One-Step Controlled Electrodeposition Fabrication of Ternary PtNiCo Nanosheets for Electrocatalytic Ammonia–Nitrogen Sensing
Abstract
1. Introduction
2. Synthesis and Measurements
2.1. The Synthesis of PtxNiyCoz Ternary Alloys
2.2. Electrochemical Measurements
3. Results and Discussion
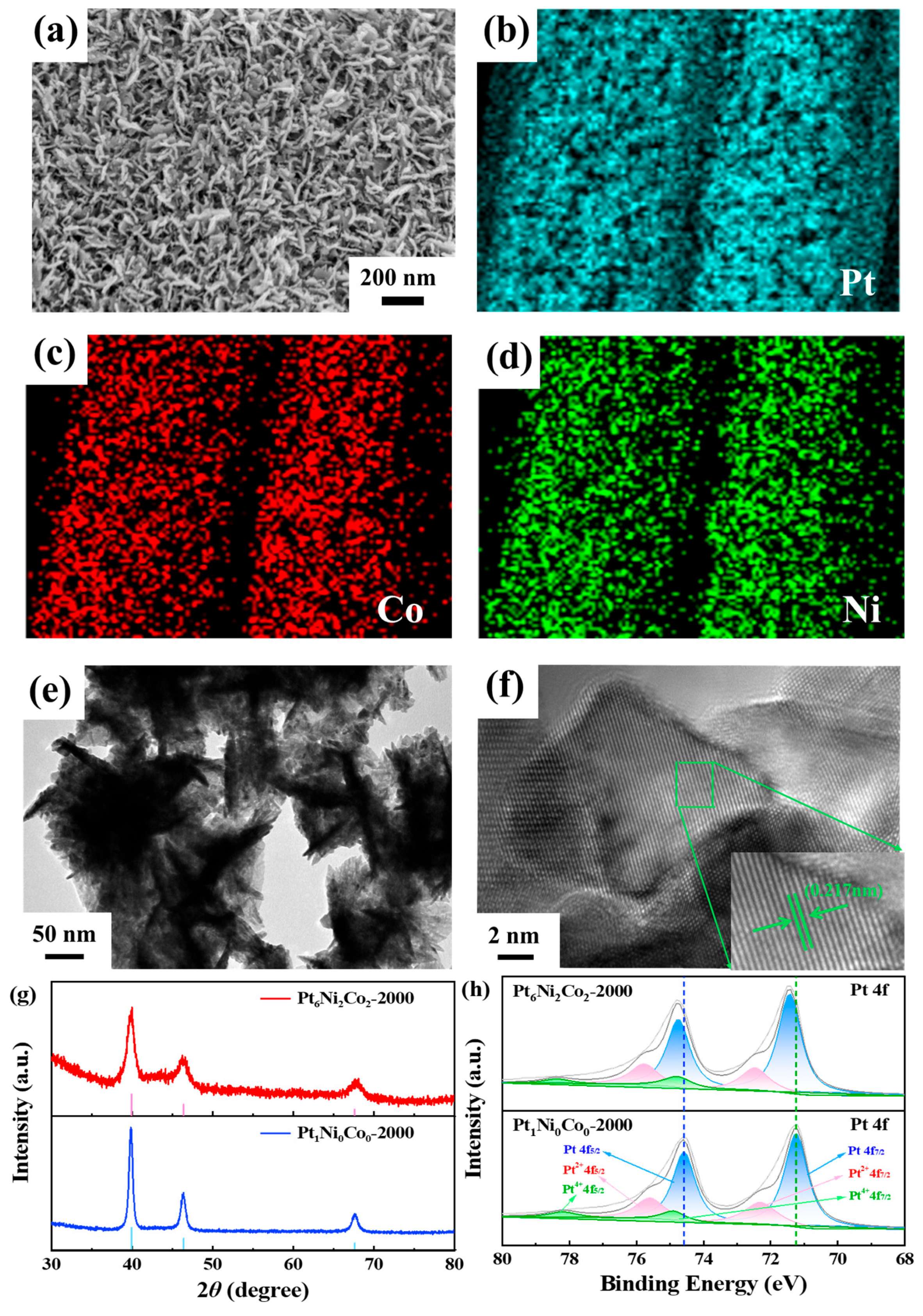
3.1. The Morphology and Structure of Pt6Ni2Co2-2000
3.2. Electrochemical Catalytic Ammonia Oxidation Reaction of PtxNiyCoz
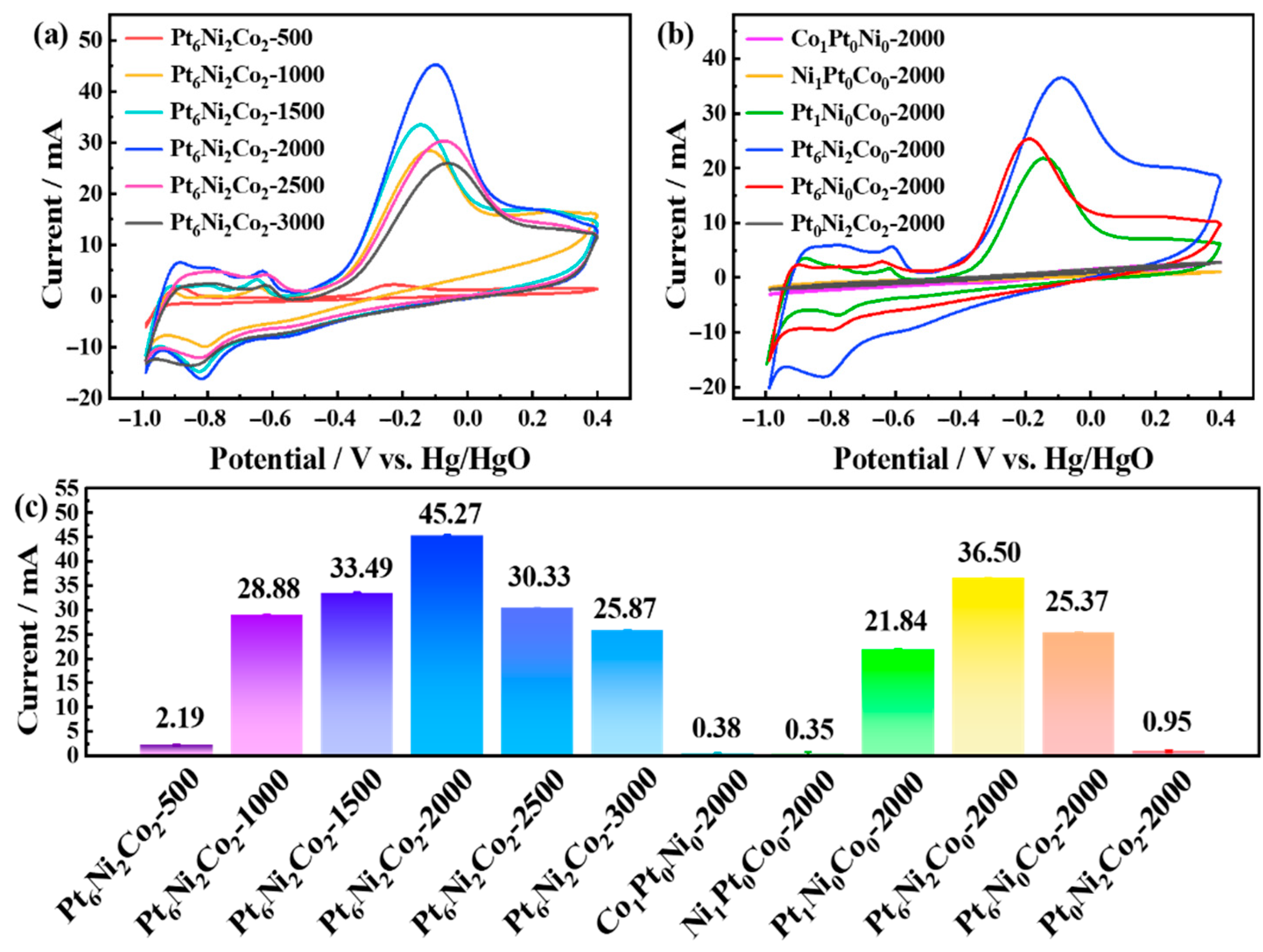
3.2.1. The Effect of the Molar Ratio (H2PtCl6·6H2O, Ni(NO3)2·6H2O and Co(NO3)2·6H2O) for Electrocatalytic Ammonia Oxidation Reactions
3.2.2. The Effect of Electrodeposition Time on Electrocatalytic Ammonia Oxidation Reactions
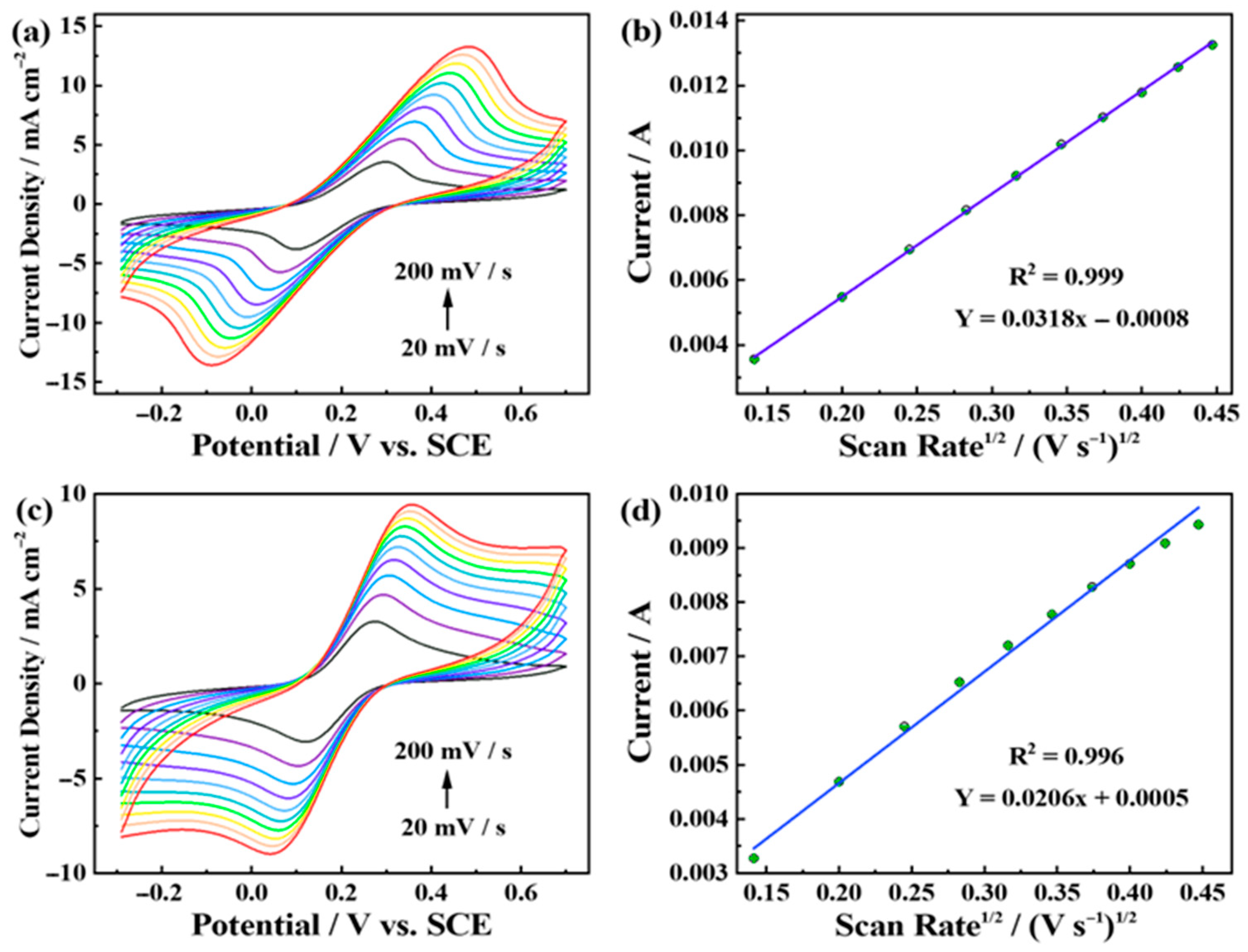
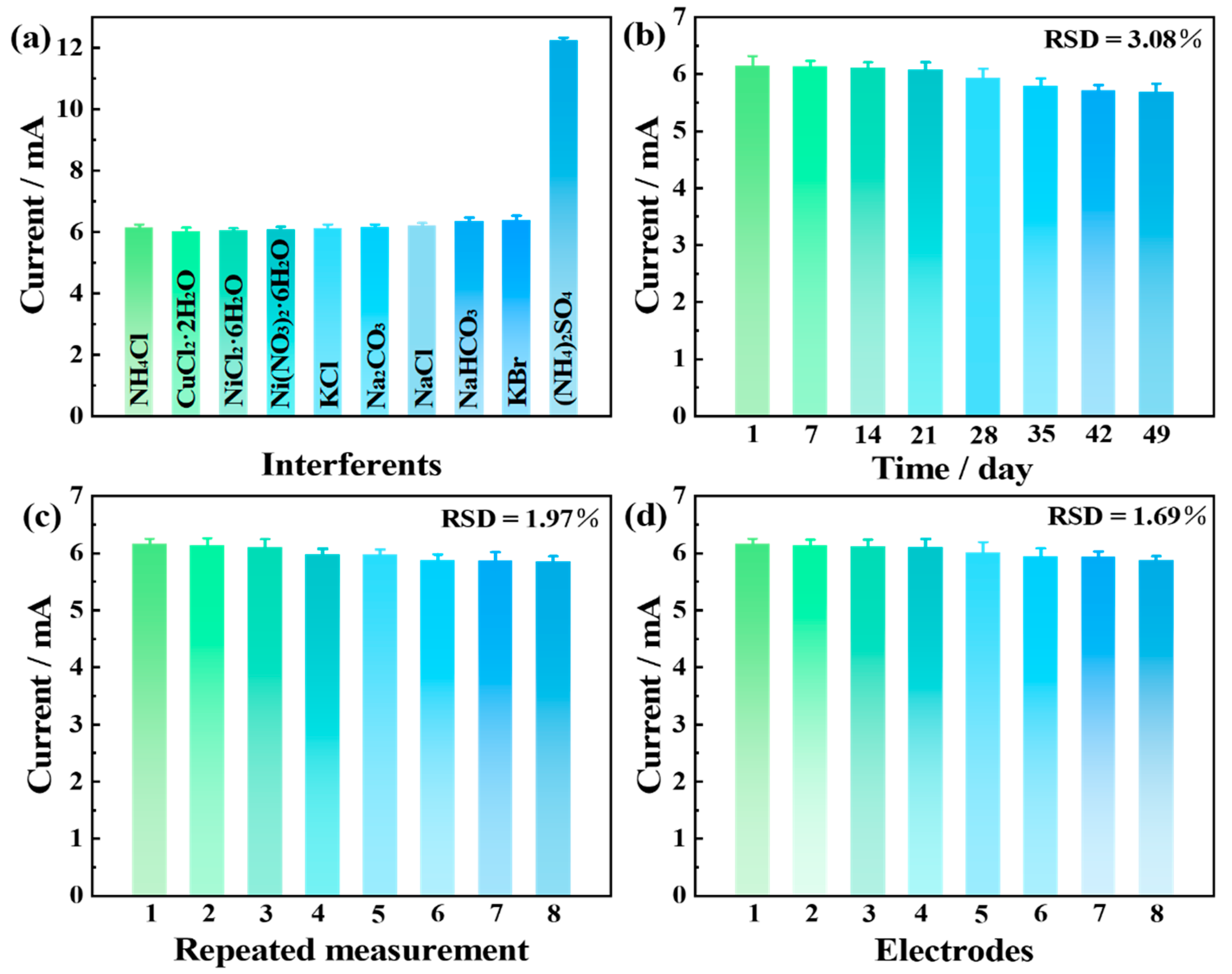
3.3. Electrochemical Active Surface Areas (ECSAs) and Stability of Pt6Ni2Co2-2000 Electrode
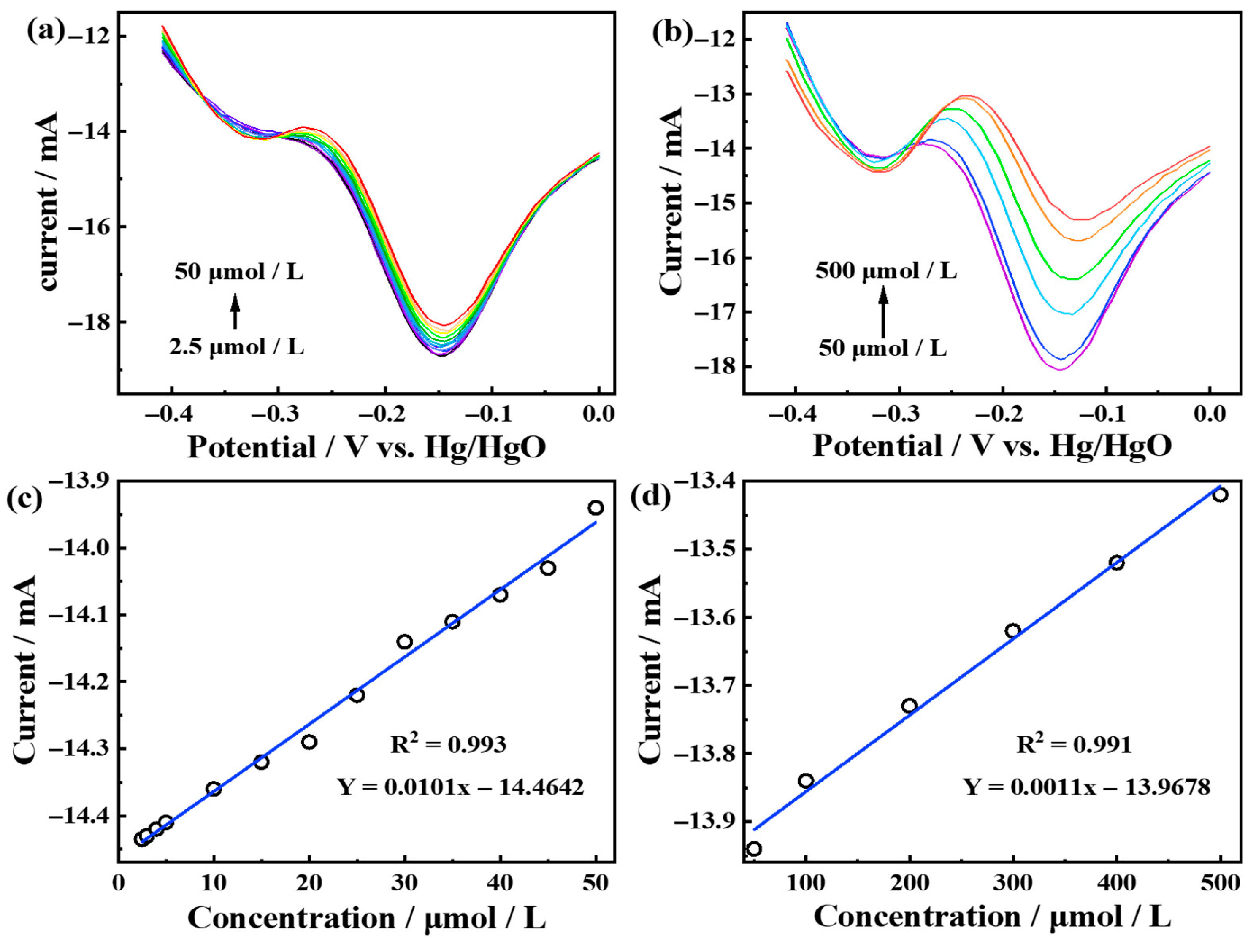
3.4. Electrochemical Detection Performance of Pt6Ni2Co2-2000 Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Yang, R.; He, C.; Xu, C.; Xu, L.; Hu, Z.H.; Wang, W. Novel insights into ammonia nitrogen removal: TiO2-based photocatalysts and potential of intimate coupling biodegradation. J. Environ. Chem. Eng. 2025, 13, 115962. [Google Scholar] [CrossRef]
- Yi, W.; Liu, G.; Wang, M.; Wang, J.; Chen, D.; Shen, J. Increased nitrogen deposition and airborne particulate matter pollution in the vicinity of intensive animal farms caused by ammonia emissions. Agric. Ecosyst. Environ. 2025, 387, 109634. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, S.; Shi, J.; Xu, S.; Gao, Y. A new particle material (CTS/ZMS) for removing ammonia and nitrate from groundwater: Performance and regeneration. Environ. Technol. 2025, 46, 1648–1665. [Google Scholar] [CrossRef]
- Flores-Zambrano, K.; Tapia, W.; Castillejo, P. Microalgae strains isolated from piggery wastewater in Ecuador: Effective nitrogen compound removal and growth potential in extremophile conditions. Biotechnol. Rep. 2025, 45, e00883. [Google Scholar] [CrossRef]
- Bhambri, A.; Karn, S.K.; Kumar, A. Ammonia transformation by potential consortium in the aquatic system. Sci. Rep. 2025, 15, 6066. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Zhang, Y.; Lai, D.; Chen, L.Q.; Zhou, L.Q.; Tao, C.L.; Fang, Z.; Zhu, R.R.; Long, W.Q.; Liu, J.W.; et al. Real-time smartphone-based multi-parameter detection of nitrite, ammonia nitrogen, and total phosphorus in water. Anal. Methods 2025, 17, 5683–5696. [Google Scholar] [CrossRef]
- Basak, H.K.; Adak, M.K.; Rajput, A.; Chakraborty, B. Low Pt loading on wolframite-type NiWO4 to excel the electrocatalytic water splitting and ammonia oxidation reaction. ACS Appl. Mater. Interfaces 2025, 17, 9391–9406. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, C.; Wang, G.; Suo, H.; He, D.; Yang, S.; Ding, J. In-situ fabrication of carbon cloth-supported polypyrrole-platinum nanosheets for the electrochemical detection of ammonia–nitrogen. Mater. Lett. 2021, 305, 130767. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Liu, X.; Han, Y.; Gu, J.; Wang, X. Two-step electrodeposition of polypyrrole nanospheres and Pt nanostars on Ni foam for electrochemical detection of ammonia–nitrogen. NANO Brief Rep. Rev. 2024, 19, 2450002. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, M.; Wang, X.; Gu, J. Enhanced electrocatalytic oxidation activity of platinum-poly (methylene blue) as a sensitive sensor for ammonia–nitrogen detection. Microchem. J. 2024, 199, 110238. [Google Scholar] [CrossRef]
- Wu, M.; Du, J.; Tao, C.; Liu, Z.; Li, Y. A tri-functionalised Pt-SnOx-based electrocatalyst for hydrogen generation via ammonia decomposition under native pH conditions. J. Colloid Interface Sci. 2019, 542, 451–459. [Google Scholar] [CrossRef]
- Bai, J.; Wang, C.; Liu, K.; Wang, H.; Liu, Y.; Liu, F.; Suo, H.; Liang, X.; Zhang, C.; Liu, F.; et al. Enhanced gas sensing performance based on the PtCu octahedral alloy nanocrystals decorated SnO2 nanoclusters. Sens. Actuators B Chem. 2021, 330, 129375. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, Y.; Wang, M.; Huan, Y.; He, Y.; Cheng, Q.; Cheng, Y.; Liu, J.; Zhou, X.; Qian, T.; et al. Awakening (220) as one more active facet of PtMo alloy via single-atom doping to boost ammonia electrooxidation in direct ammonia fuel cell. Adv. Funct. Mater. 2023, 33, 2306204. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, L. Nanostructured platinum-iridium alloy microelectrode for ammonia determination. Electroanalysis 2017, 29, 2019–2026. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Liu, G.; Ling, C.; Chen, B.; Huang, J.; Liu, X.; Li, B.; Wang, A.-L.; Hu, Z.; et al. Crystal phase-controlled growth of PtCu and PtCo alloys on 4H Au nanoribbons for electrocatalytic ethanol oxidation reaction. Nano Res. 2020, 12, 1970–1975. [Google Scholar] [CrossRef]
- Wang, G.; Ma, G.; Gao, J.; He, D.; Zhao, C.; Suo, H. Enhanced sensitivity of electrochemical sensors for ammonia-nitrogen via in-situ synthesis PtNi nanoleaves on carbon cloth. Sensors 2024, 24, 387. [Google Scholar] [CrossRef]
- Batgi, S.U.; Yapici, B.; Sahin, O.G. Electrocatalytic activity of carbon nanotube-supported Pt-based bimetallic catalysts for borohydride electro-oxidation. J. Electron. Mater. 2024, 53, 7846–7857. [Google Scholar] [CrossRef]
- Xing, Q.; Yan, W.; Wei, C.; Xiao, Z.; Jin, Z.; Liu, X.; Ren, J.; Chen, Y.; Li, X. Controlled preparation and anti-sulfate electrocatalysis of self-assembled multidimensional PtZn quasi-cubic nanodendrites. Adv. Mater. Interfaces 2022, 9, 2101944. [Google Scholar] [CrossRef]
- Minakshi, M.; Aughterson, R.; Sharma, P.; Sunda, A.P.; Ariga, K.; Shrestha, L.K. Micelle-Assisted Electrodeposition of γ-MnO2 on Lead Anodes: Structural and Electrochemical Insights. ChemNanoMat 2025, 2500270. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Y.; Zhang, Y.; Han, Y.; Zhang, Y.; Gu, J.; Zhang, L. Electrochemical fabrication of polyRhodamine B film and Pt nanosheets on Ni foam for enhancing electrochemical detection activities of ammonia-nitrogen. J. Electroanal. Chem. 2025, 996, 119433. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, X.; Liu, J.; Gu, J.; Suo, H.; Zhao, C.; Wang, X. Achieving Ni@(Pt/Ni(OH)2) ternary nanoflowers derived from Ni nanoflowers for electrochemical ammonia–nitrogen detection in the aqueous environment. Microchem. J. 2024, 200, 110401. [Google Scholar] [CrossRef]
- Cai, J.; Wei, L.; Liu, J.; Xue, C.; Chen, Z.; Hu, Y.; Zang, Y.; Wang, M.; Shi, W.; Qin, T.; et al. Two-dimensional crystalline platinum oxide. Nat. Mater. 2024, 23, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, W.; Yu, H.; Wang, W.; Xu, Y.; Shen, L.-L.; Zhang, G.-R.; Mei, D. Enhanced electrochemical oxidation of 5-hydroxymethylfurfural over tailored nickel nanoparticle assembly. Appl. Catal. B Environ. Energy 2024, 353, 124086. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, Z.; Wang, J.; Wang, J.; Huang, H.; Feng, J.; Yan, H.; Zhao, M.; Liu, X.; Liu, W.; et al. Unlocking the Potential of Oxide-Based Catalysts for CO2 Photo-Hydrogenation: Oxygen Vacancies Promoted C–O Bond Cleavage in Key Intermediates. Adv. Mater. 2025, 37, 2408906. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, J.; Cui, G.; Zhao, C.; Suo, H.; He, D. A novel electrochemical ammonia–nitrogen sensor based on carbon cloth-supported hierarchical Pt nanosheets-Ni(OH)2 nanosheets nanocomposites. Chem. Eng. Sci. 2021, 239, 116634. [Google Scholar] [CrossRef]
- Dharmalingam, S.T.; Dar, M.A.; Gul, R.; Sundaram, M.M.; Alnaser, I.A.; Sivasubramanian, R. Nano-Octahedron Cobalt Oxide Decorated Graphene Nanocomposites for the Selective/Simultaneous Detection of Dopamine. Adv. Mater. Interfaces 2025, 12, 2400981. [Google Scholar] [CrossRef]
- Bari, B.; Tyagi, P.; Udeochu, U. Cyclic voltammetry insights into Ni/Al-carbonate hydrotalcite catalysis for methanol oxidation. Sci. Rep. 2025, 15, 13365. [Google Scholar] [CrossRef]
- Zhang, J.W.; Zhang, X.; Gao, J.; Zhao, C.; Suo, H. Electrochemical surface functionalization engineering of carbon cloth for ultrasensitive electrochemical detection of nitrite. Microchem. J. 2024, 197, 109795. [Google Scholar] [CrossRef]
- Hu, D.M.; Sun, T.T.; Dong, J.; Xu, Q.M.; Xu, L.B. Engineering platinum–nickel nano-alloys in hierarchically ordered porous cobalt-nitrogen-doped carbon for highly efficient electrocatalytic oxygen reduction. Mater. Lett. 2022, 327, 133052. [Google Scholar] [CrossRef]
- Aligholizadeh K, N.A.; Alzate-Carvajal, N.; Nallayagari, A.R.; Monyoncho, E.A.; Zulevi, B.; Serov, A.; Baranova, E.A. In-situ polarization modulation IRRAS investigation of ammonia electrooxidation on Pt-Ir and Pt-Ru nanoparticles prepared on engineered catalyst supports. Electrochim. Acta 2024, 507, 145046. [Google Scholar]
- Qin, R.M.; Zhang, Y.H.; Xu, H.G.; Nie, P.C. Gold Nanoparticle-Modified Electrodes for Electrochemical Sensing of Ammonia Nitrogen in Water. ACS Appl. Nano Mater. 2024, 7, 577–593. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, X.; Wu, Y.L.; Khan, H.U.; Xiao, F.; Xia, B.Y.; Sun, Y.M. Functionalized MXene fiber electrode for the electrochemical sensing of urinary ammonia. Adv. Sens. Energy Mater. 2024, 3, 100091. [Google Scholar] [CrossRef]
- GZhou, F.; Wang, G.D.; Zhao, X.; He, D.; Zhao, C.; Suo, H. Electrochemical detection of ammonia in water using NiCu carbonate hydroxide-modified carbon cloth electrodes: A simple sensing method. Sensors 2024, 24, 4824. [Google Scholar]
- Zhang, L.; Liu, T.Y.; Ren, R.H.; Zhang, J.W.; He, D.; Zhao, C.; Suo, H. In situ synthesis of hierarchical platinum nanosheets-polyaniline array on carbon cloth for electrochemical detection of ammonia. J. Hazard. Mater. 2020, 392, 122. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Han, Y.; Huang, Y.; Gu, J.; Wang, X.; Zhao, C. One-Step Controlled Electrodeposition Fabrication of Ternary PtNiCo Nanosheets for Electrocatalytic Ammonia–Nitrogen Sensing. Chemosensors 2025, 13, 335. https://doi.org/10.3390/chemosensors13090335
Zhang L, Han Y, Huang Y, Gu J, Wang X, Zhao C. One-Step Controlled Electrodeposition Fabrication of Ternary PtNiCo Nanosheets for Electrocatalytic Ammonia–Nitrogen Sensing. Chemosensors. 2025; 13(9):335. https://doi.org/10.3390/chemosensors13090335
Chicago/Turabian StyleZhang, Liang, Yue Han, Yingying Huang, Jiali Gu, Xinyue Wang, and Chun Zhao. 2025. "One-Step Controlled Electrodeposition Fabrication of Ternary PtNiCo Nanosheets for Electrocatalytic Ammonia–Nitrogen Sensing" Chemosensors 13, no. 9: 335. https://doi.org/10.3390/chemosensors13090335
APA StyleZhang, L., Han, Y., Huang, Y., Gu, J., Wang, X., & Zhao, C. (2025). One-Step Controlled Electrodeposition Fabrication of Ternary PtNiCo Nanosheets for Electrocatalytic Ammonia–Nitrogen Sensing. Chemosensors, 13(9), 335. https://doi.org/10.3390/chemosensors13090335



