Application of Photocured Polymer Ion Selective Membranes for Solid-State Chemical Sensors
Abstract
:1. Introduction
2. Possible Problems of Photocurable Polymers as Membrane Matrices
2.1. Use of Porphyrins and Plasticizers with Nitro(–NO2) Functional Groups
2.2. Photobleaching of Membrane Components

2.3. Copolymerisable Plasticizers
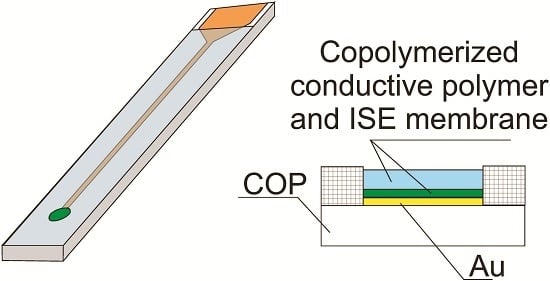
3. Solid Contact Electrodes Based on Copolymerized Conducting Polymer and Photocurable Membranes
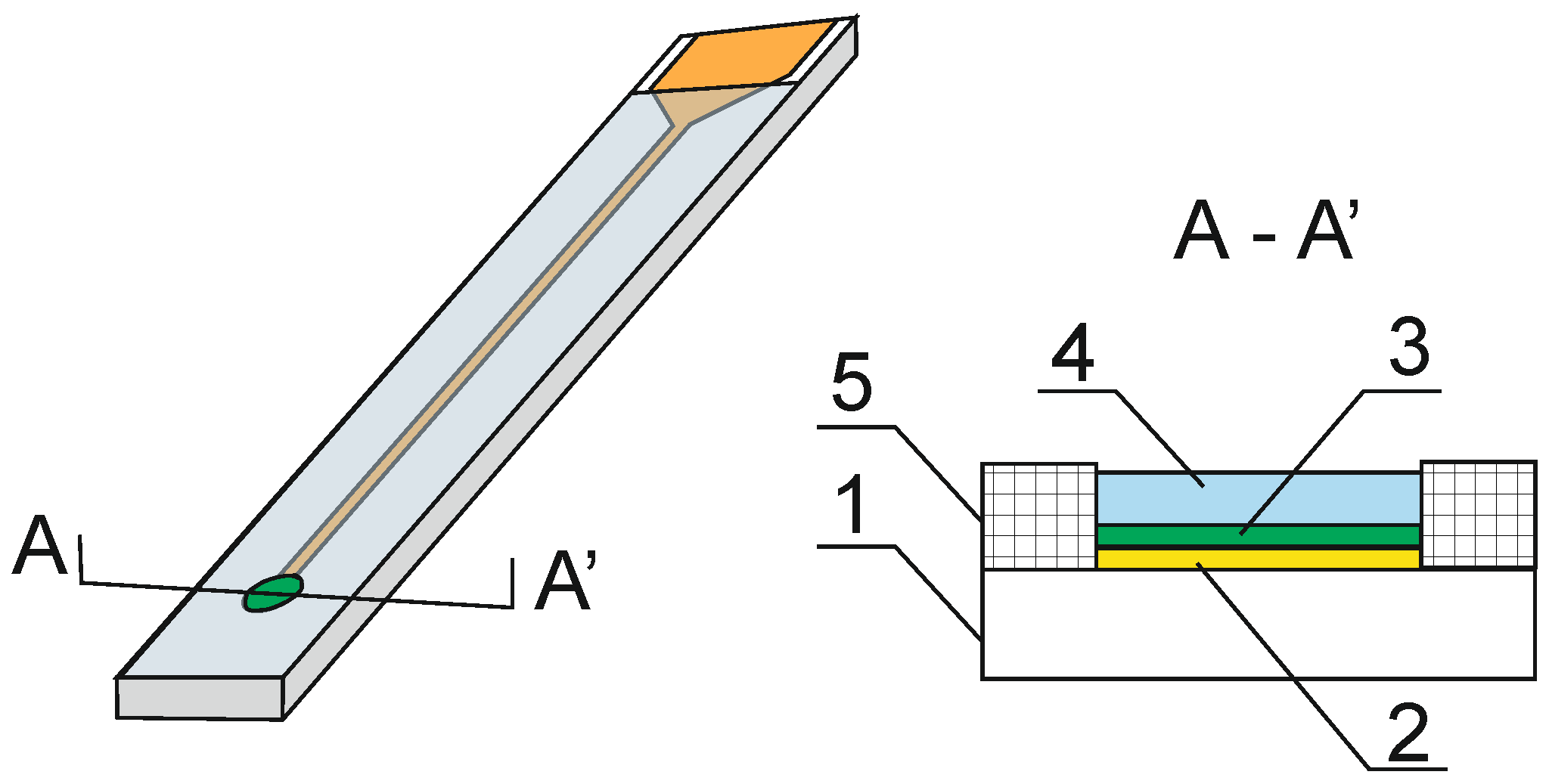
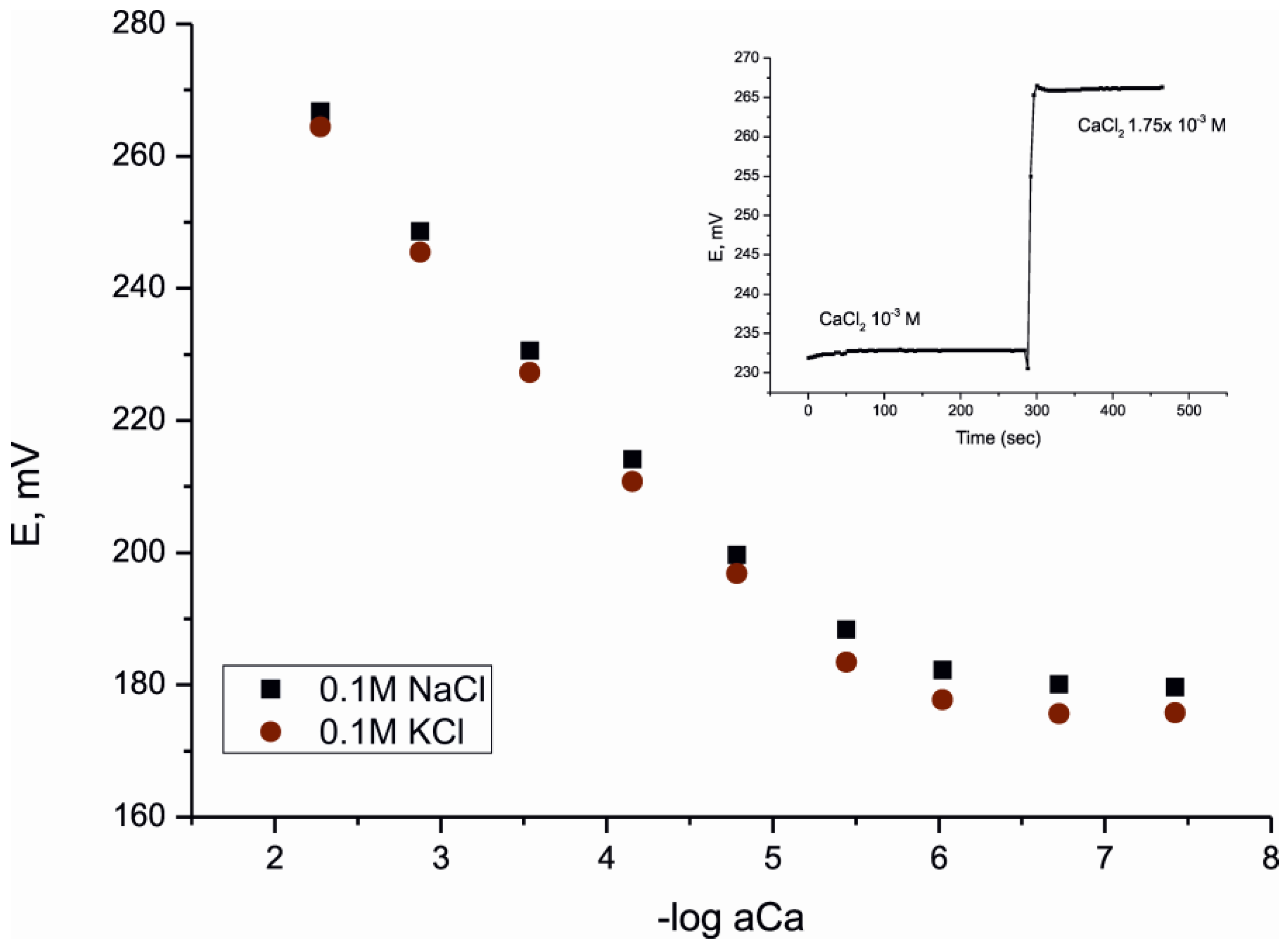
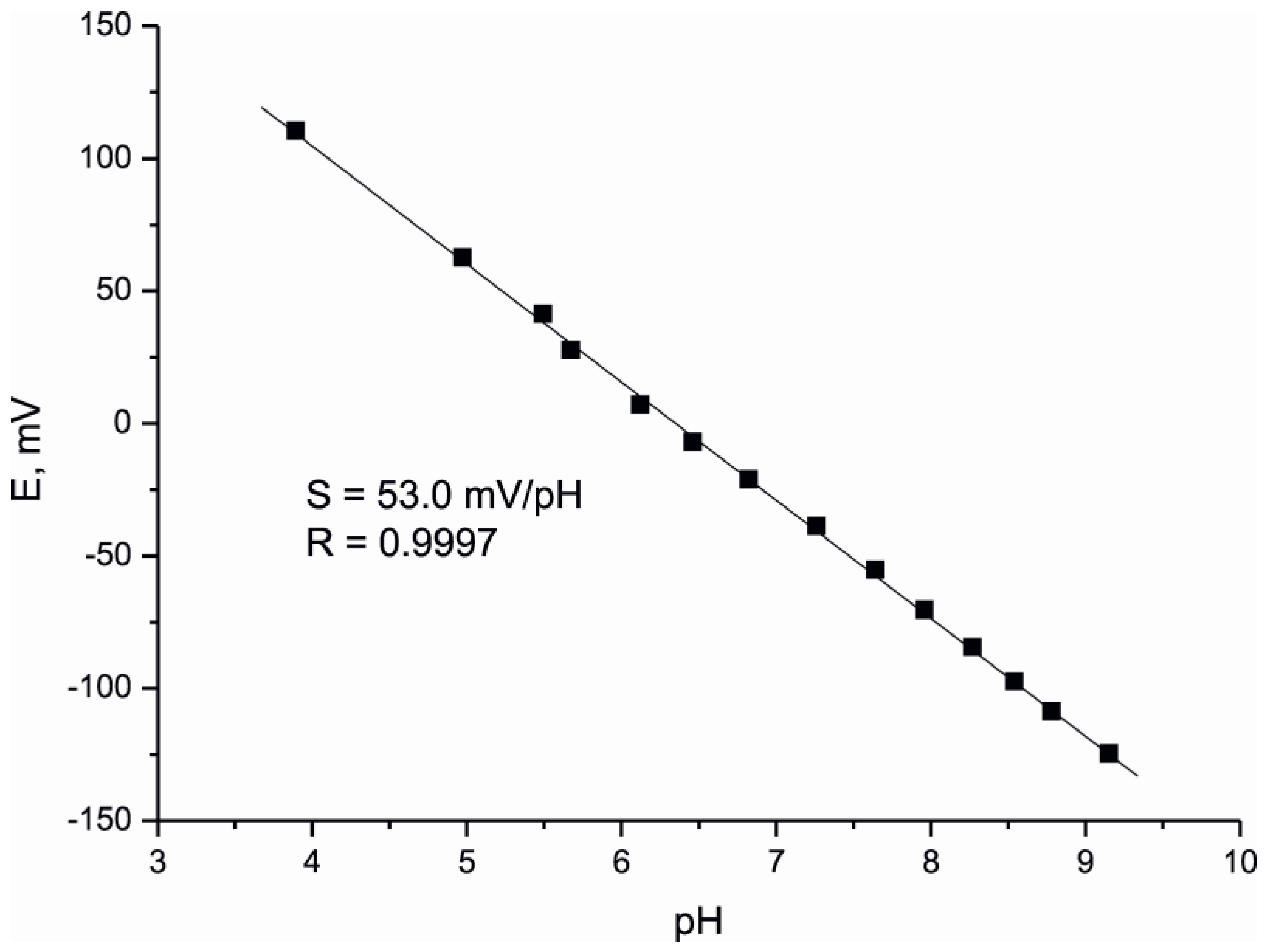
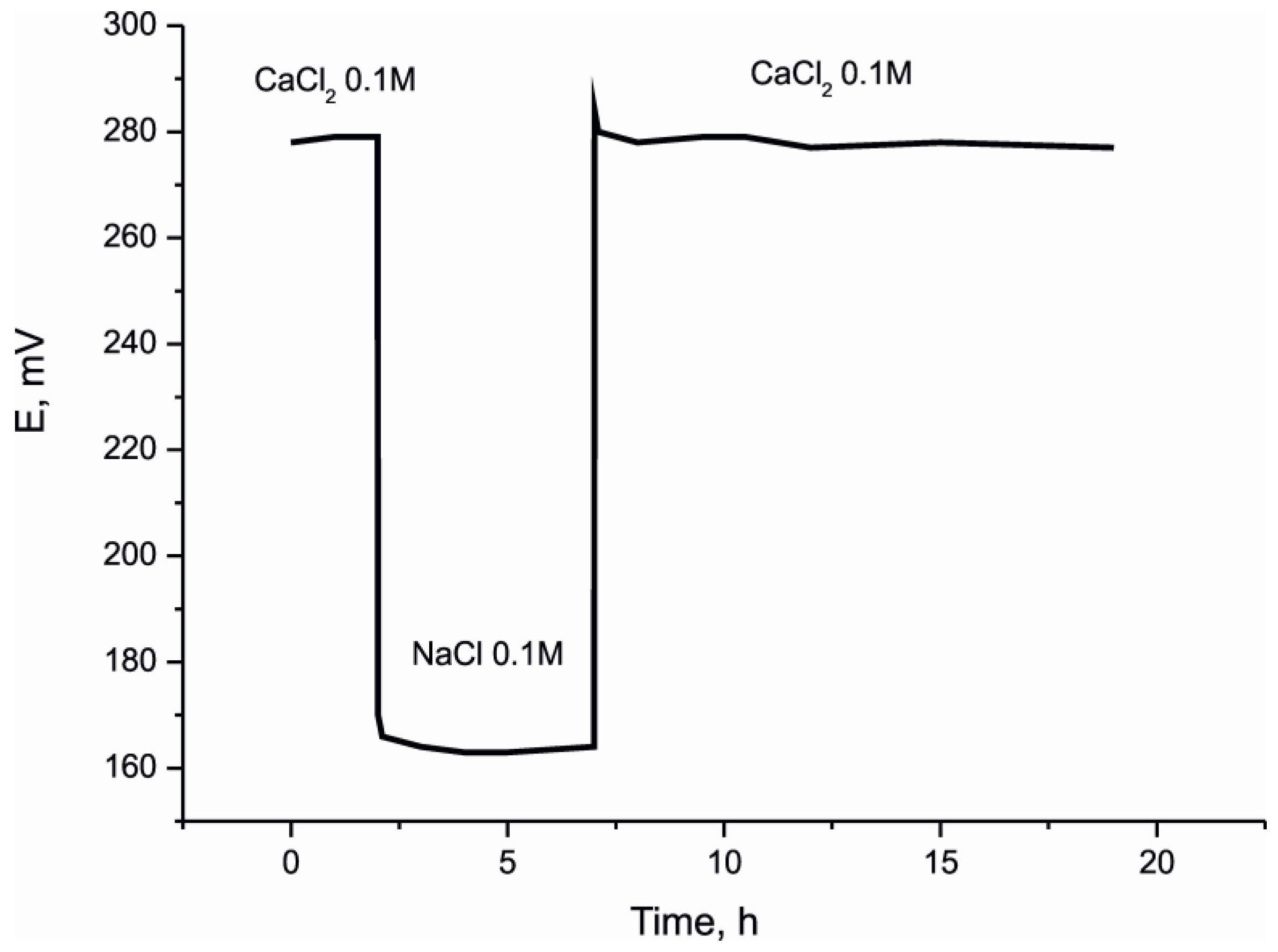
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric ion sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef]
- Abramova, N.; Bratov, A. Photocurable polymers for ion selective field effect transistors. 20 years of application. Sensors 2009, 9, 7097–7110. [Google Scholar] [PubMed]
- He, N.; Lindfors, T. Determination of Water Uptake of Polymeric Ion-Selective Membranes with the Coulometric Karl Fischer and FT-IR-Attenuated Total Reflection Techniques. Anal. Chem. 2013, 85, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Lindfors, T.; Szuecs, J.; Sundfors, F.; Gyurcsanyi, R.E. Polyaniline Nanoparticle-Based Solid-Contact Silicone Rubber Ion-Selective Electrodes for Ultratrace Measurements. Anal. Chem. 2010, 82, 9425–9432. [Google Scholar] [CrossRef] [PubMed]
- Veder, J.-P.; de Marco, R.; Clarke, G.; Chester, R.; Nelson, A.; Prince, K.; Pretsch, E.; Bakkert, E. Elimination of undesirable water layers in solid-contact polymeric ion-selective electrodes. Anal. Chem. 2008, 80, 6731–6740. [Google Scholar] [CrossRef] [PubMed]
- Ivanova-Mitseva, P.K.; Fragkou, V.; Lakshmi, D.; Whitcombe, M.J.; Davis, F.; Guerreiro, A.; Crayston, J.A.; Ivanova, D.K.; Mitsev, P.A.; Piletska, E.V.; et al. Conjugated Polymers with Pendant Iniferter Units: Versatile Materials for Grafting. Macromolecules 2011, 44, 1856–1865. [Google Scholar]
- Kim, J.; You, J.; Kim, E. Flexible Conductive Polymer Patterns from Vapor Polymerizable and Photo-Cross-Linkable EDOT. Macromolecules 2010, 43, 2322–2327. [Google Scholar] [CrossRef]
- Lakshmi, D.; Whitcombe, M.J.; Chianella, I.; Piletska, E.V.; Guerreiro, A.; Subrahmanyam, S.; Davis, F.; Brito, P.S.; Fowler, S.A.; Piletsky, S.A. Chimeric polymers formed from a monomer capable of free radical, oxidative and electrochemical polymerisation. Chem. Commun. 2009, 19, 2759–2761. [Google Scholar] [CrossRef] [PubMed]
- Grygolowicz-Pawlak, E.; Palys, B.; Biesiada, K.; Olszyna, A.R.; Malinowska, E. Covalent binding of sensor phases—A recipe for stable potentials of solid-state ion-selective sensors. Anal. Chim. Acta 2008, 625, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Abramova, N.; Bratov, A. Photocurable polymer membrane ion sensor and their application for multicomponent analysis. In Multisensor Systems for Chemical Analysis; Lvova, L., Kirsanov, D., di Natale, C., Legin, A., Eds.; Pan Stanford Publiching Pte. Ltd.: Singapore, Singapore, 2014; pp. 41–68. [Google Scholar]
- Lindner, E.; Gyurcsanyi, R.E. Quality control criteria for solid-contact, solvent polymeric membrane ion-selective electrodes. J. Solid State Electrochem. 2009, 13, 51–68. [Google Scholar] [CrossRef]
- Verkerk, U.H.; van den Vlekkert, H.H.; Reinhoudt, D.N.; Engbersen, J.; Honig, G.; Holterman, H. Development of chemically modified ISFETs as durable sensors for continuous fkow measurements. Sens. Actuators B Chem. 1993, 13, 221–225. [Google Scholar] [CrossRef]
- Reinhoudt, D.N.; Engbersen, J.; Brzozka, Z.; van den Vlekkert, H.H.; Honig, G.; Holterman, H.; Verkerk, U. Development of durable K+-selective chemically modified field effect transistor with functionalized polysiloxane membranes. Anal. Chem. 1994, 66, 3618–3623. [Google Scholar] [CrossRef]
- Harrison, D.J.; Teclemariam, A.; Cunningham, L.L. Photopolimerization of plastisizers in ion-sensitive membranes of solid-state sensors. Anal. Chem. 1989, 61, 246–251. [Google Scholar] [CrossRef]
- Cardwell, T.J.; Cattrall, R.W.; Iles, P.J.; Hamilton, I.C. Photocured polymers in ion-selective membranes. 2.A calcium electrode for flow-injection analysis. Anal. Chim. Acta 1985, 177, 239–242. [Google Scholar] [CrossRef]
- Arnaut, L.G. Design of porphyrin-based photosensitizers for photodynamic therapy. In Advances in Inorganic Chemistry, Vol 63: Inorganic Photochemistry; VanEldik, R., Stochel, G., Eds.; Academic Press: Waltham, MA, USA, 2011; Volume 63, pp. 187–233. [Google Scholar]
- Alexander, P.W.; Dimitrakopoulos, T.; Hibbert, D.B. A Photo Cured Coated Wire Calcium Ion Selective Electrode for Use in Flow Injection Potentiometry. Talanta 1997, 44, 1397–1405. [Google Scholar] [CrossRef]
- Dumschat, C.; Fromer, R.; Rautschek, H.; Muller, H.; Timpe, H.-J. Photolithographically pattenrnable nitrate-sensitive acrylate-based membrane. Anal. Chim. Acta 1991, 243, 179–182. [Google Scholar] [CrossRef]
- Pappas, S.P. Radiation Curing: Science and Technology; Plenum Press: New York, NY, USA, 1992. [Google Scholar]
- Rosatzin, T.; Bakker, E.; Suzuki, K.; Simon, W. Lipophilic and immobilized anionic additives in solvent polymeric membranes of cation-selective chemical sensors. Anal. Chim. Acta 1993, 280, 197–208. [Google Scholar] [CrossRef]
- Abramova, N.; Bratov, A. Photocurable pH-sensitive membrane for ion-selective field effect transistors. Talanta 2010, 81, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.; Xu, A.; Pretsch, E. Optimum composition of neutral carrier based pH electrodes. Anal. Chim. Acta 1994, 295, 253–262. [Google Scholar] [CrossRef]
- Van den Berg, A.; Verney-Norberg, E.; Grisel, A. An ISFET-based calcium sensor using photopolymerized polysiloxane membrane. Sens. Actuators B Chem. 1991, 4, 235–238. [Google Scholar] [CrossRef]
- Van der Wal, P.D.; van den Berg, A.; de Rooij, N.F. Universal approach for the fabrication of Ca2+, K+ and NO3− sensitive membrane-ISFETs. Sens. Actuators B Chem. 1994, 18, 200–207. [Google Scholar] [CrossRef]
- Heng, L.Y.; Hall, E.A.H. Producing ‘self-plasticizing’ ion-selective membranes. Anal. Chem. 2000, 72, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Heng, L.Y.; Hall, E.A.H. Assessing a photocured self-plasticised acrylic membrane recipe for Na+ and K+ ion selective electrodes. Anal. Chim. Acta 2001, 443, 25–40. [Google Scholar] [CrossRef]
- Abramova, N.; Ipatov, A.; Levichev, S.; Bratov, A. Integrated multi-sensor chip with photocured polymer membranes containing copolymerised plasticizer for direct pH, potassium, sodium and chloride ions determination in blood serum. Talanta 2009, 79, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Bratov, A.; Abramova, N.; Dominguez, C. Investigation of chloride sensitive ISFETs with different membrane compositions suitable for medical applications. Anal. Chim. Acta 2004, 514, 99–106. [Google Scholar] [CrossRef]
- Abramova, N.; Bratov, A.; Ipatov, A.; Levichev, S.; Aris, A.; Rodriguez, E. New flow-through analytical system based on ion-selective field effect transistors with optimised calcium selective photocurable membrane for bovine serum analysis. Talanta 2013, 113, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Moral, J.; Abramova, N.; Bratov, A. Solid contact electrodes with copolimerized conductive layer and membrane matrix. Talanta 2015. submitted. [Google Scholar]
- Lindfors, T.; Hoefler, L.; Jagerszki, G.; Gyurcsanyi, R.E. Hyphenated FT-IR-Attenuated Total Reflection and Electrochemical Impedance Spectroscopy Technique to Study the Water Uptake and Potential Stability of Polymeric Solid-Contact Ion-Selective Electrodes. Anal. Chem. 2011, 83, 4902–4908. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, Z.; Granholm, K.; Sokalski, T.; Lewenstam, A. All-solid-state electrochemical platform for potentiometric measurements. Sens. Actuators B Chem. 2015, 207, 895–899. [Google Scholar] [CrossRef]
- Fibbioli, M.; Morf, W.E.; Badertscher, M.; de Rooij, N.F.; Pretsch, E. Potential drifts of solid-contacted ion-selective electrodes due to zero-current ion fluxes through the sensor membrane. Electroanalysis 2000, 12, 1286–1292. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abramova, N.; Bratov, A. Application of Photocured Polymer Ion Selective Membranes for Solid-State Chemical Sensors. Chemosensors 2015, 3, 190-199. https://doi.org/10.3390/chemosensors3020190
Abramova N, Bratov A. Application of Photocured Polymer Ion Selective Membranes for Solid-State Chemical Sensors. Chemosensors. 2015; 3(2):190-199. https://doi.org/10.3390/chemosensors3020190
Chicago/Turabian StyleAbramova, Natalia, and Andrey Bratov. 2015. "Application of Photocured Polymer Ion Selective Membranes for Solid-State Chemical Sensors" Chemosensors 3, no. 2: 190-199. https://doi.org/10.3390/chemosensors3020190
APA StyleAbramova, N., & Bratov, A. (2015). Application of Photocured Polymer Ion Selective Membranes for Solid-State Chemical Sensors. Chemosensors, 3(2), 190-199. https://doi.org/10.3390/chemosensors3020190