Development of a Textile Solid-Phase Extraction Method Employing the Marine Sulfated Polysaccharide Ulvan for the Extraction and Analysis of Cationic Dyes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation and Characterization of Ulvan
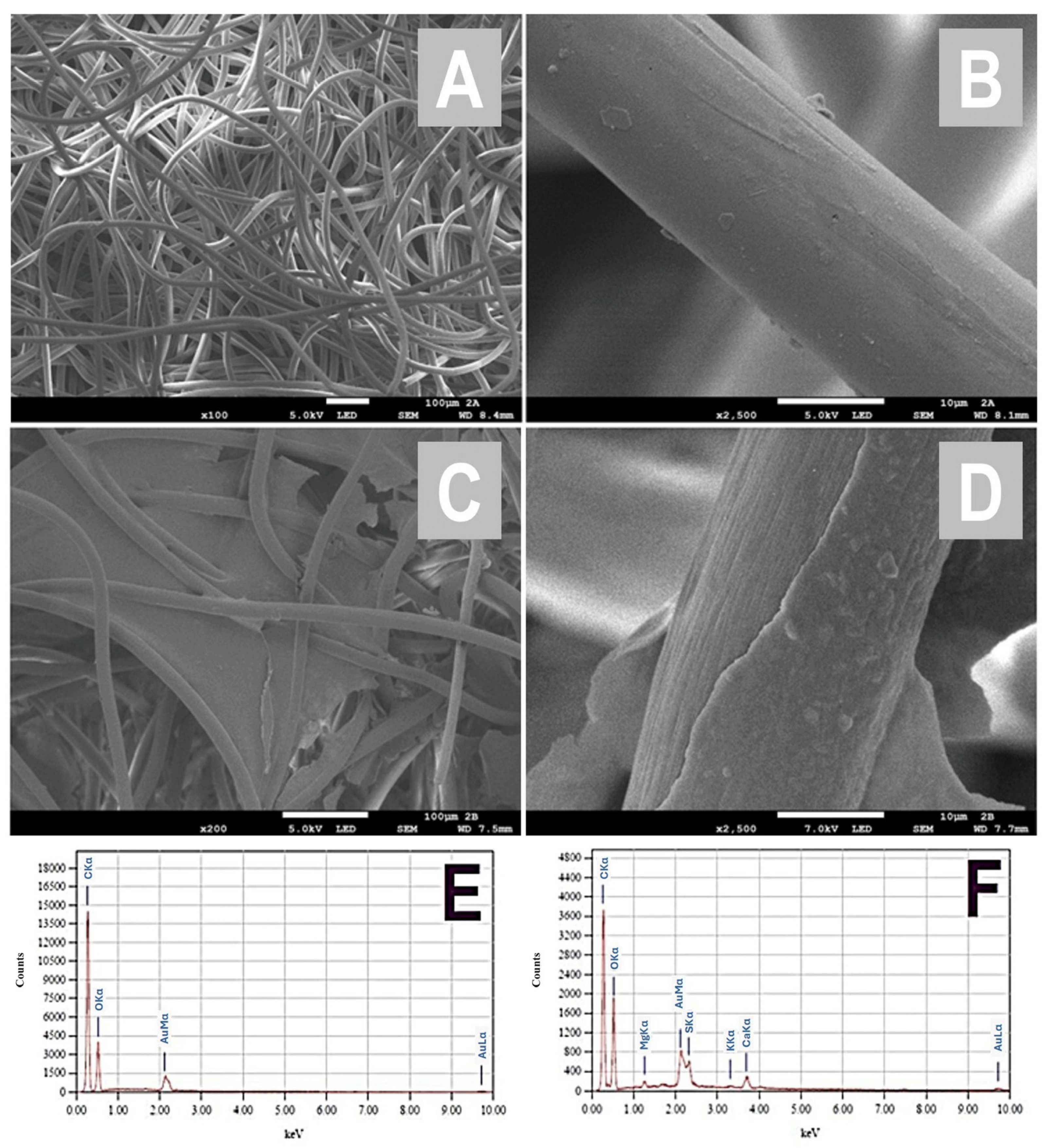
2.3. Optimized Preparation of Ulvan-Modified Nonwoven Textile
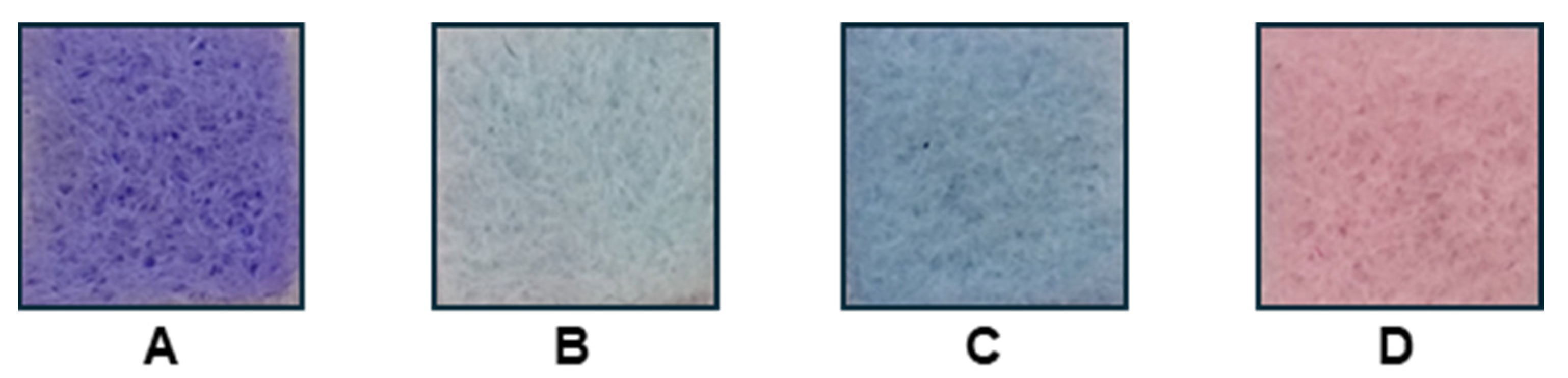
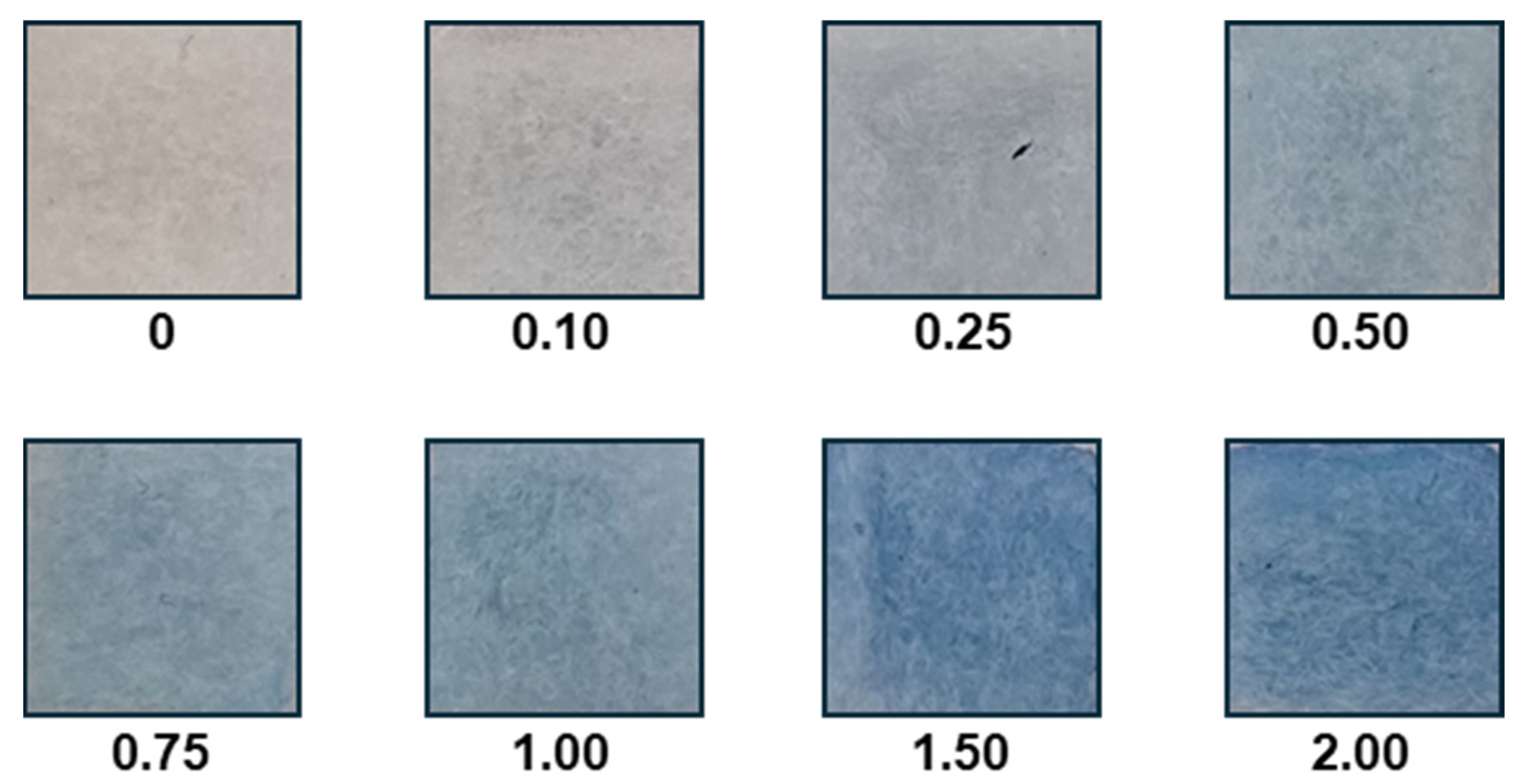
2.4. Textile Solid-Phase Extraction
2.5. Image Analysis
2.6. Other Procedures
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Mosaffa, E.; Patel, R.I.; Banerjee, A.; Basak, B.B.; Oroujzadeh, M. Comprehensive analysis of cationic dye removal from synthetic and industrial wastewater using a semi-natural curcumin grafted biochar/poly acrylic acid composite hydrogel. RSC Adv. 2024, 14, 7745–7762. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.S.; Kumar, P.S.; Vo, D.-V.N. Critical review on hazardous pollutants in water environment: Occurrence, monitoring, fate, removal technologies and risk assessment. Sci. Total Environ. 2021, 797, 149134. [Google Scholar] [CrossRef] [PubMed]
- Safarikova, M.; Safarik, I. Magnetic solid-phase extraction. J. Magn. Magn. Mater. 1999, 194, 108–112. [Google Scholar] [CrossRef]
- Safarik, I.; Prochazkova, J. Color catcher sheets for the construction of low-cost, planar optical sensors. Fibers Polym. 2024, 25, 4099–4107. [Google Scholar] [CrossRef]
- Safarik, I.; Mullerova, S.; Pospiskova, K. Semiquantitative determination of food acid dyes by magnetic textile solid phase extraction followed by image analysis. Food Chem. 2019, 274, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Kraithong, S.; Bunyameen, N.; Theppawong, A.; Ke, X.; Lee, S.; Zhang, X.; Huang, R. Potentials of Ulva spp.-derived sulfated polysaccharides as gelling agents with promising therapeutic effects. Int. J. Biol. Macromol. 2024, 273, 132882. [Google Scholar] [CrossRef] [PubMed]
- Anisha, G.S.; Augustianath, T.; Padmakumari, S.; Singhania, R.R.; Pandey, A.; Patel, A.K. Ulvan from green macroalgae: Bioactive properties advancing tissue engineering, drug delivery systems, food industry, agriculture and water treatment. Bioresour. Technol. Rep. 2023, 22, 101457. [Google Scholar] [CrossRef]
- Lakshmi, D.S.; Sankaranarayanan, S.; Gajaria, T.K.; Li, G.; Kujawski, W.; Kujawa, J.; Navia, R. A short review on the valorization of green seaweeds and ulvan: Feedstock for chemicals and biomaterials. Biomolecules 2020, 10, 991. [Google Scholar] [CrossRef]
- Sulastri, E.; Zubair, M.S.; Lesmana, R.; Mohammed, A.F.A.; Wathoni, N. Development and characterization of ulvan polysaccharides-based hydrogel films for potential wound dressing applications. Drug Des. Devel. Ther. 2021, 15, 4213–4226. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, Z.; Yao, L.; Feng, T.; Song, S.; Sun, M. Insights into the edible and biodegradable ulvan-based films and coatings for food packaging. Foods 2023, 12, 1622. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Li, H.; Fan, L.; Du, C.; Zhang, J.; Guan, H.; Wang, P.; Li, R. Metal-ion-binding properties of ulvan extracted from Ulva clathrata and structural characterization of its complexes. Carbohydr. Polym. 2021, 272, 118508. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, N.; Steinhagen, S.; Toth, G.; Pavia, H.; Edlund, U. Ulvan dialdehyde-gelatin hydrogels for removal of heavy metals and methylene blue from aqueous solution. Carbohydr. Polym. 2020, 249, 116841. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gao, B.; Yue, Q.; Wang, Y.; Li, Q.; Dong, H.; Yan, H. Study of Enteromorpha polysaccharides as a new-style coagulant aid in dye wastewater treatment. Carbohydr. Polym. 2014, 103, 179–186. [Google Scholar] [CrossRef]
- Kikionis, S.; Koromvoki, M.; Tagka, A.; Polichronaki, E.; Stratigos, A.; Panagiotopoulos, A.; Kyritsi, A.; Karalis, V.; Vitsos, A.; Rallis, M.; et al. Ulvan-based nanofibrous patches enhance wound healing of skin trauma resulting from cryosurgical treatment of keloids. Mar. Drugs 2022, 20, 551. [Google Scholar] [CrossRef]
- Safarik, I.; Prochazkova, J. Semiquantitative color catcher and smartphone-based analysis of synthetic food dyes in alcohol containing beverages. Talanta 2023, 262, 124686. [Google Scholar] [CrossRef]
- Kahu, S.Y.; Raut, R.B.; Bhurchandi, K.M. Review and evaluation of color spaces for image/video compression. Color. Res. Appl. 2019, 44, 8–33. [Google Scholar] [CrossRef]
- Ibrahim, M.I.A.; Amer, M.S.; Ibrahim, H.A.H.; Zaghloul, E.H. Considerable production of ulvan from Ulva lactuca with special emphasis on its antimicrobial and anti-fouling properties. Appl. Biochem. Biotechnol. 2022, 194, 3097–3118. [Google Scholar] [CrossRef] [PubMed]
- Alsukaibi, A.K.D. Various approaches for the detoxification of toxic dyes in wastewater. Processes 2022, 10, 1968. [Google Scholar] [CrossRef]
- Nelis, J.L.D.; Tsagkaris, A.S.; Dillon, M.J.; Hajslova, J.; Elliott, C.T. Smartphone-based optical assays in the food safety field. TrAC Trends Anal. Chem. 2020, 129, 115934. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.H.; Thangavel, B.; Sharipov, M.; Chen, K.; Shin, J.H. Smartphone-based portable bio-chemical sensors: Exploring recent advancements. Chemosensors 2023, 11, 468. [Google Scholar] [CrossRef]

| Dye | Color Space | Value | Equation and Coefficients | R2 | RSD [%] |
|---|---|---|---|---|---|
| Crystal violet | RGB | R | y = 30.407x2 − 96.734x + 147.03 | 0.9353 | 0–13.05 |
| CMY | C | y = −11.717x2 + 37.378x + 42.617 | 0.9345 | 0–14.43 | |
| Malachite green | RGB | R | y = 9.6046x2 − 39.641x + 177.38 | 0.9752 | 0–4.72 |
| CMY | C | y = −3.5776x2 + 15.304x + 30.425 | 0.9719 | 0–9.95 | |
| Methylene blue | RGB | R | y = 21.329x2 − 83.615x + 177.13 | 0.9882 | 0–4.28 |
| RGB | G | y = 8.9023x2 − 40.215x + 170.49 | 0.9851 | 0.46–2.24 | |
| CMY | C | y = −8.3361x2 + 32.997x + 30.254 | 0.9879 | 0–8.08 | |
| CIE XYZ | X | y = 6.7135x2 − 23.838x + 39.272 | 0.9789 | 1.21–7.85 | |
| Safranin O | RGB | G | y = 19.136x2 − 60.789x + 168.93 | 0.9767 | 0.92–6.10 |
| LAB | L | y = −5.8564x2 + 21.573x + 4.746 | 0.9738 | 7.52–16.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safarik, I.; Prochazkova, J.; Kikionis, S.; Roussis, V.; Ioannou, E. Development of a Textile Solid-Phase Extraction Method Employing the Marine Sulfated Polysaccharide Ulvan for the Extraction and Analysis of Cationic Dyes. Chemosensors 2025, 13, 75. https://doi.org/10.3390/chemosensors13020075
Safarik I, Prochazkova J, Kikionis S, Roussis V, Ioannou E. Development of a Textile Solid-Phase Extraction Method Employing the Marine Sulfated Polysaccharide Ulvan for the Extraction and Analysis of Cationic Dyes. Chemosensors. 2025; 13(2):75. https://doi.org/10.3390/chemosensors13020075
Chicago/Turabian StyleSafarik, Ivo, Jitka Prochazkova, Stefanos Kikionis, Vassilios Roussis, and Efstathia Ioannou. 2025. "Development of a Textile Solid-Phase Extraction Method Employing the Marine Sulfated Polysaccharide Ulvan for the Extraction and Analysis of Cationic Dyes" Chemosensors 13, no. 2: 75. https://doi.org/10.3390/chemosensors13020075
APA StyleSafarik, I., Prochazkova, J., Kikionis, S., Roussis, V., & Ioannou, E. (2025). Development of a Textile Solid-Phase Extraction Method Employing the Marine Sulfated Polysaccharide Ulvan for the Extraction and Analysis of Cationic Dyes. Chemosensors, 13(2), 75. https://doi.org/10.3390/chemosensors13020075







