Abstract
A major objective in recent years has been the use of membrane sensors for the purpose of monitoring and recognizing environmental pollutants in pharmaceuticals. Ketoprofen (KTP) is likely to be found in the environment, particularly in surface water bodies like rivers, because of its extensive use in medicine. The photodegradability of KTP and the prolonged exposure of river water to sunlight may facilitate its photodegradation. To measure KTP along with its main photo-degradation products, three membrane electrodes were fabricated using different plasticizers. Dioctyl phthalate (DOP), dibutyl sebacate (DBS), and o-nitrophenyloctyl ether (o-NPOE) membrane electrodes were constructed for the selective analysis of the investigated medication. The fabricated sensors were prepared using tetraoctyl ammonium chloride as an ion-pairing agent. A linear range of 1 × 10−5 M to 1 × 10−1 M was shown by the electrodes. The slopes (in mV/decade) for the DOP, DBS, and o-NPOE membranes were −58.80 ± 0.90, −57.90 ± 0.80, and −56.80 ± 1.10, respectively. All test parameters were refined to enhance electrochemical performance. The synthesized membranes were successfully utilized to accurately measure KTP amidst its primary photodegradants. The fabricated sensors were effectively utilized to measure KTP in river water samples without requiring pre-treatment processes.
1. Introduction
Ketoprofen (KTP) is a common nonsteroidal anti-inflammatory drug known for its pain-relieving, fever-reducing, and inflammation-fighting effects. KTP functions by suppressing the body’s synthesis of prostaglandin. Despite being effective, it is associated with several harmful effects, such as stomach ulcers, sudden kidney failure, breathing problems, and skin sensitivity to sunlight, which includes reactions after taking it by mouth and allergic responses after applying it to the skin [1].
Phototoxicity is not linked to the immune response; thus, anyone who takes the medicine could be harmed by UV radiation from the sun. On the other hand, photoallergic reactions are a type of hypersensitivity condition of the immune system, and the severity of the symptoms does not depend on the dose or the intensity of the UV radiation. Therefore, photoallergic reactions can come from more than only topical KTP formulations. They can also come from drinking water, where KTP is often found [2,3].
The breakdown of the light-sensitive KTP molecule creates free radicals that can harm human cells and attach to nearby skin proteins, leading to a skin reaction (photo-allergy) [4]. The photosensitivity reactions are linked to the benzophenone chromophore integrated into the KTP structure [5,6]. Besides the main compound, there are temporary reactive forms and final breakdown products created when KTP quickly breaks down under UV light. These products still have the benzophenone chromophore and cause the photo-sensitization seen in living organisms by triggering oxidative stress. The compounds at issue comprise 3-ethylbenzophenone (EBP) and 3-acetylbenzophenone (ABP), recognized as the principal photodegradates of KTP [7,8]. Figure 1 illustrates the structure of intact KTP and its two main photodegradation products.
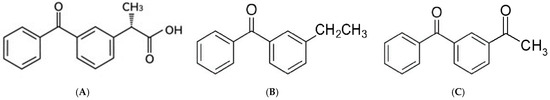
Figure 1.
Chemical structure of ketoprofen (A), 3-ethyl benzophenone (B), and 3-acetyl benzophenone (C).
The overconsumption and application of KTP have resulted in its frequent appearance in aquatic ecosystems. Prolonged exposure of surface water, such as rivers, to sunlight enhances the likelihood of KTP photo-degradation, given that KTP is a readily photo-degradable pharmaceutical. The coexistence of KTP, its photo-degradates, and transition free radicals poses a significant threat to both ecological systems and human health [3]. Therefore, it is very important to use sensitive and effective ways to keep an eye on the levels and concentrations of KTP in the aquatic environment. Likewise, these methods should be able to find KTP even when there are other compounds that are similar in structure, especially its main photo-degradation products, which are expected to be found in rivers and other surface waters.
The term “photo-stability of pharmaceuticals” describes how light affects the stability of a drug’s active components and/or finished product. Therefore, photo-stability evaluates how light affects the stability of drugs. Photo-sensitive pharmaceuticals can be influenced by both natural and artificial light sources. Sunlight can induce interactions between the medicine’s molecules and endogenous substances, converting the medication into hazardous by-products that may lead to the production of various oxygenated free radicals. A minimal amount of light exposure can significantly impact photosensitive medications; hence, considerable light exposure is not necessarily required. The generation of phototoxic compounds ultimately impacts the effectiveness of KTP as a photosensitive therapeutic agent. Photo-degradation concurrently reduces therapeutic efficacy [5].
Many methodologies, including gas chromatography (GC) [9], high-performance liquid chromatography (HPLC) [10,11,12,13], capillary electrophoresis [14], and UV-spectrophotometry [15], have assessed the extensive utilization of KTP. Liquid chromatography is the primary method employed for the stability—indicating assessment of KTP in various sample types [16,17,18,19].
Many of the methods employed for KTP determination are costly, time-intensive, and difficult to use for routine applications. These methods are not ideal for colourful or turbid solutions, require trained workers, and include sample manipulations, extraction, and derivatization processes [20]. In contrast, electrochemical methods—particularly potentiometric membrane sensors—offer straightforward, inexpensive alternatives to chemical analysis that are both safe and environmentally friendly [21].
Ion-selective electrodes (ISEs) are simple devices that use membranes to accurately measure how active ions are in a solution. Numerous ISEs have been utilized for pharmaceutical analysis. Choosing the right ion pairing agent (counter ion) and the best membrane matrix is very important when fabricating ISEs [22]. The determination of KTP was achieved through the use of ISEs [23,24], but the constructed electrodes were restricted to assessing the researched drug in its dosage form. The performance was not optimal, as indicated by the relatively high detection limits of 1 × 10−5 M [23] and 1.2 × 10−5 M [24]. Furthermore, the reported studies did not serve as a stability-indicating assay, as they did not quantify the studied drug in the presence of any of its degradation products. Table 1 is a comparative tool containing different techniques applied for the determination of the studied drug, declaring their performance characteristics.

Table 1.
A comparative table for different techniques applied for the determination of KTP in different samples.
This study developed solid-contact electrodes to measure KTP in the presence of its main photo-degradation products in river water using a tetraoctylammonium—ketoprofen complex. Solid-contact electrodes generally exhibit superior characteristics, including sensing performance, relative to conventional liquid contact ion-selective electrodes; this is due to their improved long-term stability and ease of miniaturization and handling. The storage and utilization of solid-contact electrodes are efficient and accessible. This sensor can remain airborne and does not require vertical storage. This device demonstrates mechanical resistance and operates as an autonomous sensor [25].
A comprehensive literature review indicated that there are presently no publications focusing on the potentiometric photo-stability investigation of the examined drug in the presence of its main photo-degradants in river water.
This study aims to fabricate, optimize, and validate solid-contact electrodes for quantifying KTP amidst its primary photo-degradants, as well as to apply the developed sensors for the accurate determination of KTP in river water.
2. Materials and Methods
2.1. Instrumentation
An IBM PC and an Electrochemistry EMF Interface system (Lawson Labs, Phoenixville Pike, Malvern, PA, USA) were used to measure the potential of the KTP electrode-reference electrode system at 22 (±1) °C. The salt bridge of the reference electrode (Orion 90-02) was filled with a 1 M lithium acetate solution. A Jenway pH meter (London, UK) was employed for the pH measurement throughout the investigation.
2.2. Chemicals, Reagents, and Standard Solutions
The pure materials KTP (CAS # 22071-15-4), EBP (CAS # 66067-43-4), and ABP (CAS # 66067-44-5) were purchased from Cayman Chemical in Ann Arbor, MI, USA. Their respective purities were 100.54%, 100.12%, and 100.74%. A collection of analytical-grade compounds was utilized. The chemicals used were tributyl phosphate (TBP) from Merck in Darmstadt, Germany, dioctyl phthalate (DOP), o-nitrophenyloctyl ether (o-NPOE) from Fluka (Darmstadt, Germany), dibutyl sebacate (DBS), high molecular weight poly(vinyl)chloride (PVC) from Aldrich (Boston, MA, USA), sodium hydroxide (NaOH), hydrochloric acid, 1-decanol, tetraoctyl ammonium chloride (TOA-Cl) from Fluka, and tetrahydrofuran (THF) from Sigma. Deionized water obtained from an Aquatron water still (London, UK) was used to prepare all aqueous solutions.
A stock solution of ketoprofen sodium salt with a concentration of 1.0 × 10−1 M was made by carefully mixing 2.5 g of KTP free acid with a sodium hydroxide solution until it became clear (pH 8.5). The final volume of the prepared solution was 100 mL. The working solutions within the concentration range of 1.0 × 10−2 to 1.0 × 10−8 M were prepared by diluting successive aliquots of the original solution of ketoprofen sodium salt at a concentration of 1.0 × 10−1 M with deionized water, which was alkalized with NaOH to a pH of 8.5.
2.3. Preparation of Ion Pair
The ion pair (KTP-TOA) was obtained by the periodic ion exchange extraction of the KTP anion from the aqueous phase to the organic phase. To make this complex, a mixture of 60% v/v TOA-Cl in 1-decanol was combined with a KTP solution that was 0.1 M. The volume of the organic and aqueous phase was 2 mL for each. Upon completion of the extraction, the organic phase was separated from the aqueous phase and subjected to deaeration until a transparent solution was obtained. The compound was preserved at +4 °C and subsequently utilized in membrane phase preparation.
2.4. Preparation of the Electrode
The electrode, about 10 cm in length, is constructed from an insulating material. The body embeds a cable to which an Ag/AgCl electrode was fused. The Teflon sensor is 1.5 cm in length, and it is affixed to the electrode body via a screw thread. The electrode’s membrane phase comprises two layers situated within a Teflon holder. The inner layer measures 13 mm in length and comprises plasticized PVC, within which the Ag/AgCl electrode is situated. The outer layer, in contact with the tested solutions, comprises the active material in addition to the components of the inner layer. The membrane phase measures 2 mm in length [23] (Scheme 1).
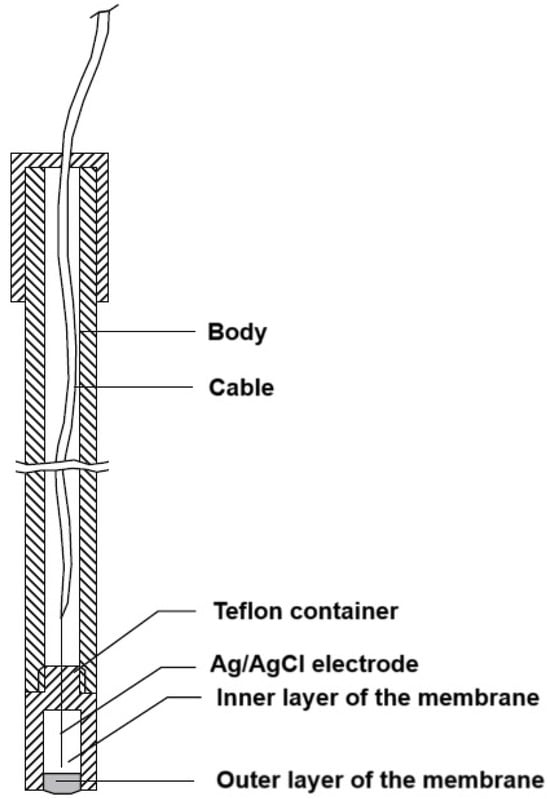
Scheme 1.
Diagrammatic representation of the fabricated KTP electrode.
The process of inner layer preparation comprises several steps. First, the components of the layer were weighed and mixed, after which the resulting mixture was deaerated. This mixture was then used to fill the Teflon sensor. Following this, the layer underwent a gelation process at 80 °C for 30 min [23]. The quantities of the components utilized in the production of KTP-fabricated electrodes are delineated in Table 2.

Table 2.
Composition of the fabricated membrane electrodes.
The outer layer membrane is prepared by weighing the layer components, dissolving the mixture in THF, positioning it in drops on the inner layer, and gelating the layer as a result of THF evaporation at a temperature of 20 °C [23]. The sensors that had been prepared were subsequently conditioned by immersing them in a 0.01 M KTP solution for 30 min.
2.5. Optimization and Calibration of the Fabricated Membranes
Following the conditioning stage, calibration was conducted by immersing the fabricated sensors in KTP standard solutions, in conjunction with a double junction Ag/AgCl electrode, across a concentration range of 1 × 10−1 to 1 × 10−8 M (pH 8.5). Deionized water was utilized to clean each membrane between measurements. The standard graphs have been drawn. The graphs were utilized to calculate the unknown drug concentrations.
Optimal membrane performance was attained through the assessment and modification of factors affecting membrane efficacy. The performance of the electrodes was evaluated following the guidelines established by IUPAC [26].
In order to assess the effect of the three plasticizers (DOP, o-NPOE, and DBS) on the membrane’s performance, three separate electrodes were built employing these materials. Identifying the plasticizer that enhanced the manufactured electrodes’ performance was the most important objective.
The effect of pH on the performance of the produced membranes was investigated by gradually adding 0.1 M sodium hydroxide or hydrochloric acid to a 1 × 10−3 M standard KTP solution. A graph was plotted to ascertain the ideal working pH by connecting the electrode potential with the pH of the solutions.
The prepared sensors were stored in the air at ambient temperatures for one year. The lifetimes were evaluated by assessing the slope of the produced electrodes every 7–14 days in freshly prepared ketoprofen solutions at pH 8.5. The accurate operational duration of the electrodes was established until they exhibited a 16% divergence from the Nernstian characteristic slope [23].
The rearranged Nicolsky equation, in its modified, separate solution form, was used to assess membrane selectivity [27]. The potentiometric selectivity coefficient (PSC) for each interfering substance was calculated by measuring how the electrodes reacted to different interferents, which included inorganic ions (Cl−, NO3−, Br−, benzoate, formate, and acetate) and similar non-steroidal anti-inflammatory drugs (ibuprofen and diclofenac sodium). The PSCs were subsequently calculated using the following formula:
Log KD,B pot = ((E1 − E2)/S) + (1 + (z1/z2)) log a
The potential measured in a solution containing 1 × 10−3 M of KTP is represented by E1. The potential obtained in a solution containing 1 × 10−3 M of the interfering chemical is shown by E2. KTP’s charge (z1) and the interference species’ charge (z2) are represented by these variables. How steep the electrode calibration plot is represented by S.
2.6. Application
2.6.1. Measuring KTP Among Its Primary Photo-Degradation Products
Different ratios of intact KTP (ranging from 90% to 10%) and its primary photo-degradants (ranging from 10% to 90%) were used to prepare laboratory-prepared combinations. The pH of the produced mixtures was adjusted to 8.5. After submerging the membranes in the mixture solutions, the potentials that emerged were used to calculate the KTP concentration, and then the recovery percent was calculated.
2.6.2. Measuring KTP in Various Samples of Water
Numerous samples of distilled and tap water have been prepared with exact amounts of KTP. The pH of the generated samples was modified to 8.5. The fabricated sensors were employed to quantify drug concentrations, and the drug recovery % was computed.
Three water samples from the River Nile in Cairo, Egypt, free of KTP, were collected from three separate locations. The samples’ pH was adjusted to 8.5. Nylon filters were employed to remove minute particle contamination from the samples. To prevent sample deterioration, the filtered solutions were stored in opaque glass vials. Quantities of pure KTP were added to KTP-free river water samples, yielding concentrations of 1 × 10−2 M, 1 × 10−3 M, and 1 × 10−4 M. The fabricated membranes were employed alongside standard curves to determine the concentrations of KTP, and the recovery percentage was calculated.
The fabricated electrodes were also applied to determine the KTP concentrations in real river water samples expected to contain KTP using the standard curves. A comparison was carried out between the results obtained by applying the proposed method and those obtained by using a reference HPLC method [10] after applying solid-phase extraction.
3. Results
3.1. Evaluation and Validation of the Fabricated Sensors
Table 3 lays out the evaluation parameters that demonstrate the effectiveness of the fabricated electrodes. A linear response was observed within the concentration range of 1 × 10−5 M to 1 × 10−1 M KTP. The slopes of the fabricated electrodes were −58.80 ± 0.90 mV/decade for the DOP electrode, −57.90 ± 0.80 mV/decade for the DBS electrode, and −56.80 ± 1.10 mV/decade for the o-NPOE electrode, as shown in Table 3. Figure 2 presents standard graphs.

Table 3.
The fabricated sensors’ electrochemical features.
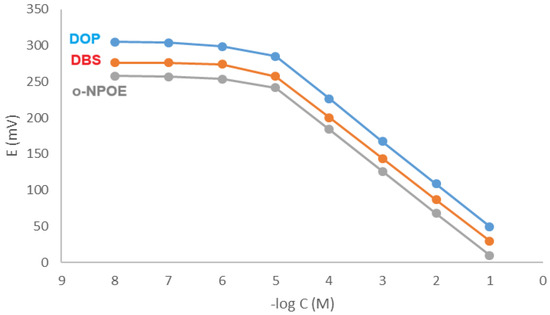
Figure 2.
Profile of the potential (in mV) versus −log concentration (in M) for KTP electrodes at pH 8.5.
The response time, optimal pH range, lifespan, and detection limit of the produced membranes were evaluated over a 12-month period, in accordance with IUPAC standards [26].
Calibration graph parameters are slightly affected by plasticizers’ chemical and physical characteristics. The slopes of the fabricated electrodes are approximately Nernstian, measuring 55–60 mV/decade. The sensitivity of the fabricated sensors was evaluated by calculating the detection limits for the fabricated sensors. The values of the detection limits are presented in Table 3. The suggested sensors have exceptional sensitivity, rendering them appropriate for application in environmental investigation.
The pH of the medium significantly influences the performance of the fabricated sensors. The fabricated electrodes demonstrated a nearly stable potential response across the pH ranges of 5.5 to 9.5 for the DOP membrane, 6.5 to 9.5 for the DBS membrane, and 6.5 to 9.0 for the o-NPOE membrane. Consequently, the sensors that have been developed can be reliably employed for measuring KTP within this pH range (Figure 3).
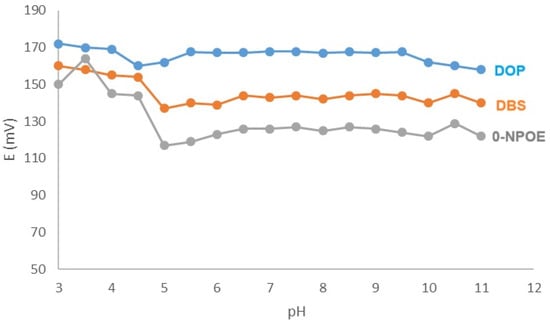
Figure 3.
Impact of pH on the membranes’ performance.
The estimated lifetimes of the membranes were 11 months for the DOP membrane and 10 months for both the DBS membrane and the o-NPOE membrane. The long lifetimes were obtained while the sensors were stored in air at room temperature. The estimates were derived by plotting the calibration graphs biweekly and consistently assessing their characteristics. The electrode was deemed functional until a deviation of 16% in its Nernstian slope was observed [23].
The response time is a critical factor that significantly impacts the membrane evaluation process. The information given shows how long it takes for the fabricated sensor to settle and keep a steady potential value (90% of the final stable potential) after a ten-fold increase in concentration [26]. The response times of the constructed sensors were 11 s for the DOP membrane, 15 s for the DBS membrane, and 18 s for the o-NPOE membrane.
The accuracy of the assay was evaluated by analyzing KTP samples with known specific concentrations using the manufactured sensors. The procedure of the analytical assay was evaluated for robustness through evaluation of its resistance to slight alterations in working conditions, including minor pH changes. The ruggedness was assessed to ascertain its reproducibility. Table 3 presents an overview of these factors.
The modified separate solution method [27] was utilized to assess the selectivity of the developed membranes. The objective was accomplished through the examination of various inorganic interferents, which included inorganic ions (Cl−, NO3−, Br−, benzoate, and formate) as well as structurally related non-steroidal anti-inflammatory drugs (ibuprofen and diclofenac sodium). The potentiometric selectivity coefficient (PSC) was subsequently computed for each interferent. Table 4 displays the calculated PSCs. The low values of the estimated PSCs may support the method’s high selectivity.

Table 4.
Membranes’ selectivity using the modified separate solution method.
3.2. Method Application
3.2.1. Measuring KTP Among Its Primary Photo-Degradants
The method’s selectivity was examined in the presence of up to 90% KTP major photo-degradants. The study focused on employing sensors to analyze various laboratory-prepared mixtures with KTP concentrations between 9 × 10−4 M and 1 × 10−4 M, alongside degradant concentrations ranging from 1 × 10−4 M to 9 × 10−4 M. Table 5 provides data demonstrating the specificity of the suggested method, and confirming its capacity for precisely determining the studied medication, even in the presence of up to 90% degradation products.

Table 5.
Analysis of laboratory-prepared mixtures for the studied drug and its primary photo-degradates.
3.2.2. Analysis of Different Water Samples
The sensors were utilized to quantify the quantity of KTP in both distilled and tap water samples containing the examined medication. Additionally, the sensors were employed to analyze various river water samples devoid of KTP and containing specified amounts of KTP. This was performed to assess the sensors’ efficacy in diverse water matrices. Table 6 presents the calculated recovery percentages.

Table 6.
Assay results of different river water samples free from KTP and loaded with definite concentrations of the studied drug.
On the other hand, three real river water samples were analyzed by the fabricated membranes. The results obtained were compared to those obtained using a reference HPLC method [10], after applying solid-phase extraction. The results are declared in Table 7.

Table 7.
Quantification of KTP in real river water samples.
4. Discussion
The extensive utilization of KTP in various human activities increases its likelihood of presence in the environment, particularly in surface waters like rivers. The exposure of river water to sunlight, along with the photolability of KTP, may increase its vulnerability to photodegradation. The development of membrane electrodes responsive to KTP is crucial for monitoring, evaluating, and quantifying KTP amid its primary photo-degradants. Potentiometric membrane sensors have been effectively employed in recent years owing to their cost-effectiveness, user-friendliness, high sensitivity, lack of sample pre-processing requirements, and ease of scalability [21].
This study explains how three membrane electrodes were fabricated to specifically sense and measure KTP in river water samples, even when its main photo-degradation products (EBP and ABP) are also present.
Different techniques, including LC-MS/MS and HPLC, were applied for the determination of the studied drug. These techniques can be considered as physical separation ones. Moreover, LC-MS/MS is characterized by its high sensitivity. On the other hand, they suffer from the complex multistep pre-treatment procedures. Also, they are costly techniques (purchase, maintenance, and infrastructure). Regarding the membrane-sensitive electrodes, they are a straightforward, environmentally friendly technology. It does not need any special pre-treatment procedures. The fabricated electrodes have acceptable sensitivity to be successfully applied for the analysis of real river water samples containing the studied drug, as demonstrated in Table 7.
For the KTP electrodes, more favourable analytical parameters were observed in those plasticized with DOP. The device exhibits the fastest response time of 11 s, a broad working pH range of 5.5 to 9.5, and a prolonged lifespan of 11 months.
The differences in membrane performance observed among the three different plasticizers may be attributed to the differences in structure and polarity that make differences in ion mobility within the membrane, which can enhance the sensor’s performance by facilitating the movement of charges needed for potentiometric measurements. However, the membrane performance is a specific relationship that depends on the overall membrane composition and the interaction between the plasticizer, the polymer matrix (PVC), and the ion-exchanger [28].
The detection limits of the manufactured membranes exhibit significant sensitivity in accordance with their intended application. They also demonstrated a rapid response time across a wide concentration range.
The effectiveness of the suggested method’s selectivity needs to be carefully checked to make sure it works well, even when there are different interfering substances in real samples. The three-dimensional structure of the ion exchanger’s receptor significantly influences the selectivity of the membrane. Plasticizers play a crucial role in membrane sensors by promoting the rapid movement of ions and ensuring that the resulting membrane exhibits desirable physical properties [28].
The fabricated electrodes were applied effectively and successfully for the quantification of KTP, even in the presence of up to 90% primary degradation products. The significant structural similarity between the examined drug and its primary photo-degradants presents a considerable challenge for the selectivity of the developed membranes, which the fabricated sensors successfully address. Furthermore, the developed sensors were utilized for the targeted quantification of the analyzed drug in various water samples containing KTP, eliminating the necessity for any sample pre-processing.
Concerning the toxicity that may arise owing to the leaching of the plasticizers into the environment, the fabricated sensors offer no significant harm to the environment because the conditioning process for the produced membranes takes about 30 min, which is shorter than the 24 h conditioning period in liquid contact electrodes. Furthermore, the produced membranes are stored in air rather than water or aqueous solutions; therefore, there is no leaching throughout the storage time. Similarly, the response time of the manufactured electrodes ranges from 11 to 18 s, allowing for a quick dipping time during sample analysis with negligible leaching.
5. Conclusions
The work’s novelty arises from the lack of previous studies on the potentiometric assessment of the target drug amid its major photo-degradants, commonly seen in river water containing KTP, due to its vulnerability to photo-degradation. The occurrence of KTP photo-degradants in water samples coming from rivers containing KTP presents a notable obstacle for its quantification, due to the substantial similarity in chemical structure between KTP and its main photo-degradation products. The lack of a sample pre-processing step demonstrates the method’s advantages over the liquid chromatographic and spectroscopic techniques employed for KTP quantification. Additionally, the proposed method is more cost-effective than those employing LC-MS/MS and UHPLC techniques due to its reduced costs per sample.
The developed electrodes effectively served their intended purpose, showcasing satisfactory selectivity and sensitivity across a broad concentration range. The DOP membrane has a more rapid response time, a broader operational pH range, and an extended lifespan compared to the DBS and o-NPOE membranes.
Author Contributions
Conceptualization, S.A.A.-G. and A.A.; data curation, S.A.A.-G. and A.A.; formal analysis, S.A.A.-G.; investigation, S.A.A.-G. and A.A.; methodology, S.A.A.-G.; resources, S.A.A.-G. and A.A.; software, S.A.A.-G. and A.A.; supervision, S.A.A.-G.; validation, S.A.A.-G.; visualization, S.A.A.-G.; writing—original draft, S.A.A.-G.; writing—review and editing, S.A.A.-G. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to Prince Sattam bin Abdulaziz University for funding this research work through the project number (PSAU/2025/03/32531).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that they have no competing interests in this manuscript.
References
- Marguery, M.C.; Chouini-Lalanne, N.; Ader, J.C.; Paillous, N. Comparison of the DNA damage photoinduced by fenofibrate and ketoprofen, two phototoxic drugs of parent structure. Photochem. Photobiol. 1998, 68, 679–684. [Google Scholar] [CrossRef]
- Kosjek, T.; Heath, E.; Krbavcic, A. Determination of non-steroidal anti-inflammatory drug (NSAIDs) residues in water samples. Environ. Int. 2005, 31, 679–685. [Google Scholar] [CrossRef]
- Vieno, N.M.; Tuhkanen, T.; Kronberg, L. Seasonal variation in the occurrence of pharmaceuticals in effluents from a sewage treatment plant and in the recipient water. Environ. Sci. Technol. 2005, 39, 8220–8226. [Google Scholar] [CrossRef]
- Lahoz, A.; Hernandez, D.; Miranda, M.A.; Perez-Prieto, J.; Morera, I.M.; Castell, J.V. Antibodies directed to drug epitopes to investigate the structure of drug-protein photoadducts. Recognition of a common photobound substructure in tiaprofenic acid/ketoprofen crossphotoreactivity. Chem. Res. Toxicol. 2001, 14, 1486–1491. [Google Scholar] [CrossRef]
- Radschuweit, A.; Ruttinger, H.H.; Nuhn, P.; Wohlrab, W.; Huschka, C. UV-induces formation of hydrogen peroxide based on the photochemisty of ketoprofen. Photochem. Photobiol. 2001, 73, 119–127. [Google Scholar] [CrossRef]
- Bosca, F.; Miranda, M.A. Photosensitizing drugs containing benzophenone chromophore. J. Photochem. Photobiol. B 1998, 43, 1–26. [Google Scholar] [CrossRef]
- Bosca, F.; Miranda, M.A.; Carganico, G.; Mauleon, D. Photochemical and photobiological properties of ketoprofen associated with the benzophenone chromophore. Photochem. Photobiol. 1994, 60, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Kosjek, T.; Perko, S.; Heath, E.; Kralj, B.; Žigon, D. Application of complementary mass spectrometric techniques to the identification of ketoprofen phototransformation products. J. Mass Spectrom. 2011, 46, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, P.; Jönsson, T.E.; Nilsson, B.; Wollheim, F. Determination of ketoprofen in plasma by extractive methylation and electron-capture gas chromatography. J. Chromatogr. A 1979, 177, 145–148. [Google Scholar] [CrossRef]
- Dvořák, J.; Hajkova, R.; Matysova, L.; Novakova, L.; Koupparis, M.A.; Solich, P. Simultaneous HPLC determination of ketoprofen and its degradation products in the presence of preservatives in pharmaceuticals. J. Pharmaceut. Biomed. Anal. 2004, 36, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Shafique, A.; Alothman, Z.A.; Akram, S.; Akram, S.; Sohail, C.S.; Mushtaq, M. An expedient and rapid High-Performance Liquid Chromatographic method for the kinetic study of Ketoprofen. J. King Saud Univ. Sci. 2020, 32, 2212–2218. [Google Scholar] [CrossRef]
- Vozniuk, O.; Kejík, Z.; Veselá, K.; Skaličková, M.; Novotný, P.; Hromádka, R.; Hajduch, J.; Martásek, P.; Jakubek, M.A. Fast HPLC/UV Method for Determination of Ketoprofen in Cellular Media. ChemistryOpen 2024, 13, e202300147. [Google Scholar] [CrossRef]
- Tsvetkova, B.; Peikova, L. HPLC determination of ketoprofen in tablet dosage forms. Trakia J. Sci. 2013, 11, 55. [Google Scholar]
- Friedberg, M.; Shihabi, Z.K. Ketoprofen analysis in serum by capillary electrophoresis. J. Chromatogr. B 1997, 695, 193–198. [Google Scholar] [CrossRef] [PubMed]
- El-Sadek, M.; El-Adl, S.; Abou-Kull, M.; Sakr, S.M. Spectrophotometric determination of ketoprofen in pharmaceutical preparations by means of charge transfer complex formation. Talanta 1993, 40, 585–588. [Google Scholar] [CrossRef]
- Lucca, L.G.; de Matos, S.P.; Weimer, P.; Teixeira, H.F.; Koester, L.S. Improved skin delivery and validation of novel stability-indicating HPLC method for ketoprofen nanoemulsion. Arab. J. Chem. 2020, 13, 4505–4511. [Google Scholar] [CrossRef]
- Yadav, N.K.; Raghuvanshi, A.; Sharma, G.; Beg, S.; Katare, O.P.; Nanda, S. QbD-based development and validation of a stability-indicating HPLC method for estimating ketoprofen in bulk drug and proniosomal vesicular system. J. Chromatogr. Sci. 2016, 54, 377–389. [Google Scholar] [CrossRef]
- Patil, P.M.; Wankhede, S.B.; Chaudhari, P.D. Stability-indicating HPTLC method for simultaneous determination of Ketoprofen, Methyl Paraben and Propyl Paraben in gel formulation. J. Pharm. Res. 2013, 6, 945–953. [Google Scholar] [CrossRef]
- Bempong, D.K.; Bhattacharyya, L. Development and validation of a stability-indicating high-performance liquid chromatographic assay for ketoprofen topical penetrating gel. J. Chromatogr. A 2005, 1073, 341–346. [Google Scholar] [CrossRef]
- Gaber, M.; Abu Shawish Hazem, M.; Khedr, A.M.; Abed-Almonem, K.I. Determination of benzalkonium chloride preservative in pharmaceutical formulation of eye and ear drops using new potentiometric sensors. Mater. Sci. Eng. C 2012, 32, 2299–2305. [Google Scholar] [CrossRef]
- Rizk, M.S.; Abdel-Haleem, F.M. Plastic membrane electrodes for the determination of flavoxate hydrochloride and cyclopentolate hydrochloride. Electrochim. Acta 2010, 55, 5592–5597. [Google Scholar] [CrossRef]
- Kharitonov, S.V. Ion-selective electrodes in medicinal drug determination. Russ. Chem. Rev. 2007, 76, 361. [Google Scholar] [CrossRef]
- Lenik, J. Properties of ion-selective electrodes with polymeric membranes for ketoprofen determination. J. Anal. Chem. 2012, 67, 543–549. [Google Scholar] [CrossRef]
- Lenik, J.; Łyszczek, R. A Potentiometric Sensor for Ketoprofen Based on a β-Cyclodextrin Derivative. J. Anal. Chem. 2022, 77, 246–256. [Google Scholar] [CrossRef]
- Urbanowicz, M.; Pijanowska, D.; Jasiński, A.; Ekman, M.; Bocheńska, M. A miniaturized solid-contact potentiometric multi sensor platform for determination of ionic profiles in human saliva. J. Solid State Electrochem. 2019, 23, 3299–3308. [Google Scholar] [CrossRef]
- Lindner, E.; Umezawa, Y. Performance evaluation criteria for preparation and measurement of macro-and microfabricated ion-selective electrodes (IUPAC Technical Report). Pure Appl. Chem. 2008, 80, 85–104. [Google Scholar] [CrossRef]
- Othman, A.M.; Rizk, N.M.H.; El-Shahawi, M.S. Polymer membrane sensors for sildenafil citrate (Viagra) determination in pharmaceutical preparations. Anal. Chim. Acta 2004, 515, 303–309. [Google Scholar] [CrossRef]
- Salvo-Comino, C.; Alonso-Pastor, L.E.; Pérez-González, C.; Pettinelli, S.; Carrero, K.C.N.; Rodríguez-Pérez, M.Á.; Rodríguez-Méndez, M.L. Impact of molecular structure and plasticization of PVC membranes in the response of solid-state ion-selective electrodes. Sens. Actuators Rep. 2025, 9, 100301. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).