3. Results and Discussion
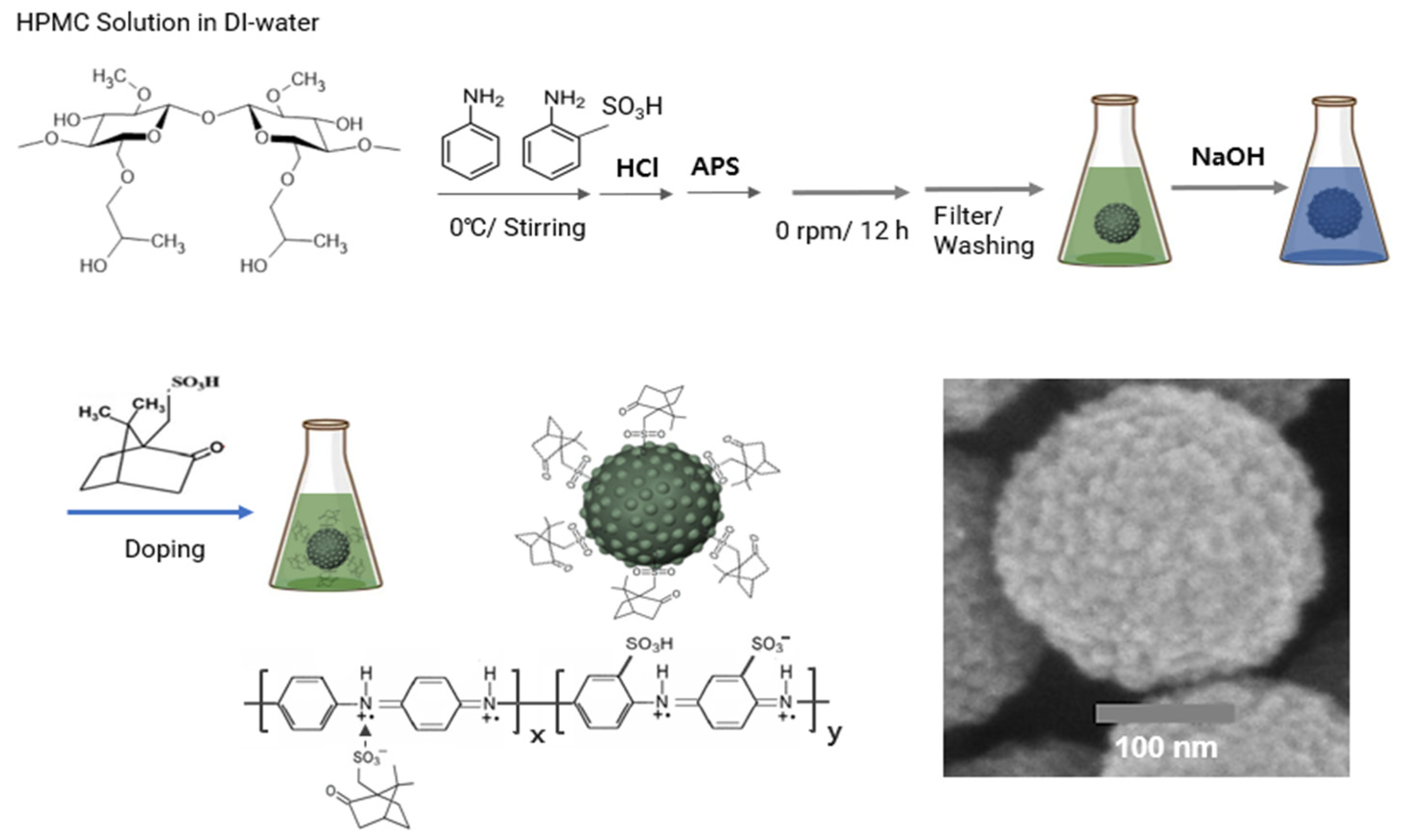
The overall synthesis procedure for CSA-capped P(ANi-co-ASNi) spherical NPs, using micelle polymerization with the HPMC surfactant in an aqueous solution, is depicted in
Scheme 1. As shown in
Scheme 1, the process began with dissolving HPMC, a surfactant essential for micelle formation, in DI water to prepare an aqueous solution. Subsequently, ANi and ASNi, substituted with a sulfonic acid (-SO
3H) group at the 2-carbon position, were added in equal molar amounts to the solution, and HCl was introduced into the previously prepared solution. During this step, the co-monomer comprising ANi and ASNi was protonated into positively charged anilinium ions (-NH
3+) via HCl. In succession, the polymerization of the co-monomer was triggered by the addition of APS, as an oxidizing agent. Afterwards, the impure ion products were neutralized and eliminated using NaOH solution, resulting in the spherical NPs of blue-colored emeraldine base structure. Finally, CSA was used for re-doping, resulting in a green-colored emeraldine salts structure-based electrically conductive NPs, as shown in the
Scheme 1. As depicted in
Scheme 1, this process yielded green P(ANi-co-ASNi) spherical NPs with an average diameter of 265 nm. These NPs were chemically capped with CSA, introducing sulfonic acid groups on the outer surface of the positively charged P(ANi-co-ASNi) chains. The P(ANi-co-ASNi) copolymer exhibits p-type semiconducting behavior, attributed to NH
+ sites within its backbone that interact with polar molecules such as acetone and H
2O via a hydrogen bonding and electrostatic forces. The CSA layer, deposited on the positively charged core, forms a porous thin film with irregularly distributed particulate domains, inducing a doping effect that enhances conductivity while serving as a protective barrier. These structural features facilitate stable adsorption and selective gas detection through both physical and chemical interactions, consistent with prior PANi-based systems [
22,
23]. Acetone vapor weakly interacts with NH
+ sites via its carbonyl group, reducing local charge density and increasing resistance due to limited hydrogen bonding and steric hindrance. In contrast, H
2O molecules form stronger hydrogen bonds and act as weak acids, increasing carrier density and lowering resistance. Notably, CSA-free nanoparticles showed ~30% higher reactivity, indicating that while CSA improves structural stability, it may attenuate gas responsiveness. The adsorption or permeation of acetone and H
2O through the CSA layer enables interaction with the P(ANi-co-ASNi) chains, providing a plausible mechanism for the observed sensing behavior.
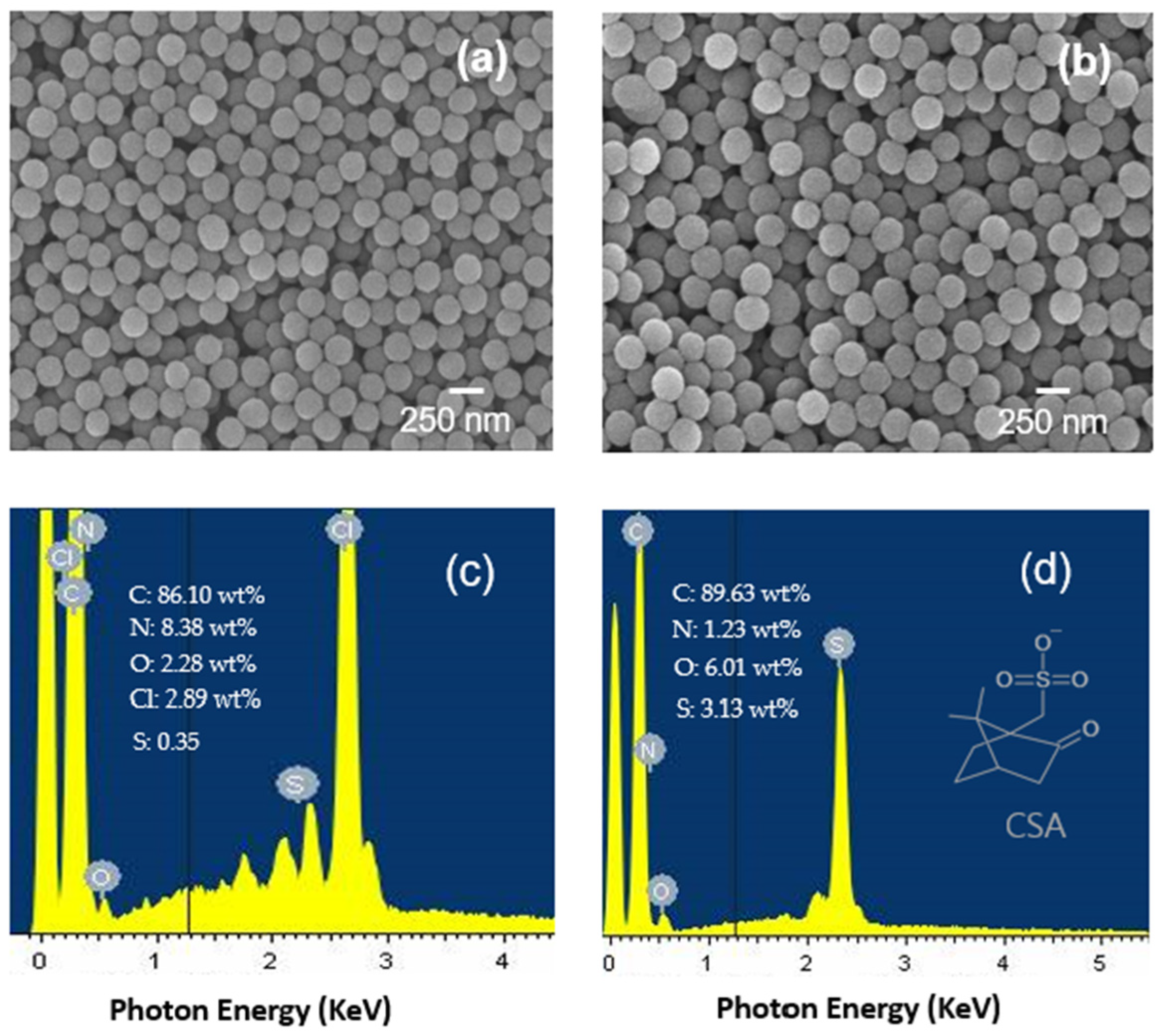
Figure 3 presents SEM images of the P(ANi-co-ASNi) NPs before and after CSA capping. Both uncapped and CSA-capped NPs exhibit well-defined spherical morphologies, with average diameters of approximately 235 nm and 265 nm, respectively. The observed increase in particle size (~30 nm) following CSA treatment is attributed to the formation of a secondary shell layer. SEM analysis reveals that the CSA shell adopts a porous thin-film structure composed of fine particulate domains irregularly distributed across the surface of the positively charged P(ANi-co-ASNi) core, as further illustrated in
Figure 1. This heterogeneous shell architecture is expected to enhance charge transport at the core–shell interface, improve resistance to ambient humidity, and facilitate interactions with organic gas molecules. Unlike conventional uniform coatings, the CSA shell in this study adheres in a non-continuous, particulate form, imparting distinct morphological features that may influence sensing performance. Electrical resistance measurements show that the pristine P(ANi-co-ASNi) NPs exhibit a resistance of approximately 1.2 MΩ, which decreases significantly to 0.6–0.8 MΩ after CSA capping. This conductivity enhancement is attributed to the sulfonic acid groups (-SO
3H) present in CSA, which are known to withdraw electrons from NH sites on the P(ANi-co-ASNi) surface. While this interaction improves conductivity, it differs from conventional electronic doping and instead reflects a molecular-level modification of charge transport properties. To further investigate the elemental composition, energy-dispersive X-ray spectroscopy (EDS) was performed (
Figure 3c,d). The presence of sulfur (S) atoms, originating from the CSA layer, was confirmed, with a measured mass percentage of 3.13% in CSA-capped P(ANi-co-ASNi) NPs. Although EDS indicates a uniform elemental distribution of sulfur, SEM images suggest that the CSA layer adheres in a structurally heterogeneous manner, highlighting the distinction between chemical composition and morphological uniformity. In contrast, the EDS spectrum of P(Ani-co-ASNi) particles without CSA (
Figure 3c) reveals only a small quantity of S atoms, amounting to 0.63%. The presence of these S atoms in the P(Ani-co-ASNi) particles is attributed to the -SO
3H group, which acts as a substituent in PASNi, a copolymer of PANi.
To investigate the changes in charge transport and molecular structure induced by CSA capping, ultraviolet/visible/near-infrared (UV/Vis/NIR) and Raman spectroscopy were employed (
Figure 4).
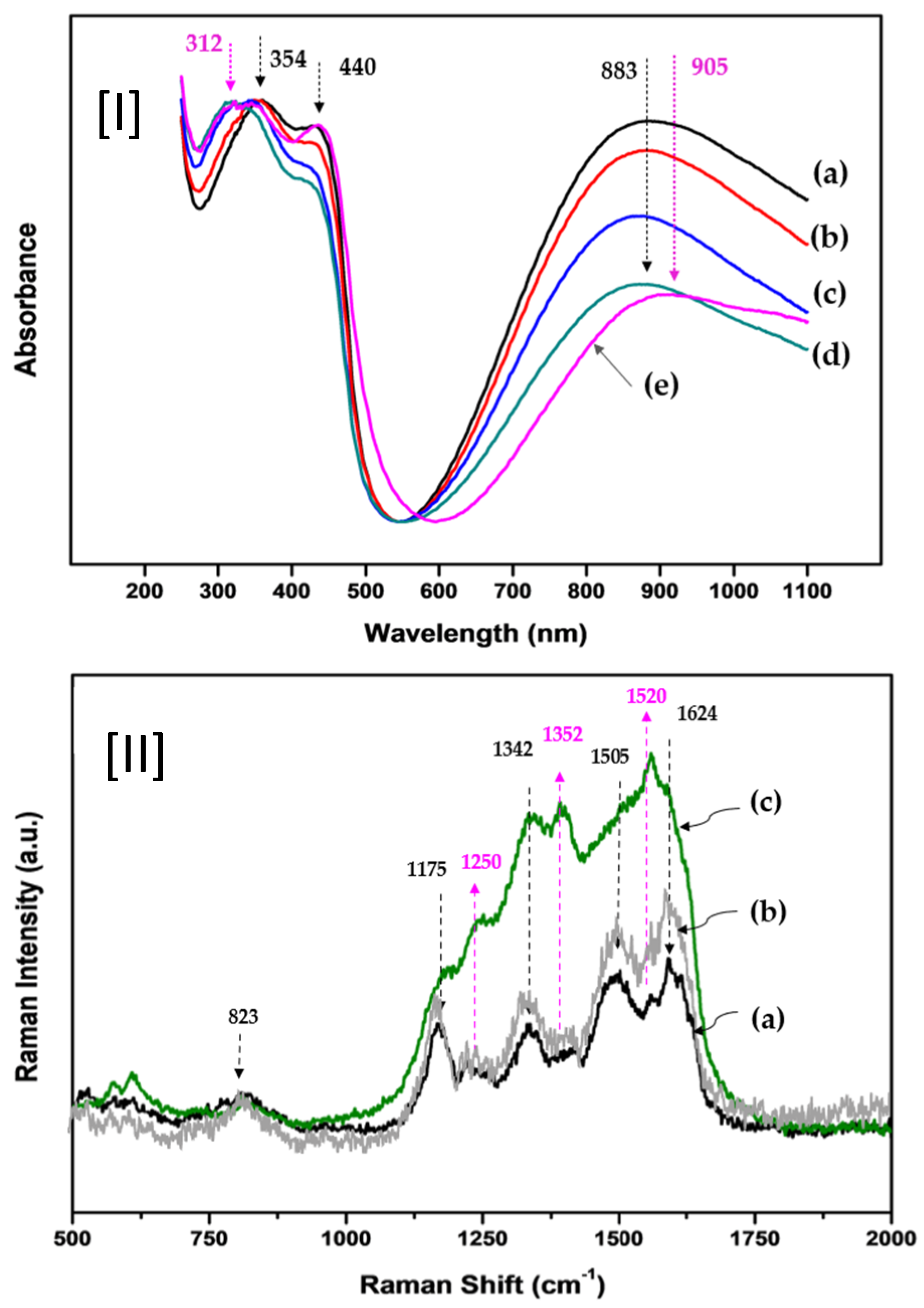
Figure 4I illustrates the UV/Vis absorption spectra of P(ANi-co-ASNi) copolymers with varying ANi-to-ASNi molar ratios. In
Figure 4I-(a), the spectrum of pure PANi (1:0 molar ratio) exhibits three distinct absorption peaks at 354, 440, and 883 nm. The peaks at 354 and 440 nm are attributed to π–π* transitions in the para-substituted benzenoid segment (-benzene-NH-benzene-), while the 883 nm peak corresponds to π–π* excitation of the quinoid structure (-N=quinone=N-) [
24,
25]. As the ASNi content increases (ratios of 0.8:0.2, 0.7:0.3, and 0.6:0.4 in
Figure 4I-(b), (c), and (d)), the absorbance intensities at 440 and 883 nm progressively decrease. This trend suggests a gradual weakening of the quinoid structure in ANi due to the incorporation of ASNi units. Interestingly, when the molar ratio reaches 0.5:0.5 in CSA-capped P(ANi-co-ASNi) (
Figure 4I-(e)), a new absorption peak emerges at 312 nm, and the original 883 nm peak shifts to 905 nm, extending into the NIR region. The peaks at 354 and 440 nm remain largely unchanged. The appearance of the 312 nm peak is attributed to self-doping effects, likely arising from electron donation by the sulfonic acid group of ASNi to the PASNi chain. Additionally, CSA capping appears to promote protonation at the imine nitrogen sites via interaction with the π-conjugated backbone, facilitating the formation of a delocalized π-conjugated system and enhancing charge mobility. Electrical resistance measurements support these spectroscopic findings. The resistance of uncapped P(ANi-co-ASNi) was approximately 1.0 MΩ, whereas CSA-capped samples exhibited reduced resistance in the range of 0.6–0.8 MΩ, indicating a conductivity enhancement of over 20%.
Raman spectroscopy was employed to elucidate the chemical bonding structures and their influence on charge transport in CSA-capped P(ANi-co-ASNi) copolymers (
Figure 4II).
Figure 4II-(a) displays the Raman spectrum of pure PANi (ANi-to-ASNi molar ratio of 1:0). A prominent band at 823 cm
−1 corresponds to the C–H out-of-plane vibration in the 1,4-distributed benzene ring. Additional peaks observed between 1250 and 1352 cm
−1 is attributed to the bending modes of the benzene ring, C–N stretching of the aromatic ring, and the stretching of the charged C–NH
+ group. The band at 1175 cm
−1 is assigned to the –SO
3H group, indicative of CSA presence. Furthermore, the C=C stretching vibrations of the quinoid and benzenoid structures appear at 1624 cm
−1 and 1505 cm
−1, respectively [
26,
27,
28,
29]. The intensity ratio of the 1624 cm
−1 and 1505 cm
−1 bands serve as a key indicator of the conjugation length and doping level within the PANi chain. A higher intensity at 1624 cm
−1 typically reflects extended conjugation and increased doping [
30].
Figure 4II-(b) and (c) present the Raman spectra of copolymers with ANi-to-ASNi molar ratios of 0.8:0.2 and 0.5:0.5, respectively. As the ASNi content increases, notable enhancements in the intensities of the bands at 1250, 1352, and 1520 cm
−1 are observed. Additionally, the absorbance in the regions around 1175 and 1624 cm
−1 becomes significantly stronger compared to pure PANi, indicating structural modifications induced by CSA and ASNi incorporation. Specifically, the peak at 1250 cm
−1 is attributed to the –SO
3H substitution group within the ASNi structure, while the 1352 cm
−1 band corresponds to the stretching of the charged C–NH
+ group in the quinoid domain of PASNi. The 1520 cm
−1 peak is assigned to the C=C/C–C stretching mode of the benzenoid structure, further confirming the presence of CSA-induced protonation and enhanced π-conjugation.
Acetone is a representative volatile organic compound (VOC) commonly encountered in industrial processes, indoor environments, and various chemical emissions. Its detection is essential for monitoring air quality and ensuring safety in occupational and environmental settings. Although trace levels of acetone (typically <1 ppm) are also known to be present in human exhaled breath due to metabolic activity [
31,
32,
33], this study focuses on general VOC sensing rather than medical breath analysis. In this work, the developed sensor material demonstrated reliable detection of acetone gas concentrations up to 1 ppm at room temperature. Furthermore, the sensor exhibited sensitivity to H
2O vapor, with measurable responses observed for concentrations up to 1%. The real-time sensing performance of CSA-capped P(ANi-co-ASNi) samples was systematically evaluated under ambient conditions to assess their responsiveness (S) to acetone and water vapor (
Figure 5).
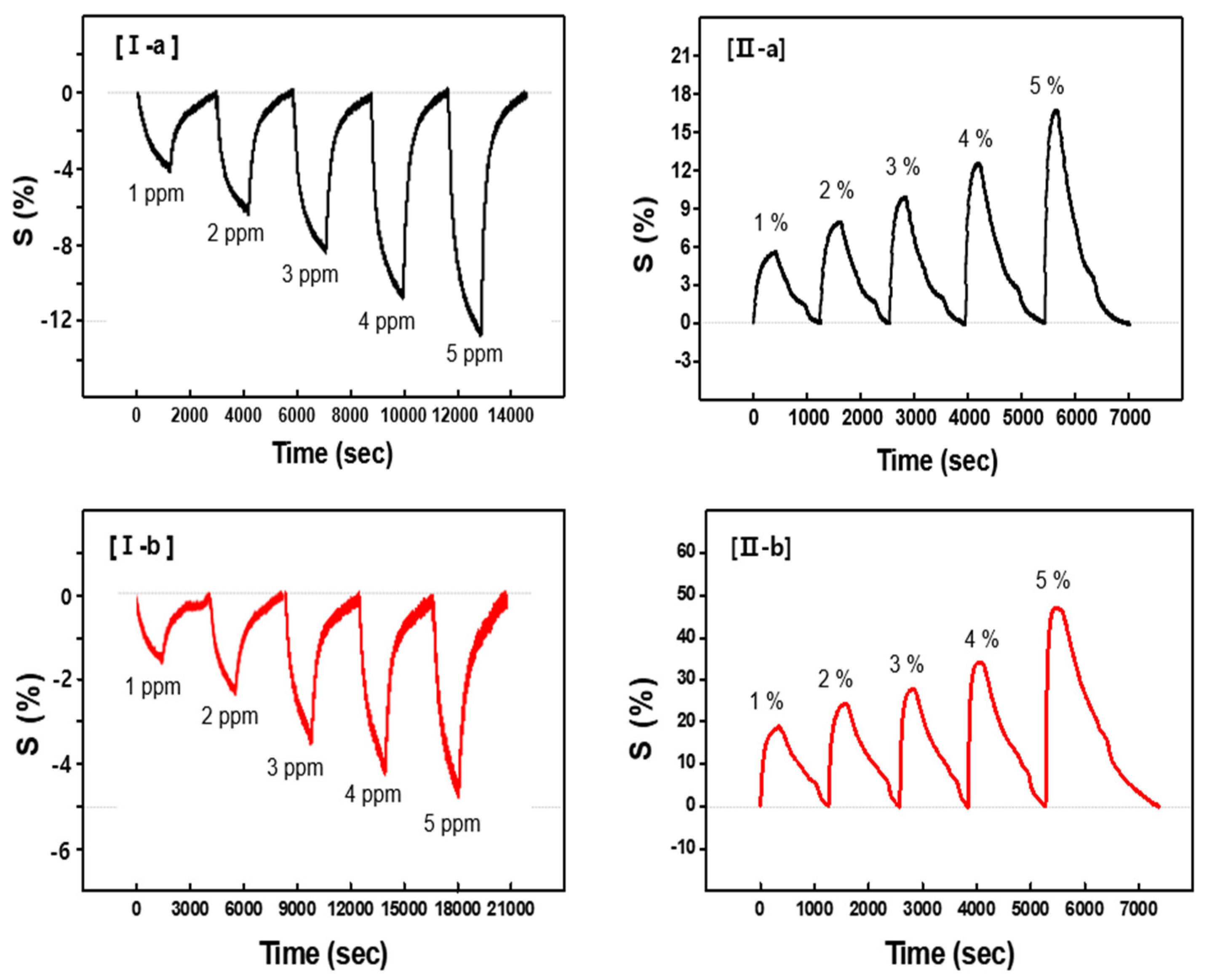
Figure 5I shows the continuous dynamic response of the CSA-capped P(ANi-co-ASNi) sensors to acetone gas concentrations ranging from 1 to 5 ppm, maintained at a constant temperature of 25 °C and 0% relative humidity (RH).
Figure 5I-a and b show the sensor performance of the CSA-capped P(ANi-co-ASNi) copolymers, with ANi-to-ASNi molar ratios of 1:0 and 0.5:0.5, respectively. The responsiveness (S%) of the CSA-capped P(ANi-co-ASNi)-based sensors to acetone gas was measured in real-time using the normalized change in resistance, ΔR/R
i = (R
i -R
0)/R
i x 100. Here, Ri denotes the initial resistance before gas exposure, R
0 denotes the real-time resistance during gas exposure, and ΔR represents the difference between R
i and R
0, which is calculated after gas exposure.
Figure 5I-a illustrates the continuous dynamic response (S) of a CSA-capped P(ANi-co-ASNi) sample with an ANi-to-ASNi molar ratio of 1:0 (pure PANi) upon exposure to acetone gas. The sensor response (S value) was quantified at acetone concentrations of 1, 2, 3, 4, and 5 ppm, with an absolute S value of 4.2 recorded at 1 ppm. Notably, increasing acetone concentration resulted in a proportional decrease in the S value, indicating reduced reactivity in the negative direction. This trend is attributed to a decline in charge density within the PANi backbone, caused by acetone adsorption. The interaction between acetone molecules and amine nitrogen (–NH
+) sites leads to the formation of hydrogen bonds with the carbonyl group of acetone molecule, thereby impeding charge transfer and increasing electrical resistance.
Figure 5I-b presents the response of CSA-capped P(ANi-co-ASNi) copolymers synthesized with an equimolar ANi-to-ASNi ratio. Compared to pure PANi, these copolymers exhibited significantly lower sensitivity to acetone, with an S value of 1.9 at 1 ppm. Despite the reduced magnitude, the sensor maintained consistent reactivity and excellent reproducibility across varying acetone concentrations. The response profile remained stable, showing proportional changes with respect to gas concentration. As defined in
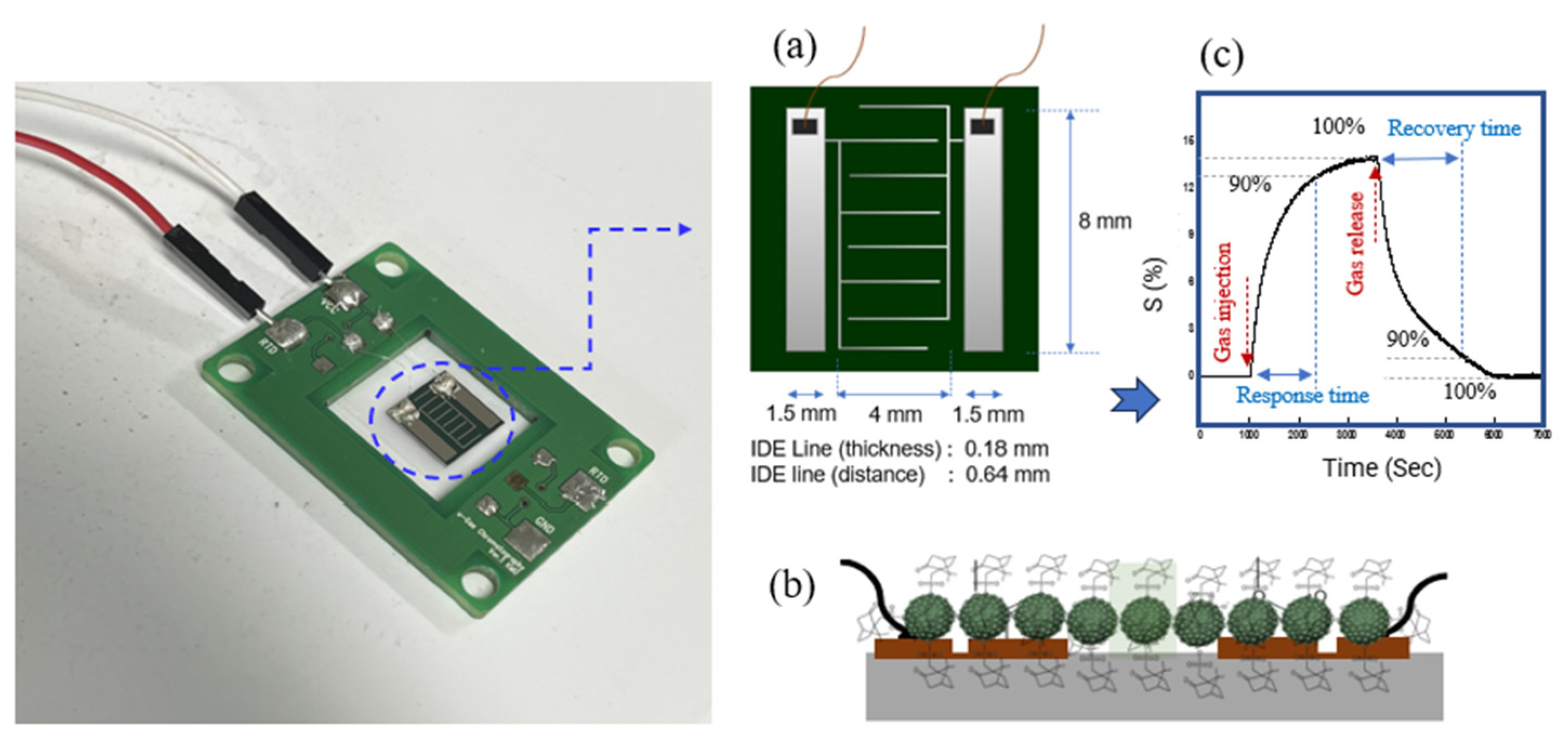
Figure 2, the response time refers to the duration required for the sensor conductance to reach 90% of its maximum value, while the recovery time denotes the interval needed for the conductance to return to a level 10% above its baseline upon exposure to ambient condition. These parameters are critical for assessing the dynamic performance and operational reliability of the sensor. CSA-capped P(ANi-co-ASNi) sensors with ANi-to-ASNi molar ratios of 1:0 and 0.5:0.5 exhibited response times of 1200 s and 1700 s, respectively, when exposed to 1 ppm acetone gas at 25 °C and 0% RH. Corresponding recovery times were measured at 1500 s and 2700 s, respectively. As illustrated in
Figure 4, the recovery time increased markedly with rising acetone concentrations, which can be attributed to the prolonged desorption process at room temperature. This delay becomes more pronounced as the quantity of adsorbed gas increases. Notably, the recovery time extension was more significant in the copolymer containing PASNi than in pure PANi, underscoring the influence of structural composition on desorption kinetics and sensor reset behavior.
Figure 5II presents the continuous dynamic response (S) of CSA-capped P(ANi-co-ASNi) sensors exposed to gaseous H
2O concentrations ranging from 1% to 5%, under controlled conditions of 25 °C and 0% RH. These results demonstrate the sensor’s sensitivity to H
2O vapor in the absence of ambient humidity. For the CSA-capped PANi sample (ANi-to-ASNi molar ratio of 1:0), an S value of approximately 5.8 was recorded at 1% H
2O vapor (
Figure 5II-a), indicating high responsiveness at low concentrations. Unlike acetone, H
2O vapor exhibited a positive correlation between concentration and S value, with the response increasing proportionally in the positive direction. This behavior is attributed to the strong interaction between H
2O molecules and NH
+ functional groups on the P(ANi-co-ASNi) chains, which enhances conduction pathways and reduces electrical resistance.
As shown in
Figure 5II-b, the CSA-capped P(ANi-co-ASNi) copolymer exhibited an S value approximately four times higher than that of pure PANi, suggesting that the incorporation of ASNi significantly enhances the material’s reactivity toward H
2O vapor. Although both acetone and H
2O molecules are capable of forming hydrogen bonds with the –NH
+ sites of the P(ANi-co-ASNi) copolymer, the resulting charge transport mechanisms differ significantly. In particular, H
2O molecules initiate a proton conduction process governed by the Grotthuss mechanism [
34,
35], wherein hydrogen bonding with –NH
+ groups lead to the formation of hydronium ions (H
3O
+) and imine sites (–N=). These H
3O
+ ions undergo self-diffusion through a dynamic hydrogen-bonded network, facilitating efficient proton hopping. This process enhances carrier mobility and reduces electrical resistance. In contrast, acetone molecules interact via dipolar hydrogen bonding, primarily through the carbonyl oxygen (–C=O), without generating mobile ionic species or enabling proton transfer. As a result, acetone exposure may lead to localized charge trapping or reduced carrier mobility, manifesting as an increase in electrical resistance (
Figure 5II-a). Therefore, despite the presence of hydrogen bonding in both cases, the distinct molecular interactions and transport pathways account for the opposite trends observed in resistance change.
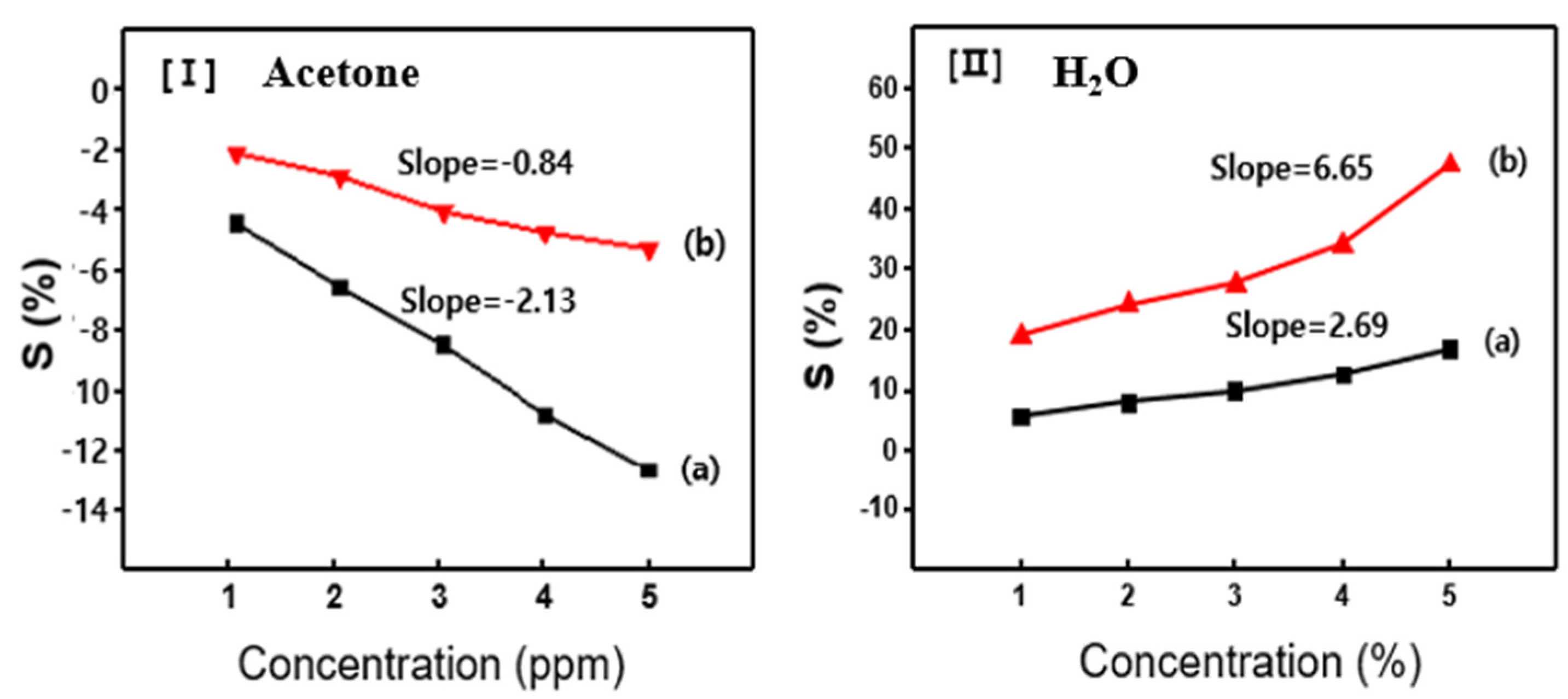
Figure 6 illustrates the variation in sensor sensitivity (S value) as a function of gas concentration, revealing a linear increase in S with rising acetone levels. This proportional response underscores the sensor’s reliability and suitability for quantitative gas analysis. Sensitivity was quantified by calculating the slope of the S value versus acetone concentration curve, expressed in ppm
−1. As shown in
Figure 6I, sensors based on pure PANi and P(ANi-co-ASNi) copolymer exhibited sensitivity values of 2.13 and 0.84 ppm
−1, respectively. These results indicate that pure PANi offers higher sensitivity to acetone, whereas the copolymer provides more stable but less reactive performance. In contrast, the sensor’s response to H
2O vapor was significantly enhanced. As shown in
Figure 6II, the CSA-capped P(ANi-co-ASNi) sensor demonstrated a sensitivity of 6.65 ppm
−1 to H
2O, notably exceeding its response to acetone (2.69 ppm
−1). This enhanced sensitivity is attributed to the hydrophilic sulfonic acid (-SO
3H) groups in the PASNi backbone, which facilitate strong adsorption of H
2O molecules. These groups promote hydrogen bonding and improve charge transfer interactions, thereby amplifying the sensor’s responsiveness to moisture. All measurements were conducted under identical environmental conditions, and each data point represents the average of at least five independent trials. To ensure analytical precision, data points within the experimental error range were excluded from the final analysis.
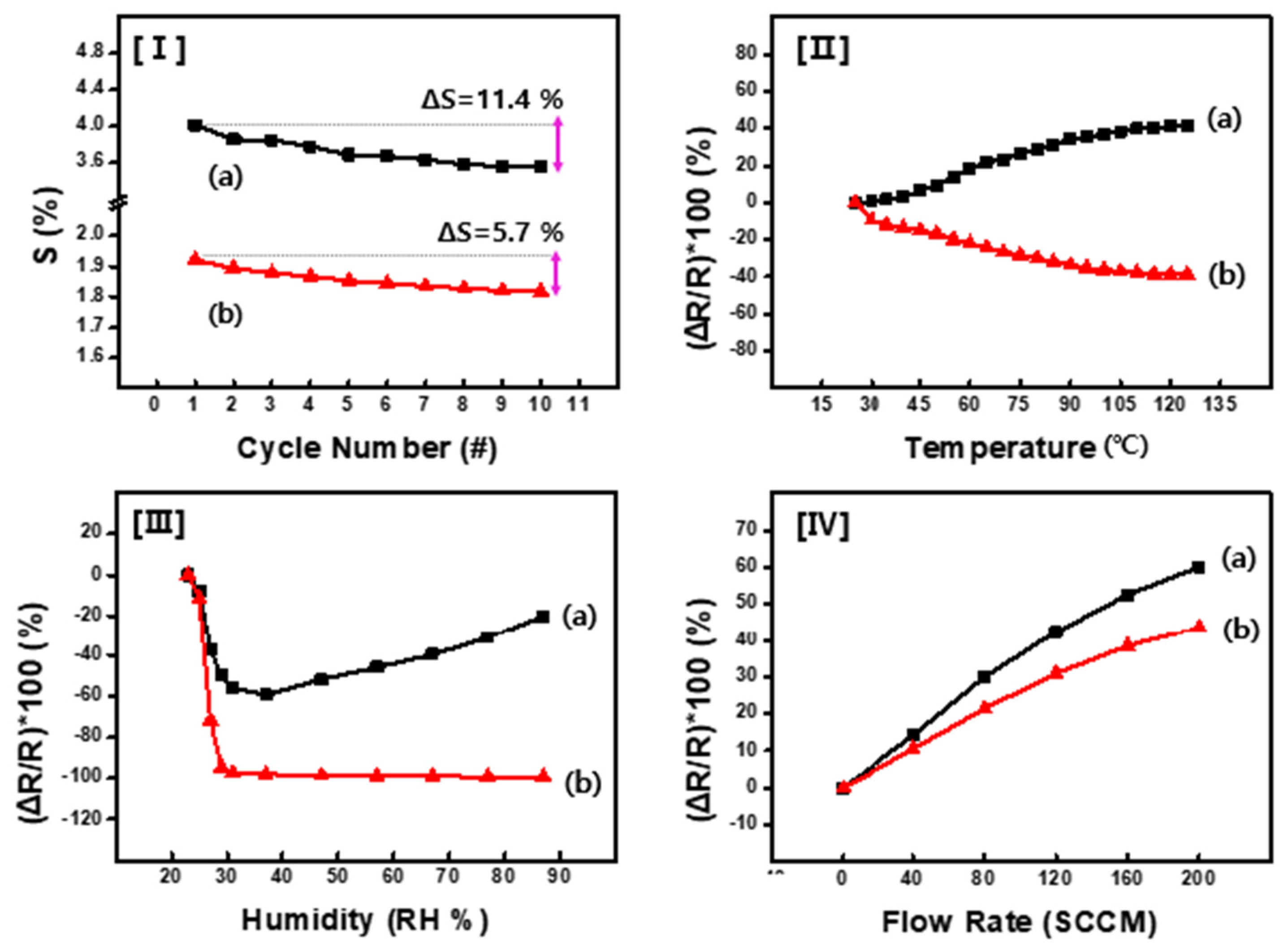
Figure 7 illustrates the sensor’s performance evaluation under a range of operational conditions, including repeated recovery cycles, and variations in temperature, humidity, and gas flow rate.
Figure 7I presents the results of cyclic testing performed on CSA-capped P(ANi-co-ASNi)-based acetone sensors to evaluate their performance consistency and long-term stability under repeated exposure. All experiments were conducted under strictly controlled conditions (25 °C, 0% RH, and 1 ppm acetone), ensuring environmental consistency across cycles. As shown in
Figure 7I-a, the PANi-based sensor exhibited a ΔS value exceeding 11% over 10 exposure cycles, indicating notable signal fluctuation. In contrast, the P(ANi-co-ASNi)-based sensor (
Figure 7I-b) showed a more moderate ΔS variation of approximately 5–6%, while maintaining a relatively high recovery rate. These results suggest that the copolymer formulation offers improved signal stability under repeated operation.
Figure 7II–IV further illustrate the sensor’s performance under varying external conditions, including temperature, relative humidity, and gas pressure. Sensor behavior was monitored by tracking changes in electrical resistance, providing a comprehensive assessment of environmental robustness.
Figure 7II-a illustrates the temperature-dependent performance of the PANi-based sensor. As the ambient temperature increased to 125 °C, the electrical resistance rose by approximately 40%. In contrast, the CSA-capped P(ANi-co-ASNi) sensor (
Figure 7II-b) exhibited a proportional decrease in resistance of roughly 40% under identical conditions. These opposing trends underscore the distinct thermal response characteristics of pure PANi versus P(ANi-co-ASNi) copolymer systems, both capped with CSA. Specifically, while CSA-capped PANi sensors show increased resistance with temperature, CSA-capped P(ANi-co-ASNi) sensors demonstrate enhanced conductivity, suggesting differing charge transport mechanisms influenced by polymer composition.
Figure 7III presents the variation in electrical resistance of the sensor under RH levels ranging from 0% to 87%. As widely [
36,
37], ambient RH significantly affects the performance of gas sensors, and the observed resistance changes further confirm the humidity sensitivity of the CSA-capped P(ANi-co-ASNi) system. As illustrated in
Figure 7III-a, the pure PANi-based sensor exhibits a proportional decrease in electrical resistance at low RH levels, particularly below 28%. This behavior is attributed to enhanced charge transport facilitated by H
2O vapor molecules interacting with the PANi backbone. However, once RH exceeds 28%, the resistance profile undergoes a distinct transition, marked by an upward trend and signal fluctuations. This inflection point is designated as the critical humidity threshold. Beyond this level, water molecules tend to aggregate on the sensor surface, forming a thin moisture layer that disrupts the interaction between the CSA capping agents and the PANi backbone, thereby increasing resistance. This effect is further amplified under low-temperature conditions, where nanoscale water films form at relatively lower RH levels, indicating a temperature-dependent modulation of humidity response. To further investigate this phenomenon, analogous experiments were conducted using the P(ANi-co-ASNi)-based sensor (
Figure 7III-b). Notably, the copolymer sensor exhibited exceptional stability, with resistance variation remaining below 5% even at RH levels exceeding 28%. This enhanced humidity tolerance is attributed to the incorporation of ASNi, which introduces -SO
3H groups at the 2-position of the benzene ring. These substituents induce a self-doped configuration within the PANi matrix, enabling the formation of conductive pathways independent of external dopants. Consequently, even under conditions conducive to H
2O condensation, the electrical resistance remains largely unaffected. This study demonstrates a clear comparative advantage over previously reported PANi-based acetone sensors operating at room temperature [
34,
35,
38].
As summarized in
Table 1, the developed sensor exhibits superior sensitivity (ppm
−1), a lower detection limit (ppm), and improved performance in terms of operating temperature, cycling stability, and high-humidity resilience. Collectively, these attributes position the CSA-capped P(ANi-co-ASNi) sensor as a promising candidate for next-generation acetone detection technologies, with potential applications in biomedical diagnostics, environmental monitoring, and industrial safety systems.
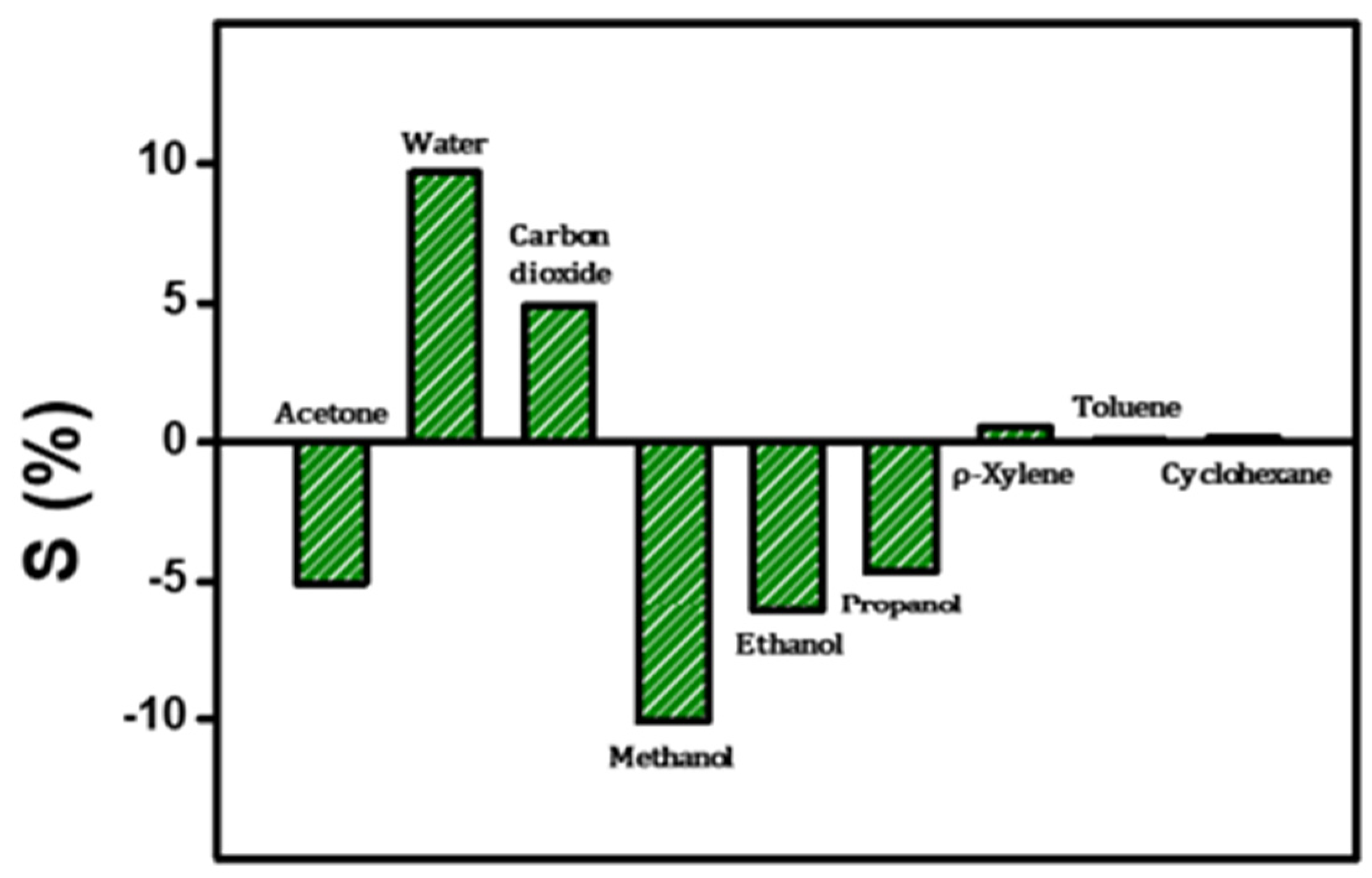
Selectivity is a key parameter in the practical application of gas sensors. To evaluate selectivity, responsiveness to various organic gases, including ethanol, methanol, H
2O, xylene, toluene, CO
2, and cyclohexane, was investigated. The selected gases are commonly encountered in clinical and industrial settings and are relatively simple and readily available compounds. Therefore, they were chosen as reference targets for comparative analysis in this study. According to the experimental results, the CSA-capped P(ANi-co-ASNi) sensor exhibited an S value of −5.2 in response to 5 ppm acetone gas and +9.6 for 5 ppm H
2O vapor. The sensor also demonstrated notable responses to other alcohol-based analytes, including ethanol (S = −7.5), methanol (S = −12), and propanol (S = −5.2) at the same concentration. In contrast, exposure to non-polar or less interactive gases such as CO
2, xylene, toluene, and cyclohexane yielded significantly lower responses, with S values of +5.5, +0.5, +0.1, and +0.2, respectively, at 5 ppm. These comparative results are summarized in
Figure 8, highlighting the sensor’s selective sensitivity toward polar and electron-accepting analytes. Interestingly, the sensor exhibited a negative response to gases such as acetone and alcohols, which function as electron acceptors, resulting in a decrease in electrical resistance. In contrast, gases with strong electron-donating characteristics—such as CO
2, toluene, xylene, H
2O, and cyclohexane—induced an increase in resistance. This behavior is attributed to the modulation of charge carrier concentration within the sensing material. Electron-accepting gases tend to extract electrons from the sensor surface, thereby reducing the carrier density and consequently lowering the conductivity. Conversely, electron-donating gases inject electrons into the sensing layer, increasing the carrier concentration and enhancing conductivity. These distinct interactions between the sensor and analyte gases underscore the material’s potential for selective gas detection based on electronic properties. While pristine polyaniline (PANI) is well known for its high selectivity toward ammonia (NH
3), SPANi alone did not exhibit reliable NH
3 sensing performance. Although a response peak was observed during preliminary tests, the sensor showed poor recovery characteristics, suggesting irreversible adsorption or strong chemisorption of NH
3 molecules. This behavior is likely attributed to the strong acid–base interactions between the sulfonic acid (–SO
3H) groups in SPANi and NH
3, which may induce permanent alterations in the conduction pathways or structural deformation of the sensing matrix. Furthermore, the hydrophilic nature of SPANi can facilitate the formation of complex hydrogen-bonding networks in humid environments, thereby impeding effective desorption of NH
3. Considering these limitations, detailed NH
3 sensing results were not included in this study. Instead, we focused on acetone sensing, where SPANi demonstrated both a pronounced response and excellent reversibility, making it a more suitable analyte for evaluating the sensing performance of SPANi-based materials.