1. Introduction
Gardnerella vaginalis is a Gram-variable bacterium primarily associated with bacterial vaginosis (BV), a common yet often asymptomatic vaginal infection in women. BV has been linked to numerous health issues, including increased susceptibility to sexually transmitted infections (STIs), preterm labor, and pelvic inflammatory disease. The accurate and timely detection of
G. vaginalis is crucial for effective management and treatment, especially because untreated cases can lead to adverse reproductive and general health outcomes [
1,
2]. Traditional diagnostic techniques for
G. vaginalis, including culture methods and PCR assays, while reliable, are often time-consuming and require laboratory resources. Hence, there is a strong clinical need for a rapid, sensitive, and cost-effective diagnostic tool to enable early detection of
G. vaginalis in point-of-care settings [
3,
4,
5,
6].
In recent years, there has been growing interest in the development of rapid and sensitive diagnostic tools that can provide timely information for both patients and healthcare providers. Advances in electrochemical sensor technology present a promising avenue for the development of effective point-of-care diagnostic devices. Electrochemical sensors offer several advantages, including ease of use, real-time response times, low cost, and the potential for miniaturization [
1,
6].
The electrochemical rapid sensor for the detection of
Gardnerella vaginalis aims to utilize specific biochemical interactions to detect the presence of this bacterium in vaginal samples. By employing various electrochemical techniques, including impedance spectroscopy and voltammetry, the sensor can achieve high sensitivity and specificity, allowing for the detection of
Gardnerella vaginalis even at low concentrations. Electrochemical sensors are analytical devices that detect specific biological or chemical analytes by measuring electrical signals resulting from redox reactions or changes in charge transfer at the electrode surface. These measurements, which include techniques such as voltammetry, amperometry, and impedance spectroscopy, provide valuable insights into analyte concentration, binding interactions, and reaction kinetics [
7,
8,
9]. These sensors are particularly advantageous for pathogen detection due to their high sensitivity, simplicity, and potential for miniaturization. Electrochemical sensors operate based on the following primary mechanisms [
2,
9,
10,
11]:
- (a)
Amperometry: Measures the current resulting from redox reactions of the analyte at the sensor’s electrode [
12].
- (b)
Potentiometry: Measures voltage changes corresponding to the concentration of the analyte.
- (c)
Electrochemical Impedance Spectroscopy: Tracks the resistance changes as the analyte binds to the sensor surface. For
G. vaginalis detection, the sensor is designed to recognize specific biomolecular markers (such as DNA sequences, proteins, or metabolites) associated with the bacterium, translating the biochemical interactions into quantifiable electrical signals [
13].
Electrochemical sensors for pathogens are typically developed using functionalized electrodes that have been modified to capture the target organism or its components specifically. The detection mechanism is based on biological recognition elements, such as antibodies, aptamers, or DNA probes, that selectively bind to
G. vaginalis [
14].
For
G. vaginalis, DNA probes targeting specific sequences within the bacterium’s genome, antibodies against
G. vaginalis surface proteins, or aptamers that specifically bind to bacterial metabolites are commonly used [
15]. The specificity of these elements ensures high accuracy and low false positives.
This work not only addresses the clinical need for rapid and reliable detection methods for bacterial vaginosis but also contributes to the broader field of biosensor development. The proposed peptide-based electrochemical sensor, functionalized with gold nanoparticles on a graphene electrode, enhances sensitivity and specificity through optimized biorecognition interactions. This study evaluates key performance parameters, including detection limit (LOD), response time, and stability under different storage conditions. Furthermore, by facilitating timely and accurate diagnosis, the developed sensor has the potential to improve patient management and outcomes by enabling healthcare providers to offer targeted treatment options, reducing complications and recurrence rates. In this study, we present the design, fabrication, and performance evaluation of the electrochemical sensor, as well as its potential applications in clinical settings. Through this innovation, we aim to enhance the diagnostic capabilities for
Gardnerella vaginalis and contribute to better reproductive health outcomes. The combined use of gold nanoparticles (AuNPs) and graphene offers several advantages over using either material alone, particularly in applications such as electrochemical sensing, catalysis, and biomedical devices (
Table 1). The synergy between these materials enhances their individual properties, leading to superior performance. Here are the key advantages:
- (a)
Enhanced Electrical Conductivity
Graphene is a highly conductive material with excellent electron mobility.
Gold nanoparticles provide additional electron transfer pathways, improving charge transfer kinetics.
Combined effect: The integration of AuNPs with graphene enhances conductivity further, making them ideal for electrochemical sensors and electronic devices.
- (b)
Increased Surface Area and Active Sites
Graphene has a large surface area, which supports high molecular adsorption.
Gold nanoparticles provide additional active sites for chemical interactions.
Combined effect: The hybrid structure increases the density of active sites, improving catalytic and sensing capabilities.
- (c)
Improved Biocompatibility and Functionalization
Gold nanoparticles are highly biocompatible and can be functionalized with biomolecules (e.g., antibodies, enzymes, DNA).
Graphene supports functionalization but may require modifications.
Combined effect: The hybrid material facilitates biomolecular interactions, making it useful in biosensors, drug delivery, and medical diagnostics.
The combination of gold nanoparticles and graphene results in a highly conductive, stable, and multifunctional material with superior properties compared to using either component alone. This hybrid structure is particularly valuable in biosensing, catalysis, energy storage, and medical applications, where enhanced performance is crucial.
2. Materials and Methods
2.1. Chemicals
Potassium hexacyanoferrate (III), methanol, sodium nitrite, and hydrochloric acid were purchased from Chempur (Piekary Śląskie, Poland). Phosphate-buffered saline was obtained from Sigma–Aldrich (Poznan, Poland). Alumina slurries, 0.3 m, were purchased from Buehler (Lake Bluff, IL, USA). Sulphuric acid, potassium hydroxide, hydrogen peroxide, ethanol, and methanol were supplied by Pol-Aura (Dywity, Poland). Graphen and gold nanoparticles were purchased from Sigma Aldrich (Poznań, Poland).
Fmoc-Rink Amide AM Resin (0.7 mmol/g) and DL-Dithiothreitol (DTT) were purchased from Iris Biotech GmbH. Fmoc (Fluorenylmethyloxycarbonyl)-protected amino acids, Fmoc-Ala-OH, Fmoc-Asn(Trt)-OH, Fmoc-Asp(OBzl)-OH, Fmoc-Tyr(tBu)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Leu-OH, and Fmoc-Thr(tBu)-OH, Fmoc-Val-OH, Fmoc-Met-OH and Fmoc-Phe-OH were purchased from CSBio (Shanghai) Ltd. (Shanghai, China), Fmoc-His(Boc)-OH from CEM Corporation, 11-mercaptoundecanoic acid (11-MUA), Oxyma pure (Ethyl cyano(hydroxyimino)acetate), N,N’-Diisopropylcarbodiimide (DIC), Piperidine, 99%, extra pure, Triisopropylsilane (TIS) from Sigma-Aldrich, N,N-Dimethylformamide, 99.8% (DMF) and Trifluoroacetic Acid (TFA) for synthesis, were gained from VWR International, LLC (Radnor, PA, USA). Ethyl dieter was collected from POCH S.A (Gliwice, Poland). Aqueous solutions were made using double-distilled sterile water (ddH2O).
2.2. Instrumentation
Potentiostat-galvanostat system (PalmSens4, Palmsens, Houten, The Netherlands) was used. Screen printed electrode (Palmsens, The Netherlands) was used as a working electrode.
3. Results
3.1. Production and Purification of Vaginolysine (VLY) Protein from Gardnerella vaginalis
The gene encoding the recombinant protein was chemically synthesized with codon optimization for expression in an
Escherichia coli host (GenScript, Piscataway, NJ, USA). This synthetic gene was inserted into the pET-51b(+) vector using the SalI and HindIII restriction sites, resulting in an N-terminal Strep-Tag II and a C-terminal 10 × His-Tag fusion. The obtained plasmid was then transformed into competent
Escherichia coli BL21(DE3) cells (#C2527, New England Biolabs, UK). Additional recombinant proteins, including Upa3, UreaD, Oppa, and Sip, served as negative controls. All recombinant proteins were induced in 1000 mL of LB broth (#2020, A&A Biotechnology, Gdansk, Poland) using 0.1 mM isopropyl β-D-thiogalactoside at an OD600 of 0.5, at 37 °C for 3 h with shaking at 180 rpm. After induction, cell cultures were centrifuged, and the resulting pellets were lysed in 50 mL of buffer containing 50 mM NaH
2PO
4, 300 mM NaCl, 10% Triton X-100, 70,000 U/mL lysozyme (#62971, Merck, Darmstadt, Germany), and 15 µg/mL DNase I (#10104159001, Merck, Germany), adjusted to pH 8.0. The recombinant proteins were subsequently isolated from the lysate using IMAC chromatography with His-Select Nickel Affinity Gel (#P6611, Merck, Germany), employing a column gravity approach with 1 mL of resin (
Table 2).
3.2. Peptide Sythesis and Modification
The peptide with a sequence of 11MUA-KKK-AHYADFYLTNVM-NH2 was synthesized on Rink Amide resin (0.08 mmol) by microwave-assisted Fmoc solid-phase peptide synthesis (SPPS). An automated microwave peptide synthesizer, Initiator+ Alstra™ (Biotage, Sweden), was utilized for peptide chain elongation. Fmoc-amino acid derivative (0.5 M, 5 equiv.), DIC (0.5 M, 5 equiv.), and Oxyma (0.5 M, 5 equiv.) in dimethylformamid (DMF) were used in two 5 min couplings at 75 °C. The fluorenylmethyloxycarbonyl (Fmoc) protecting group was removed using a 20% piperidine solution in DMF at room temperature (1 × 3 min, 1 × 10 min). 11-MUA (11-mercaptoundecanoic acid) (0.5 M, 5 equiv.) was introduced at the N-terminal end of the peptide under standard coupling conditions during synthesis (1 × 7 min, 75 °C). The 11-MUA-labeled peptide was cleaved from Rink Amide resin for 2 h using the cleavage cocktail consisting of TFA/H2O/DTT/TIS (88/5/5/2) before being precipitated with anhydrous, cold diethyl ether and lyophilized. The modified peptide was analyzed using an analytical reverse-phase high-performance liquid chromatography (RP-HPLC) method to assess its purity and identity. A Prominence-i LC-2030C Plus system (Shimadzu, Kyoto, Japan) with a UV detector (λ = 224 nm) equipped with a Jupiter Proteo column (90Å, 4.6 × 250 mm, particle size 4 µm) was used. The method was 60 min long with a linear gradient method starting from 5 to 95% solvent B at a flow rate of 1 mL/min, where solvent A was water and B was acetonitrile as eluents containing 0.1% TFA. Following mass spectrometry measurement, the expected molecular weight was confirmed, indicating that the peptide was successfully synthesized and modified.
Afterward, a semi-preparative reverse phase HPLC system (Shimadzu/Prominence Modular HPLC, column: Luna C8(2), 5 µm 100 Å, 21.2 × 250 mm, with UV detection at λ = 224 nm, using the gradient method from 30 to 70% solvent B for 60 min at a flow rate of 15 mL/min, where solvent A was water and B was 80% acetonitrile as eluents containing 0.1% TFA) was utilized to purify the crude product.
The effective synthesis and purification were validated by an ESI mass spectrometer. A single quadrupole mass spectrometer LCMS 2020 Shimadzu (Kyoto, Japan) operated in positive ion mode (+) was used to perform analysis in the following conditions. The ionization source was set to a temperature of 250 °C, with a nebulizing gas flow rate of 1.5 L/min. The samples were injected into the ESI source at a flow rate of 0.4 mL/min, using a mobile phase composed of 60% acetonitrile in 0.1% formic acid (FA).
Characterization of the 11-MUA-labeled peptide: white solid; synthetic yield: 89%; HPLC purity > 97%; Rt: 23.88 min. ESI MS of peptide estimated value: 2026.9 (g/mol); observed value (m/z): 508 [M+4H]4+, 677 [M+3H]3+, 1015 [M+2H]2+.
3.3. Biomaterials Preparation and Identification by Reference Method
The Biotechnology Laboratory isolated recombinant proteins at the Institute of Biotechnology and Molecular Medicine following standard procedures. For verifying the efficacy of the produced antibodies, a control experiment was conducted. The possibility of detection of Gardnerella vaginalis protein using anti-protein Gardnerella vaginalis antibodies was confirmed in the ELISA test (A qPCR reaction detected the presence of the bacteria and their concentrations (CFU).
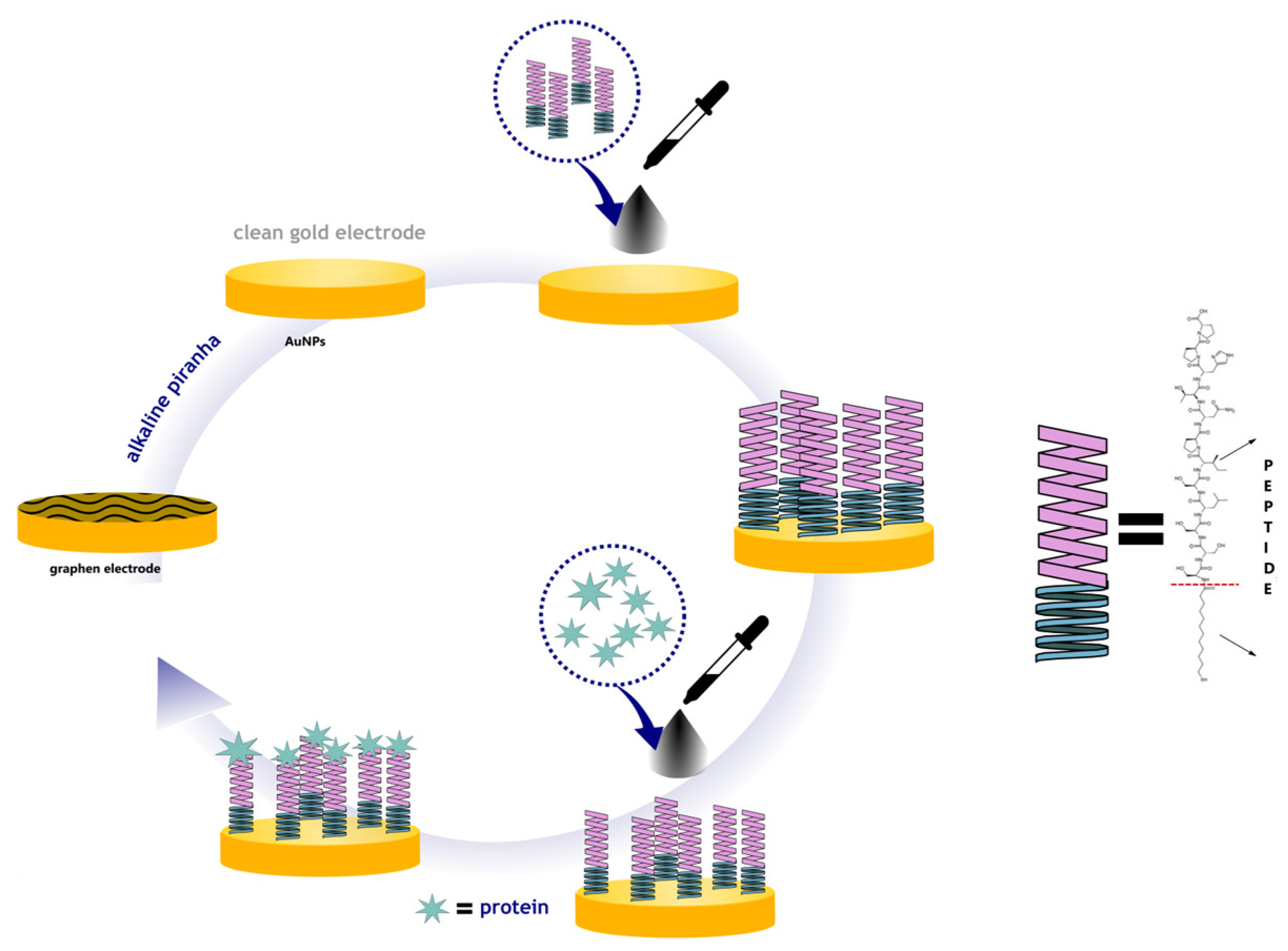
3.4. Procedure of Electrode Modification
Prior to electrochemical measurements, the commercial carbon electrodes were modified with graphene and Au nanoparticles in order to achieve a novel sensor with improved sensitivity to
Gardnerella vaginalis. Graphene dispersion was prepared by mixing 5 mg GPH (0.2% in acetic acid, pH 5). The gold nanoparticles (AuNPs) were prepared through the reduction of HAuCl4 with trisodium citrate solution in aqueous solution. Gold nanoparticles (AuNPs) can be synthesized by various methods, but in this work the Turkevich method (citrate reduction) was used. The size of the nanoparticles depends on the synthesis conditions, such as the ratio of reagents, temperature, and reaction time [
18]. The synthesis reaction can be summarized as follows:
Using the drop-and-dry method, chemically modified screen-printed carbon electrodes (SPCEs) were developed. A micropipette was used to deposit 10 μL of the nanomaterials’ composite dispersion onto the SPCEs, which were then dried at room temperature in a desiccator. The sensitive elements of the modified electrodes contained either GPH (GPH sensor) or GPH combined with AuNPs (GPH-AuNP sensor). After preparation, the electrodes were stored at 4 °C (
Figure 1).
To prepare for detection, 10.0 μL of a Pept solution (1000 × diluted in PBS) was applied to the working electrode. The device was then oven-dried at 37 °C until the solvent had fully evaporated. Following this step, 20.0 μL of protein solution was added for detection. Between each construction and detection step, the electrode was gently rinsed with PBS solution (0.1 mol L−1, pH 7.4). To prepare the device for detection, 10.0 μL of a Pept solution (1000 × diluted in PBS) was applied to the working electrode and oven-dried at 37 °C until the solvent had completely evaporated. Next, 20.0 μL of the protein solution was added for the detection step. Between each construction and detection step, the electrode was gently washed with PBS solution (0.1 mol L−1, pH 7.4).
3.5. Electrochemical Measurements
All the electrochemical tests: the cyclic voltammetry and electrochemical impedance spectroscopy (EIS) were conducted using a Palmsens 4 potentiostat/galvanostat system (Methrom, Autolab, Utrecht, The Netherlands) in the standard three-electrode configuration. Crabon electrode was used as a working electrode modified with a film from the dispersion of graphene and AuNPs, Ag|AgCl (3.0 mol L−1 KCl) as a reference electrode, and wire of platinum as a counter electrode (Pt).
All the electrochemical tests were carried out in 5 mM K3[Fe(CN)6]/K4[Fe(CN)6] in 0.01 M PBS that was previously deaerated. In cases of the electrochemical impedance spectroscopy measurements (EIS), the frequency ranged from 10 kHz to 1 Hz with 40 points. The amplitude of the AC signal was 10 mV. Each potential was held constant for 60 s before each measurement to obtain steady-state conditions. Obtained data were subjected to the analysis using EIS Spectrum Analyzer according to the proposed electric equivalent circuit (EEQC). The procedure described above is a standard measurement procedure used in biosensing.
3.6. Immunosensor Fabrication
A large set of GCE and GCE with graphene film samples was tested and studied during the optimization of process parameters to obtain an efficient modification procedure. Each surface was washed in an ultrasonic cleaner for 5 min in methanol and ddH2O and dried in an argon stream before the modification procedure.
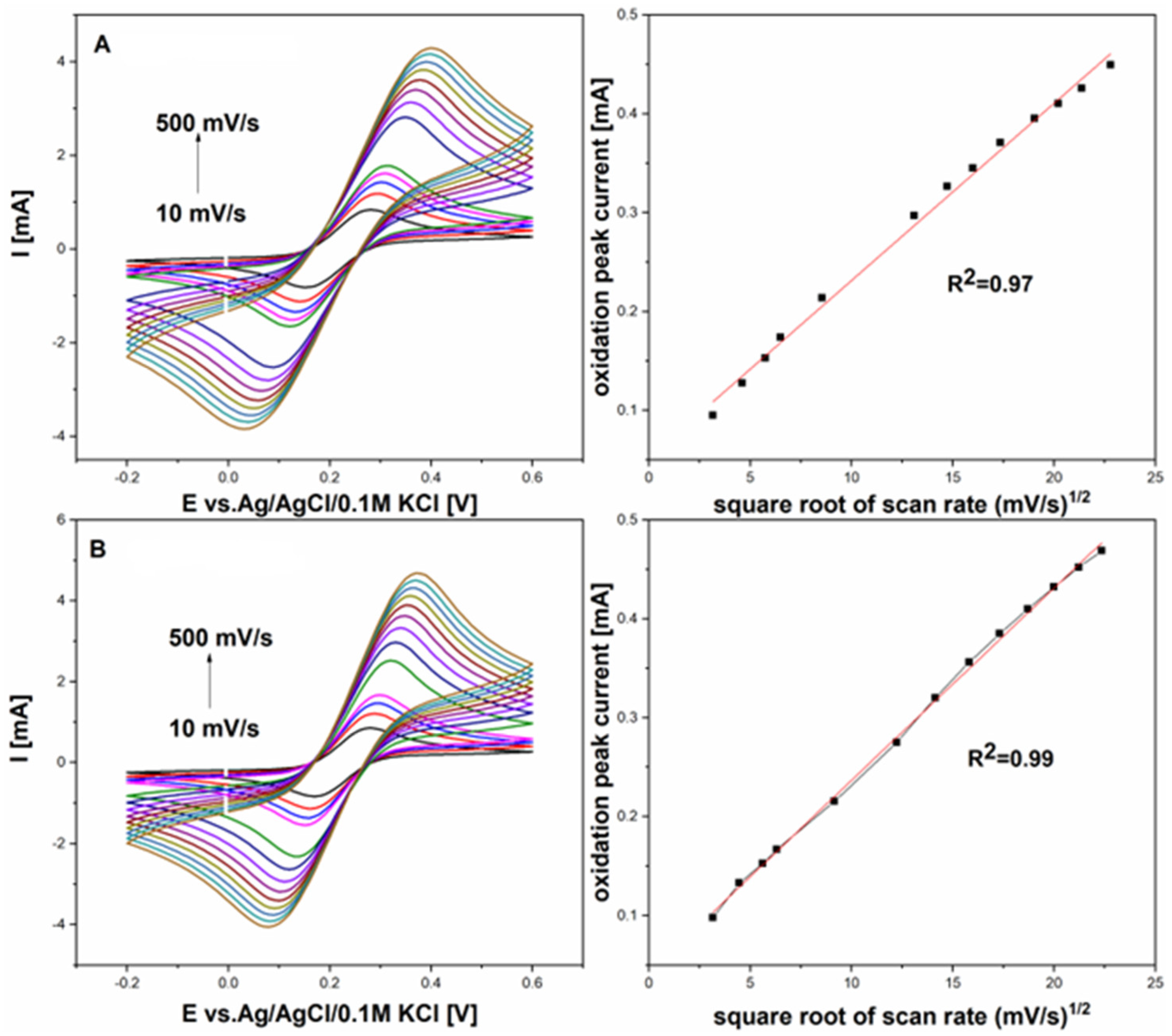
The electroactive area of the GCE and GCE with graphene film was determined using various scan rates (from 10 to 500 mV/s) in 5 mM K
3[Fe(CN)
6]/K
4[Fe(CN)
6] prepared in 0.01 M PBS with an equimolar mixture of redox probe, as shown in
Figure 2. This estimation was made by applying the following Randles–Ševčík equation:
where I
pI is the peak current (anodic or cathodic), nnn represents the number of electrons transferred (
n = 1), A is the electroactive surface area, D is the diffusion coefficient (D = 7.6 × 10
−6 cm
2/s for the redox probe in 0.01 M PBS), and C is the concentration of the redox probe.
Figure 2 compares cyclic voltammograms between the graphene electrode and the graphene electrode modified with gold nanoparticles, measured in a 5 mM K
3[Fe(CN)
6]/K
4[Fe(CN)
6] solution in 0.01 M PBS at scan rates from 10 to 500 mV/s. The difference in peak potentials (ΔEp) for the GPH electrode with AuNPs was 200 mV, while it was 243 mV for the GPH alone, indicating enhanced reversibility for the redox pair with the AuNP-modified GPH electrode. These findings demonstrate that GPH with AuNPs provides significantly better electrochemical performance than GPH alone.
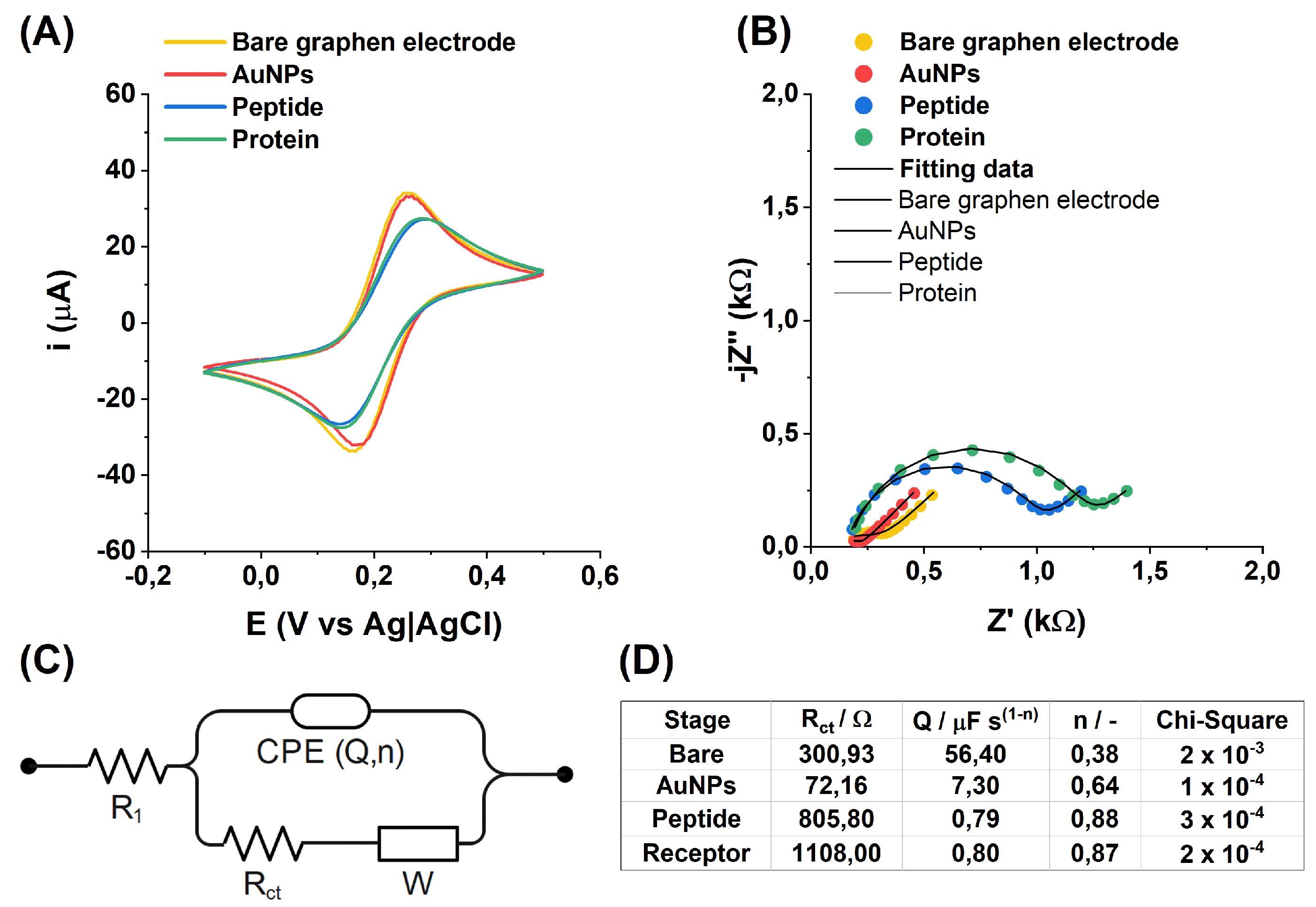
In
Figure 3A, the changes recorded during the successive steps of sensor modification are easily observable through the CV. Following the previously discussed alterations after incubation in AuNPs, a noticeable decrease of 0.5 µA in the current peak heights from the oxidation and reduction of [Fe(CN)
6]
3−/
4−, along with an increase in their separation by 0.04 mV, is evident after peptide addition. For the EIS data analysis (
Figure 3B), an electric equivalent circuit was built (
Figure 3C), a derivative of Randles circuit with parallel capacitance replaced by the constant phase element (CPE).
To enhance data presentation, the impedance spectra are displayed over a frequency range from 10 kHz to 8 Hz. For improved analysis of the EIS data, the spectra were fitted to an equivalent electrical circuit (EEC), as depicted in
Figure 3C, with the fitting results summarized in
Table 3. The chi-square parameter was used to assess the goodness of fit.
3.7. Electrochemical Characterization of the Immunosensor
The correctness of successive modifications of the electrode was verified using the EIS technique. Electrochemical measurements were conducted in a buffer solution prepared in PBS with a pH of 7.4, containing 5 mM K3[Fe(CN)6] and 5 mM K4[Fe(CN)6]. This redox system was specifically chosen because it facilitates the analysis of changes in electron transfer kinetics on the electrode surface. Such changes are indicative of modifications occurring at each stage. By comparing the results obtained after each modification step, it is possible to detect even subtle variations and assess whether each step was performed correctly. This approach ensures the reliability and accuracy of the modification process, providing a robust method for step-by-step validation. EIS measurements inform about changes in resistance that occur on the electrode surface.
3.8. Biosensor Selectivity, Repeatability and Stability Studies
One of the key requirements for developing any immunosensor is ensuring its stability under normal conditions [
19]. However, it is equally important to have the ability to store the immunosensor before use while maintaining its functionality. Unfortunately, electrochemical biosensors containing biological components often exhibit limited stability under standard conditions due to irreversible structural changes that may occur over time.
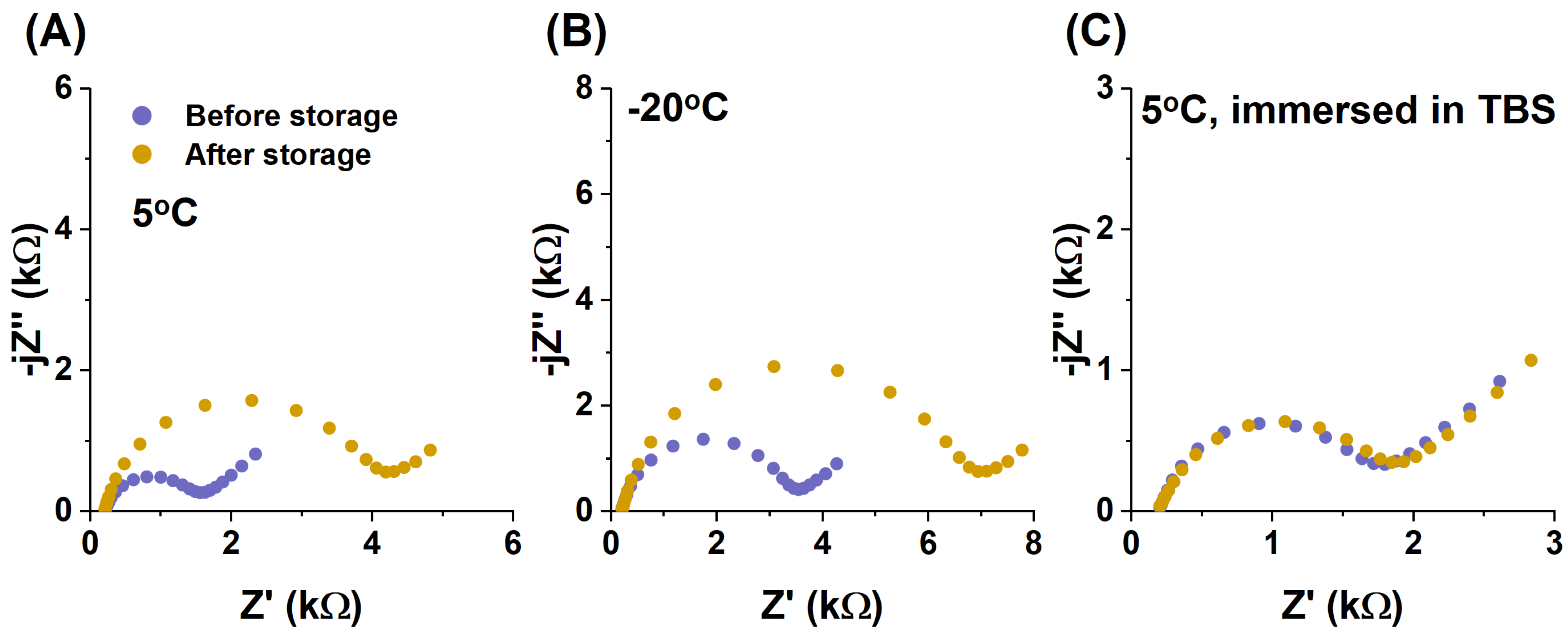
To evaluate the stability of the prepared biosensor, impedance spectra of the fully prepared electrode (ready for target protein detection) were recorded. Following this, the electrode was rinsed with deionized water and dried using an argon stream. Next, the prepared samples were stored under three different conditions:
At 5 °C, without immersion in any buffer;
At 20 °C, without immersion in any buffer;
At 5 °C, immersed in TBS buffer for 1 h.
After the designated storage period, the electrodes were once again rinsed with deionized water, dried with argon, and their impedance spectra were recorded for a second time. To ensure reliability, all tests were conducted on multiple samples, and the results are presented in
Figure 4.
This study provides insights into the effects of different storage conditions on biosensor stability, helping to optimize conditions for long-term usability.
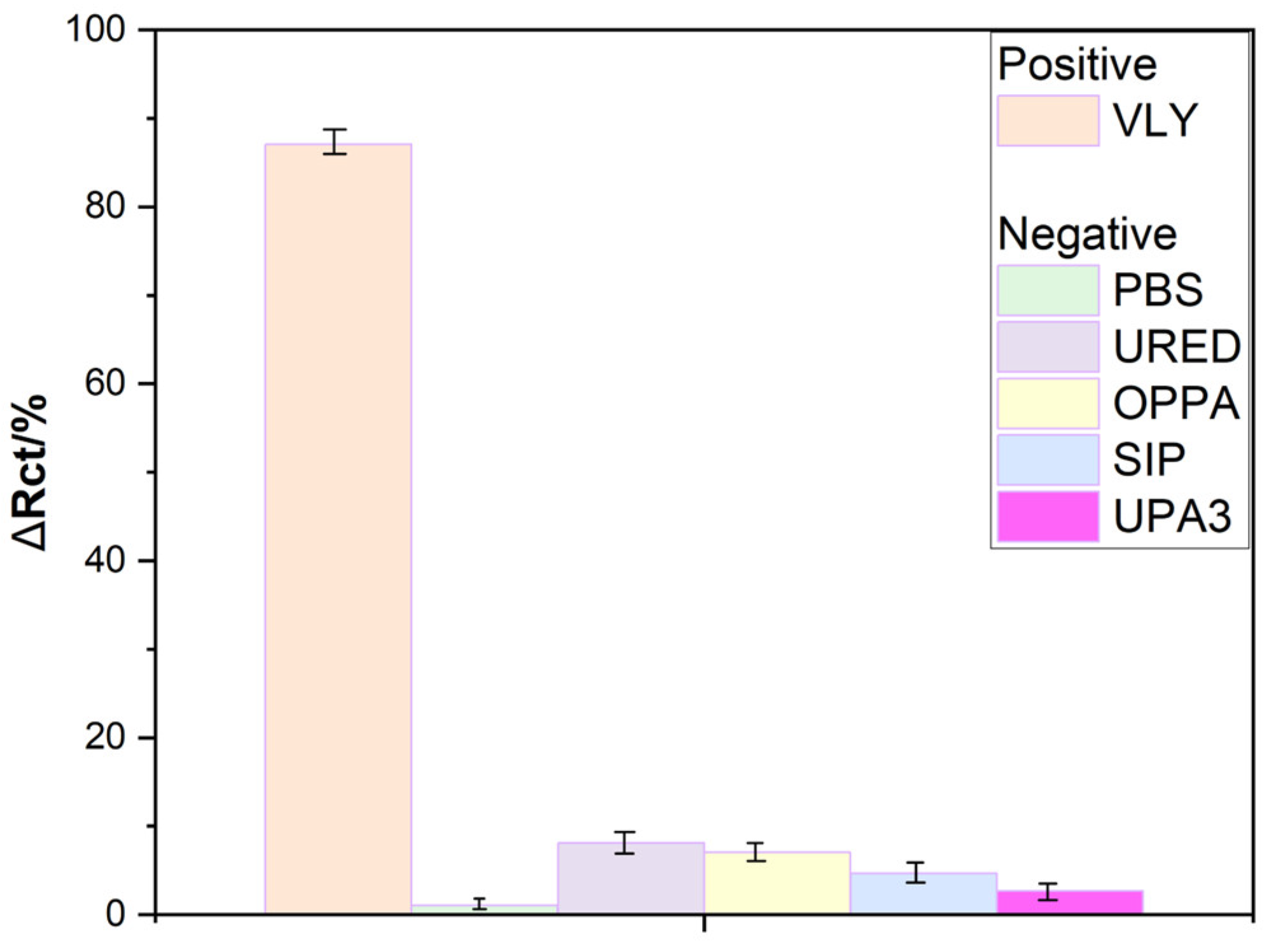
To examine the selectivity of the presented peptide-based sensor, potentially interfering samples included Mycoplasma hominis, Ureaplasma, and Streptococcus agalactiae, along with PBS buffer. The concentration of bacteria was maintained within the same order of magnitude to ensure comparable results. EIS spectra were recorded after a 2 min incubation period.
As shown in
Figure 5, none of the negative controls exhibited a significant increase in impedance; for individual samples, the percentage change in Rct remained below 10%. This establishes 10% as the threshold for differentiating between positive and negative samples.
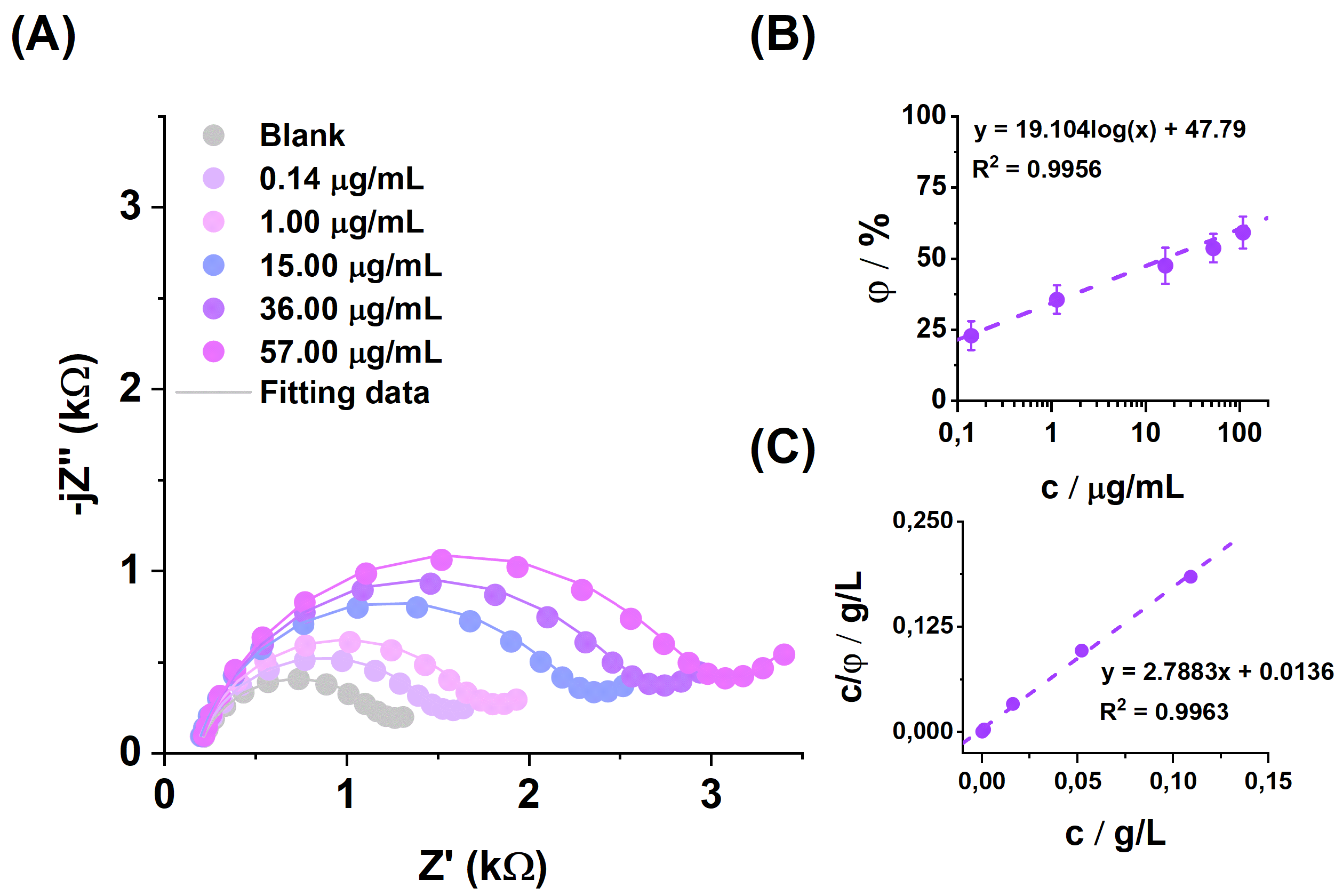
Figure 6 shows the impedance spectrum recorded during additions of the positive protein (
Gardnerella vaginalis) solution with increasing concentrations. All
Rct change values were calculated from the following equation:
where
Rct Test is for the sample, and
Rct Basic is for the fully prepared peptide-based sensor.
The EIS was used to investigate the metrological performance of the biosensor detecting the
Gardnerella vaginalis bacteria by spotting the solutions with different concentrations on the surface of electrodes and incubating them for an optimal time (
Figure 6).
The limit of detection was calculated from the relation LOD = 3 × SD/slope, where SD is the standard deviation in the low concentration range.
The calculated LOD value is 0.02305 μg/mL. To gain further insight into the process by which the VLY protein attaches to the surface of the modified electrode, the Langmuir adsorption isotherm, as illustrated in
Figure 6, can be constructed. This approach provides a deeper understanding of the adsorption mechanism and enables the determination of binding parameters essential for characterizing the interaction.
4. Conclusions
The developed electrochemical peptide-based sensor demonstrated high sensitivity, selectivity, and stability in detecting vaginolysine as a positive marker protein for Gardnerella vaginalis, making it a viable candidate for rapid and reliable diagnostic applications. The strategic use of cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) provided crucial insights into the electrode modifications and confirmed effective antibody binding and electron transfer properties. The EDC/NHS functionalization protocol enabled stable and precise immobilization of anti-Gardnerella vaginalis peptide on the gold nanoparticle-modified graphene surface, significantly enhancing the sensor’s sensitivity.
The integration of gold nanoparticles on the graphene electrode surface played a pivotal role in amplifying signal response due to their excellent conductivity and large surface-to-volume ratio, allowing for the detection of low pathogen concentrations. The peptide-based sensor exhibited rapid and specific binding to Gardnerella vaginalis antigens, with minimal cross-reactivity, underscoring its high selectivity. These properties position the sensor as a promising tool for point-of-care diagnostics in clinical environments, potentially advancing the early detection and management of Gardnerella vaginalis infections.