A UV-Photon-Energy-Integrated Gas Sensor Based on Pt-Nanoparticle-Decorated TiO2 Nanorods for Room-Temperature Hydrogen Detection
Abstract
1. Introduction
2. Materials and Methods
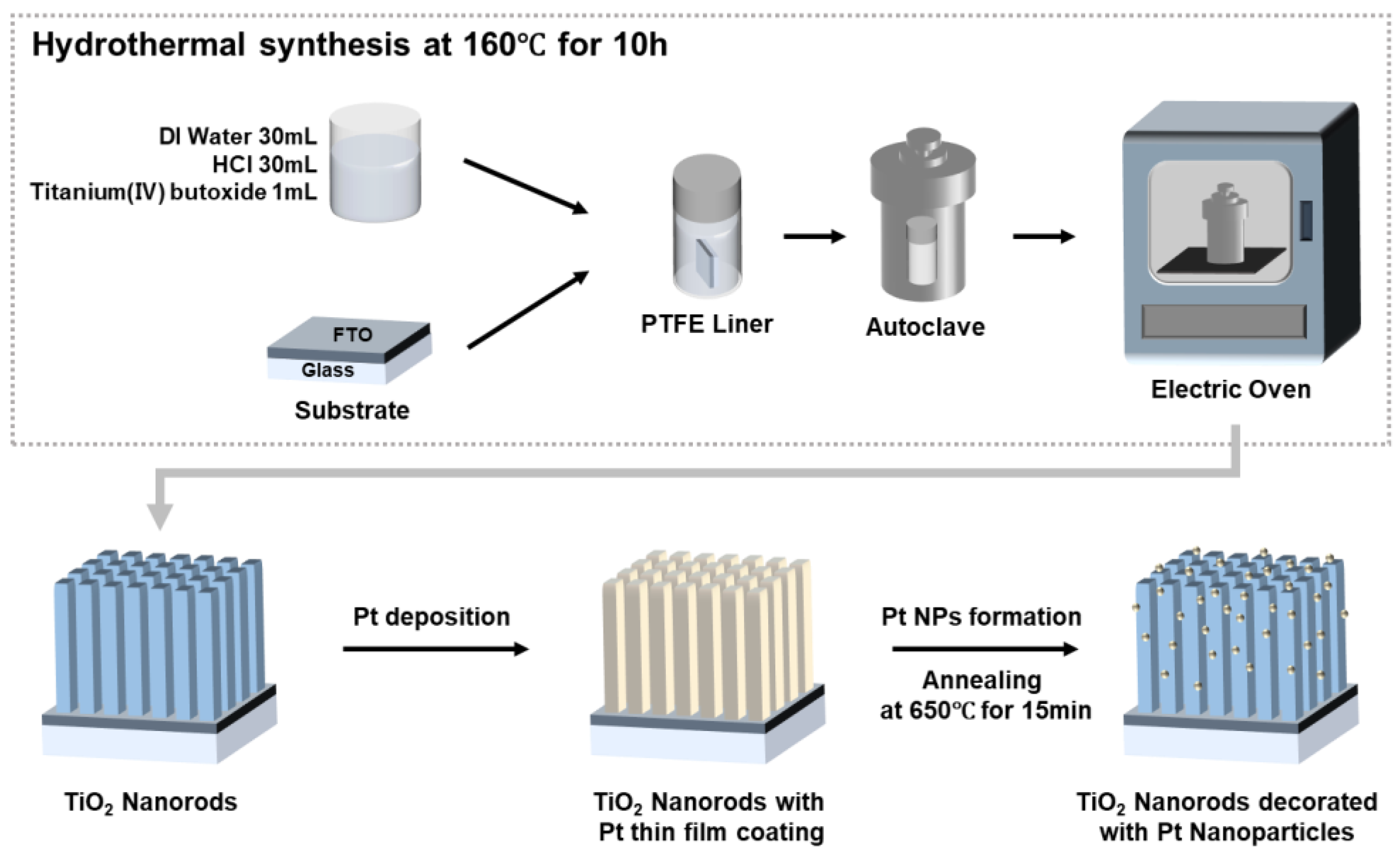
2.1. Synthesis of TiO2 NRs
2.2. Synthesis of Pt NPs
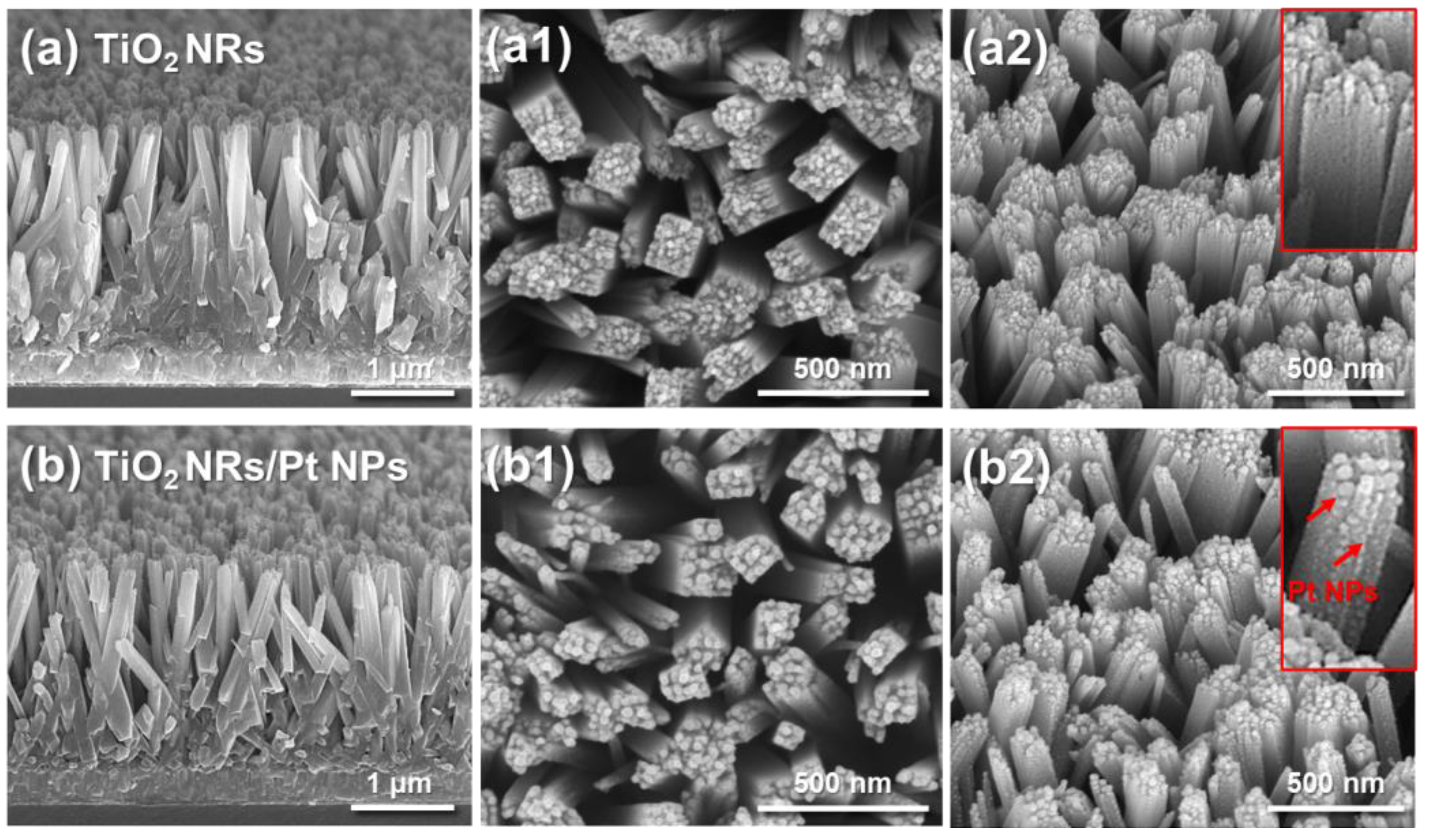
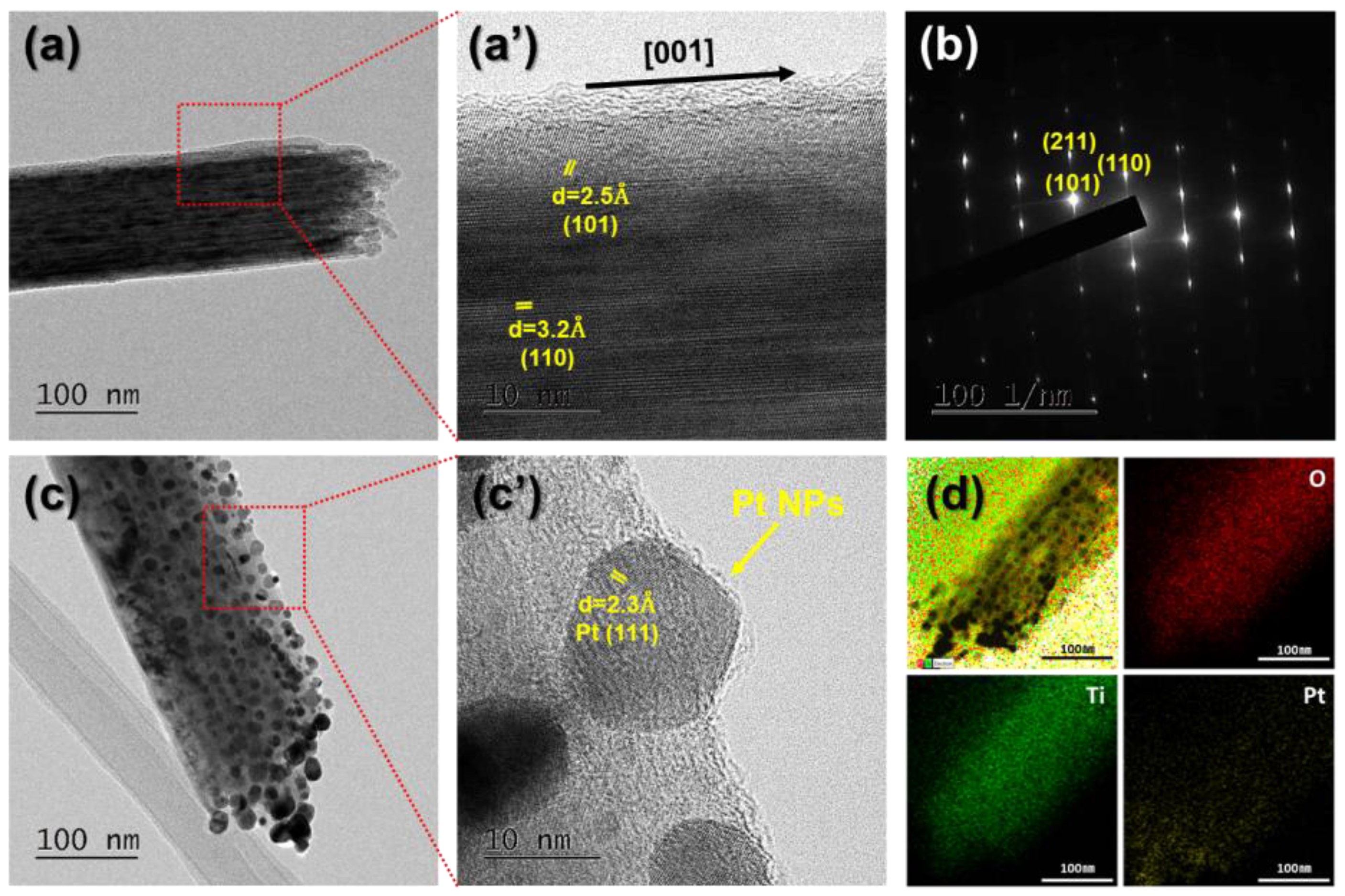
2.3. Structural and Morphological Characterization
2.4. H2 Gas-Sensing Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EDS | Energy-dispersive X-ray spectroscopy |
| FE-SEM | Field-emission secondary electron microscope |
| FE-TEM | Field-emission transmission electron microscope |
| HR | High resolution |
| LED | Light-emitting diode |
| NP | Nanoparticle |
| NR | Nanorod |
| RT | Room temperature |
| SAED | Selected-area electron diffraction |
| TF | Thin film |
| UV | Ultraviolet |
| XRD | X-ray diffraction |
References
- Singla, M.K.; Nijhawan, P.; Oberoi, A.S. Hydrogen fuel and fuel cell technology for cleaner future: A review. Environ. Sci. Pollut. Res. 2021, 28, 15607–15626. [Google Scholar] [CrossRef] [PubMed]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Yuea, M.; Lamberta, H.; Pahonb, E.; Rocheb, R.; Jemeia, S.; Hissela, D. Hydrogen energy systems: Acritical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Dhimish, M.; Vieira, R.G.; Badran, G. Investigating the stability and degradation of hydrogen PEM fuel cell. Int. J. Hydrogen Energy 2021, 46, 37017–37028. [Google Scholar] [CrossRef]
- Hua, Z.; Zheng, Z.; Pahon, E.; Péra, M.-C.; Gao, F. A review on lifetime prediction of proton exchange membrane fuel cells system. J. Power Sources 2022, 529, 231256. [Google Scholar] [CrossRef]
- Şakar, B.C. Influence of the Cu doping on the physical and H2 gas sensing properties of TiO2. Int. J. Hydrogen Energy 2024, 50, 1197–1208. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Faglia, G.; Sberveglieri, G. TiO2 Nanotubes: Recent Advances in Synthesis and Gas Sensing Properties. Sensors 2013, 13, 14813–14838. [Google Scholar] [CrossRef]
- Li, Z.; Yao, Z.; Haidry, A.A.; Plecenik, T.; Xie, L.; Sun, L.; Fatima, Q. Resistive-type hydrogen gas sensor based on TiO2: A review. Int. J. Hydrogen Energy 2018, 43, 21114–21132. [Google Scholar] [CrossRef]
- Vijaykumar, A.; Mondal, A.; Deb, S.; Ajitha, B.; Reddy, Y.A.K. Development of room-temperature operable TiO2−x-based hydrogen gas sensor with light irradiation. Appl. Surf. Sci. 2024, 670, 160664. [Google Scholar] [CrossRef]
- Kanth, S.; Domadiya, D.N.; Betty, C.A.; Kumar, S.; Choudhury, S. Highly selective hydrogen sensing by applying characteristic frequency at room temperature: Case study on TiO2–PdO hydrogen sensor. Int. J. Hydrogen Energy 2025, 106, 231–242. [Google Scholar] [CrossRef]
- Kumar, A.; Deep, A.; Kumar, P.; Singh, N.B. A room-temperature hydrogen sensor based on Pd nanoparticles decorated TiO2 nanotubes. Ceram. Int. 2014, 40, 12555–12560. [Google Scholar]
- Weyrauch, I.; Hefler, E.L.; Breuch, R.; Kaul, P.; Mathur, S.; Konstantynovski, K. Recent Developments in the Design of Photoactivated Metal Oxide Gas Sensors and Application of Plasmonic Nanoparticles in Hydrogen-Sensing Devices. Phys. Status Solidi A 2025, 240, 2400633. [Google Scholar] [CrossRef]
- Guo, Y.-Y.; Zheng, X.-H.; Bo, L.-B.; Gu, Z.-Q.; Zhang, C.; Liu, Y.-F. UV-activated Gas Sensor Based on Ordered Mesoporous ZnO–TiO2 Heterogeneous Composites for Trace NO2 Detection at Room Temperature. Talanta 2025, 285, 127415. [Google Scholar] [CrossRef] [PubMed]
- Thathsara, T.; Harrison, C.J.; Schönauer-Kamin, D.; Mansfeld, U.; Moos, R.; Malherbe, F.M.; Hocking, R.K.; Shafiei, M. Pd Nanoparticles Decorated Hollow TiO2 Nanospheres for Highly Sensitive and Selective UV-Assisted Hydrogen Gas Sensors. ACS Appl. Energy Mater. 2024, 7, 14. [Google Scholar] [CrossRef]
- Cui, S.; Sun, Y.; Chen, C.; Hong, H.; Huang, J. Preparation of Pd Nanoparticles Modified Hollow TiO2 Dodecahedrons for Highly Selective Hydrogen Detection. Sens. Actuators A Phys. 2025, 382, 116104. [Google Scholar] [CrossRef]
- Deb, S.; Mondal, A.; Kumar Reddy, Y.A. Review on Development of Metal-Oxide and 2-D Material-Based Gas Sensors under Light-Activation. Curr. Opin. Solid State Mater. Sci. 2024, 30, 101160. [Google Scholar] [CrossRef]
- Thathsara, T.; Harrison, C.J.; Hocking, R.K.; Shafiei, M. Pd- and PdO-Decorated TiO2 Nanospheres: Hydrogen Sensing Properties under Visible Light Conditions at Room Temperature. Chemosensors 2023, 11, 409. [Google Scholar] [CrossRef]
- Wang, J.; Shen, H.; Xia, Y.; Komarneni, S. Light-Activated Room-Temperature Gas Sensors Based on Metal Oxide Nanostructures: A Review on Recent Advances. Ceram. Int. 2021, 47, 7353–7368. [Google Scholar] [CrossRef]
- Lee, S.; Lee, G.H.; Choi, M.; Park, G.; Kim, D.; Lee, S.; Lee, J.-O.; Cho, D. Photoactivated Metal Oxide-Based Chemiresistors: Revolutionizing Gas Sensing with Ultraviolet Illumination. J. Sens. Sci. Technol. 2024, 33, 274–287. [Google Scholar] [CrossRef]
- Prathan, A.; Sanglao, J.; Wang, T.; Bhoomanee, C.; Ruankham, P.; Gardchareon, A.; Wongratanaphisan, D. Controlled structure and growth mechanism behind hydrothermal growth of TiO2 nanorods. Sci. Rep. 2020, 10, 8065. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Qomaruddin, M.; Casals, O.; Wasisto, H.S.; Waag, A.; Prades, J.D.; Fàbrega, C. Visible-Light-Driven Room Temperature NO2 Gas Sensor Based on Localized Surface Plasmon Resonance: The Case of Gold Nanoparticle Decorated Zinc Oxide Nanorods (ZnO NRs). Chemosensors 2022, 10, 28. [Google Scholar] [CrossRef]
- Fan, S.-W.; Srivastava, A.K.; Dravid, V.P. UV-activated room-temperature gas sensing mechanism of polycrystalline ZnO. Appl. Phys. Lett. 2009, 95, 142106. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Y.; Liu, J.; Li, H.-Y.; Hu, Z.; Luo, X.; Gao, N.; Zhang, B.; Jiang, J.; Zhong, A.; et al. Sensitive H2 Gas Sensors Based on SnO2 Nanowires. Sens. Actuators B Chem. 2021, 345, 130334. [Google Scholar] [CrossRef]
- Fomekong, R.L.; Kelm, K.; Saruhan, B. High-Temperature Hydrogen Sensing Performance of Ni-Doped TiO2 Prepared by Co-Precipitation Method. Sensors 2020, 20, 5992. [Google Scholar] [CrossRef]
- Steinebach, H.; Kannan, S.; Rieth, L.; Solzbacher, F. H2 Gas Sensor Performance of NiO at High Temperatures in Gas Mixtures. Sens. Actuators B Chem. 2010, 151, 162–168. [Google Scholar] [CrossRef]
- Fang, T.; Mo, T.; Xu, X.; Tao, H.; Wang, H.; Yu, B.; Zhao, Z.-J. Pd-Decorated SnO2 Nanofilm Integrated on Silicon Nanowires for Enhanced Hydrogen Sensing. Sensors 2025, 25, 655. [Google Scholar] [CrossRef]
- Hussain, M.; Jeong, W.; Kang, I.-S.; Choi, K.-K.; Jaffery, S.H.A.; Ali, A.; Hussain, T.; Ayaz, M.; Hussain, S.; Jung, J. Highly Fast Response of Pd/Ta2O5/SiC and Pd/Ta2O5/Si Schottky Diode-Based Hydrogen Sensors. Sensors 2021, 21, 1042. [Google Scholar] [CrossRef]
- Stolarczyk, A.; Jarosz, T.; Procek, M. Room Temperature Hydrogen Gas Sensing via Reversible Hydrogenation of Electrochemically Deposited Polycarbazole on Interdigitated Pt Transducers. Sensors 2019, 19, 1098. [Google Scholar] [CrossRef]
- Girma, H.G.; Park, K.H.; Ji, D.; Kim, Y.; Lee, H.M.; Jeon, S.; Jung, S.-H.; Kim, J.Y.; Noh, Y.-Y.; Lim, B. Room-Temperature Hydrogen Sensor with High Sensitivity and Selectivity Using Chemically Immobilized Monolayer Single-Walled Carbon Nanotubes. Adv. Funct. Mater. 2023, 33, 2213381. [Google Scholar] [CrossRef]
- Barala, S.; Kumar, A.; Kwoka, M.; Gupta, A.; Kumar, M. Spillover Effect in Pd Anchored NiO-ZnO Nanostructures Improves Hydrogen Gas Sensor’s Performance. Sens. Actuators B Chem. 2025, 433, 137534. [Google Scholar] [CrossRef]
- Roland, U.; Hebestreit, A.; Taoussanis, A.; Eiserbeck, M.; Holzer, F.; Wotzka, A.; Wohlrab, S. Cost-effective Selective Hydrogen Sensor Based on the Combination of Catalytic Spillover Effect and Impedance Measurement. Int. J. Hydrogen Energy 2023, 48, 37550–37562. [Google Scholar] [CrossRef]
- Yao, Y.; Ji, F.; Yin, M.; Ren, X.; Ma, Q.; Yan, J.; Liu, S.F. Ag Nanoparticle-Sensitized WO3 Hollow Nanosphere for Localized Surface Plasmon Enhanced Gas Sensors. ACS Appl. Mater. Interfaces 2016, 8, 18165–18172. [Google Scholar] [CrossRef]
- Vaidyanathan, A.; Mondal, B.; Rout, C.S.; Chakraborty, B. Plasmonic Gas Sensors Based on Nanomaterials: Mechanisms and Recent Developments. J. Phys. D Appl. Phys. 2024, 57, 263002. [Google Scholar] [CrossRef]

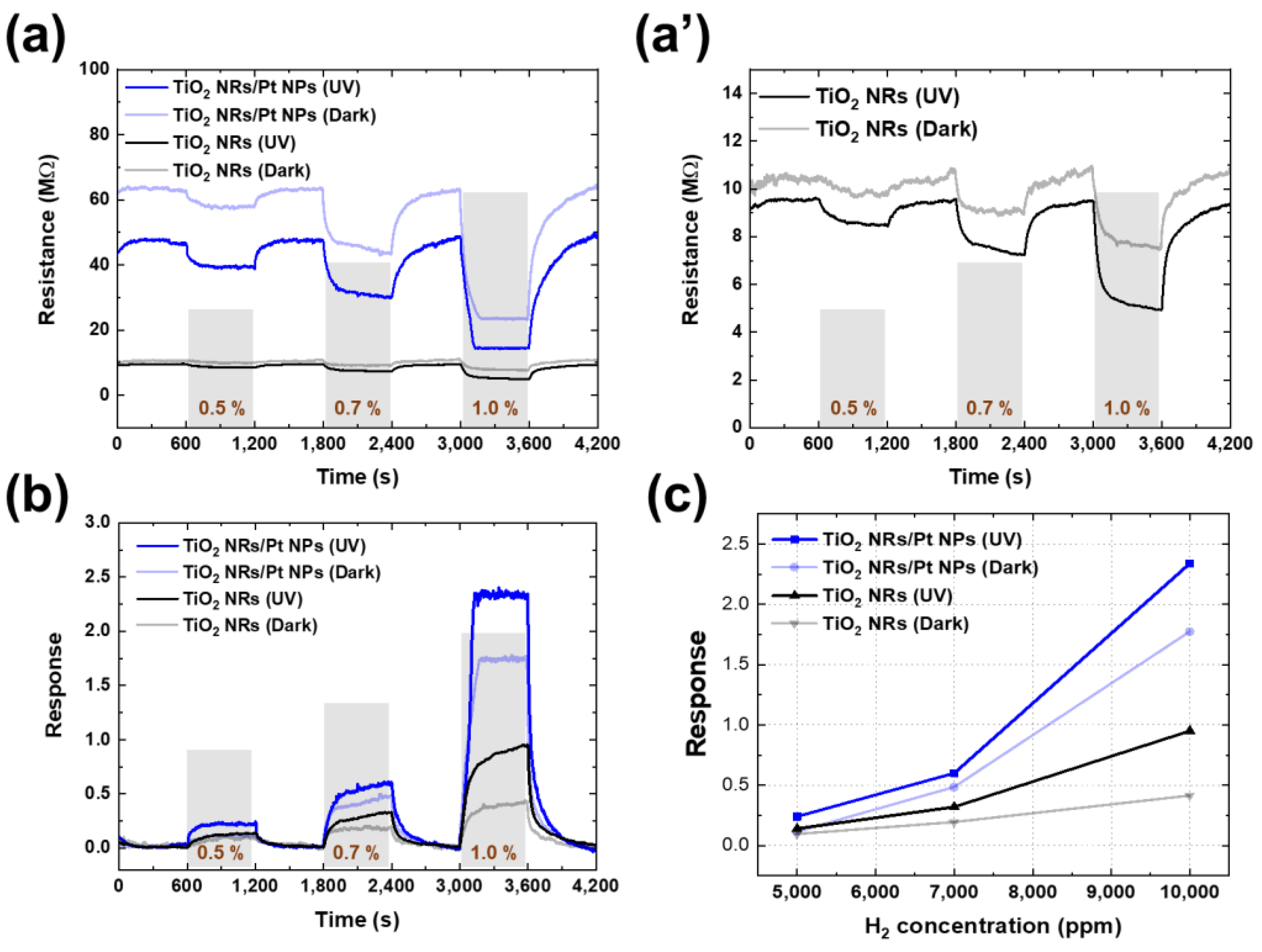
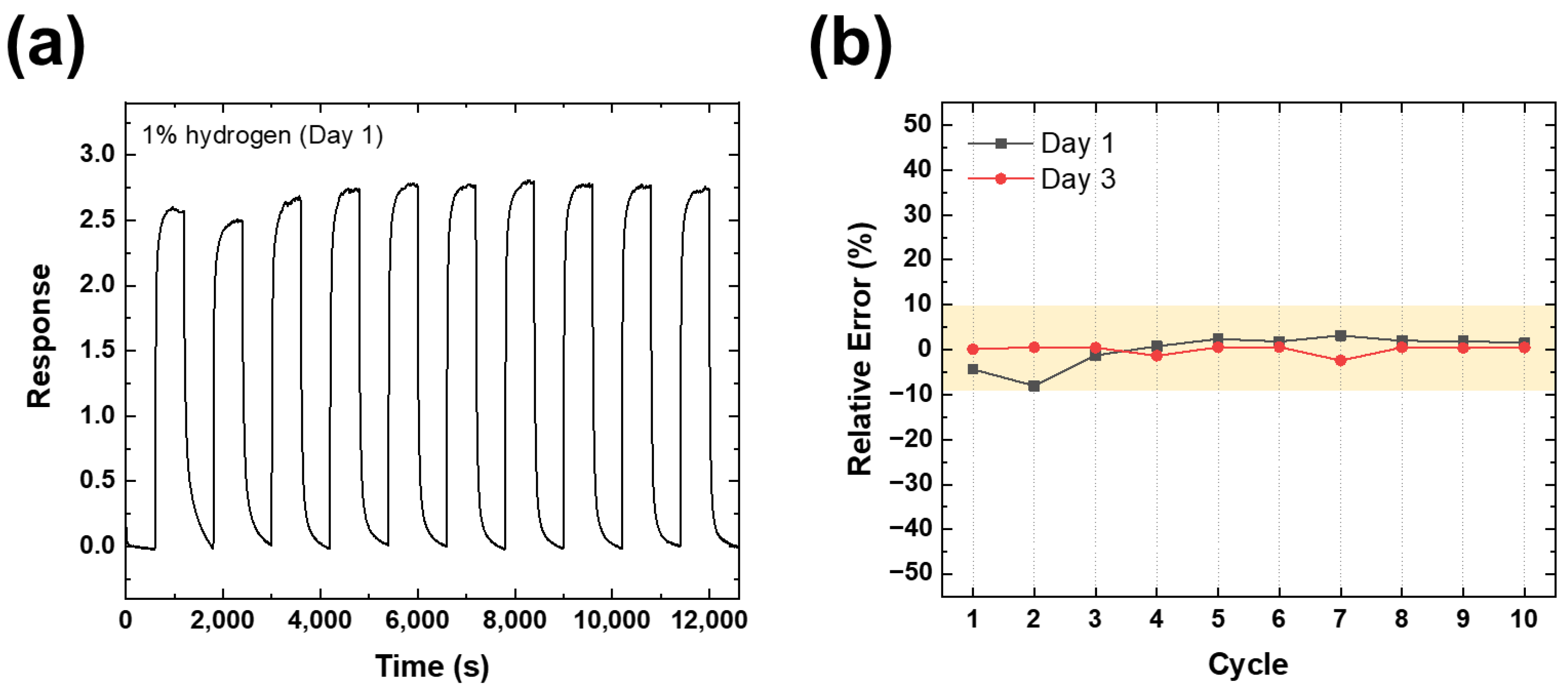
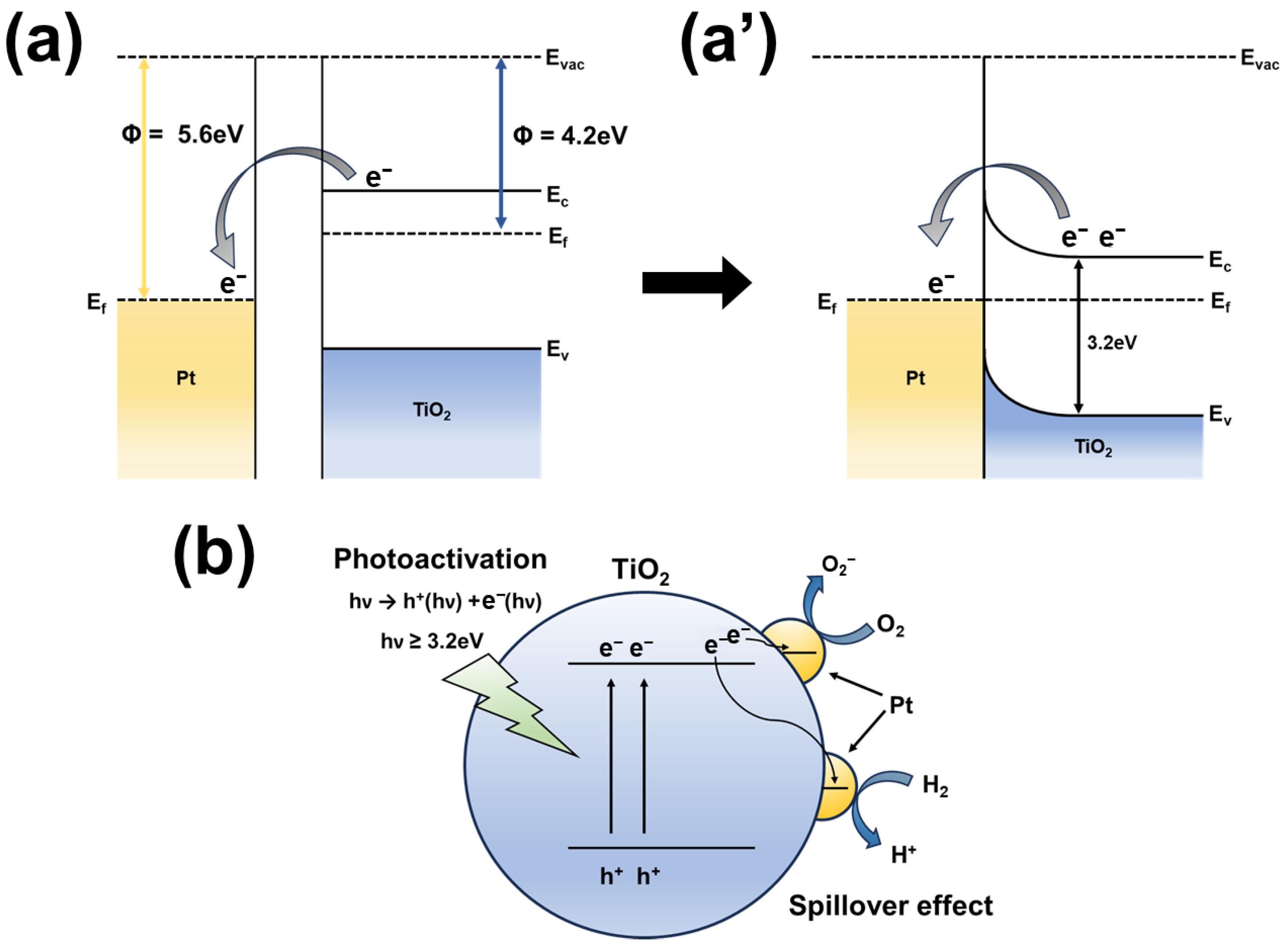
| Gas Concentration | TiO2 NRs/Pt NP (UV) | TiO2 NRs/Pt NP (Dark) | TiO2 NRs (UV) | TiO2 NRs (Dark) |
|---|---|---|---|---|
| 0.5% | 0.24 | 0.11 | 0.14 | 0.09 |
| 0.7% | 0.60 | 0.48 | 0.32 | 0.19 |
| 1.0% | 2.40 | 1.77 | 0.95 | 0.41 |
| No. | Material | Operating Temp. | H2 Concentration | Sensitivity (Response Formula) | Catalyst | UV | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Ni-doped TiO2 | 600 °C | 0.1% | 72% ((Ra − Rg)/Rg) | None | No | [26] |
| 2 | NiO film | 600 °C | 0.5% | 55 ((Ra − Rg)/Rg) | None | No | [27] |
| 3 | SnO2 film/Pd | 300 °C | 1.5% | 9 (Ra/Rg) | Pd | No | [28] |
| 4 | Pd/Ta2O5 diodes | 300 °C | 0.5% | 1000 (Ra/Rg) | Pd | No | [29] |
| 5 | PCz/IDE-Pt | RT | 1% | 281% ((Ra − Rg)/Rg) | Pt | No | [30] |
| 6 | PMMA-Pd-SWNT | RT | 1% | 285 (Ra/Rg) | Pd | No | [31] |
| 7 | TiO2 NRs/Pt NPs | RT | 1% | 2.4 ((Ra − Rg)/Rg) | Pt | Yes | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.-E.; Kim, S.; Yoon, J.; Lee, J.; Park, I.-K.; Kim, K.-K. A UV-Photon-Energy-Integrated Gas Sensor Based on Pt-Nanoparticle-Decorated TiO2 Nanorods for Room-Temperature Hydrogen Detection. Chemosensors 2025, 13, 177. https://doi.org/10.3390/chemosensors13050177
Yang J-E, Kim S, Yoon J, Lee J, Park I-K, Kim K-K. A UV-Photon-Energy-Integrated Gas Sensor Based on Pt-Nanoparticle-Decorated TiO2 Nanorods for Room-Temperature Hydrogen Detection. Chemosensors. 2025; 13(5):177. https://doi.org/10.3390/chemosensors13050177
Chicago/Turabian StyleYang, Ju-Eun, Sohyeon Kim, Jeonghye Yoon, Jeongmin Lee, Il-Kyu Park, and Kyoung-Kook Kim. 2025. "A UV-Photon-Energy-Integrated Gas Sensor Based on Pt-Nanoparticle-Decorated TiO2 Nanorods for Room-Temperature Hydrogen Detection" Chemosensors 13, no. 5: 177. https://doi.org/10.3390/chemosensors13050177
APA StyleYang, J.-E., Kim, S., Yoon, J., Lee, J., Park, I.-K., & Kim, K.-K. (2025). A UV-Photon-Energy-Integrated Gas Sensor Based on Pt-Nanoparticle-Decorated TiO2 Nanorods for Room-Temperature Hydrogen Detection. Chemosensors, 13(5), 177. https://doi.org/10.3390/chemosensors13050177






