Abstract
Ensuring food safety and quality has become increasingly critical due to the complexities introduced by globalization, industrialization, and extended supply chains. Traditional analytical methods for food quality control, such as chromatography and mass spectrometry, while accurate, face limitations including high costs, lengthy analysis times, and limited suitability for on-site rapid monitoring. Electrochemical sensors integrated with molecularly imprinted polymers (MIPs) have emerged as promising alternatives, combining high selectivity and sensitivity with portability and affordability. MIPs, often termed ‘plastic antibodies,’ are synthetic receptors capable of selective molecular recognition, tailored specifically for target analytes. This review comprehensively discusses recent advancements in MIP-based electrochemical sensing platforms, highlighting their applications in detecting various food quality markers. It particularly emphasizes the detection of antioxidants—both natural (e.g., vitamins, phenolics) and synthetic (e.g., BHA, TBHQ), artificial sweeteners (e.g., aspartame, acesulfame-K), colorants (e.g., azo dyes, anthocyanins), traditional contaminants (e.g., pesticides, heavy metals), and toxicants such as mycotoxins (e.g., aflatoxins, ochratoxins). The synthesis methods, including bulk, precipitation, surface imprinting, sol–gel polymerization, and electropolymerization (EP), are critically evaluated for their effectiveness in creating highly selective binding sites. Furthermore, the integration of advanced nanomaterials, such as graphene, carbon nanotubes, and metallic nanoparticles, into these platforms to enhance sensitivity, selectivity, and stability is examined. Practical challenges, including sensor reusability, regeneration strategies, and adaptability to complex food matrices, are addressed. Finally, the review provides an outlook on future developments and practical considerations necessary to transition these innovative MIP electrochemical sensors from laboratory research to widespread adoption in industry and regulatory settings, ultimately ensuring comprehensive food safety and consumer protection.
1. Introduction
Food safety and quality control are of paramount importance in the modern food industry. With globalization and industrialization, the diversity of food products and potential contaminants has expanded dramatically [1]. Consumers and regulators demand assurance that foods are free from harmful substances such as pesticides, toxins, adulterants, and other contaminants [2,3]. Globalization has lengthened and complicated food supply chains, introducing more points at which contamination can occur [4]. Food products can encounter hazards at every stage: on the farm through overuse of pesticides and veterinary drugs, during processing via inadvertent introduction of allergens or harmful additives, from packaging through migration of plasticizers or heavy metals, or in storage due to microbial growth and toxin production. High-profile incidents such as the melamine adulteration of milk powder and the presence of Sudan dyes in spices underscore the consequences of undetected contaminants [5,6]. These events have spurred stricter regulations and oversight, fueling demand for rapid screening tools that can be deployed outside traditional laboratories [7]. Consumers are increasingly aware and concerned about food safety, putting pressure on industry and regulators to ensure rigorous testing at all steps [8]. Regulatory agencies worldwide are enacting stricter limits and more frequent monitoring requirements, further motivating the development of rapid, reliable sensing technologies [9,10].
Traditional analytical methods (e.g., chromatography, mass spectrometry, immunoassays) provide high accuracy but often require expensive instrumentation, skilled personnel, and lengthy procedures [11,12,13]. These centralized lab-based tests are not always practical for rapid, on-site decision making in the food supply chain. There is thus a critical need for cost-effective, rapid, and portable detection methods that can be deployed in the field for real-time monitoring. Electrochemical sensors have emerged as powerful tools for food analysis due to their sensitivity, speed, and portability [14,15]. In particular, coupling electrochemical transducers with molecularly imprinted polymers (MIPs) has gained significant attention. MIPs are synthetic receptors with tailor-made binding sites complementary to target analyte molecules, often described as “plastic antibodies.” When used as recognition units in sensors, MIPs can confer high selectivity towards the analyte, even in complex food matrices [16]. MIP-based electrochemical sensors combine the selectivity of biochemical recognition with the robustness and low cost of polymer materials [17,18,19]. Over the past decade, the number of publications on MIP-modified electrochemical sensors for food safety applications has increased substantially, highlighting their growing prominence.
While recent valuable reviews have focused on MIP electrochemical sensors, they often center on specific advancements, such as material-centric strategies or are confined to a single analyte class, such as food contaminants [20]. In contrast, the distinct contribution and novelty of this manuscript lie in its novel and comprehensive classification of the field, structured entirely around the diverse applications within total food quality control.
To provide a holistic overview, we have explicitly structured this review into five distinct application-driven pillars, which are reflected in the subsequent sections:
Food Quality & Nutritional Markers (Section 3): Targeting natural and synthetic antioxidants (e.g., vitamins, phenolics, BHA, TBHQ) that are crucial for product quality, authenticity, and nutritional labeling.
Food Additive Monitoring Sweeteners (Section 4): Focusing on the detection of artificial sweeteners (e.g., aspartame, acesulfame-K) for regulatory compliance and identification of mislabeling.
Food Additive Monitoring Colorants (Section 5): Reviewing sensors for both natural (e.g., anthocyanins) and synthetic (e.g., azo dyes) colorants used for quality assurance.
Traditional Food Safety Contaminants (Section 6): Addressing legacy public health hazards, including pesticides, heavy metals, and mycotoxins (e.g., aflatoxins, ochratoxins).
Emerging Contaminants & Toxicants (Section 7): Focusing on newly recognized chemical threats, such as endocrine disruptors from packaging (e.g., bisphenols, PFAS) and intentional adulterants (e.g., melamine, Sudan dyes).
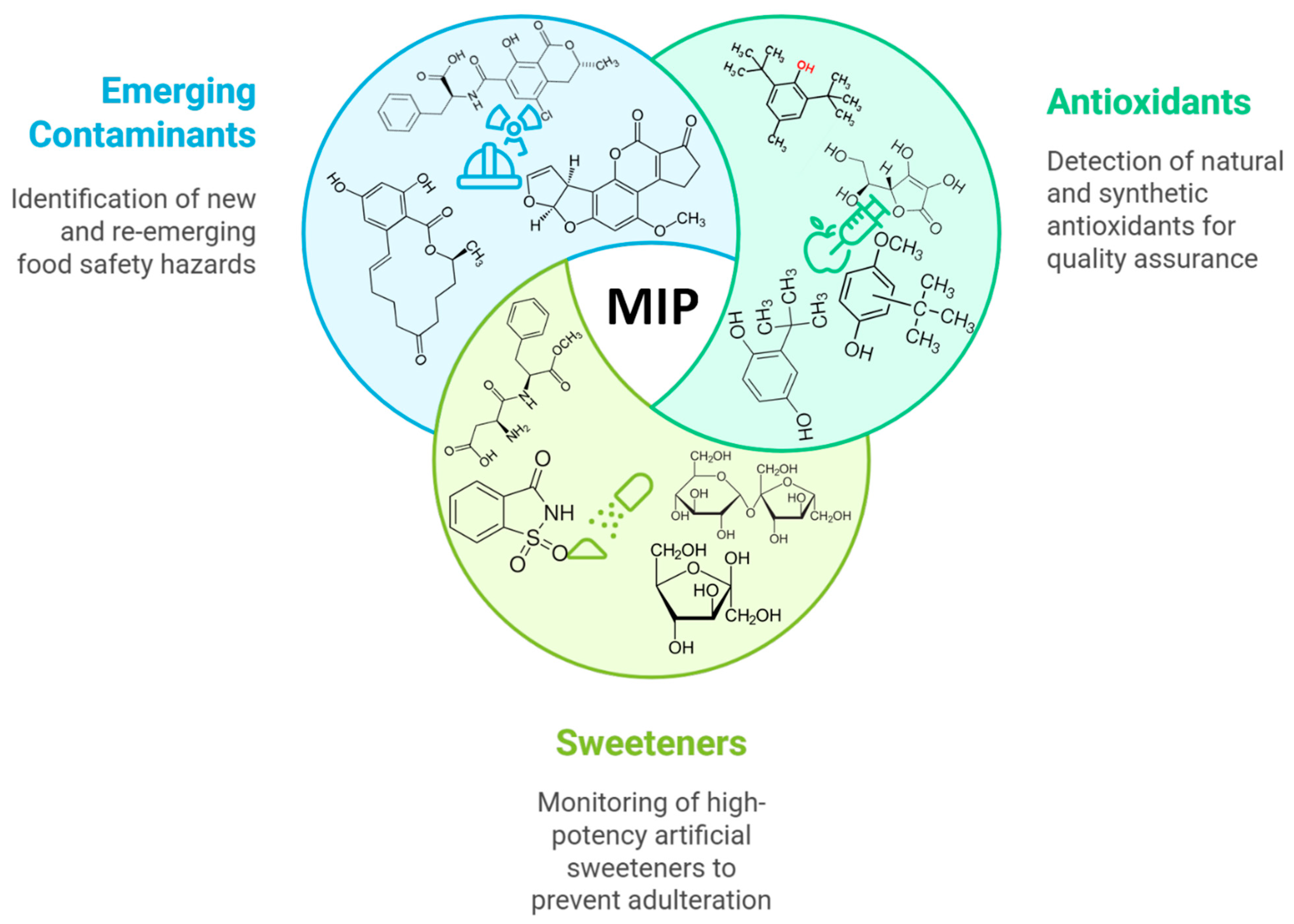
This five-part classification (visualized in Figure 1) provides a unique framework that bridges the gap between nutritional analysis, additive regulation, and contaminant screening. Following an overview of fundamentals (Section 2), each thematic section critically evaluates recent sensor advancements, material integrations, and performance in real food matrices. Finally, Section 8 addresses overarching challenges and future perspectives necessary to transition these technologies from the laboratory to industrial and regulatory adoption. We believe this comprehensive, application-centric structure offers a new and valuable perspective for researchers in food science, analytical chemistry, and sensor development.
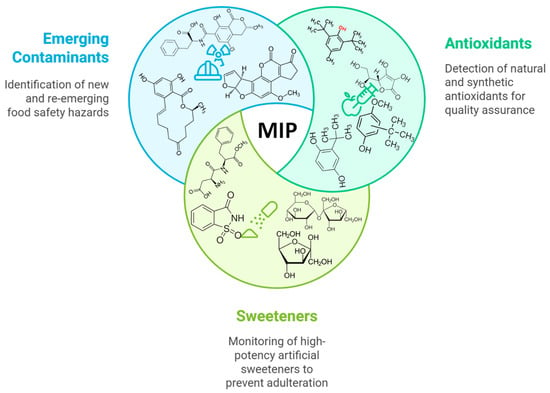
Figure 1.
Schematic illustration of the key areas of interest in food quality control using MIP electrochemical platforms: antioxidants, sweeteners, and emerging contaminants.
2. Fundamentals of MIP-Based Electrochemical Sensors
2.1. Principles and Fabrication
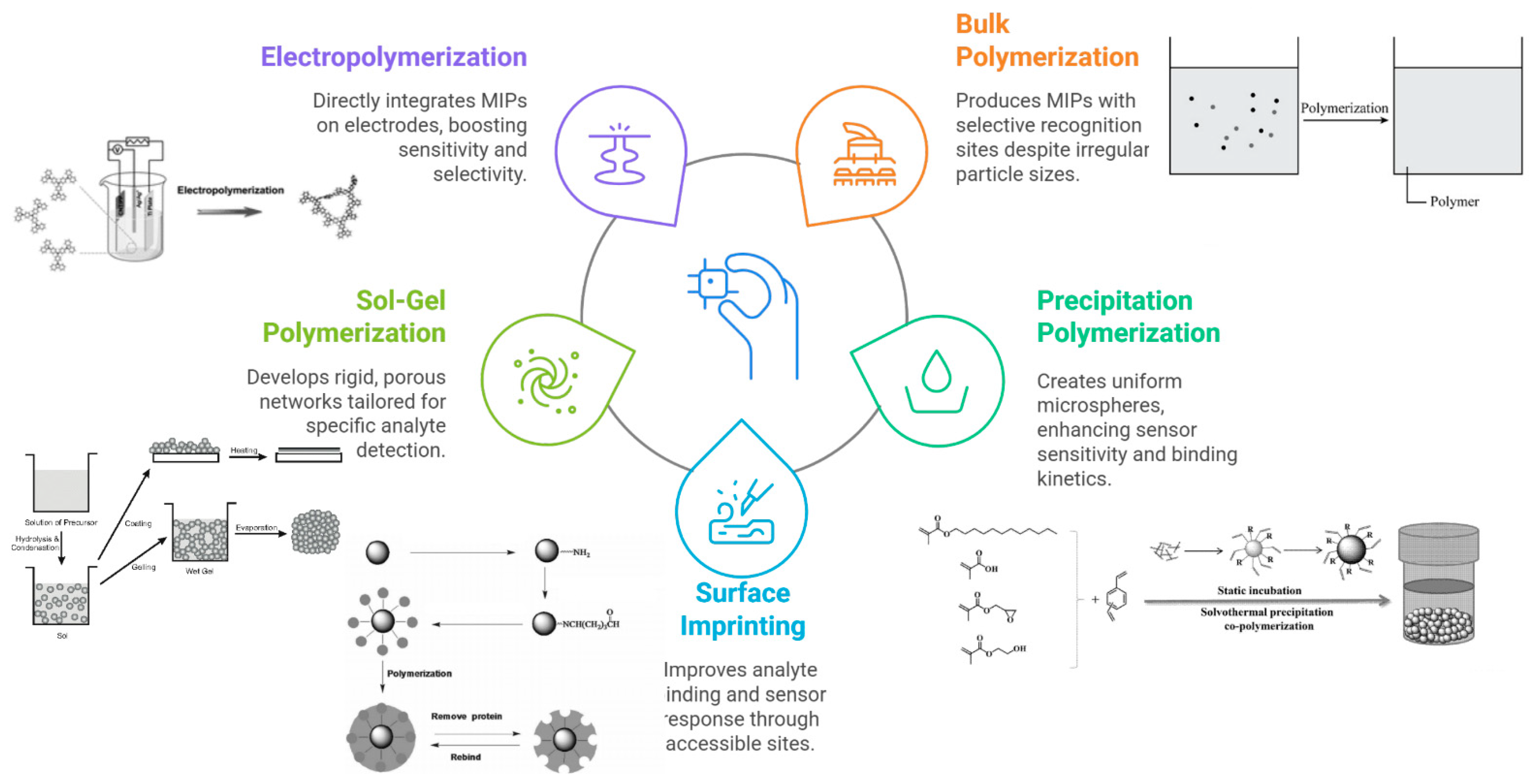
Molecular imprinting is a technique to create synthetic polymer receptors with high affinity for a target molecule (the “template”). In a typical imprinting process, functional monomers self-assemble around the template via covalent or non-covalent interactions; a cross-linking monomer polymerizes to form a rigid network, and then the template is removed, leaving behind cavities complementary in shape and functionality to the target analyte. These MIPs can bind the target selectively, much like antibodies or enzymes, but with greater chemical and thermal stability. MIPs can be tailored for a vast array of targets ranging from small molecules to proteins and even whole cells. Figure 2 and Table 1 show several polymerization methods are employed to synthesize MIPs.
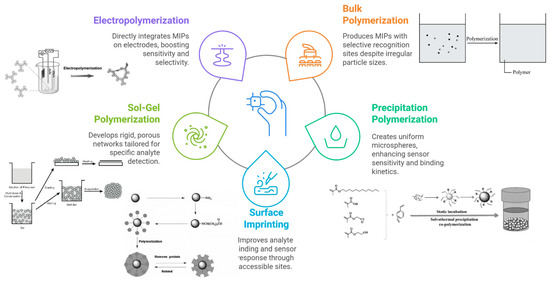
Figure 2.
Schematic representation of the main polymerization strategies used to synthesize MIPs for electrochemical sensing applications.

Table 1.
Common fabrication methods for MIP used in electrochemical sensors.
Bulk polymerization (BP) is a common approach where polymerization occurs in a homogeneous solution containing monomers, cross-linker, initiator, and template. In bulk imprinting, phase separation of the growing cross-linked network in a poor/low-polarity porogen yields a macroporous monolith whose pore architecture is governed by the porogen’s solvency parameter and the cross-linking density. Strong pre-organization of template–monomer complexes (typically hydrogen bonding or ionic pairing) reduces site heterogeneity; excessive cross-linker increases rigidity and stability but can bury sites and slow mass transport. Mechanistically, the interplay of (i) monomer–template association constants, (ii) polymerization rate (initiator level/temperature), and (iii) porogen-driven spinodal vs. nucleation-and-growth demixing dictates binding-site fidelity and accessibility after grinding. Recent analyses show cross-link density is a primary knob controlling mechanical robustness and rebinding kinetics, while porogen choice determines hierarchical porosity and diffusion paths.
Precipitation polymerization (PP) is a versatile technique carried out in a large excess of solvent, causing the polymerized material to form micro- or nano-spheres directly in suspension. Under high-dilution conditions, primary nuclei form once oligomer solubility is exceeded; particle growth proceeds via capture of soluble oligomers and limited aggregation, producing monodisperse micro/nanoparticles with surface-exposed sites. Compared with bulk gels, precipitation particles exhibit narrower site-energy distributions and faster binding kinetics because template-adjacent domains are closer to the surface and diffusion paths are shorter. Control variables include total monomer concentration (governing critical nucleation), cross-linking ratio (fixing rigidity vs. site collapse), and porogen dielectric constant.
Surface imprinting and core–shell (SI) approaches involve creating the imprinted polymer layer on the surface of a support (such as silica beads, magnetic nanoparticles, or electrode surfaces). When an imprinted layer is grown around a core (e.g., silica, Fe3O4, Au), cavities are located within a thin shell; diffusion times scale with shell thickness, so thin, hydrophilic shells minimize kinetic barriers and nonspecific adsorption in aqueous food matrices. Mechanistically, grafting density and surface-initiated polymerization rates control shell continuity and the fraction of fully formed cavities. Dummy-template strategies are often preferred here to avoid template bleeding from thin films into analytical matrices.
Another technique, sol–gel polymerization, uses inorganic precursors (like alkoxysilanes) to create a porous silica network encapsulating the template. Sol–gel MIPs can be highly rigid and often prove compatible with water-rich matrices, though controlling the final film porosity can be challenging. For instance, Garg et al. [21] employed a sol–gel approach using tetraethyl orthosilicate (TEOS) and 3-aminopropyltrimethoxysilane (APTMS) to build a selective MIP layer for detecting hypoxanthine in meat. By integrating curcumin-coated iron oxide nanospheres and multiwalled carbon nanotubes, they enlarged the sensor’s surface area and enhanced electron transport, ultimately achieving sensitive and specific recognition even in complex sample environments. In a similar strategy, Liu and co-workers [22] created a sol–gel MIP sensor for acrylamide detection, with TEOS as the cross-linker and APTMS as the functional monomer. Their electrode was first modified with Au nanoparticles, carbon nanotubes, and chitosan to improve conductivity and mechanical stability. Then, the sol–gel mixture containing the template (acrylamide) was polymerized on this conductive substrate, producing a thin yet robust imprinted film. Once the template was extracted, the resulting sol–gel MIP exhibited multiple binding cavities that matched acrylamide’s size and arrangement of functional groups. Both studies highlight key advantages of sol–gel polymerization, such as relatively straightforward preparation steps, stable silica-based networks, and the possibility of fine-tuning the surface chemistry by selecting suitable monomers and cross-linkers. Still, optimizing the sol–gel conditions (e.g., pH, catalyst amount, and precursor ratios) is essential to achieve uniform film thickness and consistent binding cavities.
EP is particularly useful for sensor fabrication. In this approach, a conducting monomer is polymerized directly onto an electrode surface by applying an electrochemical potential in the presence of the template. Common electropolymerizable monomers include anilines, phenols, pyrrole, and o-phenylenediamine, among others. EP allows fine control of film thickness (by limiting the charge or time of polymerization) and often produces MIP films with the template binding sites oriented at the electrode interface. This yields high sensitivity, since the target binding can directly modulate the electrochemical signal [23]. A potential drawback is that electropolymerized MIP films may have fewer binding sites than bulk-synthesized particles due to the constrained polymer growth on the surface. However, EP is very reproducible and easily integrated with microfabrication, making it attractive for developing disposable sensor devices. Recent studies have shown that electropolymerized MIPs can achieve remarkable detection limits when combined with various nanomaterials. For instance, Roushani and Zalpour [24] demonstrated the selective detection of Asulam using an in situ dopamine EP-based electrochemical MIP sensor, achieving ultra-trace sensitivity through direct EP on a glassy carbon electrode (GCE). In another example, Bougrini and co-workers [25] devised a MIP sensor via EP of a microporous-metal–organic framework on a gold electrode surface for tetracycline detection in honey, demonstrating how mesoporous structures can facilitate a high density of imprinting sites and enhance mass transport. Moreover, recent work by Wu et al. incorporated multiwalled carbon nanotubes to reinforce the conductive matrix of polypyrrole-based MIPs, leading to a stable and highly selective sensor for the detection of the carcinogenic dye amaranth [26]. These studies highlight the versatility of EP in creating thin yet robust MIP films with strong, selective interactions toward target analytes. An important advantage of this methodology is that the generation of the polymeric layer is straightforward and does not require complicated chemical crosslinkers, thereby reducing synthetic steps. Meanwhile, the ability to finely tune the thickness of the MIP film by controlling EP conditions helps mitigate the reduced number of accessible binding sites, which can otherwise hinder recognition efficiency.
Regardless of the fabrication method, removal of the template after polymerization is a crucial step to “activate” the MIP. Adequate washing or solvent extraction yields binding sites that can rebind the target. For food applications, it is also important that any residual template or unreacted monomers are thoroughly removed to avoid contaminating the sample or causing background signals.
2.2. Electrochemical Transduction Mechanisms
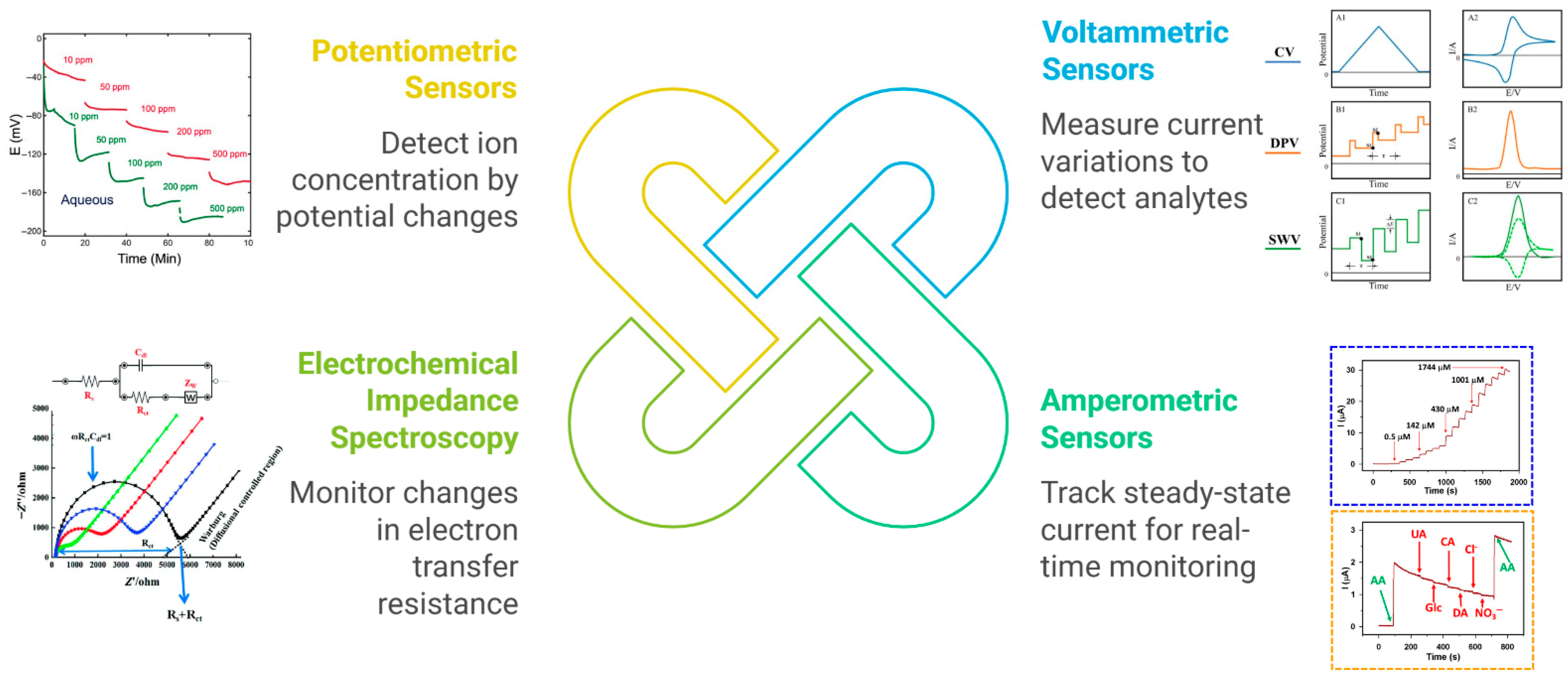
When integrated into an electrochemical sensor, the MIP serves as the selective recognition element, while the electrode transduces the binding event into a measurable electrical signal. Crucially, specific molecular interactions within the MIP—electrostatic pairing, hydrogen bonding, and π–π stacking—alter interfacial charge transport (film permeability, local double-layer structure, and the heterogeneous electron-transfer rate constant), which is what the electrode ultimately reads. Several transduction modes are commonly employed (Figure 3):
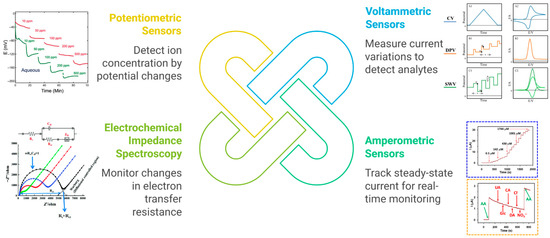
Figure 3.
Schematic overview of the principal electrochemical transduction modes in MIP-based sensors for food quality control.
Voltammetric sensors measure current as a function of the applied potential, enabling sensitive detection of food-relevant analytes. In a voltammetric MIP sensor, target binding modulates redox-probe access and k0 at the interface—for charged targets via Donnan exclusion/accumulation of [Fe(CN)6]3−/4−; for neutral H-bonded complexes via increased film density and lower local permittivity; and for aromatic targets via π–π charge-transfer complexation that can either facilitate direct electron transfer (DET) or shift oxidation potentials. These effects manifest as systematic changes in peak currents and, when modeled as a Randles circuit, as concomitant changes in Rct in EIS. Both differential pulse voltammetry (DPV) and square-wave voltammetry (SWV) are regularly employed to follow the decreased faradaic current of a redox marker (e.g., ferricyanide) as analytes bind within the polymer layer. When the molecule of interest itself is electroactive, the MIP can pre-concentrate it on the electrode, allowing direct measurement of its oxidation or reduction signal [27]. These techniques have proven valuable for food quality control. For instance, researchers used a nanomaterial-modified voltammetric platform to detect caffeic acid in dietary supplements with high sensitivity, capitalizing on carbonaceous nanofibers to improve electron transfer [28]. Likewise, carbon paste electrodes coupled with ionic liquids have facilitated simultaneous determination of vitamins such as B6 and C in real samples, demonstrating precise, rapid, and low-cost quantification [29]. In both examples, the synergy of electrode surface modification and advanced electroanalytical techniques results in excellent selectivity, low detection limits, and resistance to matrix interferences. The incorporation of an MIP layer can further bolster specificity by tailor-fitting binding cavities to the analyte’s structure.
Amperometric sensors operate by fixing the electrode at a constant potential and tracking the resulting steady-state current as the target analyte undergoes oxidation or reduction. This straightforward, single-potential mode is particularly advantageous for real-time monitoring in flow-through systems or continuous assays. Recent studies highlight how adding functional nanomaterials to MIP-based amperometric platforms can substantially amplify signals and improve detection limits. For instance, strontium molybdate embedded in graphitic carbon nitride has been applied toward amperometric sensing of food contaminants such as chloramphenicol, demonstrating ultralow detection limits and high sensitivity in complex matrices [30]. In another approach, a poly(bromocresol purple) film coated onto a carbon nanotube-modified carbon paste electrode provided a robust, fouling-resistant surface for the amperometric detection of tyrosine in milk and blood serum [31]. These examples illustrate how coupling MIP technology with nanomaterial-modified electrodes maximizes surface area, enhances electron transfer, and mitigates interferences from non-target species—each of which is vital for precise analyte measurement in food-quality applications. When the MIP layer selectively binds the target (e.g., phenolic antioxidant or residual antibiotic) and the electrode’s potential is held at an optimal oxidation or reduction value, the current directly correlates with the analyte concentration.
Electrochemical impedance spectroscopy (EIS) offers a powerful strategy for monitoring how target analytes modulate electron transfer at electrode interfaces. By immobilizing selective receptors within a MIP, the impedance increases once the analyte occupies the binding sites, effectively blocking the electrode surface. This principle extends beyond electroactive molecules: even non-electroactive species can be tracked via EIS simply by observing shifts in charge-transfer resistance. For example, a sensitive immunosensor for aflatoxin B1 (AFB1) in olive oil used a carbon nanotube/ionic liquid composite film to anchor antibodies [32]. Upon binding AFB1, the impedance rose significantly, enabling detection limits as low as 0.03 ng/mL and showing feasibility for on-site screening. Meanwhile, a portable EIS platform for detecting the foodborne bacterium Listeria monocytogenes in milk illustrates another application of impedimetric analysis [33]. In that work, gold interdigitated microelectrodes were functionalized with anti-Listeria antibodies and integrated into a microfluidic system for sample handling. The device achieved rapid detection of even very low bacterial counts (down to five cells per milliliter) after just one hour of incubation, with minimal matrix interference from the milk. Both examples highlight how EIS-based MIP sensors can identify pathogens or contaminants in complex media by capturing the target molecule on a functionalized electrode and reading the resulting impedance change.
Potentiometric sensors excel at detecting ions in complex matrices by monitoring potential changes at zero current. A prime example involves detecting metal cations like Cu(II) through carbon-paste or polymer-membrane electrodes embedded with selective ligands. One recent study employed fenoprofen as the key ionophore in a carbon paste electrode for copper determination, producing a Nernstian slope near 30 mV/decade and allowing accurate Cu(II) quantification in real food samples such as lentils, spinach, and mushroom extracts [34]. Another application highlights the determination of a cationic spoilage marker—tyramine—via a solid-state potentiometric sensor constructed by coating a plasticized PVC cocktail onto a glassy carbon substrate. This sensor displayed near-Nernstian response (approximately 57 mV/decade) to tyramine over a wide linear range and minimal interference from other ions. Impressively, the sensor enabled direct analysis of tyramine in aged cheese and pickled fish without elaborate sample preparation [35]. Both approaches underscore the versatility and robustness of potentiometric platforms for food safety: once the analyte binds in a charged state, the electrode’s voltage shifts in proportion to ion activity. Such zero-current detection is particularly advantageous for labile or low-level species, as the measurement itself does not perturb the analyte concentration. Potentiometric MIP sensors expand upon this by imprinting ion-specific cavities into a polymer matrix, ensuring high recognition and minimal cross-reactivity.
Each transduction method has its merits. Voltammetry and amperometry often achieve lower detection limits (LODs in the nM or μg/L range) because of signal amplification through redox processes. EIS and potentiometry allow label-free detection of a broad range of analytes, including those that are not easily oxidized or reduced, albeit with typically slightly higher LODs than voltammetric methods. Some sensors combine multiple techniques (e.g., measuring both DPV and EIS) to glean more information or to cross-verify the detection. Beyond purely electrochemical readouts, photoelectrochemical (PEC) MIP sensors have emerged in which light-excited semiconductors translate binding events into photocurrent changes; by separating optical excitation from the bias at the electrode, PEC often suppresses background and matrix fouling. Recent food-relevant examples include MIP-PEC assays for aflatoxin B1 and bisphenol A with sub-ppb performance in real samples [36]. Optoelectronic formats such as electrochemiluminescence (ECL) and SPR with MIPs likewise provide sensitive, label-free or luminescent outputs compatible with complex food matrices [37]. Finally, dual-mode MIPs (e.g., electrochemical–colorimetric or ECL–colorimetric) deliver ratiometric confirmation to reduce false positives, as shown for aflatoxin B1 and pesticide screening [38].
At the polymer/electrolyte/electrode interface, specific recognition forces inside the MIP couple to electrochemical readouts through three dominant pathways:
- (i)
- Electrostatic recognition → redox-probe gating (DPV/SWV/EIS). Binding of ionic analytes or formation of charged complexes establishes a Donnan potential within the MIP that excludes (for like-charged) or enriches (for oppositely charged) outer-sphere redox markers, e.g., [Fe(CN)6]3−/4−. The resulting change in probe concentration and in the heterogeneous electron-transfer rate constant k0 increases the semicircle radius in Nyquist plots and lowers DPV/SWV peak currents. Energy-diagrammatically, the driving-force alignment is unchanged, but the effective barrier width and access of the probe to the electrode are altered by the bound charge cloud.
- (ii)
- Hydrogen-bonding recognition → microenvironment densification and dielectric modulation. Multiple H-bonds around the cavity compact the polymer locally and reduce permittivity, decreasing redox-probe diffusivity and k0. This appears as higher Rct (EIS) and suppressed faradaic responses (DPV/SWV). For non-electroactive targets, the signal is entirely indirect; for electroactive targets, the same densification may preconcentrate the analyte in the cavity and yield a net “signal-on” response via DET at suitable potentials.
- (iii)
- π–π stacking recognition → charge-transfer complexation and band/level alignment. Aromatic targets can stack with π-conjugated hosts (polypyrrole/graphene), forming weak charge-transfer complexes that increase local carrier density or lower the overpotential for direct oxidation of phenolics. In energy-level terms, binding perturbs the interfacial density of states and reduces the tunneling barrier, which can shift peak potentials and increase current—opposite in sign to purely blocking effects. This pathway rationalizes “signal-on” voltammetry observed for many phenolics on PPy/graphene-MIPs.
These three pathways are consistent with contemporary MIP electrochemical reviews that attribute impedimetric signals to binding-induced changes in film permeability and Rct, and voltammetric signals to a balance of probe-gating versus DET/preconcentration effects. We adopt the standard Randles-circuit language and recommend reporting both DPV/SWV and EIS (with proper fitting) to confirm the operative transduction route.
2.3. Materials and Surface Modifications
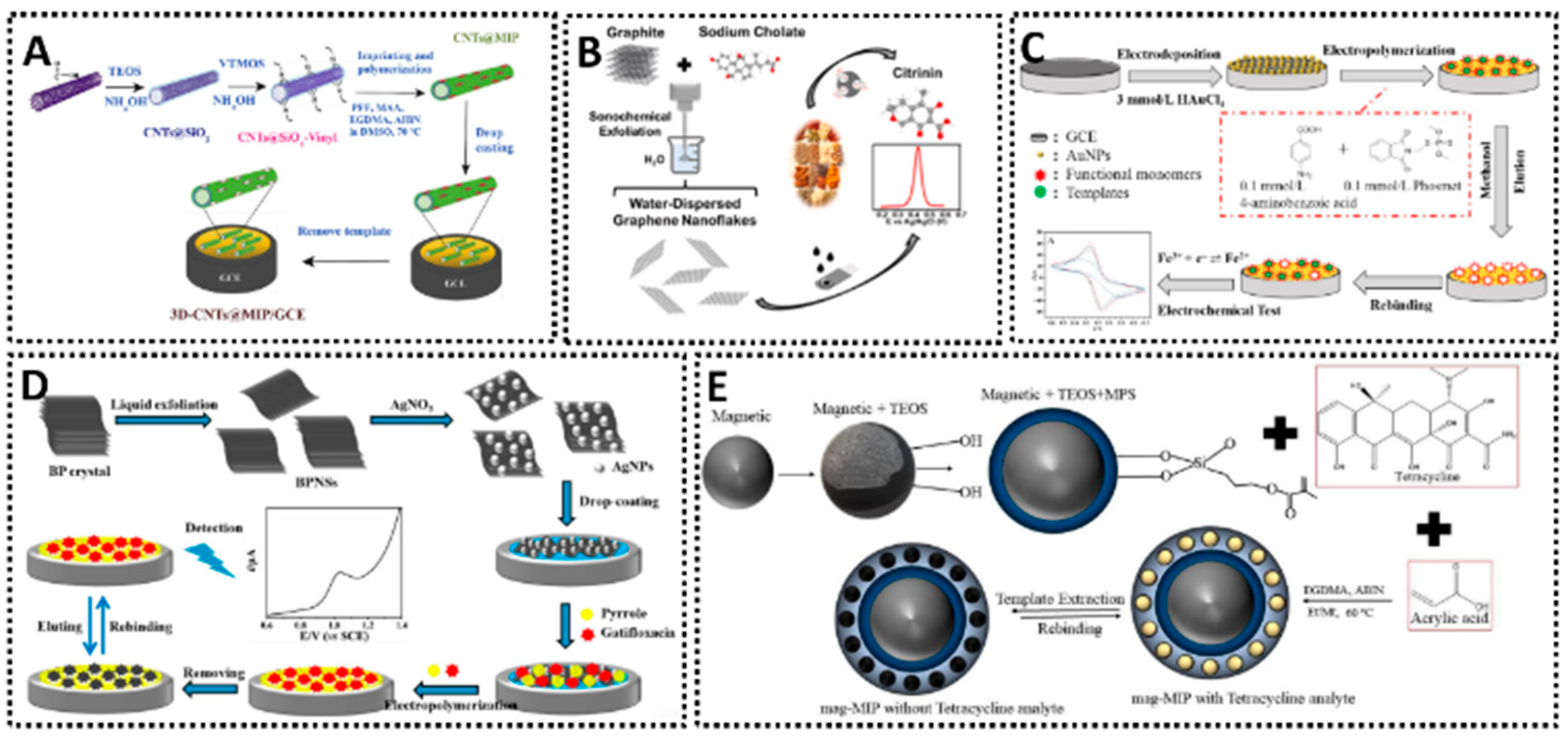
Incorporating nanomaterials and optimizing surface chemistry are pivotal for enhancing the performance of MIP sensors. Conductive nanomaterials such as carbon nanotubes (CNTs), graphene, and metallic nanoparticles (AuNPs, AgNPs) are frequently used to modify electrodes before depositing the MIP, as they can significantly boost electrochemical transduction and the overall sensitivity. CNTs are particularly effective due to their exceptional electrical conductivity, high mechanical strength, and large surface-area-to-volume ratio. When integrated onto an electrode, CNTs often form a porous, 3D nanocomposite network. This 3D structure dramatically increases the available active-site density for MIP polymerization, allowing for a higher loading of recognition sites. Furthermore, this porosity enhances diffusion kinetics by facilitating easier access of the analyte to the binding cavities and the underlying electrode surface. The inherent graphitic structure of CNTs also promotes rapid electron transfer kinetics, which is essential for amplifying the electrochemical signal. For instance, CNTs functionalized with MIPs have been applied in the selective detection of profenofos in food, leveraging the large specific surface area and the strong π–π interactions that promote fast electron transfer [39]. This approach demonstrated good accuracy and sensitivity for pesticide analysis, highlighting how 3D CNTs can effectively anchor a polymeric imprinting film (Figure 4A). Similarly, graphene and its derivatives are used for their unique 2D structure, which provides an enormous theoretical specific surface area (up to ~2630 m2/g) and outstanding electrical conductivity. This exceptionally large surface drastically increases the active-site density for MIP grafting. The flat, sp2-hybridized carbon plane acts as a highly conductive pathway, accelerating electron transfer between the MIP-bound analyte (or redox probe) and the electrode. Its strong π–π stacking capabilities also aid in anchoring the MIP’s monomers and template. In one study, graphene nanoflakes exfoliated in aqueous media were employed as a sensing layer for citrinin, achieving reliable electrochemical responses and underscoring the versatile nature of graphene when combined with MIPs [40]. Such 2D materials provide a large electroactive area for MIP films, enhancing analyte binding and electron transfer (Figure 4B). Metallic nanoparticles also play a crucial role in boosting electrode performance. With gold nanoparticles (AuNPs), for example, researchers have fabricated electrochemical sensors capable of detecting neutral pesticides like phosmet at nanomolar concentrations (Figure 4C) [41], benefiting from the improved electron transfer and catalytic activity AuNPs provide. Meanwhile, silver nanoparticles (AgNPs) serve a similar function, as shown in a MIP-based sensor incorporating AgNP-decorated black phosphorus nanosheets for antibiotic detection (Figure 4D) [42]. There, the synergistic effects of AgNPs and conductive supports provided better electron transport pathways and higher template recognition capacity, enhancing the voltammetric responses for the analyte. In both cases, metallic nanostructures are key to creating more active sites for polymer anchoring and to enabling faster charge transfers. Equally noteworthy is the combination of MIPs with magnetic nanoparticles (MNPs). By embedding iron oxide cores into a MIP matrix, researchers have produced magnetic MIP-based platforms for the selective detection of antibiotics in milk [43]. This approach allows one to exploit a magnet for quick and efficient separation or pre-concentration, making the electrochemical assay both sensitive and easy to handle (Figure 4E). The magnetic MIPs maintain specificity by virtue of their imprinted cavities, while the magnetic core simplifies electrode preparation and sample clean-up. As a result, the analyst can rapidly focus target molecules onto the sensing interface, improving both limit of detection and overall assay throughput.
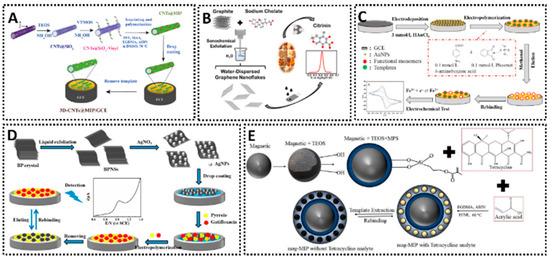
Figure 4.
(A) 3D MIP-coated CNTs for detection of profenofos [39]. (B) Liquid-phase exfoliated graphene coupled MIP for detection of citrinin [40]. (C) AuNPs-MIP electrochemical sensor for detection of phosmet residues [41]. (D) AgNPs-MIP-black phosphorus electrochemical sensor for detection of gatifloxacin [42]. (E) MNPs/MIP-based electrochemical sensor for detection of tetracycline [43].
The choice of functional monomer and cross-linker in the MIP formulation is also critical and often empirically optimized. Methacrylic acid (MAA) and acrylamide are popular monomers for non-covalent imprinting because they can form hydrogen bonds or electrostatic interactions with many templates. Cross-linkers like ethylene glycol dimethacrylate (EGDMA) provide rigidity to preserve site shape. In electropolymerized MIPs, common monomers include aniline, pyrrole, phenol, and o-phenylenediamine, selected for their ability to form polymers under electrooxidation conditions and for functional groups that can interact with the template. For instance, o-phenylenediamine was electropolymerized in the presence of acesulfame-K (a sweetener) to create an imprinted poly(o-phenylenediamine) film sensor [23]. The resulting sensor showed high selectivity for acesulfame-K over other sweeteners due to the specific cavities formed.
2.4. Addressing the Aqueous Matrix Challenge in Food Sensing
A primary challenge for the practical application of MIPs in food quality control is that most food samples are aqueous, whereas traditional molecular imprinting is overwhelmingly performed in non-polar organic solvents. This incompatibility stems from fundamental thermodynamic and kinetic challenges. During synthesis, the non-covalent interactions (especially hydrogen bonds and electrostatic forces) that form the pre-polymerization complex between the template and functional monomers are disrupted by water, a highly polar and protic solvent [44]. Water molecules effectively compete for these interaction sites, leading to poorly defined, low-affinity, and heterogeneous binding cavities within the final polymer.
During the sensing step (rebinding), the aqueous environment remains problematic. Water molecules can hydrate both the analyte and the polymer’s functional groups, weakening the desired template–MIP interactions [45]. Furthermore, traditional hydrophobic polymer matrices (e.g., based on EGDMA) can undergo non-specific swelling in water, which distorts the imprinted sites. This not only reduces binding affinity but also increases non-specific binding from other matrix components, compromising selectivity [46,47].
To overcome these significant hurdles, several innovative strategies have been developed to create “water-compatible MIPs” (W-MIPs) that exhibit high affinity and selectivity directly in aqueous media.
Aqueous-phase polymerization: One major approach is to perform the polymerization directly in water. This strategy shifts the primary driving force for complex formation away from hydrogen bonding and towards hydrophobic interactions [48]. By selecting water-soluble functional monomers and cross-linkers, the hydrophobic template and hydrophobic moieties on the monomers are “pushed” together by the surrounding water (the hydrophobic effect), forming a stable complex that is then “locked in” by polymerization. This results in binding sites that are optimized for recognizing the template from an aqueous environment, as rebinding is also driven by the same favorable hydrophobic interactions [49].
Hydrophilic core–shell imprinting: Another successful strategy is surface imprinting, particularly on hydrophilic supports. By creating only a thin (nanometers-thick) imprinted layer on a hydrophilic core (e.g., silica nanoparticles, hydrogel beads), the bulk properties of the material are dominated by the water-compatible core [50]. This approach, as seen in the work by Rezaei et al. [51] for L-tryptophan, improves particle dispersibility, minimizes non-specific swelling, and ensures that the binding sites are highly accessible at the polymer-water interface, leading to faster binding kinetics.
Advanced porogens (e.g., DES): The challenge of water competition during synthesis can also be addressed by using novel porogens that stabilize the monomer-template complex. Deep Eutectic Solvents (DES) are a prime example. As highlighted by Surapong et al. [52], DES can act as both solvent and porogen, forming strong hydrogen bonds with the template-monomer complex. This effectively “shields” the complex from water interference and facilitates a high-fidelity imprinting process even in a high-polarity medium. The resulting “DES-MDMIP” for organophosphorus pesticides demonstrated excellent affinity and reduced non-specific interactions in water-rich fruit and vegetable samples.
Stimuli-responsive polymers: Finally, stimuli-responsive or “smart” polymers offer a dynamic solution. These MIPs are designed to change their conformation (e.g., swell or shrink) in response to external triggers like pH or temperature [53]. This property can be harnessed to facilitate template removal and rebinding in water. For example, a MIP might bind an analyte strongly at one pH (e.g., in the sample) but release it completely at another pH (e.g., for regeneration), making the sensor more robust for aqueous applications [54,55].
The development of these W-MIPs is critical for transitioning sensors from the laboratory to real-world food analysis, enabling direct, sensitive, and selective detection in complex media like juices, milk, and beverages without requiring prohibitive sample pre-treatment.
2.5. Design Considerations and Improvements
In designing MIP sensors, several practical considerations come into play. One important aspect is template selection and removal. The template molecule must be stable and inert during polymerization; it should not react or polymerize itself, which would interfere with cavity formation. In some cases, a structurally analogous dummy template is used to avoid using a toxic or expensive target during polymer synthesis. After polymerization, rigorous washing is required to remove the template and any unreacted monomers, ensuring that the sensor does not leach these compounds into food samples. Effective template removal also frees up the maximum number of recognition sites. Another consideration is practical reusability and stability. Beyond solvent rinsing, several groups now employ brief electro-regeneration to expel bound analyte without harming the cavity network. For example, a sol–gel MIP/meat-freshness sensor achieved in situ regeneration by applying 0.9 V for 20 s, enabling at least 10 repeated measurements with essentially recovered baseline and only slight drift over 30 days of room-temperature storage [21]. Similarly, electrosynthesized ultra-thin MIP films on graphene retained function over four adsorption–desorption cycles with only ~10–17% signal decrease; a chitosan protective film improved layer integrity during use [56]. In oily food matrices, o-phenylenediamine-based MIP electrodes combining AuNPs/MWCNTs have delivered stable TBHQ determinations with 98.44–102.09% recoveries and ≤2.16% RSD, indicating fouling-tolerant operation under real conditions [57]. Collectively, these practices (electro- or mild solvent regeneration; thin protective coatings) are emerging as standard routes to robust, reusable MIP electrodes.
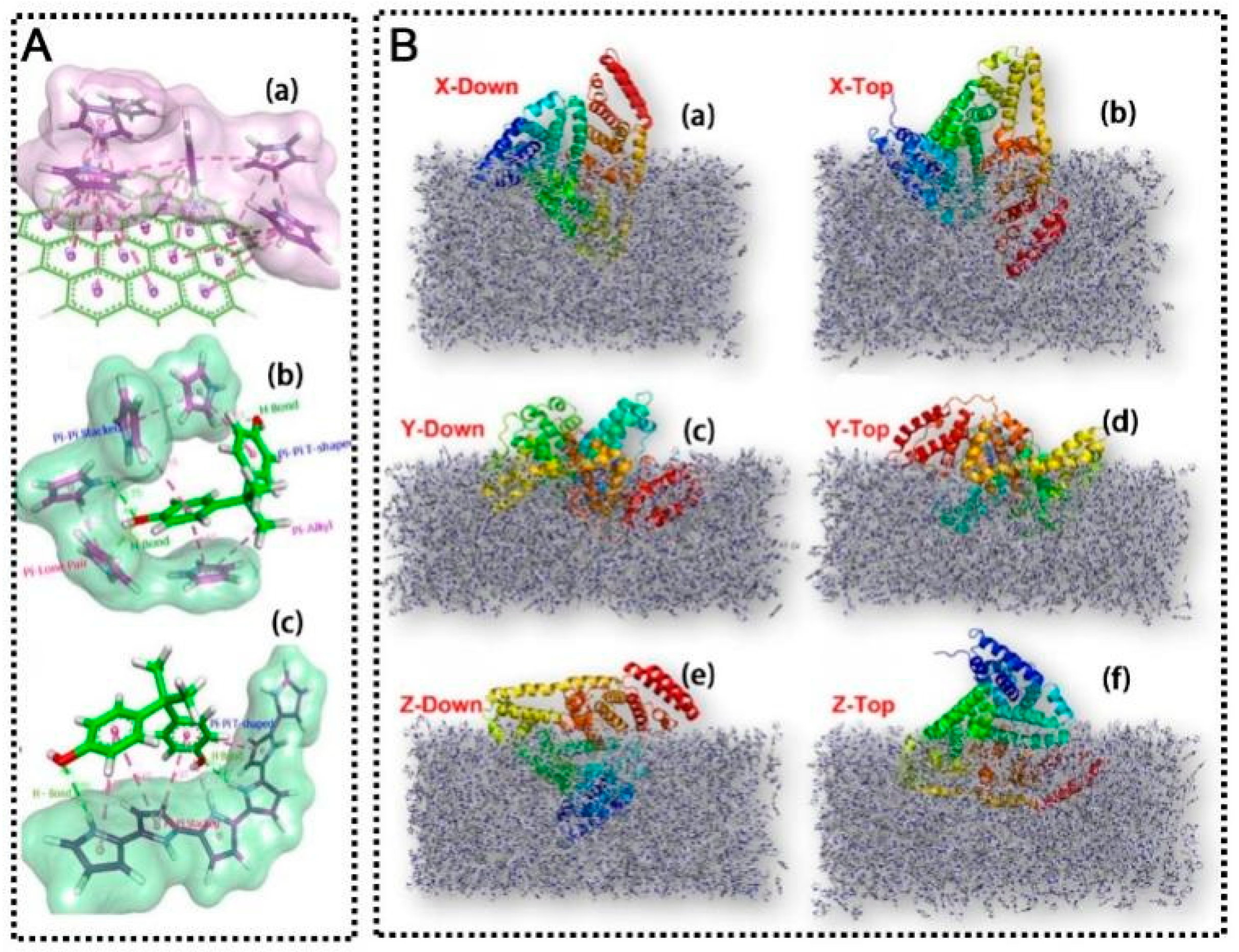
The performance of an MIP sensor can sometimes be improved by computational design of the imprinting process. Computational modeling (e.g., density functional theory calculations or molecular dynamics) can predict which functional monomers will form the strongest interactions with a given template, helping to screen candidate monomer mixtures before synthesis [58,59]. This approach can save time in optimizing MIP composition and has been used to tailor MIPs for complex targets. For example, Karthika et al. [60] employed DFT calculations at B3LYP/6-31 + G(d,p) level to predict molecular-level interactions between bisphenol A (BPA) BPA and polypyrrole-based MIP. The molecular electrostatic potential map revealed proton accepting (O30 and O32 atoms) and donating (H31 and H33 atoms) sites of BPA, while pyrrole showed strong electrophilic nature through its -NH group (Figure 5A). These complementary interactions facilitated the formation of energetically favorable BPA–pyrrole complexes with a binding energy of −2.43 kcal/mol, leading to highly selective BPA detection in milk samples. In another study, Liang et al. [61] utilized molecular dynamics simulations to optimize a BSA-imprinted polypyrrole sensor. The calculations demonstrated that BSA remained firmly embedded in the polymer matrix regardless of its starting orientation, with the most favorable Z-Top direction showing the lowest adsorption energy (−1507.8 kcal/mol) and largest contact surface area (14,347 Å2) (Figure 5B). The simulations revealed that cation/π interactions and hydrogen bonds were the dominant forces stabilizing the BSA-polymer complex, with 38 hydrogen bonds forming a potent network involving 31 amino acid residues. This computational guidance resulted in an MIP sensor with excellent sensitivity (LOD of 4.5 × 10−2 pg/mL) and selectivity for BSA detection in dairy products. Beyond DFT/MD screening, machine-learning (ML) models are now being used to predict imprinting quality and to optimize synthesis windows across monomer/solvent/pH spaces before benchtop trials. In a study using lab-generated datasets, gradient-boosted ensembles predicted the imprinting factor with R2 ≈ 0.87, reducing trial-and-error in monomer/solvent choice and process conditions [62].
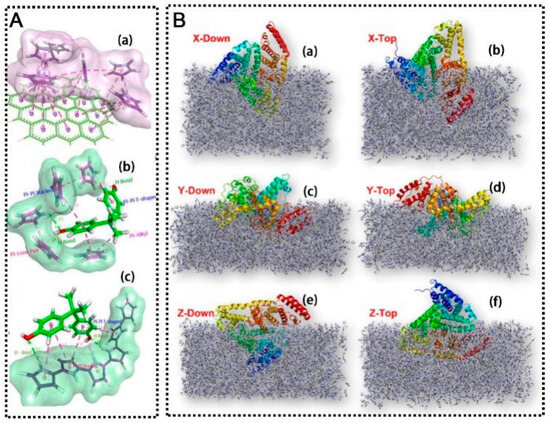
Figure 5.
(A) (a) π-π interaction between graphene with pyrrole molecules. (b) Energy minimized monomer–template complexes. (c) Auto Dock docked pose of PPy with BPA [60]. (B) Global views of the BSA protein embedded in the MIP along the (a) X-Down direction, (b) X-Top direction, (c) Y-Down direction, (d) Y-Top direction, (e) Z-Down direction, and (f) Z-Top direction [61].
Finally, integration with advanced materials (as discussed in Section 2.3) is an active area of improvement. By incorporating conductive or catalytic nanomaterials into MIPs, researchers have developed hybrid sensing layers that not only recognize the analyte but also amplify the detection signal. For instance, nanozymes (nanoparticles with enzyme-mimicking catalytic activity) have been combined with MIPs to generate an electrochemical or colorimetric response upon target binding, effectively creating a signal-on sensor. One compelling example involves using a cobalt-based zeolitic imidazolate framework (ZIF-67) as a nanozyme for detecting ethyl carbamate [63], an emerging contaminant in fermented beverages. Here, the ZIF-67 nanoparticles exhibit peroxidase-like activity, catalyzing the dissociation of hydrogen peroxide into hydroxyl radicals that amplify electrochemical signals. Once the target analyte (in this case, ethyl carbamate) is selectively bound by the MIP, it blocks the catalytic sites of the ZIF-67 nanozyme, thus reducing the overall current response. This “on-off” mechanism underscores the versatility of nanozymes in enabling rapid, sensitive detection while maintaining the specificity associated with molecular imprinting.
3. MIP Electrochemical Sensors for Antioxidants in Foods
Food antioxidants—whether naturally occurring compounds such as polyphenols, vitamins, and flavonoids, or synthetic preservatives like butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tert-butylhydroquinone (TBHQ)—are critical to food quality, shelf life, and human health. Due to their protective function against oxidative stress, antioxidants help prevent lipid oxidation, preserve color and flavor, and contribute to nutritional value. Accurate and selective detection of antioxidants in complex food matrices is therefore a central task in quality control, authenticity verification, and regulatory compliance. Traditional methods (e.g., DPPH, FRAP, and ORAC assays) measure total antioxidant capacity rather than the presence or concentration of specific antioxidants [27]; thus, MIP electrochemical sensors have gained traction as powerful tools for selective recognition and quantification of individual antioxidant molecules.
Table 2 lists a range of MIP-based electrochemical sensors targeting antioxidants commonly encountered in foods, including both natural (e.g., ascorbic acid, phenolic acids, flavonoids) and synthetic compounds (e.g., BHA, TBHQ). In edible oils, a TBHQ MIP/AuNPs/MWCNTs/o-PD sensor achieved 98.44–102.09% recoveries with ≤2.16% RSD, evidencing stable, low-fouling performance. Antioxidants may be found in foods at concentrations ranging from nanomolar to millimolar levels, depending on their chemical structures and the particular food matrix. For instance, polyphenolic compounds in tea or wine often appear in the micromolar range, while synthetic antioxidants may be incorporated at higher concentrations in frying oils or processed snacks but remain subject to regulatory limits. Given the complexity of food matrices—such as emulsions, high-fat environments, or sugary beverages—MIP-based sensors are well-suited for antioxidant detection because they rely on specific cavities that selectively bind to the target analyte. This recognition mechanism reduces interference from other antioxidants, sugars, or lipids, and allows the measurement of individual antioxidant levels in multi-component environments. To facilitate comparison across analytes and platforms, we additionally normalize all reported LODs to nM, standardize matrices (oils, beverages, powders, etc.), and group electrodes (GCE, SPE/SPCE, Au, ITO, carbon paste).

Table 2.
Selected MIP-based electrochemical sensors for antioxidants in food samples.
3.1. MIP-Based Sensors for Natural Antioxidants
Naturally occurring antioxidants include vitamins (like vitamin C), phenolic acids (caffeic acid, gallic acid), and flavonoids (catechin, quercetin, luteolin), among many others. These compounds contribute to the health benefits and quality attributes of fruits, vegetables, teas, herbs, and wines. Their selective detection is important for verifying label claims about antioxidant content, for authenticating premium food products, and for understanding the contribution of these compounds to human nutrition.
Apigenin is a flavone widely present in parsley, celery, and chamomile. A ZnO NPs/TrpMA@MIP-GCE sensor [64] fabricated via SI reached an exceptionally low LOD of 24.7 fM, with a linear range between 0.1 and 1.0 pM. The synergistic effect of ZnO NPs and the MIP on the GCE delivered both enhanced surface area and highly specific recognition sites. The sensor was successfully validated in parsley and celery extracts, demonstrating its ability to handle real food matrices and detect ultra-trace levels of this flavone.
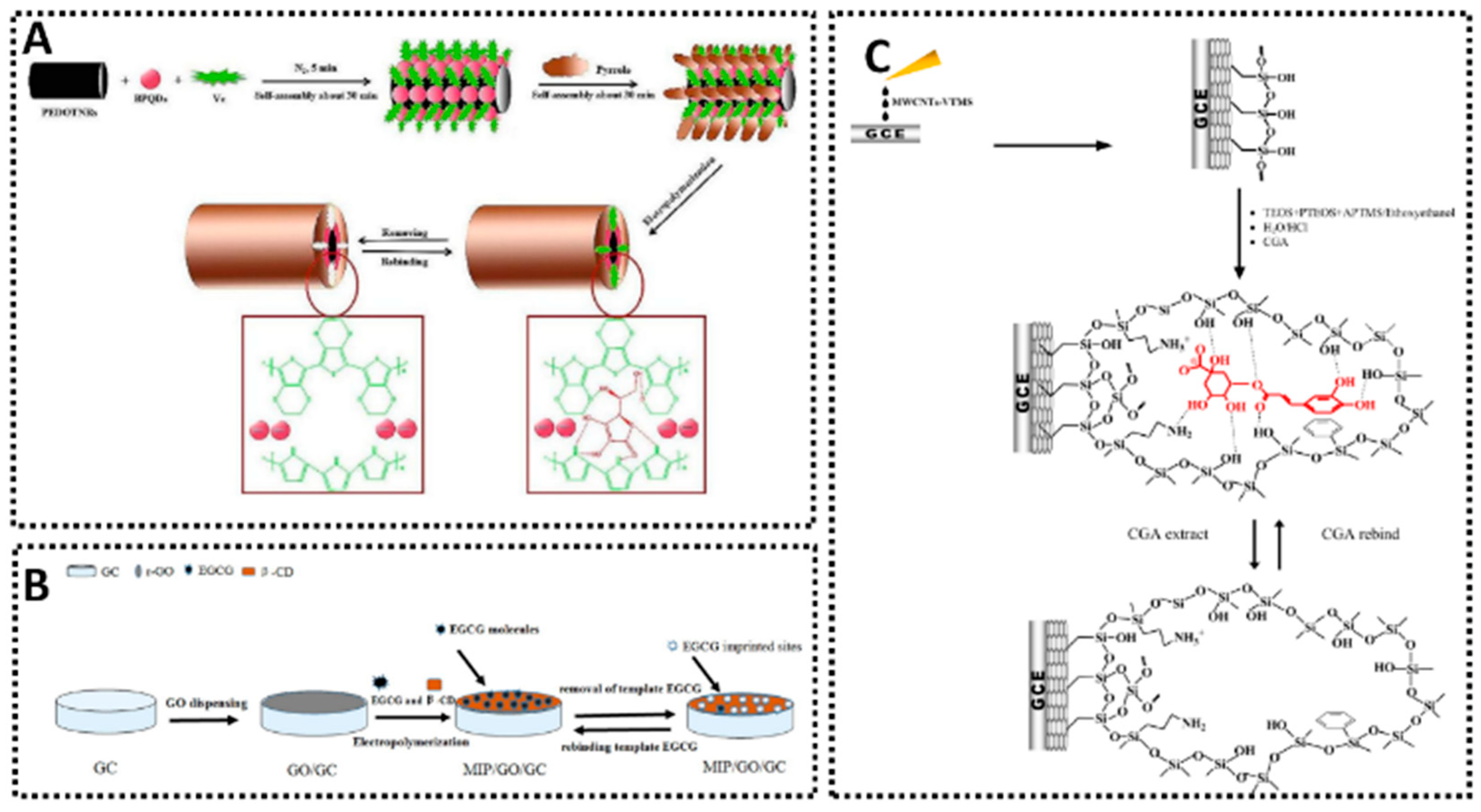
Vitamin C, abundant in fruits and vegetables and often added to beverages and dietary supplements, is a key antioxidant that can be challenging to detect electrochemically in the presence of common interfering species such as sugars. Several MIP sensors focus on this compound, employing different electrode designs and polymerization strategies. The e-MIP/SPC sensor [65] offered a dual linear range of 0.03–2.4 mM and 0.002–0.1 mM and achieved an LOD of 0.0012 mM in commercial vitamin C tablets. The GC/PPy-MIPox sensor [66], using polypyrrole EP on glassy carbon, covered 0.005–2 mM with an LOD of 3 μM and was tested successfully in orange juice. Another electropolymerized approach (AA-MIP/SPCE) [67] exhibited an LOD of 0.11 μM across a two-phase linear range of 0.45–13.52 μM and 13.52–409.10 μM, again validated in orange juice. SGI with a GE/sol–gel/MIP sensor [68] reached an LOD of 0.035 μM for vitamin C in pharmaceutical tablets. Meanwhile, an approach combining polypyrrole-based MIPs, boron phosphide quantum dots, and PEDOT nanotube arrays (Figure 6A) [69] achieved an LOD of 0.0033 mM for the 0.01–4 mM range in soft drinks. These developments confirm that careful electrode modification and imprinting provide excellent selectivity and precision for vitamin C analysis in diverse food products.
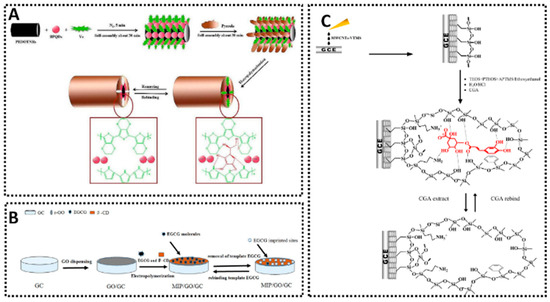
Figure 6.
Examples of MIP-based electrochemical for different natural antioxidants sensing. (A) PPy-BPQDs-MIPs/PEDOTNRs/GCE for Vitamin C detection [69]. (B) MIP-Ni(OH)2/GCE for epigallocatechin gallate detection [90]. (C) MIS/MWCNTs-VTMS/GCE for chlorogenic acid detection [82].
Caffeic acid is common in coffee, certain fruits, and wines. MIP sensors illustrate the versatility of imprinting for this compound. MIP/SPCE devices [76,78], produced by BP, showed detection limits of around 0.06–0.13 mM with linear ranges reaching up to 1.11 mM, enabling caffeic acid quantification in wine. Although these LODs are comparatively higher, the concentration levels in wine samples often remain within the sensor’s operational range. Another sensor using SGI (MIS/AuE) [77] provided an LOD of 0.15 μM, covering 0.5–60 μM. This sensor was also validated in both red and white wines, highlighting how sol–gel-based MIP layers can be designed to withstand the complex interference common to fermented beverages.
Catechins, including (+)-catechin and epigallocatechin gallate (EGCG), are abundant in teas and noted for their potent antioxidant activity. A bulk-polymerized MIP/GCE [79] covered 5–100 µM of (+)-catechin with an LOD of 37 nM and was tested in green tea. Another bulk-polymerized sensor (MWCNT/MIP/GCE) [80], incorporating multi-walled carbon nanotubes, spanned 1–300 μM with an LOD of 0.17 μM. The data confirm that carbon nanotubes improve electron transfer and provide sensor stability, enabling direct testing in tea matrices. EGCG detection is shown to be particularly efficient with surface imprinting strategies. MIP/GO/GCE reached an LOD of 8.78 nM (linear range 30 nM–10 μM) in a variety of teas [119], and MIP-Ni(OH)2/GCE (Figure 6B) pushed the LOD further down to 7 nM over 10–200 μM [90]. A membrane-based MIP [91] was validated in commercial tea drinks, and although it did not provide a detailed LOD, the sensor effectively covered 0.03–1 µg/mL. These findings collectively suggest that whether using carbon nanomaterials or metal hydroxides, the imprinting of catechins can achieve remarkable sensitivity in tea extracts and related botanical samples.
Chlorogenic acid detection is illustrated by different approaches. One example, MIP/Bi2S3/Ti3C2TX MXene/FTO [81], utilized a surface imprinting method that enabled an LOD of 2.4 nM over a 0.1412–22.59 μM range and was demonstrated in tea, juice, and coffee. MIS/MWCNTs-VTMS/GCE (Figure 6C) [82] also had a relatively low LOD of 0.032 μM, while Au/MSL/MIS [83] featured an LOD of 0.148 μM. Another method (MIPpy/PGE) [84], using electropolymerized polypyrrole on pencil graphite electrodes, extended the detection range to 1 μM–10 mM, suitable for coffee samples with high chlorogenic acid content. Many other naturally occurring phenolic and flavonoid compounds have been detection using MIP-based electrochemical sensor, such as ferulic acid [92], gallic acid [93,94,95,96,97,98,99], luteolin [100,101,102], p-coumaric acid [103], dodecyl gallate [89], quercetin [105,106,107,108], rutin [113,114], syringic acid [115], resveratrol [109,110,111,112], and curcumin [86,87,88]. Taken together, these examples confirm that both surface and bulk imprinting approaches, combined with careful electrode modification using nanomaterials, can consistently yield sensors with high specificity, low detection limits, and robust performance in complex real samples, including teas, coffees, wines, herbs, fruit juices, and nutraceutical supplements.
3.2. MIP-Based Sensors for Synthetic Antioxidants
The development of MIP-based sensors for synthetic antioxidants reflects the growing need to ensure compliance with regulations and to verify that additives remain within safe limits. Synthetic antioxidants such as BHA, BHT, TBHQ, and propyl gallate are key preservatives in oils, snacks, and other processed foods, but they require strict monitoring due to potential health concerns at high concentrations.
BHA is widely employed in frying oils, potato chips, and mayonnaise, making accurate measurement in oily or emulsion-rich matrices essential. Several MIP-based sensors have been designed to achieve high sensitivity in these challenging samples. A sensor combining MWCNT, gold nanoparticles, and MIP (MWCNT/GNP/MIP/GCE) [71] demonstrated two distinct linear ranges (0.01–5 μM and 5–1000 μM) with a 6 nM LOD, tested in real mayonnaise and oil samples. Another variant, MIP/AuNPs/SPCE [72], showed a linear range of 0.056–111.11 μM and an LOD of 5.6 nM in chewing gum, mayonnaise, and potato chips, indicating that the use of screen-printed electrodes can simplify sensor fabrication and analysis. Further modifications, including PdAuNPs/ERGO (MIP/PdAuNPs/ERGO/GCE) [120] and MIP/GNP/MWCNT/GCE [73], cover broad linear ranges up to 1000 μM, with LODs around 0.277 μM and 5 nM, respectively, validated in various edible oils. One ultra-sensitive approach, MIP/MoS2/AgNPs-CS/GCE (Figure 7A) [64], exhibited an LOD of 7.9 nM over 1 nM to 0.1 mM. Finally, sensors like MIP-MWCNT/GCE [74] and MIPs/GCE [75] also successfully measured BHA in oil-based samples, demonstrating the adaptability of MIP electrochemical platforms to highly hydrophobic environments and their ability to meet regulatory-level detection needs.
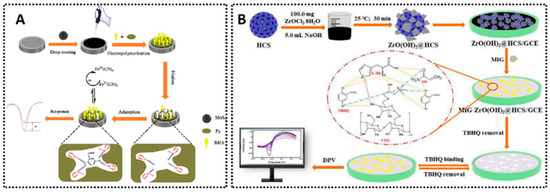
Figure 7.
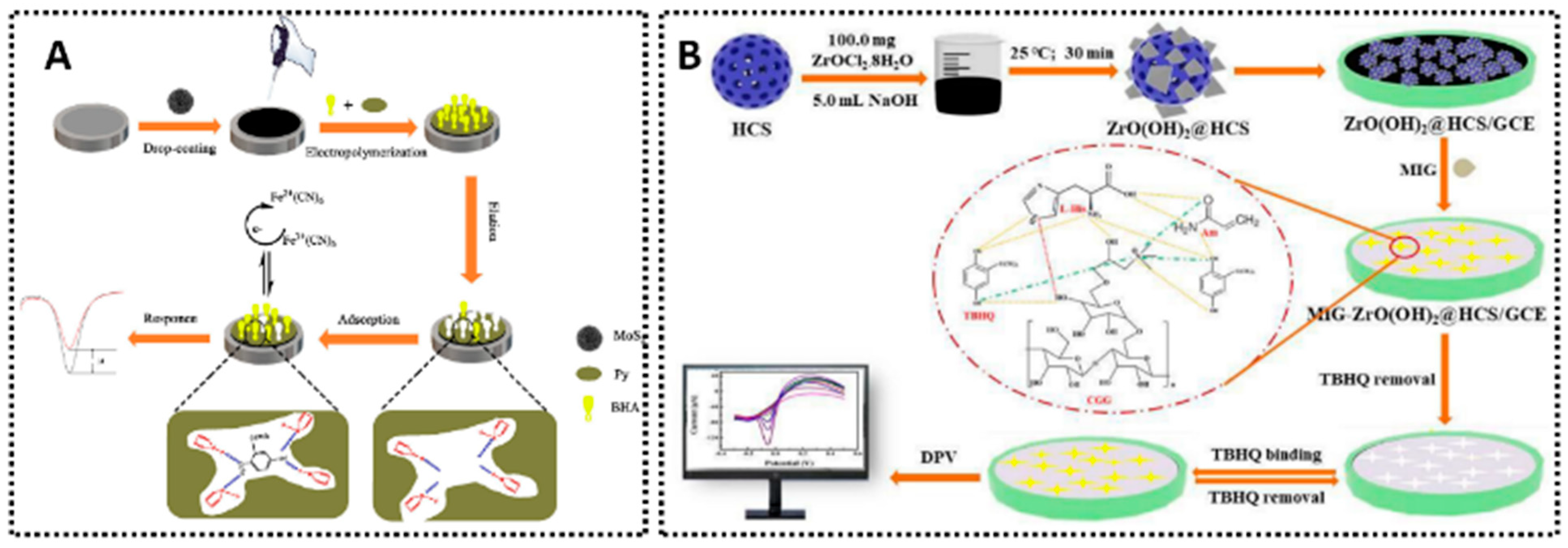
Examples of MIP-based electrochemical for different synthetic antioxidants sensing. (A) MIP/MoS2/AgNPs-CS/GCE for BHA detection [64]. (B) MIG-ZrO(OH)2@HCS/GCE for TBHQ detection [118].
TBHQ is another essential synthetic antioxidant for stabilizing fats and oils and is often regulated in fried foods. MIP-MWCNT/GCE [74] employed surface imprinting, achieving an LOD of 0.85 μM across 2.84–150 μM. It was tested in soybean oil, mayonnaise, margarine, and biodiesel, showcasing broad matrix compatibility. Another design, MIP/MoS2/EACC [116], delivered an extremely wide concentration window, from 1 μM up to 120 mM, with an LOD of 0.72 nM. MIP/AuNPs/EGP [117] extended its range to 80 nM–1 μM and 1–100 μM, providing an LOD of 12 nM validated in edible oil. A sol–gel approach (MIG-ZrO(OH)2@HCS/GCE, Figure 7B) [118] produced an LOD of 6.7 nM from 0.025 to 100 μM, demonstrating reliable detection in peanut oil, milk powder, and fried meat products. Collectively, these TBHQ sensors illustrate how MIP technology can be adapted to highly diverse food matrices and can reach sub-ppb detection levels.
Propyl gallate, an ester of gallic acid, is also used to prevent oxidation in various oily or emulsified foods. MIP/GNP/MWCNT/GCE [104] covers 0.01–5 μM and 5–1000 μM, with a 6 nM LOD. Real-sample analysis in mayonnaise, black cumin oil, and soybean oil showed high recovery values, indicating that the synergistic effects of gold nanoparticles and multi-walled carbon nanotubes can be effective in enhancing both sensitivity and reproducibility. The successful application in multiple types of oils suggests that sensor fabrication strategies tested for BHA or TBHQ can often be extended to other structurally related preservatives.
3.3. Analysis of Sensor Performance
The evidence summarized in Table 2 provides a strong quantitative basis for the “high sensitivity” attributed to MIP electrochemical sensors. A statistical analysis of the reported data reveals that these platforms consistently achieve detection limits relevant for regulatory and quality control. Of the sensors listed for antioxidants, over 60% report LODs in the nanomolar (nM) range or lower. Notably, a significant subset of these studies has successfully pushed detection capabilities into the picomolar (pM) and even femtomolar (fM) regime. Examples include the 24.7 fM LOD for apigenin, 0.22 nM for dodecyl gallate, and 0.235 pM for quercetin. This demonstrates a clear and consistent trend of ultra-sensitive detection. While a “mean” LOD is statistically complex to compute given the wide-ranging analyte concentrations and diverse electrode systems, the median LOD for these advanced sensors often falls in the low nanomolar range. This high sensitivity is frequently attributed to the synergistic use of molecular imprinting with signal-amplifying nanomaterials (such as graphene, CNTs, and metallic nanoparticles) which enhance both the electroactive surface area and the transduction of the binding event. These sensors also capitalize on the specificity afforded by imprinting, which results in minimal interference from other matrix components.
One of the principal advantages of these sensors lies in their ability to target individual antioxidant compounds selectively. This capacity is valuable for verifying the authenticity and nutritional labeling of high-value products such as premium teas, specialty coffees, olive oils, or wines. It also benefits manufacturers seeking to maintain consistent antioxidant content for extended shelf life. Another important factor is scalability: many sensors rely on straightforward polymerization methods amenable to large-scale production, particularly those that employ screen-printed electrodes or disposable formats.
4. MIP Electrochemical Sensors for Sweeteners
MIP electrochemical sensors for sweeteners in food products have gained attention due to the need for accurate, selective, and on-site detection methods that can be applied to both artificial and natural sweeteners. Conventional analytical techniques such as high-performance liquid chromatography (HPLC) or ion chromatography (IC) have long been used to determine sweetener content in beverages, confectionery, and other processed foods, often coupled with UV or mass spectrometric detection [121,122]. These methods provide high accuracy but require costly instrumentation, time-consuming sample preparation, and skilled personnel. As a complementary strategy, MIP-based electrochemical sensors can offer rapid, portable, and cost-effective analysis with strong molecular recognition. While the literature on MIP electrochemical sensors for sweeteners is comparatively modest, a number of studies have demonstrated the feasibility of detecting both artificial high-potency sweeteners (e.g., aspartame, acesulfame-K) and naturally occurring sugars (e.g., sucrose, fructose, D-arabinose, D-xylose). Table 3 presents selected examples, showing the diversity of target sweeteners and illustrating how different MIP fabrication methods and electrode materials can be adapted to meet specific detection needs. To move beyond a catalog of case studies, we comparatively evaluate sweetener sensors on five axes: imprinting route (electropolymerization vs. bulk/precipitation), film architecture (surface-imprinted/core–shell vs. bulk), transduction (DPV/SWV vs. EIS/potentiometry), food-matrix robustness (high-sugar, acidic, or fatty systems), and validation quality (use of independent calibration, spike-recovery in real foods, and ruggedness). Electropolymerized MIPs generally offer tighter thickness control and better transducer coupling, but can exhibit fewer accessible sites and greater susceptibility to over-oxidation in acidic beverages; bulk/precipitation MIPs provide higher site density yet require careful dispersion to avoid ohmic drop. These trade-offs are well documented for e-MIPs in recent critical reviews and tutorials and motivate standardized reporting of film growth charge, site accessibility, and template bleed tests (pre- and post-wash). Regulatory context also matters: analytical performance should be interpreted against intake/maximum-level frameworks (e.g., JECFA acceptable daily intake (ADI) considerations for food additives such as TBHQ and Codex/EU contaminant limits for toxins). Although sweeteners themselves are additives, their quantification shares the same validation logic used for contaminants (trueness, precision, working range, LOD/LOQ, matrix effects) defined by IUPAC and regulatory guidance; hence we explicitly benchmark reported LODs against relevant decision thresholds and require in-matrix figures of merit rather than buffer-only performance. Recent sweetener-focused MIP electrochemical sensors demonstrate that practical LODs are already compatible with beverage labeling checks. For acesulfame-K, a glassy-carbon MIP (electropolymerized) achieved an LOD in the low-submicromolar range with validation in soft drinks, confirming specificity versus common co-formulants [123]. For aspartame, MIP–MWCNT platforms have reported LODs down to a few tens of nanomolar and successful spike-recovery in sports beverages, underscoring matrix robustness [124]. Beyond high-potency sweeteners, MIP electroanalysis has also expanded to non-nutritive sucralose with carbon-dot-assisted architectures reaching ultralow LODs, suggesting a path to harmonized screening across sweetener classes [125]. Together, these examples align the sweetener subsection with the antioxidants part by emphasizing imprinting route, nanocarbon assistance, and real-sample validation.
Artificial sweeteners have been a particular focus of research because of their intense sweetness and widespread use in diet beverages, sugar-free candies, and tabletop sweeteners [126]. Their regulation in many jurisdictions requires analytical approaches that can confirm compliance with permissible concentration levels or detect undeclared usage [127]. Aspartame, an ester of the dipeptide phenylalanine and aspartic acid, is one of the most common artificial sweeteners and has attracted considerable attention in MIP sensor development. One motivation for monitoring aspartame is that it can hydrolyze under certain conditions, producing compounds such as phenylalanine or methanol, which can impact product labeling or pose health concerns for specific populations (for instance, individuals with phenylketonuria) [128]. MIP-based electrochemical sensors provide the possibility of selectively recognizing aspartame even in the presence of its breakdown products and other co-formulated sweeteners, offering a simpler alternative to chromatographic separation.
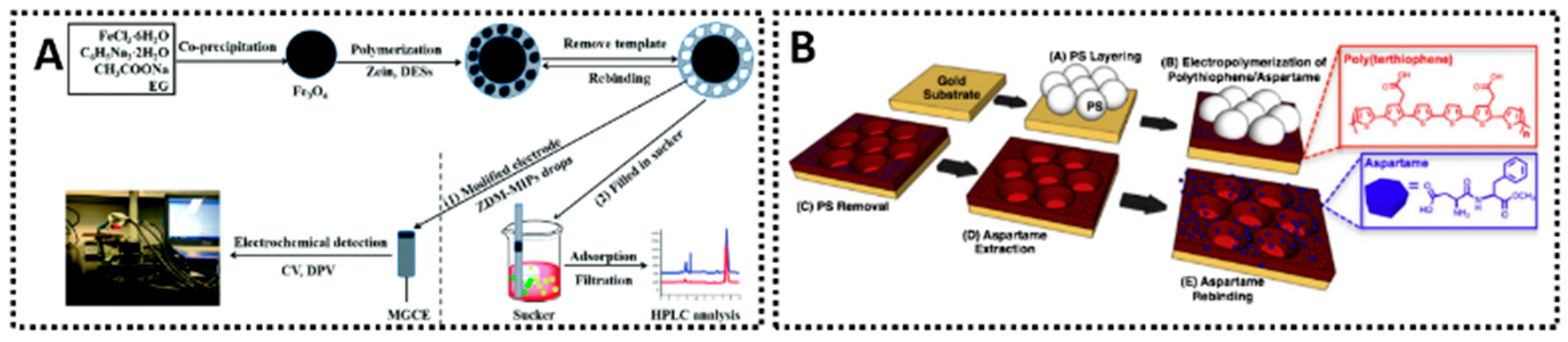
Several aspartame sensors have been reported. One advanced example of an aspartame sensor employed a magnetic MIP synthesized with the help of a deep eutectic solvent (DES) and the biopolymer zein [129]. In this design, a DES (choline chloride–ethylene glycol mixture) acted as a green solvent and co-monomer system to imprint aspartame, and zein (a corn protein) served as a benign cross-linker. The resulting magnetic MIP particles (Zein-DES MIP) were attached to a graphite electrode under a magnetic field, forming a modified electrode (Figure 8A). Using DPV, the sensor exhibited a linear range of 0.1–50 μg/mL for aspartame and achieved recoveries of about 85–107% in spiked soft drinks. The use of DES and biopolymer was advantageous for imprinting aspartame, which is polar and traditionally challenging to imprint in nonpolar media. By imprinting in a more water-like environment, the MIP’s binding sites were well-formed for operation in aqueous samples like beverages. Additionally, the magnetic feature allowed easy renewal of the sensing surface–after each measurement, the MIP-coated magnetic microbeads could be removed and fresh ones introduced, reducing any issues of surface fouling. Other simpler MIP sensor setups for aspartame have also been reported. For example, a MIP film of polypyrrole electropolymerized on a carbon electrode has been used to detect aspartame in sports beverages, yielding an LOD in the low micromolar range and good selectivity against common co-additives [124]. In comparative tests, the MIP-coated sensor outperformed a non-imprinted polymer sensor in complex matrices–where the latter suffered false signals due to interfering species, the MIP sensor maintained a clear response to aspartame. This highlights the value of imprinting: it introduces molecular recognition that significantly cleans up the electrochemical signal. An additional noteworthy approach was presented by Tiu and co-workers, who developed a microsphere-patterned, MIP polythiophene platform for aspartame detection [130]. Their strategy used polystyrene microbeads as a sacrificial template (via so-called “colloidal sphere lithography”) to create a macroporous, thin-film MIP based on a carboxyl-functionalized terthiophene. During EP, the aspartame template became embedded in the conductive polymer matrix (Figure 8B). After dissolving the microbead template and extracting aspartame with methanol, the resulting sensor exhibited a highly ordered “inverse opal” structure that provided enhanced surface area and easy analyte access.
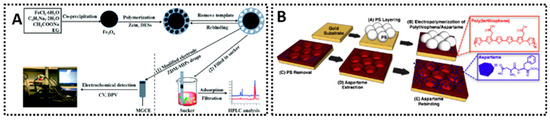
Figure 8.
Two MIP-based electrochemical sensors for aspartame detection. (A) Zein magnetic MIP modified MGCE sensor [129]. (B) Microsphere-patterned MIP sensor [130].
In contrast to aspartame, acesulfame potassium (acesulfame-K) has been the subject of far fewer MIP sensor studies. Acesulfame-K is commonly found in beverages, baked goods, and various sugar-free products. It is highly stable to heat and does not undergo metabolic degradation, so its presence can extend into wastewater streams. The single example by Singh et al. [123] involves an electropolymerized poly(o-phenylenediamine) film on a gold electrode, imprinted with acesulfame-K. Despite the limited electroactivity of acesulfame-K at low potentials, the sensor relied on monitoring changes in a ferricyanide probe, with the polymer matrix selectively binding acesulfame-K and reducing the current flow. The device achieved a linear range of 0.1–17 µM and an LOD of 0.35 µM. The authors validated the sensor’s applicability by testing it on diet cola, candy, and tabletop sweeteners, obtaining near-quantitative recoveries. This underscores the viability of MIP-based sensing for acesulfame-K despite the paucity of examples in the literature.
Beyond artificial sweeteners, natural sugar-based sweeteners have also attracted interest for MIP sensor development. Although these sugars are not used in minute concentrations like high-potency artificial sweeteners, their quantification is essential for product labeling, nutritional analysis, and authenticity verification. Adulteration with inexpensive sweeteners in products such as fruit juices or honey can be detected by monitoring the presence or concentration of specific sugars. Table 3 highlights several studies targeting sugars such as D-arabinose, D-xylose, fructose, and sucrose, demonstrating that MIP-based platforms can offer an alternative to enzymatic or chromatography-based assays. For instance, an electropolymerized MIP on carbon/few-walled carbon nanotubes (C/FMWCNT) was employed to detect D-arabinose in sugarcane bagasse hydrolysates [131]. This approach showcased a remarkable LOD of 4.25 pM over a linear range of 0.01–0.1 nM, thereby enabling sensitive detection of this pentose sugar even in relatively complex biomass-derived samples. The same group also applied a similar strategy for D-xylose, achieving an LOD as low as 4.5 pM [131]. Another study targeting D-xylose used a reduced graphene oxide (RGO)-MIP on a GCE, displaying two distinct linear ranges (0.1–1 pM and 1–10 pM) with an LOD of 80 fM [132]. Rather than emphasizing extreme LODs in buffered model systems, we compare performance under food-matrix stressors (high ionic strength, sugars/acids, proteins/colloids). For fructose and sucrose sensors, graphene-assisted e-MIPs reach ultralow LODs in buffer; however, their selectivity and stability can deteriorate in juices due to fouling and competitive hydrogen-bond donors. Water-compatible imprinting has emerged as a rational route to preserve affinity in aqueous, sugar-rich matrices and to reduce template bleed—issues repeatedly flagged in critical MIP assessments. We therefore re-analyze sugar-sensor papers for (i) use of water-compatible chemistries, (ii) recovery in undiluted juices/honey, and (iii) carry-over across at least three regeneration cycles.

Table 3.
Selected MIP-based electrochemical sensors for sweeteners.
Table 3.
Selected MIP-based electrochemical sensors for sweeteners.
| Target Sweetener | Sensing Platform | MIP Fabrication Method | Functional Monomer(s) | Linear Range | LOD (nM) | Real Sample | Reference |
|---|---|---|---|---|---|---|---|
| Acesulfame-K | GCE/MIP-o-PD | EP | o-PD | 0.1–17.0 μM | 350 | Cola drink; Candy; Tabletop sweetener | [133] |
| Aspartame | MIP/AS/MWCNT/GCE | BP | NR | 8 nM–6 µM | 22 | Sports beverages | [134] |
| ZDM-MIPs-MGCE | SI | Deep eutectic solvent | 0.34–169.9 μM | - | Soft drinks | [129] | |
| CSNP-RGO/MIP-EQCM | EP | Chitosan | 10–100 μM | 240 | Soft drinks; Sugarfree tablets | [135] | |
| P(3-TAA)/MIP-QCM | EP | 3-thiopheneacetic acid | 12.5–200 μM | 31,750 | Soft drinks | [130] | |
| D-arabinose | C/FMWCNT/MIP | EP | o-PD | 0.01–0.1 nM | 0.00425 | Sugarcane bagasse hydrolysates | [131] |
| D-xylose | GCE/RGO-MIP | EP | Phenol | 0.1–1 pM and 1–10 pM | 0.00008 | Sugarcane bagasse | [132] |
| C/FMWCNT/MIP | EP | o-PD | 0.01–0.1 nM | 0.0045 | Sugarcane bagasse hydrolysates | [131] | |
| Fructose | GCE/rGO-MIP | EP | Phenylboronic acid derivative | 10–150 fM | 0.0000032 | Orange juice; Apple juice; Grape juice | [136] |
| Sucrose | MIP/MWCNTs/GCE | EP | o-PD | 0.01–2.5 mM and 2.5–10.0 mM | 3000 | Raw sugar beet juice; Thin juice; Thick juice; Molasses | [137] |
Fructose is another abundant sugar of high commercial importance, present in fruit juices, soft drinks, and many sweeteners such as high-fructose corn syrup. A study by Zhao and co-workers [136] used a GCE modified with reduced graphene oxide to fabricate an electropolymerized MIP sensitive to fructose. The sensor showed an impressive LOD of 3.2 fM across a linear range of 10–150 fM, demonstrating that MIP-based methods can reach ultratrace levels of detection. Real sample analysis included orange, apple, and grape juices, where the sensor successfully quantified fructose content, consistent with reference methods. The high surface area and superior electrical conductivity of RGO are believed to facilitate fast electron transfer, while the imprinted cavities impart specificity to fructose in the presence of similar sugars and matrix constituents.
Sucrose, one of the most widely consumed sugars globally, has also been addressed by MIP-based electrochemical sensors. A notable example by Gupta et al. [137] describes an MIP on multi-walled carbon nanotubes deposited on a GCE. The method enabled detection across a wide dynamic range (0.01–2.5 mM and 2.5–10 mM), with a reported LOD of 0.003 mM. Real sample tests included various stages of sugar beet processing—raw juice, thin juice, thick juice, and even molasses—demonstrating the sensor’s capability to handle complex, viscous matrices. While the absolute limits of detection for sucrose might not be as low as those for the monosaccharides or artificial sweeteners, this level of performance is often sufficient for industrial sugar monitoring, where concentrations are relatively high. These findings confirm that MIP-based technology can be extended to larger carbohydrates by selecting functional monomers with affinity for hydroxyl-rich targets and employing robust electrode materials to manage fouling.
A consistent theme across these studies is that sweeteners—whether artificial or natural—often display weak direct electrochemical signals at the potentials typically used for analysis. Consequently, MIP sensors frequently rely on indirect measurements of a redox probe in solution, such as [Fe(CN)6]3−/[Fe(CN)6]4−, whose electrochemical response is modulated by analyte binding in the polymer layer. The polymer–analyte interaction changes the permeability of the film, either physically blocking or altering the ionic environment near the electrode surface. By correlating signal changes to sweetener concentration, researchers can achieve reproducible, selective determination. Additionally, the imprinting process confers an enhanced level of selectivity: even in the presence of structurally similar interferents, the sensor can maintain target specificity if the functional monomer–template interactions were appropriately chosen. Cross-linkers, co-monomers, and imprinting solvents are all crucial parameters that help define the sensitivity and specificity of the final MIP. Another important consideration in food analysis is matrix complexity. In sweetened beverages or sugarcane hydrolysates, there may be multiple sugars, artificial sweeteners, organic acids, colorants, and preservatives. MIP sensors must be tested in these real-world contexts to confirm that the imprinting is sufficiently selective. The reports reveal that most investigators do indeed validate MIP performance in products or byproducts, often comparing the results to a reference method. Good correlation between MIP-based results and classical methods supports the notion that MIPs can be incorporated into more routine, possibly field-based or semi-automatic measurement protocols. Regeneration of the MIP surface (e.g., by washing or exposing the sensor to a mild solution that disrupts the analyte–polymer interaction) can facilitate multiple reuse cycles, improving the cost-effectiveness of the approach.
Given that sweeteners, whether artificial or natural, can vary in polarity, molecular weight, and functional groups, the success of an MIP sensor depends critically on selecting monomers or polymerization conditions that best complement the target analyte. Non-covalent imprinting is the most common route, as it typically uses hydrogen bonding and electrostatic interactions. However, for polar sweeteners like aspartame, more hydrophilic imprinting environments may be required, including ionic liquids or deep eutectic solvents that stabilize the analyte-monomer complexes. The presence of nanomaterials such as carbon nanotubes or graphene derivatives can enhance sensor performance by increasing electron transfer rates and accessible surface area for imprinting. Many of the studies in Table 3 exploit hybrid materials—whether it is MWCNTs, few-walled nanotubes, or RGO—to fortify the mechanical stability and sensitivity of the sensor platform. Overall, despite the relatively small number of publications, the existing work on MIP electrochemical sensors for sweeteners underscores the broad adaptability of imprinting techniques for a range of molecular targets. Artificial sweeteners like aspartame and acesulfame-K have been successfully detected at low micromolar or even nanomolar levels, relevant to regulatory thresholds for “diet” or “sugar-free” products. Natural sugars—including sucrose, fructose, and the pentoses derived from biomass—can also be measured precisely, assisting in authenticity testing, process control, and labeling compliance. Although many of these studies remain in proof-of-concept or laboratory-scale demonstrations, they reflect promising directions for simpler and faster quality control assays.
5. MIP Electrochemical Sensors for Colorants
Food colorants play a significant role in the appearance, acceptability, and commercial value of various food products, spanning confectionery, beverages, dairy, and processed foods. They are deliberately added to enhance or restore the color lost during processing, as well as to provide consumers with visually appealing items that match their expectations. However, certain synthetic colorants have been associated with adverse health effects, prompting regulatory bodies worldwide to establish permissible limits and demand rigorous monitoring [138]. Moreover, color adulteration and the unauthorized use of non-food-grade dyes pose ongoing concerns, reinforcing the need for reliable, sensitive, and selective detection techniques. Traditional methods for colorant analysis typically involve chromatographic or spectrophotometric techniques (such as HPLC, LC-MS, and UV-Vis spectroscopy), which yield high accuracy but often require complex sample preparation, time-consuming procedures, and skilled personnel [139]. In response, MIP-based electrochemical sensors have emerged as appealing alternatives. By harnessing the intrinsic molecular selectivity of MIPs, these sensors offer portable, cost-effective, and on-site detection of colorants at trace levels, maintaining reproducibility in real sample matrices (Table 4).
Synthetic azo dyes have historically been used to impart vibrant colors to food and beverages. While their usage is permitted up to certain levels in many countries, they have been associated with potential risks such as allergic reactions, hypersensitivity, and behavioral issues in children. Amaranth (also known as E123) is a representative example that has attracted scrutiny from regulatory authorities. Several MIP electrochemical sensors have been developed to detect amaranth selectively in drinks and confectionery. One noteworthy sensor employs a MIP film on MWCNT-modified GCE, created via EP [26]. This sensor achieved a remarkably low LOD of 0.4 nM, enabling trace-level detection of amaranth in commercial juice samples such as watermelon, grape, and orange juice. The MIP sensor’s high selectivity can be attributed to hydrogen bonding and π–π interactions between the polymer matrix and the aromatic ring structure of amaranth. Another platform used a ZnO–MWCNT-modified screen-printed carbon electrode, also fabricated by EP, showing a similarly broad linear detection range for Amaranth and application in Robitussin Junior syrup and Acyclovir Arena capsules [140]. These examples underscore the adaptability of MIP sensors to various real samples. In a more advanced design, Huang et al. [141] introduced a surface imprinting strategy using polydopamine (PDA) as the imprinting layer around Pd–Cu nanoparticles supported on graphene, which were themselves wrapped in a poly(diallyldimethylammonium chloride) (PDDA) matrix. The resulting MIP-PDA composite on a GCE demonstrated an LOD of 2 nM, signifying excellent sensitivity for amaranth in commercial soft drinks. By imprinting at the surface (core–shell imprinting), analyte access to recognition sites was improved, resulting in faster response times. Furthermore, such hierarchical structures can mitigate sensor fouling by other food components, ensuring consistent performance over multiple measurements. These strategies for Amaranth detection exemplify how MIP-based electrochemical sensing integrates nanomaterials, EP, or sol–gel imprinting to achieve robust performance in complex food matrices. Comparable progress is evident for other widely regulated dyes. A single-monomer dual-template MIP on conductive substrates enabled simultaneous determination of tartrazine and brilliant blue with LODs of ~2–3 nM, illustrating multiplexing potential without sacrificing selectivity [142]. Poly(3-aminophenylboronic acid) films on carbon fiber paper have also yielded sub-micromolar detection of azo dyes with rapid diffusion kinetics, supporting fieldable formats.
Another set of colorants receiving continuous interest is the anthocyanins, naturally occurring pigments responsible for red, purple, or blue hues in fruits, vegetables, and flowers. Anthocyanins are generally considered safe; on the contrary, they are often touted for antioxidant properties. Nonetheless, accurate quantification is key for authenticity testing of natural food products, as adulteration or mislabeling of anthocyanin-rich juices, jams, or dietary supplements can impact both consumer trust and potential health claims. A MIP sensor reported by Tsogas et al. [143] used a BP approach on a GCE to detect anthocyanins across a wide range of commercial products—from energy bars and sports drinks to gummies and fruit snacks. The sensor exhibited a linear range from 1 nM to 10 μM, with a notably low LOD of 0.3 nM, demonstrating that MIPs can selectively target bioactive pigments despite the presence of sugars, preservatives, and other matrix interferences. This is particularly valuable in verifying label claims regarding the total anthocyanin content, as well as in detecting counterfeit goods that exploit synthetic dyes in lieu of natural anthocyanins.
Although natural dyes generally have a better safety profile, synthetic dyes like sunset yellow (E110) and tartrazine (E102) remain widely used in sodas, candies, and desserts due to their brightness and stability. Concerns about allergic responses or intolerance have driven stringent regulations and labeling requirements for these azo colorants, making their detection a priority for routine quality control. The data in Table 4 reveals a wealth of MIP-based sensors targeting these two colorants, reflecting a keen research interest in reliable analytical devices. For example, a highly sensitive sunset yellow sensor was realized by Chen et al. [144] using silica core–shell imprinting. By coating Fe3O4@SiO2 nanoparticles with a PDA imprinting layer (Figure 9A), the authors achieved both magnetic responsiveness and strong molecular recognition, leading to an LOD in the nanomolar range and accurate detection in soft drinks and candy. Meanwhile, a related approach by Xu and coworkers [145] utilized MWCNTs enveloped by a PDA-based MIP film to yield an LOD of 1.4 nM for sunset yellow in jelly, fruit drinks, chocolate, and instant juice powders, showing the sensor’s broad applicability. Comparable work has been reported for tartrazine, a synthetic lemon-yellow dye used in carbonated beverages, confectioneries, and snacks. George et al. [142] demonstrated that a molecularly imprinted poly(3-aminophenylboronic acid) film on carbon fiber paper (Figure 9B) could detect both brilliant blue and tartrazine in non-alcoholic beverages and various food products. By incorporating the boronic acid moieties, the sensor capitalized on enhanced hydrogen bonding and electrostatic interactions between the polymer matrix and the sulfonic groups of tartrazine. Their results indicated a linear range down to 0.02 µM, with an LOD as low as 0.010 µM for tartrazine. In another example, a MIP sensor based on EP on a GCE was applied to carbonated beverages and candy, achieving an LOD of 30 nM [146]. These findings show how slight modifications in the functional monomer selection (e.g., phenylboronic acid, phenothiazine derivatives, polydopamine) can increase affinity for tartrazine by complementing its chemical functionality. To tackle issues such as matrix complexity and high sugar or protein content in foods, some researchers opt for doping MIPs with conductive nanomaterials that promote signal transduction even in complex media. For instance, Nafion or ionic liquids can enhance charge transfer and reduce potential interferences, while carbon-based nanomaterials like graphene and carbon nanotubes can impart high surface areas conducive to better imprinting site distribution. An example is the MGO/β-cyclodextrin/ionic liquid/AuNPs system reported by Kang et al. [147] for Sunset Yellow. The β-cyclodextrin macrocycle improved recognition through host–guest inclusion, whereas the ionic liquid boosted conductivity and stabilized the MIP film. Such designs showcase how researchers integrate multiple functional components to overcome challenging sample conditions encountered in real food matrices.
Colorants such as indigo carmine, brilliant blue, Rhodamine B, and chrysoidine have also been studied using MIP-based electrochemical approaches. Indigo carmine (E132) is commonly employed in candies and dairy products, necessitating accurate monitoring to ensure it remains within allowable limits. A sensor by Mohammadi et al. [148] used an electropolymerized MIP on a CPE to detect indigo carmine, demonstrating nanomolar sensitivity in candy and ice cream. Similarly, Rhodamine B, sometimes illegally used to color chili powders and sauces, has been targeted by a MIP–SPCE–MSPE platform [149], highlighting the crucial role of MIP sensors in detecting potentially harmful additives. Meanwhile, chrysoidine, another unauthorized dye, has been detected using a β-cyclodextrin-enhanced AuNPs system created through EP [150]. These studies exemplify how MIPs can be applied in the fight against food fraud and adulteration, ensuring that manufacturers adhere to approved colorants and maintain consumer safety.
Despite the promising developments, several challenges remain for MIP sensors targeting food colorants. One issue is the wide structural diversity among colorants—ranging from small synthetic azo dyes to large anthocyanin structures—meaning that the imprinting chemistry must be carefully optimized for each dye. Functional monomers must complement the dye’s functional groups (e.g., sulfonate, hydroxyl, amino) via electrostatic, hydrogen-bonding, or π–π interactions. Another consideration is the complexity of food matrices, which often contain sugars, proteins, fats, and other potential interferents. Although MIPs offer a high degree of selectivity, some dyes have similar structural motifs. For example, many synthetic colorants share azo linkages and aromatic rings, which can complicate the generation of absolutely unique imprinting cavities. Strategies to overcome this include multi-template imprinting (for simultaneous detection of several dyes) or employing advanced nanocomposite matrices to differentiate binding sites more effectively. Sensor stability in acidic or alkaline sample conditions is also critical, especially for colorants in pickled products, yogurts, or strongly acidic beverages. Tailoring the polymer’s pH tolerance and mechanical stability through cross-linkers or protective layers can improve sensor durability. In a broader regulatory context, colorants in food products must often remain below specified ppm or ppb levels depending on local legislation and the type of dye. For instance, the European Union and the U.S. Food and Drug Administration (FDA) impose maximum permissible limits or require explicit labeling for dyes like Tartrazine and Sunset Yellow. MIP sensors that achieve LODs in the low nanomolar range can be highly advantageous in verifying compliance with such regulations. Additionally, MIP-based electroanalysis, if robustly validated and standardized, can reduce reliance on more labor-intensive chromatographic methods. In practical applications, synergy between MIP sensors and partial automation (e.g., flow injection analysis) or coupling with microfluidic channels could yield fully integrated systems for high-throughput screening of colorants. The possibility of recycling MIP-modified electrodes through simple washing or electrochemical regeneration bolsters their suitability for repeated tests and continuous monitoring in industrial processes.
Figure 9.
Examples of MIP-based electrochemical for different colorants sensing. (A) Fe3O4@SiO2@PDA NPs for sunset yellow detection [144]. (B) Poly(3-aminophenylboronic acid) film on carbon fiber paper for tartrazine and brilliant blue detection [151].
Figure 9.
Examples of MIP-based electrochemical for different colorants sensing. (A) Fe3O4@SiO2@PDA NPs for sunset yellow detection [144]. (B) Poly(3-aminophenylboronic acid) film on carbon fiber paper for tartrazine and brilliant blue detection [151].
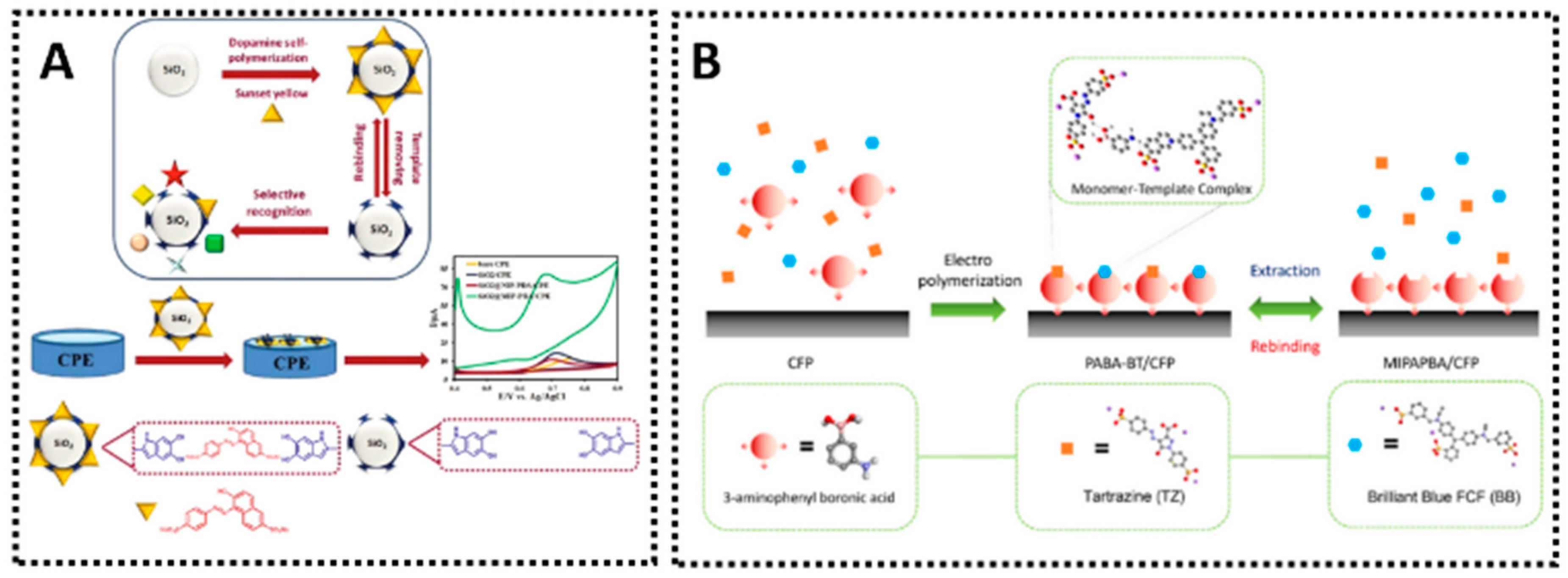

Table 4.
Selected MIP-based electrochemical sensors for colorants.
Table 4.
Selected MIP-based electrochemical sensors for colorants.
| Target Colorants | Sensing Platform | MIP Fabrication Method | Functional Monomer(s) | Linear Range | LOD (nM) | Real Sample | Reference |
|---|---|---|---|---|---|---|---|
| Amaranth | MIP/MWCNT/GCE | EP | Pyrrole | 0.007–1.0 μM and 0.4–17.0 μM | 0.4 | Watermelon juice; Grape juice; Orange juice | [26] |
| MIP/ZnO-MWCNT/SPCE | EP | Melamine | 0.01–1 μM and 1–1000 μM | 3 | Robitussin Junior syrup and Acyclovir Arena capsules | [140] | |
| PDDA-Gr-(Pd-Cu)@MIP-PDA/GCE | SI | Dopamine | 0.006–10 μM | 2 | Soft drink | [141] | |
| CMIG/GCE | SGI | Chitosan | 0.02–150 μM | 3 | Milk powder; White vinegar; Carbonated drinks | [152] | |
| MIES | EP | Aniline | 0.05–50.0 μM | 50 | Grape-flavored drink; Watermelon-flavored drink; Peach-flavored drink | [153] | |
| Anthocyanins | MIP/GCE | BP | Acrylamide | 1 nM–10 μM | 0.3 | Energy bars; Gels; powders; Protein bars; Sports drinks; Gummy chews; Recovery mix; Fruit snacks; Protein shakes | [143] |
| MIP/MWCNTs/GCE | EP | Chitosan | 2.0–968.2 μM | 487.3 | Berry fruits; Tap water | [154] | |
| DMMIPs | SI | Methacrylic acid | 18.4–184 μM | 19 | Blueberries; Grape peel | [155] | |
| Brilliant blue | MIPAPBA/CFP | EP | 3-Aminophenylboronic acid | 0.02–0.34 μM | 8.3 | Non-alcoholic beverages; Dry fruits; Frozen green peas | [151] |
| Chrysoidine | MGO/β-CD@AuNPs | EP | Pyrrole | 0.05–5.00 μM | 17 | Tap water | [150] |
| Indigo carmine | MIP@CPE | EP | o-PD | 5.10–0.13 μM | 42.9 | Candy; Ice Cream | [148] |
| Rhodamine B | MIP-SPCE-MSPE | SI | Acrylamide | 0.0125–0.25 µM | 3.01 | Chili powder; Tomato sauce | [149] |
| Sunset yellow | MIP/f-MWCNTs/GCE | EP | Acrylamide | 0.05–100 μM | 5.0 | Candy; Orange-flavored jelly powder; Peach juice powder; Candy-coated chocolate; Beverages | [156] |
| RMIECs | SGI | 3-aminopropyltriethoxysilane | 0.01–100 μM | 6.82 | Mirinda Orange; Fanta Orange | [157] | |
| SiO2@MIP-PDA/CPE | SI | Dopamine | 0.004–9.1 μM | 1.5 | Fruit drink (Fanta); Orange-flavored candy; Orange-flavored jelly powder; Cheese snack; Orange juice | [144] | |
| MWCNT@MIP-PDA | SI | Dopamine | 0.0022–4.64 μM | 1.4 | Jelly; Fruit drinks (Fanta and Mirinda); Chocolate; Instant juice powder; Ice cream; Candy | [145] | |
| MIP-rGO-IL/GCE | BP | 1-(α-methyl acrylate)-3-allylimidazolium bromide | 10 nM–1.4 μM and 1.4–16 μM | 4.0 | Fruit juice; Mirinda drink; Orange juice | [158] | |
| Au/RGO/GCE | EP | NR | 0.002–109.14 μM | 2 | Fanta; Xiang Cheng Duo; Mirinda | [159] | |
| Fe3O4@SiO2-NPs@MIP/Gr/GCE | SI | Methacrylic acid | 0.0085–30.0 μM | 5.5 | Candy; Orange-flavored jelly powder; Peach juice powder; Candy-coated chocolate; Soft drink | [160] | |
| MMIP/CPE | SI | Methylene succinic acid | 1.51–1510 μM | 86.242 | Water | [161] | |
| GO/AgNPs-MIPs/GCE | PP | Methacrylic-family monomer | 0.1–12 μM | 20 | Fanta drink; Mirinda drink; Orange juice; Mango juice | [162] | |
| MGO/β-CD/IL/AuNPs | PP | β-Cyclodextrin | 0.005–2 μM | 2 | Mirinda drink; Minute Maid; Carbonated beverages; Fruit juice; Candy | [147] | |
| EC-SPME | EP | NR | 1.25–3750 μM | 340 | Orange-flavored jelly powder; Peach juice powder; Beverage | [163] | |
| Tartrazine | MIPAPBA/CFP | EP | 3-Aminophenylboronic acid | 0.02–0.34 μM | 10 | Non-alcoholic beverages; Dry fruits; Frozen green peas | [151] |
| MIP/GCE | EP | Copolymer of m-dihydroxybenzene + o-PD | 0.1–50 μM | 30 | Carbonated beverages; Fruit juice; Candy | [146] | |
| MIP/Co3O4/GCE | EP | Acrylamide | 0.08–10 μM | 33 | Sports drinks | [164] | |
| MIPMet/CFP | EP | Amino-acid monomer | 0.6–160 μM | 27 | Saffron powder; Packaged fruit juices | [165] | |
| MIG-CuS@COOH-MWCNTs/GCE | SGI | NR | 0.03–125 μM | 5 | White vinegar; Vanilla ice cream | [166] | |
| CPE/ZnO/MIP-PArg | EP | L-Arginine | 0.008–0.112 μM and 0.25–5.0 μM | 2.7 | Soft drinks; Orange-flavored jelly powder | [167] | |
| MIP-MWNTs-IL@PtNPs/GCE | PP | Methacrylic-family monomer | 0.03–20 μM | 8 | Fanta; Mirinda; Orange powder | [168] | |
| GO–PtCo@MIPDA | SI | Dopamine | 0.003–0.180 μM and 0.180–3.950 μM | 1.1 | Orangeade; Yellow wine; Ice cream; Jelly; Instant juice powder; Candy; Cookies | [169] | |
| MIP-PmDB/PoPD-GCE | EP | Copolymer of m-dihydroxybenzene + o-PD | 0.005–1.1 μM | 3.5 | Soft drinks | [170] |
6. MIP Sensors for Traditional Contaminants
Traditional contaminants, such as pesticides, heavy metals, and mycotoxins, continue to pose significant threats to global food safety despite longstanding regulatory scrutiny and monitoring efforts. These substances can enter the food chain through various pathways—pesticides via agricultural applications, heavy metals from polluted water or soil, and mycotoxins through fungal growth on stored crops. Chronic exposure to these contaminants can lead to severe health risks, including endocrine disruption, neurotoxicity, and carcinogenic effects. As international regulations tighten and consumer demand for safer food grows, effective detection methods are essential for ensuring compliance and protecting public health. Conventional techniques, including HPLC and mass spectrometry, remain the gold standards; however, they often involve labor-intensive protocols, expensive instrumentation, and specialized personnel. In contrast, MIP-based electrochemical sensors offer a potentially transformative approach, combining strong selectivity with ease of use, portability, and rapid analysis. By tailoring the polymeric imprint towards specific analytes, these sensors achieve highly selective binding sites, enabling reliable detection even in complex food matrices. Furthermore, the adaptability of MIPs to a wide range of electrode platforms allows for their integration into point-of-need devices, thereby reducing both cost and analysis time. In the following sections, we explore the latest advancements in MIP sensor technologies targeting these traditional contaminants. Representative performance benchmarks include PFOS monitoring with MIP-modified electrodes achieving LODs around 0.05 nM thereby illustrating regulatory relevance for perfluoroalkyl contaminants [50]. For mycotoxins, impedimetric and DPV MIP sensors targeting aflatoxin B1 and zearalenone routinely reach sub-nanomolar LODs in cereals and juices, aligning with statutory limits and demonstrating robustness in complex grain and beverage matrices
6.1. Pesticides
The widespread application of pesticides in modern agriculture has been a significant factor in boosting crop yields and protecting plants against pests, thus contributing to global food supply. Nevertheless, the resulting presence of pesticide residues in food and water sources has raised concerns about environmental safety and public health, leading to strict regulatory limits on permissible concentrations in various commodities. Consuming foods contaminated with even low levels of pesticides can pose risks, including acute toxicity, endocrine disruption, and other chronic health effects. By tailoring the functional monomers and cross-linkers to specific pesticide structures, MIPs can create binding sites complementary in shape and functionality to the target analyte. This selective recognition, integrated onto electrochemical transducers, enables pesticide detection in complex media such as agricultural produce, environmental water, or processed food products. The design flexibility of MIPs allows them to be adapted to various pesticide families, including herbicides (e.g., atrazine, glyphosate, paraquat), insecticides (e.g., carbofuran, carbendazim, chlorpyrifos, imidacloprid), and organophosphates (e.g., malathion, methyl parathion, paraoxon). Table 5 provides a detailed overview of selected MIP-based electrochemical sensors for pesticides, highlighting various electrode materials, polymerization strategies, and performance metrics such as linear range, LOD, and validation in real samples.
Although a comprehensive presentation of every entry in Table 5 would be excessively lengthy, a few representative examples illustrate how the imprinting concept can be applied to different pesticide chemistries and sample matrices. Atrazine serves as a well-known herbicide widely used in corn and sorghum production. Given its potential to contaminate surface and drinking water, monitoring atrazine is crucial for environmental and food safety. Several MIP electrochemical sensors have been developed for this herbicide, showcasing different approaches. One example employs polyvinyl chloride (PVC) as a support material along with a bulk polymerized MIP, resulting in a linear detection range of 0.286–0.1879 μM and an LOD of 4.99 nM, validated with both drinking and surface water samples [171]. Another approach, based on EP (MICP), detects atrazine over an extremely wide range (0.1–15,000 μM) with an LOD of 100 nM [172]. Notably, MIPs for atrazine have also been integrated with various electrode platforms, including GCE [173] or gold film electrodes (GFE) [174], demonstrating the adaptability of imprinting to different sensor configurations. These examples underscore not only the range of detection methods, but also the capability to incorporate MIPs into portable devices that operate directly in real-world matrices without extensive sample pretreatment.
Carbofuran, a potent carbamate insecticide, also has drawn attention due to its toxicity and regulatory restrictions. MIP-based electrochemical sensors for carbofuran typically exploit EP of monomers such as pyrrole or aniline in the presence of the pesticide template. One illustration is a sensor constructed by electropolymerizing the MIP onto a gold nanoparticle-modified GCE (MIP/AuNPs/GCE), achieving a remarkable detection range of 0.05–400 μM and an LOD of 24 nM, validated in cowpea and pakchoi [175]. A more sophisticated approach uses a microfluidic lab-on-a-chip (MIP/LOC) platform capable of detecting carbofuran down to 0.067 nM and covering a linear range of 0.2–50 μM in a variety of fruit and vegetable matrices (Chinese cabbage, chili, lettuce, tomato, apple, banana, tangerine, watermelon). Incorporating the MIP into microfluidic devices brings potential for automated sample handling and integrated on-site monitoring. Yet another compelling design (MIECS) uses EP and demonstrates high sensitivity (LOD = 0.33 nM) across a range of 0.001–10.0 μM, successfully testing tangerine, potato, cowpea, and cornmeal [176]. These studies highlight that by carefully selecting functional monomers, electrode surfaces, and polymerization conditions, it is possible to engineer MIP sensors that have robust performance in challenging agricultural samples, including those with complex matrices and solid or semi-solid textures.
Another pesticide commonly encountered in the agri-food chain is carbendazim, a fungicide often used on fruits and vegetables to control fungal diseases. Its residues, however, are tightly regulated because of concerns about possible endocrine-disrupting effects. Research efforts have focused on creating MIP sensors for carbendazim that combine high sensitivity with strong selectivity and durability in diverse matrices. In a notable example, a MIP was electropolymerized on Co3O4 nanoparticles supported on carbon nanotubes (Co3O4NPs@CNTs/GCE), achieving detection down to 2.5 nM, with linear ranges spanning 0.010–2.0 μM and 2.0–10 μM [177]. The sensor was validated in tomatoes, cucumbers, pears, and grapes, thereby evidencing its applicability to multiple fruit and vegetable types. Another work integrated cobalt and nitrogen co-doped hollow carbon (Co,N-HC@CNTs) into an electrode design, obtaining a slightly improved LOD (1.67 nM) over a range of 0.005–10.0 μM [178]. These examples emphasize how incorporating nanomaterials and electrocatalytic mediators into the MIP sensor can enhance electron transfer, reduce interference, and boost overall detection performance. Further, more intricate architectures have emerged: polydopamine and hierarchical metal–organic frameworks (MOFs) are being used to imprint carbendazim onto electrodes. For instance, a polydopamine/hierarchical Al-MOF hybrid with gold nanoparticles (PDA/-@CABA/H-Al-MOF@AuNPs/SPE) enabled detection down to 0.08 nM (Figure 10A) [179]. This approach shows how functional nanomaterials can synergistically improve MIP formation, sensitivity, and electron transport pathways, while still preserving molecular selectivity.
Beyond these individual cases, organophosphorus pesticides (OPs) are a large class of insecticides that includes widely used compounds such as chlorpyrifos, malathion, methyl parathion, paraoxon, and profenofos. Their residues can persist on produce or infiltrate water supplies. MIP-based electrochemical sensors have the potential to overcome some limitations of acetylcholinesterase (AChE)-based biosensors, which, although highly sensitive, can suffer from enzyme denaturation and require special storage conditions. Chlorpyrifos is one of the most common OP insecticides, and a variety of imprinting strategies have been explored to detect it. An impressive illustration is the MIP-aptasensor, constructed via electropolymerization, providing an LOD of 0.35 fM in apples and lettuce [180]. By combining the aptamer approach with MIP technology, the resulting sensor achieves extraordinary sensitivity, exploiting both the molecular recognition of the aptamer and the binding cavities of the imprint. Another example, MIP-PPy/Au-μE, uses polypyrrole to imprint chlorpyrifos and attained an LOD of 0.93 fM across a linear range from 1 fM to 1 μM (Figure 10B) [181]. These ultralow detection limits suggest that MIP-based sensors can perform on par with, or even surpass, traditional biosensors, despite the absence of biological receptors. Indeed, the robust synthetic polymer structures are advantageous in terms of sensor shelf life and reusability. Real-sample validations in cucumber juice, pomegranate juice, or other produce extracts illustrate that the MIP’s selectivity can hold up in complex mediums.
Glyphosate is another important herbicide to highlight. As the active ingredient in many weed-control products, it has become a topic of global concern due to debates about its health and environmental risks. Its strong polarity and limited electroactivity complicate direct electrochemical detection, prompting interest in developing MIP platforms that use indirect detection strategies. Electropolymerized polypyrrole-based MIPs, for instance, have been reported to sense glyphosate with LODs in the nanomolar or even picomolar range, often by monitoring changes in a redox probe such as ferricyanide. One such configuration, MIPPy/AuSPE, demonstrates a linear range of 0.029–0.294 μM and an LOD of 9.47 nM in surface water samples [182]. Another variant incorporates graphene oxide (MIP-GO) and exhibits an LOD of 30 nM for glyphosate in corn, cornfield water, and tap water [183]. In the quest for ultratrace detection, certain researchers have turned to advanced material designs: for example, a CS-MIPs/CMA/Au sensor that applies a surface imprinting (core–shell) approach attained an LOD as low as 6.02 fM [184]. These examples illustrate that, by adapting polymerization strategies and harnessing the surface area of nanomaterials (carbon nanotubes, graphene derivatives, metallic nanoparticles), glyphosate sensors can reach sub-nanomolar or even picomolar sensitivities. This performance is relevant not only for regulatory compliance in foods (such as cereals and vegetables) but also for environmental monitoring of water bodies adjacent to agricultural fields.
Organophosphates like paraoxon, malathion, and methyl parathion represent additional serious concerns for food safety, given that they can linger on leafy vegetables and fruit surfaces if not properly washed or degraded. MIP electrochemical sensors for paraoxon have been built by BP or EP onto carbon paste or nanocomposite-modified GCE, achieving LODs in the low nanomolar range. For malathion, MIP sensors have shown detection down to a few nanomolar levels as well. For instance, MIP(p-Dop)-PNT-PGE integrated polydopamine with nanotube-based materials and achieved an LOD of 4.59 nM [185]. The PP approach was used in another work (MIP-SPE), detecting malathion from 60.6 to 3030 μM with an LOD of 3.03 nM in tap water, soil, and cabbage [186]. Methyl parathion, specifically, has been imprinted in various formats: one sensor (Magnetic-MIP/GCE, Figure 10C) obtained an LOD of 5.5 nM validated in tuna and catfish [187], while another (SMIPMs/CPE) reached 0.34 nM in soil, romaine, and spinach [188]. These sensors consistently demonstrate the capacity to function across a variety of produce or environmental contexts, and many show promise for miniaturization or integration into portable devices for in-field testing.
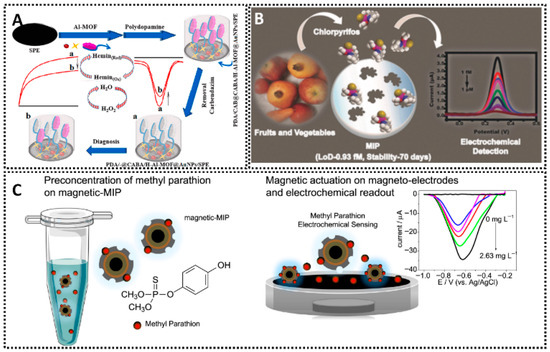
Figure 10.
Examples of MIP-based electrochemical for different pesticides sensing. (A) Dual-action electrochemical MIP-aptasensor for carbendazim detection [179]. (B) EP of pyrrole onto gold microelectrodes followed by electrodeposition for chlorpyrifos detection [181]. (C) Magnetic-MIP by magnetic actuation on m-GEC electrodes for methyl parathion detection [187].
Figure 10.
Examples of MIP-based electrochemical for different pesticides sensing. (A) Dual-action electrochemical MIP-aptasensor for carbendazim detection [179]. (B) EP of pyrrole onto gold microelectrodes followed by electrodeposition for chlorpyrifos detection [181]. (C) Magnetic-MIP by magnetic actuation on m-GEC electrodes for methyl parathion detection [187].
Paraquat is a non-selective herbicide known for its acute toxicity to humans and animals, fueling the need for rigorous monitoring in water, vegetables, and fruits. MIP-based electrochemical sensors for paraquat often exploit either sol–gel imprinting or EP to imprint the quaternary ammonium groups. A sol–gel-based sensor (MIP-SB) exhibits a linear range of 0.08–3.36 μM and an LOD of 21.1 nM in lettuce [189]. A separate approach using EP on a gold nanocluster/chitosan support (MIP-AuNC-CS/GCE) allows detection down to 2.3 nM [190]. Both examples emphasize the advantage of carefully tuning the polymerization method to complement the highly polar nature of paraquat. Moreover, these MIPs must also exhibit selective binding in the presence of structurally similar cationic species that might coexist in food matrices or natural waters.
Further illustrating the breadth of pesticides and detection strategies, investigators have imprinted molecules such as profenofos, fenitrothion, and fenamiphos. Profenofos MIPs demonstrate sub-nanomolar or nanomolar detection limits, validated in produce like sweet pepper, spring onion, or Chinese cabbage. Fenitrothion sensors leverage nanomaterials such as manganese dioxide nanowires on MXene (MnO2NWs@Mo2TiC2) to achieve an LOD of 0.3 nM [191], or silver nanoparticles embedded in a sol–gel matrix for detection of 0.005 nM [192]. Fenamiphos imprinting strategies involve surface imprinting on Au@MOF-235@g-C3N4 nanocomposites [193] or combining cobalt oxide with MOF-74 [194], demonstrating how hierarchical porous structures can support high loading of pesticide templates and expedite mass transfer to binding sites.
In real food applications, matrix effects can be quite severe. Vegetables, fruits, juices, cereals, and other products contain sugars, proteins, organic acids, pigments, and other components that can interfere with electrochemical detection. To tackle these challenges, MIPs must exhibit high specificity, often supported by additional sample pretreatment steps. Nonetheless, many of the research examples in Table 5 report impressive recovery rates (often 80–110%) for spiked samples with minimal or straightforward sample preparation, confirming that molecular imprinting can offer a degree of recognition sufficient to handle real-world complexity. Furthermore, reusability and stability over multiple cycles represent additional benefits of synthetic polymeric receptors compared to biological recognition elements like enzymes or antibodies. MIP sensors can often be regenerated by washing with a mild solvent (e.g., methanol or aqueous buffers) that disrupts the template–polymer interactions, restoring the binding cavities for the next detection cycle. This feature can bring down operating costs and make the sensor more attractive for routine on-site or in-line screening.

Table 5.
Selected MIP-based electrochemical sensors for pesticides.
Table 5.
Selected MIP-based electrochemical sensors for pesticides.
| Target Pesticides | Sensing Platform | MIP Fabrication Method | Functional Monomer(s) | Linear Range | LOD (nM) | Real Sample | Reference |
|---|---|---|---|---|---|---|---|
| Atrazine | PVC/MIP | BP | Methacrylic acid | 0.286–0.1879 μM | 4.99 | Drinking water; Surface water | [171] |
| MICP | EP | PEDOT-co-thiophene acetic acid | 0.1–15,000 μM | 100 | - | [172] | |
| MIP-SPPC | SI | Methacrylic acid | 0.5–50 μM | 400 | Tap water | [195] | |
| MIP/GFE | BP | Methacrylic acid | 5.0–140 μM | 1 | Drinking water | [174] | |
| MIP/GCE | PP | Methacrylic acid | 0.046–0.46 μM | 0.92 | Spring water | [173] | |
| Carbofuran | MIP | EP | Methacrylic acid | 0.05–10 μM | 16 | River water | [196] |
| MIP/AuNPs/GCE | EP | Methacrylic acid | 0.05–400 μM | 24 | Cowpea; Pakchoi | [175] | |
| MIECS | EP | 4-Hydroxythiophenol | 0.001–10.0 μM | 0.33 | Tangerine; Potato; Cowpea; Cornmeal | [176] | |
| MIP/LOC | BP | Methacrylic acid | 0.2–50 μM | 0.067 | Chinese cabbage; Chili; Lettuce; Tomato; Apple; Banana; Tangerine; Watermelon | [197] | |
| MIP-CNTs-Fe3O4@Au/CPE | EP | o-PD | 0.1–100 μM | 3.8 | Cabbage; Celery; Chili; Onion; Peppermint | [198] | |
| Carbaryl | IL@MMIPs | SI | Methacrylic acid | 0.22–66.5 μM | 13.3 | Apple; Rice | [199] |
| Carbendazim | Co3O4NPs@CNTs/GCE | EP | β-cyclodextrin and thionine | 0.010–2.0 μM and 2.0–10 μM | 2.5 | Tomatoes; Cucumbers; Pears; Grapes | [177] |
| MIP/Co,N-HC@CNTs/GCE | EP | 3,4-ethylenedioxythiophene | 0.005–10.0 μM | 1.67 | Tomato; Orange; Apple | [178] | |
| PDA/-@CABA/H-Al-MOF@AuNPs/SPE | EP | Dopamine | 0.0003–0.01 μM | 0.08 | Tap water; Apple juice; Tomato juice | [179] | |
| MIP/AuNP-rGO/GCE | EP | o-PD | 0.002–70 μM | 0.68 | Grape juice; Apple juice | [200] | |
| MIP/MWCNT | BP | 1-vinyl imidazole | 10.0–100.0 μM | 5.23 | River water; Industrial wastewater | [201] | |
| MIP/C-ZIF67@Ni/GCE | EP | Methacrylic acid | 0.4–1 μM | 0.134 | Soil; River water | [202] | |
| HKUST-1@MIP-GE | BP | Methacrylic acid | 0.01–50.00 μM | 2.0 | Apple juice; Cucumber juice; Tomato juice; Tangerine juice | [203] | |
| MIP/N, S–Mo2C/GCE | EP | o-PD | 0.001–8 μM | 0.67 | Grape; Apple; Tomato; Eggplant; Cucumber | [204] |
6.2. Heavy Metals Ions
Heavy metal contamination in food systems has emerged as a significant public health concern, driven by factors such as industrial pollution, agricultural runoff, and the mismanagement of waste streams. Metals such as lead (Pb), mercury (Hg), cadmium (Cd), and arsenic (As) can accumulate in various foodstuffs, including cereals, fruits, vegetables, and seafood, posing risks that range from acute toxicity to chronic disorders affecting the nervous, renal, and reproductive systems. Regulatory agencies worldwide have thus imposed stringent limits on permissible levels of heavy metals in foods, demanding rapid, reliable, and cost-effective analytical tools. While techniques such as atomic absorption spectroscopy (AAS), inductively coupled plasma mass spectrometry (ICP-MS), and X-ray fluorescence (XRF) remain the gold standards for metal detection, they often require extensive sample preparation, expensive instrumentation, and trained personnel. MIP electrochemical sensors offer an alternative that can be adapted for on-site or in-field screening of metal contaminants in both environmental and food matrices, leveraging the specificity of imprinted cavities within a polymeric matrix. In contrast to molecular targets such as small organic colorants or antioxidant molecules, imprinting heavy metals entails designing polymeric recognition sites for ionic species that often have variable oxidation states and hydration spheres. Common strategies involve the use of ion-imprinted polymers (IIPs)—a specialized class of MIPs—that rely on coordination interactions between metal ions and functional monomers or chelating ligands. During polymerization, the metal ion coordinates with functional sites, and upon extraction, vacant imprinted cavities remain, displaying selective rebinding of the target ion. Table 6 provides selected examples of MIP-based electrochemical sensors for heavy metals such as As, Cd, Cu, Cr, Hg, and Pb, highlighting their sensing platforms, imprinting methods, linear ranges, detection limits, and tested real samples. Although many of these examples focus on water or wastewater as the primary sample—reflecting the ubiquitous route of contamination—similar electrochemical principles extend to food matrices where sample pretreatment strategies (e.g., acidic digestion for solid foods, simple filtration for beverages) can be adapted.
Arsenic (As) remains one of the most notorious contaminants in water and rice-based products, especially in certain regions with high geological arsenic presence. Several groups have developed IIP sensors targeting arsenic in both groundwater and food-relevant contexts. For instance, an As(III)-MIM@MOF/AuNPs/GCE sensor [205] employed a surface imprinting (core–shell) technique using a MOF and AuNPs (Figure 11A). The resulting electrode showed a broad linear range (0.01–30,000 μM) and a very LOD of 0.3 nM, making it suitable for monitoring trace levels of arsenic. Although the authors validated their platform in tap water and river water, the same approach can be extended to detect arsenic in rice or fruit juices following appropriate sample treatment. The synergy between MOFs and AuNPs improved the sensor’s conductivity and provided a high surface area to accommodate multiple binding sites, thereby enhancing sensitivity.
Figure 11.
Examples of MIP-based electrochemical for different heavy metal ions sensing. (A) As(III)-MIP@MOF for As(III) detection [205]. (B) CS/AuNPs/GR/GCE IIP for Cd(II) detection [206].
Cadmium (Cd) is another prevalent heavy metal linked to industrial activities, fertilizer usage, and battery disposal. Cd contamination in cereals and vegetables is a recurring issue. One example from Table 6 is the CS/AuNPs/GR/GCE sensor [206], which uses an electropolymerized chitosan layer on a graphene-modified GCE (Figure 11B). The presence of AuNPs further improves electron transfer and amplifies the current response. This sensor displays a linear range of 0.1–0.9 μM and an LOD of 0.162 nM. Of particular note is its demonstration in tap water, river water, and even milk, suggesting applicability to liquid food matrices with minimal processing. Chitosan’s chelating ability (through its amine and hydroxyl groups) is especially beneficial for binding metal ions, and its biocompatibility is an added advantage for sensors geared toward food applications. Another approach for Cd detection is illustrated by the IIP/GO@GCE system [207], which uses a BP strategy and integrates GO. With a wide linear range (0.073–2400 μM) and an ultralow LOD of 0.07 nM, this sensor underscores the role of graphene-based materials in enhancing surface area and electron transfer. Although tested primarily on human hair and blood serum, an analogous protocol can be repurposed for detecting Cd in processed foods (e.g., dairy products, canned goods) known to accumulate this metal. The functionalization of GO allows for stronger interactions with the polymer matrix, stabilizing the imprinted cavities formed around Cd ions.
Copper (Cu) contamination can occur through corroded pipes, agricultural fungicides, and certain food additives. Excess Cu intake has been linked to gastrointestinal disturbances and, in rare cases, liver damage. Several IIP-based electrochemical sensors have been reported for Cu2+. For instance, a MIECS sensor [208] constructed by EP demonstrates a linear range of 0.5–30 μM and an LOD of 42.4 nM, tested in water and beverages like citric fruit juice and beer. Even though Cu is an essential micronutrient in the human diet, controlling its concentration is critical to prevent toxicity. The sensor’s successful application to both fruit juice and beer exemplifies how minimal preprocessing steps (e.g., simple filtration or dilution) can facilitate direct electrochemical measurements of Cu in diverse liquid food samples. Another interesting example is the Cu(II)-IIP sensor [209], which claims an extremely wide detection range (0.01–100,000 μM) and an LOD of 32 nM. The authors validated it in coins, multivitamins, and various water types, but its principles are immediately transferable to the analysis of food products where copper complexes or high copper residues are a concern.
Chromium (Cr) generally appears in two main oxidation states in environmental and food systems: Cr(III) and Cr(VI). Cr(III) is considered an essential trace element in human nutrition, but Cr(VI) is highly toxic and carcinogenic. In some circumstances, Cr(III) can oxidize to Cr(VI), complicating risk assessment in foods such as vegetables irrigated with contaminated water. The Pt/MWCNT-IIP sensor [210] exemplifies a surface imprinting approach for Cr3+, achieving detection in wastewater with an LOD of 51 nM. Another sensor, IIP-S/Au [211], focuses on Cr(VI) and exploits EP to achieve an exceptionally low LOD of 0.64 nM. Although validated in tap water and river water, these platforms can be extended to measure Cr content in processed foods, given the potential for cross-contamination during production. The ionic nature of Cr species makes ion-imprinting a particularly suitable route, as specialized chelating monomers can stabilize specific oxidation states, thereby enhancing selectivity.
Mercury (Hg) stands out among heavy metals for its high toxicity, especially in the organic form (methylmercury) prevalent in seafood. One exemplary approach is the RGO–IIP sensor [212], which utilizes reduced graphene oxide and surface imprinting to detect Hg2+ over a wide range of 0.35–400 μM. This sensor boasts an LOD of 0.1 nM and has been tested in tap water, aqueduct water, wastewater, and river water. The large surface area and robust electron transfer properties of RGO are key factors in enhancing the sensor’s performance. The same principle can be employed to monitor mercury levels in fish extracts, a pressing need due to bioaccumulation in marine species. Another advanced design is IIP/g-C3N4/CPE [213], which achieves an impressive LOD of 18 pM over a range of 0.06–25.0 nM. While demonstrated in tap water and sea water, the underlying materials (graphitic carbon nitride and a carbon paste electrode) could similarly be used for fish tissue digestion samples, enabling mercury quantification in a format more accessible than large-scale spectrometric methods.
Finally, lead (Pb) is a historically well-documented toxic metal with wide-ranging effects on neurological development, especially in children, and on the cardiovascular system in adults. Lead contamination may arise in canned products, soil-grown staples, and water distribution systems that use lead pipes. Multiple Pb sensors in Table 6 highlight different polymerization strategies. For instance, GCE-IIP-PAN/MWCNT [214] uses PP and MWCNTs to detect Pb2+ in a range of 2.41–57.97 μM with an LOD of 0.77 nM. Although tested in tap water and mineral water, its principle can be readily adapted to measure lead in processed drinks or even infant formula, a critical area of food safety. Another highly sensitive approach, IIP-CPE [215], offers a linear range of 0.1–1000 μM and an exceptionally low LOD of 0.03 nM. The authors validated their sensor in diverse matrices, including distilled water, tap water, Caspian Sea water, and wastewater. In a food context, the same design could be applied to evaluate lead in bottled beverages or extracts of grain products. The combination of MWCNTs or graphene structures with an ion-imprinted polymer frequently demonstrates enhanced conductivity, surface area, and mechanical stability. These factors collectively contribute to lower detection limits and improved reusability—important attributes for routine food testing labs and possible in-field screening.
Although many of these sensors are validated in environmental water samples, the analytical principles remain directly transferable to the food sector. Typical steps in adapting an IIP electrochemical sensor for use in a complex food matrix might involve digesting the sample in acid to liberate metal ions, filtering or centrifuging to remove solid debris, and adjusting pH or ionic strength to suit the sensor’s working conditions. Once in solution, the metal ions can be directly measured by DPV or SWV, with the imprinted cavities specifically rebinding the target metals and modulating the electrochemical response. The sensors can often be regenerated by treating them with an appropriate chelating agent or mild acid/base to remove bound metal ions, extending operational lifespan and reducing per-test costs. Given the global emphasis on food safety, there is clear momentum toward incorporating MIP electrochemical sensors in regulatory frameworks or as complementary screening tools. Their advantages—portability, short response time, and relatively simple fabrication—are appealing for both field inspectors and small-to-medium enterprises looking to maintain quality standards. However, to move beyond proof-of-concept, further effort is necessary to ensure sensor reproducibility, mass-producible fabrication, and robust performance in real-world conditions (e.g., temperature fluctuations, presence of complex matrix constituents). The research detailed in Table 6 illustrates the immense progress in designing IIP-based electrodes that achieve ultra-trace detection limits, often below parts-per-billion or even parts-per-trillion levels. These developments promise a future where the on-site electrochemical screening of heavy metals in diverse foods—ranging from canned vegetables and cereals to fish and dairy products—can be achieved at relatively low cost, thus safeguarding public health and meeting stringent regulatory thresholds.

Table 6.
Selected MIP-based electrochemical sensors for heavy metals.
Table 6.
Selected MIP-based electrochemical sensors for heavy metals.
| Target Heavy Metals | Sensing Platform | MIP Fabrication Method | Functional Monomer(s) | Linear Range | LOD (nM) | Real Sample | Reference |
|---|---|---|---|---|---|---|---|
| As3+ | MIP/NPG/ITO | EP | m-phenylenediamine | 0.02–9.0 μM | 0.0071 | Groundwater; Tap water | [216] |
| IIP/NPG/GE | EP | o-PD | 0.02–9.0 μM | 7.1 | Groundwater; Tap water | [217] | |
| As(III)-MIM@MOF/AuNPs/GCE | SI | Methacrylic acid | 0.01–30,000 μM | 0.3 | Tap water; River water | [205] | |
| Cd2+ | CPE-IIP | BP | Vinyl pyridine | 0.001–0.5 μM | 0.52 | Tap water; River water | [218] |
| CS/AuNPs/GR/GCE | EP | Chitosan | 0.1–0.9 μM | 0.162 | Tap water; River water; Milk | [206] | |
| IIP/rGO/GCE | EP | Pyrrole | 8.9–890 μM | 2.31 | Lake and river water | [219] | |
| IIP/ERGO/GCE | EP | o-PD | 0.0089–0.445 μM | 1.16 | Lake and river water | [220] | |
| IIP-Cd | EP | Chitosan | 0.01–0.1 μM | 3.51 | Tap water; Lake water | [221] | |
| IIP/GO@GCE | BP | Acrylamide + Methacrylic acid | 0.073–2400 μM | 0.07 | Human hair; Blood serum | [207] | |
| (Cd-IIP)/AuNPs/Au | SI | 3-mercaptopropyl trimethoxysilane | 8.89–444.5 μM | 1.96 | River water; Watsons water | [222] | |
| Cu2+ | CS/GO-IIP | SI | Chitosan | 0.5–100 μM | 150 | Tap water; River water | [223] |
| MIECS | EP | Acridine orange | 0.5–30 μM | 42.4 | Running water; Citric fruit juice; Rainwater; Beer | [208] | |
| CILE | PP | NR | 0.025–1.25 μM | 9.4 | River water; Mineral water; Tap water | [224] | |
| MMIP | SGI | Cysteine | 0.01 μM–1.0 mM | 10 | Spiked water; Serum | [225] | |
| IINPs/GCE | SI | NR | 0.06–1.9 μM | 20 | Tap water; River water; Seawater | [226] | |
| MIP-CP | BP | 4-vinyl pyridine | 0.07–1.0 μM and 1.0–100 μM | 23 | River water | [227] | |
| Cu(II)-IIP | BP | N-methacryloyl-L-histidine | 0.01–100,000 μM | 32 | Coin; Multivitamin; Tap water; River water; Lake water | [209] | |
| SPCE | EP | 4-aminophenylacetic acid | 0.01–1.2 μM | 1.71 | Drinking water; Tap water; Marine water | [228] | |
| Cr3+ | Pt/MWCNT-IIP | SI | Methacrylic acid | 19.23–96.15 μM | 51 | Wastewater | [210] |
| CPE-MWCNT/IIP | BP | NR | 1.0 to 100,000 μM | 590 | Sea water; River water; Soil | [229] | |
| Cr6+ | IIP-S/Au | EP | Chitosan | 0.001–10 μM | 0.64 | Tap water; River water | [211] |
| IP-NPs/CPE | PP | Meta-acrylate acid | 0.0001–0.1 μM and 0.1–1.0 μM | 0.03 | Ambient water | [230] | |
| Hg2+ | IIP–MWCNT–GCE | PP | Methacrylic acid | 0.01–700 μM | 5 | Wastewater; Groundwater | [231] |
| MIP/IDEs | BP | Vinylpyrrolidone | 50–450 μM | 4.5 | - | [232] | |
| RGO–IIP | SI | Methacrylic acid | 0.35–400 μM | 0.1 | Tap water; Aqueduct water; Wastewater; River water | [212] | |
| IIP-CPE | BP | Methacrylic acid | 0.004–1.3μM | 1.95 | Tap water; River water; Industrial wastewater; Metallurgy wastewater; Dental amalgam waste; Tuna fish; Shrimp; Human hair | [233] | |
| GQDTU-IIP | BP | Thiourea-derivatized graphene quantum dot | 0.06–23 μM | 23.5 | River water; Tap water | [234] | |
| IIP/g-C3N4/CPE | PP | Itaconic acid | 0.06–25.0 nM | 0.0018 | Tap water; Sea water | [213] | |
| IIP-CPE | BP | Methacryloyl-(l)-cysteine | 0.0025–5.0 μM | 0.52 | Tap water; River water | [235] | |
| IIP-CPE | SI | NR | 1.0–8000 μM | 0.2 | River water; Wastewater; Potato; Carrot; Lettuce | [236] | |
| Pb2+ | GCE-IIP-PAN/MWCNT | PP | Methacrylic acid | 2.41–57.97 μM | 0.77 | Tap water; Mineral water; Saline (physiological serum) | [214] |
| IIP/MWCNT-CPE | PP | Itaconic acid | 0.01–0.50 μM and 1–80 μM | 3.8 | Caspian Sea water; Tejen River water | [237] | |
| MWCNT-IIP/PE | SI | Acrylamide | 4.83–24.14 μM | 20 | Lake water; Mining effluent; Food sample; Cosmetics | [238] | |
| IIP-CP | PP | Methacrylic acid | 0.001–0.81 μM | 0.6 | Tap water; River water; Edible refined salt; Wastewater | [239] | |
| IIP-CPE | PP | 4-vinylpyridine | 0.1–1000 μM | 0.03 | Distilled water; Tap water; Caspian Sea water; Wastewater | [215] | |
| IIP-CPE | PP | 4-vinylpyridine | 0.001–0.75 μM | 0.013 | Flour; Rice; Tap water; Yudai River water | [240] | |
| SAMs/Au | SI | NR | 0.3–50 μM | 0.2 | River water | [241] | |
| MIP-CPE | SI | 2-methacryloyl-amido cysteine | 5–100 μM | 91.2 | Honey | [242] |
6.3. Mycotoxins
Mycotoxins are a group of structurally diverse secondary metabolites produced predominantly by molds such as Aspergillus, Fusarium, and Penicillium species. These compounds can contaminate a wide range of agricultural commodities, including cereals, nuts, fruits, spices, and animal feed. Factors such as temperature, humidity, and poor storage conditions can favor fungal growth and mycotoxin production. Due to their toxicological profiles—ranging from carcinogenic and hepatotoxic to nephrotoxic and immunosuppressive—mycotoxins pose serious public health risks and have compelled many regulatory agencies worldwide to establish strict maximum permissible limits in food and feed. Traditional methods for mycotoxin detection include chromatography-based approaches, which, although accurate and reliable, often require complex sample preparation, sophisticated instrumentation, and trained personnel. Consequently, considerable effort has gone into developing portable, cost-effective, and field-deployable tools for rapid mycotoxin analysis. Within this context, electrochemical sensors based on MIPs have emerged as promising analytical platforms because they offer excellent selectivity, low detection limits, and the potential for miniaturization while maintaining robust performance in complex matrices. Table 7 shows the selected MIP-based electrochemical sensors for mycotoxins.
Among the mycotoxins, aflatoxin B1 is arguably one of the most widely studied due to its potent carcinogenic properties and frequent occurrence in staple commodities such as peanuts, corn, and milk products. Many MIP-based electrochemical sensors have been reported to detect aflatoxin B1 with high sensitivity and selectivity, often employing EP to fabricate the imprinted layer on an electrode surface. For example, Li et al. [243] fabricated a MIP-Apt/Cu2O NCs/GCE sensor that utilized an aptamer in conjunction with the MIP matrix, a hybrid strategy that can enhance recognition and signal transduction (Figure 12A). Electropolymerizing the functional monomer in the presence of Cu2O nanocubes helped create a porous and conductive matrix, leading to a linear detection range from 0.00005 to 0.04 μM. With a LOD of 0.012 nM, the sensor was successfully validated in milk samples, showcasing the potential to address stringent regulatory limits for AFB1 in dairy products. Another noteworthy example involves a MIP-A/ITO platform for detecting both aflatoxin B1 and fumonisins B1 [244]. This study highlights the ability to imprint more than one mycotoxin type using similar electrode configurations, facilitating multiplexing capabilities in food analysis. The reported linear range for AFB1 was 0.001–1.25 μM, with an LOD of 0.313 nM. The sensor’s effectiveness was confirmed through analysis of corn, cereals, and fruits, all known to be vulnerable to aflatoxin contamination. Such versatility indicates that, by carefully tailoring the polymerization conditions, it is feasible to produce a single sensing device capable of discriminating between multiple mycotoxins in complex agricultural matrices.
Figure 12.
Examples of MIP-based electrochemical for different mycotoxins sensing. (A) Steps of the Apt-MIP nanohybrid preparation for aflatoxin B1 diagnosis [243]. (B) Preparation procedure of BN-HPC and the fabrication and working process of MIP/[APMIm]Br/BN-HPC/GCE for citrinin sensing [245].
In addition to aflatoxins, citrinin—produced by several species of Penicillium and Monascus—is another frequent contaminant in cereals and fermented products. Citrinin can cause nephrotoxic effects and poses risks in products like red yeast rice, which is often consumed as a dietary supplement. MIP-based sensors targeting citrinin typically rely on nanomaterial-enhanced electrodes to amplify electrochemical signals. A representative example is the MIP/BN-HPC/GCE sensor [245] constructed via EP on a boron nitride–highly porous carbon (BN-HPC)-modified GCE (Figure 12B). This composite matrix improves surface area and electron transfer, resulting in a linear range of 0.003–40.7 μM and an LOD of 0.1 nM. The sensor was tested in red yeast rice, rice, and wheat samples, underscoring the device’s ability to operate in diverse food matrices. Another compelling study used MIPs/Gr-MWCNTs-IL/GCE [246] for simultaneous detection of citrinin and patulin, highlighting the practicality of dual or multi-analyte detection platforms. By combining graphene, multi-walled carbon nanotubes, and ionic liquids, the sensor benefited from a highly conductive and accessible surface, enabling detection in the sub-nanomolar range.
Deoxynivalenol, also known as vomitoxin, is a trichothecene mycotoxin predominantly found in cereals such as wheat, barley, corn, and oats. It can cause gastrointestinal disorders and immunotoxic effects, and its presence in grains is regulated in numerous countries. Conventional deoxynivalenol analysis can involve time-intensive immunoassays or chromatographic methods, but MIP electrochemical sensors offer a rapid alternative. One such example is the MIP/SPGE sensor [247] that uses a screen-printed gold electrode for EP of the MIP layer. The sensor demonstrated a linear range of 0.01–10 μM and an LOD of 6.2 nM. Importantly, it was validated in various cereal flours, demonstrating robust performance in real food samples despite the complexity of powdered grain matrices. Another innovative approach employed Mn-CeO2 nanoparticles [248] as part of a composite with the MIP layer (Figure 13A). Such nanomaterial integration can further enhance sensitivity by improving the electrode’s catalytic properties and conductivity.
Fumonisins, particularly fumonisin B1 (FB1), are another class of mycotoxins frequently detected in maize-based products. They have been associated with diseases such as esophageal cancer and neural tube defects, making reliable detection in foodstuffs crucial. Several MIP sensors for fumonisin B1 have been reported, one notable instance being the MIP-F/ITO platform [244]—where “F” denotes fumonisin imprinting. Similarly to the dual-purpose sensor targeting aflatoxin B1 on ITO substrates, the MIP-F/ITO approach yielded an LOD of 0.322 nM for fumonisin B1 across a detection range of 0.001–1.25 μM. The sensor was validated for corn, cereals, and fruits, demonstrating the practicality of combining a single electrode platform with multiple MIPs for multi-toxin screening.
Ochratoxin A is a potent nephrotoxin commonly found in wine, coffee, and cereals. Its toxic effects include carcinogenicity, nephrotoxicity, and teratogenicity, prompting strict international regulations. MIP-based electrochemical sensors for ochratoxin A often include advanced nanocomposites to achieve lower detection limits. For example, a MIP/AgNPs/POM/rGO/GCE sensor [249] was fabricated using AgNPs, polyoxometalates (POM), and rGO. This multi-component matrix not only boosted electrocatalytic activity, but also helped form high-quality imprinting sites. With an LOD of 0.016 nM and a linear range of 0.05–1.5 μM, the sensor demonstrated strong applicability in grape juice and wine—both of which are prone to Aspergillus contamination during grape processing and storage. Another illustrative example is the MIP/Apt/AuNPs/ZIF-67 sensor [250] employing a core–shell surface imprinting method (Figure 13B). The integration of aptamers and metallic nanoparticles can further enhance binding specificity and electron transfer, achieving an LOD as low as 0.853 nM in complex cereal matrices.
Patulin, primarily produced by Penicillium and Aspergillus species, is a mycotoxin often found in apple-based products and other fruits. Its acute toxicity includes gastrointestinal disturbances, while chronic exposure may pose long-term risks. Various MIP sensors have been reported for patulin detection, several of which incorporate carbon-based nanomaterials. For instance, MIPs/Gr-MWCNTs-IL/GCE [246] again demonstrates the synergy of graphene, carbon nanotubes, and ionic liquids for enhanced conductivity and surface imprinting. With an LOD of 0.08 nM, this sensor displays remarkable sensitivity, validated in commercial apple and pear juice samples. Another intriguing design is an origami 3D-ePAD [251] employing a surface imprinting approach on a paper-based device (Figure 13C). This platform promises low-cost, disposable sensing with minimal reagent consumption—a significant advantage for resource-limited settings or in-field analysis. The sensor achieved a detection range of 0.001–25 μM, with an LOD of 0.2 nM. Its successful application to apples, tomatoes, grapes, oranges, and Chinese pears demonstrates the broad utility of this technology in monitoring patulin across multiple fruit types.
Finally, zearalenone, produced by Fusarium species, is an estrogenic mycotoxin often implicated in reproductive disorders in livestock and potentially humans. It frequently contaminates cereal grains such as corn, wheat, and rice. MIP-based sensors for zearalenone follow similar design principles, commonly using EP of functional monomers on nanomaterial-modified electrodes to achieve high sensitivity. One example is the MIP/CuHCF/rGNR–rGO/GCE sensor [252] that employs copper hexacyanoferrate (CuHCF) and reduced graphene nanoribbons (rGNR)–rGO composites. By leveraging the redox behavior of CuHCF and the large surface area of graphene derivatives, the resulting MIP sensor showed a linear range of 0.79–1586 μM with an LOD of 0.29 nM. Although the higher end of the range is quite large, the sub-nanomolar detection limit is highly relevant for food safety applications. Another example, MIP/AuSPE [253], uses BP on a gold screen-printed electrode, achieving an LOD of 0.12 nM over a broad range from 0.00035 to 350 μM. Such a wide detection window is particularly advantageous in screening samples that may exhibit extreme variations in contamination levels, from trace amounts up to outright spoilage.
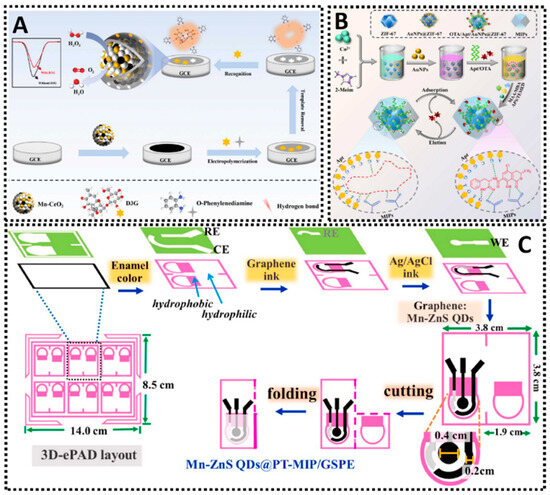
Figure 13.
Examples of MIP-based electrochemical for different mycotoxins sensing. (A) Mn-doped CeO2 nanozyme-MIP for deoxynivalenol sensing [248]. (B) Fabrication of MIPs/Apt/AuNPs@ZIF-67 for ochratoxin A sensing [250]. (C) Fabrication of Origami 3D-ePAD for patulin sensing [251].
Figure 13.
Examples of MIP-based electrochemical for different mycotoxins sensing. (A) Mn-doped CeO2 nanozyme-MIP for deoxynivalenol sensing [248]. (B) Fabrication of MIPs/Apt/AuNPs@ZIF-67 for ochratoxin A sensing [250]. (C) Fabrication of Origami 3D-ePAD for patulin sensing [251].

Table 7.
Selected MIP-based electrochemical sensors for mycotoxins.
Table 7.
Selected MIP-based electrochemical sensors for mycotoxins.
| Target Mycotoxin | Sensing Platform | MIP Fabrication Method | Functional Monomer(s) | Linear Range | LOD (nM) | Real Sample | Reference |
|---|---|---|---|---|---|---|---|
| Aflatoxins B1 | MIP-MOF | EP | p-aminothiophenol | 0.0000032–3.2 μM | 0.001 | Rice | [254] |
| PANI@MIP/CNC-CNT | EP | Aniline | 0–25 μM | 3 | Milk | [255] | |
| MAA-MIP@CPE | BP | Methacrylic acid | 0.052–0.2 μM | 5.9 | Corn; Wheat | [256] | |
| MIP-Apt/Cu2O NCs/GCE | EP | Aniline | 0.00005–0.04 μM | 0.012 | Milk | [243] | |
| MIP-A/ITO | EP | Aniline | 0.001–1.25 μM | 0.313 | Corn; Cereals; Fruits | [244] | |
| MIP/PC | EP | Pyrrole | 0.005–0.1 μM | 1.7 | - | [257] | |
| Citrinin | MIP/PtNPs/POM/rGO/GCE | EP | Pyrrole | 0.001–0.1 μM | 0.2 | Rye | [258] |
| MIP/BN-HPC/GCE | EP | Thionine | 0.003–40.7 μM | 0.1 | Red yeast rice; Rice; Wheat | [245] | |
| MIPs/Gr-MWCNTs-IL/GCE | EP | Methacrylic acid | 0.0015–0.018 μM | 0.61 | Takdaneh apple juice; Sunich apple juice; Sundis apple juice; Mihan pear nectar | [246] | |
| MIP/Nb2C-MWCNTs/GCE | EP | o-toluidine | 0.04–10.0 μM | 3.6 | Wine; Flour; Corn | [259] | |
| MIP/PdNPs/BZ/GQDs/GCE | EP | Pyrrole | 0.001–0.005 μM | 0.2 | Chicken egg | [260] | |
| Deoxynivalenol | MIP/SPGE | EP | o-PD | 0.01–10 μM | 6.2 | Corn flour; Wheat flour; Rice flour; Oat flour | [247] |
| Mn-CeO2/MIP | EP | o-PD | 0.034–170 μM | 0.01 | Barley; Wheat | [248] | |
| P-Arg-MIP/COOH-MWCNTs | EP | L-arginine | 0.1–70 μM | 70 | Wheat flour | [261] | |
| Fumonisins B1 | MIP-F/ITO | EP | 0.001–1.25 μM | 0.322 | Corn; Cereals; Fruits | [244] | |
| Ochratoxin A | MIP/MWCNT/GCE | EP | Pyrrole | 0.050–1.0 μM | 4.1 | Beer; White wine; Red wine | [262] |
| MIP-RECS | EP | Ionic liquid | 0.5–15 μM | 14 | Chinese liquor; Beer; Red wine | [263] | |
| MIP/AgNPs/POM/rGO/GCE | EP | Phenol | 0.05–1.5 μM | 0.016 | Grape juice; Wine | [249] | |
| MIP/MnCO3NS/CF/GCE | EP | Pyrrole | 0.01–1.0 μM | 2.0 | Apple juice | [264] | |
| MIP/Apt/AuNPs/ZIF-67 | SI | Methacrylic acid | 2.56–25.6μM | 0.853 | Wheat; Rice; Maize; Soybean | [250] | |
| MIP/ZIF-8 | SI | Allobarbital | 0–98.4 μM | 0.049 | Cereals | [265] | |
| Patulin | MIPs/Gr-MWCNTs-IL/GCE | EP | Methacrylic acid | 0.0005–0.013 μM | 0.08 | Takdaneh apple juice; Sunich apple juice; Sundis apple juice; Mihan pear nectar | [246] |
| MIP/Au@Cu-MOF/N-GQDs/GCE | EP | Aniline | 0.0045–315 μM | 0.0032 | Apple juice | [266] | |
| MIP/Fe3O4/GO/GCE | BP | Methacrylic acid | 0.001–250.0 nM | 0.333 | Apple juice; Commercial pear juice | [267] | |
| MIP-Au/CS-CDs/GCE | EP | o-PD | 0.001–1 μM | 0.757 | Apple juice | [268] | |
| MIP/Au@PANI/SeS2@Co MOF | EP | p-aminobenzoic acid | 0.001–0.1 μM | 0.66 | Apple juice | [269] | |
| MIP/PtPd-NPC/GCE | EP | 4-aminothiophenol | 0.049–49 μM | 0.037 | Apple juice; Grape juice | [270] | |
| Origami 3D-ePAD | SI | Methacrylic acid | 0.001–25 μM | 0.2 | Apple; Tomato; Grape; Orange; Chinese pear | [251] | |
| Zearalenone | MIP/g-C3N4NS/BSA@MnO2/GCE | EP | o-PD | 0.001–0.01 μM | 0.25 | Rice | [271] |
| MIP/SPGE | EP | o-PD | 0.0082–0.655 μM | 7.85 | Corn flakes | [272] | |
| SPE-CC-ZEN/MIP | EP | o-PD | 0.001–0.5 μM | 1 | Corn; Rice; Wheat | [273] | |
| MIP-RECS | EP | p-aminothiophenol | 0.05–13 μM | 12.7 | Human serum | [274] | |
| MIP/CuHCF/rGNR–rGO/GCE | EP | o-PD | 0.79–1586 μM | 0.29 | Corn meal | [252] | |
| MIP/AuNPs/rGNRs/GCE | EP | o-PD | 0.0033–1.65 μM | 1.07 | Maize flour | [275] | |
| MIP/rGO@rGNR/GCE | EP | o-PD | 0.0016–1.6 μM | 0.62 | Corn meal | [276] | |
| MIP/AuSPE | BP | Methacrylic acid | 0.00035 to 350 μM | 0.12 | Maize | [253] |
7. MIP Sensors for Emerging Contaminants and Toxicants
Emerging contaminants in the food context refer to chemical hazards that have only recently gained attention or for which new regulatory limits are being considered. These include pollutants like endocrine-disrupting chemicals leaching from packaging (e.g., bisphenols, phthalates), perfluorinated “forever chemicals,” adulterants such as melamine, novel process-generated toxins, and certain natural toxins whose incidence in food is rising due to environmental changes. Traditional contaminants like pesticides, heavy metals, and well-known mycotoxins are extensively monitored by established methods; here we focus on contaminants that are comparatively newer challenges or require improved detection methods. MIP electrochemical sensors have been at the forefront of detecting many of these substances, often achieving the low detection limits needed to meet stringent safety standards (Table 8).
7.1. Endocrine Disruptors and Packaging Leachates
The prevalence of endocrine disruptors and packaging leachates such as bisphenols, phthalates, and per- and polyfluoroalkyl substances (PFAS) in food and beverage products has emerged as a significant concern due to their potential impact on human health. These contaminants originate from packaging materials or environmental contamination, subsequently migrating into foods and beverages. Consequently, stringent regulatory limits have been established globally to safeguard consumer health, requiring sensitive, selective, and reliable detection methods. MIP-based electrochemical sensors offer promising analytical tools, combining high selectivity, sensitivity, simplicity, and the possibility for on-site application.
Bisphenol A (BPA), a widely studied endocrine disruptor, is extensively utilized in plastic manufacturing and epoxy resins, commonly found in food containers, beverage cans, and thermal paper. Despite BPA’s regulated limits (e.g., the European Union’s migration limit is 0.05 mg/kg food), its analogs like bisphenol S (BPS) have increasingly replaced BPA, creating a similar toxicological concern. Several notable MIP electrochemical platforms have been developed for sensitive BPA detection. For instance, an impactful study reported a MIP sensor developed through EP on rGO-modified GCE (Figure 14A) [60]. This approach provided a linear detection range from 0.5 to 750 nM, achieving a remarkably low detection limit of 0.2 nM, making it ideal for monitoring BPA contamination in tap water, bottled milk, and bottled water samples. Another example involved a carbon paste electrode modified with MWCNTs and a bulk polymerized MIP coating (MIP/MWCNT/CPE), which achieved a detection limit of 0.08 nM in various water samples, including those stored in baby bottles [277]. These examples underline the versatility and adaptability of MIP-based sensors for detecting BPA at levels significantly below regulatory thresholds. BPA analogs such as BPS have also been targeted by advanced MIP electrochemical sensors. An innovative approach involved surface imprinting on a core–shell structured material (MA-Tyr@MIP/GCE), achieving an ultra-low detection limit of 0.171 nM, capable of effectively determining BPS contamination in bottled water and human serum samples [278]. This further highlights the flexibility of imprinting strategies for structurally related analytes.
Phthalates, commonly employed as plasticizers, are notorious for contaminating food, especially those high in fat, due to their lipophilic properties. Dibutyl phthalate (DBP), a prominent phthalate contaminant, has raised considerable concern regarding its endocrine-disrupting potential. A representative MIP electrochemical sensor was developed utilizing a SGI technique on a composite of silica nanoparticles, AuNPs, and MWCNT-modified GCE. This sensor exhibited a broad linear range of 0.043–43.48 μM and an impressively low detection limit of 5.09 nM, allowing its effective application to tap water and traditional Chinese spirits (Baijiu) samples [279]. Another inventive design integrated aptamer technology into a MIP platform employing Cu-based MOFs on ITO electrodes (Figure 14B). This sensor demonstrated remarkable sensitivity, achieving a detection limit of 0.035 nM for DBP in bottled water samples, indicating the powerful synergy between aptamer recognition elements and molecular imprinting techniques [280].
Per- and polyfluoroalkyl substances (PFAS), commonly termed “forever chemicals,” have emerged as critical environmental pollutants due to their persistence and bioaccumulative nature. They can infiltrate the food chain via contaminated water sources or food packaging, such as grease-resistant papers. Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) are prominent representatives requiring sensitive analytical methods. One compelling example is a gold nanostar (AuNS)-modified electrode coated with a PFOS-imprinted polymer via EP (Figure 14C). This sensor utilized DPV with a ferrocene-carboxylic acid redox probe, achieving an exceptionally low detection limit of 0.015 nM (approximately 6 ppt), suitable for environmental monitoring of tap water samples [281]. Another ultra-sensitive sensor applied EP to fabricate a MIP-based acetylene black electrode (MIP-ACET), detecting PFOS with an extraordinary detection limit of 0.3 fM, clearly demonstrating the potential for trace-level detection in complex aqueous matrices [282]. Similarly, sophisticated sensors have been designed for PFOA detection. For instance, an electrode modified with cobalt and iron-loaded carbon nanofibers (Co/Fe@CNF) utilized EP to fabricate PFOA-specific MIPs, providing a detection limit of approximately 1.073 nM in wastewater samples [283]. Another example involved poly(3,4-ethylenedioxythiophene) functionalized with TEMPO radicals (PEDOT-TEMPO) imprinted specifically for PFOA detection (Figure 14D), achieving a detection limit of 0.28 nM, suitable for environmental surface water monitoring [284]. These methodologies emphasize the role of innovative material designs, such as conductive polymers and nanostructured supports, in enhancing sensitivity and selectivity.
Figure 14.
Examples of MIP-based electrochemical for different endocrine disruptors and packaging leachates sensing. (A) Fabrication of MIP-rGO/GCE for BPA sensing [60]. (B) Fabrication of Cu-based MOFs on ITO electrodes for DBP sensing [280]. (C) Fabrication of MIP/AuNS/GCE for PFOS sensing [281]. (D) Fabrication of PEDOT-TEMPO-MIP for PFOA sensing [284].
Figure 14.
Examples of MIP-based electrochemical for different endocrine disruptors and packaging leachates sensing. (A) Fabrication of MIP-rGO/GCE for BPA sensing [60]. (B) Fabrication of Cu-based MOFs on ITO electrodes for DBP sensing [280]. (C) Fabrication of MIP/AuNS/GCE for PFOS sensing [281]. (D) Fabrication of PEDOT-TEMPO-MIP for PFOA sensing [284].
Real sample applications demonstrate the robust performance of MIP-based sensors across diverse food matrices, including tap and bottled water, beverages, milk products, canned foods, and packaging extracts. Crucially, sensor performance evaluations against conventional analytical techniques (such as chromatography coupled with mass spectrometry) confirm reliability and accuracy, thereby endorsing MIPs as feasible candidates for routine monitoring or field-deployable testing methods. Overall, significant advances in MIP electrochemical sensors for detecting endocrine disruptors and packaging leachates underscore their potential as practical, cost-effective analytical tools capable of addressing public health concerns. Continued developments in material science, imprinting technology, and sensor miniaturization promise further enhancements in detection limits, specificity, and applicability to complex food and environmental matrices, contributing meaningfully to improved food safety and public health protection.

Table 8.
Selected MIP-based electrochemical sensors for endocrine disruptors and packaging leachates.
Table 8.
Selected MIP-based electrochemical sensors for endocrine disruptors and packaging leachates.
| Analyte | Sensing Platform | MIP Fabrication Method | Functional Monomer(s) | Linear Range | LOD (nM) | Real Sample | Reference |
|---|---|---|---|---|---|---|---|
| BPA | MIP-ERGO/GCE | EP | Pyrrole | 0.5–750 nM | 0.2 | Tap water; PC bottled water; PC bottled milk; Bovine milk | [60] |
| Au@MIP | EP | p-aminobenzoic acid | 0.5–100 μM | 52 | Tap water; Milk; Orange juice; Mineral water bottle | [285] | |
| MIP/MWCNT/CPE | BP | 2-hydroxyethyl methacrylate | 0.1–100 μM | 0.08 | Tap water; Stored water in a baby bottle; Household filtered drinking water; Soft drink | [277] | |
| MIPs/GNPs-MWCNTs | SI | 3-aminopropyltriethoxysilane | 0.113–8210 μM | 3.6 | Honey; Tap water; Grape juice | [286] | |
| MIP/GQDs/B-g-C3N4/GCE | EP | Pyrrole | 0.01–1.0 μM | 3.0 | Orange juice | [287] | |
| MIP/Fe3O4NPs/BDD | SI | 3-aminopropyltriethoxysilane | 5–73 μM | 380 | Tap water; Canned corn; Canned chickpeas; Tomato paste with basil; Milk; Bee | [288] | |
| CMOF-MIPIL | SI | 1-allyl-3-ethylimidazolium bromide | 0.005–5.0 μM | 4.0 | Lake water; River water; Plastic bottle; Fresh liquid milk | [289] | |
| MIP–GR/ABPE | SI | Chitosan | 0.008–1.0 μM and 1.0–20 μM | 6.0 | Plastic bottled drinking water; Canned beverages | [290] | |
| BMMIPs@MGCE | SI | NR | 0.8–8.0 μM | 133 | Tap water; Municipal sewage; Tea drink; Milk; Cabbage; Soil | [291] | |
| MMIP-CuMOFs/RGO/GCE | SI | 3-aminopropyltriethoxysilane | 0.5–500 μM | 0.18 | Milk | [292] | |
| MIP-NG-GCE | EP | o-PD | 8–6000 μM | 138 | PC water bottle | [293] | |
| PPY/-@p-63/AuNP/GCE | EP | Pyrrole | 0.5 fM–5 pM | 0.0000080 | Fresh milk; Milk powder; Tap water; Pretreated water in a baby glass | [294] | |
| GO/APTES–MIP | SI | 3-aminopropyl-triethoxysilane | 0.006–0.1 μM and 0.2–20 μM | 3 | Milk; Mineralized water | [295] | |
| MIP/SPE | BP | 4-vinylpyridine | 0.0047–0.008 μM | 3.2 | Bottled water; Water effluents | [296] | |
| SPCE@CB/MIP | SI | Acrylonitrile | 0.1–10 μM | 66 | Tap water | [297] | |
| SPCE/PEDOT/BMIMBr | EP | 3,4-ethylenedioxythiophene | 0.1–500 μM | 20 | Polycarbonate water bottles; Plastic juice bottles | [298] | |
| MIP-AuNPs-MCA-rGO/CILE | EP | NR | 0.004–18.0 μM | 1.1 | PVC food package; PVC bottle; PC baby bottle; PC water bottle | [299] | |
| MIP/GCE | SI | Acetylene black | 0.1–400 μM | 0.02 | Baby feeding bottles | [300] | |
| MIPs/AuNPs/GCE | EP | 4-aminothiophenol | 0.015–55 μM | 1.1 | Milk; PC nursing bottle; Soil; PVC food package; PVC drinking cup | [301] | |
| μPAD | SI | Bisphenol A | 0.0044–0.88 μM | 2.06 | Sea water; Canned food liquids; Polycarbonate plastic packaged water | [302] | |
| AuNPs/MIP-PGE | PP | N-methacryloyl-(L)-cysteine methyl ester | 1.5–7.5 μM | 161 | Drinking water | [303] | |
| BPS | MA-Tyr@MIP/GCE | SI | N-methacryloyl-L-tyrosine | 0.001–0.01 μM | 0.171 | Human serum; Plastic bottled water | [278] |
| DBP | SiO2@MIP/AuNPs/MWCNTs/GCE | SI | Methacrylic acid | 0.043–43.48 μM | 5.09 | Tap water; Chinese Baijiu | [279] |
| MIP PPY/PGE | EP | Pyrrole | 0.01–1.0 μM | 4.5 | - | [304] | |
| MIP-DBP-CTS/F-CC3/GCE | EP | Chitosan | 0–1.8 μM | 2.6 | Rice wine | [305] | |
| MIP-Aptamer[DBP]/Cu3(BTC)2/Cu2O/ITO | EP | 3-aminopropyltriethoxysilane | 0.0001–0.001 μM | 0.035 | Bottled water | [280] | |
| MMISPE | SI | Methacrylic acid | 0.043–4340 μM | 0.19 | Soybean milk; Milk | [306] | |
| MGO@AuNPs-MIPs/GCE | SI | Methacrylic acid | 2.5–5000 μM | 0.8 | Wine drinks; Ultrapure water | [307] | |
| PFOS | MIP/Au | EP | o-PD | 0.1–1500 nM | 0.04 | Distilled water; Tap water; Bottled mineral water | [308] |
| Au/MIP/SPE | EP | o-PD | 0.1–1.5 μM | 0.004 | Water samples | [309] | |
| MIP/AuNS/GCE | EP | o-PD | 0.025–5.0 μM and 5.0–500 μM | 0.015 | Tap water | [281] | |
| MIP/GCE | EP | o-PD | 0.05–0.5 nM and 1–500 nM | 0.05 | - | [50] | |
| MIP-ACET | EP | o-PD | 1.25 pM–1.25 nM | 0.0000003 | Tap water | [282] | |
| MOFMMIP/CPE | BP | Pyrrole | 0.002–165 μM | 0.7 | Tap water; River water; Well water | [310] | |
| CNW/MIP | EP | o-PD | 0.024–0.24 μM | 2.89 | Tap water; Wastewater; Landfill leachate | [311] | |
| PFOA | MIP Co/Fe@CNF | EP | Acrylamide | 0.01–90 μM | 1.073 | Wastewater | [283] |
| PEDOT-TEMPO-MIP | EP | 3,4-ethylenedioxythiophene-TEMPO | 0.001–1.0 μM | 0.28 | Surface water | [284] | |
| βCD-MB MIP | EP | β-cyclodextrin | 0.01–100 μM | 1.57 | Groundwater | [312] |
7.2. Adulterants
Food adulteration scandals have driven the need for rapid screening of unexpected chemicals. Melamine is a nitrogen-rich organic compound frequently used as an adulterant in food products to falsely elevate apparent protein content, posing significant health risks due to its nephrotoxicity. Its presence in dairy products, pet food, and infant formula has triggered global concern and regulatory scrutiny. Electrochemical sensors employing MIPs offer highly selective recognition of melamine, distinguishing it effectively from structurally similar compounds such as cyanuric acid. MIP-based electrochemical platforms typically exhibit excellent sensitivity and rapid detection capabilities, effectively addressing the demand for reliable on-site screening methods. The electrochemical signals generated upon melamine binding allow for quantitative analysis, facilitating detection at trace levels. For instance, Zhao et al. [313] developed a melamine-imprinted electrochemical sensor utilizing graphene/ionic liquid composites. By exploiting multiple-hydrogen bonding interactions between melamine and 6-aminouracil, the research team synthesized grape grain-structured MIPs, enhancing the specific surface area and improving electrochemical signal response. This sensor exhibited remarkable sensitivity, achieving a detection limit as low as 1.57 × 10−3 mg/kg, significantly lower than regulatory safety limits. Another approach was reported by Limthin et al. [314], where they developed an advanced electrochemical sensor by integrating CuO/g-C3N4 nanocomposites with MIP specifically for melamine detection. The inclusion of CuO nanoparticles significantly enhanced the photoelectrochemical properties of graphitic carbon nitride, improving electron transfer and reducing recombination rates. The CuO/g-C3N4/MIP sensor demonstrated a remarkably low detection limit of 0.42 nM, with a linear detection range of 2.5 to 50 nM and high sensitivity of 4.172 nA/nM. Esmaeily et al. [315] introduced an innovative electrochemical preconcentration method utilizing EP on a GCE modified with an overoxidized poly-(para-aminophenol) film (Figure 15A). This strategy involved converting non-electroactive melamine into electroactive poly(melamine), significantly enhancing detection sensitivity. The sensor achieved an impressive limit of detection of 0.34 μM, well below regulatory thresholds, and proved effective in analyzing real milk samples, demonstrating both robustness and practicality.
Sudan dyes are synthetic azo colorants illegally added to foods, particularly spices and chili powders, to enhance visual appeal despite their recognized carcinogenicity and genotoxicity. The complexity and similarity among different Sudan dye structures present significant analytical challenges, requiring sensors capable of precise discrimination. MIP electrochemical sensors have emerged as a promising solution due to their unique ability to selectively recognize individual dye molecules based on size, shape, and functional group interactions. These sensors demonstrate high selectivity and sensitivity, providing efficient detection even in complex food matrices with substantial background interference. Chen et al. [316] developed a MIP sol–gel polymer sensor embedded with AuNPs for the selective and sensitive electrochemical determination of Sudan I. The sol–gel polymer was synthesized using APTES, TEOS, chitosan, and AuNPs, with Sudan I serving as the template molecule. Upon removal of the template, linear sweep voltammetric measurements demonstrated a linear detection range from 0.1 to 10 μM and a remarkably low detection limit of 2 nM. The embedded AuNPs enhanced electron transfer rates and amplified the electrochemical response, thereby significantly improving sensor sensitivity and performance in practical applications, such as the analysis of spiked ketchup samples. Similarly, Gao et al. [317] designed an advanced composite MIP using bifunctional monomer oligomers—pentenyl (lipoic acyl)-isoleucyl-chitosan oligosaccharide (P(L)ICO) and pentenyl–asparaginyl–chitosan oligosaccharide (PASCO)—for highly selective electrochemical monitoring of Sudan I. AuNPs deposited on a GCE facilitated the formation of a self-assembled P(L)ICO monolayer, subsequently polymerized with PASCO and ethylene glycol dimethacrylate in the presence of Sudan I. After template removal, the sensor displayed exceptional selectivity and sensitivity with a detection limit as low as 4 nM and maintained high specificity towards Sudan I, even in the presence of structurally similar compounds like Sudan II, III, IV, and azobenzene.
Malachite green, a synthetic triarylmethane dye, has been illegally used in aquaculture for its antimicrobial properties, despite significant toxicological concerns including carcinogenicity, mutagenicity, and teratogenicity. Given the strict regulations and public health implications, sensitive and selective detection methods are essential. Electrochemical sensors incorporating MIPs offer superior selectivity and sensitivity for detecting malachite Green in seafood and aquaculture products. These MIP-based sensors leverage electrochemical responses resulting from specific binding interactions, enabling quantitative detection at trace concentrations. For instance, Xu et al. [318] developed a composite sensor combining malachite green-imprinted polypyrrole films and AuNPs deposited onto a GCE (MG-MIPPy/AuNPs/GCE). This composite electrode demonstrated a linear detection range from 2.74 nM to 2.74 μM and an exceptionally low detection limit of 35.7 pM. Importantly, the sensor also exhibited strong selectivity against structurally similar compounds such as crystal violet and phenolphthalein, as well as various common ions. In another innovative approach, Fan and colleagues [319] fabricated a sensor by directly growing mixed-valence CuFe2O4/Cu0 hierarchical nanostructures on carbon fiber paper via a hydrothermal method. This three-dimensional hierarchical electrode structure offered increased surface area and rapid analyte penetration, significantly enhancing the electrode’s sensing performance. It exhibited remarkable sensitivity for malachite green, achieving a detection range of 0.1–300 µM and a low detection limit of 0.033 µM, alongside excellent performance in mixed solutions and practical food samples validated against HPLC.
Chloramphenicol is a broad-spectrum antibiotic widely banned for use in food-producing animals due to severe side effects, including bone marrow suppression and potential carcinogenicity in humans. Nevertheless, illicit use persists, necessitating robust detection methods capable of reliably identifying this contaminant in food products. MIP-based electrochemical sensors offer significant advantages in detecting chloramphenicol owing to their high specificity, rapid response, and cost-effective analysis. These sensors exploit selective interactions between chloramphenicol and polymer recognition sites, translating binding events into quantifiable electrochemical signals suitable for routine food monitoring. For instance, a recent study developed an electrochemical sensor using chitosan-MWCNTs/AuNPs to enhance sensitivity through improved electron transfer and expanded electroactive surface area [320]. In this system, chloramphenicol molecules were imprinted into an o-PD polymer matrix deposited on a GCE modified with CS-MWCNTs/AuNPs. This sensor exhibited a linear detection range of 0.1–1000 ng/mL and a remarkably low detection limit of 0.033 ng/mL, demonstrating excellent selectivity, stability, and reproducibility. Importantly, successful chloramphenicol detection in real milk samples underscored its practical applicability in food quality control settings. In another approach, researchers combined MIPs with aptamers (Apt) for a dual-recognition electrochemical sensor aimed at improving detection specificity and sensitivity (Figure 15B) [321]. This innovative strategy utilized AuNPs alongside CS-MWCNTs to further boost electrical conductivity. The resulting sensor achieved an exceptionally broad detection range from 10−8 to 10−2 g/L and an extremely low limit of detection of 3.3 × 10−9 g/L. The dual-recognition mechanism, incorporating both MIP-generated cavities and aptamer-mediated binding sites, significantly enhanced the sensor’s resistance to interference from structurally similar antibiotics, thus ensuring precise chloramphenicol detection even in complex food matrices like milk and honey. Additionally, an electrochemical sensor based on MIP Eriochrome black T (EBT) polymer has also demonstrated efficacy for chloramphenicol detection in aquaculture water [322]. Fabricated directly on commercial SPE via in situ electro-polymerization, this sensor displayed reliable responses with a linear detection range spanning from 10 nM upwards. This approach’s simplicity and scalability make it particularly suitable for on-site monitoring applications in aquaculture surveillance.
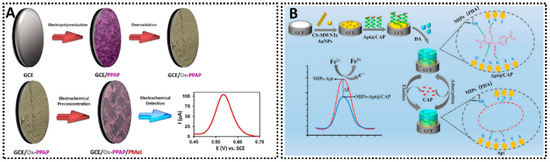
Figure 15.
Examples of MIP-based electrochemical for different adulterants sensing. (A) Fabrication of GCE/Ox-PPAP for melamine sensing [315]. (B) Fabrication of MIPs-Apt/CS-MWCNTs/AuNPs for chloramphenicol sensing [321].
Figure 15.
Examples of MIP-based electrochemical for different adulterants sensing. (A) Fabrication of GCE/Ox-PPAP for melamine sensing [315]. (B) Fabrication of MIPs-Apt/CS-MWCNTs/AuNPs for chloramphenicol sensing [321].
8. Challenges and Future Perspectives
MIP-based electrochemical sensors have shown great promise in the laboratory for detecting a wide spectrum of food-related analytes. To transition these advances into routine food quality control and industrial use, several challenges must be addressed, and future developments pursued.
One challenge is ensuring that MIP sensors can be produced consistently with the same performance. Polymer imprinting can sometimes yield batch-to-batch variability in binding site distribution or capacity. To quantitatively assess this, studies typically evaluate the RSD. The reviewed literature demonstrates strong precision; for a single sensor, intra-assay RSDs (representing multiple measurements) often fall below 5%. More critically for practical use, inter-assay (day-to-day) and inter-electrode (sensor-to-sensor) reproducibility typically yields RSDs in the 5–10% range, which is considered acceptable for many screening applications. This is especially critical if sensors are to be mass-produced (e.g., SPE with MIP coatings). Achieving robust, low-RSD mass production will require standardized synthesis protocols and possibly prefabricated MIP particles that can be deposited uniformly. Some progress in this area includes the use of automated or controlled polymerization techniques to yield more uniform MIP nanoparticles, and quality control through spectroscopic or chromatographic characterization of binding.
Food samples are complex, and matrix components can interfere with sensor readings. Fats, proteins, and other macromolecules may non-specifically foul the sensor surface or alter the signal. As noted in a recent review, each food matrix presents unique challenges–for instance, meat and fish samples have high protein/fat content that can cause biofouling, while fruit juices contain sugars and acids that can shift sensor baselines. To mitigate these issues, antifouling surface coatings can be integrated with MIPs (e.g., polyethylene glycol-based layers that prevent protein adhesion). Additionally, dilution or simple cleanup steps (filtration, centrifugation) are often employed before measurement. Many reported sensors have been tested in spiked samples with minimal preparation and still achieved good recoveries, indicating that MIPs inherently reject a lot of interference due to their selective binding. Nonetheless, when moving to unprocessed field samples, robust calibration and matrix-matched standards will be needed for quantitative work.
While MIPs are qualitatively described as “highly selective,” this can be quantified using two key metrics: the Imprinting Factor (IF) and the selectivity factor (k). The IF, calculated as the ratio of the MIP sensor’s response to the target versus a non-imprinted polymer’s (NIP) response (IF = SignalMIP/SignalNIP), measures the success of the imprinting process itself. Many studies in this review report high IFs, such as the 4.2 value for an amaranth sensor [26] or >4.0 for a BPA sensor [60], confirming the generation of specific, high-affinity binding sites.
More relevant for practical applications is the selectivity factor (k), which quantifies cross-reactivity against interfering compounds. A sensor is highly selective when k values are low. For example, the BHA sensor by Motia et al. [71] demonstrated excellent selectivity with k values of 0.04, 0.07, and 0.09 against TBHQ, ascorbic acid, and propyl gallate, respectively. Similarly, a TBHQ sensor [118] showed selectivity coefficients below 0.05 for numerous analogs. While not infallible, these quantitative data demonstrate that MIPs can be tailored—by choosing different functional monomers or imprinting strategies—to discriminate between very similar molecules and minimize false positives. For example, imprinting BPA versus BPS (a BPA analog) might involve using a dummy template or a selective porogen to favor cavities for one over the other. Careful validation of each sensor against likely interferents is necessary. In practice, some degree of cross-reactivity might be tolerable if the sensor is used for screening (with positives confirmed by a gold-standard method), but ideally MIP sensors should be as specific as necessary for standalone analysis.
For field deployment, sensors should be incorporated into user-friendly devices. The electronics for potentiostat control and readout have become remarkably compact–handheld or smartphone-based potentiostats are now available. MIP electrochemical sensors are well-suited to such devices because the assays are typically simple (immerse sensor in sample, then measure a current or impedance). There are already examples of fully portable devices: for instance, a mycotoxin sensor on a disposable chip integrated with a pocket-sized reader. Future designs might embed MIP sensors into food packaging or processing lines for continuous monitoring (e.g., a flow cell with a MIP sensor measuring a product stream in real time). The stability of MIPs (they can often be stored dry for months without losing activity) favors their use in such deployed scenarios, as they do not require special storage like biological reagents.
In conclusion, molecularly imprinted electrochemical sensors are poised for translation if three concrete thrusts are prioritized. First, scalable electropolymerization on mass-manufacturable transducers should be pursued to deliver disposable, calibration-robust chips and patches compatible with pocket potentiostats; recent demonstrations of EP-MIPs on SPCEs and roll-to-roll printed wearable patches substantiate this pathway. Second, data-driven design should complement DFT/MD workflows: machine-learning models that predict imprinting quality and monomer–template affinity can narrow monomer/porogen spaces, shorten optimization cycles, and improve selectivity in complex food matrices. Third, integration with microfluidic and wearable platforms (including PEC/electrochemical dual-mode readouts and smartphone interfaces) will enable on-line, low-volume sample handling and on-body/at-line monitoring for contaminants, additives, and quality markers. These strategies directly address throughput, matrix effects, and usability, and we anticipate they will accelerate certification-grade validation and routine deployment in food quality control.
Author Contributions
Conceptualization, L.F. and S.Z.; methodology, L.F. and S.Z.; software, L.Z. and J.Z.; validation, L.Z. and J.Z.; formal analysis, L.Z. and J.Z.; writing—original draft preparation, L.Z., S.Z. and J.Z.; writing—review and editing, L.F.; supervision, L.F. and S.Z.; project administration, L.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mazur, F.; Han, Z.; Tjandra, A.D.; Chandrawati, R. Digitalization of Colorimetric Sensor Technologies for Food Safety. Adv. Mater. 2024, 36, 2404274. [Google Scholar] [CrossRef]
- Li, T.; Shang, D.; Gao, S.; Wang, B.; Kong, H.; Yang, G.; Shu, W.; Xu, P.; Wei, G. Two-Dimensional Material-Based Electrochemical Sensors/Biosensors for Food Safety and Biomolecular Detection. Biosensors 2022, 12, 314. [Google Scholar] [CrossRef]
- Chang, K.; Zhao, Y.; Wang, M.; Xu, Z.; Zhu, L.; Xu, L.; Wang, Q. Advances in Metal-Organic Framework-Plasmonic Metal Composites Based SERS Platforms: Engineering Strategies in Chemical Sensing, Practical Applications and Future Perspectives in Food Safety. Chem. Eng. J. 2023, 459, 141539. [Google Scholar] [CrossRef]
- Lei, Y.; Cheng, J.; Dong, H.; Wang, P. Functional Porous Material-Based Sensors for Food Safety. Coord. Chem. Rev. 2024, 501, 215566. [Google Scholar] [CrossRef]
- Yu, Z.; Jung, D.; Park, S.; Hu, Y.; Huang, K.; Rasco, B.A.; Wang, S.; Ronholm, J.; Lu, X.; Chen, J. Smart Traceability for Food Safety. Crit. Rev. Food Sci. Nutr. 2022, 62, 905–916. [Google Scholar] [CrossRef]
- Han, Y.; Yang, W.; Luo, X.; He, X.; Zhao, H.; Tang, W.; Yue, T.; Li, Z. Carbon Dots Based Ratiometric Fluorescent Sensing Platform for Food Safety. Crit. Rev. Food Sci. Nutr. 2022, 62, 244–260. [Google Scholar] [CrossRef]
- Khan, A.; Ezati, P.; Kim, J.-T.; Rhim, J.-W. Biocompatible Carbon Quantum Dots for Intelligent Sensing in Food Safety Applications: Opportunities and Sustainability. Mater. Today Sustain. 2023, 21, 100306. [Google Scholar] [CrossRef]
- Yao, Z.; Coatsworth, P.; Shi, X.; Zhi, J.; Hu, L.; Yan, R.; Güder, F.; Yu, H.-D. Paper-Based Sensors for Diagnostics, Human Activity Monitoring, Food Safety and Environmental Detection. Sens. Diagn. 2022, 1, 312–342. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, W.; Zhao, Y.; Liu, H.; Sun, B. Single, Dual and Multi-Emission Carbon Dots Based Optosensing for Food Safety. Trends Food Sci. Technol. 2021, 111, 388–404. [Google Scholar] [CrossRef]
- Cheng, W.; Tang, X.; Zhang, Y.; Wu, D.; Yang, W. Applications of Metal-Organic Framework (MOF)-Based Sensors for Food Safety: Enhancing Mechanisms and Recent Advances. Trends Food Sci. Technol. 2021, 112, 268–282. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, W.; Zhu, C.; Hu, L. Recent Advances in Photoelectrochemical Sensing for Food Safety. Anal. Chem. 2024, 96, 8855–8867. [Google Scholar] [CrossRef]
- Fu, W.; Fu, X.; Li, Z.; Liu, Z.; Li, X. Advances in Smartphone Assisted Sensors for On-Site Detection of Food Safety Based on Fluorescence on-off-on Mode: A Review. Chem. Eng. J. 2024, 489, 151225. [Google Scholar] [CrossRef]
- Wei, Q.; Zhu, X.; Zhang, D.; Liu, H.; Sun, B. Innovative Nanomaterials Drive Dual and Multi-Mode Sensing Strategies in Food Safety. Trends Food Sci. Technol. 2024, 151, 104636. [Google Scholar] [CrossRef]
- Zhou, H.; Qiu, H.; Zhang, J.; Fang, Y.; Cui, B.; Shen, Y. Design, Preparation, and Application of Molecularly Imprinted Nanomaterials for Food Safety Analysis with Electrochemistry. Coord. Chem. Rev. 2024, 500, 215523. [Google Scholar] [CrossRef]
- Sun, Y.-H.; Yang, L.; Ji, X.-X.; Wang, Y.-Z.; Liu, Y.-L.; Fu, Y.; Ye, F. Efficient Detection of Flusilazole by an Electrochemical Sensor Derived from MOF MIL-53(Fe) for Food Safety. Food Chem. 2024, 440, 138244. [Google Scholar] [CrossRef]
- Mutlu, E.; Şenocak, A.; Demirbaş, E.; Koca, A.; Akyüz, D. Selective and Sensitive Molecularly Imprinted Polymer-Based Electrochemical Sensor for Detection of Deltamethrin. Food Chem. 2025, 463, 141121. [Google Scholar] [CrossRef]
- Parihar, A.; Sharma, P.; Choudhary, N.K.; Khan, R.; Mostafavi, E. Internet-of-Things-Integrated Molecularly Imprinted Polymer-Based Electrochemical Nano-Sensors for Pesticide Detection in the Environment and Food Products. Environ. Pollut. 2024, 351, 124029. [Google Scholar] [CrossRef]
- Yan, X.; Almajidi, Y.Q.; Uinarni, H.; Bokov, D.O.; Mansouri, S.; Fenjan, M.N.; Saxena, A.; Zabibah, R.S.; Hamzah, H.F.; Oudah, S.K. Bio(Sensors) Based on Molecularly Imprinted Polymers and Silica Materials Used for Food Safety and Biomedical Analysis: Recent Trends and Future Prospects. Talanta 2024, 276, 126292. [Google Scholar] [CrossRef]
- Geng, L.; Huang, J.; Fang, M.; Wang, H.; Liu, J.; Wang, G.; Hu, M.; Sun, J.; Guo, Y.; Sun, X. Recent Progress of the Research of Metal-Organic Frameworks-Molecularly Imprinted Polymers (MOFs-MIPs) in Food Safety Detection Field. Food Chem. 2024, 458, 140330. [Google Scholar] [CrossRef]
- Ayerdurai, V.; Cieplak, M.; Kutner, W. Molecularly Imprinted Polymer-Based Electrochemical Sensors for Food Contaminants Determination. TrAC Trends Anal. Chem. 2023, 158, 116830. [Google Scholar] [CrossRef]
- Garg, D.; Verma, N. Monika Molecularly Imprinted Polymer-Based Electrochemical Sensor for Rapid and Selective Detection of Hypoxanthine. Biosensors 2022, 12, 1157. [Google Scholar] [CrossRef]
- Liu, X.; Mao, L.-G.; Wang, Y.-L.; Shi, X.-B.; Liu, Y.; Yang, Y.; He, Z. Electrochemical Sensor Based on Imprinted Sol-Gel Polymer on Au NPs-MWCNTs-CS Modified Electrode for the Determination of Acrylamide. Food Anal. Methods 2016, 9, 114–121. [Google Scholar] [CrossRef]
- Ramajayam, K.; Ganesan, S.; Ramesh, P.; Beena, M.; Kokulnathan, T.; Palaniappan, A. Molecularly Imprinted Polymer-Based Biomimetic Systems for Sensing Environmental Contaminants, Biomarkers, and Bioimaging Applications. Biomimetics 2023, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Roushani, M.; Zalpour, N. Selective Detection of Asulam with In-Situ Dopamine Electropolymerization Based Electrochemical MIP Sensor. React. Funct. Polym. 2021, 169, 105069. [Google Scholar] [CrossRef]
- Bougrini, M.; Florea, A.; Cristea, C.; Sandulescu, R.; Vocanson, F.; Errachid, A.; Bouchikhi, B.; El Bari, N.; Jaffrezic-Renault, N. Development of a Novel Sensitive Molecularly Imprinted Polymer Sensor Based on Electropolymerization of a Microporous-Metal-Organic Framework for Tetracycline Detection in Honey. Food Control 2016, 59, 424–429. [Google Scholar] [CrossRef]
- Wu, Y.; Li, G.; Tian, Y.; Feng, J.; Xiao, J.; Liu, J.; Liu, X.; He, Q. Electropolymerization of Molecularly Imprinted Polypyrrole Film on Multiwalled Carbon Nanotube Surface for Highly Selective and Stable Determination of Carcinogenic Amaranth. J. Electroanal. Chem. 2021, 895, 115494. [Google Scholar] [CrossRef]
- Elhachem, M.; Cayot, P.; Abboud, M.; Louka, N.; Maroun, R.G.; Bou-Maroun, E. The Importance of Developing Electrochemical Sensors Based on Molecularly Imprinted Polymers for a Rapid Detection of Antioxidants. Antioxidants 2021, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Bounegru, A.V.; Apetrei, C. Voltammetric Sensors Based on Nanomaterials for Detection of Caffeic Acid in Food Supplements. Chemosensors 2020, 8, 41. [Google Scholar] [CrossRef]
- Baghizadeh, A.; Karimi-Maleh, H.; Khoshnama, Z.; Hassankhani, A.; Abbasghorbani, M. A Voltammetric Sensor for Simultaneous Determination of Vitamin C and Vitamin B6 in Food Samples Using ZrO2 Nanoparticle/Ionic Liquids Carbon Paste Electrode. Food Anal. Methods 2015, 8, 549–557. [Google Scholar] [CrossRef]
- Vinoth, S.; Govindasamy, M.; Wang, S.-F.; ALOthman, Z.A.; Alshgari, R.A.; Ouladsmane, M. Fabrication of Strontium Molybdate Incorporated with Graphitic Carbon Nitride Composite: High-Sensitive Amperometric Sensing Platform of Food Additive in Foodstuffs. Microchem. J. 2021, 167, 106307. [Google Scholar] [CrossRef]
- Shrestha, S.; Mascarenhas, R.J.; D’Souza, O.J.; Satpati, A.K.; Mekhalif, Z.; Dhason, A.; Martis, P. Amperometric Sensor Based on Multi-Walled Carbon Nanotube and Poly (Bromocresol Purple) Modified Carbon Paste Electrode for the Sensitive Determination of L-Tyrosine in Food and Biological Samples. J. Electroanal. Chem. 2016, 778, 32–40. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Hu, C.; Wu, H.; Yang, Y.; Huang, C.; Jia, N. Highly Sensitive Electrochemical Impedance Spectroscopy Immunosensor for the Detection of AFB1 in Olive Oil. Food Chem. 2015, 176, 22–26. [Google Scholar] [CrossRef]
- Chiriacò, M.S.; Parlangeli, I.; Sirsi, F.; Poltronieri, P.; Primiceri, E. Impedance Sensing Platform for Detection of the Food Pathogen Listeria Monocytogenes. Electronics 2018, 7, 347. [Google Scholar] [CrossRef]
- El Badry Mohamed, M.; Frag, E.Y.; El Brawy, M.H. Rapid Potentiometric Sensor for Determination of Cu(II) Ions in Food Samples. Microchem. J. 2021, 164, 106065. [Google Scholar] [CrossRef]
- Draz, M.E.; Darwish, H.W.; Darwish, I.A.; Saad, A.S. Solid-State Potentiometric Sensor for the Rapid Assay of the Biologically Active Biogenic Amine (Tyramine) as a Marker of Food Spoilage. Food Chem. 2021, 346, 128911. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H.; Zhang, M.; Zeng, B.; Zhao, F. Molecularly Imprinted Photoelectrochemical Sensor for Aflatoxin B1 Detection Based on Organic/Inorganic Hybrid Nanorod Arrays. Sens. Actuators B Chem. 2021, 339, 129900. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, L.; Mei, X.; Liu, J.; Yao, Z.; Li, Y. Dual-Mode Sensing Platform for Electrochemiluminescence and Colorimetry Detection Based on a Closed Bipolar Electrode. Anal. Chem. 2021, 93, 12367–12373. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, W.; Yang, Q.; Wu, W.; Hou, X. An Electrochemical/Colorimetric Dual-Mode Sensor for the Detection of AFB1 in Agricultural Products Based on CoFe2O4@Au Nanoparticles and Smartphone Imaging. Food Chem. 2025, 474, 143201. [Google Scholar] [CrossRef]
- Amatatongchai, M.; Sroysee, W.; Sodkrathok, P.; Kesangam, N.; Chairam, S.; Jarujamrus, P. Novel Three-Dimensional Molecularly Imprinted Polymer-Coated Carbon Nanotubes (3D-CNTs@MIP) for Selective Detection of Profenofos in Food. Anal. Chim. Acta 2019, 1076, 64–72. [Google Scholar] [CrossRef]
- Elfadil, D.; Silveri, F.; Palmieri, S.; Della Pelle, F.; Sergi, M.; Del Carlo, M.; Amine, A.; Compagnone, D. Liquid-Phase Exfoliated 2D Graphene Nanoflakes Electrochemical Sensor Coupled to Molecularly Imprinted Polymers for the Determination of Citrinin in Food. Talanta 2023, 253, 124010. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Z.; Zhang, A.; Peng, Y.; Zhou, H.; Guo, Y.; Lu, D.; Xie, L.; Shi, S. Gold Nanoparticle-Mediated Molecularly Imprinted Electrochemical Sensor MIP/AuNPs/GCE for Highly Sensitive and Selective Detection of Neutral Phosmet Residues in Fruits and Vegetables. Microchem. J. 2024, 201, 110728. [Google Scholar] [CrossRef]
- Wu, J.; Xia, Y.; Wang, T.; Zhang, Y.; Li, G. Efficient Voltammetric Platform Combining a Molecularly Imprinted Polymer and Silver-Nanoparticle-Decorated Black Phosphorus Nanosheets for Selective Determination of Gatifloxacin. Food Chem. X 2025, 25, 102094. [Google Scholar] [CrossRef]
- Zeb, S.; Wong, A.; Khan, S.; Hussain, S.; Sotomayor, M.D.P.T. Using Magnetic Nanoparticles/MIP-Based Electrochemical Sensor for Quantification of Tetracycline in Milk Samples. J. Electroanal. Chem. 2021, 900, 115713. [Google Scholar] [CrossRef]
- Janiak, D.S.; Kofinas, P. Molecular Imprinting of Peptides and Proteins in Aqueous Media. Anal. Bioanal. Chem. 2007, 389, 399–404. [Google Scholar] [CrossRef]
- Zare, E.N.; Fallah, Z.; Le, V.T.; Doan, V.-D.; Mudhoo, A.; Joo, S.-W.; Vasseghian, Y.; Tajbakhsh, M.; Moradi, O.; Sillanpää, M. Remediation of Pharmaceuticals from Contaminated Water by Molecularly Imprinted Polymers: A Review. Environ. Chem. Lett. 2022, 20, 2629–2664. [Google Scholar] [CrossRef] [PubMed]
- Sergeyeva, T.; Piletska, O.; Piletsky, S. Rationally Designed Molecularly Imprinted Polymer Membranes as Antibody and Enzyme Mimics in Analytical Biotechnology. BBA Adv. 2023, 3, 100070. [Google Scholar] [CrossRef]
- Wu, T.; Li, Y.; Zhang, Z.; Zhang, D.; Dragoi, E.; Karimi-Maleh, H. 3D Print-Based Polypyrrole /TiVCTx/UiO-66 Composites for Effective Adsorption of Combined Pollutants in Water Media. J. Nanostruct. Chem. 2025, 15, 152503. [Google Scholar] [CrossRef]
- Silvestri, D.; Barbani, N.; Cristallini, C.; Giusti, P.; Ciardelli, G. Molecularly Imprinted Membranes for an Improved Recognition of Biomolecules in Aqueous Medium. J. Membr. Sci. 2006, 282, 284–295. [Google Scholar] [CrossRef]
- Malik, M.I.; Shaikh, H.; Mustafa, G.; Bhanger, M.I. Recent Applications of Molecularly Imprinted Polymers in Analytical Chemistry. Sep. Purif. Rev. 2019, 48, 179–219. [Google Scholar] [CrossRef]
- Kazemi, R.; Potts, E.I.; Dick, J.E. Quantifying Interferent Effects on Molecularly Imprinted Polymer Sensors for Per- and Polyfluoroalkyl Substances (PFAS). Anal. Chem. 2020, 92, 10597–10605. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Ashraf, N.; Zohuri, G.H. A Smart Electrochemical Sensor Based upon Hydrophilic Core–Shell Molecularly Imprinted Polymer for Determination of L-Tryptophan. Microchem. J. 2023, 185, 108260. [Google Scholar] [CrossRef]
- Surapong, N.; Santaladchaiyakit, Y.; Burakham, R. A Water-Compatible Magnetic Dual-Template Molecularly Imprinted Polymer Fabricated from a Ternary Biobased Deep Eutectic Solvent for the Selective Enrichment of Organophosphorus in Fruits and Vegetables. Food Chem. 2022, 384, 132475. [Google Scholar] [CrossRef]
- Puoci, F.; Iemma, F.; Picci, N. Stimuli-Responsive Molecularly Imprinted Polymers for Drug Delivery: A Review. Curr. Drug Deliv. 2008, 5, 85–96. [Google Scholar] [CrossRef]
- Ge, Y.; Butler, B.; Mirza, F.; Habib-Ullah, S.; Fei, D. Smart Molecularly Imprinted Polymers: Recent Developments and Applications. Macromol. Rapid Commun. 2013, 34, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Go, Y.; Behmaneshfar, A.; Sadrnia, A.; Zhang, Z.; Dang, R. Developing a New Model for Optimizing Response in Design of Experiments Considering Consumable Factors: Removal of Pollutant from Wastewater with Fe3O4/Graphene Nano-Sorbent. J. Nanostruct. Chem. 2025, 15, 152507. [Google Scholar] [CrossRef]
- Xie, T.; Zhang, M.; Chen, P.; Zhao, H.; Yang, X.; Yao, L.; Zhang, H.; Dong, A.; Wang, J.; Wang, Z. A Facile Molecularly Imprinted Electrochemical Sensor Based on Graphene: Application to the Selective Determination of Thiamethoxam in Grain. RSC Adv. 2017, 7, 38884–38894. [Google Scholar] [CrossRef]
- Mohammed Albayatı, S.H.; Üstündağ, Z.; Soylu, P. A Novel Molecularly Imprinted Electrochemical Sensor for the Ultrasensitive Detection of Tert-Butylhydroquinone in Edible Oils. Anal. Biochem. 2023, 682, 115348. [Google Scholar] [CrossRef] [PubMed]
- Gholivand, M.B.; Torkashvand, M.; Malekzadeh, G. Fabrication of an Electrochemical Sensor Based on Computationally Designed Molecularly Imprinted Polymers for Determination of Cyanazine in Food Samples. Anal. Chim. Acta 2012, 713, 36–44. [Google Scholar] [CrossRef]
- Niu, Z.; Shi, Y.; Liu, S.; Lv, Y.; Wang, S. DFT-Assisted Design of a Electrochemical Sensor Based on MIP/CNT/MoS2-CoNi for the Detection of Sulfamethazine in Meat. J. Food Compos. Anal. 2025, 140, 107261. [Google Scholar] [CrossRef]
- Karthika, P.; Shanmuganathan, S.; Viswanathan, S.; Delerue-Matos, C. Molecularly Imprinted Polymer-Based Electrochemical Sensor for the Determination of Endocrine Disruptor Bisphenol-A in Bovine Milk. Food Chem. 2021, 363, 130287. [Google Scholar] [CrossRef]
- Liang, A.; Lv, T.; Pan, B.; Zhu, Z.; Haotian, R.; Xie, Y.; Sun, L.; Zhang, J.; Luo, A. Dynamic Simulation and Experimental Studies of Molecularly Imprinted Label-Free Sensor for Determination of Milk Quality Marker. Food Chem. 2024, 449, 139238. [Google Scholar] [CrossRef]
- Yarahmadi, B.; Hashemianzadeh, S.M.; Milani Hosseini, S.M.-R. Machine-Learning-Based Predictions of Imprinting Quality Using Ensemble and Non-Linear Regression Algorithms. Sci. Rep. 2023, 13, 12111. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Tang, Q.; Guo, Y.; Zhang, Z.; Zhang, W.; Zou, X.; Sun, Z. Molecularly Imprinted Electrochemical Sensor for Ethyl Carbamate Detection in Baijiu Based on “on-off” Nanozyme-Catalyzing Process. Food Chem. 2024, 453, 139626. [Google Scholar] [CrossRef]
- Yayla, S.; Hurkul, M.M.; Cetinkaya, A.; Uzun, L.; Ozkan, S.A. Selective Apigenin Assay in Plant Extracts and Herbal Supplement with Molecularly Imprinted Polymer-Based Electrochemical Sensor. Talanta 2025, 281, 126895. [Google Scholar] [CrossRef]
- Alberti, G.; Zanoni, C.; Magnaghi, L.R.; Biesuz, R. Ascorbic Acid Sensing by Molecularly Imprinted Electrosynthesized Polymer (e-MIP) on Screen-Printed Electrodes. Chemosensors 2023, 11, 348. [Google Scholar] [CrossRef]
- Tonelli, D.; Ballarin, B.; Guadagnini, L.; Mignani, A.; Scavetta, E. A Novel Potentiometric Sensor for L-Ascorbic Acid Based on Molecularly Imprinted Polypyrrole. Electrochim. Acta 2011, 56, 7149–7154. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Chen, H.; Chen, Y.; Huang, G. Disposable Electrochemical Ascorbic Acid Sensor Based on Molecularly Imprinted Poly(o-Phenylenediamine)-Modified Dual Channel Screen-Printed Electrode for Orange Juice Analysis. Food Anal. Methods 2014, 7, 1557–1563. [Google Scholar] [CrossRef]
- Prasad, B.B.; Kumar, D.; Madhuri, R.; Tiwari, M.P. Ascorbic Acid Imprinted Polymer-Modified Graphite Electrode: A Diagnostic Sensor for Hypovitaminosis C at Ultra Trace Ascorbic Acid Level. Sens. Actuators B Chem. 2011, 160, 418–427. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Xu, J.; Wen, Y. Electropolymerized Molecularly Imprinted Polypyrrole Decorated with Black Phosphorene Quantum Dots onto Poly(3,4-Ethylenedioxythiophene) Nanorods and Its Voltammetric Sensing of Vitamin C. J. Electroanal. Chem. 2018, 814, 153–160. [Google Scholar] [CrossRef]
- Albayatı, S.H.M.; Soylu, P. Butylated Hydroxyanisole Nanomolar Detection Using a Molecularly Imprinted Electrochemical Sensor in Food Samples. J. Appl. Electrochem. 2024, 54, 879–891. [Google Scholar] [CrossRef]
- Motia, S.; Bouchikhi, B.; El Bari, N. An Electrochemical Molecularly Imprinted Sensor Based on Chitosan Capped with Gold Nanoparticles and Its Application for Highly Sensitive Butylated Hydroxyanisole Analysis in Foodstuff Products. Talanta 2021, 223, 121689. [Google Scholar] [CrossRef]
- Yue, X.; Luo, X.; Zhou, Z.; Bai, Y. Selective Electrochemical Determination of Tertiary Butylhydroquinone in Edible Oils Based on an In-Situ Assembly Molecularly Imprinted Polymer Sensor. Food Chem. 2019, 289, 84–94. [Google Scholar] [CrossRef]
- Han, S.; Ding, Y.; Teng, F.; Yao, A.; Leng, Q. Molecularly Imprinted Electrochemical Sensor Based on 3D-Flower-like MoS2 Decorated with Silver Nanoparticles for Highly Selective Detection of Butylated Hydroxyanisole. Food Chem. 2022, 387, 132899. [Google Scholar] [CrossRef]
- dos Santos Moretti, E.; de Oliveira, F.M.; Scheel, G.L.; DalĺAntônia, L.H.; Borsato, D.; Kubota, L.T.; Segatelli, M.G.; Tarley, C.R.T. Synthesis of Surface Molecularly Imprinted Poly(Methacrylic Acid-Hemin) on Carbon Nanotubes for the Voltammetric Simultaneous Determination of Antioxidants from Lipid Matrices and Biodiesel. Electrochim. Acta 2016, 212, 322–332. [Google Scholar] [CrossRef]
- Cui, M.; Liu, S.; Lian, W.; Li, J.; Xu, W.; Huang, J. A Molecularly-Imprinted Electrochemical Sensor Based on a Graphene–Prussian Blue Composite-Modified Glassy Carbon Electrode for the Detection of Butylated Hydroxyanisole in Foodstuffs. Analyst 2013, 138, 5949–5955. [Google Scholar] [CrossRef] [PubMed]
- Elhachem, M.; Bou-Maroun, E.; Abboud, M.; Cayot, P.; Maroun, R.G. Optimization of a Molecularly Imprinted Polymer Synthesis for a Rapid Detection of Caffeic Acid in Wine. Foods 2023, 12, 1660. [Google Scholar] [CrossRef]
- Leite, F.R.F.; Santos, W.d.J.R.; Kubota, L.T. Selective Determination of Caffeic Acid in Wines with Electrochemical Sensor Based on Molecularly Imprinted Siloxanes. Sens. Actuators B Chem. 2014, 193, 238–246. [Google Scholar] [CrossRef]
- Elhachem, M.; Bou-Maroun, E.; Abboud, M.; Maroun, R.G.; Cayot, P. Combination of Screen-Printed Carbon Electrode and Molecularly Imprinted Polymers for the Selective Determination of Phenolic Compounds in Wine. Antioxidants 2022, 11, 2036. [Google Scholar] [CrossRef] [PubMed]
- Nandy Chatterjee, T.; Das, D.; Banerjee Roy, R.; Tudu, B.; Sabhapondit, S.; Tamuly, P.; Pramanik, P.; Bandyopadhyay, R. Molecular Imprinted Polymer Based Electrode for Sensing Catechin (+C) in Green Tea. IEEE Sens. J. 2018, 18, 2236–2244. [Google Scholar] [CrossRef]
- Das, D.; Nag, S.; Adaval, A.; Hazarika, A.K.; Sabhapondit, S.; Bhattacharyya, A.R.; Tudu, B.; Bandopadhyay, R.; Banerjee Roy, R. Amine Functionalized MWCNTs Modified MIP-Based Electrode for Detection of Epicatechin in Tea. IEEE Sens. J. 2022, 22, 10323–10330. [Google Scholar] [CrossRef]
- Qiu, Z.; Fan, D.; Xue, X.; Guo, S.; Lin, Y.; Chen, Y.; Tang, D. Molecularly Imprinted Polymer Functionalized Bi2S3/Ti3C2TX MXene Nanocomposites for Photoelectrochemical/Electrochemical Dual-Mode Sensing of Chlorogenic Acid. Chemosensors 2022, 10, 252. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Miguel, E.M.; Silva, J.d.S.; Silva, C.B.d.; Goulart, M.O.F.; Kubota, L.T.; Gonzaga, F.B.; Santos, W.J.R.; Lima, P.R. Application of a Nanostructured Platform and Imprinted Sol-Gel Film for Determination of Chlorogenic Acid in Food Samples. Talanta 2016, 156–157, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Santos, W.d.J.R.; Santhiago, M.; Yoshida, I.V.P.; Kubota, L.T. Novel Electrochemical Sensor for the Selective Recognition of Chlorogenic Acid. Anal. Chim. Acta 2011, 695, 44–50. [Google Scholar] [CrossRef]
- Koirala, K.; Sevilla, F.B.; Santos, J.H. Biomimetic Potentiometric Sensor for Chlorogenic Acid Based on Electrosynthesized Polypyrrole. Sens. Actuators B Chem. 2016, 222, 391–396. [Google Scholar] [CrossRef]
- Yan, L.; Yin, Y.; Lv, P.; Zhang, Z.; Wang, J.; Long, F. Synthesis and Application of Novel 3D Magnetic Chlorogenic Acid Imprinted Polymers Based on a Graphene–Carbon Nanotube Composite. J. Agric. Food Chem. 2016, 64, 3091–3100. [Google Scholar] [CrossRef]
- Ahmed, A.H.M.T.; Naskar, H.; Banerjee, S.; Ghatak, B.; Das, N.; Tudu, B.; Bandyopadhyay, R. Electrochemical Sensor Based on Molecularly Imprinted Polymer Embedded Graphite Electrode for Detecting Curcumin. Sens. Actuators Phys. 2022, 344, 113748. [Google Scholar] [CrossRef]
- Mohammadinejad, A.; Abouzari-Lotf, E.; Aleyaghoob, G.; Rezayi, M.; Kazemi Oskuee, R. Application of a Transition Metal Oxide/Carbon-Based Nanocomposite for Designing a Molecularly Imprinted Poly (l-Cysteine) Electrochemical Sensor for Curcumin. Food Chem. 2022, 386, 132845. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhai, H.Y.; Pan, Y.F.; Li, K. A Simple and Sensitive Sensor Based on a Molecularly Imprinted Polymer-Modified Carbon Paste Electrode for the Determination of Curcumin in Foods. RSC Adv. 2017, 7, 22913–22918. [Google Scholar] [CrossRef]
- Pedroso, M.M.; Foguel, M.V.; Silva, D.H.S.; Sotomayor, M.d.P.T.; Yamanaka, H. Electrochemical Sensor for Dodecyl Gallate Determination Based on Electropolymerized Molecularly Imprinted Polymer. Sens. Actuators B Chem. 2017, 253, 180–186. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, L.; Hu, Y.; Peng, X.; Du, J. A Novel Electrochemical Sensor Based on a Molecularly Imprinted Polymer for the Determination of Epigallocatechin Gallate. Food Chem. 2017, 221, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-C.; Chang, C.-C.; Chiu, H.-S.; Jiang, S.-F.; Lee, M.-H.; Chung, C.-L.; Liu, B.-D.; Huang, C.-Y.; Lin, H.-Y. A Portable Electrochemical Sensor for Caffeine and (-)Epigallocatechin Gallate Based on Molecularly Imprinted Poly(Ethylene-Co-Vinyl Alcohol) Recognition Element. J. Nanosci. Nanotechnol. 2011, 11, 10633–10638. [Google Scholar] [CrossRef]
- Buffon, E.; Stradiotto, N.R. A Molecularly Imprinted Polymer on Reduced Graphene Oxide-Gold Nanoparticles Modified Screen-Printed Electrode for Selective Determination of Ferulic Acid in Orange Peels. Microchem. J. 2021, 167, 106339. [Google Scholar] [CrossRef]
- Shojaei, S.; Nasirizadeh, N.; Entezam, M.; Koosha, M.; Azimzadeh, M. An Electrochemical Nanosensor Based on Molecularly Imprinted Polymer (MIP) for Detection of Gallic Acid in Fruit Juices. Food Anal. Methods 2016, 9, 2721–2731. [Google Scholar] [CrossRef]
- Zanoni, C.; Dallù, L.V.; Costa, C.; Cutaia, A.; Alberti, G. A Screen-Printed Voltammetric Sensor Modified with Electropolymerized Molecularly Imprinted Polymer (eMIP) to Determine Gallic Acid in Non-Alcoholic and Alcoholic Beverages. Polymers 2024, 16, 1076. [Google Scholar] [CrossRef]
- Jara-Ulloa, P.; Salgado-Figueroa, P.; Moscoso, R.; Squella, J.A. Polypyrrole Molecularly Imprinted Modified Glassy Carbon Electrode for the Recognition of Gallic Acid. J. Electrochem. Soc. 2013, 160, H243. [Google Scholar] [CrossRef]
- Qin, F.; Hu, T.; You, L.; Chen, W.; Jia, D.; Hu, N.; Qi, W. Electrochemical Detection of Gallic Acid in Green Tea Using Molecularly Imprinted Polymers on TiO2@CNTs Nanocomposite Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2022, 17, 220426. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, Q.; Chen, T.; Wu, W.; Tang, X.; Wang, G.; Feng, J.; Zhang, W. Facile Potentiometric Sensing of Gallic Acid in Edible Plants Based on Molecularly Imprinted Polymer. J. Food Sci. 2020, 85, 2622–2628. [Google Scholar] [CrossRef]
- Das, D.; Biswas, D.; Hazarika, A.K.; Sabhapondit, S.; Roy, R.B.; Tudu, B.; Bandyopadhyay, R. CuO Nanoparticles Decorated MIP-Based Electrode for Sensitive Determination of Gallic Acid in Green Tea. IEEE Sens. J. 2021, 21, 5687–5694. [Google Scholar] [CrossRef]
- Ye, C.; Chen, X.; Xu, J.; Xi, H.; Wu, T.; Deng, D.; Zhang, J.; Huang, G. Highly Sensitive Detection to Gallic Acid by Polypyrrole-Based MIES Supported by MOFs-Co2+@Fe3O4. J. Electroanal. Chem. 2020, 859, 113839. [Google Scholar] [CrossRef]
- You, Z.; Fu, Y.; Xiao, A.; Liu, L.; Huang, S. Magnetic Molecularly Imprinting Polymers and Reduced Graphene Oxide Modified Electrochemical Sensor for the Selective and Sensitive Determination of Luteolin in Natural Extract. Arab. J. Chem. 2021, 14, 102990. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, B.; Yang, L.; Zhao, F.; Zeng, B. Electrochemical Determination of Luteolin Using Molecularly Imprinted Poly-Carbazole on MoS2/Graphene-Carbon Nanotubes Nanocomposite Modified Electrode. Electrochim. Acta 2017, 258, 1413–1420. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Duan, X.; Zhu, Y.; Xue, T.; Rao, L.; Wen, Y.; Tian, Q.; Cai, Y.; Xu, Q.; et al. A Novel Nanozyme Comprised of Electro-Synthesized Molecularly Imprinted Conducting PEDOT Nanocomposite with Graphene-like MoS2 for Electrochemical Sensing of Luteolin. Microchem. J. 2021, 168, 106418. [Google Scholar] [CrossRef]
- Buffon, E.; Stradiotto, N.R. Disposable p-Coumaric Acid Sensor Containing Reduced Graphene Oxide, Nickel Nanoparticles and Biodegradable Molecularly Imprinted Polymer for Fruit Peel Analysis. J. Food Compos. Anal. 2023, 118, 105186. [Google Scholar] [CrossRef]
- Albayatı, S.H.M.; Soylu, P. A Simple Molecularly Imprinted Electrochemical Sensor for Determination of Propyl Gallate in Food Samples. Anal. Biochem. 2024, 688, 115477. [Google Scholar] [CrossRef]
- Hurkul, M.M.; Cetinkaya, A.; Yayla, S.; Kaya, S.I.; Budak, F.; Tok, K.C.; Gumustas, M.; Uzun, L.; Ozkan, S.A. Highly Selective and Sensitive Molecularly Imprinted Sensors for the Electrochemical Assay of Quercetin in Methanol Extracts of Rubus sanctus and Fragaria vesca. Talanta 2024, 273, 125883. [Google Scholar] [CrossRef]
- Yang, L.; Xu, B.; Ye, H.; Zhao, F.; Zeng, B. A Novel Quercetin Electrochemical Sensor Based on Molecularly Imprinted Poly(Para-Aminobenzoic Acid) on 3D Pd Nanoparticles-Porous Graphene-Carbon Nanotubes Composite. Sens. Actuators B Chem. 2017, 251, 601–608. [Google Scholar] [CrossRef]
- Yao, Z.; Yang, X.; Liu, X.; Yang, Y.; Hu, Y.; Zhao, Z. Electrochemical Quercetin Sensor Based on a Nanocomposite Consisting of Magnetized Reduced Graphene Oxide, Silver Nanoparticles and a Molecularly Imprinted Polymer on a Screen-Printed Electrode. Microchim. Acta 2017, 185, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zong, L.; Geng, G.; Li, Y.; Zhang, Y. Enhancing Determination of Quercetin in Honey Samples through Electrochemical Sensors Based on Highly Porous Polypyrrole Coupled with Nanohybrid Modified GCE. Sens. Actuators B Chem. 2018, 257, 1099–1109. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Q.; Yang, J.; Chen, S.; Hu, F.; Hu, Y.; Lin, H. Synergistic Effects of Layer-by-Layer Films for Highly Selective and Sensitive Electrochemical Detection of Trans-Resveratrol. Food Chem. 2021, 338, 127851. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yang, J.; Li, S.; Qin, Y.; Mo, Q.; Chen, L.; Li, X. Highly Sensitive Molecular Imprinted Voltammetric Sensor for Resveratrol Assay in Wine via Polyaniline/Gold Nanoparticles Signal Enhancement and Polyacrylamide Recognition. J. Electroanal. Chem. 2021, 895, 115455. [Google Scholar] [CrossRef]
- Mugo, S.M.; Edmunds, B.J.; Berg, D.J.; Gill, N.K. An Integrated Carbon Entrapped Molecularly Imprinted Polymer (MIP) Electrode for Voltammetric Detection of Resveratrol in Wine. Anal. Methods 2015, 7, 9092–9099. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Zhang, J.; Li, Y.; Zhang, Y.; Li, Y.; Ye, B.-C. Novel Electrochemical Sensing Platform Based on Integration of Molecularly Imprinted Polymer with Au@Ag Hollow Nanoshell for Determination of Resveratrol. Talanta 2019, 196, 479–485. [Google Scholar] [CrossRef]
- Hurkul, M.M.; Yayla, S.; Cetinkaya, A.; Kaya, S.I.; Uzun, L.; Ozkan, S.A. A Novel Electrochemical Sensor Based on a Molecularly Imprinted Polymer for Highly Selective and Sensitive Determination of Rutin from Herbal Supplements and Plant Extracts. Anal. Methods 2024, 16, 1480–1488. [Google Scholar] [CrossRef]
- Yang, L.; Yang, J.; Xu, B.; Zhao, F.; Zeng, B. Facile Preparation of Molecularly Imprinted Polypyrrole-Graphene-Multiwalled Carbon Nanotubes Composite Film Modified Electrode for Rutin Sensing. Talanta 2016, 161, 413–418. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Nag, S.; Das, D.; Roy, R.B. Detection of Syringic Acid in Food Extracts Using Molecular Imprinted Polyacrylonitrile Infused Graphite Electrode. J. Food Compos. Anal. 2024, 132, 106280. [Google Scholar] [CrossRef]
- Chi, H.; Yang, C.; Liu, G. An Electrochemical Sensor Based on Electrochemically Activated Carbon Cloth and Poly (o-Aminothiophenol) Cross-Linked Nanogold Imprinted Layer for the Determination of Tert-Butylhydroquinone. Food Chem. 2024, 452, 139548. [Google Scholar] [CrossRef]
- Fan, L.; Hao, Q.; Kan, X. Three-Dimensional Graphite Paper Based Imprinted Electrochemical Sensor for Tertiary Butylhydroquinone Selective Recognition and Sensitive Detection. Sens. Actuators B Chem. 2018, 256, 520–527. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, R.; Sun, Y.; Waterhouse, G.I.N.; Qiao, X.; Xu, Z. Development of ZrO(OH)2@HCS Co-Modified Molecularly Imprinted Gel-Based Electrochemical Sensing Platform for Sensitive and Selective Detection of Tert-Butylhydroquinone in Foods. Food Chem. 2024, 460, 140600. [Google Scholar] [CrossRef]
- Nandy Chatterjee, T.; Das, D.; Banerjee Roy, R.; Tudu, B.; Hazarika, A.K.; Sabhapondit, S.; Tamuly, P.; Bandyopadhyay, R. Development of a Nickel Hydroxide Nanopetal Decorated Molecular Imprinted Polymer Based Electrode for Sensitive Detection of Epigallocatechin-3-Gallate in Green Tea. Sens. Actuators B Chem. 2019, 283, 69–78. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Wang, L. Porous carbon derived from ZIF-8 modified molecularly imprinted electrochemical sensor for the detection of tert-butyl hydroquinone (TBHQ) in edible oil. Food Chem. 2021, 365, 130462. [Google Scholar] [CrossRef]
- Sezgin, B.; Arli, G.; Can, N.Ö. Simultaneous HPLC-DAD Determination of Seven Intense Sweeteners in Foodstuffs and Pharmaceuticals Using a Core-Shell Particle Column. J. Food Compos. Anal. 2021, 97, 103768. [Google Scholar] [CrossRef]
- Jinadasa, B.K.K.K.; Elliott, C.; Yeh, T.-S. Recent Applications of Mass Spectrometry in Sweetener Analysis. J. Food Compos. Anal. 2023, 122, 105418. [Google Scholar] [CrossRef]
- Balgobind, K.; Kanchi, S.; Sharma, D.; Bisetty, K.; Sabela, M.I. Hybrid of ZnONPs/MWCNTs for electrochemical detection of aspartame in food and beverage samples. J. Electroanal. Chem. 2016, 774, 51–57. [Google Scholar] [CrossRef]
- Su, J.; Su, X. A Multi-Walled Carbon Nanotubes-Based Molecular Imprinting Electrochemical Sensor for the Detection of Aspartame in Sports Beverage. J. Food Meas. Charact. 2024, 18, 618–624. [Google Scholar] [CrossRef]
- Mansour, A.H.; Mortada, W.I.; Awad, F.S.; Molouk, A.F.S.; Khalifa, M.E.; Abdallah, A.B. Carbon Dot-Based Imprinted Electrochemical Sensor for Ultrasensitive and Selective Detection of Sucralose in Real Samples. Mater. Chem. Front. 2025, 9, 3075–3085. [Google Scholar] [CrossRef]
- O’Connor, D.; Pang, M.; Castelnuovo, G.; Finlayson, G.; Blaak, E.; Gibbons, C.; Navas-Carretero, S.; Almiron-Roig, E.; Harrold, J.; Raben, A.; et al. A Rational Review on the Effects of Sweeteners and Sweetness Enhancers on Appetite, Food Reward and Metabolic/Adiposity Outcomes in Adults. Food Funct. 2021, 12, 442–465. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Jin, S.; Lin, W. The Role of Information in Nudging Chinese Consumers from Choosing Sugar to Alternative Sweeteners. China Econ. Rev. 2024, 87, 102233. [Google Scholar] [CrossRef]
- Shaher, S.A.A.; Mihailescu, D.F.; Amuzescu, B. Aspartame Safety as a Food Sweetener and Related Health Hazards. Nutrients 2023, 15, 3627. [Google Scholar] [CrossRef]
- Tan, L.; Li, Q.-Y.; Li, Y.-J.; Ma, R.-R.; He, J.-Y.; Jiang, Z.-F.; Yang, L.-L.; Wang, C.-Z.; Luo, L.; Zhang, Q.-H.; et al. Specific Adsorption and Determination of Aspartame in Soft Drinks with a Zein Magnetic Molecularly Imprinted Modified MGCE Sensor. RSC Adv. 2021, 11, 13486–13496. [Google Scholar] [CrossRef]
- Tiu, B.D.B.; Pernites, R.B.; Tiu, S.B.; Advincula, R.C. Detection of Aspartame via Microsphere-Patterned and Molecularly Imprinted Polymer Arrays. Colloids Surf. Physicochem. Eng. Asp. 2016, 495, 149–158. [Google Scholar] [CrossRef]
- Sousa, M.S.P.; de Sá, A.C.; de Oliveira, J.P.J.; da Silva, M.J.; Santos, R.J.; Paim, L.L. Impedimetric Sensor for Pentoses Based on Electrodeposited Carbon Nanotubes and Molecularly Imprinted Poly-o-Phenylenediamine. ECS J. Solid State Sci. Technol. 2020, 9, 041006. [Google Scholar] [CrossRef]
- Pompeu Prado Moreira, L.F.; Buffon, E.; Stradiotto, N.R. Electrochemical Sensor Based on Reduced Graphene Oxide and Molecularly Imprinted Poly(Phenol) for d-Xylose Determination. Talanta 2020, 208, 120379. [Google Scholar] [CrossRef]
- Singh, R.; Singh, M. Molecularly Imprinted Electrochemical Sensor for Highly Selective and Sensitive Determination of Artificial Sweetener Acesulfame-K. Talanta Open 2023, 7, 100194. [Google Scholar] [CrossRef]
- Çakir, S.; Coskun, E.; Biçer, E.; Çakir, O. Electrochemical study of the complexes of aspartame with Cu(II), Ni(II), and Zn(II) ions in the aqueous medium. Carbohydr. Res. 2003, 338, 1217–1222. [Google Scholar] [CrossRef]
- Srivastava, J.; Gupta, N.; Kushwaha, A.; Umrao, S.; Srivastava, A.; Singh, M. Highly Sensitive and Selective Estimation of Aspartame by Chitosan Nanoparticles–Graphene Nanocomposite Tailored EQCM-MIP Sensor. Polym. Bull. 2019, 76, 4431–4449. [Google Scholar] [CrossRef]
- Moreira, L.F.P.P.; Buffon, E.; de Sá, A.C.; Stradiotto, N.R. Fructose Determination in Fruit Juices Using an Electrosynthesized Molecularly Imprinted Polymer on Reduced Graphene Oxide Modified Electrode. Food Chem. 2021, 352, 129430. [Google Scholar] [CrossRef]
- Shekarchizadeh, H.; Ensafi, A.A.; Kadivar, M. Selective Determination of Sucrose Based on Electropolymerized Molecularly Imprinted Polymer Modified Multiwall Carbon Nanotubes/Glassy Carbon Electrode. Mater. Sci. Eng. C 2013, 33, 3553–3561. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhu, J.; Zheng, Y.; Fu, L. Electrochemical Sensing Strategies for Synthetic Orange Dyes. Molecules 2024, 29, 5026. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, S.; Lv, Y.; Chen, F.; Fu, L. Acid Red Dyes and the Role of Electrochemical Sensors in Their Determination. Microchem. J. 2024, 207, 111705. [Google Scholar] [CrossRef]
- Georgescu-State, R.; van Staden, J.K.F.; Staden, R.-I.S.; State, R.N. Electrochemical Platform Based on Molecularly Imprinted Polymer with Zinc Oxide Nanoparticles and Multiwalled Carbon Nanotubes Modified Screen-Printed Carbon Electrode for Amaranth Determination. Microchim. Acta 2023, 190, 229. [Google Scholar] [CrossRef]
- Li, L.; Zheng, H.; Guo, L.; Qu, L.; Yu, L. A Sensitive and Selective Molecularly Imprinted Electrochemical Sensor Based on Pd-Cu Bimetallic Alloy Functionalized Graphene for Detection of Amaranth in Soft Drink. Talanta 2019, 197, 68–76. [Google Scholar] [CrossRef]
- George, A.; Bharath, M.; Ghosh, M.; Varghese, A. Single-Monomer Dual Templated MIP Based Electrochemical Sensor for Tartrazine and Brilliant Blue FCF. Electrochim. Acta 2024, 475, 143682. [Google Scholar] [CrossRef]
- Wang, J.; Qin, Y. Molecularly Imprinted Sensors for the Determination of Anthocyanins in Food Products. Int. J. Electrochem. Sci. 2024, 19, 100673. [Google Scholar] [CrossRef]
- Bonyadi, S.; Ghanbari, K. Development of Highly Sensitive and Selective Sensor Based on Molecular Imprinted Polydopamine-Coated Silica Nanoparticles for Electrochemical Determination of Sunset Yellow. Microchem. J. 2021, 167, 106322. [Google Scholar] [CrossRef]
- Yin, Z.-Z.; Cheng, S.-W.; Xu, L.-B.; Liu, H.-Y.; Huang, K.; Li, L.; Zhai, Y.-Y.; Zeng, Y.-B.; Liu, H.-Q.; Shao, Y.; et al. Highly Sensitive and Selective Sensor for Sunset Yellow Based on Molecularly Imprinted Polydopamine-Coated Multi-Walled Carbon Nanotubes. Biosens. Bioelectron. 2018, 100, 565–570. [Google Scholar] [CrossRef]
- Wang, Z.; Shan, Y.; Xu, L.; Wu, G.; Lu, X. Development and Application of the Tartrazine Voltammetric Sensors Based on Molecularly Imprinted Polymers. Int. J. Polym. Anal. Charact. 2017, 22, 83–91. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Duan, H.; Wang, Y.; Bu, Y.; Luo, C. Based on Magnetic Graphene Oxide Highly Sensitive and Selective Imprinted Sensor for Determination of Sunset Yellow. Talanta 2016, 147, 169–176. [Google Scholar] [CrossRef]
- Shalapy, A.E.; Molouk, A.F.S.; Awad, F.S.; Khalifa, M.E.; Abdallah, A.B. Flexible Imprinted Electrochemical Sensing Platform for Ultra-Trace Detection of Indigo Carmine Dye in Coloured Food Additive for Candy and Ice Cream. Inorg. Chem. Commun. 2025, 174, 113951. [Google Scholar] [CrossRef]
- Benmassaoud, Y.; Murtada, K.; Salghi, R.; Zougagh, M.; Ríos, Á. Surface Polymers on Multiwalled Carbon Nanotubes for Selective Extraction and Electrochemical Determination of Rhodamine B in Food Samples. Molecules 2021, 26, 2670. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Luo, C.; Sun, M.; Li, L.; Duan, H. Ultrasensitive Molecularly Imprinted Electrochemical Sensor Based on Magnetism Graphene Oxide/β-Cyclodextrin/Au Nanoparticles Composites for Chrysoidine Analysis. Electrochim. Acta 2014, 130, 519–525. [Google Scholar] [CrossRef]
- Jacinto, C.; Maza Mejía, I.; Khan, S.; López, R.; Sotomayor, M.D.; Picasso, G. Using a smartphone-based colorimetric device with molecularly imprinted polymer for the quantification of tartrazine in soda drinks. Biosensors 2023, 13, 639. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Wang, R.; Waterhouse, G.I.N.; Xu, Z. One-Pot Synthesis of a Novel Conductive Molecularly Imprinted Gel as the Recognition Element and Signal Amplifier for the Selective Electrochemical Detection of Amaranth in Foods. Biosens. Bioelectron. 2023, 228, 115185. [Google Scholar] [CrossRef]
- Han, Q.; Wang, X.; Yang, Z.; Zhu, W.; Zhou, X.; Jiang, H. Fe3O4@rGO Doped Molecularly Imprinted Polymer Membrane Based on Magnetic Field Directed Self-Assembly for the Determination of Amaranth. Talanta 2014, 123, 101–108. [Google Scholar] [CrossRef]
- Yang, L.; Chen, S.; Zhao, L.; Chen, W.; Huang, W.; Li, X.; Zhang, H. Extraction and Electrochemical Sensing of Anthocyanins in Berry Fruits by Use of Carbon Nanotube-Based Electrode. J. Food Meas. Charact. 2024, 18, 7019–7028. [Google Scholar] [CrossRef]
- Jiang, Z.-F.; Li, Q.; Li, Q.-Y.; Xu, H.-X.; He, J.-Y.; Wang, C.-Z.; Zhou, L.-D.; Zhang, Q.-H.; Luo, L.; Yuan, C.-S. Fast Exhaustive Enrichment and Electrochemical Quantitative Detection of Anthocyanins from Natural Products by Using Dual Responsive and Dummy Molecularly Imprinted Polymers. Microchem. J. 2022, 179, 107545. [Google Scholar] [CrossRef]
- Arvand, M.; Zamani, M.; Sayyar Ardaki, M. Rapid Electrochemical Synthesis of Molecularly Imprinted Polymers on Functionalized Multi-Walled Carbon Nanotubes for Selective Recognition of Sunset Yellow in Food Samples. Sens. Actuators B Chem. 2017, 243, 927–939. [Google Scholar] [CrossRef]
- Wu, L.; Wu, T.; Zeng, W.; Zhou, S.; Zhang, W.; Ma, J. A New Ratiometric Molecularly Imprinted Electrochemical Sensor for the Detection of Sunset Yellow Based on Gold Nanoparticles. Food Chem. 2023, 413, 135600. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, F.; Zeng, B. Preparation and Application of Sunset Yellow Imprinted Ionic Liquid Polymer—Ionic Liquid Functionalized Graphene Composite Film Coated Glassy Carbon Electrodes. Electrochim. Acta 2014, 115, 247–254. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Wang, H.; Yang, P.; Du, Y. Highly Sensitive Electrochemical Determination of Sunset Yellow Based on Gold Nanoparticles/Graphene Electrode. Anal. Chim. Acta 2015, 893, 41–48. [Google Scholar] [CrossRef]
- Arvand, M.; Erfanifar, Z.; Ardaki, M.S. A New Core@Shell Silica-Coated Magnetic Molecular Imprinted Nanoparticles for Selective Detection of Sunset Yellow in Food Samples. Food Anal. Methods 2017, 10, 2593–2606. [Google Scholar] [CrossRef]
- Malik, S.; Khan, A.; Khan, H.; Rahman, G.; Ali, N.; Khan, S.; Sotomayor, M.D.P.T. Biomimetic Electrochemical Sensors Based on Core-Shell Imprinted Polymers for Targeted Sunset Yellow Estimation in Environmental Samples. Biosensors 2023, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Guo, W.; Liu, Y.; Liu, Z.; Qiu, J.; Peng, J. A Novel Electrochemical Sensor Based on Graphene Oxide Decorated with Silver Nanoparticles–Molecular Imprinted Polymers for Determination of Sunset Yellow in Soft Drinks. Food Anal. Methods 2017, 10, 2293–2301. [Google Scholar] [CrossRef]
- Kamalabadi, M.; Razavi-Mashouf, M.M.; Madrakian, T.; Ghoorchian, A.; Afkhami, A. Electrochemically Controlled Solid Phase Microextraction Based on Nanostructured Polypyrrole Film for Selective Extraction of Sunset Yellow in Food Samples. J. Iran. Chem. Soc. 2021, 18, 3127–3135. [Google Scholar] [CrossRef]
- Su, J.; Su, X. Determination of Tartrazine in Sports Drinks by a Disposable Electrochemical Sensor Modified with Co2O3. J. Food Meas. Charact. 2023, 17, 5856–5863. [Google Scholar] [CrossRef]
- George, A.; Rose Cherian, A.; Jacob, B.; Varghese, A.; Maiyalagan, T. Design Optimisation and Fabrication of Amino Acid Based Molecularly Imprinted Sensor for the Selective Determination of Food Additive Tartrazine. Food Chem. 2023, 404, 134673. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Waterhouse, G.I.N.; Gao, H.; Xu, Z. Highly Selective Molecularly Imprinted Gel-Based Electrochemical Sensor with CuS@COOH-MWCNTs Signal Amplification for Simultaneous Detection of Vanillin and Tartrazine in Foods. Sens. Actuators B Chem. 2023, 377, 133045. [Google Scholar] [CrossRef]
- Bonyadi, S.; Ghanbari, K. Application of Molecularly Imprinted Polymer and ZnO Nanoparticles as a Novel Electrochemical Sensor for Tartrazine Determination. Microchem. J. 2023, 187, 108398. [Google Scholar] [CrossRef]
- Zhao, L.; Zeng, B.; Zhao, F. Electrochemical Determination of Tartrazine Using a Molecularly Imprinted Polymer—Multiwalled Carbon Nanotubes—Ionic Liquid Supported Pt Nanoparticles Composite Film Coated Electrode. Electrochim. Acta 2014, 146, 611–617. [Google Scholar] [CrossRef]
- Cheng, S.; Tang, D.; Zhang, Y.; Xu, L.; Liu, K.; Huang, K.; Yin, Z. Specific and Sensitive Detection of Tartrazine on the Electrochemical Interface of a Molecularly Imprinted Polydopamine-Coated PtCo Nanoalloy on Graphene Oxide. Biosensors 2022, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, Y.; Zuo, J.; Zhang, J.; Zhu, L.; Zhang, J. Rapid and Sensitive Determination of Tartrazine Using a Molecularly Imprinted Copolymer Modified Carbon Electrode (MIP-PmDB/PoPD-GCE). J. Electroanal. Chem. 2017, 785, 90–95. [Google Scholar] [CrossRef]
- Che Lah, N.F.; Ahmad, A.L.; Low, S.C.; Zaulkiflee, N.D. Isotherm and Electrochemical Properties of Atrazine Sensing Using PVC/MIP: Effect of Porogenic Solvent Concentration Ratio. Membranes 2021, 11, 657. [Google Scholar] [CrossRef]
- Pardieu, E.; Cheap, H.; Vedrine, C.; Lazerges, M.; Lattach, Y.; Garnier, F.; Remita, S.; Pernelle, C. Molecularly Imprinted Conducting Polymer Based Electrochemical Sensor for Detection of Atrazine. Anal. Chim. Acta 2009, 649, 236–245. [Google Scholar] [CrossRef]
- da Costa Gonzaga, M.L.; de Albuquerque Oliveira, M.; Furtado, R.F.; Alves, C.R. Synthesis and Application of Poly(Methacrylic Acid-Co-Ethylene Glycol Dimethacrylate) as Molecularly Imprinted Polymer in Electrochemical Sensor for Atrazine Detection. J. Solid State Electrochem. 2024, 29, 3169–3179. [Google Scholar] [CrossRef]
- Che Lah, N.F.; Ahmad, A.L.; Low, S.C.; Shoparwe, N.F. The Role of Porogen-Polymer Complexation in Atrazine Imprinted Polymer to Work as an Electrochemical Sensor in Water. J. Environ. Chem. Eng. 2019, 7, 103500. [Google Scholar] [CrossRef]
- Qi, P.; Wang, J.; Wang, X.; Wang, Z.; Xu, H.; Di, S.; Wang, Q.; Wang, X. Sensitive and Selective Detection of the Highly Toxic Pesticide Carbofuran in Vegetable Samples by a Molecularly Imprinted Electrochemical Sensor with Signal Enhancement by AuNPs. RSC Adv. 2018, 8, 25334–25341. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Qin, L.; Li, D.; Qin, S.; Han, J.; Gao, W.; Jia, Y. Carbofuran-Imprinted Sensor Based on a Modified Electrode and Prepared via Combined Multiple Technologies: Preparation Process, Performance Evaluation, and Application. Electrochim. Acta 2022, 404, 139600. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, X.; Zhang, Q.; Song, H.; Kang, Q.; Shen, D. Differential Ratiometric Molecularly Imprinted Electrochemical Method for Methyl-Parathion and Carbendazim in Foods. Food Chem. 2025, 474, 143186. [Google Scholar] [CrossRef]
- Xue, S.; Zou, J.; Li, J.; Xu, J.; Chen, H.; Wang, L.; Gao, Y.; Duan, X.; Lu, L. Electrochemical Detection of Carbendazim Using Molecularly Imprinted Poly(3,4-Ethylenedioxythiophene) on Co,N Co-Doped Hollow Carbon nanocage@CNTs-Modified Electrode. Food Chem. 2024, 456, 140063. [Google Scholar] [CrossRef]
- Khosropour, H.; Keramat, M.; Laiwattanapaisal, W. A Dual Action Electrochemical Molecularly Imprinted Aptasensor for Ultra-Trace Detection of Carbendazim. Biosens. Bioelectron. 2024, 243, 115754. [Google Scholar] [CrossRef] [PubMed]
- Roushani, M.; Nezhadali, A.; Jalilian, Z. An Electrochemical Chlorpyrifos Aptasensor Based on the Use of a Glassy Carbon Electrode Modified with an Electropolymerized Aptamer-Imprinted Polymer and Gold Nanorods. Microchim. Acta 2018, 185, 551. [Google Scholar] [CrossRef]
- Nagabooshanam, S.; Roy, S.; Deshmukh, S.; Wadhwa, S.; Sulania, I.; Mathur, A.; Krishnamurthy, S.; Bharadwaj, L.M.; Roy, S.S. Microfluidic Affinity Sensor Based on a Molecularly Imprinted Polymer for Ultrasensitive Detection of Chlorpyrifos. ACS Omega 2020, 5, 31765–31773. [Google Scholar] [CrossRef]
- Geana, E.-I.; Ciucure, C.T.; Soare, A.; Enache, S.; Ionete, R.E.; Dinu, L.A. Electrochemical Detection of Glyphosate in Surface Water Samples Based on Modified Screen-Printed Electrodes. Nanomaterials 2024, 14, 948. [Google Scholar] [CrossRef]
- Ren, X.; Zeng, H.; Zhang, Q.; Cai, H.; Yang, W. Electrochemical Sensor Based on Molecularly Imprinted Polymer and Graphene Oxide Nanocomposite for Monitoring Glyphosate Content in Corn. Int. J. Electrochem. Sci. 2022, 17, 221292. [Google Scholar] [CrossRef]
- Zouaoui, F.; Bourouina-Bacha, S.; Bourouina, M.; Zine, N.; Errachid, A.; Jaffrezic-Renault, N. Mathematical Modelling of Glyphosate Molecularly Imprinted Polymer-Based Microsensor with Multiple Phenomena. Molecules 2022, 27, 493. [Google Scholar] [CrossRef]
- Yaman, Y.T.; Bolat, G.; Abaci, S.; Saygin, T.B. Peptide Nanotube Functionalized Molecularly Imprinted Polydopamine Based Single-Use Sensor for Impedimetric Detection of Malathion. Anal. Bioanal. Chem. 2022, 414, 1115–1128. [Google Scholar] [CrossRef]
- Zuo, H.G.; Zhu, J.X.; Zhan, C.R.; Shi, L.; Xing, M.; Guo, P.; Ding, Y.; Yang, H. Preparation of Malathion MIP-SPE and Its Application in Environmental Analysis. Environ. Monit. Assess. 2015, 187, 394. [Google Scholar] [CrossRef]
- Hassan, A.H.A.; Moura, S.L.; Ali, F.H.M.; Moselhy, W.A.; Taboada Sotomayor, M.d.P.; Pividori, M.I. Electrochemical Sensing of Methyl Parathion on Magnetic Molecularly Imprinted Polymer. Biosens. Bioelectron. 2018, 118, 181–187. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Zhang, Y.; Gu, M.; Wang, D.; Dang, Y.; Ye, B.-C.; Li, Y. A Robust Electrochemical Sensing Platform Using Carbon Paste Electrode Modified with Molecularly Imprinted Microsphere and Its Application on Methyl Parathion Detection. Biosens. Bioelectron. 2018, 106, 71–77. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, L.; Ran, J.; Wang, S.; Dong, N. Specific Recognition of Cationic Paraquat in Environmental Water and Vegetable Samples by Molecularly Imprinted Stir-Bar Sorptive Extraction Based on Monohydroxylcucurbit [7]Uril–Paraquat Inclusion Complex. Microchim. Acta 2020, 187, 578. [Google Scholar] [CrossRef]
- Shan, X.; de Dieu Habimana, J.; Ji, J.; Sun, J.; Pi, F.; Zhang, Y.; Sun, X. A Molecularly Imprinted Electrochemical Sensor Based on Au Nanocross-Chitosan Composites for Detection of Paraquat. J. Solid State Electrochem. 2019, 23, 1211–1220. [Google Scholar] [CrossRef]
- Yola, B.B.; Kotan, G.; Akyıldırım, O.; Atar, N.; Yola, M.L. Electrochemical Determination of Fenitrothion Pesticide Based on Ultrathin Manganese Oxide Nanowires/Molybdenum Titanium Carbide MXene Ionic Nanocomposite and Molecularly Imprinting Polymer. Microchim. Acta 2024, 191, 230. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, J.; Li, H.; Xiang, L.; Huang, Z. Fabrication of an Electrochemical Sensor with an Electrode Modified by the Two-Separated Steps of Silver Nanoparticle Sensitizing and Molecularly Imprinted Polymer Coating for the Selective and Ultrasensitive Determination of Fenitrothion. Monatshefte Für Chem.—Chem. Mon. 2023, 154, 553–561. [Google Scholar] [CrossRef]
- Mehmandoust, M.; Erk, N.; Naser, M.; Soylak, M. Molecularly Imprinted Polymer Film Loaded on the Metal–Organic Framework with Improved Performance Using Stabilized Gold-Doped Graphite Carbon Nitride Nanosheets for the Single-Step Detection of Fenamiphos. Food Chem. 2023, 404, 134627. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Yola, M.L.; Atar, N.; Orooji, Y.; Karimi, F.; Senthil Kumar, P.; Rouhi, J.; Baghayeri, M. A Novel Detection Method for Organophosphorus Insecticide Fenamiphos: Molecularly Imprinted Electrochemical Sensor Based on Core-Shell Co3O4@MOF-74 Nanocomposite. J. Colloid Interface Sci. 2021, 592, 174–185. [Google Scholar] [CrossRef]
- Alberti, G.; Zanoni, C.; Spina, S.; Magnaghi, L.R.; Biesuz, R. MIP-Based Screen-Printed Potentiometric Cell for Atrazine Sensing. Chemosensors 2022, 10, 339. [Google Scholar] [CrossRef]
- Khadem, M.; Faridbod, F.; Norouzi, P.; Foroushani, A.R.; Ganjali, M.R.; Yarahmadi, R.; Shahtaheri, S.J. Voltammetric Determination of Carbofuran Pesticide in Biological and Environmental Samples Using a Molecularly Imprinted Polymer Sensor, a Multivariate Optimization. J. Anal. Chem. 2020, 75, 669–678. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Luo, J.; Xu, Z.; Ma, X. A Microfluidic Chip Containing a Molecularly Imprinted Polymer and a DNA Aptamer for Voltammetric Determination of Carbofuran. Microchim. Acta 2018, 185, 295. [Google Scholar] [CrossRef]
- Amatatongchai, M.; Sroysee, W.; Jarujamrus, P.; Nacapricha, D.; Lieberzeit, P.A. Selective Amperometric Flow-Injection Analysis of Carbofuran Using a Molecularly-Imprinted Polymer and Gold-Coated-Magnetite Modified Carbon Nanotube-Paste Electrode. Talanta 2018, 179, 700–709. [Google Scholar] [CrossRef]
- Chen, S.; Fu, J.; Fu, Z.; Li, Y.; Su, X.; Zou, L.; He, L.; Liu, S.; Ao, X.; Yang, Y. Preparation and Characterization of Magnetic Molecular Imprinted Polymers with Ionic Liquid for the Extraction of Carbaryl in Food. Anal. Bioanal. Chem. 2020, 412, 1049–1062. [Google Scholar] [CrossRef]
- Lian, W.; Zhang, X.; Han, Y.; Li, X.; Liu, H. A Molecularly Imprinted Electrochemical Sensor for Carbendazim Detection Based on Synergy Amplified Effect of Bioelectrocatalysis and Nanocomposites. Polymers 2025, 17, 92. [Google Scholar] [CrossRef]
- Maia Júnior, F.F.; Sales Junior, R.; Barbosa, G.F.; Hussain, S.; Jara-Cornejo, E.; Khan, S. Design and Fabrication of a Biomimetic Smart Material for Electrochemical Detection of Carbendazim Pesticides in Real Samples with Enhanced Selectivity. Biosensors 2024, 14, 304. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Ren, H.; Li, X.; Chen, S.; Ye, B.-C. A Novel Electrochemical Sensor Based on Molecularly Imprinted Polymer-Modified C-ZIF67@Ni for Highly Sensitive and Selective Determination of Carbendazim. Talanta 2022, 237, 122909. [Google Scholar] [CrossRef]
- Beigmoradi, F.; Rohani Moghadam, M.; Bazmandegan-Shamili, A.; Masoodi, H.R. Electrochemical Sensor Based on Molecularly Imprinted Polymer Coating on Metal–Organic Frameworks for the Selective and Sensitive Determination of Carbendazim. Microchem. J. 2022, 179, 107633. [Google Scholar] [CrossRef]
- Feng, S.; Li, Y.; Zhang, R.; Li, Y. A Novel Electrochemical Sensor Based on Molecularly Imprinted Polymer Modified Hollow N, S-Mo2C/C Spheres for Highly Sensitive and Selective Carbendazim Determination. Biosens. Bioelectron. 2019, 142, 111491. [Google Scholar] [CrossRef]
- Fu, K.; Sun, H.; Chen, X.; Liu, L.; Cao, Y.; Zhao, J.; Li, S.; Ma, W. Computer-Aided Design and Preparation of Surface Arsenite Molecularly Imprinted Polymers for Selective Adsorption and Highly Sensitive Detection of As(Ⅲ). J. Hazard. Mater. 2024, 480, 136386. [Google Scholar] [CrossRef]
- Wu, S.; Li, K.; Dai, X.; Zhang, Z.; Ding, F.; Li, S. An Ultrasensitive Electrochemical Platform Based on Imprinted Chitosan/Gold Nanoparticles/Graphene Nanocomposite for Sensing Cadmium (II) Ions. Microchem. J. 2020, 155, 104710. [Google Scholar] [CrossRef]
- Abdallah, A.B.; El-kholany, M.R.; Molouk, A.F.S.; Awad Ali, T.; El-Shafei, A.A.; Khalifa, M.E. Selective and Sensitive Electrochemical Sensors Based on an Ion Imprinting Polymer and Graphene Oxide for the Detection of Ultra-Trace Cd(Ii) in Biological Samples. RSC Adv. 2021, 11, 30771–30780. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, L.; Wei, G.; Zhang, Y.; Zeng, Y. Highly Sensitive and Doubly Orientated Selective Molecularly Imprinted Electrochemical Sensor for Cu2+. Biosens. Bioelectron. 2015, 69, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Topcu, C.; Yilmaz, R.R.; Atasoy, B.H.; Ocsoy, I.; Yilmaz, V. A Novel Composite Electrochemical Sensor Doped Ion-Imprinted Polymer Based on N-Methacryloyl-L-Histidine/Ethylene Glycol Dimethacrylate for Ultrasensitive Determination of Copper (II) Ions in Food Supplements. Chem. Pap. 2024, 78, 8245–8260. [Google Scholar] [CrossRef]
- Aravind, A.; Mathew, B. Electrochemical Sensor Based on Nanostructured Ion Imprinted Polymer for the Sensing and Extraction of Cr(III) Ions from Industrial Wastewater. Polym. Int. 2018, 67, 1595–1604. [Google Scholar] [CrossRef]
- Wu, S.; Dai, X.; Cheng, T.; Li, S. Highly Sensitive and Selective Ion-Imprinted Polymers Based on One-Step Electrodeposition of Chitosan-Graphene Nanocomposites for the Determination of Cr(VI). Carbohydr. Polym. 2018, 195, 199–206. [Google Scholar] [CrossRef]
- Ghanei-Motlagh, M.; Taher, M.A.; Heydari, A.; Ghanei-Motlagh, R.; Gupta, V.K. A Novel Voltammetric Sensor for Sensitive Detection of Mercury(II) Ions Using Glassy Carbon Electrode Modified with Graphene-Based Ion Imprinted Polymer. Mater. Sci. Eng. C 2016, 63, 367–375. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Rahmani, A.R.; Shokoohi, R.; Farmany, A.; Khazaei, M. A Highly Sensitive and Selective Electrochemical Mercury(II) Sensor Based on Nanoparticles of Hg(II)-Imprinted Polymer and Graphitic Carbon Nitride (g-C3N4). Int. J. Electrochem. Sci. 2019, 14, 6420–6430. [Google Scholar] [CrossRef]
- Tarley, C.R.T.; Basaglia, A.M.; Segatelli, M.G.; Prete, M.C.; Suquila, F.A.C.; de Oliveira, L.L.G. Preparation and Application of Nanocomposite Based on Imprinted Poly(Methacrylic Acid)-PAN/MWCNT as a New Electrochemical Selective Sensing Platform of Pb2+ in Water Samples. J. Electroanal. Chem. 2017, 801, 114–121. [Google Scholar] [CrossRef]
- Bojdi, M.K.; Mashhadizadeh, M.H.; Behbahani, M.; Farahani, A.; Davarani, S.S.H.; Bagheri, A. Synthesis, Characterization and Application of Novel Lead Imprinted Polymer Nanoparticles as a High Selective Electrochemical Sensor for Ultra-Trace Determination of Lead Ions in Complex Matrixes. Electrochim. Acta 2014, 136, 59–65. [Google Scholar] [CrossRef]
- Ma, W.; Chang, Q.; Shi, X.; Tong, Y.; Zhou, L.; Ye, B.; Lu, J.; Zhao, J. Novel Electrochemical Sensor Based on Integration of Nanoporous Gold with Molecularly Imprinted Polymer for Detection of Arsenic Ion (III). J. Electrochem. 2020, 26, 8. [Google Scholar]
- Ma, W.; Chang, Q.; Zhao, J.; Ye, B.-C. Novel Electrochemical Sensing Platform Based on Ion Imprinted Polymer with Nanoporous Gold for Ultrasensitive and Selective Determination of As3+. Microchim. Acta 2020, 187, 571. [Google Scholar] [CrossRef]
- Alizadeh, T.; Ganjali, M.R.; Nourozi, P.; Zare, M.; Hoseini, M. A Carbon Paste Electrode Impregnated with Cd2+ Imprinted Polymer as a New and High Selective Electrochemical Sensor for Determination of Ultra-Trace Cd2+ in Water Samples. J. Electroanal. Chem. 2011, 657, 98–106. [Google Scholar] [CrossRef]
- Hu, S.; Gao, G.; Liu, Y.; Hu, J.; Song, Y.; Zou, X. An Electrochemical Sensor Based on Ion Imprinted PPy/rGO Composite for Cd(II) Determination in Water. Int. J. Electrochem. Sci. 2019, 14, 10714–10730. [Google Scholar] [CrossRef]
- Wang, J.; Hu, J.; Hu, S.; Gao, G.; Song, Y. A Novel Electrochemical Sensor Based on Electropolymerized Ion Imprinted PoPD/ERGO Composite for Trace Cd(II) Determination in Water. Sensors 2020, 20, 1004. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Liang, Y. Application of Chitosan-N-Doped Graphene Oxide Ion-Imprinted Sensor in Cd (II) Ions Detection. Diam. Relat. Mater. 2021, 119, 108591. [Google Scholar] [CrossRef]
- Yin, F.; Zhou, D.; Mo, Y.; Liu, X.; Li, G.; Gao, J.; Yuan, M.; Wu, X.; Hao, L.; Xu, F. Core–Shell Mesoporous Silica-Based Ion-Imprinted Electrochemical Sensor for the Determination of Trace Cd2+ in Water. J. Appl. Electrochem. 2024, 54, 1125–1135. [Google Scholar] [CrossRef]
- Wei, P.; Zhu, Z.; Song, R.; Li, Z.; Chen, C. An Ion-Imprinted Sensor Based on Chitosan-Graphene Oxide Composite Polymer Modified Glassy Carbon Electrode for Environmental Sensing Application. Electrochim. Acta 2019, 317, 93–101. [Google Scholar] [CrossRef]
- Karimi, Z.; Shamsipur, M.; Besharati-Seidani, A. Electrochemical Determination of Trace Cu2+ in Water Samples by Using Carbon Ionic Liquid Electrode Modified with Molecularly Imprinted Polymers. Adv. Nanochem. 2021, 3, 49–55. [Google Scholar]
- Ma, Y.; Zhang, B.; Wei, S.; Xu, J.; Wang, J.; Li, T. Histidine Detection and Signal Amplification Based on Magnetic Molecularly Imprinted Particle and Cu2+ Coordination. Sens. Actuators B Chem. 2022, 358, 131487. [Google Scholar] [CrossRef]
- Di Masi, S.; Garcia Cruz, A.; Canfarotta, F.; Cowen, T.; Marote, P.; Malitesta, C.; Piletsky, S.A. Synthesis and Application of Ion-Imprinted Nanoparticles in Electrochemical Sensors for Copper (II) Determination. ChemNanoMat 2019, 5, 754–760. [Google Scholar] [CrossRef]
- Zhihua, W.; Xiaole, L.; Jianming, Y.; Yaxin, Q.; Xiaoquan, L. Copper(II) Determination by Using Carbon Paste Electrode Modified with Molecularly Imprinted Polymer. Electrochim. Acta 2011, 58, 750–756. [Google Scholar] [CrossRef]
- Costa, M.; Di Masi, S.; Garcia-Cruz, A.; Piletsky, S.A.; Malitesta, C. Disposable Electrochemical Sensor Based on Ion Imprinted Polymeric Receptor for Cd(II) Ion Monitoring in Waters. Sens. Actuators B Chem. 2023, 383, 133559. [Google Scholar] [CrossRef]
- Alizadeh, T.; Mirzaee, S.; Rafiei, F. All-Solid-State Cr(III)-Selective Potentiometric Sensor Based on Cr(III)-Imprinted Polymer Nanomaterial/MWCNTs/Carbon Nanocomposite Electrode. Int. J. Environ. Anal. Chem. 2017, 97, 1283–1297. [Google Scholar] [CrossRef]
- Bojdi, M.K.; Behbahani, M.; Feyzabadi, Z.B. Material Design of a Chromium Imprinted Polymer and Its Application as a Highly Selective Electrochemical Sensor for Determining Chromium Ion at Trace Levels. ChemistrySelect 2021, 6, 11939–11947. [Google Scholar] [CrossRef]
- Rajabi, H.R.; Roushani, M.; Shamsipur, M. Development of a Highly Selective Voltammetric Sensor for Nanomolar Detection of Mercury Ions Using Glassy Carbon Electrode Modified with a Novel Ion Imprinted Polymeric Nanobeads and Multi-Wall Carbon Nanotubes. J. Electroanal. Chem. 2013, 693, 16–22. [Google Scholar] [CrossRef]
- Yasinzai, M.; Mustafa, G.; Asghar, N.; Ullah, I.; Zahid, M.; Lieberzeit, P.A.; Han, D.; Latif, U. Ion-Imprinted Polymer-Based Receptors for Sensitive and Selective Detection of Mercury Ions in Aqueous Environment. J. Sens. 2018, 2018, 8972549. [Google Scholar] [CrossRef]
- Shirzadmehr, A.; Afkhami, A.; Madrakian, T. A New Nano-Composite Potentiometric Sensor Containing an Hg2+-Ion Imprinted Polymer for the Trace Determination of Mercury Ions in Different Matrices. J. Mol. Liq. 2015, 204, 227–235. [Google Scholar] [CrossRef]
- Soman, S.; P. V., A.; R., K. Covalently Modified Graphene Quantum Dot Using a Thiourea Based Imprinted Polymer for the Selective Electrochemical Sensing of Hg(II) Ions. J. Polym. Res. 2021, 28, 359. [Google Scholar] [CrossRef]
- Alizadeh, T.; Ganjali, M.R.; Zare, M. Application of an Hg2+ Selective Imprinted Polymer as a New Modifying Agent for the Preparation of a Novel Highly Selective and Sensitive Electrochemical Sensor for the Determination of Ultratrace Mercury Ions. Anal. Chim. Acta 2011, 689, 52–59. [Google Scholar] [CrossRef]
- Afkhami, A.; Madrakian, T.; Soltani-Shahrivar, M.; Ahmadi, M.; Ghaedi, H. Selective and Sensitive Electrochemical Determination of Trace Amounts of Mercury Ion in Some Real Samples Using an Ion Imprinted Polymer Nano-Modifier. J. Electrochem. Soc. 2015, 163, B68. [Google Scholar] [CrossRef]
- Alizadeh, T.; Hamidi, N.; Ganjali, M.R.; Rafiei, F. An Extraordinarily Sensitive Voltammetric Sensor with Picomolar Detection Limit for Pb2+ Determination Based on Carbon Paste Electrode Impregnated with Nano-Sized Imprinted Polymer and Multi-Walled Carbon Nanotubes. J. Environ. Chem. Eng. 2017, 5, 4327–4336. [Google Scholar] [CrossRef]
- Sebastian, M.; Mathew, B. Ion Imprinting Approach for the Fabrication of an Electrochemical Sensor and Sorbent for Lead Ions in Real Samples Using Modified Multiwalled Carbon Nanotubes. J. Mater. Sci. 2018, 53, 3557–3572. [Google Scholar] [CrossRef]
- Alizadeh, T.; Amjadi, S. Preparation of Nano-Sized Pb2+ Imprinted Polymer and Its Application as the Chemical Interface of an Electrochemical Sensor for Toxic Lead Determination in Different Real Samples. J. Hazard. Mater. 2011, 190, 451–459. [Google Scholar] [CrossRef]
- Luo, X.; Huang, W.; Shi, Q.; Xu, W.; Luan, Y.; Yang, Y.; Wang, H.; Yang, W. Electrochemical Sensor Based on Lead Ion-Imprinted Polymer Particles for Ultra-Trace Determination of Lead Ions in Different Real Samples. RSC Adv. 2017, 7, 16033–16040. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, Y.; Wang, C.; Sun, L.; Lu, X.; Lu, X. Preparation of Electrochemical Sensor for Lead(II) Based on Molecularly Imprinted Film. Int. Vac. Congr. IVC-18 2012, 258, 2017–2021. [Google Scholar] [CrossRef]
- Karagözlü, M.; Aşır, S.; Abu Shama, N.; Göktürk, I.; Yılmaz, F.; Türkmen, D.; Denizli, A.; Özgören, M. Development of Molecularly Imprinted Magnetic Amino Acid-Based Nanoparticles for Voltammetric Analysis of Lead Ions in Honey. Polymers 2024, 16, 1782. [Google Scholar] [CrossRef]
- Roushani, M.; Farokhi, S.; Rahmati, Z. Development of a Dual-Recognition Strategy for the Aflatoxin B1 Detection Based on a Hybrid of Aptamer-MIP Using a Cu2O NCs/GCE. Microchem. J. 2022, 178, 107328. [Google Scholar] [CrossRef]
- Singh, A.K.; Lakshmi, G.B.V.S.; Fernandes, M.; Sarkar, T.; Gulati, P.; Singh, R.P.; Solanki, P.R. A Simple Detection Platform Based on Molecularly Imprinted Polymer for AFB1 and FuB1 Mycotoxins. Microchem. J. 2021, 171, 106730. [Google Scholar] [CrossRef]
- Hu, X.; Liu, Y.; Xia, Y.; Zhao, F.; Zeng, B. A Novel Ratiometric Electrochemical Sensor for the Selective Detection of Citrinin Based on Molecularly Imprinted Poly(Thionine) on Ionic Liquid Decorated Boron and Nitrogen Co-Doped Hierarchical Porous Carbon. Food Chem. 2021, 363, 130385. [Google Scholar] [CrossRef]
- Jalalvand, A.R.; Farshadnia, M.; Jalili, F.; Jalili, C. A Novel, Intelligent and Computer-Assisted Electrochemical Sensor for Extraction and Simultaneous Determination of Patulin and Citrinin in Apple and Pear Fruit Samples. Chemom. Intell. Lab. Syst. 2024, 252, 105188. [Google Scholar] [CrossRef]
- Radi, A.-E.; Eissa, A.; Wahdan, T. Impedimetric Sensor for Deoxynivalenol Based on Electropolymerised Molecularly Imprinted Polymer on the Surface of Screen-Printed Gold Electrode. Int. J. Environ. Anal. Chem. 2021, 101, 2586–2597. [Google Scholar] [CrossRef]
- Nie, D.; Zhu, X.; Liu, M.; Cheng, M.; Fan, K.; Zhao, Z.; Huang, Q.; Zhang, X.; Han, Z. Molecularly Imprinted Polymer-Based Electrochemical Sensor for Rapid Detection of Masked Deoxynivalenol with Mn-Doped CeO2 Nanozyme as Signal Amplifier. J. Hazard. Mater. 2024, 477, 135366. [Google Scholar] [CrossRef]
- Yola, M.L.; Gupta, V.K.; Atar, N. New Molecular Imprinted Voltammetric Sensor for Determination of Ochratoxin A. Mater. Sci. Eng. C 2016, 61, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Liu, J.; Zhang, W.; Wang, H.; Huang, J.; Wang, G.; Hu, M.; Dong, H.; Sun, J.; Fang, M.; et al. Preparation of Dual Recognition Adsorbents Based on Molecularly Imprinted Polymers and Aptamer for Highly Sensitive Recognition and Enrichment of Ochratoxin A. J. Hazard. Mater. 2024, 476, 135112. [Google Scholar] [CrossRef]
- Sodkrathok, P.; Karuwan, C.; Kamsong, W.; Tuantranont, A.; Amatatongchai, M. Patulin-Imprinted Origami 3D-ePAD Based on Graphene Screen-Printed Electrode Modified with Mn–ZnS Quantum Dot Coated with a Molecularly Imprinted Polymer. Talanta 2023, 262, 124695. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xie, H.; Li, X.; Zhu, Y.; Huang, L.; Zhong, M.; Chen, L. Construction of a Self–Reporting Molecularly–Imprinted Electrochemical Sensor Based on CuHCF Modified by rGNR–rGO for the Detection of Zearalenone. Food Chem. 2024, 448, 139154. [Google Scholar] [CrossRef]
- Guo, K. Design and Fabrication of a Molecularly Imprinted Electrochemical Sensor with High Sensitivity for Zearalenone Assessment in Maize. Int. J. Electrochem. Sci. 2024, 19, 100612. [Google Scholar] [CrossRef]
- Jiang, M.; Braiek, M.; Florea, A.; Chrouda, A.; Farre, C.; Bonhomme, A.; Bessueille, F.; Vocanson, F.; Zhang, A.; Jaffrezic-Renault, N. Aflatoxin B1 Detection Using a Highly-Sensitive Molecularly-Imprinted Electrochemical Sensor Based on an Electropolymerized Metal Organic Framework. Toxins 2015, 7, 3540–3553. [Google Scholar] [CrossRef]
- Wood, M.; Mugo, S.M. A MIP-Enabled Stainless-Steel Hypodermic Needle Sensor for Electrochemical Detection of Aflatoxin B1. Anal. Methods 2022, 14, 2063–2071. [Google Scholar] [CrossRef]
- Hernández-García, F.; Cruz-Navarro, J.A.; García-Serrano, J.; Franco-Guzmán, M.; Islas, G.; Alvarez-Romero, G.A. Development of a Voltammetric Methodology Based on a Methacrylic Molecularly Imprinted Polymer-Modified Carbon-Paste Electrode for the Determination of Aflatoxin B1. Separations 2024, 11, 246. [Google Scholar] [CrossRef]
- Chen, L.; Mamat, X.; Aisa, H.A. Determination of Aflatoxin B1 by Biomass Derived Porous Carbon Modified Electrode with Molecularly Imprinted Polymer. Electroanalysis 2023, 35, e202200371. [Google Scholar] [CrossRef]
- Atar, N.; Yola, M.L.; Eren, T. Sensitive Determination of Citrinin Based on Molecular Imprinted Electrochemical Sensor. Appl. Surf. Sci. 2016, 362, 315–322. [Google Scholar] [CrossRef]
- Huang, H.; Ouyang, W.; Feng, K.; Camarada, M.B.; Liao, T.; Tang, X.; Liu, R.; Hou, D.; Liao, X. Rational Design of Molecularly Imprinted Electrochemical Sensor Based on Nb2C-MWCNTs Heterostructures for Highly Sensitive and Selective Detection of Ochratoxin a. Food Chem. 2024, 456, 140007. [Google Scholar] [CrossRef] [PubMed]
- Akyıldırım, O.; Kardaş, F.; Beytur, M.; Yüksek, H.; Atar, N.; Yola, M.L. Palladium Nanoparticles Functionalized Graphene Quantum Dots with Molecularly Imprinted Polymer for Electrochemical Analysis of Citrinin. J. Mol. Liq. 2017, 243, 677–681. [Google Scholar] [CrossRef]
- Li, W.; Diao, K.; Qiu, D.; Zeng, Y.; Tang, K.; Zhu, Y.; Sheng, Y.; Wen, Y.; Li, M. A Highly-Sensitive and Selective Antibody-like Sensor Based on Molecularly Imprinted Poly(L-Arginine) on COOH-MWCNTs for Electrochemical Recognition and Detection of Deoxynivalenol. Food Chem. 2021, 350, 129229. [Google Scholar] [CrossRef]
- Pacheco, J.G.; Castro, M.; Machado, S.; Barroso, M.F.; Nouws, H.P.A.; Delerue-Matos, C. Molecularly Imprinted Electrochemical Sensor for Ochratoxin A Detection in Food Samples. Sens. Actuators B Chem. 2015, 215, 107–112. [Google Scholar] [CrossRef]
- Hu, X.; Xia, Y.; Liu, Y.; Chen, Y.; Zeng, B. An Effective Ratiometric Electrochemical Sensor for Highly Selective and Reproducible Detection of Ochratoxin A: Use of Magnetic Field Improved Molecularly Imprinted Polymer. Sens. Actuators B Chem. 2022, 359, 131582. [Google Scholar] [CrossRef]
- Mavioğlu Kaya, M.; Deveci, H.A.; Kaya, İ.; Atar, N.; Yola, M.L. The Electrochemical Detection of Ochratoxin A in Apple Juice via MnCO3 Nanostructures Incorporated into Carbon Fibers Containing a Molecularly Imprinting Polymer. Biosensors 2023, 13, 760. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Luo, Y.; Zhou, K.; Qiu, X.; Guo, H. A Novel Molecularly Imprinted Polymer Material for the Recognition of Ochratoxin A. J. Polym. Res. 2022, 29, 158. [Google Scholar] [CrossRef]
- Hatamluyi, B.; Rezayi, M.; Beheshti, H.R.; Boroushaki, M.T. Ultra-Sensitive Molecularly Imprinted Electrochemical Sensor for Patulin Detection Based on a Novel Assembling Strategy Using Au@Cu-MOF/N-GQDs. Sens. Actuators B Chem. 2020, 318, 128219. [Google Scholar] [CrossRef]
- Afzali, Z.; Mohadesi, A.; Ali Karimi, M.; Fathirad, F. A Highly Selective and Sensitive Electrochemical Sensor Based on Graphene Oxide and Molecularly Imprinted Polymer Magnetic Nanocomposite for Patulin Determination. Microchem. J. 2022, 177, 107215. [Google Scholar] [CrossRef]
- Guo, W.; Pi, F.; Zhang, H.; Sun, J.; Zhang, Y.; Sun, X. A Novel Molecularly Imprinted Electrochemical Sensor Modified with Carbon Dots, Chitosan, Gold Nanoparticles for the Determination of Patulin. Biosens. Bioelectron. 2017, 98, 299–304. [Google Scholar] [CrossRef]
- Selvam, S.P.; Kadam, A.N.; Maiyelvaganan, K.R.; Prakash, M.; Cho, S. Novel SeS2-Loaded Co MOF with Au@PANI Comprised Electroanalytical Molecularly Imprinted Polymer-Based Disposable Sensor for Patulin Mycotoxin. Biosens. Bioelectron. 2021, 187, 113302. [Google Scholar] [CrossRef]
- Hu, X.; Xia, Y.; Liu, Y.; Zhao, F.; Zeng, B. Determination of Patulin Using Dual-Dummy Templates Imprinted Electrochemical Sensor with PtPd Decorated N-Doped Porous Carbon for Amplification. Microchim. Acta 2021, 188, 148. [Google Scholar] [CrossRef]
- Rehman, N.Ç.; Özdemir, N.; Boyacıoğlu, H.; Yola, M.L. A Novel Molecularly Imprinted Electrochemical Sensor Based on Graphitic Carbon Nitride Nanosheets Decorated Bovine Serum albumin@MnO2 Nanocomposite for Zearalenone Detection. J. Food Compos. Anal. 2024, 125, 105857. [Google Scholar] [CrossRef]
- Radi, A.-E.; Eissa, A.; Wahdan, T. Molecularly Imprinted Impedimetric Sensor for Determination of Mycotoxin Zearalenone. Electroanalysis 2020, 32, 1788–1794. [Google Scholar] [CrossRef]
- Küçük, D.; Üner, G.; İpek, S.L.; Caglayan, M.O.; Üstündağ, Z. An Impedimetric Determination of Zearalenone on MIP-Modified Carboceramic Electrode. Toxicon 2024, 250, 108115. [Google Scholar] [CrossRef]
- Hu, X.; Tang, Y.; Xia, Y.; Liu, Y.; Zhao, F.; Zeng, B. Antifouling Ionic Liquid Doped Molecularly Imprinted Polymer-Based Ratiometric Electrochemical Sensor for Highly Stable and Selective Detection of Zearalenone. Anal. Chim. Acta 2022, 1210, 339884. [Google Scholar] [CrossRef]
- Zhou, B.; Xie, H.; Zhou, S.; Sheng, X.; Chen, L.; Zhong, M. Construction of AuNPs/Reduced Graphene Nanoribbons Co-Modified Molecularly Imprinted Electrochemical Sensor for the Detection of Zearalenone. Food Chem. 2023, 423, 136294. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, X.; Xie, H.; Li, Y.; Li, X.; Zhou, B. A Molecularly Imprinted Electrochemical Sensor Based on rGO@rGNR Modification for Zearalenone Determination. J. Food Compos. Anal. 2025, 141, 107361. [Google Scholar] [CrossRef]
- Metwally, M.G.; Shehab, O.R.; Ibrahim, H.; El Nashar, R.M. Electrochemical Detection of Bisphenol A in Plastic Bottled Drinking Waters and Soft Drinks Based on Molecularly Imprinted Polymer. J. Environ. Chem. Eng. 2022, 10, 107699. [Google Scholar] [CrossRef]
- Kaya, S.I.; Corman, M.E.; Uzun, L.; Ozkan, S.A. A Porous Molecularly Imprinted Electrochemical Sensor for Specific Determination of Bisphenol S from Human Serum and Bottled Water Samples in Femtomolar Level. Anal. Bioanal. Chem. 2022, 414, 2775–2785. [Google Scholar] [CrossRef]
- Wang, S.; Pan, M.; Liu, K.; Xie, X.; Yang, J.; Hong, L.; Wang, S. A SiO2@MIP Electrochemical Sensor Based on MWCNTs and AuNPs for Highly Sensitive and Selective Recognition and Detection of Dibutyl Phthalate. Food Chem. 2022, 381, 132225. [Google Scholar] [CrossRef]
- Yu, L.; Shen, Y.; Gao, P.; Zhang, Q.; Hu, X.; Xu, Q. A Novel Molecularly Imprinted Photoelectrochemical Aptasensor Based on Dual Recognition Mechanism for Ultratrace Detection of Plasticizer Dibutyl Phthalate. Chem. Eng. J. 2023, 472, 144925. [Google Scholar] [CrossRef]
- Lu, D.; Zhu, D.Z.; Gan, H.; Yao, Z.; Luo, J.; Yu, S.; Kurup, P. An Ultra-Sensitive Molecularly Imprinted Polymer (MIP) and Gold Nanostars (AuNS) Modified Voltammetric Sensor for Facile Detection of Perfluorooctance Sulfonate (PFOS) in Drinking Water. Sens. Actuators B Chem. 2022, 352, 131055. [Google Scholar] [CrossRef]
- Amin, N.; Chen, J.; He, Q.; Schwartz, J.S.; Wu, J.J. Ultra-Sensitive and Rapid Detection of Perfluorooctanesulfonic Acid by a Capacitive Molecularly-Imprinted-Polymer Sensor Integrated with AC Electrokinetic Acceleration. Sens. Actuators B Chem. 2024, 420, 136464. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, R.; Chen, F.; Jing, L.; Tian, Z.; Li, Z.; Wang, J.; Hou, C. Molecularly Imprinted MOFs-Driven Carbon Nanofiber for Sensitive Electrochemical Detection and Targeted Electro-Fenton Degradation of Perfluorooctanoic Acid. Sep. Purif. Technol. 2023, 310, 123257. [Google Scholar] [CrossRef]
- Hafeez, S.; Khanam, A.; Cao, H.; Chaplin, B.P.; Xu, W. Novel Conductive and Redox-Active Molecularly Imprinted Polymer for Direct Quantification of Perfluorooctanoic Acid. Environ. Sci. Technol. Lett. 2024, 11, 871–877. [Google Scholar] [CrossRef]
- Han, E.; Pan, Y.; Li, L.; Cai, J. Bisphenol A Detection Based on Nano Gold-Doped Molecular Imprinting Electrochemical Sensor with Enhanced Sensitivity. Food Chem. 2023, 426, 136608. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, X.; Lin, Q.; He, X.; Xing, X.; Huai, H.; Lian, W.; Zhu, H. Electrochemical Sensor Based on Imprinted Sol–Gel and Nanomaterials for Sensitive Determination of Bisphenol A. Food Control 2011, 22, 786–791. [Google Scholar] [CrossRef]
- Deveci, H.A.; Mavioğlu Kaya, M.; Kaya, İ.; Bankoğlu Yola, B.; Atar, N.; Yola, M.L. Bisphenol A Imprinted Electrochemical Sensor Based on Graphene Quantum Dots with Boron Functionalized G-C3N4 in Food Samples. Biosensors 2023, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Kaassamani, R.A.; Sawan, S.; Jaffrezic-Renault, N.; Maalouf, R. A Novel Electrochemical Sensor Based on Iron Oxide Nanoparticles Coated with Molecularly Imprinted Polymers for Bisphenol A Detection. Microchem. J. 2025, 208, 112632. [Google Scholar] [CrossRef]
- Lei, X.; Deng, Z.; Zeng, Y.; Huang, S.; Yang, Y.; Wang, H.; Guo, L.; Li, L. A Novel Composite of Conductive Metal Organic Framework and Molecularly Imprinted Poly (Ionic Liquid) for Highly Sensitive Electrochemical Detection of Bisphenol A. Sens. Actuators B Chem. 2021, 339, 129885. [Google Scholar] [CrossRef]
- Deng, P.; Xu, Z.; Kuang, Y. Electrochemical Determination of Bisphenol A in Plastic Bottled Drinking Water and Canned Beverages Using a Molecularly Imprinted Chitosan–Graphene Composite Film Modified Electrode. Food Chem. 2014, 157, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Xiao, W.W.; Wang, J.Y.; Xiong, X.H. Rapid Isolation and Determination of Bisphenol A in Complicated Matrices by Magnetic Molecularly Imprinted Electrochemical Sensing. Anal. Bioanal. Chem. 2021, 413, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Sheng, X.; Cao, J.; Xie, H.; Li, X.; Huang, L.; Yang, M.; Zhong, M.; Liu, Y.-N. A Novel Electrochemical Sensor Based on Dual-Functional MMIP-CuMOFs for Both Target Recognition and Signal Reporting and Its Application for Sensing Bisphenol A in Milk. Food Chem. 2024, 437, 137756. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.; Liu, S.; Lin, Q.; He, X.; Xing, X.; Lian, W. Electrochemical Sensor for Bisphenol A Detection Based on Molecularly Imprinted Polymers and Gold Nanoparticles. J. Appl. Electrochem. 2011, 41, 1323–1328. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Amini, M.; Rezaei, B. Molecularly Imprinted Electrochemical Aptasensor for the Attomolar Detection of Bisphenol A. Microchim. Acta 2018, 185, 265. [Google Scholar] [CrossRef]
- Dadkhah, S.; Ziaei, E.; Mehdinia, A.; Baradaran Kayyal, T.; Jabbari, A. A Glassy Carbon Electrode Modified with Amino-Functionalized Graphene Oxide and Molecularly Imprinted Polymer for Electrochemical Sensing of Bisphenol A. Microchim. Acta 2016, 183, 1933–1941. [Google Scholar] [CrossRef]
- Rebocho, S.; Cordas, C.M.; Viveiros, R.; Casimiro, T. Development of a Ferrocenyl-Based MIP in Supercritical Carbon Dioxide: Towards an Electrochemical Sensor for Bisphenol A. J. Supercrit. Fluids 2018, 135, 98–104. [Google Scholar] [CrossRef]
- Lamaoui, A.; María Palacios-Santander, J.; Amine, A.; Cubillana-Aguilera, L. Computational Approach and Ultrasound Probe–Assisted Synthesis of Magnetic Molecularly Imprinted Polymer for the Electrochemical Detection of Bisphenol A. Mater. Sci. Eng. B 2022, 277, 115568. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Su, Y.-L.; Wu, B.-H.; Cheng, S.-H. Reusable Electrochemical Sensor for Bisphenol A Based on Ionic Liquid Functionalized Conducting Polymer Platform. Talanta 2016, 147, 103–110. [Google Scholar] [CrossRef]
- Jalilian, R.; Ezzatzadeh, E.; Taheri, A. A Novel Self-Assembled Gold Nanoparticles-Molecularly Imprinted Modified Carbon Ionic Liquid Electrode with High Sensitivity and Selectivity for the Rapid Determination of Bisphenol A Leached from Plastic Containers. J. Environ. Chem. Eng. 2021, 9, 105513. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Athira, V.S.; Chithra Sekhar, V. Electrochemical Sensing and Nano Molar Level Detection of Bisphenol-A with Molecularly Imprinted Polymer Tailored on Multiwalled Carbon Nanotubes. Polymer 2018, 146, 312–320. [Google Scholar] [CrossRef]
- Zhao, W.-R.; Kang, T.-F.; Lu, L.-P.; Shen, F.-X.; Cheng, S.-Y. A Novel Electrochemical Sensor Based on Gold Nanoparticles and Molecularly Imprinted Polymer with Binary Functional Monomers for Sensitive Detection of Bisphenol A. J. Electroanal. Chem. 2017, 786, 102–111. [Google Scholar] [CrossRef]
- Mars, A.; Mejri, A.; Hamzaoui, A.H.; Elfil, H. Molecularly Imprinted Curcumin Nanoparticles Decorated Paper for Electrochemical and Fluorescence Dual-Mode Sensing of Bisphenol A. Microchim. Acta 2021, 188, 94. [Google Scholar] [CrossRef]
- Yılmaz, F.; Shama, N.A.; Aşır, S.; Çobanoğulları, H.; Yolaç, E.; Kiraz, A.; Göktürk, I.; Denizli, A.; Türkmen, D. Gold Nanoparticle-Modified Molecularly Imprinted Polymer-Coated Pencil Graphite Electrodes for Electrochemical Detection of Bisphenol A. ACS Omega 2025, 10, 740–753. [Google Scholar] [CrossRef]
- Bolat, G.; Yaman, Y.T.; Abaci, S. Molecularly Imprinted Electrochemical Impedance Sensor for Sensitive Dibutyl Phthalate (DBP) Determination. Sens. Actuators B Chem. 2019, 299, 127000. [Google Scholar] [CrossRef]
- Zhou, Q.; Guo, M.; Wu, S.; Fornara, D.; Sarkar, B.; Sun, L.; Wang, H. Electrochemical Sensor Based on Corncob Biochar Layer Supported Chitosan-MIPs for Determination of Dibutyl Phthalate (DBP). J. Electroanal. Chem. 2021, 897, 115549. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, L.; Cai, R.; Chen, H. A Sensitive and Selective Molecularly Imprinted Sensor Combined with Magnetic Molecularly Imprinted Solid Phase Extraction for Determination of Dibutyl Phthalate. Biosens. Bioelectron. 2013, 49, 367–373. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Li, L.; Duan, H.; Luo, C. Electrochemical Sensor Based on Magnetic Graphene Oxide@gold Nanoparticles-Molecular Imprinted Polymers for Determination of Dibutyl Phthalate. Talanta 2015, 131, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Karimian, N.; Stortini, A.M.; Moretto, L.M.; Costantino, C.; Bogialli, S.; Ugo, P. Electrochemosensor for Trace Analysis of Perfluorooctanesulfonate in Water Based on a Molecularly Imprinted Poly(o-Phenylenediamine) Polymer. ACS Sens. 2018, 3, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.; Cristofori, D.; Bottari, F.; Cattaruzza, E.; De Wael, K.; Moretto, L.M. Redesigning an Electrochemical MIP Sensor for PFOS: Practicalities and Pitfalls. Sensors 2019, 19, 4433. [Google Scholar] [CrossRef]
- Rezaei, M.; Ghanavati, M.; Mohammadi, N.; Khani, S.; Nasirimoghadam, S.; Smiley, E.; Basiryanmahabadi, A. A New Sensitive Layer Based on Clcinated Zn/Ti-MOF/Magnetic Molecularly Imprinted Polypyrrole: Application to Preconcentration and Electrochemical Determination of Perfluorooctane Sulfonic Acid by Magnetic Carbon Paste Electrode. Talanta 2024, 276, 126229. [Google Scholar] [CrossRef] [PubMed]
- Pierpaoli, M.; Szopińska, M.; Olejnik, A.; Ryl, J.; Fudala-Ksiażek, S.; Łuczkiewicz, A.; Bogdanowicz, R. Engineering Boron and Nitrogen Codoped Carbon Nanoarchitectures to Tailor Molecularly Imprinted Polymers for PFOS Determination. J. Hazard. Mater. 2023, 458, 131873. [Google Scholar] [CrossRef]
- Zhu, X.; Cheng, C.; Qin, X.; Wang, Y. β-Cyclodextrin Imprinted Film Embedded with Methylene Blue: A Host-Guest Sensitive Electrochemical Strategy for PFAS Detection. J. Hazard. Mater. 2025, 485, 136870. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, X.; Hao, J. Melamine-Imprinted Electrochemical Sensor of Graphene/Ionic Liquid Composites and Its Use for the Detection of Melamine in Dairy Products. ChemPhysMater 2022, 1, 195–202. [Google Scholar] [CrossRef]
- Limthin, D.; Leepheng, P.; Tunhoo, B.; Klamchuen, A.; Suramitr, S.; Thiwawong, T.; Phromyothin, D. Enhancement in Sensitivity and Selectivity of Electrochemical Technique with CuO/g-C3N4 Nanocomposite Combined with Molecularly Imprinted Polymer for Melamine Detection. Polymers 2024, 16, 1800. [Google Scholar] [CrossRef]
- Esmaeily, Z.; Madrakian, T.; Afkhami, A.; Ghoorchian, A.; Ghasemzadeh-Mohammadi, V. Electropolymerization as an Electrochemical Preconcentration Approach for the Determination of Melamine in Milk Samples. Electrochim. Acta 2021, 390, 138897. [Google Scholar] [CrossRef]
- Chen, S.; Du, D.; Huang, J.; Zhang, A.; Tu, H.; Zhang, A. Rational Design and Application of Molecularly Imprinted Sol–Gel Polymer for the Electrochemically Selective and Sensitive Determination of Sudan I. Talanta 2011, 84, 451–456. [Google Scholar] [CrossRef]
- Gao, Q.; Zang, Y.; Xie, J.; Chen, L.; Xu, J.; Huang, H.; Xue, H. Bifunctional Monomer Oligomers-Based Composite Molecularly Imprinted Membranes for the Electrochemical Monitoring of Sudan I. Analyst 2022, 147, 3764–3772. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, Z.; Chen, W.; Wang, E.; Li, Y. Preparation and Application of Malachite Green Molecularly Imprinted/Gold Nanoparticle Composite Film–Modified Glassy Carbon Electrode. Ionics 2019, 25, 1177–1185. [Google Scholar] [CrossRef]
- Fan, Y.; Zuo, Y.; Liu, J.; Wang, C.; Zhao, X.; Ma, J.; Wang, M. Fabrication of 3D CuFe2O4/Cu0 Hierarchical Nanostructures on Carbon Fiber Paper by Simple Hydrothermal Method for Efficient Detection of Malachite Green, Sunset Yellow and Tartrazine in Food Samples. Food Chem. 2024, 459, 140378. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Huang, J.; Zhai, H.; Shen, Z.; Han, J.; Yu, Y.; Fang, H.; Li, F.; Sun, X.; Guo, Y. Molecularly Imprinted Electrochemical Sensor Based on Multi-Walled Carbon Nanotubes for Specific Recognition and Determination of Chloramphenicol in Milk. Microchem. J. 2022, 182, 107887. [Google Scholar] [CrossRef]
- Geng, L.; Sun, J.; Liu, M.; Huang, J.; Dong, J.; Guo, Z.; Guo, Y.; Sun, X. Molecularly Imprinted Polymers-Aptamer Electrochemical Sensor Based on Dual Recognition Strategy for High Sensitivity Detection of Chloramphenicol. Food Chem. 2024, 437, 137933. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.R.; Tavares, A.P.M.; Sales, M.G.F. In-Situ Generated Molecularly Imprinted Material for Chloramphenicol Electrochemical Sensing in Waters down to the Nanomolar Level. Sens. Actuators B Chem. 2018, 256, 420–428. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).