Factors Influencing the Surface Functionalization of Citrate Stabilized Gold Nanoparticles with Cysteamine, 3-Mercaptopropionic Acid or l-Selenocystine for Sensor Applications
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Synthesis and Characterization of Citrate-Capped AuNPs
2.3. Modification and Purification of Cysteamine, 3-Mercaptopropionic Acid and l-Selenocystine Capped AuNPs
2.4. Characterization of AuNPs Capped with Different Agents
3. Results and Discussion
3.1. Surface Characterization of Citrate-Stabilized AuNPs
3.2. Optimization of the Conditions for the Functionalization of Citrate-Stabilized AuNPs
Effect of pH on Functionalization
3.3. Functionalization of AuNPs
3.3.1. Characterization of Capped-AuNPs by FTIR
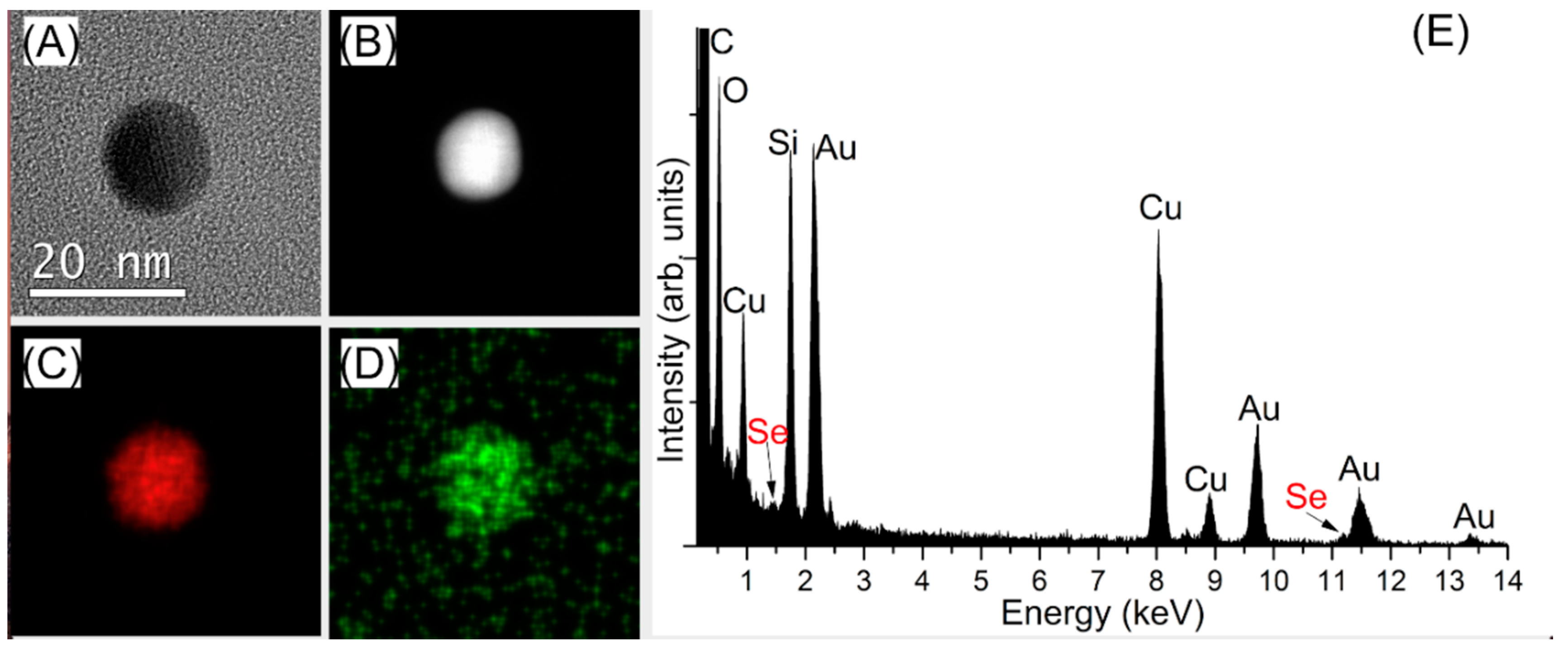
3.3.2. Characterization of the Capped-AuNPs by TEM
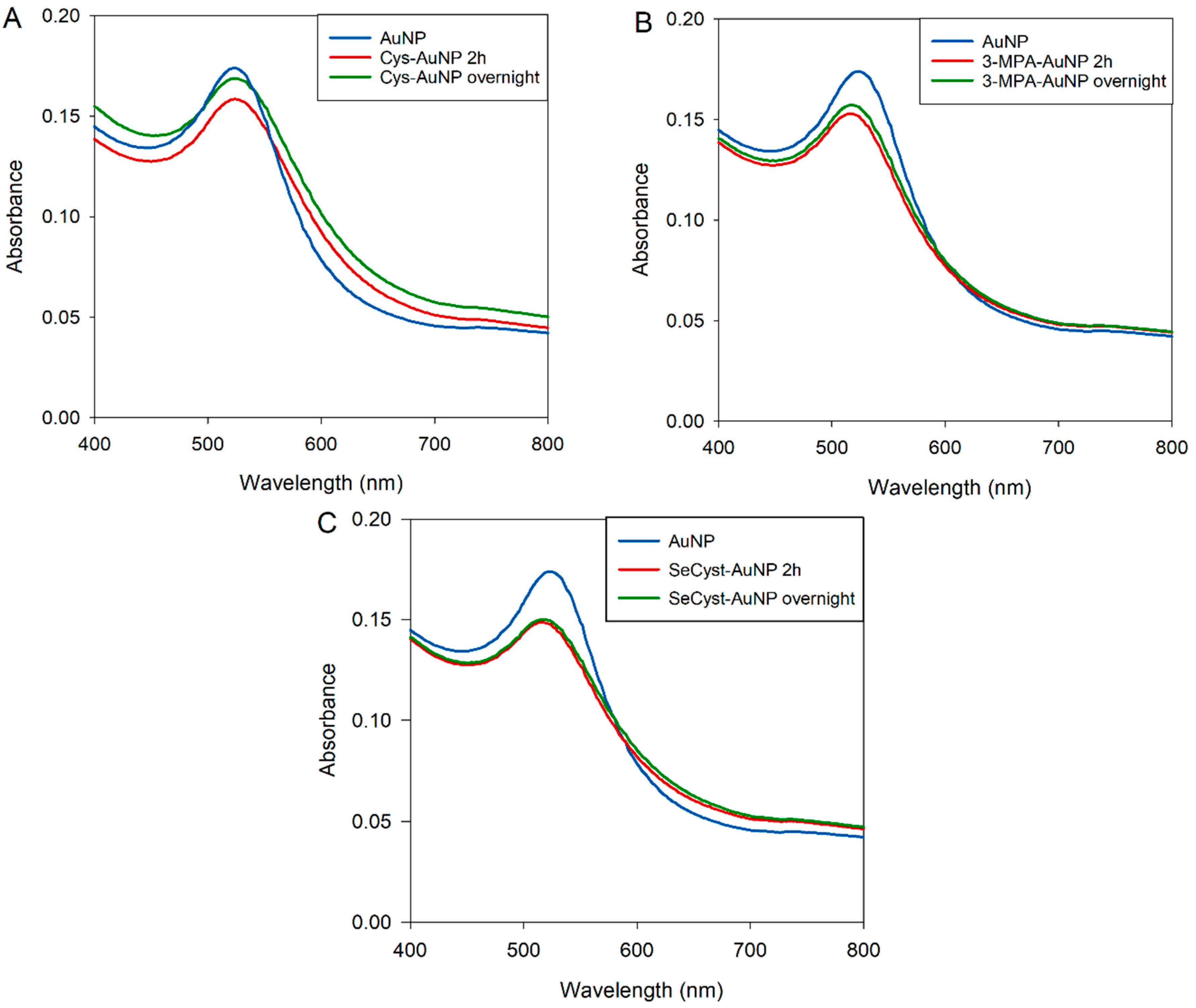
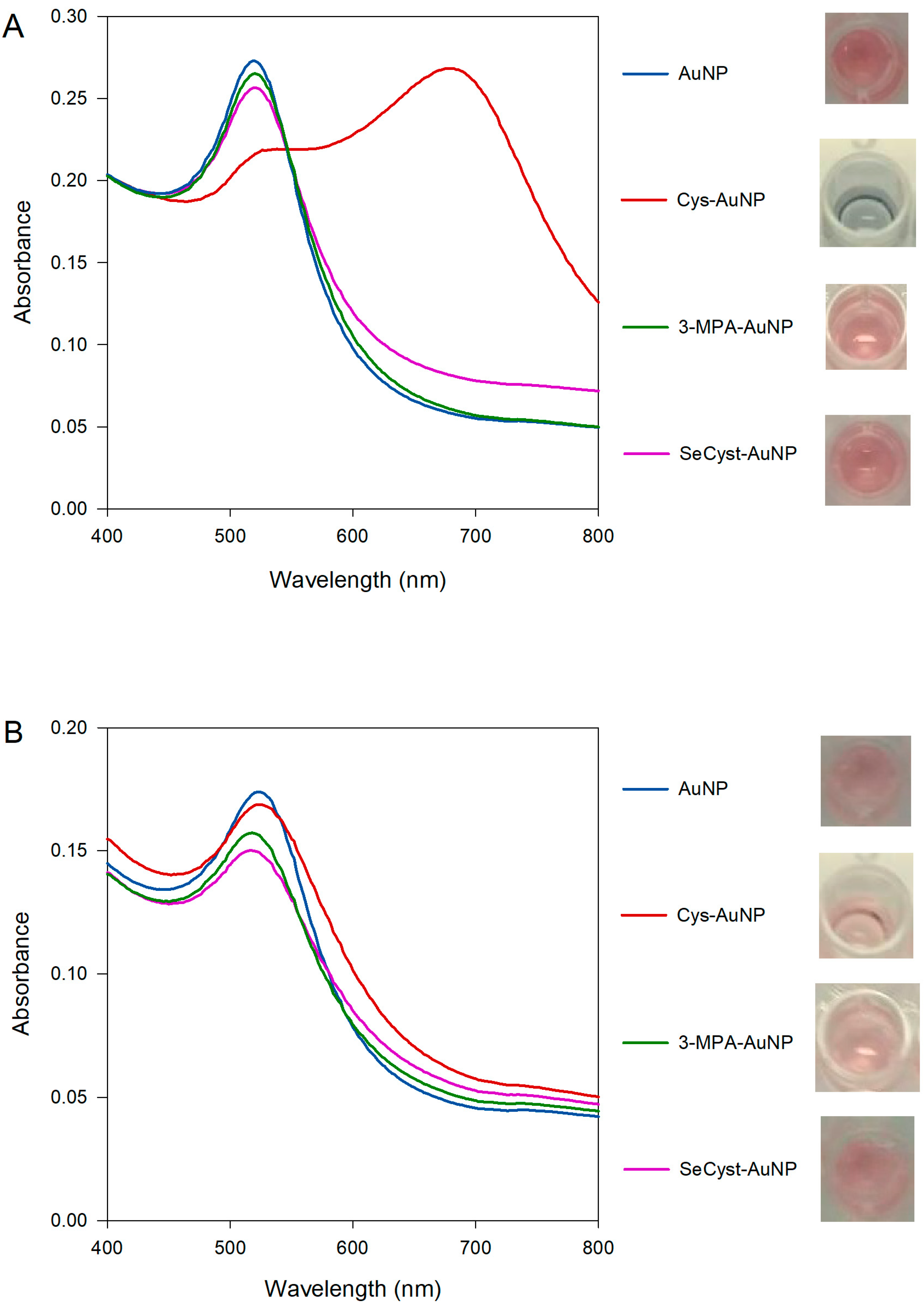
3.3.3. Characterization of Capped-AuNPs by UV-Visible Spectroscopy
4. Conclusions
5. Future Research
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery application. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Kong, F.-Y.; Zhang, J.-W.; Li, R.-F.; Wang, Z.-X.; Wang, W.-J.; Wang, W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef]
- Li, Y.; Schluesener, H.J.; Xu, S. Gold nanoparticle-based biosensors. Gold Bull. 2010, 43, 29–41. [Google Scholar] [CrossRef]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.N.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold nanoparticle-based colorimetric biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef]
- Nune, S.K.; Gunda, P.; Thallapally, P.K.; Lin, Y.-Y.; Forrest, M.L.; Berkland, C.J. Nanoparticles for biomedical imaging. Expert Opin. Drug Deliv. 2009, 6, 1175–1194. [Google Scholar] [CrossRef]
- Wong, A.C.; Wright, D.W.; Conrad, J.A. General Methods in Biomarker Research and Their Applications; Springer: Dordrecht, The Netherlands, 2014; pp. 1–26. [Google Scholar]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Meng, F.; Hou, N.; Jin, Z.; Sun, B.; Li, W.; Xiao, X.; Wang, C.; Li, M.; Liu, J. Sub-ppb detection of acetone using Au-modified flower-like hierarchical ZnO structures. Sens. Actuators B Chem. 2015, 219, 209–217. [Google Scholar] [CrossRef]
- Bagheri, S.; Yasemi, M.; Safaie-Qamsari, E.; Rashidiani, J.; Abkar, M.; Hassani, M.; Mirhosseini, S.A.; Kooshki, H. Using gold nanoparticles in diagnosis and treatment of melanoma cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 462–471. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Pradhan, N. Gold nanoparticles as efficient sensors in colorimetric detection of toxic metal ions: A review. Sens. Actuators B Chem. 2017, 238, 888–902. [Google Scholar] [CrossRef]
- Wang, C.; Yu, C. Detection of chemical pollutants in water using gold nanoparticles as sensors: A review. Rev. Anal. Chem. 2013, 32, 1–14. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef]
- Kang, B.; Austin, L.A.; El-Sayed, M.A. Observing Real-Time Molecular Event Dynamics of Apoptosis in Living Cancer Cells using Nuclear-Targeted Plasmonically Enhanced Raman Nanoprobes. ACS Nano 2014, 8, 4883–4892. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Academic Press, Elsevier: Oxford, UK, 2013. [Google Scholar]
- Ivanov, M.R.; Bednar, H.R.; Haes, A.J. Investigations of the mechanism of gold nanoparticle stability and surface functionalization in capillary electrophoresis. ACS Nano 2009, 3, 386–394. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Ojea-Jiménez, I.; Romero, F.M.; Bastús, N.G.; Puntes, V.J. Small gold nanoparticles synthesized with sodium citrate and heavy water: Insights into the reaction mechanism. Phys. Chem. C 2010, 114, 1800–1804. [Google Scholar] [CrossRef]
- Sivaraman, S.K.; Kumar, S.; Santhanam, V.J. Monodisperse sub-10 nm gold nanoparticles by reversing the order of addition in Turkevich method—The role of chloroauric acid. Colloid Interface Sci. 2011, 361, 543–547. [Google Scholar] [CrossRef]
- Grabar, K.C.; Hommer, M.B.; Natan, M.J.; Freeman, R.G. Preparation and characterization of Au colloid monolayers. Anal. Chem. 1995, 67, 735–743. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Oh, S.; Baba, R.; Zhou, H.; Hwang, S.; Lee, J.; Park, E.Y. Synthesis of Gold Nanoparticles with Buffer-Dependent Variations of Size and Morphology in Biological Buffers. Nanoscale Res. Lett. 2016, 11, 1–11. [Google Scholar] [CrossRef]
- Zhao, P.; Li, N.; Astruc, D. State of the art in gold nanoparticle synthesis. Coord. Chem. Rev. 2013, 257, 638–665. [Google Scholar] [CrossRef]
- Havaldar, D.V.; Patil, R.V.; Moholkar, D.N.; Magdum, P.S.; Vadrale, A.P.; Pawar, K.D. Differently synthesized gold nanoparticles respond differently to functionalization with L-amino acids. Particuology 2020, 52, 97–104. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Ji, X.; Song, X.; Li, J.; Bai, Y.; Yang, W.; Peng, X.J. Size Control of Gold Nanocrystals in Citrate Reduction: The Third Role of Citrate. Am. Chem. Soc. 2007, 129, 13939–13948. [Google Scholar] [CrossRef]
- Rohiman, A.; Anshori, I.; Surawijaya, A.; Idris, I. Study of Colloidal Gold Synthesis Using Turkevich Method. AIP Conf. Proc. 2011, 1415, 39–42. [Google Scholar]
- Kim, J.-H.; Lavin, B.W.; Burnett, R.D.; Boote, B.W. Controlled synthesis of gold nanoparticles by fluorescent light irradiation. Nanotechnology 2011, 22, 285602. [Google Scholar] [CrossRef]
- Su, C.-H.; Wu, P.-L.; Yeh, C.-S. Sonochemical Synthesis of Well-Dispersed Gold Nanoparticles at the Ice Temperature. J. Phys. Chem. B 2003, 107, 14240–14243. [Google Scholar] [CrossRef]
- Volkert, A.A.; Subramaniam, V.; Haes, A.J. Implications of citrate concentration during the seeded growth synthesis of gold nanoparticles. Chem. Commun. 2011, 47, 478–480. [Google Scholar] [CrossRef]
- Patungwasa, W.; Hodak, J.H. pH tunable morphology of the gold nanoparticles produced by citrate reduction. Mater. Chem. Phys. 2008, 108, 45–54. [Google Scholar] [CrossRef]
- Dong, J.; Carpinone, P.L.; Pyrgiotakis, G.; Demokritou, P.; Moudgil, B.M. Synthesis of Precision Gold Nanoparticles Using Turkevich Method. Kona. 2020, 37, 224–232. [Google Scholar] [CrossRef]
- Yee, C.K.; Ulman, A.; Ruiz, J.D.; Parikh, A.; White, H.; Rafailovich, M. Alkyl Selenide-and Alkyl Thiolate-Functionalized Gold Nanoparticles: Chain Packing and Bond Nature. Langmuir 2003, 19, 9450–9458. [Google Scholar] [CrossRef]
- Romashov, L.V.; Ananikov, V.P. Self-Assembled Selenium Monolayers: From Nanotechnology to Materials Science and Adaptive Catalysis. Chem. Eur. J. 2013, 19, 17640–17660. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Shumaker-Parry, J.S. Strong Resistance of Citrate Anions on Metal Nanoparticles to Desorption under Thiol Functionalization. ACS Nano 2015, 9, 1665–1682. [Google Scholar] [CrossRef] [PubMed]
- Chong, G.; Hernandez, R.J. Adsorption Dynamics and Structure of Polycations on Citrate-Coated Gold Nanoparticles. J. Phys. Chem. C 2018, 122, 19962–19969. [Google Scholar] [CrossRef]
- Wei, H.; Leng, W.; Song, J.; Liu, C.; Willner, M.R.; Huang, Q.; Zhou, W.; Vikesland, P.J. Real-Time Monitoring of Ligand Exchange Kinetics on Gold Nanoparticle Surfaces Enabled by Hot Spot-Normalized Surface-Enhanced Raman Scattering. Environ. Sci. Technol. 2019, 53, 575–585. [Google Scholar] [CrossRef]
- Perera, G.S.; Athukorale, S.A.; Perez, F.; Pittman, C.U.; Zhang, D.J. Facile displacement of citrate residues from gold nanoparticle surfaces. J Colloid Interface Sci. 2018, 511, 335–343. [Google Scholar] [CrossRef]
- Stolarczyk, E.U.; Leś, A.; Łaszcz, M.; Kubiszewski, M.; Strzempek, W.; Menaszek, E.; Fusaro, M.; Sidoryk, K.; Stolarczyk, K. The ligand exchange of citrates to thioabiraterone on gold nanoparticles for prostate cancer therapy. Int. J. Pharm. 2020, 583, 119319. [Google Scholar] [CrossRef]
- Rani, M.; Moudgil, L.; Singh, B.; Kaushal, A.; Mittal, A.; Saini, G.S.S.; Tripathi, S.K.; Singh, G.; Kaura, A. Understanding the mechanism of replacement of citrate from the surface of gold nanoparticles by amino acids: A theoretical and experimental investigation and their biological application. RSC Adv. 2016, 6, 17373–17383. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Size and Temperature Dependence of the Plasmon Absorption of Colloidal Gold Nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M. Tailoring Surface Plasmons through the Morphology and Assembly of Metal Nanoparticles. Langmuir 2006, 22, 32–41. [Google Scholar] [CrossRef]
- Jain, P.K.; El-Sayed, M.A. Surface Plasmon Resonance Sensitivity of Metal Nanostructures: Physical Basis and Universal Scaling in Metal Nanoshells. J. Phys. Chem. C 2007, 111, 17451–17454. [Google Scholar] [CrossRef]
- Doyen, M.; Bartik, K.; Bruylants, G.J. UV–Vis and NMR study of the formation of gold nanoparticles by citrate reduction: Observation of gold–citrate aggregates. J. Colloid Interface Sci. 2013, 399, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.K.; Yang, L.; Yung, L.Y.L.; Ong, C.N.; Ong, W.Y.; Yu, L.E. Characterization, purification, and stability of gold nanoparticles. Biomaterials 2010, 31, 9023–9030. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Shumaker-Parry, J.S. Structural Study of Citrate Layers on Gold Nanoparticles: Role of Intermolecular Interactions in Stabilizing Nanoparticles. J. Am. Chem. Soc. 2014, 136, 1907–1921. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, S.; Kitano, T.; Ito, K. Infrared and ultraviolet spectroscopic studies on intramolecular hydrogen bonding in an alternating copolymer of isobutylene and maleic acid. Macromolecules 1991, 24, 6030–6036. [Google Scholar] [CrossRef]
- Ojea-Jiménez, I.; Puntes, V.J. Instability of Cationic Gold Nanoparticle Bioconjugates: The Role of Citrate Ions. J. Am. Chem. Soc. 2009, 131, 13320–13327. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Spectral Properties and Relaxation Dynamics of Surface Plasmon Electronic Oscillations in Gold and Silver Nanodots and Nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Shaporenko, A.; Cyganik, P.; Buck, M.; Terfort, A.; Zharnikov, M.J. Self-Assembled Monolayers of Aromatic Selenolates on Noble Metal Substrates. J. Phys. Chem. B 2005, 109, 13630–13638. [Google Scholar] [CrossRef]
- Wall, S.B.; Oh, J.Y.; Diers, A.R.; Landar, A. Oxidative modification of proteins: An emerging mechanism of cell signaling. Front. Physiol. 2012, 3, 369. [Google Scholar] [CrossRef]
- Tielens, F.; Santos, E.J. AuS and SH Bond Formation/Breaking during the Formation of Alkanethiol SAMs on Au(111): A Theoretical Study. J. Phys. Chem. C 2010, 114, 9444–9452. [Google Scholar] [CrossRef]
- Kankate, L.; Turchanin, A.; Gölzhäuser, A. On the Release of Hydrogen from the S−H groups in the Formation of Self-Assembled Monolayers of Thiols. Langmuir 2009, 25, 10435–10438. [Google Scholar] [CrossRef]
- Xue, Y.; Li, X.; Li, H.; Zhang, W. Quantifying thiol-gold interactions towards the efficient strength control. Nat. Commun. 2014, 5, 4348. [Google Scholar] [CrossRef] [PubMed]
- Helios, K.; Pietraszko, A.; Zierkiewicz, W.; Wójtowicz, H.; Michalska, D. The crystal structure, infrared, Raman and density functional studies of bis (2-aminophenyl) diselenide. Polyhedron 2011, 30, 2466–2472. [Google Scholar] [CrossRef]
- Underwood, S.; Mulvaney, P. Effect of the Solution Refractive Index on the Color of Gold Colloids. Langmuir 1994, 10, 3427–3430. [Google Scholar] [CrossRef]
| Chemical Shift (ppm) | Pattern | Coupling Constant J (Hz) | Assignment | Samples |
|---|---|---|---|---|
| 3.44 | singlet | - | Acetoacetic acid | Synthesized AuNPs |
| 2.59, 2.55 | doublet | 15 | Citrate | Synthesized AuNPs, trisodium citrate |
| 2.47, 2.44 | doublet | 12 | Citrate | Synthesized AuNPs, trisodium citrate |
| 2.74, 2.70 | doublet | 15 | Other “forms” of citrate [43] | Trisodium citrate |
| 2.30, 2.26 | doublet | 15 | Other “forms” of citrate | Trisodium citrate |
| 2.14 | singlet | - | Acetone | Synthesized AuNPs, trisodium citrate |
| 1.82 | singlet | - | Acetic acid | Synthesized AuNPs, purchased AuNPs |
| 3.67, 3.65, 3.63 | triplet | 8, 8 | α-ketoglutaric acid | Purchased AuNPs |
| 3.056, 3.044, 3.036, 3.026, 3.015 | quintet | 4.8, 3.2, 4, 4.4 | - | Purchased AuNPs |
| 2.758, 2.747, 2.738, 2.730, 2.717 | quintet | 4.4, 3.6, 3.2, 5.2 | - | Purchased AuNPs |
| 2.54, 2.52, 2.51 | triplet | 6, 6 | α-ketoglutaric acid | Purchased AuNPs |
| 1.19 | singlet | - | - | Purchased AuNPs |
| Synthesized AuNPs | Wavelength (nm) | Purchased AuNPs | Wavelength (nm) |
|---|---|---|---|
| Cit-AuNP | 520 | Cit-AuNP | 524 |
| Cys-AuNP | 538, 678 | Cys-AuNP | 525 |
| 3-MPA-AuNP | 518 | 3-MPA-AuNP | 517 |
| SeCyst-AuNP | 524 | SeCyst-AuNP | 516 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakellari, G.I.; Hondow, N.; Gardiner, P.H.E. Factors Influencing the Surface Functionalization of Citrate Stabilized Gold Nanoparticles with Cysteamine, 3-Mercaptopropionic Acid or l-Selenocystine for Sensor Applications. Chemosensors 2020, 8, 80. https://doi.org/10.3390/chemosensors8030080
Sakellari GI, Hondow N, Gardiner PHE. Factors Influencing the Surface Functionalization of Citrate Stabilized Gold Nanoparticles with Cysteamine, 3-Mercaptopropionic Acid or l-Selenocystine for Sensor Applications. Chemosensors. 2020; 8(3):80. https://doi.org/10.3390/chemosensors8030080
Chicago/Turabian StyleSakellari, Georgia I., Nicole Hondow, and Philip H.E. Gardiner. 2020. "Factors Influencing the Surface Functionalization of Citrate Stabilized Gold Nanoparticles with Cysteamine, 3-Mercaptopropionic Acid or l-Selenocystine for Sensor Applications" Chemosensors 8, no. 3: 80. https://doi.org/10.3390/chemosensors8030080
APA StyleSakellari, G. I., Hondow, N., & Gardiner, P. H. E. (2020). Factors Influencing the Surface Functionalization of Citrate Stabilized Gold Nanoparticles with Cysteamine, 3-Mercaptopropionic Acid or l-Selenocystine for Sensor Applications. Chemosensors, 8(3), 80. https://doi.org/10.3390/chemosensors8030080







