Electrochemical Detection of Diclofenac Using a Screen-Printed Electrode Modified with Graphene Oxide and Phenanthroline
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Apparatus and Methods
2.3. Pharmaceuticals Product Analysis
2.4. Pharmaceutical Sample Preparation
3. Results
3.1. FT-IR and SEM Characterization of the Sensor Surface
3.2. Preliminary Studies in PBS and Ferrocyanide/Ferricyanide Redox Probe
3.3. Electrochemical Detection of Diclofenac in Aqueous Solution
3.4. Calibration Curve Registration
3.5. Diclofenac Detection in Pharmaceutical Products
3.6. Stability, Reproducibility, Repeatability, and Interference Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brogden, R.N.; Heel, R.C.; Pakes, G.E.; Speight, T.M.; Avery, G.S. Diclofenac Sodium: A Review of Its Pharmacological Properties and Therapeutic Use in Rheumatic Diseases and Pain of Varying Origin. Drugs 1980, 20, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, J.; Kołodziejczyk, M. Diclofenac in the Treatment of Pain in Patients with Rheumatic Diseases. Reumatologia 2018, 56, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Dutta, N.K.; Mazumdar, K.; Dastidar, S.G.; Park, J.-H. Activity of Diclofenac Used Alone and in Combination with Streptomycin against Mycobacterium Tuberculosis in Mice. Int. J. Antimicrob. Agents 2007, 30, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Eman, F.A.; Rehab, M.A.E.-B.; Abo, B.F.A.; Nancy, G.F.; Neveen, A.A.; Gamal, F.M.G. Evaluation of Antibacterial Activity of Some Non-Steroidal Anti-Inflammatory Drugs against Escherichia Coli Causing Urinary Tract Infection. Afr. J. Microbiol. Res. 2016, 10, 1408–1416. [Google Scholar] [CrossRef]
- O’brien, W.M. Adverse Reactions to Nonsteroidal Anti-Inflammatory Drugs Diclofenac Compared with Other Nonsteroidal Anti-Inflammatory Drugs. Am. J. Med. 1986, 80, 70–80. [Google Scholar] [CrossRef]
- Derry, P.; Derry, S.; Moore, R.A.; McQuay, H.J. Single Dose Oral Diclofenac for Acute Postoperative Pain in Adults. In Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; p. CD004768.pub2. [Google Scholar]
- Ku, E.C.; Wsvary, J.M.; Cash, W.D. Diclofenac Sodium (GP 45840, Voltaren), a Potent Inhibitor of Prostaglandin Synthetase. Biochem. Pharmacol. 1975, 24, 641–643. [Google Scholar] [CrossRef]
- Thomas, G.J.; Herranz, P.; Cruz, S.B.; Parodi, A. Treatment of Actinic Keratosis through Inhibition of Cyclooxygenase-2: Potential Mechanism of Action of Diclofenac Sodium 3% in Hyaluronic Acid 2.5%. Dermatol. Ther. 2019, 32, e12800. [Google Scholar] [CrossRef]
- Bort, R.; Ponsoda, X.; Jover, R.; Gómez-Lechón, M.J.; Castell, J.V. Diclofenac Toxicity to Hepatocytes: A Role for Drug Metabolism in Cell Toxicity. J. Pharmacol. Exp. Ther. 1999, 288, 65–72. [Google Scholar] [CrossRef]
- Selvaraj, S.; Oh, J.-H.; Yoon, S.; Borlak, J. Diclofenac Disrupts the Circadian Clock and through Complex Cross-Talks Aggravates Immune-Mediated Liver Injury—A Repeated Dose Study in Minipigs for 28 Days. Int. J. Mol. Sci. 2023, 24, 1445. [Google Scholar] [CrossRef]
- Siu, K.; Lee, W. Maternal Diclofenac Sodium Ingestion and Severe Neonatal Pulmonary Hypertension. J. Paediatr. Child Health 2004, 40, 152–153. [Google Scholar] [CrossRef]
- Zenker, M.; Klinge, J.; Krüger, C.; Singer, H.; Scharf, J. Severe Pulmonary Hypertension in a Neonate Caused by Premature Closure of the Ductus Arteriosus Following Maternal Treatment with Diclofenac: A Case Report. J. Perinat. Med. 1998, 26, 231–234. [Google Scholar] [PubMed]
- Kornowski, R.; Pines, A.; Levo, Y. Ischemic Stroke Following an Intramuscular Injection of Diclofenac: Case Report. Angiology 1995, 46, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Arendt-Nielsen, L.; Hauge, E.M.; Soerensen, H.T.; Pedersen, L. Dose-Dependency of Diclofenac’s Cardiovascular Risks: A Series of Nationwide Emulated Trials. Eur. Heart J. 2022, 43, ehac544.2730. [Google Scholar] [CrossRef]
- Fatta-Kassinos, D.; Hapeshi, E.; Achilleos, A.; Meric, S.; Gros, M.; Petrovic, M.; Barcelo, D. Existence of Pharmaceutical Compounds in Tertiary Treated Urban Wastewater That Is Utilized for Reuse Applications. Water Resour. Manag. 2011, 25, 1183–1193. [Google Scholar] [CrossRef]
- Elkady, E.F. Simultaneous Determination of Diclofenac Potassium and Methocarbamol in Ternary Mixture with Guaifenesin by Reversed Phase Liquid Chromatography. Talanta 2010, 82, 1604–1607. [Google Scholar] [CrossRef]
- Song, J.; Sun, P.; Ji, Z.; Li, J. Flow Injection Determination of Diclofenac Sodium Based on Its Sensitizing Effect on the Chemiluminescent Reaction of Acidic Potassium Permanganate–Formaldehyde. Luminescence 2015, 30, 32–37. [Google Scholar] [CrossRef]
- Sebaiy, M.; El-Shanawany, A.; Baraka, M.; Abdel-Aziz, L.; Isbell, T.; Colyer, C. Determination of Morphine and Its Metabolites in Human Urine by Capillary Electrophoresis with Laser Induced Fluorescence Detection Employing On-Column Labeling with a New Boronic Acid Functionalized Squarylium Cyanine Dye. Separations 2016, 3, 1. [Google Scholar] [CrossRef]
- Matin, A.A.; Farajzadeh, M.A.; Jouyban, A. A Simple Spectrophotometric Method for Determination of Sodium Diclofenac in Pharmaceutical Formulations. Il Farm. 2005, 60, 855–858. [Google Scholar] [CrossRef]
- Bhattacharya, S.S.; Banerjee, S.; Ghosh, A.K.; Chattopadhyay, P.; Verma, A.; Ghosh, A. A RP-HPLC Method for Quantification of Diclofenac Sodium Released from Biological Macromolecules. Int. J. Biol. Macromol. 2013, 58, 354–359. [Google Scholar] [CrossRef]
- Kaale, E.; Nyamweru, B.C.; Manyanga, V.; Chambuso, M.; Layloff, T. The Development and Validation of a Thin Layer Chromatography Densitometry Method for the Analysis of Diclofenac Sodium Tablets. Int. J. Chem. Anal. Sci. 2013, 4, 73–79. [Google Scholar] [CrossRef]
- Gashu, M.; Aragaw, B.A.; Tefera, M.; Abebe, A. Cobalt(II) Bis-(1,10-Phenanthroline) Complex Electropolymerized Glassy Carbon Electrode and Its Electrocatalytic Sensing of Diclofenac in Pharmaceuticals and Biological Samples. Colloids Surf. A Physicochem. Eng. Asp. 2024, 693, 133974. [Google Scholar] [CrossRef]
- Karuppiah, C.; Cheemalapati, S.; Chen, S.-M.; Palanisamy, S. Carboxyl-Functionalized Graphene Oxide-Modified Electrode for the Electrochemical Determination of Nonsteroidal Anti-Inflammatory Drug Diclofenac. Ionics 2015, 21, 231–238. [Google Scholar] [CrossRef]
- Kimuam, K.; Rodthongkum, N.; Ngamrojanavanich, N.; Chailapakul, O.; Ruecha, N. Single Step Preparation of Platinum Nanoflowers/Reduced Graphene Oxide Electrode as a Novel Platform for Diclofenac Sensor. Microchem. J. 2020, 155, 104744. [Google Scholar] [CrossRef]
- El-Wekil, M.M.; Alkahtani, S.A.; Ali, H.R.H.; Mahmoud, A.M. Advanced Sensing Nanomaterials Based Carbon Paste Electrode for Simultaneous Electrochemical Measurement of Esomeprazole and Diclofenac Sodium in Human Serum and Urine Samples. J. Mol. Liq. 2018, 262, 495–503. [Google Scholar] [CrossRef]
- Minta, D.; González, Z.; Melendi-Espina, S.; Gryglewicz, G. Easy-to-Prepare Graphene-Based Inkjet-Printed Electrodes for Diclofenac Electrochemical Sensing. Prog. Org. Coat. 2023, 185, 107942. [Google Scholar] [CrossRef]
- Oztekin, Y.; Yazicigil, Z.; Solak, A.O.; Ustundag, Z.; Kilic, Z.; Bilge, S. Surface Modification and Characterization of Phenanthroline Nanofilms on Carbon Substrate. Surf. Interface Anal. 2011, 43, 923–930. [Google Scholar] [CrossRef]
- Shaari, H.A.H.; Ramli, M.M.; Mohtar, M.N.; Razali, N.H.F. Characterization and Conductivity of Graphene Oxide (GO) Dispersion in Different Solvents. In AIP Conference Proceedings; AIP Publishing: Arau, Malaysia, 2021; p. 060001. [Google Scholar]
- Tamiru, G.; Abebe, A.; Abebe, M.; Liyew, M. Synthesis, Structural Investigation and Biological Application of New Mono- and Binuclear Cobalt (II) Mixed-Ligand Complexes Containing 1,10-Phenanthroline, Acetamide and Ethylenediamine. Eth. J. Sci. Technol. 2019, 12, 69. [Google Scholar] [CrossRef]
- Zhang, Z.; Schniepp, H.C.; Adamson, D.H. Characterization of Graphene Oxide: Variations in Reported Approaches. Carbon 2019, 154, 510–521. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Voltammetric Sensors Based on Nanomaterials for Detection of Caffeic Acid in Food Supplements. Chemosensors 2020, 8, 41. [Google Scholar] [CrossRef]
- Choi, W.-K. Electrochemical Characterizations of the Reducibility and Persistency of Electrolyzed Reduced Water Produced from Purified Tap Water. Int. J. Electrochem. Sci. 2014, 9, 10. [Google Scholar] [CrossRef]
- Gayathri, P.; Senthil Kumar, A. Electrochemical Behavior of the 1,10-Phenanthroline Ligand on a Multiwalled Carbon Nanotube Surface and Its Relevant Electrochemistry for Selective Recognition of Copper Ion and Hydrogen Peroxide Sensing. Langmuir 2014, 30, 10513–10521. [Google Scholar] [CrossRef] [PubMed]
- Heyrovský, M.; Vavřička, S. On the pH-Dependence of Polarographic Half-Wave Potentials. J. Electroanal. Chem. Interfacial Electrochem. 1972, 36, 223–233. [Google Scholar] [CrossRef]
- Shimizu, K.; Hutcheson, R.; Engelmann, M.D.; Francis Cheng, I. Cyclic Voltammetric and Aqueous Equilibria Model Study of the pH Dependant Iron(II/III)Ethylenediamminetetraacetate Complex Reduction Potential. J. Electroanal. Chem. 2007, 603, 44–50. [Google Scholar] [CrossRef]
- Gunache (Roșca), R.O.; Bounegru, A.V.; Apetrei, C. Determination of Atorvastatin with Voltammetric Sensors Based on Nanomaterials. Inventions 2021, 6, 57. [Google Scholar] [CrossRef]
- Heineman, W.R.; Kissinger, P.T.; Wehmeyer, K.R. Editors’ Choice—Review—From Polarography to Electrochemical Biosensors: The 100-Year Quest for Selectivity and Sensitivity. J. Electrochem. Soc. 2021, 168, 116504. [Google Scholar] [CrossRef]
- Sundaresan, P.; Lee, T.Y. Facile Synthesis of Exfoliated Graphite-Supported Cobalt Ferrite (Co1.2Fe1.8O4) Nanocomposite for the Electrochemical Detection of Diclofenac. Microchem. J. 2022, 181, 107777. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Fernandes, P.M.V.; Pereira, C.M.; Silva, F. Electrochemical Sensors and Biosensors for Determination of Catecholamine Neurotransmitters: A Review. Talanta 2016, 160, 653–679. [Google Scholar] [CrossRef]
- Goyal, R.N.; Chatterjee, S.; Agrawal, B. Electrochemical Investigations of Diclofenac at Edge Plane Pyrolytic Graphite Electrode and Its Determination in Human Urine. Sens. Actuators B Chem. 2010, 145, 743–748. [Google Scholar] [CrossRef]
- Cid-Cerón, M.M.; Guzmán-Hernández, D.S.; Ramírez-Silva, M.T.; Galano, A.; Romero-Romo, M.; Palomar-Pardavé, M. New insigths on the kinetics and mechanism of the electrochemical oxidation of diclofenac in neutral aqueous medium. Electrochim. Acta 2016, 199, 92–98. [Google Scholar] [CrossRef]
- Aguilar-Lira, G.Y.; Álvarez-Romero, G.A.; Zamora-Suárez, A.; Palomar-Pardavé, M.; Rojas-Hernández, A.; Rodríguez-Ávila, J.A.; Páez-Hernández, M.E. New Insights on Diclofenac Electrochemistry Using Graphite as Working Electrode. J. Electroanal. Chem. 2017, 794, 182–188. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Sensitive Detection of Hydroxytyrosol in Extra Virgin Olive Oils with a Novel Biosensor Based on Single-Walled Carbon Nanotubes and Tyrosinase. Int. J. Mol. Sci. 2022, 23, 9132. [Google Scholar] [CrossRef] [PubMed]
- Slim, C.; Tlili, N.; Richard, C.; Griveau, S.; Bedioui, F. Amperometric Detection of Diclofenac at a Nano-Structured Multi-Wall Carbon Nanotubes Sensing Films. Inorg. Chem. Commun. 2019, 107, 107454. [Google Scholar] [CrossRef]
- Hanabaratti, R.M.; Gowda, J.I.; Tuwar, S.M. Development of a sensor by electro-polymerization of erichrome black-t on glassy carbon electrode and determination of an anti-inflammatory drug diclofenac. Int. J. Pharm. Pharm. Sci. 2018, 11, 81–87. [Google Scholar] [CrossRef]
- Seguro, I.; Pacheco, J.G.; Delerue-Matos, C. Low Cost, Easy to Prepare and Disposable Electrochemical Molecularly Imprinted Sensor for Diclofenac Detection. Sensors 2021, 21, 1975. [Google Scholar] [CrossRef]
- Das, S.; Chakravorty, A.; Luktuke, S.; Raj, A.; Mini, A.A.; Ramesh, K.; Grace, A.N.; Pandey, S.K.; Raghavan, V. Graphene/Gadolinium Oxide Composite Modified Screen-Printed Electrochemical Sensor for Detection of Diclofenac Sodium. Results Chem. 2023, 6, 101189. [Google Scholar] [CrossRef]
- Eteya, M.M.; Rounaghi, G.H.; Deiminiat, B. Fabrication of a New Electrochemical Sensor Based on Au Pt Bimetallic Nanoparticles Decorated Multi-Walled Carbon Nanotubes for Determination of Diclofenac. Microchem. J. 2019, 144, 254–260. [Google Scholar] [CrossRef]
- Baezzat, M.R.; Tavakkoli, N.; Zamani, H. Construction of a New Electrochemical Sensor Based on MoS2 Nanosheets Modified Graphite Screen Printed Electrode for Simultaneous Determination of Diclofenac and Morphine. Anal. Bioanal. Chem. Res. 2022, 9, 153–162. [Google Scholar] [CrossRef]
- De Carvalho, R.C.; Betts, A.J.; Cassidy, J.F. Diclofenac Determination Using CeO2 Nanoparticle Modified Screen-Printed Electrodes—A Study of Background Correction. Microchem. J. 2020, 158, 105258. [Google Scholar] [CrossRef]
- Das, S.; Chakravorty, A.; Raj, A.; Luktuke, S.; Appu Mini, A.; Awasthi, S.; Sankar Sana, S.; Kumar Pandey, S.; Raghavan, V. Graphene/MWCNT/Copper-Nanoparticle Fabricated Printed Electrode for Diclofenac Detection in Milk and Drinking Water: Electrochemical and in-Silico Analysis. J. Mol. Liq. 2024, 411, 125750. [Google Scholar] [CrossRef]
- Fard, G.P.; Alipour, E.; Ali Sabzi, R.E. Modification of a Disposable Pencil Graphite Electrode with Multiwalled Carbon Nanotubes: Application to Electrochemical Determination of Diclofenac Sodium in Some Pharmaceutical and Biological Samples. Anal. Methods 2016, 8, 3966–3974. [Google Scholar] [CrossRef]
- Arvand, M.; Gholizadeh, T.M.; Zanjanchi, M.A. MWCNTs/Cu(OH)2 Nanoparticles/IL Nanocomposite Modified Glassy Carbon Electrode as a Voltammetric Sensor for Determination of the Non-Steroidal Anti-Inflammatory Drug Diclofenac. Mater. Sci. Eng. C 2012, 32, 1682–1689. [Google Scholar] [CrossRef]
- Killedar, L.; Ilager, D.; Shetti, N.P.; Aminabhavi, T.M.; Raghava Reddy, K. Synthesis of Ruthenium Doped Titanium Dioxide Nanoparticles for the Electrochemical Detection of Diclofenac Sodium. J. Mol. Liq. 2021, 340, 116891. [Google Scholar] [CrossRef]
- Goyal, R.N.; Chatterjee, S.; Rana, A.R.S. The Effect of Modifying an Edge-Plane Pyrolytic Graphite Electrode with Single-Wall Carbon Nanotubes on Its Use for Sensing Diclofenac. Carbon 2010, 48, 4136–4144. [Google Scholar] [CrossRef]
- Beitollahi, H.; Garkani Nejad, F.; Tajik, S.; Di Bartolomeo, A. Screen-Printed Graphite Electrode Modified with Graphene-Co3O4 Nanocomposite: Voltammetric Assay of Morphine in the Presence of Diclofenac in Pharmaceutical and Biological Samples. Nanomaterials 2022, 12, 3454. [Google Scholar] [CrossRef]
- Yeola, C.A.; Sonawane, V.N.; Sonawane, V.N.; Surana, K.R.; Patil, D.M.; Sonawane, D.D. Development and Validation of Simple UV- Spectrophotometric Method for Estimation of Diclofenac Sodium. Asian J. Pharm. Anal. 2023, 13, 183–189. [Google Scholar] [CrossRef]
- Rathod, A.C.; Dudhe, A.R.; Gajare, K.H. Development of Analytical Technique for Dichlofenac Sodium and Ibuprofen Utilizing UV Visible Spectroscopy and AUC Method. Int. J. Multidiscip. Res. 2023, 5, 2570. [Google Scholar] [CrossRef]
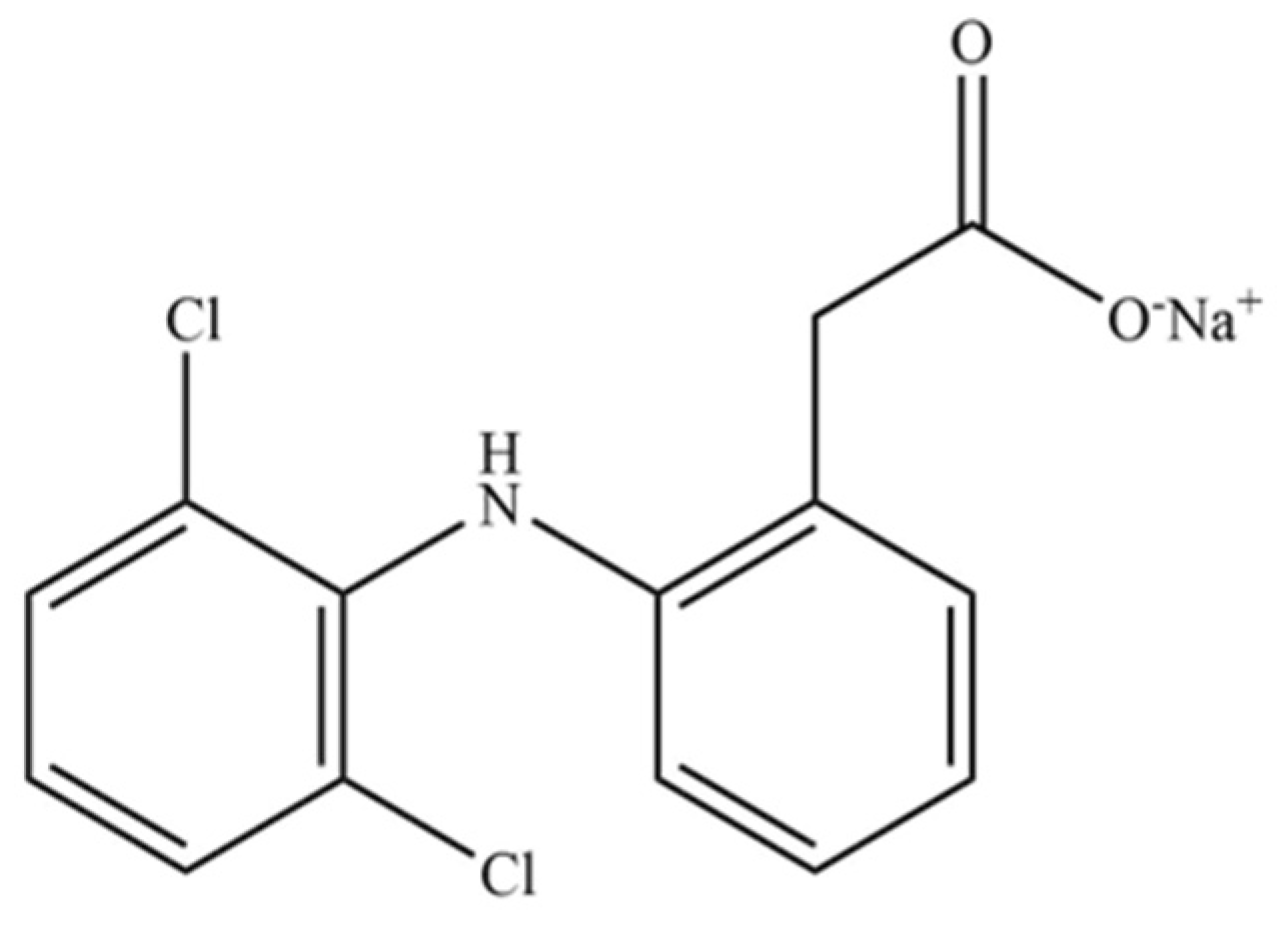
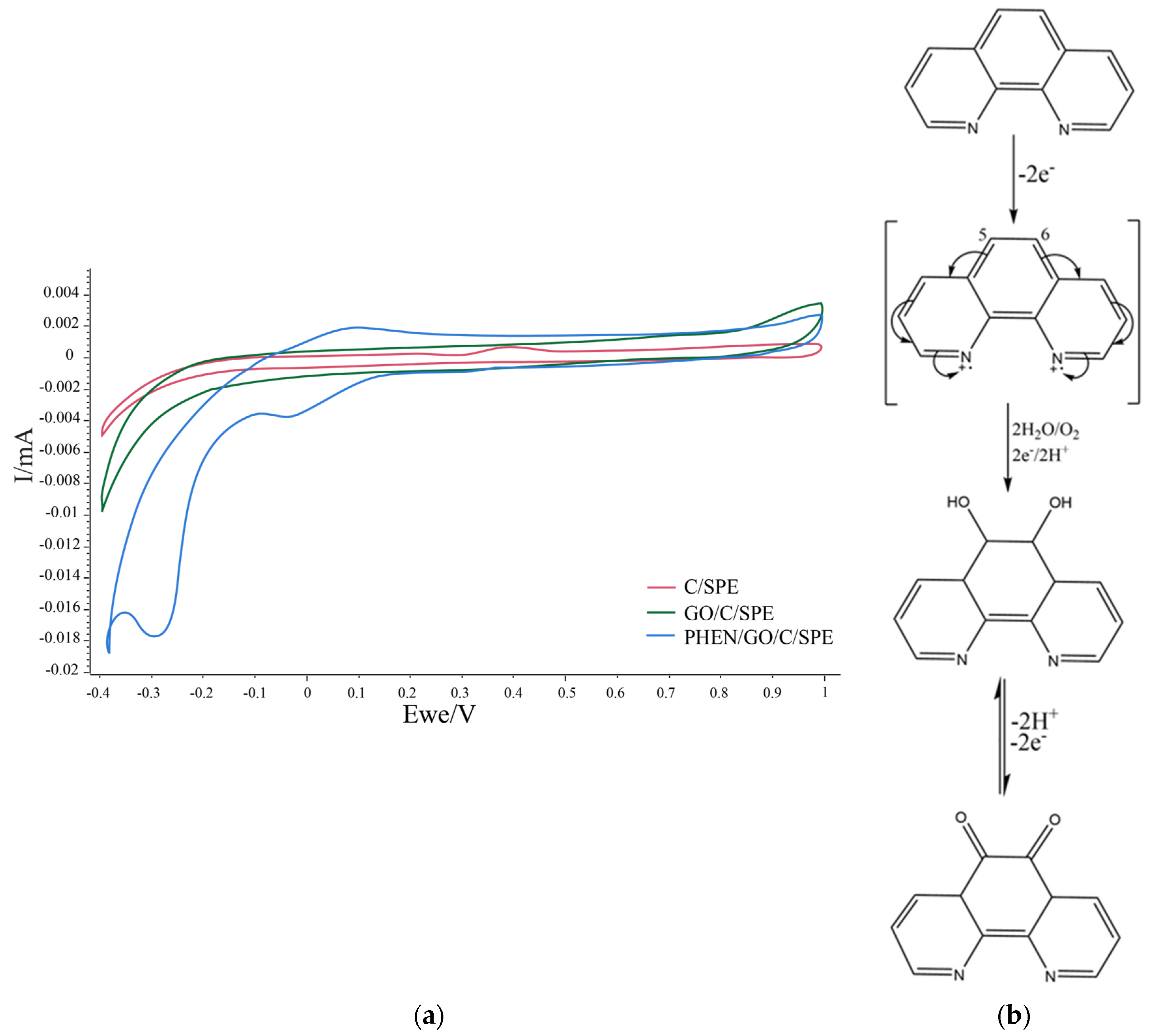

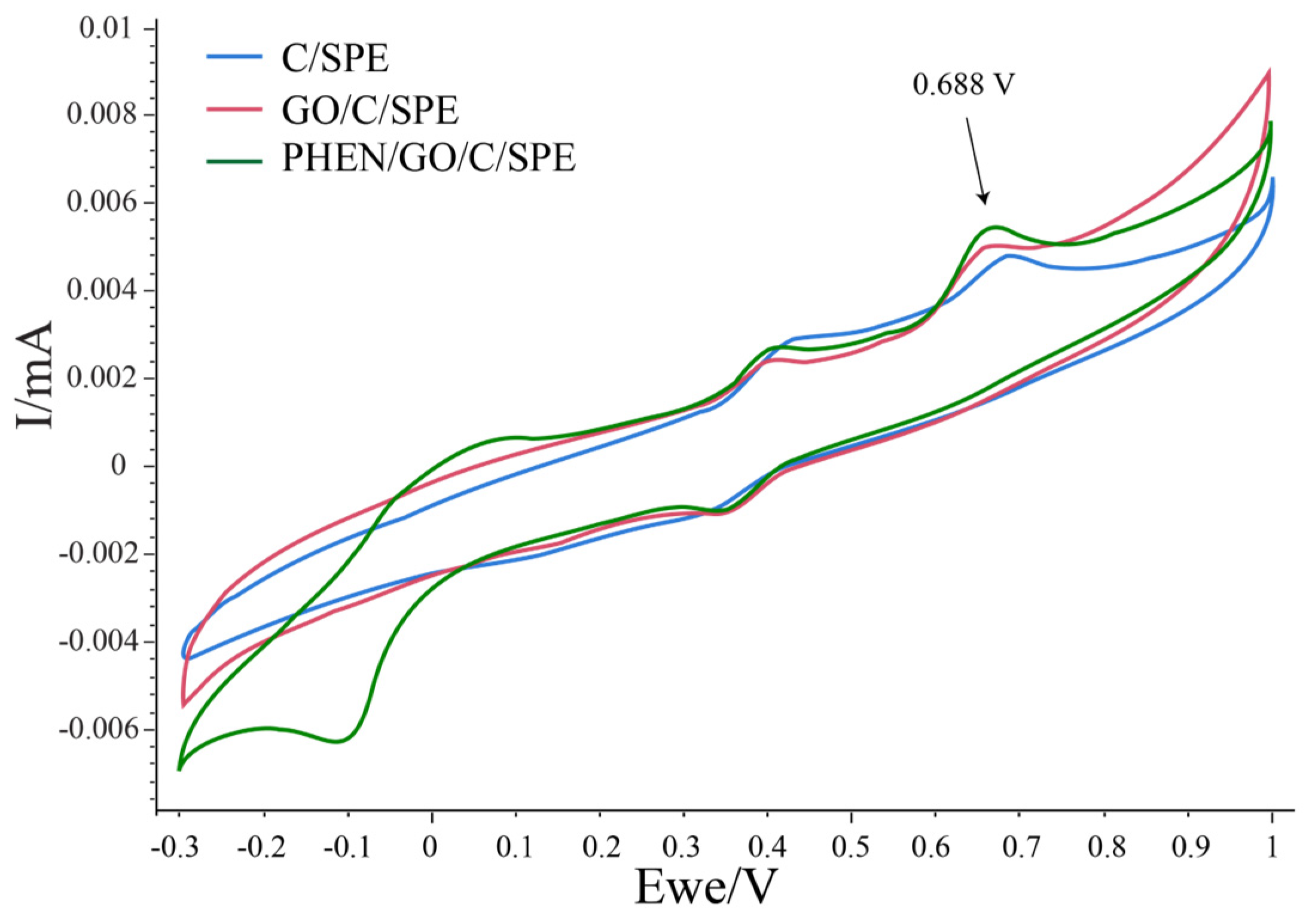
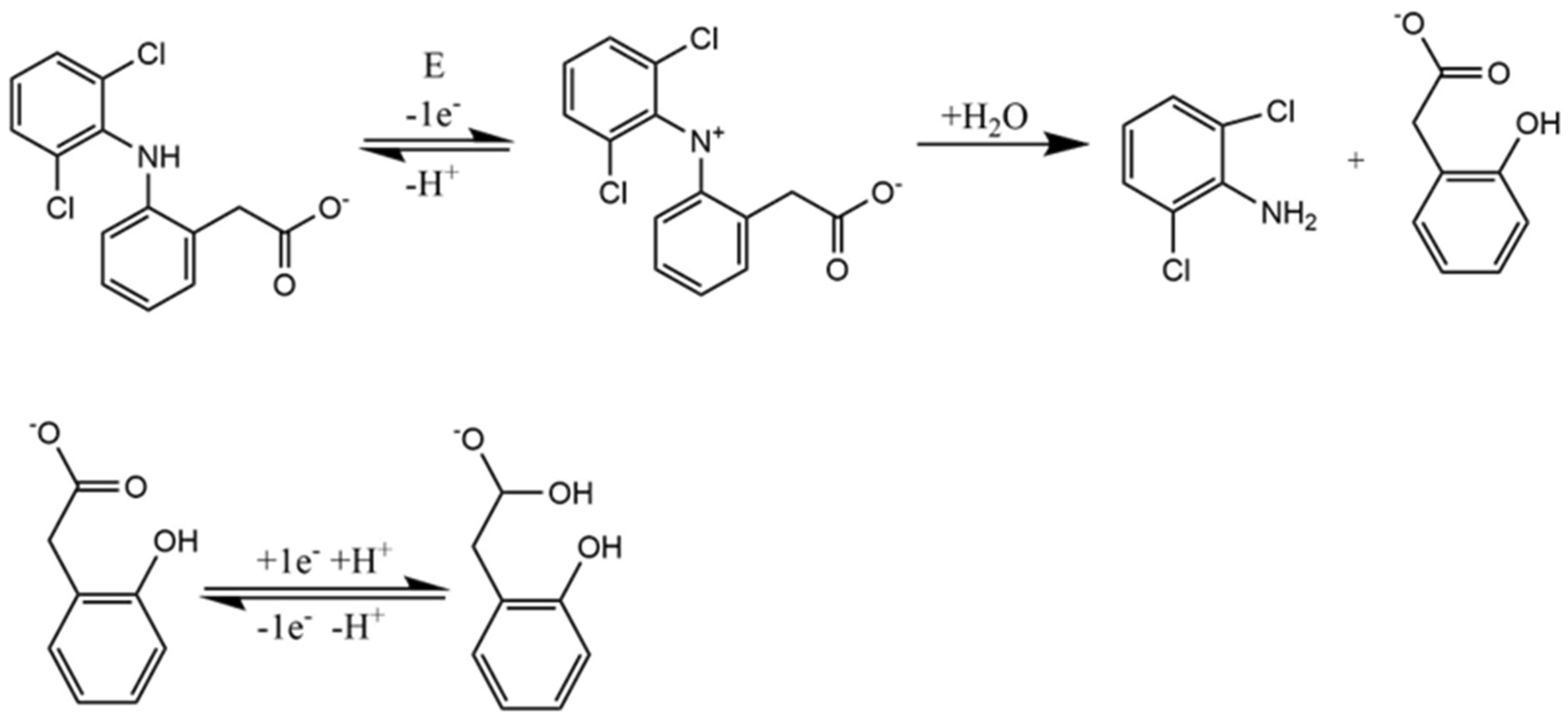
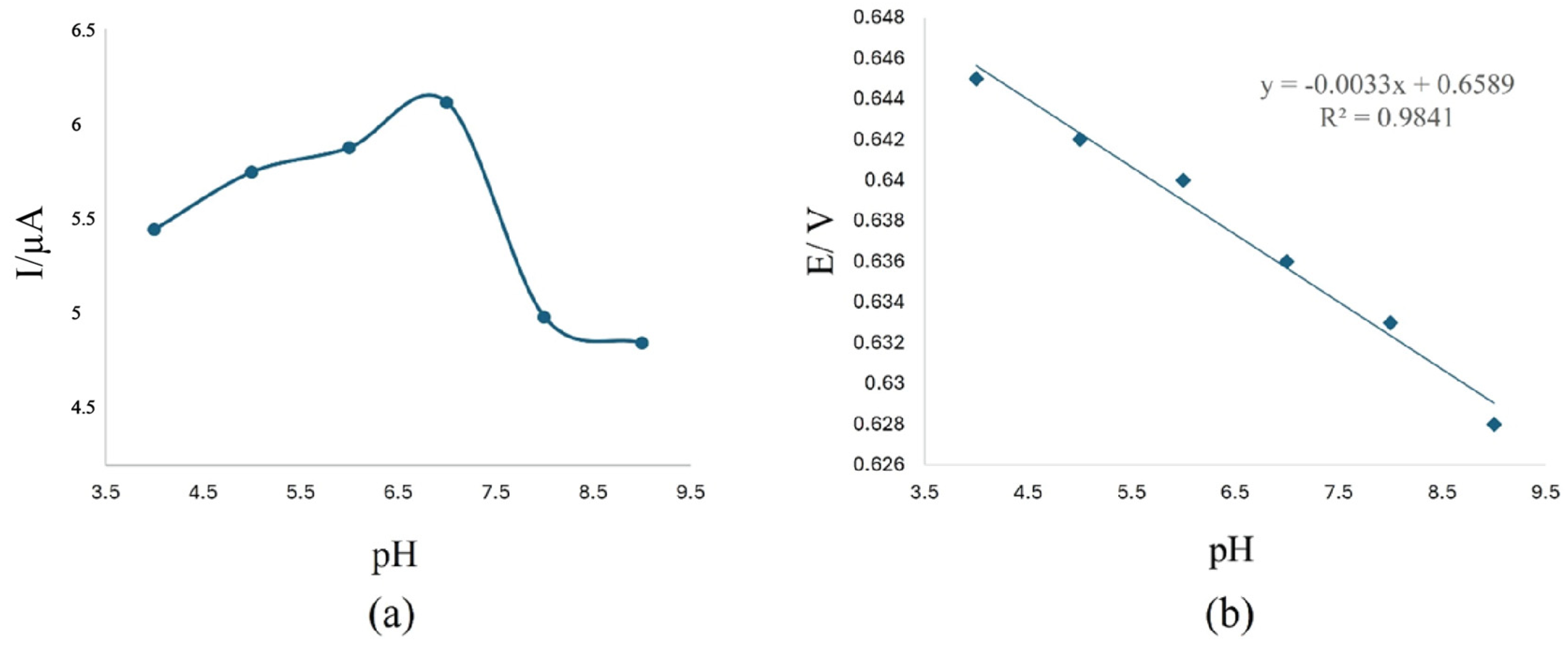

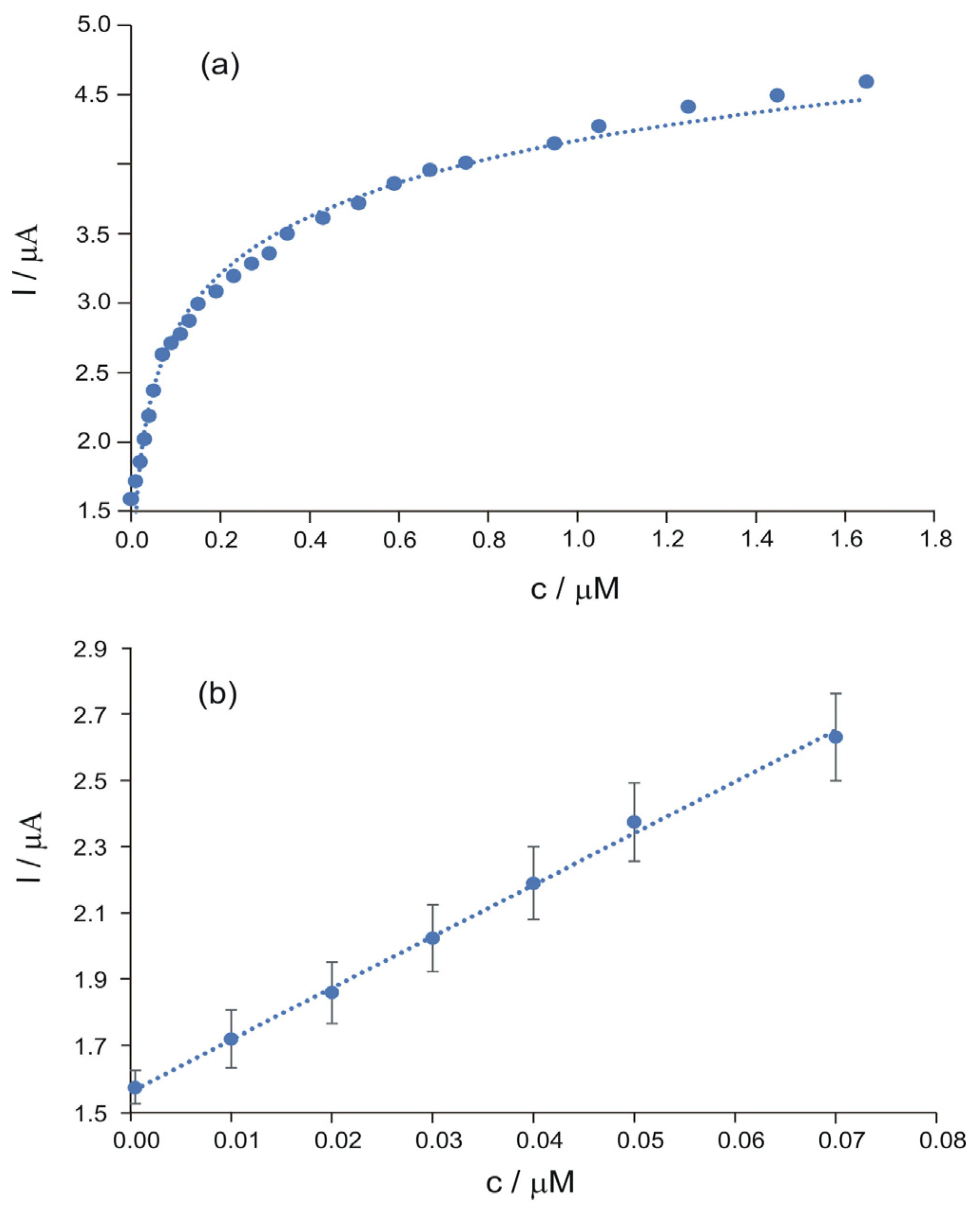
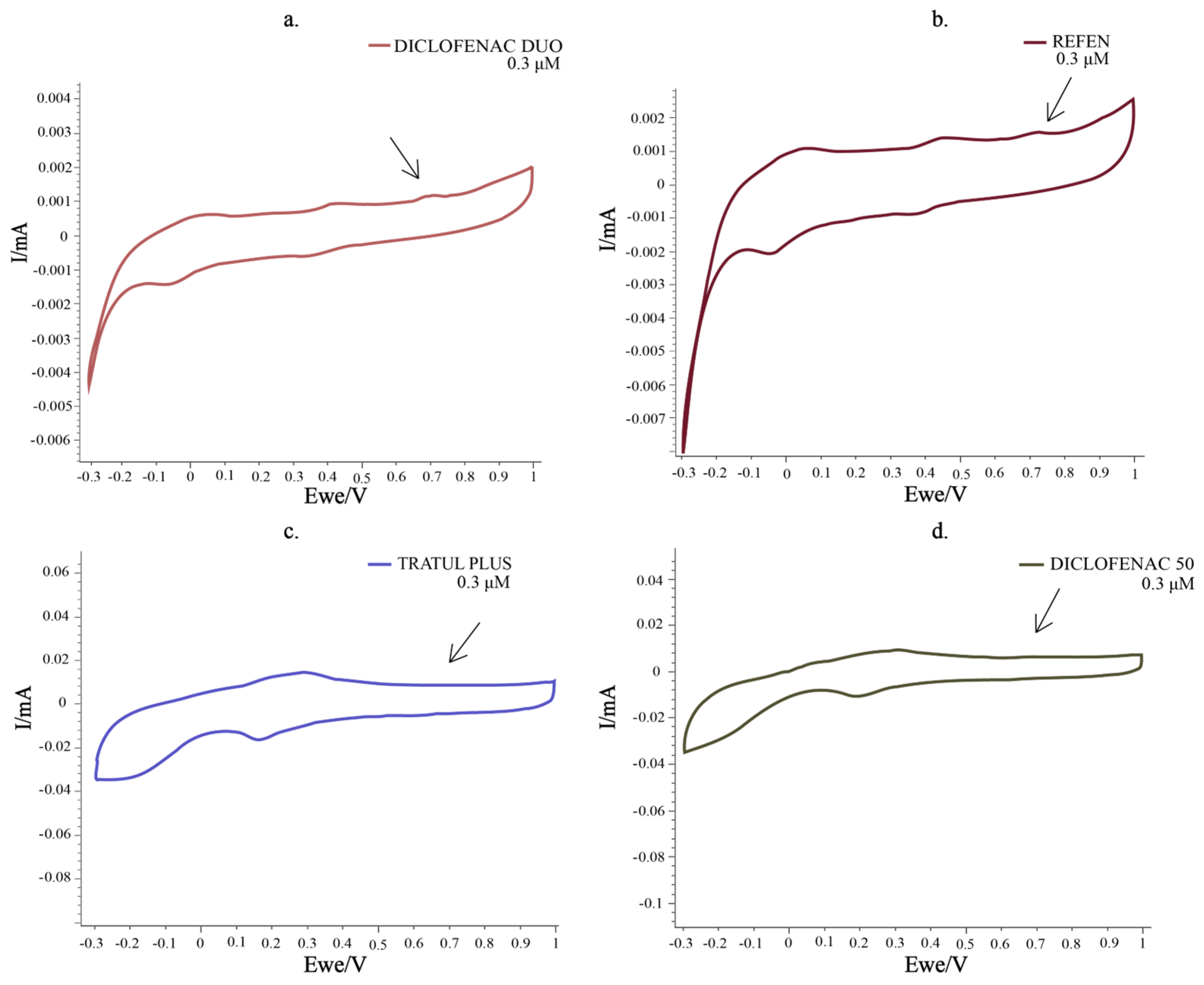
| Pharmaceutical Product | Administration | Dosage Form | Active Compound | List of Ingredients from the Manufacturer |
|---|---|---|---|---|
| Diclofenac 50 | Oral | gastro-resistant tablets | Diclofenac sodium 50 mg | lactose monohydrate, povidone, corn starch, anhydrous colloidal silica, magnesium stearate, talcum, methacrylic acid ethyl acrylate copolymer 30%, triethyl citrate, titanium dioxide (E171), quinoline yellow (E104), indigotin (E132), simethicone emulsion 30%, and sodium hydroxide. |
| Diclofenac Duo | Oral | capsules with extended release | Diclofenac sodium 75 mg | microcrystalline cellulose (MCC), polyvidone K25, colloidal silicon dioxide, methacrylic acid-ethyl acrylate copolymer (1:1), propylene glycol, talcum, poly[ethylacrylate-co-methylmethacrylate-co-(2-trimethylammonioethyl methacrylate chloride] 1:2:0,1, poly[ethylacrylate-co-(2-trimethylammonioethyl) methacrylate chloride] 1:2:0.2, dibutyl phthalate, indigotin, titanium dioxide (E171), gelatin, antifoam emulsion, shellac. |
| Tratul Plus | Oral | gastro-resistant capsules | Diclofenac sodium 50 mg | thiamine hydrochloride (vitamin B1), pyridoxine hydrochloride (vitamin B6), cyanocobalamin (vitamin B12) povidone, methacrylic acid-ethyl acrylate copolymer (1:1), triethyl citrate, talcum, iron oxide red (E172), iron oxide yellow (E172), titanium dioxide (E171), gelatin. |
| Refen | Injectable | solution | Diclofenac sodium 25 mg/mL | benzyl alcohol, mannitol, sodium hydroxide, propylene glycol, water for injection formulation. |
| Electrode | 1Epa (V) | 2Epc (V) | 3E1/2 (V) | 4∆E (V) | 5Ipa (mA) | 6Ipc (mA) | Ipc/Ipa |
|---|---|---|---|---|---|---|---|
| C/SPE | 0.470 | 0.139 | 0.304 | 0.331 | 0.0159 | −0.0150 | 0.943 |
| GO/C/SPE | 0.416 | 0.184 | 0.300 | 0.232 | 0.0091 | −0.0086 | 0.945 |
| PHEN/GO/C/SPE | 0.419 | 0.201 | 0.310 | 0.218 | 0.0186 | −0.0167 | 0.897 |
| Electrode | Active Area (cm2) | Geometric Area (cm2) | R2 |
|---|---|---|---|
| C/SPE | 0.635 | 0.1256 | 0.9998 |
| GO/C/SPE | 0.3908 | 0.9946 | |
| PHEN/GO/C/SPE | 1.0258 | 0.9973 |
| Sensor | Linear Equation | R2 | ᴦ (mol/cm2) | Epa (V) |
|---|---|---|---|---|
| C/SPE | Ipa = 0.0000085·v + 0.0045 | 0.9900 | 3.265 × 10−12 | 0.676 |
| GO/C/SPE | Ipa = 0.00001·v + 0.0038 | 0.9925 | 6.242 × 10−12 | 0.666 |
| PHEN/GO/C/SPE | Ipa = 0.00001·v + 0.0053 | 0.9904 | 2.401 × 10−11 | 0.688 |
| Sensor | Linear Equation | R2 | LOD (μM) | LOQ (μM) |
|---|---|---|---|---|
| PHEN/GO/C/SPE | Ipa (μA) = 0.1559·c(μM) + 1.6 | 0.9968 | 0.00153 | 0.00508 |
| Sensor/Sensitive Material | Method | Linearity Range (μM) | LOD (μM) |
|---|---|---|---|
| MIP [46] | CV | 0.1–10 | 3.5 |
| Graphene/Gadolinium oxide [47] | CV | 5.86–66.6 | 0.028 |
| Au-PtNPs/f-MWCNTs/AuE [48] | DPV | 0.5–1000 | 0.3 |
| MoS2 Nanosheets Modified Graphite [49] | CV | 0.05–600 | 0.03 |
| CeO2-SPCE [50] | DPV | 0.05–1 2–250 | 0.4 |
| Graphene/MWCNT/copper-nanoparticle [51] | DPV | 17.41–206.45 | 0.057 |
| Pt nanoflowers/reduced GO [24] | DPV | 0.1−100 | 0.04 |
| MWCNTs [52] | DPV | 0.047–12.95 | 0.017 |
| MWCNTs/Cu(OH)2 NPs/IL/GCE [53] | DPV | 0.18–119 | 0.04 |
| GO/COOH [23] | CV, LSV | 1.2–400 | 0.09 |
| Ru/TiO2 [54] | CV | 0.291 | 11.48 |
| SWCNT modified EPPGE [55] | SWV | 0.02–1.5 | 0.02 |
| Graphene—Co3O4/SPGE [56] | DPV | 0.02–575 | 0.007 |
| Carbon modified with graphene oxide and phenanthroline—this work | CV | 0.01–0.07 | 0.00153 |
| Pharmaceutical Product | Diclofenac Sodium Content | |||||
|---|---|---|---|---|---|---|
| Indicated by Producer | Conc. of the Sample (M) | Determined by UV (M) ± RSD (%) | Recovery (%) | Determined by Sensor (M) ± RSD (%) | Recovery (%) | |
| Diclofenac 50 (tablet) | 50 mg/caps | 1.69 × 10−6 3.38 × 10−6 5.12 × 10−6 | 1.65 × 10−6 ± 0.01 3.13 × 10−6 ± 0.03 4.20 × 10−6 ± 0.02 | 97.63 92.60 82.03 | 1.58 × 10−6 ± 1.19 2.95 × 10−6 ± 0.88 4.14 × 10−6 ± 0.97 | 93.49 87.47 80.85 |
| Diclofenac Duo (capsule) | 75 mg/caps | 2.53 × 10−6 5.06 × 10−6 7.59 × 10−6 | 2.43 × 10−6 ± 0.11 4.20 × 10−6 ± 0.08 6.23 × 10−6 ± 0.18 | 96.04 83.00 82.08 | 2.46 × 10−6 ± 0.19 3.95 × 10−6 ± 0.77 6.32 × 10−6 ± 0.47 | 97.23 78.06 83.26 |
| Tratul Plus (capsule) | 50 mg/caps | 1.69 × 10−6 3.38 × 10−6 5.12 × 10−6 | 1.47 × 10−6 ± 0.21 4.27 × 10−6 ± 0.19 5.40 × 10−6 ± 0.09 | 86.98 126.33 105.46 | 1.50 × 10−6 ± 0.36 4.26 × 10−6 ± 0.71 5.73 × 10−6 ± 0.26 | 88.75 126.03 111.91 |
| Refen (injectable solution) | 75 mg/3 mL | 2.35 × 10−6 4.70 × 10−6 7.05 × 10−6 | 2.00 × 10−6 ± 0.07 4.19 × 10−6 ± 0.12 6.20 × 10−6 ± 0.13 | 85.10 89.15 87.94 | 2.02 × 10−6 ± 0.82 4.23 × 10−6 ± 0.65 6.25 × 10−6 ± 0.36 | 85.95 90.00 85.46 |
| Interfering Compound | Concentration (M) | Recovery (%) | Coefficient of Variation (%) | Tolerance Limit (M) |
|---|---|---|---|---|
| Acetaminophen | 10−5 | 98.73 | 2.5 | 2 × 10−4 |
| Phenylbutazone | 99.75 | 3.0 | 5 × 10−4 | |
| Ibuprofen | 101.32 | 1.8 | 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Măghinici, A.-R.; Bounegru, A.-V.; Apetrei, C. Electrochemical Detection of Diclofenac Using a Screen-Printed Electrode Modified with Graphene Oxide and Phenanthroline. Chemosensors 2025, 13, 55. https://doi.org/10.3390/chemosensors13020055
Măghinici A-R, Bounegru A-V, Apetrei C. Electrochemical Detection of Diclofenac Using a Screen-Printed Electrode Modified with Graphene Oxide and Phenanthroline. Chemosensors. 2025; 13(2):55. https://doi.org/10.3390/chemosensors13020055
Chicago/Turabian StyleMăghinici, Ana-Raluca, Alexandra-Virginia Bounegru, and Constantin Apetrei. 2025. "Electrochemical Detection of Diclofenac Using a Screen-Printed Electrode Modified with Graphene Oxide and Phenanthroline" Chemosensors 13, no. 2: 55. https://doi.org/10.3390/chemosensors13020055
APA StyleMăghinici, A.-R., Bounegru, A.-V., & Apetrei, C. (2025). Electrochemical Detection of Diclofenac Using a Screen-Printed Electrode Modified with Graphene Oxide and Phenanthroline. Chemosensors, 13(2), 55. https://doi.org/10.3390/chemosensors13020055







