Development of Polyphenol–Metal Film-Modified Colored Porous Microspheres for Enhanced Monkeypox Antigen Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Instrumentation and Apparatus
2.3. Synthesis of SMA-MAA Copolymer Microspheres
2.4. Preparation of Porous Microspheres
2.5. Staining of Microspheres
2.6. Encapsulation of Metal-Colored Microspheres
2.7. Conjugation of Colored Microspheres with Antibodies and LFIA Detection
3. Results and Discussion
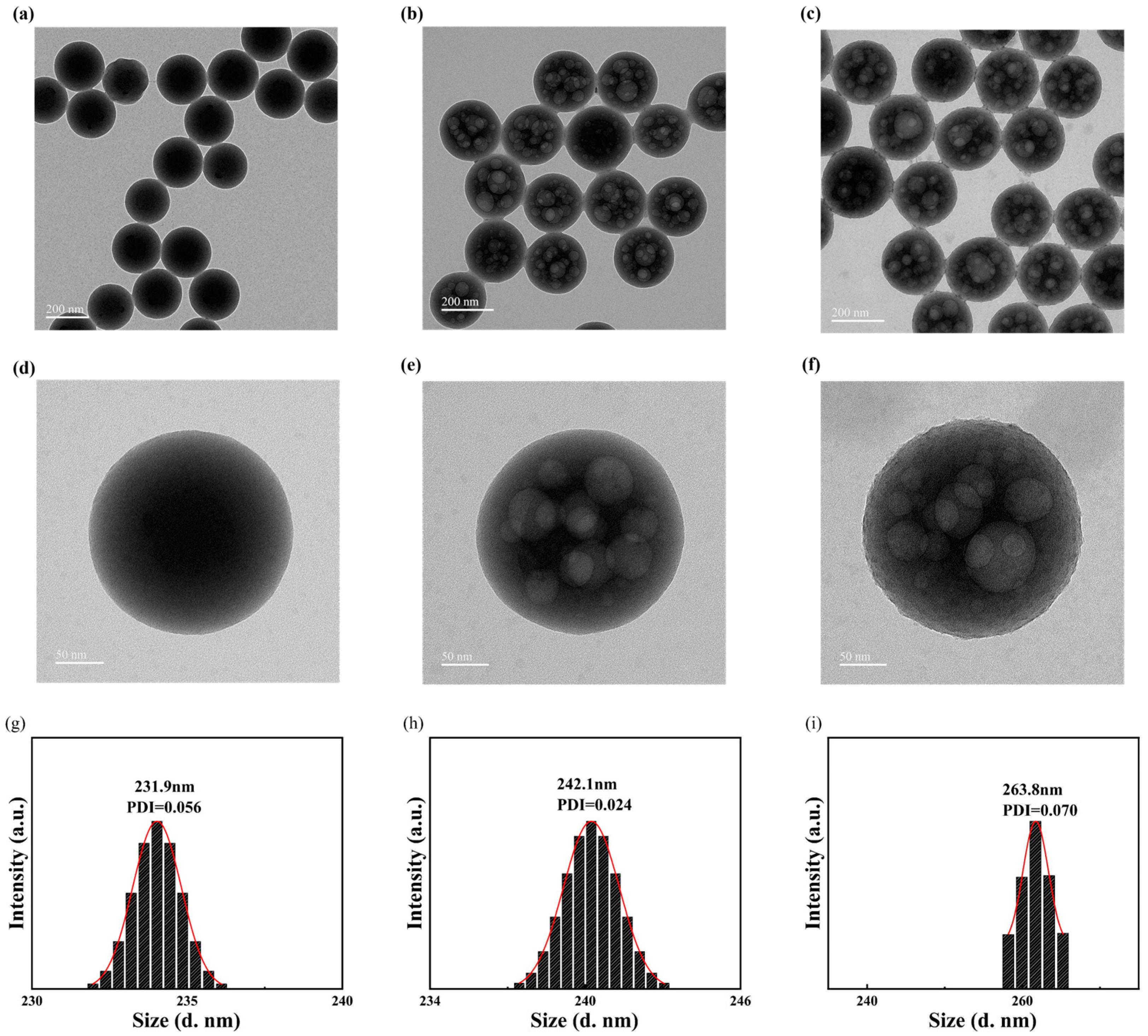
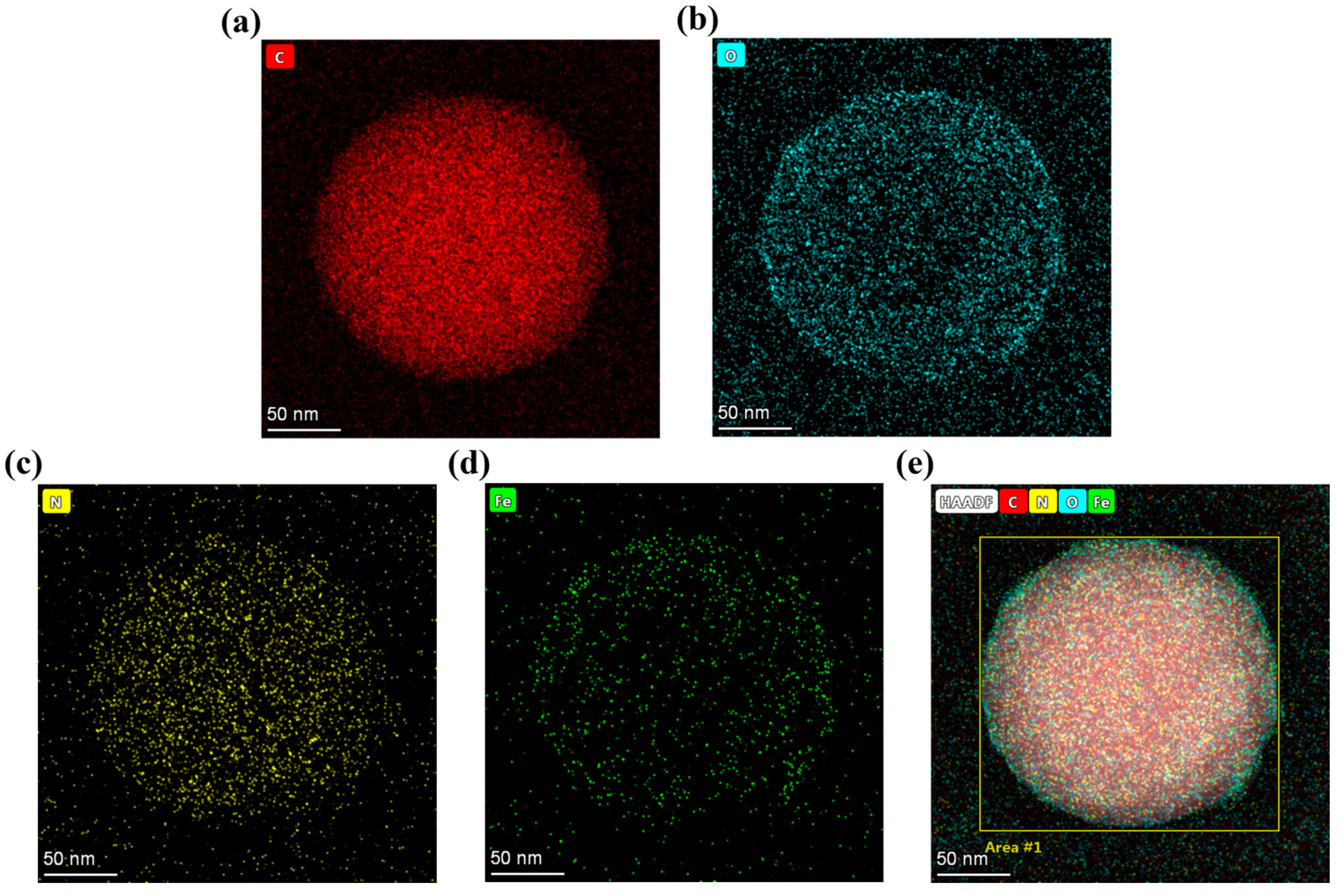
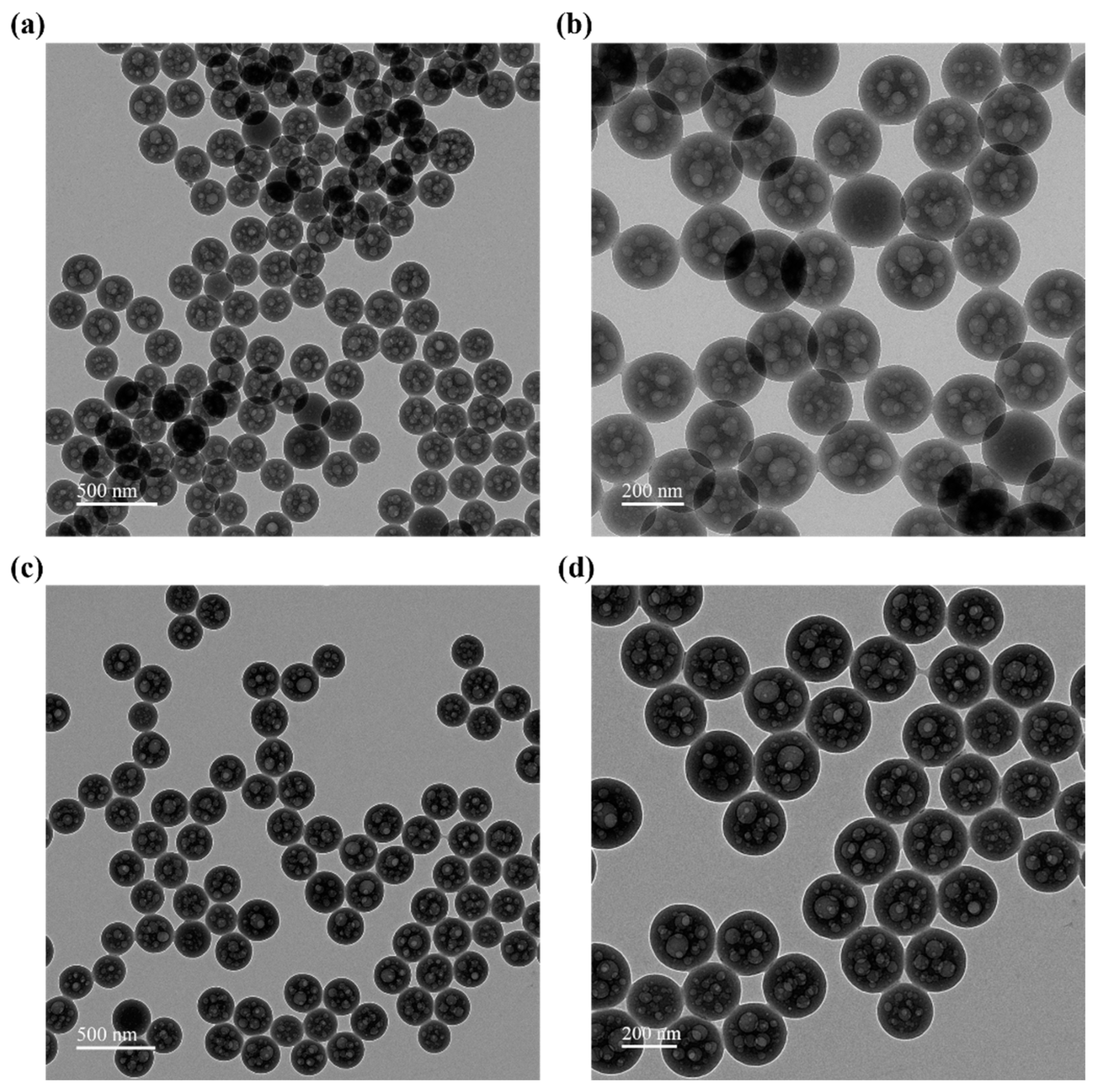
3.1. Characterization of SMA-MAA Microspheres
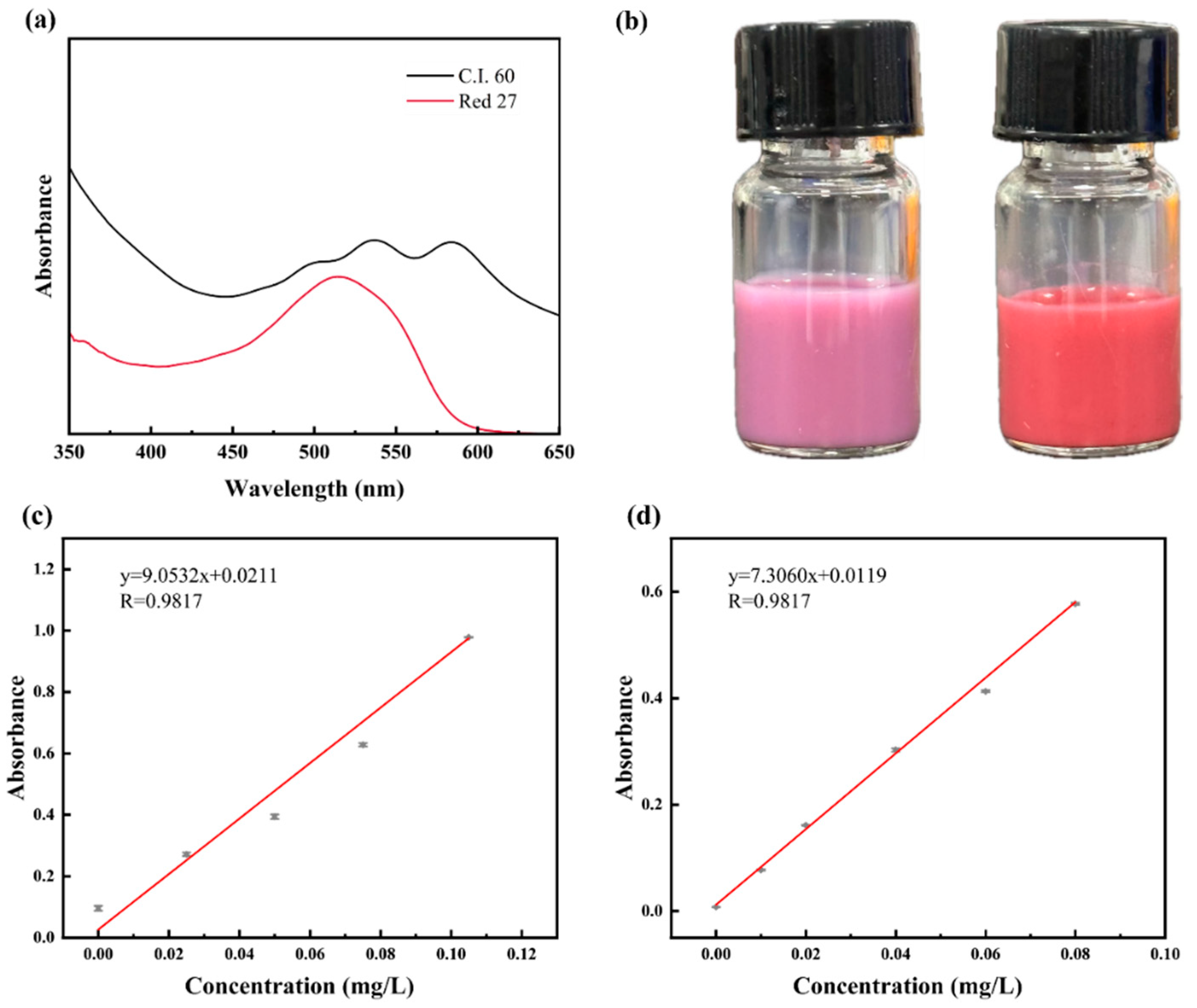
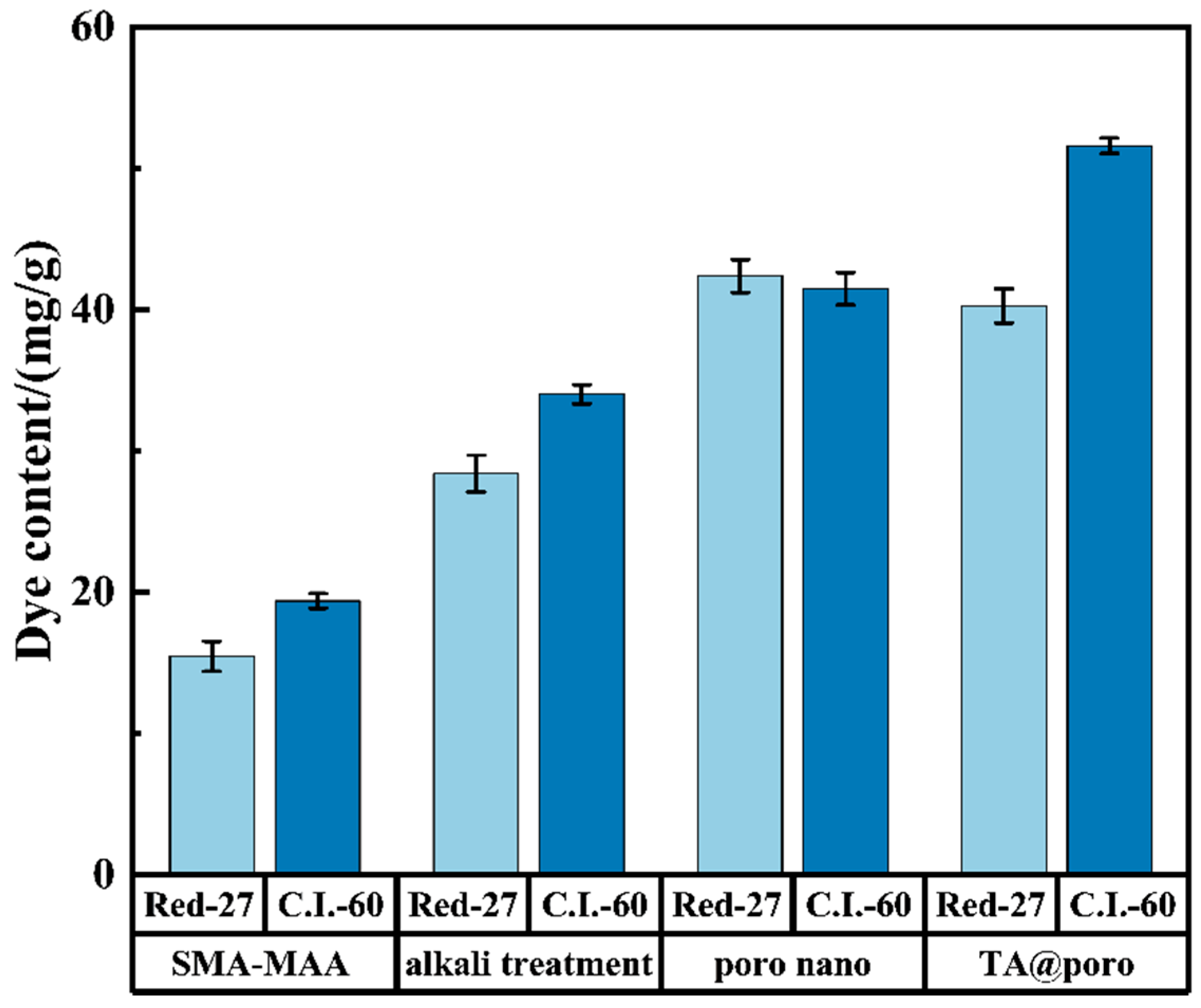
3.2. Dye Content of Colored Microspheres
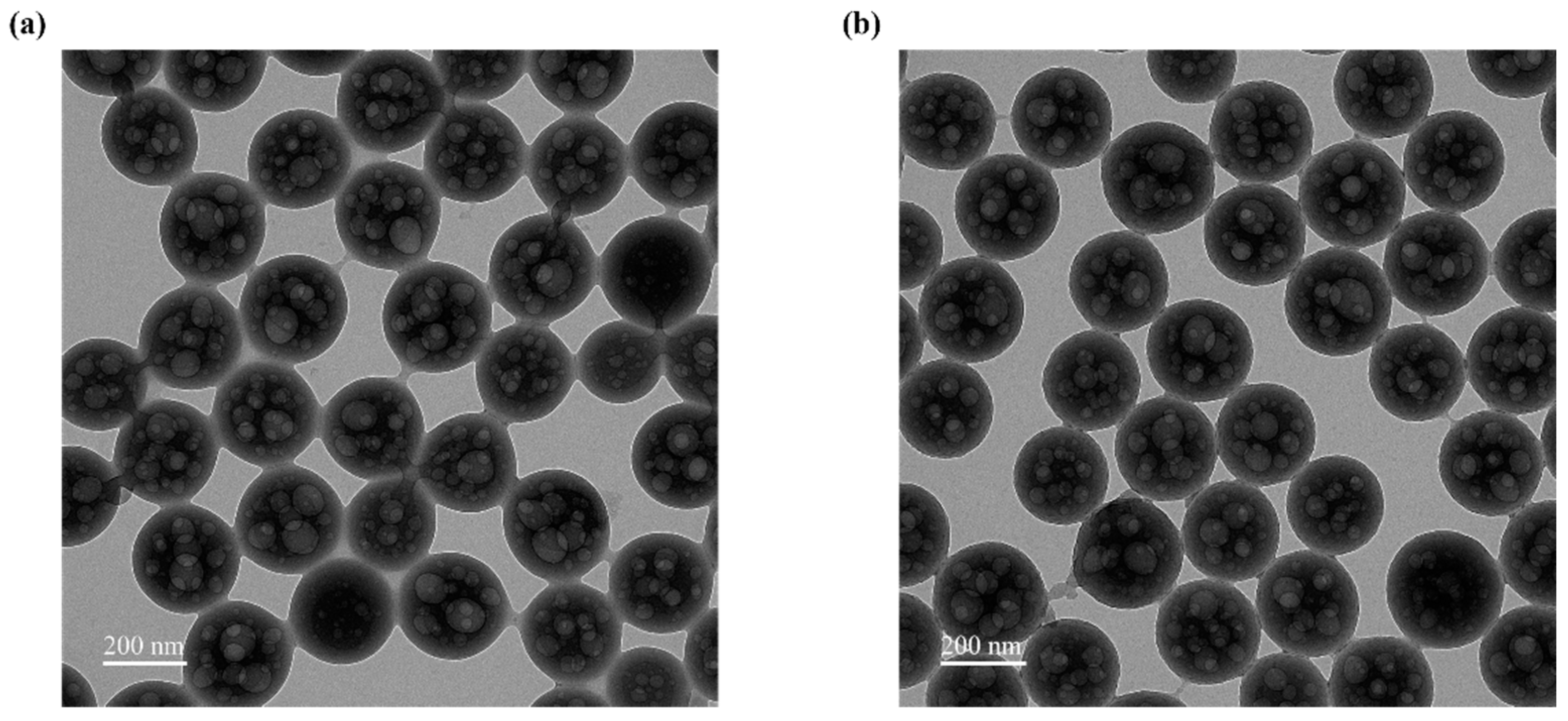
3.3. Effect of Microsphere Treatment and TA on Dye Loading
3.4. Effects of Iron Ion and TA Introduction on the System
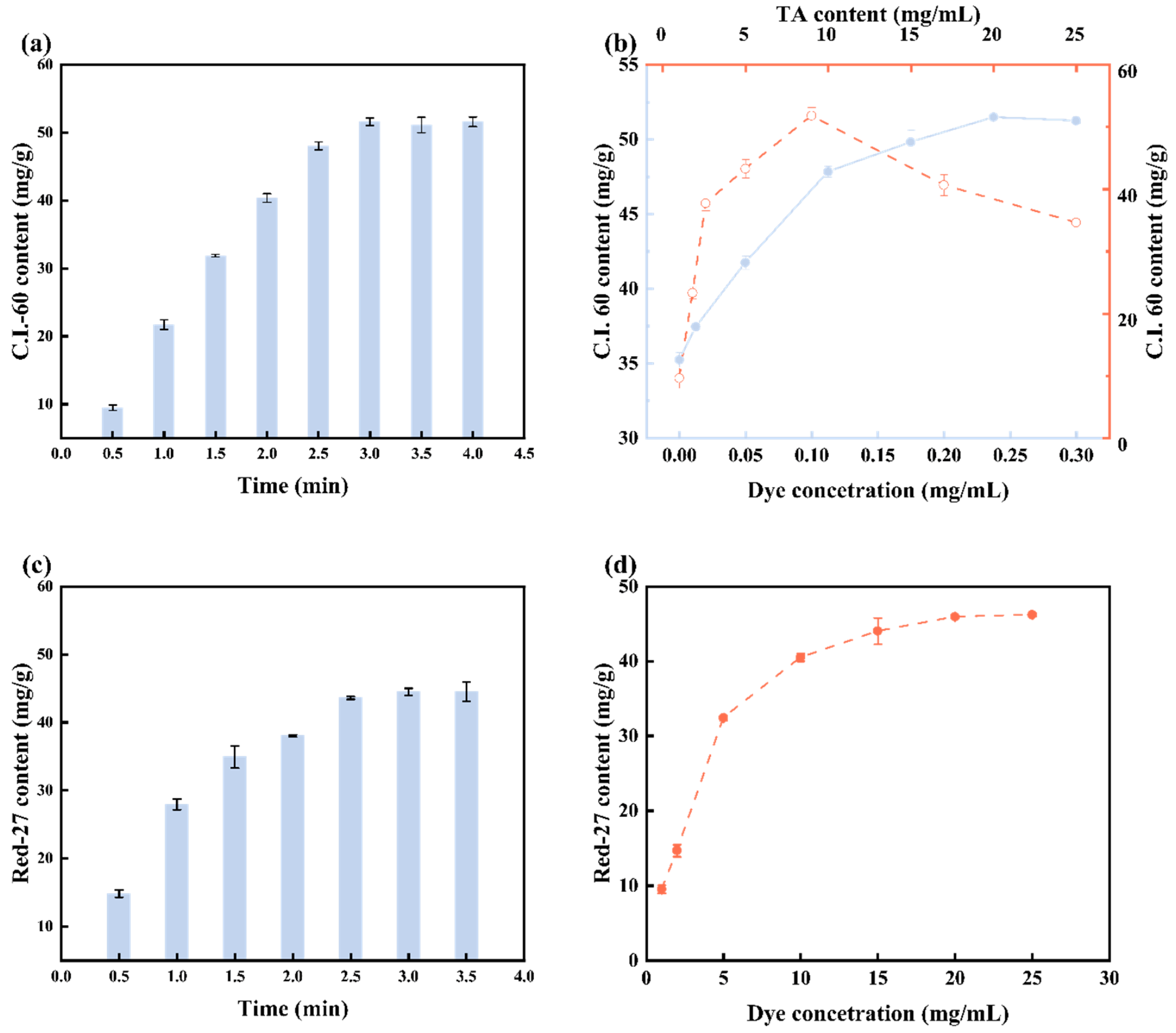
3.5. Optimization of Dyeing Parameters
3.6. Monkeypox Antigen Immunoassay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SMA-MAA | polystyrene-maleic anhydride microspheres |
| C.I.-60 | Disperse Red 60 |
| Red-27 | Solvent Red 27 |
| TA | Tannic acid |
| MPF | Metal–polyphenol film |
References
- Wu, Y.; Xiao, Y.; Ju, Y.; Sun, Y.; He, J.; Liu, T.; He, M.; Wang, K.; Yang, Y. Multi-Mode Sensing Platform for Gallic Acid Determination Based on the Peroxidase-like Activity of Magnetic Fe3O4@Co/Ni/Al-LDH Nanospheres. LWT 2024, 203, 116366. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Li, Z.; Liu, F.; Jing, W. Machine Learning Assisted Multi-Signal Nanozyme Sensor Array for the Antioxidant Phenolic Compounds Intelligent Recognition. Food Chem. 2025, 471, 142826. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Z.; Zhang, X.; Duan, Q.; Zhang, Y.; Midgley, A.C.; Jiao, L.; Liu, R.; Zhu, M.; Kong, D.; et al. Assembly of Genetically Engineered Ionizable Protein Nanocage-Based Nanozymes for Intracellular Superoxide Scavenging. Nat. Commun. 2025, 16, 1123. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, J.; Zhang, C.; Guo, S.; Chen, Y.; Wang, Y.; Huang, X.; Zeng, Q. Ultra-High Capacity and Selectivity for Uranium Fixation by Carbon Nanosphere Supported Hydroxyapatite Nanorod Adsorbent. J. Colloid Interface Sci. 2025, 688, 478–489. [Google Scholar] [CrossRef]
- Ma, Y.; Remón, J.; Luo, L.; Ding, W.; Jiang, Z.; Shi, B. Natural-Based UV-Shielding Additives to Protect Photosensitive Pesticides: Production of Nanoparticles from the Co-Self-Assembly of Lignin and Tannin. Nano Today 2025, 60, 102550. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, H.; Wang, M.; Fang, K.; Song, Y.; Liu, Q.; Chen, W. Controlled Micro-Scale Ink Droplet Spreading on Cotton Fabrics via Cellulose-Based Coatings for Greener Textile Inkjet Printing. Ind. Crops Prod. 2024, 213, 118488. [Google Scholar] [CrossRef]
- Zhang, S.; Fang, K.; Liu, X.; Liu, D.; Qiao, X.; Wang, J.; Sun, F. Enhanced Hydrophobicity and UV Resistance of Cotton Fabrics through the Synergistic Effect of Raspberry-Shaped Colored Nanoparticles and Polymethylhydrosiloxane. Appl. Surf. Sci. 2023, 637, 157849. [Google Scholar] [CrossRef]
- Dong, T.; Sun, G.; Yang, Z.; Jiao, Y.; Li, J.; Lin, Z.; Zhang, X.; Bian, Z.; Liu, A. Sensitive Dual-Channel Lateral Flow Immunoassay Tagged with High Brightness Latex Microsphere for Simultaneous Detection of Respiratory Viral Antigens. Chem. Eng. J. 2024, 497, 154826. [Google Scholar] [CrossRef]
- Chen, Z.; Lai, K.; Wang, A.; Ji, H.; Yu, S.; Fang, Z.; Liu, D.; Peng, J.; Lai, W. Electron Transfer-Driven Nanozymes Boost Biosensor Sensitivity via a Synergistic Signal Amplification Strategy. ACS Nano 2025, 19, 9282–9291. [Google Scholar] [CrossRef]
- Cai, Y.; Dong, T.; Bian, Z.; Liu, H.; Liu, X.; Liu, A. Metal-organic frameworks based fluorescent sensing: Mechanisms and detection applications. Coord. Chem. Rev. 2025, 529, 216470. [Google Scholar] [CrossRef]
- Xie, S.; Zhao, S.; Jiang, X.; Zhu, Y.; Liu, J.; Zhang, B.; Zhao, H. Adsorption Plasticizer by Nanosphere Adsorbent of Persimmon Tannin Binding Bovine Serum Protein. Food Chem. 2025, 464, 141653. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Guan, T.; Su, H.; Zhong, Y.; Cao, F.; Zhang, Y.; Xia, X.; Wang, X.; Bao, N.; Tu, J. Synergistic Interfacial Bonding in Reduced Graphene Oxide Fiber Cathodes Containing Polypyrrole@sulfur Nanospheres for Flexible Energy Storage. Angew. Chem. Int. Ed. 2022, 61, e202212151. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Ye, S.; Kong, C.; Webb, K.; Yi, C.; Zhang, S.; Zhang, Q.; Fourkas, J.T.; Nie, Z. Polymeric Ligand-Mediated Regioselective Bonding of Plasmonic Nanoplates and Nanospheres. J. Am. Chem. Soc. 2020, 142, 17282–17286. [Google Scholar] [CrossRef]
- Tan, L.; He, C.; Chu, X.; Chu, Y.; Ding, Y. Charge-Reversal ZnO-Based Nanospheres for Stimuli-Responsive Release of Multiple Agents towards Synergistic Cancer Therapy. Chem. Eng. J. 2020, 395, 125177. [Google Scholar] [CrossRef]
- Fernandes, R.D.V.; Gomes, P.; Zille, A.; Souto, A.P. The Influence of Chemical Reaction Conditions upon Poly(Styrene-methyl Methacrylate-acrylic Acid) Synthesis: Variations in Nanoparticle Size, Colour and Deposition Methods. Color. Technol. 2020, 136, 101–109. [Google Scholar] [CrossRef]
- Li, S.; Xiao, Y.; Shan, G.; Jia, L. A New Strategy for Structural Color Fabrics: Transfer of Photonic Crystal Coatings from Slides to Fabrics. Dye. Pigment. 2024, 226, 112119. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Li, Z.; Wang, G.; Wang, Y.; Li, P.; Yue, X. Facile Fabrication of Eccentric Hollow Molecularly Imprinted Polymers Microspheres via Miniemulsion One-Step Seed Swelling Polymerization. J. Environ. Chem. Eng. 2024, 12, 113405. [Google Scholar] [CrossRef]
- Zhao, X.; Cui, Y.; He, Y.; Wang, S.; Wang, J. Synthesis of Multi-Mode Quantum Dots Encoded Molecularly Imprinted Polymers Microspheres and Application in Quantitative Detection for Dopamine. Sens. Actuators B Chem. 2020, 304, 127265. [Google Scholar] [CrossRef]
- Du, W.; Wu, P.; Zhao, Z.; Zhang, X. Facile Preparation and Characterization of Temperature-Responsive Hydrophilic Crosslinked Polymer Microspheres by Aqueous Dispersion Polymerization. Eur. Polym. J. 2020, 128, 109610. [Google Scholar] [CrossRef]
- Zhan, Y.; Fu, W.; Xing, Y.; Ma, X.; Chen, C. Advances in Versatile Anti-Swelling Polymer Hydrogels. Mater. Sci. Eng. C 2021, 127, 112208. [Google Scholar] [CrossRef]
- Chen, H.; Zhan, J.; Man, L.; Deng, H.; Zhou, H.; Hao, L.; Zhou, X. High Foliar Retention Tannic Acid/Fe3+ Functionalized Ti-Pillared Montmorillonite Pesticide Formulation with pH-Responsibility and High UV Stability. Appl. Surf. Sci. 2023, 620, 156838. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, N.; Ray, S.S. Recent Advances in Carbon Nanomaterial-Based Adsorbents for Water Purification. Coord. Chem. Rev. 2020, 405, 213111. [Google Scholar]
- Zhang, W.; Roy, S.; Ezati, P.; Yang, D.-P.; Rhim, J.-W. Tannic Acid: A Green Crosslinker for Biopolymer-Based Food Packaging Films. Trends Food Sci. Technol. 2023, 136, 11–23. [Google Scholar] [CrossRef]
- Rahim, M.A.; Ejima, H.; Cho, K.L.; Kempe, K.; Müllner, M.; Best, J.P.; Caruso, F. Coordination-Driven Multistep Assembly of Metal–Polyphenol Films and Capsules. Chem. Mater. 2014, 26, 1645–1653. [Google Scholar] [CrossRef]
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J.; et al. Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. [Google Scholar] [CrossRef]
- Ye, L.; Lei, X.; Xu, X.; Xu, L.; Kuang, H.; Xu, C. Gold-Based Paper for Antigen Detection of Monkeypox Virus. Analyst 2023, 148, 985–994. [Google Scholar] [CrossRef]
- Stefano, J.S.; Silva, L.R.G.E.; Kalinke, C.; Oliveira, P.R.D.; Crapnell, R.D.; Brazaca, L.C.; Bonacin, J.A.; Campuzano, S.; Banks, C.E.; Janegitz, B.C. Human Monkeypox Virus: Detection Methods and Perspectives for Diagnostics. TrAC Trends Anal. Chem. 2023, 167, 117226. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, X.; Liu, K.; Li, Y.; Wang, T.; Fang, Z.; Chen, C.; Tan, X.; Lin, Y.; Xu, Q.; et al. A Comparative Evaluation of Three Diagnostic Assays for the Detection of Human Monkeypox. Viruses 2024, 16, 1286. [Google Scholar] [CrossRef]
- Guo, J.; Chen, S.; Tian, S.; Liu, K.; Ma, X.; Guo, J. A Sensitive and Quantitative Prognosis of C-Reactive Protein at Picogram Level Using Mesoporous Silica Encapsulated Core-Shell up-Conversion Nanoparticle Based Lateral Flow Strip Assay. Talanta 2021, 230, 122335. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Wang, X.; Xu, J.; Wang, D.; Zhang, W. Synthesis of Porous Poly(Styrene-Co-Acrylic Acid) Microspheres through One-Step Soap-Free Emulsion Polymerization: Whys and Wherefores. J. Colloid Interface Sci. 2012, 368, 220–225. [Google Scholar] [CrossRef]
- Zhou, Z.; Rui, Z.; Feng, Y.-N.; Wang, Q.; Lu, T.-D. Alkali-Assisted Metal-Phenolic Network Assembly for Structural Regulation of Nanofiltration Membranes. J. Membr. Sci. 2025, 720, 123771. [Google Scholar] [CrossRef]
- Song, Y.; Fang, K.; Bukhari, M.N.; Zhang, K.; Tang, Z. Improved Inkjet Printability of Dye-Based Inks through Enhancing the Interaction of Dye Molecules and Polymer Nanospheres. J. Mol. Liq. 2021, 324, 114702. [Google Scholar] [CrossRef]
- Zheng, Q.; Fang, K.; Song, Y.; Wang, L.; Hao, L.; Ren, Y. Enhanced Interaction of Dye Molecules and Fibers via Bio-Based Acids for Greener Coloration of Silk/Polyamide Fabric. Ind. Crops Prod. 2023, 195, 116418. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Y.; Zheng, Q.; Wu, J.; Zhang, H.; Fu, R.; Ren, Y. Color and Functionality Construction of Cellulose Towels with Biological Monascus Pigment Based on a Nano-Suspension System. Int. J. Biol. Macromol. 2024, 282, 137351. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Bai, Y.; Zhao, D.; Wang, Q. Aggregation of Carboxyl-Modified Polystyrene Nanoplastics in Water with Aluminum Chloride: Structural Characterization and Theoretical Calculation. Water Res. 2022, 208, 117884. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhou, S.; Chen, T.; Li, J.; Shen, H.; Chai, Y.; Li, L.S. Quantitative and rapid detection of C-reactive protein using quantum dot-based lateral flow test strip. Anal. Chim. Acta 2018, 1008, 1–7. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, P.; Xing, X.; Lv, Q.; Jiang, Y.; Jiang, Y.; Wang, Z. Improving the fluorescence intensity of lanthanide-doped microspheres via incorporation of lauryl methacrylate: Synthesis and their application in C-reactive protein detection. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 653, 130065. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.-Z.; Zhang, C.-F.; Ding, S.-N. Development of Polyphenol–Metal Film-Modified Colored Porous Microspheres for Enhanced Monkeypox Antigen Detection. Chemosensors 2025, 13, 142. https://doi.org/10.3390/chemosensors13040142
Zhang W-Z, Zhang C-F, Ding S-N. Development of Polyphenol–Metal Film-Modified Colored Porous Microspheres for Enhanced Monkeypox Antigen Detection. Chemosensors. 2025; 13(4):142. https://doi.org/10.3390/chemosensors13040142
Chicago/Turabian StyleZhang, Wei-Zhi, Chen-Fei Zhang, and Shou-Nian Ding. 2025. "Development of Polyphenol–Metal Film-Modified Colored Porous Microspheres for Enhanced Monkeypox Antigen Detection" Chemosensors 13, no. 4: 142. https://doi.org/10.3390/chemosensors13040142
APA StyleZhang, W.-Z., Zhang, C.-F., & Ding, S.-N. (2025). Development of Polyphenol–Metal Film-Modified Colored Porous Microspheres for Enhanced Monkeypox Antigen Detection. Chemosensors, 13(4), 142. https://doi.org/10.3390/chemosensors13040142






