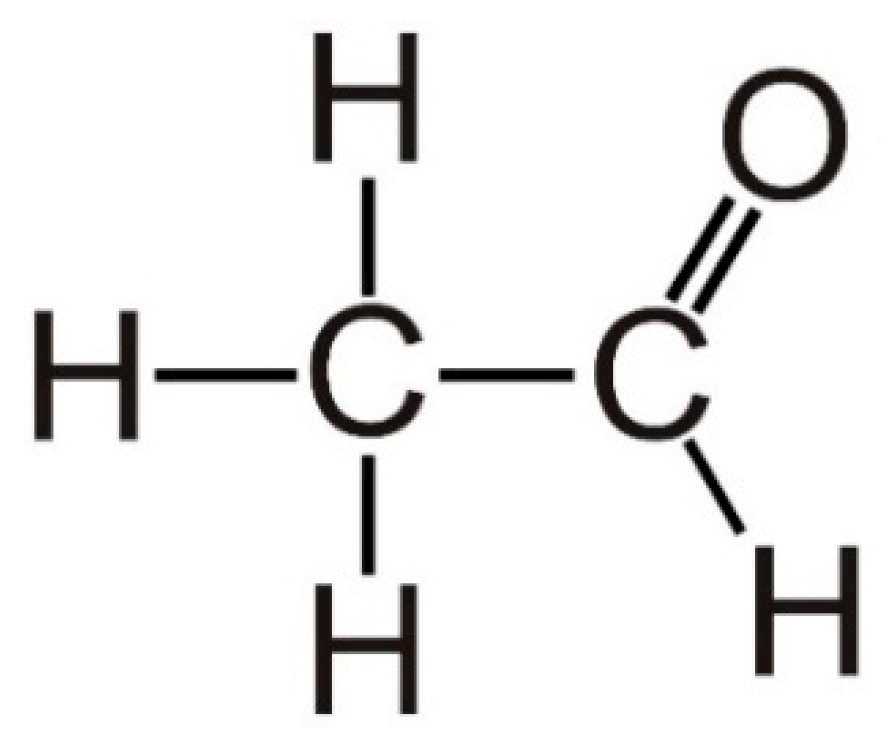
Nanostructured Semiconducting Metal Oxide Gas Sensors for Acetaldehyde Detection
Abstract
1. Introduction
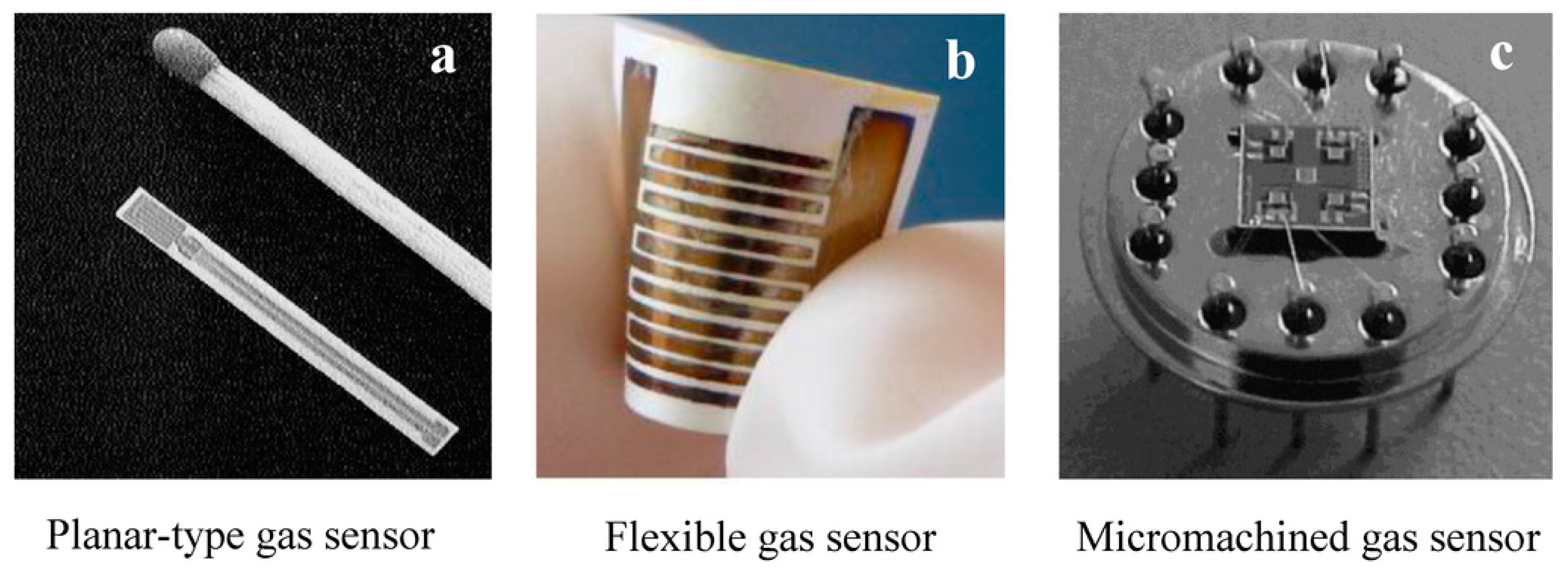
2. Chemiresistive Gas Sensors
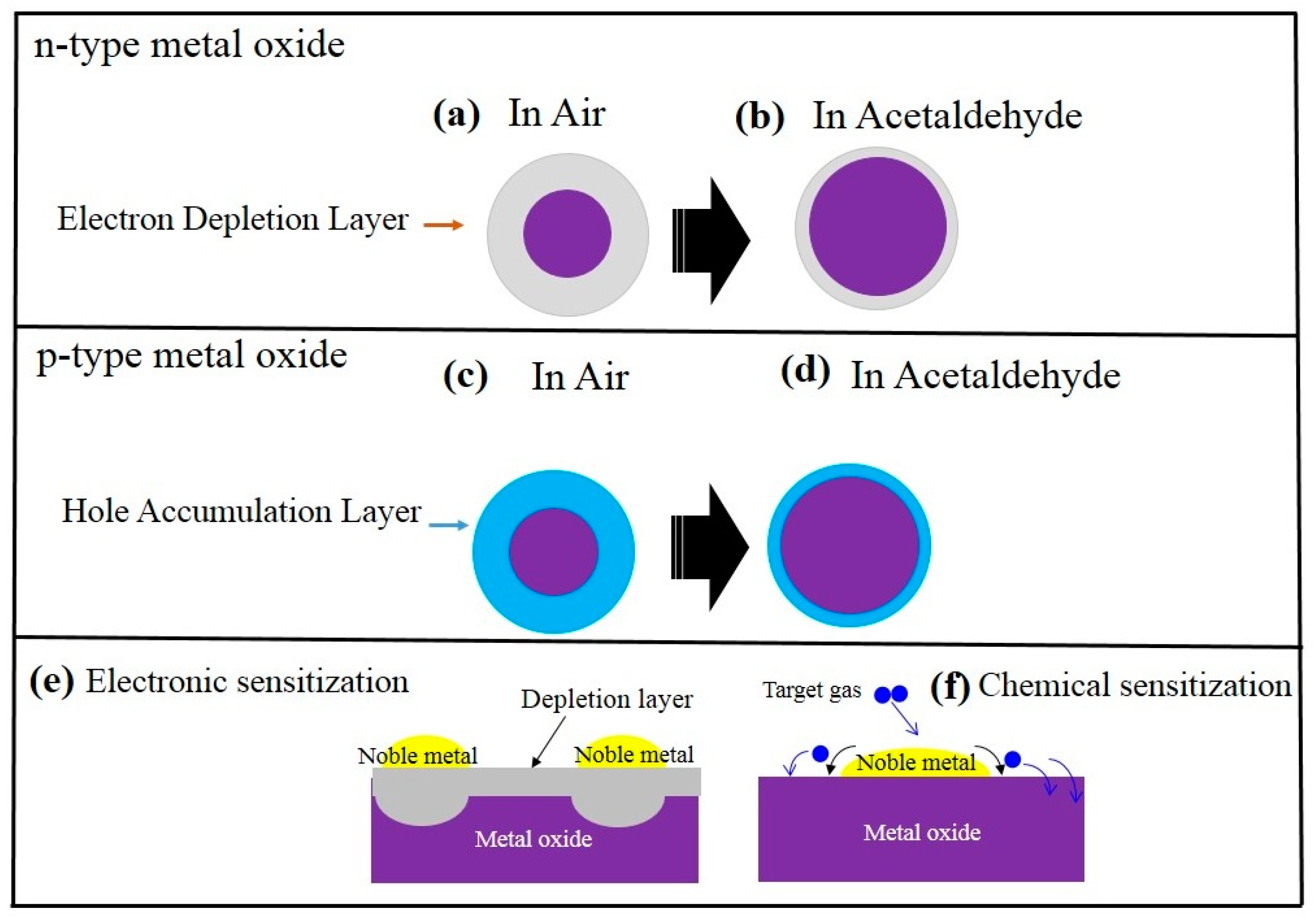
2.1. General Sensing Mechanism
3. Acetaldehyde Detection Using Chemiresistive-Based Gas Sensors
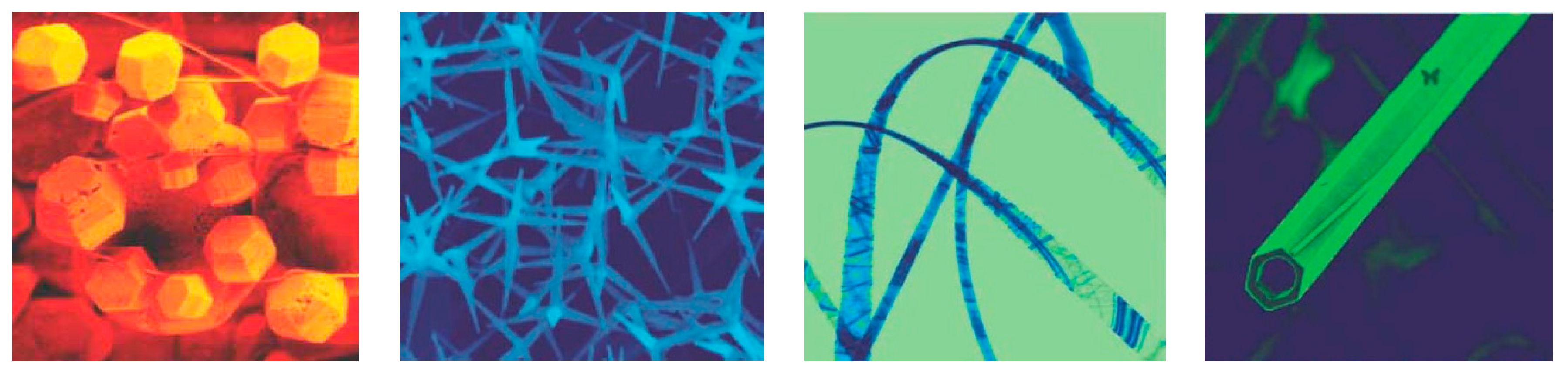
3.1. Morphology-Engineered Nanostructures as Acetaldehyde Gas Sensors
3.1.1. Nanoparticle-Based Acetaldehyde Gas Sensors
3.1.2. Effect of UV Light
3.1.3. Noble Metal Loading
3.1.4. Composites
3.1.5. Doped Nanostructure as Acetaldehyde Gas Sensors
4. Conclusions and Outlooks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qi, Y.; Shen, L.; Zhang, J.; Yao, J.; Lu, R.; Miyakoshi, T. Species and release characteristics of VOCs in furniture coating process. Environ. Pollut. 2019, 245, 810–819. [Google Scholar] [CrossRef]
- Hou, H.; Liu, H.; Gao, F.; Shang, M.; Wang, L.; Xu, L.; Yang, W. Packaging BiVO4 nanoparticles in ZnO microbelts for efficient photoelectrochemical hydrogen production. Electrochim. Acta 2018, 283, 497–508. [Google Scholar] [CrossRef]
- Katsumata, K.I.; Motoyoshi, R.; Matsushita, N.; Okada, K. Preparation of graphitic carbon nitride (g-C3N4)/WO3 composites and enhanced visible-light-driven photodegradation of acetaldehyde gas. J. Hazard. Mater. 2013, 260, 475–482. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Jiang, L.-M.; Dai, Y.-H.; Wu, M.-J.; Chen, X.-M.; Cai, C.-Q. Determination of formaldehyde and acetaldehyde in cigarette adhesives by high performance liquid chromatography. Chin. J. Anal. Lab. 2010, 5, 69–72. [Google Scholar]
- Calestani, D.; Mosca, R.; Zanichelli, M.; Villani, M.; Zappettini, A. Aldehyde detection by ZnO tetrapod-based gas sensors. J. Mater. Chem. 2011, 21, 15532–15536. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, Z.; Zhang, X. A novel gaseous acetaldehyde sensor utilizing cataluminescence on nanosized BaCO3. Sens. Actuators B Chem. 2004, 99, 30–35. [Google Scholar] [CrossRef]
- Delikhoon, M.; Fazlzadeh, M.; Sorooshian, A.; Baghani, A.N.; Golaki, M.; Ashournejad, Q.; Barkhordari, A. Characteristics and health effects of formaldehyde and acetaldehyde in an urban area in Iran. Environ. Pollut. 2018, 242, 938–951. [Google Scholar] [CrossRef]
- Santana, F.O.; Campos, V.P.; Cruz, L.P.; Luz, S.R. Formaldehyde and acetaldehyde in the atmosphere of Salvador-Ba, Brazil, using passive sampling. Microchem. J. 2017, 134, 78–86. [Google Scholar] [CrossRef]
- Stefanov, B.I.; Topalian, Z.; Granqvist, C.G.; Österlund, L. Acetaldehyde adsorption and condensation on anatase TiO2: Influence of acetaldehyde dimerization. J. Mol. Catal. A Chem. 2014, 381, 77–88. [Google Scholar] [CrossRef]
- Kovács, I.; Farkas, A.P.; Szitás, Á.; Kónya, Z.; Kiss, J. Adsorption, polymerization and decomposition of acetaldehyde on clean and carbon-covered Rh (111) surfaces. Surf. Sci. 2017, 664, 129–136. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Salaspuro, M. ALDH2-deficiency as genetic epidemiologic and biochemical model for the carcinogenicity of acetaldehyde. Reg. Tox. Pharm. 2017, 86, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Raktim Pal Kim, K.-H.; Hong, Y.-J.; Jeon, E.-C. The pollution status of atmospheric carbonyls in a highly industrialized area. J. Hazard. Mater. 2008, 153, 1122–1135. [Google Scholar]
- Salaspuro, M. Key role of local acetaldehyde in upper GI tract carcinogenesis. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 491–499. [Google Scholar] [CrossRef]
- Giberti, A.; Carotta, M.C.; Fabbri, B.; Gherardi, S.; Guidi, V.; Malagù, C. High-sensitivity detection of acetaldehyde. Sens. Actuators B Chem. 2012, 174, 402–405. [Google Scholar] [CrossRef]
- Haussmann, H.J. Use of hazard indices for a theoretical evaluation of cigarette smoke composition. Chem. Res. Toxicol. 2012, 25, 794–810. [Google Scholar] [CrossRef]
- Sad, M.E.; Peña, L.G.; Padró, C.L.; Apesteguía, C.R. Selective synthesis of acetaldehyde from lactic acid on acid zeolites. Catal. Today 2018, 302, 203–209. [Google Scholar] [CrossRef]
- Balagurunathan, B.; Tan, L.; Zhao, H. Metabolic engineering of Escherichia coli for acetaldehyde overproduction using pyruvate decarboxylase from Zymomonas mobilis. Enzym. Microb. Technol. 2018, 109, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.; Španěl, P.; Smith, D. A longitudinal study of ethanol and acetaldehyde in the exhaled breath of healthy volunteers using selected-ion flow-tube mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kanjanasiranont, N.; Prueksasit, T.; Morknoy, D. Inhalation exposure and health risk levels to BTEX and carbonyl compounds of traffic policeman working in the inner city of Bangkok, Thailand. Atmos. Environ. 2017, 152, 111–120. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, L.; Li, Y.; Zhang, Y.; Cao, C.; Yang, J.; Qi, F. Acetaldehyde oxidation at low and intermediate temperatures: An experimental and kinetic modeling investigation. Combust. Flame 2018, 191, 431–441. [Google Scholar] [CrossRef]
- Smith, D.; Wang, T.; Sulé-Suso, J.; Španěl, P.; Haj, A.E. Quantification of acetaldehyde released by lung cancer cells in vitro using selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Tryba, B.; Jafari, S.; Sillanpää, M.; Nitta, A.; Ohtani, B.; Morawski, A.W. Influence of TiO2 structure on its photocatalytic activity towards acetaldehyde decomposition. Appl. Surf. Sci. 2019, 470, 376–385. [Google Scholar] [CrossRef]
- Itoh, T.; Matsubara, I.; Shin, W.; Izu, N.; Nishibori, M. Preparation of layered organic–inorganic nanohybrid thin films of molybdenum trioxide with polyaniline derivatives for aldehyde gases sensors of several tens ppb level. Sens. Actuators B Chem. 2008, 128, 512–520. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, M.; Dong, S. A self-powered acetaldehyde sensor based on biofuel cell. Anal. Chem. 2012, 84, 10345–10349. [Google Scholar] [CrossRef] [PubMed]
- Verissimo, M.I.; Gamelas, J.A.; Simoes, M.M.; Evtuguin, D.V.; Gomes, M.T.S. Quantifying acetaldehyde in cider using a Mn (III)-substituted polyoxotungstate coated acoustic wave sensor. Sens. Actuators B Chem. 2018, 255, 2608–2613. [Google Scholar] [CrossRef]
- Mani, G.K.; Rayappan, J.B.B. ZnO nanoarchitectures: Ultrahigh sensitive room temperature acetaldehyde sensor. Sens. Actuators B.Chem. 2016, 223, 343–351. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef]
- Gao, F.; Boussaid, F.; Xuan, W.; Tsui, C.Y.; Bermak, A. Dual transduction surface acoustic wave gas sensor for VOC discrimination. IEEE Electron. Device Lett. 2018, 39, 1920–1923. [Google Scholar] [CrossRef]
- Zhao, Y.; Zaghloul, M.; Lilach, Y.; Benkstein, K.; Semancik, S. Metal Organic Framework-Coated Optical VOC Gas Sensor. In Proceedings of the 2018 IEEE Photonics Conference (IPC), Reston, VA, USA, 30 September–4 October 2018. [Google Scholar]
- Rizzo, G.; Arena, A.; Bonavita, A.; Donato, N.; Neri, G.; Saitta, G. Gasochromic response of nanocrystalline vanadium pentoxide films deposited from ethanol dispersions. Thin Solid Films 2010, 518, 7124–7127. [Google Scholar] [CrossRef]
- Rajanna, K. Development of thermoelectric gas sensors for volatile organic compounds. In Proceedings of the SENSORS, 2006 IEEE, Daegu, Korea, 22–25 October 2006; pp. 716–718. [Google Scholar]
- Mori, M.; Nishimura, H.; Itagaki, Y.; Sadaoka, Y. Potentiometric VOC detection in air using 8YSZ-based oxygen sensor modified with SmFeO3 catalytic layer. Sens. Actuators B Chem. 2009, 142, 141–146. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. SnO2 (n)-NiO (p) composite nanowebs: Gas sensing properties and sensing mechanisms. Sens. Actuators B Chem. 2018, 258, 204–214. [Google Scholar] [CrossRef]
- Mirzaei, A.; Park, S.; Sun, G.J.; Kheel, H.; Lee, C.; Lee, S. Fe2O3/Co3O4 composite nanoparticle ethanol sensor. J. Korean Phys. Soc. 2016, 69, 373–380. [Google Scholar] [CrossRef]
- Mirzaei, A.; Park, S.; Kheel, H.; Sun, G.J.; Ko, T.; Lee, S.; Lee, C. Acetone Sensors Based on In2O3-Co3O4 Composite Nanoparticles. J. Nanosci. Nanotechnol. 2017, 17, 4087–4090. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Mirzaei, A.; Neri, G. Microwave-assisted synthesis of metal oxide nanostructures for gas sensing application: A review. Sen. Actuators B Chem. 2016, 237, 749–775. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Neri, G. Metal-core@ metal oxide-shell nanomaterials for gas-sensing applications: A review. J. Nanoparticle Res. 2015, 17, 371. [Google Scholar] [CrossRef]
- Neri, G. First fifty years of chemoresistive gas sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.H.; Kim, H.W.; Kim, S.S. Resistive-based gas sensors for detection of benzene, toluene and xylene (BTX) gases: A review. J. Mater. Chem. C 2018, 6, 4342–4370. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, S.S.; Kim, H.W. Resistance-based H2S gas sensors using metal oxide nanostructures: A review of recent advances. J. Hazard. Mater. 2018, 357, 314–331. [Google Scholar] [CrossRef]
- Leonardi, S. Two-dimensional zinc oxide nanostructures for gas sensor applications. Chemosensors 2017, 5, 17. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.H.; Kim, H.W.; Kim, S.S. How shell thickness can affect the gas sensing properties of nanostructured materials: Survey of literature. Sens. Actuators B Chem. 2018, 258, 270–294. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. A novel gas sensor based on Ag/Fe2O3 core-shell nanocomposites. Ceram. Int. 2016, 42, 18974–18982. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.H.; Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Realization of H2S sensing by Pd-functionalized networked CuO nanowires in self-heating mode. Sens. Actuators B Chem. 2019, 299, 126965. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Kim, J.H.; Zheng, Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Synthesis and selective sensing properties of rGO/metal-coloaded SnO2 nanofibers. J. Electron. Mater. 2017, 46, 3531–3541. [Google Scholar] [CrossRef]
- Choi, M.S.; Bang, J.H.; Mirzaei, A.; Oum, W.; Na, H.G.; Jin, C.; Kim, S.S.; Kim, H.W. Promotional effects of ZnO-branching and Au-functionalization on the surface of SnO2 nanowires for NO2 sensing. J. Alloy. Comp. 2019, 786, 27–39. [Google Scholar] [CrossRef]
- Yang, P.; Lau, C.; Liang, J.Y.; Lu, J.Z.; Liu, X. Zeolite-based cataluminescence sensor for the selective detection of acetaldehyde. Lum. J. Biol. Chem. Lum. 2007, 22, 473–479. [Google Scholar] [CrossRef]
- Abideen, Z.U.; Kim, J.H.; Lee, J.H.; Kim, J.Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Electrospun metal oxide composite nanofibers gas sensors: A review. J. Korean Ceram. Soc. 2017, 54, 366–379. [Google Scholar] [CrossRef]
- Wang, Z.L. Nanostructures of zinc oxide. Mater. Today 2004, 7, 26–33. [Google Scholar] [CrossRef]
- Ahmad, R.; Majhi, S.M.; Zhang, X.; Swager, T.M.; Salama, K.N. Recent progress and perspectives of gas sensors based on vertically oriented ZnO nanomaterials. Adv. Colloid Interface Sci. 2019, 270, 1–27. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Low power-consumption CO gas sensors based on Au-functionalized SnO2-ZnO core-shell nanowires. Sens. Actuators B Chem. 2018, 267, 597–607. [Google Scholar] [CrossRef]
- Gao, R.; Cheng, X.; Gao, S.; Zhang, X.; Xu, Y.; Zhao, H.; Huo, L. Highly selective detection of saturated vapors of abused drugs by ZnO nanorod bundles gas sensor. Appl. Surf. Sci. 2019, 485, 266–273. [Google Scholar] [CrossRef]
- Yoo, R.; Li, D.; Rim, H.J.; Cho, S.; Lee, H.S.; Lee, W. High sensitivity in Al-doped ZnO nanoparticles for detection of acetaldehyde. Sens. Actuators B Chem. 2018, 266, 883–888. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, W.; Hu, W.; Zhou, Q.; He, K.; Xu, K.; Yang, Y.; Yu, T.; Yuan, C. Synthesis and gas-sensing properties of ZnO@ NiCo2O4 core@ shell nanofibers. Mater. Res. Bull. 2019, 114, 1–9. [Google Scholar] [CrossRef]
- Suematsu, K.; Watanabe, K.; Tou, A.; Sun, Y.; Shimanoe, K. Ultraselective toluene-gas sensor: Nanosized gold loaded on zinc oxide nanoparticles. Anal. Chem. 2018, 90, 1959–1966. [Google Scholar] [CrossRef]
- Rai, P.; Yu, Y.T. Citrate-assisted hydrothermal synthesis of single crystalline ZnO nanoparticles for gas sensor application. Sens. Actuators B Chem. 2012, 173, 58–65. [Google Scholar] [CrossRef]
- Srinivasan, P.; Rayappan, J.B.B. Investigations on room temperature dual sensitization of ZnO nanostructures towards fish quality biomarkers. Sens. Actuators B Chem 2019. In press. [Google Scholar] [CrossRef]
- Fu, X.; Liu, J.; Han, T.; Zhang, X.; Meng, F.; Liu, J. A three-dimensional hierarchical CdO nanostructure: Preparation and its improved gas-diffusing performance in gas sensor. Sens. Actuators B Chem. 2013, 184, 260–267. [Google Scholar] [CrossRef]
- Rai, P.; Song, H.M.; Kim, Y.S.; Song, M.K.; Oh, P.R.; Yoon, J.M.; Yu, Y.T. Microwave assisted hydrothermal synthesis of single crystalline ZnO nanorods for gas sensor application. Mater. Lett. 2012, 68, 90–93. [Google Scholar] [CrossRef]
- Rai, P.; Raj, S.; Ko, K.J.; Park, K.K.; Yu, Y.T. Synthesis of flower-like ZnO microstructures for gas sensor applications. Sens. Actuators B Chem. 2013, 178, 107–112. [Google Scholar] [CrossRef]
- Patil, V.B.; Adhyapak, P.V.; Patil, P.S.; Suryavanshi, S.S.; Mulla, I.S. Hydrothermally synthesized tungsten trioxide nanorods as NO2 gas sensors. Ceram. Int. 2015, 41, 3845–3852. [Google Scholar] [CrossRef]
- Zhang, S.L.; Lim, J.O.; Huh, J.S.; Noh, J.S.; Lee, W. Two-step fabrication of ZnO nanosheets for high-performance VOCs gas sensor. Curr. Appl. Phys. 2013, 13, S156–S161. [Google Scholar] [CrossRef]
- Mani, G.K.; Rayappan, J.B.B. Novel and facile synthesis of randomly interconnected ZnO nanoplatelets using spray pyrolysis and their room temperature sensing characteristics. Sens. Actuators B Chem. 2014, 198, 125–133. [Google Scholar] [CrossRef]
- Subbiah, D.K.; Mani, G.K.; Babu, K.J.; Das, A.; Rayappan, J.B.B. Nanostructured ZnO on cotton fabrics–A novel flexible gas sensor & UV filter. J. Clean. Prod. 2018, 194, 372–382. [Google Scholar]
- Patil, P.; Nakate, U.T.; Nakate, Y.T.; Ambare, R.C. Acetaldehyde sensing properties using ultrafine CuO nanoparticles. Mater. Sci. Semicond. Proc. 2019, 101, 76–81. [Google Scholar] [CrossRef]
- Chava, R.K.; Cho, H.Y.; Yoon, J.M.; Yu, Y.T. Fabrication of aggregated In2O3 nanospheres for highly sensitive acetaldehyde gas sensors. J. Alloy. Comp. 2019, 772, 834–842. [Google Scholar] [CrossRef]
- Srinath, A.K.; Sankaranarayanan, L.; Pandeeswari, R.; Jeyaprakash, B.G. Thin films of α-Mn2O3 for resistance-based sensing of acetaldehyde vapor at ambient temperature. Microchim. Acta 2015, 182, 1619–1626. [Google Scholar] [CrossRef]
- Chu, X.; Hu, T.; Gao, F.; Dong, Y.; Sun, W.; Bai, L. Gas sensing properties of graphene-WO3 composites prepared by hydrothermal method. Mater. Sci. Eng. B 2015, 193, 97–104. [Google Scholar] [CrossRef]
- Fu, Q.; Ai, M.; Duan, Y.; Lu, L.; Tian, X.; Sun, D.; Sun, Y. Synthesis of uniform porous NiO nanotetrahedra and their excellent gas-sensing performance toward formaldehyde. RSC Adv. 2017, 7, 52312–52320. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, C.; De, Q.; Gu, F.; Han, D. One-step synthesis of Co-doped In2O3 nanorods for high response of formaldehyde sensor at low temperature. ACS Sens. 2018, 3, 468–475. [Google Scholar] [CrossRef]
- Saidi, T.; Palmowski, D.; Babicz-Kiewlicz, S.; Welearegay, T.G.; El Bari, N.; Ionescu, R.; Smulko, J.; Bouchikhi, B. Exhaled breath gas sensing using pristine and functionalized WO3 nanowire sensors enhanced by UV-light irradiation. Sens. Actuators B Chem. 2018, 273, 1719–1729. [Google Scholar] [CrossRef]
- Espid, E.; Taghipour, F. UV-LED photo-activated chemical gas sensors: A review. Crit. Rev. Solid State Mater. Sci. 2017, 42, 416–432. [Google Scholar] [CrossRef]
- De Lacy Costello, B.P.J.; Ewen, R.J.; Ratcliffe, N.M.; Richards, M. The characteristics of novel low-cost sensors for volatile biomarker detection. J. Breath Res. 2008, 2, 037017. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Han, J.; Zhang, Y.; Sun, Y.A.; Xie, B. Studies on alcohol sensing mechanism of ZnO based gas sensors. Sens. Actuators B Chem. 2008, 132, 334–339. [Google Scholar] [CrossRef]
- Shimizu, Y.; Yamaguchi, K.; Fukunaga, K.; Takao, Y.; Hyodo, T.; Egashira, M. Acetaldehyde gas-sensing properties and surface chemistry of SnO2-based sensor materials. J. Electrochem. Soc. 1999, 146, 1222–1226. [Google Scholar] [CrossRef]
- Majhi, S.M.; Lee, H.J.; Choi, H.N.; Cho, H.Y.; Kim, J.S.; Lee, C.R.; Yu, Y.T. Construction of novel hybrid PdO-ZnO p-n heterojunction nanostructures as a high-response sensor for acetaldehyde gas. CrystEngComm 2019, 21, 5084–5094. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2009, 110, 132–145. [Google Scholar] [CrossRef]
- Deng, S.; Liu, X.; Chen, N.; Deng, D.; Xiao, X.; Wang, Y. A highly sensitive VOC gas sensor using p-type mesoporous Co3O4 nanosheets prepared by a facile chemical coprecipitation method. Sens. Actuators B Chem. 2016, 233, 615–623. [Google Scholar] [CrossRef]
- Cho, S.Y.; Yoo, H.W.; Kim, J.Y.; Jung, W.B.; Jin, M.L.; Kim, J.S.; Jung, H.T. High-resolution p-type metal oxide semiconductor nanowire array as an ultrasensitive sensor for volatile organic compounds. Nano Lett. 2016, 16, 4508–4515. [Google Scholar] [CrossRef]
- Wang, L.; Deng, J.; Lou, Z.; Zhang, T. Nanoparticles-assembled Co3O4 nanorods p-type nanomaterials: One-pot synthesis and toluene-sensing properties. Sens. Actuators B Chem. 2014, 201, 1–6. [Google Scholar] [CrossRef]
- Gunasekaran, E.; Ezhilan, M.; Mani, G.K.; Shankar, P.; Kulandaisamy, A.J.; Rayappan, J.B.B.; Babu, K.J. Fluorine doped ZnO thin film as acetaldehyde sensor. Semicond. Sci. Technol. 2018, 33, 095005. [Google Scholar] [CrossRef]
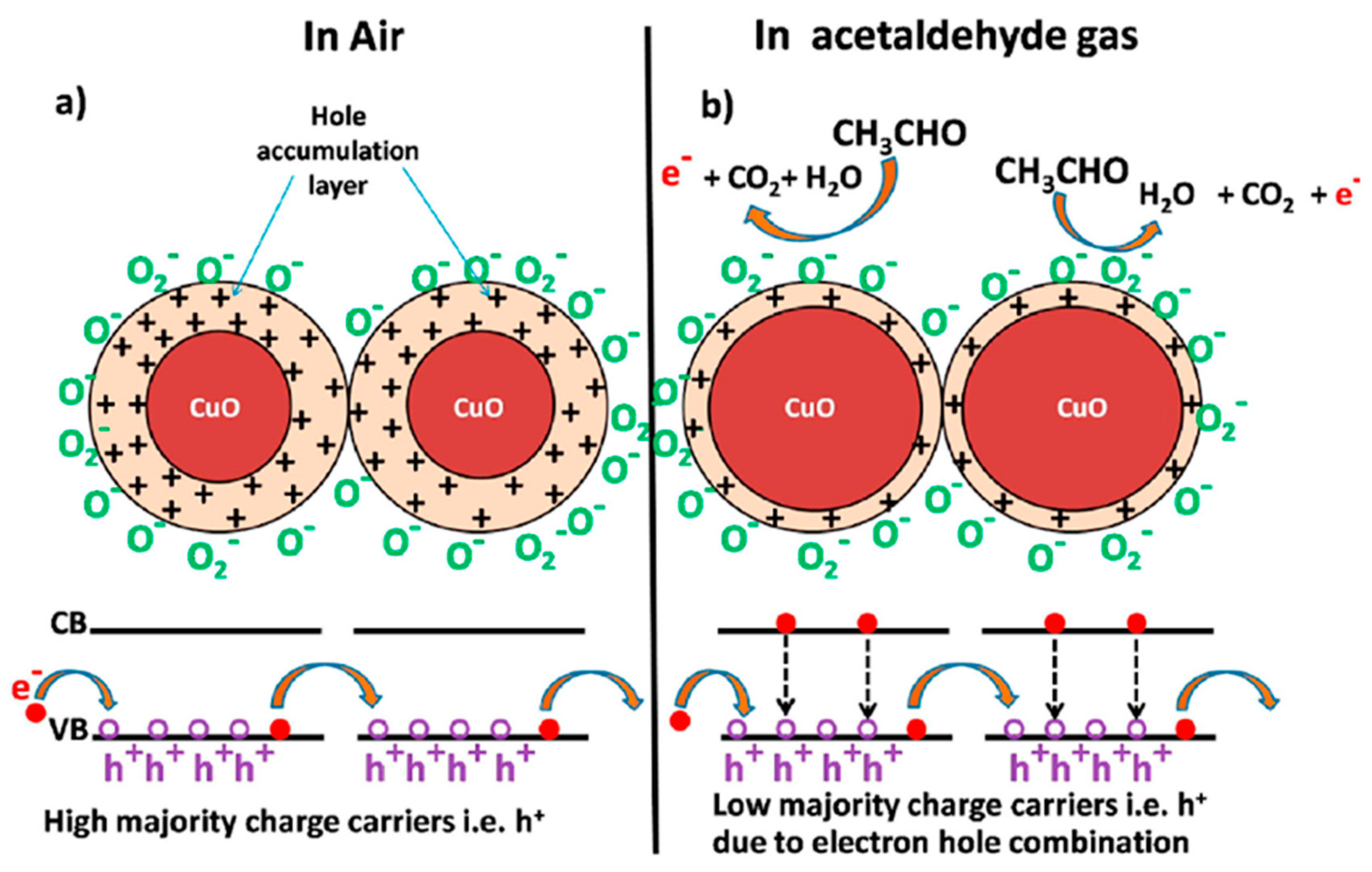
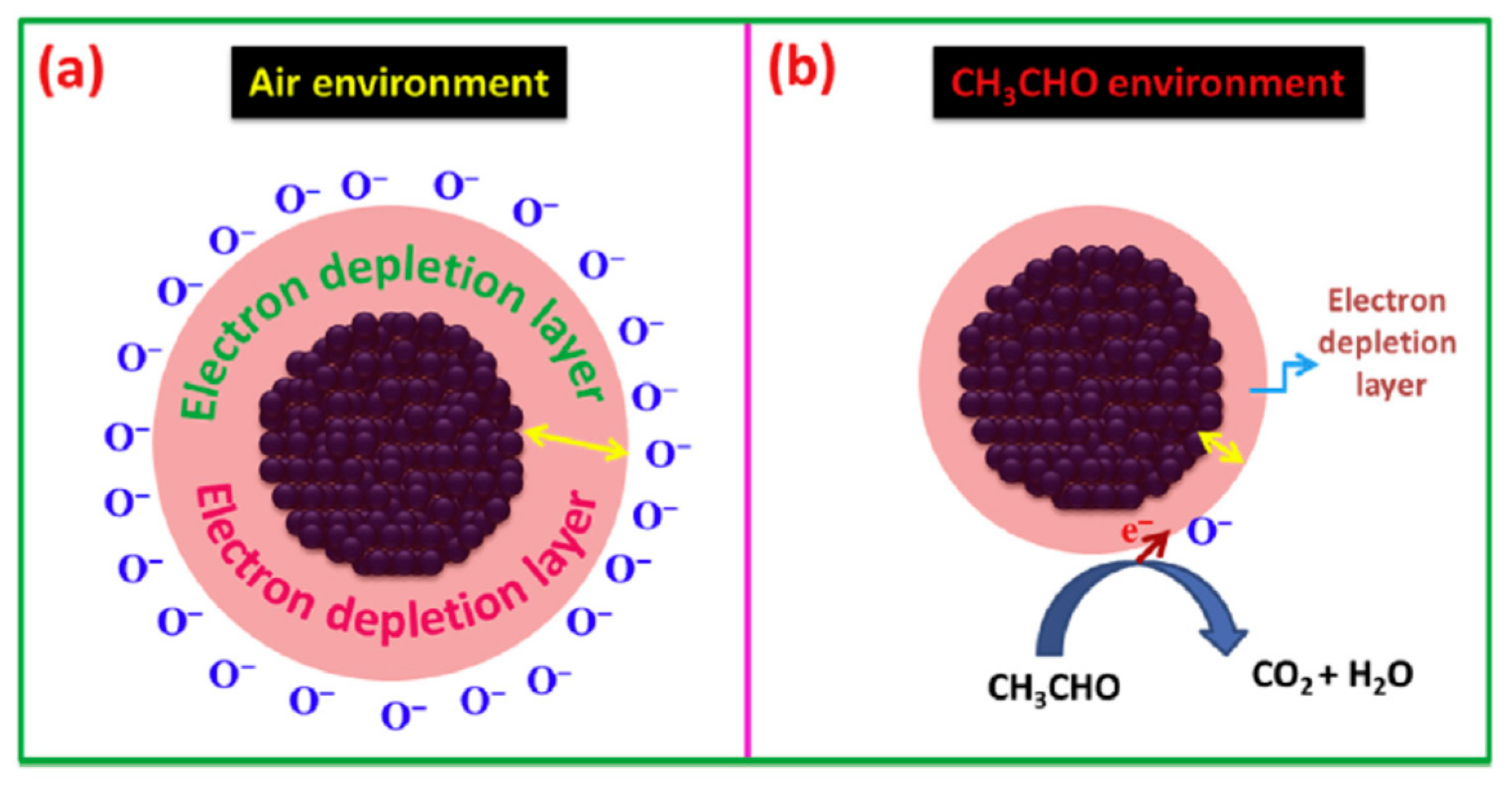
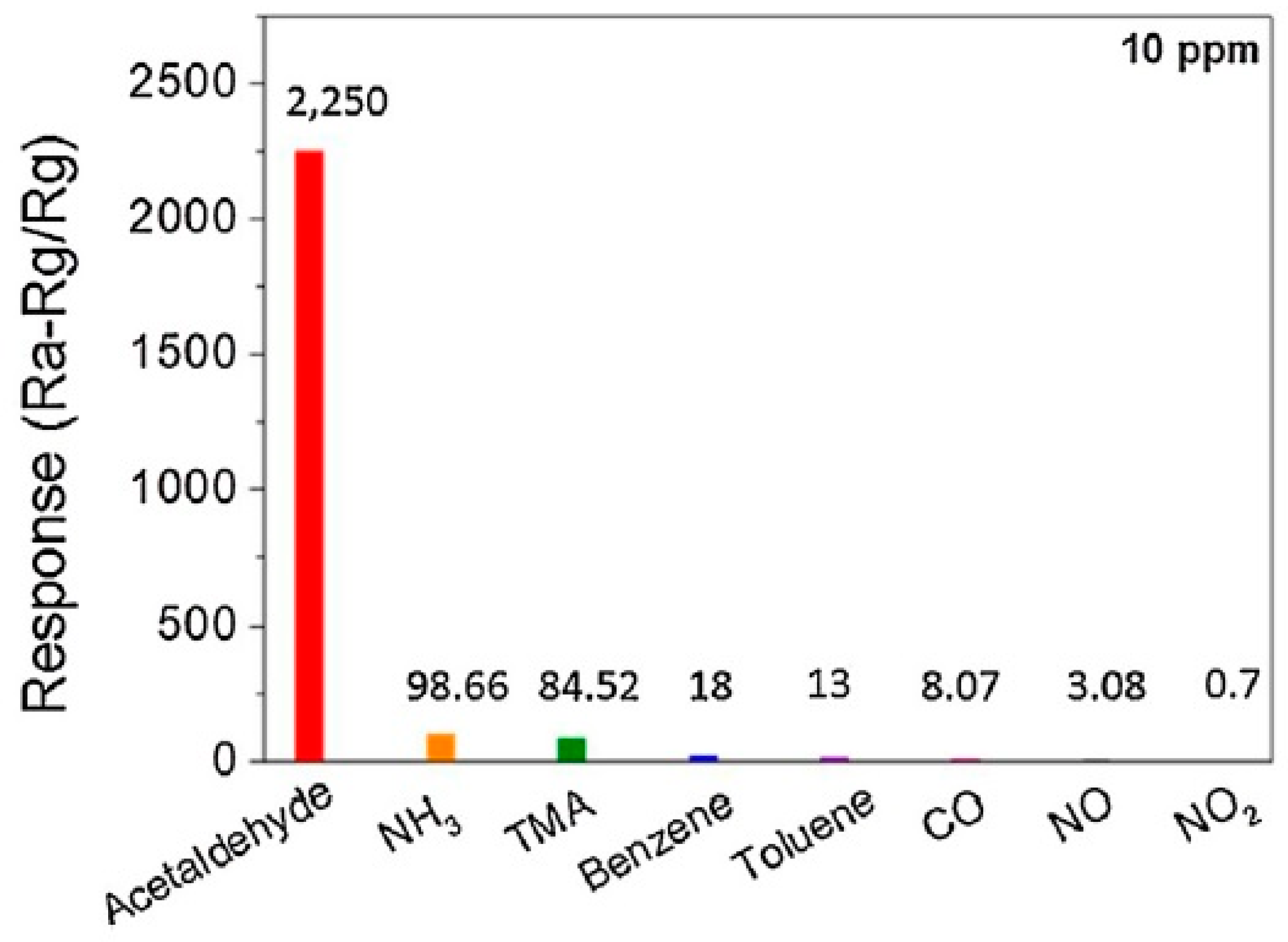
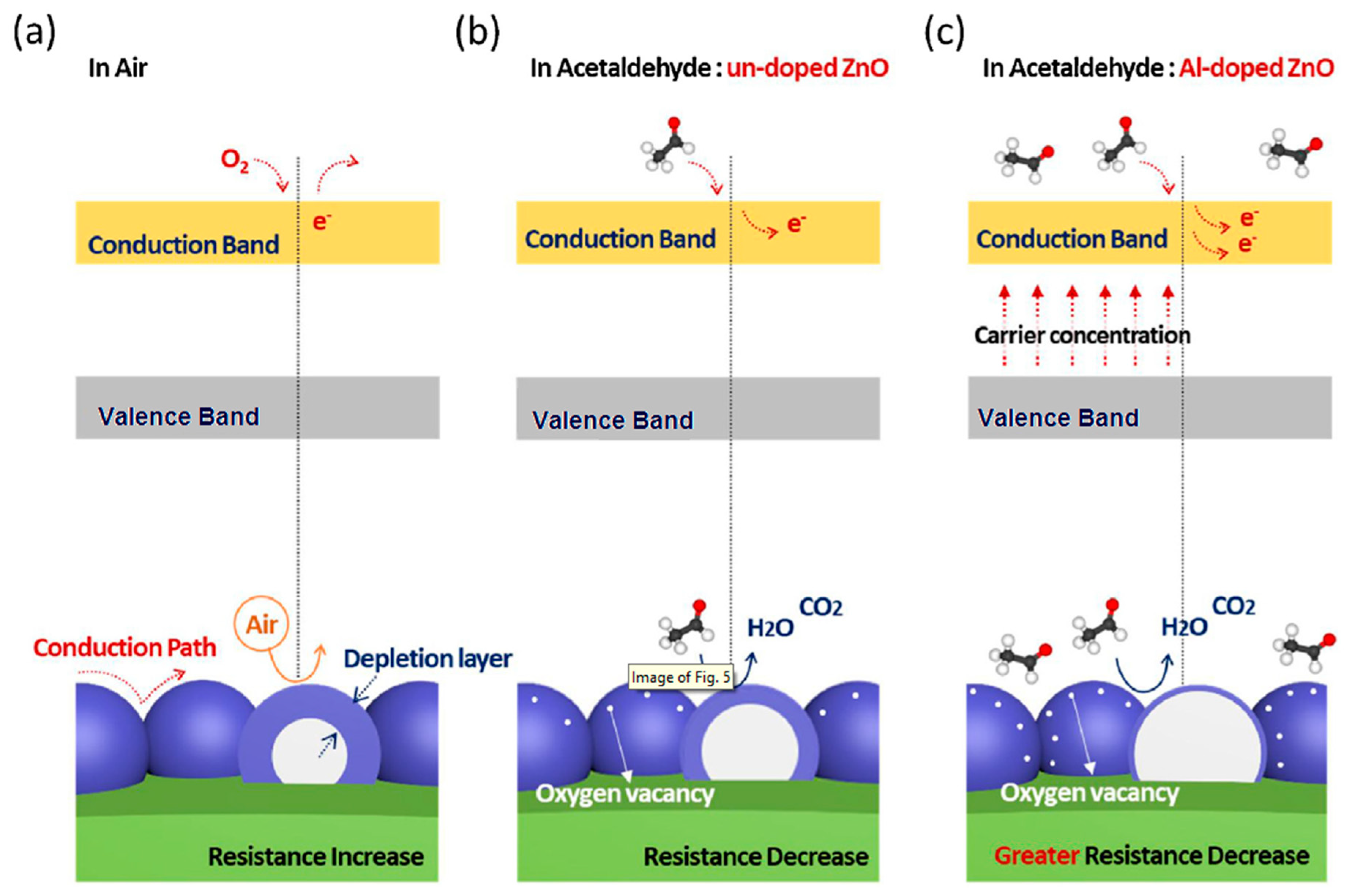
| Morphology | Concentration (ppm) | Operating Temperature (°C) | Response (Ra/Rg) | Ref. |
|---|---|---|---|---|
| Al-doped ZnO | 10 | 500 | 2250 | [55] |
| ZnO powders | 2 | 450 | 5.73 | [14] |
| ZnO-NiCo2O4 nanofibers | 100 | 250 | ~3 | [56] |
| 0.15 mol% Au-ZnO NPs | 100 | 377 | ~7 | [57] |
| ZnO particles | 250 | 400 | ∼45 | [58] |
| ZnO tetrapods | 50 | 47.5 | [5] | |
| ZnO nanoaggregates | 200 | 25 | ~8 | [59] |
| ZnO sheets | 1 | 220 | 77 | [60] |
| ZnO rods | 250 | 400 | 5.30 | [61] |
| ZnO flowers | 8 | [62] | ||
| ZnO petals | 100 | 25 | 14 | |
| ZnO branched nanorods | 10 | 2.85 | [26] | |
| Co-doped ZnO branched nanorods | 10 | 800 |
| Sensing Material | Gas Conc. (ppm) | T (°C) | Response (Ra/Rg) | Ref. |
|---|---|---|---|---|
| 1 wt % Ru/10 wt % WO3/SnO2 | 100 | 200 | 11 | [65] |
| CuO NPs | 100 | 200 | 23.29 (ΔR/Ra) | [67] |
| 0.1 wt % graphene-WO3 | 1000 | 100 | 5 | [70] |
| In2O3 nanospheres | 100 | 300 | 11.06 | [68] |
| NiO nanotetrahedra | 50 | 250 | 4 | [71] |
| Au@SnO2 core-shell NPs | 500 | 300 | 0.85 | [72] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirzaei, A.; Kim, H.W.; Kim, S.S.; Neri, G. Nanostructured Semiconducting Metal Oxide Gas Sensors for Acetaldehyde Detection. Chemosensors 2019, 7, 56. https://doi.org/10.3390/chemosensors7040056
Mirzaei A, Kim HW, Kim SS, Neri G. Nanostructured Semiconducting Metal Oxide Gas Sensors for Acetaldehyde Detection. Chemosensors. 2019; 7(4):56. https://doi.org/10.3390/chemosensors7040056
Chicago/Turabian StyleMirzaei, Ali, Hyoun Woo Kim, Sang Sub Kim, and Giovanni Neri. 2019. "Nanostructured Semiconducting Metal Oxide Gas Sensors for Acetaldehyde Detection" Chemosensors 7, no. 4: 56. https://doi.org/10.3390/chemosensors7040056
APA StyleMirzaei, A., Kim, H. W., Kim, S. S., & Neri, G. (2019). Nanostructured Semiconducting Metal Oxide Gas Sensors for Acetaldehyde Detection. Chemosensors, 7(4), 56. https://doi.org/10.3390/chemosensors7040056








