Design, Fabrication and Validation of Chemical Sensors for Detecting Hydrocarbons to Facilitate Oil Spillage Remediation
Abstract
1. Introduction
2. Theoretical and Empirical Background
2.1. Theory of Swelling Mechanism
2.2. Mechanism of Electrically Conducting Polymer Nanocomposites
3. Materials and Methods
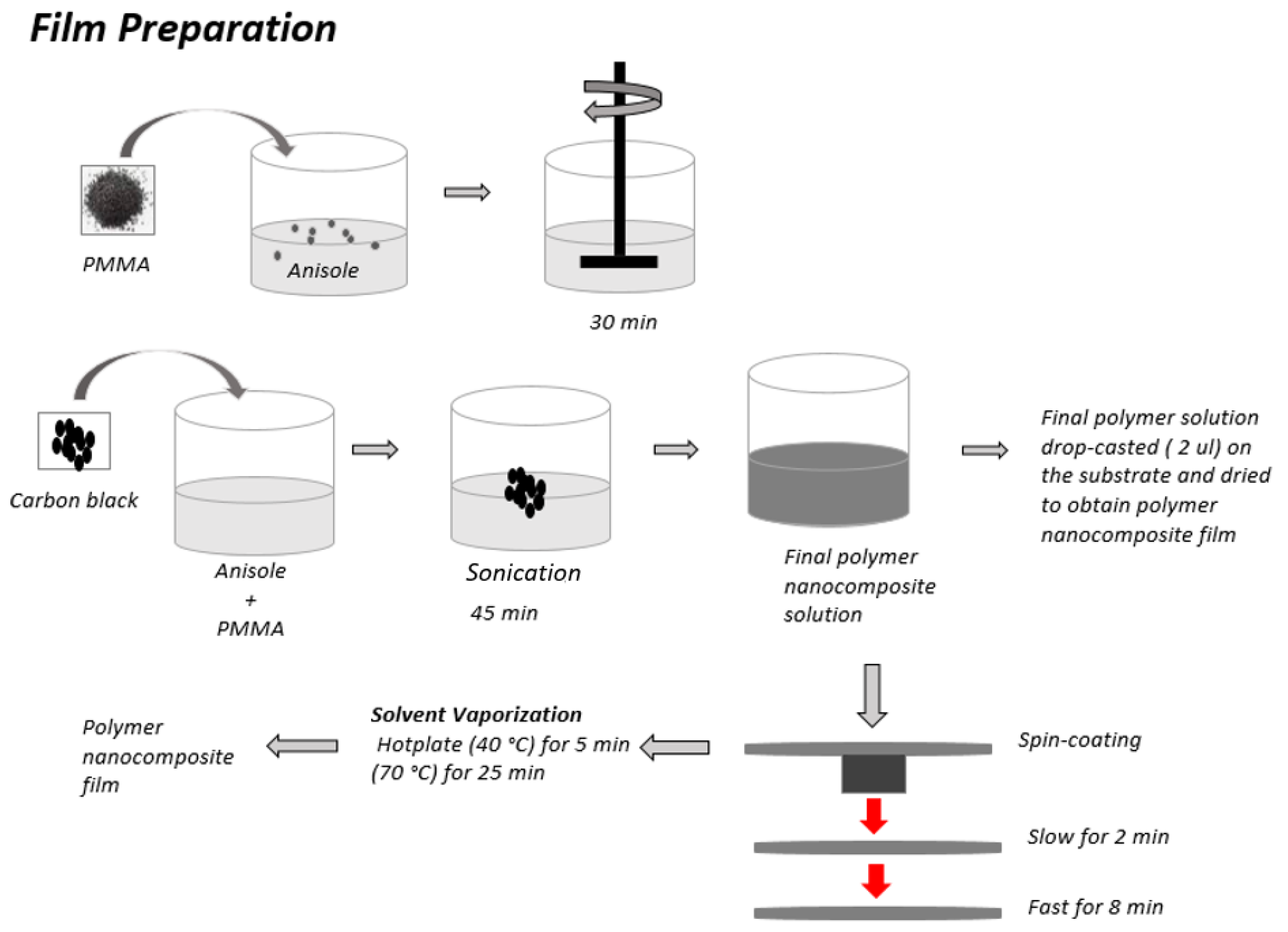
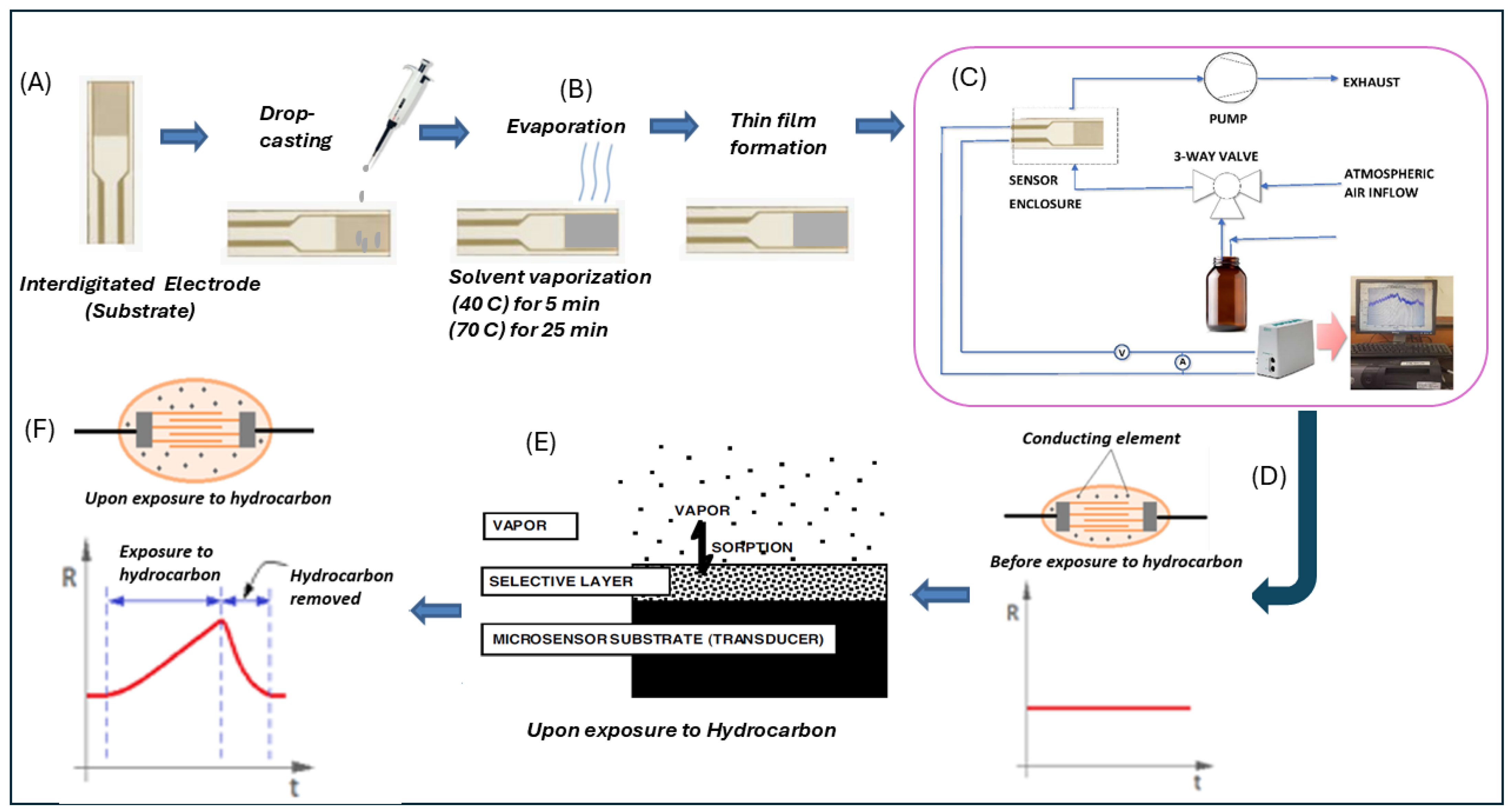
3.1. Preparation of Conducting Polymer Composite
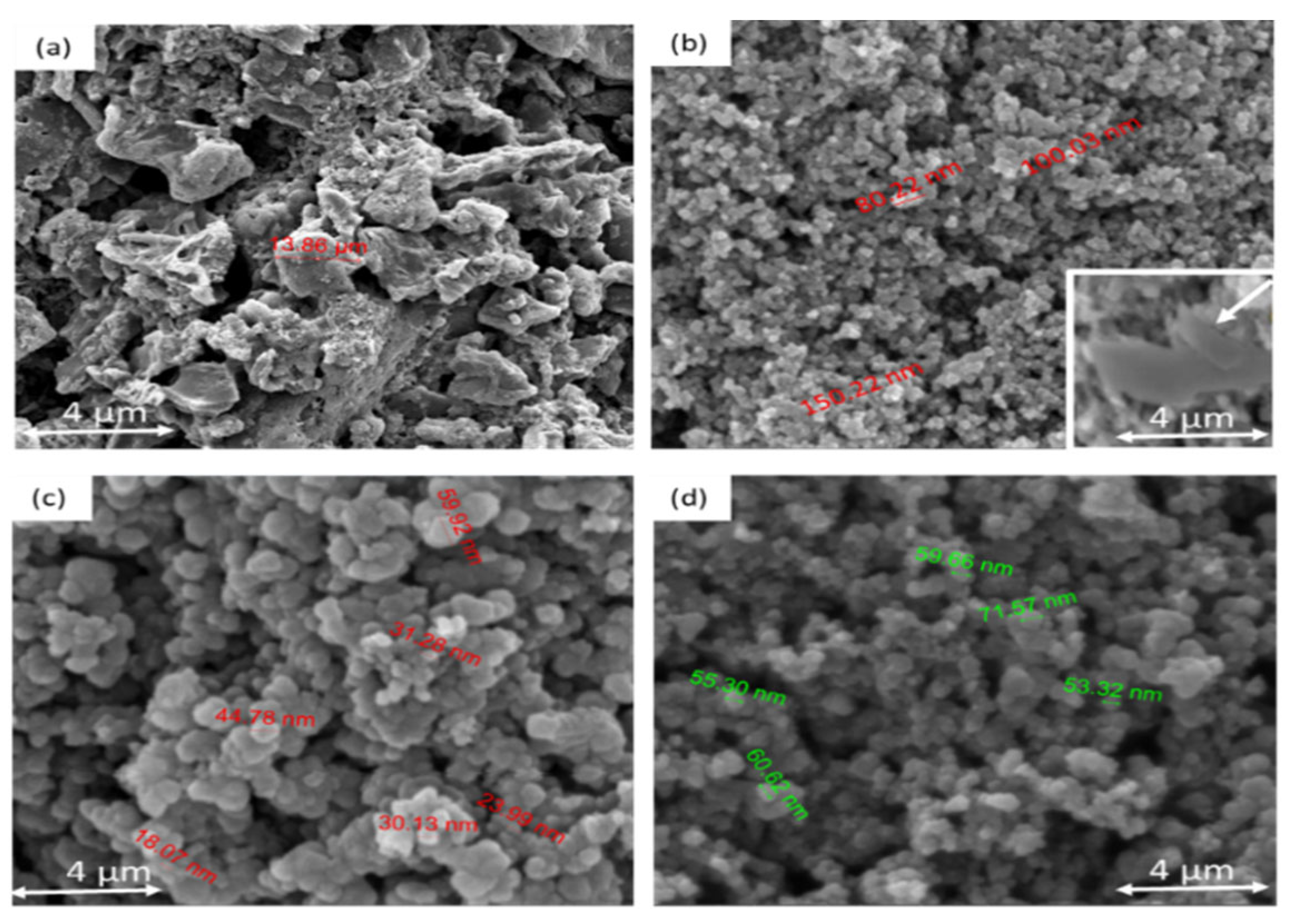
3.2. Investigation of the Morphology of the Composites Using SEM
4. Results and Discussion
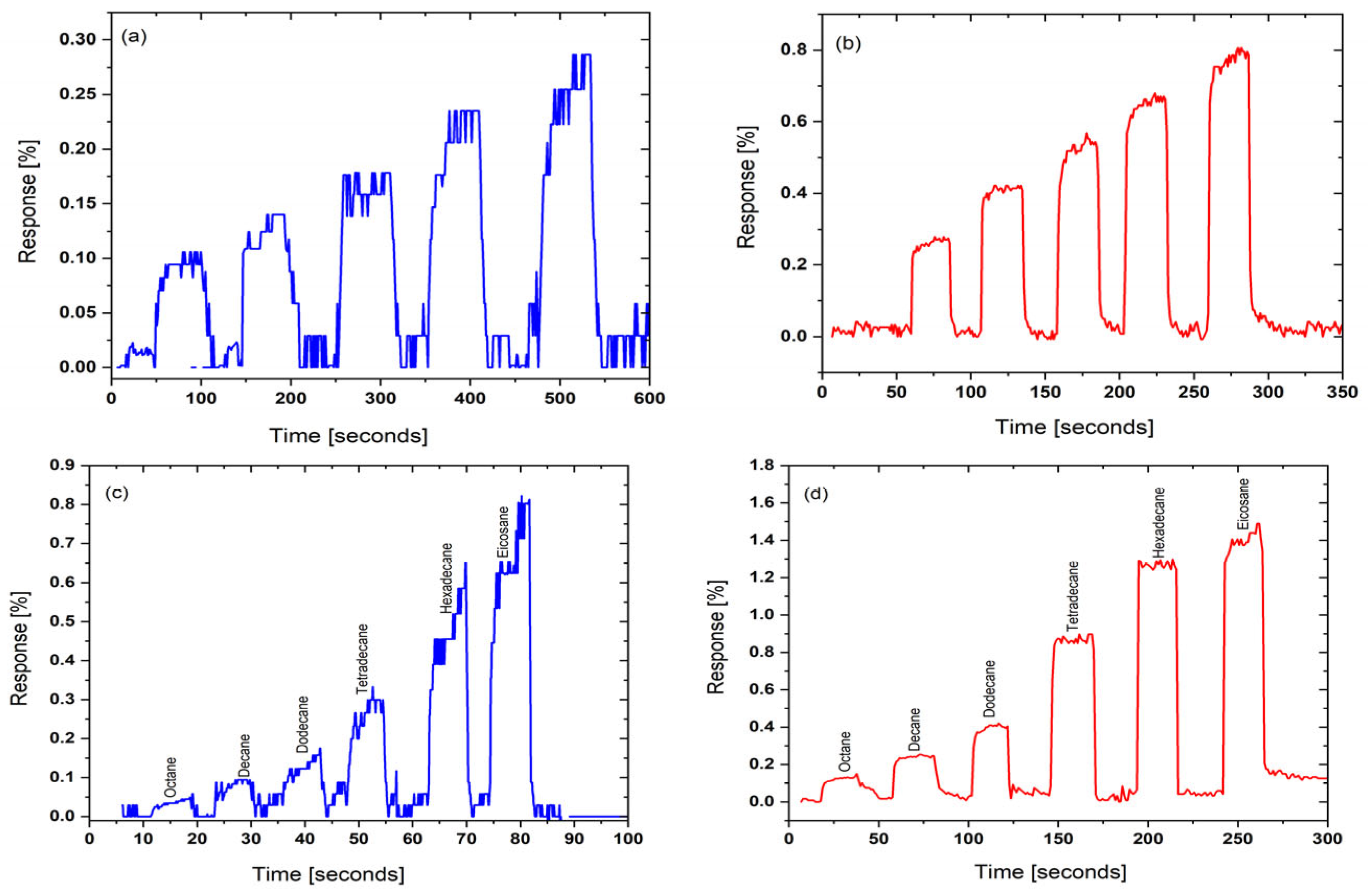
4.1. Sensor Performance
4.2. Calculation of Concentration Using the Antoine Equation
4.3. Concentration–Response Analysis
4.4. Limit of Detection (LOD)
5. Conclusions
6. Future Research Opportunities
- The integration of chemiresistors within an automated multisensor array system. This will allow sensor probes to be electrically connected to chambers deployed at different depths for real-time monitoring;
- Fickian diffusion models can be used for analyzing the responses of films with parallel arrangements of swellable resistors, as well as statistical techniques like Fisher linear discriminant analysis, principal component analysis, and artificial neural network algorithms to facilitate discriminations between mixtures;
- The integration of automated GPS loggers can facilitate the precise positioning and mapping of large sites, whilst automatic re-zeroing before each measurement could ensure baseline stability and the accuracy of measurements;
- For practical applications, a single sensor may not adequately discriminate between the wide range of hydrocarbons in contaminated soils. Thus, there might be a need for further research into chemiresistors within a multisensor array environment, utilizing electronic nose technology for petroleum hydrocarbon detection and classification.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Presidency Ministry (Spain). Royal Decree 9/2005 of 14 January Which Establishes a List of Potentially Soil Contaminating Activities and Criteria and Standards for Declaring That Sites Are Contaminated; Official State Bulletin: Madrid, Spain, 2005; pp. 1833–1843. [Google Scholar]
- Weisman, W. Analysis of Petroleum Hydrocarbons in Environmental Media. Total Petroleum Hydrocarbon Criteria Working Group (TPHCWG) Series, 1; Amherst Scientific Publishers: Amherst, MA, USA, 1998; Available online: https://books.google.co.uk/books?id=CckOAQAAMAAJ (accessed on 10 April 2025).
- Dutch Ministry of Housing Spatial Planning and the Environment (VROM). Soil Remediation Circular 2009. Staatscourant 3 April 2012, Nr. 6563; Ministry of Housing Spatial Planning and the Environment: The Hague, The Netherlands, 2012. [Google Scholar]
- Alinnor, I.J.; Ogukwe, C.E.; Nwagbo, N.C. Characteristic Level of Total Petroleum Hydrocarbon in Soil and Groundwater of Oil Impacted Area in the Niger Delta Region, Nigeria. J. Environ. Earth Sci. 2014, 4, 188–195. [Google Scholar]
- Lieutenant, C.; Ross, N.M.; USAF, BSC for The Air Force Center For Environmental Excellence. Using Risk-Based Standards Will Shorten Cleanup Time At Petroleum Contaminated Sites; 1994. Available online: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/2860096 (accessed on 1 April 2025).
- Falih, K.T.; Razali, S.F.M.; Maulud, K.N.A.; Rahman, N.A.; Abba, S.I.; Yaseen, Z.M. Assessment of petroleum contamination in soil, water, and atmosphere: A comprehensive review. Int. J. Environ. Sci. Technol. 2024, 21, 8803–8832. [Google Scholar] [CrossRef]
- Mitkidou, S.; Kokkinos, N.; Emmanouilidou, E.; Yohannah, Y.; Spanos, T.; Chatzichristou, C.; Ene, A. Investigation of Petroleum Hydrocarbon Fingerprints of Water and Sediment Samples of the Nestos River Estuary in Northern Greece. Appl. Sci. 2022, 12, 1636. [Google Scholar] [CrossRef]
- Mace, G.B.; Deborah, N.V.; Ron, A.H.; Yim, U.H. Long-Term Ecological Impacts from Oil Spills: Comparison of Exxon Valdez, Hebei Spirit, and Deepwater Horizon. Environ. Sci. Technol. 2020, 54, 6456–6467. [Google Scholar] [CrossRef]
- Response to Oil Spills. Understanding Oil Spills and Oil Spill Response; United States Environmental Protection Agency: Washington, DC, USA, 1999; Volume 7, pp. 37–44.
- Majors, R.E. Sample Preparation Fundamentals for Chromatography; Agile Technology: Aliso Viejo, CA, USA, 2013; p. 5991-3326EN. [Google Scholar]
- Almeida, C.M.M. Overview of sample preparation and chromatographic methods to analysis pharmaceutical active compounds in waters matrices. Separations 2021, 8, 16. [Google Scholar] [CrossRef]
- Yin, J.; Wu, M.; Lin, R.; Li, X.; Ding, H.; Han, L.; Yang, W.; Song, X.; Li, W.; Qu, H. Application and development trends of gas chromatography–ion mobility spectrometry for traditional Chinese medicine, clinical, food and environmental analysis. Microchem. J. 2021, 168, 106527. [Google Scholar] [CrossRef]
- Tovar-Lopez, F.J. Recent Progress in Micro- and Nanotechnology-Enabled Sensors for Biomedical and Environmental Challenges. Sensors 2023, 23, 47–49. [Google Scholar] [CrossRef]
- Petrosense. Portable Hydrocarbon Analyzer PHA-100Plus. Available online: https://www.petro-online.com/news/analytical-instrumentation/11/petrosense/chile-selects-portable-hydrocarbon-analyser-to-improve-environmental-performance/30813 (accessed on 5 April 2025).
- PETROSENSE® PHA-100Plus—Portable Hydrocarbon Analyzer Training Guide. Environ. Protect. 2011, 75243, 1–48.
- FCI Environmental Inc. PetroSense® CMS-4000 Continuous Monitoring System. Brochure 2005. p. 2. Available online: https://www.airmet.com.au/assets/documents/product/165/file_1438308455_752.pdf (accessed on 4 April 2024).
- Khudur, L.S.; Ball, A.S. RemScan: A tool for monitoring the bioremediation of Total Petroleum Hydrocarbons in contaminated soil. MethodsX 2018, 5, 705–709. [Google Scholar] [CrossRef]
- Chýlková, J.; Cuhorka, J.; Mikulášek, P. The effect of surfactants upon spectrophotometric monitoring of the efficiency of removal of crude petroleum products from waste water by means of pressure-driven membrane processes. Wseas Trans. Environ. Dev. 2014, 10. [Google Scholar]
- Arrieta, M.; Swanson, B.; Fogg, L.; Bhushan, A. Review of linear and nonlinear models in breath analysis by Cyranose 320. J. Breath Res. 2023, 17, 36005. [Google Scholar] [CrossRef]
- Yaqoob, U. Chemical Gas Sensors: Recent Developments, Challenges. Sensors 2021, 21, 2877. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Shi, X.; Yao, Z.; Zhi, J.; Hu, L.; Yan, R.; Shi, C.; Yu, H.-D.; Huang, W. Fully paper-integrated hydrophobic and air permeable piezoresistive sensors for high-humidity and underwater wearable motion monitoring. NPJ Flex Electron 2023, 7, 13. [Google Scholar] [CrossRef]
- Farzi, G.; Gheysipour, M. Encapsulation with Polymers; Elsevier: Amsterdam, The Netherlands, 2023; Volume 2. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Xu, Y.; Wen, L.; Dong, J.; Wang, L. Enhancement of the Surface Hydrophilicity of Poly (Vinyl Chloride). Using Hyperbranched Polylysine with Polydopamine. Coatings 2024, 1, 103. [Google Scholar] [CrossRef]
- Liu, X.M. Mechanical response of composite materials prepared with polyurethane elastomers and polyvinyl chloride films. J. Mech. Behav. Biomed. Mater. 2023, 146, 106006. [Google Scholar] [CrossRef]
- Rotaru, I.M.; Dobrotă, D.; Miriţoiu, C.M.; Dimulescu, C.S. Optimization of the composition of polyvinyl chloride based composite materials with rubber matrices and fly ash additions respectively. Polym. Test. 2023, 129, 108280. [Google Scholar] [CrossRef]
- Vergadou, N.; Theodorou, D.N. Molecular modeling investigations of sorption and diffusion of small molecules in Glassy polymers. Membranes 2019, 9, 98. [Google Scholar] [CrossRef]
- Saleem, M.; Asfour, A.A.; De Kee, D.; Harison, B. Diffusion of organic penetrant through lowdensity polyethylene (LDPE) films: Effect of size and shape of the penetrant molecules. J. Appl. Polym. Sci. 1989, 37, 617–625. [Google Scholar] [CrossRef]
- Park, J.K.; Nibras, M. Mass flux of organic chemicals through polyethylene geomembranes. Water Environ. Res. 1993, 65, 227–237. [Google Scholar] [CrossRef]
- Tammaro, D.; Lombardi, L.; Scherillo, G.; Di Maio, E.; Ahuja, N.; Giuseppe, M. Modelling sorption thermodynamics and mass transport of n-hexane in a propylene-ethylene elastomer. Polymers 2021, 13, 1157. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Naik, H.G. Chemical compatibility testing of geomembranes- Sorption/desorption, diffusion and swelling phenomena. Geomembr. Geotext. 1998, 16, 333–354. [Google Scholar] [CrossRef]
- Frisch, H.L. Diffusion in Polymers. In Jounal of Applied Polymer Science; Crank, J., Park, G.S., Eds.; Academic Press: London, UK, 1968; Volume 14, p. 452. [Google Scholar]
- Crank, J. The Mathematics of Diffusion; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- Comyn, J. Polymer Permeability; Elsevier: Essex, UK, 1985. [Google Scholar]
- Neogi, P. Diffusion in Polymers; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Kang, M.J.; Heo, Y.J.; Jin, F.L.; Park, S.J. A review: Role of interfacial adhesion between carbon blacks and elastomeric materials. Carbon Lett. 2016, 18, 1–10. [Google Scholar] [CrossRef]
- Jovanović, V.; Samaržija-Jovanović, S.; Budinski-Simendić, J.; Marković, G.; Marinović-Cincović, M. Composites based on carbon black reinforced NBR/EPDM rubber blends. Compos. Part B Eng. 2013, 45, 333–340. [Google Scholar] [CrossRef]
- Araby, S.; Meng, Q.; Zhang, L.; Zaman, I.; Majewski, P.; Ma, J. Elastomeric composites based on carbon nanomaterials. Nanotechnology 2015, 26, 112001. [Google Scholar] [CrossRef]
- Griffini, G.; Suriano, R.; Turri, S. Correlating mechanical and electrical properties of filler-loaded polyurethane fluoroelastomers: The influence of carbon black. Polym. Eng. Sci. 2012, 52, 2543–2551. [Google Scholar] [CrossRef]
- Korai, Y.; Wang, Y.G.; Yoon, S.H.; Ishida, S.; Mochida, I.; Nakagawa, Y.; Matsumura, Y. Effects of carbon black addition on preparation of meso-carbon microbeads. Carbon 1997, 35, 875–884. [Google Scholar] [CrossRef]
- Kanno, K.; Yoon, K.; Fernandez, J.; Mochida, I.; Fortin, F.; Korai, Y. Modifications to carbonization of mesophase pitch by addition of carbon blacks. Carbon 1997, 35, 1627–1637. [Google Scholar] [CrossRef]
- Ulfah, I.M.; Fidyaningsih, R.; Rahayu, S.; Fitriani, D.A.; Saputra, D.A.; Winarto, D.A.; Wisojodharmo, L.A. Influence of carbon black and silica filler on the rheological and mechanical properties of natural rubber compound. Procedia Chem. 2015, 16, 258–264. [Google Scholar] [CrossRef]
- Kim, J. Conductive carbon black filled composite (I): The effect of carbon block on the conductivity. Elastomers Compos 1998, 33, 355–362. [Google Scholar]
- Wang, J.; Vincent, J.; Quarles, C.A. Review of positron annihilation spectroscopy studies of rubber with carbon black filler. Nucl. Instrum. Methods Phys. Res. 2005, 241, 271–275. [Google Scholar] [CrossRef]
- Tzounis, L.; Debnath, S.; Rooj, S.; Fischer, D.; Mäder, E.; Das, A.; Stamm, M.; Heinrich, G. High performance natural rubber composites with a hierarchical reinforcement structure of carbon nanotube modified natural fibers. Mater. Des. 2014, 58, 1–11. [Google Scholar]
- Omnès, B.; Thuillier, S.; Pilvin, P.; Grohens, Y.; Gillet, S. Effective properties of carbon black filled natural rubber: Experiments and modeling. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1141–1149. [Google Scholar]
- Askeland, D.; Fulay, P.; Wright, W. The Science of Engineering and Materials, 6th ed.; Cengage Learning: Boston, MA, USA, 2011; Volume 26. [Google Scholar] [CrossRef]
- Murphy, J. Chapter 7—Modifying Specific Properties: Appearance—Black and White Pigmentation. In Additives for Plastics Handbook, 2nd ed.; Murphy, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 73–92. Available online: https://api.semanticscholar.org/CorpusID:138851324 (accessed on 1 April 2025).
- Sisk, B.C.; Lewis, N.S. Vapor sensing using polymer/carbon black composites in the percolative conduction regime. Langmuir 2006, 22, 7928–7935. [Google Scholar] [CrossRef]
- Li, J.; Ma, P.C.; Chow, W.S.; To, C.K.; Tang, B.Z.; Kim, J.K. Correlations between percolation threshold, dispersion state, and aspect ratio of carbon nanotubes. Adv. Funct. Mater. 2007, 17, 3207–3215. [Google Scholar] [CrossRef]
- Parnian, P.; D’Amore, A. Investigating the Electrical and Mechanical Properties of Polystyrene (PS)/Untreated SWCNT Nanocomposite Films. J. Compos. Sci. 2024, 8, 49. [Google Scholar] [CrossRef]
- Doleman, B.J.; Lonergan, M.C.; Severin, E.J.; Vaid, T.P.; Lewis, N.S. Quantitative Study of the Resolving Power of Arrays of Carbon Black Polymer Composites in Various Vapor Sensing Tasks. Anal. Chem. 1998, 70, 4177–4190. [Google Scholar] [PubMed]
- Welker, R.W. Basics and Sampling of Particles for Size Analysis and Identification; Elsevier: Amsterdam, The Netherlands, 2012; Volume 4. [Google Scholar] [CrossRef]
- Lapuerta, M.; Canoira, L. The Suitability of Fatty Acid Methyl Esters (FAME) as Blending Agents in Jet A-1; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Fink, J. Dispersions, Emulsions, and Foams. In Petroleum Engineer’s Guide to Oil Field Chemicals and Fluids, Gulf Professional Publishing; Fink, J.K., Ed.; Gulf Professional Publishing: Houston, TX, USA, 2012; pp. 663–694. [Google Scholar] [CrossRef]
- Frisch, K.C.; Klempner, D. Advances in Interpenetrating Polymer Networks. Polym. Int. 1995, 38, 105–106. [Google Scholar]
- Burke, J. Part 2—The Hildebrand Solubilty Parameter; American Institute for Conservation: Washington, DC, USA, 2020; pp. 12–24. [Google Scholar]
- Synovec, R. Chemical Separation Techniques, Angel Kelley. Available online: https://slideplayer.com/slide/11702144/ (accessed on 12 October 2022).
- Freund, M.S.; Lewis, N.S. A chemically diverse conducting polymer-based electronic nose. Proc Natl. Acad. Sci. USA 1995, 92, 2652–2656. [Google Scholar]
- Lonergan, M.C.; Severin, E.J.; Doleman, B.J.; Beaber, S.A.; Grubbs, R.H.; Lewis, N.S. Array-based vapor sensing using chemically sensitive, carbon black-Polymer resistors. Chem. Mater. 1996, 8, 2298–2312. [Google Scholar] [CrossRef]
- Brighenti, R.; Cosma, M.P. Swelling mechanism in smart polymers responsive to mechano-chemical stimuli. J. Mech. Phys. Solids 2020, 143, 104011. [Google Scholar] [CrossRef]
- Dobrokhotov, V.; Larin, A.; Sowell, D. Vapor trace recognition using a single nonspecific chemiresistor. Sensors 2013, 137, 9016–9028. [Google Scholar] [CrossRef]
- Tierney, M.; Kim, H. Electrochemical gas sensor with extremely fast response times. Anal. Chem. 1993, 65, 3435–3440. [Google Scholar] [CrossRef]
- Stetter, J.; Li, J. Amperometric gas sensors—A review. Chem. Rev. 2008, 108, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Severin, E.; Doleman, B.; Lewis, N. An investigation of the concentration dependence and response to analyte mixtures of carbon black/insulating organic polymer composite vapor detectors. Anal.Chem. 2000, 72, 658–668. [Google Scholar]
- Ho, C.; Hughes, R. In-Situ Chemiresistor Sensor Package for Real-Time Detection of Volatile Organic Compounds in Soil and Groundwater. Sensors 2002, 2, 23–34. [Google Scholar] [CrossRef]
- Prager, S.; Long, F.A. Diffusion of Hydrocarbons in Polyisobutylene. J. Am. Chem. Soc. 1951, 73, 4072–4075. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas sensors based on conducting polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Gardner, J.; Bartlett, P.N. Sensors and Sensory Systems for an Electronic Nose, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar] [CrossRef]
- Janata, J.; Josowicz, M. Chemical Modulation of Work Function as a Transduction Mechanism for Chemical Sensors. Acc. Chem. 1998, 31, 241–248. [Google Scholar] [CrossRef]
- Clingerman, M.L. Development and Modeling of Electrically Conductive Composite Materials, Michigan Technological University. 2001. Available online: http://www.chem.mtu.edu/org/ctc/pdf/mlcdissertation.pdf (accessed on 24 July 2022).
- Demain, A.; Issi, J. The Effect of Fiber Concentration on the Thermal Conductivity of a Polycarbonate/Pitch-Based Carbon Fiber Composite. Compos. Mater. 1993, 27, 668–683. [Google Scholar]
- Donnet, J.-B.; Bansal, R.C.; Wang, M.-J. Carbon Black, 2nd ed.; Marcel Dekker: New York, NY, USA, 1993. [Google Scholar]
- Chung, K.T.; Sabo, A.; Pica, A.P. Electrical permittivity and conductivity of carbon black-polyvinyl chloride composites. J. Appl. Phys. 1982, 53, 6867–6879. [Google Scholar]
- Sichel, E.K. Carbon Black-Polymer Composites: The Physics of Electrically Conducting Composites; Marcel Dekker: New York, NY, USA, 1982. [Google Scholar]
- Janzen, J. On the critical conductive filler loading in antistatic composites. J. Appl. Phys. 1975, 46, 966–969. [Google Scholar] [CrossRef]
- Liu, H.; Thostenson, E.T. 6.11 Conductive Nanocomposites for Multifunctional Sensing Applications; Comprehensive Composite Materials II; Elsevier: Amsterdam, The Netherlands, 2018; Volume 65. [Google Scholar] [CrossRef]
- Li, C.; Thostenson, E.T.; Chou, T.W. Dominant role of tunneling resistance in the electrical conductivity of carbon nanotube-based composites. Appl. Phys. Lett. 2007, 91, 223114:1–223114:3. [Google Scholar]
- Natarajan, B.; Orloff, N.D.; Ashkar, R.; Doshi, S.; Twedt, K.; Krishnamurthy, A.; Davis, C.; Forster, A.M.; Thostenson, E.; Obrzut, J.; et al. Multiscale metrologies for process optimization of carbon nanotube polymer composites. Carbon 2016, 108, 381–393. [Google Scholar] [CrossRef]
- Oskouyi, A.B.; Sundararaj, U.; Mertiny, P. Tunneling conductivity and piezoresistivity of composites containing randomly dispersed conductive nano-platelets. Materials 2014, 7, 2501–2521. [Google Scholar] [CrossRef]
- Dalven, R. Introduction to Applied Solid State Physics; Plenum Press: New York, NY, USA, 1980. [Google Scholar]
- Paredes-Madrid, L.; Palacio, C.A.; Matute, A.; Vargas, C.A.P. Underlying physics of conductive polymer composites and force sensing resistors (FSRs) under static loading conditions. Sensors 2017, 10, 1334. [Google Scholar] [CrossRef]
- Patel, S.V.; Yelton, G.W.; Hughes, R.C. Effect of hydroxyl concentration of chemical sensitivity of polyvinyl alcohol/carbon-black composite chemiresistors. In Chemical Sensors IV: Proceedings of the Symposium; Butter, M.A., Ed.; Electrochemical Society: Pennington, NJ, USA, 1999; Volume 4, p. 456. [Google Scholar]
- Fox, L.P. The Conductive Video Disc. RCA Rev. 1978, 39, 116–228. [Google Scholar]
- Skórczewska, K.; Lewandowski, K.; Wilczewski, S. Novel Composites of Poly(vinyl chloride) with Carbon Fibre/Carbon Nanotube Hybrid Filler. Materials 2022, 15, 5625. [Google Scholar] [CrossRef]
- Gao, T.; Woodka, M.D.; Brunschwig, B.S.; Lewis, N.S. Chemiresistors for Array-Based Vapor Sensing Using Composites of Carbon Black with Low Volatility Organic Molecules. Chem. Mater. 2006, 18, 5193–5202. [Google Scholar] [CrossRef]
- Lewis, N.; Electronic Nose: Nathan Lewis Research Group. Division of Chemistry and Chemical Engineering Caltech. California Institute of Technology. 2013. Available online: https://cce.caltech.edu/people/nathan-s-nate-lewis (accessed on 23 May 2021).
- Briglin, S.M.; Lewis, N.S. Characterization of the Temporal Response Profile of Carbon Black-Polymer Composite Detectors to Volatile Organic Vapors. J. Phys. Chem. 2003, 107, 11031–11042. [Google Scholar] [CrossRef]
- Lewis, N.; Maldonado, S.; García-Berríos, E.; Bruce, B.; Woodka, M. Detection of organic vapors and NH3(g) using thin-film carbon black–metallophthalocyanine composite chemiresistors. Sens. Actuators B Chem. 2008, 134, 521–531. [Google Scholar]
- Dafu, W.; Tiejun, Z.; Yi, X. Resistivity-volume resistivity expansion characteristics of carbon black-loaded polyethylene. J. Appl. Polym. Sci. 2000, 77, 53–58. [Google Scholar] [CrossRef]
- Alexander, G.M. Anomalous temperature dependence of the electrical conductivity of carbon-poly(methyl methacrylate) composites. Mater. Res. Bull. 1999, 34, 603–611. [Google Scholar] [CrossRef]
- Mallette, J.G.; Marques, A. Carbon black filled PET/PMMA blends: Electrical and morphological studies. Polym. Eng. Sci. 2000, 20, 2272–2278. [Google Scholar] [CrossRef]
- Lobova, T.; Shvetsova, G.; Kiparisov, S.; Smirnov, Y.; Terenin, E. An Ultrasonic Method of Mixing Powders of Refractory Metal Dichalcogenides with Galliumbase Low-Melting-Point Alloys; Plenum Publishing Corporation: New York, NY, USA, 1977; Volume 16, pp. 42–45. [Google Scholar]
- Wohltjen, H.; Barger, W.R.; Snow, A.W.; Jarvis, N.L. A vapor-sensitive chemiresistor fabricated with planar microelectrodes and a Langmuir-Blodgett organic semiconductor film. IEEE Trans. Electron Devices 1985, 32, 1170–1174. [Google Scholar] [CrossRef]
- Feng, Y.; Li, D.; Liu, J.; He, W. Carbon-based materials in microbial fuel cells. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 49–74. [Google Scholar] [CrossRef]
- Doleman, B.; Severin, E.; Lewis, N. Trends in odor intensity for human and electronic noses: Relative roles of odorant vapor pressure vs. molecularly specific odorant binding. Proc. Natl. Acad. Sci. USA 1998, 95, 5442–5447. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S. Comparisons between mammalian and artificial olfaction based on arrays of carbon black-polymer composite vapor detectors. Acc. Chem. Res. 2004, 37, 663–672. [Google Scholar] [CrossRef]
- Hopkins, A.R.; Lewis, N.S. Detection and Classification Characteristics of Arrays of Carbon Black/Organic Polymer Composite Chemiresistive Vapor Detectors for the Nerve Agent Simulants Dimethylmethylphosphonate and Diisopropylmethylphosponate. Chem. Biol. Sens. III 2002, 4722, 86–97. [Google Scholar] [CrossRef]
- Zhang, H.; Hackam, R. Electrical Surface Resistance, Hydrophobicity and Diffusion Phenomena in PVC. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 73–83. [Google Scholar] [CrossRef]
- Edwards, P.; Anker, L.; Jurs, P. Quantitative structure-property relationship studies of the odor threshold of odor active compounds. Chem. Senses 1991, 16, 447–465. [Google Scholar] [CrossRef]
- Edwards, P.; Jurs, P. Correlation of odor intensities with structural properties of odorants. Chem. Senses 1989, 14, 281–291. [Google Scholar] [CrossRef]
- BS ISO 3534-1:2006; Statistics. Vocabulary and Symbols: General Statistical Terms and Terms Used in Probability. British Standards Institute: London, UK, 2006.

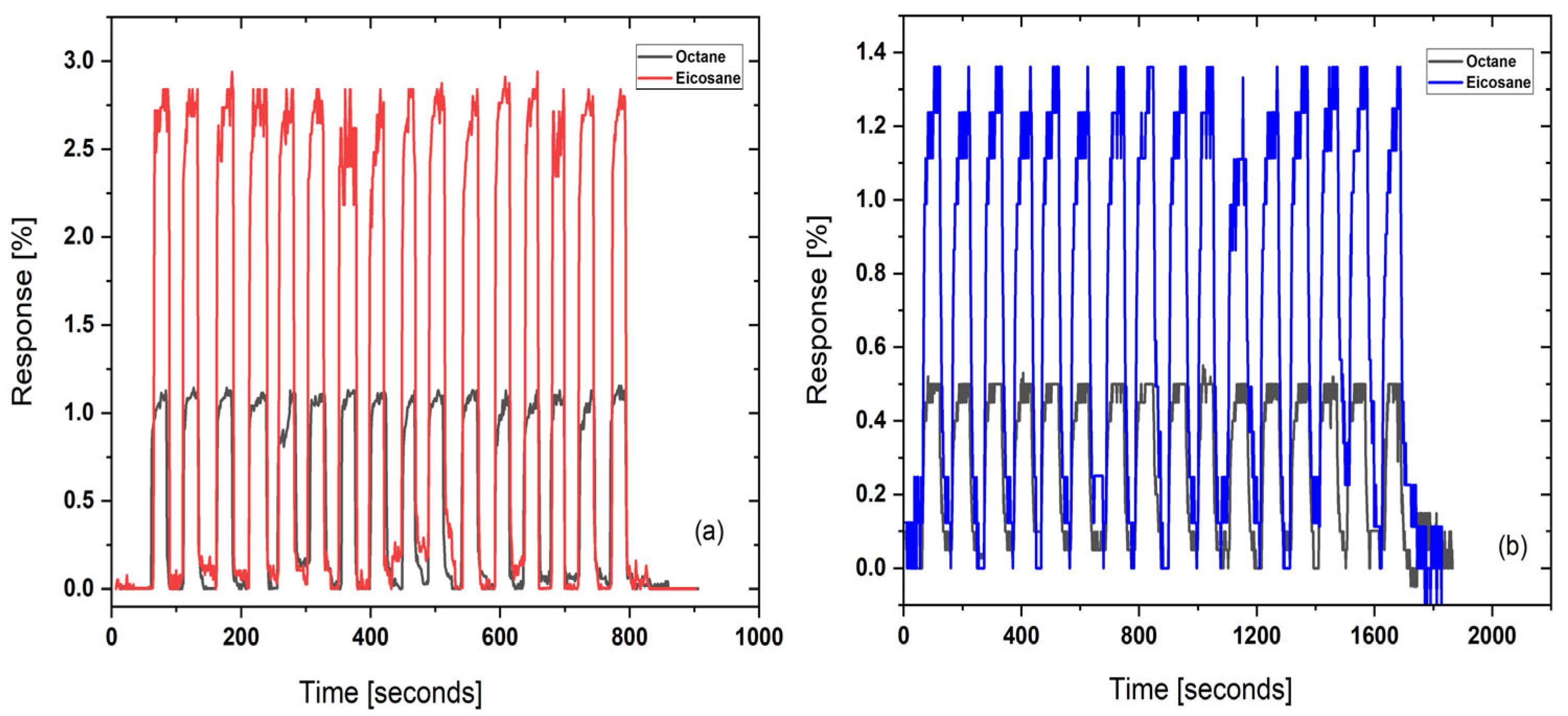
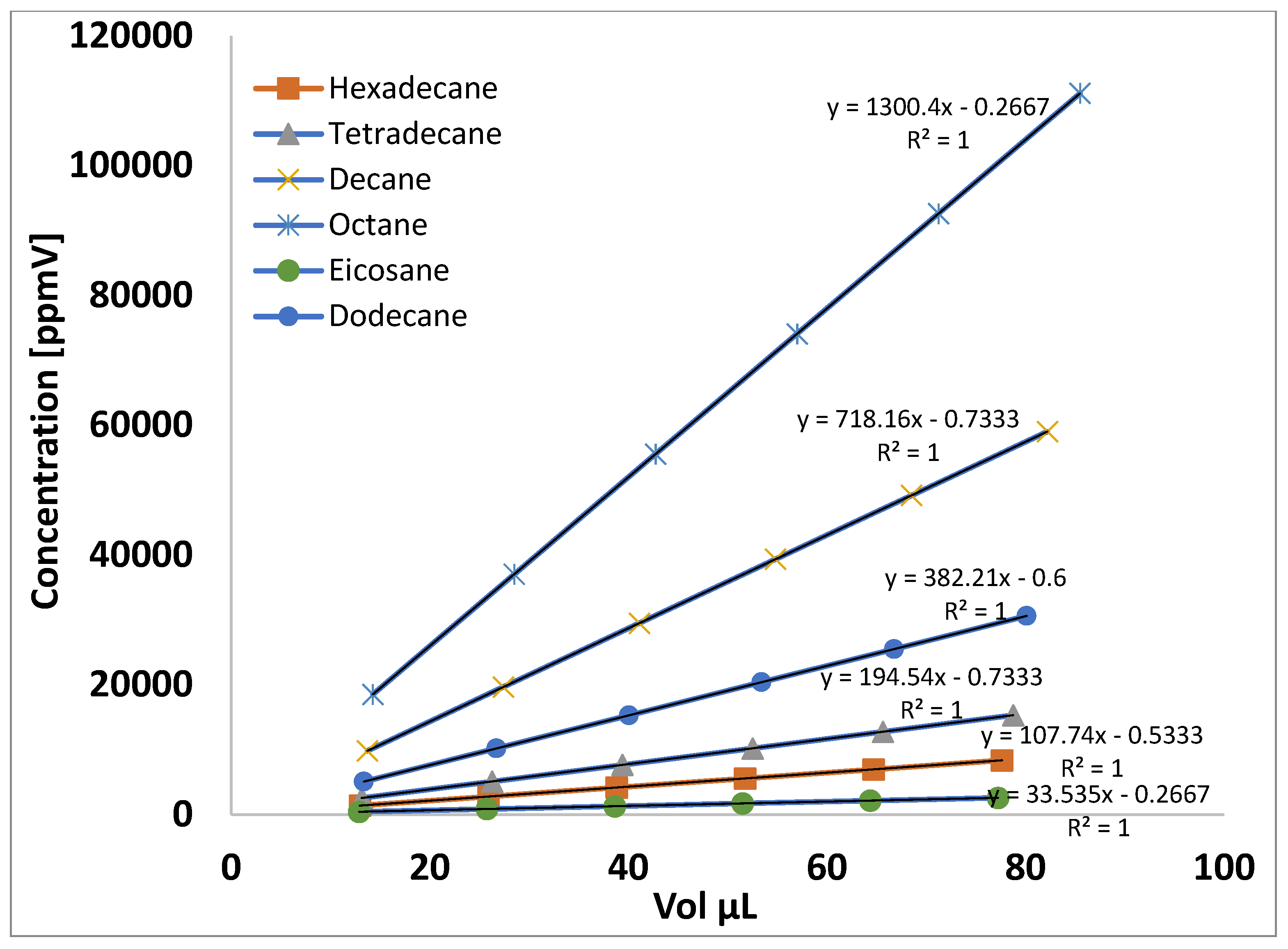
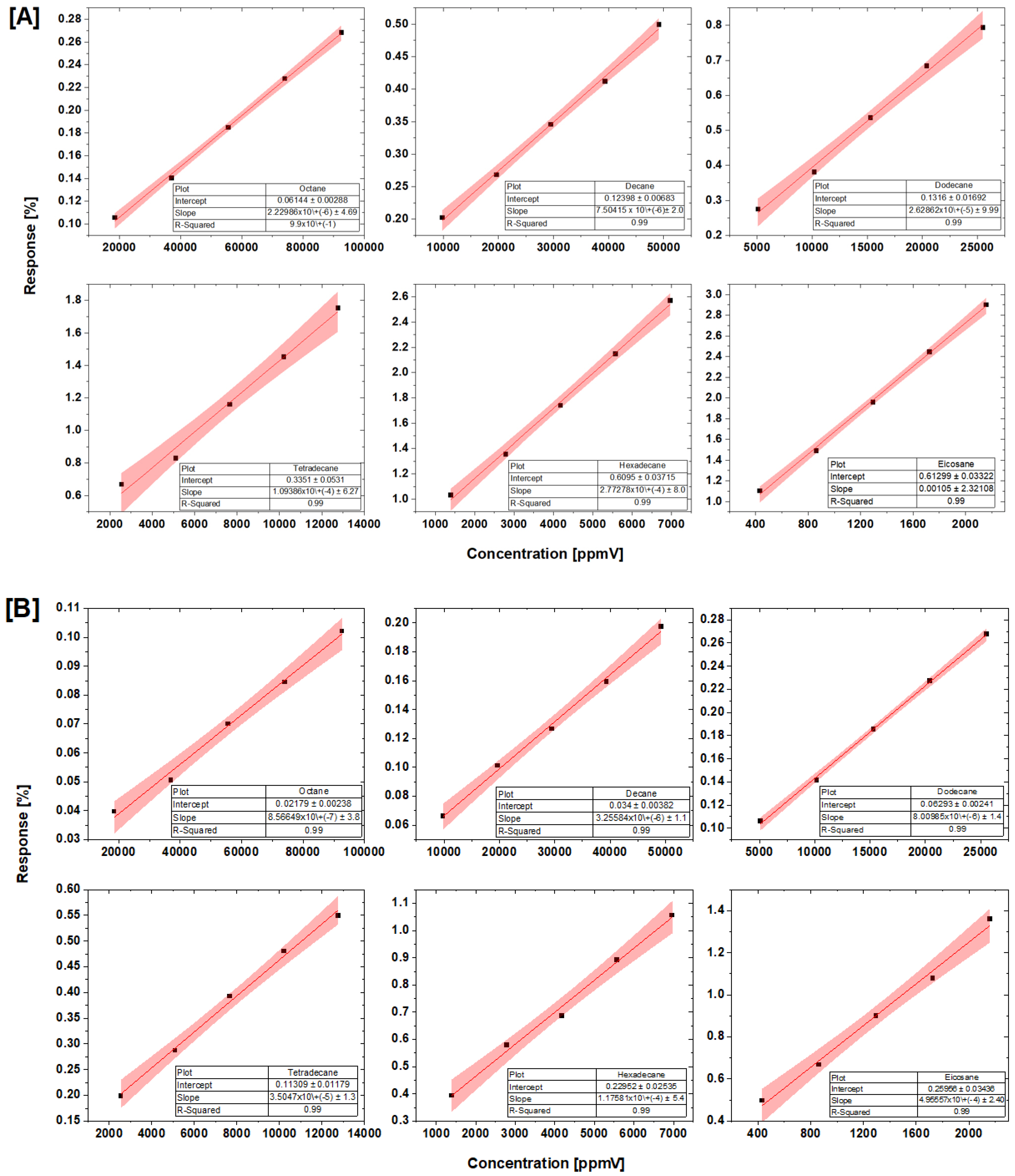
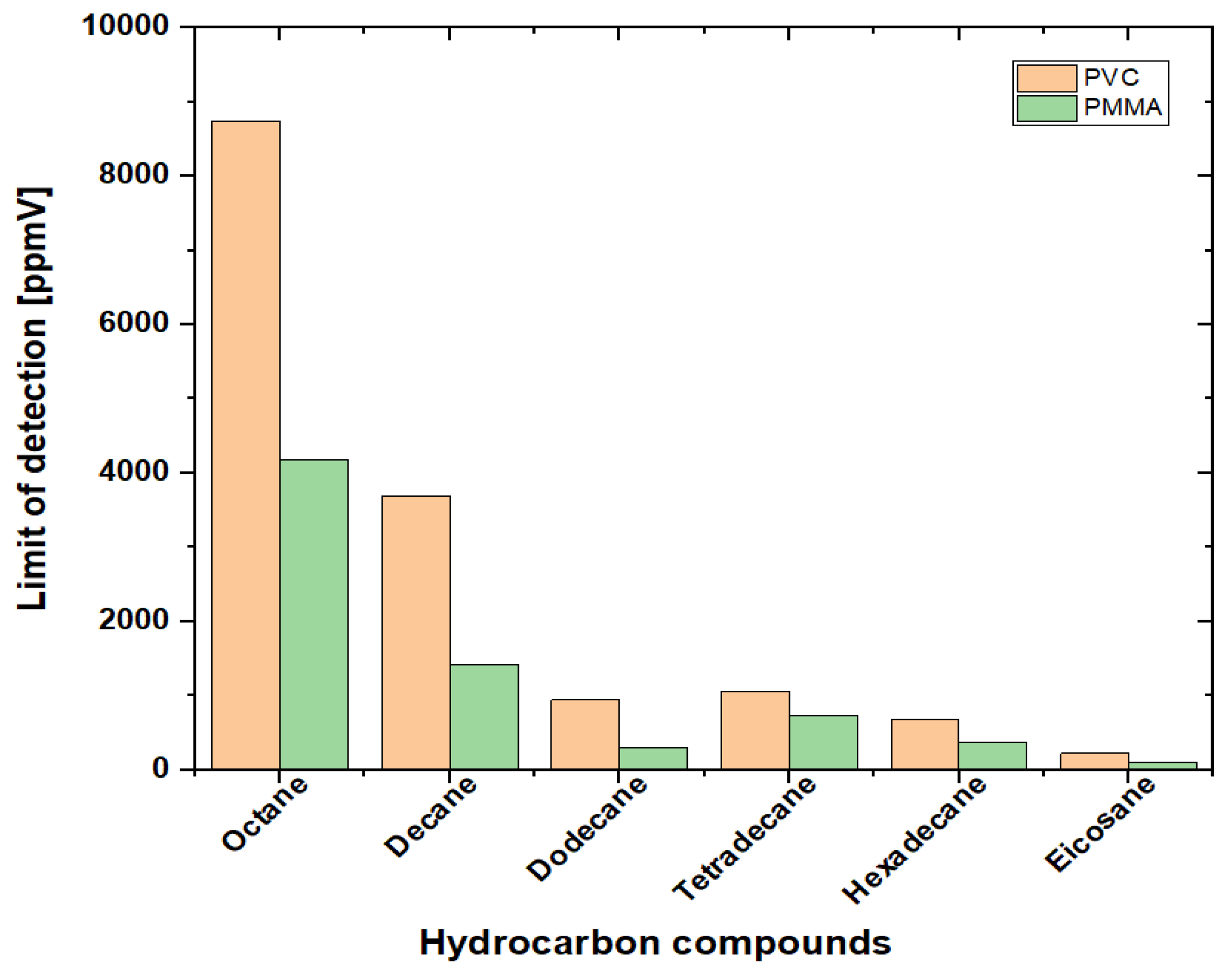
| Hydrocarbons | A | B | C | tmin | tmax |
|---|---|---|---|---|---|
| Octane | 7.14462 | 1498.96 | 225.878 | −56.77 | 295.68 |
| Decane | 7.21745 | 1693.93 | 216.459 | −29.66 | 345.83 |
| Dodecane | 7.22883 | 1807.47 | 199.381 | 9.58 | 385.05 |
| Tetradecane | 7.26165 | 1914.86 | 183.519 | 5.86 | 419.25 |
| Hexadecane | 7.36235 | 2094.08 | 180.407 | 18.17 | 447.45 |
| Eicosane | 7.27683 | 2208.52 | 158.612 | 36.44 | 494.89 |
| Temperature (°C) | Hydrocarbon Compounds | Vapor Pressure (mmHg) |
|---|---|---|
| 70 | Octane | 7.99 |
| 70 | Decane | 3.68 |
| 70 | Dodecane | 1.68 |
| 70 | Tetradecane | 0.75 |
| 70 | Hexadecane | 0.37 |
| 70 | Eicosane | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eze-Idehen, P.; Persaud, K. Design, Fabrication and Validation of Chemical Sensors for Detecting Hydrocarbons to Facilitate Oil Spillage Remediation. Chemosensors 2025, 13, 140. https://doi.org/10.3390/chemosensors13040140
Eze-Idehen P, Persaud K. Design, Fabrication and Validation of Chemical Sensors for Detecting Hydrocarbons to Facilitate Oil Spillage Remediation. Chemosensors. 2025; 13(4):140. https://doi.org/10.3390/chemosensors13040140
Chicago/Turabian StyleEze-Idehen, Perpetual, and Krishna Persaud. 2025. "Design, Fabrication and Validation of Chemical Sensors for Detecting Hydrocarbons to Facilitate Oil Spillage Remediation" Chemosensors 13, no. 4: 140. https://doi.org/10.3390/chemosensors13040140
APA StyleEze-Idehen, P., & Persaud, K. (2025). Design, Fabrication and Validation of Chemical Sensors for Detecting Hydrocarbons to Facilitate Oil Spillage Remediation. Chemosensors, 13(4), 140. https://doi.org/10.3390/chemosensors13040140





