2,1,3-Benzothiadiazoles Are Versatile Fluorophore Building Blocks for the Design of Analyte-Sensing Optical Devices
Abstract
1. Introduction
2. Fluorescence Response Mechanisms in Analyte Sensing Optical Devices
3. Target Analytes for Detection Using BTD-Based Optical Devices
3.1. Cationic Species
3.2. Anionic Species
3.3. Neutral Analytes
4. Synthesis of BTD-Based Compounds
4.1. BTD-Br2 and Their Modifications
4.1.1. Extrusion
4.1.2. Replacement of Bromine of BTD-Br2
4.1.3. Synthesis and Modification of 4-Bromo-7-nitrobenzo[c][1,2,5]thiadiazole (61)
4.2. The Strategical Synthesis of 5,6-Dinitro-BTD Derivatives
4.3. 5,6-Diamino-BTD and Its Derivatives
5. Design and Sensing Fluorescence Mechanisms in BTD-Based Optical Molecular Devices
6. Optical Sensing of Cation Based on BTD
7. Optical Sensing of Anion Based on BTD
8. Optical Sensing of Neutral Analytes Based on BTD
9. Optical Sensing of Multi-Analytes Based on BTD Core
10. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-HG | 2-Hydroxyglutarate |
| A–A–A | Acceptor–acceptor–acceptor |
| AIE | Aggregation-induced emission |
| AML | Acute myeloid leukemia |
| ASE | Amplified spontaneous emission |
| APTES | 3-(Aminopropyl)triethoxysilane |
| Ar | Generic aryl group |
| BOC | tert-Butyloxycarbonyl |
| BODIPY | 4,4-Difluoro-4-bora-3a,4a-diaza-s-indacene |
| BSA | Bovine serum albumin |
| BTD | 2,1,3-Benzothiadiazole |
| CHEF | Chelation-enhanced fluorescence |
| CHEQ | Chelation-enhanced fluorescence quenching |
| COD | 1,5-Cyclooctadiene |
| Cp* | 1,2,3,4,5-Pentamethylcyclopentadiene |
| CT | Charge transfer |
| CTAB | Cetyltrimethylammonium bromide |
| CV | Cyclic voltammetry |
| Cys | Cysteine |
| D-(A)–A–(A)-D | Donor (acceptor)–acceptor–(acceptor) donor |
| D–A | Donor−acceptor |
| dba | Dibenzylideneacetone |
| DCE | 1,2-Dichloroethane |
| DFT | Density functional theory |
| DLS | Dynamic light scattering |
| DMA | N,N-dimethylacetamide |
| DMF | N,N-dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| DNA | Deoxyribonucleic acid |
| DNT | 2,4-Dinitrotoluene |
| dppf | 1,1′-Bis(diphenylphosphino)ferrocene |
| EDA | Ethylenediamine |
| ELF | Electron localization function |
| EPR | Electron paramagnetic resonance |
| ESIPT | Excited-state intramolecular proton transfer |
| ESP | Electrostatic potential analysis |
| EE | Electron exchange |
| FESEM | Field emission scanning electron microscopy |
| FRET | Fluorescence resonance energy transfer |
| FT-IR | Fourier-transform infrared spectroscopy |
| fw | Volume fraction |
| GSH | Glutathione |
| GO | Glyoxal |
| HEPES | 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid |
| HCy | Homocysteine |
| HRMS | High-resolution mass spectrometry |
| ICT | Internal (or intramolecular) charge transfer |
| IRR | Intramolecular rotations |
| JAF | J-aggregate formation |
| LOD | Limit of detection |
| LOL | Localized orbital locator |
| LUMO | Lowest Unoccupied Molecular Orbital |
| MGO | Methylglyoxal |
| MLCT | Metal-to-ligand charge transfer |
| MW | Microwave |
| NIR | Near-infrared |
| NIR-I | First near-infrared (400–900 nm) |
| NIR-II | Second near-infrared (1000−1700 nm) |
| NMR | Nuclear magnetic resonance |
| NP | p-Nitrophenol |
| NBS | N-Bromosuccinimide |
| o-DBC | 1,2-Dichlorobenzene |
| OLED | Organic light-emitting diodes |
| o-tol | o-Tolyl |
| PA | Picric acid |
| PAS | Sodium pyruvate |
| PBS | Phosphate-buffered saline |
| PES | Potential energy surface analysis |
| PET | Photoinduced electron transfer |
| Phen | 1,10-Phenanthroline |
| PHT | Photoinduced hole transfer |
| PGO | Phenylglyoxal |
| PPA | Phenylpyruvic acid |
| ppb | Part per billion |
| ppm | Part per million |
| R2 | Coefficient of determination |
| rt | Room temperature |
| RIR | Restriction of intramolecular rotation |
| ROS | Reactive oxygen species |
| SEM | Scanning electron microscopy |
| TBAF | Tetra-n-butylammonium fluoride |
| TBDPD-Cl | tert-Butyl(chloro)diphenylsilane |
| TCNE | Tetracyanoethylene |
| TCNQ | 7,7,8,8-Tetracyanoquinodimethane |
| TEA | Triethanolamine |
| TICT | Twisted internal (or intramolecular) charge transfer |
| THF | Tetrahydrofuran |
| TLV | Threshold limit value |
| TMEDA | Tetramethylethylenediamine |
| TMS | Tetramethylsilane |
| TMSCl | Trimethylsilyl chloride |
| TNT | 2,4,6-Trinitrotoluene |
| TPA | Two-photon absorption cross-section |
| WHO | World Health Organization |
| UV | Ultraviolet |
| XRD | X-ray diffraction |
| α-CyD | α-Cyclodextrin |
| α-KA | α-Ketoglutarate |
| λmax | Maximum wavelength |
| λem | Maximum emission wavelength |
| λabs | Maximum absorption wavelength |
| Φ | Fluorescence quantum yield |
References
- Mohammed, E.; Mohammed, T.; Mohammed, A. Optimization of Instrument Conditions for the Analysis for Mercury, Arsenic, Antimony and Selenium by Atomic Absorption Spectroscopy. MethodsX 2018, 5, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Wysocka, I. Determination of Rare Earth Elements Concentrations in Natural Waters—A Review of ICP-MS Measurement Approaches. Talanta 2021, 221, 121636. [Google Scholar] [CrossRef] [PubMed]
- Ammann, A.A. Inductively Coupled Plasma Mass Spectrometry (ICP MS): A Versatile Tool. J. Mass Spectrom. 2007, 42, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Michalski, R. Ion Chromatography Applications in Wastewater Analysis. Separations 2018, 5, 16. [Google Scholar] [CrossRef]
- Dimeski, G.; Badrick, T.; St John, A. Ion Selective Electrodes (ISEs) and interferences—A review. Clin. Chim. Acta 2010, 411, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Ying, Y.; Ping, J. Recent Advances in Solid-Contact Ion-Selective Electrodes: Functional Materials, Transduction Mechanisms, and Development Trends. Chem. Soc. Rev. 2020, 49, 4405–4465. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Singh, R. Anion Selective Electrodes: A Brief Compilation. Microchem. J. 2019, 149, 104045. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Ye, R.; Duan, Q. Advances on Water Quality Detection by UV-Vis Spectroscopy. Appl. Sci. 2020, 10, 6874. [Google Scholar] [CrossRef]
- Xu, G.; Song, P.; Xia, L. Examples in the Detection of Heavy Metal Ions Based on Surface-Enhanced Raman Scattering Spectroscopy. Nanophotonics 2021, 10, 4419–4445. [Google Scholar] [CrossRef]
- Khongkaew, P.; Phechkrajang, C.; Cruz, J.; Cárdenas, V.; Rojsanga, P. Quantitative Models for Detecting the Presence of Lead in Turmeric Using Raman Spectroscopy. Chemom. Intell. Lab. Syst. 2020, 200, 103994. [Google Scholar] [CrossRef]
- Ji, W.; Li, L.; Zhang, Y.; Wang, X.; Ozaki, Y. Recent Advances in Surface-Enhanced Raman Scattering-Based Sensors for the Detection of Inorganic Ions: Sensing Mechanism and Beyond. J. Raman Spectrosc. 2021, 52, 468–481. [Google Scholar] [CrossRef]
- Monakhova, Y.B.; Kuballa, T.; Tschiersch, C.; Diehl, B.W.K. Rapid NMR Determination of Inorganic Cations in Food Matrices: Application to Mineral Water. Food Chem. 2017, 221, 1828–1833. [Google Scholar] [CrossRef] [PubMed]
- Hafer, E.; Holzgrabe, U.; Kraus, K.; Adams, K.; Hook, J.M.; Diehl, B. Qualitative and Quantitative 1H NMR Spectroscopy for Determination of Divalent Metal Cation Concentration in Model Salt Solutions, Food Supplements, and Pharmaceutical Products by Using EDTA as Chelating Agent. Magn. Reson. Chem. 2020, 58, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G. A Review of the Identification and Detection of Heavy Metal Ions in the Environment by Voltammetry. Talanta 2018, 178, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Bansod, B.K.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A Review on Various Electrochemical Techniques for Heavy Metal Ions Detection with Different Sensing Platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and Removal of Heavy Metal Ions: A Review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Bacon, J.R.; Butler, O.T.; Cairns, W.R.L.; Cavoura, O.; Cook, J.M.; Davidson, C.M.; Mertz-Kraus, R. Atomic Spectrometry Update-a Review of Advances in Environmental Analysis. J. Anal. At. Spectrom. 2021, 36, 10–55. [Google Scholar] [CrossRef]
- Kettler, H.; White, K.; Hawkes, S. Mapping the Landscape of Diagnostics for Sexually Transmitted Infections: Key Findings and Recommandations; Unicef/Undp/World Bank/Who: Geneva, Switzerland, 2004; pp. 1–44. [Google Scholar]
- Peeling, R.W.; Holmes, K.K.; Mabey, D.; Ronald, A. Rapid Tests for Sexually Transmitted Infections (STIs): The Way Forward. Sex Transm. Infect. 2006, 82, 1–6. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef]
- Jungreis, E. Spot Test Analysis: Clinical, Environmental, Forensic, and Geochemical Applications, 2nd ed.; Wiley-Blackwell: New York, NY, USA, 1997. [Google Scholar]
- Edwards, R. Immunodiagnostics: A Practical Approach, 1st ed.; Oxford University Press: Oxford, UK, 1999; ISBN 0199635889. [Google Scholar]
- Anslyn, E.V. Supramolecular Analytical Chemistry. J. Org. Chem. 2007, 72, 687–699. [Google Scholar] [CrossRef]
- Wang, B.; Anslyn, E.V. (Eds.) Chemosensors: Principles, Strategies, and Applications; Wiley: Hoboken, NJ, USA, 2011; ISBN 0854046356. [Google Scholar]
- Boiocchi, M.; Boca, L.D.; Esteban-Gómez, D.; Fabbrizzi, L.; Licchelli, M.; Monzani, E. Anion-Induced Urea Deprotonation. Chem.—Eur. J. 2005, 11, 3097–3104. [Google Scholar] [CrossRef] [PubMed]
- Buske, J.L.O.; Nicoleti, C.R.; Cavallaro, A.A.; Machado, V.G. 4-(Pyren-1-Ylimino)Methylphenol and Its Silylated Derivative as Chromogenic Chemosensors Highly Selective for Fluoride or Cyanide. J. Braz. Chem. Soc. 2015, 26, 2507–2519. [Google Scholar] [CrossRef]
- Nandi, L.G.; Nicoleti, C.R.; Marini, V.G.; Bellettini, I.C.; Valandro, S.R.; Cavalheiro, C.C.S.; Machado, V.G. Optical Devices for the Detection of Cyanide in Water Based on Ethyl(Hydroxyethyl)Cellulose Functionalized with Perichromic Dyes. Carbohydr. Polym. 2017, 157, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Nicoleti, C.R.; Garcia, D.N.; Da Silva, L.E.; Begnini, I.M.; Rebelo, R.A.; Joussef, A.C.; Machado, V.G. Synthesis of 1,8-Naphthyridines and Their Application in the Development of Anionic Fluorogenic Chemosensors. J. Fluoresc. 2012, 22, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Schramm, A.D.S.; Nicoleti, C.R.; Stock, R.I.; Heying, R.S.; Bortoluzzi, A.J.; Machado, V.G. Anionic Optical Devices Based on 4-(Nitrostyryl)Phenols for the Selective Detection of cyanide in Acetonitrile and Cyanide in Water. Sens. Actuators B Chem. 2017, 240, 1036–1048. [Google Scholar] [CrossRef]
- Ferreira, N.L.; de Cordova, L.M.; Schramm, A.D.S.; Nicoleti, C.R.; Machado, V.G. Chromogenic and Fluorogenic Chemodosimeter Derived from Meldrum’s Acid Detects Cyanide and Sulfide in Aqueous Medium. J. Mol. Liq. 2019, 282, 142–153. [Google Scholar] [CrossRef]
- Souto, F.T.; Jonatan, J.L.; Nicoleti, C.R.; Dreyer, J.P.; da, S. Heying, R.; Bortoluzzi, A.J.; Machado, V.G. Chromogenic Chemodosimeter Based on a Silylated Azo Compound Detects Cyanide in Water and Cassava. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 260, 119950. [Google Scholar] [CrossRef] [PubMed]
- Quang, D.T.; Kim, J.S. Fluoro- and Chromogenic Chemodosimeters for Heavy Metal Ion Detection in Solution and Biospecimens. Chem. Rev. 2010, 110, 6280–6301. [Google Scholar] [CrossRef] [PubMed]
- Chemate, S.; Erande, Y.; Mohbiya, D.; Sekar, N. Acridine Derivative as a “Turn on” Probe for Selective Detection of Picric Acid: Via PET Deterrence. RSC Adv. 2016, 6, 84319–84325. [Google Scholar] [CrossRef]
- Dai, Q.; Gao, C.; Liu, Y.; Liu, H.; Xiao, B.; Chen, C.; Chen, J.; Yuan, Z.; Jiang, Y. Highly Sensitive and Selective “Naked Eye” Sensing of Cu(II) by a Novel Acridine-Based Sensor Both in Aqueous Solution and on the Test Kit. Tetrahedron 2018, 74, 6459–6464. [Google Scholar] [CrossRef]
- Lee, S.C.; Park, S.; So, H.; Lee, G.; Kim, K.-T.; Kim, C. An Acridine-Based Fluorescent Sensor for Monitoring ClO− in Water Samples and Zebrafish. Sensors 2020, 20, 4764. [Google Scholar] [CrossRef] [PubMed]
- Carlos, F.d.S.; da Silva, L.A.; Zanlorenzi, C.; Nunes, F.S. A Novel Macrocycle Acridine-Based Fluorescent Chemosensor for Selective Detection of Cd2+ in Brazilian Sugarcane Spirit and Tobacco Cigarette Smoke Extract. Inorg. Chim. Acta. 2020, 508, 119634. [Google Scholar] [CrossRef]
- Carlos, F.d.S.; Monteiro, R.F.; da Silva, L.A.; Zanlorenzi, C.; Nunes, F.S. A Highly Selective Acridine-Based Fluorescent Probe for Detection of Al3+ in Alcoholic Beverage Samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 231, 118119. [Google Scholar] [CrossRef]
- Nunes, M.C.; Carlos, F.; Fuganti, O.; Galindo, D.D.M.; De Boni, L.; Abate, G.; Nunes, F.S. Turn-on Fluorescence Study of a Highly Selective Acridine-Based Chemosensor for Zn2+ in Aqueous Solutions. Inorg. Chim. Acta 2020, 499, 119191. [Google Scholar] [CrossRef]
- Attia, G.; Rahali, S.; Teka, S.; Fourati, N.; Zerrouki, C.; Seydou, M.; Chehimi, S.; Hayouni, S.; Mbakidi, J.P.; Bouquillon, S.; et al. Anthracene Based Surface Acoustic Wave Sensors for Picomolar Detection of Lead Ions. Correlation between Experimental Results and DFT Calculations. Sens. Actuators B Chem. 2018, 276, 349–355. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, B. Recent Development in Anthracene Possessing Chemosensors for Cations and Anions. Microchem. J. 2020, 158, 105131. [Google Scholar] [CrossRef]
- Prusti, B.; Chakravarty, M. Electron-Rich Anthracene-Based Twisted π-System as a Highly Fluorescent Dye: Easy Recognition of Solvents and Volatile Organic Compounds. Dyes Pigm. 2020, 181, 108543. [Google Scholar] [CrossRef]
- Tümay, S.O.; Irani-nezhad, M.H.; Khataee, A. Development of Dipodal Fluorescence Sensor of Iron for Real Samples Based on Pyrene Modified Anthracene. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 261, 120017. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Gupta, A.; Kaur, N. A Novel, Anthracene-Based Naked Eye Probe for Detecting Hg2+ Ions in Aqueous as Well as Solid State Media. Microchem. J. 2020, 153, 104508. [Google Scholar] [CrossRef]
- Kim, D.; Yamamoto, K.; Ahn, K.H. A BODIPY-Based Reactive Probe for Ratiometric Fluorescence Sensing of Mercury Ions. Tetrahedron 2012, 68, 5279–5282. [Google Scholar] [CrossRef]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent Indicators Based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K. Recent Advances in the Application of BODIPY in Bioimaging and Chemosensing. J. Mater. Chem. C Mater. 2019, 7, 11361–11405. [Google Scholar] [CrossRef]
- Wang, L.; Ding, H.; Ran, X.; Tang, H.; Cao, D. Recent Progress on Reaction-Based BODIPY Probes for Anion Detection. Dyes Pigm. 2020, 172, 107857. [Google Scholar] [CrossRef]
- Shi, W.J.; Huang, Y.; Liu, W.; Xu, D.; Chen, S.T.; Liu, F.; Hu, J.; Zheng, L.; Chen, K. A BODIPY-Based “OFF-ON” Fluorescent Probe for Fast and Selective Detection of Hypochlorite in Living Cells. Dyes Pigm. 2019, 170, 107566. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Y.; Shao, S. A BODIPY Based Fluorescent Chemosensor for Cu(II) Ions and Homocysteine/Cysteine. Sens. Actuators B Chem. 2012, 171–172, 872–877. [Google Scholar] [CrossRef]
- Cao, D.; Liu, Z.; Verwilst, P.; Koo, S.; Jangjili, P.; Kim, J.S.; Lin, W. Coumarin-Based Small-Molecule Fluorescent Chemosensors. Chem. Rev. 2019, 119, 10403–10519. [Google Scholar] [CrossRef]
- Devendhiran, T.; Kumarasamy, K.; Lin, M.C.; Yang, Y.X. Synthesis and Physical Studies of Coumarin-Based Chemosensor for Cyanide Ions. Inorg. Chem. Commun. 2021, 134, 108951. [Google Scholar] [CrossRef]
- Sun, X.Y.; Liu, T.; Sun, J.; Wang, X.J. Synthesis and Application of Coumarin Fluorescence Probes. RSC Adv. 2020, 10, 10826–10847. [Google Scholar] [CrossRef] [PubMed]
- Janeková, H.; Gašpar, J.; Gáplovský, A.; Stankovičová, H. Selective Fluoride Chemosensors Based on Coumarin Semicarbazones. J. Photochem. Photobiol. A Chem. 2021, 410, 113168. [Google Scholar] [CrossRef]
- Şenol, A.M.; Onganer, Y.; Meral, K. An Unusual “off-on” Fluorescence Sensor for Iron(III) Detection Based on Fluorescein–Reduced Graphene Oxide Functionalized with Polyethyleneimine. Sens. Actuators B Chem. 2017, 239, 343–351. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Y.; Wang, R.; Wang, B.; Chang, H.; Chen, J.; Yang, G.; He, H. Fluorescein-Based Fluorescent Sensor with High Selectivity for Mercury and Its Imaging in Living Cells. Inorg. Chem. Commun. 2018, 89, 46–50. [Google Scholar] [CrossRef]
- Keerthana, S.; Sam, B.; George, L.; Sudhakar, Y.N.; Varghese, A. Fluorescein Based Fluorescence Sensors for the Selective Sensing of Various Analytes. J. Fluoresc. 2021, 31, 1251–1276. [Google Scholar] [CrossRef]
- Rathod, R.V.; Bera, S.; Maity, P.; Mondal, D. Mechanochemical Synthesis of a Fluorescein-Based Sensor for the Selective Detection and Removal of Hg2+ Ions in Industrial Effluents. ACS Omega 2020, 5, 4982–4990. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, M. Recent Development in Fluorescein Derivatives. J. Mol. Struct. 2021, 1224, 129085. [Google Scholar] [CrossRef]
- Das, B.; Jana, A.; Das Mahapatra, A.; Chattopadhyay, D.; Dhara, A.; Mabhai, S.; Dey, S. Fluorescein Derived Schiff Base as Fluorimetric Zinc (II) Sensor via ‘Turn on’ Response and Its Application in Live Cell Imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 212, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Liu, X.; Zhou, L.; He, H.; Zhou, P.; Duan, C.; Peng, X. A Fluorescein Derivative-Based Fluorescent Sensor for Selective Recognition of Copper(II) Ions. J. Photochem. Photobiol. A Chem. 2018, 355, 67–71. [Google Scholar] [CrossRef]
- Hou, L.; Feng, J.; Wang, Y.; Dong, C.; Shuang, S.; Wang, Y. Single Fluorescein-Based Probe for Selective Colorimetric and Fluorometric Dual Sensing of Al3+ and Cu2+. Sens. Actuators B Chem. 2017, 247, 451–460. [Google Scholar] [CrossRef]
- Ciardelli, G.; Ranieri, N. The Treatment and Reuse of Wastewater in the Textile Industry by Means of Ozonation and Electroflocculation. Water Res. 2001, 35, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Chulanova, E.A.; Irtegova, I.G.; Vasilieva, N.V.; Bagryanskaya, I.Y.; Gritsan, N.P.; Zibarev, A.V. Novel Long-Lived π-Heterocyclic Radical Anion: A Hybrid of 1,2,5-Thiadiazo- and 1,2,3-Dithiazolidyls. Mendeleev Commun. 2015, 25, 336–338. [Google Scholar] [CrossRef]
- Prakash, S.M.; Jayamoorthy, K.; Srinivasan, N.; Dhanalekshmi, K.I. Fluorescence tuning of 2-(1H-Benzimidazol-2-yl)phenol-ESIPT process. J. Lumin. 2016, 172, 304–308. [Google Scholar] [CrossRef]
- Cho, N.; Song, K.; Lee, J.K.; Ko, J. Facile Synthesis of Fluorine-Substituted Benzothiadiazole-Based Organic Semiconductors and Their Use in Solution-Processed Small-Molecule Organic Solar Cells. Chem.—Eur. J. 2012, 18, 11433–11439. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, R.; Nájera, C. Recent Advances in Sonogashira Reactions. Chem. Soc. Rev. 2011, 40, 5084–5121. [Google Scholar] [CrossRef] [PubMed]
- Jindal, G.; Vashisht, P.; Kaur, N. Benzimidazole appended optical sensors for ionic species: Compilation of literature reports from 2017 to 2022. Results Chem. 2022, 4, 100551. [Google Scholar] [CrossRef]
- Dias, G.G.; Rodrigues, M.O.; Paz, E.R.S.; Nunes, M.P.; Araujo, M.H.; Rodembusch, F.S.; Da Silva Júnior, E.N. Aryl-Phenanthro[9,10-d]Imidazole: A Versatile Scaffold for the Design of Optical-Based Sensors. ACS Sens. 2022, 7, 2865–2919. [Google Scholar] [CrossRef] [PubMed]
- Padghan, S.D.; Wang, C.Y.; Liu, W.C.; Sun, S.S.; Liu, K.M.; Chen, K.Y. A Naphthalene-Based Colorimetric and Fluorometric Dual-Channel Chemodosimeter for Sensing Cyanide in a Wide pH Range. Dyes Pigm. 2020, 183, 108724. [Google Scholar] [CrossRef]
- Muniyasamy, H.; Chinnadurai, C.; Nelson, M.; Chinnamadhaiyan, M.; Ayyanar, S. Triazole-Naphthalene Based Fluorescent Chemosensor for Highly Selective Naked Eye Detection of Carbonate Ion and Real Sample Analyses. Inorg. Chem. Commun. 2021, 133, 108883. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Jin, S.; Zhang, Y.; Chen, X.; Zhang, Z.; Shu, Q. Naphthalene Based Lab-on-a-Molecule for Fluorimetric and Colorimetric Sensing of F− and CN− and Nitroaromatic Explosives. Sens. Actuators B Chem. 2017, 242, 994–998. [Google Scholar] [CrossRef]
- Xiao, N.; Zhang, C. Selective Monitoring of Cu(II) with a Fluorescence–on Naphthalene–Based Probe in Aqueous Solution. Inorg. Chem. Commun. 2019, 107, 107467. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, X.; Gu, W.; Cheng, T.; Wang, B.; Jiang, Y.; Shen, J. A Novel Naphthalene-Based Fluorescent Probe for Highly Selective Detection of Cysteine with a Large Stokes Shift and Its Application in Bioimaging. New J. Chem. 2018, 42, 18109–18116. [Google Scholar] [CrossRef]
- Hagimori, M.; Taniura, M.; Mizuyama, N.; Karimine, Y.; Kawakami, S.; Saji, H.; Mukai, T. Synthesis of a Novel Pyrazine-Pyridone Biheteroaryl-Based Fluorescence Sensor and Detection of Endogenous Labile Zinc Ions in Lung Cancer Cells. Sensors 2019, 19, 2049. [Google Scholar] [CrossRef]
- Dhanushkodi, M.; Vinoth Kumar, G.G.; Balachandar, B.K.; Sarveswari, S.; Gandhi, S.; Rajesh, J. A Simple Pyrazine Based Ratiometric Fluorescent Sensor for Ni2+ Ion Detection. Dyes Pigm. 2020, 173, 107897. [Google Scholar] [CrossRef]
- Shi, F.; Cui, S.; Liu, H.; Pu, S. A High Selective Fluorescent Sensor for Cu2+ in Solution and Test Paper Strips. Dyes Pigm. 2020, 173, 107914. [Google Scholar] [CrossRef]
- Guo, F.-F.; Wang, B.-B.; Wu, W.-N.; Bi, W.-Y.; Xu, Z.-H.; Fan, Y.-C.; Bian, L.-Y.; Wang, Y. A Pyrazine-Containing Hydrazone Derivative for Sequential Detection of Al3+ and F−. J. Mol. Struct. 2022, 1251, 132073. [Google Scholar] [CrossRef]
- Prabakaran, G.; David, C.I.; Nandhakumar, R. A review on pyrene based chemosensors for the specific detection on d-transition metal ions and their various applications. J. Environ. Chem. Eng. 2023, 11, 109701. [Google Scholar] [CrossRef]
- Kowser, Z.; Rayhan, U.; Akther, T.; Redshaw, C.; Yamato, T. A brief review on novel pyrene based fluorometric and colorimetric chemosensors for the detection of Cu2+. Mater. Chem. Front. 2021, 5, 2173–2200. [Google Scholar] [CrossRef]
- Kinik, F.P.; Ortega-Guerrero, A.; Ongari, D.; Ireland, C.P.; Smit, B. Pyrene-based metal organic frameworks: From synthesis to applications. Chem. Soc. Rev. 2021, 50, 3143–3177. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Mishra, G.; Pathak, A.K.; Pandey, S.; Awasthi, C.; Pandey, M.D.; Behera, K. Pyrene-Appended Luminescent Probes for Selective Detection of Toxic Heavy Metals and Live Cell Applications. ChemistrySelect 2024, 9, e202303914. [Google Scholar] [CrossRef]
- Zimmermann-Dimer, L.M.; Reis, D.C.; Machado, C.; Machado, V.G. Chromogenic Anionic Chemosensors Based on Protonated Merocyanine Solvatochromic Dyes in Trichloromethane and in Trichloromethane-Water Biphasic System. Tetrahedron 2009, 65, 4239–4248. [Google Scholar] [CrossRef]
- Zimmermann-Dimer, L.M.; Machado, V.G. Chromogenic Anionic Chemosensors Based on Protonated Merocyanine Solvatochromic Dyes: Influence of the Medium on the Quantitative and Naked-Eye Selective Detection of Anionic Species. Dyes Pigm. 2009, 82, 187–195. [Google Scholar] [CrossRef]
- Guerra, J.P.T.A.; Lindner, A.; Nicoleti, C.R.; Marini, V.G.; Silva, M.; Machado, V.G. Synthesis of Anionic Chemodosimeters Based on Silylated Pyridinium N-Phenolate Betaine Dyes. Tetrahedron Lett. 2015, 56, 4733–4736. [Google Scholar] [CrossRef]
- Machado, V.G.; Stock, R.I.; Reichardt, C. Pyridinium N-Phenolate Betaine Dyes. Chem. Rev. 2014, 114, 10429–10475. [Google Scholar] [CrossRef] [PubMed]
- Nicoleti, C.R.; Nandi, L.G.; Ciancaleoni, G.; Machado, V.G. Spectrometric and Kinetics Studies Involving Anionic Chromogenic Chemodosimeters Based on Silylated Imines in Acetonitrile or Acetonitrile-Water Mixtures. RSC Adv. 2016, 6, 101853–101861. [Google Scholar] [CrossRef]
- Huang, C.; Fan, J.; Peng, X.; Lin, Z.; Guo, B.; Ren, A.; Cui, J.; Sun, S. Highly Selective and Sensitive Twin-Cyano-Stilbene-Based Two-Photon Fluorescent Probe for Mercury (II) in Aqueous Solution with Large Two-Photon Absorption Cross-Section. J. Photochem. Photobiol. A Chem. 2008, 199, 144–149. [Google Scholar] [CrossRef]
- Zhu, M.Q.; Gu, Z.; Zhang, R.; Xiang, J.N.; Nie, S. A Stilbene-Based Fluoroionophore for Copper Ion Sensing in Both Reduced and Oxidized Environments. Talanta 2010, 81, 678–683. [Google Scholar] [CrossRef]
- Stock, R.I.; Dreyer, J.P.; Nunes, G.E.; Bechtold, I.H.; Machado, V.G. Optical Chemosensors and Chemodosimeters for Anion Detection Based on Merrifield Resin Functionalized with Brooker’s Merocyanine Derivatives. ACS Appl. Polym. Mater. 2019, 1, 1757–1768. [Google Scholar] [CrossRef]
- Dreyer, J.P.; Stock, R.I.; Nandi, L.G.; Bellettini, I.C.; Machado, V.G. Electrospun Blends Comprised of Poly(Methyl Methacrylate) and Ethyl(Hydroxyethyl)Cellulose Functionalized with Perichromic Dyes. Carbohydr. Polym. 2020, 236, 115991. [Google Scholar] [CrossRef] [PubMed]
- Nandi, L.G.; Nicoleti, C.R.; Bellettini, I.C.; Machado, V.G. Optical Chemosensor for the Detection of Cyanide in Water Based on Ethyl(Hydroxyethyl)Cellulose Functionalized with Brooker’s Merocyanine. Anal. Chem. 2014, 86, 4653–4656. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, Z.; Mu, L.; Zeng, X.; Redshaw, C.; Wei, G. A Quinoline-Based Fluorometric and Colorimetric Dual-Modal pH Probe and Its Application in Bioimaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 188, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Mikata, Y.; Nodomi, Y.; Kizu, A.; Konno, H. Quinoline-Attached Triazacyclononane (TACN) Derivatives as Fluorescent Zinc Sensors. Dalton Trans. 2014, 43, 1684–1690. [Google Scholar] [CrossRef]
- Loya, M.; Hazarika, S.I.; Pahari, P.; Atta, A.K. Fluorometric Detection of Cu2+ and Ni2+ by a Quinoline-Based Glucopyranose Derivative via the Excimer of Quinoline Subunit. J. Mol. Struct. 2021, 1241, 130634. [Google Scholar] [CrossRef]
- Ranee, S.J.; Sivaraman, G.; Pushpalatha, A.M.; Muthusubramanian, S. Quinoline Based Sensors for Bivalent Copper Ions in Living Cells. Sens. Actuators B Chem. 2018, 255, 630–637. [Google Scholar] [CrossRef]
- Paisuwan, W.; Rashatasakhon, P.; Ruangpornvisuti, V.; Sukwattanasinitt, M.; Ajavakom, A. Dipicolylamino Quinoline Derivative as Novel Dual Fluorescent Detecting System for Hg2+ and Fe3+. Sens. Biosens. Res. 2019, 24, 100283. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, C.Y.; Liu, J.; Dong, D. Recent Developments in Rhodamine Salicylidene Hydrazone Chemosensors. Anal. Methods 2016, 8, 2863–2871. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhou, L.; He, H.; Yin, J.; Gao, Q.; Wei, J.; Duan, C.; Peng, X. A Novel Rhodamine B-Based “off-on’’ Fluorescent Sensor for Selective Recognition of Copper (II) Ions. Talanta 2018, 184, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.; Lim, C.S.; Kim, H.M.; Ghosh, K. New Six-Membered pH-Insensitive Rhodamine Spirocycle in Selective Sensing of Cu2+ through C-C Bond Cleavage and Its Application in Cell Imaging. ACS Omega 2017, 2, 8167–8176. [Google Scholar] [CrossRef] [PubMed]
- Arumugaperumal, R.; Srinivasadesikan, V.; Lin, M.C.; Shellaiah, M.; Shukla, T.; Lin, H.C. Facile Rhodamine-Based Colorimetric Sensors for Sequential Detections of Cu(II) Ions and Pyrophosphate (P2O74−) Anions. RSC Adv. 2016, 6, 106631–106640. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, E.; Ding, P.; Tang, J.; Zhang, D.; Zhao, Y.; Ye, Y. Two Rhodamine Based Chemosensors for Sn4+ and the Application in Living Cells. Sens. Actuators B Chem. 2015, 221, 688–693. [Google Scholar] [CrossRef]
- da Silva, L.C.; Machado, V.G.; Menezes, F.G. Quinoxaline-Based Chromogenic and Fluorogenic Chemosensors for the Detection of Metal Cations. Chem. Pap. 2021, 75, 1775–1793. [Google Scholar] [CrossRef]
- Dey, S.K.; Al Kobaisi, M.; Bhosale, S.V. Functionalized Quinoxaline for Chromogenic and Fluorogenic Anion Sensing. ChemistryOpen 2018, 7, 934–952. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Hashemi, Z.; Biparva, P. A Multifunctional Quinoxaline-Based Chemosensor for Colorimetric Detection of Fe3+ and Highly Selective Fluorescence Turn-off Response of Cu2+ and Their Practical Application. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 302, 123092. [Google Scholar] [CrossRef]
- Neto, B.A.D.; Lapis, A.A.M.; Mancilha, F.S.; Batista, E.L., Jr.; Netz, P.A.; Rominger, F.; Basso, L.A.; Santos, D.S.; Dupont, J. On the Selective Detection of Duplex Deoxyribonucleic Acids by 2,1,3-Benzothiadiazole Fluorophores. Mol. Biosyst. 2010, 6, 967. [Google Scholar] [CrossRef] [PubMed]
- Neto, B.A.D.; Correa, J.R.; Spencer, J. Fluorescent Benzothiadiazole Derivatives as Fluorescence Imaging Dyes: A Decade of New Generation Probes. Chem.—Eur. J. 2022, 28, e202103262. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Xu, J.; Peng, F.; Peng, S.; Liao, J.; Zhao, H.; Li, L.; Zeng, X.; Yu, H. Novel Dual Acceptor (D-D′-A′-π-A) Dye-Sensitized Solar Cells Based on the Triarylamine Structure and Benzothiadiazole Double Electron Withdrawing Unit. New J. Chem. 2021, 45, 4443–4452. [Google Scholar] [CrossRef]
- Fang, H.; Gao, H.; Wang, T.; Zhang, B.; Xing, W.; Cheng, X. Benzothiadiazole-Based D-π-A-π-D Fluorophores: Synthesis, Self-Assembly, Thermal and Photophysical Characterization. Dyes Pigm. 2017, 147, 190–198. [Google Scholar] [CrossRef]
- Frizon, T.E.A.; Valdivia Martínez, J.C.; Westrup, J.L.; Duarte, R.d.C.; Zapp, E.; Domiciano, K.G.; Rodembusch, F.S.; Dal-Bó, A.G. 2,1,3-Benzothiadiazole-Based Fluorophores. Synthesis, Electrochemical, Thermal and Photophysical Characterization. Dyes Pigm. 2016, 135, 26–35. [Google Scholar] [CrossRef]
- Aguiar, L.d.O.; Regis, E.; Tuzimoto, P.; Girotto, E.; Bechtold, I.H.; Gallardo, H.; Vieira, A.A. Investigation of Thermal and Luminescent Properties in 4,7-Diphenylethynyl-2,1,3-Benzothiadiazole Systems. Liq. Cryst. 2018, 45, 49–58. [Google Scholar] [CrossRef]
- Casey, A.; Ashraf, R.S.; Fei, Z.; Heeney, M. Thioalkyl-Substituted Benzothiadiazole Acceptors: Copolymerization with Carbazole Affords Polymers with Large Stokes Shifts and High Solar Cell Voltages. Macromolecules 2014, 47, 2279–2288. [Google Scholar] [CrossRef]
- Ishi-I, T.; Sakai, M.; Shinoda, C. Benzothiadiazole-Based Dyes That Emit Red Light in Solution, Solid, and Liquid State. Tetrahedron 2013, 69, 9475–9480. [Google Scholar] [CrossRef]
- Patalag, L.J.; Werz, D.B. Benzothiadiazole Oligoene Fatty Acids: Fluorescent Dyes with Large Stokes Shifts. Beilstein J. Org. Chem. 2016, 12, 2739–2747. [Google Scholar] [CrossRef]
- Thomas, A.M.; Sujatha, A.; Anilkumar, G. Recent Advances and Perspectives in Copper-Catalyzed Sonogashira Coupling Reactions. RSC Adv. 2014, 4, 21688–21698. [Google Scholar] [CrossRef]
- Jagtap, S. Heck Reaction—State of the Art. Catalysts 2017, 7, 267. [Google Scholar] [CrossRef]
- Carsten, B.; He, F.; Son, H.J.; Xu, T.; Yu, L. Stille Polycondensation for Synthesis of Functional Materials. Chem. Rev. 2011, 111, 1493–1528. [Google Scholar] [CrossRef] [PubMed]
- Bellina, F.; Carpita, A.; Rossi, R. Palladium Catalysts for the Suzuki Cross-Coupling Reaction: An Overview of Recent Advances. Synthesis 2004, 2419–2440. [Google Scholar] [CrossRef]
- Dias, G.G.; Pinho, P.V.B.; Duarte, H.A.; Resende, J.M.; Rosa, A.B.B.; Correa, J.R.; Neto, B.A.D.; Da Silva Júnior, E.N. Fluorescent Oxazoles from Quinones for Bioimaging Applications. RSC Adv. 2016, 6, 76053–76063. [Google Scholar] [CrossRef]
- Srivastav, N.; Singh, R.; Kaur, V. Carbastannatranes: A Powerful Coupling Mediators in Stille Coupling. RSC Adv. 2015, 5, 62202–62213. [Google Scholar] [CrossRef]
- Martín-Matute, B.; Szabó, K.J.; Mitchell, T.N. Organotin Reagents in Cross-Coupling Reactions. In Metal Catalyzed Cross-Coupling Reactions and More; de Meijere, A., Bräse, S., Oestreich, M., Eds.; Wiley-VCH: Weinheim, Germany, 2013; Volume 1, pp. 423–474. [Google Scholar] [CrossRef]
- Levashov, A.S.; Buryi, D.S.; Goncharova, O.V.; Konshin, V.V.; Dotsenko, V.V.; Andreev, A.A. Tetraalkynylstannanes in the Stille Cross Coupling Reaction: A New Effective Approach to Arylalkynes. New J. Chem. 2017, 41, 2910–2918. [Google Scholar] [CrossRef]
- Hall, D.G. Structure, Properties, and Preparation of Boronic Acid Derivatives. Overview of Their Reactions and Applications. In Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine; Hall, D.G., Ed.; Wiley-VCH: Weinheim, Germany, 2005; ISBN 9783527606542. [Google Scholar]
- Lennox, A.J.J.; Lloyd-Jones, G.C. Selection of Boron Reagents for Suzuki-Miyaura Coupling. Chem. Soc. Rev. 2014, 43, 412–443. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.; Elagamy, A.; Althagafi, I.; Pratap, R. Synthesis of Alkynes from Non-Alkyne Sources. Org. Biomol. Chem. 2020, 18, 3797–3817. [Google Scholar] [CrossRef]
- Li, J.; Meng, J.; Huang, X.; Cheng, Y.; Zhu, C. A Highly Selective Fluorescent Sensor for Hg2+ Based on the Water-Soluble Poly(p-Phenyleneethynylene). Polymer 2010, 51, 3425–3430. [Google Scholar] [CrossRef]
- Huang, X.; Xu, Y.; Zheng, L.; Meng, J.; Cheng, Y. A Highly Selective and Sensitive Fluorescence Chemosensor Based on Optically Active Polybinaphthyls for Hg2+. Polymer 2009, 50, 5996–6000. [Google Scholar] [CrossRef]
- Pu, K.Y.; Liu, B. Fluorescence Turn-on Responses of Anionic and Cationic Conjugated Polymers toward Proteins: Effect of Electrostatic and Hydrophobic Interactions. J. Phys. Chem. B 2010, 114, 3077–3084. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.J.; Lin, J.Y.; Hu, Z.J.; Hsiao, V.K.S.; Chung, M.Y.; Wu, J.Y. Luminescent Zinc(II) Coordination Polymers of Bis(Pyridin-4-Yl)Benzothiadiazole and Aromatic Polycarboxylates for Highly Selective Detection of Fe(III) and High-Valent Oxyanions. Cryst. Growth Des. 2021, 21, 2056–2067. [Google Scholar] [CrossRef]
- Dey, S.; Singh, B.; Dasgupta, S.; Dutta, A.; Indra, A.; Lahiri, G.K. Ruthenium-Benzothiadiazole Building Block Derived Dynamic Heterometallic Ru-Ag Coordination Polymer and Its Enhanced Water-Splitting Feature. Inorg. Chem. 2021, 60, 9607–9620. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wu, W.C.; Chen, C.Y.; Strovas, T.; Li, Y.; Jin, Y.; Su, F.; Meldrum, D.R.; Jen, A.K.Y. 2,1,3-Benzothiadiazole (BTD)-Moiety-Containing Red Emitter Conjugated Amphiphilic Poly(Ethylene Glycol)-Block-Poly(ε-Caprolactone) Copolymers for Bioimaging. J. Mater. Chem. 2010, 20, 1728–1736. [Google Scholar] [CrossRef]
- Harris, J.D.; Stihl, M.; Schmidt, H.W.; Carter, K.R. Dithienobenzimidazole-Containing Conjugated Donor–Acceptor Polymers: Synthesis and Characterization. J. Polym. Sci. A Polym. Chem. 2019, 57, 60–69. [Google Scholar] [CrossRef]
- Wang, L.; Xia, Q.; Liu, R.; Qu, J. Real-Time Imaging of Cancer Cell Generations and Monitoring Tumor Growth Using a Nucleus-Targeted Red Fluorescent Probe. J. Mater. Chem. B 2018, 6, 2340–2346. [Google Scholar] [CrossRef]
- Wang, L.; Xia, Q.; Hou, M.; Yan, C.; Xu, Y.; Qu, J.; Liu, R. A Photostable Cationic Fluorophore for Long-Term Bioimaging. J. Mater. Chem. B 2017, 5, 9183–9188. [Google Scholar] [CrossRef]
- Souza, V.S.; Corrêa, J.R.; Carvalho, P.H.P.R.; Zanotto, G.M.; Matiello, G.I.; Guido, B.C.; Gatto, C.C.; Ebeling, G.; Gonçalves, P.F.B.; Dupont, J.; et al. Appending Ionic Liquids to Fluorescent Benzothiadiazole Derivatives: Light up and Selective Lysosome Staining. Sens. Actuators B Chem. 2020, 321, 128530. [Google Scholar] [CrossRef]
- Raitz, I.; De Souza Filho, R.Y.; De Andrade, L.P.; Correa, J.R.; Neto, B.A.D.; Pilli, R.A. Preferential Mitochondrial Localization of a Goniothalamin Fluorescent Derivative. ACS Omega 2017, 2, 3774–3784. [Google Scholar] [CrossRef]
- Qin, W.; Li, K.; Feng, G.; Li, M.; Yang, Z.; Liu, B.; Tang, B.Z. Bright and Photostable Organic Fluorescent Dots with Aggregation-Induced Emission Characteristics for Noninvasive Long-Term Cell Imaging. Adv. Funct. Mater. 2014, 24, 635–643. [Google Scholar] [CrossRef]
- Pennakalathil, J.; Jahja, E.; Özdemir, E.S.; Konu, Ö.; Tuncel, D. Red Emitting, Cucurbituril-Capped, pH-Responsive Conjugated Oligomer-Based Nanoparticles for Drug Delivery and Cellular Imaging. Biomacromolecules 2014, 15, 3366–3374. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Liu, J.; Zhang, R.; Liu, B. Cell Imaging Using Red Fluorescent Light-up Probes Based on an Environment-Sensitive Fluorogen with Intramolecular Charge Transfer Characteristics. Chem. Commun. 2014, 50, 9497–9500. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.A.R.; Correa, J.R.; De Andrade, L.P.; Assumpção, J.A.F.; De Souza Cintra, G.A.; Freitas-Junior, L.H.; Da Silva, W.A.; De Oliveira, H.C.B.; Neto, B.A.D. From Live Cells to Caenorhabditis Elegans: Selective Staining and Quantification of Lipid Structures Using a Fluorescent Hybrid Benzothiadiazole Derivative. ACS Omega 2018, 3, 3874–3881. [Google Scholar] [CrossRef] [PubMed]
- Palamà, I.; Di Maria, F.; Viola, I.; Fabiano, E.; Gigli, G.; Bettini, C.; Barbarella, G. Live-Cell-Permeant Thiophene Fluorophores and Cell-Mediated Formation of Fluorescent Fibrils. J. Am. Chem. Soc. 2011, 133, 17777–17785. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.F.D.; Santos, D.C.B.D.; Lapis, A.A.M.; Corrêa, J.R.; Gomes, A.F.; Gozzo, F.C.; Moreira, P.F.; De Oliveira, V.C.; Quina, F.H.; Neto, B.A.D. On the Use of 2,1,3-Benzothiadiazole Derivatives as Selective Live Cell Fluorescence Imaging Probes. Bioorganic Med. Chem. Lett. 2010, 20, 6001–6007. [Google Scholar] [CrossRef] [PubMed]
- Passos, S.T.A.; Souza, G.C.; Brandão, D.C.; Machado, D.F.S.; Grisolia, C.K.; Correa, J.R.; da Silva, W.A.; Neto, B.A.D. Plasma Membrane Staining with Fluorescent Hybrid Benzothiadiazole and Coumarin Derivatives: Tuning the Cellular Selection by Molecular Design. Dyes Pigm. 2021, 186, 109005. [Google Scholar] [CrossRef]
- Carvalho, P.H.P.R.; Correa, J.R.; Guido, B.C.; Gatto, C.C.; De Oliveira, H.C.B.; Soares, T.A.; Neto, B.A.D. Designed Benzothiadiazole Fluorophores for Selective Mitochondrial Imaging and Dynamics. Chem.—Eur. J. 2014, 20, 15360–15374. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Feng, L.; Yang, P. 2, 1, 3-Benzothiadiazole Derivative Small Molecule Fluorophores for NIR-II Bioimaging. Adv. Funct. Mater. 2024, 34, 2310818. [Google Scholar] [CrossRef]
- Neto, B.A.D.; Carvalho, P.H.P.R.; Correa, J.R. Benzothiadiazole Derivatives as Fluorescence Imaging Probes: Beyond Classical Scaffolds. Acc. Chem. Res. 2015, 48, 1560–1569. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, Z.; Zhao, Z.; Zhang, H.; Ma, D.; Lam, J.W.Y.; Qin, A.; Tang, B.Z. In Situ Generation of Red-Emissive AIEgens from Commercial Sources for Nondoped OLEDs. ACS Omega 2018, 3, 16347–16356. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, J.; Qu, J.; Qian, P.-C.; Wong, W.-Y. Recent progress of electronic materials based on 2,1,3-benzothiadiazole and its derivatives: Synthesis and their application in organic light-emitting diodes. Sci. China Chem. 2021, 64, 341–357. [Google Scholar] [CrossRef]
- Ni, F.; Wu, Z.; Zhu, Z.; Chen, T.; Wu, K.; Zhong, C.; An, K.; Wei, D.; Ma, D.; Yang, C. Teaching an Old Acceptor New Tricks: Rationally Employing 2,1,3-Benzothiadiazole as Input to Design a Highly Efficient Red Thermally Activated Delayed Fluorescence Emitter. J. Mater. Chem. C Mater. 2017, 5, 1363–1368. [Google Scholar] [CrossRef]
- Liu, T.; Chen, X.; Zhao, J.; Wei, W.; Mao, Z.; Wu, W.; Jiao, S.; Liu, Y.; Yang, Z.; Chi, Z. Hybridized Local and Charge-Transfer Excited State Fluorophores Enabling Organic Light-Emitting Diodes with Record High Efficiencies Close to 20%. Chem. Sci. 2021, 12, 5171–5176. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xie, G.; Zhong, C.; Gong, S.; Yang, C. Boosting the Efficiency of Near-Infrared Fluorescent OLEDs with an Electroluminescent Peak of Nearly 800 Nm by Sensitizer-Based Cascade Energy Transfer. Adv. Funct. Mater. 2018, 28, 1706088. [Google Scholar] [CrossRef]
- Gudeika, D.; Miasojedovas, A.; Bezvikonnyi, O.; Volyniuk, D.; Gruodis, A.; Jursenas, S.; Grazulevicius, J.V. Differently Substituted Benzothiadiazoles as Charge-Transporting Emitters for Fluorescent Organic Light-Emitting Diodes. Dyes Pigm. 2019, 166, 217–225. [Google Scholar] [CrossRef]
- Collier, G.S.; Brown, L.A.; Boone, E.S.; Kaushal, M.; Ericson, M.N.; Walter, M.G.; Long, B.K.; Kilbey, S.M. Linking Design and Properties of Purine-Based Donor-Acceptor Chromophores as Optoelectronic Materials. J. Mater. Chem. C Mater. 2017, 5, 6891–6898. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Wang, C.; Zhou, X.; Xu, Y.; Hanif, M.; Qiu, X.; Hu, D.; Ma, D.; Ma, Y. Highly Efficient Deep-Red/near-Infrared D-A Chromophores Based on Naphthothiadiazole for OLEDs Applications. Dyes Pigm. 2020, 173, 107960. [Google Scholar] [CrossRef]
- Zhang, X.; Yamaguchi, R.; Moriyama, K.; Kadowaki, M.; Kobayashi, T.; Ishi-i, T.; Thiemann, T.; Mataka, S. Highly Dichroic Benzo-2,1,3-Thiadiazole Dyes Containing Five Linearly π-Conjugated Aromatic Residues, with Fluorescent Emission Ranging from Green to Red, in a Liquid Crystal Guest-Host System. J. Mater. Chem. 2006, 16, 736–740. [Google Scholar] [CrossRef]
- Zhang, X.; Gorohmaru, H.; Kadowaki, M.; Kobayashi, T. Benzo-2,1,3-Thiadiazole-Based, Highly Dichroic Fluorescent Dyes for Fluorescent Host–Guest Liquid Crystal Displays. J. Mater. Chem. 2004, 14, 1901–1904. [Google Scholar] [CrossRef]
- Vieira, A.A.; Cristiano, R.; Bortoluzzi, A.J.; Gallardo, H. Luminescent 2,1,3-Benzothiadiazole-Based Liquid Crystalline Compounds. J. Mol. Struct. 2008, 875, 364–371. [Google Scholar] [CrossRef]
- Benevides, T.O.; Regis, E.; Nicoleti, C.R.; Bechtold, I.H.; Vieira, A.A. Phase-Dependent Photoluminescence of Non-Symmetric 2,1,3-Benzothiadiazole Liquid Crystals. Dyes Pigm. 2019, 163, 300–307. [Google Scholar] [CrossRef]
- Zhao, Z.; Yin, Z.; Chen, H.; Zheng, L.; Zhu, C.; Zhang, L.; Tan, S.; Wang, H.; Guo, Y.; Tang, Q.; et al. High-Performance, Air-Stable Field-Effect Transistors Based on Heteroatom-Substituted Naphthalenediimide-Benzothiadiazole Copolymers Exhibiting Ultrahigh Electron Mobility up to 8.5 Cm V−1 S−1. Adv. Mater. 2017, 29, 1602410. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.Y.; Zhang, J.; Long, G.; Wang, Z.; Zhang, Q. Solution-Processable Thiadiazoloquinoxaline-Based Donor-Acceptor Small Molecules for Thin-Film Transistors. J. Mater. Chem. C Mater. 2016, 4, 3809–3814. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, W.; Zhou, Y.; Wei, C.; Huang, J.; Wang, Q.; Wang, L.; Yu, G. Chalcogenophene-Sensitive Charge Carrier Transport Properties in A-D-A′′-D Type NBDO-Based Copolymers for Flexible Field-Effect Transistors. Macromolecules 2018, 51, 8662–8671. [Google Scholar] [CrossRef]
- Bulumulla, C.; Gunawardhana, R.; Kularatne, R.N.; Hill, M.E.; McCandless, G.T.; Biewer, M.C.; Stefan, M.C. Thieno[3,2- b] Pyrrole-Benzothiadiazole Banana-Shaped Small Molecules for Organic Field-Effect Transistors. ACS Appl. Mater. Interfaces 2018, 10, 11818–11825. [Google Scholar] [CrossRef] [PubMed]
- Baek, N.S.; Hau, S.K.; Yip, H.-L.; Acton, O.; Chen, K.-S.; Jen, A.K.-Y. High Performance Amorphous Metallated π-Conjugated Polymers for Field-Effect Transistors and Polymer Solar Cells. Chem. Mater. 2008, 20, 5734–5736. [Google Scholar] [CrossRef]
- Livi, F.; Gobalasingham, N.S.; Thompson, B.C.; Bundgaard, E. Analysis of Diverse Direct Arylation Polymerization (DArP) Conditions toward the Efficient Synthesis of Polymers Converging with Stille Polymers in Organic Solar Cells. J. Polym. Sci. A Polym. Chem. 2016, 54, 2907–2918. [Google Scholar] [CrossRef]
- Heuvel, R.; van Franeker, J.J.; Janssen, R.A.J. Energy Level Tuning of Poly(Phenylene-Alt-Dithienobenzothiadiazole)s for Low Photon Energy Loss Solar Cells. Macromol. Chem. Phys. 2017, 218, 1600502. [Google Scholar] [CrossRef] [PubMed]
- Gobalasingham, N.S.; Ekiz, S.; Pankow, R.M.; Livi, F.; Bundgaard, E.; Thompson, B.C. Carbazole-Based Copolymers via Direct Arylation Polymerization (DArP) for Suzuki-Convergent Polymer Solar Cell Performance. Polym. Chem. 2017, 8, 4393–4402. [Google Scholar] [CrossRef]
- Gautam, P.; Misra, R.; Siddiqui, S.A.; Sharma, G.D. Unsymmetrical Donor-Acceptor-Acceptor-π-Donor Type Benzothiadiazole-Based Small Molecule for a Solution Processed Bulk Heterojunction Organic Solar Cell. ACS Appl. Mater. Interfaces 2015, 7, 10283–10292. [Google Scholar] [CrossRef]
- Du, C.; Li, W.; Li, C.; Bo, Z. Ethynylene-Containing Donor-Acceptor Alternating Conjugated Polymers: Synthesis and Photovoltaic Properties. J. Polym. Sci. A Polym. Chem. 2013, 51, 383–393. [Google Scholar] [CrossRef]
- Cariello, M.; Ahn, S.; Park, K.W.; Chang, S.K.; Hong, J.; Cooke, G. An Investigation of the Role Increasing π-Conjugation Has on the Efficiency of Dye-Sensitized Solar Cells Fabricated from Ferrocene-Based Dyes. RSC Adv. 2016, 6, 9132–9138. [Google Scholar] [CrossRef]
- Ashraf, R.S.; Gilot, J.; Janssen, R.A.J. Fused Ring Thiophene-Based Poly(Heteroarylene Ethynylene)s for Organic Solar Cells. Sol. Energy Mater. 2010, 94, 1759–1766. [Google Scholar] [CrossRef]
- Gu, H.; Liu, W.; Li, H.; Sun, W.; Du, J.; Fan, J.; Peng, X. 2,1,3-Benzothiadiazole Derivative AIEgens for Smart Phototheranostics. Coord. Chem. Rev. 2022, 473, 214803. [Google Scholar] [CrossRef]
- Li, H.; Lin, H.; Lv, W.; Gai, P.; Li, F. Equipment-Free and Visual Detection of Multiple Biomarkers via an Aggregation Induced Emission Luminogen-Based Paper Biosensor. Biosens. Bioelectron. 2020, 165, 112336. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, B.; Shen, Y.; Cong, H. Design of NIR-II High Performance Organic Small Molecule Fluorescent Probes and Summary of Their Biomedical Applications. Coord. Chem. Rev. 2022, 468, 214609. [Google Scholar] [CrossRef]
- Galdino, N.M.; Souza, V.S.; Rodembusch, F.S.; Bussamara, R.; Scholten, J.D. Biosensors Based on Graphene Oxide Functionalized with Benzothiadiazole-Derived Ligands for the Detection of Cholesterol. ACS Appl. Biol. Mater. 2023, 6, 2651–2666. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; He, F.; Yao, L.; Gu, C.; Xu, H.; Xie, Z.; Wu, H.; Ma, Y. Chemistry and Materials Based on 5,5′-Bibenzo[c][1,2,5]Thiadiazole. Chem. Commun. 2013, 49, 5730–5732. [Google Scholar] [CrossRef]
- Misra, R.; Gautam, P.; Mobin, S.M. Aryl-Substituted Unsymmetrical Benzothiadiazoles: Synthesis, Structure, and Properties. J. Org. Chem. 2013, 78, 12440–12452. [Google Scholar] [CrossRef]
- Kato, S.I.; Matsumoto, T.; Shigeiwa, M.; Gorohmaru, H.; Maeda, S.; Ishi-i, T.; Mataka, S. Novel 2,1,3-Benzothiadiazole-Based Red-Fluorescent Dyes with Enhanced Two-Photon Absorption Cross-Sections. Chem.—Eur. J. 2006, 12, 2303–2317. [Google Scholar] [CrossRef]
- Hamer, S.; Röhricht, F.; Jakoby, M.; Howard, I.A.; Zhang, X.; Näther, C.; Herges, R. Synthesis of Dipolar Molecular Rotors as Linkers for Metal-Organic Frameworks. Beilstein J. Org. Chem. 2019, 15, 1331–1338. [Google Scholar] [CrossRef]
- Krüger, R.; Iepsen, B.; Larroza, A.M.E.; Fronza, M.G.; Silveira, C.H.; Bevilacqua, A.C.; Köhler, M.H.; Piquini, P.C.; Lenardão, E.J.; Savegnago, L.; et al. Symmetrical and Unsymmetrical 4,7-Bis-Arylvinyl-Benzo-2,1,3-Chalcogenodiazoles: Synthesis, Photophysical and Electrochemical Properties and Biomolecular Interaction Studies. Eur. J. Org. Chem. 2020, 2020, 348–361. [Google Scholar] [CrossRef]
- Behramand, B.; Molin, F.; Gallardo, H. Dyes and Pigments Synthesis, Characterization and Photophysical/Electrochemical Properties. Dyes Pigm. 2012, 95, 600–605. [Google Scholar] [CrossRef]
- Xu, B.; Wu, X.; Li, H.; Tong, H.; Wang, L. Selective Detection of TNT and Picric Acid by Conjugated Polymer Film Sensors with Donor-Acceptor Architecture. Macromolecules 2011, 44, 5089–5092. [Google Scholar] [CrossRef]
- Malik, A.H.; Hussain, S.; Iyer, P.K. Aggregation-Induced FRET via Polymer-Surfactant Complexation: A New Strategy for the Detection of Spermine. Anal. Chem. 2016, 88, 7358–7364. [Google Scholar] [CrossRef] [PubMed]
- Zhan, R.; Liu, B. Benzothiadiazole-Containing Conjugated Polyelectrolytes for Biological Sensing and Imaging. Macromol. Chem. Phys. 2015, 216, 131–144. [Google Scholar] [CrossRef]
- Dong, Y.; Koken, B.; Ma, X.; Wang, L.; Cheng, Y.; Zhu, C. Polymer-Based Fluorescent Sensor Incorporating 2,2′-Bipyridyl and Benzo[2,1,3]Thiadiazole Moieties for Cu2+ Detection. Inorg. Chem. Commun. 2011, 14, 1719–1722. [Google Scholar] [CrossRef]
- Bouffard, J.; Swager, T.M. Fluorescent Conjugated Polymers That Incorporate Substituted 2,1,3-Benzooxadiazole and 2,1,3-Benzothiadiazole Units. Macromolecules 2008, 41, 5559–5562. [Google Scholar] [CrossRef]
- Wang, D.Y.; Wang, W.J.; Wang, R.; Xi, S.C.; Dong, B. A Fluorescent Covalent Triazine Framework Consisting of Donor–Acceptor Structure for Selective and Sensitive Sensing of Fe3+. Eur. Polym. J. 2021, 147, 110297. [Google Scholar] [CrossRef]
- Udhayakumari, D. Mechanistic Innovations in Fluorescent Chemosensors for Detecting Toxic Ions: PET, ICT, ESIPT, FRET and AIE Approaches. J. Fluoresc. 2024, 1–30. [Google Scholar] [CrossRef]
- Mako, T.L.; Racicot, J.M.; Levine, M. Supramolecular Luminescent Sensors. Chem. Rev. 2019, 119, 322–477. [Google Scholar] [CrossRef] [PubMed]
- Daly, B.; Ling, J.; De Silva, A.P. Current Developments in Fluorescent PET (Photoinduced Electron Transfer) Sensors and Switches. Chem. Soc. Rev. 2015, 44, 4203–4211. [Google Scholar] [CrossRef] [PubMed]
- Escudero, D. Revising Intramolecular Photoinduced Electron Transfer (PET) from First-Principles. Acc. Chem. Res. 2016, 49, 1816–1824. [Google Scholar] [CrossRef]
- Martínez-Máñez, R.; Sancenón, F. Fluorogenic and Chromogenic Chemosensors and Reagents for Anions. Chem. Rev. 2003, 103, 4419–4476. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Karmakar, M.; Bhatta, S.R.; Thakur, A. A Detailed Insight into Anion Sensing Based on Intramolecular Charge Transfer (ICT) Mechanism: A Comprehensive Review of the Years 2016 to 2021. Coord. Chem. Rev. 2021, 448, 214167. [Google Scholar] [CrossRef]
- Thompson, D.W.; Ito, A.; Meyer, T.J. [Ru(Bpy)3]2+* and Other Remarkable Metal-to-Ligand Charge Transfer (MLCT) Excited States. Pure Appl. Chem. 2013, 85, 1257–1305. [Google Scholar] [CrossRef]
- Sasaki, S.; Drummen, G.P.C.; Konishi, G.I. Recent Advances in Twisted Intramolecular Charge Transfer (TICT) Fluorescence and Related Phenomena in Materials Chemistry. J. Mater. Chem. C Mater. 2016, 4, 2731–2743. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zheng, K.; Zhu, S. FRET-Based Small-Molecule Fluorescent Probes: Rational Design and Bioimaging Applications. Acc. Chem. Res. 2013, 46, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Fabbrizzi, L.; Licchelli, M.; Pallavicini, P.; Parodi, L.; Taglietti, A. Fluorescent Sensors for and with Transition Metals. In Perspectives in Supramolecular Chemistry; Sauvage, J.P., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1999; pp. 93–134. [Google Scholar]
- Sedgwick, A.C.; Wu, L.; Han, H.H.; Bull, S.D.; He, X.P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-State Intramolecular Proton-Transfer (ESIPT) Based Fluorescence Sensors and Imaging Agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tang, B.Z. Aggregation-Induced Emission Luminogens for Activity-Based Sensing. Acc. Chem. Res. 2019, 52, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Tang, B.Z. Fluorescent Sensors Based on Aggregation-Induced Emission: Recent Advances and Perspectives. ACS Sens. 2017, 2, 1382–1399. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, E.; Lam, J.W.Y.; Tang, B.Z. AIE Luminogens: Emission Brightened by Aggregation. Mater. Today 2015, 18, 365–377. [Google Scholar] [CrossRef]
- Alam, P.; Leung, N.L.C.; Zhang, J.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. AIE-Based Luminescence Probes for Metal Ion Detection. Coord. Chem. Rev. 2021, 429, 213693. [Google Scholar] [CrossRef]
- Kim, D.; Ryu, H.G.; Ahn, K.H. Recent Development of Two-Photon Fluorescent Probes for Bioimaging. Org. Biomol. Chem. 2014, 12, 4550–4566. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Cho, B.R. Two-Photon Fluorescent Probes for Metal Ions. Chem. Asian J. 2011, 6, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Pourret, O. On the Necessity of Banning the Term “Heavy Metal” from the Scientific Literature. Sustainability 2018, 10, 2879. [Google Scholar] [CrossRef]
- Pourret, O.; Hursthouse, A. It’s Time to Replace the Term “Heavy Metals” with “Potentially Toxic Elements” When Reporting Environmental Research. Int. J. Environ. Res. Public Health 2019, 16, 4446. [Google Scholar] [CrossRef]
- Duffus, J.H. “Heavy Metals”—A Meaningless Term? (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Bradley, M.; Barst, B.; Basu, N. A Review of Mercury Bioavailability in Humans and Fish. Int. J. Environ. Res. Public Health 2017, 14, 169. [Google Scholar] [CrossRef] [PubMed]
- Egeland, G.M.; Middaugh, J.P. Balancing Fish Consumption Benefits with Mercury Exposure. Science 1997, 278, 1904–1905. [Google Scholar] [CrossRef] [PubMed]
- Oregaard, G.; Sørensen, S.J. High Diversity of Bacterial Mercuric Reductase Genes from Surface and Sub-Surface Floodplain Soil (Oak Ridge, USA). ISME J. 2007, 1, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, L.; Wang, L.; Wu, Q.; Wang, F.; Hao, J. A Review of Atmospheric Mercury Emissions, Pollution and Control in China. Front. Environ. Sci. Eng. 2014, 8, 631–649. [Google Scholar] [CrossRef]
- Wang, Q.; Kim, D.; Dionysiou, D.D.; Sorial, G.A.; Timberlake, D. Sources and Remediation for Mercury Contamination in Aquatic Systems—A Literature Review. Environ. Pollut. 2004, 131, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, X.; Guo, G.; Yan, Z. Status and Environmental Management of Soil Mercury Pollution in China: A Review. J. Environ. Manag. 2021, 277, 111442. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, R.A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Harris, H.H.; Pickering, I.J.; George, G.N. The Chemical Form of Mercury in Fish. Science 2003, 301, 1203. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.M.; Lippard, S.J. Tools and Tactics for the Optical Detection of Mercuric Ion. Chem. Rev. 2008, 108, 3443–3480. [Google Scholar] [CrossRef]
- Klein, G.L. Aluminum Toxicity to Bone: A Multisystem Effect? Osteoporos. Sarcopenia 2019, 5, 2–5. [Google Scholar] [CrossRef]
- Greger, J.L.; Sutherland, J.E. Aluminum Exposure and Metabolism. Crit. Rev. Clin. Lab. Sci. 1997, 34, 439–474. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Esposito, S. Aluminum in Vaccines: Does It Create a Safety Problem? Vaccine 2018, 36, 5825–5831. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.S.; Zhuang, L.; Bottema, J.; Wittebrood, A.J.; De Smet, P.; Haszler, A.; Vieregge, A. Recent Development in Aluminium Alloys for the Automotive Industry. Mater. Sci. Eng. A. 2000, 280, 37–49. [Google Scholar] [CrossRef]
- Wu, X.; Guo, Z.; Wu, Y.; Zhu, S.; James, T.D.; Zhu, W. Near-Infrared Colorimetric and Fluorescent Cu2+ Sensors Based on Indoline-Benzothiadiazole Derivatives via Formation of Radical Cations. ACS Appl. Mater. Interfaces 2013, 5, 12215–12220. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D. Aluminium, Antiperspirants and Breast Cancer. J. Inorg. Biochem. 2005, 99, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Al-fartusie, F.S.; Mohssan, S.N. Essential Trace Elements and Their Vital Roles in Human Body. Indian J. Adv. Chem. Sci. 2017, 5, 127–136. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological Relevance and Mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Brocato, J.; Costa, M. Oral Chromium Exposure and Toxicity. Curr. Environ. Health Rep. 2015, 2, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.C.; Steinbeisser, S.; Zane, G.M.; Wall, J.D.; Fields, M.W. Cr(VI) Reduction and Physiological Toxicity Are Impacted by Resource Ratio in Desulfovibrio Vulgaris. Appl. Microbiol. Biotechnol. 2018, 102, 2839–2850. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Choudhury, N.; Saha, B.; De, P. Recent Progress in Polymer-Based Optical Chemosensors for Cu2+ and Hg2+ Ions: A Comprehensive Review. Eur. Polym. J. 2021, 145, 110233. [Google Scholar] [CrossRef]
- Domaille, D.W.; Que, E.L.; Chang, C.J. Synthetic Fluorescent Sensors for Studying the Cell Biology of Metals. Nat. Chem. Biol. 2008, 4, 168–175. [Google Scholar] [CrossRef]
- Kumawat, L.K.; Mergu, N.; Asif, M.; Gupta, V.K. Novel Synthesized Antipyrine Derivative Based “Naked Eye” Colorimetric Chemosensors for Al3+ and Cr3+. Sens. Actuators B Chem. 2016, 231, 847–859. [Google Scholar] [CrossRef]
- Bagchi, D.; Stohs, S.J.; Downs, B.W.; Bagchi, M.; Preuss, H.G. Cytotoxicity and Oxidative Mechanisms of Different Forms of Chromium. Toxicology 2002, 180, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Denkhaus, E.; Salnikow, K. Nickel Essentiality, Toxicity, and Carcinogenicity. Crit. Rev. Oncol. Hematol. 2002, 42, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chae, P.S.; Kumar, S. A Dual-Responsive Anthrapyridone-Triazole-Based Probe for Selective Detection of Ni2+ and Cu2+: A Mimetic System for Molecular Logic Gates Based on Color Change. Dyes Pigm. 2020, 174, 108092. [Google Scholar] [CrossRef]
- Gao, G.; Cao, Y.; Liu, W.; Li, D.; Zhou, W.; Liu, J. Fluorescent Sensors for Sodium Ions. Anal. Methods 2017, 9, 5570–5579. [Google Scholar] [CrossRef]
- Jaitovich, A.; Bertorello, A.M. Intracellular Sodium Sensing: SIK1 Network, Hormone Action and High Blood Pressure. Biochim. Biophys. Acta Mol. Basis Dis. 2010, 1802, 1140–1149. [Google Scholar] [CrossRef]
- Grant, F.D.; Romero, J.R.; Jeunemaitre, X.; Hunt, S.C.; Hopkins, P.N.; Hollenberg, N.H.; Williams, G.H. Low-Renin Hypertension, Altered Sodium Homeostasis, and an α-Adducin Polymorphism. Hypertension 2002, 39, 191–196. [Google Scholar] [CrossRef]
- Gale, P.A.; Caltagirone, C. Anion Sensing by Small Molecules and Molecular Ensembles. Chem. Soc. Rev. 2015, 44, 4212–4227. [Google Scholar] [CrossRef]
- Zimmermann-Dimer, L.M.; Machado, V.G. Chromogenic and Fluorogenic Chemosensors for Detection of Anionic Analites. Quim. Nova 2008, 31, 2134–2146. [Google Scholar] [CrossRef]
- Figueroa, L.E.S.; Moragues, M.E.; Climent, E.; Agostini, A.; Martínez-Máñez, R.; Sancenón, F. Chromogenic and Fluorogenic Chemosensors and Reagents for Anions. A Comprehensive Review of the Years 2010-2011. Chem. Soc. Rev. 2013, 42, 3489–3613. [Google Scholar] [CrossRef]
- Chakraborty, S.; Paul, S.; Roy, P.; Rayalu, S. Detection of Cyanide Ion by Chemosensing and Fluorosensing Technology. Inorg. Chem. Commun. 2021, 128, 108562. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Yoon, J. Recent Progress in the Development of Fluorometric and Colorimetric Chemosensors for Detection of Cyanide Ions. Chem. Soc. Rev. 2014, 43, 4312–4324. [Google Scholar] [CrossRef]
- Vogel, S.N.; Sultan, T.R. Cyanide Poisoning. Clin. Toxicol. 1964, 8, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Dasgupta, P.K. Analytica Chimica Acta Recent Developments in Cyanide Detection: A Review. Anal. Chim. Acta 2010, 673, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Balomajumder, C.; Agarwal, V.K. Enzymatic Mechanism and Biochemistry for Cyanide Degradation: A Review. J. Hazard. Mater. 2010, 176, 1–13. [Google Scholar] [CrossRef]
- Pati, P.B. Organic Chemodosimeter for Cyanide: A Nucleophilic Approach. Sens. Actuators B Chem. 2016, 222, 374–390. [Google Scholar] [CrossRef]
- Clarkson, J.J.; McLoughlin, J. Role of Fluoride in Oral Health Promotion. Int. Dent. J. 2000, 50, 119–128. [Google Scholar] [CrossRef]
- Pollick, H. The Role of Fluoride in the Prevention of Tooth Decay. Pediatr. Clin. N. Am. 2018, 65, 923–940. [Google Scholar] [CrossRef]
- Gai, L.; Mack, J.; Lu, H.; Nyokong, T.; Li, Z.; Kobayashi, N.; Shen, Z. Organosilicon Compounds as Fluorescent Chemosensors for Fluoride Anion Recognition. Coord. Chem. Rev. 2015, 285, 24–51. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.F.; Yoon, J. Fluorescence and Colorimetric Chemosensors for Fluoride-Ion Detection. Chem. Rev. 2014, 114, 5511–5571. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Chen, W.; Cheng, Y.; Hakuna, L.; Strongin, R.; Wang, B. Thiol Reactive Probes and Chemosensors. Sensors 2012, 12, 15907–15946. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A.; Choi, Y.B.; Takahashi, H.; Zhang, D.; Li, W.; Godzik, A.; Bankston, L.A. Cysteine Regulation of Protein Function—As Exemplified by NMDA-Receptor Modulation. Trends Neurosci. 2002, 25, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of Its Protective Roles, Measurement, and Biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Munday, R.; Winterbourn, C.C. Reduced Glutathione in Combination with Superoxide Dismutase as an Important Biological Antioxidant Defence Mechanism. Biochem. Pharmacol. 1989, 38, 4349–4352. [Google Scholar] [CrossRef] [PubMed]
- Szabõ, C. Hydrogen Sulphide and Its Therapeutic Potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Y.; Peng, X.; Yoon, J. Fluorescent and Colorimetric Probes for Detection of Thiols. Chem. Soc. Rev. 2010, 39, 2120–2135. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Miao, F.J.P.; Lin, D.C.H.; Schwandner, R.T.; Wang, Z.; Gao, J.; Chen, J.L.; Tlan, H.; Ling, L. Citric Acid Cycle Intermediates as Ligands for Orphan G-Protein-Coupled Receptors. Nature 2004, 429, 188–193. [Google Scholar] [CrossRef]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Fu, X.; Chin, R.M.; Vergnes, L.; Hwang, H.; Deng, G.; Xing, Y.; Pai, M.Y.; Li, S.; Ta, L.; Fazlollahi, F.; et al. 2-Hydroxyglutarate Inhibits ATP Synthase and MTOR Signaling. Cell Metab. 2015, 22, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.S.; Patel, J.; Wise, D.R.; Abdel-Wahab, O.; Bennett, B.D.; Coller, H.A.; Cross, J.R.; Fantin, V.R.; Hedvat, C.V.; Perl, A.E.; et al. The Common Feature of Leukemia-Associated IDH1 and IDH2 Mutations Is a Neomorphic Enzyme Activity Converting α-Ketoglutarate to 2-Hydroxyglutarate. Cancer Cell 2010, 17, 225–234. [Google Scholar] [CrossRef]
- Das, A.; Alam, M.; Gogoi, C.; Dalapati, R.; Biswas, S. Rational Design of a Functionalized Aluminum Metal-Organic Framework as a Turn-off Fluorescence Sensor for α-Ketoglutaric Acid. Dalton Trans. 2020, 49, 16928–16934. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.L.; Chen, L.H.; Nan, F.J. Discovery of a Novel Calcium-Sensitive Fluorescent Probe for α-Ketoglutarate. Acta Pharmacol. Sin. 2017, 38, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.H.; Hao, Y.; Chen, W.; Zhang, Y.; Xu, M.; Yang, M.; Liu, Y.N. Recent Progress in the Development of Fluorescent Probes for Hydrazine. Luminescence 2018, 33, 816–836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Yang, Y.S.; Wang, W.; Jiao, Q.C.; Zhu, H.L. Fluorescent Sensors for the Detection of Hydrazine in Environmental and Biological Systems: Recent Advances and Future Prospects. Coord. Chem. Rev. 2020, 417, 213367. [Google Scholar] [CrossRef]
- Hu, X.; Cao, H.; Dong, W.; Tang, J. Ratiometric Fluorescent Sensing of Ethanol Based on Copper Nanoclusters with Tunable Dual Emission. Talanta 2021, 233, 122480. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Liu, K.; Liu, H.; Wang, G.; Liu, T.; Miao, R.; Peng, H.; Fang, Y. Film-Based Fluorescent Sensor for Monitoring Ethanol-Water-Mixture Composition via Vapor Sampling. Anal. Chem. 2018, 90, 14088–14093. [Google Scholar] [CrossRef]
- Da Rocha, D.R. Oxalyl Chloride: A Versatile Reagent in Organic Synthesis. Synlett 2007, 2007, 1172–1173. [Google Scholar] [CrossRef][Green Version]
- Boersch, C.; Merkul, E.; Müller, T.J.J. Catalytic Syntheses of N-Heterocyclic Ynones and Ynediones by in Situ Activation of Carboxylic Acids with Oxalyl Chloride. Angew. Chem. Int. Ed. 2011, 50, 10448–10452. [Google Scholar] [CrossRef]
- Gupta, N.K.; Pashigreva, A.; Pidko, E.A.; Hensen, E.J.M.; Mleczko, L.; Roggan, S.; Ember, E.E.; Lercher, J.A. Bent Carbon Surface Moieties as Active Sites on Carbon Catalysts for Phosgene Synthesis. Angew. Chem. Int. Ed. 2016, 128, 1760–1764. [Google Scholar] [CrossRef]
- Rosenthal, T.; Baum, G.L.; Frand, U.; Molho, M. Poisoning Caused by Inhalation of Hydrogen Chloride, Phosphorus Oxychloride, Phosphorus Pentachloride, Oxalyl Chloride, and Oxalic Acid. Chest 1978, 73, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Barbee, S.J.; Stone, J.J.; Hilaski, R.J. Acute Inhalation Toxicology of Oxalyl Chloride. Am. Ind. Hyg. Assoc. J. 1995, 56, 74–76. [Google Scholar] [CrossRef]
- Gangopadhyay, A.; Ali, S.S.; Kumar, A. A Powerful Turn-On Fluorescent Probe for Phosgene: A Primary Amide Strategically Attached to an Anthracene Fluorophore. ChemistrySelect 2019, 4, 8968–8972. [Google Scholar] [CrossRef]
- Tan, J.; Li, Z.; Lu, Z.; Chang, R.; Sun, Z.; You, J. Recent Progress in the Development of Chemodosimeters for Fluorescence Visualization of Phosgene. Dyes Pigm. 2021, 193, 109540. [Google Scholar] [CrossRef]
- Tang, L.; Sun, Y.; Zhong, K.; Jin, L. A TCF-Based Colorimetric and Fluorescent Probe for Highly Selective Detection of Oxalyl Chloride. Tetrahedron Lett. 2020, 61, 152470. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Design of Fluorescent Sensors Based on Azaheterocyclic Push-Pull Systems towards Nitroaromatic Explosives and Related Compounds: A Review. Dyes Pigm. 2020, 180, 108414. [Google Scholar] [CrossRef]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva, C. Multi-Phase Semicrystalline Microstructures Drive Exciton Dissociation in Neat Plastic Semiconductors. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef]
- Akhgari, F.; Fattahi, H.; Oskoei, Y.M. Recent Advances in Nanomaterial-Based Sensors for Detection of Trace Nitroaromatic Explosives. Sens. Actuators B Chem. 2015, 221, 867–878. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, W.; He, Q.; Cheng, J. Recent Progress in Thin Film Fluorescent Probe for Organic Amine Vapour. Sci. China Chem. 2016, 59, 3–15. [Google Scholar] [CrossRef]
- Ahmed, M.; Latif, N.; Khan, R.A.; Ahmad, A.; Schetinger, M.R.; Rocha, J.B. Toxicological Effect of N, N, N′, N′-Tetramethylethylene on Rat Brain Acetylcholinesterase. Toxicol. Ind. Health 2014, 30, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Danchuk, A.I.; Komova, N.S.; Mobarez, S.N.; Doronin, S.Y.; Burmistrova, N.A.; Markin, A.V.; Duerkop, A. Optical Sensors for Determination of Biogenic Amines in Food. Anal. Bioanal. Chem. 2020, 412, 4023–4036. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Chopra, S.; Singh, G.; Raj, P.; Bhasin, A.; Sahoo, S.K.; Kuwar, A.; Singh, N. Chemosensors for Biogenic Amines and Biothiols. J. Mater. Chem. B 2018, 6, 4872–4902. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Bin, H.; Zhang, Y.; Li, Y. Synthesis and Optoelectronic Properties of New D-A Copolymers Based on Fluorinated Benzothiadiazole and Benzoselenadiazole. Polym. Chem. 2014, 5, 567–577. [Google Scholar] [CrossRef]
- Cevher, S.C.; Hizalan, G.; Alemdar Yilmaz, E.; Cevher, D.; Udum Arslan, Y.; Toppare, L.; Yıldırım, E.; Cirpan, A. A Comprehensive Study: Theoretical and Experimental Investigation of Heteroatom and Substituent Effects on Frontier Orbitals and Polymer Solar Cell Performances. J. Polym. Sci. 2020, 58, 2792–2806. [Google Scholar] [CrossRef]
- Bin, H.; Angunawela, I.; Ma, R.; Nallapaneni, A.; Zhu, C.; Leenaers, P.J.; Saes, B.W.H.; Wienk, M.M.; Yan, H.; Ade, H.; et al. Effect of Main and Side Chain Chlorination on the Photovoltaic Properties of Benzodithiophene: Alt -Benzotriazole Polymers. J. Mater. Chem. C Mater. 2020, 8, 15426–15435. [Google Scholar] [CrossRef]
- Mo, D.; Lin, L.; Chao, P.; Lai, H.; Zhang, Q.; Tian, L.; He, F. Chlorination: Vs. Fluorination: A Study of Halogenated Benzo [c] [1,2,5]Thiadiazole-Based Organic Semiconducting Dots for near-Infrared Cellular Imaging. New J. Chem. 2020, 44, 7740–7748. [Google Scholar] [CrossRef]
- Shi, S.; Wang, H.; Chen, P.; Uddin, M.A.; Wang, Y.; Tang, Y.; Guo, H.; Cheng, X.; Zhang, S.; Woo, H.Y.; et al. Cyano-Substituted Benzochalcogenadiazole-Based Polymer Semiconductors for Balanced Ambipolar Organic Thin-Film Transistors. Polym. Chem. 2018, 9, 3873–3884. [Google Scholar] [CrossRef]
- Macé, Y.; Bony, E.; Delvaux, D.; Pinto, A.; Mathieu, V.; Kiss, R.; Feron, O.; Quetin-Leclercq, J.; Riant, O. Cytotoxic Activities and Metabolic Studies of New Combretastatin Analogues. Med. Chem. Res. 2015, 24, 3143–3156. [Google Scholar] [CrossRef]
- Milata, V.; Vaculka, M. 4-Amino and 5-Aminobenzothiadiazoles in Gould–Jacobs Reaction. Monatsh. Chem. 2019, 150, 711–719. [Google Scholar] [CrossRef]
- Kim, J.; Yun, M.H.; Kim, G.H.; Kim, J.Y.; Yang, C. Replacing 2,1,3-Benzothiadiazole with 2,1,3-Naphthothiadiazole in PCDTBT: Towards a Low Bandgap Polymer with Deep HOMO Energy Level. Polym. Chem. 2012, 3, 3276–3281. [Google Scholar] [CrossRef]
- Doyranlı, C.; Çolak, B.; Lacinel, G.; Can, M.; Koyuncu, F.B.; Koyuncu, S. Effect of the Planar Center Moiety for a Donor-Acceptor Polymeric Electrochrome. Polymer 2017, 108, 423–431. [Google Scholar] [CrossRef]
- Xiao, J.; Xiao, X.; Zhao, Y.; Wu, B.; Liu, Z.; Zhang, X.; Wang, S.; Zhao, X.; Liu, L.; Jiang, L. Synthesis, Physical Properties and Self-Assembly Behavior of Azole-Fused Pyrene Derivatives. Nanoscale 2013, 5, 5420–5425. [Google Scholar] [CrossRef] [PubMed]
- Kini, G.P.; Lee, S.K.; Shin, W.S.; Moon, S.J.; Song, C.E.; Lee, J.C. Achieving a Solar Power Conversion Efficiency Exceeding 9% by Modifying the Structure of a Simple, Inexpensive and Highly Scalable Polymer. J. Mater. Chem. A Mater. 2016, 4, 18585–18597. [Google Scholar] [CrossRef]
- Kini, G.P.; Oh, S.; Abbas, Z.; Rasool, S.; Jahandar, M.; Song, C.E.; Lee, S.K.; Shin, W.S.; So, W.W.; Lee, J.C. Effects on Photovoltaic Performance of Dialkyloxy-Benzothiadiazole Copolymers by Varying the Thienoacene Donor. ACS Appl. Mater. Interfaces 2017, 9, 12617–12628. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, Y.; Zhang, Q.; Nakatani, K.; Tian, H.; Zhu, W. Aromaticity-Controlled Thermal Stability of Photochromic Systems Based on a Six-Membered Ring as Ethene Bridges: Photochemical and Kinetic Studies. Chem.—Eur. J. 2012, 18, 11685–11694. [Google Scholar] [CrossRef] [PubMed]
- Toscani, A.; Marín-Hernández, C.; Robson, J.A.; Chua, E.; Dingwall, P.; White, A.J.P.; Sancenón, F.; de la Torre, C.; Martínez-Máñez, R.; Wilton-Ely, J.D.E.T. Highly Sensitive and Selective Molecular Probes for Chromo-Fluorogenic Sensing of Carbon Monoxide in Air, Aqueous Solution and Cells. Chem.—Eur. J. 2019, 25, 2069–2081. [Google Scholar] [CrossRef]
- Larsen, C.B.; Van Der Salm, H.; Shillito, G.E.; Lucas, N.T.; Gordon, K.C. Tuning the Rainbow: Systematic Modulation of Donor-Acceptor Systems through Donor Substituents and Solvent. Inorg. Chem. 2016, 55, 8446–8458. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Si, Y.; Wu, Z.; Zhang, K.; Li, J.; Yin, Y. Alkyne/Ruthenium(Ii) Complex-Based Ratiometric Surface-Enhanced Raman Scattering Nanoprobe for in Vitro and Ex Vivo Tracking of Carbon Monoxide. Anal. Chem. 2020, 92, 924–931. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, Z.; Huang, K.; Lu, P.; Wang, Y. Butterfly-Shaped π-Extended Benzothiadiazoles as Promising Emitting Materials for White OLEDs. J. Mater. Chem. C Mater. 2019, 7, 6706–6713. [Google Scholar] [CrossRef]
- Nakanishi, T.; Shirai, Y.; Han, L. Synthesis and Optical Properties of Photovoltaic Materials Based on the Ambipolar Dithienonaphthothiadiazole Unit. J. Mater. Chem. A Mater. 2015, 3, 4229–4238. [Google Scholar] [CrossRef]
- Efrem, A.; Lim, C.J.; Lu, Y.; Ng, S.C. Synthesis and Characterization of Dithienobenzothiadiazole-Based Donor-Acceptor Conjugated Polymers for Organic Solar Cell Applications. Tetrahedron Lett. 2014, 55, 4849–4852. [Google Scholar] [CrossRef]
- Arroyave, F.A.; Richard, C.A.; Reynolds, J.R. Efficient Synthesis of Benzo[1,2- b:6,5- b’]Dithiophene-4,5-Dione (BDTD) and Its Chemical Transformations into Precursors for π-Conjugated Materials. Org. Lett. 2012, 14, 6138–6141. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, C.; Zhou, L.; Liang, X.; Li, Y.; Sheng, G.; Du, Z.; Tang, J. An Effective Strategy to Design a Large Bandgap Conjugated Polymer by Tuning the Molecular Backbone Curvature. Macromol. Rapid Commun. 2021, 42, 2000757. [Google Scholar] [CrossRef] [PubMed]
- Neidlein, R.; Knecht, D. Bromierung Methylsubstituierter 2,1,3-Benzothiadiazole Und 2,1,3-Benzoselenadiazole. Chem. Ber. 1987, 120, 1593–1595. [Google Scholar] [CrossRef]
- Neidlein, R.; Knecht, D. Oxidationsreaktionen methyl-substituierter 2,1,3-Benzothiadiazole und 2,1,3-Benzoselenadiazole mit Selen-dioxid. Helv. Chim. Acta 1987, 70, 997–1000. [Google Scholar] [CrossRef]
- Ratha, R.; Singh, A.; Raju, T.B.; Iyer, P.K. Insight into the Synthesis and Fabrication of 5,6-Alt-Benzothiadiazole-Based D–π–A-Conjugated Copolymers for Bulk-Heterojunction Solar Cell. Polym. Bull. 2018, 75, 2933–2951. [Google Scholar] [CrossRef]
- Ratha, R.; Afroz, M.A.; Gupta, R.K.; Iyer, P.K. Functionalizing Benzothiadiazole with Non-Conjugating Ester Groups as Side Chains in a Donor-Acceptor Polymer Improves Solar Cell Performance. New J. Chem. 2019, 43, 4242–4252. [Google Scholar] [CrossRef]
- Mahesh, K.; Karpagam, S.; Putnin, T.; Le, H.; Bui, T.T.; Ounnunkad, K.; Goubard, F. Role of Cyano Substituents on Thiophene Vinylene Benzothiadiazole Conjugated Polymers and Application as Hole Transporting Materials in Perovskite Solar Cells. J. Photochem. Photobiol. A Chem. 2019, 371, 238–247. [Google Scholar] [CrossRef]
- Vanelle, P.; Liegeois, C.T.; Meuche, J.; Maldonado, J.; Crozet, M.P. An Original Way for Synthesis of New Nitro-Benzothiadiazole Derivatives. Heterocycles 1997, 45, 955–962. [Google Scholar] [CrossRef]
- Pilgram, K.; Zupan, M.; Skiles, R. Bromination of 2,1,3-benzothiadiazoles. J. Heterocycl. Chem. 1970, 7, 629–633. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, X.; Zhang, Y. Samarium Diiodide Mediated Regeneration of 1,2-Benzenediamine and Preparation of Benzimidazolin-2-Ones from 2,1,3-Benzothiadiazoles. J. Chem. Res. 2005, 2005, 21–22. [Google Scholar] [CrossRef]
- Biegger, P.; Schaffroth, M.; Tverskoy, O.; Rominger, F.; Bunz, U.H.F. A Stable Bis(Benzocyclobutadiene)-Annelated Tetraazapentacene Derivative. Chem.—Eur. J. 2016, 22, 15896–15901. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Kreis, F.; Popp, D.; Hübner, O.; Kaifer, E.; Himmel, H.J. 1,2,4,5-Tetrakis(Tetramethylguanidino)-3,6-Diethynyl-Benzenes: Fluorescent Probes, Redox-Active Ligands and Strong Organic Electron Donors. Chem.—Eur. J. 2020, 26, 10336–10347. [Google Scholar] [CrossRef] [PubMed]
- Prashad, M.; Liu, Y.; Repic, O. A Practical Method for the Reduction of 2,1,3-Benzothiadiazoles to 1,2-Benzenediamines with Magnesium and Methanol. Tetrahedron Lett. 2001, 42, 2277–2279. [Google Scholar] [CrossRef]
- Neto, B.A.D.S.; Lopes, A.S.; Wüst, M.; Costa, V.E.U.; Ebeling, G.; Dupont, J. Reductive Sulfur Extrusion Reaction of 2,1,3-Benzothiadiazole Compounds: A New Methodology Using NaBH4/CoCl2·6H2O(Cat) as the Reducing System. Tetrahedron Lett. 2005, 46, 6843–6846. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; He, X.; Tu, G. All-Thiophene-Substituted: N-Heteroacene Electron-Donor Materials for Efficient Organic Solar Cells. J. Mater. Chem. A Mater. 2016, 4, 13519–13524. [Google Scholar] [CrossRef]
- de Moliner, F.; King, A.; Dias, G.G.; de Lima, G.F.; de Simone, C.A.; Júnior, E.N.d.S.; Vendrell, M. Quinone-Derived π-Extended Phenazines as New Fluorogenic Probes for Live-Cell Imaging of Lipid Droplets. Front. Chem. 2018, 6, 339. [Google Scholar] [CrossRef]
- Ileri, M.; Hacioglu, S.O.; Toppare, L. The Effect of Para- and Meta-Substituted Fluorine on Optical Behavior of Benzimidazole Derivatives. Electrochim. Acta 2013, 109, 214–220. [Google Scholar] [CrossRef]
- Kwan, C.S.; Wang, T.; Chan, S.M.; Cai, Z.; Leung, K.C.F. Selective Detection of Sulfide in Human Lung Cancer Cells with a Blue-Fluorescent “ON-OFF-ON” Benzimidazole-Based Chemosensor Ensemble. Dalton Trans. 2020, 49, 5445–5453. [Google Scholar] [CrossRef]
- Keshtov, M.L.; Kuklin, S.A.; Ostapov, I.E.; Chen, F.C.; Khokhlov, A.R. Novel Regular D–A-Conjugated Polymers Based on 2,6-Bis(6-Fluoro-2-Hexyl-2H-Benzotriazol-4-Yl)-4,4-Bis(2-Ethylhexyl)-4H-Silolo[3,2-b:4,5-B′]Dithiophene Derivatives: Synthesis, Optoelectronic, and Electrochemical Properties. Dokl. Chem. 2016, 470, 274–278. [Google Scholar] [CrossRef]
- Fu, Y.; Qiu, F.; Zhang, F.; Mai, Y.; Wang, Y.; Fu, S.; Tang, R.; Zhuang, X.; Feng, X. A Dual-Boron-Cored Luminogen Capable of Sensing and Imaging. Chem. Commun. 2015, 51, 5298–5301. [Google Scholar] [CrossRef] [PubMed]
- Mancilha, F.S.; Da Silveira Neto, B.A.; Lopes, A.S.; Moreira, P.F.; Quina, F.H.; Gonçalves, R.S.; Dupont, J. Are Molecular 5,8-π-Extended Quinoxaline Derivatives Good Chromophores for Photoluminescence Applications? Eur. J. Org. Chem. 2006, 2006, 4924–4933. [Google Scholar] [CrossRef]
- Shome, S.; Singh, S.P. Access to Small Molecule Semiconductors: Via C-H Activation for Photovoltaic Applications. Chem. Commun. 2018, 54, 7322–7325. [Google Scholar] [CrossRef] [PubMed]
- He, C.Y.; Wu, C.Z.; Qing, F.L.; Zhang, X. Direct (Het)Arylation of Fluorinated Benzothiadiazoles and Benzotriazole with (Het)Aryl Iodides. J. Org. Chem. 2014, 79, 1712–1718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, W.; Rojas, A.J.; Jucov, E.V.; Timofeeva, T.V.; Parker, T.C.; Barlow, S.; Marder, S.R. Controllable Direct Arylation: Fast Route to Symmetrical and Unsymmetrical 4,7-Diaryl-5,6-Difluoro-2,1,3-Benzothiadiazole Derivatives for Organic Optoelectronic Materials. J. Am. Chem. Soc. 2013, 135, 16376–16379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Parker, T.C.; Chen, W.; Williams, L.R.; Khrustalev, V.N.; Jucov, E.V.; Barlow, S.; Timofeeva, T.V.; Marder, S.R. C-H-Activated Direct Arylation of Strong Benzothiadiazole and Quinoxaline-Based Electron Acceptors. J. Org. Chem. 2016, 81, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Pilgram, K.; Zupan, M. Anomalous Nitration in the 2,1,3-Benzothiadiazole Series. J. Org. Chem. 1971, 36, 207–209. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Liu, C.; Yang, W.; Li, Y. Strong Charge-Transfer Chromophores from [2+2] Cycloadditions of TCNE and TCNQ to Peripheral Donor-Substituted Alkynes. Eur. J. Org. Chem. 2011, 2011, 6445–6451. [Google Scholar] [CrossRef]
- Chen, S.; Qin, Z.; Liu, T.; Wu, X.; Li, Y.; Liu, H.; Song, Y.; Li, Y. Aggregation-Induced Emission on Benzothiadiazole Dyads with Large Third-Order Optical Nonlinearity. Phys. Chem. Chem. Phys. 2013, 15, 12660–12666. [Google Scholar] [CrossRef]
- Jenkinson, D.R.; Cadby, A.J.; Jones, S. The Synthesis and Photophysical Analysis of a Series of 4-Nitrobenzochalcogenadiazoles for Super-Resolution Microscopy. Chem.—Eur. J. 2017, 23, 12585–12592. [Google Scholar] [CrossRef] [PubMed]
- Komin, A.P.; Carmack, M. The Chemistry of 1,2,5-thiadiazoles V. Synthesis of 3,4-diamino-1,2,5-thiadiazole and [1,2,5] Thiadiazolo[3,4-b ]Pyrazines. J. Heterocycl. Chem. 1976, 13, 13–22. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Banerjee, D.; Arockiam, P.B.; Junge, H.; Junge, K.; Pohl, M.M.; Radnik, J.; Brückner, A.; Beller, M. Highly Selective Transfer Hydrogenation of Functionalised Nitroarenes Using Cobalt-Based Nanocatalysts. Green Chem. 2015, 17, 898–902. [Google Scholar] [CrossRef]
- Formenti, D.; Ferretti, F.; Topf, C.; Surkus, A.E.; Pohl, M.M.; Radnik, J.; Schneider, M.; Junge, K.; Beller, M.; Ragaini, F. Co-Based Heterogeneous Catalysts from Well-Defined A-Diimine Complexes: Discussing the Role of Nitrogen. J. Catal. 2017, 351, 79–89. [Google Scholar] [CrossRef]
- Li, W.; Artz, J.; Broicher, C.; Junge, K.; Hartmann, H.; Besmehn, A.; Palkovits, R.; Beller, M. Superior Activity and Selectivity of Heterogenized Cobalt Catalysts for Hydrogenation of Nitroarenes. Catal. Sci. Technol. 2019, 9, 157–162. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Surkus, A.E.; Junge, H.; Pohl, M.M.; Radnik, J.; Rabeah, J.; Huan, H.; Schünemann, V.; Brückner, A.; Beller, M. Nanoscale Fe2O3-Based Catalysts for Selective Hydrogenation of Nitroarenes to Anilines. Science 2013, 342, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesh, R.V.; Stemmler, T.; Surkus, A.E.; Junge, H.; Junge, K.; Beller, M. Hydrogenation Using Iron Oxide-Based Nanocatalysts for the Synthesis of Amines. Nat. Protoc. 2015, 10, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesh, R.V.; Natte, K.; Junge, H.; Beller, M. Nitrogen-Doped Graphene-Activated Iron-Oxide-Based Nanocatalysts for Selective Transfer Hydrogenation of Nitroarenes. ACS Catal. 2015, 5, 1526–1529. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Prashad, M.; Repič, O.; Blacklock, T.J. A Practical and Chemoselective Reduction of Nitroarenes to Anilines Using Activated Iron. Adv. Synth. Catal. 2005, 347, 217–219. [Google Scholar] [CrossRef]
- Sharma, U.; Verma, P.K.; Kumar, N.; Kumar, V.; Bala, M.; Singh, B. Phosphane-Free Green Protocol for Selective Nitro Reduction with an Iron-Based Catalyst. Chem.—Eur. J. 2011, 17, 5903–5907. [Google Scholar] [CrossRef]
- Sharma, U.; Kumar, P.; Kumar, N.; Kumar, V.; Singh, B. Highly Chemo- and Regioselective Reduction of Aromatic Nitro Compounds Catalyzed by Recyclable Copper(II) as Well as Cobalt(II) Phthalocyanines. Adv. Synth. Catal. 2010, 352, 1834–1840. [Google Scholar] [CrossRef]
- Sharma, U.; Kumar, N.; Verma, P.K.; Kumar, V.; Singh, B. Zinc Phthalocyanine with PEG-400 as a Recyclable Catalytic System for Selective Reduction of Aromatic Nitro Compounds. Green Chem. 2012, 14, 2289–2293. [Google Scholar] [CrossRef]
- Verma, P.K.; Bala, M.; Thakur, K.; Sharma, U.; Kumar, N.; Singh, B. Iron and Palladium(II) Phthalocyanines as Recyclable Catalysts for Reduction of Nitroarenes. Catal. Lett. 2014, 144, 1258–1267. [Google Scholar] [CrossRef]
- Sharma, S.; Bhattacherjee, D.; Das, P. Supported Rhodium Nanoparticles Catalyzed Reduction of Nitroarenes, Arylcarbonyls and Aryl/Benzyl Sulfoxides Using Ethanol/Methanol as In Situ Hydrogen Source. Adv. Synth. Catal. 2018, 360, 2131–2137. [Google Scholar] [CrossRef]
- Pedrajas, E.; Sorribes, I.; Gushchin, A.L.; Laricheva, Y.A.; Junge, K.; Beller, M.; Llusar, R. Chemoselective Hydrogenation of Nitroarenes Catalyzed by Molybdenum Sulphide Clusters. ChemCatChem 2017, 9, 1128–1134. [Google Scholar] [CrossRef]
- Li, G.; Zheng, S.; Wang, L.; Zhang, X. Metal-Free Chemoselective Hydrogenation of Nitroarenes by N-Doped Carbon Nanotubes via in Situ Polymerization of Pyrrole. ACS Omega 2020, 5, 7519–7528. [Google Scholar] [CrossRef] [PubMed]
- Bashirov, D.A.; Sukhikh, T.S.; Kuratieva, N.V.; Chulanova, E.A.; Yushina, I.V.; Gritsan, N.P.; Konchenko, S.N.; Zibarev, A.V. Novel Applications of Functionalized 2,1,3-Benzothiadiazoles for Coordination Chemistry and Crystal Engineering. RSC Adv. 2014, 4, 28309–28316. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Khisamov, R.M.; Bashirov, D.A.; Komarov, V.Y.; Molokeev, M.S.; Ryadun, A.A.; Benassi, E.; Konchenko, S.N. Tuning of the Coordination and Emission Properties of 4-Amino-2,1,3-Benzothiadiazole by Introduction of Diphenylphosphine Group. Cryst. Growth Des. 2020, 20, 5796–5807. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Komarov, V.Y.; Konchenko, S.N.; Benassi, E. The Hows and Whys of Peculiar Coordination of 4-Amino-2,1,3-Benzothiadiazole. Polyhedron 2018, 139, 33–43. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Bashirov, D.A.; Shuvaev, S.; Komarov, V.Y.; Kuratieva, N.V.; Konchenko, S.N.; Benassi, E. Noncovalent Interactions and Photophysical Properties of New Ag(I) Complexes with 4-Amino-2,1,3-Benzothiadiazole. Polyhedron 2018, 141, 77–86. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Ogienko, D.S.; Bashirov, D.A.; Kuratieva, N.V.; Komarov, V.Y.; Rakhmanova, M.I.; Konchenko, S.N. New Red-Luminescent Cadmium Coordination Polymers with 4-Amino-2,1,3-Benzothiadiazole. J. Coord. Chem. 2016, 69, 3284–3293. [Google Scholar] [CrossRef]
- Khisamov, R.; Sukhikh, T.; Bashirov, D.; Ryadun, A.; Konchenko, S. Structural and Photophysical Properties of 2,1,3-Benzothiadiazole Based Phosph(III)Azane and Its Complexes. Molecules 2020, 25, 2428. [Google Scholar] [CrossRef] [PubMed]
- Hanson, R.H.; Meloan, C.E. A Study of the Metal Complexes of 2,1,3-Benzoselenadiazole, 2,1,3-Benzothiadiazole and Their Derivatives—II. Inorg. Nucl. Chem. Lett. 1971, 7, 467–472. [Google Scholar] [CrossRef]
- Nagarajan, S.; Majumder, S.; Sharma, U.; Rajendran, S.; Kumar, N.; Chatterjee, S.; Singh, B. Synthesis and Anti-Angiogenic Activity of Benzothiazole, Benzimidazole Containing Phthalimide Derivatives. Bioorganic Med. Chem. Lett. 2013, 23, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Tichenor, M.S.; Keith, J.M.; Jones, W.M.; Pierce, J.M.; Merit, J.; Hawryluk, N.; Seierstad, M.; Palmer, J.A.; Webb, M.; Karbarz, M.J.; et al. Heteroaryl Urea Inhibitors of Fatty Acid Amide Hydrolase: Structure-Mutagenicity Relationships for Arylamine Metabolites. Bioorganic Med. Chem. Lett. 2012, 22, 7357–7362. [Google Scholar] [CrossRef] [PubMed]
- Keith, J.M.; Apodaca, R.; Xiao, W.; Seierstad, M.; Pattabiraman, K.; Wu, J.; Webb, M.; Karbarz, M.J.; Brown, S.; Wilson, S.; et al. Thiadiazolopiperazinyl Ureas as Inhibitors of Fatty Acid Amide Hydrolase. Bioorganic Med. Chem. Lett. 2008, 18, 4838–4843. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Alsaid, M.S.; El-Gaby, M.S.A.; Safwat, N.A.; Elaasser, M.M.; Soliman, A.M. Biological Evaluation of Some New N-(2,6-Dimethoxypyrimidinyl) Thioureido Benzenesulfonamide Derivatives as Potential Antimicrobial and Anticancer Agents. Eur. J. Med. Chem. 2016, 124, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.D.; Houari, Y.; Carvalho, P.H.P.R.; Neto, B.A.D.; Jacquemin, D. ESIPT or Not ESIPT? Revisiting Recent Results on 2,1,3-Benzothiadiazole under the TD-DFT Light. RSC Adv. 2014, 4, 14189–14192. [Google Scholar] [CrossRef]
- Suzuki, T.; Tsuji, T. Preparation, Structure, and Amphoteric Redox Properties of p-Phenylenediamine-Type Dyes Fused with a Chalcogenadiazole Unit. J. Org. Chem. 2001, 4, 8954–8960. [Google Scholar] [CrossRef]
- Brzozowski, Z.; Saączewski, F.; Gdaniec, M. Synthesis, Structural Characterization and in Vitro Antitumor Activity of 4-Dimethylaminopyridinium (6-Chloro-1,1-Dioxo-1,4,2-Benzodithiazin-3-yl) Methanides. Eur. J. Med. Chem. 2003, 38, 991–999. [Google Scholar] [CrossRef]
- Brzozowski, Z.; Saączewski, F. A New Type of Mixed Anhydride and Its Applications to the Synthesis of 7-Substituted 8-Chloro-5,5-Dioxoimidazo[1,2-b][1,4,2]Benzodithiazines with in Vitro Antitumor Activity. J. Med. Chem. 2002, 45, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, Z.; Saączewski, F.; Neamati, N. Synthesis, Antitumor and Anti-HIV Activities of Benzodithiazine-Dioxides. Bioorganic Med. Chem. 2006, 14, 2985–2993. [Google Scholar] [CrossRef] [PubMed]
- Aronov, A.M.; Munagala, N.R.; Ortiz De Montellano, P.R.; Kuntz, I.D.; Wang, C.C. Rational Design of Selective Submicromolar Inhibitors of Tritrichomonas Foetus Hypoxanthine-Guanine-Xanthine Phosphoribosyltransferase. Biochemistry 2000, 39, 4684–4691. [Google Scholar] [CrossRef]
- Robertson, L.A.; Shkrob, I.A.; Agarwal, G.; Zhao, Y.; Yu, Z.; Assary, R.S.; Cheng, L.; Moore, J.S.; Zhang, L. Fluorescence-Enabled Self-Reporting for Redox Flow Batteries. ACS Energy Lett. 2020, 5, 3062–3068. [Google Scholar] [CrossRef]
- Neumann, J.; Elangovan, S.; Spannenberg, A.; Junge, K.; Beller, M. Improved and General Manganese-Catalyzed N-Methylation of Aromatic Amines Using Methanol. Chem. Eur. J. 2017, 23, 5410–5413. [Google Scholar] [CrossRef] [PubMed]
- Bala, M.; Verma, P.K.; Sharma, U.; Kumar, N.; Singh, B. Iron Phthalocyanine as an Efficient and Versatile Catalyst for N-Alkylation of Heterocyclic Amines with Alcohols: One-Pot Synthesis of 2-Substituted Benzimidazoles, Benzothiazoles and Benzoxazoles. Green Chem. 2013, 15, 1687–1693. [Google Scholar] [CrossRef]
- Ferraro, V.; Girotto, M.; Bortoluzzi, M. N,N-Dimethyl-4-amino-2,1,3-benzothiadiazole: Synthesis and Luminescent Solvatochromism. Chem. Proc. 2022, 8, 87. [Google Scholar] [CrossRef]
- Bertrand, H.C.; Clède, S.; Guillot, R.; Lambert, F.; Policar, C. Luminescence Modulations of Rhenium Tricarbonyl Complexes Induced by Structural Variations. Inorg. Chem. 2014, 53, 6204–6223. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Somanathan, N. Poly(Thiophenes) Functionalised with Thiazole Heterocycles as Electroluminescent Polymers. J. Mater. Chem. 2006, 16, 2990–3000. [Google Scholar] [CrossRef]
- Sokolova, A.S.; Yarovaya, O.I.; Shernyukov, A.V.; Gatilov, Y.V.; Razumova, Y.V.; Zarubaev, V.V.; Tretiak, T.S.; Pokrovsky, A.G.; Kiselev, O.I.; Salakhutdinov, N.F. Discovery of a New Class of Antiviral Compounds: Camphor Imine Derivatives. Eur. J. Med. Chem. 2015, 105, 263–273. [Google Scholar] [CrossRef]
- Gao, R.; Canney, D.J. A Versatile and Practical Microwave-Assisted Synthesis of Sterically Hindered N-Arylpiperazines. J. Org. Chem. 2010, 75, 7451–7453. [Google Scholar] [CrossRef] [PubMed]
- Mundla, S.R.; Wilson, L.J.; Klopfenstein, S.R.; Seibel, W.L.; Nikolaides, N.N. A Novel Method for the Efficient Synthesis of 2-Arylamino-2-Imidazolines. Tetrahedron Lett. 2000, 41, 6563–6566. [Google Scholar] [CrossRef]
- Tortolani, D.R.; Poss, M.A. A Convenient Synthesis to N-Aryl-Substituted 4-Piperidones. Org. Lett. 1999, 1, 1261–1262. [Google Scholar] [CrossRef]
- Wu, T.Y.; Li, J.L. Electrochemical Synthesis, Optical, Electrochemical and Electrochromic Characterizations of Indene and 1,2,5-Thiadiazole-Based Poly(2,5-Dithienylpyrrole) Derivatives. RSC Adv. 2016, 6, 15988–15998. [Google Scholar] [CrossRef]
- Broncová, G.; Shishkanova, T.V.; Dendisová, M.; Člupek, M.; Kubáč, D.; Matějka, P. Poly(4-Amino-2,1,3-Benzothiadiazole) Films: Preparation, Characterization and Applications. Chem. Pap. 2017, 71, 359–366. [Google Scholar] [CrossRef]
- Al Mamari, H.H.; Al Awaimri, N.; Al Lawati, Y. N-Benzo[c][1,2,5]Thiazol-4-Yl-3-Trifluoromethylbenzamide Hamad. Molbank 2019, 2019, M1075. [Google Scholar] [CrossRef]
- Dalal, A.; Singh, P.; Babu, S.A. One-Pot, Solvent-Free Pd(II)-Catalyzed Direct β-C-H Arylation of Carboxamides Involving Anhydrides as Substrates via in Situ Installation of Directing Group. Tetrahedron 2019, 75, 1246–1257. [Google Scholar] [CrossRef]
- Reddy, C.; Bisht, N.; Parella, R.; Babu, S.A. 4-Amino-2,1,3-Benzothiadiazole as a Removable Bidentate Directing Group for the Pd(II)-Catalyzed Arylation/Oxygenation of Sp2/Sp3 β-C-H Bonds of Carboxamides. J. Org. Chem. 2016, 81, 12143–12168. [Google Scholar] [CrossRef]
- Kato, S.I.; Furuya, T.; Kobayashi, A.; Nitani, M.; Ie, Y.; Aso, Y.; Yoshihara, T.; Tobita, S.; Nakamura, Y. π-Extended Thiadiazoles Fused with Thienopyrrole or Indole Moieties: Synthesis, Structures, and Properties. J. Org. Chem. 2012, 77, 7595–7606. [Google Scholar] [CrossRef]
- Mo, D.; Chen, H.; Zhou, J.; Tang, N.; Han, L.; Zhu, Y.; Chao, P.; Lai, H.; Xie, Z.; He, F. Alkyl Chain Engineering of Chlorinated Acceptors for Elevated Solar Conversion. J. Mater. Chem. A Mater. 2020, 8, 8903–8912. [Google Scholar] [CrossRef]
- Luo, Z.; Ma, R.; Xiao, Y.; Liu, T.; Sun, H.; Su, M.; Guo, Q.; Li, G.; Gao, W.; Chen, Y.; et al. Conformation-Tuning Effect of Asymmetric Small Molecule Acceptors on Molecular Packing, Interaction, and Photovoltaic Performance. Small 2020, 16, 2001942. [Google Scholar] [CrossRef] [PubMed]
- Bhanvadia, V.J.; Machhi, H.K.; Soni, S.S.; Zade, S.S.; Patel, A.L. Design and Development of Dithienopyrrolobenzothiadiazole (DTPBT)-Based Rigid Conjugated Polymers with Improved Hole Mobilities. Polymer 2020, 211, 123089. [Google Scholar] [CrossRef]
- Zhang, J.; Han, Y.; Zhang, W.; Ge, J.; Xie, L.; Xia, Z.; Song, W.; Yang, D.; Zhang, X.; Ge, Z. High-Efficiency Thermal-Annealing-Free Organic Solar Cells Based on an Asymmetric Acceptor with Improved Thermal and Air Stability. ACS Appl. Mater. Interfaces 2020, 12, 57271–57280. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Ma, X.; An, Q.; Gao, J.; Zhong, C.; Zhang, F.; Yang, C. An Asymmetrical Fused-Ring Electron Acceptor Designed by a Cross-Conceptual Strategy Achieving 15.6% Efficiency. J. Mater. Chem. A Mater. 2020, 8, 14583–14591. [Google Scholar] [CrossRef]
- Cheung, A.M.H.; Yu, H.; Luo, S.; Wang, Z.; Qi, Z.; Zhou, W.; Arunagiri, L.; Chang, Y.; Yao, H.; Ade, H.; et al. Incorporation of Alkylthio Side Chains on Benzothiadiazole-Based Non-Fullerene Acceptors Enables High-Performance Organic Solar Cells with over 16% Efficiency. J. Mater. Chem. A Mater. 2020, 8, 23239–23247. [Google Scholar] [CrossRef]
- Chai, G.; Chang, Y.; Peng, Z.; Jia, Y.; Zou, X.; Yu, D.; Yu, H.; Chen, Y.; Chow, P.C.Y.; Wong, K.S.; et al. Enhanced Hindrance from Phenyl Outer Side Chains on Nonfullerene Acceptor Enables Unprecedented Simultaneous Enhancement in Organic Solar Cell Performances with 16.7% Efficiency. Nano Energy 2020, 76, 105087. [Google Scholar] [CrossRef]
- Lin, F.; Jiang, K.; Kaminsky, W.; Zhu, Z.; Jen, A.K.Y. A Non-Fullerene Acceptor with Enhanced Intermolecular π-Core Interaction for High-Performance Organic Solar Cells. J. Am. Chem. Soc. 2020, 142, 15246–15251. [Google Scholar] [CrossRef]
- Xiao, J.; Yan, T.; Lei, T.; Li, Y.; Han, Y.; Cao, L.; Song, W.; Tan, S.; Ge, Z. Organic Solar Cells Based on Non-Fullerene Acceptors of Nine Fused-Ring by Modifying End Groups. Org. Electron. 2020, 81, 105662. [Google Scholar] [CrossRef]
- Xu, Y.; Yao, H.; Ma, L.; Wu, Z.; Cui, Y.; Hong, L.; Zu, Y.; Wang, J.; Woo, H.Y.; Hou, J. Organic Photovoltaic Cells with High Efficiencies for Both Indoor and Outdoor Applications. Mater. Chem. Front. 2021, 5, 893–900. [Google Scholar] [CrossRef]
- Zhao, J.; Zhong, D.; Zhou, S. NIR-I-to-NIR-II Fluorescent Nanomaterials for Biomedical Imaging and Cancer Therapy. J. Mater. Chem. B 2018, 6, 349–365. [Google Scholar] [CrossRef]
- Hong, G.; Antaris, A.L.; Dai, H. Near-Infrared Fluorophores for Biomedical Imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- He, S.; Song, J.; Qu, J.; Cheng, Z. Crucial Breakthrough of Second Near-Infrared Biological Window Fluorophores: Design and Synthesis toward Multimodal Imaging and Theranostics. Chem. Soc. Rev. 2018, 47, 4258–4278. [Google Scholar] [CrossRef] [PubMed]
- Alifu, N.; Zebibula, A.; Qi, J.; Zhang, H.; Sun, C.; Yu, X.; Xue, D.; Lam, J.W.Y.; Li, G.; Qian, J.; et al. Single-Molecular Near-Infrared-II Theranostic Systems: Ultrastable Aggregation-Induced Emission Nanoparticles for Long-Term Tracing and Efficient Photothermal Therapy. ACS Nano 2018, 12, 11282–11293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, C.; Zhou, S.; Wang, R.; Fan, Q.; Liu, D.; Wu, W.; Jiang, X. Improving Quantum Yield of a NIR-II Dye by Phenylazo Group. Adv. Healthc. Mater. 2020, 9, 1901470. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Qu, C.; Ma, X.; Chen, H.; Kandawa-Schulz, M.; Song, W.; Miao, W.; Wang, Y.; Cheng, Z. Tuning the near Infrared II Emitting Wavelength of Small Molecule Dyes by Single Atom Alteration. Chem. Commun. 2020, 56, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Z.; Liu, C.; Zhang, X.; Fan, Q.; Wu, W.; Jiang, X. NIR-II Dye-Labeled Cylindrical Polymer Brushes for in Vivo Imaging. ACS Macro Lett. 2019, 8, 1623–1628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, Z.; Xu, L.; Xu, Y.; Lin, Y.; Zhang, Y.; Sun, Y.; Yang, G. Rational Design of a Multifunctional Molecular Dye with Single Dose and Laser for Efficiency NIR-II Fluorescence/Photoacoustic Imaging Guided Photothermal Therapy. Anal. Chem. 2019, 91, 12476–12483. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, Y.; Yang, Y.; Yang, Y.; Zhang, K.; Guo, L.; Ge, H.; Chen, X.; Liu, J.; Feng, H. Molecular Engineering of an Organic NIR-II Fluorophore with Aggregation-Induced Emission Characteristics for In Vivo Imaging. Small 2019, 15, 1805549. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Song, G.; Liu, Y.; Han, X.; Li, Z.; Wang, Y.; Huan, S.; Zhang, X.B.; Tan, W. Nitric Oxide-Activated “Dual-Key-One-Lock” Nanoprobe for in Vivo Molecular Imaging and High-Specificity Cancer Therapy. J. Am. Chem. Soc. 2019, 141, 13572–13581. [Google Scholar] [CrossRef]
- Li, Q.; Ding, Q.; Li, Y.; Zeng, X.; Liu, Y.; Lu, S.; Zhou, H.; Wang, X.; Wu, J.; Meng, X.; et al. Novel Small-Molecule Fluorophores for: In Vivo NIR-IIa and NIR-IIb Imaging. Chem. Commun. 2020, 56, 3289–3292. [Google Scholar] [CrossRef]
- Li, S.; Deng, Q.; Zhang, Y.; Li, X.; Wen, G.; Cui, X.; Wan, Y.; Huang, Y.; Chen, J.; Liu, Z.; et al. Rational Design of Conjugated Small Molecules for Superior Photothermal Theranostics in the NIR-II Biowindow. Adv. Mater. 2020, 32, 2001146. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ding, F.; Zhou, Z.; Li, C.; Pu, M.; Xu, Y.; Zhan, Y.; Lu, X.; Li, H.; Yang, G.; et al. Rhomboidal Pt(II) Metallacycle-Based NIR-II Theranostic Nanoprobe for Tumor Diagnosis and Image-Guided Therapy. Proc. Natl. Acad. Sci. USA 2019, 116, 1968–1973. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Lou, Y.; Su, L.; Chen, B.; Guo, Z.; Gao, S.; Zhang, W.; Chen, T.; Song, J.; Yang, H. Single Wavelength Laser Excitation Ratiometric NIR-II Fluorescent Probe for Molecule Imaging in Vivo. Anal. Chem. 2020, 92, 6111–6120. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wei, G.; Zhang, S.; Zheng, B.; Xu, J.; Chen, G.; Li, M.; Song, S.; Fu, W.; Xiao, Z.; et al. Albumin Tailoring Fluorescence and Photothermal Conversion Effect of Near-Infrared-II Fluorophore with Aggregation-Induced Emission Characteristics. Nat. Commun. 2019, 10, 2206. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Chen, Z.; Kim, W.Y.; Sharma, A.; Li, C.; Ouyang, Q.; Zhu, H.; Yang, G.; Sun, Y.; Kim, J.S. A Nano-Cocktail of an NIR-II Emissive Fluorophore and Organoplatinum(II) Metallacycle for Efficient Cancer Imaging and Therapy. Chem. Sci. 2019, 10, 7023–7028. [Google Scholar] [CrossRef]
- Marco, A.B.; Cortizo-Lacalle, D.; Gozalvez, C.; Olano, M.; Atxabal, A.; Sun, X.; Melle-Franco, M.; Hueso, L.E.; Mateo-Alonso, A. An Electron-Conducting Pyrene-Fused Phenazinothiadiazole. Chem. Commun. 2015, 51, 10754–10757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, F.; Li, Q.; Zhang, Z.; He, X.; Chen, Z.; Shi, J.; Tu, G. Novel N-Heteroacene Small Molecules as Electron Donors for Organic Bulk Heterojunction Photovoltaics. Org. Electron. 2018, 57, 93–97. [Google Scholar] [CrossRef]
- Simayi, R.; Murat, A.; Imerhasan, M.; Mijit, M.; Mahmut, M. Low Band Gap Polymers Based on the Electrochemical Polymerization of Phenazine: Studies on the Color Changing Ability in near-Infrared Region. J. Polym. Res. 2020, 27, 293. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, Q.; Zhang, Z.; Tang, L.; Shen, X.; Xue, S.; Yang, W. New Frog-Type Dibenzo[a,c][1,2,5]Thiadiazolo[3,4-i]Phenazine Heterocyclic Derivatives with Aggregation-Enhanced One- and Two-Photon Excitation NIR Fluorescence. Dyes Pigm. 2018, 153, 233–240. [Google Scholar] [CrossRef]
- Brownell, L.V.; Robins, K.A.; Jeong, Y.; Lee, Y.; Lee, D.C. Highly Systematic and Efficient HOMO-LUMO Energy Gap Control of Thiophene-Pyrazine-Acenes. J. Phys. Chem. C 2013, 117, 25236–25247. [Google Scholar] [CrossRef]
- Qi, J.; Sun, C.; Li, D.; Zhang, H.; Yu, W.; Zebibula, A.; Lam, J.W.Y.; Xi, W.; Zhu, L.; Cai, F.; et al. Aggregation-Induced Emission Luminogen with near-Infrared-II Excitation and near-Infrared-I Emission for Ultradeep Intravital Two-Photon Microscopy. ACS Nano 2018, 12, 7936–7945. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cheng, T.; Yin, C.; Zhou, S.; Fan, Q.; Wu, W.; Jiang, X. Phenothiazine versus Phenoxazine: Structural Effects on the Photophysical Properties of NIR-II AIE Fluorophores. ACS Appl. Mater. Interfaces 2020, 12, 43466–43473. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yin, C.; Wang, R.; Fan, Q.; Wu, W.; Jiang, X. Second Near-Infrared Aggregation-Induced Emission Fluorophores with Phenothiazine Derivatives as the Donor and 6,7-Diphenyl-[1,2,5]Thiadiazolo[3,4-g]Quinoxaline as the Acceptor for in Vivo Imaging. ACS Appl. Mater. Interfaces 2020, 12, 20281–20286. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Wen, G.; Liu, C.; Yang, B.; Lin, S.; Huang, J.; Zhao, P.; Wong, S.H.D.; Zhang, K.; Chen, X.; et al. Organic Semiconducting Polymer Nanoparticles for Photoacoustic Labeling and Tracking of Stem Cells in the Second Near-Infrared Window. ACS Nano 2018, 12, 12201–12211. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, H.; Liu, J.; Chen, C.; Liu, B. NIR-II Light Activated Photosensitizer with Aggregation-Induced Emission for Precise and Efficient Two-Photon Photodynamic Cancer Cell Ablation. Adv. Funct. Mater. 2020, 30, 2002546. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.P.; Kang, L.; Zhao, Y. Novel Mitochondrial-Targeted Thiadiazolo[3,4-g] Quinoxaline Dyes as Efficient Photosensitizers for Ultra-Low Dose Operable Photodynamic Therapy. Chem. Commun. 2019, 55, 11390–11393. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Prieto, P.; Carrillo, J.R.; Rodríguez, A.M.; De Cozar, A.; Boj, P.G.; Díaz-García, M.A.; Ramírez, M.G. Design, Synthesis and Amplified Spontaneous Emission of 1,2,5-Benzothiadiazole Derivatives. J. Mater. Chem. C Mater. 2019, 7, 9996–10007. [Google Scholar] [CrossRef]
- Kato, S.I.; Watanabe, K.; Tamura, M.; Ueno, M.; Nitani, M.; Ie, Y.; Aso, Y.; Yamanobe, T.; Uehara, H.; Nakamura, Y. Tetraalkoxyphenanthrene-Fused Thiadiazoloquinoxalines: Synthesis, Electronic, Optical, and Electrochemical Properties, and Self-Assembly. J. Org. Chem. 2017, 82, 3132–3143. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Li, M.; Chen, W.; Wan, X.; Chen, Y.; Zhang, Q. Novel Donor-Acceptor Polymers Based on 7-Perfluorophenyl-6H-[1,2,5]Thiadiazole[3,4-g]Benzoimidazole for Bulk Heterojunction Solar Cells. RSC Adv. 2015, 5, 50137–50145. [Google Scholar] [CrossRef]
- Hu, B.; Wang, C.; Zhang, J.; Qian, K.; Lee, P.S.; Zhang, Q. Organic Memory Effect from Donor-Acceptor Polymers Based on 7-Perfluorophenyl-6H-[1,2,5]Thiadiazole[3,4-g]Benzoimidazole. RSC Adv. 2015, 5, 77122–77129. [Google Scholar] [CrossRef]
- Mahmut, M.; Awut, T.; Nurulla, I.; Mijit, M. Synthesis and Spectroelectrochemical Investigation of Low-Bandgap Polymer: Integrated Quinoxaline and Benzimidazole in One Electron Acceptor Unit. J. Appl. Polym. Sci. 2014, 131, 1–7. [Google Scholar] [CrossRef]
- Dietrich, B.; Viout, P.; Lehn, J.-M. Macrocyclic Chemistry: Aspects of Organic and Inorganic Supramolecular Chemistry; VCH: Weinheim, Germany, 1993; ISBN 3-527-28330-7. [Google Scholar]
- Lehn, J. Supramolecular Chemistry: Concepts and Perspectives, 1st ed.; VCH: Weinheim, Germany; New York, NY, USA; Basel, Switzerland; Cambridge, UK; Tokyo, Japan, 1995; ISBN 978-3-527-29311-7. [Google Scholar]
- Fitzpatrick, D.W.; Ulrich, H.J. Macrocyclic Chemistry: New Research Developments, 1st ed.; Nova Science Publishers: New York, NY, USA, 2010; ISBN 1608768961. [Google Scholar]
- Gokel, G.W.; Korzeniowski, S.H. Macrocyclic Polyether Syntheses; Springer: Berlin/Heidelberg, Germany, 1982; ISBN 3540113177. [Google Scholar]
- He, H.; Meng, X.; Deng, L.; Sun, Q.; Huang, X.; Lan, N.; Zhao, F. A Novel Benzothiadiazole-Based and NIR-Emissive Fluorescent Sensor for Detection of Hg2+ and Its Application in Living Cell and Zebrafish Imaging. Org. Biomol. Chem. 2020, 18, 6357–6363. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wang, J.; Li, X.; Sun, Q.; Tu, Q.; Liu, X.; He, H.; Zhao, F. A Large Stokes Shift Probe Based Enhanced ICT Strategy for Hg2+-Detection in Cancer Cells and Zebrafish. Microchem. J. 2021, 169, 106551. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Zuo, H.; Wang, C.; Shen, Y. A New Colorimetric and Ratiometric Chemodosimeter for Mercury (II) Based on Triphenylamine and Benzothiadiazolenovel. J. Photochem. Photobiol. A Chem. 2017, 332, 293–298. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Zuo, H.; Wang, C.; Shen, Y. A Novel Near-Infrared Chemosensor for Mercury Ion Detection Based on D-A Structure of Triphenylamine and Benzothiadiazole. Tetrahedron 2017, 73, 2824–2830. [Google Scholar] [CrossRef]
- Zou, Q.; Tian, H. Chemodosimeters for Mercury(II) and Methylmercury(I) Based on 2,1,3-Benzothiadiazole. Sens. Actuators B Chem. 2010, 149, 20–27. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; He, J.; Zhang, J.; Zhou, H.; Gao, C. A Novel Red-Emitting Fluorescent Probe for the Highly Selective Detection of Hg2+ Ion with AIE Mechanism. Chem. Phys. 2020, 539, 110944. [Google Scholar] [CrossRef]
- Tian, X.M.; Yao, S.L.; Wu, J.; Xie, H.; Zheng, T.F.; Jiang, X.J.; Wu, Y.; Mao, J.; Liu, S.J. Two Benzothiadiazole-Based Fluorescent Sensors for Selective Detection of Cu2+ and OH– Ions. Polyhedron 2019, 171, 523–529. [Google Scholar] [CrossRef]
- Yu, B.; Yuan, R.; He, T.; Liang, L.; Huang, K. A Benzothidiazole-Based Reversible Fluorescent Probe with Excellent Performances in Selectively and Sensitively Sensing of Hg2+ in Water. J. Fluoresc. 2022, 32, 2077–2086. [Google Scholar] [CrossRef]
- Souto, F.T.; Dias, G.G. Input Selection Drives Molecular Logic Gate Design. Analytica 2023, 4, 456–499. [Google Scholar] [CrossRef]
- Dias, G.G.; Souto, F.T. Architecture of Molecular Logic Gates: From Design to Application as Optical Detection Devices. Organics 2024, 5, 114–162. [Google Scholar] [CrossRef]
- Lu, N.; Jiang, T.; Tan, H.; Hang, Y.; Yang, J.; Wang, J.; Qu, X.; Hua, J. A Red Fluorescent Turn-on Chemosensor for Al3+ Based on a Dimethoxy Triphenylamine Benzothiadiazole Derivative with Aggregation-Induced Emission. Anal. Methods 2017, 9, 2689–2695. [Google Scholar] [CrossRef]
- Maisonneuve, S.; Fang, Q.; Xie, J. Benzothiadiazoyl-Triazoyl Cyclodextrin: A Selective Fluoroionophore for Ni(II). Tetrahedron 2008, 64, 8716–8720. [Google Scholar] [CrossRef]
- Li, C.; Tang, J.; Xie, J. Synthesis of Crosslinking Amino Acids by Click Chemistry. Tetrahedron 2009, 65, 7935–7941. [Google Scholar] [CrossRef]
- Ruan, Y.B.; Maisonneuve, S.; Li, C.; Tang, J.; Xie, J. Cooperative Recognition of Cu2+ Based on Amino Acids Tethered Benzothiadiazoyl-Bistriazoles. Front. Chem. 2010, 5, 208–213. [Google Scholar] [CrossRef]
- Wang, Z.C.; Tan, Y.Z.; Yu, H.; Bao, W.H.; Tang, L.L.; Zeng, F. A Benzothiadiazole-Based Self-Assembled Cage for Cadmium Detection. Molecules 2023, 28, 1841. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Li, T.Y.; Pan, Y.; Han, X.; Guo, Y.; Shi, L.; Song, M.-P. Covalent Organic Nanocage with Aggregation Induced Emission Property and Detection for Hg2+ as Fluorescence Sensors. Dyes Pigm. 2023, 219, 111584. [Google Scholar] [CrossRef]
- Moser, M.; Thorley, K.J.; Moruzzi, F.; Ponder, J.F.; Maria, I.P.; Giovannitti, A.; Inal, S.; McCulloch, I. Highly Selective Chromoionophores for Ratiometric Na+ Sensing Based on an Oligoethyleneglycol Bridged Bithiophene Detection Unit. J. Mater. Chem. C Mater. 2019, 7, 5359–5365. [Google Scholar] [CrossRef]
- Yoshio, M.; Noguchi, H. Crown Ethers for Chemical Analysis: A Review. Anal. Lett. 1982, 15, 1197–1276. [Google Scholar] [CrossRef]
- Tsukanov, A.V.; Dubonosov, A.D.; Bren, V.A.; Minkin, V.I. Organic Chemosensors with Crown-Ether Groups (Review). Chem. Heterocycl. Compd. 2008, 44, 899–923. [Google Scholar] [CrossRef]
- Lohr, H.G.; Vögtle, F. Chromo- and Fluoroionophores. A New Class of Dye Reagents. Acc. Chem. Res. 1985, 18, 65–72. [Google Scholar] [CrossRef]
- Qian, J.; Huang, N.; Lu, Q.; Wen, C.; Xia, J. A Novel D-A-D-Typed Rod-like Fluorescent Material for Efficient Fe(Ⅲ) and Cr(Ⅵ) Detection: Synthesis, Structure and Properties. Sens. Actuators B Chem. 2020, 320, 128377. [Google Scholar] [CrossRef]
- Ruan, Y.B.; Yu, Y.; Li, C.; Bogliotti, N.; Tang, J.; Xie, J. Triazolyl Benzothiadiazole Fluorescent Chemosensors: A Systematic Investigation of 1,4- or 1,5-Disubstituted Mono- and Bis-Triazole Derivatives. Tetrahedron 2013, 69, 4603–4608. [Google Scholar] [CrossRef]
- Li, C.; Henry, E.; Mani, N.K.; Tang, J.; Brochon, J.C.; Deprez, E.; Xie, J. Click Chemistry to Fluorescent Amino Esters: Synthesis and Spectroscopic Studies. Eur. J. Org. Chem. 2010, 2010, 2395–2405. [Google Scholar] [CrossRef]
- Çimen, O.; Dinçalp, H.; Varlikli, C. Studies on UV-Vis and Fluorescence Changements in Co2+ and Cu2+ Recognition by a New Benzimidazole-Benzothiadiazole Derivative. Sens. Actuators B Chem. 2015, 209, 853–863. [Google Scholar] [CrossRef]
- Uauy, R.; Olivares, M.; Gonzalez, M. Essentiality of Copper in Humans. Am. J. Clin. Nutr. 2018, 67, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Little, C.; Aakre, S.E.; Rumsby, M.G.; Gwarsha, K. Effect of Co2+-Substitution on the Substrate Specificity of Phospholipase C from Bacillus Cereus during Attack on Two Membrane Systems. Biochem. J. 1982, 207, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Zhang, X.B.; Jin, Z.; Han, R.; Shen, G.L.; Yu, R.Q. A Fluorescent Chemosensor for Cobalt Ions Based on a Multi-Substituted Phenol-Ruthenium(II) Tris(Bipyridine) Complex. Anal. Chim. Acta 2006, 580, 143–148. [Google Scholar] [CrossRef]
- Choe, D.; Kim, C. A Benzothiadiazole-Based Colorimetric Chemosensor for Detecting Cu2+ and Sequential H2S in Practical Samples. Inorg. Chim. Acta 2022, 543, 121180. [Google Scholar] [CrossRef]
- Moro, A.V.; Ferreira, P.C.; Migowski, P.; Rodembusch, F.S.; Dupont, J.; Lüdtke, D.S. Synthesis and Photophysical Properties of Fluorescent 2,1,3- Benzothiadiazole-Triazole-Linked Glycoconjugates: Selective Chemosensors for Ni(II). Tetrahedron 2013, 69, 201–206. [Google Scholar] [CrossRef]
- Bryant, J.J.; Lindner, B.D.; Bunz, U.H.F. Water-Soluble Bis-Triazolyl Benzochalcogendiazole Cycloadducts as Tunable Metal Ion Sensors. J. Org. Chem. 2013, 78, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Musikavanhu, B.; Zhu, D.; Tang, M.; Xue, Z.; Wang, S.; Zhao, L. A Naphthol Hydrazone Schiff Base Bearing Benzothiadiazole Unit for Fluorescent Detection of Fe3+ in PC3 Cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 289, 122242. [Google Scholar] [CrossRef]
- Udhayakumari, D. Detection of Toxic Fluoride Ion via Chromogenic and Fluorogenic Sensing. A Comprehensive Review of the Year 2015–2019. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 228, 117817. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, X.; Kim, H.N.; Yoon, J. Sensors for the Optical Detection of Cyanide Ion. Chem. Soc. Rev. 2010, 39, 127–137. [Google Scholar] [CrossRef]
- Deng, K.; Wang, L.; Xia, Q.; Liu, R.; Qu, J. A Turn-on Fluorescent Chemosensor Based on Aggregation-Induced Emission for Cyanide Detection and Its Bioimaging Applications. Sens. Actuators B Chem. 2019, 296, 126645. [Google Scholar] [CrossRef]
- Wang, B.; Pan, H.; Jia, J.; Ge, Y.Q.; Cai, W.Q.; Wang, J.W.; Zhao, C.H. Highly Emissive Dimesitylboryl-Substituted 2,1,3-Benzothiadiazole Derivatives: Photophysical Properties and Efficient Fluorescent Sensor for Fluoride Anions. Tetrahedron 2014, 70, 5488–5493. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Pan, H.; Fu, G.L.; Lin, J.M.; Zhao, C.H. A Highly Emissive Cruciform Triarylborane as a Ratiometric and Solid State Fluorescence Sensor for Fluoride Ions. Tetrahedron Lett. 2011, 52, 3832–3835. [Google Scholar] [CrossRef]
- Pan, H.; Fu, G.L.; Zhao, Y.H.; Zhao, C.H. Through-Space Charge-Transfer Emitting Biphenyls Containing a Boryl and an Amino Group at the o, o ′-Positions. Org. Lett. 2011, 13, 4830–4833. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lai, G.; Li, Z.; Lu, Y.; Leng, T.; Shen, Y.; Wang, C. Novel 2,1,3-Benzothiadiazole Derivatives Used as Selective Fluorescent and Colorimetric Sensors for Fluoride Ion. Dyes Pigm. 2016, 124, 268–276. [Google Scholar] [CrossRef]
- Yu, Y.; Dong, C. An Efficient Colorimetric and Fluorescent Probe for the Detection of Fluoride Ions Based on a Benzothiadiazole Derivative. Anal. Methods 2015, 7, 9604–9608. [Google Scholar] [CrossRef]
- Chen, P.; Bai, W.; Bao, Y. Fluorescent Chemodosimeters for Fluoride Ions via Silicon-Fluorine Chemistry: 20 Years of Progress. J. Mater. Chem. C Mater. 2019, 7, 11731–11746. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Zuo, H.; Wang, C.; Shen, Y. A Novel Colorimetric and Fluorescent Sensor for Cyanide Anions Detection Based on Triphenylamine and Benzothiadiazole. Tetrahedron 2016, 72, 1244–1248. [Google Scholar] [CrossRef]
- Wu, H.; Chen, M.; Xu, Q.; Zhang, Y.; Liu, P.; Li, W.; Fan, S. Switching to a “Turn-on” Fluorescent Probe for Selective Monitoring of Cyanide in Food Samples and Living Systems. Chem. Commun. 2019, 55, 15137–15140. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Li, Y.; Zhang, M.; Wang, L.; Hu, Y.; Yin, K.; Fan, S.; Wu, H. Aggregation-Induced Emission Activity of Sensor TBM-C1 Hybrid of Methoxy-Triphenylamine (OMe-TPA) and Dicyanovinyl for Cyanide Detection in Aqueous THF: Mechanistic Insights and Potential Applications. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 312, 124058. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Hu, Z.; Peng, C.; Liu, B.; Li, W.; Gao, C. Rational Design of a Colorimetric and Fluorescence Turn-on Chemosensor with Benzothiazolium Moiety for Cyanide Detection in Aqueous Solution. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 224, 117409. [Google Scholar] [CrossRef] [PubMed]
- Elavarasan, K.; Saravanan, C.; Selvam, N.P.; Easwaramoorthi, S. Benzothiadiazole-Based Diarylamines as a Fluoride Sensor: Prevention of Fluoride Induced Decomposition of Receptor Molecule by Complex Formation with Cu2+. ChemistrySelect 2018, 3, 10085–10090. [Google Scholar] [CrossRef]
- Yu, Y.; Bogliotti, N.; Maisonneuve, S.; Tang, J.; Xie, J. Fluorescent Dyad for Cooperative Recognition of Copper Cation and Halogen Anion. Tetrahedron Lett. 2013, 54, 1877–1883. [Google Scholar] [CrossRef]
- Lee, D.; Kim, G.; Yin, J.; Yoon, J. An Aryl-Thioether Substituted Nitrobenzothiadiazole Probe for the Selective Detection of Cysteine and Homocysteine. Chem. Commun. 2015, 51, 6518–6520. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, J.; Qu, W.; Zhong, X.; Liu, H.; Ren, J.; He, H.; Zhang, X.; Wang, S. Development of a Novel Benzothiadiazole-Based Fluorescent Turn-on Probe for Highly Selective Detection of Glutathione over Cysteine/Homocysteine. Sens. Actuators B Chem. 2018, 266, 528–533. [Google Scholar] [CrossRef]
- Yang, C.F.; Zeng, L.Y.; Ning, B.K.; Wang, J.Y.; Zhang, H.; Zhang, Z.H. Development of a Fast-Responsive and Turn on Fluorescent Probe with Large Stokes Shift for Specific Detection of Cysteine in Vivo. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 225, 117482. [Google Scholar] [CrossRef]
- Whalley, D.M.; Greaney, M.F. Recent Advances in the Smiles Rearrangement: New Opportunities for Arylation. Synthesis 2022, 54, 1908–1918. [Google Scholar] [CrossRef]
- Truce, W.E.; Kreider, E.M.; Brand, W.W. The Smiles and Related Rearrangements of Aromatic Systems. In Organic Reactions; Wiley: Hoboken, NJ, USA, 2011; pp. 99–215. [Google Scholar]
- Zhang, Q.; Hu, X.; Dai, X.; Sun, J.; Gao, F. A Photostable Reaction-Based A-A-A Type Two-Photon Fluorescent Probe for Rapid Detection and Imaging of Sulfur Dioxide. J. Mater. Chem. B 2021, 9, 3554–3562. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Guo, Z.; Jin, P.; Jiao, C.; Tian, H.; Zhu, W. Optimizing the Chemical Recognition Process of a Fluorescent Chemosensor for α-Ketoglutarate. Ind. Eng. Chem. Res. 2015, 54, 2886–2893. [Google Scholar] [CrossRef]
- Gui, K.; Mutkins, K.; Schwenn, P.E.; Krueger, K.B.; Pivrikas, A.; Wolfer, P.; Stingelin Stutzmann, N.; Burn, P.L.; Meredith, P. A Flexible N-Type Organic Semiconductor for Optoelectronics. J. Mater. Chem. 2012, 22, 1800–1806. [Google Scholar] [CrossRef]
- Zhang, G.; Loch, A.S.; Kistemaker, J.C.M.; Burn, P.L.; Shaw, P.E. Dicyanovinyl-Based Fluorescent Sensors for Dual Mechanism Amine Sensing. J. Mater. Chem. C Mater. 2020, 8, 13723–13732. [Google Scholar] [CrossRef]
- Silla Santos, M.H. Biogenic Amines: Their Importance in Foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Shalaby, A.R. Significance of Biogenic Amines to Food Safety and Human Health. Food Res. Int. 1996, 29, 675–690. [Google Scholar] [CrossRef]
- Loch, A.S.; Burn, P.L.; Shaw, P.E. Fluorescent Sensors for the Detection of Free-Base Illicit Drugs—Effect of Tuning the Electronic Properties. Sens. Actuators B Chem. 2023, 387, 133766. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Cheng, K.; Yang, X.; Li, Q.Y.; Zhang, H.; Ma, Z.; Lu, H.; Wu, H.; Wang, X.J. A Benzothiadiazole-Based Fluorescent Sensor for Selective Detection of Oxalyl Chloride and Phosgene. Org. Chem. Front. 2017, 4, 1719–1725. [Google Scholar] [CrossRef]
- Maji, R.; Mahapatra, A.K.; Maiti, K.; Mondal, S.; Ali, S.S.; Sahoo, P.; Mandal, S.; Uddin, M.R.; Goswami, S.; Quah, C.K.; et al. A Highly Sensitive Fluorescent Probe for Detection of Hydrazine in Gas and Solution Phases Based on the Gabriel Mechanism and Its Bioimaging. RSC Adv. 2016, 6, 70855–70862. [Google Scholar] [CrossRef]
- Santos, C.O.; Passos, S.T.A.; Sorto, J.E.P.; Machado, D.F.S.; Correa, J.R.; da Silva Júnior, E.N.; Rodrigues, M.O.; Neto, B.A.D. Sensitive Hydrazine Detection and Quantification with a Fluorescent Benzothiadiazole Sensor: Selective Lipid Droplets and in Vivo Imaging. Org. Biomol. Chem. 2023, 21, 4606–4619. [Google Scholar] [CrossRef] [PubMed]
- Fiuza, R.M.d.; Padilha, J.; Maqueira, L.; Aucélio, R.Q.; Limberger, J. Synthesis and Application of a Highly Fluorescent Styryl-Benzothiadiazole Derivative as a Chemosensor for Ethanol in Hydroalcoholic Solutions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 271, 120913. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.M.; Iyer, S.K. D–π–A–π–D-Configured Imidazole-Tethered Benzothiadiazole-Based Sensor for the Ratiometric Discrimination of Picric Acid: Applications in Latent Fingerprint Imaging. J. Org. Chem. 2024, 89, 5392–5400. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Ye, K.; Sun, J.; Zhan, Y.; Jia, J.; Xue, P.; Zhang, G.; Zhang, Z.; Lu, R. Branched Benzothiadiazole-Cored Oligomers with Terminal Carbazoles: Synthesis and Fluorescence Probing Nitroaromatics. Dyes Pigm. 2015, 116, 36–45. [Google Scholar] [CrossRef]
- Prentø, P. Staining of Macromolecules: Possible Mechanisms and Examples. Biotech. Histochem. 2009, 84, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Dias, G.G.; King, A.; De Moliner, F.; Vendrell, M.; Da Silva Júnior, E.N. Quinone-Based Fluorophores for Imaging Biological Processes. Chem. Soc. Rev. 2018, 47, 12–27. [Google Scholar] [CrossRef]
- Cheng, Z.; Valença, W.O.; Dias, G.G.; Scott, J.; Barth, N.D.; de Moliner, F.; Souza, G.B.P.; Mellanby, R.J.; Vendrell, M.; da Silva Júnior, E.N. Natural Product-Inspired Profluorophores for Imaging NQO1 Activity in Tumour Tissues. Bioorganic Med. Chem. 2019, 27, 3938–3946. [Google Scholar] [CrossRef] [PubMed]
- Neto, B.A.D.; Lapis, A.A.M.; Mancilha, F.S.; Vasconcelos, I.B.; Thum, C.; Basso, L.A.; Santos, D.S.; Dupont, J. New Sensitive Fluorophores for Selective DNA Detection. Org. Lett. 2007, 9, 4001–4004. [Google Scholar] [CrossRef]
- Krüger, R.; Larroza, A.; Fronza, M.G.; Tisoco, I.; Savegnago, L.; Reis, J.S.; Back, D.F.; Iglesias, B.A.; Alves, D. Bis-Triazolylchalcogenium-Functionalized Benzothiadiazole Derivatives as Light-up Sensors for DNA and BSA. J. Org. Chem. 2021, 86, 17866–17883. [Google Scholar] [CrossRef]
- Ebersol, C.P.; Debia, N.P.; Zimba, H.C.; Moraes, E.S.; Lüdtke, D.S.; Rodembusch, F.S.; Moro, A.V. Photoactive Benzothiadiazole-N-Heterocycle Derivatives: Synthesis, Photophysics and Water Sensing in Organic Solvents. New J. Chem. 2024, 48, 4680–4689. [Google Scholar] [CrossRef]
- Qi, Y.L.; Chen, L.L.; Guo, L.; Cao, Y.Y.; Wang, H.R.; Yang, Y.S.; Lu, Y.D.; Zhu, H.L. Multifunctional Fluorescent Probes “Killing Two Birds with One Stone”—Recent Progress and Outlook. Appl. Mater. Today 2020, 21, 100877. [Google Scholar] [CrossRef]
- Kolanowski, J.L.; Liu, F.; New, E.J. Fluorescent Probes for the Simultaneous Detection of Multiple Analytes in Biology. Chem. Soc. Rev. 2018, 47, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Cui, G.; Li, H.; Liu, Y.; Tian, Y.; Yan, S. Multifunctional Optical Sensing Probes Based on Organic-Inorganic Hybrid Composites. J. Mater. Chem. B 2016, 4, 5194–5216. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, H.; Zhang, X.; Chen, S.; You, T.; Xu, F. Recent Studies on Cellulose-Based Fluorescent Smart Materials and Their Applications: A Comprehensive Review. Carbohydr. Polym. 2021, 267, 118135. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, M.; Espinosa, A.; Tárraga, A.; Molina, P. Multifunctional Benzothiadiazole-Based Small Molecules Displaying Solvatochromism and Sensing Properties toward Nitroarenes, Anions, and Cations. ChemistryOpen 2014, 3, 242–249. [Google Scholar] [CrossRef]
- Qiu, C.Q.; Li, L.Q.; Yao, S.L.; Liu, S.J.; Xu, H.; Zheng, T.F. Two Benzothiadiazole-Based Compounds as Multifunctional Fluorescent Sensors for Detection of Organic Amines and Anions. Polyhedron 2021, 199, 115100. [Google Scholar] [CrossRef]

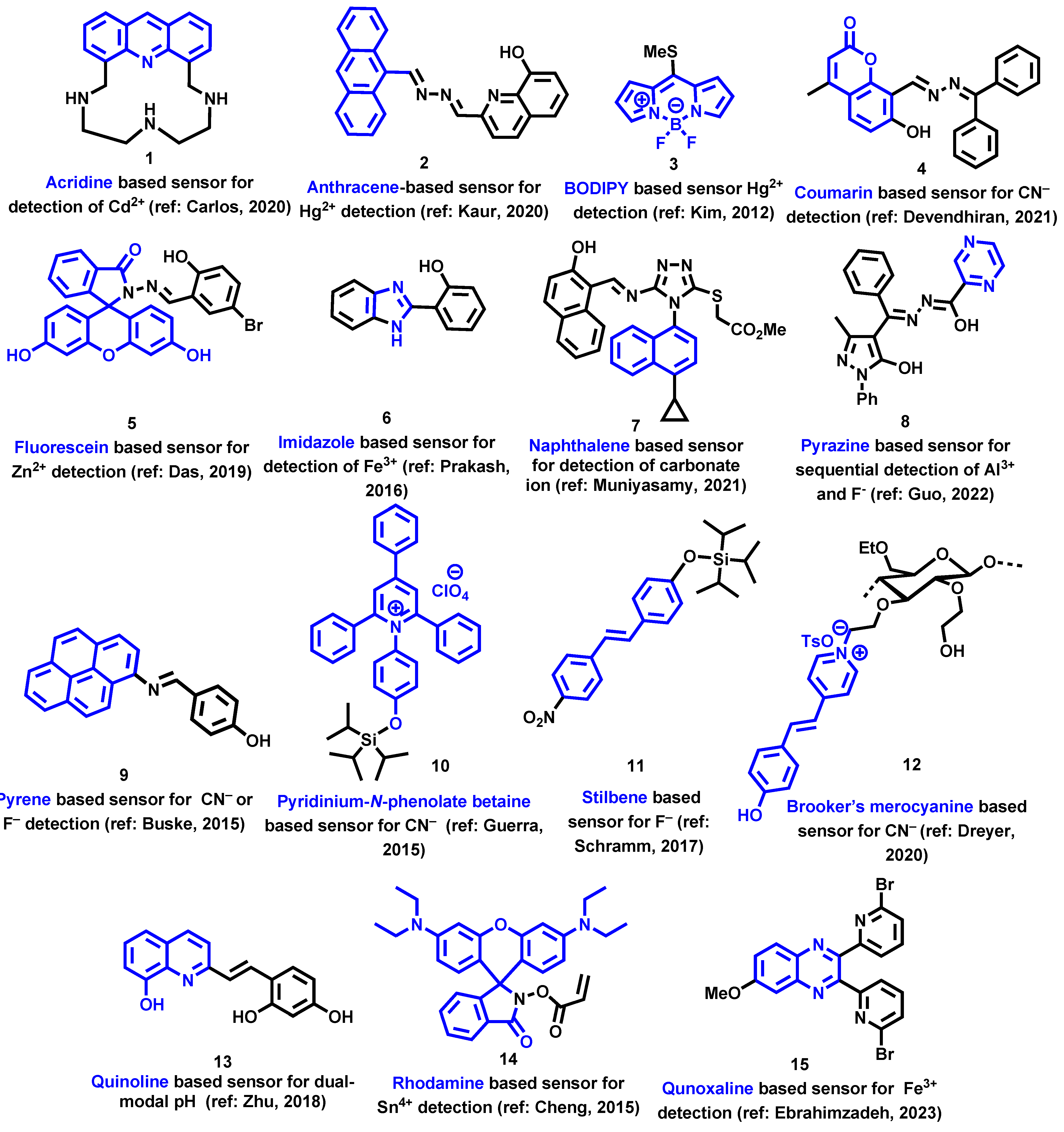
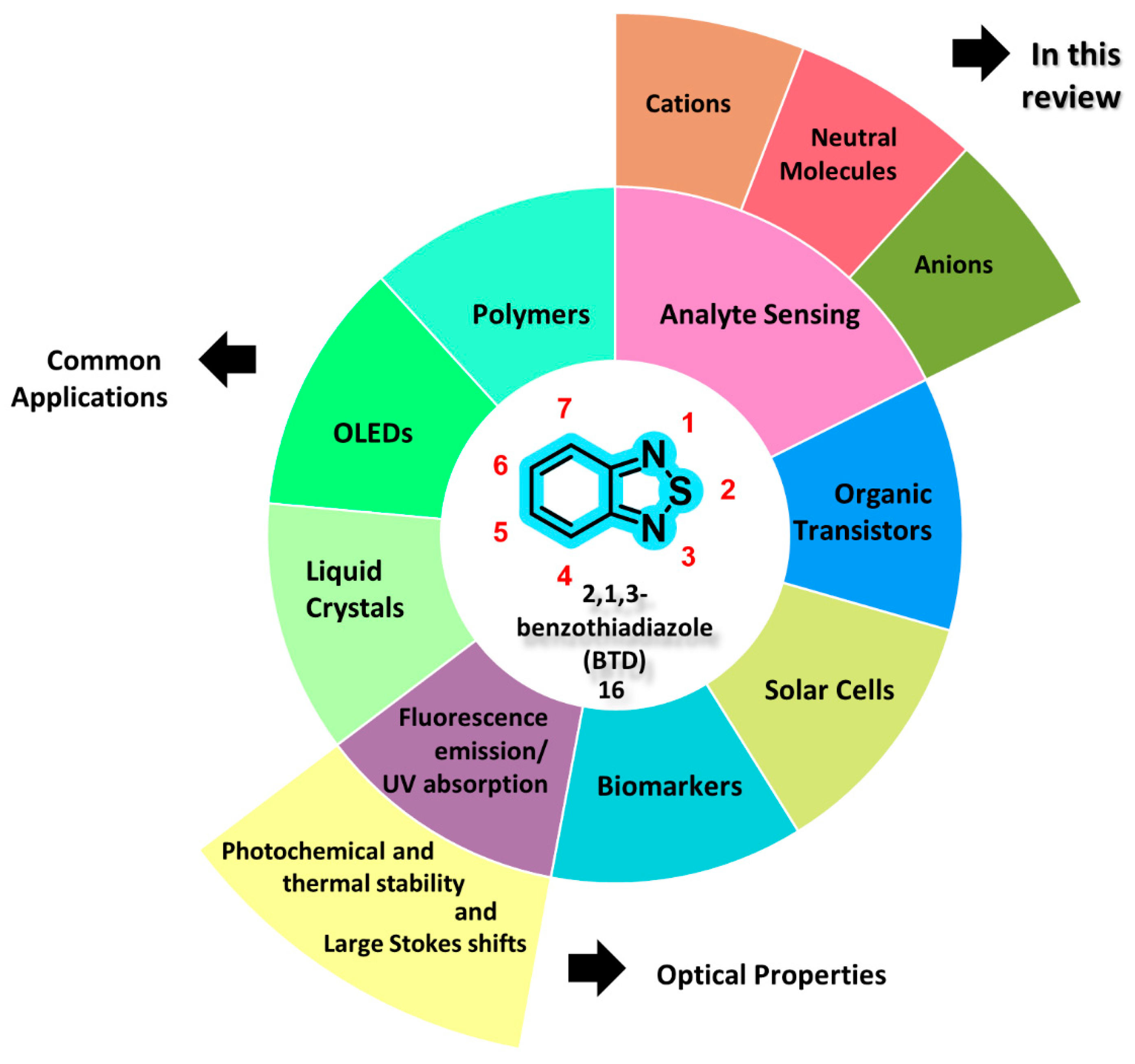
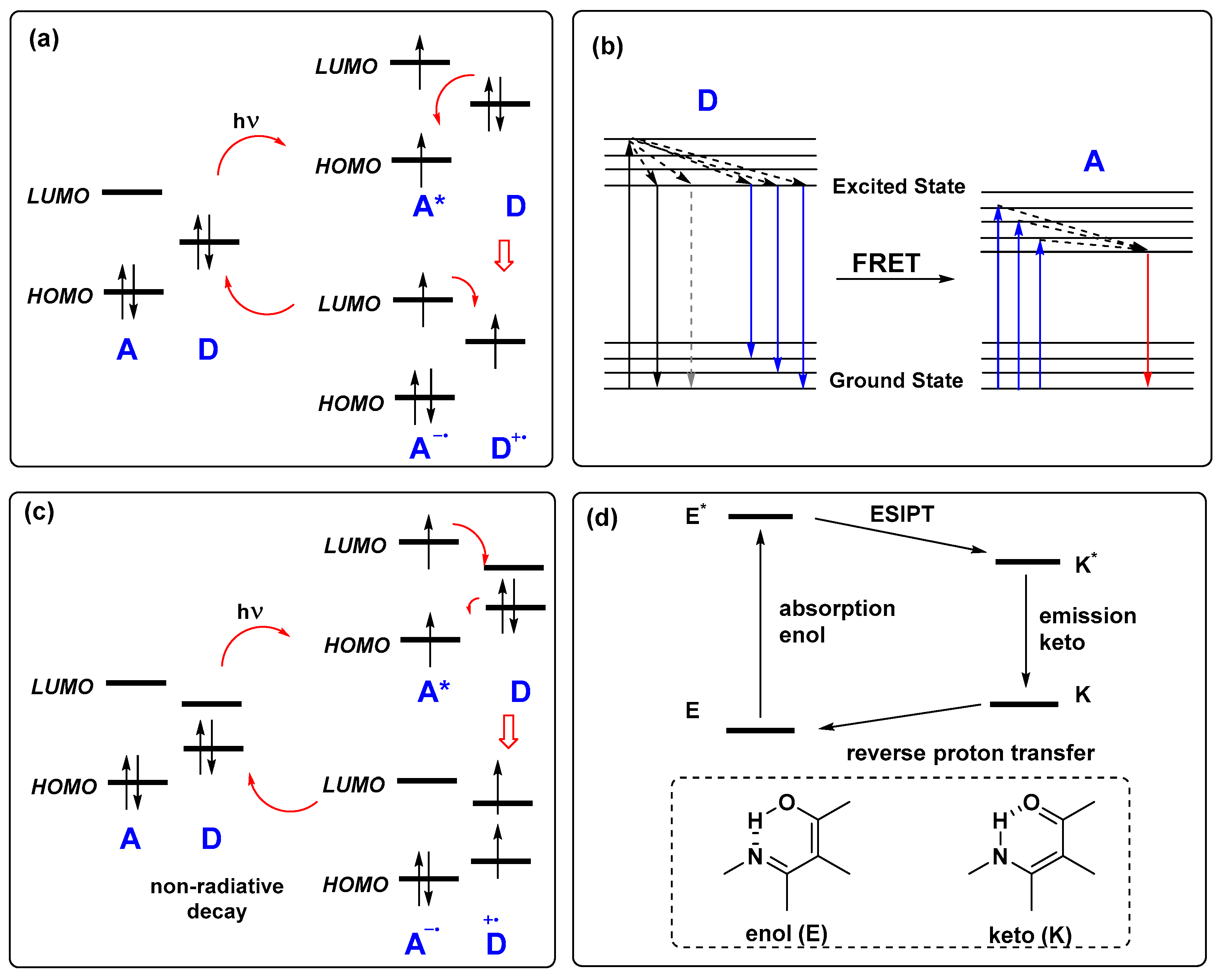
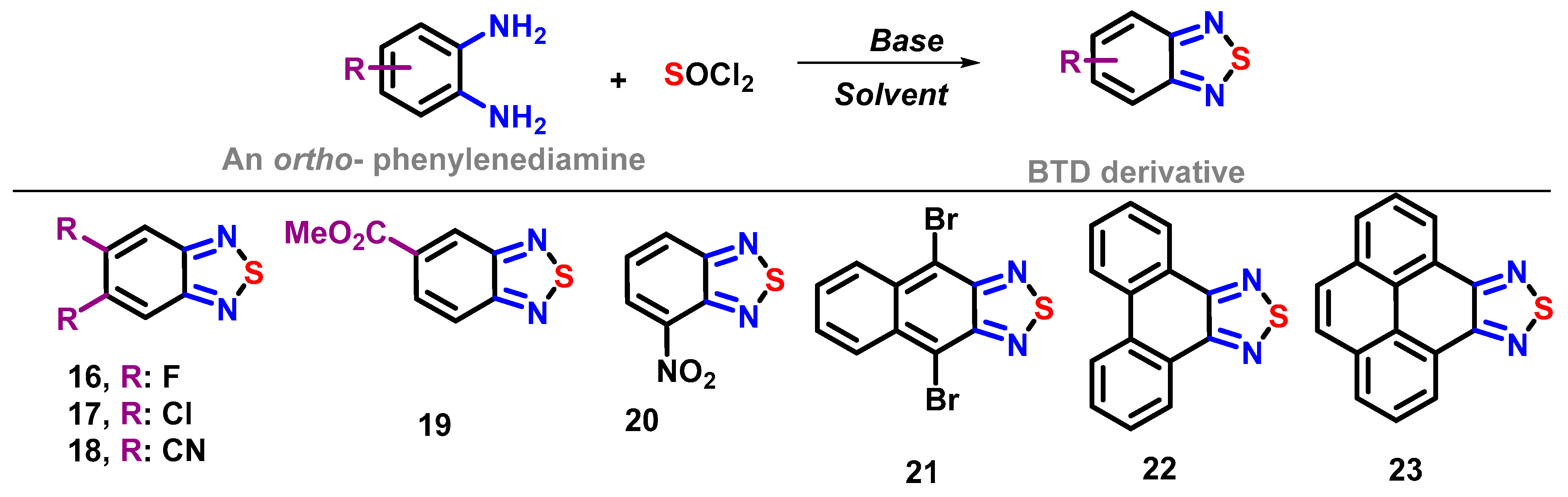
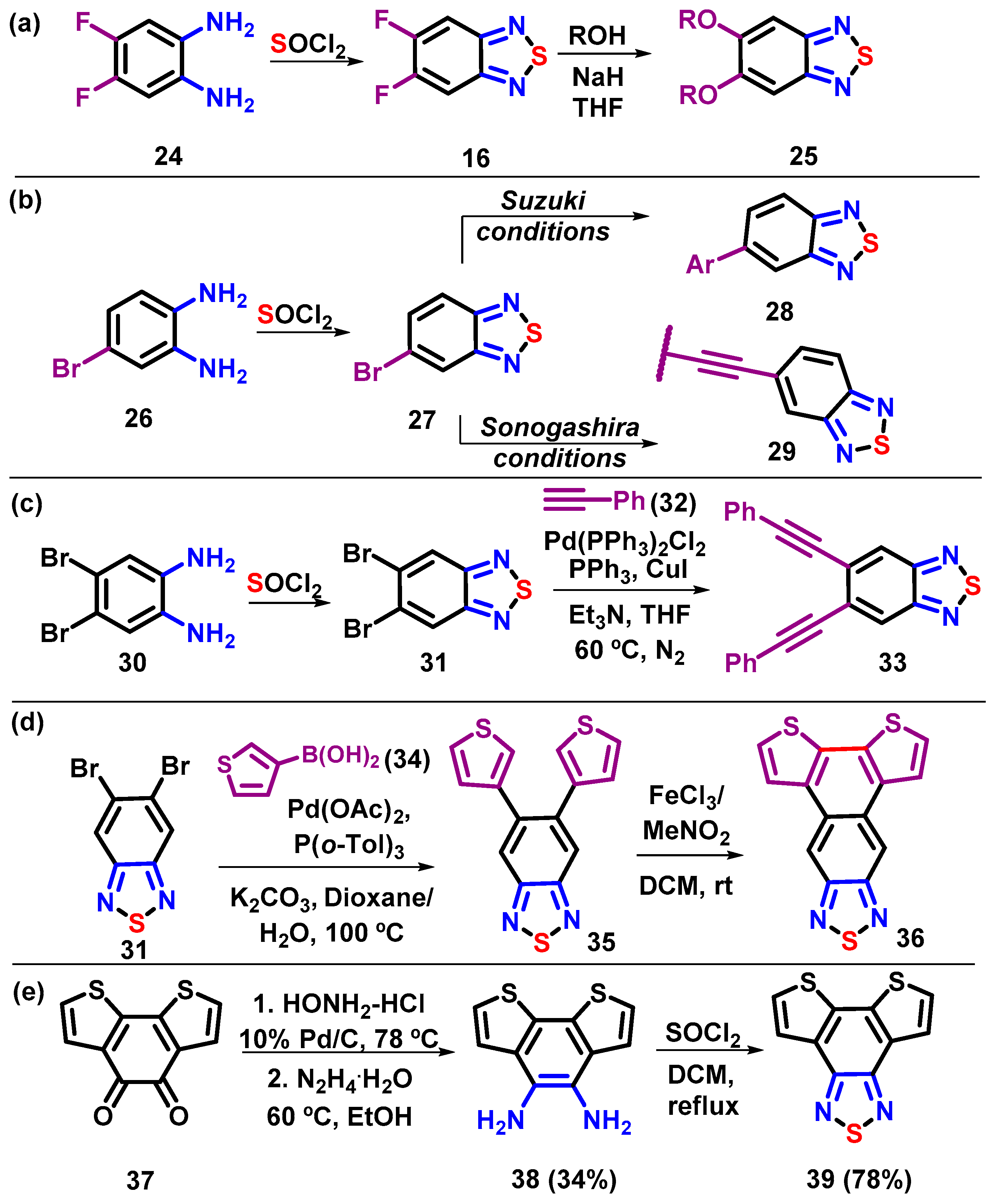
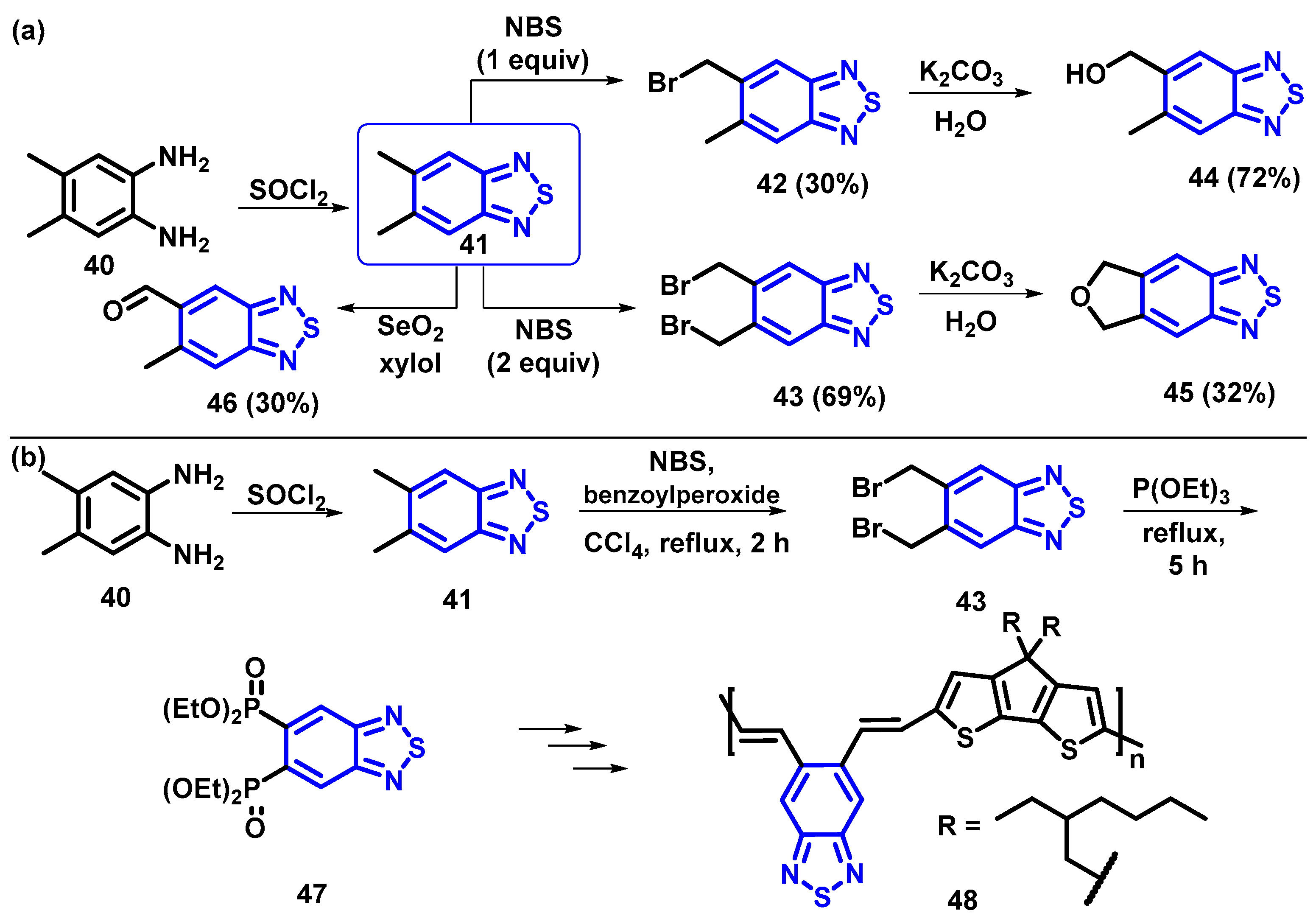


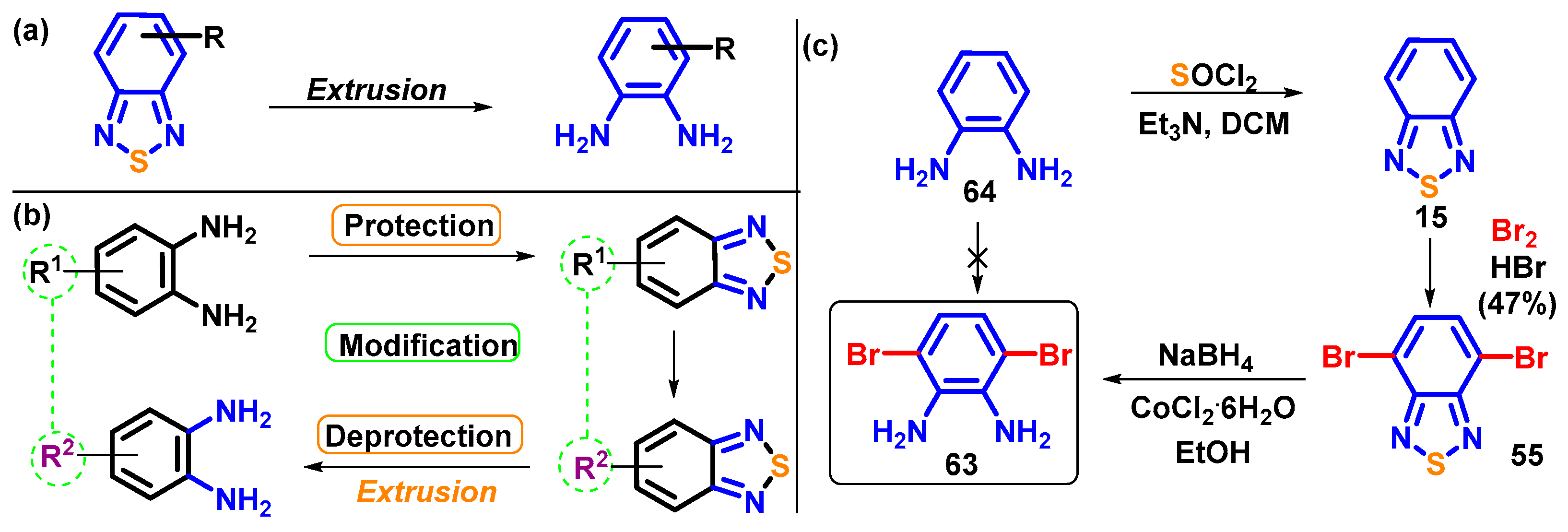
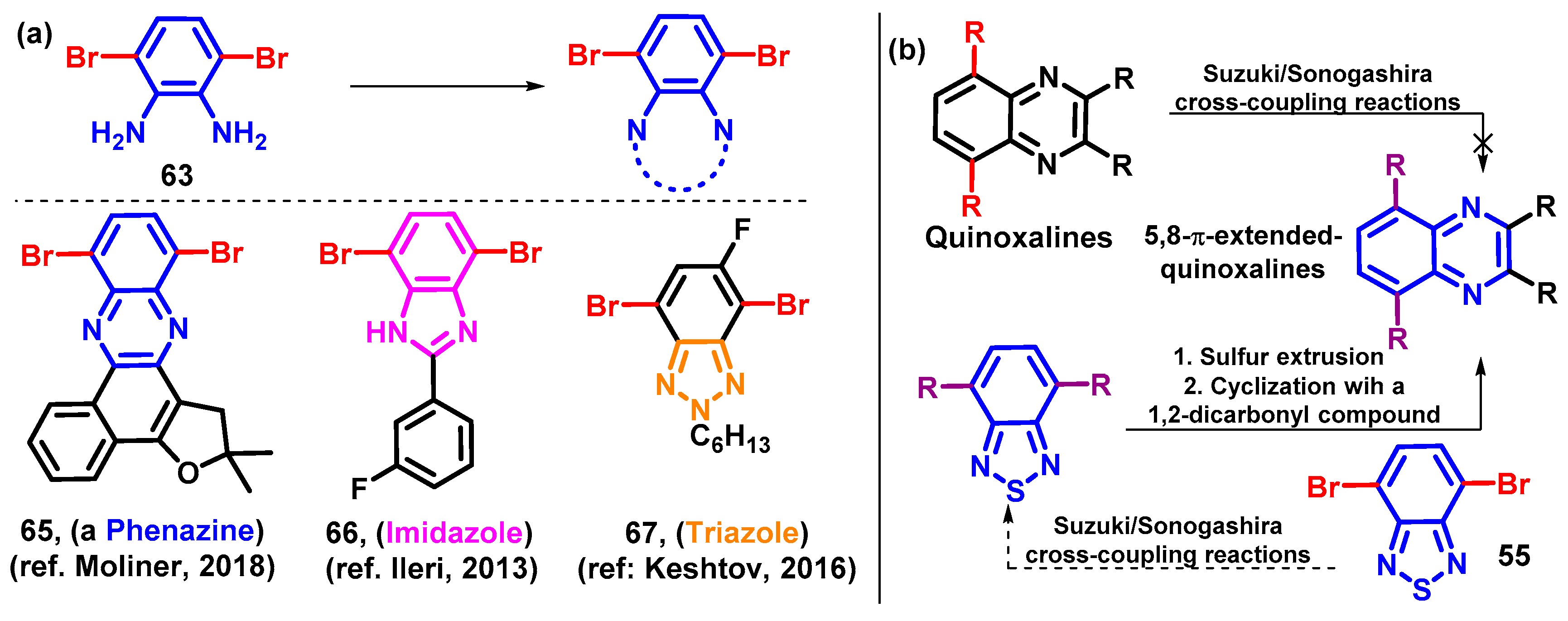

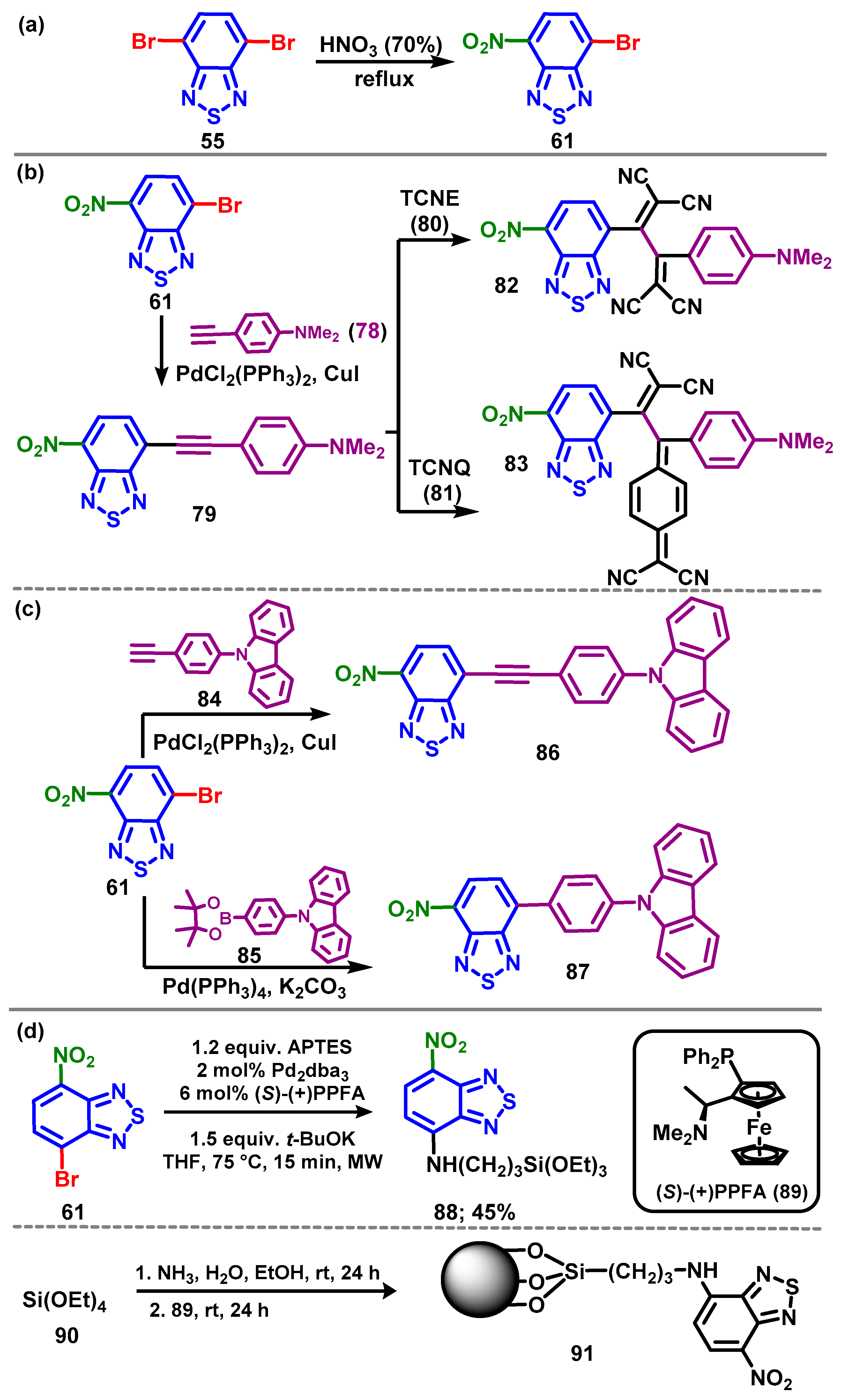
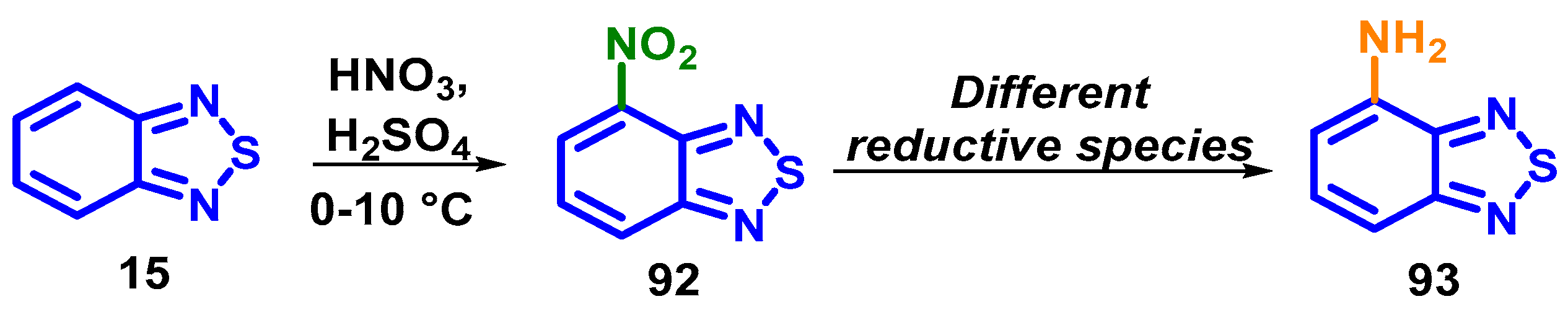
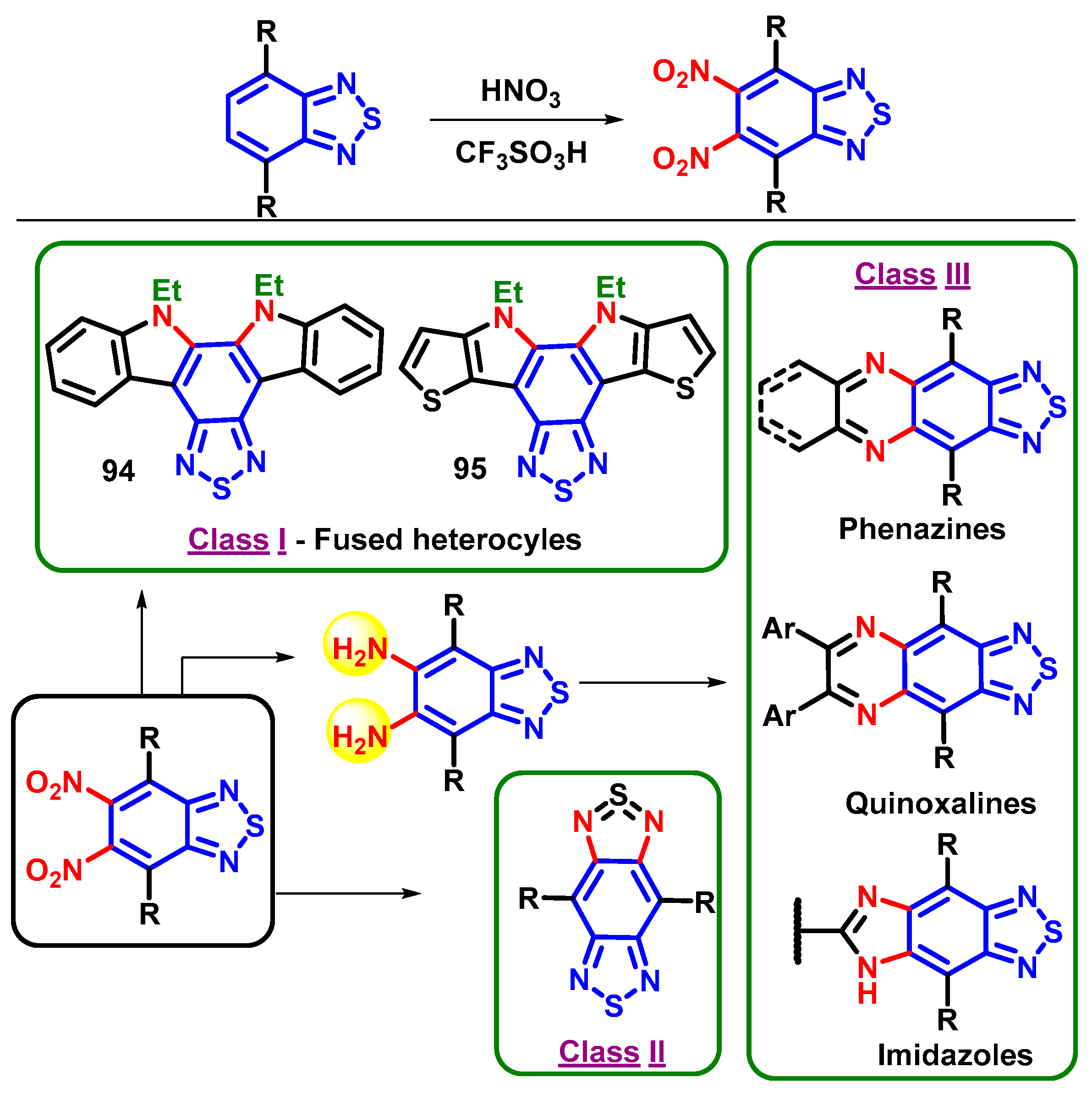

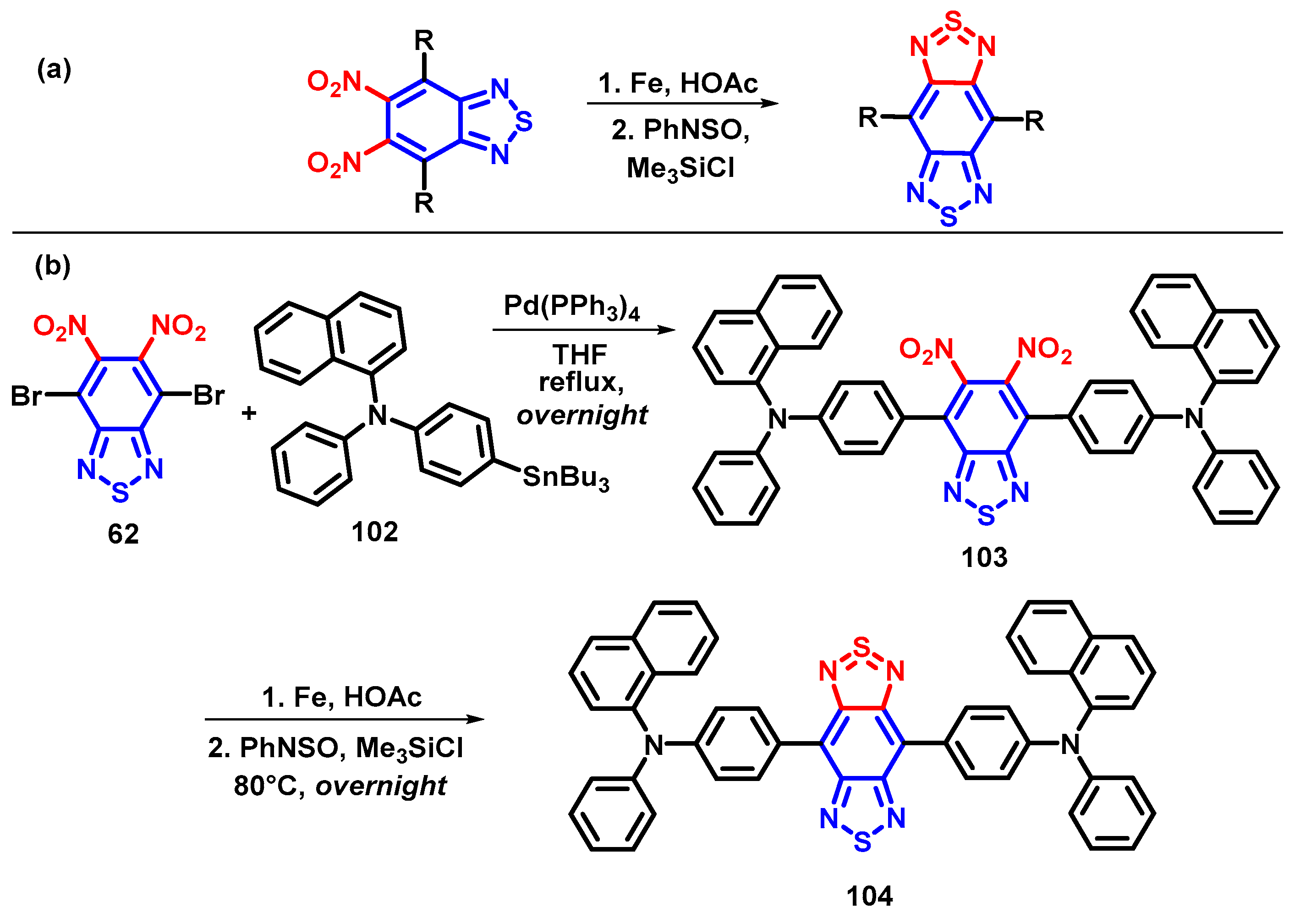

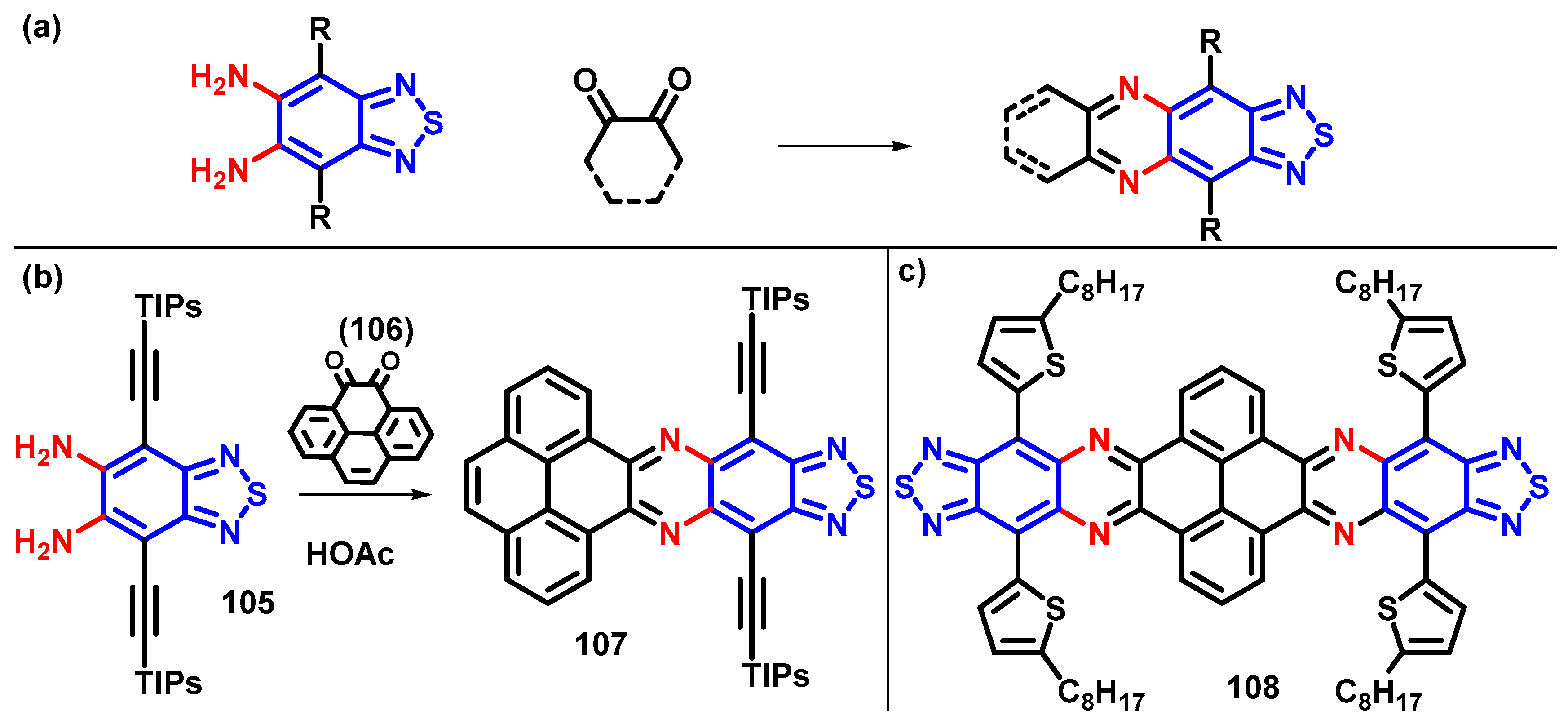

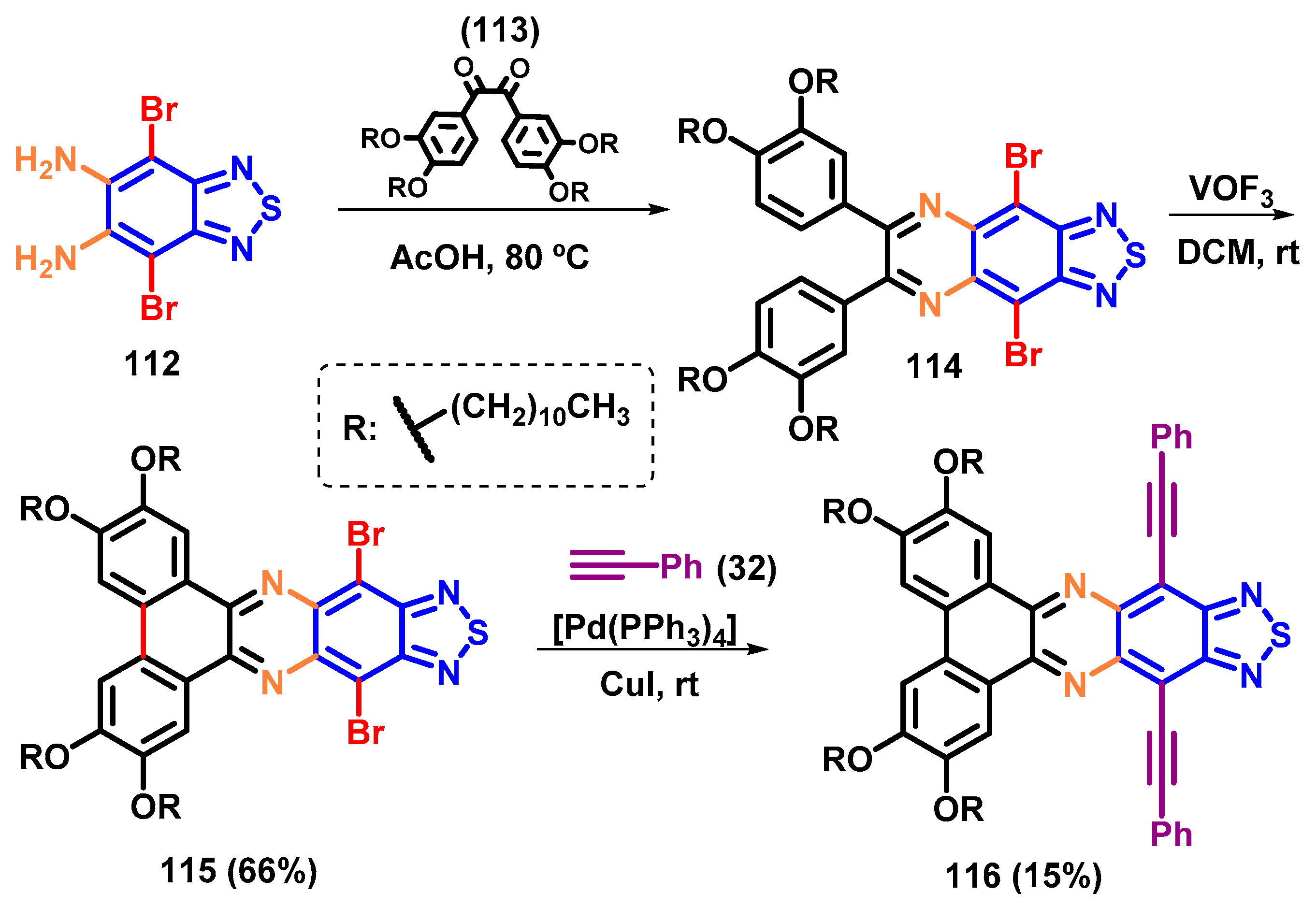
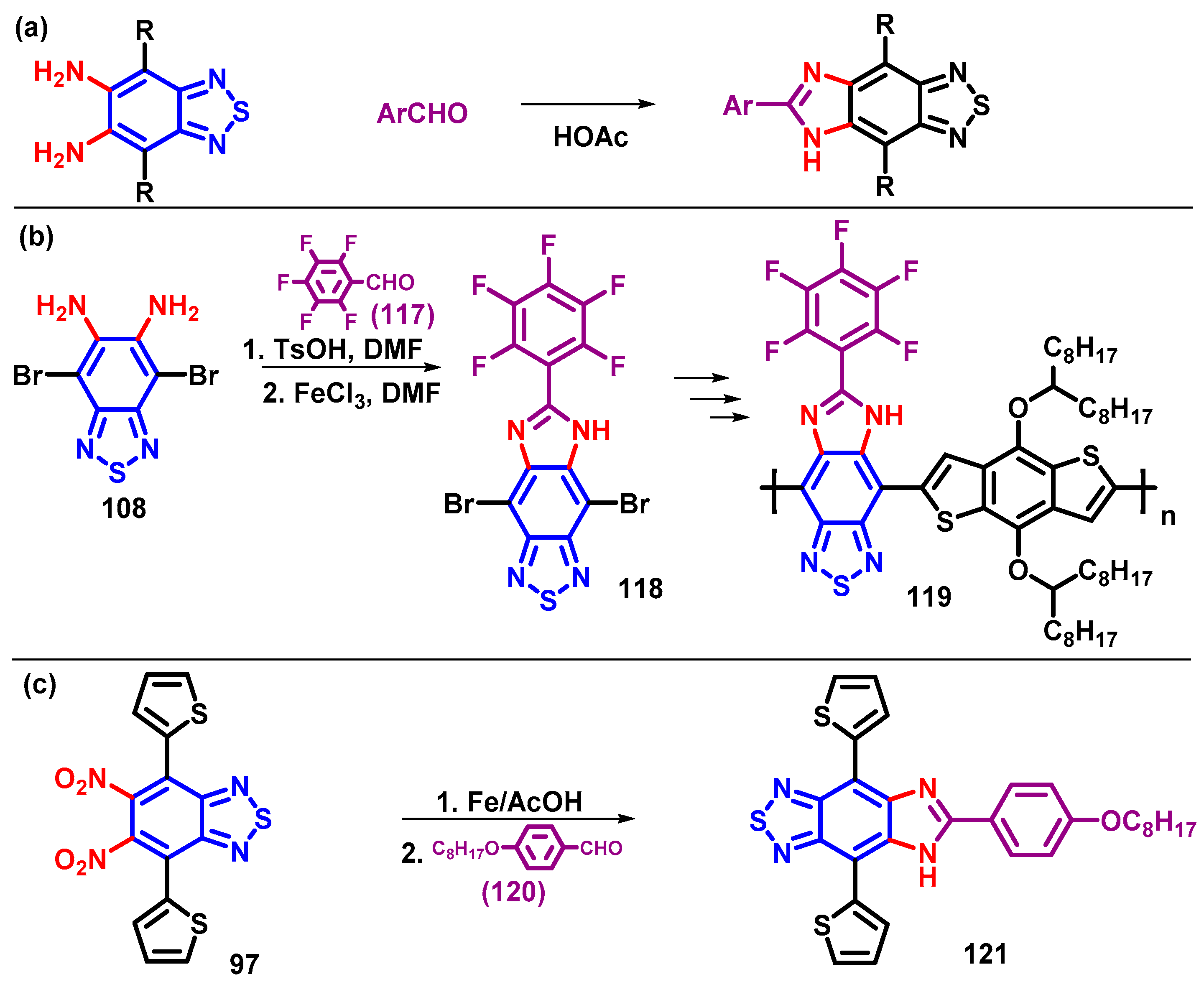
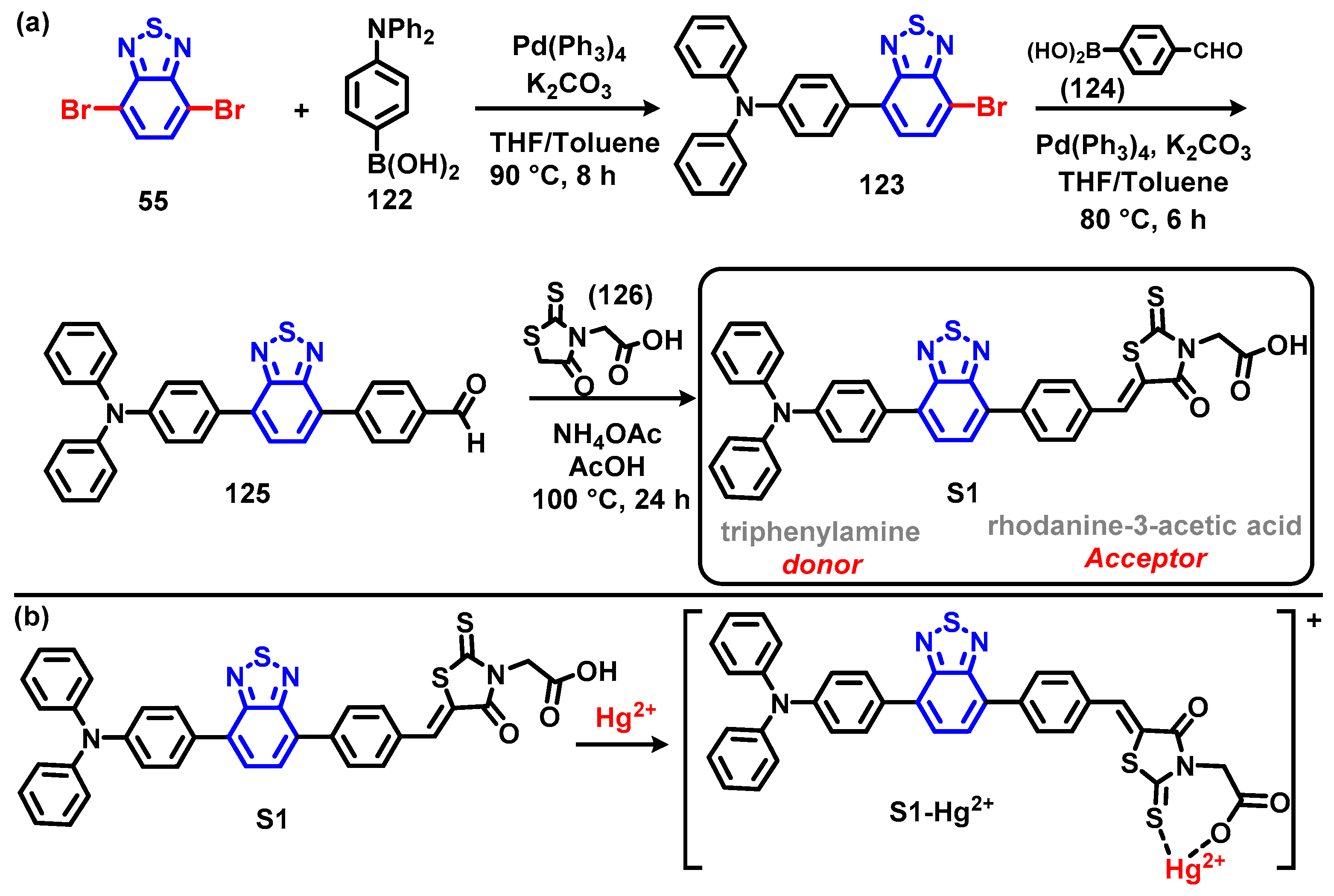
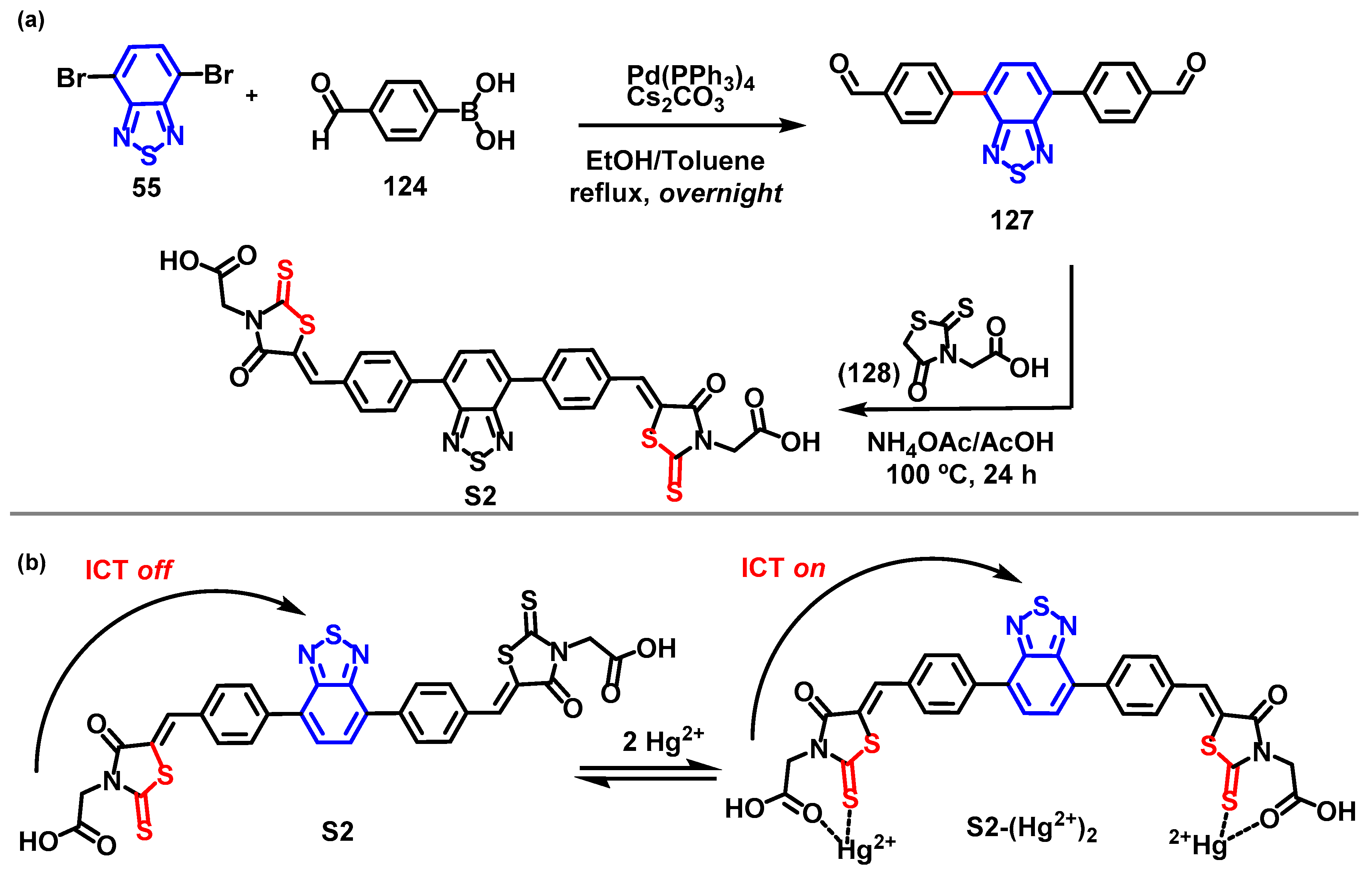
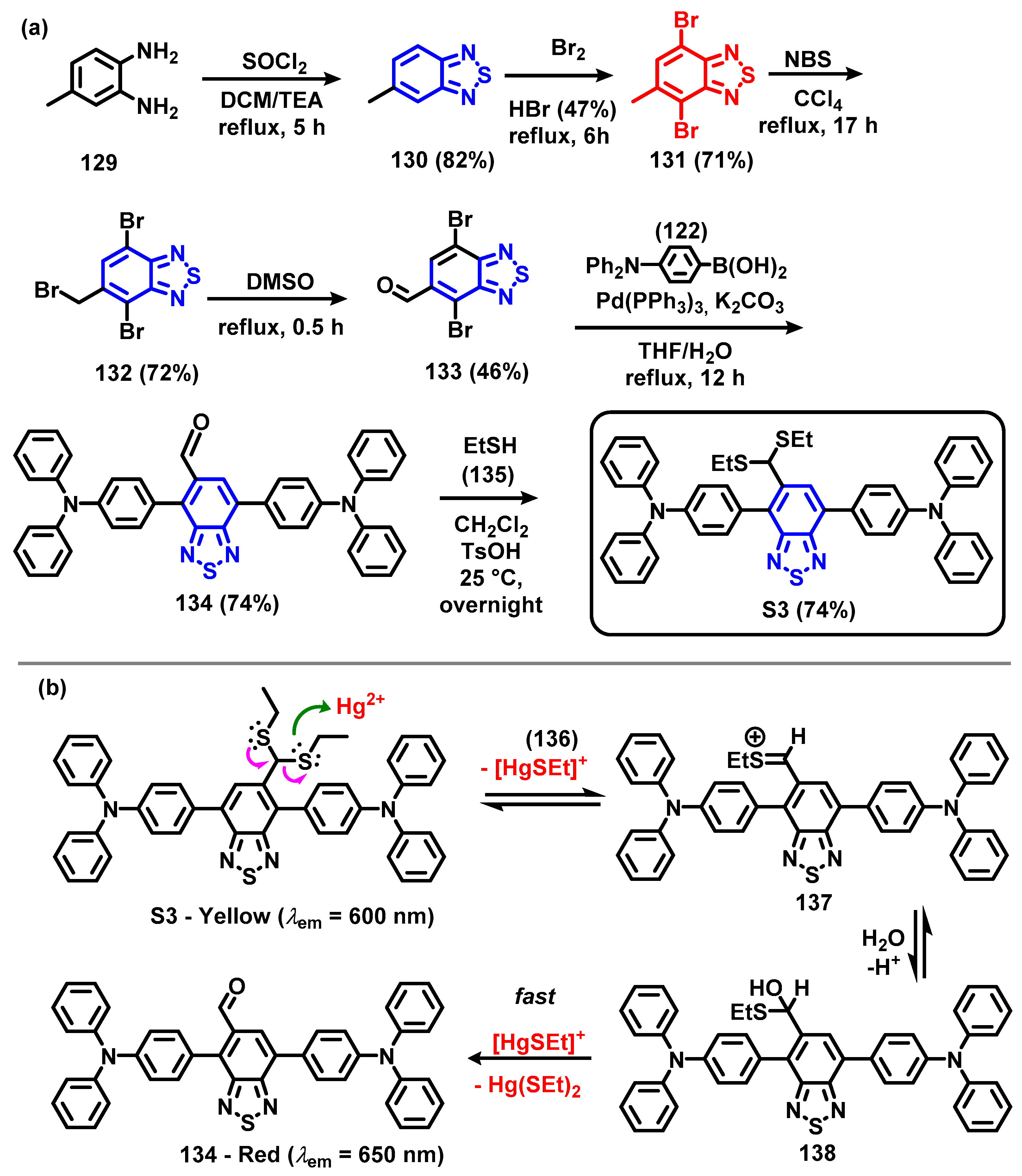


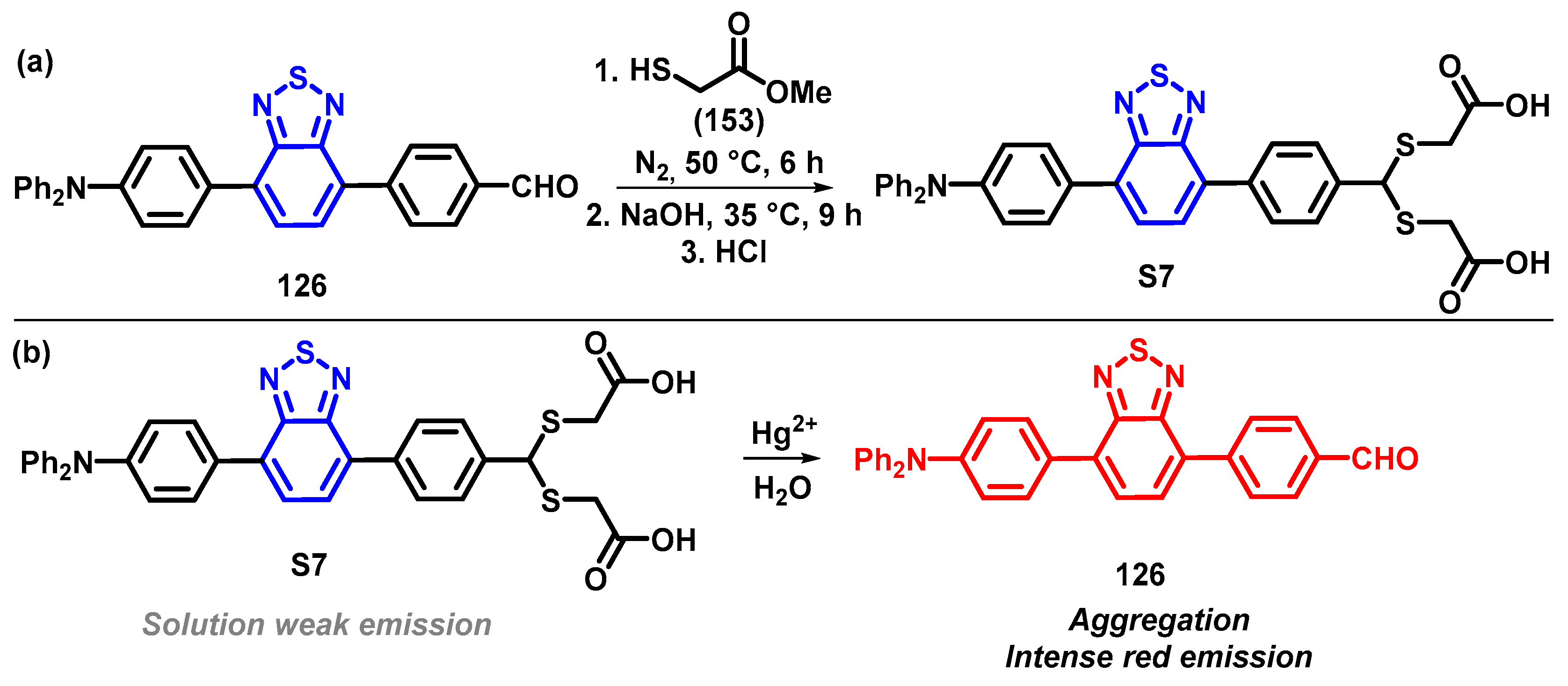
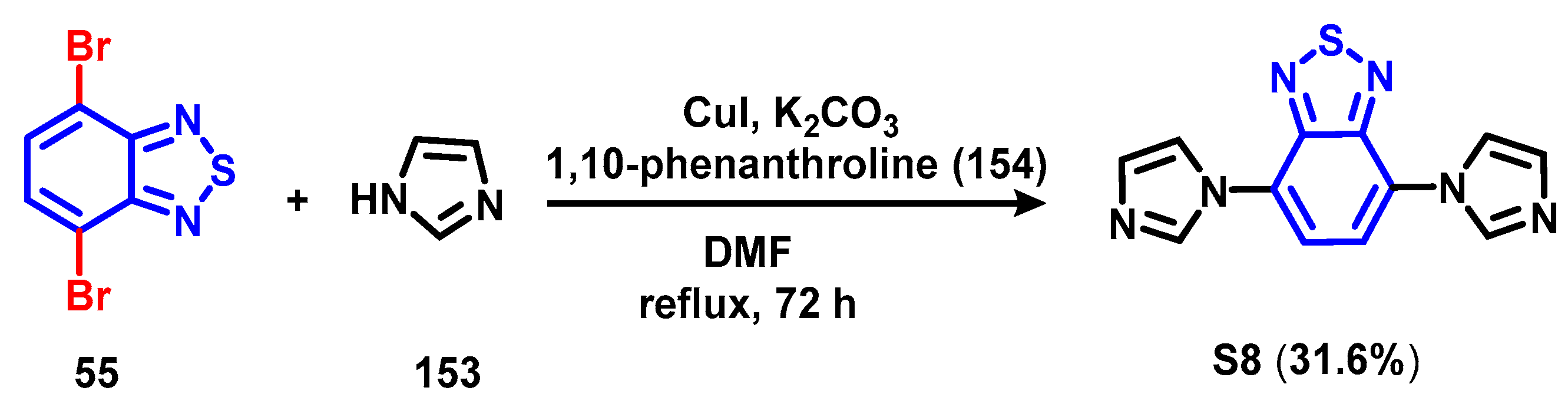
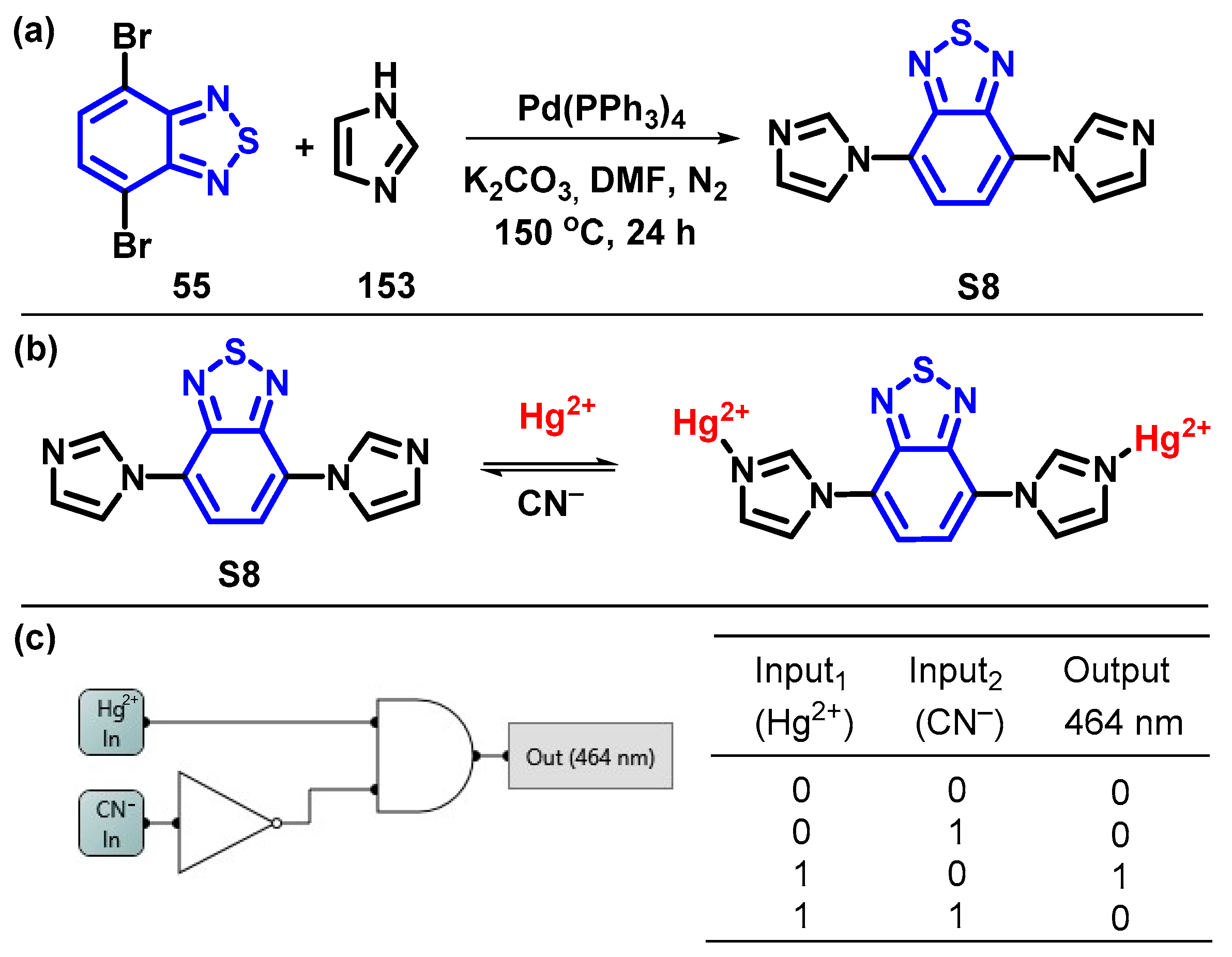

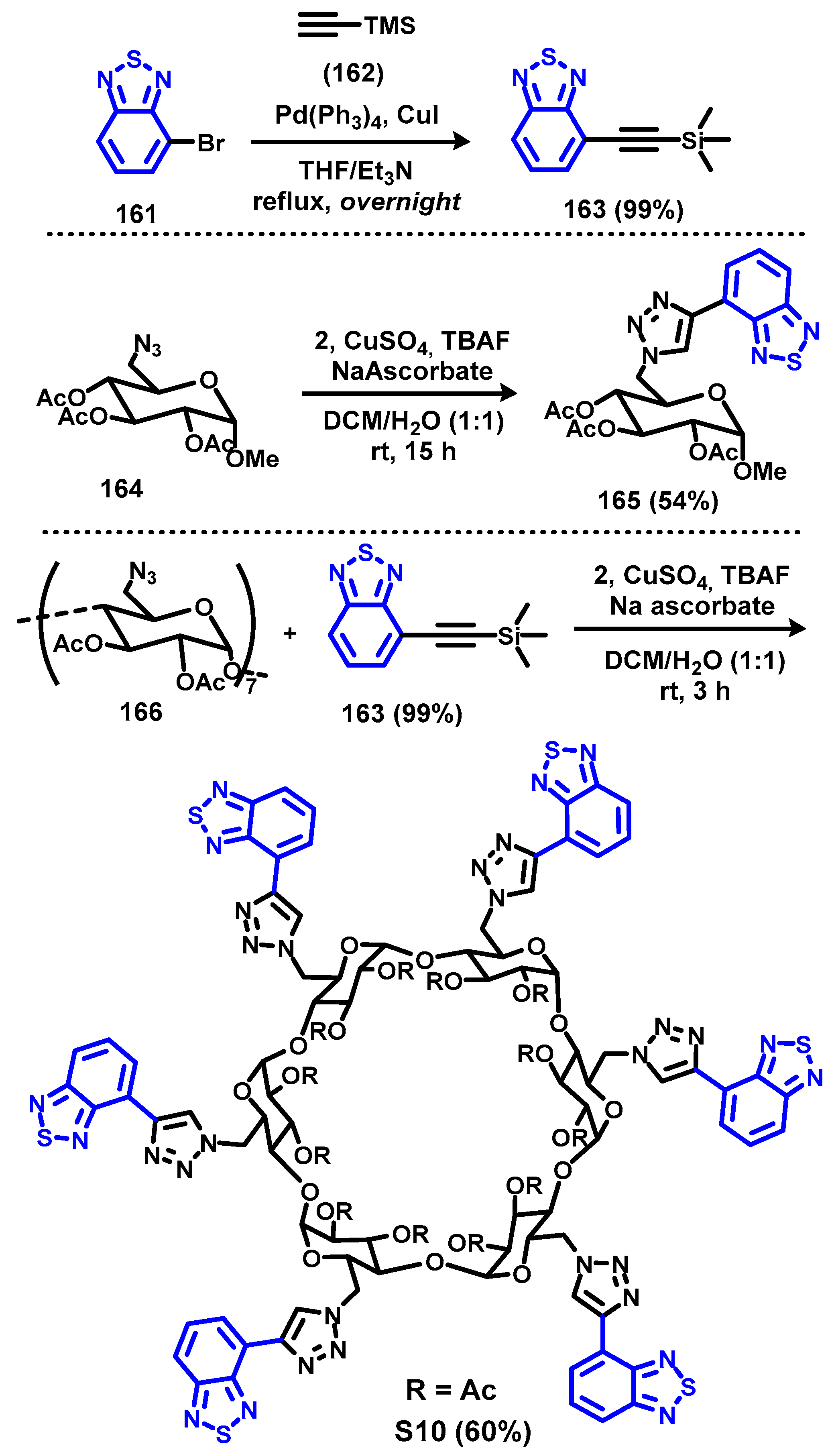
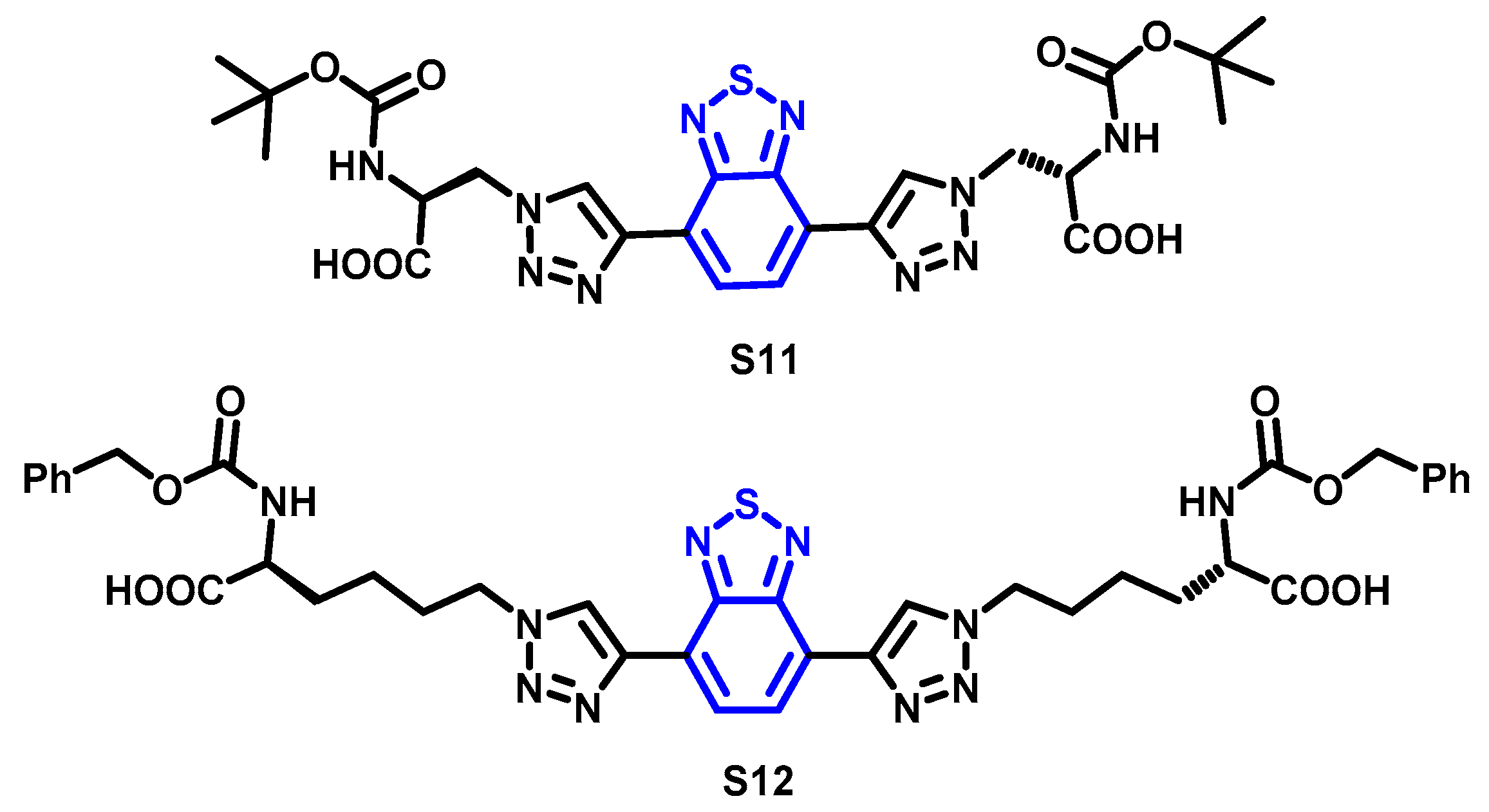

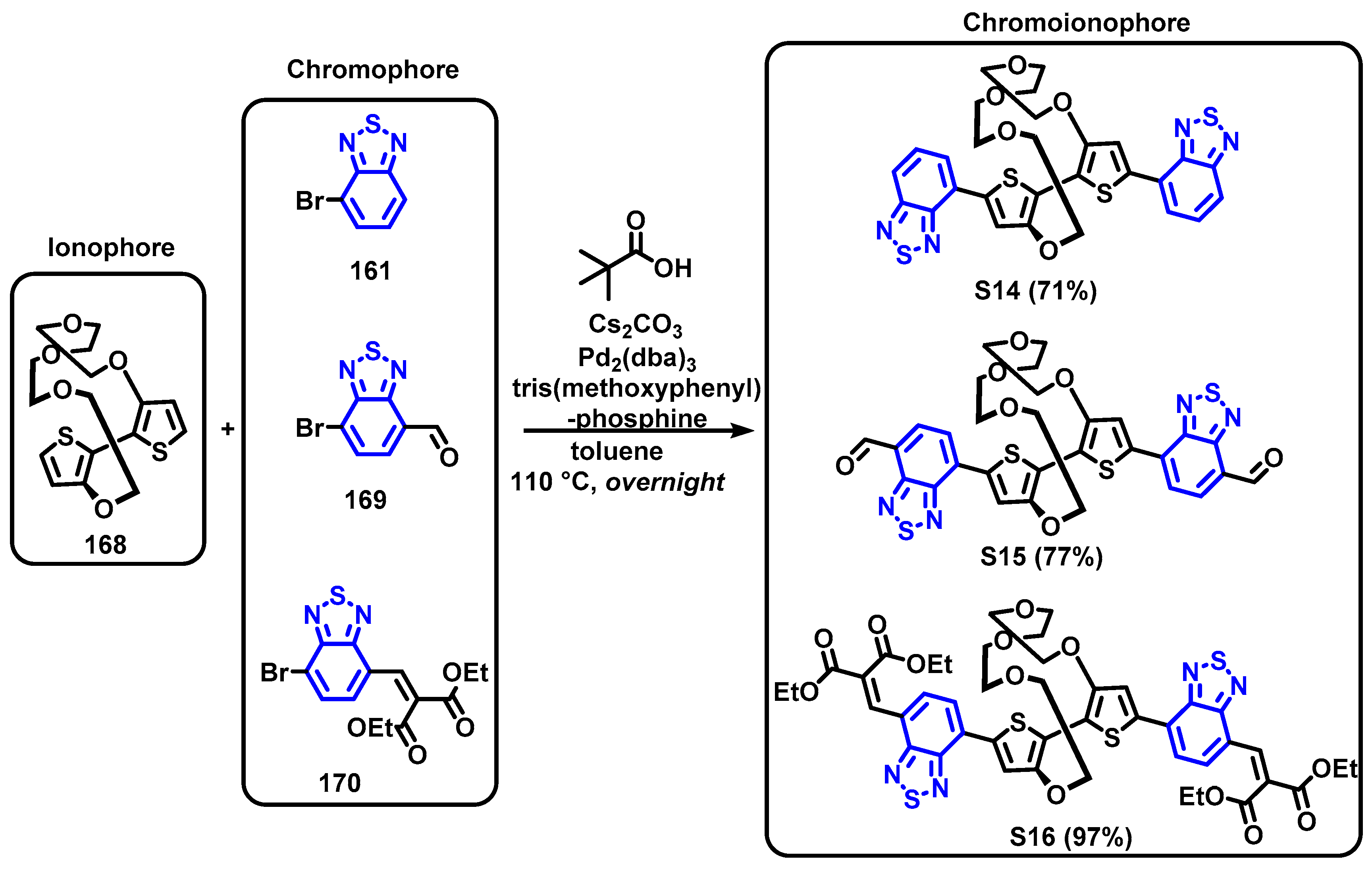

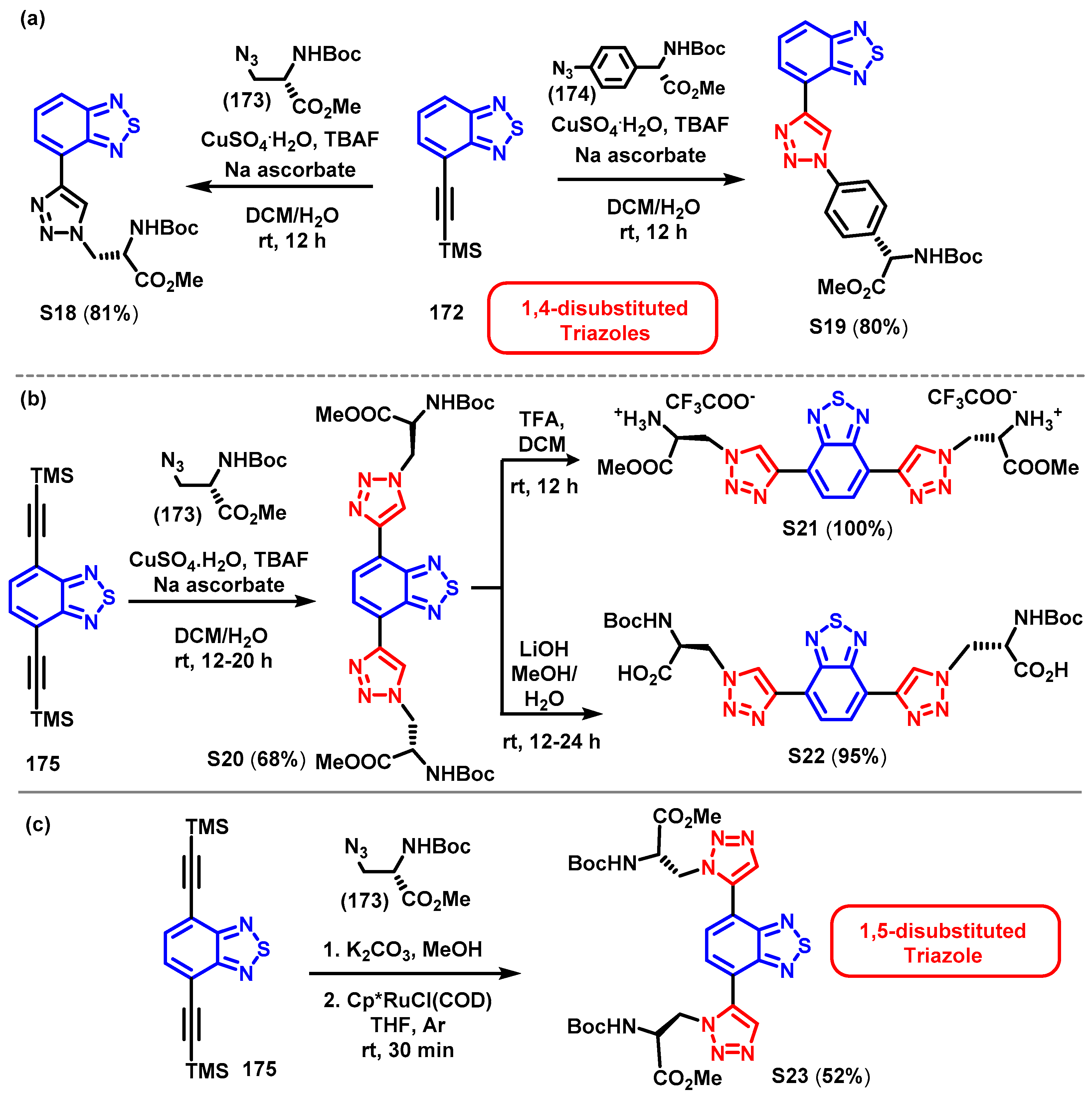
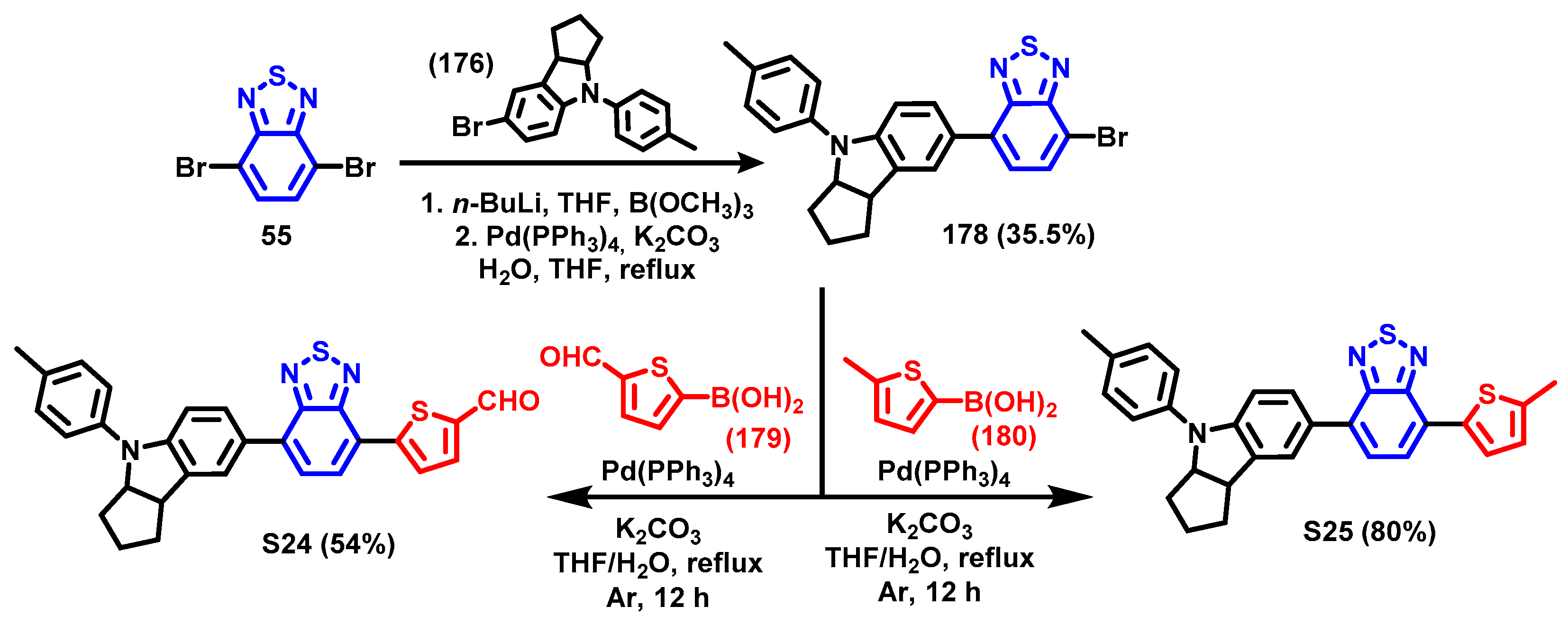
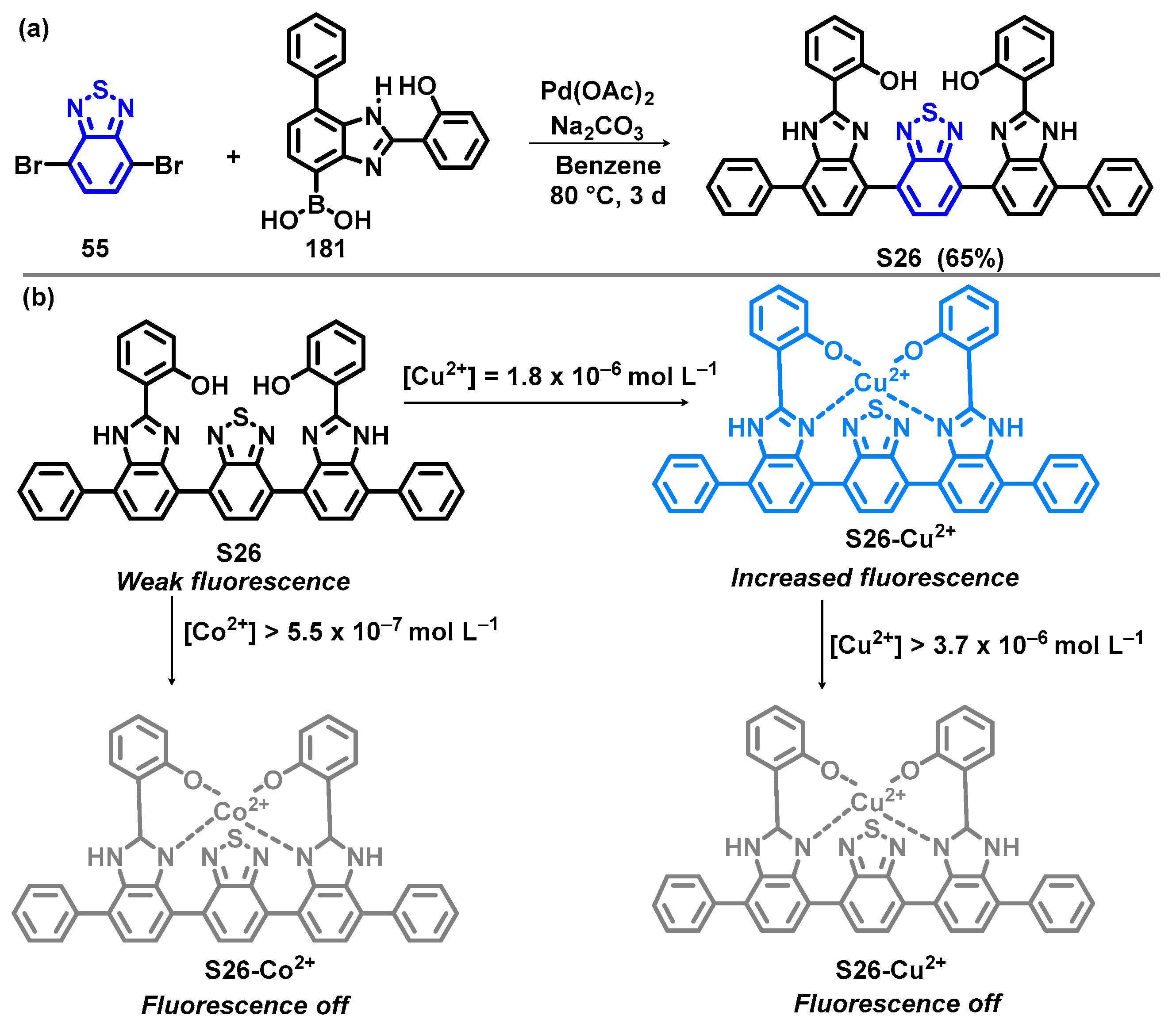
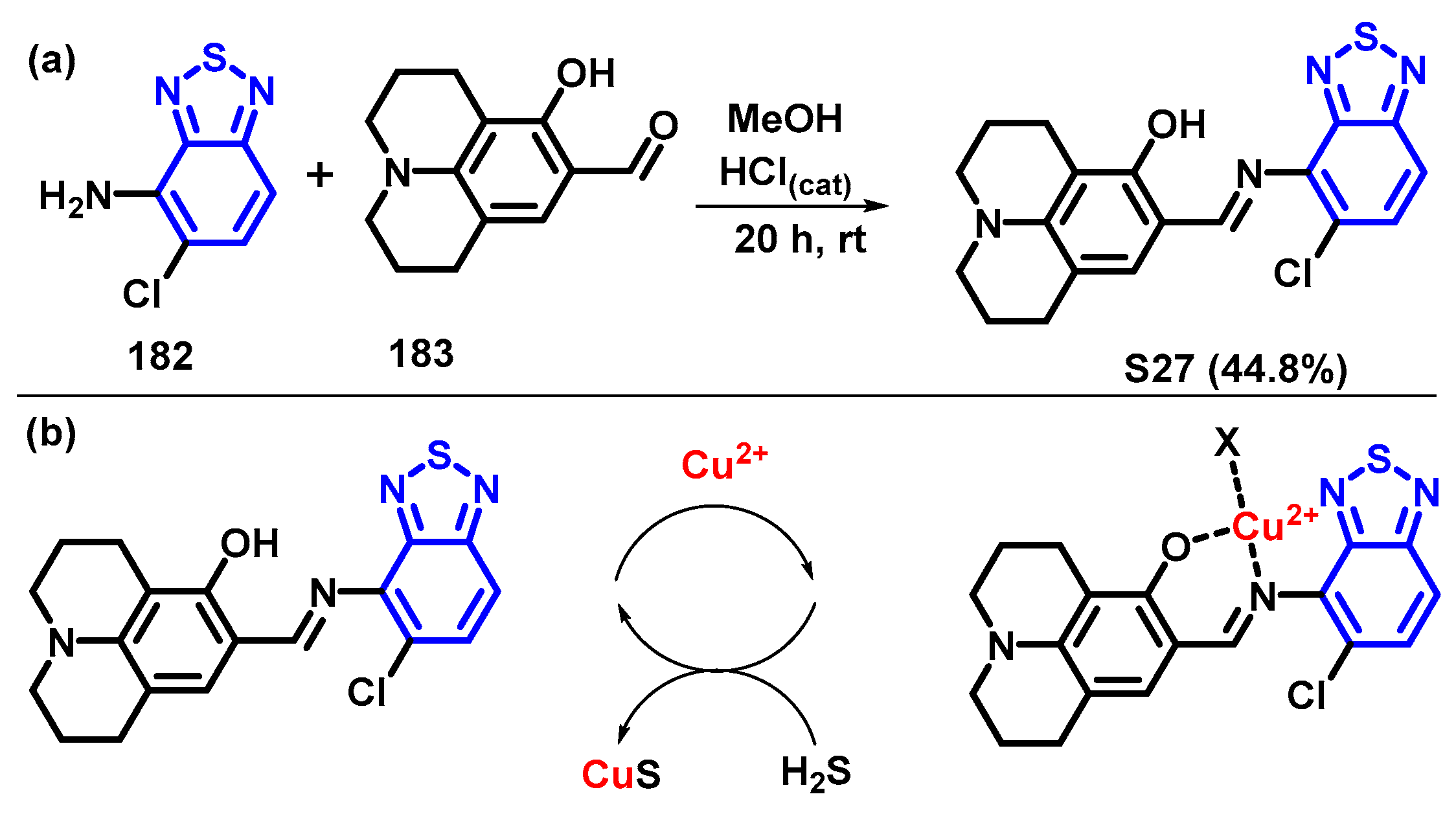
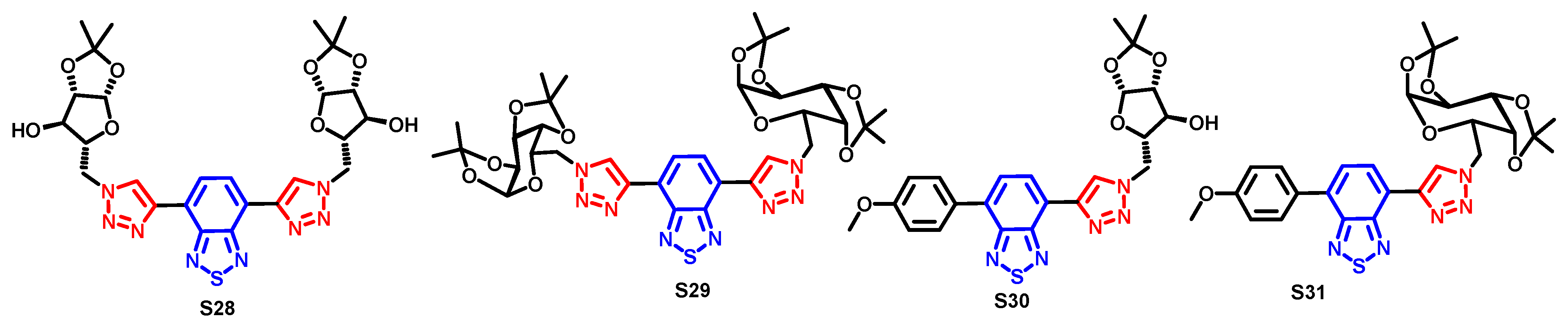
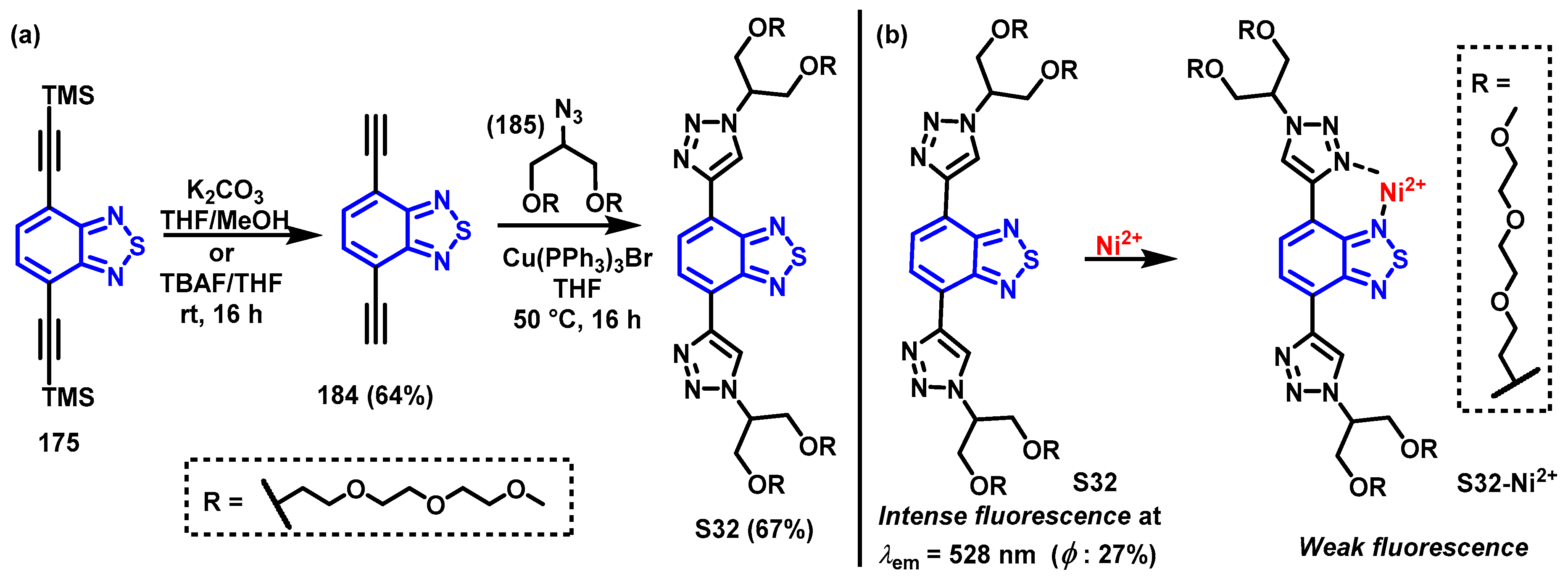
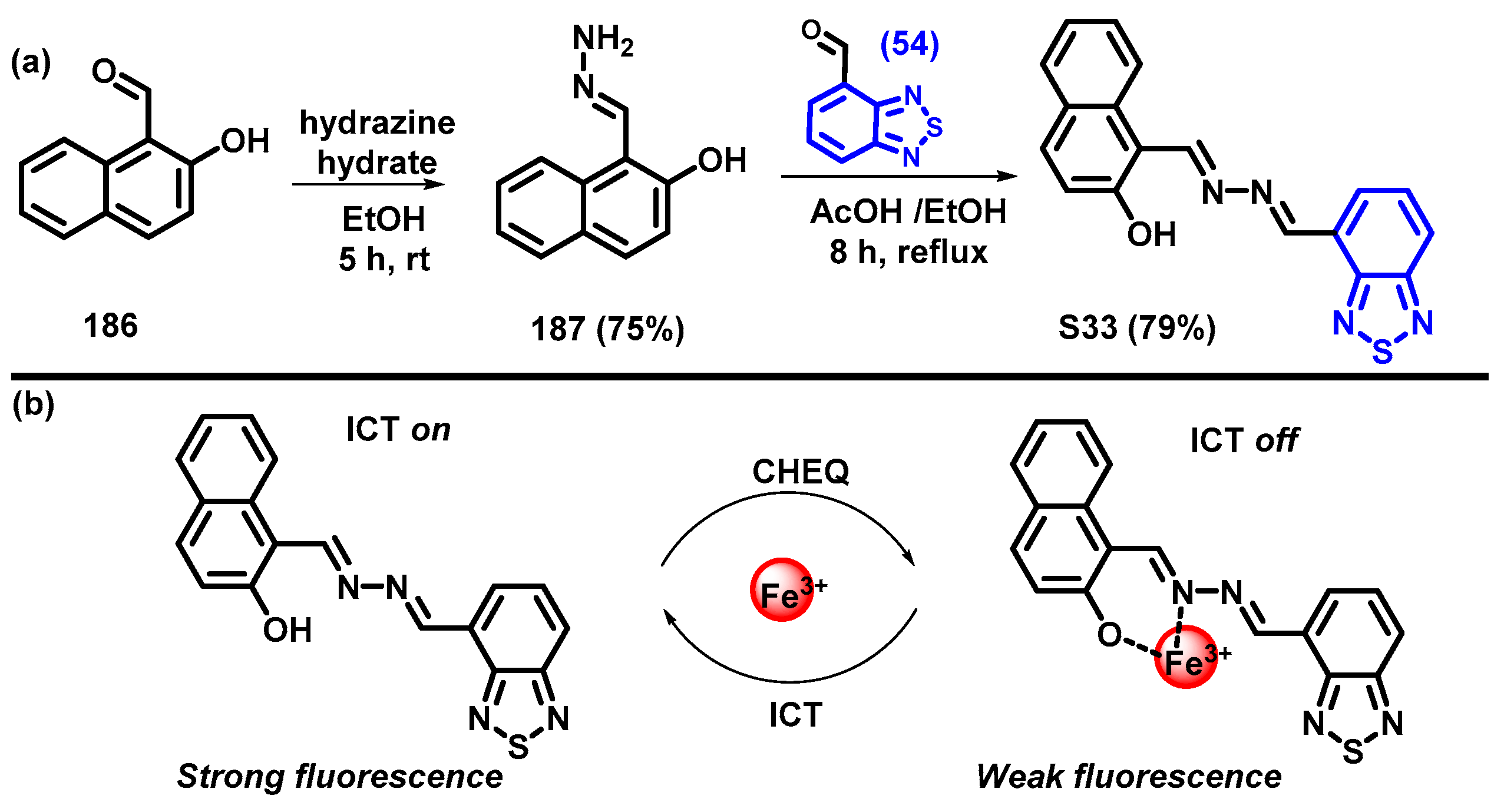
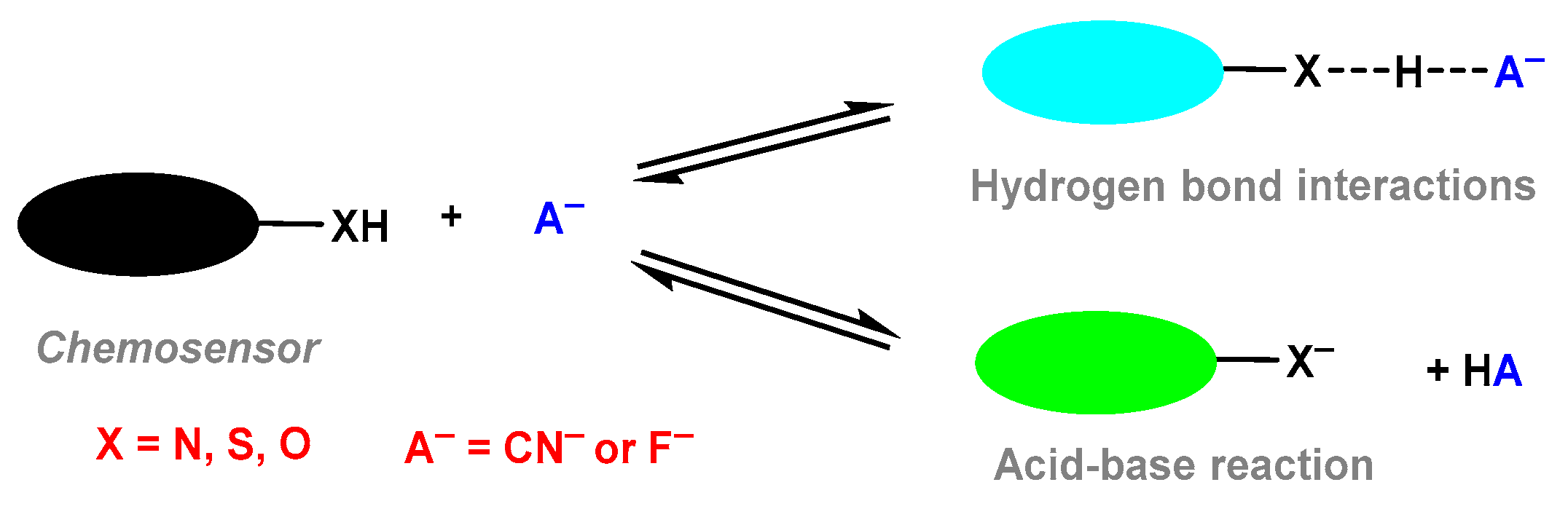
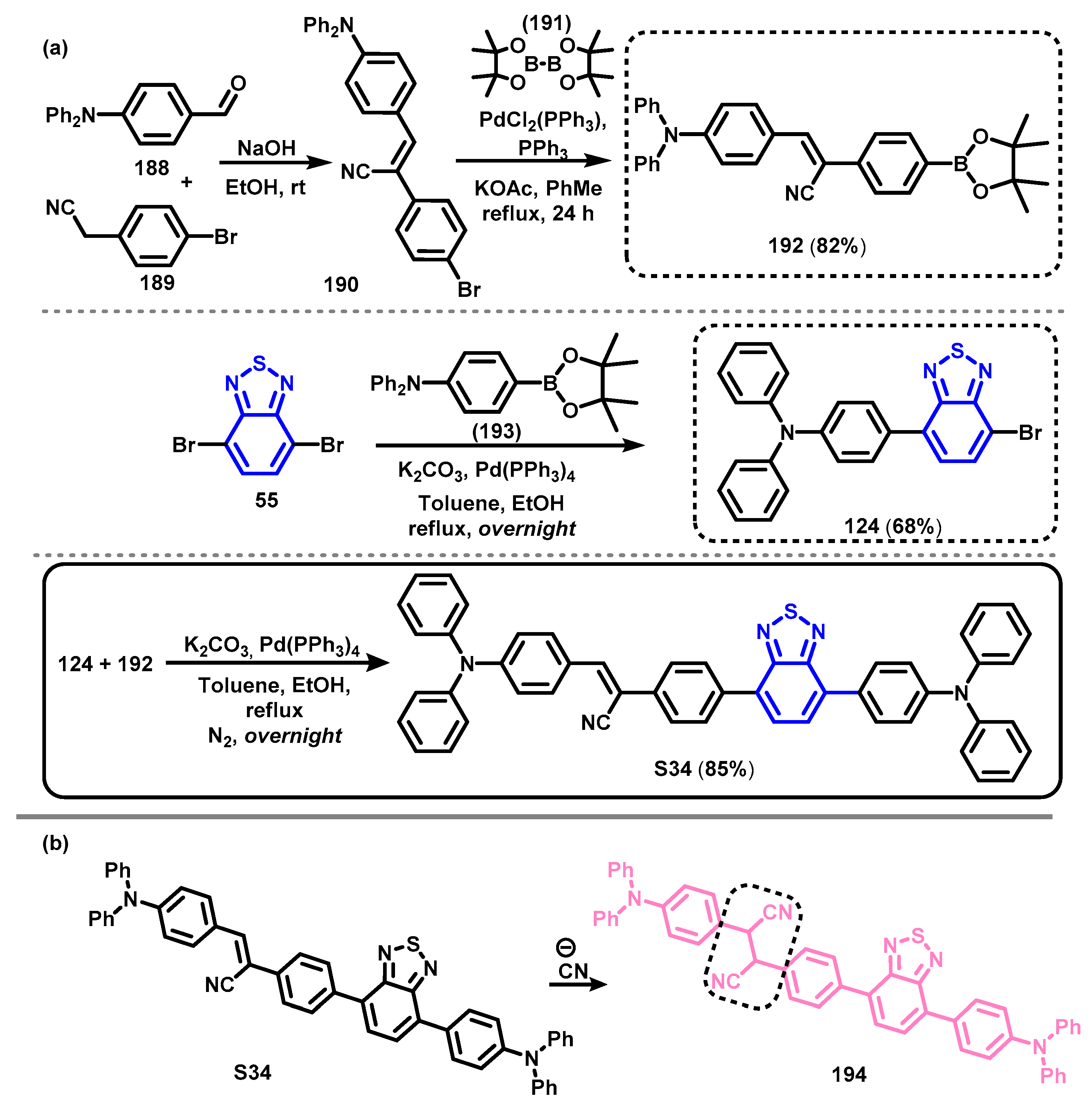
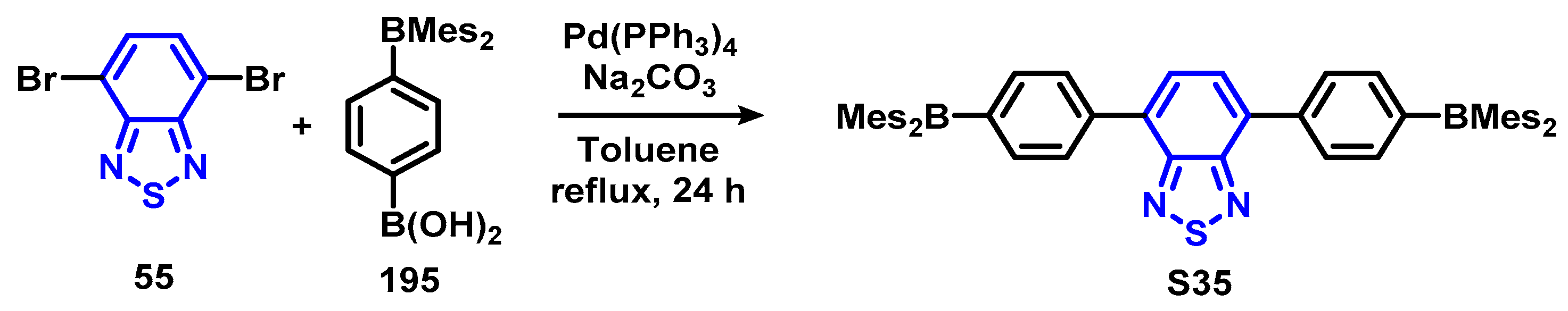
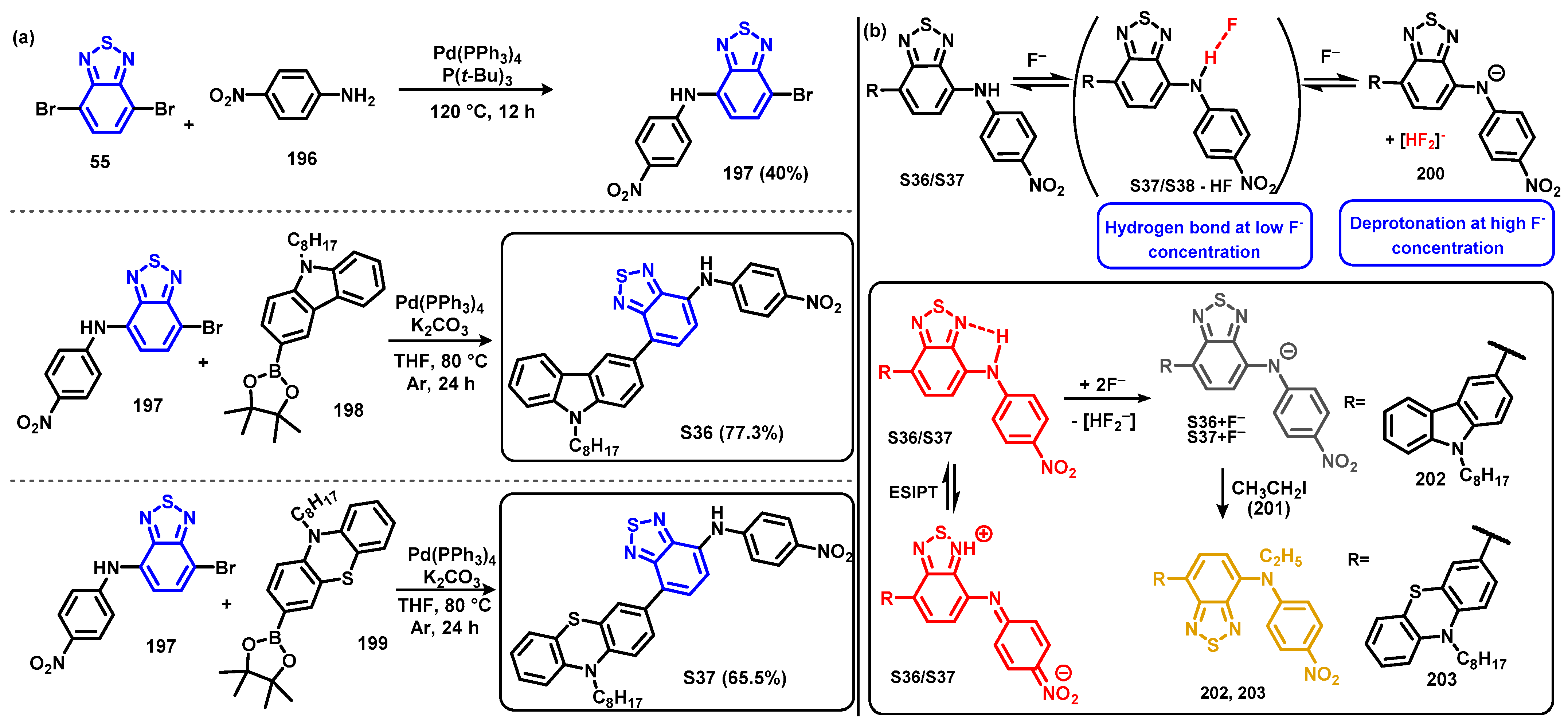
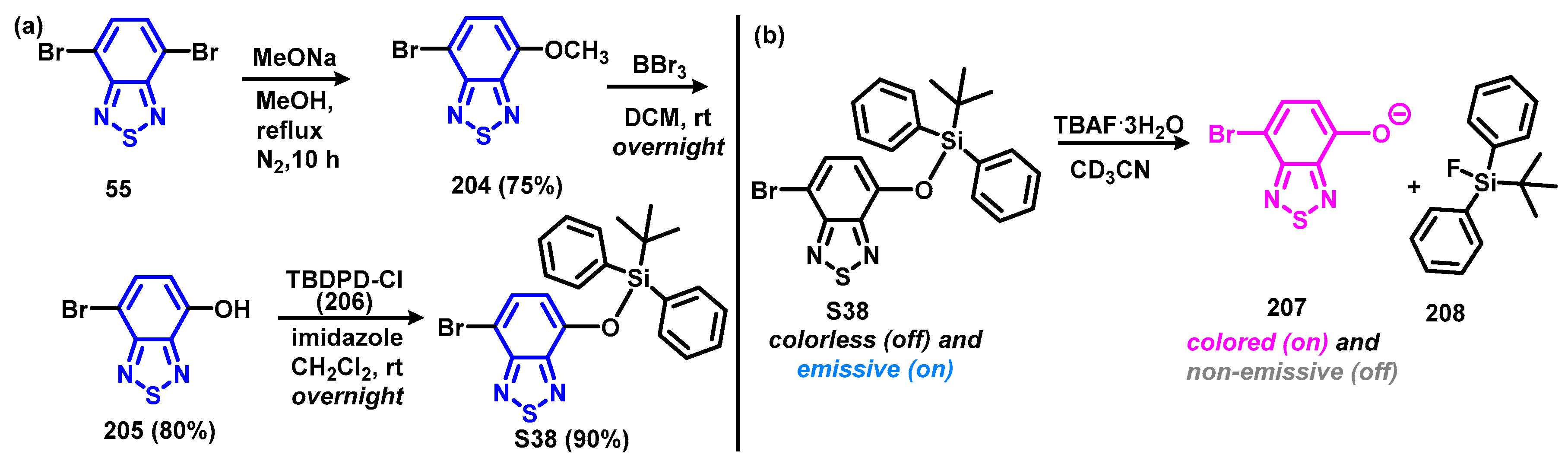


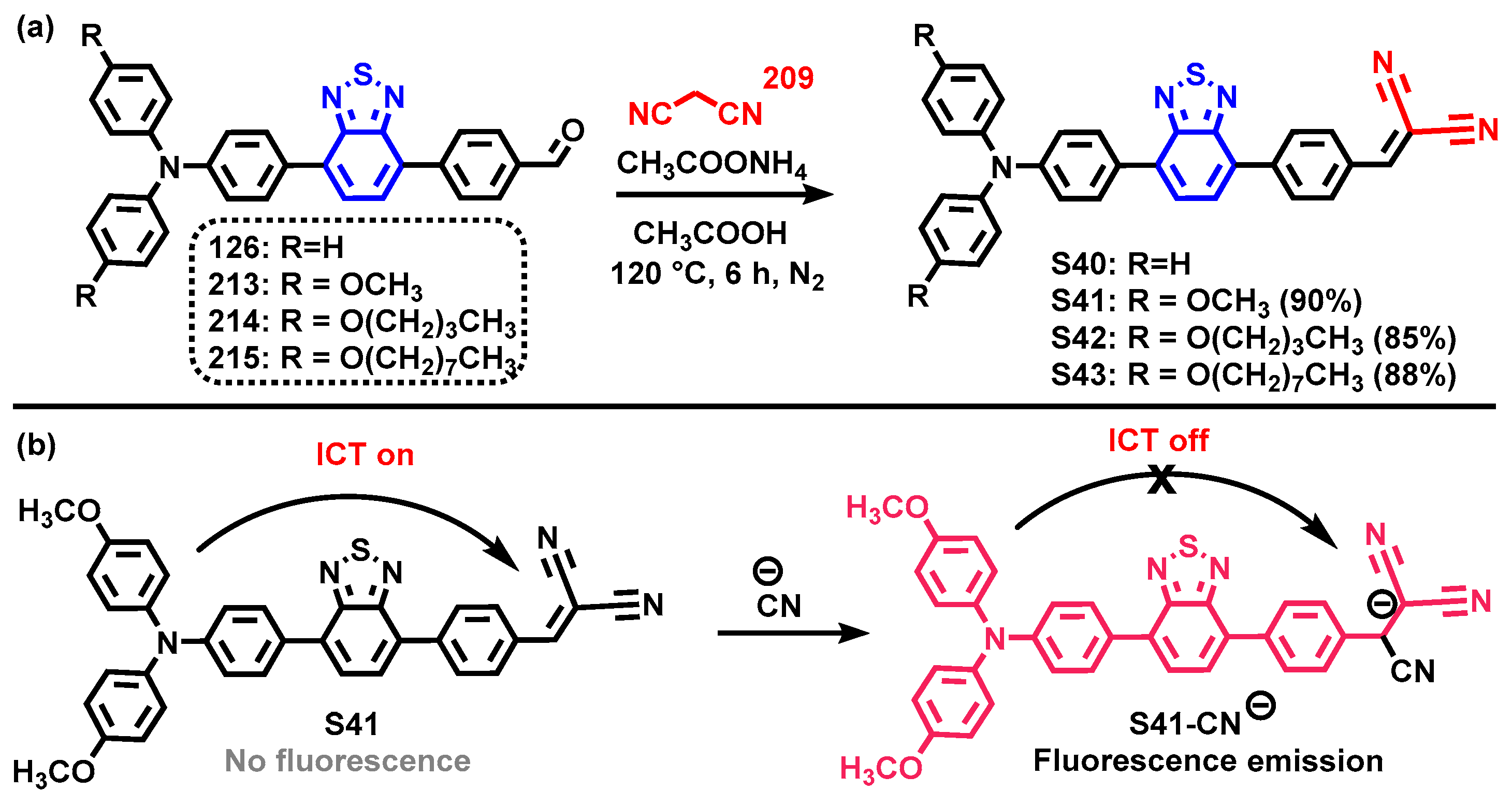

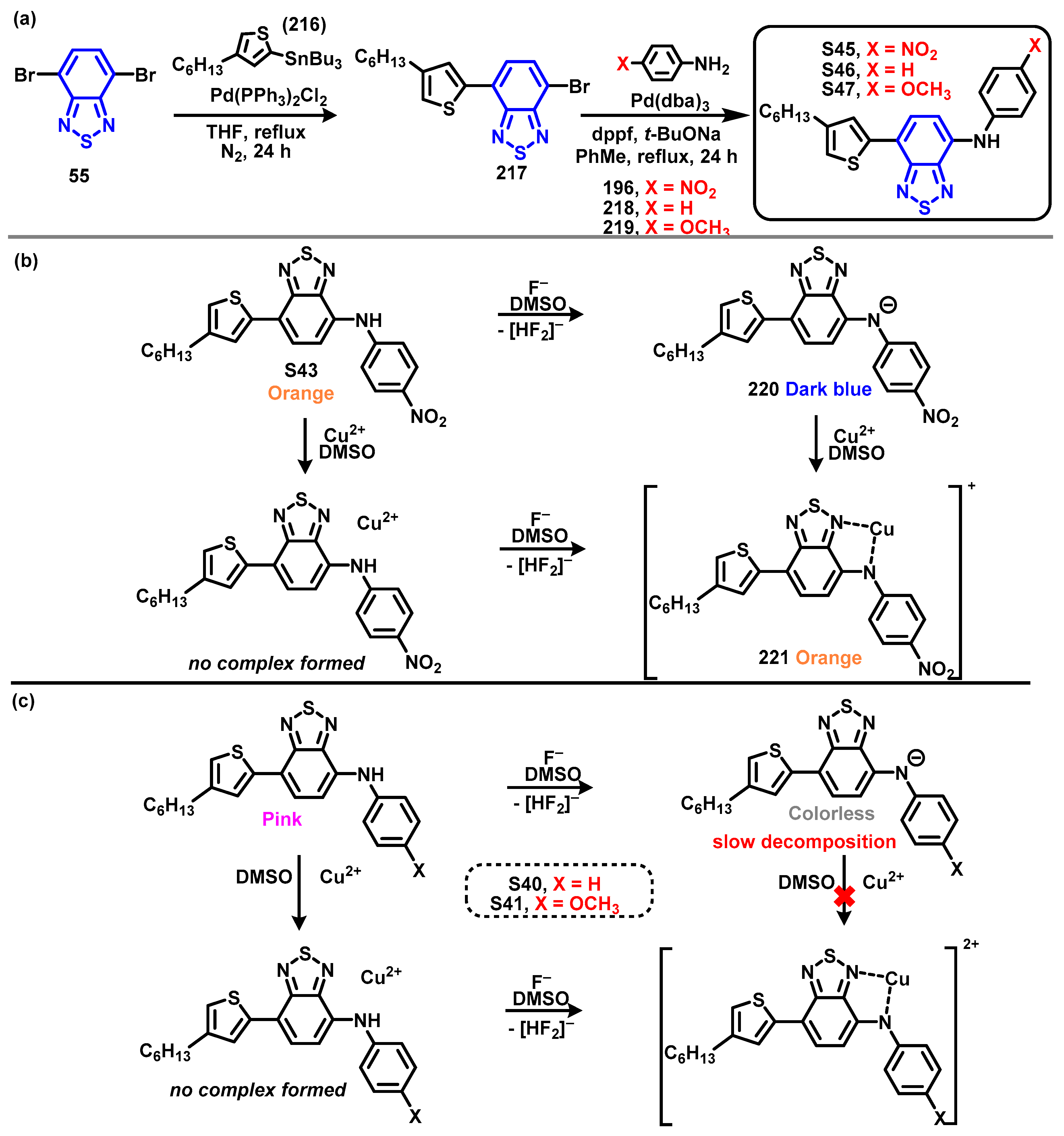
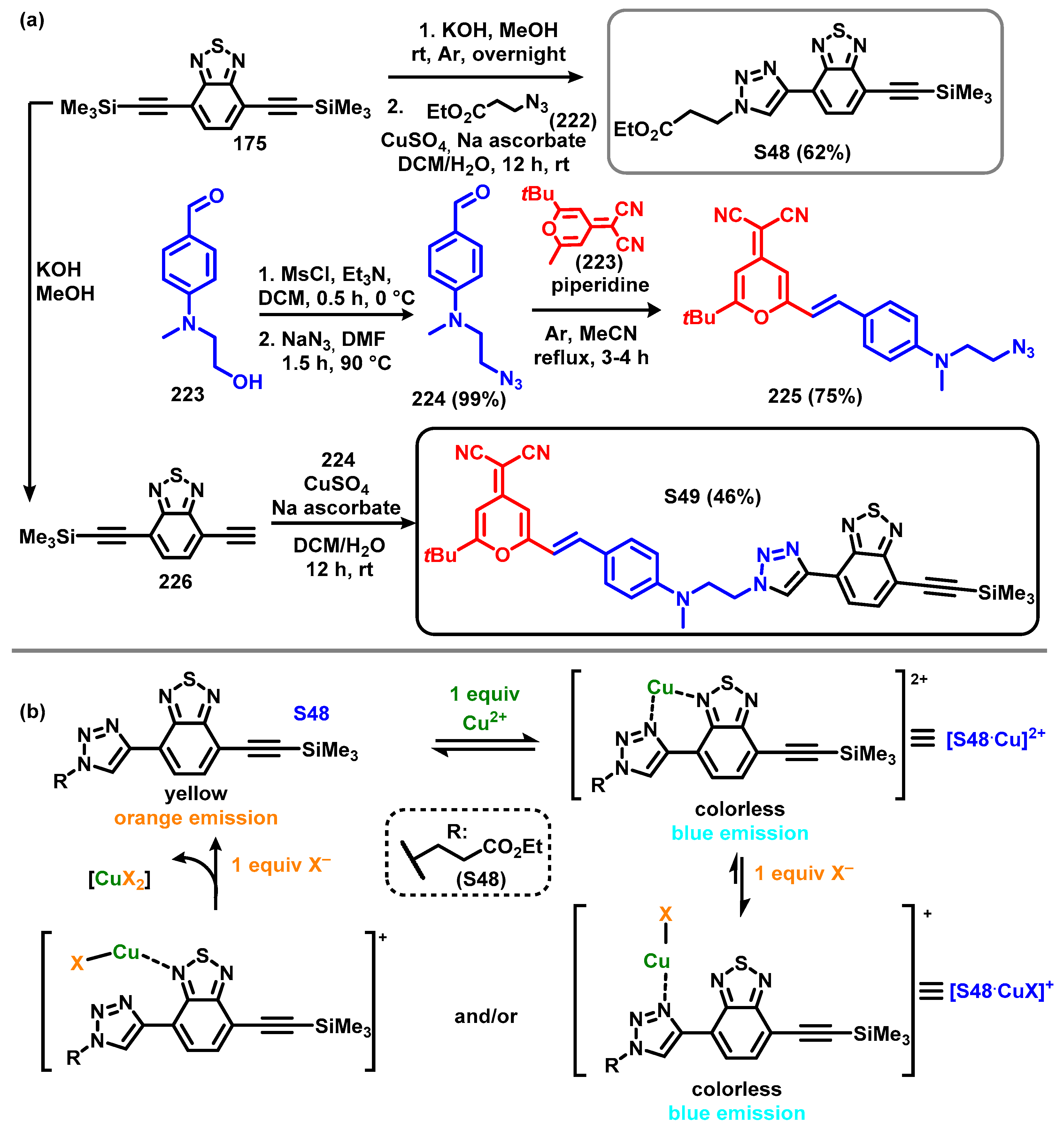
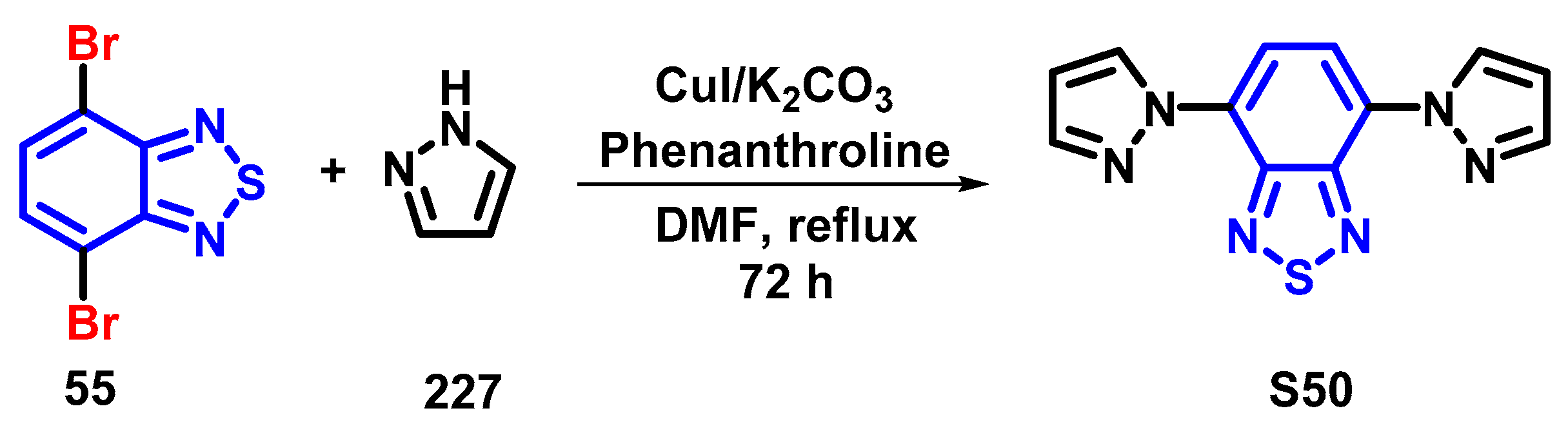

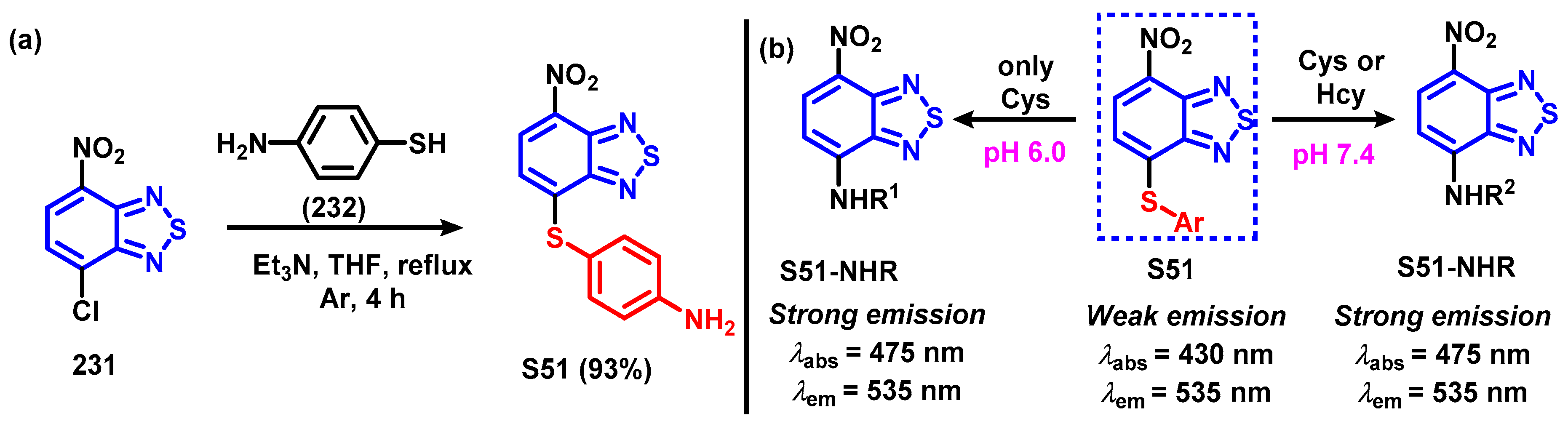

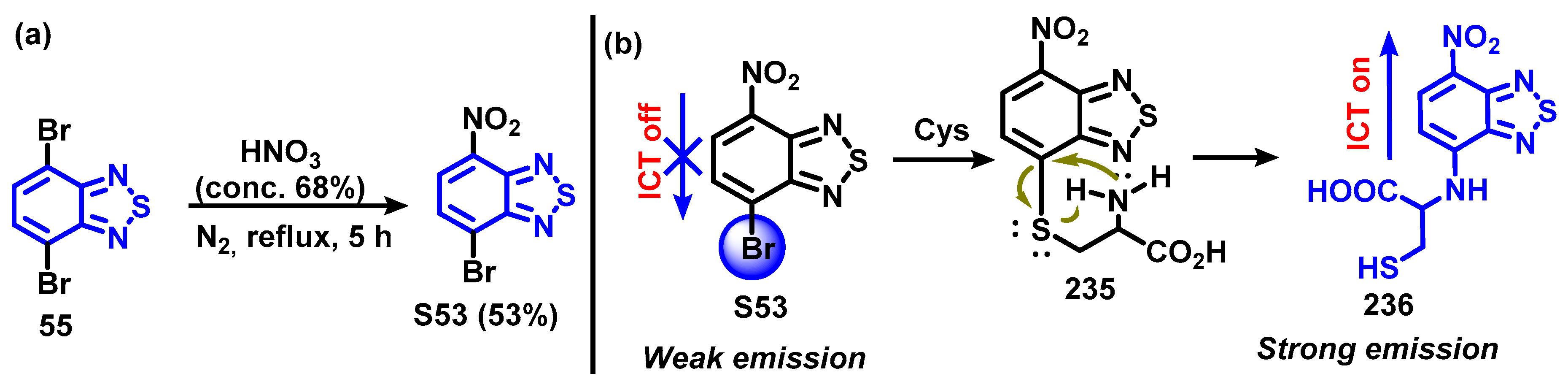
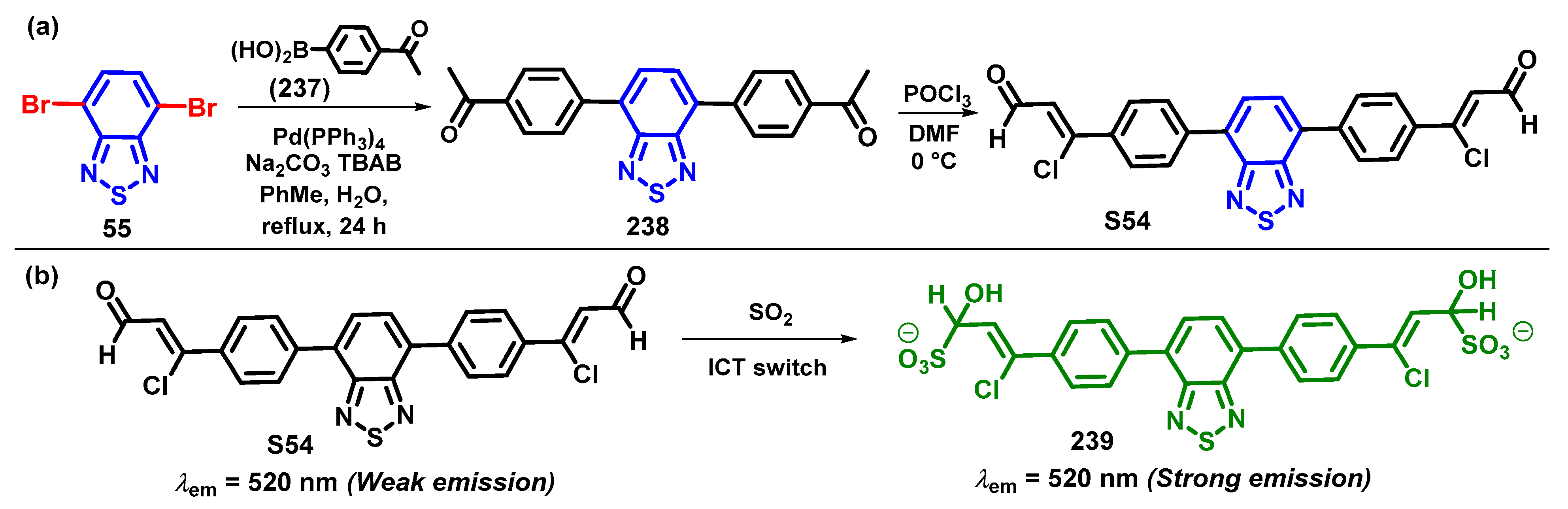
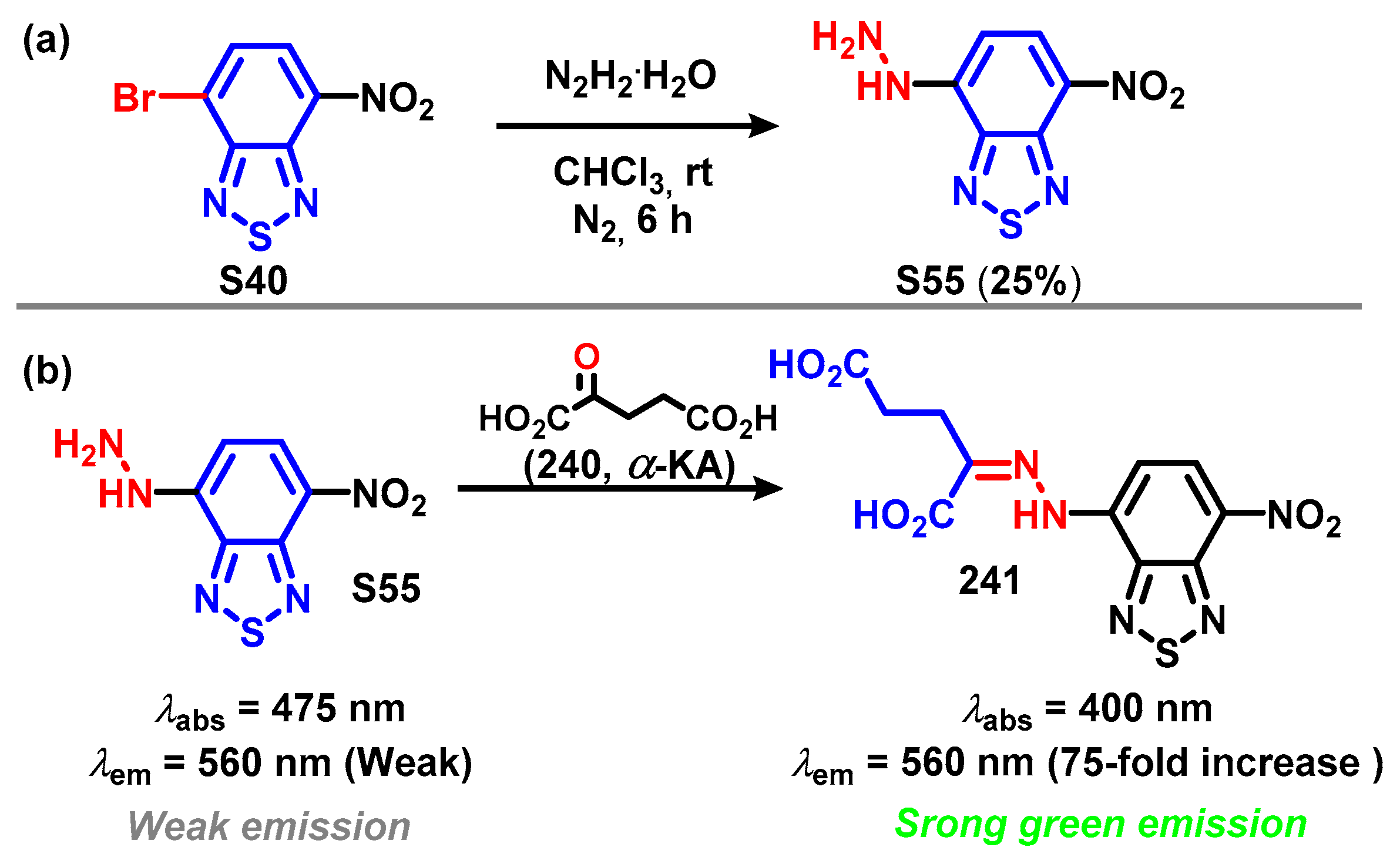
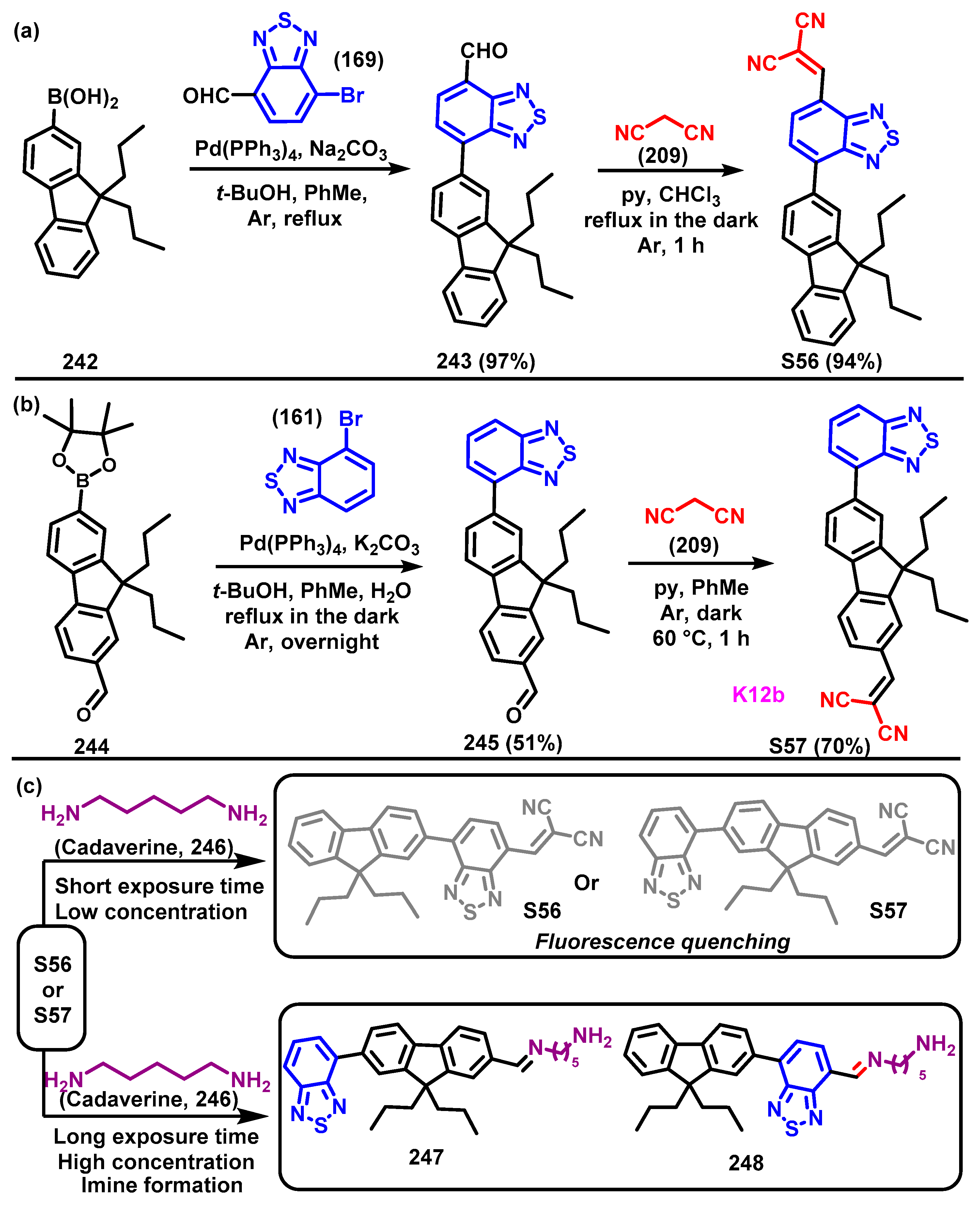
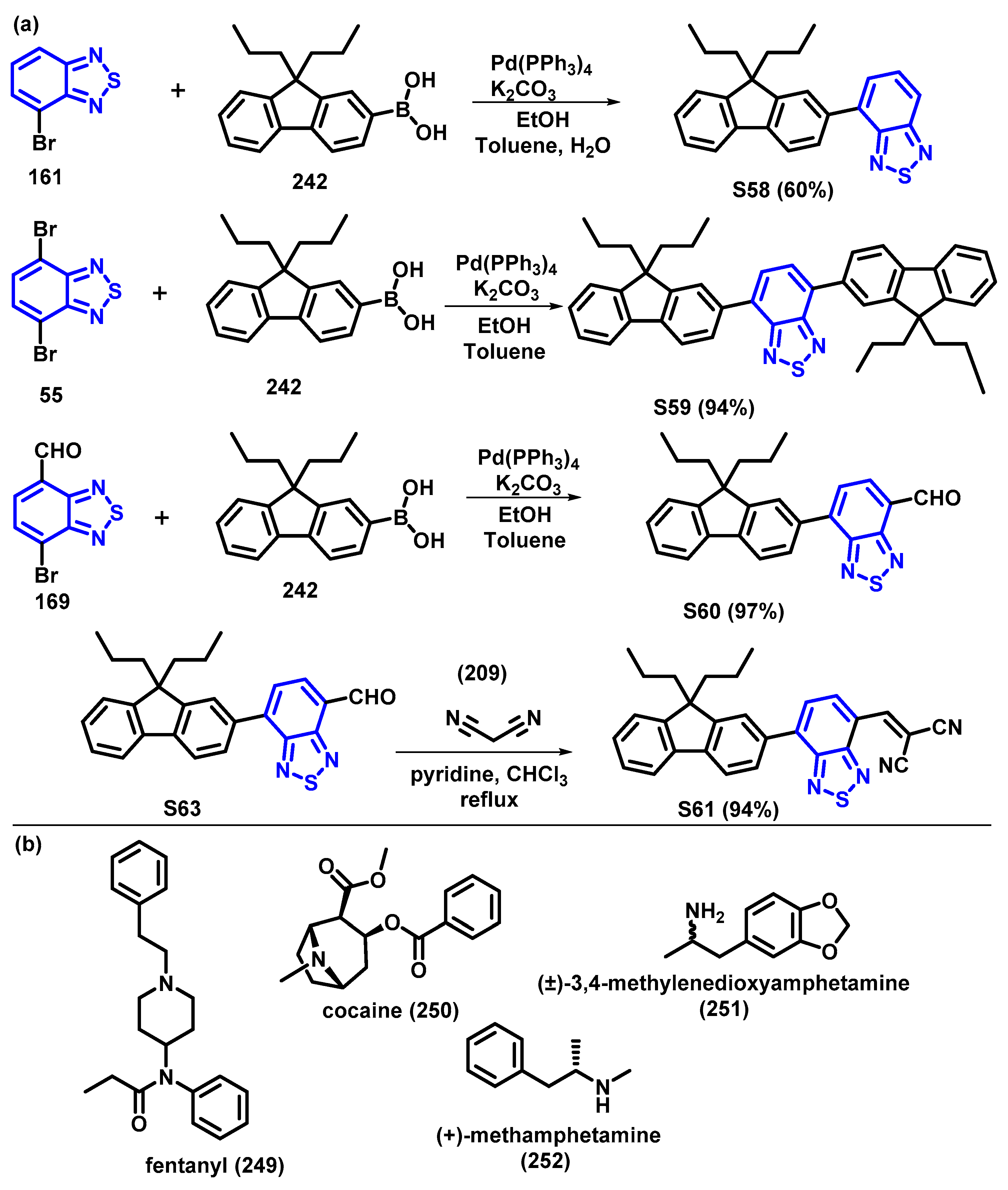


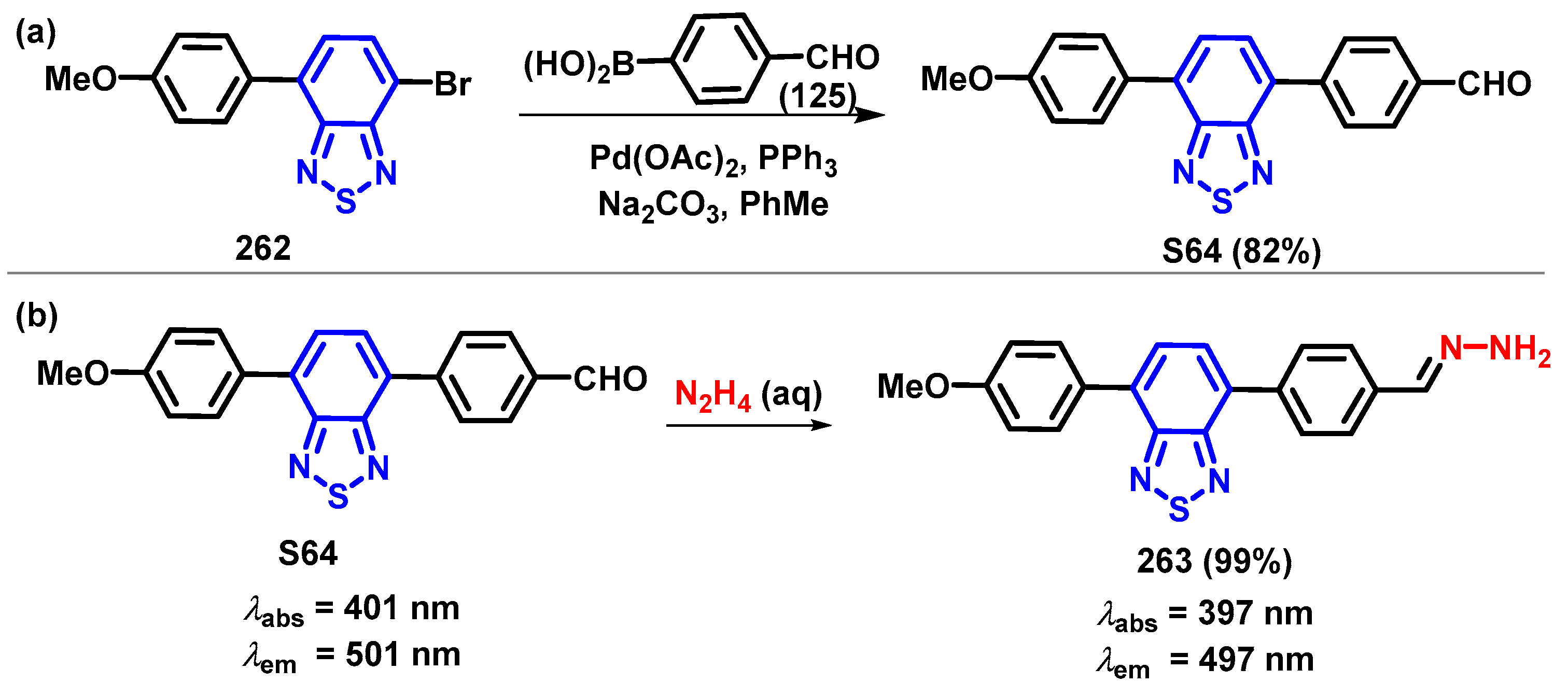
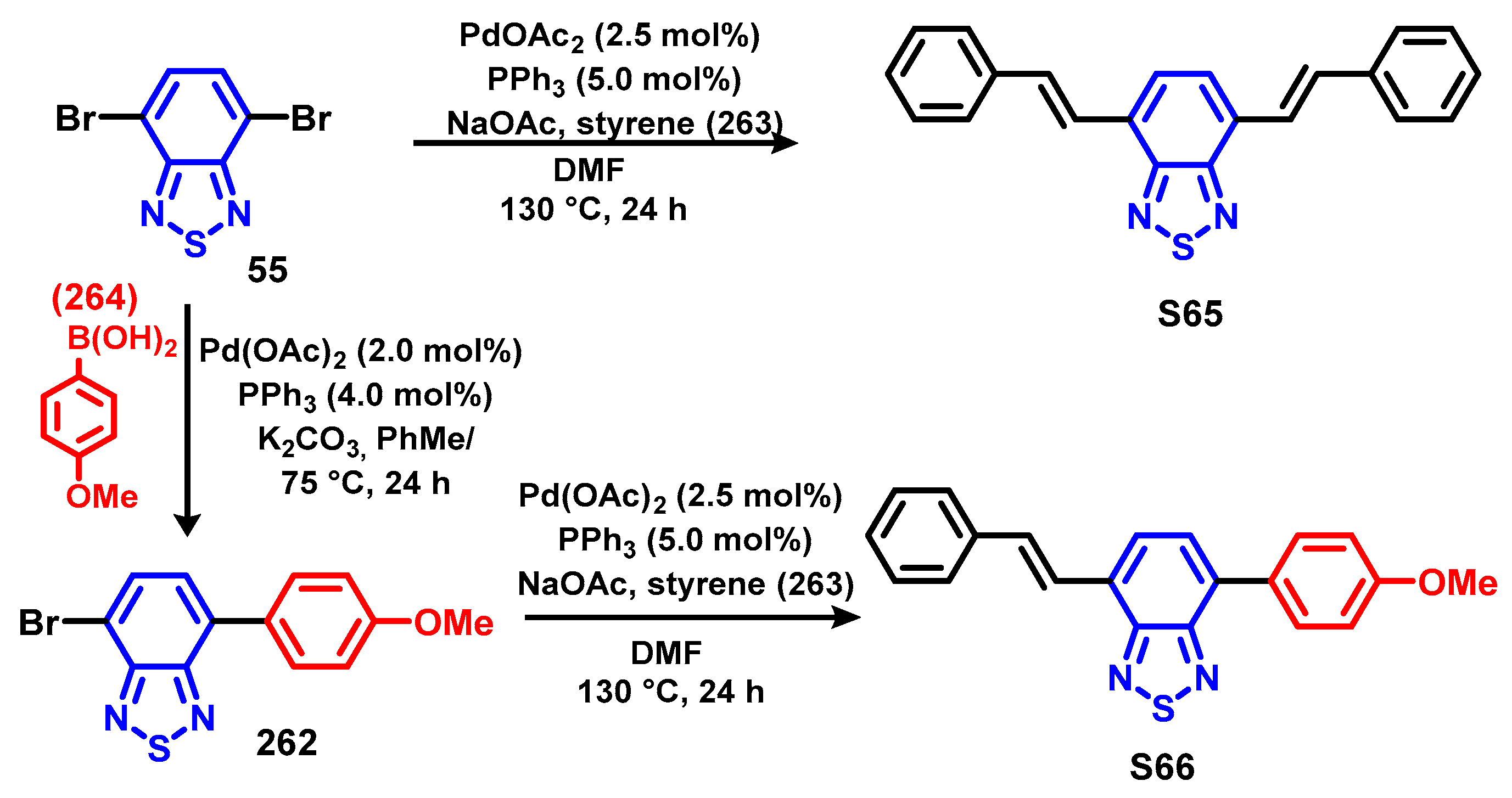

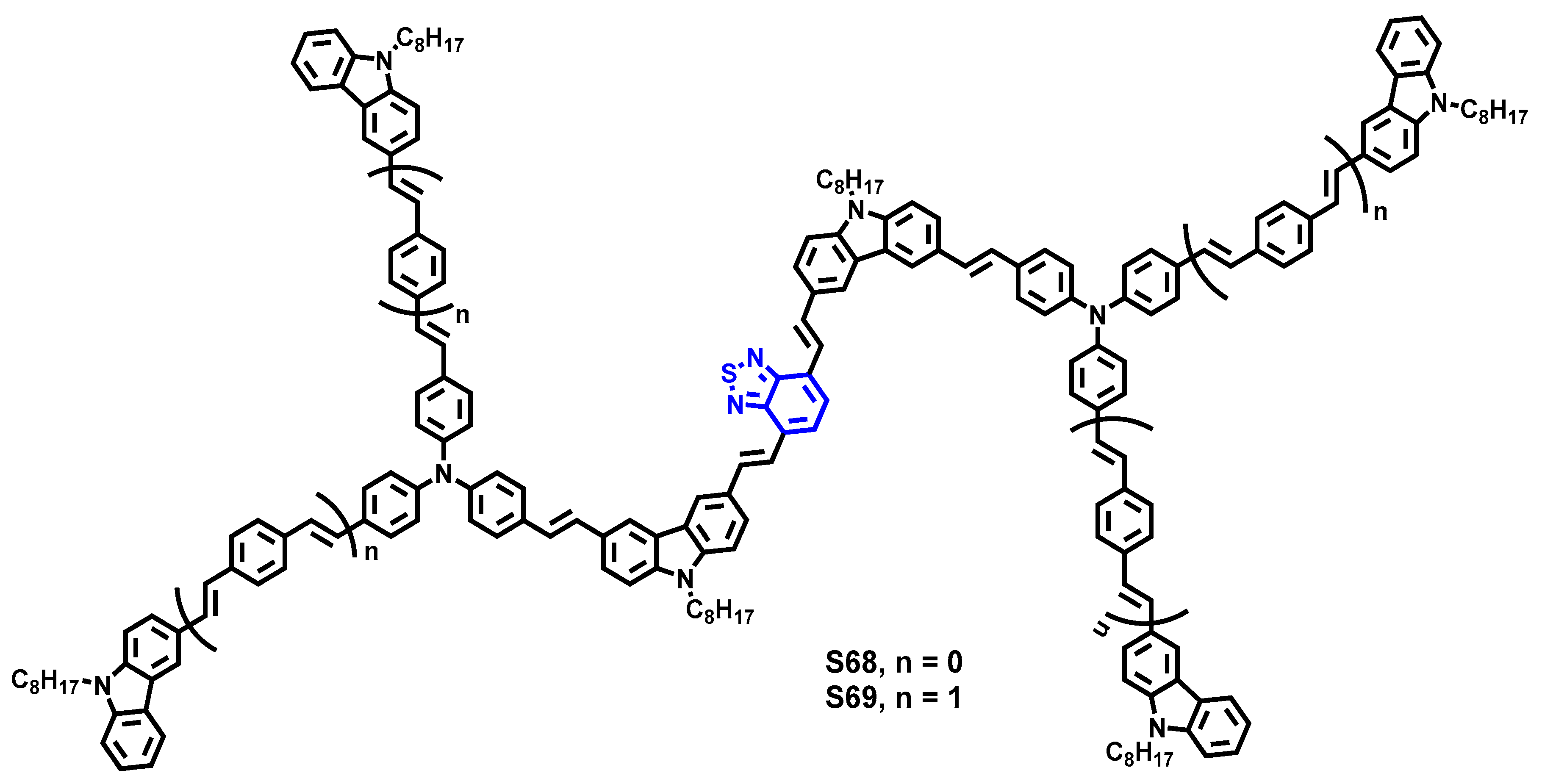
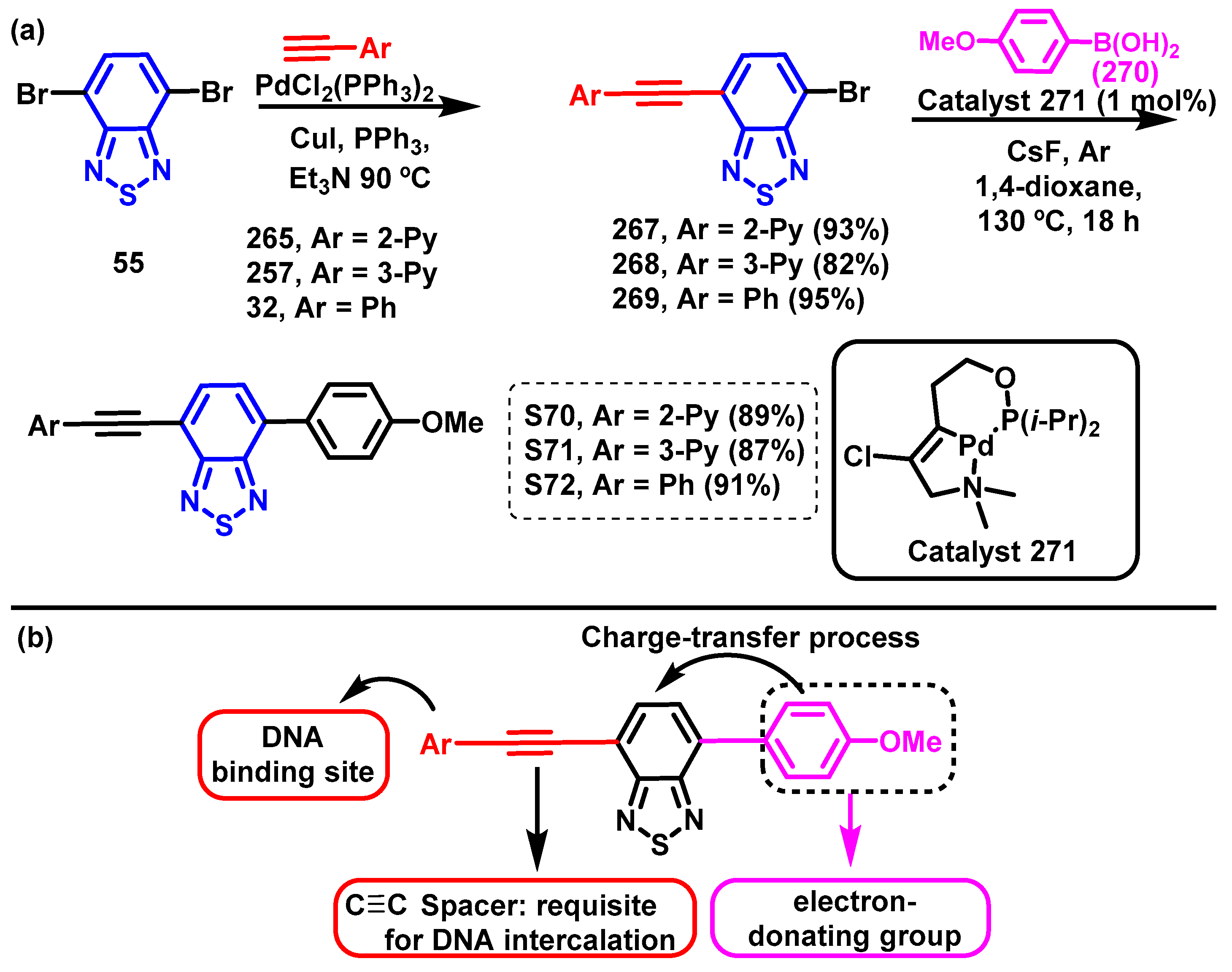

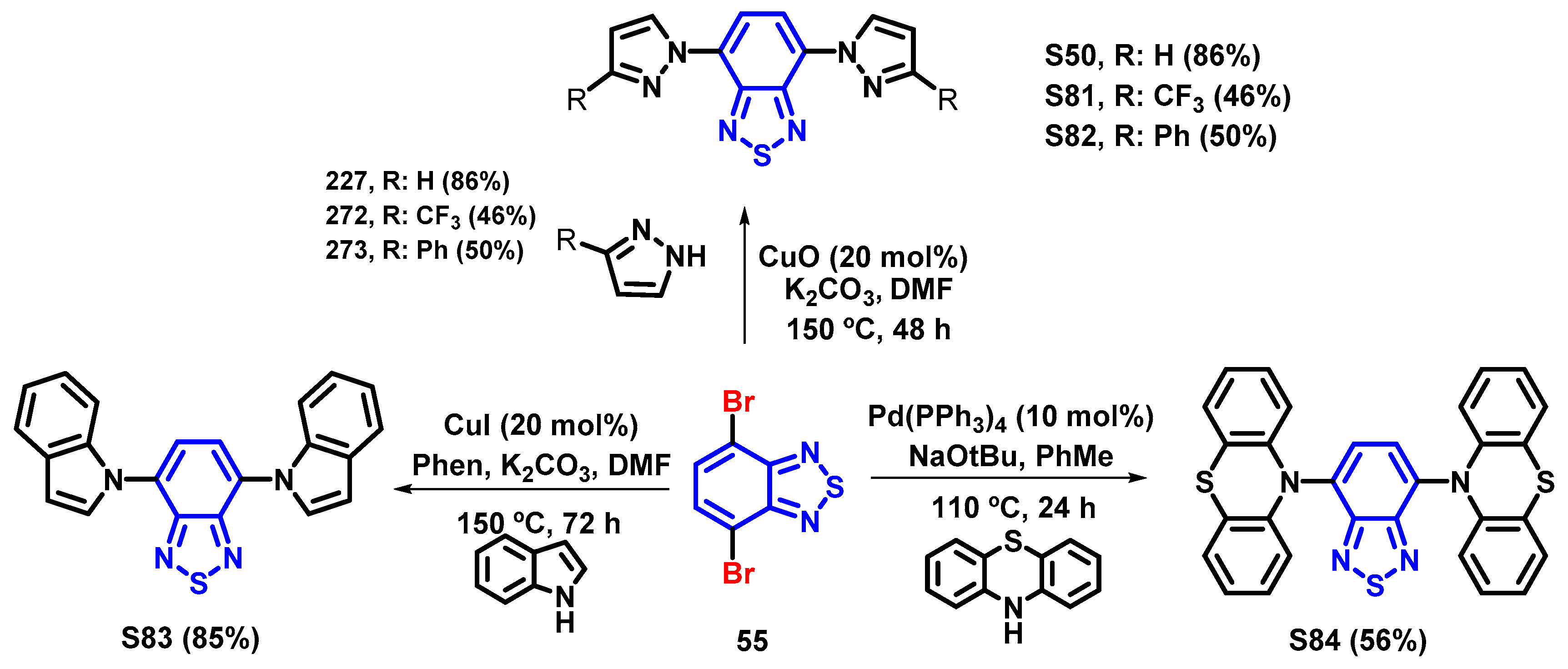
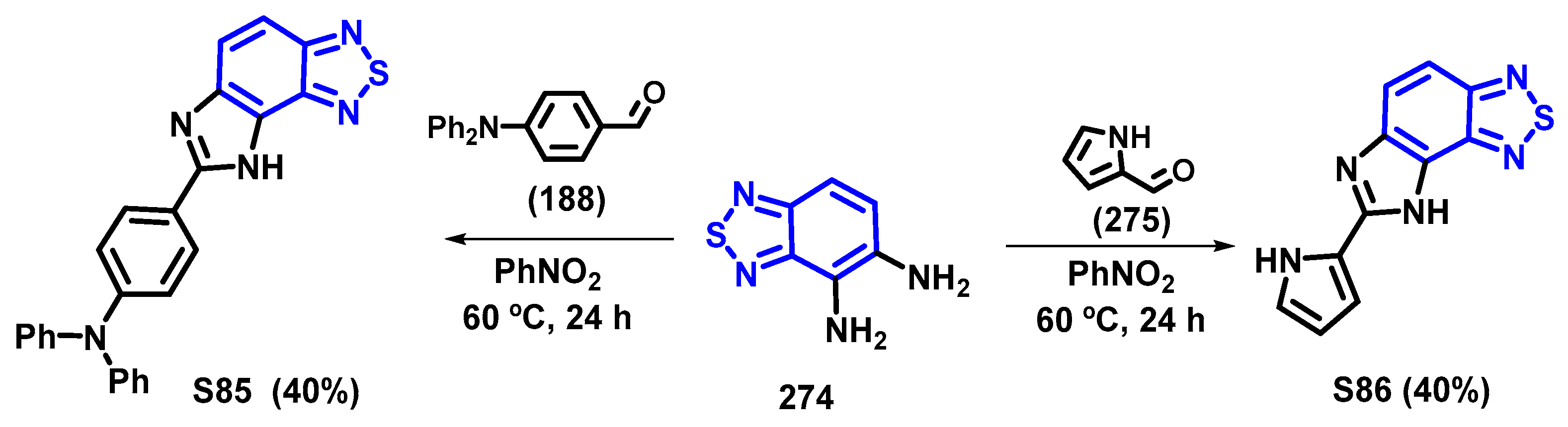
| Molecule | Analyte | Solvent Medium | Mechanism | LOD | Ref. |
|---|---|---|---|---|---|
| S1 | Hg2+ | THF/H2O (9:1, v/v) | S,O-chelation D–A structure | 0.0131 µmol L−1 | [423] |
| S2 | Hg2+ | Acetone-H2O (8:2, v/v) | S,O-chelation D–A structure | 0.393 µmol L−1 | [424] |
| S3 | Hg2+ | THF/H2O (99:1, v/v) | ICT D–A–D structure | 0.089 µmol L−1 | [425] |
| S4 | Hg2+ | THF/H2O (99:1, v/v) | ICT D-π-A-π-D structure | 0.36 µmol L−1 | [426] |
| S5 | Hg2+ CH3Hg+ | CH3CN/H2O (1:1, v/v) | PET | 0.16 µmol L−1 0.8 µmol L−1 | [427] |
| S6 | Hg2+ CH3Hg+ | CH3CN/H2O (1:1, v/v) | PET | 0.5 µmol L−1 1.0 µmol L−1 | [427] |
| S7 | Hg2+ | HEPES buffer (10 mmol L−1, pH = 7.4, with 3% DMSO) | ICT and AIE | 0.090 µmol L−1 | [428] |
| S8 | Cu2+ | DMF | Transference of electrons from imidazole to the empty d-orbital of Cu2+ | 0.11 µmol L−1 | [429] |
| S8 | Hg2+ | Water | ICT | 0.00093 µmol L−1 | [430] |
| S9 | Al3+ | HEPES buffer (50 vol% DMSO, pH = 7.0) | AIE | 0.15 µmol L−1 | [433] |
| S10 | Ni2+ | CH3CN | PET or CT | - | [434] |
| S11 | Cu2+ | HEPES buffer (pH 7.4) | Metal complexation | - | [436] |
| S12 | Cu2+ | HEPES buffer pH 7.4 | Metal complexation | - | [436] |
| S13 | Cd2+ | CHCl3/MeCN (10:1) | - | - | [437] |
| S13 | Hg2+ | Trichloromethane | Metal complexation AIE | - | [438] |
| S14 | Na+ | THF:CH3CN (1:1, v/v) | Conformational twist | 14.6 µmol L−1 | [439] |
| S15 | Na+ | THF:CH3CN (1:1, v/v) | Conformational twist | 17.2 µmol L−1 | [439] |
| S16 | Na+ | THF:CH3CN (1:1, v/v) | Conformational twist | 21.7 µmol L−1 | [439] |
| S17 | Fe3+ Cr6+ | THF | Thermodynamic stability of the newly rigid chain D–A–D structure | 3.04 μmol L−1 0.0435 µmol L−1 | [443] |
| S18 | Ni2+, Hg2+, Cu2+, Co2+ | CH3CN | Metal complexation | - | [444] |
| S19 | Cu2+ | HEPES buffer (pH 7.4) | Metal complexation | - | [444] |
| S20 | Ni2+, Hg2+ Cu2+, Co2+ | CH3CN | Metal complexation | - | [444] |
| S21 | Ni2+, Hg2+ Cu2+, Co2+ | CH3CN | Metal complexation | - | [444] |
| S22 | Not selective | CH3CN | Metal complexation | - | [444] |
| S23 | Hg2+ | CH3CN | Metal complexation | [444] | |
| S24 | Cu2+ | CH3CN | ICT | - | [221] |
| S25 | Cu2+ | CH3CN | ICT | - | [221] |
| S26 | Co2+ Cu2+ Co2+ Cu2+ Co2+–F– | EtOH PhCN | Metal-to-ligand charge transfer (MLCT) | 0.407 µmol L−1 0.550 µmol L−1 0.550 µmol L−1 3.70 µmol L−1 | [446] |
| S27 | Cu2+ H2S | MeOH/PBS solution (9:1, v/v) | - | 0.55 μmol L−1 0.55 μmol L−1 | [450] |
| S28 | Ni2+ | CH3CN | PET or CT | - | [451] |
| S29 | Ni2+ | CH3CN | PET or CT | - | [451] |
| S30 | Ni2+ | CH3CN | PET or CT | - | [451] |
| S31 | Ni2+ | CH3CN | PET or CT | - | [451] |
| S32 | Ag+ Cu2+ Ni2+ | H2O | Metal complexation | 3.80 μmol L−1 0.27 μmol L−1 0.56 μmol L−1 | [452] |
| S33 | Fe3+ | 0.2 mol L−1 HEPES buffer/pH 7.2 in 2 vol% DMSO | ICT | 0.036 μmol L−1 | [453] |
| S34 | CN− | PBS solution | AIE and ICT | 0.35 μmol L−1 | [456] |
| S35 | F− | THF | Intramolecular CT | - | [457] |
| S36 | F− | DMSO | ESIPT | 0.86 µmol L−1 | [460] |
| S37 | F− | DMSO | ESIPT | 4.25 µmol L−1 | [460] |
| S38 | F− | CH3CN/Tris–HCl buffer (9:1, v/v, pH 7.5) | Cleavage of Si–O bonds | 1.7 µmol L−1 | [461] |
| S39 | CN− | THF/H2O (99:1, v/v) | ICT | 0.014 µmol L−1 | [463] |
| S40 | CN− | THF | ICT | 0.087 μmol L−1 | [464] |
| S41 | CN− | THF/H2O (v/v, 1/9) | ICT | 0.077 μmol L−1 | [465] |
| S42 | CN− | THF | ICT | - | [465] |
| S43 | CN− | THF | ICT | - | [465] |
| S44 | CN− | THF/H2O (2:8, v/v) | AIE | 0.134 µmol L−1 | [466] |
| S45 | F− | DMSO | Deprotonation | - | [467] |
| S46 | F− | DMSO | Deprotonation | - | [467] |
| S47 | F− | DMSO | Deprotonation | - | [467] |
| S48 | Cu2+ F− Br− | CH3CN | Deprotection TMS group | - | [468] |
| S49 | Cu2+ F− Br− | CH3CN | Deprotection TMS group | Cu2+: 1.31 (λem 608/486 nm) to 2.28 ppb (λem 486/608 nm); Br−: 69.0 (λem 605/490 nm) to 88.2 ppb (λem 490/605 nm); F−: 0.13 ppb | [468] |
| S50 | HO– | DMF | Electron transfer–hydrogen bonding interaction | 55 µmol L−1 | [429] |
| S50 | TEA TMEDA | DMF | Aggregation Aggregation | 0.400 µmol L−1 0.278 µmol L−1 | [498] |
| S51 | Cys Hcy | HEPES (0.01 mol L−1, pH 7.4) /1% DMSO | PET | 0.1 µmol L−1 0.1 µmol L−1 | [469] |
| S52 | GSH Cys Hcy | PBS solution (10 mmol L−1, pH 7.4/1 mmol L−1 CTAB) | ICT | 0.089 µmol L−1 - - | [470] |
| S53 | Cys | PBS solution (containing 20% DMSO, pH 7.4) | ICT | - | [471] |
| S54 | SO2 | PBS solution (pH 7.4) containing 5% DMSO | ICT | 0.190 µmol L−1 | [474] |
| S55 | α-KA | PBS solution (pH 5.7, 1 mmol L−1 CTAB) | ICT | - | [475] |
| S56 | Biogenic amines (cadaverine) | DCM Thin films | PHT | - 130 ppb | [477] |
| S57 | Biogenic amines (cadaverine) | DCM Thin films | PHT | - 610 ppb | [477] |
| S58 | (+)-Methamphetamine and fentanyl | Films | PHT | - | [480] |
| S59 | Fentanyl | Films | PHT | - | [480] |
| S60 | (+)-Methamphetamine, (±)-3,4-methylenedioxy-amphetamine, cocaine, and fentanyl | Films | PHT | - | [480] |
| S61 | (+)-Methamphetamine, (±)-3,4-methylenedioxy-amphetamine, cocaine, and fentanyl | Films | PHT | - | [480] |
| S62 | Oxalyl chloride Phosgene | DCM | ICT | 0.003 µmol L−1 0.020 µmol L−1 | [481] |
| S63 | Hydrazine | H2O/DMSO (4:6, v/v) solution (10 mmol L−1 HEPES buffer, pH 7.4) | PET | 0.0847 µmol L−1 | [482] |
| S64 | Hydrazine | Water | ICT | 0.340 µmol L−1 | [483] |
| S65 | Not tested | - | - | - | [484] |
| S66 | EtOH in water | EtOH/water | ICT | [484] | |
| S67 | PA | ACN/H2O (8:2 v/v) | ICT D–π–A–π–D structure | 0.0079 nmol L−1 | [485] |
| S68 | TNT DNT | Toluene | ICT | 13 µmol L−1 20 µmol L−1 | [486] |
| S69 | TNT DNT | Toluene | ICT | 15 µmol L−1 18 µmol L−1 | [486] |
| S70 | DNA | PBS solution (pH 7.0) | ICT | 1 ppm | [490] |
| S71 | DNA | PBS solution (pH 7.0) | ICT | 1 ppm | [490] |
| S72 | DNA | PBS solution (pH 7.0) | ICT | 1 ppm | [490] |
| S73 | CT-DNA BSA | DMSO (5%)/Tris-HCl buffer (pH 7.4) | - | 1.05 µmol L−1 7.10 µmol L−1 | [491] |
| S74 | CT-DNA BSA | DMSO (5%)/Tris-HCl buffer (pH 7.4) | - | 9.45 µmol L−1 7.30 µmol L−1 | [491] |
| S75 | CT-DNA BSA | DMSO (5%)/Tris-HCl buffer (pH 7.4) | - | 7.65 µmol L−1 0.10 µmol L−1 | [491] |
| S76 | CT-DNA BSA | DMSO (5%)/Tris-HCl buffer (pH 7.4) | - | 7.72 µmol L−1 7.27 µmol L−1 | [491] |
| S77 | CT-DNA BSA | DMSO (5%)/Tris-HCl buffer (pH 7.4) | - | 0.30 µmol L−1 0.65 µmol L−1 | [491] |
| S78 | CT-DNA BSA | DMSO (5%)/Tris-HCl buffer (pH 7.4) | - | 2.52 µmol L−1 7.52 µmol L−1 | [491] |
| S79 | CT-DNA BSA | DMSO (5%)/Tris-HCl buffer (pH 7.4) | - | 6.60 µmol L−1 6.55 µmol L−1 | [491] |
| S80 | CT-DNA BSA | DMSO (5%)/Tris-HCl buffer (pH 7.4) | - | 1.40 µmol L−1 2.22 µmol L−1 | [491] |
| S81 | Water in acetone | Water/acetone | CT ligand-to-ligand charge transfer (LLCT) | - | [492] |
| S82 | Water in acetone | Water/acetone | CT LLCT | - | [492] |
| S83 | Water in acetone | Water/acetone | CT LLCT | - | [492] |
| S84 | Water in acetone | Water/acetone | CT LLCT | - | [492] |
| S85 | Hg2+ AcO− p-nitrophenol picric acid | CH3CN | Coordination bond interaction Hydrogen-bonding interactions Hydrogen-bonding interactions | 0.13 µg mL−1 1.07 mg mL−1 - | [497] |
| S86 | Hg2+ AcO− p-nitrophenol picric acid | CH3CN | Coordination bond interaction Coordination bond interaction Hydrogen-bonding interactions Hydrogen-bonding interactions | 159 µg mL−1 - - 317 µg mL−1 | [497] |
| S87 | TEA EDA TMEDA MnO4− ClO− | DMF | Aggregation Aggregation Aggregation Hydrogen bond Hydrogen bond | 0.129 µmol L−1 0.635 µmol L−1 0.320 µmol L−1 9.820 µmol L−1 12.740 µmol L−1 | [498] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, G.G.; Souto, F.T.; Machado, V.G. 2,1,3-Benzothiadiazoles Are Versatile Fluorophore Building Blocks for the Design of Analyte-Sensing Optical Devices. Chemosensors 2024, 12, 156. https://doi.org/10.3390/chemosensors12080156
Dias GG, Souto FT, Machado VG. 2,1,3-Benzothiadiazoles Are Versatile Fluorophore Building Blocks for the Design of Analyte-Sensing Optical Devices. Chemosensors. 2024; 12(8):156. https://doi.org/10.3390/chemosensors12080156
Chicago/Turabian StyleDias, Gleiston Gonçalves, Francielly Thaís Souto, and Vanderlei Gageiro Machado. 2024. "2,1,3-Benzothiadiazoles Are Versatile Fluorophore Building Blocks for the Design of Analyte-Sensing Optical Devices" Chemosensors 12, no. 8: 156. https://doi.org/10.3390/chemosensors12080156
APA StyleDias, G. G., Souto, F. T., & Machado, V. G. (2024). 2,1,3-Benzothiadiazoles Are Versatile Fluorophore Building Blocks for the Design of Analyte-Sensing Optical Devices. Chemosensors, 12(8), 156. https://doi.org/10.3390/chemosensors12080156







