Recent Development of Electrospun Nanostructured Fibers as Colorimetric Probes for Metal Ion Sensing: A Review
Abstract
1. Introduction
| S. No | Metal Ions | Acceptable Limits (mg/L) | Maximum Permissible pH Levels | Effects |
|---|---|---|---|---|
| 1. | Silver (Ag+) | 0.1 | 6.0–9.0 | Neuronal disorder, mental fatigue, rheumatism, and gastroenteritis |
| 2. | Mercury (Hg2+) | 0.001 | Effects on digestive system, hypertension, and impaired neurologic development | |
| 3. | Copper (Cu2+) | 2.0 | Nephrology disorders, allergies, and anemia | |
| 4. | Chromium (Cr3+/6+) | 0.05 | Dermatitis, carcinogenicity, and reproductive and embryonic damage | |
| 5. | Lead (Pb2+) | 0.01 | Effect on blood–brain barrier (BBB), kidney damage, and neurological disorders | |
| 6. | Iron (Fe3+) | 3 | Hemochromatosis and damage of heart and liver | |
| 7. | Cadmium (Cd2+) | 0.003 | Osteoporosis, renal toxicity, hypertension, and lung cancer | |
| 8. | Arsenic (As3+/5+) | 0.01 | Effect on central nervous system, gastrointestinal diseases, and cardiovascular and pulmonary diseases |
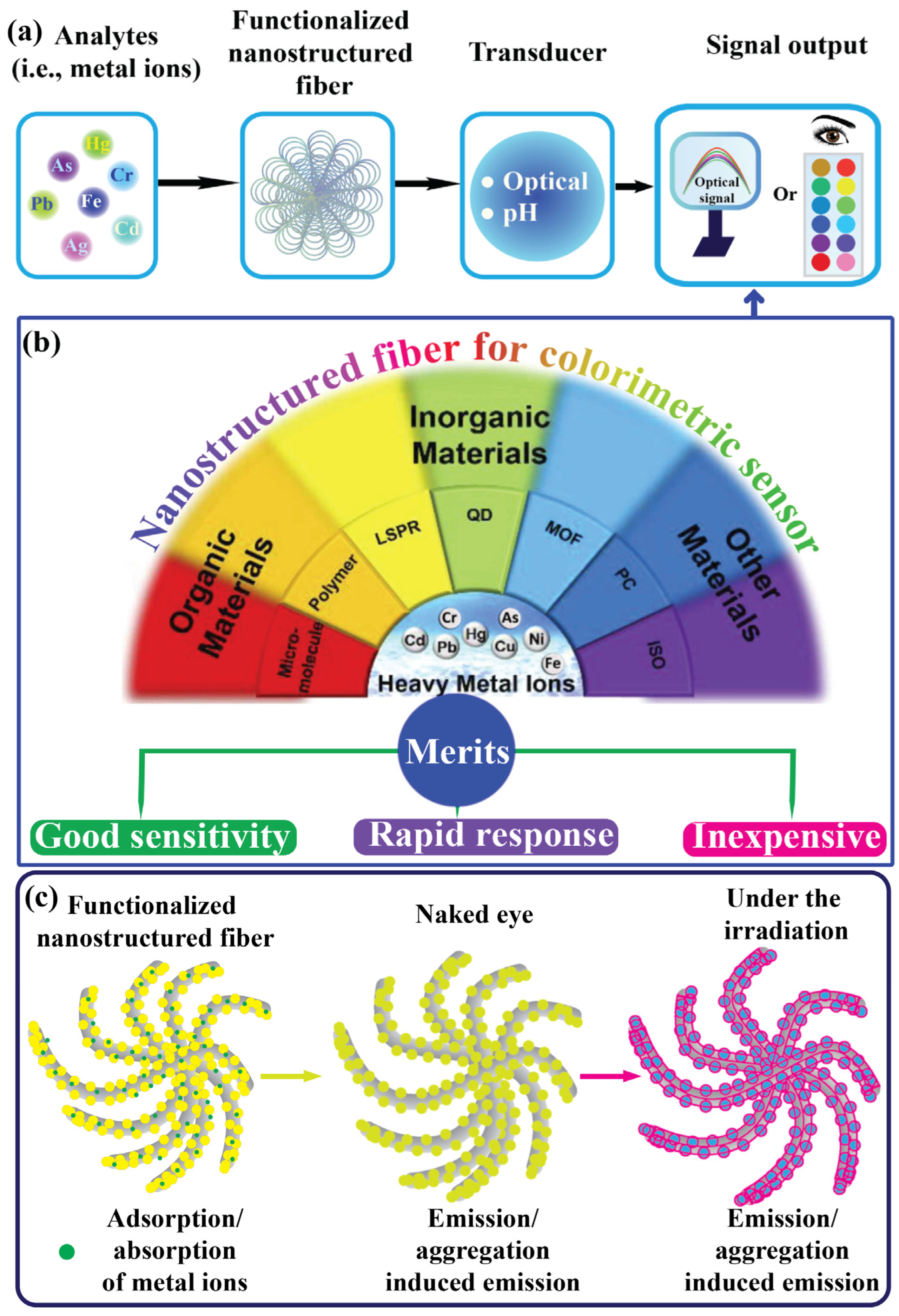
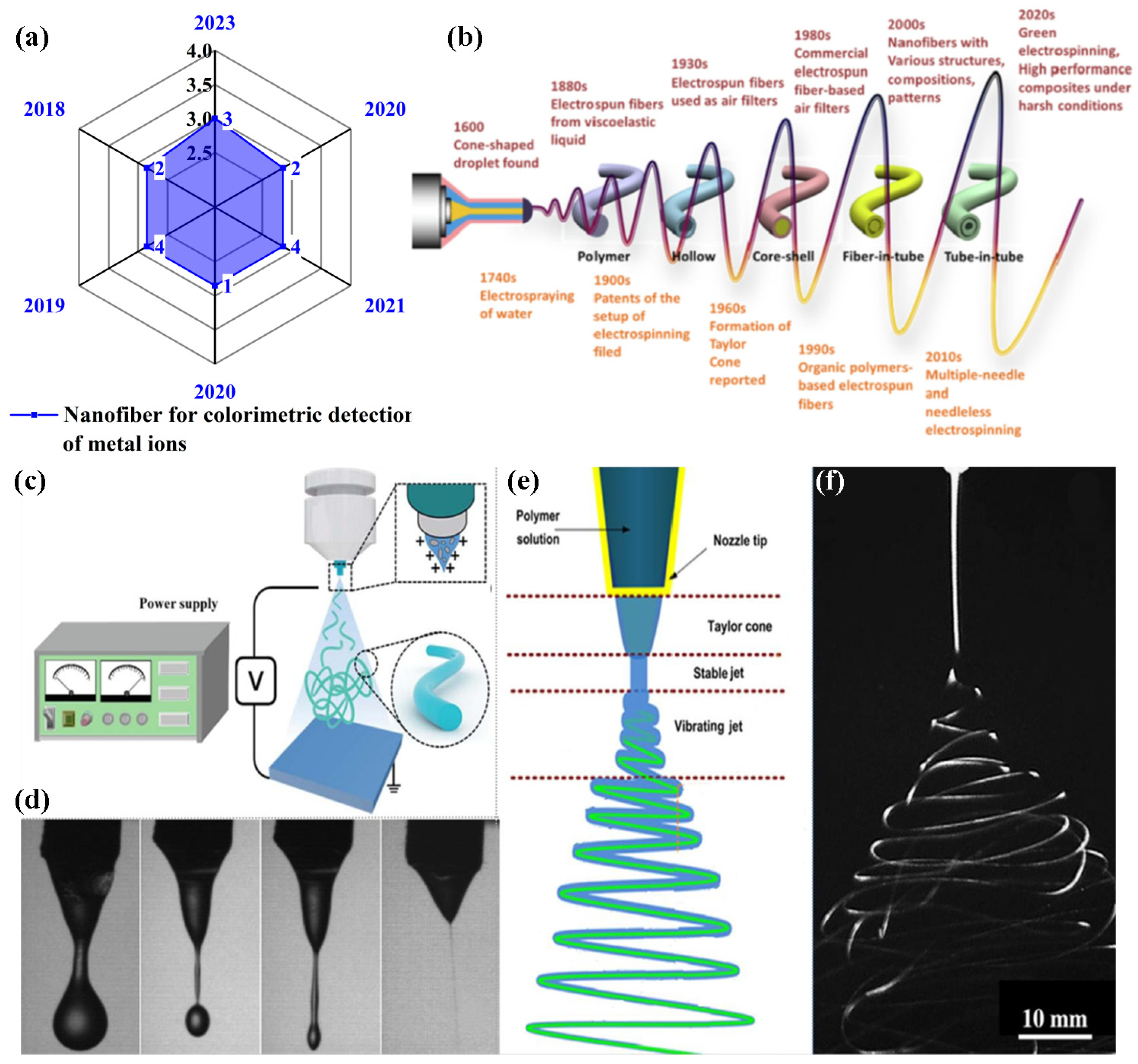
2. Mechanism of Electrospinning Technique
3. Preparation of Nanostructure Nanofibers
4. Colorimetric-Based Detection of Metal Ions
5. Detection of Cu2+ Metal Ions
6. Detection of Fe Metal Ions
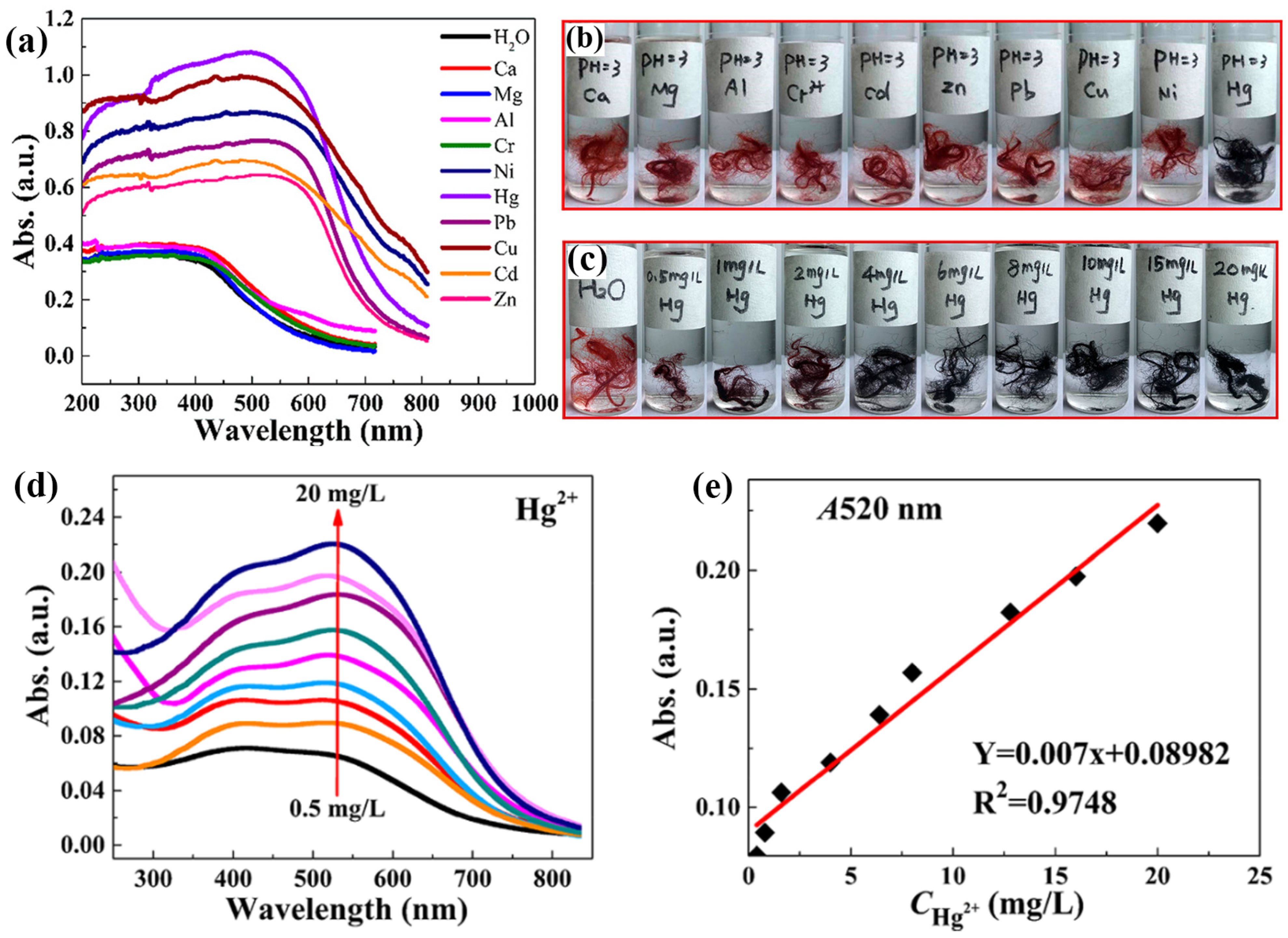
7. Detection of Hg Metal Ions
8. Detection of Cr, Pb, Ag, and Cs Metal Ions
9. Non-Metal Ion Sensing Using Nanostructured Fibers
10. Summary
11. Conclusions and Future Prospective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hara, T.O.; Singh, B. Electrochemical biosensors for detection of pesticides and heavy metal toxicants in water: Recent trends and progress. ACS ES T Water 2021, 1, 462–478. [Google Scholar] [CrossRef]
- Riu, J.; Giussani, B. Electrochemical biosensors for the detection of pathogenic bacteria in food. TrAC Trends Anal. Chem. 2020, 126, 115863. [Google Scholar] [CrossRef]
- Kirchhain, A.; Bonini, A.; Vivaldi, F.; Poma, N.; Di Francesco, F. Latest developments in non-faradic impedimetric biosensors: Towards clinical applications. TrAC Trends Anal. Chem. 2020, 133, 116073. [Google Scholar] [CrossRef]
- Dincer, C.; Bruch, R.; Costa-Rama, E.; Fernández-Abedul, M.T.; Merkoçi, A.; Manz, A.; Urban, G.A.; Güder, F. Disposable sensors in diagnostics, food, and environmental monitoring. Adv. Mater. 2019, 31, 1806739. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Q.; Lv, M.; Chen, L. Microorganism remediation strategies towards heavy metals. Chem. Eng. J. 2019, 360, 1553–1563. [Google Scholar] [CrossRef]
- Miranda, L.S.; Deilami, K.; Ayoko, G.A.; Egodawatta, P.; Goonetilleke, A. Influence of land use class and configuration on water-sediment partitioning of heavy metals. Sci. Total Environ. 2022, 804, 150116. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, X.; Chen, Z.; Zhou, X.; Lu, X.; Liu, J. Pollution characteristics and source analysis of heavy metals in surface sediments of Luoyuan Bay, Fujian. Environ. Res. 2022, 203, 111911. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.; Qi, J.; You, J.; Ma, J.; Chen, L. Colorimetric detection of heavy metal ions with various chromogenic materials: Strategies and applications. J. Hazard. Mater. 2023, 441, 129889. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Mustafa, F.S.; Omer, K.M.; Hama, S.; Hamarawf, R.F.; Rahman, K.O. Heavy metal pollution in the aquatic environment: Efficient and low-cost removal approaches to eliminate their toxicity: A review. RSC Adv. 2023, 13, 17595–17610. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Thai, V.-P.; Tran, D.N.; Kosugi, K.; Takahashi, K.; Sasaki, T.; Kikuchi, T. One-Step Synthesis of N-Doped Graphene Quantum Dots via Plasma Contacting Liquid for Multiple Heavy Metal Ion Detection. ACS Appl. Nano Mater. 2024, 7, 12664–12672. [Google Scholar] [CrossRef]
- Jagannathan, M.; Badhulika, S. Graphitic carbon nitride and its nanocomposites-based sensors for detection of pharmaceutical effluents in food, ecological and biological samples: A mini-review. Sens. Actuators Rep. 2023, 7, 100183. [Google Scholar] [CrossRef]
- Peivasteh-Roudsari, L.; Barzegar-Bafrouei, R.; Sharifi, K.A.; Azimisalim, S.; Karami, M.; Abedinzadeh, S.; Asadinezhad, S.; Tajdar-Oranj, B.; Mahdavi, V.; Alizadeh, A.M. Origin, dietary exposure, and toxicity of endocrine-disrupting food chemical contaminants: A comprehensive review. Heliyon 2023, 9, e18140. [Google Scholar] [CrossRef]
- Abd Elnabi, M.K.; Elkaliny, N.E.; Elyazied, M.M.; Azab, S.H.; Elkhalifa, S.A.; Elmasry, S.; Mouhamed, M.S.; Shalamesh, E.M.; Alhorieny, N.A.; Abd Elaty, A.E. Toxicity of heavy metals and recent advances in their removal: A review. Toxics 2023, 11, 580. [Google Scholar] [CrossRef] [PubMed]
- Marghade, D.; Mehta, G.; Shelare, S.; Jadhav, G.; Nikam, K.C. Arsenic contamination in Indian groundwater: From origin to mitigation approaches for a sustainable future. Water 2023, 15, 4125. [Google Scholar] [CrossRef]
- Sazakli, E. Human Health Effects of Oral Exposure to Chromium: A Systematic Review of the Epidemiological Evidence. Int. J. Environ. Res. Public Health 2024, 21, 406. [Google Scholar] [CrossRef]
- Mulenga, M.; Ouma, K.O.; Monde, C.; Syampungani, S. Aquatic mercury pollution from artisanal and small-scale gold mining in sub-Saharan Africa: Status, Impacts, and Interventions. Water 2024, 16, 756. [Google Scholar] [CrossRef]
- Brown, M.; Patel, P.; Nash, E.; Dikid, T.; Blanton, C.; Forsyth, J.; Fontaine, R.; Sharma, P.; Keith, J.; Babu, B. Prevalence of elevated blood lead levels and risk factors among children living in Patna, Bihar, India 2020. PLOS Glob. Public Health 2022, 2, e0000743. [Google Scholar] [CrossRef] [PubMed]
- Mendil, D. Mineral and trace metal levels in some cheese collected from Turkey. Food Chem. 2006, 96, 532–537. [Google Scholar] [CrossRef]
- Perelonia, K.B.S.; Benitez, K.C.D.; Banicod, R.J.S.; Tadifa, G.C.; Cambia, F.D.; Montojo, U.M. Validation of an analytical method for the determination of cadmium, lead and mercury in fish and fishery resources by graphite furnace and Cold Vapor Atomic Absorption Spectrometry. Food Control 2021, 130, 108363. [Google Scholar] [CrossRef]
- Xue, J.; Gong, S.; Wang, X.; Fan, Y.; Li, X. Determination of Hg, As, Pb, and Cd in orchard soils by sequential injection vapor generation atomic fluorescence spectrometry. Anal. Lett. 2012, 45, 2257–2268. [Google Scholar] [CrossRef]
- Ding, R.; Cheong, Y.H.; Ahamed, A.; Lisak, G. Heavy metals detection with paper-based electrochemical sensors. Anal. Chem. 2021, 93, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, M.; Dhinasekaran, D.; Rajendran, A.R.; Cho, S. A Review of Electroactive Nanomaterials in the Detection of Nitrogen-Containing Organic Compounds and Future Applications. Biosensors 2023, 13, 989. [Google Scholar] [CrossRef] [PubMed]
- Karthik, D.; Vijayarekha, K. Chemometric identification of a few heavy metals, pesticides and plasticides in edible sunflower oil for health risk assessment. Int. J. Food Prop. 2018, 21, 1442–1448. [Google Scholar] [CrossRef]
- Ichinoki, S.; Kitahata, N.; Fujii, Y. Selective determination of mercury (II) ion in water by solvent extraction followed by reversed-phase HPLC. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 1785–1798. [Google Scholar] [CrossRef]
- Hassan, A.H.; Zeinhom, M.M.; Shaban, M.; Korany, A.M.; Gamal, A.; Abdel-Atty, N.S.; Al-Saeedi, S.I. Rapid and sensitive in situ detection of heavy metals in fish using enhanced Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 313, 124082. [Google Scholar] [CrossRef] [PubMed]
- Makam, P.; Shilpa, R.; Kandjani, A.E.; Periasamy, S.R.; Sabri, Y.M.; Madhu, C.; Bhargava, S.K.; Govindaraju, T. SERS and fluorescence-based ultrasensitive detection of mercury in water. Biosens. Bioelectron. 2018, 100, 556–564. [Google Scholar] [CrossRef]
- Sarfo, D.K.; Izake, E.L.; O’Mullane, A.P.; Ayoko, G.A. Molecular recognition and detection of Pb (II) ions in water by aminobenzo-18-crown-6 immobilised onto a nanostructured SERS substrate. Sens. Actuators B Chem. 2018, 255, 1945–1952. [Google Scholar] [CrossRef]
- Cheng, F.; Xu, H.; Wang, C.; Gong, Z.; Tang, C.; Fan, M. Surface enhanced Raman scattering fiber optic sensor as an ion selective optrode: The example of Cd2+ detection. RSC Adv. 2014, 4, 64683–64687. [Google Scholar] [CrossRef]
- Sun, X.; Li, B.; Qi, A.; Tian, C.; Han, J.; Shi, Y.; Lin, B.; Chen, L. Improved assessment of accuracy and performance using a rotational paper-based device for multiplexed detection of heavy metals. Talanta 2018, 178, 426–431. [Google Scholar] [CrossRef]
- Sener, G.; Uzun, L.; Denizli, A. Colorimetric sensor array based on gold nanoparticles and amino acids for identification of toxic metal ions in water. ACS Appl. Mater. Interfaces 2014, 6, 18395–18400. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Meng, G.; Tang, H.; Ye, Y.; Sun, R.; Chen, M.; Wang, K.-M. A novel cationic iridium (iii) complex with a thiorhodamine-based auxiliary ligand: Application for luminescent and colorimetric detection of Hg2+ in an aqueous solution. New J. Chem. 2017, 41, 8312–8319. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Zou, C.; Zhou, J.; Yang, M.; Zhang, S.; Huo, D.; Hou, C. Colorimetric detection of Cr6+ ions based on surface plasma resonance using the catalytic etching of gold nano-double cone@ silver nanorods. Anal. Chim. Acta 2021, 1149, 238141. [Google Scholar] [CrossRef] [PubMed]
- Senthamizhan, A.; Balusamy, B.; Uyar, T. Recent progress on designing electrospun nanofibers for colorimetric biosensing applications. Curr. Opin. Biomed. Eng. 2020, 13, 1–8. [Google Scholar] [CrossRef]
- Soundharraj, P.; Jagannathan, M.; Dhinasekaran, D.; Thiruvarasu, P. Fluorescent zinc titanate as an effective sensing platform for urea detection. Mater. Today Proc. 2022, 50, 101–106. [Google Scholar] [CrossRef]
- Jagannathan, M.; Dhinasekaran, D.; Soundharraj, P.; Rajendran, S.; Vo, D.-V.N.; Prakasarao, A.; Ganesan, S. Green synthesis of white light emitting carbon quantum dots: Fabrication of white fluorescent film and optical sensor applications. J. Hazard. Mater. 2021, 416, 125091. [Google Scholar] [CrossRef]
- Andersson, M.; Jia, Q.; Abella, A.; Lee, X.-Y.; Landreh, M.; Purhonen, P.; Hebert, H.; Tenje, M.; Robinson, C.V.; Meng, Q. Biomimetic spinning of artificial spider silk from a chimeric minispidroin. Nat. Chem. Biol. 2017, 13, 262–264. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C. One-Dimensional Nanostructures: Electrospinning Technique and Unique Nanofibers; Springer: Berlin/Heidelber, Germany, 2013. [Google Scholar]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Mercante, L.A.; Pavinatto, A.; Pereira, T.S.; Migliorini, F.L.; dos Santos, D.M.; Correa, D.S. Nanofibers interfaces for biosensing: Design and applications. Sens. Actuators Rep. 2021, 3, 100048. [Google Scholar] [CrossRef]
- Ram Thimmiah, B.; Nallathambi, G. Thiol Functionalized Aloe Vera Fibre Filter: A Simple Portable Water Filter For Heavy Metal Ion Removal. ChemistrySelect 2023, 8, e202203747. [Google Scholar] [CrossRef]
- Fernández-Ramos, M.D.; Bastida-Armesto, M.; Blanc-García, R.; Capitán-Vallvey, L.F.; Medina-Castillo, A.L. Design of colorimetric nanostructured sensor phases for simple and fast quantification of low concentrations of acid vapors. Microchim. Acta 2023, 190, 160. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Song, Y.; Xu, L.; Wang, J.; Yang, Z.; Zhao, Y.; Chen, F.; Liu, B.; Liu, L.; Chen, D. Functional Electrospun Nanofibrous Hybrid Materials for Colorimetric Sensors: A Review. ACS Omega 2024, 9, 5157–5174. [Google Scholar] [CrossRef] [PubMed]
- Terra, I.A.; Mercante, L.A.; Andre, R.S.; Correa, D.S. Fluorescent and colorimetric electrospun nanofibers for heavy-metal sensing. Biosensors 2017, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Balusamy, B.; Senthamizhan, A.; Uyar, T. Functionalized electrospun nanofibers as a versatile platform for colorimetric detection of heavy metal ions in water: A review. Materials 2020, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Balusamy, B.; Senthamizhan, A.; Uyar, T. Functionalized electrospun nanofibers as colorimetric sensory probe for mercury detection: A review. Sensors 2019, 19, 4763. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Rowland, C.E.; Schaller, R.D.; Korgel, B.A. Synthesis and ligand exchange of thiol-capped silicon nanocrystals. Langmuir 2015, 31, 6886–6893. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.S.; Jaimes, R.F.; Borysow, W.; Gomes, O.F.; Salcedo, W.J. Portable multispectral colorimeter for metallic ion detection and classification. Sensors 2017, 17, 1730. [Google Scholar] [CrossRef] [PubMed]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, X.; Zhang, M.; Ge, M.; Wang, J.; Tang, Y.; Zhang, Y.; Mi, J.; Cai, W.; Lai, Y. Rational design of electrospun nanofibers for gas purification: Principles, opportunities, and challenges. Chem. Eng. J. 2022, 446, 137099. [Google Scholar] [CrossRef]
- Mitchell, G.R. Electrospinning: Principles, Practice and Possibilities; Royal Society of Chemistry: Cambridge, UK, 2015. [Google Scholar]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Xu, X.; Lu, T.; Wang, X.; Yang, L.; Jing, X. BCNU-loaded PEG–PLLA ultrafine fibers and their in vitro antitumor activity against Glioma C6 cells. J. Control. Release 2006, 114, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Razak, S.; Wahab, I.; Fadil, F.; Dahli, F.; Md Khudzari, A.; Adeli, H. A review of electrospun conductive polyaniline based nanofiber composites and blends: Processing, features, applications, and future directions. Adv. Mater. Sci. Eng. 2005, 2015, 356286. [Google Scholar]
- Katsogiannis, K.A.G.; Vladisavljević, G.T.; Georgiadou, S. Porous electrospun polycaprolactone (PCL) fibres by phase separation. Eur. Polym. J. 2015, 69, 284–295. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, G.; Wang, P. Visualized fibrous adsorbent prepared by the microwave-assisted method for both detection and removal of heavy metal ions. ACS Sustain. Chem. Eng. 2018, 7, 1159–1168. [Google Scholar] [CrossRef]
- Abedalwafa, M.A.; Li, Y.; Li, D.; Sanbhal, N.; Yang, J.; Wang, L. Aminated polyacrylonitrile nanofibers with immobilized gold-silver core-shell nanoparticles for use in a colorimetric test strip for copper (II). Microchim. Acta 2018, 185, 402. [Google Scholar] [CrossRef]
- Rao, P.G.; Saritha, B.; Siva Rao, T. Colorimetric and turn-on fluorescence Chemosensor for Hg2+ ion detection in aqueous media. J. Fluoresc. 2019, 29, 353–360. [Google Scholar] [CrossRef]
- Kumar, D.; Talreja, N. Nickel nanoparticles-doped rhodamine grafted carbon nanofibers as colorimetric probe: Naked eye detection and highly sensitive measurement of aqueous Cr3+ and Pb2+. Korean J. Chem. Eng. 2019, 36, 126–135. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Yu, Q.; Jia, L.; Wan, L.Y. Poly (aspartic acid) electrospun nanofiber hydrogel membrane-based reusable colorimetric sensor for Cu (II) and Fe (III) detection. ACS Omega 2019, 4, 14633–14639. [Google Scholar] [CrossRef]
- Shah, K.J.; Imae, T. Selective gas capture ability of gas-adsorbent-incorporated cellulose nanofiber films. Biomacromolecules 2016, 17, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Rahmawati, A.; Shih, C.-F.; Imae, T. Film sensor of a ligand-functionalized cellulose nanofiber for the selective detection of copper and cesium ions. Polym. J. 2020, 52, 1235–1243. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, S.Y.; Jung, J.M.; Kim, C. A new Schiff-base chemosensor for selective detection of Cu2+ and Co2+ and its copper complex for colorimetric sensing of S2− in aqueous solution. Photochem. Photobiol. Sci. 2017, 16, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- ReddyPrasad, P.; Imae, T. Selective detection of copper ion in water by tetradentate ligand sensor. J. Taiwan Inst. Chem. Eng. 2017, 72, 194–199. [Google Scholar] [CrossRef]
- Kim, K.B.; Kim, H.; Song, E.J.; Kim, S.; Noh, I.; Kim, C. A cap-type Schiff base acting as a fluorescence sensor for zinc (II) and a colorimetric sensor for iron (II), copper (II), and zinc (II) in aqueous media. Dalton Trans. 2013, 42, 16569–16577. [Google Scholar] [CrossRef]
- Shao, H.; Yin, D.; Li, D.; Ma, Q.; Yu, W.; Dong, X. Simultaneous visual detection and removal of Cu2+ with electrospun self-supporting flexible amidated polyacrylonitrile/branched polyethyleneimine nanofiber membranes. ACS Appl. Mater. Interfaces 2021, 13, 49288–49300. [Google Scholar] [CrossRef]
- Gao, R.; Xu, G.; Zheng, L.; Xie, Y.; Tao, M.; Zhang, W. A highly selective and sensitive reusable colorimetric sensor for Ag+ based on thiadiazole-functionalized polyacrylonitrile fiber. J. Mater. Chem. C 2016, 4, 5996–6006. [Google Scholar] [CrossRef]
- Wang, L.; Guo, W.; Zhu, H.; He, H.; Wang, S. Preparation and properties of a dual-function cellulose nanofiber-based bionic biosensor for detecting silver ions and acetylcholinesterase. J. Hazard. Mater. 2021, 403, 123921. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Metal–organic frameworks supported on nanofibers to remove heavy metals. J. Mater. Chem. A 2018, 6, 4550–4555. [Google Scholar] [CrossRef]
- Sherlala, A.; Raman, A.; Bello, M.; Asghar, A. A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere 2018, 193, 1004–1017. [Google Scholar] [CrossRef]
- Fakhre, N.A.; Ibrahim, B.M. The use of new chemically modified cellulose for heavy metal ion adsorption. J. Hazard. Mater. 2018, 343, 324–331. [Google Scholar] [CrossRef]
- Kaur, B.; Kaur, N.; Kumar, S. Colorimetric metal ion sensors–a comprehensive review of the years 2011–2016. Coord. Chem. Rev. 2018, 358, 13–69. [Google Scholar] [CrossRef]
- Parizadeh, P.; Moeinpour, F.; Mohseni-Shahri, F.S. Anthocyanin-induced color changes in bacterial cellulose nanofibers for the accurate and selective detection of Cu (II) in water samples. Chemosphere 2023, 326, 138459. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Kumaresan, S. Coumarin Xanthene Combined Probe for the Multi-Color Detection of Metal Ions and Electrospun Fibers Developed for Real-Time Monitoring. J. Fluoresc. 2023, 33, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.E.; El-Newehy, M.H.; Aldalbahi, A.; Salem, W.M.; Khattab, T.A. Immobilization of anthocyanin extract from red-cabbage into electrospun polyvinyl alcohol nanofibers for colorimetric selective detection of ferric ions. J. Environ. Chem. Eng. 2021, 9, 105072. [Google Scholar] [CrossRef]
- Gouda, M.; Abd El-Lateef, H.M.; Abou Taleb, M.F.; Khalaf, M.M. Immobilization of natural extract from Humulus lupulus L. in electrospun polyvinyl alcohol nanofibrous membrane for colorimetric determination of ferric. J. Mol. Liq. 2024, 399, 124447. [Google Scholar] [CrossRef]
- Wu, Z.; Shen, H.; Hu, J.; Fu, Q.; Yao, C.; Yu, S.; Xiao, W.; Tang, Y. Aptamer-based fluorescence-quenching lateral flow strip for rapid detection of mercury (II) ion in water samples. Anal. Bioanal. Chem. 2017, 409, 5209–5216. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Yang, X. Colorimetric biosensing of mercury (II) ion using unmodified gold nanoparticle probes and thrombin-binding aptamer. Biosens. Bioelectron. 2010, 25, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, M.A.; Gruünhut, M.; Pistonesi, M.F.; Di Nezio, M.S.; Centurión, M.E. Automatic flow-batch system for cold vapor atomic absorption spectroscopy determination of mercury in honey from Argentina using online sample treatment. J. Agric. Food Chem. 2012, 60, 4812–4817. [Google Scholar] [CrossRef] [PubMed]
- Diviš, P.; Reichstädter, M.; Gao, Y.; Leermakers, M.; Křikala, J. Determination of mercury in fish sauces by thermal decomposition gold amalgamation atomic absorption spectroscopy after preconcentration by diffusive gradients in thin films technique. Foods 2020, 9, 1858. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chi, E.-Z.; Zhao, X.-H.; Zhang, Q. A simple and rapid “signal on” fluorescent sensor for detecting mercury (II) based on the molecular beacon aptamer. Foods 2022, 11, 1847. [Google Scholar] [CrossRef]
- Izadi, S.; Tashkhourian, J.; Hafshejani, S.A.H. Ecofriendly ratiometric colorimetric determination of mercury (II) ion in environmental water samples using gallic acid-capped gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 308, 123778. [Google Scholar] [CrossRef]
- Patel, M.R.; Upadhyay, M.D.; Ghosh, S.; Basu, H.; Singhal, R.K.; Park, T.J.; Kailasa, S.K. Synthesis of multicolor silver nanostructures for colorimetric sensing of metal ions (Cr3+, Hg2+ and K+) in industrial water and urine samples with different spectral characteristics. Environ. Res. 2023, 232, 116318. [Google Scholar] [CrossRef] [PubMed]
- Sheikhzadeh, E.; Naji-Tabasi, S.; Verdian, A.; Kolahi-Ahari, S. Equipment-free and visual detection of Pb2+ ion based on curcumin-modified bacterial cellulose nanofiber. J. Iran. Chem. Soc. 2022, 19, 283–290. [Google Scholar] [CrossRef]
- Jang, S.; Son, S.U.; Kang, B.; Kim, J.; Lim, J.; Seo, S.; Kang, T.; Jung, J.; Lee, K.-S.; Kim, H. Electrospun nanofibrous membrane-based colorimetric device for rapid and simple screening of amphetamine-type stimulants in drinks. Anal. Chem. 2022, 94, 3535–3542. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Yeang, B.J.; Park, Y.K.; Choi, H.J.; Kim, J.H.; Kang, Y.S.; Bae, Y.; Kim, J.Y.; Lim, S.J.; Lee, W. Washable colorimetric nanofiber nonwoven for ammonia gas detection. Polymers 2020, 12, 1585. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-H.; Kim, Y.K.; Jeong, S.M.; Choi, C.; Son, K.Y.; Lee, S.-K.; Lim, S.K. Wearable colorimetric sensing fiber based on polyacrylonitrile with PdO@ ZnO hybrids for the application of detecting H2 leakage. Text. Res. J. 2020, 90, 2198–2211. [Google Scholar] [CrossRef]
- Zhang, J.; Hurren, C.; Lu, Z.; Wang, D. Nanofiber-based colorimetric platform for point-of-care detection of E. coli. Chem. Eng. J. 2023, 463, 142357. [Google Scholar] [CrossRef]
- Li, K.; Yu, R.-H.; Shi, C.-M.; Tao, F.-R.; Li, T.-D.; Cui, Y.-Z. Electrospun nanofibrous membrane based on AIE-active compound for detecting picric acid in aqueous solution. Sens. Actuators B Chem. 2018, 262, 637–645. [Google Scholar] [CrossRef]
- Wang, Y.; He, J.; Sun, H.; Zou, L.; Li, Y.V. Preparation of Polyvinyl Alcohol Fluorescent Nanofiber Mats Modified by Hyperbranched Poly (Phenylalanine-Lysine) via Electrospinning for Metal Ions Detection. J. Polym. Environ. 2023, 31, 131–148. [Google Scholar] [CrossRef]
- Dewi, I.R.; Rujiralai, T.; Putson, C.; Cheewasedtham, W. A novel double metal-dithizone functionalized polyurethane electrospun nanofiber and film for colorimetric determination of hexavalent chromium. RSC Adv. 2023, 13, 2852–2859. [Google Scholar] [CrossRef]
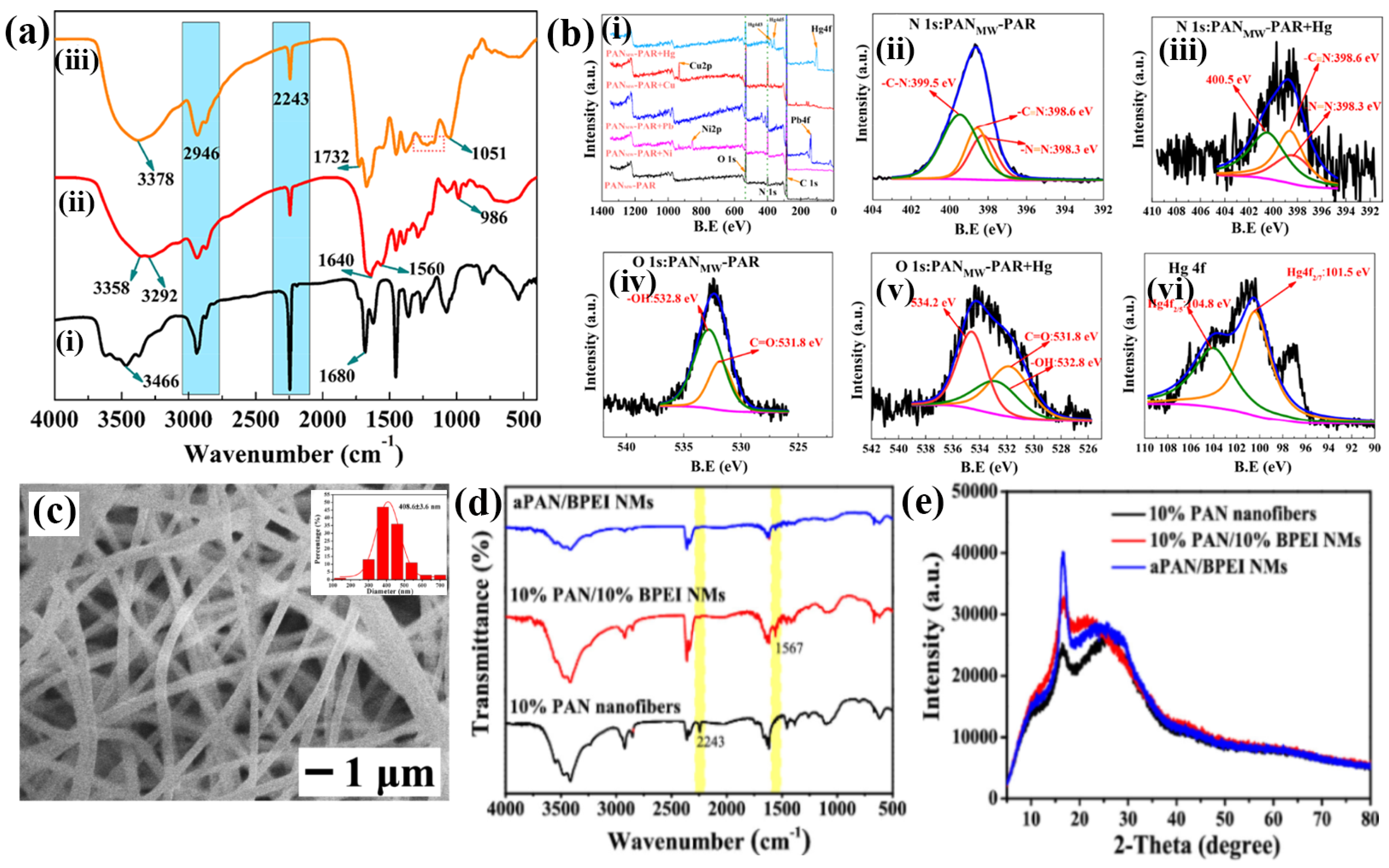
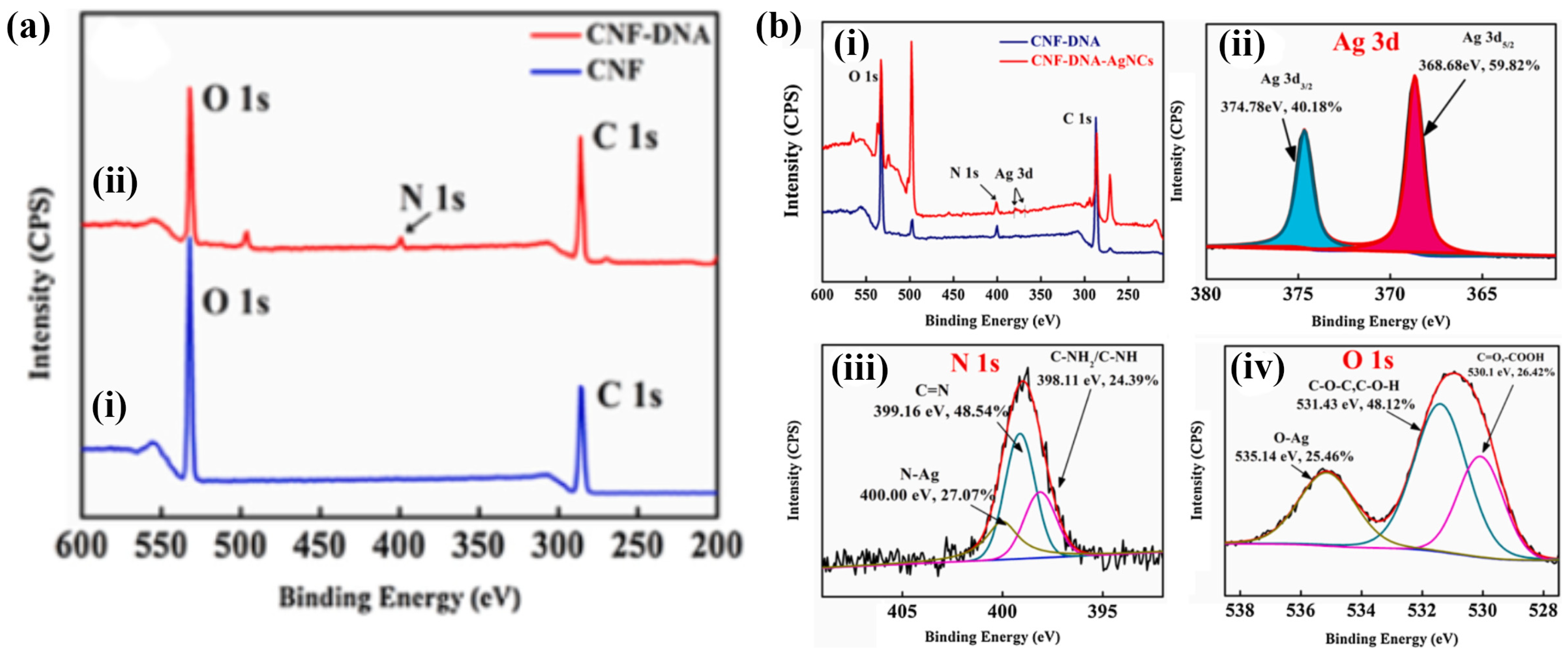



| S. No | Fibers | Linear Range | Detection Limit | Repeatability | Analyte | Interferences | Recovery Percentage (%) | Real Samples | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1. | PASP–ENHM | 0.1–100 mg/L for Cu2+ and 0.1 to 10 mg/L for Fe3+ | 0.3 mg/L for Cu2+ and 0.1 mg/L for Fe3+ | Several times | Cu2+ and Fe3+ | Ni2+, Pb2+, Co2+, Ca2+, Mg2+, Sn2+, and Zn2+, | [60] | ||
| 2. | TOCNF | 5–600 ppm | 5 ppm | Cu2+ and Cs+ | Ni2+, Zn2+, Hg2+, Co2+, Pb2+, and Cd2+ | [62] | |||
| 3. | Au/Ag@APANNFM | 0.05–1 μM | 50 nM | 6 cycles | Cu2+ | Zn2+, Mn2+, Li+, Na+, Mg+, Ca2+, Cd2+, K+, Cr3+, Al3+, Ag+, Pb2+, Co2+, Ni2+, Fe3+, Hg2+, and their Cu2+complex | Drinking water | [57] | |
| 4. | aPAN/BPEI NMs | 50–700 μM | 11.5 and 4.8 μM | 6 times | Cu2+ | Ca2+, Cd2+, Al3+, K+, Ba2+, Fe3+, Ni2+, Li+, Co2+, Zn2+ Cu2+, Mn2+, and anions | 96.5–117.2 (RSD = 0.64–1.29%) | Tap water | [66] |
| 5. | BCNF-ANT | 1.0 to 1000 ppm | 10–400 ppm and 20–300 ppm | Cu2+ | Pb2+, Co2+, Zn2+, Ni2+, Al3+, Ba2+, Hg2+, Mg2+, and Na+ | Freshwater | [73] | ||
| 6. | RhBC combined polyurethane electrospun nanofibers | Cu2+ | Al3+, Co2+, Zn2+, Pb2+, Cu2+ Ag+, Hg2+, Li+, Ni2+, K+, Fe2+, Ca2+, Na+, Mg2+, and Cs+ ions | - | [74] | ||||
| 7. | PVA/HBPL FNM | µM to 100 µM | 50 µM | 3 times | Cu2+ | Na+, K+, Ca2+, Mg2+, Zn2+, Ba2+, Cu2+, Fe3+, Co2+, and Sn2+ | [90] | ||
| 8. | Ni-CNF-RhB | 0.1–10 ppm | 203 nM for Cr3+ and 132 nM for Pb2+ | 6 cycles | Cr3+ and Pb2+ | Fe3+, Co2+, Mn2+, Cu2+, Hg2+, As5+, Ni2+, and Cd2+ | [59] | ||
| 9. | BCNF-CU | 0.9–9000 µM | 9 µM and 0.9 µM | Pb2+ | Cd2+, Ni2+, Ba2+, Mg2+, Zn2+, and Ca2+ | Real rice | [84] | ||
| 10. | MWPAN-PAR | 0.5–20 mg/L | 35 μg/L | 10 cycles | Hg2+ | Ca2+, Mg2+, and Al3+ | 98.26–105.52% (RSD = 0.15–0.62%) | Wastewater | [56] |
| 11. | CNF-DNA | 0–20 nM | 1 ×10−6 nM | Ag+ | Hg2+, Cd2+, Pb2+, Mg2+, Ba2+, Zn2+, Mn2+, and H2O | [68] | |||
| 12. | DTZ-Co2+/PU-MPF | 0.01–5.0 mg/L | 0.018 mg/L | 5 times | Cr6+ | Ca2+, Mg2+, Zn2+, Cu2+, Fe3+, Fe2+, Mn2+, Ni2+, Pb2+, As3+, Se2+, Cd2+, and Hg2+ | 80.0 to 137.5% (RSD = 4.7%) | Vegetable (i.e., palm) oil | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagannathan, M.; Yohan, R.K.; Cho, S. Recent Development of Electrospun Nanostructured Fibers as Colorimetric Probes for Metal Ion Sensing: A Review. Chemosensors 2024, 12, 129. https://doi.org/10.3390/chemosensors12070129
Jagannathan M, Yohan RK, Cho S. Recent Development of Electrospun Nanostructured Fibers as Colorimetric Probes for Metal Ion Sensing: A Review. Chemosensors. 2024; 12(7):129. https://doi.org/10.3390/chemosensors12070129
Chicago/Turabian StyleJagannathan, Mohanraj, Ravi Kumar Yohan, and Sungbo Cho. 2024. "Recent Development of Electrospun Nanostructured Fibers as Colorimetric Probes for Metal Ion Sensing: A Review" Chemosensors 12, no. 7: 129. https://doi.org/10.3390/chemosensors12070129
APA StyleJagannathan, M., Yohan, R. K., & Cho, S. (2024). Recent Development of Electrospun Nanostructured Fibers as Colorimetric Probes for Metal Ion Sensing: A Review. Chemosensors, 12(7), 129. https://doi.org/10.3390/chemosensors12070129






