Recent Progress with Bismuth Sulfide for Room-Temperature Gas Sensing
Abstract
1. Introduction
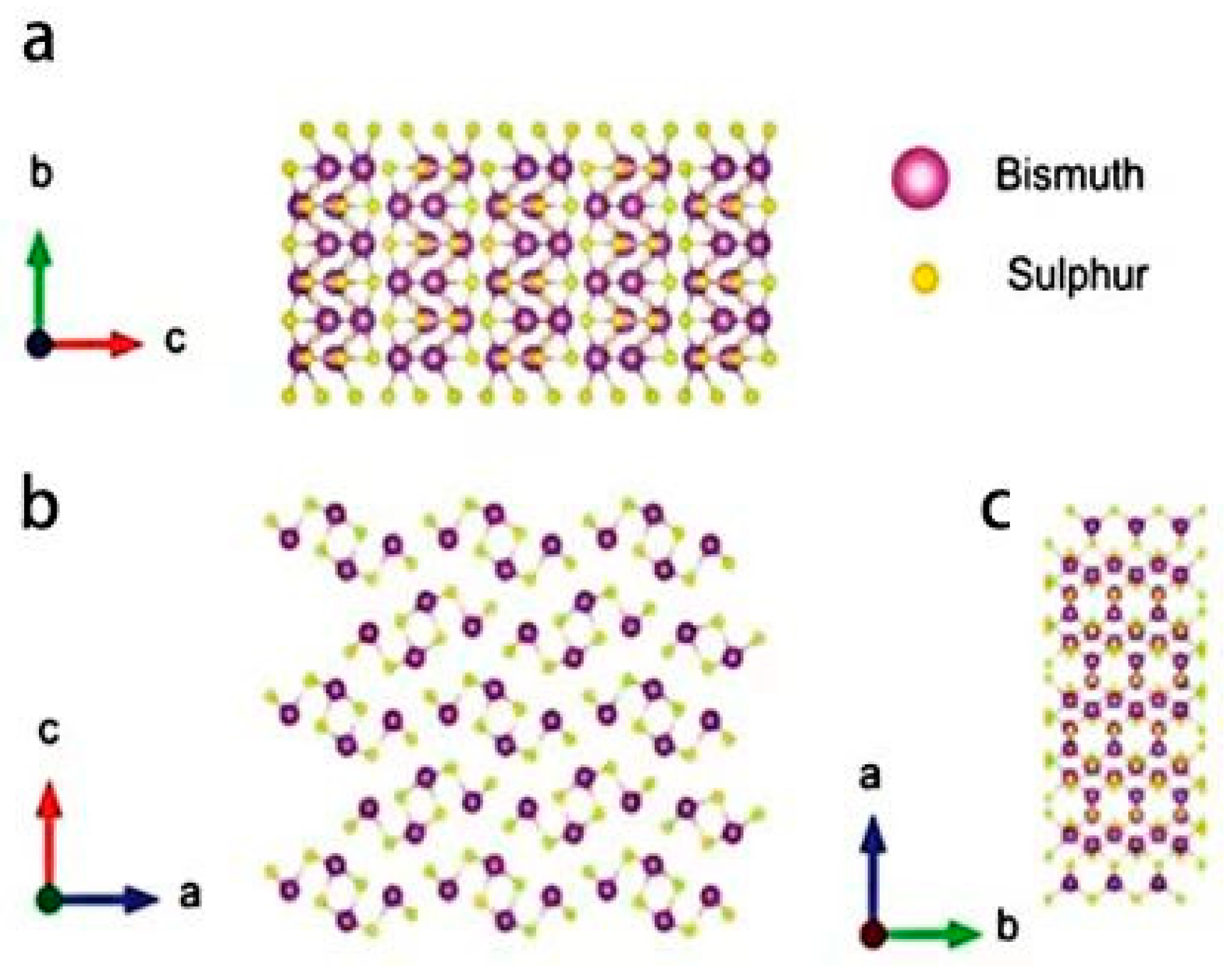
2. Bi2S3: Rising Material for Gas-Sensing Applications
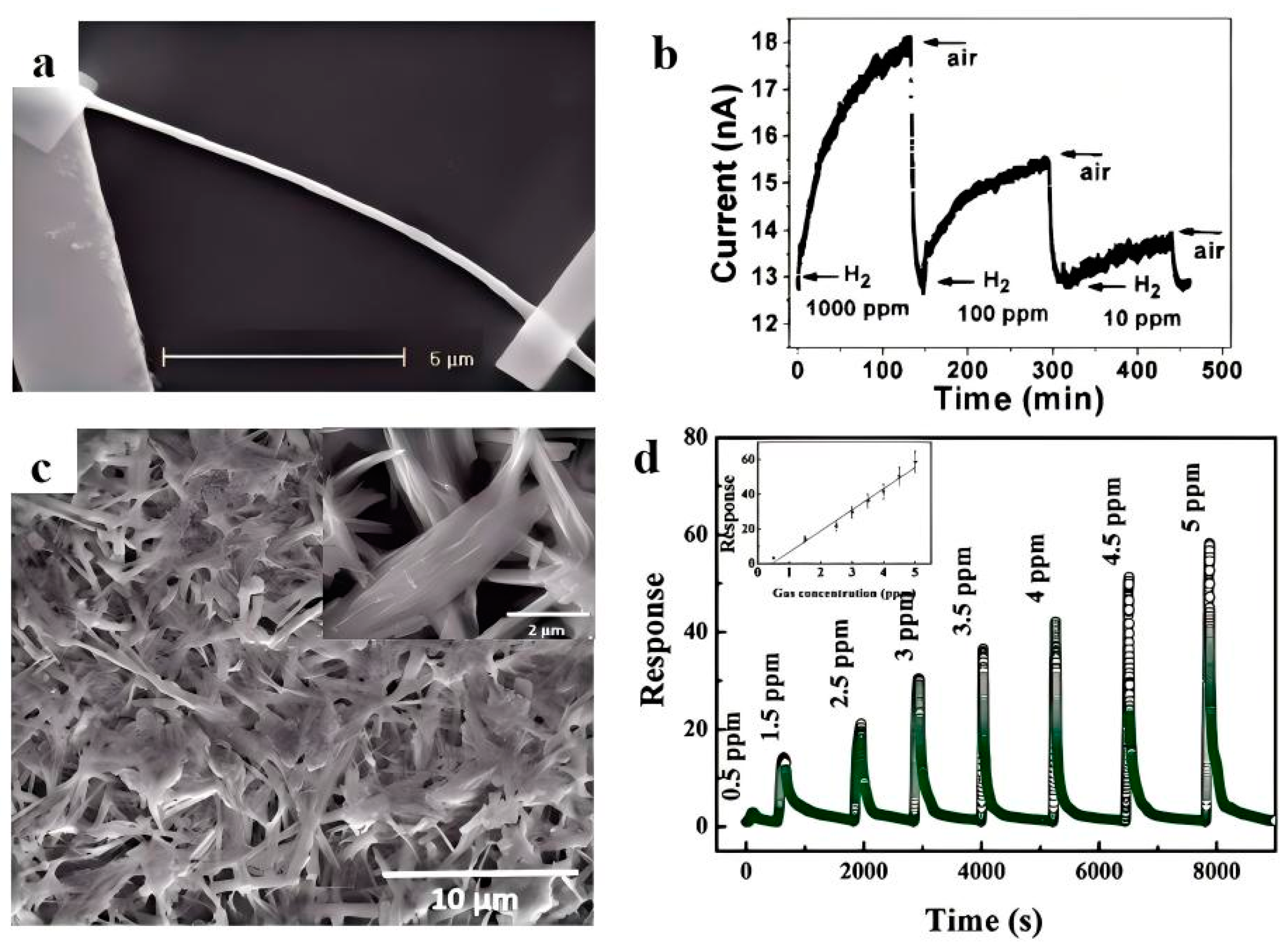
2.1. Gas Sensors Constructed with Pristine Bi2S3
2.2. Strategies for Enhancing Room-Temperature Sensing Performance
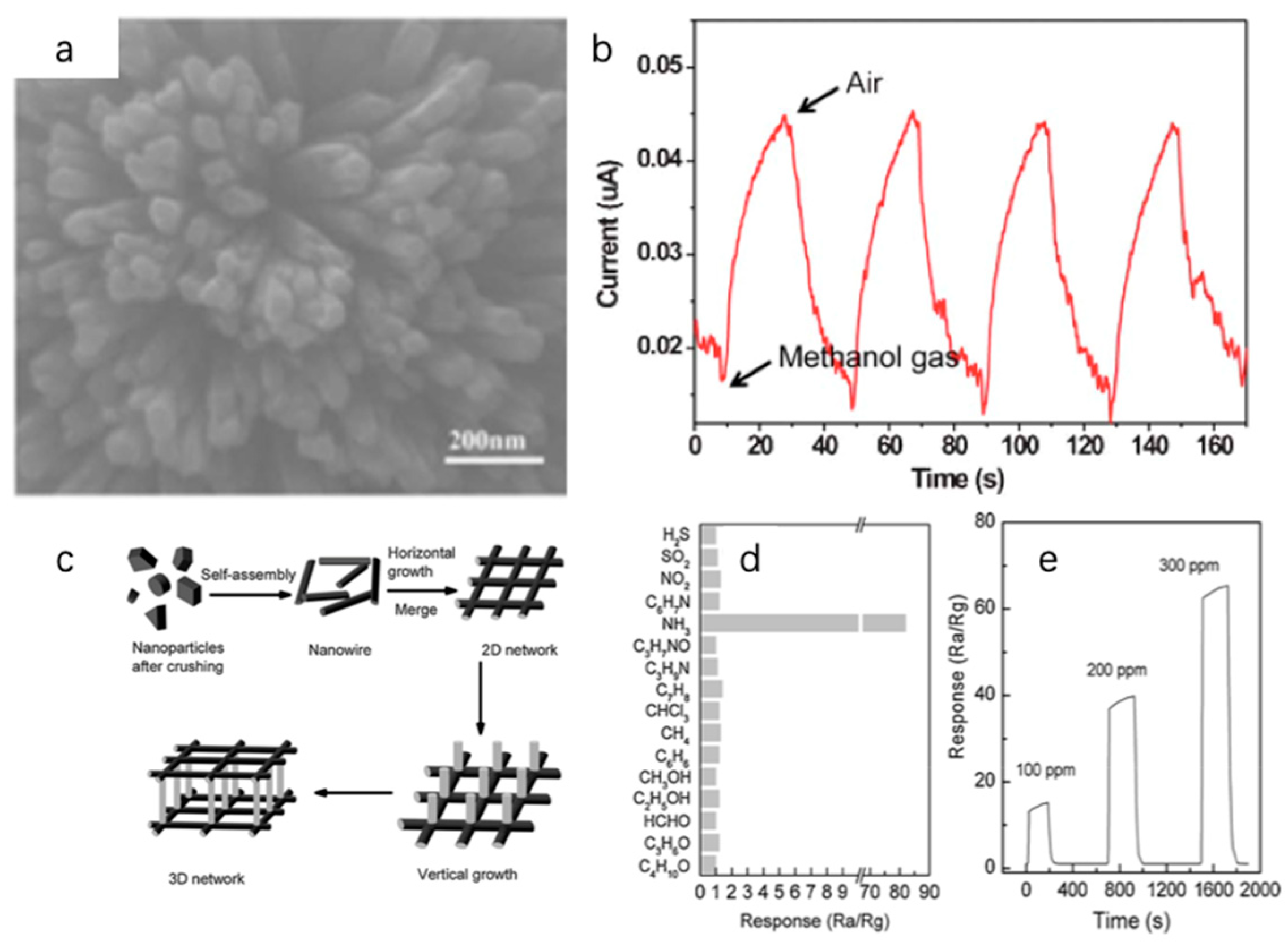
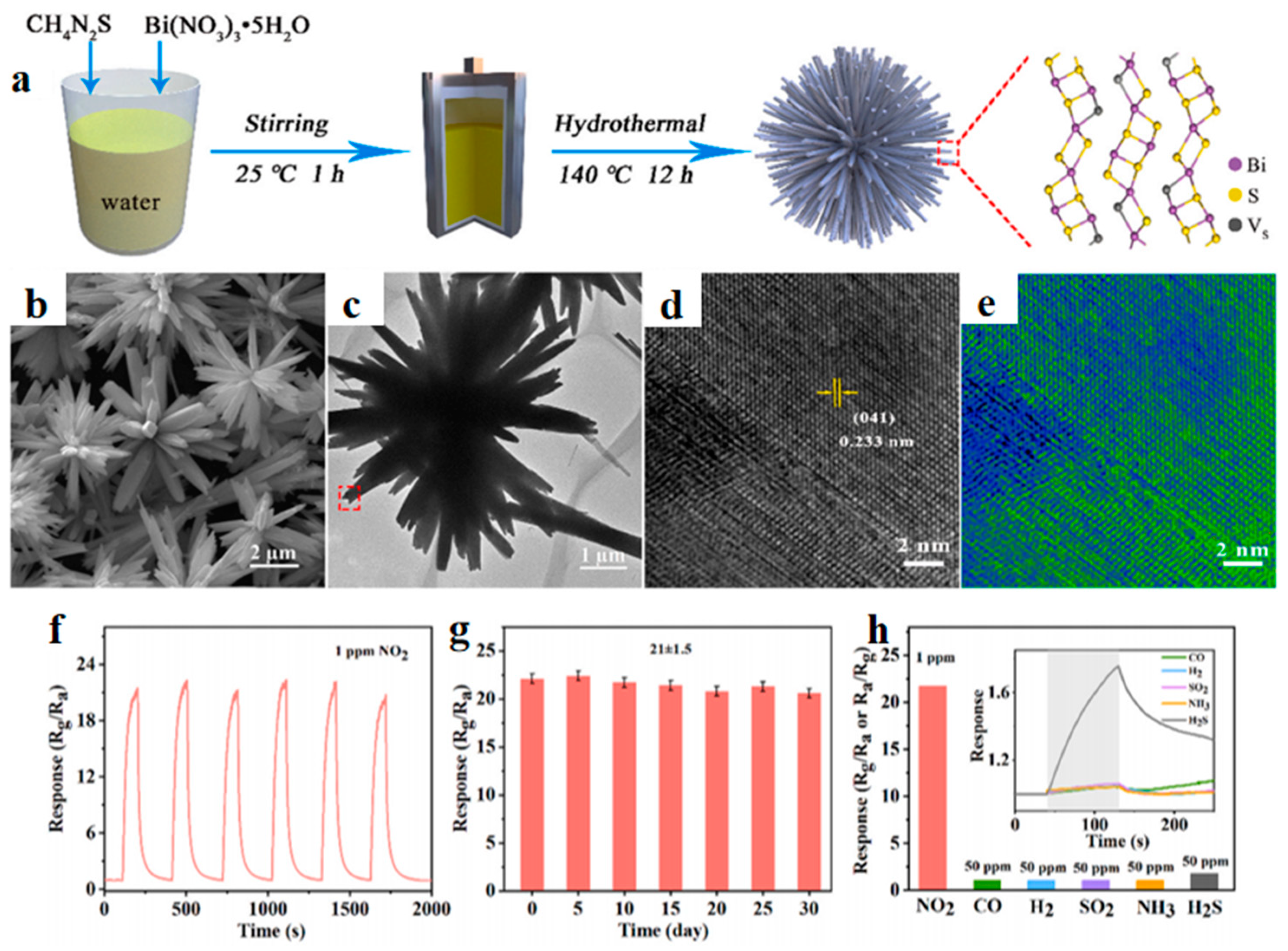
2.2.1. Morphological Design
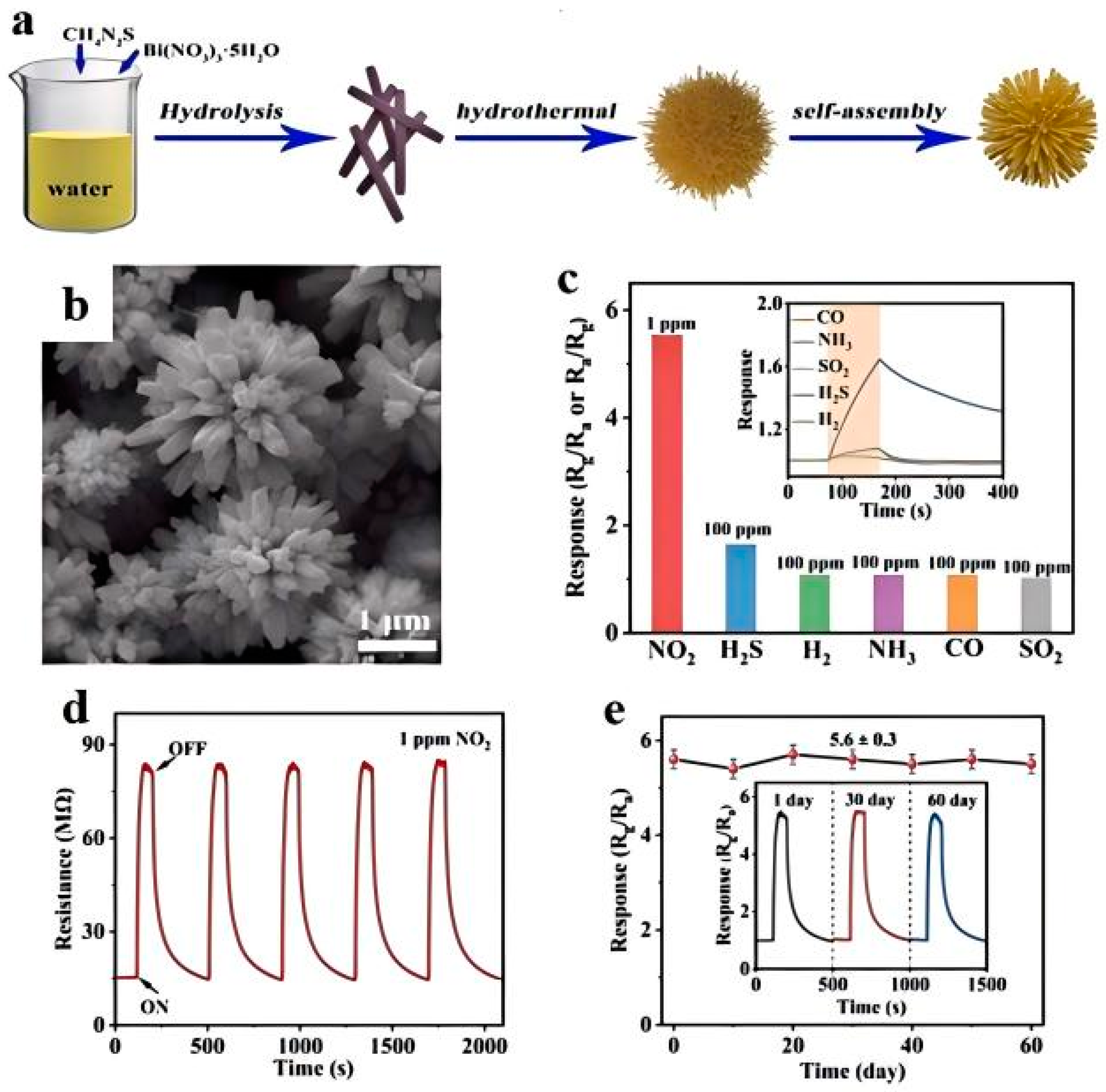
2.2.2. Defect Engineering
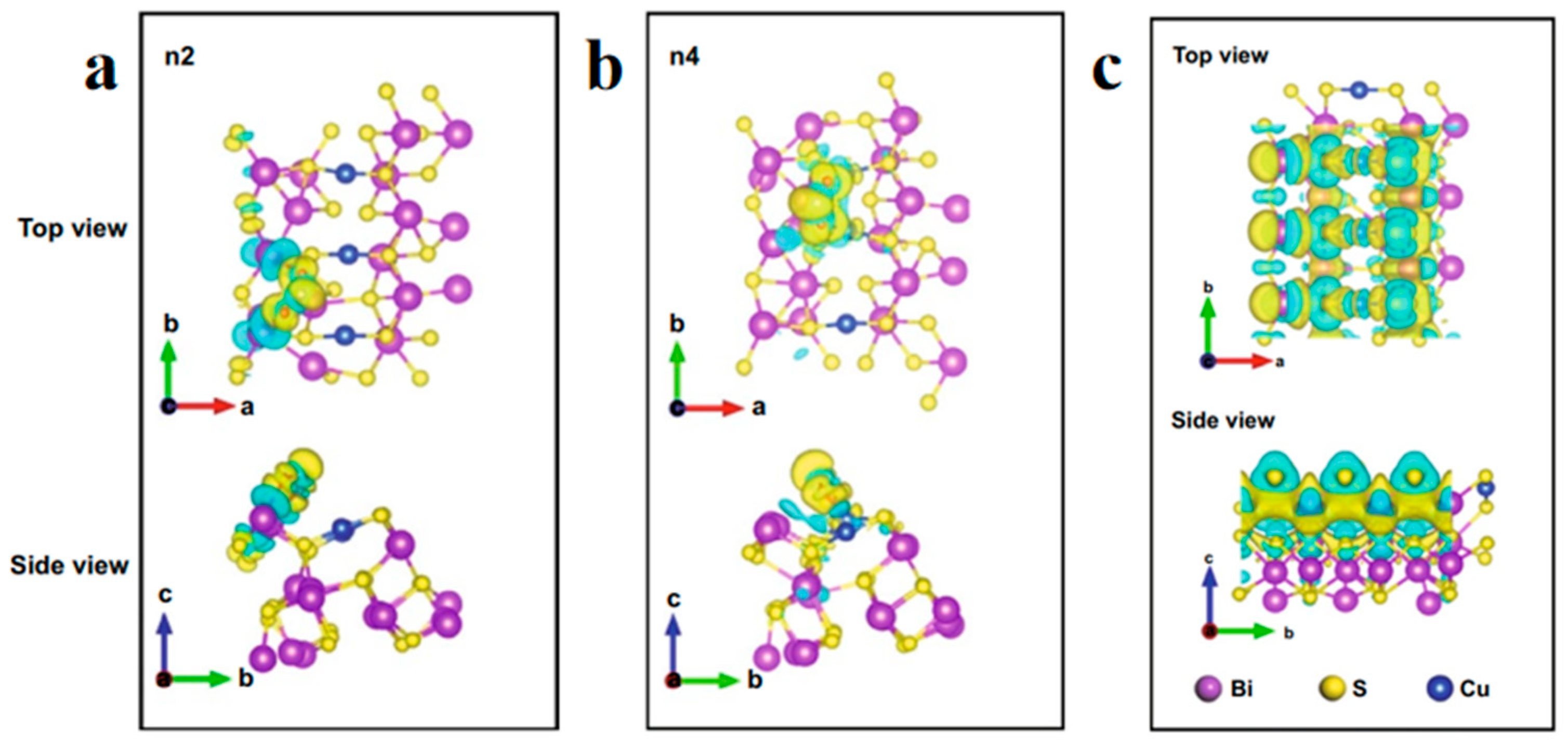
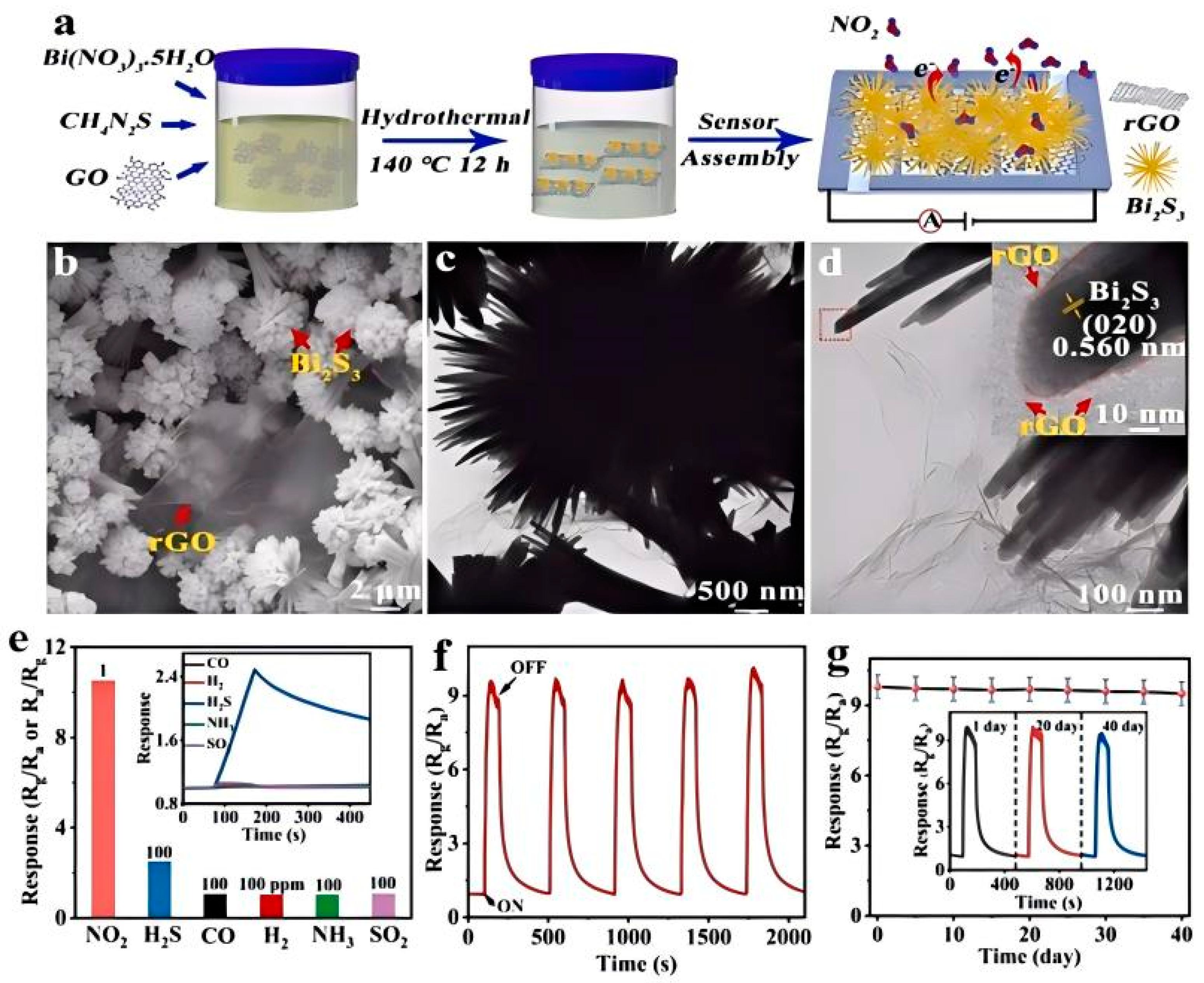
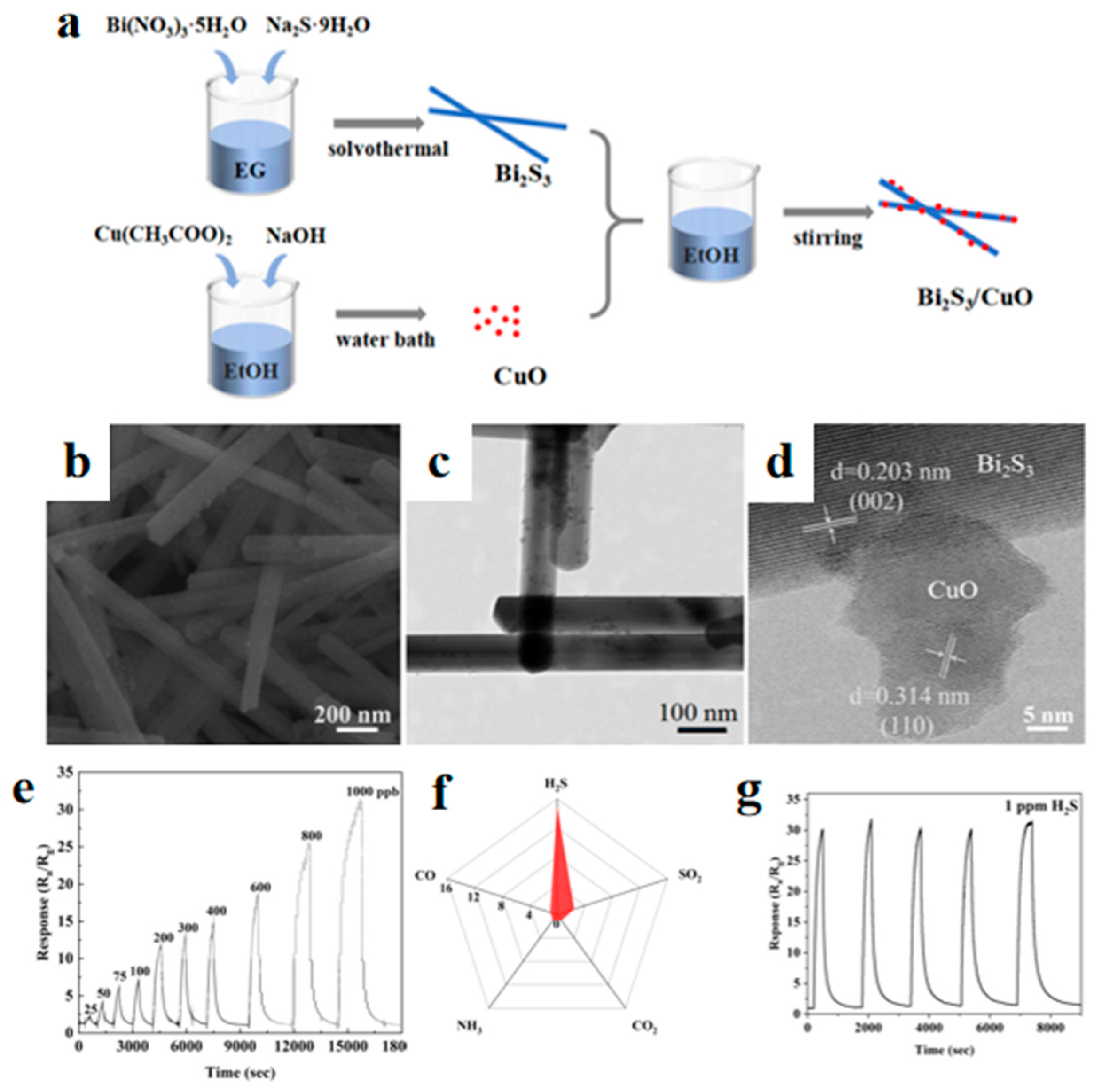
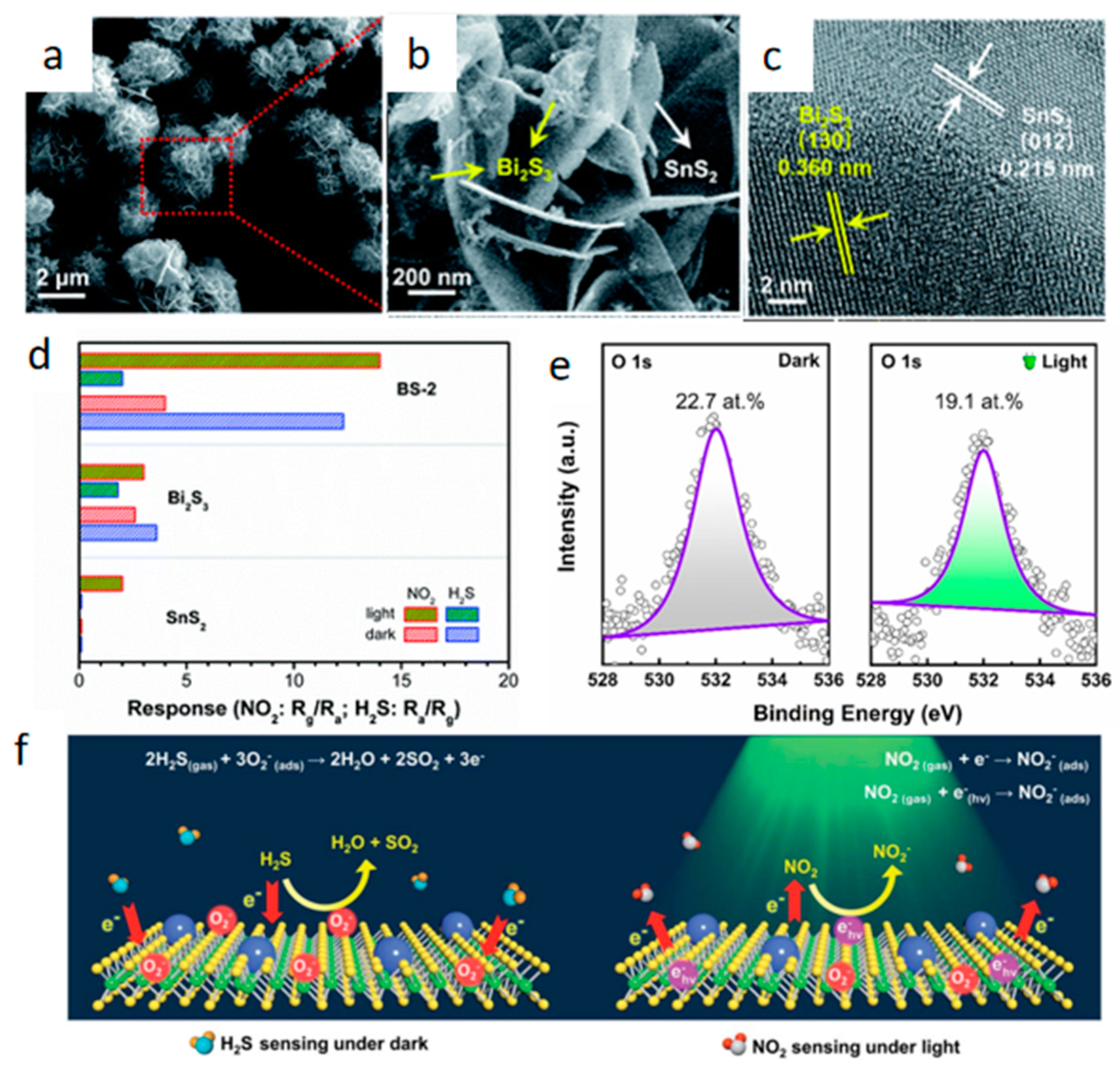
2.2.3. Heterostructures Construction
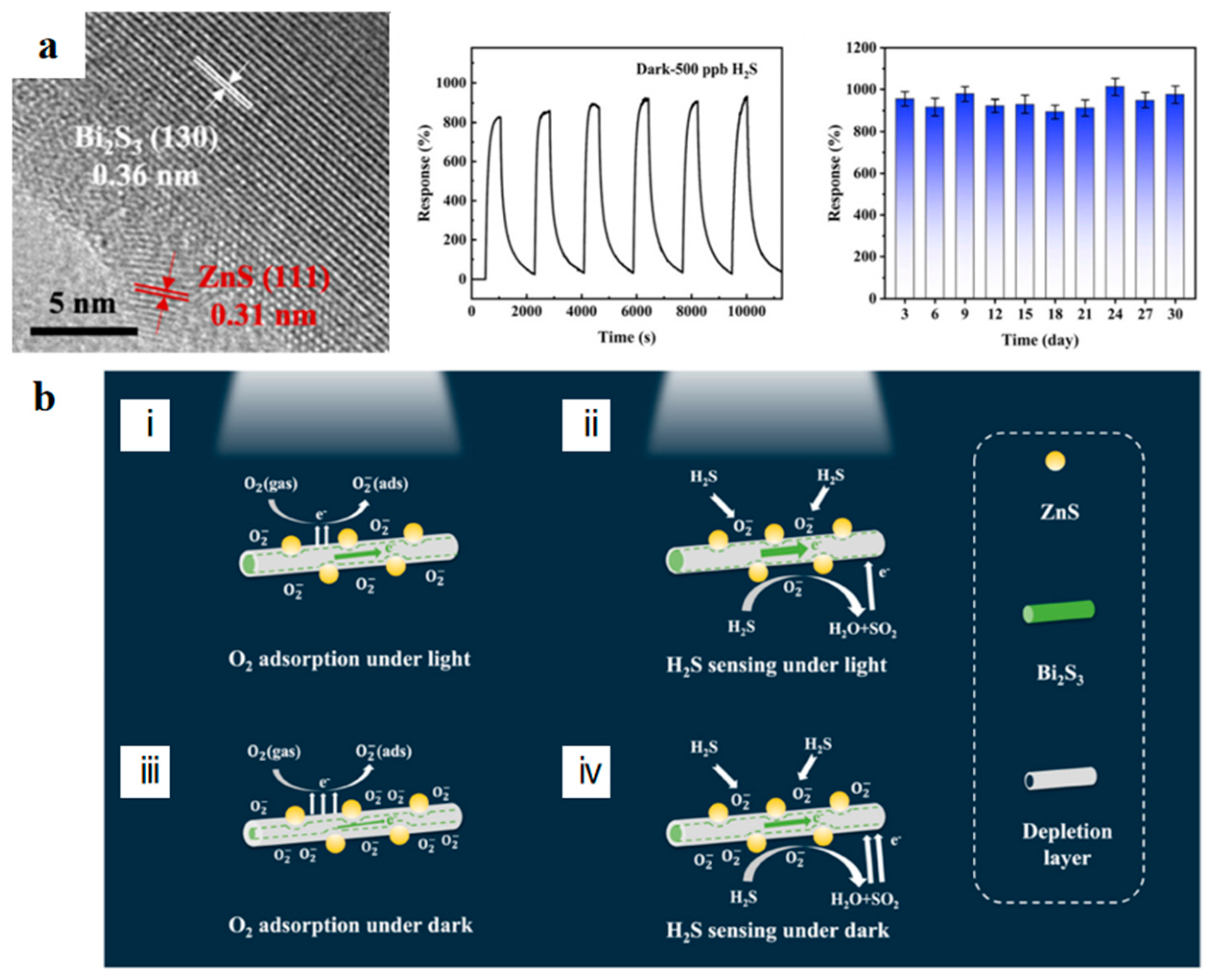
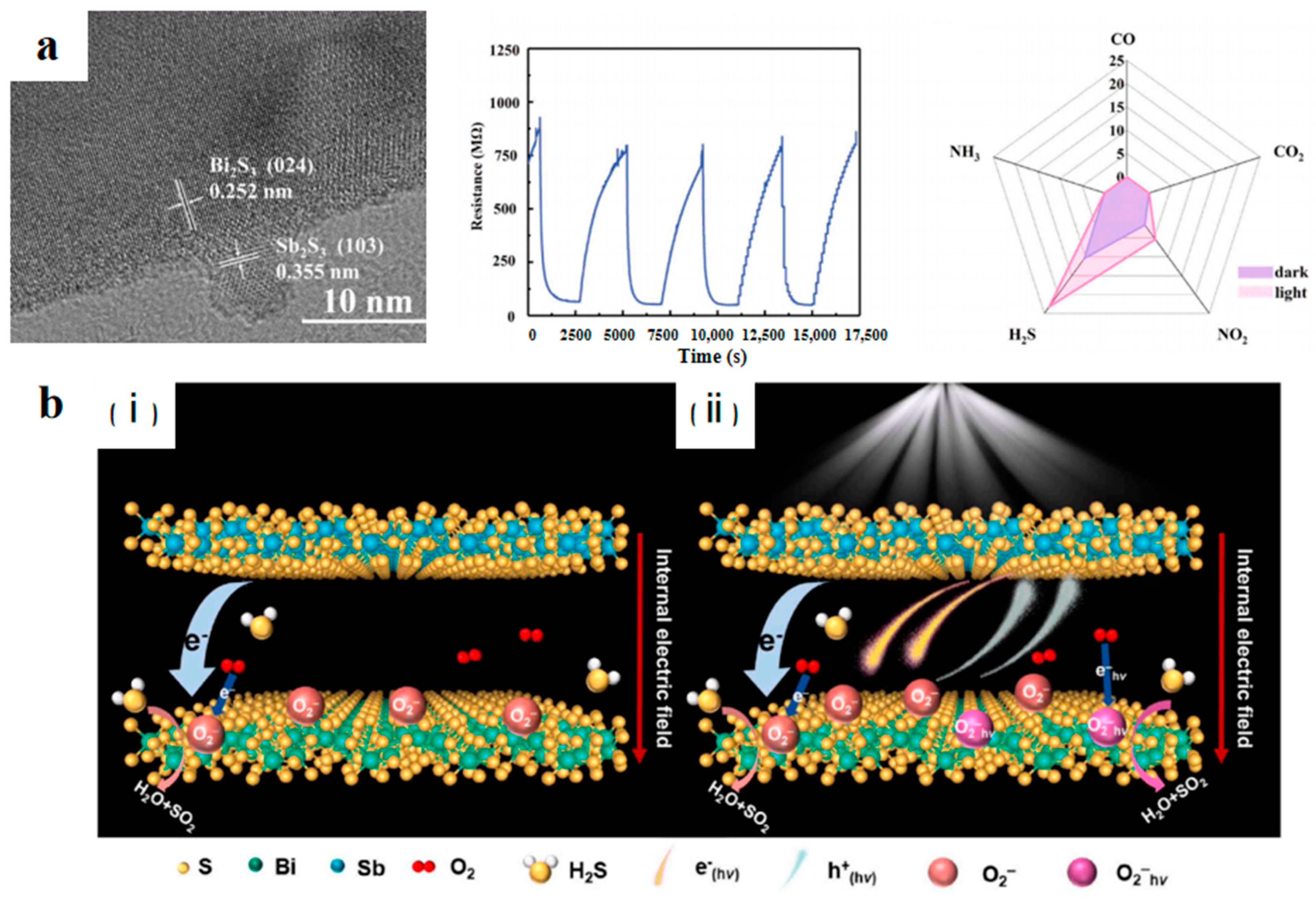
2.2.4. Light Irradiation
3. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Wang, H.; Lustig, W.P.; Li, J. Sensing and Capture of Toxic and Hazardous Gases and Vapors by Metal-Organic Frameworks. Chem. Soc. Rev. 2018, 47, 4729–4756. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, J.; Chen, J.; Zhang, L.; Zhang, Y.; Pei, X.-L. Pd-modified SmFeO3 with hollow tubular structure under light shows extremely high acetone gas sensitivity. Rare Met. 2022, 42, 545–557. [Google Scholar] [CrossRef]
- Yao, L.; Zhao, Z.; Chen, C.; Shao, Z.; Wu, L. Fabrication of BI2S3/ZnO nanocomposites for TEA gas sensors operating at room temperature: Towards seafood freshness detection during storage. Microchem. J. 2024, 207, 112239. [Google Scholar]
- Wang, Y.; Tang, C.; Su, M.; Ji, Y.; Xie, L.; Yang, Q.; Du, A.; Zhou, Y.; Yang, J. Highly sensitive, humidity-tolerant and flexible NO2 sensors based on nanoplate Bi2Se3 film. Chin. Chem. Lett. 2023, 34, 107981. [Google Scholar] [CrossRef]
- Wang, T.-T.; Xing, B.-S.; Guo, C.-Y.; Hao, J.-Y.; Wang, Y.; Huo, L.-H.; Cheng, X.-L.; Xu, Y.-M. Recent advances of emerging tin disulfide for room temperature gas sensing. Rare Met. 2023, 42, 3897–3913. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Tang, T.; Cheng, Y.; Cheng, L.; Wang, X.; Haidry, A.A.; Jannat, A.; Ou, J.Z. Room-Temperature Optoelectronic NO2 Sensing Using Two-Dimensional Gallium Oxyselenides. ACS Appl. Nano Mater. 2024, 7, 3229–3238. [Google Scholar] [CrossRef]
- Cheng, X.; Yao, Y.; Zheng, S.; Wan, Y.; Wei, C.; Yang, G.; Yuan, Y.; Tsai, H.-S.; Wang, Y.; Hao, J. Te@Se Core–Shell Heterostructures with Tunable Shell Thickness for Ultra-Stable NO2 Detection. ACS Sens. 2025, 10, 283–291. [Google Scholar] [CrossRef]
- Tang, T.; Li, Z.; Liu, Y.Y.; Chen, Y.L.; Cheng, Y.F.; Liang, Y.; Zhuang, J.H.; Hu, X.Y.; Jannat, A.; Ou, R.; et al. Ultrathin two-dimensional titanium oxysulfide for enhanced sensitivity and stability of room temperature NO2 sensing. Ceram. Int. 2025, 51, 3216–3223. [Google Scholar] [CrossRef]
- Huang, H.; Pan, Z.; Wang, J.; Wang, T.; Yang, W.; Yu, H.; Li, F.; Dong, X.; Yang, Y. Ag nanoparticles sensitized ZnO/MoS2 composites to detect ppb-level NO2 and automobile exhaust gas. Sens. Actuators B Chem. 2025, 433, 137569. [Google Scholar] [CrossRef]
- Yu, M.; Li, J.; Yin, D.; Zhou, Z.; Wei, C.; Wang, Y.; Hao, J. Enhanced oxygen anions generation on Bi2S3/Sb2S3 heterostructure by visible light for trace H2S detection at room temperature. J. Hazard. Mater. 2024, 476, 134932. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, J.; Pan, Z.; Wang, T.; Yu, H.; Li, F.; Dong, X.; Liu, Z.; Yang, Y. rGO doped MOFs-derived CuO nanocomposites as flexible room-temperature sensors for ppb-level H2S detection. Sens. Actuators B Chem. 2025, 422, 136596. [Google Scholar] [CrossRef]
- Shen, S.-K.; Xin, Y.-Y.; Zhang, X.-F.; Zhou, X.; Deng, Z.-P.; Xu, Y.-M.; Huo, L.-H.; Gao, S. Corn stigma template-assisted synthesis of single crystal MoO3 elliptical nanosheets for chemiresistive detection of dibutylamine vapor. Sens. Actuators B Chem. 2024, 408, 135480. [Google Scholar] [CrossRef]
- Li, J.; Zheng, M.; Yang, M.; Zhang, X.; Cheng, X.; Zhou, X.; Gao, S.; Xu, Y.; Huo, L. Three-in-one Ni doped porous SnO2 nanorods sensor: Controllable oxygen vacancies content, surface site activation and low power consumption for highly selective NO2 monitoring. Sens. Actuators B Chem. 2023, 382, 133550. [Google Scholar] [CrossRef]
- Gai, L.-Y.; Lai, R.-P.; Dong, X.-H.; Wu, X.; Luan, Q.-T.; Wang, J.; Lin, H.-F.; Ding, W.-H.; Wu, G.-L.; Xie, W.-F. Recent advances in ethanol gas sensors based on metal oxide semiconductor heterojunctions. Rare Met. 2022, 41, 1818–1842. [Google Scholar] [CrossRef]
- Sun, X.; Gao, R.; Wu, Y.; Zhang, X.; Cheng, X.; Gao, S.; Xu, Y.; Huo, L. Novel in-situ deposited V2O5 nanorods array film sensor with enhanced gas sensing performance to n-butylamine. Chem. Eng. J. 2023, 459, 141505. [Google Scholar] [CrossRef]
- Goswami, P.; Gupta, G. Recent progress of flexible NO2 and NH3 gas sensors based on transition metal dichalcogenides for room temperature sensing. Mater. Today Chem. 2022, 23, 100726. [Google Scholar] [CrossRef]
- Zhang, L.; Khan, K.; Zou, J.; Zhang, H.; Li, Y. Recent Advances in Emerging 2D Material-Based Gas Sensors: Potential in Disease Diagnosis. Adv. Mater. Interfaces 2019, 6, 1901329. [Google Scholar] [CrossRef]
- Milone, A.; Monteduro, A.G.; Rizzato, S.; Leo, A.; Di Natale, C.; Kim, S.S.; Maruccio, G. Advances in Materials and Technologies for Gas Sensing from Environmental and Food Monitoring to Breath Analysis. Adv. Sustain. Syst. 2022, 7, 2200083. [Google Scholar] [CrossRef]
- Rosolina, S.M.; Carpenter, T.S.; Xue, Z.-L. Bismuth-Based, Disposable Sensor for the Detection of Hydrogen Sulfide Gas. Anal. Chem. 2016, 88, 1553–1558. [Google Scholar] [CrossRef]
- Potyrailo, R.A. Multivariable Sensors for Ubiquitous Monitoring of Gases in the Era of Internet of Things and Industrial Internet. Chem. Rev. 2016, 116, 11877–11923. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Kim, J.S.; Lee, J.H. Rational Design of Semiconductor-Based Chemiresistors and their Libraries for Next-Generation Artificial Olfaction. Adv. Mater. 2020, 32, 2002075. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Guo, C.; Zhang, X.; Xu, Y.; Cheng, X.; Gao, S.; Huo, L. Recent Development of Hierarchical Metal Oxides Based Gas Sensors: From Gas Sensing Performance to Applications. Adv. Sustain. Syst. 2022, 6, 2100370. [Google Scholar] [CrossRef]
- Wu, R.; Hao, J.; Wang, Y. Recent Advances in Engineering of 2D Layered Metal Chalcogenides for Resistive-Type Gas Sensor. Small 2024, 20, 2404821. [Google Scholar] [CrossRef]
- Cui, S.; Pu, H.; Wells, S.A.; Wen, Z.; Mao, S.; Chang, J.; Hersam, M.C.; Chen, J. Ultrahigh sensitivity and layer-dependent sensing performance of phosphorene-based gas sensors. Nat. Commun. 2015, 6, 8632. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.-N.; Zhang, Y.-J.; Duan, Z.-H.; Wang, S.; Liu, C.; Jiang, Y.-D.; Tai, H.-L. A review on Ti3C2Tx-based nanomaterials: Synthesis and applications in gas and humidity sensors. Rare Met. 2020, 40, 1459–1476. [Google Scholar] [CrossRef]
- Qiu, L.; Huo, Y.; Pan, Z.; Wang, T.; Yu, H.; Liu, X.; Tong, X.; Yang, Y. Resister-type sensors based on Ti3C2Tx MXene decorated In2O3 p-n heterojunction for ppb-level NO2 detection at room temperature. J. Environ. Chem. Eng. 2025, 13, 115249. [Google Scholar] [CrossRef]
- Sang, D.K.; Wang, H.; Guo, Z.; Xie, N.; Zhang, H. Recent Developments in Stability and Passivation Techniques of Phosphorene toward Next-Generation Device Applications. Adv. Funct. Mater. 2019, 29, 1903419. [Google Scholar] [CrossRef]
- Lee, E.; Yoon, Y.S.; Kim, D.-J. Two-Dimensional Transition Metal Dichalcogenides and Metal Oxide Hybrids for Gas Sensing. ACS Sens. 2018, 3, 2045–2060. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Huo, M.; Ho, S.-H.; Tsai, H.-S. Metallic group VB transition metal dichalcogenides for electrochemical energy storage. Mater. Today Chem. 2022, 26, 101241. [Google Scholar] [CrossRef]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Agrawal, A.V.; Kumar, N.; Kumar, M. Strategy and Future Prospects to Develop Room-Temperature-Recoverable NO2 Gas Sensor Based on Two-Dimensional Molybdenum Disulfide. Nano-Micro Lett. 2021, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Z.; Zhan, Q.; Liu, D.; Zhao, P.; Cheng, Y. Recent advances of substitutionally doped tin dichalcogenides. J. Mater. Chem. C 2022, 10, 7771–7782. [Google Scholar] [CrossRef]
- Tang, H.; Sacco, L.N.; Vollebregt, S.; Ye, H.; Fan, X.; Zhang, G. Recent advances in 2D/nanostructured metal sulfide-based gas sensors: Mechanisms, applications, and perspectives. J. Mater. Chem. A 2020, 8, 24943–24976. [Google Scholar] [CrossRef]
- Hermawan, A.; Septiani, N.L.W.; Taufik, A.; Yuliarto, B.; Suyatman; Yin, S. Advanced Strategies to Improve Performances of Molybdenum-Based Gas Sensors. Nano-Micro Lett. 2021, 13, 207. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, F.; Zhang, Y.; Hu, J. Review on the advancement of SnS2 in photocatalysis. J. Mater. Chem. A 2023, 11, 7331–7343. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Ansari, S.A.; Kim, S.-H. Fundamentals and recent progress of Sn-based electrode materials for supercapacitors: A comprehensive review. J. Energy Storage 2022, 53, 105187. [Google Scholar] [CrossRef]
- Guo, Y.; Ao, Y.; Wang, P.; Wang, C. Mediator-free direct dual-Z-scheme Bi2S3/BiVO4/MgIn2S4 composite photocatalysts with enhanced visible-light-driven performance towards carbamazepine degradation. Appl. Catal. B Environ. 2019, 254, 479–490. [Google Scholar] [CrossRef]
- Dong, Y.; Hu, M.; Zhang, Z.; Zapien, J.A.; Wang, X.; Lee, J.-M. Hierarchical self-assembled Bi2S3 hollow nanotubes coated with sulfur-doped amorphous carbon as advanced anode materials for lithium ion batteries. Nanoscale 2018, 10, 13343–13350. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Y.; Hu, Q. Controllable Epitaxial-like Growth of Bi2S3/MoS2 Hybrid Aerogel Nanostructures for Sensitive NO2 Detection at Room Temperature. ACS Appl. Nano Mater. 2023, 6, 8260–8269. [Google Scholar] [CrossRef]
- Wang, T.; Liu, J.; Zhang, Y.; Liang, Q.; Wu, R.; Tsai, H.-S.; Wang, Y.; Hao, J. Bifunctional gas sensor based on Bi2S3/SnS2 heterostructures with improved selectivity through visible light modulation. J. Mater. Chem. A 2022, 10, 4306–4315. [Google Scholar] [CrossRef]
- Afsar, M.F.; Rafiq, M.A.; Jamil, A.; Fareed, S.; Siddique, F.; Tok, A.I.Y.; Hasan, M.M.U. Development of High-Performance Bismuth Sulfide Nanobelts Humidity Sensor and Effect of Humid Environment on its Transport Properties. ACS Omega 2019, 4, 2030–2039. [Google Scholar] [CrossRef]
- Ali, S.; Kazmi, S.M.T.; Sher, F.; Nigah, A.; Bilal, M.; Rafiq, M.A. Fast, Sensitive, and Selective Hydrogen Sensing of Silver-Doped Bismuth Sulfide Nanobelts. J. Electron. Mater. 2024, 53, 2753–2762. [Google Scholar] [CrossRef]
- Fang, L.; Qiu, Y.; Zhai, T.; Wang, F.; Lan, M.; Huang, K.; Jing, Q. Flower-like nanoarchitecture assembled from Bi2S3 nanorod/MoS2 nanosheet heterostructures for high-performance supercapacitor electrodes. Colloids Surf. A Physicochem. Eng. Asp. 2017, 535, 41–48. [Google Scholar] [CrossRef]
- Furukawa, R.; Hasegawa, T.; Okawa, A.; Yin, S. One-pot facile synthesis of rod-like Bi2S3 particles in deep eutectic solvent and their ppb-level selective H2S gas detection. Dalton Trans. 2024, 53, 18116–18121. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.F.; Cao, S.; Zhang, J.; Tang, H.; Guo, S.; Tian, Y.; Liu, Q. Topotactic Transformations of Superstructures: From Thin Films to Two-Dimensional Networks to Nested Two-Dimensional Networks. J. Am. Chem. Soc. 2011, 133, 8211–8215. [Google Scholar] [CrossRef]
- Dhar, N.; Syed, N.; Mohiuddin, M.; Jannat, A.; Zavabeti, A.; Zhang, B.Y.; Datta, R.S.; Atkin, P.; Mahmood, N.; Esrafilzadeh, D.; et al. Exfoliation Behavior of van der Waals Strings: Case Study of Bi2S3. ACS Appl. Mater. Interfaces 2018, 10, 42603–42611. [Google Scholar] [CrossRef]
- Messalea, K.A.; Zavabeti, A.; Mohiuddin, M.; Syed, N.; Jannat, A.; Atkin, P.; Ahmed, T.; Walia, S.; McConville, C.F.; Kalantar-Zadeh, K.; et al. Two-Step Synthesis of Large-Area 2D Bi2S3 Nanosheets Featuring High In-Plane Anisotropy. Adv. Mater. Interfaces 2020, 7, 2001131. [Google Scholar] [CrossRef]
- Yao, K.; Gong, W.W.; Hu, Y.F.; Liang, X.L.; Chen, Q.; Peng, L.M. Individual Bi2S3 Nanowire-Based Room-Temperature H2 Sensor. J. Phys. Chem. C 2008, 112, 4. [Google Scholar]
- Yang, X.; Tian, S.; Li, R.; Wang, W.; Zhou, S. Use of single-crystalline Bi2S3 nanowires as room temperature ethanol sensor synthesized by hydrothermal approach. Sens. Actuators B Chem. 2017, 241, 210–216. [Google Scholar] [CrossRef]
- Kan, H.; Li, M.; Song, Z.; Liu, S.; Zhang, B.; Liu, J.; Li, M.-Y.; Zhang, G.; Jiang, S.; Liu, H. Highly sensitive response of solution-processed bismuth sulfide nanobelts for room-temperature nitrogen dioxide detection. J. Colloid Interface Sci. 2017, 506, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Xie, X.; Zhang, M.; Liao, N. First-principles investigation of Bi2S3 as sensitive and selective NO2 sensor upon humidity exposure. J. Mater. Sci. 2023, 58, 2198–2208. [Google Scholar] [CrossRef]
- Luo, J.; Feng, X.; Kan, H.; Li, H.; Fu, C. One-Dimensional Bi2S3 Nanobelts-Based Surface Acoustic Wave Sensor for NO2 Detection at Room Temperature. IEEE Sens. J. 2021, 21, 1404–1408. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, M.; Zhang, H.; Wang, B.; Chen, C.; Li, J.; Wang, Y.; Hao, J. Room temperature gas sensor based on rGO/Bi2S3 heterostructures for ultrasensitive and rapid NO2 detection. Chem. Eng. J. 2024, 490, 151872. [Google Scholar] [CrossRef]
- Kan, H.; Yang, W.; Guo, Z.; Li, M. Highly sensitive room-temperture NO2 gas sensor based on Bi2S3 nanorods. J. Mater. Sci. Mater. Electron. 2024, 35, 331. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, C.; Wang, Y.; Hao, J. Nanorods Assembled Hierarchical Bi2S3 for Highly Sensitive Detection of Trace NO2 at Room Temperature. Chemosensors 2024, 12, 8. [Google Scholar] [CrossRef]
- Cheng, Y.; Chang, Y.; Feng, Y.; Jian, H.; Tang, Z.; Zhang, H. Deep-Level Defect Enhanced Photothermal Performance of Bismuth Sulfide–Gold Heterojunction Nanorods for Photothermal Therapy of Cancer Guided by Computed Tomography Imaging. Angew. Chem. Int. Ed. 2017, 57, 246–251. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, T. Recent Progress of Nanostructured Sensing Materials from 0D to 3D: Overview of Structure–Property-Application Relationship for Gas Sensors. Small Methods 2021, 5, 2100515. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, M. Synthesis, characterization, and hydrophobic properties of Bi2S3 hierarchical nanostructures. Mater. Res. Bull. 2011, 46, 555–562. [Google Scholar] [CrossRef]
- Jia, T.; Wang, X.; Long, F.; Li, J.; Kang, Z.; Fu, F.; Sun, G.; Chen, J. Facile Synthesis, Characterization, and Visible-light Photocatalytic Activities of 3D Hierarchical Bi2S3 Architectures Assembled by Nanoplatelets. Crystals 2016, 6, 140. [Google Scholar] [CrossRef]
- Fu, T.-X. Gas sensor based on three dimensional Bi2S3 nanowires network for ammonia detection at room temperature. Mater. Res. Bull. 2018, 99, 460–465. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Zhang, J.; Zhou, M. Facile synthesis of hierarchical Bi2S3 nanostructures for photodetector and gas sensor. RSC Adv. 2012, 2, 6258–6261. [Google Scholar] [CrossRef]
- Yang, Y.; Xin, T.; Liu, C.; Zhang, T.; Hao, W.; Wang, Y.; Hao, J. Urchin-like Bi2S3 nanostructures with rich sulfur vacancies for ppb-level NO2 sensing. J. Alloys Compd. 2023, 930, 167467. [Google Scholar] [CrossRef]
- Shen, G.; Chen, D.; Tang, K.; Li, F.; Qian, Y. Large-scale synthesis of uniform urchin-like patterns of Bi2S3 nanorods through a rapid polyol process. Chem. Phys. Lett. 2003, 370, 334–337. [Google Scholar] [CrossRef]
- Lu, J.; Han, Q.; Yang, X.; Lu, L.; Wang, X. Microwave-assisted synthesis and characterization of 3D flower-like Bi2S3 superstructures. Mater. Lett. 2007, 61, 2883–2886. [Google Scholar] [CrossRef]
- Tang, J.; Alivisatos, A.P. Crystal Splitting in the Growth of Bi2S3. Nano Lett. 2006, 6, 2701–2706. [Google Scholar] [PubMed]
- Han, D.; Du, M.-H.; Dai, C.-M.; Sun, D.; Chen, S. Influence of defects and dopants on the photovoltaic performance of Bi2S3: First-principles insights. J. Mater. Chem. A 2017, 5, 6200–6210. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, Z.; Wu, D.; Ye, L.; Wang, T.; Wang, B.; An, T.; Wong, P.K. Defect-Type-Dependent Near-Infrared-Driven Photocatalytic Bacterial Inactivation by Defective Bi2S3 nanorods. ChemSusChem 2019, 12, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, J.; Wang, T.; Zheng, S.; Lv, W.; Chen, X.; Yang, J.; Zeng, M.; Hu, N.; Su, Y.; et al. High-Performance Wearable Sensor Inspired by the Neuron Conduction Mechanism through Gold-Induced Sulfur Vacancies. ACS Sens. 2022, 7, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Lee, N.-E. Gas sensing with heterostructures based on two-dimensional nanostructured materials: A review. J. Mater. Chem. C 2019, 7, 13367–13383. [Google Scholar] [CrossRef]
- Ma, S.; Yang, D.; Li, B.; Guan, Y.; Wu, M.; Wu, J.; Guo, Y.; Sheng, L.; Liu, L.; Yao, T. An interfacial C-S bond bridged S-scheme ZnS/C3N5 for photocatalytic H2 evolution: Opposite internal-electric-field of ZnS/C3N4, increased field strength, and accelerated surface reaction. J. Colloid Interface Sci. 2024, 664, 960–971. [Google Scholar] [CrossRef]
- Ghuge, R.S.; Shinde, M.D.; Rane, S.B. Bismuth-Based Gas Sensors: A Comprehensive Review. J. Electron. Mater. 2021, 50, 6060–6072. [Google Scholar] [CrossRef]
- Chen, X.; Wang, T.; Shi, J.; Lv, W.; Han, Y.; Zeng, M.; Yang, J.; Hu, N.; Su, Y.; Wei, H.; et al. A Novel Artificial Neuron-Like Gas Sensor Constructed from CuS Quantum Dots/Bi2S3 Nanosheets. Nano-Micro Lett. 2021, 14, 8. [Google Scholar] [CrossRef]
- Pei, Y.; Li, X.; Chu, H.; Ge, Y.; Dong, P.; Baines, R.; Pei, L.; Ye, M.; Shen, J. Anion-exchange engineering of cookie-like Bi2S3/Bi2 MoO6 heterostructure for enhanced photocatalytic activities and gas-sensing properties. Talanta 2017, 165, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhi, M.; Hang, D.; Ren, Q.; Zhang, P.; Chen, C.; Chen, Q.; Li, Q.; Zhang, Z.; Yan, J.; et al. Ultrasensitive NO2 gas sensor based on MoS2 modified urchin-like Bi2S3 heterojunction. Phys. E Low-Dimens. Syst. Nanostruct. 2023, 147, 115575. [Google Scholar] [CrossRef]
- Zhao, Z.; Su, Z.; Lv, Z.; Shi, P.; Jin, G.; Wu, L. Room temperature gas sensors for NH3 detection based on the heterojunction of 2D Ti3C2Tx MXenes and Bi2S3. Microchim. Acta 2024, 191, 687. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, D.; Sun, Q.; Zheng, S.; Sun, J.; Wang, Y. Hierarchical SnS2/SnO2 nanoheterojunctions with increased active-sites and charge transfer for ultrasensitive NO2 detection. Nanoscale 2018, 10, 7210–7217. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mao, J.; Yin, D.; Zhang, T.; Liu, C.; Hao, W.; Wang, Y.; Hao, J. Synergy of S-vacancy and heterostructure in BiOCl/Bi2S3−x boosting room-temperature NO2 sensing. J. Hazard. Mater. 2023, 455, 131591. [Google Scholar] [CrossRef]
- Hu, C.; Yu, M.; Zhou, Z.; Wei, C.; Wang, Y.; Hao, J. Rapid room-temperature H2S detection based on Bi2S3/CuO heterostructures: The synergy of increased surface-adsorbed oxygen and a heterojunction effect. Inorg. Chem. Front. 2025, 12, 578–587. [Google Scholar] [CrossRef]
- Cheng, Y.; Ren, B.; Xu, K.; Jeerapan, I.; Chen, H.; Li, Z.; Ou, J.Z. Recent progress in intrinsic and stimulated room-temperature gas sensors enabled by low-dimensional materials. J. Mater. Chem. C 2021, 9, 3026–3051. [Google Scholar] [CrossRef]
- Lim, K.; Jo, Y.M.; Yoon, J.W.; Kim, J.S.; Lee, D.J.; Moon, Y.K.; Yoon, J.W.; Kim, J.H.; Choi, H.J.; Lee, J.H. A Transparent Nanopatterned Chemiresistor: Visible-Light Plasmonic Sensor for Trace-Level NO2 Detection at Room Temperature. Small 2021, 17, 2100438. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.M.; Eom, T.H.; Cho, S.H.; Kim, T.; Jang, H.W. Light-activated gas sensing: A perspective of integration with micro-LEDs and plasmonic nanoparticles. Mater. Adv. 2021, 2, 827–844. [Google Scholar] [CrossRef]
- Liu, D.; Tang, Z.; Zhang, Z. Comparative study on NO2 and H2S sensing mechanisms of gas sensors based on WS2 nanosheets. Sens. Actuators B Chem. 2020, 303, 127114. [Google Scholar] [CrossRef]
- Liu, J.; Xin, T.; Yang, Z.; Hao, W.; Wang, Y.; Hao, J. Bi2S3/ZnS heterostructures for H2S sensing in the dark: The synergy of increased surface-adsorbed oxygen and charge transfer. Inorg. Chem. Front. 2022, 9, 4921–4929. [Google Scholar] [CrossRef]
- Li, X.; Quan, W.; Yang, R.; Li, X.; Wu, J.; Fan, C.; Lv, W.; Liu, X.; Zeng, M.; Yang, J.; et al. Bismuth Sulfide Nanorod/Vanadium Carbide MXene as Nitrogen Dioxide Gas Sensor Operating at Room Temperature under Ultraviolet-Assisted Recovery. ACS Appl. Nano Mater. 2024, 7, 23882–23893. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, R.; Lei, H.; Han, M.; Hao, J. Recent Progress with Bismuth Sulfide for Room-Temperature Gas Sensing. Chemosensors 2025, 13, 120. https://doi.org/10.3390/chemosensors13040120
Ma R, Lei H, Han M, Hao J. Recent Progress with Bismuth Sulfide for Room-Temperature Gas Sensing. Chemosensors. 2025; 13(4):120. https://doi.org/10.3390/chemosensors13040120
Chicago/Turabian StyleMa, Renping, Haoxin Lei, Mingyang Han, and Juanyuan Hao. 2025. "Recent Progress with Bismuth Sulfide for Room-Temperature Gas Sensing" Chemosensors 13, no. 4: 120. https://doi.org/10.3390/chemosensors13040120
APA StyleMa, R., Lei, H., Han, M., & Hao, J. (2025). Recent Progress with Bismuth Sulfide for Room-Temperature Gas Sensing. Chemosensors, 13(4), 120. https://doi.org/10.3390/chemosensors13040120





