A Miniaturized Device Based on Cobalt Oxide Nanoparticles for the Quantification of Uric Acid in Artificial and Human Sweat
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Solutions and Samples
2.2. Ink Based on Co3O4 Nanoparticles and Uox Immobilization
2.3. Preparation of the Electrodes
2.4. Electrochemical Techniques
2.5. Interference Tests
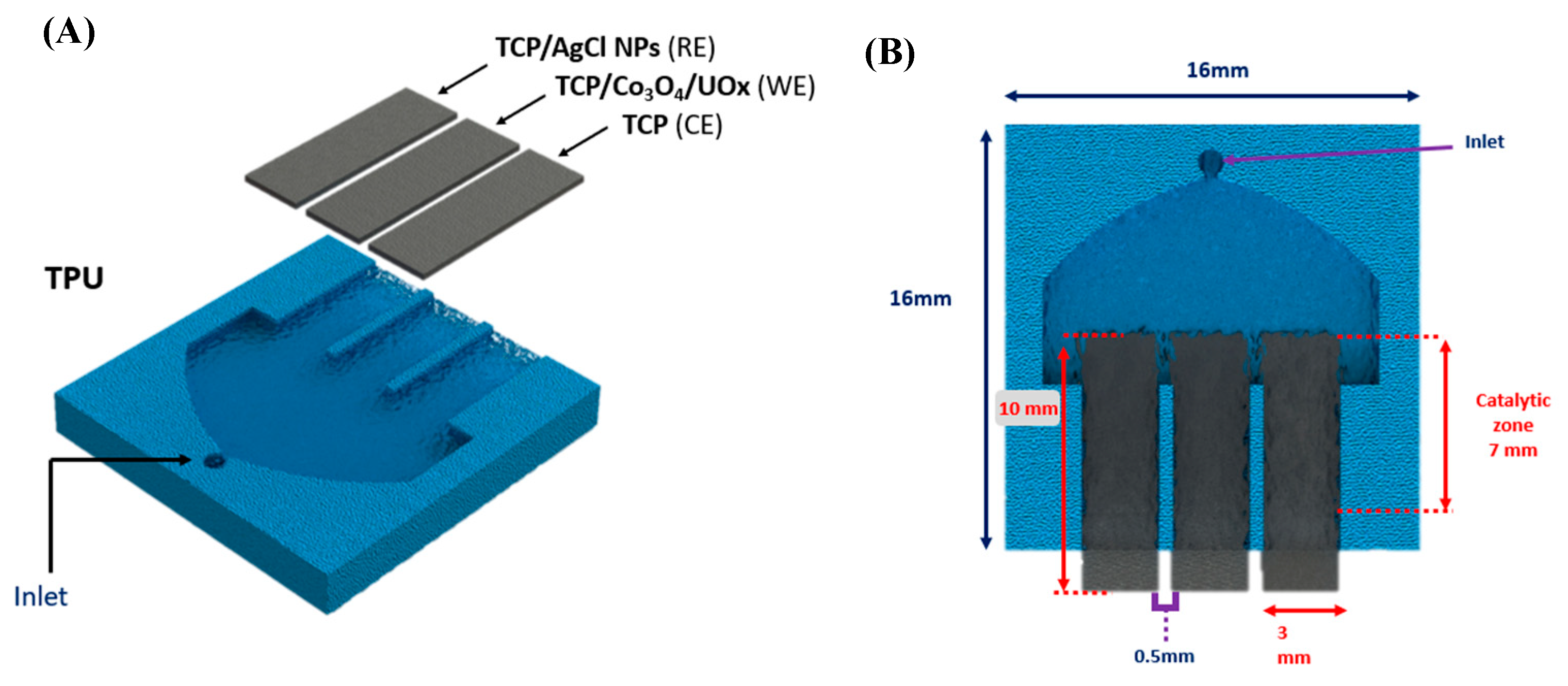
2.6. Construction of the Miniaturized Device
3. Results and Discussion
3.1. Physicochemical Characterization
3.1.1. X-Ray Diffraction (XRD)
3.1.2. Scanning Electron Microscopy (SEM)
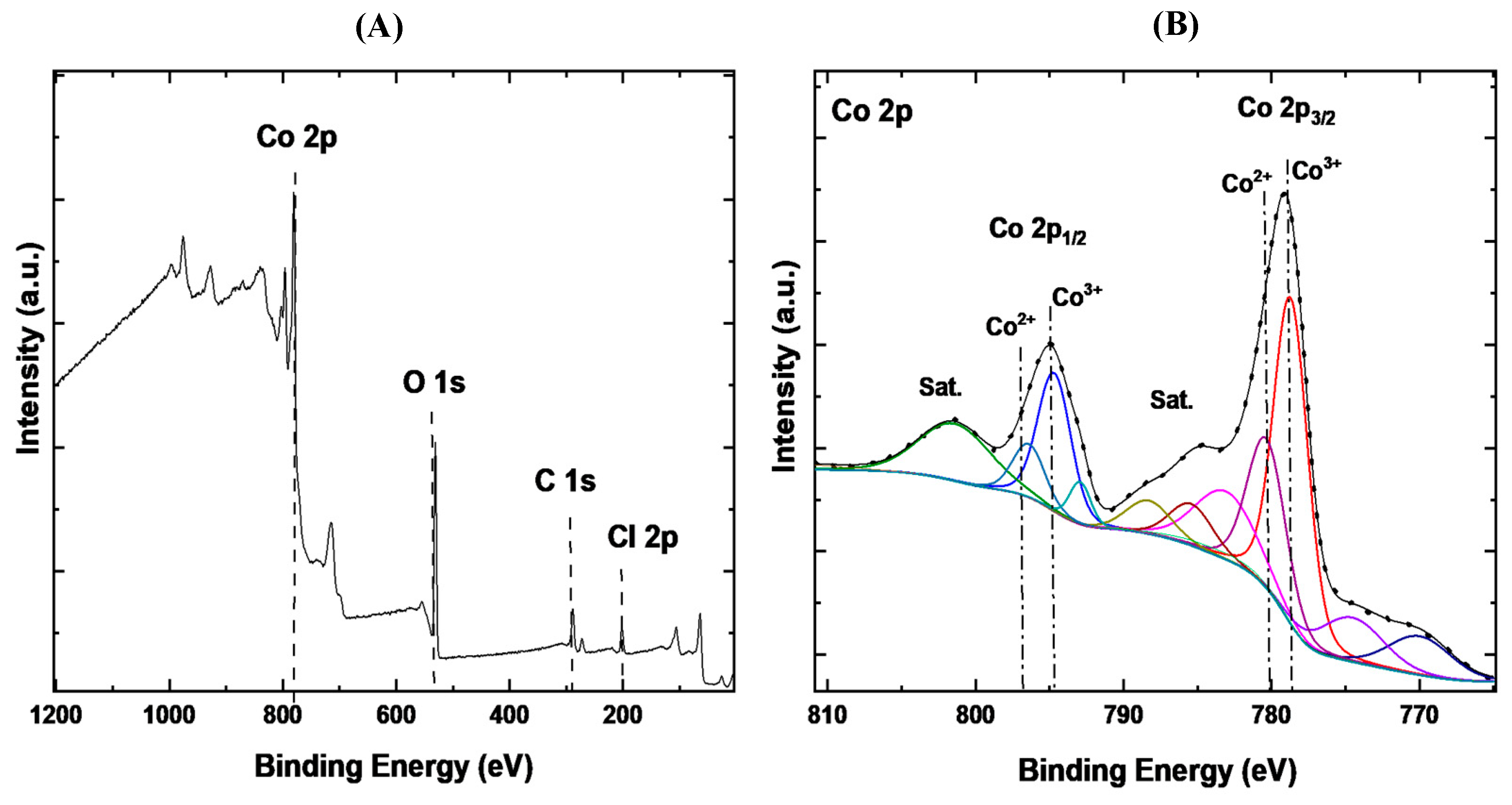
3.1.3. X-Ray Photoelectron Spectroscopy (XPS) Analysis
3.2. Electrochemical Characterization
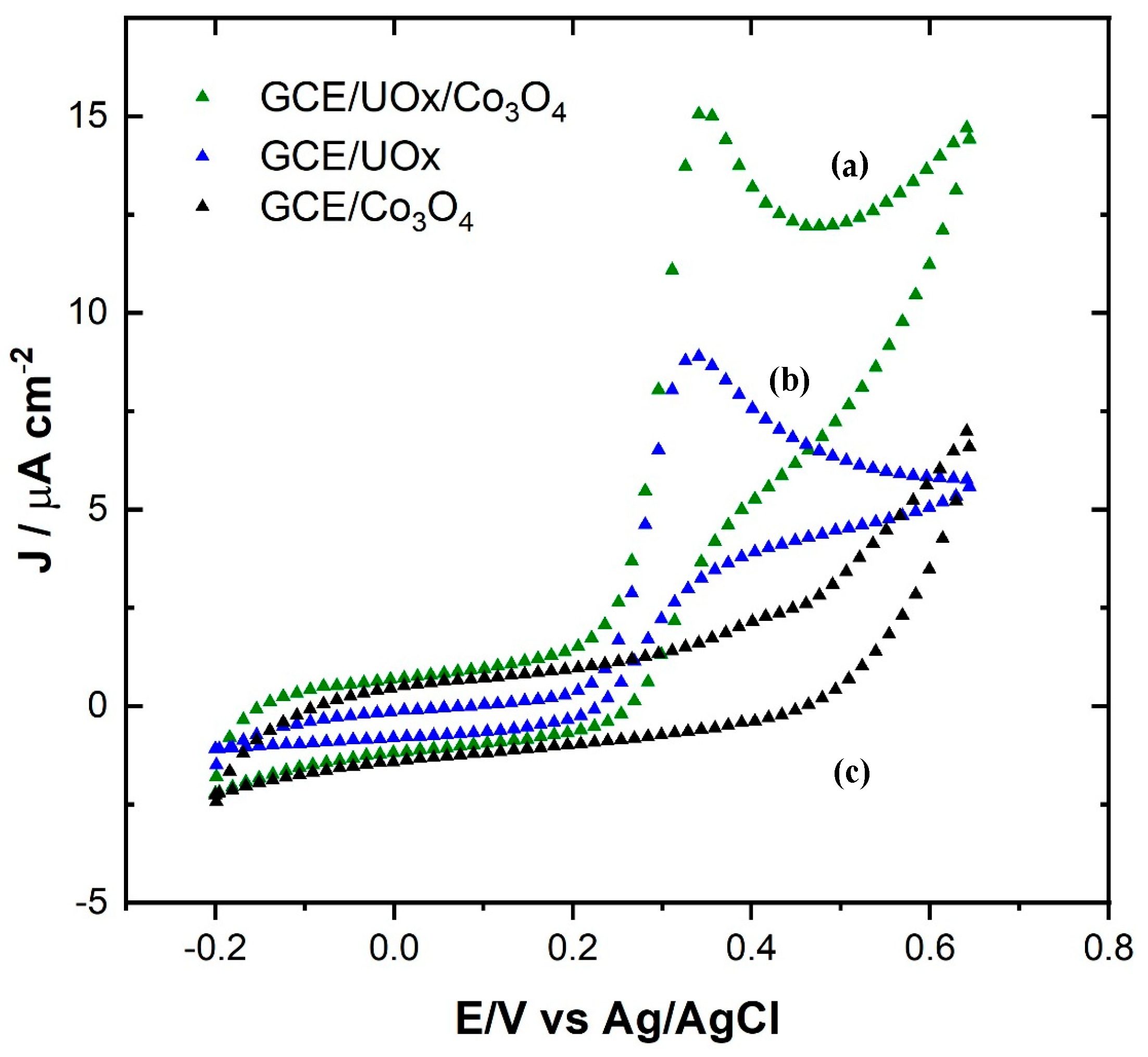
3.2.1. Electrocatalytic Activity in the Half-Cell Configuration
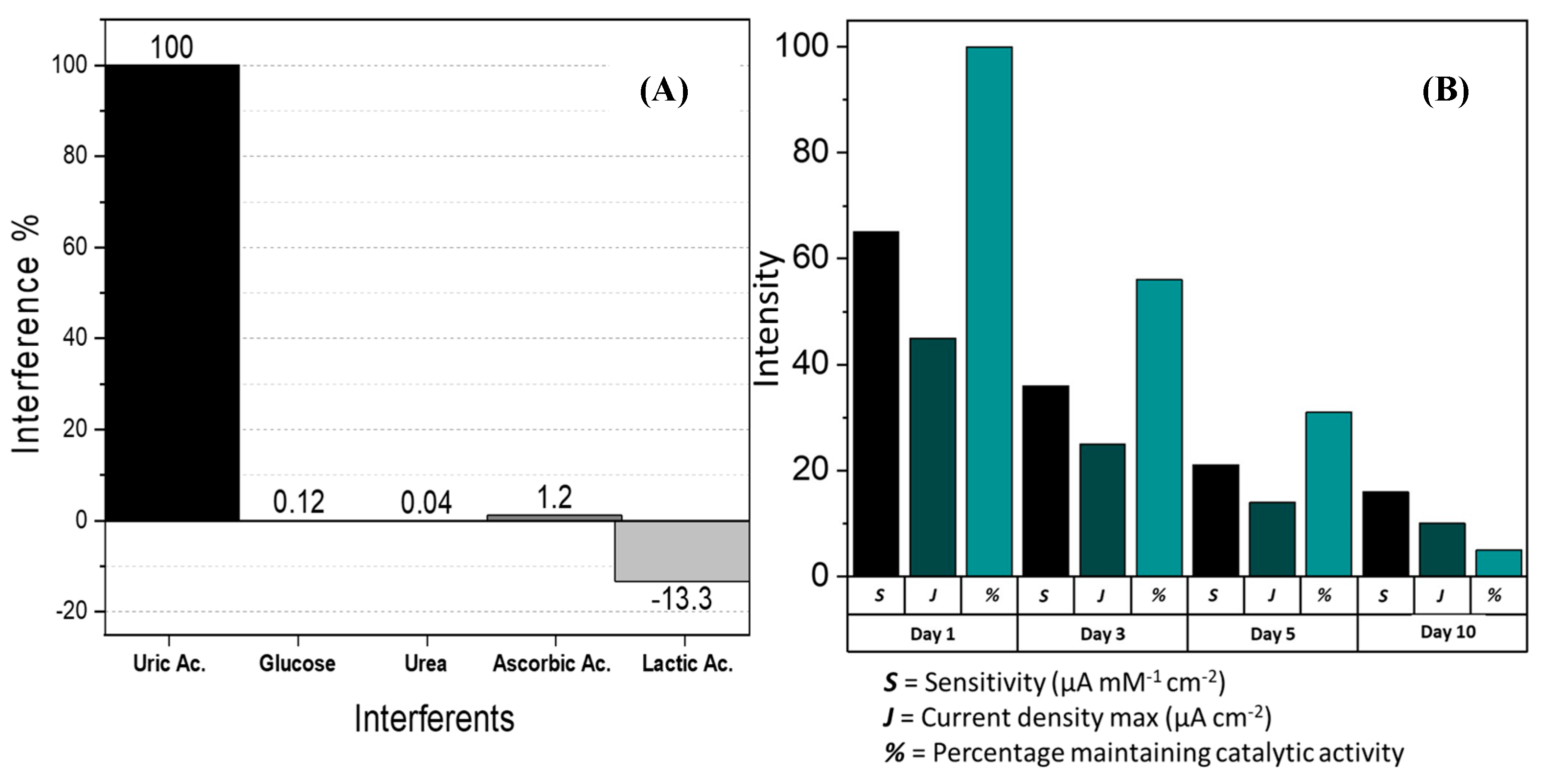
3.2.2. Interferents and Stability Tests of GCE/Co3O4/UOx
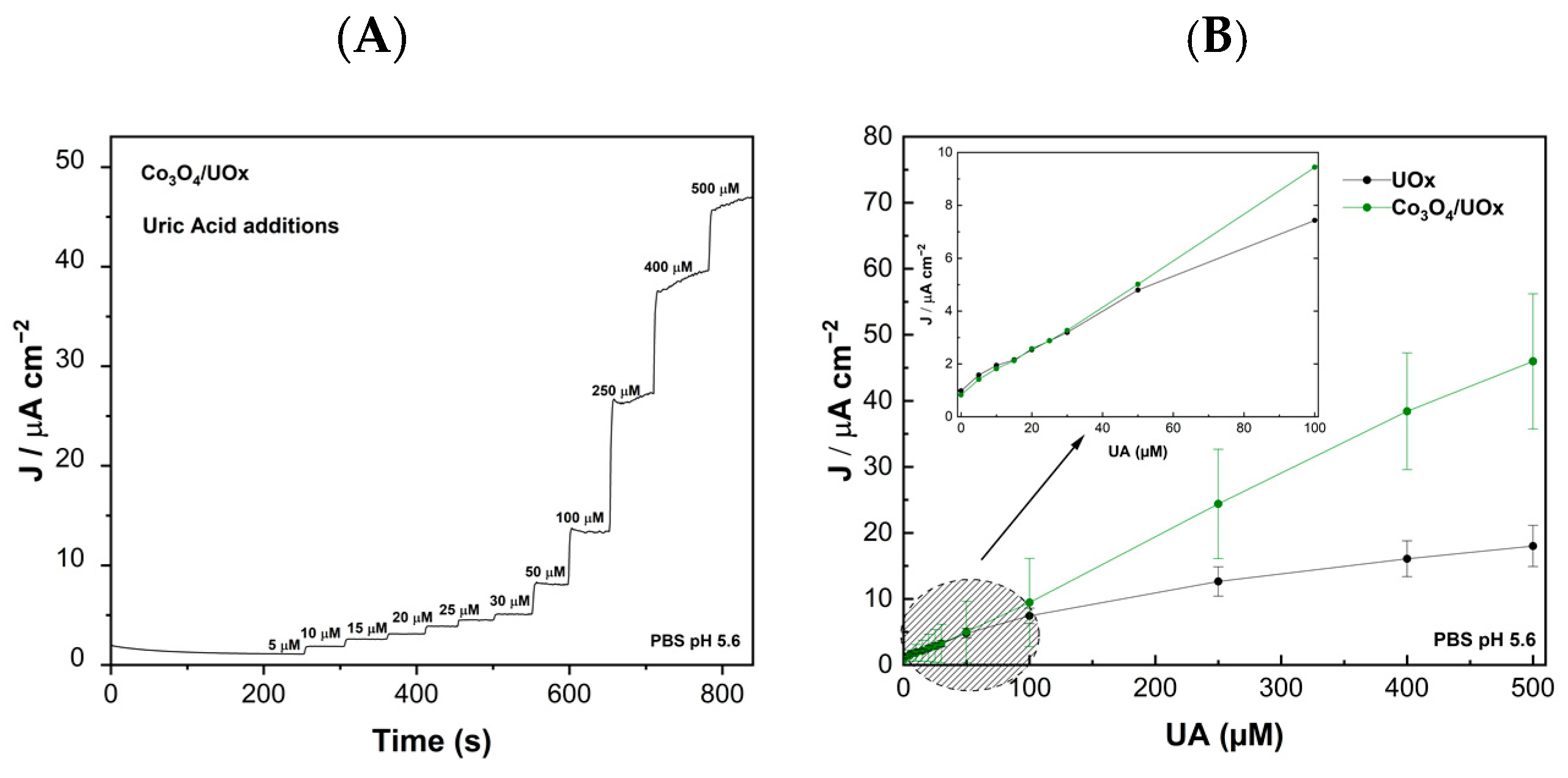
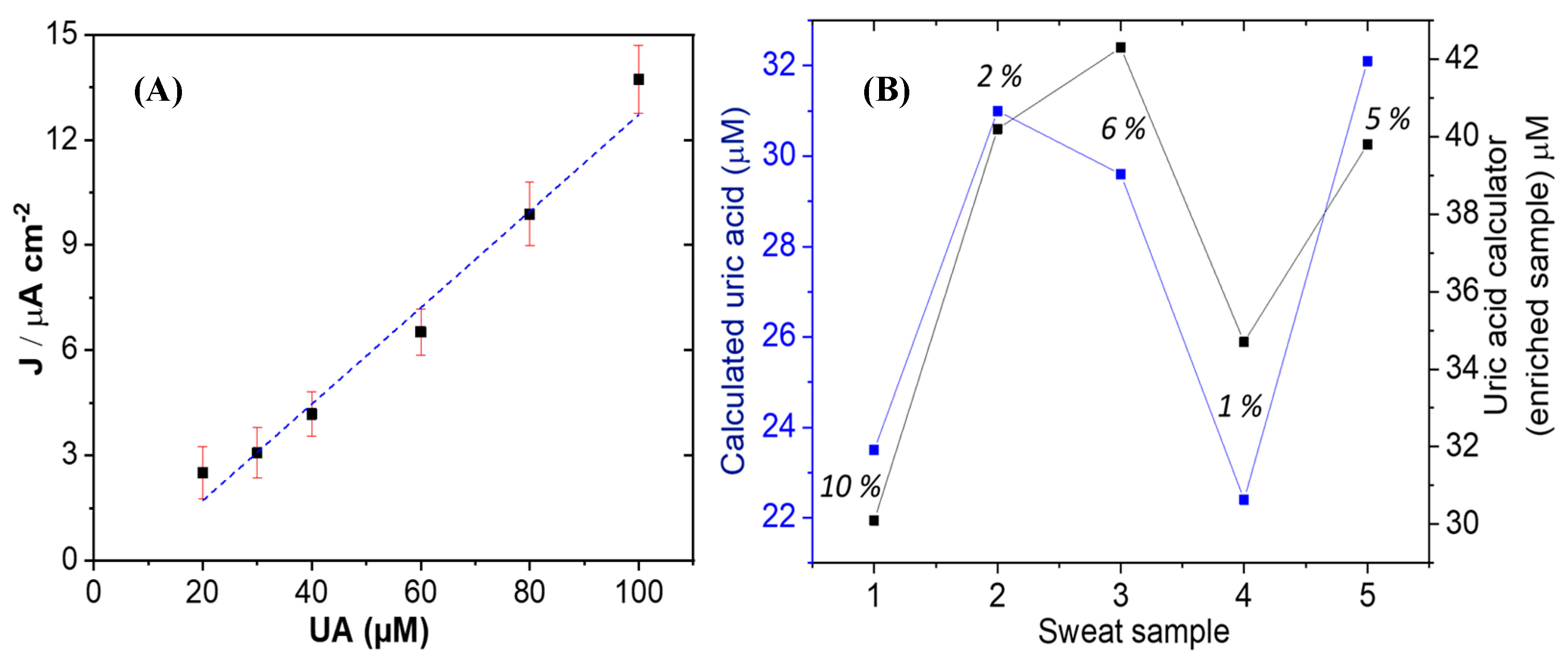
3.2.3. Electrocatalytic Reaction of GCE/Co3O4/UOx Using the Miniaturized Device in Artificial Sweat and Real Sweat
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, R.J.; Kang, D.-H.; Feig, D.; Kivlighn, S.; Kanellis, J.; Watanabe, S.; Tuttle, K.R.; Rodriguez-Iturbe, B.; Herrera-Acosta, J.; Mazzali, M. Is There a Pathogenetic Role for Uric Acid in Hypertension and Cardiovascular and Renal Disease? Hypertension 2003, 41, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Dutt, V.E.; Mottola, H.A. Determination of Uric Acid at the Microgram Level by a Kinetic Procedure Based on a Pseudo-Induction Period. Anal. Chem. 1974, 46, 1777–1781. [Google Scholar] [CrossRef] [PubMed]
- Raj, C.R.; Ohsaka, T. Voltammetric Detection of Uric Acid in the Presence of Ascorbic Acid at a Gold Electrode Modified with a Self-Assembled Monolayer of Heteroaromatic Thiol. J. Electroanal. Chem. 2003, 540, 69–77. [Google Scholar] [CrossRef]
- Alderman, M.; Aiyer, K.J.V. Uric Acid: Role in Cardiovascular Disease and Effects of Losartan. Curr. Med. Res. Opin. 2004, 20, 369–379. [Google Scholar] [CrossRef]
- Huang, S.-H.; Shih, Y.-C.; Wu, C.-Y.; Yuan, C.-J.; Yang, Y.-S.; Li, Y.-K.; Wu, T.-K. Detection of Serum Uric Acid Using the Optical Polymeric Enzyme Biochip System. Biosens. Bioelectron. 2004, 19, 1627–1633. [Google Scholar] [CrossRef]
- Aafria, S.; Kumari, P.; Sharma, S.; Yadav, S.; Batra, B.; Rana, J.S.; Sharma, M. Electrochemical Biosensing of Uric Acid: A Review. Microchem. J. 2022, 182, 107945. [Google Scholar] [CrossRef]
- Ma, C.-B.; Shang, X.; Zhu, Z.; Zheng, L.; Sun, M.; Cao, M.; Bai, J.; Du, Y.; Zhou, M. Evolving Health Monitoring: Nanoscale Flexible Electronics for Noninvasive Uric Acid Analysis in Sweat. TrAC Trends Anal. Chem. 2024, 179, 117889. [Google Scholar] [CrossRef]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of Uric Acid Metabolism and Excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Erden, P.E.; Kılıç, E. A Review of Enzymatic Uric Acid Biosensors Based on Amperometric Detection. Talanta 2013, 107, 312–323. [Google Scholar] [CrossRef]
- Cook, J.D.; Strauss, K.A.; Caplan, Y.H.; LoDico, C.P.; Bush, D.M. Urine PH: The Effects of Time and Temperature after Collection. J. Anal. Toxicol. 2007, 31, 486–496. [Google Scholar] [CrossRef]
- Huang, C.T.; Chen, M.L.; Huang, L.L.; Mao, I.F. Uric Acid and Urea in Human Sweat. Chin. J. Physiol. 2002, 45, 109–116. [Google Scholar] [PubMed]
- Xu, Z.; Song, J.; Liu, B.; Lv, S.; Gao, F.; Luo, X.; Wang, P. A Conducting Polymer PEDOT:PSS Hydrogel Based Wearable Sensor for Accurate Uric Acid Detection in Human Sweat. Sens. Actuators B Chem. 2021, 348, 130674. [Google Scholar] [CrossRef]
- Promphet, N.; Rattanawaleedirojn, P.; Siralertmukul, K.; Soatthiyanon, N.; Potiyaraj, P.; Thanawattano, C.; Hinestroza, J.P.; Rodthongkum, N. Non-Invasive Textile Based Colorimetric Sensor for the Simultaneous Detection of Sweat PH and Lactate. Talanta 2019, 192, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, K.; Kimura, M.; Ikarashi, R.; Yamaguchi, A.; Watanabe, T. Uric Acid Concentration in Saliva and Its Changes with the Patients Receiving Treatment for Hyperuricemia. Metabolomics 2012, 8, 484–491. [Google Scholar] [CrossRef]
- Georgakopoulos, C.D.; Lamari, F.N.; Karathanasopoulou, I.N.; Gartaganis, V.S.; Pharmakakis, N.M.; Karamanos, N.K. Tear Analysis of Ascorbic Acid, Uric Acid and Malondialdehyde with Capillary Electrophoresis. Biomed. Chromatogr. 2009, 24, 852–857. [Google Scholar] [CrossRef]
- Horwath-Winter, J.; Kirchengast, S.; Meinitzer, A.; Wachswender, C.; Faschinger, C.; Schmut, O. Determination of Uric Acid Concentrations in Human Tear Fluid, Aqueous Humour and Serum. Acta Ophthalmol. 2009, 87, 188–192. [Google Scholar] [CrossRef]
- Martinez-Pérez, D.; Ferrer, M.L.; Mateo, C.R. A Reagent Less Fluorescent Sol–Gel Biosensor for Uric Acid Detection in Biological Fluids. Anal. Biochem. 2003, 322, 238–242. [Google Scholar] [CrossRef]
- Ravariu, C.; Parvulescu, C.C.; Manea, E.; Tucureanu, V. Optimized Technologies for Cointegration of MOS Transistor and Glucose Oxidase Enzyme on a Si-Wafer. Biosensors 2021, 11, 497. [Google Scholar] [CrossRef]
- Robescu, M.S.; Bavaro, T. A Comprehensive Guide to Enzyme Immobilization: All You Need to Know. Molecules 2025, 30, 939. [Google Scholar] [CrossRef]
- Uchiyama, S.; Sakamoto, H. Immobilization of Uricase to Gas Diffusion Carbon Felt by Electropolymerization of Aniline and Its Application as an Enzyme Reactor for Uric Acid Sensor. Talanta 1997, 44, 1435–1439. [Google Scholar] [CrossRef]
- Kang, L.; He, D.; Bie, L.; Jiang, P. Nanoporous Cobalt Oxide Nanowires for Non-Enzymatic Electrochemical Glucose Detection. Sens. Actuators B Chem. 2015, 220, 888–894. [Google Scholar] [CrossRef]
- Hou, Y.; Ndamanisha, J.C.; Guo, L.; Peng, X.; Bai, J. Synthesis of Ordered Mesoporous Carbon/Cobalt Oxide Nanocomposite for Determination of Glutathione. Electrochim. Acta 2009, 54, 6166–6171. [Google Scholar] [CrossRef]
- Numan, A.; Shahid, M.M.; Omar, F.S.; Ramesh, K.; Ramesh, S. Facile Fabrication of Cobalt Oxide Nanograin-Decorated Reduced Graphene Oxide Composite as Ultrasensitive Platform for Dopamine Detection. Sens. Actuators B Chem. 2017, 238, 1043–1051. [Google Scholar] [CrossRef]
- Puspalak, A.; Chinnadurai, P.; Prathibha, R.; Kumar, M.P.; Manjushree, S.G.; UdayaKumar, V.; Adarakatti, P.S. Cobalt Oxide Nanoparticles Based Carbon Electrode for the Detection of Residual Nitrite in the Soil of Agricultural Fields. Mater. Res. Innov. 2023, 27, 100–109. [Google Scholar] [CrossRef]
- Li, S.-J.; Du, J.-M.; Zhang, J.-P.; Zhang, M.-J.; Chen, J. A Glassy Carbon Electrode Modified with a Film Composed of Cobalt Oxide Nanoparticles and Graphene for Electrochemical Sensing of H2O2. Microchim. Acta 2014, 181, 631–638. [Google Scholar] [CrossRef]
- Akyilmaz, E. A Biosensor Based on Urate Oxidase–Peroxidase Coupled Enzyme System for Uric Acid Determination in Urine. Talanta 2003, 61, 73–79. [Google Scholar] [CrossRef]
- Kuwabata, S.; Nakaminami, T.; Ito, S.; Yoneyama, H. Preparation and Properties of Amperometric Uric Acid Sensors. Sens. Actuators B Chem. 1998, 52, 72–77. [Google Scholar] [CrossRef]
- Arora, K.; Tomar, M.; Gupta, V. Highly Sensitive and Selective Uric Acid Biosensor Based on RF Sputtered NiO Thin Film. Biosens. Bioelectron. 2011, 30, 333–336. [Google Scholar] [CrossRef]
- Arora, K.; Sumana, G.; Saxena, V.; Gupta, R.K.; Gupta, S.K.; Yakhmi, J.V.; Pandey, M.K.; Chand, S.; Malhotra, B.D. Improved Performance of Polyaniline-Uricase Biosensor. Anal. Chim. Acta 2007, 594, 17–23. [Google Scholar] [CrossRef]
- Zhao, C.; Wan, L.; Wang, Q.; Liu, S.; Jiao, K. Highly Sensitive and Selective Uric Acid Biosensor Based on Direct Electron Transfer of Hemoglobin-Encapsulated Chitosan-Modified Glassy Carbon Electrode. Anal. Sci. 2009, 25, 1013–1017. [Google Scholar] [CrossRef]
- Kanyong, P.; Pemberton, R.M.; Jackson, S.K.; Hart, J.P. Development of a Sandwich Format, Amperometric Screen-Printed Uric Acid Biosensor for Urine Analysis. Anal. Biochem. 2012, 428, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, L.; Zhu, Z.; Zhang, J.; Zhu, J. Novel Uric Acid Sensor Based on Enzyme Electrode Modified by ZnO Nanoparticles and Multiwall Carbon Nanotubes. Anal. Lett. 2009, 42, 775–789. [Google Scholar] [CrossRef]
- John, J.F.; Dhinasekaran, D.; Subashchandran, S. Cobalt Doped NiFe2O4 on 3D Nickel Foam Substrate for Electrochemical Detection of Uric Acid. Surf. Interfaces 2024, 44, 103629. [Google Scholar] [CrossRef]
- Wang, L.; Pan, L.; Han, X.; Ha, M.N.; Li, K.; Yu, H.; Zhang, Q.; Li, Y.; Hou, C.; Wang, H. A Portable Ascorbic Acid in Sweat Analysis System Based on Highly Crystalline Conductive Nickel-Based Metal-Organic Framework (Ni-MOF). J. Colloid Interface Sci. 2022, 616, 326–337. [Google Scholar] [CrossRef]
- Baker, L.B.; Wolfe, A.S. Physiological Mechanisms Determining Eccrine Sweat Composition. Eur. J. Appl. Physiol. 2020, 120, 719–752. [Google Scholar] [CrossRef]
- Eggenstein, C.; Borchardt, M.; Diekmann, C.; Gründig, B.; Dumschat, C.; Cammann, K.; Knoll, M.; Spener, F. A Disposable Biosensor for Urea Determination in Blood Based on an Ammonium-Sensitive Transducer. Biosens. Bioelectron. 1999, 14, 33–41. [Google Scholar] [CrossRef]
- Al-Tamer, Y.Y.; Hadi, E.A.; Al-Badrani, I. Sweat Urea, Uric Acid and Creatinine Concentrations in Uraemic Patients. Urol. Res. 1997, 25, 337–340. [Google Scholar] [CrossRef]
- Su, D.; Dou, S.; Wang, G. Single Crystalline Co3O4 Nanocrystals Exposed with Different Crystal Planes for Li-O2 Batteries. Sci. Rep. 2014, 4, 5767. [Google Scholar] [CrossRef]
- Cardenas-Flechas, L.J.; Barba-Ortega, J.J.; Joya, M.R. Analysis and Evaluation of Structural Properties of Co3O4 Microparticles Obtained at Low Temperature. Cerâmica 2022, 68, 52–59. [Google Scholar] [CrossRef]
- Karuppiah, C.; Thirumalraj, B.; Alagar, S.; Piraman, S.; Li, Y.-J.J.; Yang, C.-C. Solid-State Ball-Milling of Co3O4 Nano/Microspheres and Carbon Black Endorsed LaMnO3 Perovskite Catalyst for Bifunctional Oxygen Electrocatalysis. Catalysts 2021, 11, 76. [Google Scholar] [CrossRef]
- Wu, W.-T.; Huang, Z.; Liu, Y.; Hong, J.; Zhang, Q. Novel Co-MOF/GO Triggered Peroxymonosulfate Activation: Insight into Singlet Oxygen Generation Mechanism from Peroxymonosulfate Self-Decomposition and Secondary Radical Conversion. Chem. Eng. J. 2023, 476, 146365. [Google Scholar] [CrossRef]
- Ruiz-Guerrero, C.D.; Estrada-Osorio, D.V.; Gutiérrez, A.; Espinosa-Lagunes, F.I.; Escalona-Villalpando, R.A.; Luna-Bárcenas, G.; Molina, A.; Arenillas, A.; Arriaga, L.G.; Ledesma-García, J. Novel Cobalt-Based Aerogels for Uric Acid Detection in Fluids at Physiological PH. Biosens. Bioelectron. 2025, 267, 116850. [Google Scholar] [CrossRef]
- Chelmea, L.; Badea, M.; Scarneciu, I.; Moga, M.A.; Dima, L.; Restani, P.; Murdaca, C.; Ciurescu, D.; Gaman, L.E. New Trends in Uric Acid Electroanalysis. Chemosensors 2023, 11, 341. [Google Scholar] [CrossRef]
- Moutcine, A.; Laghlimi, C.; Ziat, Y.; El Bahraoui, S.; Belkhanchi, H.; Jouaiti, A. Advanced Design of Chemically Modified Electrodes for the Electrochemical Analysis of Uric Acid and Xanthine. J. Pharm. Biomed. Anal. 2025, 253, 116536. [Google Scholar] [CrossRef]
- Cheng, J.; Wu, Y.; Zhu, L.; Lin, S.; Liu, Y.; Huang, K.; Xu, W. Uric Acid Biosensors Based on Molecular Recognition: Classifications, Advances, and Prospects. TrAC Trends Anal. Chem. 2024, 179, 117887. [Google Scholar] [CrossRef]
- Zhou, F.; Lim, H.N.; Ibrahim, I.; Endot, N.A.; Malek, E.A.; Gowthaman, N.S.K. Simultaneous Electrochemical Detection of Dopamine and Uric Acid via Au@Cu-Metal Organic Framework. Chempluschem 2024, 89, e202300686. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Wang, C.; Zeng, X.; Lei, J.; Hou, J.; Huo, D.; Hou, C. Co Single-Atom Nanozymes for the Simultaneous Electrochemical Detection of Uric Acid and Dopamine in Biofluids. ACS Appl. Nano. Mater. 2024, 7, 6273–6283. [Google Scholar] [CrossRef]
- Wang, L.; Gao, N.; Cai, Z.; Grushevskaya, H.; He, H.; He, Y.; Chang, G. Alumina Inorganic Molecularly Imprinted Polymer Modified Multi-Walled Carbon Nanotubes for Uric Acid Detection in Sweat. Microchim. Acta 2024, 191, 247. [Google Scholar] [CrossRef]
- Fang, B.; Zhao, Z.; Ma, J.; Li, H.; Yan, F.; Tao, X. In Vitro Detection of Lactate and Uric Acid Based on Adaptive Graphene Oxide Membranes. Small Sci. 2024, 4, 2300264. [Google Scholar] [CrossRef]
- Jiang, D.; Zhu, Y.; Sun, Z.; Zhu, Z.; He, Q.; Huang, X.; Yang, Y.; Ge, Y.; Zhang, Q.; Wang, Y. A Silver Nanowires@Prussian Blue Composite Aerogel-Based Wearable Sensor for Noninvasive and Dynamic Monitoring of Sweat Uric Acid. Chem. Eng. J. 2024, 486, 150220. [Google Scholar] [CrossRef]
- Albaqami, M.D.; Medany, S.S.; Nafady, A.; Ibupoto, M.H.; Willander, M.; Tahira, A.; Aftab, U.; Vigolo, B.; Ibupoto, Z.H. The Fast Nucleation/Growth of Co 3 O 4 Nanowires on Cotton Silk: The Facile Development of a Potentiometric Uric Acid Biosensor. RSC Adv. 2022, 12, 18321–18332. [Google Scholar] [CrossRef]

| Fluid | Concentration (µM) | pH | Reference |
|---|---|---|---|
| Blood | 90–420 | 7.4 | [8] |
| Urine | 1400–4400 | 4.5–8.0 (5–6) | [9,10] |
| Sweat | 24.5 | 4.0–6.8 | [11,12,13] |
| Saliva | 201.1 ± 2.5 | 6.7–7.5 | [14] |
| Tears | 107.2–118.9 | 7.0–7.7 | [15,16] |
| Fluid | RE | Support | LOD | S | LR | Reference |
|---|---|---|---|---|---|---|
| Sweat | UOx/PSS | PEDOT hydrogel | 1.2 μM | 0.875 μA mM−1 cm−2 | 2–250 μM | [12] |
| Urine | UOx | Teflon membrane | 0.1 μM | - | 0.1–0.5 μM | [26] |
| Biological fluids | UOx | Sol–gel matrix | 20 nM | - | 20 nM–1 μM | [17] |
| Urine | UOx | Porous carbon | 4 μM | 2.5 μA mM−1 cm−2 | 10.0–400 μM | [20] |
| Blood | UOx | Au electrode | - | 143.0 nA μM−1 cm−2 | 6 μM | [27] |
| Blood | UOx | Pt/Ti/NiO cover on glass | 0.11 mM | 1278.48 μA mM−1 cm−2 | 0.05–1 mM | [28] |
| Blood | UOx | Polyaniline screen | 0.01 mM | 47.2 mA mM−1 10.66 mA mM−1 | 0.01–0.05 mM 0.1–0.6 mM | [29] |
| Urine | UOx | Chitosan | 0.85 μM | 29.5 μA mM−1 cm−2 | 2.0–30 μM | [30] |
| Urine | UOx | Carbon electrode | 0.015 mM | 2.10 μA mM−1 cm−2 | 0.015–0.25 mM | [31] |
| Urine | UOx | ZNO/MWCNT modified electrode | 2.0 mM | 393.0 mA M−1 cm−2 | 5.0–1000 μM | [32] |
| Electrode | Km (µM) | S (µA mM−1 cm−2) | LOD (µM) | LOQ (µM) |
|---|---|---|---|---|
| GCE/Co3O4/UOx | 30.9 | 65 | 3.7 ± 0.5 | 12.3 |
| GCE/UOx | 10.8 | 45 | 5.3 ± 0.1 | 17.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Guerrero, C.D.; Estrada-Osorio, D.V.; Gutiérrez, A.; Espinosa-Lagunes, F.I.; Luna-Barcenas, G.; Escalona-Villalpando, R.A.; Arriaga, L.G.; Ledesma-García, J. A Miniaturized Device Based on Cobalt Oxide Nanoparticles for the Quantification of Uric Acid in Artificial and Human Sweat. Chemosensors 2025, 13, 114. https://doi.org/10.3390/chemosensors13030114
Ruiz-Guerrero CD, Estrada-Osorio DV, Gutiérrez A, Espinosa-Lagunes FI, Luna-Barcenas G, Escalona-Villalpando RA, Arriaga LG, Ledesma-García J. A Miniaturized Device Based on Cobalt Oxide Nanoparticles for the Quantification of Uric Acid in Artificial and Human Sweat. Chemosensors. 2025; 13(3):114. https://doi.org/10.3390/chemosensors13030114
Chicago/Turabian StyleRuiz-Guerrero, Carlos D., Dulce V. Estrada-Osorio, Alejandro Gutiérrez, Fabiola I. Espinosa-Lagunes, Gabriel Luna-Barcenas, Ricardo A. Escalona-Villalpando, Luis G. Arriaga, and Janet Ledesma-García. 2025. "A Miniaturized Device Based on Cobalt Oxide Nanoparticles for the Quantification of Uric Acid in Artificial and Human Sweat" Chemosensors 13, no. 3: 114. https://doi.org/10.3390/chemosensors13030114
APA StyleRuiz-Guerrero, C. D., Estrada-Osorio, D. V., Gutiérrez, A., Espinosa-Lagunes, F. I., Luna-Barcenas, G., Escalona-Villalpando, R. A., Arriaga, L. G., & Ledesma-García, J. (2025). A Miniaturized Device Based on Cobalt Oxide Nanoparticles for the Quantification of Uric Acid in Artificial and Human Sweat. Chemosensors, 13(3), 114. https://doi.org/10.3390/chemosensors13030114





