques to Manufacture Micro-Hydrogel Particles for the Food Industry and Their Applications. J. Food Eng. 2013, 119, 781–792. [Google Scholar] [CrossRef]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.-F.; Adler, H.-J.P. Review on Hydrogel-Based PH Sensors and Microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef] [Green Version]
- Rong, Q.; Lei, W.; Liu, M. Conductive Hydrogels as Smart Materials for Flexible Electronic Devices. Chem. Eur. J. 2018, 24, 16930–16943. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, P.; Chen, J.; Sun, Z.; Zhao, B. Electrically Conductive Hydrogels for Flexible Energy Storage Systems. Prog. Polym. Sci. 2019, 88, 220–240. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Orooji, Y.; Karimi, F.; Alizadeh, M.; Baghayeri, M.; Rouhi, J.; Tajik, S.; Beitollahi, H.; Agarwal, S.; Gupta, V.K. A Critical Review on the Use of Potentiometric Based Biosensors for Biomarkers Detection. Biosens. Bioelectron. 2021, 184, 113252. [Google Scholar] [CrossRef]
- Guiseppi-Elie, A.; Wilson, A.M.; Brown, K.E. Electroconductive Hydrogels: Novel Materials for the Controlled Electrorelease of Bioactive Peptides. Am. Chem. Soc. Polym. Prepr. Div. Polym. Chem. 1997, 38, 608–609. [Google Scholar]
- Small, C.; Too, C.; Wallace, G. Responsive Conducting Polymer-Hydrogel Composites. Polym. Gels Netw. 1997, 5, 251–265. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Karimi, F.; Malekmohammadi, S.; Zakariae, N.; Esmaeili, R.; Rostamnia, S.; Yola, M.L.; Atar, N.; Movaghgharnezhad, S.; Rajendran, S.; et al. An Amplified Voltammetric Sensor Based on Platinum Nanoparticle/Polyoxometalate/Two-Dimensional Hexagonal Boron Nitride Nanosheets Composite and Ionic Liquid for Determination of N-Hydroxysuccinimide in Water Samples. J. Mol. Liq. 2020, 310, 113185. [Google Scholar] [CrossRef]
- Fu, L.; Liu, Z.; Ge, J.; Guo, M.; Zhang, H.; Chen, F.; Su, W.; Yu, A. (001) Plan Manipulation of α-Fe2O3 Nanostructures for Enhanced Electrochemical Cr(VI) Sensing. J. Electroanal. Chem. 2019, 841, 142–147. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, Q.; Zheng, Y.; Fu, L.; Zhu, J.; Karimi-Maleh, H. An Electrochemical Fingerprint Approach for Direct Soy Sauce Authentic Identification Using a Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2020, 15, 10093–10103. [Google Scholar] [CrossRef]
- Fu, L.; Zheng, Y.; Zhang, P.; Zhang, H.; Wu, M.; Zhang, H.; Wang, A.; Su, W.; Chen, F.; Yu, J.; et al. An Electrochemical Method for Plant Species Determination and Classification Based on Fingerprinting Petal Tissue. Bioelectrochemistry 2019, 129, 199–205. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Alizadeh, M.; Orooji, Y.; Karimi, F.; Baghayeri, M.; Rouhi, J.; Tajik, S.; Beitollahi, H.; Agarwal, S.; Gupta, V.K.; et al. Guanine-Based DNA Biosensor Amplified with Pt/SWCNTs Nanocomposite as Analytical Tool for Nanomolar Determination of Daunorubicin as an Anticancer Drug: A Docking/Experimental Investigation. Ind. Eng. Chem. Res. 2021, 60, 816–823. [Google Scholar] [CrossRef]
- Clark, L.C., Jr.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Updike, S.J.; Hicks, G.P. The Enzyme Electrode. Nature 1967, 214, 986–988. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, Y.; Zhang, J.; Karimi-Maleh, H.; Xu, Y.; Zhou, Q.; Fu, L.; Wu, W. Characterization of the Electrochemical Profiles of Lycoris Seeds for Species Identification and Infrageneric Relationships. Anal. Lett. 2020, 53, 2517–2528. [Google Scholar] [CrossRef]
- Fu, L.; Wang, A.; Lai, G.; Su, W.; Malherbe, F.; Yu, J.; Lin, C.-T.; Yu, A. Defects Regulating of Graphene Ink for Electrochemical Determination of Ascorbic Acid, Dopamine and Uric Acid. Talanta 2018, 180, 248–253. [Google Scholar] [CrossRef]
- Fu, L.; Zheng, Y.; Zhang, P.; Zhang, H.; Xu, Y.; Zhou, J.; Zhang, H.; Karimi-Maleh, H.; Lai, G.; Zhao, S.; et al. Development of an Electrochemical Biosensor for Phylogenetic Analysis of Amaryllidaceae Based on the Enhanced Electrochemical Fingerprint Recorded from Plant Tissue. Biosens. Bioelectron. 2020, 159, 112212. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, M.; Zhou, J.; Xu, Y.; Li, Z.; Yao, Y.; Fu, L. Development of Electrochemical Sensor for Fast Liquor Authentication. Sens. Mater. 2020, 32, 2941–2948. [Google Scholar]
- Fu, L.; Su, W.; Chen, F.; Zhao, S.; Zhang, H.; Karimi-Maleh, H.; Yu, A.; Yu, J.; Lin, C.-T. Early Sex Determination of Ginkgo Biloba Based on the Differences in the Electrocatalytic Performance of Extracted Peroxidase. Bioelectrochemistry 2021, 140, 107829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, R.; Li, Z.; Zhang, M.; Wang, Q.; Xu, Y.; Fu, L.; Du, J.; Zheng, Y.; Zhu, J. Electroanalytical Study of Infrageneric Relationship of Lagerstroemia Using Glassy Carbon Electrode Recorded Voltammograms. Rev. Mex. Ing. Quím. 2020, 19, 281–291. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Orooji, Y.; Ayati, A.; Qanbari, S.; Tanhaei, B.; Karimi, F.; Alizadeh, M.; Rouhi, J.; Fu, L.; Sillanpää, M. Recent Advances in Removal Techniques of Cr(VI) Toxic Ion from Aqueous Solution: A Comprehensive Review. J. Mol. Liq. 2021, 329, 115062. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ayati, A.; Davoodi, R.; Tanhaei, B.; Karimi, F.; Malekmohammadi, S.; Orooji, Y.; Fu, L.; Sillanpää, M. Recent Advances in Using of Chitosan-Based Adsorbents for Removal of Pharmaceutical Contaminants: A Review. J. Clean. Prod. 2021, 291, 125880. [Google Scholar] [CrossRef]
- Cass, A.E.; Davis, G.; Francis, G.D.; Hill, H.A.O.; Aston, W.J.; Higgins, I.J.; Plotkin, E.V.; Scott, L.D.; Turner, A.P. Ferrocene-Mediated Enzyme Electrode for Amperometric Determination of Glucose. Anal. Chem. 1984, 56, 667–671. [Google Scholar] [CrossRef]
- Fan, B.; Wang, Q.; Wu, W.; Zhou, Q.; Li, D.; Xu, Z.; Fu, L.; Zhu, J.; Karimi-Maleh, H.; Lin, C.-T. Electrochemical Fingerprint Biosensor for Natural Indigo Dye Yielding Plants Analysis. Biosensors 2021, 11, 155. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Q.; Zhang, M.; Zheng, Y.; Wu, M.; Lan, Z.; Pu, J.; Zhang, H.; Chen, F.; Su, W. Electrochemical Sex Determination of Dioecious Plants Using Polydopamine-Functionalized Graphene Sheets. Front. Chem. 2020, 8, 92. [Google Scholar] [CrossRef]
- Fu, L.; Xie, K.; Wu, D.; Wang, A.; Zhang, H.; Ji, Z. Electrochemical Determination of Vanillin in Food Samples by Using Pyrolyzed Graphitic Carbon Nitride. Mater. Chem. Phys. 2020, 242, 122462. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Karimi, F.; Fu, L.; Sanati, A.L.; Alizadeh, M.; Karaman, C.; Orooji, Y. Cyanazine Herbicide Monitoring as a Hazardous Substance by a DNA Nanostructure Biosensor. J. Hazard. Mater. 2021, 423, 127058. [Google Scholar] [CrossRef]
- Power, A.C.; Gorey, B.; Chandra, S.; Chapman, J. Carbon Nanomaterials and Their Application to Electrochemical Sensors: A Review. Nanotechnol. Rev. 2018, 7, 19–41. [Google Scholar] [CrossRef]
- Putzbach, W.; Ronkainen, N.J. Immobilization Techniques in the Fabrication of Nanomaterial-Based Electrochemical Biosensors: A Review. Sensors 2013, 13, 4811–4840. [Google Scholar] [CrossRef]
- Munteanu, R.-E.; Moreno, P.S.; Bramini, M.; Gaspar, S. 2D Materials in Electrochemical Sensors for in Vitro or in Vivo Use. Anal. Bioanal. Chem. 2021, 413, 701–725. [Google Scholar] [CrossRef]
- Baig, N.; Saleh, T.A. Electrodes Modified with 3D Graphene Composites: A Review on Methods for Preparation, Properties and Sensing Applications. Microchim. Acta 2018, 185, 1–21. [Google Scholar] [CrossRef]
- Herrmann, A.; Haag, R.; Schedler, U. Hydrogels and Their Role in Biosensing Applications. Adv. Healthc. Mater. 2021, 10, 2100062. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, X.; Wang, C.; Xiang, K.; Deng, M.; Yin, H. PAMPS/MMT Composite Hydrogel Electrolyte for Solid-State Supercapacitors. J. Alloys Compd. 2017, 709, 596–601. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, H.; Xiao, Y.; Yu, X.; Li, X. PAMPS/PVA/MMT Semi-Interpenetrating Polymer Network Hydrogel Electrolyte for Solid-State Supercapacitors. Int. J. Electrochem. Sci. 2019, 14, 1817–1829. [Google Scholar] [CrossRef]
- Diao, W.; Wu, L.; Ma, X.; Zhuang, Z.; Li, S.; Bu, X.; Fang, Y. Highly Stretchable, Ionic Conductive and Self-recoverable Zwitterionic Polyelectrolyte-based Hydrogels by Introducing Multiple Supramolecular Sacrificial Bonds in Double Network. J. Appl. Polym. Sci. 2019, 136, 47783. [Google Scholar] [CrossRef]
- Contin, A.; Frasca, S.; Vivekananthan, J.; Leimkühler, S.; Wollenberger, U.; Plumeré, N.; Schuhmann, W. A PH Responsive Redox Hydrogel for Electrochemical Detection of Redox Silent Biocatalytic Processes. Control of Hydrogel Solvation. Electroanalysis 2015, 27, 938–944. [Google Scholar] [CrossRef]
- Pinyou, P.; Ruff, A.; Pöller, S.; Ma, S.; Ludwig, R.; Schuhmann, W. Design of an Os Complex-Modified Hydrogel with Optimized Redox Potential for Biosensors and Biofuel Cells. Chem.-Eur. J. 2016, 22, 5319–5326. [Google Scholar] [CrossRef]
- Wu, Y.; Jiao, L.; Xu, W.; Gu, W.; Zhu, C.; Du, D.; Lin, Y. Polydopamine-Capped Bimetallic AuPt Hydrogels Enable Robust Biosensor for Organophosphorus Pesticide Detection. Small 2019, 15, 1900632. [Google Scholar] [CrossRef]
- Yadavalli, V.K.; Koh, W.-G.; Lazur, G.J.; Pishko, M.V. Microfabricated Protein-Containing Poly(Ethylene Glycol) Hydrogel Arrays for Biosensing. Sens. Actuators B Chem. 2004, 97, 290–297. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, J.; Cheng, G.; Dong, S. Amperometric Enzyme Electrode for the Determination of Hydrogen Peroxide Based on Sol–Gel/Hydrogel Composite Film. Anal. Chim. Acta 2000, 407, 111–118. [Google Scholar] [CrossRef]
- Yan, J.; Pedrosa, V.A.; Enomoto, J.; Simonian, A.L.; Revzin, A. Electrochemical Biosensors for On-Chip Detection of Oxidative Stress from Immune Cells. Biomicrofluidics 2011, 5, 032008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Xue, H.; Carr, L.R.; Wang, J.; Jiang, S. Zwitterionic Poly(Carboxybetaine) Hydrogels for Glucose Biosensors in Complex Media. Biosens. Bioelectron. 2011, 26, 2454–2459. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Zhang, Y.; Rasooly, A.; Yang, M. Electrochemical Biosensing Platform Using Hydrogel Prepared from Ferrocene Modified Amino Acid as Highly Efficient Immobilization Matrix. Anal. Chem. 2014, 86, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Pedrosa, V.A.; Simonian, A.L.; Revzin, A. Immobilizing Enzymes onto Electrode Arrays by Hydrogel Photolithography to Fabricate Multi-Analyte Electrochemical Biosensors. ACS Appl. Mater. Interfaces 2010, 2, 748–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muya, F.N.; Baker, P.G.L.; Iwuoha, E.I. Polysulfone Hydrogel Nanocomposite Alkaline Phosphatase Biosensor for the Detection of Vanadium. Electrocatalysis 2020, 11, 374–382. [Google Scholar] [CrossRef]
- De Wael, K.; Bashir, Q.; Van Vlierberghe, S.; Dubruel, P.; Heering, H.A.; Adriaens, A. Electrochemical Determination of Hydrogen Peroxide with Cytochrome c Peroxidase and Horse Heart Cytochrome c Entrapped in a Gelatin Hydrogel. Bioelectrochemistry 2012, 83, 15–18. [Google Scholar] [CrossRef]
- Zhou, J.; Liao, C.; Zhang, L.; Wang, Q.; Tian, Y. Molecular Hydrogel-Stabilized Enzyme with Facilitated Electron Transfer for Determination of H2O2 Released from Live Cells. Anal. Chem. 2014, 86, 4395–4401. [Google Scholar] [CrossRef]
- Cha, W.; Meyerhoff, M.E. S-Nitrosothiol Detection via Amperometric Nitric Oxide Sensor with Surface Modified Hydrogel Layer Containing Immobilized Organoselenium Catalyst. Langmuir 2006, 22, 10830–10836. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Fau, M.; Karbarz, M.; Donten, M.; Stojek, Z.; Nowicka, A.M. Hydrogel with Chains Functionalized with Carboxyl Groups as Universal 3D Platform in DNA Biosensors. Biosens. Bioelectron. 2014, 54, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Cizek, K.; Prior, C.; Thammakhet, C.; Galik, M.; Linker, K.; Tsui, R.; Cagan, A.; Wake, J.; Belle, J.L.; Wang, J. Integrated Explosive Preconcentrator and Electrochemical Detection System for 2,4,6-Trinitrotoluene (TNT) Vapor. Anal. Chim. Acta 2010, 661, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Chen, X.; Wang, K.; Wei, N.; Sun, Z.; Lin, X.; Chen, Y.; Du, M. Electrochemical DNA Biosensor Based on Aldehyde-Agarose Hydrogel Modified Glassy Carbon Electrode for Detection of PML/RARa Fusion Gene. Sens. Actuators B Chem. 2011, 160, 1458–1463. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, W.; Xu, L. Surface Grafted Cross-Linked Poly(Ionic Liquid) Hydrogel for Electrocatalytic Oxidation of Cysteine. Eur. Polym. J. 2020, 136, 109928. [Google Scholar] [CrossRef]
- Wang, C.; Ye, X.; Wang, Z.; Wu, T.; Wang, Y.; Li, C. Molecularly Imprinted Photo-Electrochemical Sensor for Human Epididymis Protein 4 Based on Polymerized Ionic Liquid Hydrogel and Gold Nanoparticle/ZnCdHgSe Quantum Dots Composite Film. Anal. Chem. 2017, 89, 12391–12398. [Google Scholar] [CrossRef]
- Reddy, S.M.; Sette, G.; Phan, Q. Electrochemical Probing of Selective Haemoglobin Binding in Hydrogel-Based Molecularly Imprinted Polymers. Electrochim. Acta 2011, 56, 9203–9208. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Deng, Y.; Hu, X.; Gao, Z.; Sun, C. Electrochemical Sensing of Trichloroacetic Acid Based on Silver Nanoparticles Doped Chitosan Hydrogel Film Prepared with Controllable Electrodeposition. Electrochim. Acta 2012, 76, 410–415. [Google Scholar] [CrossRef]
- Li, S.; Tao, Y.; Maryum, P.; Wang, Q.; Zhu, J.; Min, F.; Cheng, H.; Zhao, S.; Wang, C. Bifunctional Polyaniline Electroconductive Hydrogels with Applications in Supercapacitor and Wearable Strain Sensors. J. Biomater. Sci. Polym. Ed. 2020, 31, 938–953. [Google Scholar] [CrossRef]
- Prabhakar, R.; Kumar, D. Influence of Dopant Ions on the Properties of Conducting Polyacrylamide/Polyaniline Hydrogels. Polym.-Plast. Technol. Eng. 2016, 55, 46–53. [Google Scholar] [CrossRef]
- Prabhakar, R.; Kumar, D. Effect of Preparation Conditions on the Conductivity of Polyaniline Impregnated Polyacrylate Conducting Hydrogel. J. Nanosci. Nanotechnol. 2017, 17, 5008–5014. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H.; Zhang, H.; Ma, L.; Wang, Z. Polyacrylamide-Phytic Acid-Polydopamine Conducting Porous Hydrogel for Rapid Detection and Removal of Copper (II) Ions. Biosens. Bioelectron. 2017, 91, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Rong, Q.; Han, H.; Feng, F.; Ma, Z. Network Nanostructured Polypyrrole Hydrogel/Au Composites as Enhanced Electrochemical Biosensing Platform. Sci. Rep. 2015, 5, 11440. [Google Scholar] [CrossRef] [Green Version]
- Shang, J.; Yan, J.; Zhang, Z.; Huang, X.; Maturavongsadit, P.; Song, B.; Jia, Y.; Ma, T.; Li, D.; Xu, K.; et al. A Hydrogel-Based Glucose Affinity Microsensor. Sens. Actuators B Chem. 2016, 237, 992–998. [Google Scholar] [CrossRef] [Green Version]
- Tee, B.C.; Wang, C.; Allen, R.; Bao, Z. An Electrically and Mechanically Self-Healing Composite with Pressure-and Flexion-Sensitive Properties for Electronic Skin Applications. Nat. Nanotechnol. 2012, 7, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Meng, Q.; Shi, G.; Arabi, S.; Ma, J.; Zhao, N.; Kuan, H.-C. Electrically Conductive, Mechanically Robust, PH-Sensitive Graphene/Polymer Composite Hydrogels. Compos. Sci. Technol. 2016, 127, 119–126. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Meng, T.; Wu, Q.; Fang, L.; Zhao, D.; Zhang, Y.; Li, D. Electrically Conductive Polyacrylamide/Carbon Nanotube Hydrogel: Reinforcing Effect from Cellulose Nanofibers. Cellulose 2019, 26, 8843–8851. [Google Scholar] [CrossRef]
- Fu, L.; Wang, A.; Lyu, F.; Lai, G.; Yu, J.; Lin, C.-T.; Liu, Z.; Yu, A.; Su, W. A Solid-State Electrochemical Sensing Platform Based on a Supramolecular Hydrogel. Sens. Actuators B Chem. 2018, 262, 326–333. [Google Scholar] [CrossRef]
- Fu, L.; Wang, A.; Lyv, F.; Lai, G.; Zhang, H.; Yu, J.; Lin, C.-T.; Yu, A.; Su, W. Electrochemical Antioxidant Screening Based on a Chitosan Hydrogel. Bioelectrochemistry 2018, 121, 7–10. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, M.; Han, F.; Wu, D.; Fu, L. Evaluation of Total Antioxidant Activity of Different Floral Sources of Honeys Using Crosslinked Hydrogels. Int. J. Electrochem. Sci. 2019, 14, 1479–1487. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, M.; Xu, Y.; Peng, X.; Zhang, M.; Wang, Q.; Du, J.; Zhang, H.; Fu, L. Electrochemical Determination of Antioxidant Activity of Different Bee Products. Int. J. Electrochem. Sci. 2019, 14, 3663–3672. [Google Scholar] [CrossRef]
- Yan, S.; Yue, Y.; Zeng, L.; Su, L.; Hao, M.; Zhang, W.; Wang, X. Preparation of Graphene Oxide-Embedded Hydrogel as a Novel Sensor Platform for Antioxidant Activity Evaluation of Scutellaria Baicalensis. Front. Chem. 2021, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, Y.; Jiang, B. Extraction and Electrochemical Analysis of Polyphenols in Plant Samples. Int. J. Electrochem. Sci. 2019, 14, 7410–7422. [Google Scholar] [CrossRef] [Green Version]
- Zheng, P.; Wang, B.; Huang, Y.; Huang, H. Flavonoids Activity Determination of Ginkgo Sample Using Electrochemical Method. Rev. Mex. Ing. Quím. 2021, 20, 77–86. [Google Scholar] [CrossRef]
- Zhong, R.; Tang, Q.; Wang, S.; Zhang, H.; Zhang, F.; Xiao, M.; Man, T.; Qu, X.; Li, L.; Zhang, W.; et al. Self-Assembly of Enzyme-Like Nanofibrous G-Molecular Hydrogel for Printed Flexible Electrochemical Sensors. Adv. Mater. 2018, 30, 1706887. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, X.; Jiang, L.; Sun, H.; Huo, D.; Hou, C. A Three-Dimensional Hydrogel-Modified Indium Tin Oxide Electrode with Enhanced Performance for in Situ Electrochemical Detection of Extracellular H2O2. Analyst 2021, 146, 5403–5412. [Google Scholar] [CrossRef]
- Palanisamy, S.; Thangavelu, K.; Chen, S.-M.; Velusamy, V.; Chen, T.-W.; Kannan, R.S. Preparation and Characterization of a Novel Hybrid Hydrogel Composite of Chitin Stabilized Graphite: Application for Selective and Simultaneous Electrochemical Detection of Dihydroxybenzene Isomers in Water. J. Electroanal. Chem. 2017, 785, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Huang, R.; Hu, N. Myoglobin in Polyacrylamide Hydrogel Films: Direct Electrochemistry and Electrochemical Catalysis. Talanta 2002, 56, 1131–1139. [Google Scholar] [CrossRef]
- Cui, H.-F.; Ye, J.-S.; Zhang, W.-D.; Sheu, F.-S. Modification of Carbon Nanotubes with Redox Hydrogel: Improvement of Amperometric Sensing Sensitivity for Redox Enzymes. Biosens. Bioelectron. 2009, 24, 1723–1729. [Google Scholar] [CrossRef]
- Derkus, B.; Emregul, K.C.; Emregul, E. Evaluation of Protein Immobilization Capacity on Various Carbon Nanotube Embedded Hydrogel Biomaterials. Mater. Sci. Eng. C 2015, 56, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Bao, J.; Huo, D.; Yang, M.; Hou, C.; Guo, J.; Chen, M.; Fa, H.; Luo, X.; Ma, Y. 3D Graphene Hydrogel—Gold Nanoparticles Nanocomposite Modified Glassy Carbon Electrode for the Simultaneous Determination of Ascorbic Acid, Dopamine and Uric Acid. Sens. Actuators B Chem. 2017, 238, 1316–1323. [Google Scholar] [CrossRef]
- Burrs, S.L.; Vanegas, D.C.; Bhargava, M.; Mechulan, N.; Hendershot, P.; Yamaguchi, H.; Gomes, C.; McLamore, E.S. A Comparative Study of Graphene–Hydrogel Hybrid Bionanocomposites for Biosensing. Analyst 2015, 140, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Han, H.; Ma, Z. Conductive Hydrogel Composed of 1,3,5-Benzenetricarboxylic Acid and Fe3+ Used as Enhanced Electrochemical Immunosensing Substrate for Tumor Biomarker. Bioelectrochemistry 2017, 114, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Wang, F.; Chen, Z. Electrochemical Glucose Sensor Based on One-Step Construction of Gold Nanoparticle–Chitosan Composite Film. Sens. Actuators B Chem. 2009, 138, 539–544. [Google Scholar] [CrossRef]
- Zanini, V.P.; López de Mishima, B.; Solís, V. An Amperometric Biosensor Based on Lactate Oxidase Immobilized in Laponite–Chitosan Hydrogel on a Glassy Carbon Electrode. Application to the Analysis of l-Lactate in Food Samples. Sens. Actuators B Chem. 2011, 155, 75–80. [Google Scholar] [CrossRef]
- Kestwal, R.M.; Bagal-Kestwal, D.; Chiang, B.-H. Fenugreek Hydrogel–Agarose Composite Entrapped Gold Nanoparticles for Acetylcholinesterase Based Biosensor for Carbamates Detection. Anal. Chim. Acta 2015, 886, 143–150. [Google Scholar] [CrossRef]
- Al-Sagur, H.; Komathi, S.; Khan, M.A.; Gurek, A.G.; Hassan, A. A Novel Glucose Sensor Using Lutetium Phthalocyanine as Redox Mediator in Reduced Graphene Oxide Conducting Polymer Multifunctional Hydrogel. Biosens. Bioelectron. 2017, 92, 638–645. [Google Scholar] [CrossRef]
- Chazaro-Ruiz, L.F.; Olvera-Sosa, M.; Vidal, G.; Rangel-Mendez, J.R.; Palestino, G.; Perez, F.; Zhang, W. Synthesis of Bamboo-like Multiwall Carbon Nanotube–Poly (Acrylic Acid-Co-Itaconic Acid)/NaOH Composite Hydrogel and Its Potential Application for Electrochemical Detection of Cadmium (II). Biosensors 2020, 10, 147. [Google Scholar] [CrossRef]
- Luo, X.-L.; Xu, J.-J.; Zhang, Q.; Yang, G.-J.; Chen, H.-Y. Electrochemically Deposited Chitosan Hydrogel for Horseradish Peroxidase Immobilization through Gold Nanoparticles Self-Assembly. Biosens. Bioelectron. 2005, 21, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Liu, A.; Zhao, M.; Dong, W.; Zhao, T.; Wang, J.; Tang, W. Bimetallic PdCu Nanoparticle Decorated Three-Dimensional Graphene Hydrogel for Non-Enzymatic Amperometric Glucose Sensor. Sens. Actuators B Chem. 2014, 190, 707–714. [Google Scholar] [CrossRef]
- Juanjuan, Z.; Ruiyi, L.; Zaijun, L.; Junkang, L.; Zhiguo, G.; Guangli, W. Synthesis of Nitrogen-Doped Activated Graphene Aerogel/Gold Nanoparticles and Its Application for Electrochemical Detection of Hydroquinone and o-Dihydroxybenzene. Nanoscale 2014, 6, 5458–5466. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ding, Y.; Li, T.; Yang, M. Redox Hydrogel Based Immunosensing Platform for the Label-Free Detection of a Cancer Biomarker. Anal. Methods 2015, 7, 411–415. [Google Scholar] [CrossRef]
- Cao, X.; Zhu, X.; He, S.; Xu, X.; Ye, Y.; Gunasekaran, S. Gold Nanoparticle-Doped Three-Dimensional Reduced Graphene Hydrogel Modified Electrodes for Amperometric Determination of Indole-3-Acetic Acid and Salicylic Acid. Nanoscale 2019, 11, 10247–10256. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Sheng, K.; Zhang, P.; Li, C.; Shi, G. Graphene Oxide/Conducting Polymer Composite Hydrogels. J. Mater. Chem. 2011, 21, 18653–18658. [Google Scholar] [CrossRef]
- Li, J.; Xie, J.; Gao, L.; Li, C.M. Au Nanoparticles–3D Graphene Hydrogel Nanocomposite To Boost Synergistically in Situ Detection Sensitivity toward Cell-Released Nitric Oxide. ACS Appl. Mater. Interfaces 2015, 7, 2726–2734. [Google Scholar] [CrossRef]
- Manickam, P.; Vashist, A.; Madhu, S.; Sadasivam, M.; Sakthivel, A.; Kaushik, A.; Nair, M. Gold Nanocubes Embedded Biocompatible Hybrid Hydrogels for Electrochemical Detection of H2O2. Bioelectrochemistry 2020, 131, 107373. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Zhang, X.; Zhang, H. 3D Porous and Redox-Active Prussian Blue-in-Graphene Aerogels for Highly Efficient Electrochemical Detection of H2O2. J. Mater. Chem. 2012, 22, 22090–22096. [Google Scholar] [CrossRef]
- Hoa, L.T.; Chung, J.S.; Hur, S.H. A Highly Sensitive Enzyme-Free Glucose Sensor Based on Co3O4 Nanoflowers and 3D Graphene Oxide Hydrogel Fabricated via Hydrothermal Synthesis. Sens. Actuators B Chem. 2016, 223, 76–82. [Google Scholar] [CrossRef]
- Lu, Z.; Wu, L.; Zhang, J.; Dai, W.; Mo, G.; Ye, J. Bifunctional and Highly Sensitive Electrochemical Non-Enzymatic Glucose and Hydrogen Peroxide Biosensor Based on NiCo2O4 Nanoflowers Decorated 3D Nitrogen Doped Holey Graphene Hydrogel. Mater. Sci. Eng. C 2019, 102, 708–717. [Google Scholar] [CrossRef]
- Hao, N.; Zhang, X.; Zhou, Z.; Qian, J.; Liu, Q.; Chen, S.; Zhang, Y.; Wang, K. Three-Dimensional Nitrogen-Doped Graphene Porous Hydrogel Fabricated Biosensing Platform with Enhanced Photoelectrochemical Performance. Sens. Actuators B Chem. 2017, 250, 476–483. [Google Scholar] [CrossRef]
- Du, C.; Yao, Z.; Chen, Y.; Bai, H.; Li, L. Synthesis of Metal Nanoparticle@ Graphene Hydrogel Composites by Substrate-Enhanced Electroless Deposition and Their Application in Electrochemical Sensors. RSC Adv. 2014, 4, 9133–9138. [Google Scholar] [CrossRef]
- Qi, Y.; Cao, Y.; Meng, X.; Cao, J.; Li, X.; Hao, Q.; Lei, W.; Li, Q.; Li, J.; Si, W. Facile Synthesis of 3D Sulfur/Nitrogen Co-Doped Graphene Derived from Graphene Oxide Hydrogel and the Simultaneous Determination of Hydroquinone and Catechol. Sens. Actuators B Chem. 2019, 279, 170–176. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of Electrically Conducting Organic Polymers: Halogen Derivatives of Polyacetylene,(CH) x. J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Edwards, J.H.; Feast, W. A New Synthesis of Poly (Acetylene). Polymer 1980, 21, 595–596. [Google Scholar] [CrossRef]
- Grubbs, R.H.; Tumas, W. Polymer Synthesis and Organotransition Metal Chemistry. Science 1989, 243, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, Y.; Wang, X.; Xing, L.; Shi, L.; Ran, R. Stable, Strain-Sensitive Conductive Hydrogel with Antifreezing Capability, Remoldability, and Reusability. ACS Appl. Mater. Interfaces 2018, 10, 44000–44010. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Pan, L.; Liu, B.; Wang, Y.; Cui, Y.; Bao, Z.; Yu, G. Nanostructured Conductive Polypyrrole Hydrogels as High-Performance, Flexible Supercapacitor Electrodes. J. Mater. Chem. A 2014, 2, 6086–6091. [Google Scholar] [CrossRef]
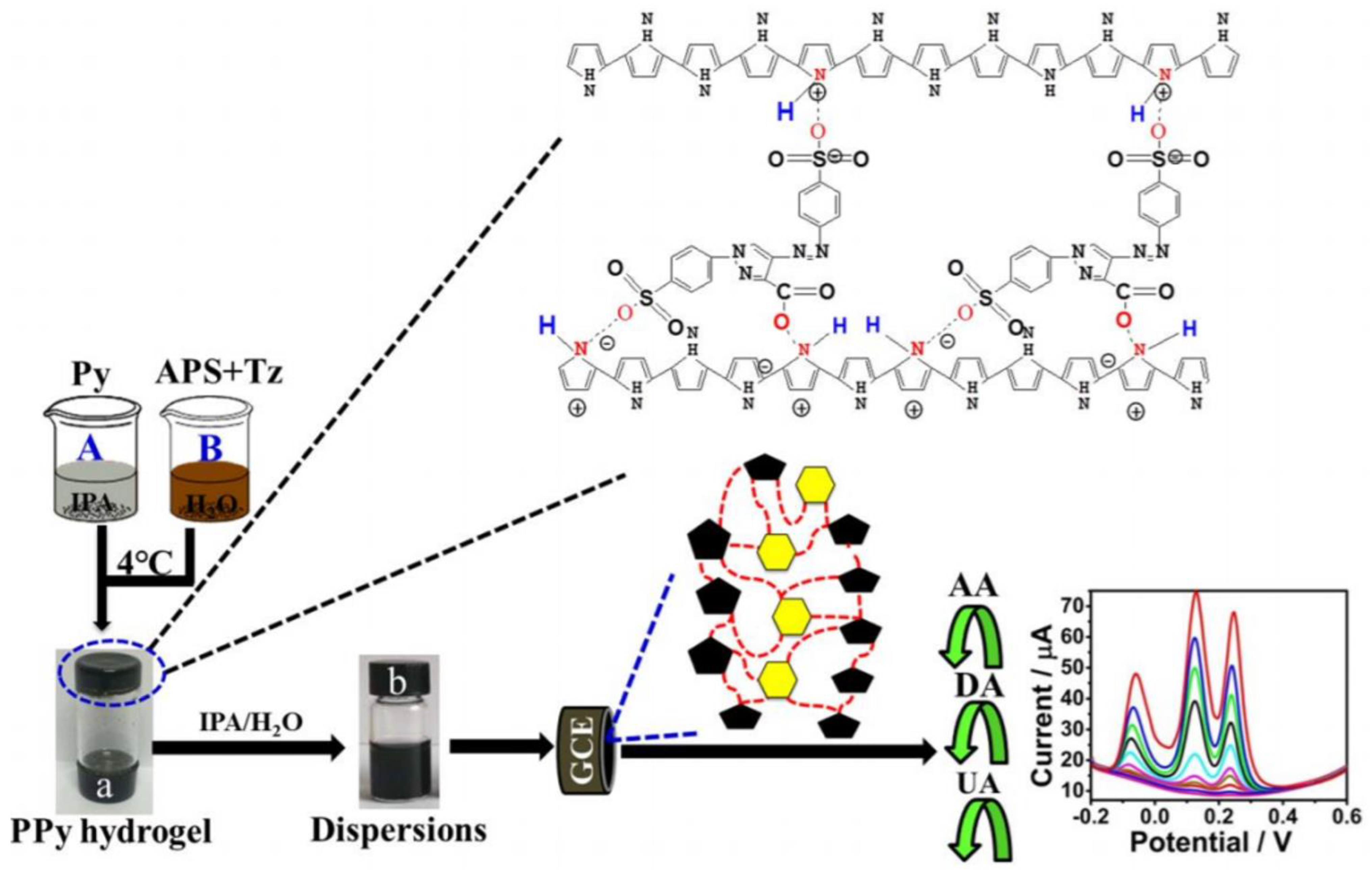
- Wang, M.; Cui, M.; Liu, W.; Liu, X. Highly Dispersed Conductive Polypyrrole Hydrogels as Sensitive Sensor for Simultaneous Determination of Ascorbic Acid, Dopamine and Uric Acid. J. Electroanal. Chem. 2019, 832, 174–181. [Google Scholar] [CrossRef]
- Ravichandran, R.; Martinez, J.G.; Jager, E.W.H.; Phopase, J.; Turner, A.P.F. Type I Collagen-Derived Injectable Conductive Hydrogel Scaffolds as Glucose Sensors. ACS Appl. Mater. Interfaces 2018, 10, 16244–16249. [Google Scholar] [CrossRef] [Green Version]
- Zhai, D.; Liu, B.; Shi, Y.; Pan, L.; Wang, Y.; Li, W.; Zhang, R.; Yu, G. Highly Sensitive Glucose Sensor Based on Pt Nanoparticle/Polyaniline Hydrogel Heterostructures. ACS Nano 2013, 7, 3540–3546. [Google Scholar] [CrossRef]
- Das, J.; Sarkar, P. Enzymatic Electrochemical Biosensor for Urea with a Polyaniline Grafted Conducting Hydrogel Composite Modified Electrode. Rsc Adv. 2016, 6, 92520–92533. [Google Scholar] [CrossRef]
- Brahim, S.; Wilson, A.M.; Narinesingh, D.; Iwuoha, E.; Guiseppi-Elie, A. Chemical and Biological Sensors Based on Electrochemical Detection Using Conducting Electroactive Polymers. Microchim. Acta 2003, 143, 123–137. [Google Scholar] [CrossRef]
- Ismail, Y.A.; Martínez, J.G.; Al Harrasi, A.S.; Kim, S.J.; Otero, T.F. Sensing Characteristics of a Conducting Polymer/Hydrogel Hybrid Microfiber Artificial Muscle. Sens. Actuators B Chem. 2011, 160, 1180–1190. [Google Scholar] [CrossRef]
- Brahim, S.; Narinesingh, D.; Guiseppi-Elie, A. Polypyrrole-Hydrogel Composites for the Construction of Clinically Important Biosensors. Biosens. Bioelectron. 2002, 17, 53–59. [Google Scholar] [CrossRef]
- Muralikrishna, S.; Nagaraju, D.H.; Balakrishna, R.G.; Surareungchai, W.; Ramakrishnappa, T.; Shivanandareddy, A.B. Hydrogels of Polyaniline with Graphene Oxide for Highly Sensitive Electrochemical Determination of Lead Ions. Anal. Chim. Acta 2017, 990, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Chen, X.; Lu, Y.; Yang, W. Self-Assembled Peptide Hydrogel as a Smart Biointerface for Enzyme-Based Electrochemical Biosensing and Cell Monitoring. ACS Appl. Mater. Interfaces 2016, 8, 25036–25042. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Wei, W.; Li, X.; Zeng, J.; Wu, L. Direct Electrochemistry and Electrocatalysis of Hemoglobin Entrapped in Semi-Interpenetrating Polymer Network Hydrogel Based on Polyacrylamide and Chitosan. Bioelectrochemistry 2007, 71, 135–141. [Google Scholar] [CrossRef]
- Biela, A.; Watkinson, M.; Meier, U.C.; Baker, D.; Giovannoni, G.; Becer, C.R.; Krause, S. Disposable MMP-9 Sensor Based on the Degradation of Peptide Cross-Linked Hydrogel Films Using Electrochemical Impedance Spectroscopy. Biosens. Bioelectron. 2015, 68, 660–667. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Xie, S.; Zhang, J.; Tang, D.; Tang, Y. An Electrochemical Impedance Biosensor for Hg2+ Detection Based on DNA Hydrogel by Coupling with DNAzyme-Assisted Target Recycling and Hybridization Chain Reaction. Biosens. Bioelectron. 2017, 98, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Su, W.; Li, Y.; Zhang, L.; Ding, X. Manufacturing of an Electrochemical Biosensing Platform Based on Hybrid DNA Hydrogel: Taking Lung Cancer-Specific MiR-21 as an Example. Biosens. Bioelectron. 2018, 103, 1–5. [Google Scholar] [CrossRef]
- Chang, Y.; Li, M.; Wu, Z.; Zhuo, Y.; Chai, Y.; Xiao, Q.; Yuan, R. Homogeneous Entropy Catalytic-Driven DNA Hydrogel as Strong Signal Blocker for Highly Sensitive Electrochemical Detection of Platelet-Derived Growth Factor. Anal. Chem. 2018, 90, 8241–8247. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Chen, G.; Wang, Z.; Zhang, Y.; Zhu, X.; Li, G. Surface-Immobilized and Self-Shaped DNA Hydrogels and Their Application in Biosensing. Chem. Sci. 2018, 9, 811–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Lyu, D.; Liu, S.; Guo, W. DNA Hydrogels and Microgels for Biosensing and Biomedical Applications. Adv. Mater. 2020, 32, 1806538. [Google Scholar] [CrossRef]
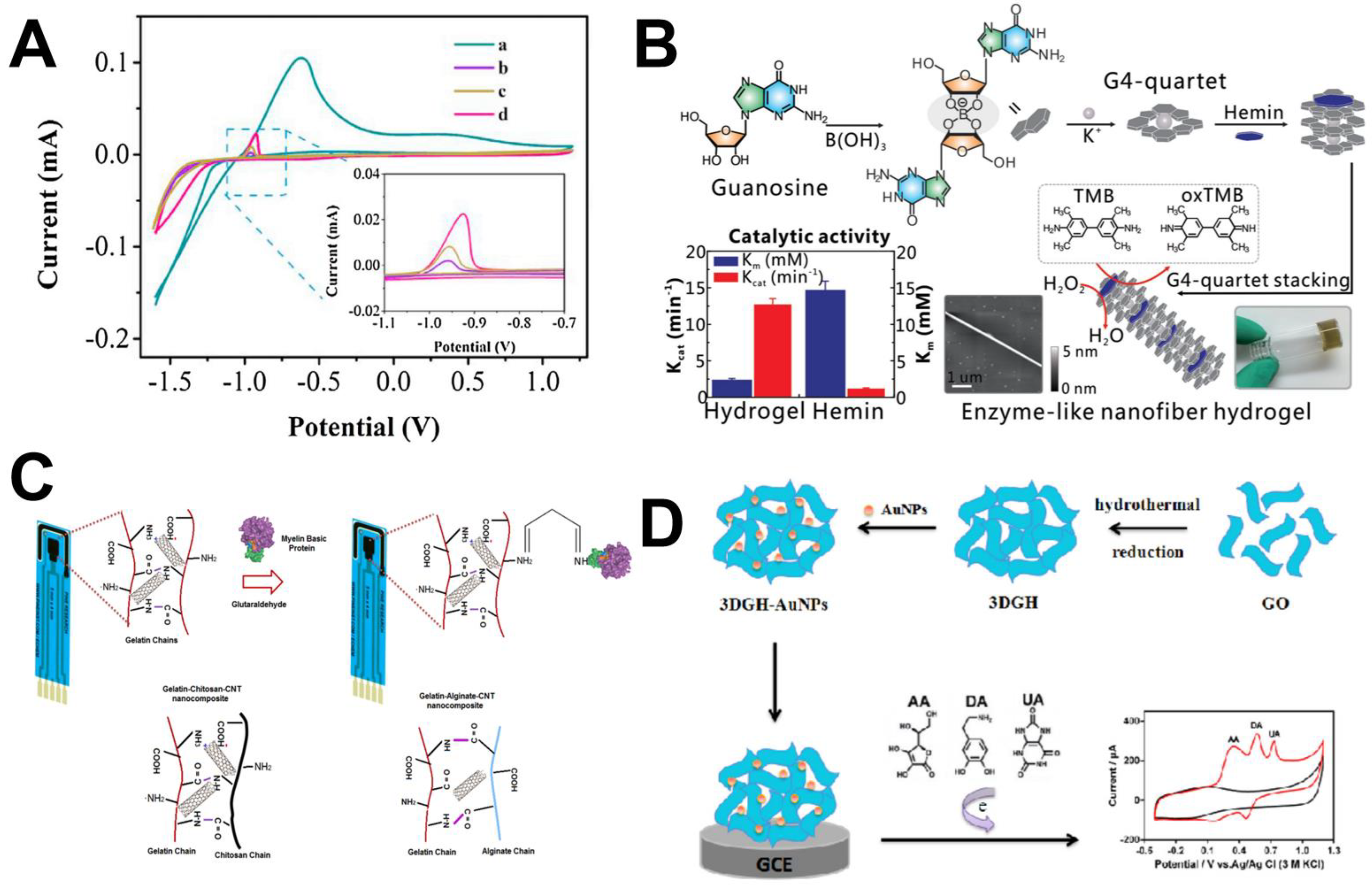
| Raw Material | Inorganic Filler | Analyte | Reference |
|---|---|---|---|
| Chitosan | Zn2+ | Antioxidant activity | [70] |
| Chitosan | Ag+ | Antioxidant activity | [71] |
| 1,3,5-benzenetricarboxylic acid | Fe2+ | Neuron-specific enolase | [85] |
| Guanosine, B(OH)3 | K+ | Glucose | [77] |
| Chitin | Graphite | Hydroquinone, catechol, resorcinol | [79] |
| Chitosan | Au NPs | Glucose | [86] |
| Chitosan | Laponite | L-lactate | [87] |
| Graphene oxide | Au NPs | AA, DA, UA | [83] |
| agarose | Au NPs | Carbamate | [88] |
| PA, PANI, lutetium phthalocyanine | Graphene oxide | Glucose | [89] |
| PVI-dmeOs | MWCNT | Glucose oxidase, lactate oxidase | [81] |
| PAM | Pyrolytic graphite | Myoglobin | [80] |
| Poly(acrylic acid-co-itaconic acid) | MWCNT | Cd2+ | [90] |
| Chitosan | Graphene | H2O2 | [84] |
| Chitosan | Au NPs | H2O2 | [91] |
| PNIPAAM | Graphene | H2O2 | [84] |
| Silk fibroin | Graphene | H2O2 | [84] |
| CNC | Graphene | H2O2 | [84] |
| Graphene oxide | PdCu NPs | Glucose | [92] |
| Nitrogen-doped activated graphene | Au NPs | Hydroquinone, o-dihydroxybenzene | [93] |
| Ferrocene (Fc) modified amino acid | Phenylalanine | Prostate specific antigen | [94] |
| Graphene oxide | Au NPs | Indole-3-acetic acid, salicylic acid | [95] |
| Pyrrole | Graphene | Ammonia gas | [96] |
| Graphene oxide | Au NPs | Nitric oxide | [97] |
| β-cyclodextrin, chitosan | Au NCs | H2O2 | [98] |
| Graphene | Prussian blue | H2O2 | [99] |
| Graphene oxide | Co3O4 | Glucose | [100] |
| Graphene oxide | NiCo2O4 | H2O2 | [101] |
| Graphene oxide | ZnO | H2O2, glucose | [102] |
| Graphene oxide | Au, Pt, Pd and Cu | UA | [103] |
| Graphene oxide | Pyrrole, Co2+ | Catechol, hydroquinone | [104] |
| Conductive Polymer | Other Material | Analyte | Reference |
|---|---|---|---|
| PPy | Collagen | Glucose | [111] |
| PANI | PtNPs | Glucose | [112] |
| PPy | Tartrazine | AA, DA, UA | [110] |
| PANI | PAM | UA | [113] |
| PPy | PANI | Glucose, urea | [114] |
| PPy | - | Concentration of the electrolyte | [115] |
| PPy | p(2-hydroxyethyl methacrylate) | Glucose | [116] |
| PANI | Graphene | Pb2+ | [117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, L.; Yu, A.; Lai, G. Conductive Hydrogel-Based Electrochemical Sensor: A Soft Platform for Capturing Analyte. Chemosensors 2021, 9, 282. https://doi.org/10.3390/chemosensors9100282
Fu L, Yu A, Lai G. Conductive Hydrogel-Based Electrochemical Sensor: A Soft Platform for Capturing Analyte. Chemosensors. 2021; 9(10):282. https://doi.org/10.3390/chemosensors9100282
Chicago/Turabian StyleFu, Li, Aimin Yu, and Guosong Lai. 2021. "Conductive Hydrogel-Based Electrochemical Sensor: A Soft Platform for Capturing Analyte" Chemosensors 9, no. 10: 282. https://doi.org/10.3390/chemosensors9100282
APA StyleFu, L., Yu, A., & Lai, G. (2021). Conductive Hydrogel-Based Electrochemical Sensor: A Soft Platform for Capturing Analyte. Chemosensors, 9(10), 282. https://doi.org/10.3390/chemosensors9100282







