Early Detection of Monilinia laxa in Nectarine (Prunus persica var. nectarina) Using Electronic Nose Technology: A Non-Destructive Diagnostic Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Volatile Compound Analysis
2.2.1. Volatile Extraction
2.2.2. Gas Chromatography/Mass Spectrometry (GC/MS) Analysis
2.3. E-Nose Analysis
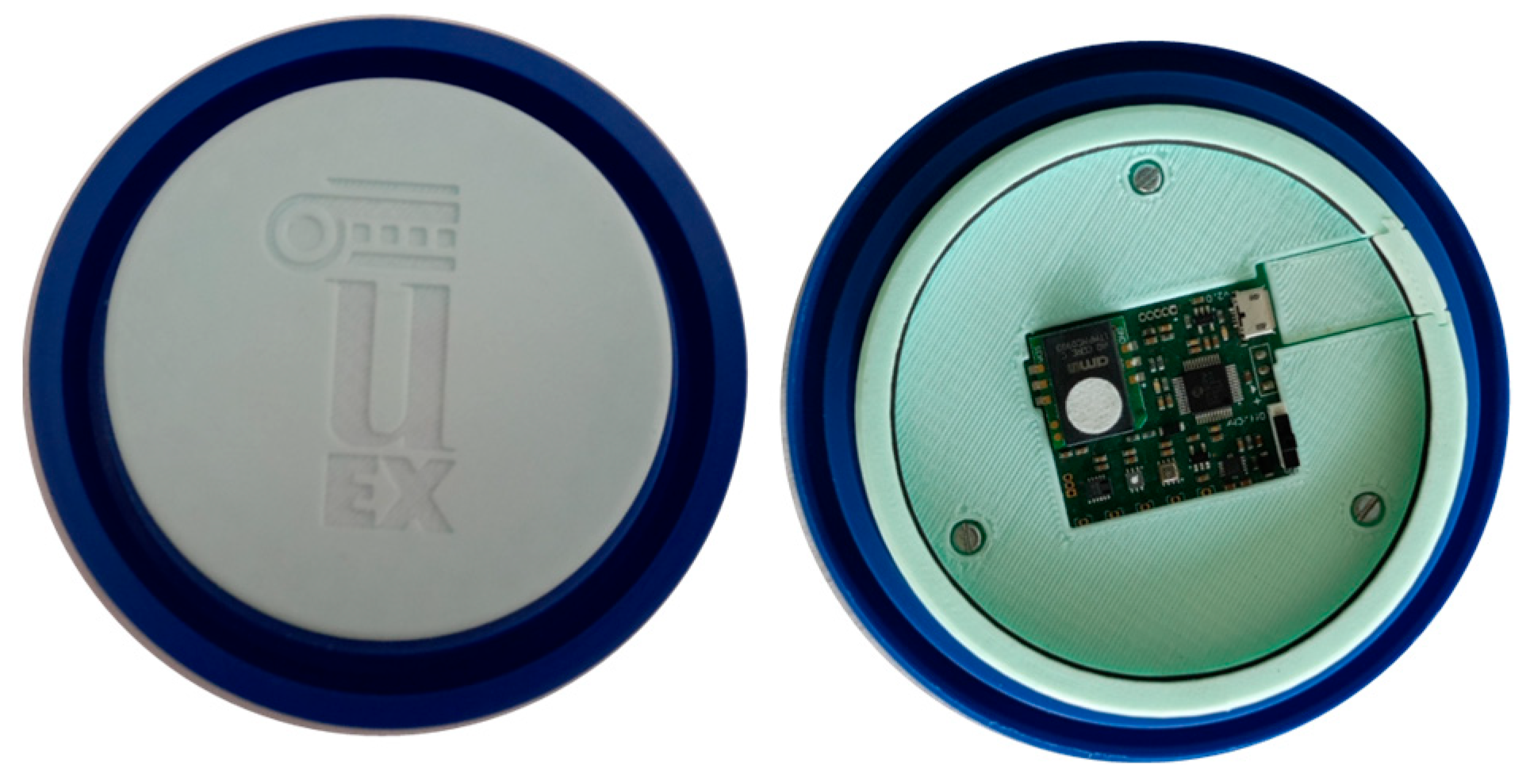
2.3.1. E-Nose System
2.3.2. Measurement Process and Data Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Volatile Organic Compounds
3.2. Relationship Between VOCs and Signals from E-Nose Sensors
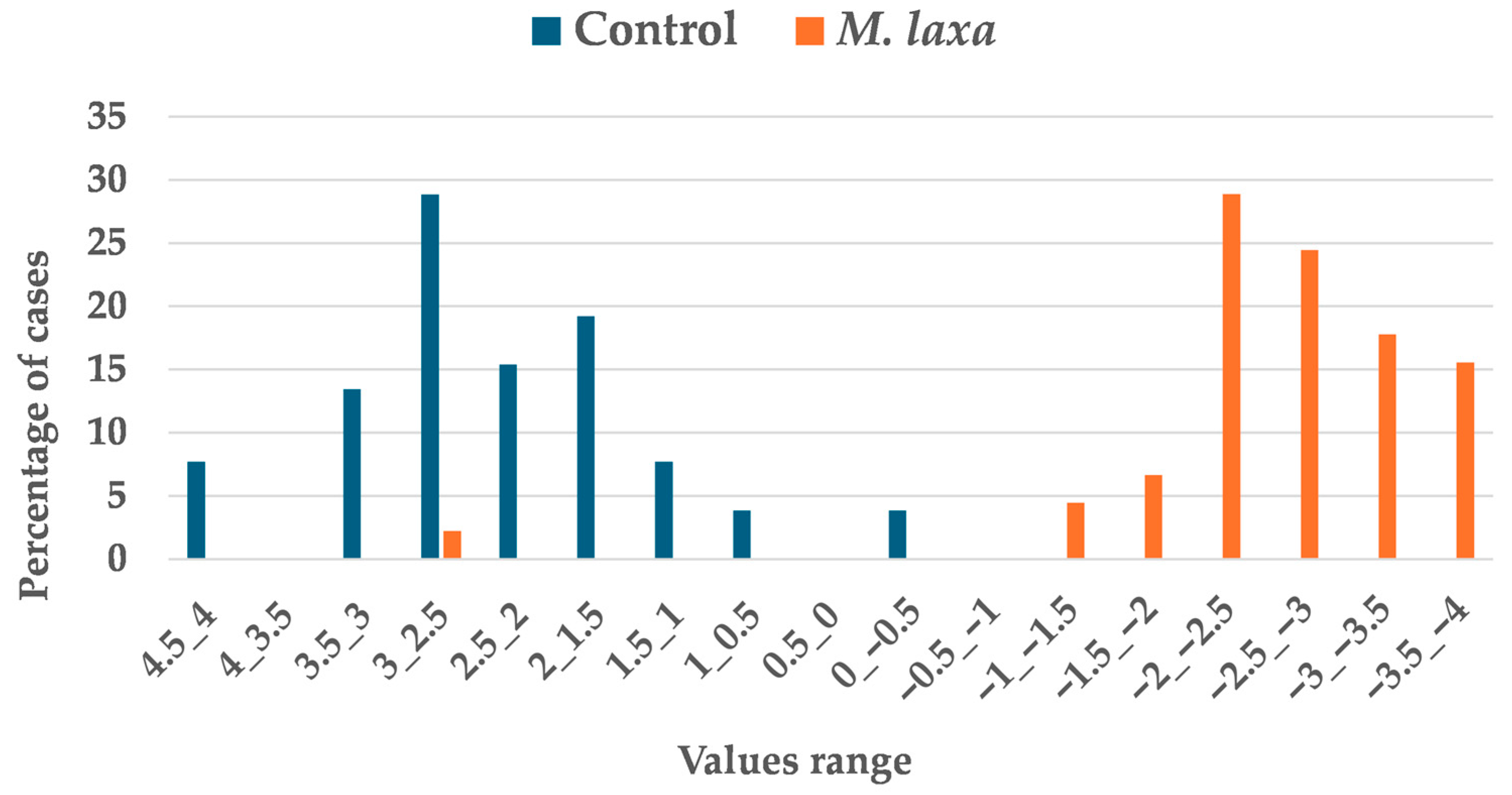
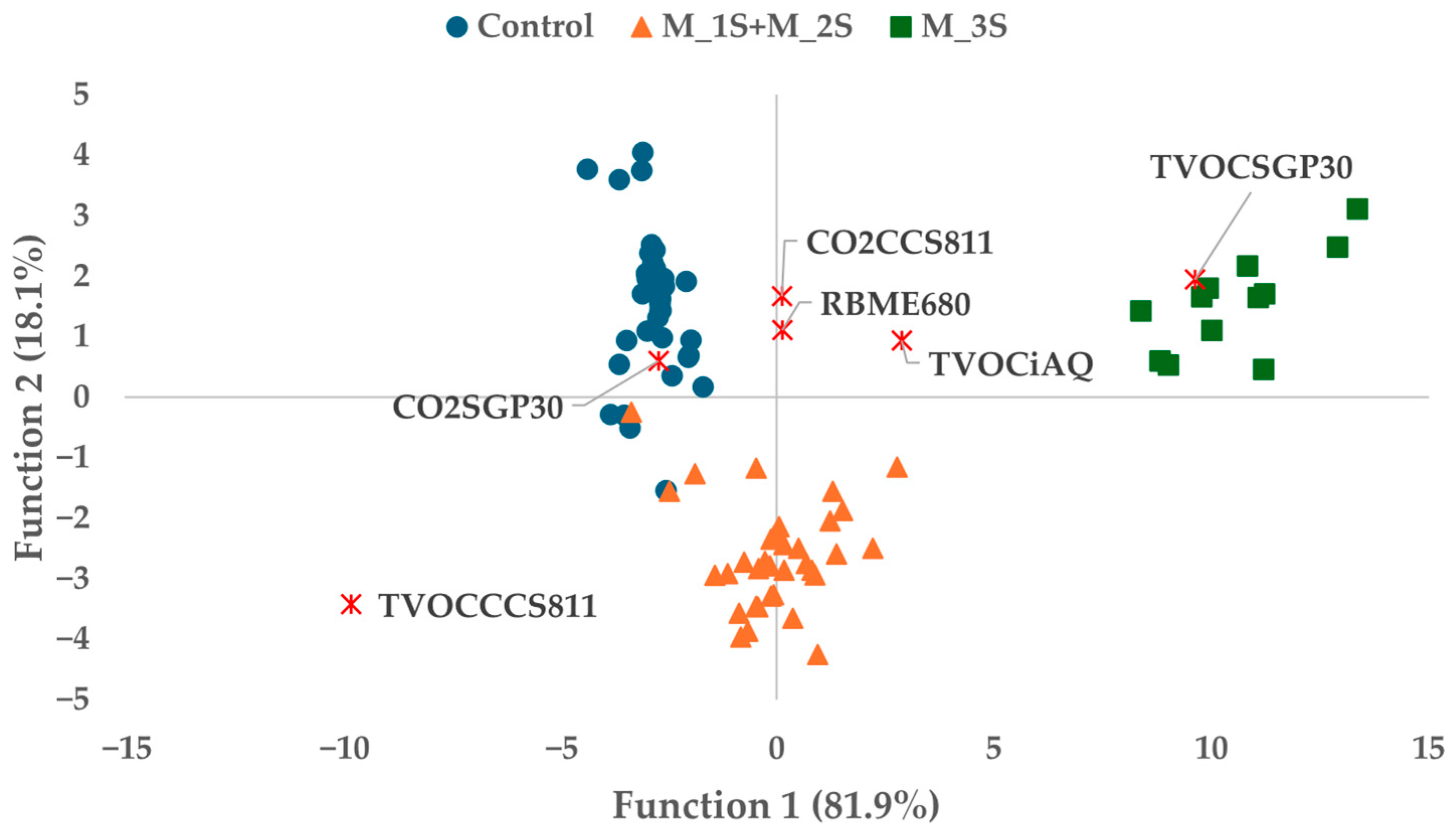
3.3. Determination of Incipient Fungal Decay of Nectarines by E-Nose During Postharvest Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC/MS | Gas Chromatography/Mass Spectrometry |
| LDA | Linear Discriminant Analysis |
| PCA | Principal Component Analysis |
| NIR | Near-Infrared Spectroscopy |
| HSI | Hyperspectral imaging |
| VOCs | Volatile organic compounds |
| PLSR | Partial Least Squares Regression |
| SVMs | Support Vector Machines |
| ANNs | Artificial Neural Networks |
| MOX | Metal Oxide Sensors |
| E-nose | Electronic nose |
| HCA | Hierarchical Cluster Analysis |
| HS-SPME | Headspace Solid-Phase Microextraction |
Appendix A
| Sensor | Manufacturer | Type | Measured Parameters (Signals) | Unit | Variable Name | Measurement Range |
|---|---|---|---|---|---|---|
| BME680 | Bosch Sensortec GmbH (Reutlingen, Germany) | MOX | Temperature, Pressure, Relative humidity, Gas resistance | °C, hPa, %RH, Ω | RBME680 | Temp: −40–85 °C; Pressure: 300–1100 hPa; Humidity: 0–100%RH |
| SGP30 | Sensirion AG (Stäfa, Switzerland) | MOX | Equivalent CO2 concentration, Total VOC, Hydrogen (resistive), Ethanol (resistive) | ppm, ppb, -,- | CO2SGP30, TVOCSGP30, H2SGP30, EtanolSGP30 | eCO2: 400–60,000 ppm; eTVOC: 0–60,000 ppb |
| CCS811 | ScioSense B.V. (Eindhoven, The Netherlands) | MOX | Equivalent CO2 concentration (eCO2), Equivalent total VOC (eTVOC), Sensor resistance | ppm, ppb, Ω | CO2CCS811, TVOCCCS811, ResohmCCS811 | eCO2: 400–29,206 ppm; eTVOC: 0–32,768 ppb |
| iAQ-Core | ScioSense B.V. (Eindhoven, The Netherlands) | MOX | Equivalent CO2 concentration, Total VOC, Sensor resistance | ppm, ppb, Ω | CO2iAQ, TVOCiAQ, RiAQCore | eCO2: 450–2000 ppm; eTVOC: 125–600 ppb |
References
- Larena, I.; Torres, R.; De Cal, A.; Liñán, M.; Melgarejo, P.; Domenichini, P.; Bellini, A.; Mandrin, J.F.; Lichou, J.; Ochoa de Eribe, X.; et al. Biological control of postharvest brown rot (Monilinia spp.) of peaches by field applications of Epicoccum nigrum. Biol. Control 2005, 32, 305–310. [Google Scholar] [CrossRef]
- Pellegrino, C.; Gullino, M.L.; Garibaldi, A.; Spadaro, D. First report of brown rot of stone fruit caused by Monilinia fructicola in Italy. Plant Dis. 2009, 93, 668. [Google Scholar] [CrossRef]
- Hilber-Bodmer, M.; Bünter, M.; Patocchi, A. First report of brown rot caused by Monilinia fructicola on apricot in a Swiss orchard. Plant Dis. 2010, 94, 643. [Google Scholar] [CrossRef]
- Ondejková, N.; Hudecová, M.; Bacigálová, K. First report on Monilinia fructicola in the Slovak Republic. Plant Prot. Sci. 2010, 46, 181. [Google Scholar] [CrossRef]
- Villarino, M.; Larena, I.; Martínez, F.; Melgarejo, P.; De Cal, A. Analysis of genetic diversity in Monilinia fructicola from the Ebro Valley in Spain using ISSR and RAPD markers. Eur. J. Plant Pathol. 2012, 132, 511–524. [Google Scholar] [CrossRef]
- Villarino, M.; Egüen, B.; Lamarca, N.; Segarra, J.; Usall, J.; Melgarejo, P.; De Cal, A. Occurrence of Monilinia laxa and M. fructigena after introduction of M. fructicola in peach orchards in Spain. Eur. J. Plant Pathol. 2013, 137, 835–845. [Google Scholar] [CrossRef]
- Rungjindamai, N.; Jeffries, P.; Xu, X.M. Epidemiology and management of brown rot on stone fruit caused by Monilinia laxa. Eur. J. Plant Pathol. 2014, 140, 1–17. [Google Scholar] [CrossRef]
- Rajkumar, P.; Wang, N.; EImasry, G.; Raghavan, G.S.V.; Gariepy, Y. Studies on banana fruit quality and maturity stages using hyperspectral imaging. J. Food Eng. 2012, 108, 194–200. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y. Electronic nose and its application in the food industry: A review. Eur. Food Res. Technol. 2024, 250, 21–67. [Google Scholar] [CrossRef]
- Chen, Q.; Song, J.; Bi, J.; Meng, X.; Wu, X. Characterization of volatile profile from ten different varieties of Chinese jujubes by HS-SPME/GC–MS coupled with E-nose. Food Res. Int. 2018, 105, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, R.; Martín-Tornero, E.; Lozano, J.; Boselli, E.; Arroyo, P.; Meléndez, F.; Martín-Vertedor, D. E-Nose discrimination of abnormal fermentations in Spanish-Style Green Olives. Molecules 2021, 26, 5353. [Google Scholar] [CrossRef]
- Chandra, A.; Raj, H.; Ranjan, H.; Kumar, A.; Kumari, P. Isolation, characterization and sporulation of fungi from decaying vegetables and fruits of local vegetable market in hazaribag india. Plant Arch. 2022, 22, 426–430. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, W.; Zhu, N.; Mao, S.; Tu, K. Early detection and classification of pathogenic fungal disease in post-harvest strawberry fruit by electronic nose and gas chromatography–mass spectrometry. Food Res. Int. 2014, 62, 162–168. [Google Scholar] [CrossRef]
- Haghbin, N.; Bakhshipour, A.; Mousanejad, S.; Zareiforoush, H. Monitoring Botrytis cinerea infection in kiwifruit using electronic nose and machine learning techniques. Food Bioproc. Technol. 2023, 16, 749–767. [Google Scholar] [CrossRef]
- Jia, W.; Liang, G.; Tian, H.; Sun, J.; Wan, C. Electronic nose-based technique for rapid detection and recognition of moldy apples. Sensors 2019, 19, 1526. [Google Scholar] [CrossRef]
- Gu, S.; Wang, Z.; Chen, W.; Wang, J. Early identification of Aspergillus spp. contamination in milled rice by E-nose combined with chemometrics. J. Sci. Food Agri. 2021, 101, 4220–4228. [Google Scholar] [CrossRef]
- Amari, A.; El Bari, N.; Bouchikhi, B. Electronic nose for anchovy freshness monitoring based on sensor array and pattern recognition methods: Principal components analysis, linear discriminant analysis and support vector machine. Int. J. Compu. 2014, 6, 61–67. [Google Scholar] [CrossRef]
- Martínez, A.; Hernández, A.; Arroyo, P.; Lozano, J.S.; de Guía Córdoba, M.; Martín, A. E-Nose Detection of Changes in Volatile Profile Associated with Early Decay of ‘Golden Delicious’ Apple by Penicillium expansum. Food Control 2024, 110907. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, J.; Gao, L. Discrimination and characterization of strawberry juice based on electronic nose and tongue: Comparison of different juice processing approaches by lda, plsr, rf, and svm. J. Agri. Food Chem. 2014, 62, 6426–6434. [Google Scholar] [CrossRef]
- Khorramifar, A.; Sharabiani, V.R.; Karami, H.; Kisalaei, A.; Lozano, J.; Rusinek, R.; Gancarz, M. Investigating changes in pH and soluble solids content of potato during the storage by electronic nose and Vis/NIR spectroscopy. Foods 2022, 11, 4077. [Google Scholar] [CrossRef]
- Sharabiani, V.R.; Khorramifar, A.; Karami, H.; Lozano, J.; Tabor, S.; Darvishi, Y.; Gancarz, M. Non-destructive test to detect adulteration of rice using gas sensors coupled with chemometrics methods. Int. Agrophys. 2023, 37, 235–244. [Google Scholar] [CrossRef]
- Cascos, G.; Lozano, J.; Montero-Fernández, I.; Marcía-Fuentes, J.A.; Aleman, R.S.; Ruiz-Canales, A.; Martín-Vertedor, D. Electronic nose and gas chromatograph devices for the evaluation of the sensory quality of green coffee beans. Foods 2023, 13, 87. [Google Scholar] [CrossRef]
- Mirzaee-Ghaleh, E.; Taheri-Garavand, A.; Ayari, F.; Lozano, J. Identification of fresh-chilled and frozen-thawed chicken meat and estimation of their shelf life using an E-nose machine coupled fuzzy KNN. Food Anal. Methods 2020, 13, 678–689. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Martín, A.; Villalobos, M.C.; Calle, A.; Serradilla, M.J.; Córdoba, M.G.; Hernández, A. Yeasts isolated from figs (Ficus carica L.) as biocontrol agents of postharvest fruit diseases. Food Microbiol. 2016, 57, 45–53. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Martín, A.; Ruiz-Moyano, S.; Hernández, A.; López-Corrales, M.; de Guía Córdoba, M. Physicochemical and sensorial characterisation of four sweet cherry cultivars grown in Jerte Valley (Spain). Food Chem. 2012, 133, 1551–1559. [Google Scholar] [CrossRef]
- Arroyo, P.; Meléndez, F.; Suárez, J.I.; Herrero, J.L.; Rodríguez, S.; Lozano, J. Electronic nose with digital gas sensors connected via Bluetooth to a smartphone for air quality measurements. Sensors 2020, 20, 786. [Google Scholar] [CrossRef] [PubMed]
- Aubert, C.; Günata, Z.; Ambid, C.; Baumes, R. Changes in physicochemical characteristics and volatile constituents of yellow-and white-fleshed nectarines during maturation and artificial ripening. J. Agri. Food Chem. 2003, 51, 3083–3091. [Google Scholar] [CrossRef]
- Muto, A.; Müller, C.T.; Bruno, L.; McGregor, L.; Ferrante, A.; Chiappetta, A.A.C.; Bitonti, M.B.; Rogers, H.J.; Spadafora, N.D. Fruit volatilome profiling through GC× GC-ToF-MS and gene expression analyses reveal differences amongst peach cultivars in their response to cold storage. Sci. Rep. 2020, 10, 18333. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Ma, R.; Yu, M. Comparison of aroma trait of the white-fleshed peach ‘hu jing mi lu’ and the yellow-fleshed peach ‘jin yuan’ based on odor activity value and odor characteristics. Horticulturae 2022, 8, 245. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Li, S.; Yang, L.; Wang, Y.; Zhao, J.; Jiang, Q. Volatile characteristics of 50 peaches and nectarines evaluated by HP–SPME with GC–MS. Food Chem. 2009, 116, 356–364. [Google Scholar] [CrossRef]
- Fanesi, B.; D’Ortenzio, A.L.; Kuhalskaya, A.; Nartea, A.; Fiorini, D.; Moumni, M.; Landi, L.; Lucci, P.; Romanazzi, G.; Pacetti, D. Identification of volatile organic compounds as markers to detect Monilinia fructicola infection in fresh peaches. Postharvest Biol. Technol. 2023, 206, 112581. [Google Scholar] [CrossRef]
- Visai, C.; Vanoli, M. Volatile compound production during growth and ripening of peaches and nectarines. Sci. Horti. 1997, 70, 15–24. [Google Scholar] [CrossRef]
- Balsells-Llauradó, M.; Echeverria, G.; Torres, R.; Vall-llaura, N.; Teixidó, N.; Usall, J. Emission of volatile organic compounds during nectarine-Monilinia laxa interaction and its relationship with fruit susceptibility to brown rot. Postharvest Biol. Technol. 2022, 192, 111997. [Google Scholar] [CrossRef]
- Engel, K.H.; Flath, R.A.; Buttery, R.G.; Mon, T.R.; Ramming, D.W.; Teranishi, R. Investigation of volatile constituents in nectarines. 1. Analytical and sensory characterization of aroma components in some nectarine cultivars. J. Agri. Food Chem. 1988, 36, 549–553. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, N.; Zhou, D.; Sun, Y.; Sun, K.; Pan, L.; Tu, K. Discrimination and growth tracking of fungi contamination in peaches using electronic nose. Food Chem. 2018, 262, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Jeleń, H.; Wasowicz, E. Volatile fungal metabolites and their relation to the spoilage of agricultural commodities. Food Rev. Int. 1998, 14, 391–426. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Bai, J.; Plotto, A.; Dea, S. Electronic noses and tongues: Applications for the food and pharmaceutical industries. Sensors 2011, 11, 4744–4766. [Google Scholar] [CrossRef]
- Li, Z.; Suslick, K.S. Portable optoelectronic nose for monitoring meat freshness. Acs Sens. 2016, 1, 1330–1335. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Lv, Z.; Zeng, Q.; Fu, X.; Chen, Q.; Luo, Z.; Luo, C.; Wang, D.; Zhang, W. Analysis of the volatile profiles of kiwifruits experiencing soft rot using E-nose and HS-SPME/GC–MS. LWT 2023, 173, 114405. [Google Scholar] [CrossRef]
- Huang, G.L.; Liu, T.T.; Mao, X.M.; Quan, X.Y.; Sui, S.Y.; Ma, J.J.; Sun, L.X.; Li, H.C.; Shao, Q.S.; Wang, Y.N. Insights into the volatile flavor and quality profiles of loquat (Eriobotrya japonica Lindl.) during shelf-life via HS-GC-IMS, E-nose, and E-tongue. Food Chem. X 2023, 20, 100886. [Google Scholar] [CrossRef]
- Djeziri, M.; Benmoussa, S.; Bendahan, M.; Seguin, J.L. Review on data-driven approaches for improving the selectivity of MOX-sensors. Microsyst. Technol. 2024, 30, 791–807. [Google Scholar] [CrossRef]
- Rezaee, Z.; Mohtasebi, S.S.; Firouz, M.S. An electronic nose system supported by machine learning techniques for rapid detection of aspergillus flavus in pistachio. J. Food Meas. Charact. 2024, 18, 5757–5765. [Google Scholar] [CrossRef]

| Mean 5 | p Values 7 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| RT 1 | CD 2 | Volatile Compounds | ID 3 | KI 4 | AAU | % | RSD 6 | Ps | Pi |
| Hydrocarbons | 3872 | 2.00 | |||||||
| 6.6 | v16 | 2,4-dimethylheptane | A | 834 | 156 | 0.08 | 160 | -- | |
| 8.4 | v18 | Ethylbenzene | C | 897 | 336 | 0.18 | 128 | --- | --- |
| 14.3 | v25 | 2,2,4,6,6-pentamethylheptane | C | 992 | 866 | 0.47 | 67 | --- | --- |
| 32.7 | v53 | Pentadecane | A | 1500 | 1473 | 0.79 | 71 | - | |
| 36.2 | v54 | Heptadecane | A | 1700 | 886 | 0.48 | 63 | + | |
| Alcohol | 14,729 | 7.93 | |||||||
| 1.9 | v2 | Propan-1-ol | A | 595 | 74 | 0.04 | 244 | -- | ++ |
| 2.9 | v6 | Pent-1-en-3-ol | B | 689 | 882 | 0.47 | 131 | +++ | |
| 3.8 | v10 | 2-methylbutan-1-ol | B | 731 | 4510 | 2.43 | 142 | +++ | |
| 4.6 | v11 | Pentan-1-ol | B | 762 | 9059 | 4.88 | 130 | +++ | |
| 21.1 | v35 | (Z)-non-3-en-1-ol | B | 1156 | 204 | 0.11 | 127 | +++ | |
| Aldehyde | 2461 | 1.33 | |||||||
| 2.6 | v5 | 3-methylbutanal | C | 658 | 86 | 0.05 | 201 | +++ | +++ |
| 5.7 | v14 | Hexanal | B | 803 | 834 | 0.45 | 310 | ||
| 19.1 | v32 | Nonanal | B | 1105 | 974 | 0.52 | 64 | -- | |
| 23 | v39 | Decanal | B | 1240 | 567 | 0.31 | 30 | -- | +++ |
| Ketone | 4241 | 2.28 | |||||||
| 3.1 | v7 | Pentan-3-one | B | 704 | 4241 | 2.28 | 89 | --- | +++ |
| Carboxilic acid | 76 | 0.04 | |||||||
| 27.7 | v45 | (4E)-3-methyl-4-decenoic acid | C | 1430 | 76 | 0.04 | 203 | -- | +++ |
| Ester | 117,912 | 63.5 | |||||||
| 1.7 | v1 | Methyl acetate | A | 522 | 1369 | 0.74 | 187 | -- | ++ |
| 2.1 | v3 | Ethyl acetate | A | 605 | 21,945 | 11.82 | 66 | +++ | --- |
| 3.4 | v8 | Propyl acetate | B | 715 | 996 | 0.54 | 105 | ||
| 3.6 | v9 | Methyl butanoate | B | 723 | 595 | 0.32 | 226 | +++ | +++ |
| 4.8 | v12 | 2-methylpropyl acetate | B | 769 | 4334 | 2.33 | 120 | ||
| 5 | v13 | Methyl 3-methylbutanoate | B | 777 | 412 | 0.22 | 166 | ||
| 5.8 | v15 | Ethyl butanoate | B | 807 | 3642 | 1.96 | 141 | +++ | +++ |
| 8.2 | v17 | Ethyl 3-methylbutanoate | B | 890 | 4481 | 2.41 | 76 | -- | +++ |
| 9.3 | v20 | 2-methylbutyl acetate | B | 913 | 2415 | 1.30 | 73 | --- | ++ |
| 10.4 | v21 | Ethyl pentanoate | B | 930 | 982 | 0.53 | 195 | +++ | +++ |
| 11.1 | v23 | Pentyl acetate | B | 941 | 9012 | 4.85 | 119 | - | +++ |
| 11.5 | v24 | Methyl hexanoate | B | 948 | 762 | 0.41 | 112 | +++ | |
| 14.9 | v27 | Ethyl hexanoate | B | 1002 | 5514 | 2.97 | 66 | --- | +++ |
| 15.2 | v28 | [(E)-hex-3-enyl] acetate | C | 1010 | 4876 | 2.63 | 99 | ||
| 15.5 | v29 | Hexyl acetate | B | 1017 | 2532 | 1.36 | 184 | -- | |
| 18.8 | v30 | Pentyl butanoate | B | 1098 | 896 | 0.48 | 131 | --- | +++ |
| 20 | v33 | Methyl octanoate | B | 1128 | 8440 | 4.54 | 100 | --- | +++ |
| 20.7 | v34 | Pentyl 3-methylbutanoate | C | 1146 | 2767 | 1.49 | 133 | + | +++ |
| 22.4 | v37 | Ethyl oct-7-enoate | C | 1190 | 1066 | 0.57 | 65 | -- | ++ |
| 22.7 | v38 | Ethyl octanoate | B | 1197 | 36,175 | 19.48 | 59 | --- | +++ |
| 24.1 | v40 | [(Z)-hex-3-enyl] 3-methylbutanoate | C | 1335 | 568 | 0.31 | 76 | --- | |
| 24.3 | v41 | Hexyl 3-methylbutanoate | C | 1343 | 142 | 0.08 | 100 | - | |
| 25.9 | v42 | Pentyl hexanoate | C | 1404 | 512 | 0.28 | 139 | --- | +++ |
| 26.1 | v43 | Propyl octanoate | C | 1407 | 84 | 0.05 | 177 | ++ | |
| 26.7 | v44 | Methyl dec-4-enoate | C | 1415 | 555 | 0.30 | 123 | +++ | |
| 27.9 | v46 | 2-methylpropyl octanoate | C | 1432 | 215 | 0.12 | 136 | --- | +++ |
| 29 | v47 | Ethyl (E)-dec-4-enoate | C | 1448 | 1214 | 0.65 | 78 | --- | +++ |
| 29.2 | v48 | Pentyl heptanoate | C | 1451 | 81 | 0.04 | 196 | -- | +++ |
| 31.1 | v49 | 3-methylbutyl octanoate | C | 1477 | 68 | 0.04 | 191 | ++ | |
| 31.2 | v50 | 2-methylbutyl octanoate | C | 1479 | 50 | 0.03 | 203 | ++ | |
| 32.3 | v52 | Pentyl octanoate | C | 1494 | 1211 | 0.65 | 135 | --- | +++ |
| Terpeniods | 25,617 | 13.79 | |||||||
| 19 | v31 | Linalool | B | 1103 | 25,469 | 13.71 | 98 | -- | |
| 22 | v36 | 2-methylisoborneol | C | 1179 | 148 | 0.08 | 254 | +++ | +++ |
| Other compunds | 16,800 | 9.13 | |||||||
| 2.2 | v4 | Unidentified compound | D | 616 | 7469 | 4.02 | 254 | --- | |
| 8.6 | v19 | 4-methylactone | B | 902 | 155 | 0.08 | 171 | -- | |
| 10.9 | v22 | Methyl (Z)-N-hydroxybenzenecarboximidate | C | 938 | 253 | 0.14 | 152 | - | |
| 14.4 | v26 | Pentylfuran | B | 994 | 7106 | 3.83 | 70 | +++ | |
| 31.8 | v51 | 2(3H)-furanone, 5-hexyldihydro | C | 1487 | 1972 | 1.06 | 70 | ||
| MOX 1,2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster | CD | VOCs | M_1 | M_4 | M_5 | M_8 | M_2 | M_3 | M_6 | M_7 | M_9 | M_10 | M_11 |
| 1 | V3 | Ethyl acetate | -- | -- | -- | -- | ++ | ++ | ++ | ++ | ++ | ++ | - |
| 3 | V16 | 2,4-dimethylheptane | - | - | - | - | + | - | |||||
| 3 | V18 | Ethylbenzene | + | + | |||||||||
| 3 | V19 | 4-methylactone | - | - | - | - | + | -- | |||||
| 3 | V22 | Methyl (Z)-N-hydroxybenzenecarboximidate | -- | -- | -- | -- | + | + | + | -- | |||
| 2 | V5 | 3-methylbutanal | + | + | + | + | -- | -- | - | -- | -- | -- | |
| 2 | V6 | Pent-1-en-3-ol | ++ | ++ | ++ | ++ | -- | -- | -- | -- | -- | -- | + |
| 2 | V7 | Pentan-3-one | ++ | ++ | ++ | ++ | -- | -- | -- | -- | -- | -- | ++ |
| 2 | V9 | Methyl butanoate | ++ | + | + | ++ | -- | -- | -- | -- | -- | -- | |
| 2 | V10 | 2-methylbutan-1-ol | ++ | ++ | ++ | ++ | -- | -- | -- | -- | -- | -- | |
| 2 | V11 | Pentan-1-ol | + | + | ++ | ++ | - | - | -- | - | -- | -- | |
| 2 | V12 | 2-methylpropyl acetate | + | + | - | - | - | - | |||||
| 2 | V15 | Ethyl butanoate | ++ | ++ | + | ++ | -- | -- | -- | -- | -- | -- | |
| 2 | V21 | Ethyl pentanoate | ++ | + | + | ++ | -- | -- | -- | -- | -- | -- | |
| 2 | V23 | Pentyl acetate | + | + | ++ | ++ | -- | - | - | + | |||
| 2 | V24 | Methyl hexanoate | ++ | ++ | ++ | ++ | -- | -- | -- | -- | -- | -- | |
| 2 | V26 | Pentylfuran | + | + | + | + | - | - | -- | - | - | - | |
| 2 | V30 | Pentyl butanoate | + | + | ++ | ++ | - | - | -- | - | -- | -- | |
| 2 | V33 | Methyl octanoate | + | + | ++ | ++ | - | - | -- | - | - | - | + |
| 2 | V34 | Pentyl 3-methylbutanoate | + | + | - | ||||||||
| 2 | V35 | (Z)-non-3-en-1-ol | ++ | ++ | ++ | ++ | -- | -- | -- | -- | -- | -- | + |
| 2 | V36 | 2-methylisoborneol | ++ | + | + | - | -- | - | -- | -- | -- | ||
| 2 | V42 | Pentyl hexanoate | + | + | + | -- | |||||||
| 2 | V44 | Methyl dec-4-enoate | ++ | ++ | ++ | ++ | -- | -- | -- | -- | -- | -- | + |
| 2 | V46 | 2-methylpropyl octanoate | + | + | ++ | ++ | -- | - | - | ||||
| 2 | V47 | Ethyl (E)-dec-4-enoate | + | ++ | ++ | ++ | -- | ++ | |||||
| 2 | V52 | Pentyl octanoate | + | + | ++ | ++ | -- | - | - | ||||
| 4 | V27 | Ethyl hexanoate | + | + | |||||||||
| 4 | V39 | Decanal | - | ||||||||||
| Correctly Classified Nectarine Counts | Total | ||
|---|---|---|---|
| Batches | Control | M. laxa | |
| Total | 52 | 46 | 98 |
| Computed classes | 51 | 45 | 96 |
| Predicted classes | 50 | 45 | 95 |
| Selected variable | CO2CCS811 | ||
| ResohmCCS811 | |||
| RBME680 | |||
| EtanolSGP30 | |||
| Correctly Classified Nectarine Counts | Total | |||
|---|---|---|---|---|
| Batches | Control | M_1S + M_2S | M_3S | |
| Total | 44 | 34 | 12 | 90 |
| Computed classes | 43 | 33 | 12 | 88 |
| Predicted classes | 43 | 31 | 12 | 86 |
| CO2CCS811 | ||||
| TVOCCCS811 | ||||
| Selected variable | TVOCSGP30 | |||
| TVOCiAQ | ||||
| RBME680 | ||||
| CO2SGP30 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, A.; Hernández, A.; Arroyo, P.; Lozano, J.; Martín, A.; Córdoba, M.d.G. Early Detection of Monilinia laxa in Nectarine (Prunus persica var. nectarina) Using Electronic Nose Technology: A Non-Destructive Diagnostic Approach. Chemosensors 2025, 13, 391. https://doi.org/10.3390/chemosensors13110391
Martínez A, Hernández A, Arroyo P, Lozano J, Martín A, Córdoba MdG. Early Detection of Monilinia laxa in Nectarine (Prunus persica var. nectarina) Using Electronic Nose Technology: A Non-Destructive Diagnostic Approach. Chemosensors. 2025; 13(11):391. https://doi.org/10.3390/chemosensors13110391
Chicago/Turabian StyleMartínez, Ana, Alejandro Hernández, Patricia Arroyo, Jesús Lozano, Alberto Martín, and María de Guía Córdoba. 2025. "Early Detection of Monilinia laxa in Nectarine (Prunus persica var. nectarina) Using Electronic Nose Technology: A Non-Destructive Diagnostic Approach" Chemosensors 13, no. 11: 391. https://doi.org/10.3390/chemosensors13110391
APA StyleMartínez, A., Hernández, A., Arroyo, P., Lozano, J., Martín, A., & Córdoba, M. d. G. (2025). Early Detection of Monilinia laxa in Nectarine (Prunus persica var. nectarina) Using Electronic Nose Technology: A Non-Destructive Diagnostic Approach. Chemosensors, 13(11), 391. https://doi.org/10.3390/chemosensors13110391







