A Coumarin-Benzothiazole Derivative as a FRET-Based Chemosensor of Adenosine 5′-Triphosphate
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis
2.3. Job Plot Analysis
2.4. Computational Studies
3. Results and Discussion
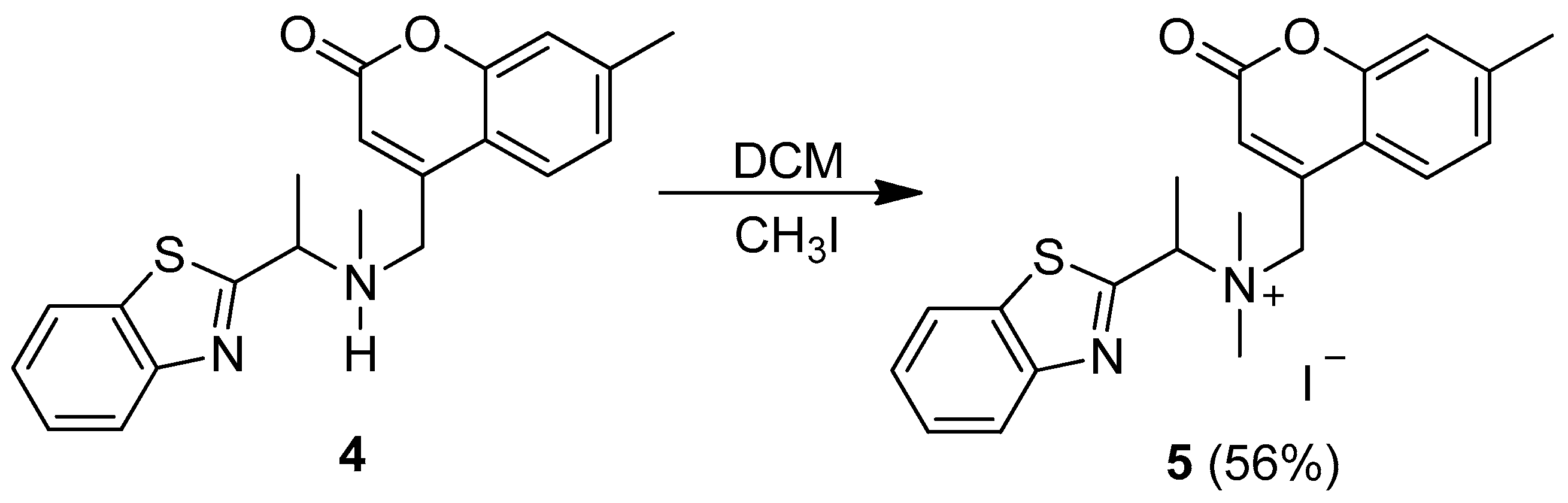
3.1. Synthesis
3.2. Design of Probe 5
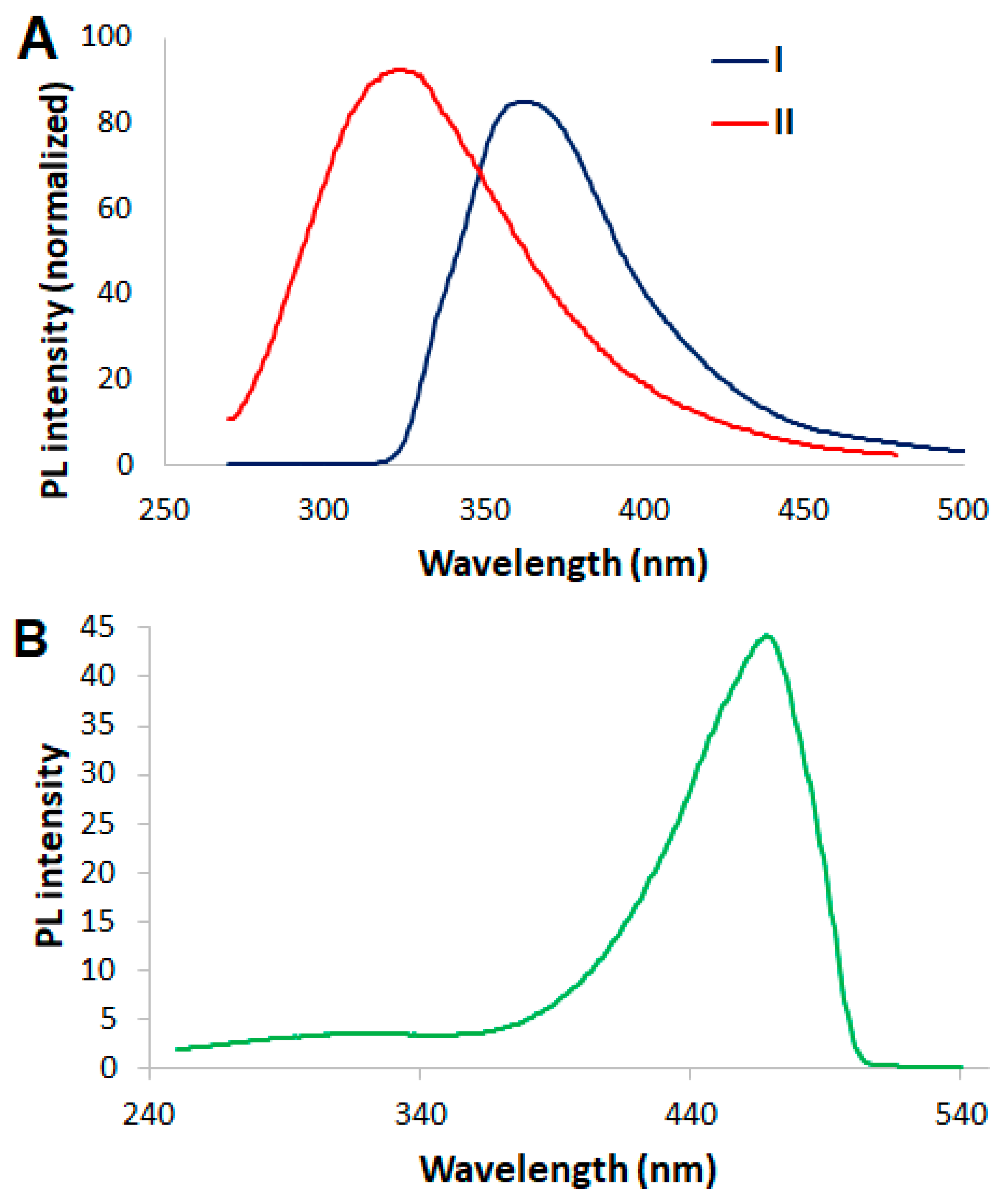
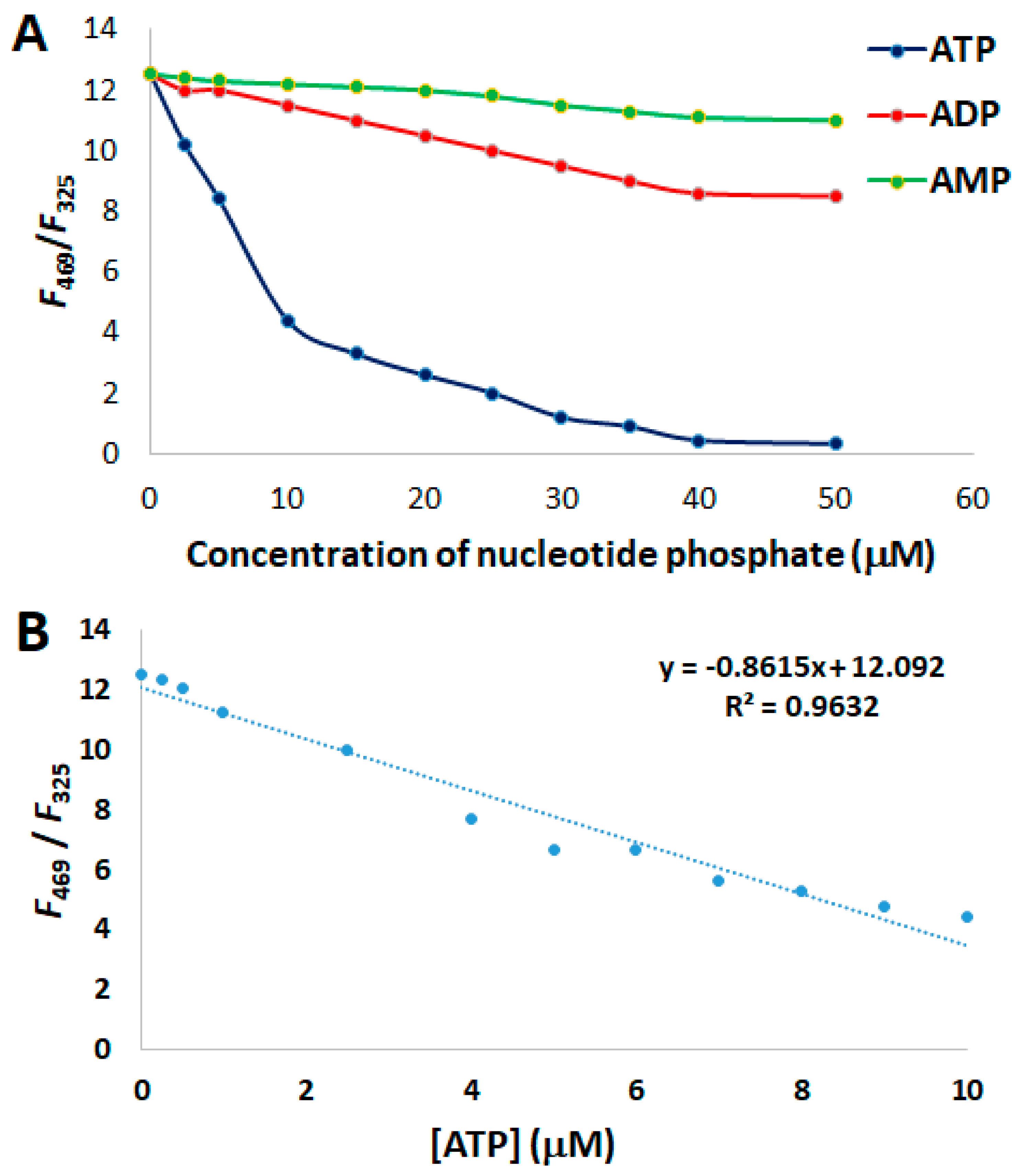
3.3. FRET Sensing of ATP by Probe 5
3.4. Aggregation of 5
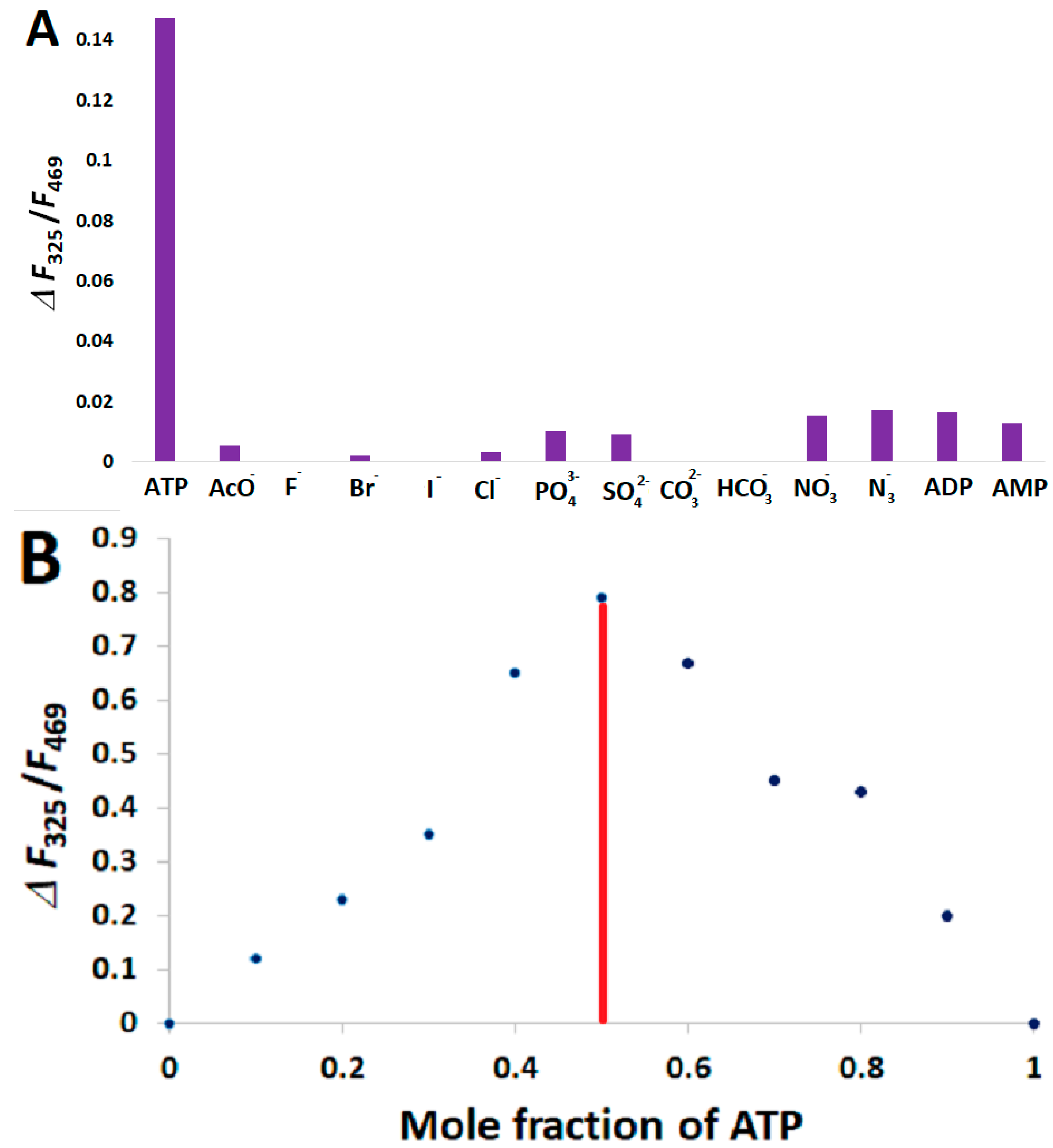
3.5. Analysis of Selectivity and Stoichiometry of Binding
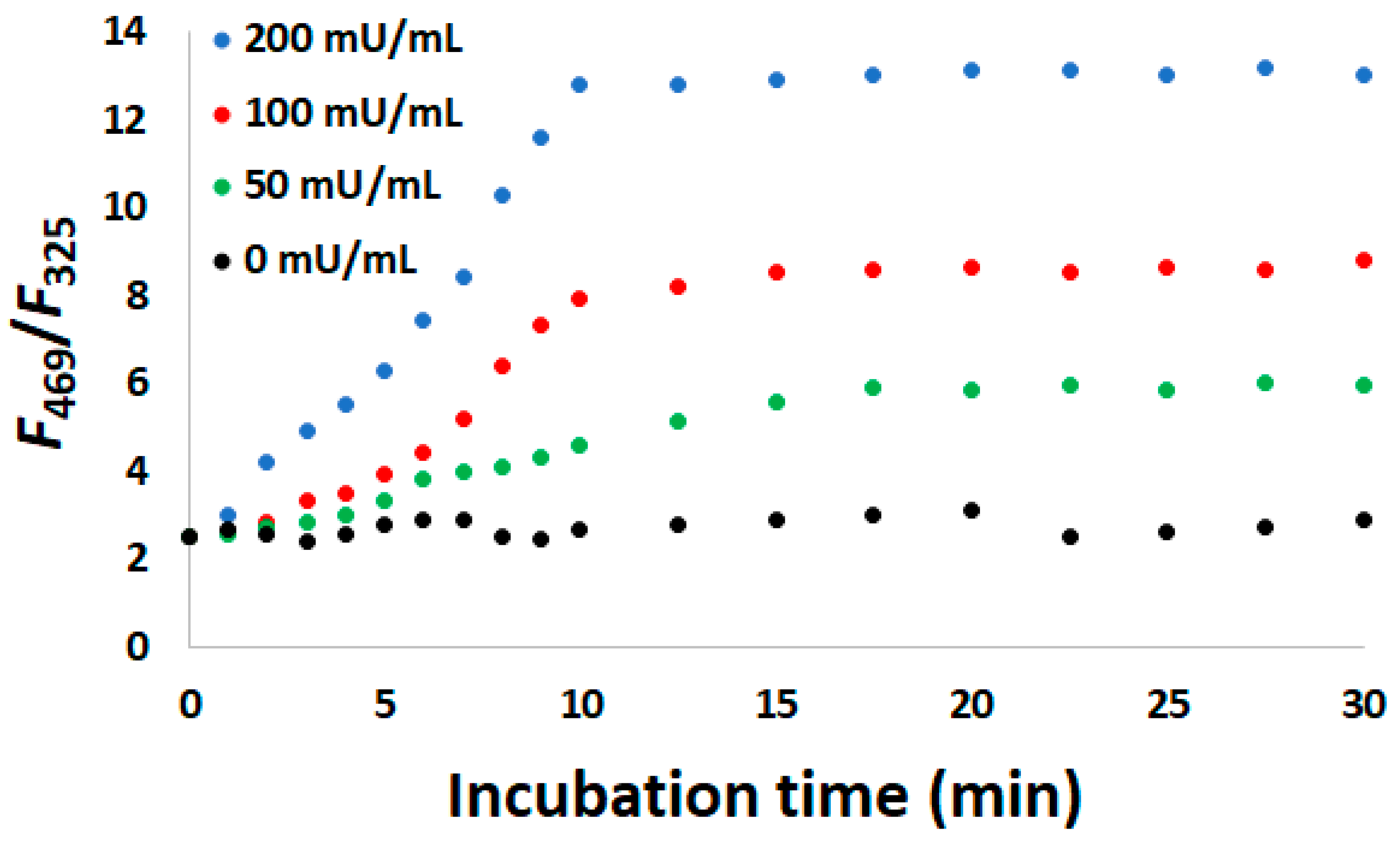
3.6. Monitoring ATP Hydrolysis Reaction
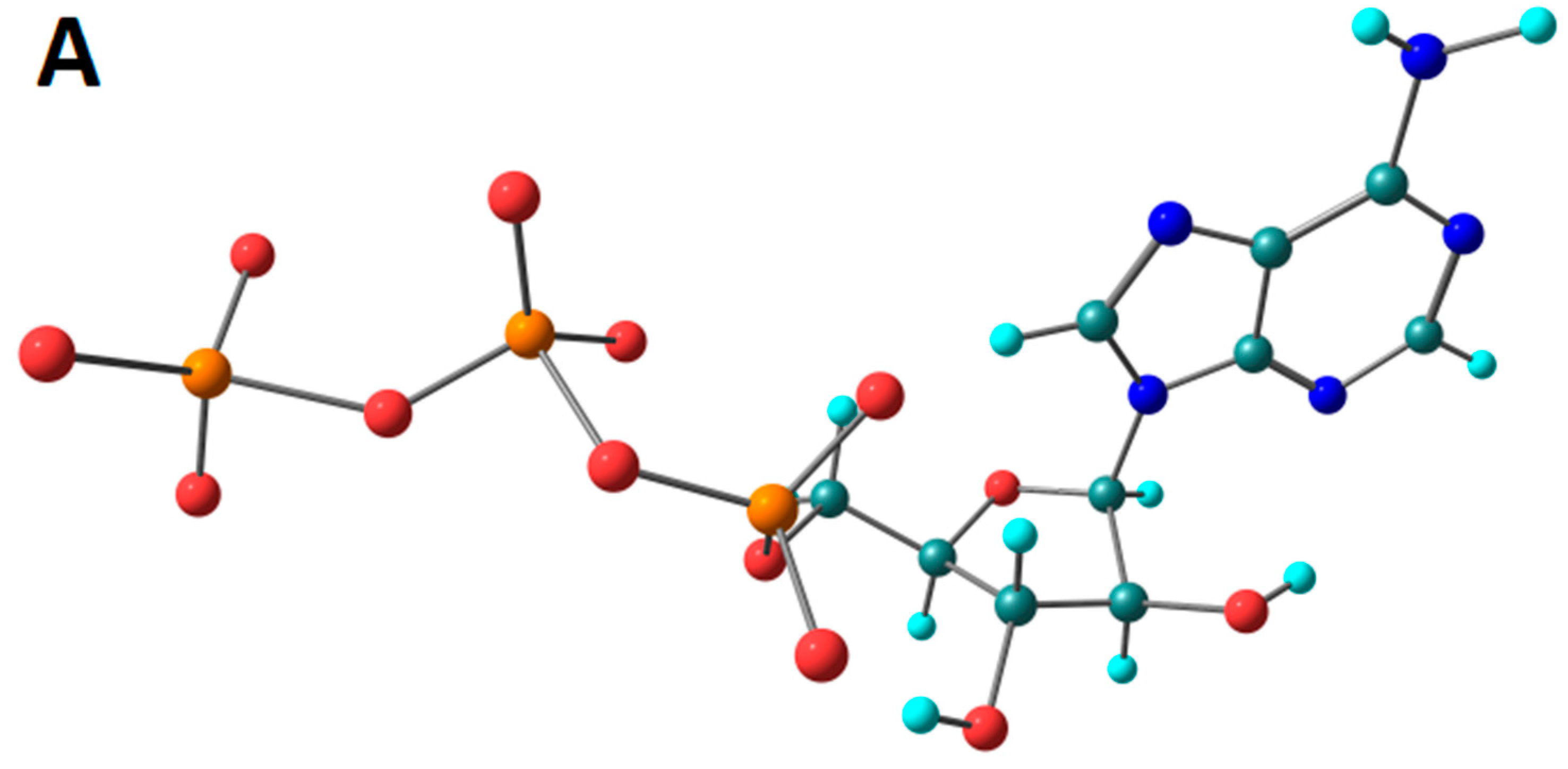
3.7. Computational Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, P.C. The contrast formation in optical microscopy. In Handbook of Biological Confocal Microscopy, 3rd ed.; Springer: Boston, MA, USA, 2006; pp. 162–206. [Google Scholar] [CrossRef]
- Jares-Erijman, E.A.; Jovin, T.M. FRET imaging. Nat. Biotechnol. 2003, 21, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, D.; Jenei, A.; Nagy, P.; Vereb, G.; Szollosi, J. Understanding FRET as a research tool for cellular studies. Int. J. Mol. Sci. 2015, 16, 6718–6756. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Tan, J.; Feng, G.; Yuan, Z.; Wu, C.; Zhang, X. Nanoscale metal-organic frameworks coated with poly(vinyl alcohol) for ratiometric peroxynitrite sensing through FRET. Chem. Sci. 2017, 8, 5101–5106. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ding, Z.; Zhang, G.; Tian, L.; Zhang, X. Construction of thermo-responsive elastin-like polypeptides (ELPs)-Aggregation-Induced-Emission (AIE) conjugates for temperature sensing. Molecules 2018, 23, 1725. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Wang, C.; Feng, G.; Zhang, X. Thermo-responsive fluorescent polymers with diverse LCSTs for ratiometric temperature sensing through FRET. Polymers 2018, 10, 283. [Google Scholar] [CrossRef]
- Kikuchi, K.; Takakusa, H.; Nagano, T. Recent advances in the design of small molecule-based FRET sensors for cell biology. Trends Anal. Chem. 2004, 23, 407–415. [Google Scholar] [CrossRef]
- Lundin, A.; Thore, A. Comparison of methods for extraction of bacterial adenine nucleotides determined by firefly assay. Appl. Microbiol. 1975, 30, 713–721. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC187260/ (accessed on 6 July 2019).
- Traut, T.W. Physiological concentrations of purines and pyrimidines. Mol. Cell Biochem. 1994, 140, 1–22. [Google Scholar] [CrossRef]
- Tsuyama, T.; Kishikawa, J.; Han, Y.-W.; Harada, Y.; Tsubouchi, A.; Noji, H.; Kakizuka, A.; Yokoyma, K.; Uemura, T.; Imamura, H. In vivo fluorescent adenosine 5′-triphosphate (ATP) imaging of Drosophila melanogaster and Caenorhabditis elegans by using a genetically encoded fluorescent ATP biosensor optimized for low temperatures. Anal. Chem. 2013, 85, 7889–7896. [Google Scholar] [CrossRef]
- Conley, J.M.; Radhakrishnan, S.; Valentino, S.A.; Tantama, M. Imaging extracellular ATP with a genetically-encoded ratiometric fluorescent sensor. PLoS ONE 2017, 12, e0187481. [Google Scholar] [CrossRef]
- Nakano, S.; Shimizu, M.; Dinh, H.; Morii, T. Highly selective dual sensing of ATP and ADP using fluorescent ribonucleopeptide sensors. Chem. Commun. 2019, 55, 1611–1614. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wen, J.; Li, H.; Sun, S.; Xu, Y. Fluorescent probes of ATP. Chin. Chem. Lett. 2017, 28, 1916–1924. [Google Scholar] [CrossRef]
- Tan, K.Y.; Li, C.Y.; Li, Y.F.; Fei, J.; Yang, B.; Fu, Y.J.; Li, F. Real-time monitoring ATP in mitochondrion of living cells: A specific fluorescent probe for ATP by dual recognition sites. Anal. Chem. 2017, 89, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.W.; Sarkar, S.; Kim, K.H.; Ahn, K.H. Molecular probes for fluorescence imaging of ATP in cells and tissues. ChemPhotoChem 2019, 3, 214–219. [Google Scholar] [CrossRef]
- Rao, A.S.; Kim, D.; Nam, H.; Jo, H.; Kim, K.H.; Ban, C.; Ahn, K.H. A turn-on two-photon fluorescent probe for ATP and ADP. Chem. Commun. 2012, 48, 3206–3208. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Z.; Yoon, J. Fluorescent and colorimetric chemosensors for detection of nucleotides, FAD and NADH: Highlighted research during 2004–2010. Chem. Soc. Rev. 2011, 40, 2222–2235. [Google Scholar] [CrossRef]
- Xue, L.; Liu, C.; Jiang, H. A ratiometric fluorescent sensor with a large Stokes shift for imaging zinc ions in living cells. Chem. Commun. 2009, 1061–1063. [Google Scholar] [CrossRef]
- Tang, J.-L.; Li, C.-Y.; Li, Y.-F.; Zou, C.-X. A ratiometric fluorescent probe with unexpected high selectivity for ATP and its application in cell imaging. Chem. Commun. 2014, 50, 15411–15414. [Google Scholar] [CrossRef]
- Bojtar, M.; Janzso-Berend, P.Z.; Mester, D.; Hessz, D.; Kallay, M.; Kubinyi, M.; Bitter, I. An uracil-linked hydroxyflavone probe for the recognition of ATP. Beilstein J. Org. Chem. 2018, 14, 747–755. [Google Scholar] [CrossRef]
- Kurishita, Y.; Kohira, T.; Ojida, A.; Hamachi, I. Rational design of FRET-based ratiometric chemosensors for in vitro and in cell fluorescence analyses of nucleoside polyphosphates. J. Am. Chem. Soc. 2010, 132, 13290–13299. [Google Scholar] [CrossRef]
- Zeng, Z.; Torriero, A.A.J.; Bond, A.M.; Spiccia, L. Fluorescent and electrochemical sensing of polyphosphate nucleotides by ferrocene functionalised with two ZnII(TACN)(pyrene)complexes. Chem. Eur. J. 2010, 16, 9154–9163. [Google Scholar] [CrossRef] [PubMed]
- Widmer, S.; Dorrestijn, M.; Camerlo, A.; Urek, S.K.; Lobnik, A.; Housecroft, C.E.; Constable, E.C.; Scherer, L.J. Coumarin meets fluorescein: A Forster resonance energy transfer enhanced optical ammonia gas sensor. Analyst 2014, 139, 4335–4342. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wen, J.; Qin, Z.; Wang, H. A new coumarin-rhodamine FRET system as an efficient ratiometric fluorescent probe for Hg2+ in aqueous solution and living cells. Dyes Pigment. 2015, 120, 208–212. [Google Scholar] [CrossRef]
- Kim, J.H.; Sumranjit, J.; Kang, H.J.; Chung, S.J. Discovery of coumarin derivatives as fluorescence acceptors for intrinsic fluorescence resonance energy transfer of proteins. Mol. Biosyst. 2014, 10, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, C.; Chang, G.; Ren, X. Facile synthesis of new coumarin-based colorimetric and fluorescence chemosensors: Highly efficient and selective detection of Pd2+ in aqueous solutions. Sens. Actuators Chem. B 2017, 240, 212–219. [Google Scholar] [CrossRef]
- Chang, C.; Wang, F.; Wei, T.; Chen, X. Benzothiazole-based fluorescent sensor for ratiometric detection of Zn(II) ions and secondary sensing PPi and its applications for biological imaging and PPase catalysis assays. Ind. Eng. Chem. Res. 2017, 56, 8797–8805. [Google Scholar] [CrossRef]
- Erdemir, S.; Tabakci, B. Selective and sensitive fluorescein-benzothiazole based fluorescent sensor for Zn2+ ion in aqueous media. J. Fluoresc. 2017, 27, 2145–2152. [Google Scholar] [CrossRef]
- Erdemir, S.; Malkondu, S.; Alici, O. A highly selective and sensitive benzothiazole-based ‘turn-on’ fluorescent sensor for Hg2+ ion. Color. Technol. 2015, 131, 32–37. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Chen, Y.; Huang, K.; Liu, Y. A label-free and ultrasensitive fluorescent sensor for dopamine detection based on double-stranded DNA templated copper nanoparticles. Sens. Actuators B 2015, 220, 146–153. [Google Scholar] [CrossRef]
- Pilla, C.; Emanuelli, T.; Frassetto, S.S.; Battastini, A.M.; Dias, R.D.; Sarkis, J.J. ATP diphosphohydrolase activity (apyrase, EC 3.6.1.5) in human blood platelets. Platelets 1996, 7, 225–230. [Google Scholar] [CrossRef]
- Moeckel, D.; Jeong, S.S.; Sun, X.; Broekman, M.J.; Nguyen, A.; Drosopoulos, J.H.F.; Marcus, A.J.; Robson, S.C.; Chen, R.; Abendschein, D. Optimizing human apyrase to treat arterial thrombosis and limit reperfusion injury without increasing bleeding risk. Sci. Transl. Med. 2014, 6, 248ra105. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabr, M.T.; Ibrahim, M.M.H.; Tripathi, A.; Prabhakar, C. A Coumarin-Benzothiazole Derivative as a FRET-Based Chemosensor of Adenosine 5′-Triphosphate. Chemosensors 2019, 7, 34. https://doi.org/10.3390/chemosensors7030034
Gabr MT, Ibrahim MMH, Tripathi A, Prabhakar C. A Coumarin-Benzothiazole Derivative as a FRET-Based Chemosensor of Adenosine 5′-Triphosphate. Chemosensors. 2019; 7(3):34. https://doi.org/10.3390/chemosensors7030034
Chicago/Turabian StyleGabr, Moustafa T., Mostafa M. H. Ibrahim, Anuj Tripathi, and Chetti Prabhakar. 2019. "A Coumarin-Benzothiazole Derivative as a FRET-Based Chemosensor of Adenosine 5′-Triphosphate" Chemosensors 7, no. 3: 34. https://doi.org/10.3390/chemosensors7030034
APA StyleGabr, M. T., Ibrahim, M. M. H., Tripathi, A., & Prabhakar, C. (2019). A Coumarin-Benzothiazole Derivative as a FRET-Based Chemosensor of Adenosine 5′-Triphosphate. Chemosensors, 7(3), 34. https://doi.org/10.3390/chemosensors7030034





