Abstract
Processes’ occurring at the Ag/AgCl/Cl–, ([Fe(CN)6]3–/4–) ions interface study results are presented. Conditions are selected for the mixed salts’ precipitate formation on the silver surface. It has been shown that the potential of a silver screen-printed electrode (AgSPE) coated with a mixed precipitate containing silver chloride/ferricyanide is stable in the presence of [Fe(CN)6]3–/4–. The electrode can serve as a quasi-reference electrode (QRE) in electrochemical measurements in media containing ions [Fe(CN)6]3–/4–. The electrode is formed during polarization of AgSPE (0.325 V vs. Ag/AgCl/KCl, 3.5 M) in a solution containing chloride- and ferri/ferrocyanides ions. The results of the obtained QRE study by potentiometry, scanning electron microscopy and cyclic voltammetry are presented. The proposed QRE was used in a sensor system to evaluate the antioxidant activity (AOA) of solutions by hybrid potentiometric method (HPM). The results of AOA assessment of fruit juices and biofluids obtained using new QRE and commercial Ag/AgCl RE with separated spaces do not differ.
1. Introduction
Processes occurring at the interface silver/solution containing [Fe(CN)6]3–/4–, and creating sensor systems capable of functioning in the presence of these ions are of interest because of the following reasons:
- [Fe(CN)6]3–/4– serves as a mediator system in assessing the antioxidant/oxidant activity (AOA/OA) of solutions in hybrid potentiometric (HPM) [1,2,3,4,5] and chronoamperometric (CA) [5,6,7] methods;
- [Fe(CN)6]3–/4– serves as a mediator system in assessing AOA/OA of solid-phase objects in contact hybrid potentiometric method (CHPM) [8,9,10,11];
- [Fe(CN)6]3–/4– is used as a redox probe to study processes in cyclic voltammetry [12,13,14,15,16] and electrochemical impedance spectroscopy [15,16,17];
- [Fe(CN)6]3–/4– is used in bio- [18,19], immuno- [20] and aptasensors [21].
AOA/OA assessment by HPM [1,2,3,4,5], CA [5,6,7] and CHPM [8,9,10,11] is of great importance in biomedical analysis, as it is a source of information on oxidative stress and health status [22,23]. Evaluation of AOA of food products [3,4,7,11], pharmaceuticals [4], dietary supplements [4] and cosmetics [8] allows to assess their quality, and to determine AOA/OA of biological media [1,2,5,6,8,9,10] to track the impact of various factors on human health and to evaluate, for example, the effectiveness of the therapy. In this regard, the development of sensors and sensor systems for AOA/OA monitoring is a very urgent and important task.
Sensor systems, as a rule, consist of two or three electrodes, one of which is always a reference electrode (RE). The correctness of measurements is determined by the stability of its potential. A classic and most commonly used RE is a silver/silver chloride electrode [24,25,26,27,28].
Ag/AgCl quasi-reference electrodes (QREs) are described in [29,30,31,32]. With all the convenience and functionality of Ag/AgCl QREs, their serious drawback is the non-stability of Ag/AgCl measuring surface from the environment in which it is located. In media containing ions that form sparingly soluble compounds with silver, potential of such electrodes changes uncontrollably [27]. If in voltammetric and amperometric methods, an admissible uncertainty in the RE potential is allowed, more stringent requirements are imposed on the stability of the RE potential in potentiometry [27,28,33]. The situation is more complicated in those cases in which concentration of components (for example, [Fe(CN)6]3–/4–) changes during analysis. This fact causes the challenge of applying Ag/AgCl QREs in sensor systems.
For the described solid-state reference electrodes (SSREs), a number of attempts have been made to protect the Ag/AgCl measuring surface from the impact of environment. To prevent AgCl dissolution, the thickness of the Ag and AgCl layers is increased [34]; protective coatings of polyurethane [35,36], Nafion [36] or graphene oxide [37] are used; an AgCl layer of a special structure (horizontally from the periphery of the template) in combination with an external protective coating of polyimide [38] is formed. In other cases, an external electrolyte-doped polymer is used [39,40,41,42], which has a dual function: it provides a relatively constant concentration of chloride anions and creates a diffusion barrier that slows down the dissolution of AgCl. A significant part of Ag/AgCl SSREs’ design includes combination of an intermediate electrolyte-doped polymer material and an external protective coating [43,44,45,46,47,48,49,50]. Encapsulation of Ag/AgCl/Cl– into strong hardening polymers such as polyvinyl chloride [51,52] or poly (n-butyl acrylate) [53,54,55] is described in recent studies. SSREs described in [26,31,32,33,35] keep the potential constant in a solution containing [Fe(CN)6]3–/4–, but protective polymer layers, in some cases, create a barrier for the penetration of substances generating the analytical signal to the measuring surface. Additionally, the complexity of such electrodes’ manufacturing makes their mass use unlikely. Thus, creation of RE or QRE for sensor systems operating in the presence of [Fe(CN)6]3–/4− still faces the problem of the reference electrode selection.
The aim of this work is to study the processes occurring at the interface silver/solution containing Cl– and [Fe(CN)6]3–/4–; searching the information needed for creation of a new QRE that maintains a constant potential in the presence of substances interacting with silver; and demonstration of the analytical applicability of this QRE as part of a sensor system designed to analyze solution’s AOA by HPM.
2. Materials and Methods
2.1. Chemicals and Materials
The following chemicals were used: К3[Fe(CN)6)], КСl, Na2HPO4 × 12H2O (CJSC Vecton, St. Petersburg, Russia); KNO3 (AO Reachim Ltd., Moscow, Russia); К4[Fe(CN)6] × 3H2O (CJSC Kupavnareaktiv, Staraya Kupavna, Russia); KH2PO4 (NevaReaktiv Ltd., St. Petersburg, Russia). These reagents were chemically pure. Other chemicals were: H2SO4 0.1 M standard solution (Ural Chemical Products Plant Ltd., Verhnyaya Pyshma, Russia), HCl 0.1 M standard solution (Ekroshim Ltd., St. Petersburg, Russia), acetone pure for analysis (Component-reaktive Ltd., Moscow, Russia), ethanol 95% (Konstanta-farm M Ltd., Moscow, Russia), FeCl3 × 6H2O 97–102% (AppliChem GmbH – An ITW Company, Darmstadt, Germany). The solvent used was deionized (DI) water with a resistivity 18 МΩ × cm.
The following materials were used: 0.5 mm thick plate made of K–96 ceramic (96% Al2O3, 2.36% SiO2, 0.56% CaO and 1.08% MgO) from JSC South Ural Radioceramics Plant (YUzhnouralsk, Russia), conductive silver paste PP–17C from RPE Delta-Pastes Ltd. (Zelenograd, Russia) and Cementit universal from Merz + Benteli AG (Niederwangen, Switzerland).
2.2. Instruments and Devices
For potentiometric measurements, pH/ions meters TA–ION (RPE Tomanalyt Ltd., Tomsk, Russia) were used. Voltammetric measurements were performed on an IVA–5 inversion voltammetric analyzer (RPIE Iva Ltd., Yekaterinburg, Russia). Surface morphology of the electrodes was studied using a JSM–6490LV electron scanning microscope (JEOL Ltd., Tokyo, Japan). Polyethylene terephthalate tubes (Chengdu Puth Medical Plastics Packaging Co., Ltd., Chengdu, China) containing a blood coagulation activator (SiO2) were used for blood collection. Whole blood serum was obtained using a CM–6M centrifuge (SIA ELMI, Riga, Latvia). Silver screen-printed electrodes (AgSPEs) were manufactured using a DEK–248 screen-printer (ASM Assembly Systems Weymouth Ltd., Weymouth, UK). DI water with a resistivity 18 MΩ × cm was obtained on an Akvalab UVOI–MF–1812 installation (JSC RPC Mediana-filter, Moscow, Russia).
2.3. Electrodes
A platinum electrode (disk diameter 3 mm) in a polyether ether ketone case type 6.1204.310 (Metrohm AG, Switzerland) was used as a control indicator electrode (Pt). Before starting work, Pt was polished using aluminum oxide powder (first used, a grain size of 0.3 μm, then 0.05 μm) deposited on a polishing cloth, and additionally subjected to a cyclic polarization in the potential range from –0.2 to 1.5 V and scanning rate 0.1 V/s in 0.1 M H2SO4 solution until a stable cyclic voltammogram was obtained [56]. After each regeneration step, Pt was washed with DI water. Control reference electrode was a silver/silver chloride electrode Ag/AgCl/KCl (3.5 M) type 6.0728.040 completed with an electrolyte vessel type 6.1245.010 (Metrohm AG, Switzerland). A silver/silver chloride electrode Ag/AgCl/KCl (3.5 M) type EVL–1M3.1 (JSC Gomel Plant of Measuring Devices, Belorussia) was used in the measurements, unless otherwise indicated. Potential of the EVL–1M3.1 at 25 °C is 0.204 ± 0.003 V relative to SHE [57]. Potential of the EVL–1M3.1 was checked daily in relation to the reference electrode (a deviation of ± 3 mV was considered acceptable). Glassy carbon rod GC–2000 3 mm/100 mm (JSC RI Grafit, Russia) was used as an auxiliary electrode in voltammetric measurements.
AgSPE was prepared by applying two layers of silver paste on a ceramic plate washed with acetone, ethanol and deionized water. Each layer of paste was hardened as follows: heated to 850 °C for 30 min, cooled to 150 °C for 30 min, dried at 150 °C for 15 min and cooled to room temperature. Double paste application provided an average silver layer thickness of 30 μm. The plate was cut and electrodes 5 × 40 mm in size were obtained. The middle part of AgSPE separating the working and contact zones was isolated with a Cementit–acetone mixture in a ratio of 1:5 by v/v, so that the electrode working area was 20 mm2 (5×4 mm). AgSPEs were modified by forming a precipitate on its surface by electrolysis of a solution containing [Fe(CN)6]3–/4– under various conditions (see Section 3.2.).
2.4. Auxiliary Solution
To modify the AgSPEs and carrying out analysis, solution containing 10 mM K3[Fe(CN)6], 0.1 mM K4[Fe(CN)6], 1 M KCl, 54.5 mM Na2HPO4 and 12.1 mM KH2PO4 (pH 7.4) was used. Its composition corresponds to that used to determine AOA of food products [4] and biofluids [1,2,5].
2.5. Potentiometric Measurements
For potentiometric studies, the measurement circuit proposed in this work and shown in Figure 1, was applied. The advantage of such a circuit over the traditional two-electrode configuration is the implementation of identical conditions for comparing the electrodes and the reduction of time, since it provides the possibility to obtain the results of two measurements in one experiment.
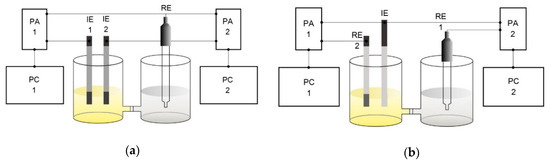
Figure 1.
Schemes for measuring potential (a) of two indicator electrodes (IE 1 and IE 2) with respect to one reference electrode (RE); (b) one indicator electrode (IE) relatively to two reference electrodes (RE 1 and RE 2). PA 1 and PA 2: potentiometric analyzers; PC 1 and PC 2: personal computers; IE 1 and IE 2: modified AgSPEs; RE = RE 1: EVL – 1M3.1; RE 2: QREmix.
Electrode stability in the auxiliary solution was evaluated by potentiometric method using measuring circuit shown in Figure 1a. Following parameters were determined:
- τ—potential stabilization (establishment) time, s;
- E1—steady-state value of potential, mV;
- ΔE/Δt—drift of the potential, mV/h;
- E2—potential value, mV, was obtained when 0.4 mL 0.1 M K4[Fe(CN)6] was added to 4 mL of auxiliary solution (the final concentration of K4[Fe(CN)6] in the cell is 9.1 mM, which models the upper value of antioxidant content in the sample).
- E3—potential value, mV, was established when electrode is returned to the initial solution.
These parameters were evaluated based on the study of four modified AgSPEs (Table 2) and four Ag/AgCl QREs (Table 4). The most stable modified AgSPE was considered as a QREmix.
Solution’s (fruit juices and biofluids) AOA were evaluated at room temperature by HPM [1,2,3,4,5]. The measuring circuit, shown in Figure 1b, was used. In the circuit, PtSPE vs. EVL–1M3.1 served as an electrode pair, and PtSPE vs. QREmix served as a sensor system.
2.6. Voltammetric and Scanning Electron Microscopy Measurements
Normal three-electron cell was used, consisting of AgSPE or Pt, EVL–1M3.1 or QREmix and GC–2000 as working electrode, reference electrode, and auxiliary electrode. Potential scanning rate was 0.05 V/s. Scanning electron microscopy (SEM) measurements were performed at 20 kV in vacuum. Data were used for electrodes surface morphology characterization (see Section 3.4).
2.7. Potentiometric Sensor System Assembly
Platinum screen-printed electrode (PtSPE) was used as an indicator in the evaluation of AOA of fruit juices and biofluids. Applicability of PtSPE in food analysis [4] and biofluids [1,2,5] has been confirmed earlier. After operation in food and biological matrices, PtSPE was regenerated by annealing at a temperature of 750 °C for 1 h [58]. The most stable modified AgSPE (i.e., QREmix) served as a reference electrode. The results obtained are presented in Section 3.6.
2.8. Sampling and Sample Preparation
Samples of apple juices of the Dobryj, Rich, J7 brands and fresh apples of Smit and Fuji varieties were purchased at a local supermarket. The juices taken from the packages opened before analysis and freshly squeezed juices were examined. Saliva was taken into a plastic container 10 min before analysis, while the respondent refrained from eating, drinking, smoking and brushing his teeth for at least an hour. Blood was collected by venipuncture at the bend of the elbow joint into a polyethylene terephthalate tube containing a blood coagulation activator (SiO2). To obtain serum, whole blood samples were centrifuged at 3500 rpm for 15 min. The resulting blood serum was frozen and stored at −18 °C. The ejaculate was taken into a plastic container by natural masturbation after 2–3 days of abstinence. The selected ejaculate samples were kept for 40 min at room temperature, and then they were frozen and stored at −18 °C. Before analysis, serum and ejaculate samples were thawed for 40 min at room temperature.
2.9. Statistical Analysis
All measurements were repeated 4 times. Statistical analysis was performed in Microsoft Excel 2010 with an accepted significance level of α = 0.05. The data are presented as X ± ΔX, where X is the average value, ΔX is the standard deviation. Validation of the results of the evaluation of AOA solutions obtained on the developed reference electrode (QREmix) was performed in relation to the results obtained on a commercial reference electrode (EVL–1M3.1), based on F- and t-tests.
3. Results and Discussion
3.1. Study of Redox Processes Occurring on Polarized AgSPE
Figure 2 shows cyclic voltammograms describing the redox processes on AgSPE in solutions of KCl, K4[Fe(CN)6], K3[Fe(CN)6], Na2HPO4 and KH2PO4, which are components of the solution used. Cyclic voltammograms for Pt are also shown for comparison.
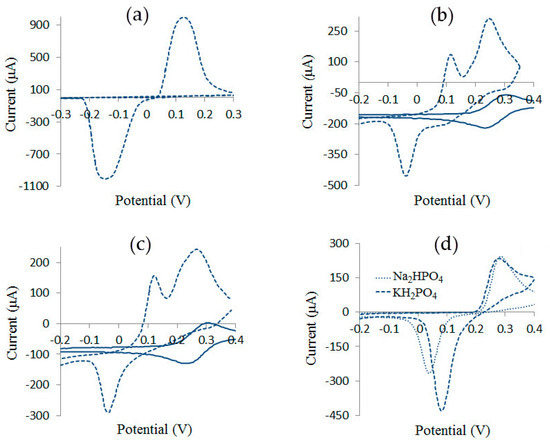
Figure 2.
Cyclic voltammograms recorded on AgSPE (dashed lines, first cycle) and Pt (solid lines) in solutions of (a) 1 M KCl, (b) 0.005 M K4[Fe(CN)6] and 1 M KNO3, (c) 0.005 M K3[Fe(CN)6] and 1 M KNO3, (d) 0.005 M Na2HPO4 with 1 M KNO3 and 0.005 M KH2PO4 with 1 M KNO3. Scanning rate 0.05 V/s.
Taking into account the low solubility of silver salts (Table 1), it should be concluded that the currents in cyclic voltammograms (Figure 2) are due to the following processes:

Table 1.
The equilibrium concentration of silver ions over solutions of sparingly soluble compound.
- Figure 2b: 4Ag0(s) + [Fe(CN)6]4–(aq.) → Ag4[Fe(CN)6](s) + 4e– (first peak at 0.05 V < Е < 0.15 V) and [Fe(CN)6]4–(s) → [Fe(CN)6]3–(s) + e– (second peak at Е > 0.15 V), which is associated with a similar process for an indifferent Pt [Fe(CN)6]4–(aq.) → [Fe(CN)6]3–(aq.) + e–;
- Figure 2d: 3Ag0(s) + PO43–(aq.) → Ag3PO4(s) + 3e– at E > 0.2 V.
In a mixture of components, redox processes considered can take place in parallel or as competing ones. Composition of the precipitate formed on AgSPE depends on concentration of anions in the solution, solubility product of silver compounds, and electrode potential. In the potential range 0.05 V < E < 0.15 V, AgCl and Ag4[Fe(CN)6] are formed together; at 0.15 V < Е < 0.2 V, AgCl and Ag3[Fe(CN)6] are formed; and at E > 0.2 V formation of three sparingly soluble compounds AgCl, Ag3[Fe(CN)6] and Ag3PO4 is possible. Since, predominantly, those poorly soluble compounds are formed, in equilibrium with which concentration of silver ions is minimal (Table 1), formation of Ag3PO4 under these conditions can be neglected.
3.2. Selection of Surface Formation Conditions of QREmix
Table 2 presents modification conditions of AgSPE. AgSPE polarization modes are selected according to the cyclic curves shown in Figure 2.

Table 2.
AgSPE modification conditions in the solution containing [Fe(CN)6]3–/4– at room temperature.
Figure 3 shows dependences of the modified AgSPE potential stabilization time (τ) on modification duration. As can be seen from Figure 3a, potential of an open-chain modified AgSPE with solution stirring stabilizes faster than without solution stirring. Minimum τ values for open-chain modified AgSPEs are observed at modification duration of 7200 s (2 h). Parameters τ, ΔE/Δt, E1, E2, E3 for AgSPE modified in an open circuit without solution stirring are poorly reproducible. Potential stabilization time of AgSPE modified in the potentiostatic mode decreases with increasing of modification duration (Figure 3b). Minimum τ values are observed after modifying electrodes for 120 s. Results in which standard deviation of one or more parameters E1, E2, and E3 exceeds 3 mV are excluded from further consideration, since they do not satisfy necessary conditions. Results in which standard deviation of the parameters E1, E2 and E3 is less than 3 mV are shown in Table 3.
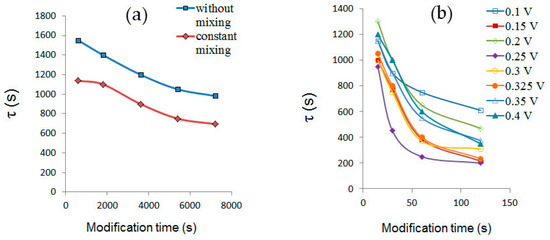
Figure 3.
AgSPE potential stabilization time (average values for n = 4 shown) versus open circuit modification time (a) and potentiostatic mode (b).

Table 3.
Influence of AgSPE modification conditions in [Fe(CN)6]3–/4– containing solution on the values of parameters characterizing electrode properties.
It follows from Table 3 that optimal conditions are: potentiostatic polarization mode at 0.325 V for 120 s. Further, the electrode formed under these conditions was considered as a new QRE and defined as QREmix, which indicated presence of a mixed precipitate on its surface. In this case, precipitate consisted mainly of AgCl and Ag3[Fe(CN)6] (see Section 3.1).
3.3. Comparison of QREmix and Ag/AgCl QRE
Conditions of Ag/AgCl QRE formation are presented in Table 4. The results of a comparative study of Ag/AgCl QRE and QREmix in solution containing [Fe(CN)6]3–/4–stability are shown in Figure 4. It can be seen from Figure 4 that QREmix is the most stable. In this case, parameter’s (τ, ΔE/Δt, E1, E2, E3) values that determine operability of the electrode are minimal. QREmix was stored in air at room temperature without direct sunlight. Parameters preserve normal values for application as a reference electrode for 7 days (Figure 4).

Table 4.
Ag/AgCl QRE types and their formation conditions.
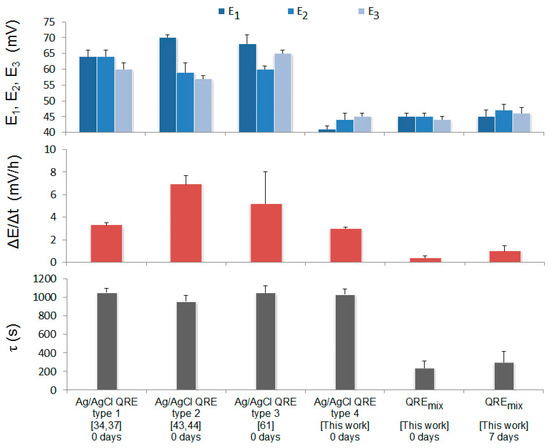
Figure 4.
Experimental values of the parameters (τ is potential stabilization time, ΔE/Δt is potential drift and E1, E2, E3 are potentials values) characteristic for QREmix and Ag/AgCl QREs depending on the electrodes storage duration before measurements. The boundaries indicate upper values of standard deviation for α = 0.05 and n = 4.
3.4. Electrode Surface Morphology
Figure 5 shows SEM images of AgSPE surface (Figure 5a) and AgSPE’s surface, which are formed at a potential of 0.325 V in non-containing and containing [Fe(CN)6]3–/4– solutions (Figure 5b,f). AgSPE surface is a conglomerate of polydisperse crystals 2–10 μm size (Figure 5a). Ag/AgCl QRE type 4 and QREmix surface (Figure 5b,c) consists of polydisperse large (0.4–1.8 μm) crystals. Fine crystalline precipitates form on AgSPEs obtained in [Fe(CN)6]3–/4– solutions (Figure 5d,f). Probably a fine-crystalline precipitate leave out, mainly of Ag3[Fe(CN)6], leave out as shown earlier (see Section 3.1), fills pores between large AgCl crystals, which leads to an improvement of QREmix stability compared to Ag/AgCl QREs.
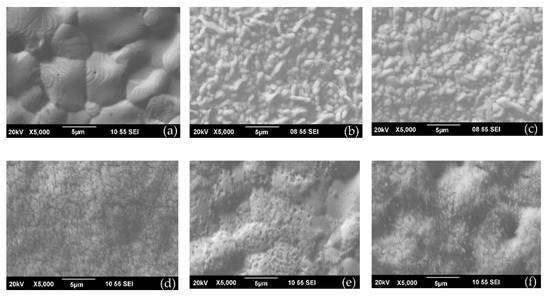
Figure 5.
SEM images of surfaces: (a) AgSPE, (b) Ag/AgCl QRE type 4, (c) QREmix and AgSPEs modified in potentiostatic mode (0.325 V, 120 s) in solutions containing (d) 5 mM K3[Fe(CN)6], 54.5 mM Na2HPO4, 12.1 mM KH2PO4 and 1 M KNO3, (e) 5 mM K4[Fe(CN)6], 54.5 mM Na2HPO4, 12.1 mM KH2PO4 and 1 M KNO3, (f) 5 mM K3[Fe(CN)6], 5 mM K4[Fe(CN)6], 54.5 mM Na2HPO4, 12.1 mM KH2PO4 and 1 M KNO3.
3.5. QREmix Characterization by Cyclic Voltammetry
Cyclic voltammograms recorded on Pt in a solution containing 1 mM [Fe(CN)6]3–/4– (1:1) and 1 M KCl using EVL–1M3.1 or QREmix as a reference electrode are shown in Figure 6. It is seen that the obtained cyclic voltammograms are almost identical and differ only in a parallel shift along the potential axis by 45 ± 3 mV (n = 4), which corresponds to the potential difference between EVL–1M3.1 and QREmix. This is one more evidence of QREmix stable operation in a solution containing [Fe(CN)6]3–/4–. It may contribute to the use of this electrode for other electrochemical applications.
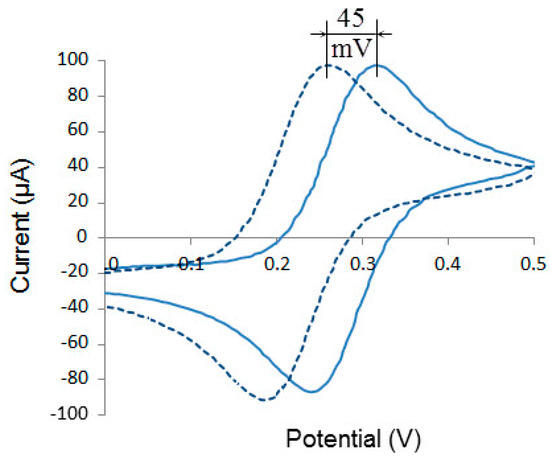
Figure 6.
Cyclic voltammograms recorded on Pt in a solution containing 1 mM K3[Fe(CN)6], 1 mM K4[Fe(CN)6] and 1 M KCl, using EVL–1M3.1 (solid line) and QREmix (dashed line) as a reference electrode.
3.6. Potentiometric Sensor System in the Determination of AOA of Solutions
The QREmix proposed was used as part of the sensor system to evaluate AOA of fruit juices and biofluids by HPM in comparison with commercial Ag/AgCl RE. Measurement circuit shown in Figure 1b (IE = PtSPE, RE 1 = EVL–1M3.1 and RE 2 = QREmix) was used. The results are presented in Table 5. It can be seen from Table 5 that values of F- and t-tests are less than theoretical ones, which proves the same reproducibility and statistically insignificant differences between the results. The data presented indicate correctness of using of QREmix in the real samples’ analysis.

Table 5.
AOA determination results of solutions (fruit juices and biofluids) obtained using the PtSPE vs. QREmix and PtSPE vs. EVL–1M3.1 (n = 4).
4. Conclusions
The results of a study of the processes occurring at the electrode/multianionic interface, some of which form sparingly soluble or complex compounds with the electrode material, are of general interest. The data obtained in this work make a definite contribution to this area: they justify research paths and provide the information needed for creating QREs for sensor systems operating in the presence of interfering ions, [Fe(CN)6]3–/4– in particular. The latter allowed us to solve a very important problem—to develop sensory systems for monitoring AOA/OA of various objects, including vital biological ones. The QRE proposed consists of an Ag screen-printed substrate electrochemically coated with a mixed precipitate containing silver chloride/ferricyanide. The sediment was studied by potentiometry, scanning electron microscopy and cyclic voltammetry. A new electrochemical scheme is proposed and used, which allows comparing electrodes in one measurement. This approach increases the accuracy of the research results and reduces time required to obtain them. Performance of the sensor system with the new QRE is illustrated by its application for determination of AOA of fruit juices and body liquids. The results obtained have good analytical characteristics, which allows us to predict the widespread use of QRE described in this paper. Additional strategies aimed at improving the stability of the developed QRE may include, but are not limited to, the use of protective coatings. The approaches and methods described may be useful for creating QREs working in the presence of other interfering substances.
Author Contributions
Conceptualization, K.Z.B.; validation, A.V.T.; formal analysis, A.V.T.; investigation, A.V.T.; resources, A.V.T.; writing—original draft preparation, K.Z.B., A.V.T. and M.B.V.; writing—review and editing, K.Z.B. and M.B.V.; visualization, A.V.T.; funding acquisition, A.V.T. All authors have read and agreed to the published version of the manuscript.
Funding
The reported study was funded by RFBR according to the research project № 18-33-00215.
Acknowledgments
The authors are grateful to the Laboratory of Structural Methods of Analysis and the Properties of Materials and Nanomaterials in the Ural Federal University named after the first President of Russia, B.N. Yeltsin, for conducting electrodes SEM.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brainina, K.Z.; Alyoshina, L.V.; Gerasimova, E.L.; Kazakov, Y.E.; Ivanova, A.V.; Beykin, Y.B.; Belyaeva, S.V.; Usatova, T.I.; Khodos, M.Y. New electrochemical method of determining blood and blood fractions antioxidant activity. Electroanalysis 2009, 21, 618–624. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Gerasimova, E.L.; Varzakova, D.P.; Balezin, S.L.; Portnov, I.G.; Makutina, V.A.; Tyrchaninova, E.V. Potentiometric method for evaluating the oxidant/antioxidant activity of seminal and follicular fluids and clinical significance of this parameter for human reproductive function. Open Chem. Biomed. Methods J. 2012, 5, 1–7. [Google Scholar] [CrossRef][Green Version]
- Brainina, K.Z.; Zaharov, A.S.; Vidrevich, M.B. Potentiometry for the determination of oxidant activity. Anal. Methods 2016, 8, 5667–5675. [Google Scholar] [CrossRef]
- Brainina, K.; Stozhko, N.; Bukharinova, M.; Khamzina, E.; Vidrevich, M. Potentiometric method of plant microsuspensions antioxidant activity determination. Food Chem. 2019, 278, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Brainina, K.Z.; Varzakova, D.P.; Kazakov, Y.E.; Vidrevich, M.B. Noninvasive electrochemical antioxidant activity estimation: Saliva analysis. Biointerface Res. Appl. Chem. 2018, 8, 3381–3387. [Google Scholar]
- Brainina, K.Z.; Varzakova, D.P.; Gerasimova, E.L. A chronoamperometric method for determining total antioxidant activity. J. Anal. Chem. 2012, 67, 364–369. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Tarasov, A.V.; Khodos, M.Y. Determination of the oxidant activity of chlorinated water by chronoamperometry. J. Anal. Chem. 2017, 72, 911–916. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Gerasimova, E.L.; Varzakova, D.P.; Kazakov, Y.E.; Galperin, L.G. Noninvasive method of determining skin antioxidant/oxidant activity: Clinical and cosmetics applications. Anal. Bioanal. Electrochem. 2013, 5, 528–542. [Google Scholar]
- Brainina, K.Z.; Markina, M.G.; Stozhko, N.Y. Optimized potentiometric assay for non-invasive investigation of skin antioxidant activity. Electroanalysis 2018, 30, 2405–2412. [Google Scholar] [CrossRef]
- Brainina, K.; Tarasov, A.; Khamzina, E.; Kazakov, Y.; Stozhko, N. Disposable potentiometric sensory system for skin antioxidant activity evaluation. Sensors 2019, 19, 2586. [Google Scholar] [CrossRef]
- Brainina, K.; Tarasov, A.; Khamzina, E.; Stozhko, N.; Vidrevich, M. Contact hybrid potentiometric method for on-site and in situ estimation of the antioxidant activity of fruits and vegetables. Food Chem. 2020, 309, 125703. [Google Scholar] [CrossRef] [PubMed]
- Lounasvuori, M.M.; Rosillo-Lopez, M.; Salzmann, C.G.; Caruana, D.J.; Holt, K.B. The influence of acidic edge groups on the electrochemical performance of graphene nanoflakes. J. Electroanal. Chem. 2015, 753, 28–34. [Google Scholar] [CrossRef]
- Tucceri, R. The charge transport process at gold electrodes modified by thick nickel hydroxide films. A study employing rotating disc electrode voltammetry in the presence of the Fe(CN)63−/4− redox couple. J. Electroanal. Chem. 2016, 782, 125–132. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Y.; Yan, K.; Zhang, J. Reversibility-dependent photovoltammetric behavior of electroactive compounds on a CdS-graphene hybrid film electrode. Chem. Eur. J. 2017, 23, 13294–13299. [Google Scholar] [CrossRef]
- Long, Y.-T.; Li, C.-Z.; Kraatz, H.-B.; Lee, J.S. AC impedance spectroscopy of native DNA and M-DNA. Biophys. J. 2003, 84, 3218–3225. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, W.; Wang, X.; Zhang, H.; Cui, X.; Wang, H. Ultrahigh capacitive performance from both Co(OH)2/graphene electrode and K3Fe(CN)6 electrolyte. Sci. Rep. 2013, 3, 2986. [Google Scholar] [CrossRef]
- Ragoisha, G.A.; Bondarenko, A.S. Potentiodynamic electrochemical impedance spectroscopy. Electrochim. Acta 2005, 50, 1553–1563. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Moreira, F.T.C.; Fernandes, R.; Sales, M.G.F. Novel and simple electrochemical biosensor monitoring attomolar levels of miRNA-155 in breast cancer. Biosens. Bioelectron. 2016, 80, 621–630. [Google Scholar] [CrossRef]
- Nik Mansor, N.N.; Leong, T.T.; Safitri, E.; Futra, D.; Ahmad, N.S.; Nasuruddin, D.N.; Itnin, A.; Zaini, I.Z.; Arifin, K.T.; Heng, L.Y.; et al. An amperometric biosensor for the determination of bacterial sepsis biomarker, secretory phospholipase group 2-IIA using a tri-enzyme system. Sensors 2018, 18, 686. [Google Scholar] [CrossRef]
- Chu, Z.; Dai, H.; Liu, Y.; Lin, Y. Development of a semiconductor-based electrochemical sensor for interferon-γ detection. Int. J. Electrochem. Sci. 2017, 12, 9141–9149. [Google Scholar] [CrossRef]
- Le, T.H.; Pham, V.P.; La, T.H.; Phan, T.B.; Le, Q.H. Electrochemical aptasensor for detecting tetracycline in milk. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 015008. [Google Scholar] [CrossRef]
- Kazakov, Y.; Khodos, M.; Vidrevich, M.; Brainina, K. Potentiometry as a tool for monitoring of antioxidant activity and oxidative stress estimation in medicine. Crit. Rev. Anal. Chem. 2019, 49, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Kazakov, Y.; Tarasov, A.; Alyoshina, L.; Brainina, K. Interplay between antioxidant activity, health and disease. Biointerface Res. Appl. Chem. 2020, 10, 4893–4901. [Google Scholar] [CrossRef]
- Guth, U.; Gerlach, F.; Decker, M.; Oelßner, W.; Vonau, W. Solid-state reference electrodes for potentiometric sensors. J. Solid State Electrochem. 2009, 13, 27–39. [Google Scholar] [CrossRef]
- Shinwari, M.W.; Zhitomirsky, D.; Deen, I.A.; Selvaganapathy, P.R.; Deen, M.J.; Landheer, D. Microfabricated reference electrodes and their biosensing applications. Sensors 2010, 10, 1679–1715. [Google Scholar] [CrossRef]
- Spitzer, P.; Wunderli, S.; Maksymiuk, K.; Michalska, A.; Kisiel, A.; Galus, Z.; Tauber, G. Reference electrodes for aqueous solutions. In Handbook of Reference Electrodes; Inzelt, G., Lewenstam, A., Scholz, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Chapter 5; pp. 86–98. [Google Scholar] [CrossRef]
- Kahlert, H. Micro-reference electrodes. In Handbook of Reference Electrodes; Inzelt, G., Lewenstam, A., Scholz, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Chapter 11; pp. 289–303. [Google Scholar] [CrossRef]
- Michalska, A.; Kisiel, A.; Maksymiuk, K. Screen-printed disposable reference electrodes. In Handbook of Reference Electrodes; Inzelt, G., Lewenstam, A., Scholz, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Chapter 13; pp. 325–330. [Google Scholar] [CrossRef]
- Desmond, D.; Lane, B.; Alderman, J.; Glennon, J.D.; Diamond, D.; Arrigan, D.W.M. Evaluation of miniaturised solid state reference electrodes on a silicon based component. Sens. Actuators B 1997, 44, 389–396. [Google Scholar] [CrossRef]
- Sun, X.; Wang, M. Fabrication and characterization of planar reference electrode for on-chip electroanalysis. Electrochim. Acta 2006, 52, 427–433. [Google Scholar] [CrossRef]
- Almeida, F.L.; Fontes, M.B.A.; Jimenez, C.; Burdallo, I. Secondary Ag/AgCl pseudo-reference electrode on silicon substrate. ECS Trans. 2008, 14, 73–82. [Google Scholar] [CrossRef]
- Cranny, A.; Harris, N.; White, N. Screen printed potentiometric chloride sensors. Procedia Eng. 2014, 87, 220–223. [Google Scholar] [CrossRef][Green Version]
- Lito, M.J.G.; Camões, M.F. Meeting the requirements of the silver/silver chloride reference electrode. J. Solut. Chem. 2009, 38, 1471–1482. [Google Scholar] [CrossRef]
- Polk, B.J.; Stelzenmuller, A.; Mijares, G.; MacCrehan, W.; Gaitan, M. Ag/AgCl microelectrodes with improved stability for microfluidics. Sens. Actuators B 2006, 114, 239–247. [Google Scholar] [CrossRef]
- Gernet, S.; Koudelka, M.; De Rooij, N.F. Fabrication and characterization of a planar electrochemical cell and its application as a glucose sensor. Sens. Actuators 1989, 18, 59–70. [Google Scholar] [CrossRef]
- Moussy, F.; Harrison, D.J. Prevention of the rapid degradation of subcutaneously implanted Ag/AgCl reference electrodes using polymer coatings. Anal. Chem. 1994, 66, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Hong, S.A.; Yang, S. A solid-state thin-film Ag/AgCl reference electrode coated with graphene oxide and its use in a pH sensor. Sensors 2015, 15, 6469–6482. [Google Scholar] [CrossRef]
- Suzuki, H.; Hiratsuka, A.; Sasaki, S.; Karube, I. Problems associated with the thin-film Ag/AgCl reference electrode and a novel structure with improved durability. Sens. Actuators B 1998, 46, 104–113. [Google Scholar] [CrossRef]
- Rehm, D.; McEnroe, E.; Diamond, D. An all solid-state reference electrode based on a potassium chloride doped vinyl ester resin. Anal. Proc. 1995, 32, 319–322. [Google Scholar] [CrossRef]
- Cosofret, V.V.; Erdosy, M.; Johnson, T.A.; Buck, R.P.; Ash, R.B.; Neuman, M.R. Microfabricated sensor arrays sensitive to pH and K+ for ionic distribution measurements in the beating heart. Anal. Chem. 1995, 67, 1647–1653. [Google Scholar] [CrossRef]
- Huang, I.-Y.; Huang, R.-S.; Lo, L.-H. Improvement of integrated Ag/AgCl thin-film electrodes by KCl-gel coating for ISFET applications. Sens. Actuators B 2003, 94, 53–64. [Google Scholar] [CrossRef]
- Mamińska, R.; Dybko, A.; Wróblewski, W. All-solid-state miniaturised planar reference electrodes based on ionic liquids. Sens. Actuators B 2006, 115, 552–557. [Google Scholar] [CrossRef]
- Nolan, M.A.; Tan, S.H.; Kounaves, S.P. Fabrication and characterization of a solid state reference electrode for electroanalysis of natural waters with ultramicroelectrodes. Anal. Chem. 1997, 69, 1244–1247. [Google Scholar] [CrossRef]
- Kwon, N.-H.; Lee, K.-S.; Won, M.-S.; Shim, Y.-B. An all-solid-state reference electrode based on the layer-by-layer polymer coating. Analyst 2007, 132, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Koudelka, M. Performance characteristics of a planar “clark-type” oxygen sensor. Sens. Actuators 1986, 9, 249–258. [Google Scholar] [CrossRef]
- Arquint, P.; van den Berg, A.; van der Schoot, B.H.; de Rooij, N.F.; Bühler, H.; Morf, W.E.; Dürselen, L.F.J. Integrated blood-gas sensor for pO2, pCO2 and pH. Sens. Actuators B 1993, 13, 340–344. [Google Scholar] [CrossRef]
- Kinlen, P.J.; Heider, J.E.; Hubbard, D.E. A solid-state pH sensor based on a Nafion-coated iridium oxide indicator electrode and a polymer-based silver chloride reference electrode. Sens. Actuators B 1994, 22, 13–25. [Google Scholar] [CrossRef]
- Simonis, A.; Lüth, H.; Wang, J.; Schöning, M.J. New concepts of miniaturised reference electrodes in silicon technology for potentiometric sensor systems. Sens. Actuators B 2004, 103, 429–435. [Google Scholar] [CrossRef]
- Liao, W.-Y.; Chou, T.-C. Fabrication of a planar-form screen-printed solid electrolyte modified Ag/AgCl reference electrode for application in a potentiometric biosensor. Anal. Chem. 2006, 78, 4219–4223. [Google Scholar] [CrossRef]
- Tymecki, Ł.; Zwierkowska, E.; Koncki, R. Screen-printed reference electrodes for potentiometric measurements. Anal. Chim. Acta 2004, 526, 3–11. [Google Scholar] [CrossRef]
- Kisiel, A.; Marcisz, H.; Michalska, A.; Maksymiuk, K. All-solid-state reference electrodes based on conducting polymers. Analyst 2005, 130, 1655–1662. [Google Scholar] [CrossRef]
- Kisiel, A.; Michalska, A.; Maksymiuk, K. Plastic reference electrodes and plastic potentiometric cells with dispersion cast poly(3,4-ethylenedioxythiophene) and poly(vinyl chloride) based membranes. Bioelectrochemistry 2007, 71, 75–80. [Google Scholar] [CrossRef]
- Kisiel, A.; Michalska, A.; Maksymiuk, K.; Hall, E.A.H. All-solid-state reference electrodes with poly(n-butyl acrylate) based membranes. Electroanalysis 2008, 20, 318–323. [Google Scholar] [CrossRef]
- Rius-Ruiz, F.X.; Kisiel, A.; Michalska, A.; Maksymiuk, K.; Riu, J.; Rius, F.X. Solid-state reference electrodes based on carbon nanotubes and polyacrylate membranes. Anal. Bioanal. Chem. 2011, 399, 3613–3622. [Google Scholar] [CrossRef] [PubMed]
- Rius-Ruiz, F.X.; Bejarano-Nosas, D.; Blondeau, P.; Riu, J.; Rius, F.X. Disposable planar reference electrode based on carbon nanotubes and polyacrylate membrane. Anal. Chem. 2011, 83, 5783–5788. [Google Scholar] [CrossRef] [PubMed]
- Swain, G.M. Solid electrode materials: Pretreatment and activation. In Handbook of Electrochemistry; Zoski, C.G., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2007; Chapter 5; p. 146. [Google Scholar] [CrossRef]
- Kahlert, H. Reference electrodes. In Electroanalytical Methods, 2nd ed.; Scholz, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Chapter III; p. 299. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Tarasov, A.V.; Kazakov, Y.E.; Vidrevich, M.B. Platinum electrode regeneration and quality control method for chronopotentiometric and chronoamperometric determination of antioxidant activity of biological fluids. J. Electroanal. Chem. 2018, 808, 14–20. [Google Scholar] [CrossRef]
- Solubility products of the hardly soluble in water compounds. In Chemist Handbook, 2nd ed.; Nikolskiy, B.P., Ed.; Chemistry: Moscow, Russia, 1965; Volume 3, pp. 229–234. (In Russian) [Google Scholar]
- Solubility product constants. In CRC Handbook of Chemistry and Physics, 89th ed.; Lide, D.R., Ed.; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2009; pp. 8-118–8-120. [Google Scholar]
- Brewer, P.J.; Leese, R.J.; Brown, R.J.C. An improved approach for fabricating Ag/AgCl reference electrodes. Electrochim. Acta 2012, 71, 252–257. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).