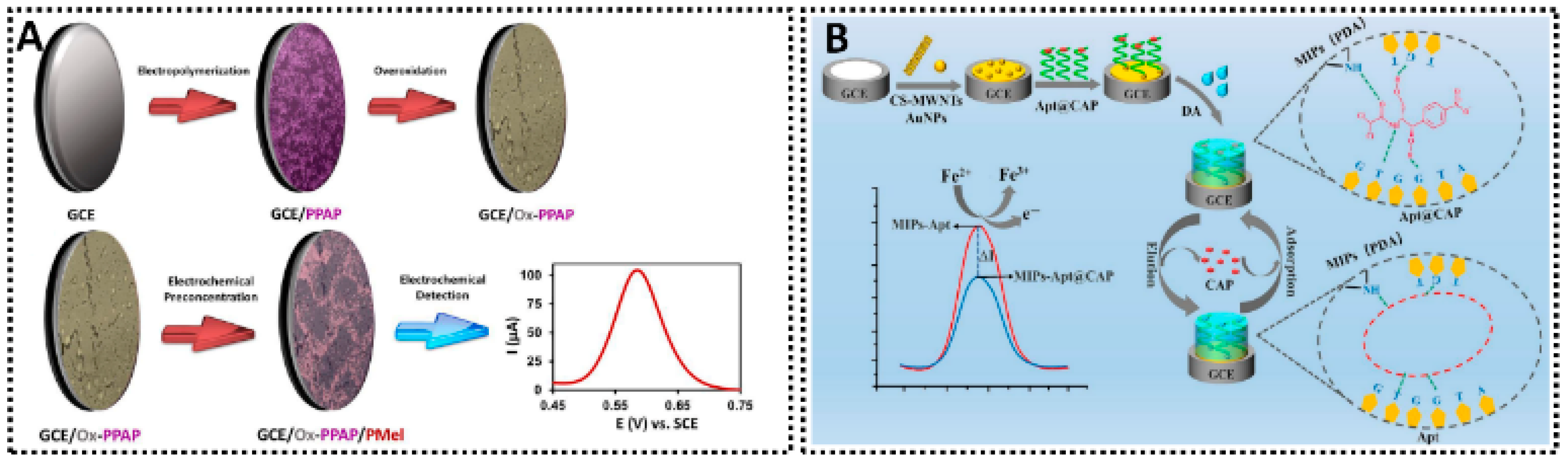
Advances in Molecularly Imprinted Electrochemical Platforms for Food Quality Control: Targeting Antioxidants, Sweeteners, Colorants, Contaminants and Toxicants
Abstract
1. Introduction
2. Fundamentals of MIP-Based Electrochemical Sensors
2.1. Principles and Fabrication
2.2. Electrochemical Transduction Mechanisms
- (i)
- Electrostatic recognition → redox-probe gating (DPV/SWV/EIS). Binding of ionic analytes or formation of charged complexes establishes a Donnan potential within the MIP that excludes (for like-charged) or enriches (for oppositely charged) outer-sphere redox markers, e.g., [Fe(CN)6]3−/4−. The resulting change in probe concentration and in the heterogeneous electron-transfer rate constant k0 increases the semicircle radius in Nyquist plots and lowers DPV/SWV peak currents. Energy-diagrammatically, the driving-force alignment is unchanged, but the effective barrier width and access of the probe to the electrode are altered by the bound charge cloud.
- (ii)
- Hydrogen-bonding recognition → microenvironment densification and dielectric modulation. Multiple H-bonds around the cavity compact the polymer locally and reduce permittivity, decreasing redox-probe diffusivity and k0. This appears as higher Rct (EIS) and suppressed faradaic responses (DPV/SWV). For non-electroactive targets, the signal is entirely indirect; for electroactive targets, the same densification may preconcentrate the analyte in the cavity and yield a net “signal-on” response via DET at suitable potentials.
- (iii)
- π–π stacking recognition → charge-transfer complexation and band/level alignment. Aromatic targets can stack with π-conjugated hosts (polypyrrole/graphene), forming weak charge-transfer complexes that increase local carrier density or lower the overpotential for direct oxidation of phenolics. In energy-level terms, binding perturbs the interfacial density of states and reduces the tunneling barrier, which can shift peak potentials and increase current—opposite in sign to purely blocking effects. This pathway rationalizes “signal-on” voltammetry observed for many phenolics on PPy/graphene-MIPs.
2.3. Materials and Surface Modifications
2.4. Addressing the Aqueous Matrix Challenge in Food Sensing
2.5. Design Considerations and Improvements
3. MIP Electrochemical Sensors for Antioxidants in Foods
3.1. MIP-Based Sensors for Natural Antioxidants
3.2. MIP-Based Sensors for Synthetic Antioxidants
3.3. Analysis of Sensor Performance
4. MIP Electrochemical Sensors for Sweeteners
| Target Sweetener | Sensing Platform | MIP Fabrication Method | Functional Monomer(s) | Linear Range | LOD (nM) | Real Sample | Reference |
|---|---|---|---|---|---|---|---|
| Acesulfame-K | GCE/MIP-o-PD | EP | o-PD | 0.1–17.0 μM | 350 | Cola drink; Candy; Tabletop sweetener | [133] |
| Aspartame | MIP/AS/MWCNT/GCE | BP | NR | 8 nM–6 µM | 22 | Sports beverages | [134] |
| ZDM-MIPs-MGCE | SI | Deep eutectic solvent | 0.34–169.9 μM | - | Soft drinks | [129] | |
| CSNP-RGO/MIP-EQCM | EP | Chitosan | 10–100 μM | 240 | Soft drinks; Sugarfree tablets | [135] | |
| P(3-TAA)/MIP-QCM | EP | 3-thiopheneacetic acid | 12.5–200 μM | 31,750 | Soft drinks | [130] | |
| D-arabinose | C/FMWCNT/MIP | EP | o-PD | 0.01–0.1 nM | 0.00425 | Sugarcane bagasse hydrolysates | [131] |
| D-xylose | GCE/RGO-MIP | EP | Phenol | 0.1–1 pM and 1–10 pM | 0.00008 | Sugarcane bagasse | [132] |
| C/FMWCNT/MIP | EP | o-PD | 0.01–0.1 nM | 0.0045 | Sugarcane bagasse hydrolysates | [131] | |
| Fructose | GCE/rGO-MIP | EP | Phenylboronic acid derivative | 10–150 fM | 0.0000032 | Orange juice; Apple juice; Grape juice | [136] |
| Sucrose | MIP/MWCNTs/GCE | EP | o-PD | 0.01–2.5 mM and 2.5–10.0 mM | 3000 | Raw sugar beet juice; Thin juice; Thick juice; Molasses | [137] |
5. MIP Electrochemical Sensors for Colorants

| Target Colorants | Sensing Platform | MIP Fabrication Method | Functional Monomer(s) | Linear Range | LOD (nM) | Real Sample | Reference |
|---|---|---|---|---|---|---|---|
| Amaranth | MIP/MWCNT/GCE | EP | Pyrrole | 0.007–1.0 μM and 0.4–17.0 μM | 0.4 | Watermelon juice; Grape juice; Orange juice | [26] |
| MIP/ZnO-MWCNT/SPCE | EP | Melamine | 0.01–1 μM and 1–1000 μM | 3 | Robitussin Junior syrup and Acyclovir Arena capsules | [140] | |
| PDDA-Gr-(Pd-Cu)@MIP-PDA/GCE | SI | Dopamine | 0.006–10 μM | 2 | Soft drink | [141] | |
| CMIG/GCE | SGI | Chitosan | 0.02–150 μM | 3 | Milk powder; White vinegar; Carbonated drinks | [152] | |
| MIES | EP | Aniline | 0.05–50.0 μM | 50 | Grape-flavored drink; Watermelon-flavored drink; Peach-flavored drink | [153] | |
| Anthocyanins | MIP/GCE | BP | Acrylamide | 1 nM–10 μM | 0.3 | Energy bars; Gels; powders; Protein bars; Sports drinks; Gummy chews; Recovery mix; Fruit snacks; Protein shakes | [143] |
| MIP/MWCNTs/GCE | EP | Chitosan | 2.0–968.2 μM | 487.3 | Berry fruits; Tap water | [154] | |
| DMMIPs | SI | Methacrylic acid | 18.4–184 μM | 19 | Blueberries; Grape peel | [155] | |
| Brilliant blue | MIPAPBA/CFP | EP | 3-Aminophenylboronic acid | 0.02–0.34 μM | 8.3 | Non-alcoholic beverages; Dry fruits; Frozen green peas | [151] |
| Chrysoidine | MGO/β-CD@AuNPs | EP | Pyrrole | 0.05–5.00 μM | 17 | Tap water | [150] |
| Indigo carmine | MIP@CPE | EP | o-PD | 5.10–0.13 μM | 42.9 | Candy; Ice Cream | [148] |
| Rhodamine B | MIP-SPCE-MSPE | SI | Acrylamide | 0.0125–0.25 µM | 3.01 | Chili powder; Tomato sauce | [149] |
| Sunset yellow | MIP/f-MWCNTs/GCE | EP | Acrylamide | 0.05–100 μM | 5.0 | Candy; Orange-flavored jelly powder; Peach juice powder; Candy-coated chocolate; Beverages | [156] |
| RMIECs | SGI | 3-aminopropyltriethoxysilane | 0.01–100 μM | 6.82 | Mirinda Orange; Fanta Orange | [157] | |
| SiO2@MIP-PDA/CPE | SI | Dopamine | 0.004–9.1 μM | 1.5 | Fruit drink (Fanta); Orange-flavored candy; Orange-flavored jelly powder; Cheese snack; Orange juice | [144] | |
| MWCNT@MIP-PDA | SI | Dopamine | 0.0022–4.64 μM | 1.4 | Jelly; Fruit drinks (Fanta and Mirinda); Chocolate; Instant juice powder; Ice cream; Candy | [145] | |
| MIP-rGO-IL/GCE | BP | 1-(α-methyl acrylate)-3-allylimidazolium bromide | 10 nM–1.4 μM and 1.4–16 μM | 4.0 | Fruit juice; Mirinda drink; Orange juice | [158] | |
| Au/RGO/GCE | EP | NR | 0.002–109.14 μM | 2 | Fanta; Xiang Cheng Duo; Mirinda | [159] | |
| Fe3O4@SiO2-NPs@MIP/Gr/GCE | SI | Methacrylic acid | 0.0085–30.0 μM | 5.5 | Candy; Orange-flavored jelly powder; Peach juice powder; Candy-coated chocolate; Soft drink | [160] | |
| MMIP/CPE | SI | Methylene succinic acid | 1.51–1510 μM | 86.242 | Water | [161] | |
| GO/AgNPs-MIPs/GCE | PP | Methacrylic-family monomer | 0.1–12 μM | 20 | Fanta drink; Mirinda drink; Orange juice; Mango juice | [162] | |
| MGO/β-CD/IL/AuNPs | PP | β-Cyclodextrin | 0.005–2 μM | 2 | Mirinda drink; Minute Maid; Carbonated beverages; Fruit juice; Candy | [147] | |
| EC-SPME | EP | NR | 1.25–3750 μM | 340 | Orange-flavored jelly powder; Peach juice powder; Beverage | [163] | |
| Tartrazine | MIPAPBA/CFP | EP | 3-Aminophenylboronic acid | 0.02–0.34 μM | 10 | Non-alcoholic beverages; Dry fruits; Frozen green peas | [151] |
| MIP/GCE | EP | Copolymer of m-dihydroxybenzene + o-PD | 0.1–50 μM | 30 | Carbonated beverages; Fruit juice; Candy | [146] | |
| MIP/Co3O4/GCE | EP | Acrylamide | 0.08–10 μM | 33 | Sports drinks | [164] | |
| MIPMet/CFP | EP | Amino-acid monomer | 0.6–160 μM | 27 | Saffron powder; Packaged fruit juices | [165] | |
| MIG-CuS@COOH-MWCNTs/GCE | SGI | NR | 0.03–125 μM | 5 | White vinegar; Vanilla ice cream | [166] | |
| CPE/ZnO/MIP-PArg | EP | L-Arginine | 0.008–0.112 μM and 0.25–5.0 μM | 2.7 | Soft drinks; Orange-flavored jelly powder | [167] | |
| MIP-MWNTs-IL@PtNPs/GCE | PP | Methacrylic-family monomer | 0.03–20 μM | 8 | Fanta; Mirinda; Orange powder | [168] | |
| GO–PtCo@MIPDA | SI | Dopamine | 0.003–0.180 μM and 0.180–3.950 μM | 1.1 | Orangeade; Yellow wine; Ice cream; Jelly; Instant juice powder; Candy; Cookies | [169] | |
| MIP-PmDB/PoPD-GCE | EP | Copolymer of m-dihydroxybenzene + o-PD | 0.005–1.1 μM | 3.5 | Soft drinks | [170] |
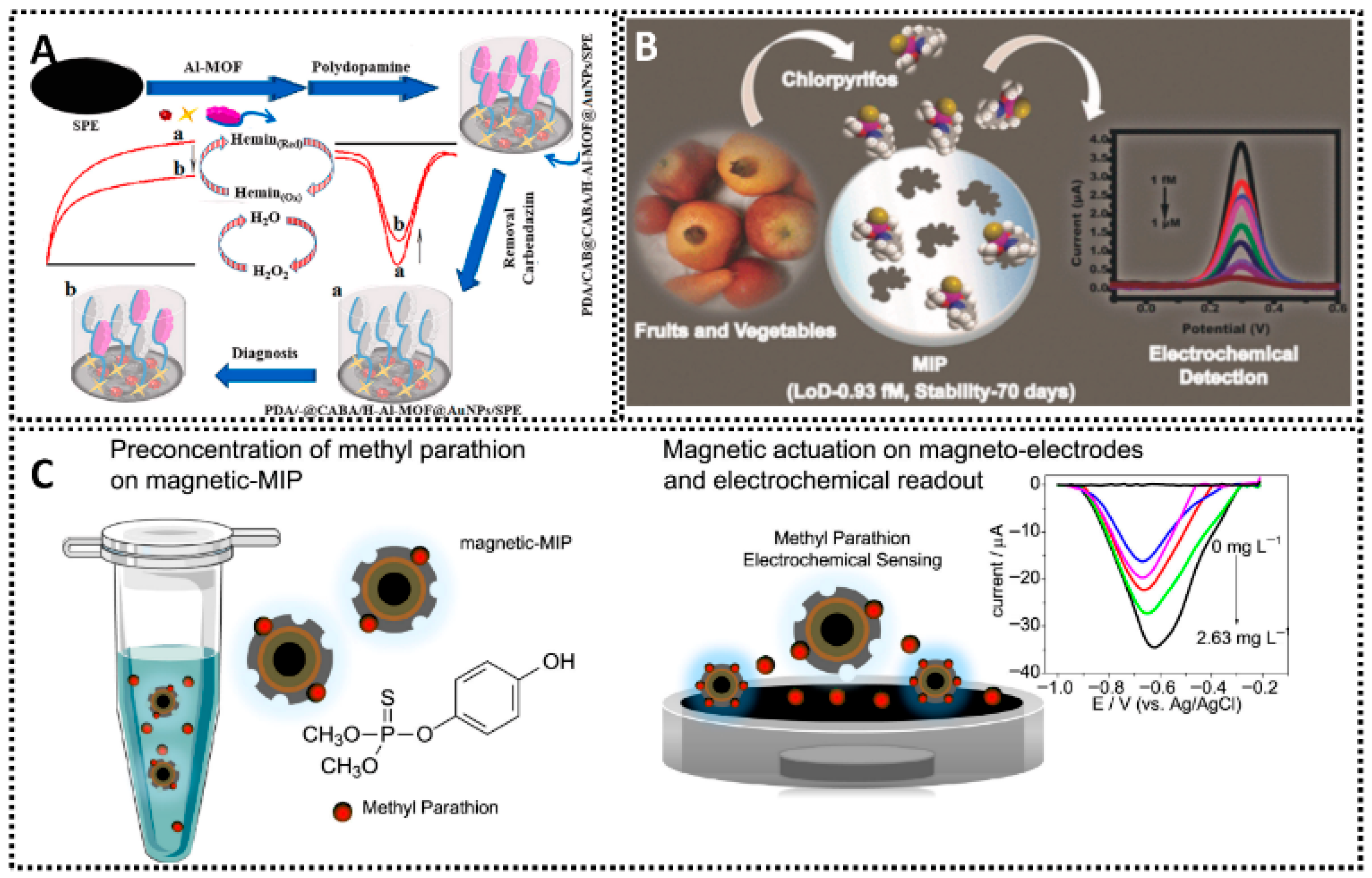
6. MIP Sensors for Traditional Contaminants
6.1. Pesticides
| Target Pesticides | Sensing Platform | MIP Fabrication Method | Functional Monomer(s) | Linear Range | LOD (nM) | Real Sample | Reference |
|---|---|---|---|---|---|---|---|
| Atrazine | PVC/MIP | BP | Methacrylic acid | 0.286–0.1879 μM | 4.99 | Drinking water; Surface water | [171] |
| MICP | EP | PEDOT-co-thiophene acetic acid | 0.1–15,000 μM | 100 | - | [172] | |
| MIP-SPPC | SI | Methacrylic acid | 0.5–50 μM | 400 | Tap water | [195] | |
| MIP/GFE | BP | Methacrylic acid | 5.0–140 μM | 1 | Drinking water | [174] | |
| MIP/GCE | PP | Methacrylic acid | 0.046–0.46 μM | 0.92 | Spring water | [173] | |
| Carbofuran | MIP | EP | Methacrylic acid | 0.05–10 μM | 16 | River water | [196] |
| MIP/AuNPs/GCE | EP | Methacrylic acid | 0.05–400 μM | 24 | Cowpea; Pakchoi | [175] | |
| MIECS | EP | 4-Hydroxythiophenol | 0.001–10.0 μM | 0.33 | Tangerine; Potato; Cowpea; Cornmeal | [176] | |
| MIP/LOC | BP | Methacrylic acid | 0.2–50 μM | 0.067 | Chinese cabbage; Chili; Lettuce; Tomato; Apple; Banana; Tangerine; Watermelon | [197] | |
| MIP-CNTs-Fe3O4@Au/CPE | EP | o-PD | 0.1–100 μM | 3.8 | Cabbage; Celery; Chili; Onion; Peppermint | [198] | |
| Carbaryl | IL@MMIPs | SI | Methacrylic acid | 0.22–66.5 μM | 13.3 | Apple; Rice | [199] |
| Carbendazim | Co3O4NPs@CNTs/GCE | EP | β-cyclodextrin and thionine | 0.010–2.0 μM and 2.0–10 μM | 2.5 | Tomatoes; Cucumbers; Pears; Grapes | [177] |
| MIP/Co,N-HC@CNTs/GCE | EP | 3,4-ethylenedioxythiophene | 0.005–10.0 μM | 1.67 | Tomato; Orange; Apple | [178] | |
| PDA/-@CABA/H-Al-MOF@AuNPs/SPE | EP | Dopamine | 0.0003–0.01 μM | 0.08 | Tap water; Apple juice; Tomato juice | [179] | |
| MIP/AuNP-rGO/GCE | EP | o-PD | 0.002–70 μM | 0.68 | Grape juice; Apple juice | [200] | |
| MIP/MWCNT | BP | 1-vinyl imidazole | 10.0–100.0 μM | 5.23 | River water; Industrial wastewater | [201] | |
| MIP/C-ZIF67@Ni/GCE | EP | Methacrylic acid | 0.4–1 μM | 0.134 | Soil; River water | [202] | |
| HKUST-1@MIP-GE | BP | Methacrylic acid | 0.01–50.00 μM | 2.0 | Apple juice; Cucumber juice; Tomato juice; Tangerine juice | [203] | |
| MIP/N, S–Mo2C/GCE | EP | o-PD | 0.001–8 μM | 0.67 | Grape; Apple; Tomato; Eggplant; Cucumber | [204] |
6.2. Heavy Metals Ions
| Target Heavy Metals | Sensing Platform | MIP Fabrication Method | Functional Monomer(s) | Linear Range | LOD (nM) | Real Sample | Reference |
|---|---|---|---|---|---|---|---|
| As3+ | MIP/NPG/ITO | EP | m-phenylenediamine | 0.02–9.0 μM | 0.0071 | Groundwater; Tap water | [216] |
| IIP/NPG/GE | EP | o-PD | 0.02–9.0 μM | 7.1 | Groundwater; Tap water | [217] | |
| As(III)-MIM@MOF/AuNPs/GCE | SI | Methacrylic acid | 0.01–30,000 μM | 0.3 | Tap water; River water | [205] | |
| Cd2+ | CPE-IIP | BP | Vinyl pyridine | 0.001–0.5 μM | 0.52 | Tap water; River water | [218] |
| CS/AuNPs/GR/GCE | EP | Chitosan | 0.1–0.9 μM | 0.162 | Tap water; River water; Milk | [206] | |
| IIP/rGO/GCE | EP | Pyrrole | 8.9–890 μM | 2.31 | Lake and river water | [219] | |
| IIP/ERGO/GCE | EP | o-PD | 0.0089–0.445 μM | 1.16 | Lake and river water | [220] | |
| IIP-Cd | EP | Chitosan | 0.01–0.1 μM | 3.51 | Tap water; Lake water | [221] | |
| IIP/GO@GCE | BP | Acrylamide + Methacrylic acid | 0.073–2400 μM | 0.07 | Human hair; Blood serum | [207] | |
| (Cd-IIP)/AuNPs/Au | SI | 3-mercaptopropyl trimethoxysilane | 8.89–444.5 μM | 1.96 | River water; Watsons water | [222] | |
| Cu2+ | CS/GO-IIP | SI | Chitosan | 0.5–100 μM | 150 | Tap water; River water | [223] |
| MIECS | EP | Acridine orange | 0.5–30 μM | 42.4 | Running water; Citric fruit juice; Rainwater; Beer | [208] | |
| CILE | PP | NR | 0.025–1.25 μM | 9.4 | River water; Mineral water; Tap water | [224] | |
| MMIP | SGI | Cysteine | 0.01 μM–1.0 mM | 10 | Spiked water; Serum | [225] | |
| IINPs/GCE | SI | NR | 0.06–1.9 μM | 20 | Tap water; River water; Seawater | [226] | |
| MIP-CP | BP | 4-vinyl pyridine | 0.07–1.0 μM and 1.0–100 μM | 23 | River water | [227] | |
| Cu(II)-IIP | BP | N-methacryloyl-L-histidine | 0.01–100,000 μM | 32 | Coin; Multivitamin; Tap water; River water; Lake water | [209] | |
| SPCE | EP | 4-aminophenylacetic acid | 0.01–1.2 μM | 1.71 | Drinking water; Tap water; Marine water | [228] | |
| Cr3+ | Pt/MWCNT-IIP | SI | Methacrylic acid | 19.23–96.15 μM | 51 | Wastewater | [210] |
| CPE-MWCNT/IIP | BP | NR | 1.0 to 100,000 μM | 590 | Sea water; River water; Soil | [229] | |
| Cr6+ | IIP-S/Au | EP | Chitosan | 0.001–10 μM | 0.64 | Tap water; River water | [211] |
| IP-NPs/CPE | PP | Meta-acrylate acid | 0.0001–0.1 μM and 0.1–1.0 μM | 0.03 | Ambient water | [230] | |
| Hg2+ | IIP–MWCNT–GCE | PP | Methacrylic acid | 0.01–700 μM | 5 | Wastewater; Groundwater | [231] |
| MIP/IDEs | BP | Vinylpyrrolidone | 50–450 μM | 4.5 | - | [232] | |
| RGO–IIP | SI | Methacrylic acid | 0.35–400 μM | 0.1 | Tap water; Aqueduct water; Wastewater; River water | [212] | |
| IIP-CPE | BP | Methacrylic acid | 0.004–1.3μM | 1.95 | Tap water; River water; Industrial wastewater; Metallurgy wastewater; Dental amalgam waste; Tuna fish; Shrimp; Human hair | [233] | |
| GQDTU-IIP | BP | Thiourea-derivatized graphene quantum dot | 0.06–23 μM | 23.5 | River water; Tap water | [234] | |
| IIP/g-C3N4/CPE | PP | Itaconic acid | 0.06–25.0 nM | 0.0018 | Tap water; Sea water | [213] | |
| IIP-CPE | BP | Methacryloyl-(l)-cysteine | 0.0025–5.0 μM | 0.52 | Tap water; River water | [235] | |
| IIP-CPE | SI | NR | 1.0–8000 μM | 0.2 | River water; Wastewater; Potato; Carrot; Lettuce | [236] | |
| Pb2+ | GCE-IIP-PAN/MWCNT | PP | Methacrylic acid | 2.41–57.97 μM | 0.77 | Tap water; Mineral water; Saline (physiological serum) | [214] |
| IIP/MWCNT-CPE | PP | Itaconic acid | 0.01–0.50 μM and 1–80 μM | 3.8 | Caspian Sea water; Tejen River water | [237] | |
| MWCNT-IIP/PE | SI | Acrylamide | 4.83–24.14 μM | 20 | Lake water; Mining effluent; Food sample; Cosmetics | [238] | |
| IIP-CP | PP | Methacrylic acid | 0.001–0.81 μM | 0.6 | Tap water; River water; Edible refined salt; Wastewater | [239] | |
| IIP-CPE | PP | 4-vinylpyridine | 0.1–1000 μM | 0.03 | Distilled water; Tap water; Caspian Sea water; Wastewater | [215] | |
| IIP-CPE | PP | 4-vinylpyridine | 0.001–0.75 μM | 0.013 | Flour; Rice; Tap water; Yudai River water | [240] | |
| SAMs/Au | SI | NR | 0.3–50 μM | 0.2 | River water | [241] | |
| MIP-CPE | SI | 2-methacryloyl-amido cysteine | 5–100 μM | 91.2 | Honey | [242] |
6.3. Mycotoxins
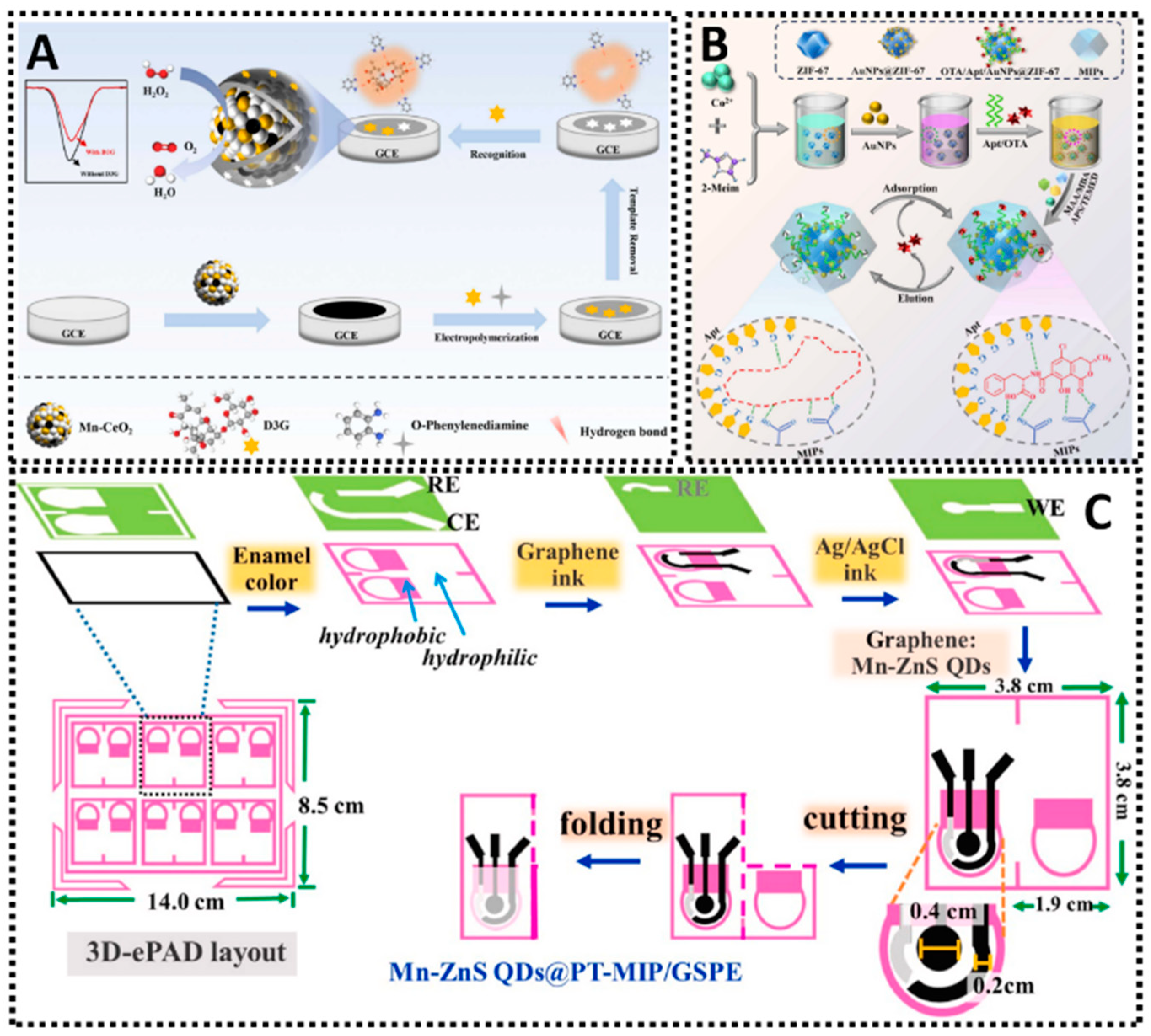
| Target Mycotoxin | Sensing Platform | MIP Fabrication Method | Functional Monomer(s) | Linear Range | LOD (nM) | Real Sample | Reference |
|---|---|---|---|---|---|---|---|
| Aflatoxins B1 | MIP-MOF | EP | p-aminothiophenol | 0.0000032–3.2 μM | 0.001 | Rice | [254] |
| PANI@MIP/CNC-CNT | EP | Aniline | 0–25 μM | 3 | Milk | [255] | |
| MAA-MIP@CPE | BP | Methacrylic acid | 0.052–0.2 μM | 5.9 | Corn; Wheat | [256] | |
| MIP-Apt/Cu2O NCs/GCE | EP | Aniline | 0.00005–0.04 μM | 0.012 | Milk | [243] | |
| MIP-A/ITO | EP | Aniline | 0.001–1.25 μM | 0.313 | Corn; Cereals; Fruits | [244] | |
| MIP/PC | EP | Pyrrole | 0.005–0.1 μM | 1.7 | - | [257] | |
| Citrinin | MIP/PtNPs/POM/rGO/GCE | EP | Pyrrole | 0.001–0.1 μM | 0.2 | Rye | [258] |
| MIP/BN-HPC/GCE | EP | Thionine | 0.003–40.7 μM | 0.1 | Red yeast rice; Rice; Wheat | [245] | |
| MIPs/Gr-MWCNTs-IL/GCE | EP | Methacrylic acid | 0.0015–0.018 μM | 0.61 | Takdaneh apple juice; Sunich apple juice; Sundis apple juice; Mihan pear nectar | [246] | |
| MIP/Nb2C-MWCNTs/GCE | EP | o-toluidine | 0.04–10.0 μM | 3.6 | Wine; Flour; Corn | [259] | |
| MIP/PdNPs/BZ/GQDs/GCE | EP | Pyrrole | 0.001–0.005 μM | 0.2 | Chicken egg | [260] | |
| Deoxynivalenol | MIP/SPGE | EP | o-PD | 0.01–10 μM | 6.2 | Corn flour; Wheat flour; Rice flour; Oat flour | [247] |
| Mn-CeO2/MIP | EP | o-PD | 0.034–170 μM | 0.01 | Barley; Wheat | [248] | |
| P-Arg-MIP/COOH-MWCNTs | EP | L-arginine | 0.1–70 μM | 70 | Wheat flour | [261] | |
| Fumonisins B1 | MIP-F/ITO | EP | 0.001–1.25 μM | 0.322 | Corn; Cereals; Fruits | [244] | |
| Ochratoxin A | MIP/MWCNT/GCE | EP | Pyrrole | 0.050–1.0 μM | 4.1 | Beer; White wine; Red wine | [262] |
| MIP-RECS | EP | Ionic liquid | 0.5–15 μM | 14 | Chinese liquor; Beer; Red wine | [263] | |
| MIP/AgNPs/POM/rGO/GCE | EP | Phenol | 0.05–1.5 μM | 0.016 | Grape juice; Wine | [249] | |
| MIP/MnCO3NS/CF/GCE | EP | Pyrrole | 0.01–1.0 μM | 2.0 | Apple juice | [264] | |
| MIP/Apt/AuNPs/ZIF-67 | SI | Methacrylic acid | 2.56–25.6μM | 0.853 | Wheat; Rice; Maize; Soybean | [250] | |
| MIP/ZIF-8 | SI | Allobarbital | 0–98.4 μM | 0.049 | Cereals | [265] | |
| Patulin | MIPs/Gr-MWCNTs-IL/GCE | EP | Methacrylic acid | 0.0005–0.013 μM | 0.08 | Takdaneh apple juice; Sunich apple juice; Sundis apple juice; Mihan pear nectar | [246] |
| MIP/Au@Cu-MOF/N-GQDs/GCE | EP | Aniline | 0.0045–315 μM | 0.0032 | Apple juice | [266] | |
| MIP/Fe3O4/GO/GCE | BP | Methacrylic acid | 0.001–250.0 nM | 0.333 | Apple juice; Commercial pear juice | [267] | |
| MIP-Au/CS-CDs/GCE | EP | o-PD | 0.001–1 μM | 0.757 | Apple juice | [268] | |
| MIP/Au@PANI/SeS2@Co MOF | EP | p-aminobenzoic acid | 0.001–0.1 μM | 0.66 | Apple juice | [269] | |
| MIP/PtPd-NPC/GCE | EP | 4-aminothiophenol | 0.049–49 μM | 0.037 | Apple juice; Grape juice | [270] | |
| Origami 3D-ePAD | SI | Methacrylic acid | 0.001–25 μM | 0.2 | Apple; Tomato; Grape; Orange; Chinese pear | [251] | |
| Zearalenone | MIP/g-C3N4NS/BSA@MnO2/GCE | EP | o-PD | 0.001–0.01 μM | 0.25 | Rice | [271] |
| MIP/SPGE | EP | o-PD | 0.0082–0.655 μM | 7.85 | Corn flakes | [272] | |
| SPE-CC-ZEN/MIP | EP | o-PD | 0.001–0.5 μM | 1 | Corn; Rice; Wheat | [273] | |
| MIP-RECS | EP | p-aminothiophenol | 0.05–13 μM | 12.7 | Human serum | [274] | |
| MIP/CuHCF/rGNR–rGO/GCE | EP | o-PD | 0.79–1586 μM | 0.29 | Corn meal | [252] | |
| MIP/AuNPs/rGNRs/GCE | EP | o-PD | 0.0033–1.65 μM | 1.07 | Maize flour | [275] | |
| MIP/rGO@rGNR/GCE | EP | o-PD | 0.0016–1.6 μM | 0.62 | Corn meal | [276] | |
| MIP/AuSPE | BP | Methacrylic acid | 0.00035 to 350 μM | 0.12 | Maize | [253] |
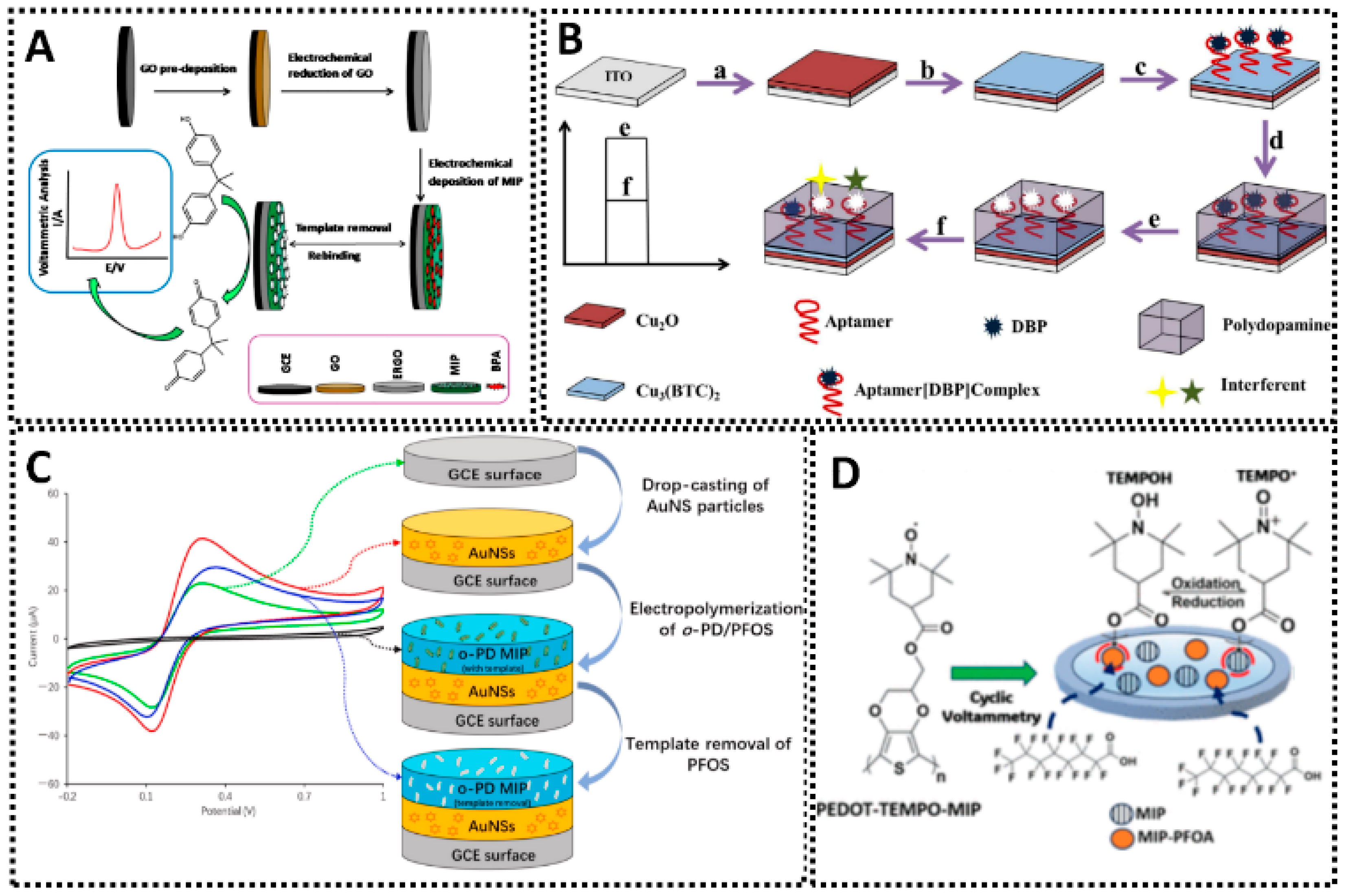
7. MIP Sensors for Emerging Contaminants and Toxicants
7.1. Endocrine Disruptors and Packaging Leachates
| Analyte | Sensing Platform | MIP Fabrication Method | Functional Monomer(s) | Linear Range | LOD (nM) | Real Sample | Reference |
|---|---|---|---|---|---|---|---|
| BPA | MIP-ERGO/GCE | EP | Pyrrole | 0.5–750 nM | 0.2 | Tap water; PC bottled water; PC bottled milk; Bovine milk | [60] |
| Au@MIP | EP | p-aminobenzoic acid | 0.5–100 μM | 52 | Tap water; Milk; Orange juice; Mineral water bottle | [285] | |
| MIP/MWCNT/CPE | BP | 2-hydroxyethyl methacrylate | 0.1–100 μM | 0.08 | Tap water; Stored water in a baby bottle; Household filtered drinking water; Soft drink | [277] | |
| MIPs/GNPs-MWCNTs | SI | 3-aminopropyltriethoxysilane | 0.113–8210 μM | 3.6 | Honey; Tap water; Grape juice | [286] | |
| MIP/GQDs/B-g-C3N4/GCE | EP | Pyrrole | 0.01–1.0 μM | 3.0 | Orange juice | [287] | |
| MIP/Fe3O4NPs/BDD | SI | 3-aminopropyltriethoxysilane | 5–73 μM | 380 | Tap water; Canned corn; Canned chickpeas; Tomato paste with basil; Milk; Bee | [288] | |
| CMOF-MIPIL | SI | 1-allyl-3-ethylimidazolium bromide | 0.005–5.0 μM | 4.0 | Lake water; River water; Plastic bottle; Fresh liquid milk | [289] | |
| MIP–GR/ABPE | SI | Chitosan | 0.008–1.0 μM and 1.0–20 μM | 6.0 | Plastic bottled drinking water; Canned beverages | [290] | |
| BMMIPs@MGCE | SI | NR | 0.8–8.0 μM | 133 | Tap water; Municipal sewage; Tea drink; Milk; Cabbage; Soil | [291] | |
| MMIP-CuMOFs/RGO/GCE | SI | 3-aminopropyltriethoxysilane | 0.5–500 μM | 0.18 | Milk | [292] | |
| MIP-NG-GCE | EP | o-PD | 8–6000 μM | 138 | PC water bottle | [293] | |
| PPY/-@p-63/AuNP/GCE | EP | Pyrrole | 0.5 fM–5 pM | 0.0000080 | Fresh milk; Milk powder; Tap water; Pretreated water in a baby glass | [294] | |
| GO/APTES–MIP | SI | 3-aminopropyl-triethoxysilane | 0.006–0.1 μM and 0.2–20 μM | 3 | Milk; Mineralized water | [295] | |
| MIP/SPE | BP | 4-vinylpyridine | 0.0047–0.008 μM | 3.2 | Bottled water; Water effluents | [296] | |
| SPCE@CB/MIP | SI | Acrylonitrile | 0.1–10 μM | 66 | Tap water | [297] | |
| SPCE/PEDOT/BMIMBr | EP | 3,4-ethylenedioxythiophene | 0.1–500 μM | 20 | Polycarbonate water bottles; Plastic juice bottles | [298] | |
| MIP-AuNPs-MCA-rGO/CILE | EP | NR | 0.004–18.0 μM | 1.1 | PVC food package; PVC bottle; PC baby bottle; PC water bottle | [299] | |
| MIP/GCE | SI | Acetylene black | 0.1–400 μM | 0.02 | Baby feeding bottles | [300] | |
| MIPs/AuNPs/GCE | EP | 4-aminothiophenol | 0.015–55 μM | 1.1 | Milk; PC nursing bottle; Soil; PVC food package; PVC drinking cup | [301] | |
| μPAD | SI | Bisphenol A | 0.0044–0.88 μM | 2.06 | Sea water; Canned food liquids; Polycarbonate plastic packaged water | [302] | |
| AuNPs/MIP-PGE | PP | N-methacryloyl-(L)-cysteine methyl ester | 1.5–7.5 μM | 161 | Drinking water | [303] | |
| BPS | MA-Tyr@MIP/GCE | SI | N-methacryloyl-L-tyrosine | 0.001–0.01 μM | 0.171 | Human serum; Plastic bottled water | [278] |
| DBP | SiO2@MIP/AuNPs/MWCNTs/GCE | SI | Methacrylic acid | 0.043–43.48 μM | 5.09 | Tap water; Chinese Baijiu | [279] |
| MIP PPY/PGE | EP | Pyrrole | 0.01–1.0 μM | 4.5 | - | [304] | |
| MIP-DBP-CTS/F-CC3/GCE | EP | Chitosan | 0–1.8 μM | 2.6 | Rice wine | [305] | |
| MIP-Aptamer[DBP]/Cu3(BTC)2/Cu2O/ITO | EP | 3-aminopropyltriethoxysilane | 0.0001–0.001 μM | 0.035 | Bottled water | [280] | |
| MMISPE | SI | Methacrylic acid | 0.043–4340 μM | 0.19 | Soybean milk; Milk | [306] | |
| MGO@AuNPs-MIPs/GCE | SI | Methacrylic acid | 2.5–5000 μM | 0.8 | Wine drinks; Ultrapure water | [307] | |
| PFOS | MIP/Au | EP | o-PD | 0.1–1500 nM | 0.04 | Distilled water; Tap water; Bottled mineral water | [308] |
| Au/MIP/SPE | EP | o-PD | 0.1–1.5 μM | 0.004 | Water samples | [309] | |
| MIP/AuNS/GCE | EP | o-PD | 0.025–5.0 μM and 5.0–500 μM | 0.015 | Tap water | [281] | |
| MIP/GCE | EP | o-PD | 0.05–0.5 nM and 1–500 nM | 0.05 | - | [50] | |
| MIP-ACET | EP | o-PD | 1.25 pM–1.25 nM | 0.0000003 | Tap water | [282] | |
| MOFMMIP/CPE | BP | Pyrrole | 0.002–165 μM | 0.7 | Tap water; River water; Well water | [310] | |
| CNW/MIP | EP | o-PD | 0.024–0.24 μM | 2.89 | Tap water; Wastewater; Landfill leachate | [311] | |
| PFOA | MIP Co/Fe@CNF | EP | Acrylamide | 0.01–90 μM | 1.073 | Wastewater | [283] |
| PEDOT-TEMPO-MIP | EP | 3,4-ethylenedioxythiophene-TEMPO | 0.001–1.0 μM | 0.28 | Surface water | [284] | |
| βCD-MB MIP | EP | β-cyclodextrin | 0.01–100 μM | 1.57 | Groundwater | [312] |
7.2. Adulterants
8. Challenges and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mazur, F.; Han, Z.; Tjandra, A.D.; Chandrawati, R. Digitalization of Colorimetric Sensor Technologies for Food Safety. Adv. Mater. 2024, 36, 2404274. [Google Scholar] [CrossRef]
- Li, T.; Shang, D.; Gao, S.; Wang, B.; Kong, H.; Yang, G.; Shu, W.; Xu, P.; Wei, G. Two-Dimensional Material-Based Electrochemical Sensors/Biosensors for Food Safety and Biomolecular Detection. Biosensors 2022, 12, 314. [Google Scholar] [CrossRef]
- Chang, K.; Zhao, Y.; Wang, M.; Xu, Z.; Zhu, L.; Xu, L.; Wang, Q. Advances in Metal-Organic Framework-Plasmonic Metal Composites Based SERS Platforms: Engineering Strategies in Chemical Sensing, Practical Applications and Future Perspectives in Food Safety. Chem. Eng. J. 2023, 459, 141539. [Google Scholar] [CrossRef]
- Lei, Y.; Cheng, J.; Dong, H.; Wang, P. Functional Porous Material-Based Sensors for Food Safety. Coord. Chem. Rev. 2024, 501, 215566. [Google Scholar] [CrossRef]
- Yu, Z.; Jung, D.; Park, S.; Hu, Y.; Huang, K.; Rasco, B.A.; Wang, S.; Ronholm, J.; Lu, X.; Chen, J. Smart Traceability for Food Safety. Crit. Rev. Food Sci. Nutr. 2022, 62, 905–916. [Google Scholar] [CrossRef]
- Han, Y.; Yang, W.; Luo, X.; He, X.; Zhao, H.; Tang, W.; Yue, T.; Li, Z. Carbon Dots Based Ratiometric Fluorescent Sensing Platform for Food Safety. Crit. Rev. Food Sci. Nutr. 2022, 62, 244–260. [Google Scholar] [CrossRef]
- Khan, A.; Ezati, P.; Kim, J.-T.; Rhim, J.-W. Biocompatible Carbon Quantum Dots for Intelligent Sensing in Food Safety Applications: Opportunities and Sustainability. Mater. Today Sustain. 2023, 21, 100306. [Google Scholar] [CrossRef]
- Yao, Z.; Coatsworth, P.; Shi, X.; Zhi, J.; Hu, L.; Yan, R.; Güder, F.; Yu, H.-D. Paper-Based Sensors for Diagnostics, Human Activity Monitoring, Food Safety and Environmental Detection. Sens. Diagn. 2022, 1, 312–342. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, W.; Zhao, Y.; Liu, H.; Sun, B. Single, Dual and Multi-Emission Carbon Dots Based Optosensing for Food Safety. Trends Food Sci. Technol. 2021, 111, 388–404. [Google Scholar] [CrossRef]
- Cheng, W.; Tang, X.; Zhang, Y.; Wu, D.; Yang, W. Applications of Metal-Organic Framework (MOF)-Based Sensors for Food Safety: Enhancing Mechanisms and Recent Advances. Trends Food Sci. Technol. 2021, 112, 268–282. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, W.; Zhu, C.; Hu, L. Recent Advances in Photoelectrochemical Sensing for Food Safety. Anal. Chem. 2024, 96, 8855–8867. [Google Scholar] [CrossRef]
- Fu, W.; Fu, X.; Li, Z.; Liu, Z.; Li, X. Advances in Smartphone Assisted Sensors for On-Site Detection of Food Safety Based on Fluorescence on-off-on Mode: A Review. Chem. Eng. J. 2024, 489, 151225. [Google Scholar] [CrossRef]
- Wei, Q.; Zhu, X.; Zhang, D.; Liu, H.; Sun, B. Innovative Nanomaterials Drive Dual and Multi-Mode Sensing Strategies in Food Safety. Trends Food Sci. Technol. 2024, 151, 104636. [Google Scholar] [CrossRef]
- Zhou, H.; Qiu, H.; Zhang, J.; Fang, Y.; Cui, B.; Shen, Y. Design, Preparation, and Application of Molecularly Imprinted Nanomaterials for Food Safety Analysis with Electrochemistry. Coord. Chem. Rev. 2024, 500, 215523. [Google Scholar] [CrossRef]
- Sun, Y.-H.; Yang, L.; Ji, X.-X.; Wang, Y.-Z.; Liu, Y.-L.; Fu, Y.; Ye, F. Efficient Detection of Flusilazole by an Electrochemical Sensor Derived from MOF MIL-53(Fe) for Food Safety. Food Chem. 2024, 440, 138244. [Google Scholar] [CrossRef]
- Mutlu, E.; Şenocak, A.; Demirbaş, E.; Koca, A.; Akyüz, D. Selective and Sensitive Molecularly Imprinted Polymer-Based Electrochemical Sensor for Detection of Deltamethrin. Food Chem. 2025, 463, 141121. [Google Scholar] [CrossRef]
- Parihar, A.; Sharma, P.; Choudhary, N.K.; Khan, R.; Mostafavi, E. Internet-of-Things-Integrated Molecularly Imprinted Polymer-Based Electrochemical Nano-Sensors for Pesticide Detection in the Environment and Food Products. Environ. Pollut. 2024, 351, 124029. [Google Scholar] [CrossRef]
- Yan, X.; Almajidi, Y.Q.; Uinarni, H.; Bokov, D.O.; Mansouri, S.; Fenjan, M.N.; Saxena, A.; Zabibah, R.S.; Hamzah, H.F.; Oudah, S.K. Bio(Sensors) Based on Molecularly Imprinted Polymers and Silica Materials Used for Food Safety and Biomedical Analysis: Recent Trends and Future Prospects. Talanta 2024, 276, 126292. [Google Scholar] [CrossRef]
- Geng, L.; Huang, J.; Fang, M.; Wang, H.; Liu, J.; Wang, G.; Hu, M.; Sun, J.; Guo, Y.; Sun, X. Recent Progress of the Research of Metal-Organic Frameworks-Molecularly Imprinted Polymers (MOFs-MIPs) in Food Safety Detection Field. Food Chem. 2024, 458, 140330. [Google Scholar] [CrossRef]
- Ayerdurai, V.; Cieplak, M.; Kutner, W. Molecularly Imprinted Polymer-Based Electrochemical Sensors for Food Contaminants Determination. TrAC Trends Anal. Chem. 2023, 158, 116830. [Google Scholar] [CrossRef]
- Garg, D.; Verma, N. Monika Molecularly Imprinted Polymer-Based Electrochemical Sensor for Rapid and Selective Detection of Hypoxanthine. Biosensors 2022, 12, 1157. [Google Scholar] [CrossRef]
- Liu, X.; Mao, L.-G.; Wang, Y.-L.; Shi, X.-B.; Liu, Y.; Yang, Y.; He, Z. Electrochemical Sensor Based on Imprinted Sol-Gel Polymer on Au NPs-MWCNTs-CS Modified Electrode for the Determination of Acrylamide. Food Anal. Methods 2016, 9, 114–121. [Google Scholar] [CrossRef]
- Ramajayam, K.; Ganesan, S.; Ramesh, P.; Beena, M.; Kokulnathan, T.; Palaniappan, A. Molecularly Imprinted Polymer-Based Biomimetic Systems for Sensing Environmental Contaminants, Biomarkers, and Bioimaging Applications. Biomimetics 2023, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Roushani, M.; Zalpour, N. Selective Detection of Asulam with In-Situ Dopamine Electropolymerization Based Electrochemical MIP Sensor. React. Funct. Polym. 2021, 169, 105069. [Google Scholar] [CrossRef]
- Bougrini, M.; Florea, A.; Cristea, C.; Sandulescu, R.; Vocanson, F.; Errachid, A.; Bouchikhi, B.; El Bari, N.; Jaffrezic-Renault, N. Development of a Novel Sensitive Molecularly Imprinted Polymer Sensor Based on Electropolymerization of a Microporous-Metal-Organic Framework for Tetracycline Detection in Honey. Food Control 2016, 59, 424–429. [Google Scholar] [CrossRef]
- Wu, Y.; Li, G.; Tian, Y.; Feng, J.; Xiao, J.; Liu, J.; Liu, X.; He, Q. Electropolymerization of Molecularly Imprinted Polypyrrole Film on Multiwalled Carbon Nanotube Surface for Highly Selective and Stable Determination of Carcinogenic Amaranth. J. Electroanal. Chem. 2021, 895, 115494. [Google Scholar] [CrossRef]
- Elhachem, M.; Cayot, P.; Abboud, M.; Louka, N.; Maroun, R.G.; Bou-Maroun, E. The Importance of Developing Electrochemical Sensors Based on Molecularly Imprinted Polymers for a Rapid Detection of Antioxidants. Antioxidants 2021, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Bounegru, A.V.; Apetrei, C. Voltammetric Sensors Based on Nanomaterials for Detection of Caffeic Acid in Food Supplements. Chemosensors 2020, 8, 41. [Google Scholar] [CrossRef]
- Baghizadeh, A.; Karimi-Maleh, H.; Khoshnama, Z.; Hassankhani, A.; Abbasghorbani, M. A Voltammetric Sensor for Simultaneous Determination of Vitamin C and Vitamin B6 in Food Samples Using ZrO2 Nanoparticle/Ionic Liquids Carbon Paste Electrode. Food Anal. Methods 2015, 8, 549–557. [Google Scholar] [CrossRef]
- Vinoth, S.; Govindasamy, M.; Wang, S.-F.; ALOthman, Z.A.; Alshgari, R.A.; Ouladsmane, M. Fabrication of Strontium Molybdate Incorporated with Graphitic Carbon Nitride Composite: High-Sensitive Amperometric Sensing Platform of Food Additive in Foodstuffs. Microchem. J. 2021, 167, 106307. [Google Scholar] [CrossRef]
- Shrestha, S.; Mascarenhas, R.J.; D’Souza, O.J.; Satpati, A.K.; Mekhalif, Z.; Dhason, A.; Martis, P. Amperometric Sensor Based on Multi-Walled Carbon Nanotube and Poly (Bromocresol Purple) Modified Carbon Paste Electrode for the Sensitive Determination of L-Tyrosine in Food and Biological Samples. J. Electroanal. Chem. 2016, 778, 32–40. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Hu, C.; Wu, H.; Yang, Y.; Huang, C.; Jia, N. Highly Sensitive Electrochemical Impedance Spectroscopy Immunosensor for the Detection of AFB1 in Olive Oil. Food Chem. 2015, 176, 22–26. [Google Scholar] [CrossRef]
- Chiriacò, M.S.; Parlangeli, I.; Sirsi, F.; Poltronieri, P.; Primiceri, E. Impedance Sensing Platform for Detection of the Food Pathogen Listeria Monocytogenes. Electronics 2018, 7, 347. [Google Scholar] [CrossRef]
- El Badry Mohamed, M.; Frag, E.Y.; El Brawy, M.H. Rapid Potentiometric Sensor for Determination of Cu(II) Ions in Food Samples. Microchem. J. 2021, 164, 106065. [Google Scholar] [CrossRef]
- Draz, M.E.; Darwish, H.W.; Darwish, I.A.; Saad, A.S. Solid-State Potentiometric Sensor for the Rapid Assay of the Biologically Active Biogenic Amine (Tyramine) as a Marker of Food Spoilage. Food Chem. 2021, 346, 128911. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H.; Zhang, M.; Zeng, B.; Zhao, F. Molecularly Imprinted Photoelectrochemical Sensor for Aflatoxin B1 Detection Based on Organic/Inorganic Hybrid Nanorod Arrays. Sens. Actuators B Chem. 2021, 339, 129900. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, L.; Mei, X.; Liu, J.; Yao, Z.; Li, Y. Dual-Mode Sensing Platform for Electrochemiluminescence and Colorimetry Detection Based on a Closed Bipolar Electrode. Anal. Chem. 2021, 93, 12367–12373. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, W.; Yang, Q.; Wu, W.; Hou, X. An Electrochemical/Colorimetric Dual-Mode Sensor for the Detection of AFB1 in Agricultural Products Based on CoFe2O4@Au Nanoparticles and Smartphone Imaging. Food Chem. 2025, 474, 143201. [Google Scholar] [CrossRef]
- Amatatongchai, M.; Sroysee, W.; Sodkrathok, P.; Kesangam, N.; Chairam, S.; Jarujamrus, P. Novel Three-Dimensional Molecularly Imprinted Polymer-Coated Carbon Nanotubes (3D-CNTs@MIP) for Selective Detection of Profenofos in Food. Anal. Chim. Acta 2019, 1076, 64–72. [Google Scholar] [CrossRef]
- Elfadil, D.; Silveri, F.; Palmieri, S.; Della Pelle, F.; Sergi, M.; Del Carlo, M.; Amine, A.; Compagnone, D. Liquid-Phase Exfoliated 2D Graphene Nanoflakes Electrochemical Sensor Coupled to Molecularly Imprinted Polymers for the Determination of Citrinin in Food. Talanta 2023, 253, 124010. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Z.; Zhang, A.; Peng, Y.; Zhou, H.; Guo, Y.; Lu, D.; Xie, L.; Shi, S. Gold Nanoparticle-Mediated Molecularly Imprinted Electrochemical Sensor MIP/AuNPs/GCE for Highly Sensitive and Selective Detection of Neutral Phosmet Residues in Fruits and Vegetables. Microchem. J. 2024, 201, 110728. [Google Scholar] [CrossRef]
- Wu, J.; Xia, Y.; Wang, T.; Zhang, Y.; Li, G. Efficient Voltammetric Platform Combining a Molecularly Imprinted Polymer and Silver-Nanoparticle-Decorated Black Phosphorus Nanosheets for Selective Determination of Gatifloxacin. Food Chem. X 2025, 25, 102094. [Google Scholar] [CrossRef]
- Zeb, S.; Wong, A.; Khan, S.; Hussain, S.; Sotomayor, M.D.P.T. Using Magnetic Nanoparticles/MIP-Based Electrochemical Sensor for Quantification of Tetracycline in Milk Samples. J. Electroanal. Chem. 2021, 900, 115713. [Google Scholar] [CrossRef]
- Janiak, D.S.; Kofinas, P. Molecular Imprinting of Peptides and Proteins in Aqueous Media. Anal. Bioanal. Chem. 2007, 389, 399–404. [Google Scholar] [CrossRef]
- Zare, E.N.; Fallah, Z.; Le, V.T.; Doan, V.-D.; Mudhoo, A.; Joo, S.-W.; Vasseghian, Y.; Tajbakhsh, M.; Moradi, O.; Sillanpää, M. Remediation of Pharmaceuticals from Contaminated Water by Molecularly Imprinted Polymers: A Review. Environ. Chem. Lett. 2022, 20, 2629–2664. [Google Scholar] [CrossRef] [PubMed]
- Sergeyeva, T.; Piletska, O.; Piletsky, S. Rationally Designed Molecularly Imprinted Polymer Membranes as Antibody and Enzyme Mimics in Analytical Biotechnology. BBA Adv. 2023, 3, 100070. [Google Scholar] [CrossRef]
- Wu, T.; Li, Y.; Zhang, Z.; Zhang, D.; Dragoi, E.; Karimi-Maleh, H. 3D Print-Based Polypyrrole /TiVCTx/UiO-66 Composites for Effective Adsorption of Combined Pollutants in Water Media. J. Nanostruct. Chem. 2025, 15, 152503. [Google Scholar] [CrossRef]
- Silvestri, D.; Barbani, N.; Cristallini, C.; Giusti, P.; Ciardelli, G. Molecularly Imprinted Membranes for an Improved Recognition of Biomolecules in Aqueous Medium. J. Membr. Sci. 2006, 282, 284–295. [Google Scholar] [CrossRef]
- Malik, M.I.; Shaikh, H.; Mustafa, G.; Bhanger, M.I. Recent Applications of Molecularly Imprinted Polymers in Analytical Chemistry. Sep. Purif. Rev. 2019, 48, 179–219. [Google Scholar] [CrossRef]
- Kazemi, R.; Potts, E.I.; Dick, J.E. Quantifying Interferent Effects on Molecularly Imprinted Polymer Sensors for Per- and Polyfluoroalkyl Substances (PFAS). Anal. Chem. 2020, 92, 10597–10605. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Ashraf, N.; Zohuri, G.H. A Smart Electrochemical Sensor Based upon Hydrophilic Core–Shell Molecularly Imprinted Polymer for Determination of L-Tryptophan. Microchem. J. 2023, 185, 108260. [Google Scholar] [CrossRef]
- Surapong, N.; Santaladchaiyakit, Y.; Burakham, R. A Water-Compatible Magnetic Dual-Template Molecularly Imprinted Polymer Fabricated from a Ternary Biobased Deep Eutectic Solvent for the Selective Enrichment of Organophosphorus in Fruits and Vegetables. Food Chem. 2022, 384, 132475. [Google Scholar] [CrossRef]
- Puoci, F.; Iemma, F.; Picci, N. Stimuli-Responsive Molecularly Imprinted Polymers for Drug Delivery: A Review. Curr. Drug Deliv. 2008, 5, 85–96. [Google Scholar] [CrossRef]
- Ge, Y.; Butler, B.; Mirza, F.; Habib-Ullah, S.; Fei, D. Smart Molecularly Imprinted Polymers: Recent Developments and Applications. Macromol. Rapid Commun. 2013, 34, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Go, Y.; Behmaneshfar, A.; Sadrnia, A.; Zhang, Z.; Dang, R. Developing a New Model for Optimizing Response in Design of Experiments Considering Consumable Factors: Removal of Pollutant from Wastewater with Fe3O4/Graphene Nano-Sorbent. J. Nanostruct. Chem. 2025, 15, 152507. [Google Scholar] [CrossRef]
- Xie, T.; Zhang, M.; Chen, P.; Zhao, H.; Yang, X.; Yao, L.; Zhang, H.; Dong, A.; Wang, J.; Wang, Z. A Facile Molecularly Imprinted Electrochemical Sensor Based on Graphene: Application to the Selective Determination of Thiamethoxam in Grain. RSC Adv. 2017, 7, 38884–38894. [Google Scholar] [CrossRef]
- Mohammed Albayatı, S.H.; Üstündağ, Z.; Soylu, P. A Novel Molecularly Imprinted Electrochemical Sensor for the Ultrasensitive Detection of Tert-Butylhydroquinone in Edible Oils. Anal. Biochem. 2023, 682, 115348. [Google Scholar] [CrossRef] [PubMed]
- Gholivand, M.B.; Torkashvand, M.; Malekzadeh, G. Fabrication of an Electrochemical Sensor Based on Computationally Designed Molecularly Imprinted Polymers for Determination of Cyanazine in Food Samples. Anal. Chim. Acta 2012, 713, 36–44. [Google Scholar] [CrossRef]
- Niu, Z.; Shi, Y.; Liu, S.; Lv, Y.; Wang, S. DFT-Assisted Design of a Electrochemical Sensor Based on MIP/CNT/MoS2-CoNi for the Detection of Sulfamethazine in Meat. J. Food Compos. Anal. 2025, 140, 107261. [Google Scholar] [CrossRef]
- Karthika, P.; Shanmuganathan, S.; Viswanathan, S.; Delerue-Matos, C. Molecularly Imprinted Polymer-Based Electrochemical Sensor for the Determination of Endocrine Disruptor Bisphenol-A in Bovine Milk. Food Chem. 2021, 363, 130287. [Google Scholar] [CrossRef]
- Liang, A.; Lv, T.; Pan, B.; Zhu, Z.; Haotian, R.; Xie, Y.; Sun, L.; Zhang, J.; Luo, A. Dynamic Simulation and Experimental Studies of Molecularly Imprinted Label-Free Sensor for Determination of Milk Quality Marker. Food Chem. 2024, 449, 139238. [Google Scholar] [CrossRef]
- Yarahmadi, B.; Hashemianzadeh, S.M.; Milani Hosseini, S.M.-R. Machine-Learning-Based Predictions of Imprinting Quality Using Ensemble and Non-Linear Regression Algorithms. Sci. Rep. 2023, 13, 12111. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Tang, Q.; Guo, Y.; Zhang, Z.; Zhang, W.; Zou, X.; Sun, Z. Molecularly Imprinted Electrochemical Sensor for Ethyl Carbamate Detection in Baijiu Based on “on-off” Nanozyme-Catalyzing Process. Food Chem. 2024, 453, 139626. [Google Scholar] [CrossRef]
- Yayla, S.; Hurkul, M.M.; Cetinkaya, A.; Uzun, L.; Ozkan, S.A. Selective Apigenin Assay in Plant Extracts and Herbal Supplement with Molecularly Imprinted Polymer-Based Electrochemical Sensor. Talanta 2025, 281, 126895. [Google Scholar] [CrossRef]
- Alberti, G.; Zanoni, C.; Magnaghi, L.R.; Biesuz, R. Ascorbic Acid Sensing by Molecularly Imprinted Electrosynthesized Polymer (e-MIP) on Screen-Printed Electrodes. Chemosensors 2023, 11, 348. [Google Scholar] [CrossRef]
- Tonelli, D.; Ballarin, B.; Guadagnini, L.; Mignani, A.; Scavetta, E. A Novel Potentiometric Sensor for L-Ascorbic Acid Based on Molecularly Imprinted Polypyrrole. Electrochim. Acta 2011, 56, 7149–7154. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Chen, H.; Chen, Y.; Huang, G. Disposable Electrochemical Ascorbic Acid Sensor Based on Molecularly Imprinted Poly(o-Phenylenediamine)-Modified Dual Channel Screen-Printed Electrode for Orange Juice Analysis. Food Anal. Methods 2014, 7, 1557–1563. [Google Scholar] [CrossRef]
- Prasad, B.B.; Kumar, D.; Madhuri, R.; Tiwari, M.P. Ascorbic Acid Imprinted Polymer-Modified Graphite Electrode: A Diagnostic Sensor for Hypovitaminosis C at Ultra Trace Ascorbic Acid Level. Sens. Actuators B Chem. 2011, 160, 418–427. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Xu, J.; Wen, Y. Electropolymerized Molecularly Imprinted Polypyrrole Decorated with Black Phosphorene Quantum Dots onto Poly(3,4-Ethylenedioxythiophene) Nanorods and Its Voltammetric Sensing of Vitamin C. J. Electroanal. Chem. 2018, 814, 153–160. [Google Scholar] [CrossRef]
- Albayatı, S.H.M.; Soylu, P. Butylated Hydroxyanisole Nanomolar Detection Using a Molecularly Imprinted Electrochemical Sensor in Food Samples. J. Appl. Electrochem. 2024, 54, 879–891. [Google Scholar] [CrossRef]
- Motia, S.; Bouchikhi, B.; El Bari, N. An Electrochemical Molecularly Imprinted Sensor Based on Chitosan Capped with Gold Nanoparticles and Its Application for Highly Sensitive Butylated Hydroxyanisole Analysis in Foodstuff Products. Talanta 2021, 223, 121689. [Google Scholar] [CrossRef]
- Yue, X.; Luo, X.; Zhou, Z.; Bai, Y. Selective Electrochemical Determination of Tertiary Butylhydroquinone in Edible Oils Based on an In-Situ Assembly Molecularly Imprinted Polymer Sensor. Food Chem. 2019, 289, 84–94. [Google Scholar] [CrossRef]
- Han, S.; Ding, Y.; Teng, F.; Yao, A.; Leng, Q. Molecularly Imprinted Electrochemical Sensor Based on 3D-Flower-like MoS2 Decorated with Silver Nanoparticles for Highly Selective Detection of Butylated Hydroxyanisole. Food Chem. 2022, 387, 132899. [Google Scholar] [CrossRef]
- dos Santos Moretti, E.; de Oliveira, F.M.; Scheel, G.L.; DalĺAntônia, L.H.; Borsato, D.; Kubota, L.T.; Segatelli, M.G.; Tarley, C.R.T. Synthesis of Surface Molecularly Imprinted Poly(Methacrylic Acid-Hemin) on Carbon Nanotubes for the Voltammetric Simultaneous Determination of Antioxidants from Lipid Matrices and Biodiesel. Electrochim. Acta 2016, 212, 322–332. [Google Scholar] [CrossRef]
- Cui, M.; Liu, S.; Lian, W.; Li, J.; Xu, W.; Huang, J. A Molecularly-Imprinted Electrochemical Sensor Based on a Graphene–Prussian Blue Composite-Modified Glassy Carbon Electrode for the Detection of Butylated Hydroxyanisole in Foodstuffs. Analyst 2013, 138, 5949–5955. [Google Scholar] [CrossRef] [PubMed]
- Elhachem, M.; Bou-Maroun, E.; Abboud, M.; Cayot, P.; Maroun, R.G. Optimization of a Molecularly Imprinted Polymer Synthesis for a Rapid Detection of Caffeic Acid in Wine. Foods 2023, 12, 1660. [Google Scholar] [CrossRef]
- Leite, F.R.F.; Santos, W.d.J.R.; Kubota, L.T. Selective Determination of Caffeic Acid in Wines with Electrochemical Sensor Based on Molecularly Imprinted Siloxanes. Sens. Actuators B Chem. 2014, 193, 238–246. [Google Scholar] [CrossRef]
- Elhachem, M.; Bou-Maroun, E.; Abboud, M.; Maroun, R.G.; Cayot, P. Combination of Screen-Printed Carbon Electrode and Molecularly Imprinted Polymers for the Selective Determination of Phenolic Compounds in Wine. Antioxidants 2022, 11, 2036. [Google Scholar] [CrossRef] [PubMed]
- Nandy Chatterjee, T.; Das, D.; Banerjee Roy, R.; Tudu, B.; Sabhapondit, S.; Tamuly, P.; Pramanik, P.; Bandyopadhyay, R. Molecular Imprinted Polymer Based Electrode for Sensing Catechin (+C) in Green Tea. IEEE Sens. J. 2018, 18, 2236–2244. [Google Scholar] [CrossRef]
- Das, D.; Nag, S.; Adaval, A.; Hazarika, A.K.; Sabhapondit, S.; Bhattacharyya, A.R.; Tudu, B.; Bandopadhyay, R.; Banerjee Roy, R. Amine Functionalized MWCNTs Modified MIP-Based Electrode for Detection of Epicatechin in Tea. IEEE Sens. J. 2022, 22, 10323–10330. [Google Scholar] [CrossRef]
- Qiu, Z.; Fan, D.; Xue, X.; Guo, S.; Lin, Y.; Chen, Y.; Tang, D. Molecularly Imprinted Polymer Functionalized Bi2S3/Ti3C2TX MXene Nanocomposites for Photoelectrochemical/Electrochemical Dual-Mode Sensing of Chlorogenic Acid. Chemosensors 2022, 10, 252. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Miguel, E.M.; Silva, J.d.S.; Silva, C.B.d.; Goulart, M.O.F.; Kubota, L.T.; Gonzaga, F.B.; Santos, W.J.R.; Lima, P.R. Application of a Nanostructured Platform and Imprinted Sol-Gel Film for Determination of Chlorogenic Acid in Food Samples. Talanta 2016, 156–157, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Santos, W.d.J.R.; Santhiago, M.; Yoshida, I.V.P.; Kubota, L.T. Novel Electrochemical Sensor for the Selective Recognition of Chlorogenic Acid. Anal. Chim. Acta 2011, 695, 44–50. [Google Scholar] [CrossRef]
- Koirala, K.; Sevilla, F.B.; Santos, J.H. Biomimetic Potentiometric Sensor for Chlorogenic Acid Based on Electrosynthesized Polypyrrole. Sens. Actuators B Chem. 2016, 222, 391–396. [Google Scholar] [CrossRef]
- Yan, L.; Yin, Y.; Lv, P.; Zhang, Z.; Wang, J.; Long, F. Synthesis and Application of Novel 3D Magnetic Chlorogenic Acid Imprinted Polymers Based on a Graphene–Carbon Nanotube Composite. J. Agric. Food Chem. 2016, 64, 3091–3100. [Google Scholar] [CrossRef]
- Ahmed, A.H.M.T.; Naskar, H.; Banerjee, S.; Ghatak, B.; Das, N.; Tudu, B.; Bandyopadhyay, R. Electrochemical Sensor Based on Molecularly Imprinted Polymer Embedded Graphite Electrode for Detecting Curcumin. Sens. Actuators Phys. 2022, 344, 113748. [Google Scholar] [CrossRef]
- Mohammadinejad, A.; Abouzari-Lotf, E.; Aleyaghoob, G.; Rezayi, M.; Kazemi Oskuee, R. Application of a Transition Metal Oxide/Carbon-Based Nanocomposite for Designing a Molecularly Imprinted Poly (l-Cysteine) Electrochemical Sensor for Curcumin. Food Chem. 2022, 386, 132845. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhai, H.Y.; Pan, Y.F.; Li, K. A Simple and Sensitive Sensor Based on a Molecularly Imprinted Polymer-Modified Carbon Paste Electrode for the Determination of Curcumin in Foods. RSC Adv. 2017, 7, 22913–22918. [Google Scholar] [CrossRef]
- Pedroso, M.M.; Foguel, M.V.; Silva, D.H.S.; Sotomayor, M.d.P.T.; Yamanaka, H. Electrochemical Sensor for Dodecyl Gallate Determination Based on Electropolymerized Molecularly Imprinted Polymer. Sens. Actuators B Chem. 2017, 253, 180–186. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, L.; Hu, Y.; Peng, X.; Du, J. A Novel Electrochemical Sensor Based on a Molecularly Imprinted Polymer for the Determination of Epigallocatechin Gallate. Food Chem. 2017, 221, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-C.; Chang, C.-C.; Chiu, H.-S.; Jiang, S.-F.; Lee, M.-H.; Chung, C.-L.; Liu, B.-D.; Huang, C.-Y.; Lin, H.-Y. A Portable Electrochemical Sensor for Caffeine and (-)Epigallocatechin Gallate Based on Molecularly Imprinted Poly(Ethylene-Co-Vinyl Alcohol) Recognition Element. J. Nanosci. Nanotechnol. 2011, 11, 10633–10638. [Google Scholar] [CrossRef]
- Buffon, E.; Stradiotto, N.R. A Molecularly Imprinted Polymer on Reduced Graphene Oxide-Gold Nanoparticles Modified Screen-Printed Electrode for Selective Determination of Ferulic Acid in Orange Peels. Microchem. J. 2021, 167, 106339. [Google Scholar] [CrossRef]
- Shojaei, S.; Nasirizadeh, N.; Entezam, M.; Koosha, M.; Azimzadeh, M. An Electrochemical Nanosensor Based on Molecularly Imprinted Polymer (MIP) for Detection of Gallic Acid in Fruit Juices. Food Anal. Methods 2016, 9, 2721–2731. [Google Scholar] [CrossRef]
- Zanoni, C.; Dallù, L.V.; Costa, C.; Cutaia, A.; Alberti, G. A Screen-Printed Voltammetric Sensor Modified with Electropolymerized Molecularly Imprinted Polymer (eMIP) to Determine Gallic Acid in Non-Alcoholic and Alcoholic Beverages. Polymers 2024, 16, 1076. [Google Scholar] [CrossRef]
- Jara-Ulloa, P.; Salgado-Figueroa, P.; Moscoso, R.; Squella, J.A. Polypyrrole Molecularly Imprinted Modified Glassy Carbon Electrode for the Recognition of Gallic Acid. J. Electrochem. Soc. 2013, 160, H243. [Google Scholar] [CrossRef]
- Qin, F.; Hu, T.; You, L.; Chen, W.; Jia, D.; Hu, N.; Qi, W. Electrochemical Detection of Gallic Acid in Green Tea Using Molecularly Imprinted Polymers on TiO2@CNTs Nanocomposite Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2022, 17, 220426. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, Q.; Chen, T.; Wu, W.; Tang, X.; Wang, G.; Feng, J.; Zhang, W. Facile Potentiometric Sensing of Gallic Acid in Edible Plants Based on Molecularly Imprinted Polymer. J. Food Sci. 2020, 85, 2622–2628. [Google Scholar] [CrossRef]
- Das, D.; Biswas, D.; Hazarika, A.K.; Sabhapondit, S.; Roy, R.B.; Tudu, B.; Bandyopadhyay, R. CuO Nanoparticles Decorated MIP-Based Electrode for Sensitive Determination of Gallic Acid in Green Tea. IEEE Sens. J. 2021, 21, 5687–5694. [Google Scholar] [CrossRef]
- Ye, C.; Chen, X.; Xu, J.; Xi, H.; Wu, T.; Deng, D.; Zhang, J.; Huang, G. Highly Sensitive Detection to Gallic Acid by Polypyrrole-Based MIES Supported by MOFs-Co2+@Fe3O4. J. Electroanal. Chem. 2020, 859, 113839. [Google Scholar] [CrossRef]
- You, Z.; Fu, Y.; Xiao, A.; Liu, L.; Huang, S. Magnetic Molecularly Imprinting Polymers and Reduced Graphene Oxide Modified Electrochemical Sensor for the Selective and Sensitive Determination of Luteolin in Natural Extract. Arab. J. Chem. 2021, 14, 102990. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, B.; Yang, L.; Zhao, F.; Zeng, B. Electrochemical Determination of Luteolin Using Molecularly Imprinted Poly-Carbazole on MoS2/Graphene-Carbon Nanotubes Nanocomposite Modified Electrode. Electrochim. Acta 2017, 258, 1413–1420. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Duan, X.; Zhu, Y.; Xue, T.; Rao, L.; Wen, Y.; Tian, Q.; Cai, Y.; Xu, Q.; et al. A Novel Nanozyme Comprised of Electro-Synthesized Molecularly Imprinted Conducting PEDOT Nanocomposite with Graphene-like MoS2 for Electrochemical Sensing of Luteolin. Microchem. J. 2021, 168, 106418. [Google Scholar] [CrossRef]
- Buffon, E.; Stradiotto, N.R. Disposable p-Coumaric Acid Sensor Containing Reduced Graphene Oxide, Nickel Nanoparticles and Biodegradable Molecularly Imprinted Polymer for Fruit Peel Analysis. J. Food Compos. Anal. 2023, 118, 105186. [Google Scholar] [CrossRef]
- Albayatı, S.H.M.; Soylu, P. A Simple Molecularly Imprinted Electrochemical Sensor for Determination of Propyl Gallate in Food Samples. Anal. Biochem. 2024, 688, 115477. [Google Scholar] [CrossRef]
- Hurkul, M.M.; Cetinkaya, A.; Yayla, S.; Kaya, S.I.; Budak, F.; Tok, K.C.; Gumustas, M.; Uzun, L.; Ozkan, S.A. Highly Selective and Sensitive Molecularly Imprinted Sensors for the Electrochemical Assay of Quercetin in Methanol Extracts of Rubus sanctus and Fragaria vesca. Talanta 2024, 273, 125883. [Google Scholar] [CrossRef]
- Yang, L.; Xu, B.; Ye, H.; Zhao, F.; Zeng, B. A Novel Quercetin Electrochemical Sensor Based on Molecularly Imprinted Poly(Para-Aminobenzoic Acid) on 3D Pd Nanoparticles-Porous Graphene-Carbon Nanotubes Composite. Sens. Actuators B Chem. 2017, 251, 601–608. [Google Scholar] [CrossRef]
- Yao, Z.; Yang, X.; Liu, X.; Yang, Y.; Hu, Y.; Zhao, Z. Electrochemical Quercetin Sensor Based on a Nanocomposite Consisting of Magnetized Reduced Graphene Oxide, Silver Nanoparticles and a Molecularly Imprinted Polymer on a Screen-Printed Electrode. Microchim. Acta 2017, 185, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zong, L.; Geng, G.; Li, Y.; Zhang, Y. Enhancing Determination of Quercetin in Honey Samples through Electrochemical Sensors Based on Highly Porous Polypyrrole Coupled with Nanohybrid Modified GCE. Sens. Actuators B Chem. 2018, 257, 1099–1109. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Q.; Yang, J.; Chen, S.; Hu, F.; Hu, Y.; Lin, H. Synergistic Effects of Layer-by-Layer Films for Highly Selective and Sensitive Electrochemical Detection of Trans-Resveratrol. Food Chem. 2021, 338, 127851. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yang, J.; Li, S.; Qin, Y.; Mo, Q.; Chen, L.; Li, X. Highly Sensitive Molecular Imprinted Voltammetric Sensor for Resveratrol Assay in Wine via Polyaniline/Gold Nanoparticles Signal Enhancement and Polyacrylamide Recognition. J. Electroanal. Chem. 2021, 895, 115455. [Google Scholar] [CrossRef]
- Mugo, S.M.; Edmunds, B.J.; Berg, D.J.; Gill, N.K. An Integrated Carbon Entrapped Molecularly Imprinted Polymer (MIP) Electrode for Voltammetric Detection of Resveratrol in Wine. Anal. Methods 2015, 7, 9092–9099. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Zhang, J.; Li, Y.; Zhang, Y.; Li, Y.; Ye, B.-C. Novel Electrochemical Sensing Platform Based on Integration of Molecularly Imprinted Polymer with Au@Ag Hollow Nanoshell for Determination of Resveratrol. Talanta 2019, 196, 479–485. [Google Scholar] [CrossRef]
- Hurkul, M.M.; Yayla, S.; Cetinkaya, A.; Kaya, S.I.; Uzun, L.; Ozkan, S.A. A Novel Electrochemical Sensor Based on a Molecularly Imprinted Polymer for Highly Selective and Sensitive Determination of Rutin from Herbal Supplements and Plant Extracts. Anal. Methods 2024, 16, 1480–1488. [Google Scholar] [CrossRef]
- Yang, L.; Yang, J.; Xu, B.; Zhao, F.; Zeng, B. Facile Preparation of Molecularly Imprinted Polypyrrole-Graphene-Multiwalled Carbon Nanotubes Composite Film Modified Electrode for Rutin Sensing. Talanta 2016, 161, 413–418. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Nag, S.; Das, D.; Roy, R.B. Detection of Syringic Acid in Food Extracts Using Molecular Imprinted Polyacrylonitrile Infused Graphite Electrode. J. Food Compos. Anal. 2024, 132, 106280. [Google Scholar] [CrossRef]
- Chi, H.; Yang, C.; Liu, G. An Electrochemical Sensor Based on Electrochemically Activated Carbon Cloth and Poly (o-Aminothiophenol) Cross-Linked Nanogold Imprinted Layer for the Determination of Tert-Butylhydroquinone. Food Chem. 2024, 452, 139548. [Google Scholar] [CrossRef]
- Fan, L.; Hao, Q.; Kan, X. Three-Dimensional Graphite Paper Based Imprinted Electrochemical Sensor for Tertiary Butylhydroquinone Selective Recognition and Sensitive Detection. Sens. Actuators B Chem. 2018, 256, 520–527. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, R.; Sun, Y.; Waterhouse, G.I.N.; Qiao, X.; Xu, Z. Development of ZrO(OH)2@HCS Co-Modified Molecularly Imprinted Gel-Based Electrochemical Sensing Platform for Sensitive and Selective Detection of Tert-Butylhydroquinone in Foods. Food Chem. 2024, 460, 140600. [Google Scholar] [CrossRef]
- Nandy Chatterjee, T.; Das, D.; Banerjee Roy, R.; Tudu, B.; Hazarika, A.K.; Sabhapondit, S.; Tamuly, P.; Bandyopadhyay, R. Development of a Nickel Hydroxide Nanopetal Decorated Molecular Imprinted Polymer Based Electrode for Sensitive Detection of Epigallocatechin-3-Gallate in Green Tea. Sens. Actuators B Chem. 2019, 283, 69–78. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Wang, L. Porous carbon derived from ZIF-8 modified molecularly imprinted electrochemical sensor for the detection of tert-butyl hydroquinone (TBHQ) in edible oil. Food Chem. 2021, 365, 130462. [Google Scholar] [CrossRef]
- Sezgin, B.; Arli, G.; Can, N.Ö. Simultaneous HPLC-DAD Determination of Seven Intense Sweeteners in Foodstuffs and Pharmaceuticals Using a Core-Shell Particle Column. J. Food Compos. Anal. 2021, 97, 103768. [Google Scholar] [CrossRef]
- Jinadasa, B.K.K.K.; Elliott, C.; Yeh, T.-S. Recent Applications of Mass Spectrometry in Sweetener Analysis. J. Food Compos. Anal. 2023, 122, 105418. [Google Scholar] [CrossRef]
- Balgobind, K.; Kanchi, S.; Sharma, D.; Bisetty, K.; Sabela, M.I. Hybrid of ZnONPs/MWCNTs for electrochemical detection of aspartame in food and beverage samples. J. Electroanal. Chem. 2016, 774, 51–57. [Google Scholar] [CrossRef]
- Su, J.; Su, X. A Multi-Walled Carbon Nanotubes-Based Molecular Imprinting Electrochemical Sensor for the Detection of Aspartame in Sports Beverage. J. Food Meas. Charact. 2024, 18, 618–624. [Google Scholar] [CrossRef]
- Mansour, A.H.; Mortada, W.I.; Awad, F.S.; Molouk, A.F.S.; Khalifa, M.E.; Abdallah, A.B. Carbon Dot-Based Imprinted Electrochemical Sensor for Ultrasensitive and Selective Detection of Sucralose in Real Samples. Mater. Chem. Front. 2025, 9, 3075–3085. [Google Scholar] [CrossRef]
- O’Connor, D.; Pang, M.; Castelnuovo, G.; Finlayson, G.; Blaak, E.; Gibbons, C.; Navas-Carretero, S.; Almiron-Roig, E.; Harrold, J.; Raben, A.; et al. A Rational Review on the Effects of Sweeteners and Sweetness Enhancers on Appetite, Food Reward and Metabolic/Adiposity Outcomes in Adults. Food Funct. 2021, 12, 442–465. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Jin, S.; Lin, W. The Role of Information in Nudging Chinese Consumers from Choosing Sugar to Alternative Sweeteners. China Econ. Rev. 2024, 87, 102233. [Google Scholar] [CrossRef]
- Shaher, S.A.A.; Mihailescu, D.F.; Amuzescu, B. Aspartame Safety as a Food Sweetener and Related Health Hazards. Nutrients 2023, 15, 3627. [Google Scholar] [CrossRef]
- Tan, L.; Li, Q.-Y.; Li, Y.-J.; Ma, R.-R.; He, J.-Y.; Jiang, Z.-F.; Yang, L.-L.; Wang, C.-Z.; Luo, L.; Zhang, Q.-H.; et al. Specific Adsorption and Determination of Aspartame in Soft Drinks with a Zein Magnetic Molecularly Imprinted Modified MGCE Sensor. RSC Adv. 2021, 11, 13486–13496. [Google Scholar] [CrossRef]
- Tiu, B.D.B.; Pernites, R.B.; Tiu, S.B.; Advincula, R.C. Detection of Aspartame via Microsphere-Patterned and Molecularly Imprinted Polymer Arrays. Colloids Surf. Physicochem. Eng. Asp. 2016, 495, 149–158. [Google Scholar] [CrossRef]
- Sousa, M.S.P.; de Sá, A.C.; de Oliveira, J.P.J.; da Silva, M.J.; Santos, R.J.; Paim, L.L. Impedimetric Sensor for Pentoses Based on Electrodeposited Carbon Nanotubes and Molecularly Imprinted Poly-o-Phenylenediamine. ECS J. Solid State Sci. Technol. 2020, 9, 041006. [Google Scholar] [CrossRef]
- Pompeu Prado Moreira, L.F.; Buffon, E.; Stradiotto, N.R. Electrochemical Sensor Based on Reduced Graphene Oxide and Molecularly Imprinted Poly(Phenol) for d-Xylose Determination. Talanta 2020, 208, 120379. [Google Scholar] [CrossRef]
- Singh, R.; Singh, M. Molecularly Imprinted Electrochemical Sensor for Highly Selective and Sensitive Determination of Artificial Sweetener Acesulfame-K. Talanta Open 2023, 7, 100194. [Google Scholar] [CrossRef]
- Çakir, S.; Coskun, E.; Biçer, E.; Çakir, O. Electrochemical study of the complexes of aspartame with Cu(II), Ni(II), and Zn(II) ions in the aqueous medium. Carbohydr. Res. 2003, 338, 1217–1222. [Google Scholar] [CrossRef]
- Srivastava, J.; Gupta, N.; Kushwaha, A.; Umrao, S.; Srivastava, A.; Singh, M. Highly Sensitive and Selective Estimation of Aspartame by Chitosan Nanoparticles–Graphene Nanocomposite Tailored EQCM-MIP Sensor. Polym. Bull. 2019, 76, 4431–4449. [Google Scholar] [CrossRef]
- Moreira, L.F.P.P.; Buffon, E.; de Sá, A.C.; Stradiotto, N.R. Fructose Determination in Fruit Juices Using an Electrosynthesized Molecularly Imprinted Polymer on Reduced Graphene Oxide Modified Electrode. Food Chem. 2021, 352, 129430. [Google Scholar] [CrossRef]
- Shekarchizadeh, H.; Ensafi, A.A.; Kadivar, M. Selective Determination of Sucrose Based on Electropolymerized Molecularly Imprinted Polymer Modified Multiwall Carbon Nanotubes/Glassy Carbon Electrode. Mater. Sci. Eng. C 2013, 33, 3553–3561. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhu, J.; Zheng, Y.; Fu, L. Electrochemical Sensing Strategies for Synthetic Orange Dyes. Molecules 2024, 29, 5026. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, S.; Lv, Y.; Chen, F.; Fu, L. Acid Red Dyes and the Role of Electrochemical Sensors in Their Determination. Microchem. J. 2024, 207, 111705. [Google Scholar] [CrossRef]
- Georgescu-State, R.; van Staden, J.K.F.; Staden, R.-I.S.; State, R.N. Electrochemical Platform Based on Molecularly Imprinted Polymer with Zinc Oxide Nanoparticles and Multiwalled Carbon Nanotubes Modified Screen-Printed Carbon Electrode for Amaranth Determination. Microchim. Acta 2023, 190, 229. [Google Scholar] [CrossRef]
- Li, L.; Zheng, H.; Guo, L.; Qu, L.; Yu, L. A Sensitive and Selective Molecularly Imprinted Electrochemical Sensor Based on Pd-Cu Bimetallic Alloy Functionalized Graphene for Detection of Amaranth in Soft Drink. Talanta 2019, 197, 68–76. [Google Scholar] [CrossRef]
- George, A.; Bharath, M.; Ghosh, M.; Varghese, A. Single-Monomer Dual Templated MIP Based Electrochemical Sensor for Tartrazine and Brilliant Blue FCF. Electrochim. Acta 2024, 475, 143682. [Google Scholar] [CrossRef]
- Wang, J.; Qin, Y. Molecularly Imprinted Sensors for the Determination of Anthocyanins in Food Products. Int. J. Electrochem. Sci. 2024, 19, 100673. [Google Scholar] [CrossRef]
- Bonyadi, S.; Ghanbari, K. Development of Highly Sensitive and Selective Sensor Based on Molecular Imprinted Polydopamine-Coated Silica Nanoparticles for Electrochemical Determination of Sunset Yellow. Microchem. J. 2021, 167, 106322. [Google Scholar] [CrossRef]
- Yin, Z.-Z.; Cheng, S.-W.; Xu, L.-B.; Liu, H.-Y.; Huang, K.; Li, L.; Zhai, Y.-Y.; Zeng, Y.-B.; Liu, H.-Q.; Shao, Y.; et al. Highly Sensitive and Selective Sensor for Sunset Yellow Based on Molecularly Imprinted Polydopamine-Coated Multi-Walled Carbon Nanotubes. Biosens. Bioelectron. 2018, 100, 565–570. [Google Scholar] [CrossRef]
- Wang, Z.; Shan, Y.; Xu, L.; Wu, G.; Lu, X. Development and Application of the Tartrazine Voltammetric Sensors Based on Molecularly Imprinted Polymers. Int. J. Polym. Anal. Charact. 2017, 22, 83–91. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Duan, H.; Wang, Y.; Bu, Y.; Luo, C. Based on Magnetic Graphene Oxide Highly Sensitive and Selective Imprinted Sensor for Determination of Sunset Yellow. Talanta 2016, 147, 169–176. [Google Scholar] [CrossRef]
- Shalapy, A.E.; Molouk, A.F.S.; Awad, F.S.; Khalifa, M.E.; Abdallah, A.B. Flexible Imprinted Electrochemical Sensing Platform for Ultra-Trace Detection of Indigo Carmine Dye in Coloured Food Additive for Candy and Ice Cream. Inorg. Chem. Commun. 2025, 174, 113951. [Google Scholar] [CrossRef]
- Benmassaoud, Y.; Murtada, K.; Salghi, R.; Zougagh, M.; Ríos, Á. Surface Polymers on Multiwalled Carbon Nanotubes for Selective Extraction and Electrochemical Determination of Rhodamine B in Food Samples. Molecules 2021, 26, 2670. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Luo, C.; Sun, M.; Li, L.; Duan, H. Ultrasensitive Molecularly Imprinted Electrochemical Sensor Based on Magnetism Graphene Oxide/β-Cyclodextrin/Au Nanoparticles Composites for Chrysoidine Analysis. Electrochim. Acta 2014, 130, 519–525. [Google Scholar] [CrossRef]
- Jacinto, C.; Maza Mejía, I.; Khan, S.; López, R.; Sotomayor, M.D.; Picasso, G. Using a smartphone-based colorimetric device with molecularly imprinted polymer for the quantification of tartrazine in soda drinks. Biosensors 2023, 13, 639. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Wang, R.; Waterhouse, G.I.N.; Xu, Z. One-Pot Synthesis of a Novel Conductive Molecularly Imprinted Gel as the Recognition Element and Signal Amplifier for the Selective Electrochemical Detection of Amaranth in Foods. Biosens. Bioelectron. 2023, 228, 115185. [Google Scholar] [CrossRef]
- Han, Q.; Wang, X.; Yang, Z.; Zhu, W.; Zhou, X.; Jiang, H. Fe3O4@rGO Doped Molecularly Imprinted Polymer Membrane Based on Magnetic Field Directed Self-Assembly for the Determination of Amaranth. Talanta 2014, 123, 101–108. [Google Scholar] [CrossRef]
- Yang, L.; Chen, S.; Zhao, L.; Chen, W.; Huang, W.; Li, X.; Zhang, H. Extraction and Electrochemical Sensing of Anthocyanins in Berry Fruits by Use of Carbon Nanotube-Based Electrode. J. Food Meas. Charact. 2024, 18, 7019–7028. [Google Scholar] [CrossRef]
- Jiang, Z.-F.; Li, Q.; Li, Q.-Y.; Xu, H.-X.; He, J.-Y.; Wang, C.-Z.; Zhou, L.-D.; Zhang, Q.-H.; Luo, L.; Yuan, C.-S. Fast Exhaustive Enrichment and Electrochemical Quantitative Detection of Anthocyanins from Natural Products by Using Dual Responsive and Dummy Molecularly Imprinted Polymers. Microchem. J. 2022, 179, 107545. [Google Scholar] [CrossRef]
- Arvand, M.; Zamani, M.; Sayyar Ardaki, M. Rapid Electrochemical Synthesis of Molecularly Imprinted Polymers on Functionalized Multi-Walled Carbon Nanotubes for Selective Recognition of Sunset Yellow in Food Samples. Sens. Actuators B Chem. 2017, 243, 927–939. [Google Scholar] [CrossRef]
- Wu, L.; Wu, T.; Zeng, W.; Zhou, S.; Zhang, W.; Ma, J. A New Ratiometric Molecularly Imprinted Electrochemical Sensor for the Detection of Sunset Yellow Based on Gold Nanoparticles. Food Chem. 2023, 413, 135600. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, F.; Zeng, B. Preparation and Application of Sunset Yellow Imprinted Ionic Liquid Polymer—Ionic Liquid Functionalized Graphene Composite Film Coated Glassy Carbon Electrodes. Electrochim. Acta 2014, 115, 247–254. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Wang, H.; Yang, P.; Du, Y. Highly Sensitive Electrochemical Determination of Sunset Yellow Based on Gold Nanoparticles/Graphene Electrode. Anal. Chim. Acta 2015, 893, 41–48. [Google Scholar] [CrossRef]
- Arvand, M.; Erfanifar, Z.; Ardaki, M.S. A New Core@Shell Silica-Coated Magnetic Molecular Imprinted Nanoparticles for Selective Detection of Sunset Yellow in Food Samples. Food Anal. Methods 2017, 10, 2593–2606. [Google Scholar] [CrossRef]
- Malik, S.; Khan, A.; Khan, H.; Rahman, G.; Ali, N.; Khan, S.; Sotomayor, M.D.P.T. Biomimetic Electrochemical Sensors Based on Core-Shell Imprinted Polymers for Targeted Sunset Yellow Estimation in Environmental Samples. Biosensors 2023, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Guo, W.; Liu, Y.; Liu, Z.; Qiu, J.; Peng, J. A Novel Electrochemical Sensor Based on Graphene Oxide Decorated with Silver Nanoparticles–Molecular Imprinted Polymers for Determination of Sunset Yellow in Soft Drinks. Food Anal. Methods 2017, 10, 2293–2301. [Google Scholar] [CrossRef]
- Kamalabadi, M.; Razavi-Mashouf, M.M.; Madrakian, T.; Ghoorchian, A.; Afkhami, A. Electrochemically Controlled Solid Phase Microextraction Based on Nanostructured Polypyrrole Film for Selective Extraction of Sunset Yellow in Food Samples. J. Iran. Chem. Soc. 2021, 18, 3127–3135. [Google Scholar] [CrossRef]
- Su, J.; Su, X. Determination of Tartrazine in Sports Drinks by a Disposable Electrochemical Sensor Modified with Co2O3. J. Food Meas. Charact. 2023, 17, 5856–5863. [Google Scholar] [CrossRef]
- George, A.; Rose Cherian, A.; Jacob, B.; Varghese, A.; Maiyalagan, T. Design Optimisation and Fabrication of Amino Acid Based Molecularly Imprinted Sensor for the Selective Determination of Food Additive Tartrazine. Food Chem. 2023, 404, 134673. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Waterhouse, G.I.N.; Gao, H.; Xu, Z. Highly Selective Molecularly Imprinted Gel-Based Electrochemical Sensor with CuS@COOH-MWCNTs Signal Amplification for Simultaneous Detection of Vanillin and Tartrazine in Foods. Sens. Actuators B Chem. 2023, 377, 133045. [Google Scholar] [CrossRef]
- Bonyadi, S.; Ghanbari, K. Application of Molecularly Imprinted Polymer and ZnO Nanoparticles as a Novel Electrochemical Sensor for Tartrazine Determination. Microchem. J. 2023, 187, 108398. [Google Scholar] [CrossRef]
- Zhao, L.; Zeng, B.; Zhao, F. Electrochemical Determination of Tartrazine Using a Molecularly Imprinted Polymer—Multiwalled Carbon Nanotubes—Ionic Liquid Supported Pt Nanoparticles Composite Film Coated Electrode. Electrochim. Acta 2014, 146, 611–617. [Google Scholar] [CrossRef]
- Cheng, S.; Tang, D.; Zhang, Y.; Xu, L.; Liu, K.; Huang, K.; Yin, Z. Specific and Sensitive Detection of Tartrazine on the Electrochemical Interface of a Molecularly Imprinted Polydopamine-Coated PtCo Nanoalloy on Graphene Oxide. Biosensors 2022, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, Y.; Zuo, J.; Zhang, J.; Zhu, L.; Zhang, J. Rapid and Sensitive Determination of Tartrazine Using a Molecularly Imprinted Copolymer Modified Carbon Electrode (MIP-PmDB/PoPD-GCE). J. Electroanal. Chem. 2017, 785, 90–95. [Google Scholar] [CrossRef]
- Che Lah, N.F.; Ahmad, A.L.; Low, S.C.; Zaulkiflee, N.D. Isotherm and Electrochemical Properties of Atrazine Sensing Using PVC/MIP: Effect of Porogenic Solvent Concentration Ratio. Membranes 2021, 11, 657. [Google Scholar] [CrossRef]
- Pardieu, E.; Cheap, H.; Vedrine, C.; Lazerges, M.; Lattach, Y.; Garnier, F.; Remita, S.; Pernelle, C. Molecularly Imprinted Conducting Polymer Based Electrochemical Sensor for Detection of Atrazine. Anal. Chim. Acta 2009, 649, 236–245. [Google Scholar] [CrossRef]
- da Costa Gonzaga, M.L.; de Albuquerque Oliveira, M.; Furtado, R.F.; Alves, C.R. Synthesis and Application of Poly(Methacrylic Acid-Co-Ethylene Glycol Dimethacrylate) as Molecularly Imprinted Polymer in Electrochemical Sensor for Atrazine Detection. J. Solid State Electrochem. 2024, 29, 3169–3179. [Google Scholar] [CrossRef]
- Che Lah, N.F.; Ahmad, A.L.; Low, S.C.; Shoparwe, N.F. The Role of Porogen-Polymer Complexation in Atrazine Imprinted Polymer to Work as an Electrochemical Sensor in Water. J. Environ. Chem. Eng. 2019, 7, 103500. [Google Scholar] [CrossRef]
- Qi, P.; Wang, J.; Wang, X.; Wang, Z.; Xu, H.; Di, S.; Wang, Q.; Wang, X. Sensitive and Selective Detection of the Highly Toxic Pesticide Carbofuran in Vegetable Samples by a Molecularly Imprinted Electrochemical Sensor with Signal Enhancement by AuNPs. RSC Adv. 2018, 8, 25334–25341. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Qin, L.; Li, D.; Qin, S.; Han, J.; Gao, W.; Jia, Y. Carbofuran-Imprinted Sensor Based on a Modified Electrode and Prepared via Combined Multiple Technologies: Preparation Process, Performance Evaluation, and Application. Electrochim. Acta 2022, 404, 139600. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, X.; Zhang, Q.; Song, H.; Kang, Q.; Shen, D. Differential Ratiometric Molecularly Imprinted Electrochemical Method for Methyl-Parathion and Carbendazim in Foods. Food Chem. 2025, 474, 143186. [Google Scholar] [CrossRef]
- Xue, S.; Zou, J.; Li, J.; Xu, J.; Chen, H.; Wang, L.; Gao, Y.; Duan, X.; Lu, L. Electrochemical Detection of Carbendazim Using Molecularly Imprinted Poly(3,4-Ethylenedioxythiophene) on Co,N Co-Doped Hollow Carbon nanocage@CNTs-Modified Electrode. Food Chem. 2024, 456, 140063. [Google Scholar] [CrossRef]
- Khosropour, H.; Keramat, M.; Laiwattanapaisal, W. A Dual Action Electrochemical Molecularly Imprinted Aptasensor for Ultra-Trace Detection of Carbendazim. Biosens. Bioelectron. 2024, 243, 115754. [Google Scholar] [CrossRef] [PubMed]
- Roushani, M.; Nezhadali, A.; Jalilian, Z. An Electrochemical Chlorpyrifos Aptasensor Based on the Use of a Glassy Carbon Electrode Modified with an Electropolymerized Aptamer-Imprinted Polymer and Gold Nanorods. Microchim. Acta 2018, 185, 551. [Google Scholar] [CrossRef]
- Nagabooshanam, S.; Roy, S.; Deshmukh, S.; Wadhwa, S.; Sulania, I.; Mathur, A.; Krishnamurthy, S.; Bharadwaj, L.M.; Roy, S.S. Microfluidic Affinity Sensor Based on a Molecularly Imprinted Polymer for Ultrasensitive Detection of Chlorpyrifos. ACS Omega 2020, 5, 31765–31773. [Google Scholar] [CrossRef]
- Geana, E.-I.; Ciucure, C.T.; Soare, A.; Enache, S.; Ionete, R.E.; Dinu, L.A. Electrochemical Detection of Glyphosate in Surface Water Samples Based on Modified Screen-Printed Electrodes. Nanomaterials 2024, 14, 948. [Google Scholar] [CrossRef]
- Ren, X.; Zeng, H.; Zhang, Q.; Cai, H.; Yang, W. Electrochemical Sensor Based on Molecularly Imprinted Polymer and Graphene Oxide Nanocomposite for Monitoring Glyphosate Content in Corn. Int. J. Electrochem. Sci. 2022, 17, 221292. [Google Scholar] [CrossRef]
- Zouaoui, F.; Bourouina-Bacha, S.; Bourouina, M.; Zine, N.; Errachid, A.; Jaffrezic-Renault, N. Mathematical Modelling of Glyphosate Molecularly Imprinted Polymer-Based Microsensor with Multiple Phenomena. Molecules 2022, 27, 493. [Google Scholar] [CrossRef]
- Yaman, Y.T.; Bolat, G.; Abaci, S.; Saygin, T.B. Peptide Nanotube Functionalized Molecularly Imprinted Polydopamine Based Single-Use Sensor for Impedimetric Detection of Malathion. Anal. Bioanal. Chem. 2022, 414, 1115–1128. [Google Scholar] [CrossRef]
- Zuo, H.G.; Zhu, J.X.; Zhan, C.R.; Shi, L.; Xing, M.; Guo, P.; Ding, Y.; Yang, H. Preparation of Malathion MIP-SPE and Its Application in Environmental Analysis. Environ. Monit. Assess. 2015, 187, 394. [Google Scholar] [CrossRef]
- Hassan, A.H.A.; Moura, S.L.; Ali, F.H.M.; Moselhy, W.A.; Taboada Sotomayor, M.d.P.; Pividori, M.I. Electrochemical Sensing of Methyl Parathion on Magnetic Molecularly Imprinted Polymer. Biosens. Bioelectron. 2018, 118, 181–187. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Zhang, Y.; Gu, M.; Wang, D.; Dang, Y.; Ye, B.-C.; Li, Y. A Robust Electrochemical Sensing Platform Using Carbon Paste Electrode Modified with Molecularly Imprinted Microsphere and Its Application on Methyl Parathion Detection. Biosens. Bioelectron. 2018, 106, 71–77. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, L.; Ran, J.; Wang, S.; Dong, N. Specific Recognition of Cationic Paraquat in Environmental Water and Vegetable Samples by Molecularly Imprinted Stir-Bar Sorptive Extraction Based on Monohydroxylcucurbit [7]Uril–Paraquat Inclusion Complex. Microchim. Acta 2020, 187, 578. [Google Scholar] [CrossRef]
- Shan, X.; de Dieu Habimana, J.; Ji, J.; Sun, J.; Pi, F.; Zhang, Y.; Sun, X. A Molecularly Imprinted Electrochemical Sensor Based on Au Nanocross-Chitosan Composites for Detection of Paraquat. J. Solid State Electrochem. 2019, 23, 1211–1220. [Google Scholar] [CrossRef]
- Yola, B.B.; Kotan, G.; Akyıldırım, O.; Atar, N.; Yola, M.L. Electrochemical Determination of Fenitrothion Pesticide Based on Ultrathin Manganese Oxide Nanowires/Molybdenum Titanium Carbide MXene Ionic Nanocomposite and Molecularly Imprinting Polymer. Microchim. Acta 2024, 191, 230. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, J.; Li, H.; Xiang, L.; Huang, Z. Fabrication of an Electrochemical Sensor with an Electrode Modified by the Two-Separated Steps of Silver Nanoparticle Sensitizing and Molecularly Imprinted Polymer Coating for the Selective and Ultrasensitive Determination of Fenitrothion. Monatshefte Für Chem.—Chem. Mon. 2023, 154, 553–561. [Google Scholar] [CrossRef]
- Mehmandoust, M.; Erk, N.; Naser, M.; Soylak, M. Molecularly Imprinted Polymer Film Loaded on the Metal–Organic Framework with Improved Performance Using Stabilized Gold-Doped Graphite Carbon Nitride Nanosheets for the Single-Step Detection of Fenamiphos. Food Chem. 2023, 404, 134627. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Yola, M.L.; Atar, N.; Orooji, Y.; Karimi, F.; Senthil Kumar, P.; Rouhi, J.; Baghayeri, M. A Novel Detection Method for Organophosphorus Insecticide Fenamiphos: Molecularly Imprinted Electrochemical Sensor Based on Core-Shell Co3O4@MOF-74 Nanocomposite. J. Colloid Interface Sci. 2021, 592, 174–185. [Google Scholar] [CrossRef]
- Alberti, G.; Zanoni, C.; Spina, S.; Magnaghi, L.R.; Biesuz, R. MIP-Based Screen-Printed Potentiometric Cell for Atrazine Sensing. Chemosensors 2022, 10, 339. [Google Scholar] [CrossRef]
- Khadem, M.; Faridbod, F.; Norouzi, P.; Foroushani, A.R.; Ganjali, M.R.; Yarahmadi, R.; Shahtaheri, S.J. Voltammetric Determination of Carbofuran Pesticide in Biological and Environmental Samples Using a Molecularly Imprinted Polymer Sensor, a Multivariate Optimization. J. Anal. Chem. 2020, 75, 669–678. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Luo, J.; Xu, Z.; Ma, X. A Microfluidic Chip Containing a Molecularly Imprinted Polymer and a DNA Aptamer for Voltammetric Determination of Carbofuran. Microchim. Acta 2018, 185, 295. [Google Scholar] [CrossRef]
- Amatatongchai, M.; Sroysee, W.; Jarujamrus, P.; Nacapricha, D.; Lieberzeit, P.A. Selective Amperometric Flow-Injection Analysis of Carbofuran Using a Molecularly-Imprinted Polymer and Gold-Coated-Magnetite Modified Carbon Nanotube-Paste Electrode. Talanta 2018, 179, 700–709. [Google Scholar] [CrossRef]
- Chen, S.; Fu, J.; Fu, Z.; Li, Y.; Su, X.; Zou, L.; He, L.; Liu, S.; Ao, X.; Yang, Y. Preparation and Characterization of Magnetic Molecular Imprinted Polymers with Ionic Liquid for the Extraction of Carbaryl in Food. Anal. Bioanal. Chem. 2020, 412, 1049–1062. [Google Scholar] [CrossRef]
- Lian, W.; Zhang, X.; Han, Y.; Li, X.; Liu, H. A Molecularly Imprinted Electrochemical Sensor for Carbendazim Detection Based on Synergy Amplified Effect of Bioelectrocatalysis and Nanocomposites. Polymers 2025, 17, 92. [Google Scholar] [CrossRef]
- Maia Júnior, F.F.; Sales Junior, R.; Barbosa, G.F.; Hussain, S.; Jara-Cornejo, E.; Khan, S. Design and Fabrication of a Biomimetic Smart Material for Electrochemical Detection of Carbendazim Pesticides in Real Samples with Enhanced Selectivity. Biosensors 2024, 14, 304. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Ren, H.; Li, X.; Chen, S.; Ye, B.-C. A Novel Electrochemical Sensor Based on Molecularly Imprinted Polymer-Modified C-ZIF67@Ni for Highly Sensitive and Selective Determination of Carbendazim. Talanta 2022, 237, 122909. [Google Scholar] [CrossRef]
- Beigmoradi, F.; Rohani Moghadam, M.; Bazmandegan-Shamili, A.; Masoodi, H.R. Electrochemical Sensor Based on Molecularly Imprinted Polymer Coating on Metal–Organic Frameworks for the Selective and Sensitive Determination of Carbendazim. Microchem. J. 2022, 179, 107633. [Google Scholar] [CrossRef]
- Feng, S.; Li, Y.; Zhang, R.; Li, Y. A Novel Electrochemical Sensor Based on Molecularly Imprinted Polymer Modified Hollow N, S-Mo2C/C Spheres for Highly Sensitive and Selective Carbendazim Determination. Biosens. Bioelectron. 2019, 142, 111491. [Google Scholar] [CrossRef]
- Fu, K.; Sun, H.; Chen, X.; Liu, L.; Cao, Y.; Zhao, J.; Li, S.; Ma, W. Computer-Aided Design and Preparation of Surface Arsenite Molecularly Imprinted Polymers for Selective Adsorption and Highly Sensitive Detection of As(Ⅲ). J. Hazard. Mater. 2024, 480, 136386. [Google Scholar] [CrossRef]
- Wu, S.; Li, K.; Dai, X.; Zhang, Z.; Ding, F.; Li, S. An Ultrasensitive Electrochemical Platform Based on Imprinted Chitosan/Gold Nanoparticles/Graphene Nanocomposite for Sensing Cadmium (II) Ions. Microchem. J. 2020, 155, 104710. [Google Scholar] [CrossRef]
- Abdallah, A.B.; El-kholany, M.R.; Molouk, A.F.S.; Awad Ali, T.; El-Shafei, A.A.; Khalifa, M.E. Selective and Sensitive Electrochemical Sensors Based on an Ion Imprinting Polymer and Graphene Oxide for the Detection of Ultra-Trace Cd(Ii) in Biological Samples. RSC Adv. 2021, 11, 30771–30780. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, L.; Wei, G.; Zhang, Y.; Zeng, Y. Highly Sensitive and Doubly Orientated Selective Molecularly Imprinted Electrochemical Sensor for Cu2+. Biosens. Bioelectron. 2015, 69, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Topcu, C.; Yilmaz, R.R.; Atasoy, B.H.; Ocsoy, I.; Yilmaz, V. A Novel Composite Electrochemical Sensor Doped Ion-Imprinted Polymer Based on N-Methacryloyl-L-Histidine/Ethylene Glycol Dimethacrylate for Ultrasensitive Determination of Copper (II) Ions in Food Supplements. Chem. Pap. 2024, 78, 8245–8260. [Google Scholar] [CrossRef]
- Aravind, A.; Mathew, B. Electrochemical Sensor Based on Nanostructured Ion Imprinted Polymer for the Sensing and Extraction of Cr(III) Ions from Industrial Wastewater. Polym. Int. 2018, 67, 1595–1604. [Google Scholar] [CrossRef]
- Wu, S.; Dai, X.; Cheng, T.; Li, S. Highly Sensitive and Selective Ion-Imprinted Polymers Based on One-Step Electrodeposition of Chitosan-Graphene Nanocomposites for the Determination of Cr(VI). Carbohydr. Polym. 2018, 195, 199–206. [Google Scholar] [CrossRef]
- Ghanei-Motlagh, M.; Taher, M.A.; Heydari, A.; Ghanei-Motlagh, R.; Gupta, V.K. A Novel Voltammetric Sensor for Sensitive Detection of Mercury(II) Ions Using Glassy Carbon Electrode Modified with Graphene-Based Ion Imprinted Polymer. Mater. Sci. Eng. C 2016, 63, 367–375. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Rahmani, A.R.; Shokoohi, R.; Farmany, A.; Khazaei, M. A Highly Sensitive and Selective Electrochemical Mercury(II) Sensor Based on Nanoparticles of Hg(II)-Imprinted Polymer and Graphitic Carbon Nitride (g-C3N4). Int. J. Electrochem. Sci. 2019, 14, 6420–6430. [Google Scholar] [CrossRef]
- Tarley, C.R.T.; Basaglia, A.M.; Segatelli, M.G.; Prete, M.C.; Suquila, F.A.C.; de Oliveira, L.L.G. Preparation and Application of Nanocomposite Based on Imprinted Poly(Methacrylic Acid)-PAN/MWCNT as a New Electrochemical Selective Sensing Platform of Pb2+ in Water Samples. J. Electroanal. Chem. 2017, 801, 114–121. [Google Scholar] [CrossRef]
- Bojdi, M.K.; Mashhadizadeh, M.H.; Behbahani, M.; Farahani, A.; Davarani, S.S.H.; Bagheri, A. Synthesis, Characterization and Application of Novel Lead Imprinted Polymer Nanoparticles as a High Selective Electrochemical Sensor for Ultra-Trace Determination of Lead Ions in Complex Matrixes. Electrochim. Acta 2014, 136, 59–65. [Google Scholar] [CrossRef]
- Ma, W.; Chang, Q.; Shi, X.; Tong, Y.; Zhou, L.; Ye, B.; Lu, J.; Zhao, J. Novel Electrochemical Sensor Based on Integration of Nanoporous Gold with Molecularly Imprinted Polymer for Detection of Arsenic Ion (III). J. Electrochem. 2020, 26, 8. [Google Scholar]
- Ma, W.; Chang, Q.; Zhao, J.; Ye, B.-C. Novel Electrochemical Sensing Platform Based on Ion Imprinted Polymer with Nanoporous Gold for Ultrasensitive and Selective Determination of As3+. Microchim. Acta 2020, 187, 571. [Google Scholar] [CrossRef]
- Alizadeh, T.; Ganjali, M.R.; Nourozi, P.; Zare, M.; Hoseini, M. A Carbon Paste Electrode Impregnated with Cd2+ Imprinted Polymer as a New and High Selective Electrochemical Sensor for Determination of Ultra-Trace Cd2+ in Water Samples. J. Electroanal. Chem. 2011, 657, 98–106. [Google Scholar] [CrossRef]
- Hu, S.; Gao, G.; Liu, Y.; Hu, J.; Song, Y.; Zou, X. An Electrochemical Sensor Based on Ion Imprinted PPy/rGO Composite for Cd(II) Determination in Water. Int. J. Electrochem. Sci. 2019, 14, 10714–10730. [Google Scholar] [CrossRef]
- Wang, J.; Hu, J.; Hu, S.; Gao, G.; Song, Y. A Novel Electrochemical Sensor Based on Electropolymerized Ion Imprinted PoPD/ERGO Composite for Trace Cd(II) Determination in Water. Sensors 2020, 20, 1004. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Liang, Y. Application of Chitosan-N-Doped Graphene Oxide Ion-Imprinted Sensor in Cd (II) Ions Detection. Diam. Relat. Mater. 2021, 119, 108591. [Google Scholar] [CrossRef]
- Yin, F.; Zhou, D.; Mo, Y.; Liu, X.; Li, G.; Gao, J.; Yuan, M.; Wu, X.; Hao, L.; Xu, F. Core–Shell Mesoporous Silica-Based Ion-Imprinted Electrochemical Sensor for the Determination of Trace Cd2+ in Water. J. Appl. Electrochem. 2024, 54, 1125–1135. [Google Scholar] [CrossRef]
- Wei, P.; Zhu, Z.; Song, R.; Li, Z.; Chen, C. An Ion-Imprinted Sensor Based on Chitosan-Graphene Oxide Composite Polymer Modified Glassy Carbon Electrode for Environmental Sensing Application. Electrochim. Acta 2019, 317, 93–101. [Google Scholar] [CrossRef]
- Karimi, Z.; Shamsipur, M.; Besharati-Seidani, A. Electrochemical Determination of Trace Cu2+ in Water Samples by Using Carbon Ionic Liquid Electrode Modified with Molecularly Imprinted Polymers. Adv. Nanochem. 2021, 3, 49–55. [Google Scholar]
- Ma, Y.; Zhang, B.; Wei, S.; Xu, J.; Wang, J.; Li, T. Histidine Detection and Signal Amplification Based on Magnetic Molecularly Imprinted Particle and Cu2+ Coordination. Sens. Actuators B Chem. 2022, 358, 131487. [Google Scholar] [CrossRef]
- Di Masi, S.; Garcia Cruz, A.; Canfarotta, F.; Cowen, T.; Marote, P.; Malitesta, C.; Piletsky, S.A. Synthesis and Application of Ion-Imprinted Nanoparticles in Electrochemical Sensors for Copper (II) Determination. ChemNanoMat 2019, 5, 754–760. [Google Scholar] [CrossRef]
- Zhihua, W.; Xiaole, L.; Jianming, Y.; Yaxin, Q.; Xiaoquan, L. Copper(II) Determination by Using Carbon Paste Electrode Modified with Molecularly Imprinted Polymer. Electrochim. Acta 2011, 58, 750–756. [Google Scholar] [CrossRef]
- Costa, M.; Di Masi, S.; Garcia-Cruz, A.; Piletsky, S.A.; Malitesta, C. Disposable Electrochemical Sensor Based on Ion Imprinted Polymeric Receptor for Cd(II) Ion Monitoring in Waters. Sens. Actuators B Chem. 2023, 383, 133559. [Google Scholar] [CrossRef]
- Alizadeh, T.; Mirzaee, S.; Rafiei, F. All-Solid-State Cr(III)-Selective Potentiometric Sensor Based on Cr(III)-Imprinted Polymer Nanomaterial/MWCNTs/Carbon Nanocomposite Electrode. Int. J. Environ. Anal. Chem. 2017, 97, 1283–1297. [Google Scholar] [CrossRef]
- Bojdi, M.K.; Behbahani, M.; Feyzabadi, Z.B. Material Design of a Chromium Imprinted Polymer and Its Application as a Highly Selective Electrochemical Sensor for Determining Chromium Ion at Trace Levels. ChemistrySelect 2021, 6, 11939–11947. [Google Scholar] [CrossRef]
- Rajabi, H.R.; Roushani, M.; Shamsipur, M. Development of a Highly Selective Voltammetric Sensor for Nanomolar Detection of Mercury Ions Using Glassy Carbon Electrode Modified with a Novel Ion Imprinted Polymeric Nanobeads and Multi-Wall Carbon Nanotubes. J. Electroanal. Chem. 2013, 693, 16–22. [Google Scholar] [CrossRef]
- Yasinzai, M.; Mustafa, G.; Asghar, N.; Ullah, I.; Zahid, M.; Lieberzeit, P.A.; Han, D.; Latif, U. Ion-Imprinted Polymer-Based Receptors for Sensitive and Selective Detection of Mercury Ions in Aqueous Environment. J. Sens. 2018, 2018, 8972549. [Google Scholar] [CrossRef]
- Shirzadmehr, A.; Afkhami, A.; Madrakian, T. A New Nano-Composite Potentiometric Sensor Containing an Hg2+-Ion Imprinted Polymer for the Trace Determination of Mercury Ions in Different Matrices. J. Mol. Liq. 2015, 204, 227–235. [Google Scholar] [CrossRef]
- Soman, S.; P. V., A.; R., K. Covalently Modified Graphene Quantum Dot Using a Thiourea Based Imprinted Polymer for the Selective Electrochemical Sensing of Hg(II) Ions. J. Polym. Res. 2021, 28, 359. [Google Scholar] [CrossRef]
- Alizadeh, T.; Ganjali, M.R.; Zare, M. Application of an Hg2+ Selective Imprinted Polymer as a New Modifying Agent for the Preparation of a Novel Highly Selective and Sensitive Electrochemical Sensor for the Determination of Ultratrace Mercury Ions. Anal. Chim. Acta 2011, 689, 52–59. [Google Scholar] [CrossRef]
- Afkhami, A.; Madrakian, T.; Soltani-Shahrivar, M.; Ahmadi, M.; Ghaedi, H. Selective and Sensitive Electrochemical Determination of Trace Amounts of Mercury Ion in Some Real Samples Using an Ion Imprinted Polymer Nano-Modifier. J. Electrochem. Soc. 2015, 163, B68. [Google Scholar] [CrossRef]
- Alizadeh, T.; Hamidi, N.; Ganjali, M.R.; Rafiei, F. An Extraordinarily Sensitive Voltammetric Sensor with Picomolar Detection Limit for Pb2+ Determination Based on Carbon Paste Electrode Impregnated with Nano-Sized Imprinted Polymer and Multi-Walled Carbon Nanotubes. J. Environ. Chem. Eng. 2017, 5, 4327–4336. [Google Scholar] [CrossRef]
- Sebastian, M.; Mathew, B. Ion Imprinting Approach for the Fabrication of an Electrochemical Sensor and Sorbent for Lead Ions in Real Samples Using Modified Multiwalled Carbon Nanotubes. J. Mater. Sci. 2018, 53, 3557–3572. [Google Scholar] [CrossRef]
- Alizadeh, T.; Amjadi, S. Preparation of Nano-Sized Pb2+ Imprinted Polymer and Its Application as the Chemical Interface of an Electrochemical Sensor for Toxic Lead Determination in Different Real Samples. J. Hazard. Mater. 2011, 190, 451–459. [Google Scholar] [CrossRef]
- Luo, X.; Huang, W.; Shi, Q.; Xu, W.; Luan, Y.; Yang, Y.; Wang, H.; Yang, W. Electrochemical Sensor Based on Lead Ion-Imprinted Polymer Particles for Ultra-Trace Determination of Lead Ions in Different Real Samples. RSC Adv. 2017, 7, 16033–16040. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, Y.; Wang, C.; Sun, L.; Lu, X.; Lu, X. Preparation of Electrochemical Sensor for Lead(II) Based on Molecularly Imprinted Film. Int. Vac. Congr. IVC-18 2012, 258, 2017–2021. [Google Scholar] [CrossRef]
- Karagözlü, M.; Aşır, S.; Abu Shama, N.; Göktürk, I.; Yılmaz, F.; Türkmen, D.; Denizli, A.; Özgören, M. Development of Molecularly Imprinted Magnetic Amino Acid-Based Nanoparticles for Voltammetric Analysis of Lead Ions in Honey. Polymers 2024, 16, 1782. [Google Scholar] [CrossRef]
- Roushani, M.; Farokhi, S.; Rahmati, Z. Development of a Dual-Recognition Strategy for the Aflatoxin B1 Detection Based on a Hybrid of Aptamer-MIP Using a Cu2O NCs/GCE. Microchem. J. 2022, 178, 107328. [Google Scholar] [CrossRef]
- Singh, A.K.; Lakshmi, G.B.V.S.; Fernandes, M.; Sarkar, T.; Gulati, P.; Singh, R.P.; Solanki, P.R. A Simple Detection Platform Based on Molecularly Imprinted Polymer for AFB1 and FuB1 Mycotoxins. Microchem. J. 2021, 171, 106730. [Google Scholar] [CrossRef]
- Hu, X.; Liu, Y.; Xia, Y.; Zhao, F.; Zeng, B. A Novel Ratiometric Electrochemical Sensor for the Selective Detection of Citrinin Based on Molecularly Imprinted Poly(Thionine) on Ionic Liquid Decorated Boron and Nitrogen Co-Doped Hierarchical Porous Carbon. Food Chem. 2021, 363, 130385. [Google Scholar] [CrossRef]
- Jalalvand, A.R.; Farshadnia, M.; Jalili, F.; Jalili, C. A Novel, Intelligent and Computer-Assisted Electrochemical Sensor for Extraction and Simultaneous Determination of Patulin and Citrinin in Apple and Pear Fruit Samples. Chemom. Intell. Lab. Syst. 2024, 252, 105188. [Google Scholar] [CrossRef]
- Radi, A.-E.; Eissa, A.; Wahdan, T. Impedimetric Sensor for Deoxynivalenol Based on Electropolymerised Molecularly Imprinted Polymer on the Surface of Screen-Printed Gold Electrode. Int. J. Environ. Anal. Chem. 2021, 101, 2586–2597. [Google Scholar] [CrossRef]
- Nie, D.; Zhu, X.; Liu, M.; Cheng, M.; Fan, K.; Zhao, Z.; Huang, Q.; Zhang, X.; Han, Z. Molecularly Imprinted Polymer-Based Electrochemical Sensor for Rapid Detection of Masked Deoxynivalenol with Mn-Doped CeO2 Nanozyme as Signal Amplifier. J. Hazard. Mater. 2024, 477, 135366. [Google Scholar] [CrossRef]
- Yola, M.L.; Gupta, V.K.; Atar, N. New Molecular Imprinted Voltammetric Sensor for Determination of Ochratoxin A. Mater. Sci. Eng. C 2016, 61, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Liu, J.; Zhang, W.; Wang, H.; Huang, J.; Wang, G.; Hu, M.; Dong, H.; Sun, J.; Fang, M.; et al. Preparation of Dual Recognition Adsorbents Based on Molecularly Imprinted Polymers and Aptamer for Highly Sensitive Recognition and Enrichment of Ochratoxin A. J. Hazard. Mater. 2024, 476, 135112. [Google Scholar] [CrossRef]
- Sodkrathok, P.; Karuwan, C.; Kamsong, W.; Tuantranont, A.; Amatatongchai, M. Patulin-Imprinted Origami 3D-ePAD Based on Graphene Screen-Printed Electrode Modified with Mn–ZnS Quantum Dot Coated with a Molecularly Imprinted Polymer. Talanta 2023, 262, 124695. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xie, H.; Li, X.; Zhu, Y.; Huang, L.; Zhong, M.; Chen, L. Construction of a Self–Reporting Molecularly–Imprinted Electrochemical Sensor Based on CuHCF Modified by rGNR–rGO for the Detection of Zearalenone. Food Chem. 2024, 448, 139154. [Google Scholar] [CrossRef]
- Guo, K. Design and Fabrication of a Molecularly Imprinted Electrochemical Sensor with High Sensitivity for Zearalenone Assessment in Maize. Int. J. Electrochem. Sci. 2024, 19, 100612. [Google Scholar] [CrossRef]
- Jiang, M.; Braiek, M.; Florea, A.; Chrouda, A.; Farre, C.; Bonhomme, A.; Bessueille, F.; Vocanson, F.; Zhang, A.; Jaffrezic-Renault, N. Aflatoxin B1 Detection Using a Highly-Sensitive Molecularly-Imprinted Electrochemical Sensor Based on an Electropolymerized Metal Organic Framework. Toxins 2015, 7, 3540–3553. [Google Scholar] [CrossRef]
- Wood, M.; Mugo, S.M. A MIP-Enabled Stainless-Steel Hypodermic Needle Sensor for Electrochemical Detection of Aflatoxin B1. Anal. Methods 2022, 14, 2063–2071. [Google Scholar] [CrossRef]
- Hernández-García, F.; Cruz-Navarro, J.A.; García-Serrano, J.; Franco-Guzmán, M.; Islas, G.; Alvarez-Romero, G.A. Development of a Voltammetric Methodology Based on a Methacrylic Molecularly Imprinted Polymer-Modified Carbon-Paste Electrode for the Determination of Aflatoxin B1. Separations 2024, 11, 246. [Google Scholar] [CrossRef]
- Chen, L.; Mamat, X.; Aisa, H.A. Determination of Aflatoxin B1 by Biomass Derived Porous Carbon Modified Electrode with Molecularly Imprinted Polymer. Electroanalysis 2023, 35, e202200371. [Google Scholar] [CrossRef]
- Atar, N.; Yola, M.L.; Eren, T. Sensitive Determination of Citrinin Based on Molecular Imprinted Electrochemical Sensor. Appl. Surf. Sci. 2016, 362, 315–322. [Google Scholar] [CrossRef]
- Huang, H.; Ouyang, W.; Feng, K.; Camarada, M.B.; Liao, T.; Tang, X.; Liu, R.; Hou, D.; Liao, X. Rational Design of Molecularly Imprinted Electrochemical Sensor Based on Nb2C-MWCNTs Heterostructures for Highly Sensitive and Selective Detection of Ochratoxin a. Food Chem. 2024, 456, 140007. [Google Scholar] [CrossRef] [PubMed]
- Akyıldırım, O.; Kardaş, F.; Beytur, M.; Yüksek, H.; Atar, N.; Yola, M.L. Palladium Nanoparticles Functionalized Graphene Quantum Dots with Molecularly Imprinted Polymer for Electrochemical Analysis of Citrinin. J. Mol. Liq. 2017, 243, 677–681. [Google Scholar] [CrossRef]
- Li, W.; Diao, K.; Qiu, D.; Zeng, Y.; Tang, K.; Zhu, Y.; Sheng, Y.; Wen, Y.; Li, M. A Highly-Sensitive and Selective Antibody-like Sensor Based on Molecularly Imprinted Poly(L-Arginine) on COOH-MWCNTs for Electrochemical Recognition and Detection of Deoxynivalenol. Food Chem. 2021, 350, 129229. [Google Scholar] [CrossRef]
- Pacheco, J.G.; Castro, M.; Machado, S.; Barroso, M.F.; Nouws, H.P.A.; Delerue-Matos, C. Molecularly Imprinted Electrochemical Sensor for Ochratoxin A Detection in Food Samples. Sens. Actuators B Chem. 2015, 215, 107–112. [Google Scholar] [CrossRef]
- Hu, X.; Xia, Y.; Liu, Y.; Chen, Y.; Zeng, B. An Effective Ratiometric Electrochemical Sensor for Highly Selective and Reproducible Detection of Ochratoxin A: Use of Magnetic Field Improved Molecularly Imprinted Polymer. Sens. Actuators B Chem. 2022, 359, 131582. [Google Scholar] [CrossRef]
- Mavioğlu Kaya, M.; Deveci, H.A.; Kaya, İ.; Atar, N.; Yola, M.L. The Electrochemical Detection of Ochratoxin A in Apple Juice via MnCO3 Nanostructures Incorporated into Carbon Fibers Containing a Molecularly Imprinting Polymer. Biosensors 2023, 13, 760. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Luo, Y.; Zhou, K.; Qiu, X.; Guo, H. A Novel Molecularly Imprinted Polymer Material for the Recognition of Ochratoxin A. J. Polym. Res. 2022, 29, 158. [Google Scholar] [CrossRef]
- Hatamluyi, B.; Rezayi, M.; Beheshti, H.R.; Boroushaki, M.T. Ultra-Sensitive Molecularly Imprinted Electrochemical Sensor for Patulin Detection Based on a Novel Assembling Strategy Using Au@Cu-MOF/N-GQDs. Sens. Actuators B Chem. 2020, 318, 128219. [Google Scholar] [CrossRef]
- Afzali, Z.; Mohadesi, A.; Ali Karimi, M.; Fathirad, F. A Highly Selective and Sensitive Electrochemical Sensor Based on Graphene Oxide and Molecularly Imprinted Polymer Magnetic Nanocomposite for Patulin Determination. Microchem. J. 2022, 177, 107215. [Google Scholar] [CrossRef]
- Guo, W.; Pi, F.; Zhang, H.; Sun, J.; Zhang, Y.; Sun, X. A Novel Molecularly Imprinted Electrochemical Sensor Modified with Carbon Dots, Chitosan, Gold Nanoparticles for the Determination of Patulin. Biosens. Bioelectron. 2017, 98, 299–304. [Google Scholar] [CrossRef]
- Selvam, S.P.; Kadam, A.N.; Maiyelvaganan, K.R.; Prakash, M.; Cho, S. Novel SeS2-Loaded Co MOF with Au@PANI Comprised Electroanalytical Molecularly Imprinted Polymer-Based Disposable Sensor for Patulin Mycotoxin. Biosens. Bioelectron. 2021, 187, 113302. [Google Scholar] [CrossRef]
- Hu, X.; Xia, Y.; Liu, Y.; Zhao, F.; Zeng, B. Determination of Patulin Using Dual-Dummy Templates Imprinted Electrochemical Sensor with PtPd Decorated N-Doped Porous Carbon for Amplification. Microchim. Acta 2021, 188, 148. [Google Scholar] [CrossRef]
- Rehman, N.Ç.; Özdemir, N.; Boyacıoğlu, H.; Yola, M.L. A Novel Molecularly Imprinted Electrochemical Sensor Based on Graphitic Carbon Nitride Nanosheets Decorated Bovine Serum albumin@MnO2 Nanocomposite for Zearalenone Detection. J. Food Compos. Anal. 2024, 125, 105857. [Google Scholar] [CrossRef]
- Radi, A.-E.; Eissa, A.; Wahdan, T. Molecularly Imprinted Impedimetric Sensor for Determination of Mycotoxin Zearalenone. Electroanalysis 2020, 32, 1788–1794. [Google Scholar] [CrossRef]
- Küçük, D.; Üner, G.; İpek, S.L.; Caglayan, M.O.; Üstündağ, Z. An Impedimetric Determination of Zearalenone on MIP-Modified Carboceramic Electrode. Toxicon 2024, 250, 108115. [Google Scholar] [CrossRef]
- Hu, X.; Tang, Y.; Xia, Y.; Liu, Y.; Zhao, F.; Zeng, B. Antifouling Ionic Liquid Doped Molecularly Imprinted Polymer-Based Ratiometric Electrochemical Sensor for Highly Stable and Selective Detection of Zearalenone. Anal. Chim. Acta 2022, 1210, 339884. [Google Scholar] [CrossRef]
- Zhou, B.; Xie, H.; Zhou, S.; Sheng, X.; Chen, L.; Zhong, M. Construction of AuNPs/Reduced Graphene Nanoribbons Co-Modified Molecularly Imprinted Electrochemical Sensor for the Detection of Zearalenone. Food Chem. 2023, 423, 136294. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, X.; Xie, H.; Li, Y.; Li, X.; Zhou, B. A Molecularly Imprinted Electrochemical Sensor Based on rGO@rGNR Modification for Zearalenone Determination. J. Food Compos. Anal. 2025, 141, 107361. [Google Scholar] [CrossRef]
- Metwally, M.G.; Shehab, O.R.; Ibrahim, H.; El Nashar, R.M. Electrochemical Detection of Bisphenol A in Plastic Bottled Drinking Waters and Soft Drinks Based on Molecularly Imprinted Polymer. J. Environ. Chem. Eng. 2022, 10, 107699. [Google Scholar] [CrossRef]
- Kaya, S.I.; Corman, M.E.; Uzun, L.; Ozkan, S.A. A Porous Molecularly Imprinted Electrochemical Sensor for Specific Determination of Bisphenol S from Human Serum and Bottled Water Samples in Femtomolar Level. Anal. Bioanal. Chem. 2022, 414, 2775–2785. [Google Scholar] [CrossRef]
- Wang, S.; Pan, M.; Liu, K.; Xie, X.; Yang, J.; Hong, L.; Wang, S. A SiO2@MIP Electrochemical Sensor Based on MWCNTs and AuNPs for Highly Sensitive and Selective Recognition and Detection of Dibutyl Phthalate. Food Chem. 2022, 381, 132225. [Google Scholar] [CrossRef]
- Yu, L.; Shen, Y.; Gao, P.; Zhang, Q.; Hu, X.; Xu, Q. A Novel Molecularly Imprinted Photoelectrochemical Aptasensor Based on Dual Recognition Mechanism for Ultratrace Detection of Plasticizer Dibutyl Phthalate. Chem. Eng. J. 2023, 472, 144925. [Google Scholar] [CrossRef]
- Lu, D.; Zhu, D.Z.; Gan, H.; Yao, Z.; Luo, J.; Yu, S.; Kurup, P. An Ultra-Sensitive Molecularly Imprinted Polymer (MIP) and Gold Nanostars (AuNS) Modified Voltammetric Sensor for Facile Detection of Perfluorooctance Sulfonate (PFOS) in Drinking Water. Sens. Actuators B Chem. 2022, 352, 131055. [Google Scholar] [CrossRef]
- Amin, N.; Chen, J.; He, Q.; Schwartz, J.S.; Wu, J.J. Ultra-Sensitive and Rapid Detection of Perfluorooctanesulfonic Acid by a Capacitive Molecularly-Imprinted-Polymer Sensor Integrated with AC Electrokinetic Acceleration. Sens. Actuators B Chem. 2024, 420, 136464. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, R.; Chen, F.; Jing, L.; Tian, Z.; Li, Z.; Wang, J.; Hou, C. Molecularly Imprinted MOFs-Driven Carbon Nanofiber for Sensitive Electrochemical Detection and Targeted Electro-Fenton Degradation of Perfluorooctanoic Acid. Sep. Purif. Technol. 2023, 310, 123257. [Google Scholar] [CrossRef]
- Hafeez, S.; Khanam, A.; Cao, H.; Chaplin, B.P.; Xu, W. Novel Conductive and Redox-Active Molecularly Imprinted Polymer for Direct Quantification of Perfluorooctanoic Acid. Environ. Sci. Technol. Lett. 2024, 11, 871–877. [Google Scholar] [CrossRef]
- Han, E.; Pan, Y.; Li, L.; Cai, J. Bisphenol A Detection Based on Nano Gold-Doped Molecular Imprinting Electrochemical Sensor with Enhanced Sensitivity. Food Chem. 2023, 426, 136608. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, X.; Lin, Q.; He, X.; Xing, X.; Huai, H.; Lian, W.; Zhu, H. Electrochemical Sensor Based on Imprinted Sol–Gel and Nanomaterials for Sensitive Determination of Bisphenol A. Food Control 2011, 22, 786–791. [Google Scholar] [CrossRef]
- Deveci, H.A.; Mavioğlu Kaya, M.; Kaya, İ.; Bankoğlu Yola, B.; Atar, N.; Yola, M.L. Bisphenol A Imprinted Electrochemical Sensor Based on Graphene Quantum Dots with Boron Functionalized G-C3N4 in Food Samples. Biosensors 2023, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Kaassamani, R.A.; Sawan, S.; Jaffrezic-Renault, N.; Maalouf, R. A Novel Electrochemical Sensor Based on Iron Oxide Nanoparticles Coated with Molecularly Imprinted Polymers for Bisphenol A Detection. Microchem. J. 2025, 208, 112632. [Google Scholar] [CrossRef]
- Lei, X.; Deng, Z.; Zeng, Y.; Huang, S.; Yang, Y.; Wang, H.; Guo, L.; Li, L. A Novel Composite of Conductive Metal Organic Framework and Molecularly Imprinted Poly (Ionic Liquid) for Highly Sensitive Electrochemical Detection of Bisphenol A. Sens. Actuators B Chem. 2021, 339, 129885. [Google Scholar] [CrossRef]
- Deng, P.; Xu, Z.; Kuang, Y. Electrochemical Determination of Bisphenol A in Plastic Bottled Drinking Water and Canned Beverages Using a Molecularly Imprinted Chitosan–Graphene Composite Film Modified Electrode. Food Chem. 2014, 157, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Xiao, W.W.; Wang, J.Y.; Xiong, X.H. Rapid Isolation and Determination of Bisphenol A in Complicated Matrices by Magnetic Molecularly Imprinted Electrochemical Sensing. Anal. Bioanal. Chem. 2021, 413, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Sheng, X.; Cao, J.; Xie, H.; Li, X.; Huang, L.; Yang, M.; Zhong, M.; Liu, Y.-N. A Novel Electrochemical Sensor Based on Dual-Functional MMIP-CuMOFs for Both Target Recognition and Signal Reporting and Its Application for Sensing Bisphenol A in Milk. Food Chem. 2024, 437, 137756. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.; Liu, S.; Lin, Q.; He, X.; Xing, X.; Lian, W. Electrochemical Sensor for Bisphenol A Detection Based on Molecularly Imprinted Polymers and Gold Nanoparticles. J. Appl. Electrochem. 2011, 41, 1323–1328. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Amini, M.; Rezaei, B. Molecularly Imprinted Electrochemical Aptasensor for the Attomolar Detection of Bisphenol A. Microchim. Acta 2018, 185, 265. [Google Scholar] [CrossRef]
- Dadkhah, S.; Ziaei, E.; Mehdinia, A.; Baradaran Kayyal, T.; Jabbari, A. A Glassy Carbon Electrode Modified with Amino-Functionalized Graphene Oxide and Molecularly Imprinted Polymer for Electrochemical Sensing of Bisphenol A. Microchim. Acta 2016, 183, 1933–1941. [Google Scholar] [CrossRef]
- Rebocho, S.; Cordas, C.M.; Viveiros, R.; Casimiro, T. Development of a Ferrocenyl-Based MIP in Supercritical Carbon Dioxide: Towards an Electrochemical Sensor for Bisphenol A. J. Supercrit. Fluids 2018, 135, 98–104. [Google Scholar] [CrossRef]
- Lamaoui, A.; María Palacios-Santander, J.; Amine, A.; Cubillana-Aguilera, L. Computational Approach and Ultrasound Probe–Assisted Synthesis of Magnetic Molecularly Imprinted Polymer for the Electrochemical Detection of Bisphenol A. Mater. Sci. Eng. B 2022, 277, 115568. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Su, Y.-L.; Wu, B.-H.; Cheng, S.-H. Reusable Electrochemical Sensor for Bisphenol A Based on Ionic Liquid Functionalized Conducting Polymer Platform. Talanta 2016, 147, 103–110. [Google Scholar] [CrossRef]
- Jalilian, R.; Ezzatzadeh, E.; Taheri, A. A Novel Self-Assembled Gold Nanoparticles-Molecularly Imprinted Modified Carbon Ionic Liquid Electrode with High Sensitivity and Selectivity for the Rapid Determination of Bisphenol A Leached from Plastic Containers. J. Environ. Chem. Eng. 2021, 9, 105513. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Athira, V.S.; Chithra Sekhar, V. Electrochemical Sensing and Nano Molar Level Detection of Bisphenol-A with Molecularly Imprinted Polymer Tailored on Multiwalled Carbon Nanotubes. Polymer 2018, 146, 312–320. [Google Scholar] [CrossRef]
- Zhao, W.-R.; Kang, T.-F.; Lu, L.-P.; Shen, F.-X.; Cheng, S.-Y. A Novel Electrochemical Sensor Based on Gold Nanoparticles and Molecularly Imprinted Polymer with Binary Functional Monomers for Sensitive Detection of Bisphenol A. J. Electroanal. Chem. 2017, 786, 102–111. [Google Scholar] [CrossRef]
- Mars, A.; Mejri, A.; Hamzaoui, A.H.; Elfil, H. Molecularly Imprinted Curcumin Nanoparticles Decorated Paper for Electrochemical and Fluorescence Dual-Mode Sensing of Bisphenol A. Microchim. Acta 2021, 188, 94. [Google Scholar] [CrossRef]
- Yılmaz, F.; Shama, N.A.; Aşır, S.; Çobanoğulları, H.; Yolaç, E.; Kiraz, A.; Göktürk, I.; Denizli, A.; Türkmen, D. Gold Nanoparticle-Modified Molecularly Imprinted Polymer-Coated Pencil Graphite Electrodes for Electrochemical Detection of Bisphenol A. ACS Omega 2025, 10, 740–753. [Google Scholar] [CrossRef]
- Bolat, G.; Yaman, Y.T.; Abaci, S. Molecularly Imprinted Electrochemical Impedance Sensor for Sensitive Dibutyl Phthalate (DBP) Determination. Sens. Actuators B Chem. 2019, 299, 127000. [Google Scholar] [CrossRef]
- Zhou, Q.; Guo, M.; Wu, S.; Fornara, D.; Sarkar, B.; Sun, L.; Wang, H. Electrochemical Sensor Based on Corncob Biochar Layer Supported Chitosan-MIPs for Determination of Dibutyl Phthalate (DBP). J. Electroanal. Chem. 2021, 897, 115549. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, L.; Cai, R.; Chen, H. A Sensitive and Selective Molecularly Imprinted Sensor Combined with Magnetic Molecularly Imprinted Solid Phase Extraction for Determination of Dibutyl Phthalate. Biosens. Bioelectron. 2013, 49, 367–373. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Li, L.; Duan, H.; Luo, C. Electrochemical Sensor Based on Magnetic Graphene Oxide@gold Nanoparticles-Molecular Imprinted Polymers for Determination of Dibutyl Phthalate. Talanta 2015, 131, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Karimian, N.; Stortini, A.M.; Moretto, L.M.; Costantino, C.; Bogialli, S.; Ugo, P. Electrochemosensor for Trace Analysis of Perfluorooctanesulfonate in Water Based on a Molecularly Imprinted Poly(o-Phenylenediamine) Polymer. ACS Sens. 2018, 3, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.; Cristofori, D.; Bottari, F.; Cattaruzza, E.; De Wael, K.; Moretto, L.M. Redesigning an Electrochemical MIP Sensor for PFOS: Practicalities and Pitfalls. Sensors 2019, 19, 4433. [Google Scholar] [CrossRef]
- Rezaei, M.; Ghanavati, M.; Mohammadi, N.; Khani, S.; Nasirimoghadam, S.; Smiley, E.; Basiryanmahabadi, A. A New Sensitive Layer Based on Clcinated Zn/Ti-MOF/Magnetic Molecularly Imprinted Polypyrrole: Application to Preconcentration and Electrochemical Determination of Perfluorooctane Sulfonic Acid by Magnetic Carbon Paste Electrode. Talanta 2024, 276, 126229. [Google Scholar] [CrossRef] [PubMed]
- Pierpaoli, M.; Szopińska, M.; Olejnik, A.; Ryl, J.; Fudala-Ksiażek, S.; Łuczkiewicz, A.; Bogdanowicz, R. Engineering Boron and Nitrogen Codoped Carbon Nanoarchitectures to Tailor Molecularly Imprinted Polymers for PFOS Determination. J. Hazard. Mater. 2023, 458, 131873. [Google Scholar] [CrossRef]
- Zhu, X.; Cheng, C.; Qin, X.; Wang, Y. β-Cyclodextrin Imprinted Film Embedded with Methylene Blue: A Host-Guest Sensitive Electrochemical Strategy for PFAS Detection. J. Hazard. Mater. 2025, 485, 136870. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, X.; Hao, J. Melamine-Imprinted Electrochemical Sensor of Graphene/Ionic Liquid Composites and Its Use for the Detection of Melamine in Dairy Products. ChemPhysMater 2022, 1, 195–202. [Google Scholar] [CrossRef]
- Limthin, D.; Leepheng, P.; Tunhoo, B.; Klamchuen, A.; Suramitr, S.; Thiwawong, T.; Phromyothin, D. Enhancement in Sensitivity and Selectivity of Electrochemical Technique with CuO/g-C3N4 Nanocomposite Combined with Molecularly Imprinted Polymer for Melamine Detection. Polymers 2024, 16, 1800. [Google Scholar] [CrossRef]
- Esmaeily, Z.; Madrakian, T.; Afkhami, A.; Ghoorchian, A.; Ghasemzadeh-Mohammadi, V. Electropolymerization as an Electrochemical Preconcentration Approach for the Determination of Melamine in Milk Samples. Electrochim. Acta 2021, 390, 138897. [Google Scholar] [CrossRef]
- Chen, S.; Du, D.; Huang, J.; Zhang, A.; Tu, H.; Zhang, A. Rational Design and Application of Molecularly Imprinted Sol–Gel Polymer for the Electrochemically Selective and Sensitive Determination of Sudan I. Talanta 2011, 84, 451–456. [Google Scholar] [CrossRef]
- Gao, Q.; Zang, Y.; Xie, J.; Chen, L.; Xu, J.; Huang, H.; Xue, H. Bifunctional Monomer Oligomers-Based Composite Molecularly Imprinted Membranes for the Electrochemical Monitoring of Sudan I. Analyst 2022, 147, 3764–3772. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, Z.; Chen, W.; Wang, E.; Li, Y. Preparation and Application of Malachite Green Molecularly Imprinted/Gold Nanoparticle Composite Film–Modified Glassy Carbon Electrode. Ionics 2019, 25, 1177–1185. [Google Scholar] [CrossRef]
- Fan, Y.; Zuo, Y.; Liu, J.; Wang, C.; Zhao, X.; Ma, J.; Wang, M. Fabrication of 3D CuFe2O4/Cu0 Hierarchical Nanostructures on Carbon Fiber Paper by Simple Hydrothermal Method for Efficient Detection of Malachite Green, Sunset Yellow and Tartrazine in Food Samples. Food Chem. 2024, 459, 140378. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Huang, J.; Zhai, H.; Shen, Z.; Han, J.; Yu, Y.; Fang, H.; Li, F.; Sun, X.; Guo, Y. Molecularly Imprinted Electrochemical Sensor Based on Multi-Walled Carbon Nanotubes for Specific Recognition and Determination of Chloramphenicol in Milk. Microchem. J. 2022, 182, 107887. [Google Scholar] [CrossRef]
- Geng, L.; Sun, J.; Liu, M.; Huang, J.; Dong, J.; Guo, Z.; Guo, Y.; Sun, X. Molecularly Imprinted Polymers-Aptamer Electrochemical Sensor Based on Dual Recognition Strategy for High Sensitivity Detection of Chloramphenicol. Food Chem. 2024, 437, 137933. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.R.; Tavares, A.P.M.; Sales, M.G.F. In-Situ Generated Molecularly Imprinted Material for Chloramphenicol Electrochemical Sensing in Waters down to the Nanomolar Level. Sens. Actuators B Chem. 2018, 256, 420–428. [Google Scholar] [CrossRef]
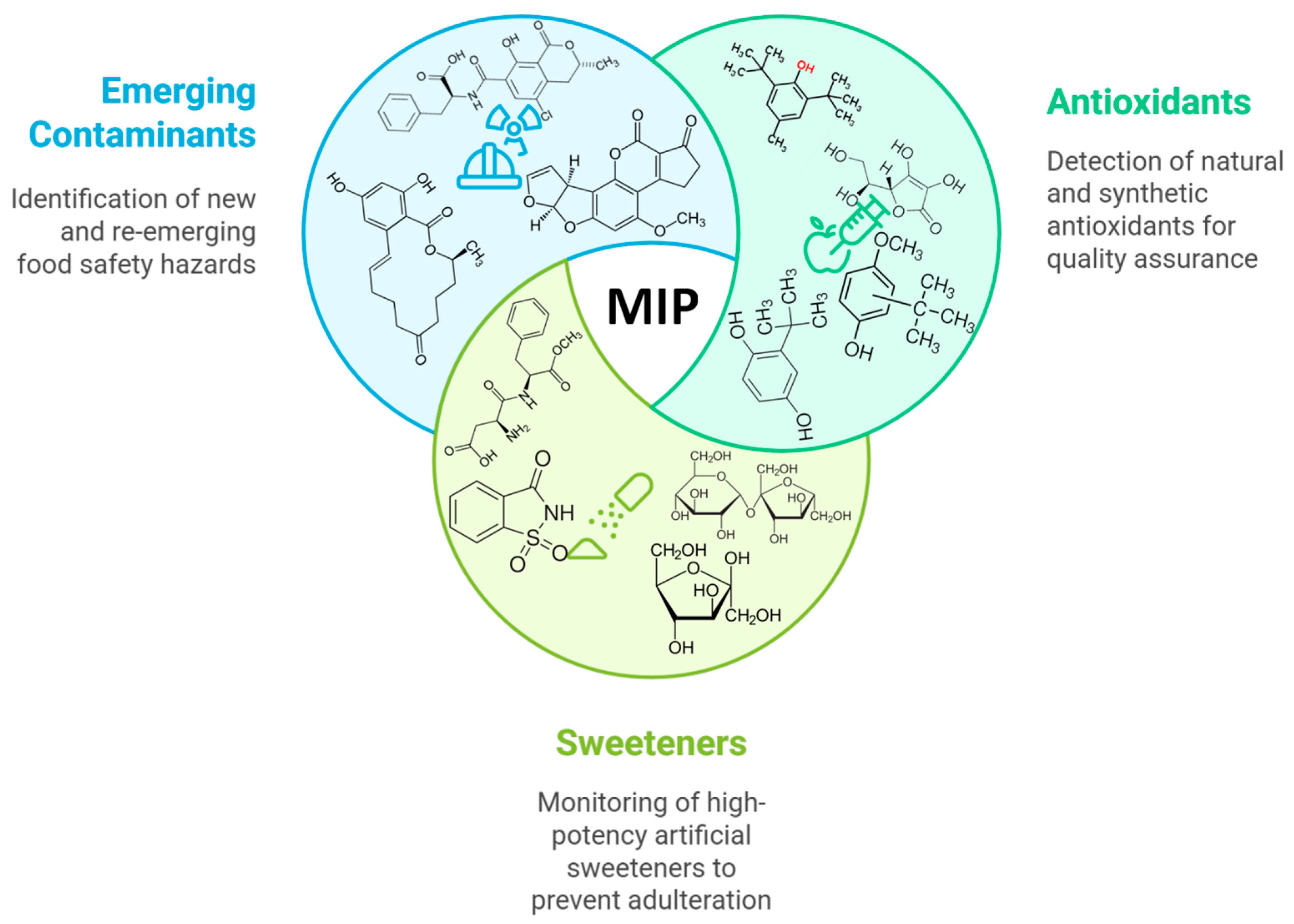
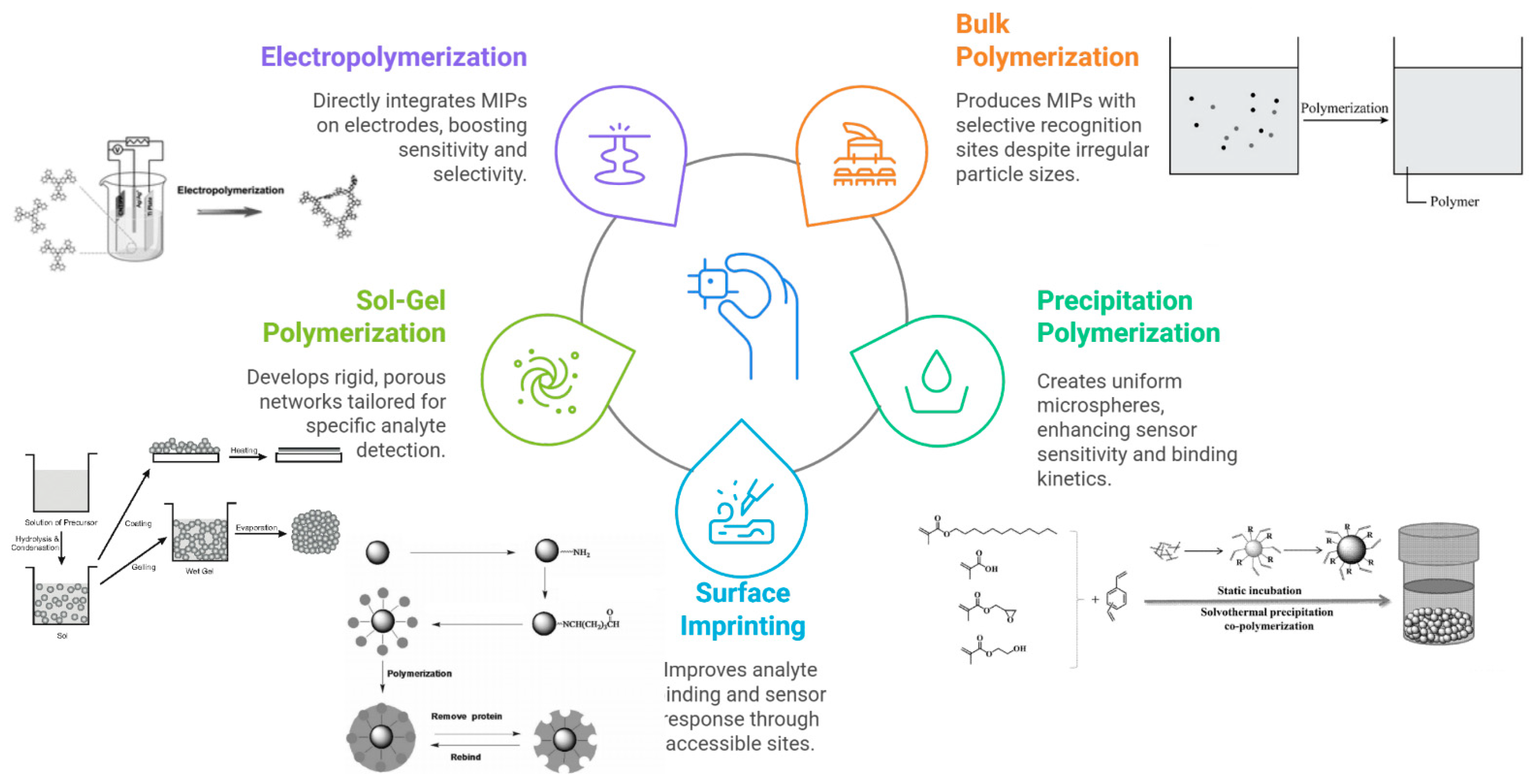

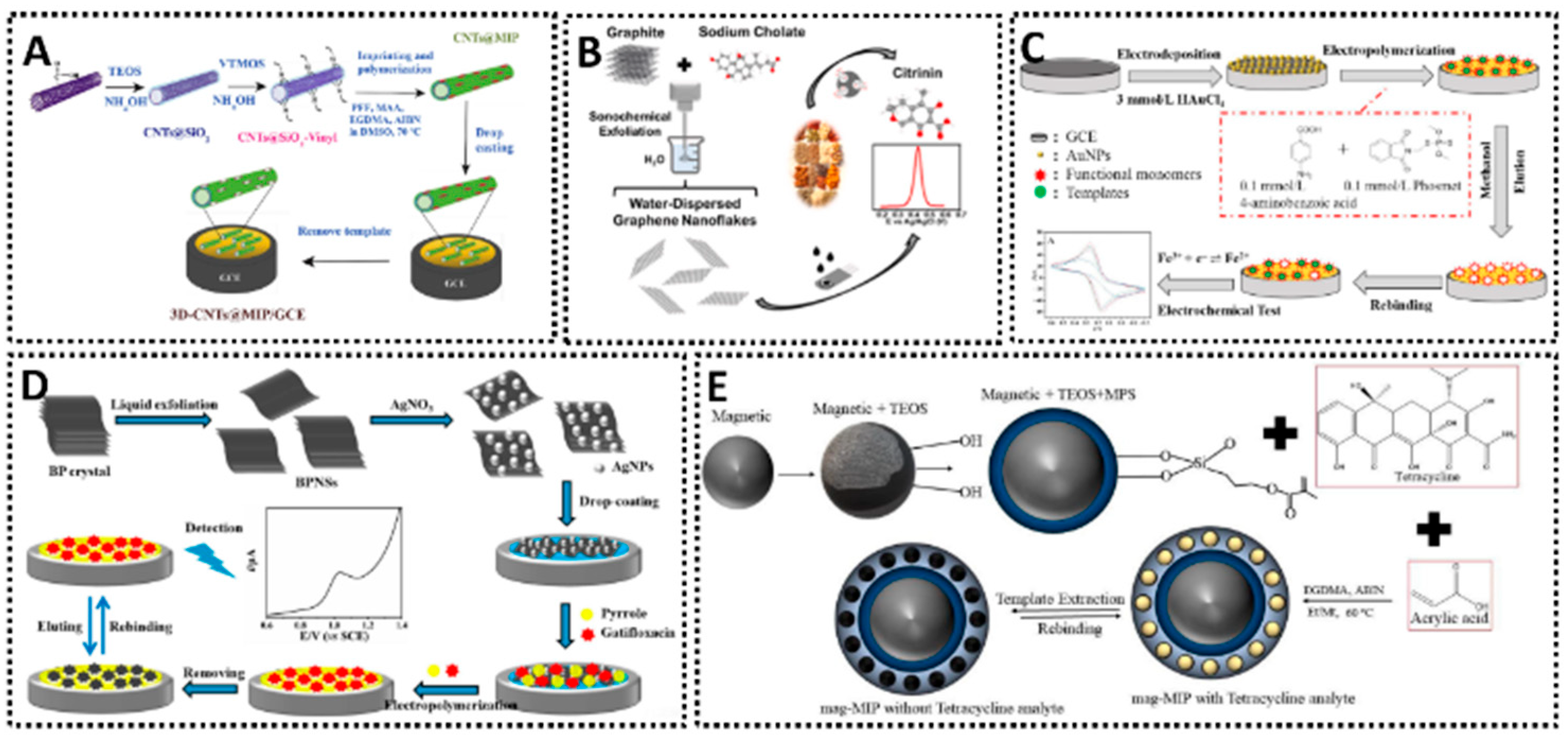
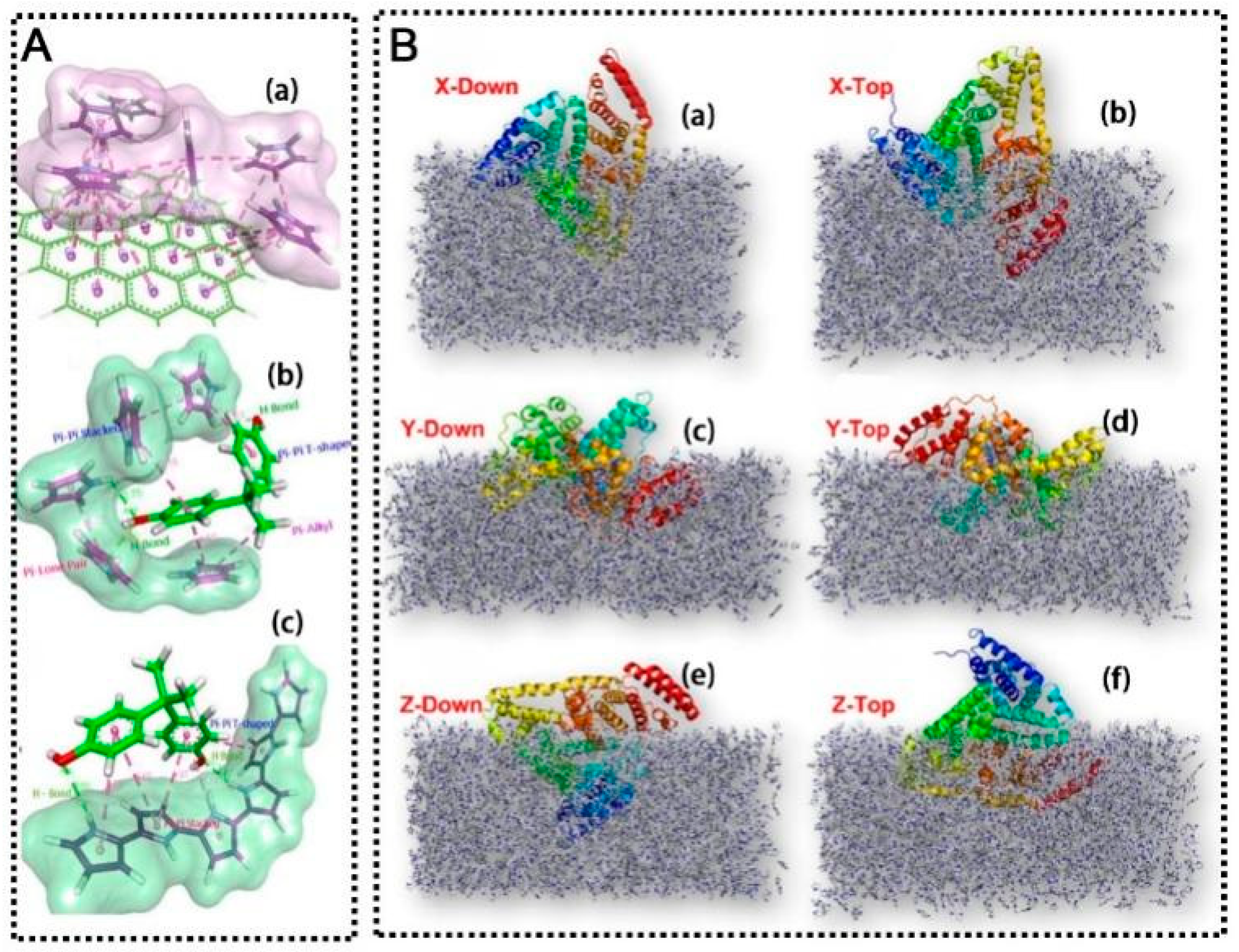

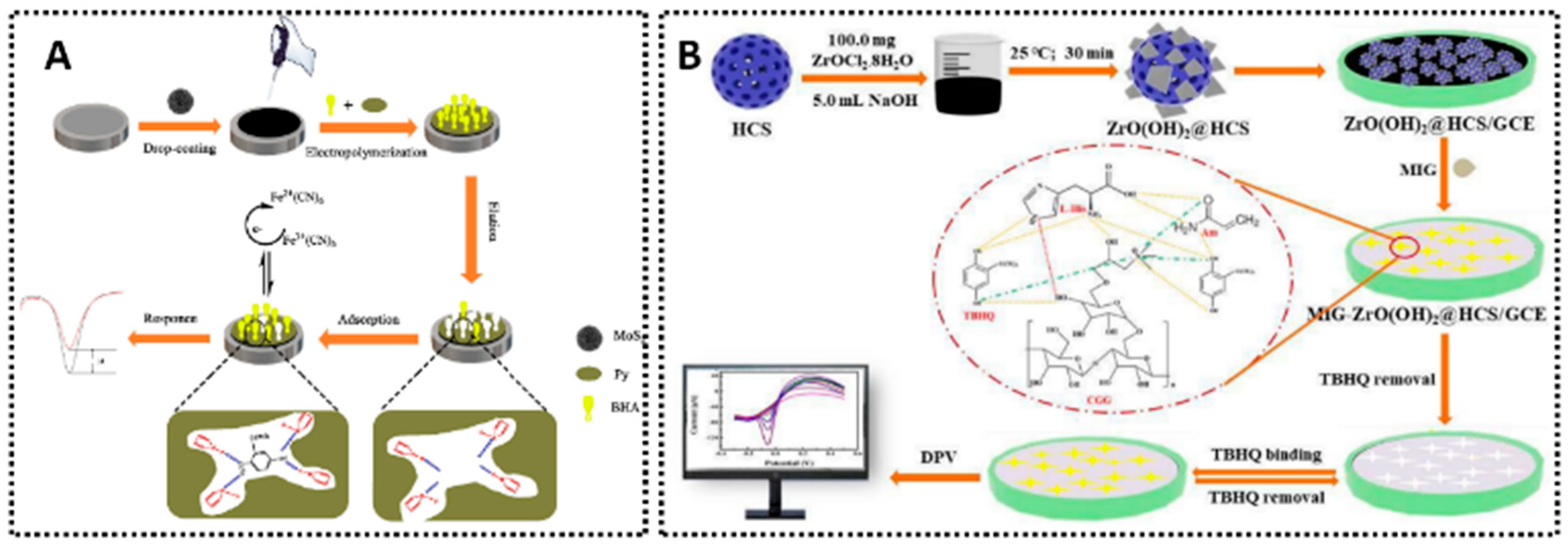
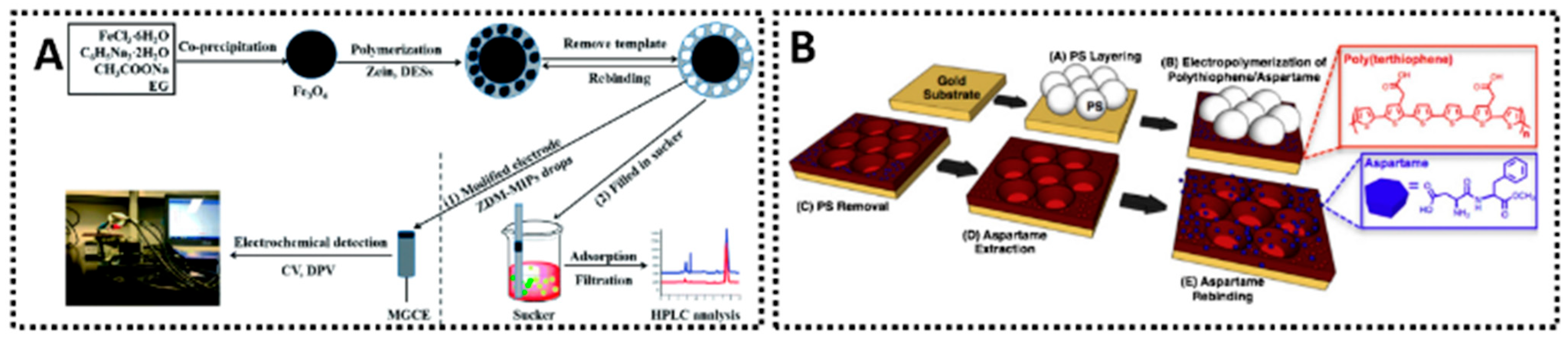
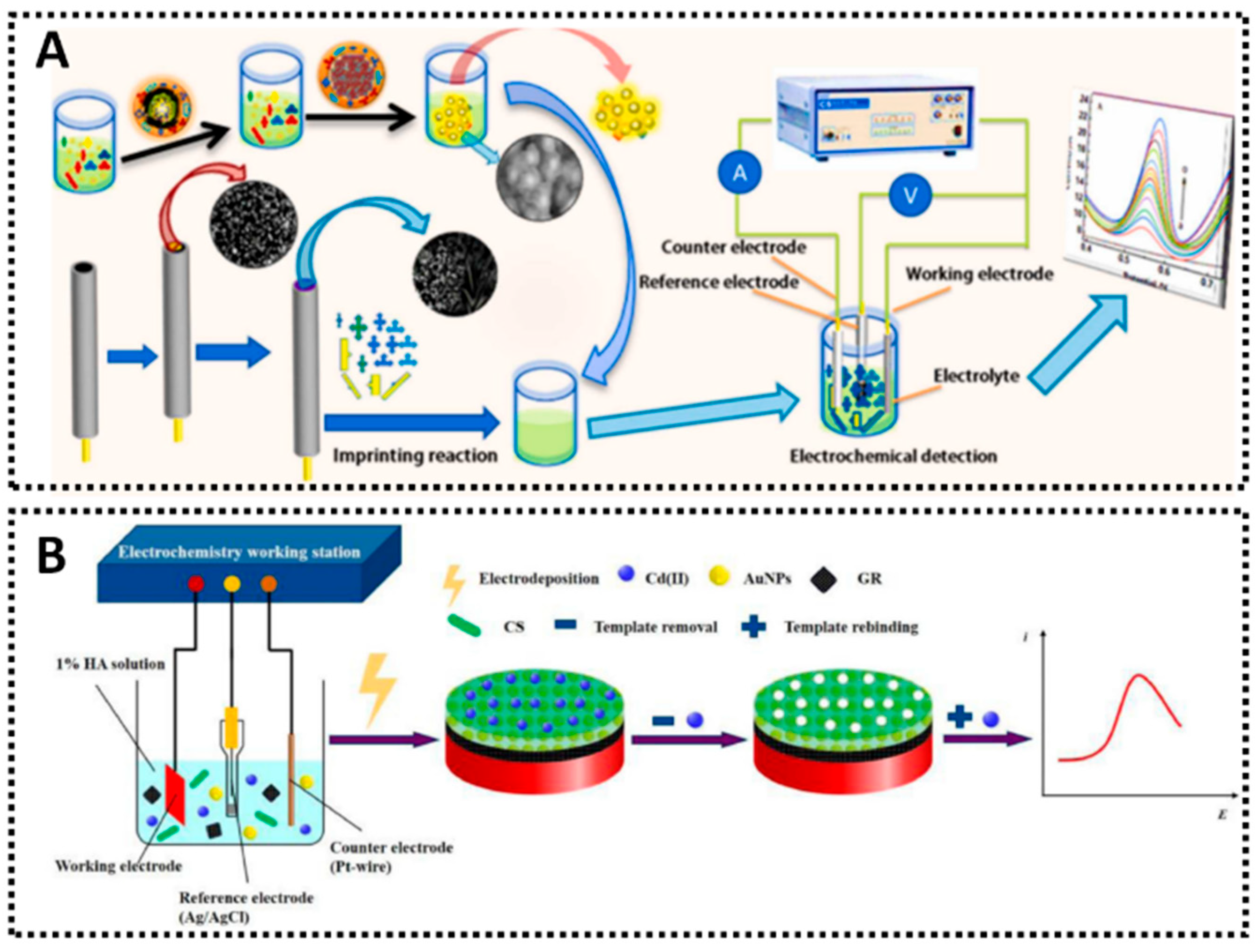

| Imprinting Method | Description and Format | Advantages | Limitations |
|---|---|---|---|
| BP | Traditional polymerization in solution; yields monolith subsequently ground into particles. | Simple setup; high binding capacity (many sites) | Irregular particles; requires post-synthesis grinding; some binding sites buried inside polymer. |
| PP | Polymerization in dilute solution causing spherical MIP nanoparticle precipitation. | Yields uniform nano-sized beads; no grinding needed. | Lower polymerization yield; requires large solvent volume. |
| SI | Imprinted polymer layer formed on surface of supports (e.g., silica or Fe3O4 nanoparticles). | Binding sites fully exposed at surface → fast kinetics and good aqueous compatibility. | Often lower total binding capacity; multi-step synthesis required to graft polymer onto support. |
| Sol–gel imprinting (SGI) | Template and functional alkoxysilanes co-polymerize into a porous silica network. | Inorganic polymer matrix is stable to heat/solvents; tunable porosity. | Fragility of gel structure; potential shrinkage on drying can collapse sites; template removal can be challenging. |
| EP | Electrochemical formation of a polymer film on an electrode in presence of template. | Direct integration onto sensor; precise film thickness control; thin films yield fast and sensitive response. | Limited choice of monomers (must be electroactive); film may contain fewer sites than bulk MIPs. |
| Target Antioxidant | Sensing Platform | MIP Fabrication Method | Functional Monomer(s) | Linear Range | LOD (nM) | Real Sample | Reference |
|---|---|---|---|---|---|---|---|
| Apigenin | ZnO NPs/TrpMA@MIP-GCE | SI | TrpMA ± HEMA co-monomer | 0.1–1.0 pM | 0.0000247 | Celery (Apium graveolens L.); Parsley (Petroselinum crispum (Mill.) Fuss) | [64] |
| Ascorbic acid | e-MIP/SPC | EP | Pyrrole | 0.03–2.4 mM and 0.002–0.1 mM | 1200 | VIVIN C® tablets; TIOBEC® 400 tablets | [65] |
| GC/PPy-MIPox | EP | Pyrrole | 0.005–2 mM | 3000 | Orange juice | [66] | |
| AA-MIP/SPCE | EP | o-Phenylenediamine (o-PD) | 0.45–13.52 μM and 13.52–409.10 μM | 110 | Orange juice | [67] | |
| GE/sol-gel/MIP | SI | 1,3,5-trisacrylamide-2,4,6,-triazine | 0.108–7.86 μM | 35 | Vitamin C tablets; Multivitamin tablets | [68] | |
| PPy-BPQDs-MIPs/PEDOTNRs/GCE | EP | Pyrrole | 0.01–4 mM | 3300 | Soft drink | [69] | |
| BHA | MWCNT/GNP/MIP/GCE | EP | o-PD | 0.01–5 μM and 5–1000 μM | 6 | Mayonnaise; Black seed oil; Soybean oil | [70] |
| MIP/AuNPs/SPCE | EP | Chitosan | 0.056–111.11 μM | 5.6 | Chewing gum; Mayonnaise; Potato chips | [71] | |
| MIP/PdAuNPs/ERGO/GCE | EP | o-PD | 3.01–361.2 μM | 277 | Arowana blend oil | [72] | |
| MIP/GNP/MWCNT/GCE | EP | o-PD | 0.1–0.6 μM and 0.6–1000 μM | 5 | Black seed oil; Walnut oil; Olive oil | [57] | |
| MIP/MoS2/AgNPs-CS/GCE | EP | Pyrrole | 1 nM to 0.1 mM | 7.9 | Potato chips; Oatmeal; Instant noodles | [73] | |
| MIP-MWCNT/GCE | SI | Fe(III)protoporphyrin(IX) (hemin) and methacrylic acid | 1.66–150.0 μM | 500 | Soybean oil; Mayonnaise; Margarine; Biodiesel | [74] | |
| MIPs/GCE | EP | Pyrrole | 90 nM–70 μM | - | Potato chips | [75] | |
| Caffeic acid | MIP/SPCE | BP | N-phenylacrylamide or methacrylic acid | 0–1.11 mM | 130,000 | Burgundy red wine | [76] |
| MIS/AuE | SGI | N-phenylacrylamide | 0.5–60 μM | 150 | Red wines; White wines | [77] | |
| MIP/SPCE | BP | APTES/TEOS-based siloxane mixture | 0.17–0.56 mM | 60,000 | Burgundy red wine | [78] | |
| (+)-Catechin | MIP/GCE | BP | N-phenylacrylamide | 5–100 µM. | 37 | Green tea | [79] |
| MWCNT/MIP/GCE | BP | NR | 1–30 μM and 30–300 μM | 170 | Green tea | [80] | |
| Chlorogenic Acid | MIP/Bi2S3/Ti3C2TX MXene/FTO | SI | Methacrylic acid (MAA) | 0.1412–22.59 μM | 2.4 | Tea; Juice; Coffee | [81] |
| MIS/MWCNTs-VTMS/GCE | SGI | VTMS-grafted MWCNTs; MIS from TEOS/PTEOS/APTMS | 0.08–100 μM | 32 | Coffee; Tomatoes; Apples | [82] | |
| Au/MSL/MIS | SGI | Silane monomer | 0.5–12 μM | 148 | Coffee; Black tea; Green tea; Mate tea | [83] | |
| MIPpy/PGE | EP | Pyrrole | 1 μM–10 mM | 1000 | Roasted coffee samples (arabica, excelsa, liberica and robusta) | [84] | |
| D MMIPs | SI | Methacrylic acid | 0.014–0.085 mM | - | Eucommia leaves extract | [85] | |
| Curcumin | PAA-MIP/G | BP | Acrylic acid (AA)/poly(acrylic acid) | 1–10 μM and 10–180 μM | 40 | Raw turmeric; Turmeric powder; Turmeric capsules | [86] |
| GCE/CuCo2O4/N-CNTs/P-GO/MIP | EP | l-cysteine | 0.1–0.8 μM and 0.8–30 μM | 30 | - | [87] | |
| MIP/CPE | PP | Methacrylic acid | 0.1–50 μM | 10.1 | Curcuma powder; Curcuma cookies | [88] | |
| Dodecyl gallate | f-MWCNT/MIP/GCE | EP | o-PD | 0.5–8 nM | 0.22 | - | [89] |
| Epigallocatechin gallate | MIP/GO/GCE | EP | β-cyclodextrins | 30 nM–10 μM | 8.78 | Tea samples (including Puer Tea, Black Tea, Red Tea, Tieguanyin tea, and White Tea) | [90] |
| MIP-Ni(OH)2/GCE | BP | NR | 10–200 μM | 7 | Green tea | [79] | |
| MIP/membrane electrode | BP | NR | 0.03 µg/mL to 1 µg/mL | - | Commercial tea drinks | [91] | |
| Ferulic acid | SPE/rGO-AuNPs-MIP | EP | Phenol | 10 nM–1 μM and 2–10 μM | 3.1 | Orange peels | [92] |
| Gallic acid | MIP/MWCNT/CPE | PP | Methacrylic acid | 0.12–380.0 μM | 47 | Apple juice; Pineapple juice; Orange juice; Commercial green tea drink | [93] |
| MIP/SPE | EP | Pyrrole | 5–70 μM | 5000 | White wine; Red wine; Rosé wine; Marsala wine; Green tea (Lipton) | [94] | |
| PPy-MIP/GCE | EP | Pyrrole | 0.1–2.0 mM | - | - | [95] | |
| MIP/TiO2@CNTs/GCE | EP | NR | 50–700 µM | 12 | Green tea | [96] | |
| MAA-MIP | BP | Methacrylic acid | 0.01–0.32 mM | 10,000 | Green tea; Pineapple; Orange; Apple; Passion fruit | [97] | |
| MIP–CuO | BP | NR | 1–100 µM and 100–900 µM | 12.6 | Green tea | [98] | |
| MIP/Fe3O4@ZIF-67/Au | EP | Pyrrole | 6–600 pM | 0.000297 | Black tea; Green tea | [99] | |
| Luteolin | Fe3O4@MIP/rGO/GCE | SI | Methacrylic acid | 2.5 pM–0.1 μM | 0.001 | Lotus leaves extract | [100] |
| MIP/MoS2/GN-CNTs/GCE | EP | Carbazole | 0.04–2.0 μM | 9.0 | Carrot; Chrysanthemum tea | [101] | |
| MoS2-MIPs/GCE | EP | 3,4-ethylenedioxythiophene | 0.3–30 μM | 40 | Gnaphalium affine | [102] | |
| p-Coumaric acid | SPE/rGO-NiNPs-MIP | EP | 3-Indoleacetic acid | 0.1–1 nM and 1–10 nM | 0.081 | Banana peel extract; Orange peel extract | [103] |
| Propyl gallate | MIP/GNP/MWCNT/GCE | - | o-PD | 0.01–5 μM and 5–1000 μM | 6 | Mayonnaise; Black cumin oil; Soybean oil | [104] |
| Quercetin | TrpMA@QUE/MIP-GCE | Surface imprinting-Photopolymerization | TrpMA | 1–25 pM | 0.000235 | Rubus sanctus (stem, leaf, and flower extracts); Fragaria vesca (fruit extracts); Commercial herbal supplements | [105] |
| MIP/Pd/pGN-CNTs/GCE | EP | p-Aminobenzoic acid | 0.01–0.50 μM | 5 | Pule’an tablets; Honeysuckle juice; Red wine | [106] | |
| SPE|MrGO-MIP | SI | Acrylamide | 0.02–250 μM | 13 | Pharmaceutical tablets | [107] | |
| MIP/MIL-101(Cr)/MoS2/GCE | EP | MAA | 0.1–10.5 μM and 10.5–700 μM | 60 | Honey | [108] | |
| Resveratrol | GCE|Gr-Au/MIPs | SI | Acrylamide | 0.01–10.0 µM | 4.4 | Red wines; Grape skins | [109] |
| PAM/PANI/AuNPs/GCE | SI | Aniline (polyaniline) and acrylamide (PAM) | 1.0–200 μM | 87 | Red wines | [110] | |
| CEMIP | SI | Methacrylic acid | 0.1–5.0 mg/L | 87.6 | Red wine | [111] | |
| MIP/Au@Ag/ITO | EP | o-PD | 20 pM–1 nM | 0.0071 | Grape seed extract | [112] | |
| Rutin | MA-Asp@RUT/MIP-GCE | Photopolymerization | Methacrylic acid–aspartic-acid-derived monomer | 1–10 pM | 0.000269 | Herbal supplement samples | [113] |
| MIP/G-MWCNTs/GCE | EP | Pyrrole | 0.01–1.0 μM | 5 | Buckwheat tea; Orange juice | [114] | |
| Syringic acid | MIP-AN@G | BP | Aniline | 10–100 μM | 320 | Cauliflower; Oregano; Black olives | [115] |
| TBHQ | MIP-MWCNT/GCE | SI | Fe(III)protoporphyrin(IX) (hemin) and methacrylic acid | 2.84–150.0 μM | 850 | Soybean oil; Mayonnaise; Margarine; Biodiesel | [74] |
| MIP/MoS2/EACC | EP | o-ATP@AuNPs | 1 μM–0.5 mM and 0.5–120 mM | 0.72 | Soybean oil; Peanut oil; Sesame oil | [116] | |
| MIP/AuNPs/EGP | EP | Pyrrole | 80 nM–1 μM and 1–100 μM | 12 | Edible oil | [117] | |
| MIG-ZrO(OH)2@HCS/GCE | SGI | Guar gum | 0.025–100 μM | 6.7 | Peanut oil; Milk powder; Snowflake chicken cutlets; Crispy fried pork; Boneless chicken filets | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhao, S.; Zhu, J.; Fu, L. Advances in Molecularly Imprinted Electrochemical Platforms for Food Quality Control: Targeting Antioxidants, Sweeteners, Colorants, Contaminants and Toxicants. Chemosensors 2025, 13, 398. https://doi.org/10.3390/chemosensors13110398
Zhang L, Zhao S, Zhu J, Fu L. Advances in Molecularly Imprinted Electrochemical Platforms for Food Quality Control: Targeting Antioxidants, Sweeteners, Colorants, Contaminants and Toxicants. Chemosensors. 2025; 13(11):398. https://doi.org/10.3390/chemosensors13110398
Chicago/Turabian StyleZhang, Lu, Shichao Zhao, Jiangwei Zhu, and Li Fu. 2025. "Advances in Molecularly Imprinted Electrochemical Platforms for Food Quality Control: Targeting Antioxidants, Sweeteners, Colorants, Contaminants and Toxicants" Chemosensors 13, no. 11: 398. https://doi.org/10.3390/chemosensors13110398
APA StyleZhang, L., Zhao, S., Zhu, J., & Fu, L. (2025). Advances in Molecularly Imprinted Electrochemical Platforms for Food Quality Control: Targeting Antioxidants, Sweeteners, Colorants, Contaminants and Toxicants. Chemosensors, 13(11), 398. https://doi.org/10.3390/chemosensors13110398