Sorbent-Based Microextraction Combined with GC-MS: A Valuable Tool in Bioanalysis
Abstract
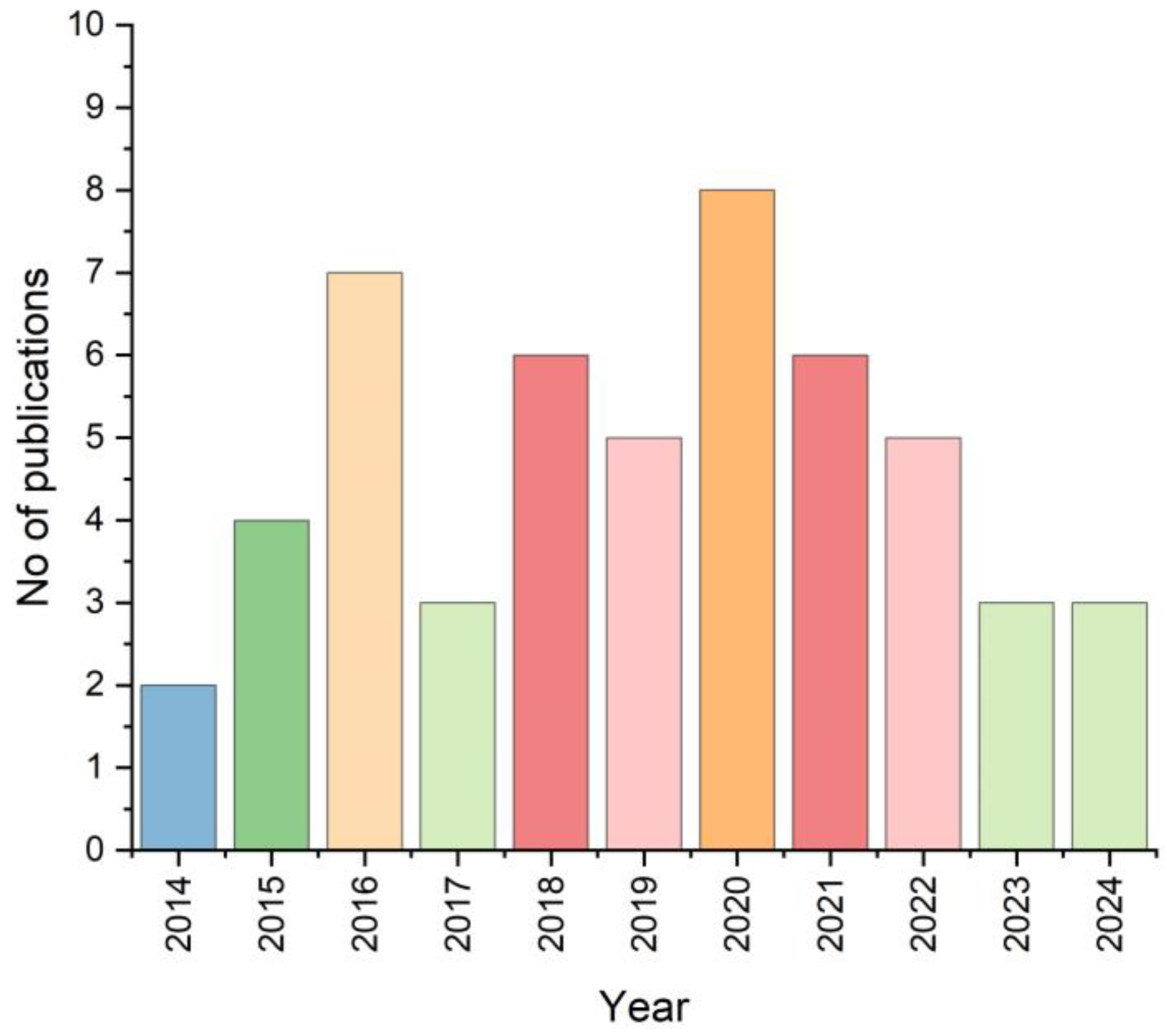
1. Introduction
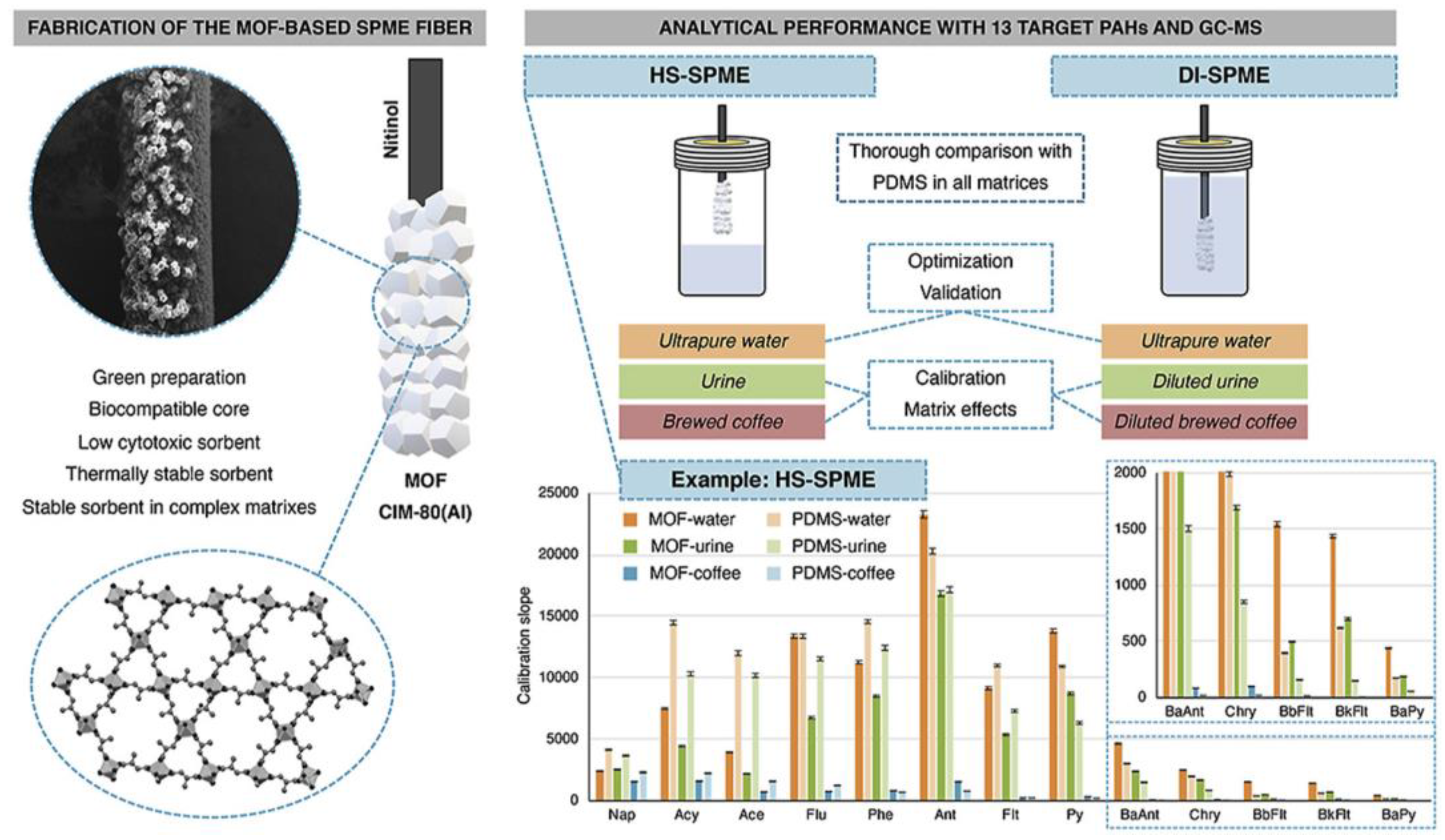
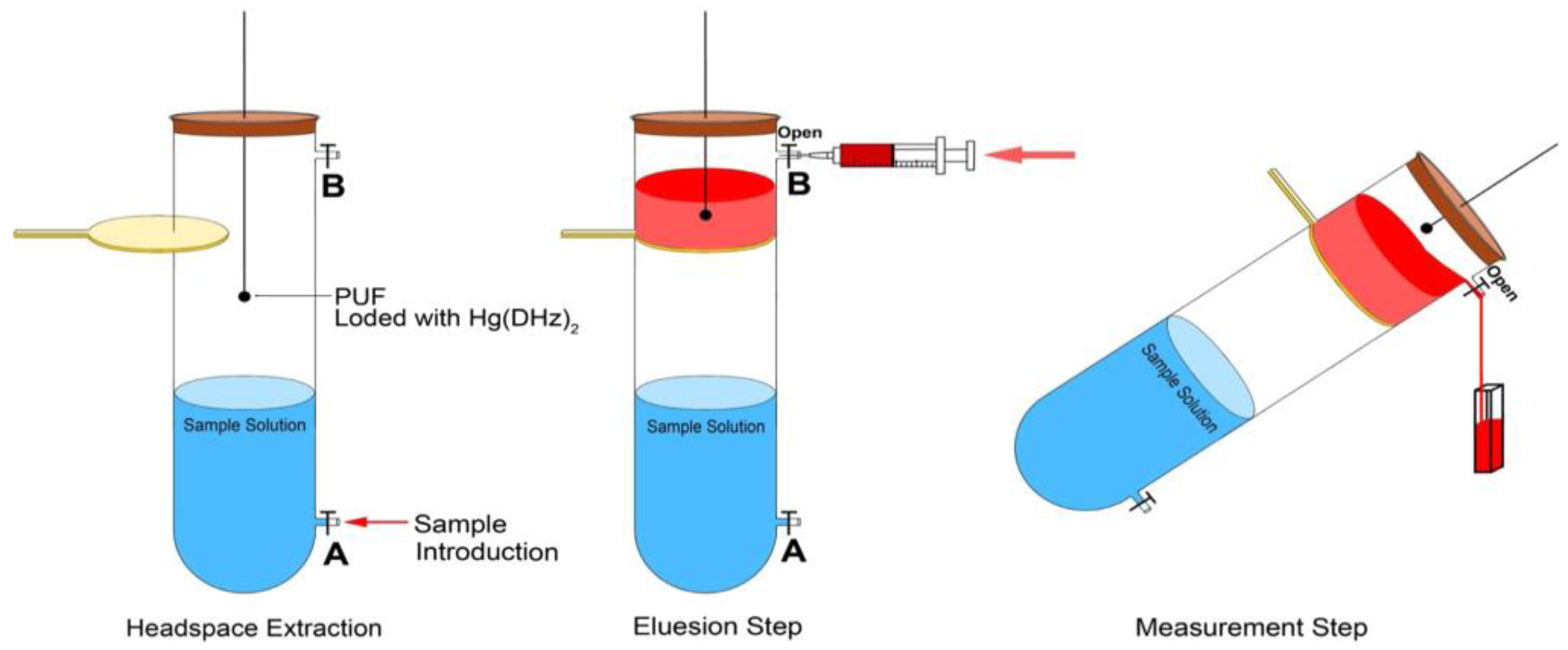
2. Solid-Phase Microextraction
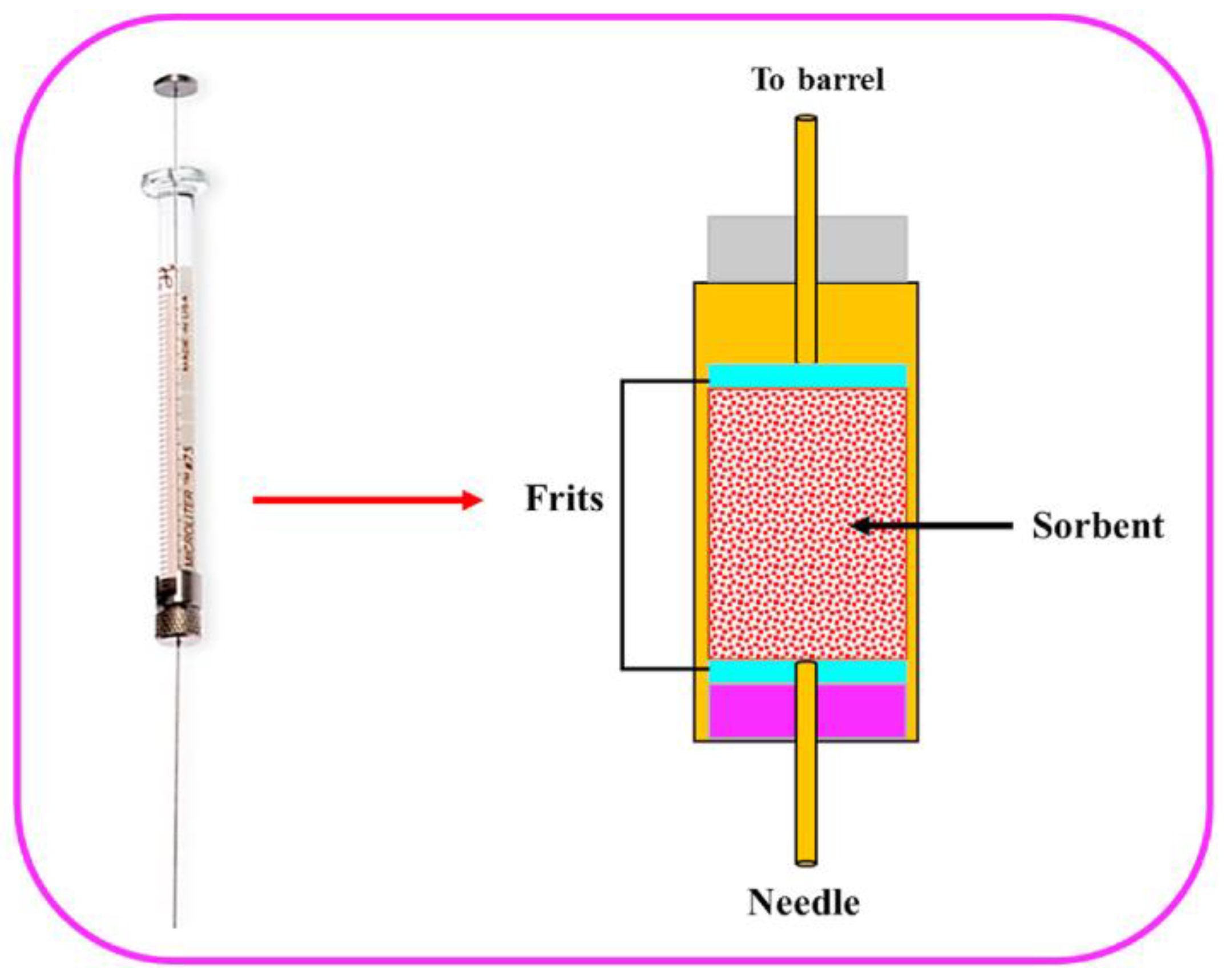
3. Microextraction by Packed Sorbent
4. Dispersive Micro Solid-Phase Extraction
5. Bar Adsorptive Microextraction
6. Other Microextraction Techniques
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Queiroz, M.E.C.; Souza, I.D.; de Oliveira, I.G.; de Grecco, C.F. In Vivo Solid Phase Microextraction for Bioanalysis. TrAC Trends Anal. Chem. 2022, 153, 116656. [Google Scholar] [CrossRef]
- Ingle, R.G.; Zeng, S.; Jiang, H.; Fang, W.J. Current Developments of Bioanalytical Sample Preparation Techniques in Pharmaceuticals. J. Pharm. Anal. 2022, 12, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Guijo, A.; Hartmann, M.F.; Wudy, S.A. Introduction to Gas Chromatography-Mass Spectrometry. Methods Mol. Biol. 2013, 1065, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2009, 39, 301–312. [Google Scholar] [CrossRef]
- Yahaya, N.; Zain NN, M.; Mohamed, A.H.; Grasianto; Kamaruzaman, S.; Miskam, M.; Jain, R.; Raoov, M.; Wan Abdullah, W.N. Nanosorbents in Solid-Phase Extraction Techniques for Bioanalysis: A Review. Microchem. J. 2024, 207, 112170. [Google Scholar] [CrossRef]
- Castañeda, F.N.; Prince, D.L.; Peirano, S.R.; Giovannoni, S.; Echevarría, R.N.; Keunchkarian, S.; Reta, M. New Sorbents for Sample Pretreatment: Development and Applications. TrAC Trends Anal. Chem. 2024, 180, 117924. [Google Scholar] [CrossRef]
- Aly, A.A.; Górecki, T. Green Approaches to Sample Preparation Based on Extraction Techniques. Molecules 2020, 25, 1719. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Z.; Li, G. Recent Advance on Microextraction Sampling Technologies for Bioanalysis. J. Chromatogr. A 2024, 1720, 464775. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, X.; Bai, Y.; Liu, H. Applications of Metal-Organic Frameworks as Advanced Sorbents in Biomacromolecules Sample Preparation. TrAC Trends Anal. Chem. 2018, 109, 154–162. [Google Scholar] [CrossRef]
- Nazario, C.E.D.; Fumes, B.H.; da Silva, M.R.; Lanças, F.M. New Materials for Sample Preparation Techniques in Bioanalysis. J. Chromatogr. B 2017, 1043, 81–95. [Google Scholar] [CrossRef]
- Arabi, M.; Ostovan, A.; Bagheri, A.R.; Guo, X.; Wang, L.; Li, J.; Wang, X.; Li, B.; Chen, L. Strategies of Molecular Imprinting-Based Solid-Phase Extraction Prior to Chromatographic Analysis. TrAC Trends Anal. Chem. 2020, 128, 115923. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, J.; Luo, W.; Han, Q.; Ding, M. Covalent Organic Frameworks Nanocomposites and Their Role in Performance Enhancement of Bioanalysis Based Biosensor. TrAC Trends Anal. Chem. 2024, 172, 117604. [Google Scholar] [CrossRef]
- Sevgen, S.; Kara, G.; Kir, A.S.; Şahin, A.; Boyaci, E. A Critical Review of Bioanalytical and Clinical Applications of Solid Phase Microextraction. J. Pharm. Biomed. Anal. 2025, 252, 116487. [Google Scholar] [CrossRef]
- Feng, X.; Kuang, Y.; Gan, L.; Zhou, S.; Zheng, J.; Ouyang, G. Solid Phase Microextraction for the Bioanalysis of Emerging Organic Pollutants. TrAC Trends Anal. Chem. 2024, 177, 117786. [Google Scholar] [CrossRef]
- Sajid, M.; Khaled Nazal, M.; Rutkowska, M.; Szczepańska, N.; Namieśnik, J.; Płotka-Wasylka, J. Solid Phase Microextraction: Apparatus, Sorbent Materials, and Application. Crit. Rev. Anal. Chem. 2019, 49, 271–288. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; Rentero, M.; Ayala, J.H.; Pasán, J.; Pino, V. Green Solid-Phase Microextraction Fiber Coating Based on the Metal-Organic Framework CIM-80(Al): Analytical Performance Evaluation in Direct Immersion and Headspace Using Gas Chromatography and Mass Spectrometry for the Analysis of Water, Urine and Brewed Coffee. Anal. Chim. Acta 2020, 1133, 137–149. [Google Scholar] [CrossRef]
- Omarova, A.; Muratuly, A.; Kazemian, H.; Baimatova, N. Metal-Organic Frameworks in Solid-Phase Microextraction. In Metal-Organic Frameworks in Analytical Sample Preparation and Sensing; Elsevier: Amsterdam, The Netherlands, 2024; pp. 187–217. [Google Scholar] [CrossRef]
- Cozzolino, R.; De Magistris, L.; Saggese, P.; Stocchero, M.; Martignetti, A.; Di Stasio, M.; Malorni, A.; Marotta, R.; Boscaino, F.; Malorni, L. Use of Solid-Phase Microextraction Coupled to Gas Chromatography-Mass Spectrometry for Determination of Urinary Volatile Organic Compounds in Autistic Children Compared with Healthy Controls. Anal. Bioanal. Chem. 2014, 406, 4649–4662. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Wang, J.; Lu, G.; Jia, Z.; Yang, J.; Shi, E. Oxidized Multiwalled Carbon Nanotubes Coated Fibers for Headspace Solid-Phase Microextraction of Amphetamine-Type Stimulants in Human Urine. Forensic Sci. Int. 2018, 290, 49–55. [Google Scholar] [CrossRef]
- Hajebi, N.; Seidi, S.; Ramezani, M.; Manouchehri, M. Electrospun Polyamide/Graphene Oxide/Polypyrrole Composite Nanofibers: An Efficient Sorbent for Headspace Solid Phase Microextraction of Methamphetamine in Urine Samples Followed by GC-MS Analysis. New J. Chem. 2020, 44, 14429–14437. [Google Scholar] [CrossRef]
- Al-Saidi, H.M.; Al-Harbi, S.A.; Aljuhani, E.H.; El-Shahawi, M.S. Headspace Sorptive Solid Phase Microextraction (HS-SPME) Combined with a Spectrophotometry System: A Simple Glass Devise for Extraction and Simultaneous Determination of Cyanide and Thiocyanate in Environmental and Biological Samples. Talanta 2016, 159, 137–142. [Google Scholar] [CrossRef]
- Azorín, C.; López-Juan, A.L.; Aparisi, F.; Benedé, J.L.; Chisvert, A. Determination of Hexanal and Heptanal in Saliva Samples by an Adapted Magnetic Headspace Adsorptive Microextraction for Diagnosis of Lung Cancer. Anal. Chim. Acta 2023, 1271, 341435. [Google Scholar] [CrossRef]
- Abdel-Rehim, M. Microextraction by Packed Sorbent (MEPS): A Tutorial. Anal. Chim. Acta 2011, 701, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rehim, M. New Trend in Sample Preparation: On-Line Microextraction in Packed Syringe for Liquid and Gas Chromatography Applications: I. Determination of Local Anaesthetics in Human Plasma Samples Using Gas Chromatography–Mass Spectrometry. J. Chromatogr. B 2004, 801, 317–321. [Google Scholar] [CrossRef]
- Abdel-Rehim, M. Recent Advances in Microextraction by Packed Sorbent for Bioanalysis. J. Chromatogr. A 2010, 1217, 2569–2580. [Google Scholar] [CrossRef]
- Firoozichahak, A.; Soleymani-ghoozhdi, D.; Alizadeh, S.; Rahimpoor, R. Microextraction by Packed Sorbents (MEPS): Fundamental Principles and Nanomaterial-Based Adsorbents. TrAC Trends Anal. Chem. 2024, 181, 118043. [Google Scholar] [CrossRef]
- Alves, G.; Rodrigues, M.; Fortuna, A.; Falcão, A.; Queiroz, J. A Critical Review of Microextraction by Packed Sorbent as a Sample Preparation Approach in Drug Bioanalysis. Bioanalysis 2013, 5, 1409–1442. [Google Scholar] [CrossRef]
- Soares, S.; Rosado, T.; Barroso, M.; Gallardo, E. Quantification of Antidepressants in Oral Fluid and Plasma Samples Using Microextraction by Packed Sorbent and Analysis by Gas Chromatography-Tandem Mass Spectrometry. Microchem. J. 2024, 204, 111031. [Google Scholar] [CrossRef]
- Simão, A.Y.; Monteiro, C.; Marques, H.; Rosado, T.; Margalho, C.; Barroso, M.; Andraus, M.; Gallardo, E. Analysis of Opiates in Urine Using Microextraction by Packed Sorbent and Gas Chromatography- Tandem Mass Spectrometry. J. Chromatogr. B 2022, 1207, 123361. [Google Scholar] [CrossRef]
- Prata, M.; Ribeiro, A.; Figueirinha, D.; Rosado, T.; Oppolzer, D.; Restolho, J.; Araújo, A.R.T.S.; Costa, S.; Barroso, M.; Gallardo, E. Determination of Opiates in Whole Blood Using Microextraction by Packed Sorbent and Gas Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2019, 1602, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Malaca, S.; Rosado, T.; Restolho, J.; Rodilla, J.M.; Rocha, P.M.M.; Silva, L.; Margalho, C.; Barroso, M.; Gallardo, E. Determination of Amphetamine-Type Stimulants in Urine Samples Using Microextraction by Packed Sorbent and Gas Chromatography-Mass Spectrometry. J. Chromatogr. B 2019, 1120, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Barroso, M.; Martinho, A.; Cruz, A.; Gallardo, E. Determination of Ketamine and Its Major Metabolite, Norketamine, in Urine and Plasma Samples Using Microextraction by Packed Sorbent and Gas Chromatography-Tandem Mass Spectrometry. J. Chromatogr. B 2015, 1004, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Simão, A.Y.; Oliveira, P.; Rosendo, L.M.; Rosado, T.; Andraus, M.; Barroso, M.; Gallardo, E. Microextraction by Packed Sorbent as a Clean-up Approach for the Determination of Ketamine and Norketamine in Hair by Gas Chromatography–Tandem Mass Spectrometry. J. Anal. Toxicol. 2023, 47, 227–235. [Google Scholar] [CrossRef]
- Rosendo, L.M.; Rosado, T.; Oliveira, P.; Simão, A.Y.; Margalho, C.; Costa, S.; Passarinha, L.A.; Barroso, M.; Gallardo, E. The Determination of Cannabinoids in Urine Samples Using Microextraction by Packed Sorbent and Gas Chromatography-Mass Spectrometry. Molecules 2022, 27, 5503. [Google Scholar] [CrossRef] [PubMed]
- Rosado, T.; Fernandes, L.; Barroso, M.; Gallardo, E. Sensitive Determination of THC and Main Metabolites in Human Plasma by Means of Microextraction in Packed Sorbent and Gas Chromatography–Tandem Mass Spectrometry. J. Chromatogr. B 2017, 1043, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Peña, J.; Casas-Ferreira, A.M.; Morales-Tenorio, M.; Moreno-Cordero, B.; Pérez-Pavón, J.L. Determination of Polyamines and Related Compounds in Saliva via in Situ Derivatization and Microextraction by Packed Sorbents Coupled to GC-MS. J. Chromatogr. B 2019, 1129, 121821. [Google Scholar] [CrossRef] [PubMed]
- Casas Ferreira, A.M.; Moreno Cordero, B.; Crisolino Pozas, Á.P.; Pérez Pavón, J.L. Use of Microextraction by Packed Sorbents and Gas Chromatography-Mass Spectrometry for the Determination of Polyamines and Related Compounds in Urine. J. Chromatogr. A 2016, 1444, 32–41. [Google Scholar] [CrossRef] [PubMed]
- García-García, S.; Matilla-González, H.; Peña, J.; Nogal Sánchez, M.; del Casas-Ferreira, A.M.; Pérez Pavón, J.L. Determination of Hydroxy Polycyclic Aromatic Hydrocarbons in Human Urine Using Automated Microextraction by Packed Sorbent and Gas Chromatography–Mass Spectrometry. Int. J. Environ. Res. Public Health 2022, 19, 13089. [Google Scholar] [CrossRef]
- Martín Santos, P.; Jiménez Carracedo, C.; del Nogal Sánchez, M.; Pérez Pavón, J.L.; Moreno Cordero, B. A Sensitive and Automatic Method Based on Microextraction by Packed Sorbents for the Determination of Polycyclic Aromatic Hydrocarbons in Saliva Samples. Microchem. J. 2020, 152, 104274. [Google Scholar] [CrossRef]
- Dhingra, G.; Bansal, P.; Dhingra, N.; Rani, S.; Malik, A.K. Development of a Microextraction by Packed Sorbent with Gas Chromatography-Mass Spectrometry Method for Quantification of Nitroexplosives in Aqueous and Fluidic Biological Samples. J. Sep. Sci. 2018, 41, 639–647. [Google Scholar] [CrossRef]
- Yang, L.; Han, Q.; Cao, S.; Yang, J.; Zhao, J.; Qin, M.; Ding, M. Self-Made Microextraction by Packed Sorbent Device for the Cleanup of Polychlorinated Biphenyls from Bovine Serum. J. Sep. Sci. 2016, 39, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Pautova, A.; Khesina, Z.; Getsina, M.; Sobolev, P.; Revelsky, A.; Beloborodova, N. Determination of Tryptophan Metabolites in Serumand Cerebrospinal Fluid Samples Using Microextraction by Packed Sorbent, Silylation and GC-MS Detection. Molecules 2020, 25, 3258. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, P.D.; Pautova, A.K.; Revelsky, A.I. Microextraction of Aromatic Microbial Metabolites by Packed Sorbent (MEPS) from Model Solutions Followed by Gas Chromatography/Mass Spectrometry Analysis of Their Silyl Derivatives. J. Anal. Chem. 2017, 72, 1426–1433. [Google Scholar] [CrossRef]
- Klimowska, A.; Wielgomas, B. Off-Line Microextraction by Packed Sorbent Combined with on Solid Support Derivatization and GC-MS: Application for the Analysis of Five Pyrethroid Metabolites in Urine Samples. Talanta 2018, 176, 165–171. [Google Scholar] [CrossRef]
- Santos, C.; Oppolzer, D.; Gonçalves, A.; Barroso, M.; Gallardo, E. Determination of Organophosphorous Pesticides in Blood Using Microextraction in Packed Sorbent and Gas Chromatography–Tandem Mass Spectrometry. J. Anal. Toxicol. 2018, 42, 321–329. [Google Scholar] [CrossRef]
- Abdel-Rehim, M.; Dahlgren, M.; Blomberg, L.; Claude, S.; Tabacchi, R. Microextraction in Packed Syringe (MEPS) Utilizing Methylcyanopropyl- Silarylene as Coating Polymer for Extraction of Drugs in Biological Samples. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 2537–2544. [Google Scholar] [CrossRef]
- Pragst, F.; Balikova, M.A. State of the Art in Hair Analysis for Detection of Drug and Alcohol Abuse. Clin. Chim. Acta 2006, 370, 17–49. [Google Scholar] [CrossRef] [PubMed]
- Jain, B.; Jain, R.; Kabir, A.; Alharthi, S.; AbdElrahman, M.; Ghoneim, M.M.; Sharma, S.; Yahaya, N.; Rashid, S. Modern Sorbent-Based Sample Preparation Methods for the Determination of Analytes of Forensic Interest: A Dual-Tool Approach for Evaluation of Greenness and Practical Utility. TrAC Trends Anal. Chem. 2025, 184, 118129. [Google Scholar] [CrossRef]
- Chung, L.W.; Liu, G.J.; Li, Z.G.; Chang, Y.Z.; Lee, M.R. Solvent-Enhanced Microwave-Assisted Derivatization Following Solid-Phase Extraction Combined with Gas Chromatography-Mass Spectrometry for Determination of Amphetamines in Urine. J. Chromatogr. B 2008, 874, 115–118. [Google Scholar] [CrossRef]
- De Souza Eller, S.C.W.; de Oliveira, F.; Yonamine, M. Measurement Uncertainty for the Determination of Amphetamines in Urine by Liquid-Phase Microextraction and Gas Chromatography-Mass Spectrometry. Forensic Sci. Int. 2016, 265, 81–88. [Google Scholar] [CrossRef]
- Ahmadi-Jouibari, T.; Fattahi, N.; Shamsipur, M. Rapid Extraction and Determination of Amphetamines in Human Urine Samples Using Dispersive Liquid-Liquid Microextraction and Solidification of Floating Organic Drop Followed by High Performance Liquid Chromatography. J. Pharm. Biomed. Anal. 2014, 94, 145–151. [Google Scholar] [CrossRef]
- Raikos, N.; Christopoulou, K.; Theodoridis, G.; Tsoukali, H.; Psaroulis, D. Determination of Amphetamines in Human Urine by Headspace Solid-Phase Microextraction and Gas Chromatography. J. Chromatogr. B 2003, 789, 9–63. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.Y.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A.P. Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. Medicines 2019, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Presley, B.C.; Gurney, S.M.R.; Scott, K.S.; Kacinko, S.L.; Logan, B.K. Metabolism and Toxicological Analysis of Synthetic Cannabinoids in Biological Fluids and Tissues. Forensic Sci. Rev. 2016, 28, 103–169. [Google Scholar]
- Lacassie, E.; Dreyfuss, M.F.; Gaulier, J.M.; Marquet, P.; Daguet, J.L.; Lachâtre, G. Multiresidue Determination Method for Organophosphorus Pesticides in Serum and Whole Blood by Gas Chromatography-Mass-Selective Detection. J. Chromatogr. B Biomed. Sci. Appl. 2001, 759, 109–116. [Google Scholar] [CrossRef]
- Valente, N.I.P.; Tarelho, S.; Castro, A.L.; Silvestre, A.; Teixeira, H.M. Analysis of Organophosphorus Pesticides in Whole Blood by GC-MS-ΜECD with Forensic Purposes. J. Forensic Leg. Med. 2015, 33, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; In, S.W.; Lee, S.K.; Choi, W.K.; Park, Y.S.; Chung, H.S. Postmortem Blood Concentrations of Organophosphorus Pesticides. Forensic Sci. Int. 2009, 184, 28–31. [Google Scholar] [CrossRef]
- Hernández, F.; Pitarch, E.; Beltran, J.; López, F.J. Headspace Solid-Phase Microextraction in Combination with Gas Chromatography and Tandem Mass Spectrometry for the Determination of Organochlorine and Organophosphorus Pesticides in Whole Numan Blood. J. Chromatogr. B 2002, 769, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Cazorla-Reyes, R.; Fernández-Moreno, J.L.; Romero-González, R.; Frenich, A.G.; Vidal, J.L.M. Single Solid Phase Extraction Method for the Simultaneous Analysis of Polar and Non-Polar Pesticides in Urine Samples by Gas Chromatography and Ultra High Pressure Liquid Chromatography Coupled to Tandem Mass Spectrometry. Talanta 2011, 85, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Novita Sari, I.; Setiawan, T.; Seock Kim, K.; Toni Wijaya, Y.; Won Cho, K.; Young Kwon, H. Metabolism and Function of Polyamines in Cancer Progression. Cancer Lett. 2021, 519, 91–104. [Google Scholar] [CrossRef]
- Sun, L.; Zhu, S.; Zheng, Z.; Sun, J.; Zhao, X.E.; Liu, H. 9-Plex Ultra High Performance Liquid Chromatography Tandem Mass Spectrometry Determination of Free Hydroxyl Polycyclic Aromatic Hydrocarbons in Human Plasma and Urine. J. Chromatogr. A 2020, 1623, 461182. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Huang, S.; Kuang, Y.; Chen, Z.; Guo, J.; Cui, S.; Zheng, J.; Ouyang, G. Facile Fabrication of Composited Solid Phase Microextraction Thin Membranes for Sensitive Detections of Trace Hydroxylated Polycyclic Aromatic Hydrocarbons in Human Urine. Anal. Chim. Acta 2021, 1158, 338422. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Guo, S.; Wang, Y.; Sun, H.; Zhao, H. Simultaneous Determination of Multiple Isomeric Hydroxylated Polycyclic Aromatic Hydrocarbons in Urine by Using Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry. J. Chromatogr. B 2021, 1184, 122983. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Luo, Y.; Bi, J.; Li, H.; Lin, J.M. Quantification of Selected Monohydroxy Metabolites of Polycyclic Aromatic Hydrocarbons in Human Urine. Sci. China Chem. 2015, 58, 1579–1584. [Google Scholar] [CrossRef]
- Carrizo, D.; Domeño, C.; Nerín, I.; Alfaro, P.; Nerín, C. Atmospheric Pressure Solid Analysis Probe Coupled to Quadrupole-Time of Flight Mass Spectrometry as a Tool for Screening and Semi-Quantitative Approach of Polycyclic Aromatic Hydrocarbons, Nitro-Polycyclic Aromatic Hydrocarbons and Oxo-Polycyclic Aromatic Hydrocarbons in Complex Matrices. Talanta 2015, 131, 175–184. [Google Scholar] [CrossRef]
- Carrizo, D.; Nerín, I.; Domeño, C.; Alfaro, P.; Nerín, C. Direct Screening of Tobacco Indicators in Urine and Saliva by Atmospheric Pressure Solid Analysis Probe Coupled to Quadrupole-Time of Flight Mass Spectrometry (ASAP-MS-Q-TOF-). J. Pharm. Biomed. Anal. 2016, 124, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, E.; Ek, S.; Colmsjö, A. Extraction of Explosives from Soil Followed by Gas Chromatography-Mass Spectrometry Analysis with Negative Chemical Ionization. J. Chromatogr. A 2012, 1222, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Tachon, R.; Pichon, V.; Barbe Le Borgne, M.; Minet, J.J. Comparison of Solid-Phase Extraction Sorbents for Sample Clean-up in the Analysis of Organic Explosives. J. Chromatogr. A 2008, 1185, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Pacheco-Fernández, I.; Taima-Mancera, I.; Díaz, J.H.A.; Pino, V. Evolution and Current Advances in Sorbent-Based Microextraction Configurations. J. Chromatogr. A 2020, 1634, 461670. [Google Scholar] [CrossRef]
- Mohebbi, A.; Yaripour, S.; Farajzadeh, M.A.; Afshar Mogaddam, M.R. Combination of Dispersive Solid Phase Extraction and Deep Eutectic Solvent–Based Air–Assisted Liquid–Liquid Microextraction Followed by Gas Chromatography–Mass Spectrometry as an Efficient Analytical Method for the Quantification of Some Tricyclic Antidepressant Drugs in Biological Fluids. J. Chromatogr. A 2018, 1571, 84–93. [Google Scholar] [CrossRef]
- Seidi, S.; Mohammadi, F.; Tajik, M.; Baharfar, M.; Mohammadi, A.; Otoufat, T. Quantitative Determination of Trace Phenazopyridine in Human Urine Samples by Hyphenation of Dispersive Solid-Phase Extraction and Liquid-Phase Microextraction Followed by Gas Chromatography/Mass Spectrometry Analysis. J. Sep. Sci. 2020, 43, 2897–2904. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.; Masrournia, M.; Es’haghi, Z.; Pordel, M. Determination of Four Antiepileptic Drugs with Solvent Assisted Dispersive Solid Phase Microextraction–Gas Chromatography–Mass Spectrometry in Human Urine Samples. Microchem. J. 2020, 159, 105542. [Google Scholar] [CrossRef]
- Sefaty, B.; Masrournia, M.; Es’haghi, Z.; Bozorgmehr, M.R. Determination of Tramadol and Fluoxetine in Biological and Water Samples by Magnetic Dispersive Solid-Phase Microextraction (MDSPME) with Gas Chromatography–Mass Spectrometry (GC-MS). Anal. Lett. 2021, 54, 884–902. [Google Scholar] [CrossRef]
- Isazad, M.; Amirzehni, M.; Akhgari, M. Highly Efficient Dispersive Liquid-Liquid Microextraction Assisted by Magnetic Porous Carbon Composite-Based Dispersive Micro Solid-Phase Extraction for Determination of Tramadol and Methadone in Urine Samples by Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2022, 1670, 462989. [Google Scholar] [CrossRef]
- Jouyban, A.; Nemati, M.; Farazajdeh, M.A.; Alizadeh Nabil, A.A.; Afshar Mogaddam, M.R. A Polymer-Based Dispersive Solid Phase Extraction Combined with Deep Eutectic Solvent Based-Dispersive Liquid–Liquid Microextraction for the Determination of Four Hydroxylated Polycyclic Aromatic Hydrocarbons from Urine Samples. J. Sep. Sci. 2021, 44, 4025–4036. [Google Scholar] [CrossRef] [PubMed]
- Neng, N.R.; Silva, A.R.M.; Nogueira, J.M.F. Adsorptive Micro-Extraction Techniques—Novel Analytical Tools for Trace Levels of Polar Solutes in Aqueous Media. J. Chromatogr. A 2010, 1217, 7303–7310. [Google Scholar] [CrossRef] [PubMed]
- Carasek, E.; Morés, L.; Merib, J. Basic Principles, Recent Trends and Future Directions of Microextraction Techniques for the Analysis of Aqueous Environmental Samples. Trends Environ. Anal. Chem. 2018, 19, e00060. [Google Scholar] [CrossRef]
- Ahmad, S.M.; Oliveira, M.N.; Neng, N.R.; Nogueira, J.M.F. A Fast and Validated High Throughput Bar Adsorptive Microextraction (HT-BAµE) Method for the Determination of Ketamine and Norketamine in Urine Samples. Molecules 2020, 25, 1438. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.M.; Nogueira, J.M.F. High Throughput Bar Adsorptive Microextraction: A Novel Cost-Effective Tool for Monitoring Benzodiazepines in Large Number of Biological Samples. Talanta 2019, 199, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.N.; Gonçalves, O.C.; Ahmad, S.M.; Schneider, J.K.; Krause, L.C.; Neng, N.R.; Caramão, E.B.; Nogueira, J.M.F. Application of Bar Adsorptive Microextraction for the Determination of Levels of Tricyclic Antidepressants in Urine Samples. Molecules 2021, 26, 3101. [Google Scholar] [CrossRef]
- Almeida, C.V.P.; Neng, N.R.; Nogueira, J.M.F.; Ruivo, J. Application of a Bar Adsorptive Microextraction Based Methodology for Doping Control of Alkylamine Stimulants in Urine Matrices. J. Chromatogr. B 2024, 1234, 124006. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Basheer, C. Stir-Bar Supported Micro-Solid-Phase Extraction for the Determination of Polychlorinated Biphenyl Congeners in Serum Samples. J. Chromatogr. A 2016, 1455, 37–44. [Google Scholar] [CrossRef]
- Mohammadiazar, S.; Sheikhi, T.; Mazoji, H.; Roostaie, A. Simultaneous Determination of Methadone and Tramadol in Serum Samples by Ultrasonic-Assisted Micro Solid Phase Extraction and Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2024, 1725, 464875. [Google Scholar] [CrossRef] [PubMed]
- Ares-Fuentes, A.M.; Lorenzo, R.A.; Fernández, P.; Fernández, A.M.; Furton, K.G.; Kabir, A.; Carro, A.M. Determination of Synthetic Opioids in Oral Fluid Samples Using Fabric Phase Sorptive Extraction and Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2022, 1663, 462768. [Google Scholar] [CrossRef] [PubMed]
- Jain, B.; Jain, R.; Jha, R.R.; Ghosh, A.; Basu, D.; Abourehab, M.A.S.; Bajaj, A.; Chauhan, V.; Kaur, S.; Sharma, S. Cellulose Paper Sorptive Extraction (CPSE): A Simple and Affordable Microextraction Method for Analysis of Basic Drugs in Blood as a Proof of Concept. J. Chromatogr. B 2023, 1214, 123551. [Google Scholar] [CrossRef]
- Manousi, N.; Kabir, A.; A Zachariadis, G. Green Bioanalytical Sample Preparation: Fabric Phase Sorptive Extraction. Bioanalysis 2021, 13, 693–710. [Google Scholar] [CrossRef] [PubMed]
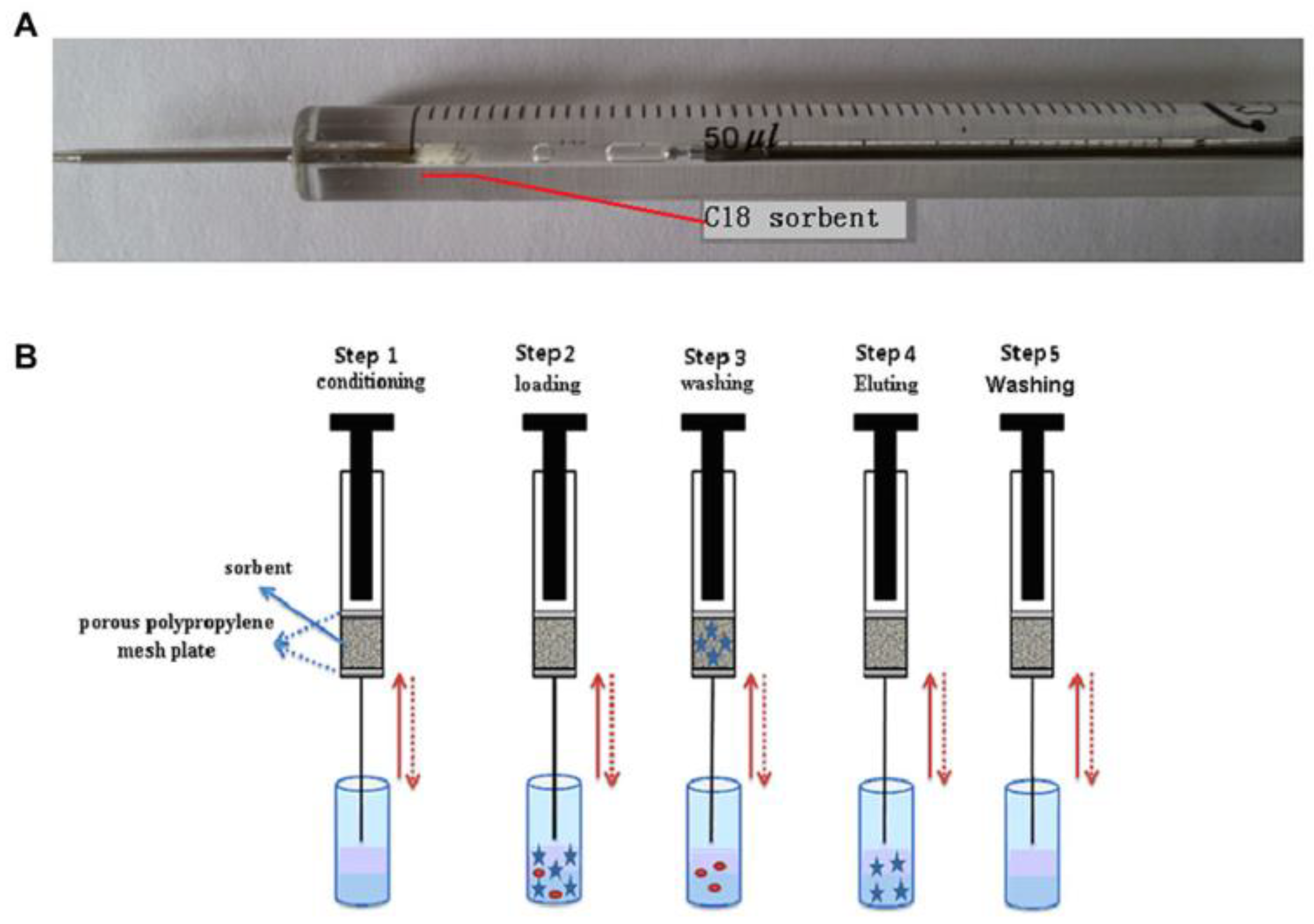
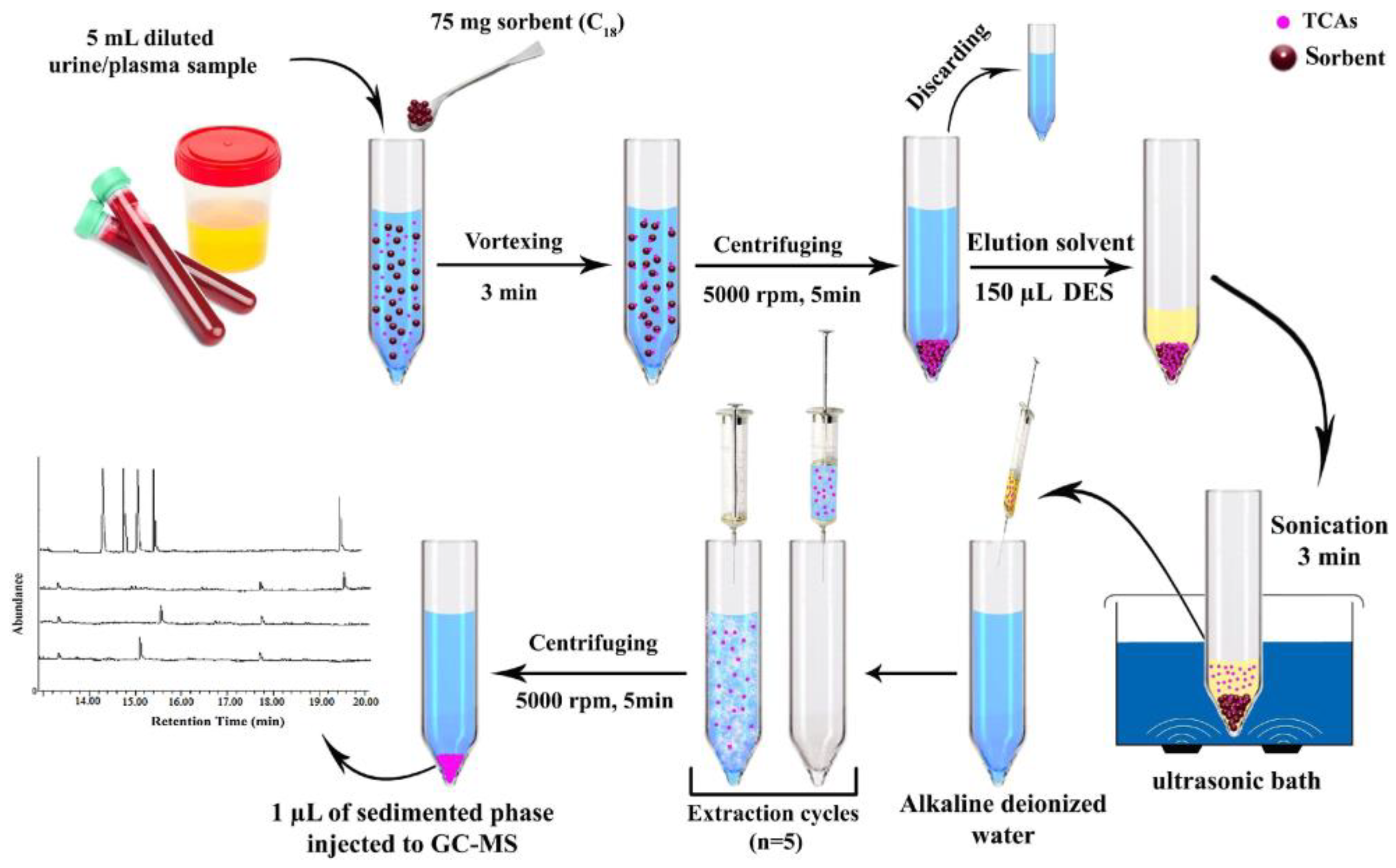
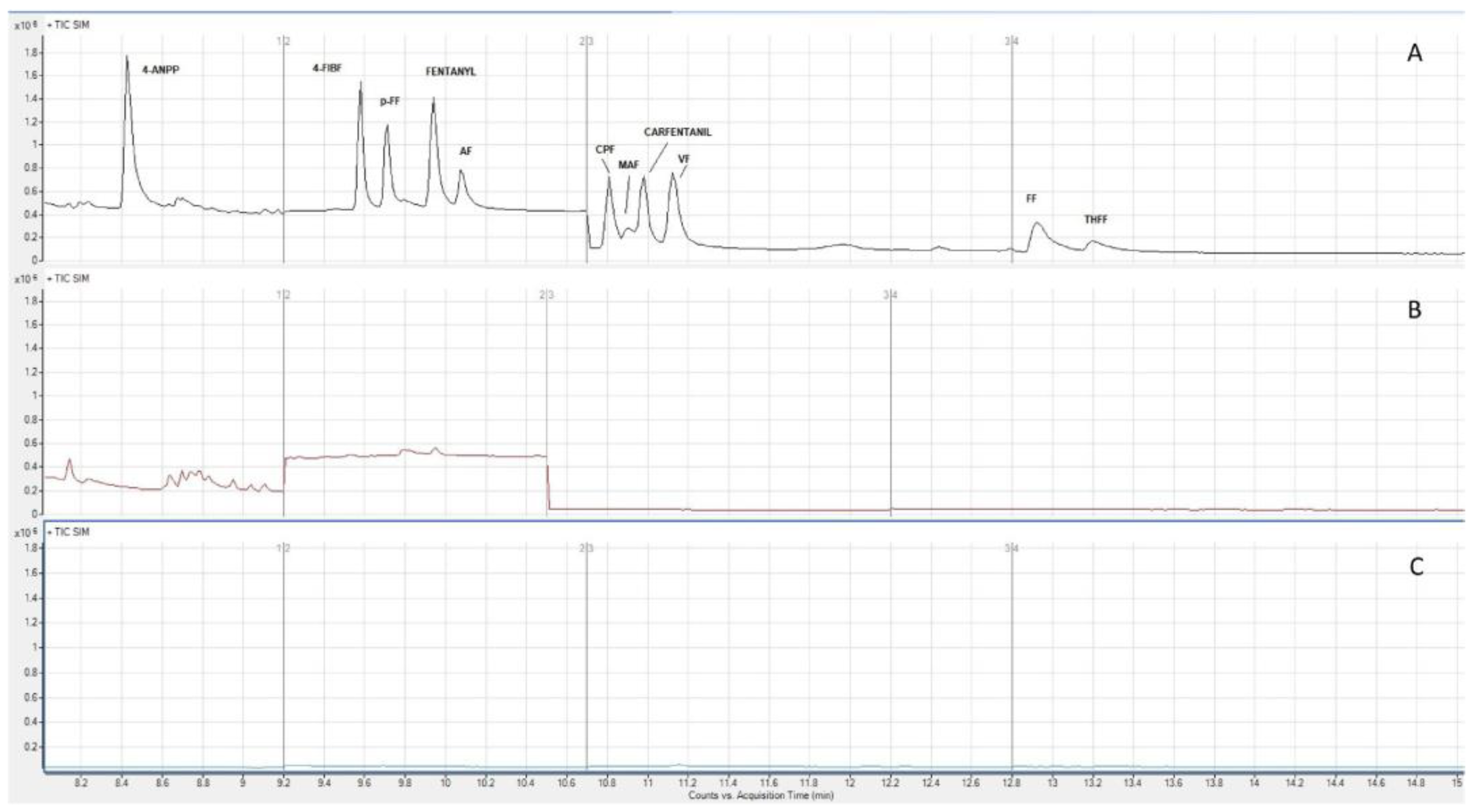
| Analyte | Sample | SPME Sorbent/ Extraction Conditions | Sample Volume (mL) | LOD (ng/mL) | % RR 1 | Reference |
|---|---|---|---|---|---|---|
| PAHs | Environmental water, urine, brewed coffee | CIM-80(Al) MOF/ET 2: 60 min at 75 °C (for HS); ET: 60 min at 50 °C (for direct immersion) | 10 mL (for HS) 19 mL (for direct immersion) | 0.0003–0.0015 | 80.1–107 (for urine) 74.6–107 (for coffee) | [16] |
| VOCs | Human urine | DVB/CAR/PDMS/ET: 30 min at 40 °C (HS) | 4 | – | – | [18] |
| Amphetamine-type stimulants | Human urine | MWCNTs-COOH/ET: 20 min at 80 °C (HS) | 5 | 0.2–1.3 | 88–107 | [19] |
| Methamphetamine | Human urine | Polyamide/graphene oxide/polypyrrole/ET: 20 min at 40 °C (HS) | 5 | 0.9 | 89.5–95.3 | [20] |
| Cyanide, Thiocyanate | Environmental and biological samples | PUF treated with Hg(II) dithizonate complex/ET: 5 min at 60 °C | 5 | 0.34 (μmol/L) | 98.7–101 (wastewater) 99.9–100.5 (saliva) | [21] |
| Hexanal, Heptanal | Saliva | CoFe2O4 magnetic nanoparticles embedded into a reversed-phase polymer/ET: 40 min at 30 °C | 1 | 0.22–0.26 | 75–135 (Hexanal) 82–133 (Heptanal) | [22] |
| Analyte | Sample | MEPS Sorbent | Extraction Time (min) | Sorbent Reusability (Extraction Cycles) | Sample Volume (μL) | Linear Range | LOD (ng/mL) | % RR 1 | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Antidepressants | Oral fluid, Plasma | M1 (4 mg; 80% C8—20% SCX) | – | 40–50 (oral fluids) 20–30 (plasma) | 150 | 10–500 ng/mL | 2–50 | 12–93%, 28–101% | [28] |
| Opiates | Urine | M1 (4 mg; 80% C8—20% SCX) | – | – | 250 | 1–1000 ng/mL | 1–10 | 17–107.8% | [29] |
| Opiates | Whole blood | M1 (4 mg; 80% C8—20% SCX) | – | – | 250 | 5–1000 ng/mL | 5 | 6.06–22.5% | [30] |
| Amphetamine-type stimulants | Urine | C18 | <3 | 100 | 200 | 25–1000 ng/mL | – | 19–71% | [31] |
| Ketamine— Norketamine | Urine, Plasma | M1 (4 mg; 80% C8—20% SCX) | – | – | 250 | 1–250 ng/mL 10–500 ng/mL | 5 ng/mL | 63–101% | [32] |
| Ketamine— Norketamine | Hair | M1 (4 mg; 80% C8—20% SCX) | – | – | 150 | 0.05–10 ng/mg | 0.1 ng/mg (KET) 0.05 ng/mg (NK) | 32–61% | [33] |
| Cannabinoids | Urine | M1 (4 mg; 80% C8—20% SCX) | – | 100 | 250 | 1–400 ng/mL | 1–10 ng/mL | 26–85% | [34] |
| Tetrahydrocannabinol and metabolites | Plasma | M1 (4 mg; 80% C8—20% SCX) | 3 | >200 | 250 | 0.1–30 ng/mL | 0.2 ng/mL | 53–78% | [35] |
| Polyamines | Saliva | C18 | – | 110 | 100 | 0–7.5 mg/L | 1.84–34 ng/mL | 89–130% | [36] |
| Polyamines | Urine | Silica-C18 | – | – | 500 | 0–400 ng mL | 0.18–2.7 ng/mL | 90–113% | [37] |
| PAHs | Urine | C18 | 9 | 80 | 300 | 1.5–375 ng/mL | 0.5–19.4 ng/mL | 88–110% | [38] |
| PAHs | Saliva | C18 | 21 | – | 1500 | 10–842 ng/mL | 4.6–79 pg/mL | 78–123% | [39] |
| Nitro explosives | Blood, Urine | Silica-C18 | 10 | 60 | 50 | 1–250 ng/mL | 0.014–0.828 ng/mL | >88% | [40] |
| Polychlorinated biphenyls | Bovine serum | C18 | 5 | – | 100 | 2–2000 ng/mL | 0.06–0.53 ng/mL | 60–91.4% | [41] |
| Tryptophan metabolites | Human serum, cerebrospinal fluid | C18 | – | – | 40 | 0.4–10 μM (serum) 0.4–7 μM (cerebrospinal fluid) | 0.2–0.4 μM | 40–60%, 40–80% | [42] |
| Aromatic microbial metabolites | Aqueous solutions | C18 | 21 | – | 50 | – | 2–3 μM | 21–102% | [43] |
| Pyrethroid metabolites | Urine | C18 | – | 70 | 500 | 0.05–25 ng/mL | – | 92–124% | [44] |
| Organophosphorus pesticides | Whole blood | C18 | – | – | 150 | 0.5–50 μg/mL | 0.5–2.5 μg/mL | 61–77% | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntorkou, M.; Zacharis, C.K. Sorbent-Based Microextraction Combined with GC-MS: A Valuable Tool in Bioanalysis. Chemosensors 2025, 13, 71. https://doi.org/10.3390/chemosensors13020071
Ntorkou M, Zacharis CK. Sorbent-Based Microextraction Combined with GC-MS: A Valuable Tool in Bioanalysis. Chemosensors. 2025; 13(2):71. https://doi.org/10.3390/chemosensors13020071
Chicago/Turabian StyleNtorkou, Marianna, and Constantinos K. Zacharis. 2025. "Sorbent-Based Microextraction Combined with GC-MS: A Valuable Tool in Bioanalysis" Chemosensors 13, no. 2: 71. https://doi.org/10.3390/chemosensors13020071
APA StyleNtorkou, M., & Zacharis, C. K. (2025). Sorbent-Based Microextraction Combined with GC-MS: A Valuable Tool in Bioanalysis. Chemosensors, 13(2), 71. https://doi.org/10.3390/chemosensors13020071







