Abstract
The colorimetric detection of metal ions has witnessed a surge in advancements, with nanostructured fibers emerging as a powerful platform for environmental monitoring and remediation applications. These fibers offer several advantages, including a high surface area, enhanced sensitivity and selectivity, non-intrusive analysis, rapid response times, robustness under harsh conditions, and user-friendly handling. This unique combination makes them particularly suitable for visible eye detection of metal ions in remote or challenging environments. This review provides a concise overview of recent developments in nanostructured fibers, and their cutting-edge fabrication methods, for the colorimetric-based detection of various heavy metal ions in real-time samples. By exploiting the unique properties of these fibers, colorimetric detection offers a promising and cost-effective approach for heavy metal ion determination. This review delves into the design principles, functionalization strategies, and detection mechanisms employed in these innovative sensors. We highlight the potential of nanostructured fibers as a well-established and efficient platform for the colorimetric detection of heavy metals, paving the way for more sustainable and accessible environmental monitoring solutions.
1. Introduction
Chemosensors are analytical devices that have garnered considerable attention from researchers and the sensor market because of their enormous potential for detecting various forms of analytes, particularly in environmental, food, and healthcare diagnostics [1,2,3]. These devices offer several advantages over traditional methods, including selectivity, sensitivity, economic feasibility, rapid response, and quick analysis [4]. Sensors comprise three essential components: (i) a biorecognition element, (ii) a transducer that converts the measurement signal, and (iii) a data acquisition system [1], as illustrated in Figure 1a. Heavy metal ion contaminants have recently become a global threat due to industrial activities. Their effluent waste products are among the most dangerous contaminants owing to their high toxicity and bioaccumulation effects [5,6,7]. These include silver ions (Ag+), mercury ions (Hg2+), copper ions (Cu2+), chromium ions (Cr2+, Cr6+), lead ions (Pb2+), iron ions (Fe2+), cadmium ions (Cd2+), and arsenic ions (As2+) [8,9,10,11]. Industrial effluents typically release heavy metal ions into the environment (e.g., water and soil), potentially affecting health via the food chain [12]. Tracking the presence of harmful heavy metal ions in ecological and food samples is crucial. These ions are highly toxic and adversely affect human health through cardiovascular dysfunction, neurotoxicity, nephrotoxicity, carcinogenicity, immunotoxicity, and DNA impairment [13,14]. For instance, in the Ganga–Meghna–Brahmaputra region, around 3000 communities have As2+ concentrations over 50 ppm, endangering approximately 6 million people. Prolonged As2+ exposure leads to severe health issues, including skin lesions, premature hair depigmentation, accelerated aging, and cancer [15]. Oral exposure to Cr2+ is associated with harmful health effects. Prenatal exposure can impair fetal development, leading to preterm delivery or low birth weight, while exposure later in life can disrupt physiological functions and metabolic pathways [16]. West Africa contributes the most to aquatic Hg2+ pollution (50.2%), followed by Central Africa (39.6%) and Southern Africa (9.6%), with Eastern Africa contributing less than 1%. Freshwater contamination was evident in ASGM (i.e., artisanal and small-scale gold mining) regions, affecting surface and groundwater, stream sediments, aquatic biota, and riparian vegetation. Significant environmental health risks to humans have been noted from fish consumption, water use, and other Hg-contaminated water resources [17]. Children in Patna and Bihar, India, face exposure to Pb2+ from various sources, particularly house dust, and locally sourced spices, which significantly elevate their blood Pb2+ levels. This underscores the importance of addressing all potential sources of Pb2+ exposure comprehensively. In this two groups of children, a 10% rise in Pb2+ content in dust, turmeric, or other spices correlated with a 0.7–2.0% increase in blood Pb2+ levels [18]. As shown in Table 1, the World Health Organization (WHO) has reported acceptable limits for heavy metal ion concentrations and pH levels. Researchers have developed various analytical approaches for detecting heavy metal ions, such as atomic absorption spectroscopy (AAS) [19,20], atomic fluorescence spectroscopy [21], electroanalytical techniques [22,23], chromatography techniques [24,25], and surface-enhanced Raman scattering (SERS) [26,27,28,29]. However, these sophisticated instruments require complex operations and trained professionals, and are typically limited to laboratory settings. Colorimetric detection methods for heavy metal ions have gained significant attention because of their advantages and plausible mechanism, including rapid recognition and response, on-site detection, and visibility to the naked eye, as illustrated in Figure 1b,c. These methods do not require complex instruments and provide fast responses, portability, and ease of use, making them suitable for the detection of real samples. Because of these advantages, researchers have proposed the development of various new materials, methodologies, mechanisms, and applications, leading to the design of new analytical methods for the detection and determination of heavy metal ions with satisfactory results. Therefore, colorimetric methods for detecting heavy metal ions using nanostructured fibers deserve systematic and concise discussion [8,30,31,32,33]. To date, remarkable progress has been made in exploring various types of nanomaterials for the design of colorimetric chemosensors to detect a variety of agents [34,35,36].
Fibers are elongated substances or continuous filaments that are ubiquitous in nature and have been a substantial part of human life since civilization. State-of-the-art techniques have emerged as significant sources for the development of synthetic fibers [37,38]. Among these, the electrospinning technique has garnered great attention due to its multifaceted and sustainable approach for fabricating ultrafine nanofibers with diameters below one micrometer. Electrospinning techniques and the engineering of electrospun nanofibers have made significant advancements in various applications [39]. The emerging fields of nanoscience and nanotechnology have unlocked ways to engineer the surface area of nanostructured fibers to accommodate and immobilize receptors. For instance, the combination of chemical substances and preparation of nanostructured fibers via electrospinning has resulted in properties such as a high surface area, facile functionalization, and adequate porosity to accommodate metal ions. As shown in Figure 1b, various types of chemical substances, such as organic compounds (e.g., micromolecules, polymers, isomers, and monomers), inorganic compounds (e.g., silver nanoparticles (NPs), gold NPs, quantum dots, and metal-organic-frameworks), and standard fluorescent probes (e.g., pigments), have been identified as efficient substances. Additionally, the reliability of these properties in nanostructured fibers makes them attractive for the development of functional nanostructured fibers [40,41,42,43]. To detect heavy metal ions, various materials are used to enhance sensing performance via surface functionalization and determine plausible sensing mechanisms [44,45]. Colorimetric sensing offers a significant advantage as it can be distinguished by the naked eye through the color change that occurs after exposure to heavy metal ions, which is crucial for real-time and on-site analysis applications [46,47]. Herein, we highlight recent developments in nanostructured fibers derived from electrospinning techniques with various compositions to enhance adsorption, adherence, and entrapment. Additionally, their application in heavy metal ion detection using nanostructured fibers as colorimetric platforms for analyte determination is discussed. The number of publications in the last five years in the Web of Science with the keywords “nanofiber for colorimetric-based sensing of metal ions”, and the timeline of electrospun nanofibers’ development are shown in Figure 2a,b.

Table 1.
Acceptable limits of heavy metal ions and pH as reported in WHO guidelines [48,49].
Table 1.
Acceptable limits of heavy metal ions and pH as reported in WHO guidelines [48,49].
| S. No | Metal Ions | Acceptable Limits (mg/L) | Maximum Permissible pH Levels | Effects |
|---|---|---|---|---|
| 1. | Silver (Ag+) | 0.1 | 6.0–9.0 | Neuronal disorder, mental fatigue, rheumatism, and gastroenteritis |
| 2. | Mercury (Hg2+) | 0.001 | Effects on digestive system, hypertension, and impaired neurologic development | |
| 3. | Copper (Cu2+) | 2.0 | Nephrology disorders, allergies, and anemia | |
| 4. | Chromium (Cr3+/6+) | 0.05 | Dermatitis, carcinogenicity, and reproductive and embryonic damage | |
| 5. | Lead (Pb2+) | 0.01 | Effect on blood–brain barrier (BBB), kidney damage, and neurological disorders | |
| 6. | Iron (Fe3+) | 3 | Hemochromatosis and damage of heart and liver | |
| 7. | Cadmium (Cd2+) | 0.003 | Osteoporosis, renal toxicity, hypertension, and lung cancer | |
| 8. | Arsenic (As3+/5+) | 0.01 | Effect on central nervous system, gastrointestinal diseases, and cardiovascular and pulmonary diseases |
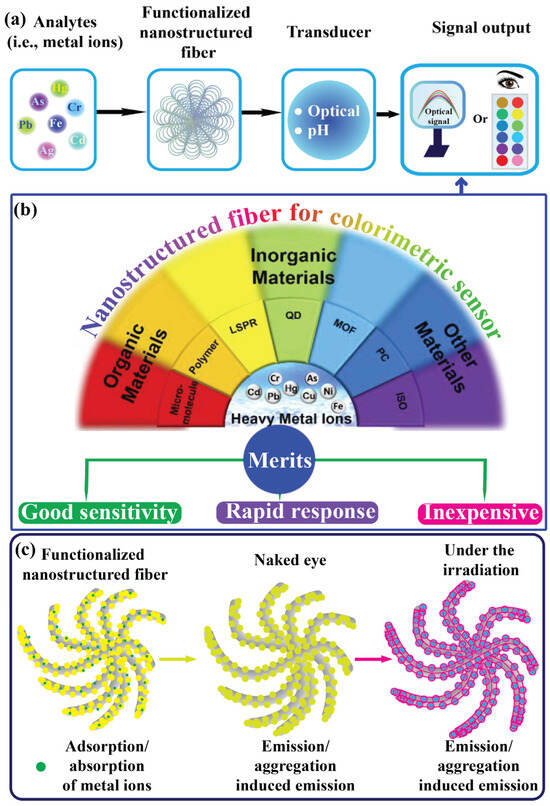
Figure 1.
(a,b) Schematic representation of colorimetric sensing materials that can be utilized for the recognition of heavy metal ions with their merits. Modified and reprinted with permission from Ref. [8]. (c) The general plausible mechanism.
Figure 1.
(a,b) Schematic representation of colorimetric sensing materials that can be utilized for the recognition of heavy metal ions with their merits. Modified and reprinted with permission from Ref. [8]. (c) The general plausible mechanism.
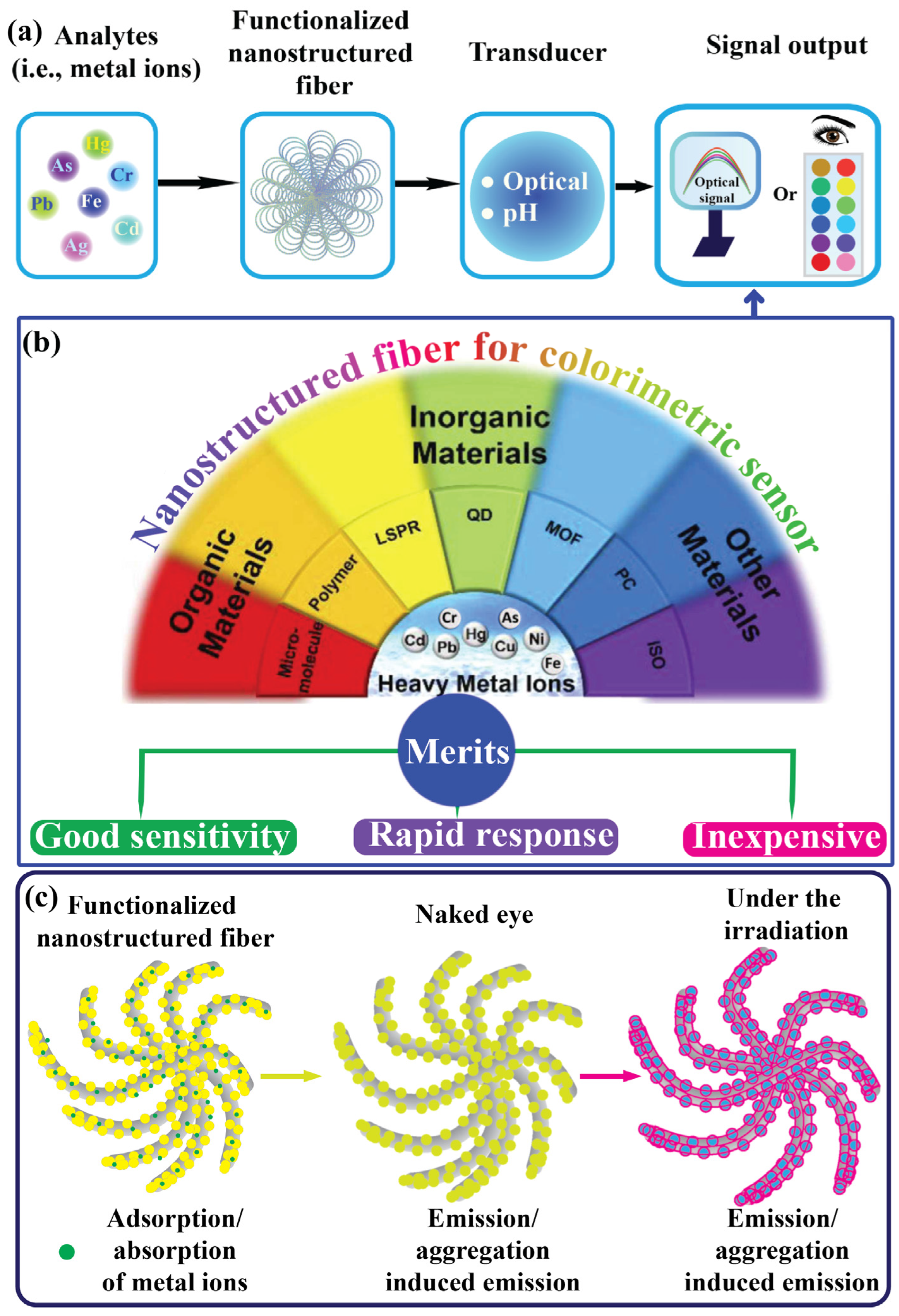
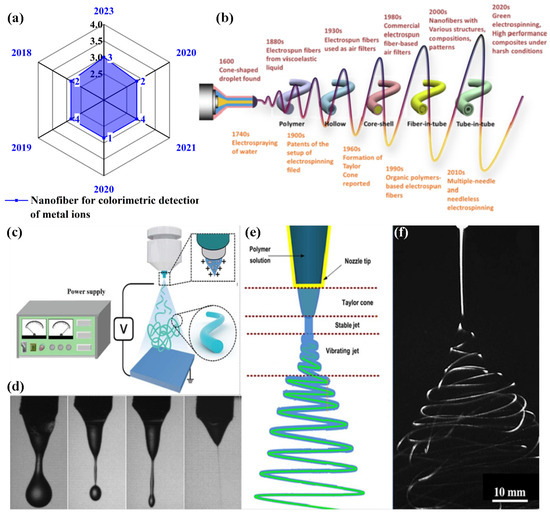
Figure 2.
(a) The number of publications in the last five years (source: Web of Science and keywords: “nanofiber for colorimetric-based detection of heavy metal ions”), (b) the history of electrospun nanofiber development, (c) the essential setup for electrospinning, (d) a solution droplet dripping without applying potential, (e) a schematic representation of the formation of a Taylor cone and electrified jet morphology, and (f) an image depicting a jet made of electrospun material with well-developed loops. (b–f) Reprinted with permission from Ref. [50].
Figure 2.
(a) The number of publications in the last five years (source: Web of Science and keywords: “nanofiber for colorimetric-based detection of heavy metal ions”), (b) the history of electrospun nanofiber development, (c) the essential setup for electrospinning, (d) a solution droplet dripping without applying potential, (e) a schematic representation of the formation of a Taylor cone and electrified jet morphology, and (f) an image depicting a jet made of electrospun material with well-developed loops. (b–f) Reprinted with permission from Ref. [50].
2. Mechanism of Electrospinning Technique
Electrospinning or electrospun devices consist of three major components: (i) a spinneret (metallic needle), (ii) a power supply that operates at a high voltage (1–50 kV), and (iii) a collection plate or ground collector (i.e., a flat plate or rotating plate collector), as illustrated in Figure 2c. As depicted in Figure 2d–f, this method begins by placing the desired polymer solution in a syringe without air bubbles for precision fabrication. A metallic needle is then attached to the syringe and the solution fluidity is controlled using a regulating pump. Furthermore, to create a Taylor cone, a liquid droplet of the polymer solution is injected into the nozzle of a metallic needle with a high-voltage power supply (1–30 kV). When a high voltage is applied, the charge distribution on the surface of the droplet becomes uniform as it undergoes electrification and stretching. During this process, the droplets experience electrostatic repulsion and Coulomb forces as their primary electrostatic forces. Electrostatic attraction counteracts the surface tension, and the peripheral electric field exerts Coulombic forces. When an electrified jet (polymer solution) is deposited on the counter electrode, it stretches and thrashes, forming continuous and uniform nanofibers. A collector plate or ground collector is used to gather uniform nanofibers with nanoscale diameters from an optimized distance. As the solvent evaporates, the polymer mixture is emitted through the needle to the collector plate, resulting in the formation of nanofibers. The nanofibers are collected from the plate for further processing [39,50,51].
The choice of polymers and solvents is crucial in the manufacturing of electrospun nanofibers, as it significantly influences their properties. For instance, the molecular weight of polymers like polyethylene oxide (PEO) impacts the viscosity of the electrospinning solution, thereby affecting the diameter of the fiber [52]. Blending polymers such as poly (lactic-co-glycolic acid) (PLGA) and polyethylene glycol (PEG) alters the mechanical properties and degradation behavior of the fibers [53]. Conductive polymers like polyaniline (PANI), electrospun in solvents of varying conductivities, can influence fiber alignment and uniformity [54]. Additionally, the choice of solvents for electrospinning polymers like polycaprolactone (PCL) can modify fiber morphology due to differing rates of solvent evaporation [55]. Researchers can utilize these studies to optimize polymer–solvent combinations designed for applications in materials science, biomedical engineering, and other related fields.
3. Preparation of Nanostructure Nanofibers
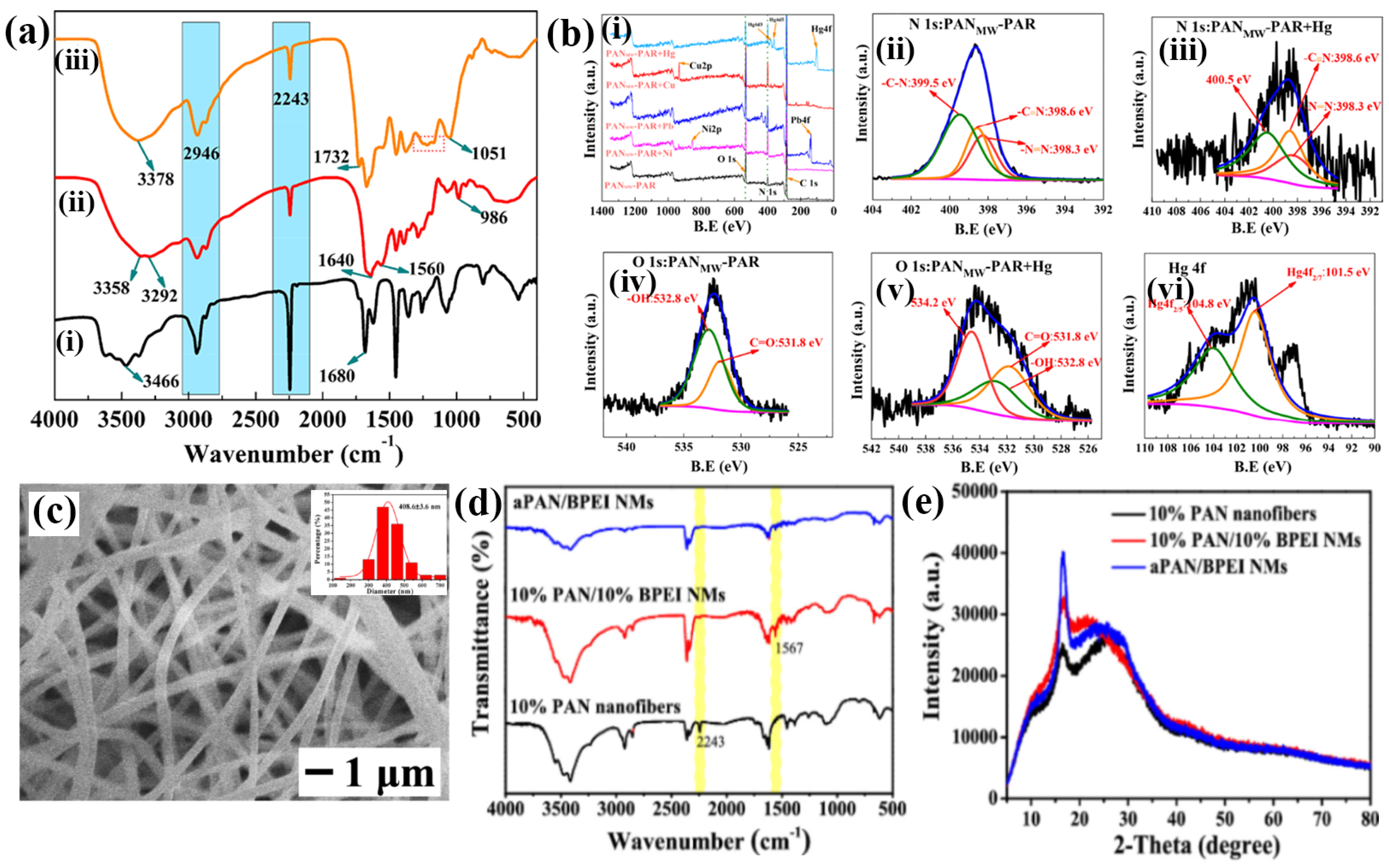
Polyacrylonitrile (PAN) fibers are considered viable substrate materials for adsorbent fabrication owing to their recyclability, extremely large surface area, high thermal and mechanical stability, and low flow resistance. Deng et al. reported that 4-(2-pyridylazo)-resorcinol (PAR)-immobilized PAN fibers, termed MWPAN-PAR fibers, were achieved using a microwave (MW)-aided irradiation (200 W) approach. The fabricated MWPAN-PAR fiber adsorbent removed and detected Hg2+ ions, as indicated by the color changes. The amination process, also known as grafting rate or percentage (GP), has proven to be an effective approach for surface modification, and MW-assisted heating has demonstrated superior advantages over conventional heating in facilitating such changes. Furthermore, the amination process is clearly evidenced by the presence of functional groups such as amines, nitriles, and imines. Electron spectroscopy for chemical analysis (ESCA) revealed oxygen (1 s), carbon (1 s), and nitrogen (1 s) peaks before adsorption, and Ni2+, Pb2+, Cu2+, and Hg2+ peaks were observed after adsorption, indicating the anchoring of PAR onto the PAN fibers before and after adsorption (Figure 3(a(i–iii),b(i–vi))) [56]. In another study, Abedalwafa et al. reported aminated PAN nanofibers immobilized with core–shell gold–silver (CS-Au-Ag) for the colorimetric detection of Cu2+. The transmission electron microscopy (TEM) image of CS-Au-Ag with a spherical shape and particle size distribution was observed via dynamic light scattering (DLS), showing a diameter of 21.0 nm. Furthermore, the amination process established a functional group, followed by the immobilization of CS-Au-Ag on the PAN fibers. The FTIR results revealed that an amine group induced a porous structure and highly precise surface area on the PAN nanofiber. Field emission scanning electron microscopic (FE-SEM) images showed that the average diameter of the PAN nanofibers was reduced to 236 nm after amination, with increased surface roughness [57]. Rao et al. developed an N-methyl imidazole unit (RIM) that functionalized a polyurethane electrospun nanofiber with an embedded rhodamine derivative, exhibiting excellent receptor activity, selectivity, and sensitivity towards Hg2+ ions as a fluorescence-on sensor [58]. Similarly, Saho et al. reported the use of facile electrospinning, followed by a hydrothermal method, to design and fabricate a self-sustaining flexible amidated polyacrylonitrile with a branched polyethyleneimine nanofiber membrane (APAN/BPEI NM). These membranes are effective adsorbents for removing Cu2+ from water and serve as strips for colorimetric-based visual detection of Cu2+. Morphological optimization was conducted on the PAN nanofibers with 10% PAN-based uneven nanofibers for further addition of BPEI NM at an optimized concentration of 10%. The obtained 10% PAN/10% BPEI NM exhibited good morphology and deformation resistance compared to the 5% and 15%, although this composition was water-soluble. To overcome this issue, BPEI was grafted with a 1:1 ratio of 10% PAN/10% BPEI NM to generate an APAN/BPEI NM that exhibited efficient flexibility and a disordered arrangement of nanofibers with diameters of 409 nm, which is suitable for sensing and adsorption studies. Fourier-transform infrared (FTIR) and X-ray diffraction (XRD) analyses confirmed the development of APAN/BPEI NMs amide bonds, and the diffraction peaks with corresponding lattice planes at 16.6° (100) and 26.5° (110) decreased and shifted, respectively, after the grafting of BPEI, as shown in Figure 3c–e. Kumar and Talreha developed an Ni-NP-doped rhodamine B (RhB)-grafted carbon nanofiber (Ni-CNF-RhB) as a colorimetric probe for the naked-eye detection of Pb2+ and Cr3+. Ni-doped phenolic beads (Ni-PhB) were prepared via suspension polymerization and crosslinking. The obtained Ni-PhB was then transferred to an electrical tubular furnace and calcined at 900 °C for 2 h under inert gas (N2) atmospheric conditions. To create porous carbon beads, the prepared Ni NPs were placed in a CVD instrument and acted as a catalyst for the growth of carbon nanofibers on the beads, resulting in Ni-CNF-PhB. RhB grafting onto Ni-CNF-PhB was then performed with physicochemical characterization using FTIR and scanning electron microscope (SEM), making it suitable for the direct, visible colorimetric-based detection of metals in an authentic sample [59]. Zhang et al. developed a poly (aspartic acid) (PASP) electrospun nanofiber hydrogel membrane (PASP-ENHM) with a high surface area as a reusable colorimetric sensor. While electrospinning is typically not suitable for preparing a cross-linked hydrogel network, the hydrolysis and cross-linking of the PASP hydrogel resulted in the preparation of larger-diameter nanofibers with significant geometric characteristics. FTIR spectra show the amino, carboxylic, and amide functional groups, which facilitate excellent metal ion interactions [60]. In addition, cellulose-based biomaterials have emerged as promising candidates for the preparation of nanofibers [61,62]. These materials are effective in converting cellulose nanofibers into uniform and ultrafine nanostructured fibers with carboxyl groups, thus providing an efficient platform for extended applications. A Schiff base ligand was introduced to chelate the metal ions and achieve selective and sensitive detection, as well as to form strong metal complexes in aqueous solution. This chelation effect increases the intramolecular and ligand-to-metal charge transfer π electrons, crucial for metal ion detection [63,64,65]. The plausible optimized parameters for electrospinning are as follows: the distance between the needle nozzle and collector plate, applied voltage, and feeding speed were to be 15–20 cm, 15–30 kV, and 1 mL h−1, respectively.
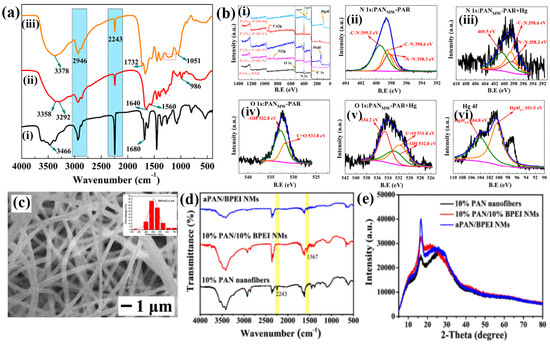
Figure 3.
(a) FTIR spectra of (i) pristine PAN fibers, (ii) MWPAN-EDA fibers, and (iii) MWPAN-PAR fibers. (b) ESCA spectrum of (i) wide spectral scan of MWPAN-PAR fibers before and after adsorption of Ni2+, Pb2+, Cu2+, and Hg2+; deconvolution analysis of (ii) nitrogen (1 s) spectra of MWPAN-PAR fibers prior and (iii) after adsorption of Hg2+; (iv) oxygen (1 s) spectra of MWPAN-PAR fibers before and (v) after adsorption of Hg2+; and (vi) comprehensive investigation of Hg 4f. (a,b) Reprinted with permission from Ref. [56]. (c) SEM image APAN/BPEI NMs (inset: histogram in diameters); (d) FTIR spectra; (e) XRD patterns. (c–e) Reprinted with permission from Ref. [66].
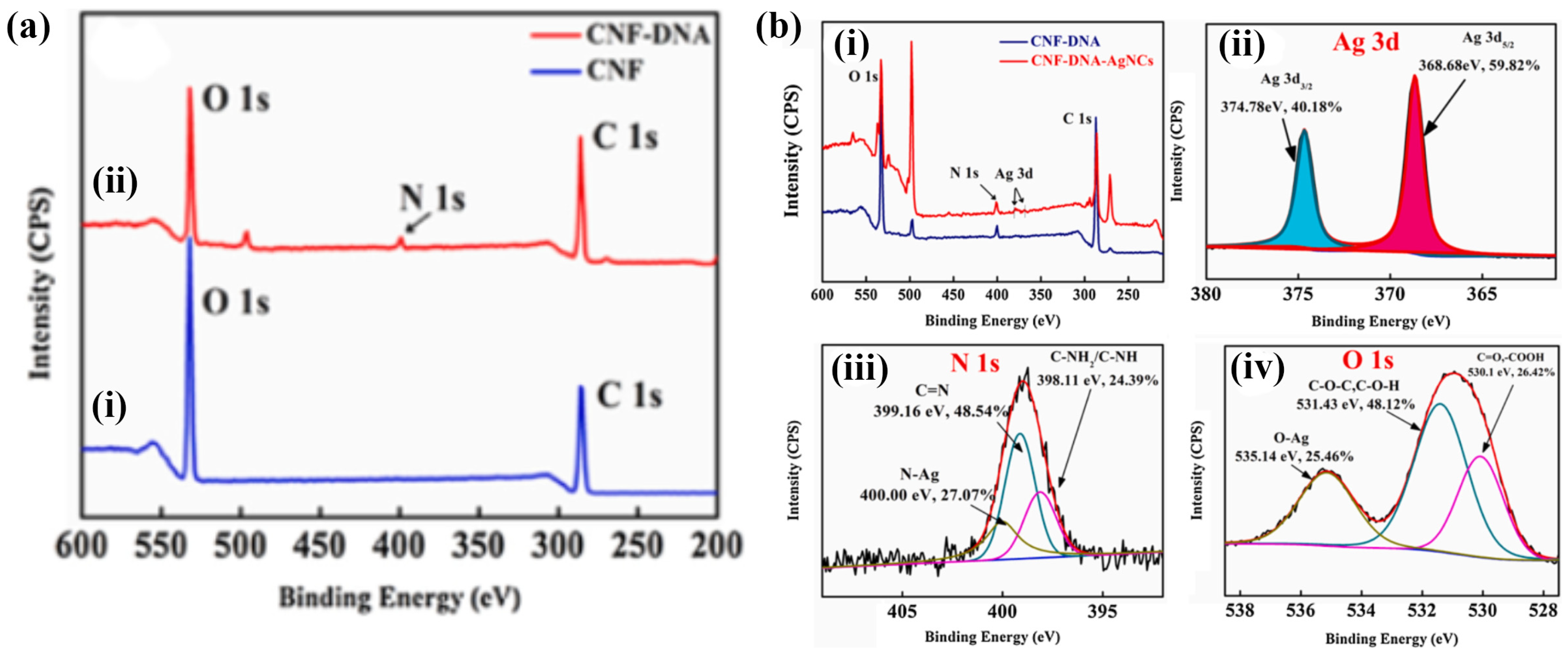
The development of a film-type ligand-functionalized cellulose nanofiber platform was demonstrated by Rahmawati et al. In their study, a sensing film platform was fabricated from ligand 3,5-bis((2-hydroxynaphthalen-1-yl) methylene) amino) benzoic acid (3,5-BHNMABA)-bound 2,2,6,6-tetramethyl-1-piperidinyloxy radical (TEMPO)-oxidized cellulose nanofibers (TOCNF) via a chemical reaction. This is described as a TOCNF/3,5-BHNMABA ligand film. The FTIR spectra aligned well with the ligand, although they provided a lower contribution because the nanocompound films contained only a small portion of the ligand. Both the TOCNF/3,5-BHNMABA (1–3) films and the TOCNF surface exhibited a smooth and uniform film surface, indicating that the immobilization of ligands in minute quantities (5% or less) did not have a substantial impact on the film morphology [62]. Wang et al. developed dual-function cellulose nanofibers (DF-CNF) from bagasse pulp to detect Ag+. DNA was grafted onto the CNF to attach acetylcholinesterase (AChE), leveraging the large number of COOH groups on the CNF surface. The CNF/DNA grafting degree was 26.566% higher than that of the CNF. Figure 4a,b show the binding energies before and after the detection of Ag+ ions. Before detection, N was absent from the CNF surface (Figure 4a(i)), whereas the N 1s ESCA peak was observed after the CNF interacted with DNA (Figure 4a(ii)). It is apparent from these results that DNA was successfully grafted onto the CNF surfaces. As shown in Figure 4b(i), an ESCA survey was used to obtain the spectral profiles of CNF/DNA and CNF/DNA/AgNCs. CNF/DNA/AgNCs displayed a peak for Ag 3d at 366.08–382.08 eV, and the deconvolution spectra agreed well with a peak at 368.68 eV (3d5/2) and a peak at 374.78 eV (3d3/2), as represented in Figure 4b(ii). This demonstrates the detection of Ag+ ions and their chelation to form CNF/DNA. Additionally, the deconvolution of the N 1s (Figure 4b(iii)) and O 1s (Figure 4b(iv)) curves indicates that the Ag+ ions bind to the NH2 group in DNA, whereas oxygen atoms play a crucial role in the interaction between CNF/DNA and Ag+ ions [67]. These types of fabrication methods for nanostructured fibers hold significant potential for designing and constructing strategies to optimize synergistic effects for detecting heavy metal ions.
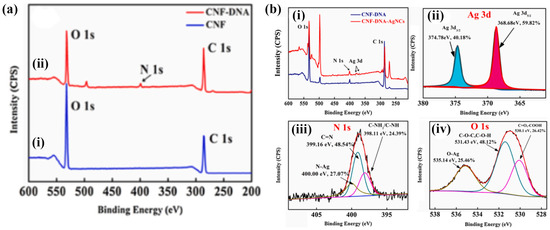
Figure 4.
(a) ESCA band for (i) CNFs (blue) and (ii) CNF/DNA (red); (b) ESCA analysis of CNF/DNA and (i) CNF/DNA/AgNCs; (ii) Ag 3d, (iii) N 1s, and (iv) O 1s bands for CNF/DNA/AgNCs. Reprinted with permission from Ref. [68].
4. Colorimetric-Based Detection of Metal Ions
The facile and innovative development of nanostructured fibers has emerged as a promising method for metal ion detection [69]. The unique properties of nanostructured materials (high surface area, tunable porosity, and functionalization) can be exploited to create highly sensitive and selective sensors. Consequently, along with strategies for the removal of heavy metals, numerous detection methods have been developed, with colorimetric sensing gaining significant attention because of its ease of use and cost-effectiveness. Colorimetric sensors allow the direct observation of metal ions in ecological systems with the naked eye, offering real-time monitoring. The simplicity of this method makes it particularly appealing for widespread environmental applications where rapid onsite detection is essential [70,71,72]. Recent advancements in nanostructured fibers have expanded their use in colorimetric analysis to detect a variety of heavy metal ions, including Ag+, Hg2+, Cu2+, Cr6+, Pb2+, Fe2+, and Cs+. These fibers can be engineered to exhibit distinct color changes upon interaction with specific metal ions, thereby enhancing visual detection. For instance, functionalizing nanofibers with specific ligands or dyes can improve their selectivity towards particular ions, thereby reducing the interference from other substances in complex environmental samples. The development of nanostructured fibers for colorimetric sensing represents a significant step forward in environmental monitoring.
5. Detection of Cu2+ Metal Ions
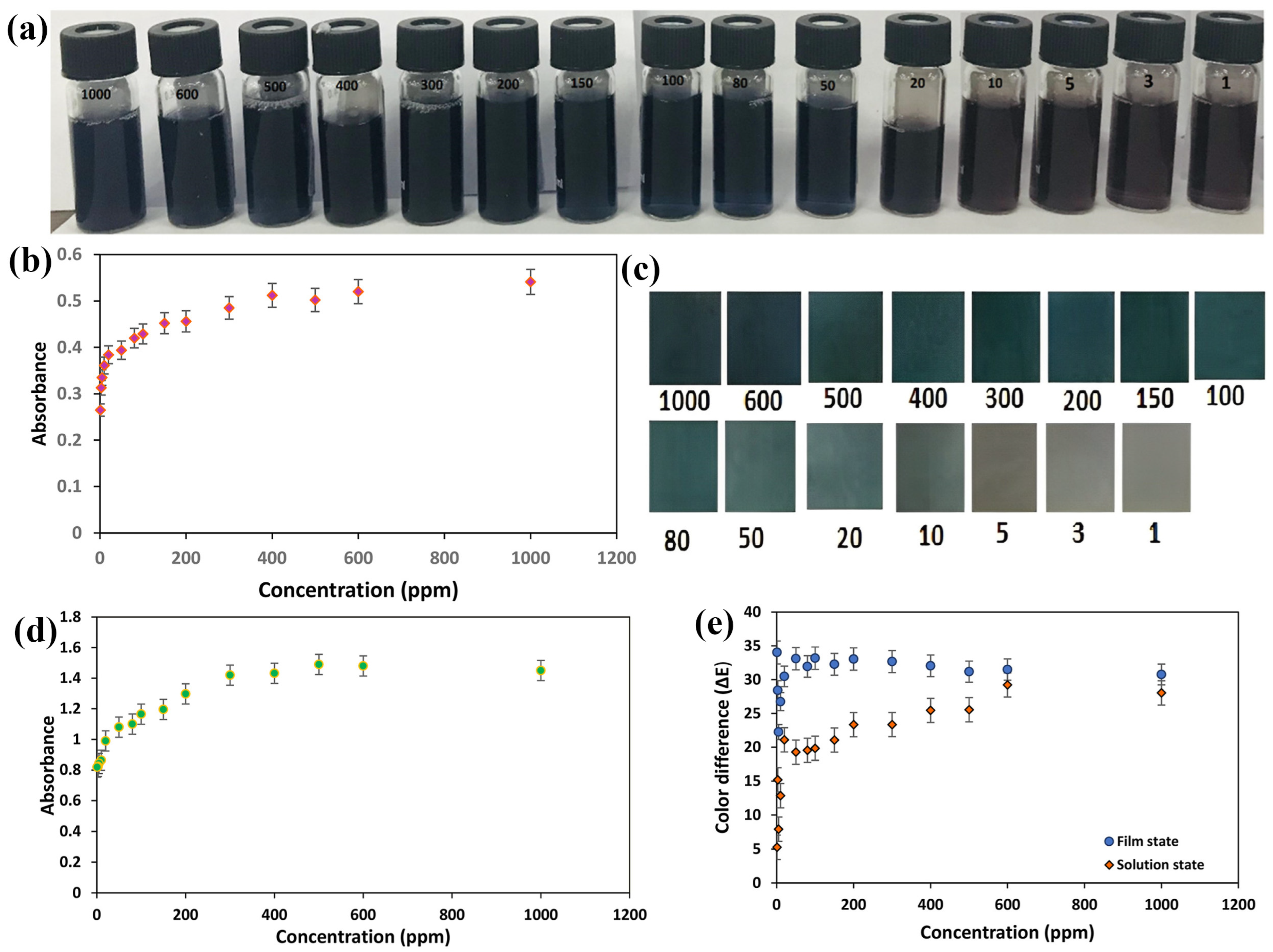
Abedalwafa et al. recently developed test strips with large surface areas and tortuous porous structures by immobilizing Au/Ag nanoparticles on aminated porous polyacrylonitrile nanofibers. Strips containing CS-Au-Ag on PAN fibers were investigated for Cu2+ sensing. Various factors, including ammonium cation (NH4+), thiosulfate (SO32−), pH, and incubation time, were found to influence the feasibility of the test strip. The optimal conditions were determined to be 0.05 M NH4+, 0.009 M S2O32−, pH 11, and 180 s incubation time. Additionally, selectivity analysis with various metal salts revealed a noticeable color shift in the Au/Ag NPs (yellow–pink–colorless), demonstrating their significant analytical potential and promising selectivity for Cu2+ detection. The sensitivity of the colorimetric strips was evaluated by spiking Cu2+ at different concentrations into the leaching liquor under optimized conditions. As the Cu2+ concentration increased, the absorption intensity of the strips increased, accompanied by a blue shift from 420 to 500 nm. This suggests a possible catalyst acceleration effect of Cu2+ on the stripping of core–shell Au/Ag NPs. The test strips exhibited a linear range of 50–1000 nM, detection limit of 50 nM, and reusability for up to six cycles. This method provides a rapid system for Cu2+ detection without the need for sophisticated equipment or specialized personnel. The use of nanotechnology in this assay, which employs catalytic leaching for heavy metal analysis, demonstrates its potential to detect in ecological and biological samples [57]. Parizadeh et al. recently developed an uncomplicated, swift, economical, sensitive, selective, and portable colorimetric-based sensor for detecting Cu2+ ions, both in solution and solid form. The detection method primarily relied on anthocyanin extracted from black eggplant peels, which served as a spectroscopic colorimetric agent and was immobilized onto bacterial cellulose nanofibers (bCNF) in the solid state. The colorimetric performances of both the anthocyanin solution and the bCNF-ANT film are depicted in Figure 5. In the solution state, as the concentration of Cu2+ ions increased from 1000 to 106 ppb (1–1000 ppm), noticeable color changes were observed in Figure 5a, ranging from brown to dark blue. These changes were attributed to the establishment of coordination bonds between phenolic anthocyanins and Cu2+, resulting in the formation of complexes with varying degrees of green color, corresponding to different copper concentrations. The total content of Cu2+ in the aqueous solution exhibits a correlation profile, as shown in Figure 5b. The absorbance intensity at 559 nm demonstrated a direct correlation with increasing Cu2+ concentration between 1000 and 106 ppb, indicating higher absorbance peak intensities at higher Cu2+ concentrations. However, concentrations below 1000 ppb showed no observable changes in absorption band intensity at 559 nm, establishing the detection limit to be less than 1000 ppb. A further increase in the Cu2+ concentration beyond 400 ppm did not result in a corresponding increase in the intensity of the absorption band at 559 nm, leading to a detection limit of 104 ppb. For the bCNF-ANT tape, visible color changes were discernible when exposed to Cu2+ concentrations ranging from 1000 to 106 ppb, as illustrated in Figure 5c. Figure 5d displays the absorbance intensities at 559 nm, indicating a detection limit ranging from 104 to 106 ppb. The image analysis was conducted according to equations. , where, , and indicate numerical values of lightness (i.e., from white to black), color changing ranges from red to green, as well as color ranges from yellow to blue, respectively, with ΔE values represented in Figure 5e. These results revealed E values > 15 at concentrations ranging from 104 to 106 ppb, underscoring the efficacy of the indicator across a broad concentration range [73].
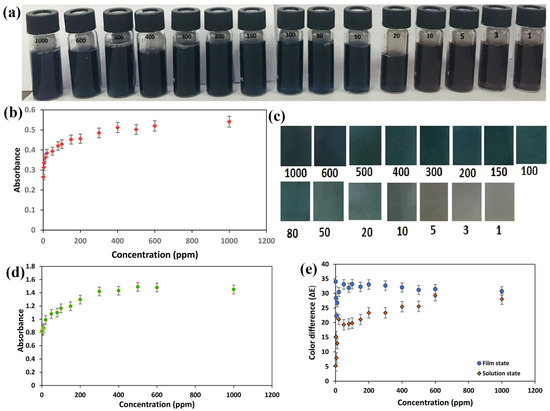
Figure 5.
Represents the changes in color in the presence of (a) anthocyanin mixture and variations in absorbance peak intensity of (b) anthocyanin mixture (red) and (c) bCNF/ANT sensor in response to various concentration (104–106 ppb of Cu(II) (d) bCNF/ANT sensor band at 559 nm upon detecting increased concentrations (104–106 ppb) of Cu2+ in the aqueous solution (green); and (e) overall color difference (ΔE) of anthocyanin medium and bCNF/ANT sensor across different concentrations (104–106 ppb) of Cu2+. (a–e) Reprinted with permission from Ref. [73].
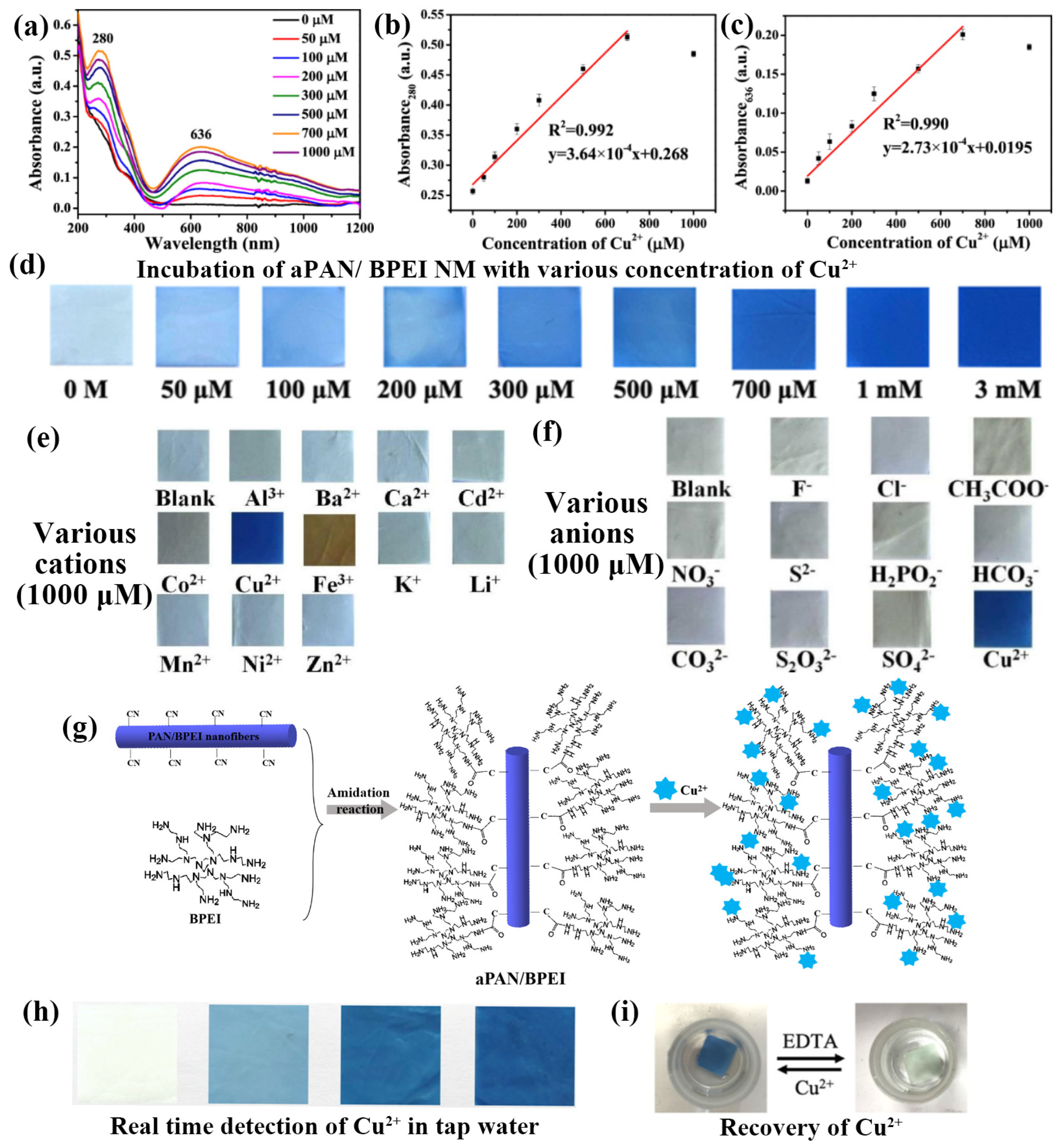
Subbiah et al. developed a novel coumarin–rhodamine (RhBC)-fused nanostructured fiber probe for selective Cu2+ ion detection. There are numerous metal ions in the perchlorate salt form (Al3+, Co2+, Zn2+, Pb2+, Ag+, Hg2+, Cu2+, Ni2+, Fe2+, Ca2+, Mg2+, K+, Cs+, Li+, and Na+) in a volume ratio of water/N, N-dimethylformamide (8:2) solution. The response of RhBC to these ions was assessed visually and via fluorescence irradiation. As expected, the addition of Cu2+ resulted in a color change from yellow to red. Based on these observations, the synthesized RhBC demonstrated selectivity for detecting Cu2+ ions visually. In addition, ultraviolet (UV)-visible measurements were employed for validation, and the fluorescence properties were monitored with various cations added to the RhBC solution alongside Cu2+ ions. RhBC exhibited minimal changes in the absorbance spectra with the studied cations, but significant responses in the UV–visible spectra upon the addition of Cu2+ ions, particularly in the presence of other cations, indicated high selectivity for Cu2+ ions. Additionally, distinct peaks at 557 nm for Cu2+ ions were observed, demonstrating effective Cu2+ ion sensing by the probe. The efficiency of RhBC in the detection Cu2+ ions was further investigated by studying the effect of pH. pH values ranging from 2 to 12 were tested to determine the effective pH range for Cu2+ detection. Interestingly, RhBC exhibits a color switch from yellow to pale red at acidic pH levels, despite the absence of metal ions. At neutral and basic pH values (7–12), RhBC appeared yellow, indicating that its spirolactam was in a closed form. Upon the addition of Cu2+ ions, significant UV–visible absorbance changes were observed at pH 6–7 (acidic pH), whereas no color change was evident at pH 8–12 (basic pH). These findings suggest that the synthesized RhBC probe is particularly effective for detecting Cu2+ ions in the neutral pH range 6–7 [74]. Shao et al. designed and developed a method for the simultaneous detection and removal of heavy metal ions using APAN/BPEI NM. They demonstrated the detection of Cu2+ using color changes. As shown in Figure 6a–c, the UV–vis absorption intensity increased with increasing concentration from 0 to 1000 × 10−6 M, with two significant peaks at 280 and 636 nm, exhibiting correlation coefficients (R2) of 0.992 and 0.990, respectively. The pictorial representation in Figure 6d shows that the absorption color for the APAN/BPEI NM changed from yellow to blue as the concentration was adjusted from 0 to 3 mM, which can be observed by the unaided eye. The evaluation of detection performance involves the assessment of selectivity. Upon the addition of other metal ions and anions at a concentration of 1000 × 10−6 M, the selectivity of APAN/BPEI NM for Cu2+ was investigated. It was observed that metal ions other than Cu2+ had no significant impact on the optical density of the APAN/BPEI NMs. Moreover, the optical images, as shown in Figure 6e, demonstrated that the coexistence of Cu2+ with different metal ions did not influence its detection. However, the addition of anions to Cu2+ resulted in either no or minimal color change due to precipitation during the reaction, particularly for the disulfur anions, as illustrated in Figure 6f. These results demonstrate that the APAN/BPEI NM is extremely selective for Cu2+. Figure 6g illustrates the use of aminated membranes for Cu2+ detection and removal. The reaction occurred with the amine groups of the APAN/BPEI NM. By supporting the flexible aPAN/BPEI NM, they can be easily separated from the solution and have been used for the detection and remediation of Cu2+. Figure 6h shows the pictorial images of the real-time and potential application studies on real tap water samples spiked with various Cu2+ concentrations and incubated. The color of the APAN/BPEI NM ranged from yellow to blue, allowing the concentration of Cu2+ to be estimated. Recovery studies are crucial for determining the economic feasibility of APAN/BPEI NM. The results showed that Ethylenediaminetetraacetic acid (EDTA) easily removed Cu2+ by forming stable EDTA-Cu2+ complexes. Subsequently, the blue APAN/BPEI NM were restored to their original yellow color. Ultimately, these results provide semi-quantitative determination of metal ions with the naked eye (Figure 6i) [66].
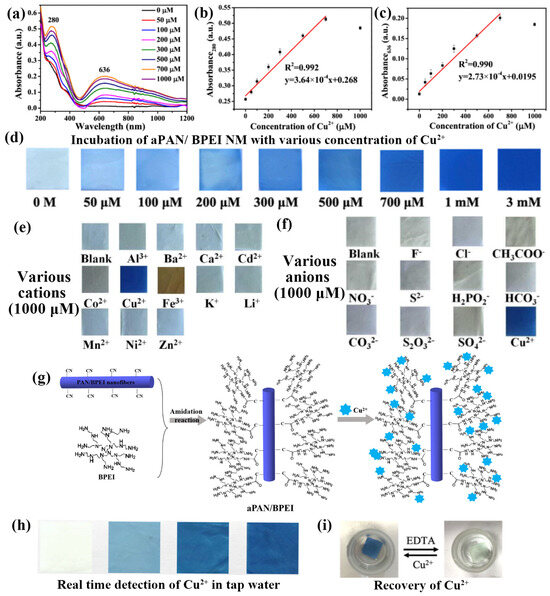
Figure 6.
(a) UVvis absorption spectrum of aPAN/BPEI NMs in Cu2+ aqueous solutions with different concentrations, (b,c) linear fit for 280 and 636 nm versus the linear concentration of Cu2+ (0–1000 × 10−6 M), (d) pictorial representation of aPAN/BPEI NM after incubation with various concentrations, (e,f) selectivity in the presence of cations and anions, (g) schematic diagram for the detection of Cu2+, (h) Realtime detection of Cu2+ in tap water, and (i) recovery studies of Cu2+ using EDTA. (a–i) Reprinted with permission from Ref. [66].
Zhang et al. developed and reported the detection of Cu2+ in aqueous solutions using a facile filtration method with a PASP-ENHM design. The PASP-ENHM sensor demonstrated exceptional sensitivity and specificity, exhibiting visible-light colorimetric recognition of Cu2+. The addition of Cu2+ caused the sensor to change color from white to blue, and the detection limit was found to be 0.3 × 10−3 g/L with a linear range from 0.1 × 10−3 to 100 × 10−3 g/L. The UV–vis absorption and reflectance spectrum intensity changed, respectively, in the presence of Cu2+. Strong absorption peaks appeared at higher concentrations, indicating superior detectability. However, the color of the sensor depended on the adsorption and surface area of the PASP-ENHM when more Cu2+ ions accumulated on the PASP-ENHM, resulting in a deeper color. Therefore, the color of the sensor is visible to the naked eye up to a certain level. The reflection spectra showed different concentrations of Cu2+ ions, indicating that at low concentrations, detection was difficult, similar to the blank samples. As the concentration of Cu2+ increased, the reflectance intensity gradually decreased to approximately 600 nm, with a saturation level of 30 × 10−3 g/L for the PASP-ENHM-based sensor. The optimal thickness of the PASP-ENHM was determined and fixed at 0.1 mm. As a result, the color changed from white to blue as the concentration of Cu2+ increased, as demonstrated and identified in the color differentiation map. This is an appropriate method for the visible detection of Cu2+ in real-time analysis. Because of its wide detection range, high sensitivity, and reusable design, the Cu2+ sensor based on the PASP-ENHM is a useful, affordable, and simple-to-use tool with potential for widespread use in recognizing Cu2+ ions in potable water [60]. The introduction of Schiff base ligands that are selective for metal ions has received considerable attention. However, for the detection of specific metal ions (e.g., Cu2+), chemical immobilization of the ligand on the TOCNF-based film was utilized, as reported by Rahmawati et al. The developed TOCNF/ligand films exhibited fluorescence emission at approximately 410 nm with an excitation wavelength of 310 nm. The fluorescence emission spectra of TOCNF/ligand film decreased as the concentrations were altered from 5 × 103 to 6 × 106 ppb (5–600 ppm). The quenching intensity was plotted against Cu2+ concentration based on the Stern–Volmer equation. Consequently, extinguishing the fluorescence led to an increase in the Stern–Volmer constant, indicating the effective sensing of Cu2+ ions. This is because Cu2+ ions are more attractive to TOCNF with a higher quantity of ligands, which enhances the selectivity for Cu2+ ions. The developed sensors have advantages such as being film-type, recoverable, and reusable and have great potential to be portable and easily accessible for on-site analysis [62].
6. Detection of Fe Metal Ions
Iron (Fe), which exists in ferrous (Fe2+) and ferric (Fe3+) forms, is a critical element for diverse biological functions, particularly oxygen transport and cellular respiration. Maintaining iron homeostasis is essential because deficiencies and excesses can have detrimental consequences. Iron deficiency, often manifested as anemia, can compromise immune function and energy production. Conversely, Fe overload can generate reactive oxygen species (ROS), leading to cellular damage and potentially contributing to chronic diseases. Therefore, reliable and selective methods for iron detection, particularly focusing on Fe2+, are essential for understanding its biological roles and environmental, and industrial applications. Regulatory agencies like the WHO establish safe limits (e.g., 0.3 mg/L Fe in drinking water) to mitigate potential health risks associated with Fe imbalance. Broad research has been continuing to determine the presence of Fe ions in various sectors. For example, Zhang et al. introduced a novel method for detecting Fe3+ ions using a repeatable colorimetric-based sensor composed of a poly (aspartic acid) (PASP) electrospun nanofiber hydrogel-membrane (ENHM). This sensor exploits the unique properties of PASP and nanofibers to achieve high selectivity and sensitivity for Fe3+ ions in aqueous solutions. The color of the PASP/ENHM combination shifted from white to yellow as the Fe3+ concentration increased, allowing detection by both visible light and spectrophotometry. The sensor boasts a detection limit that was found to be 0.1 mg/L for Fe3+ and exhibits excellent selectivity, with minimal interference from other metal ions such as Ca2+, Zn2+, and Mg2+. This makes the PASP-ENHM sensor a valuable tool for real-time on-site monitoring of metal ion contamination in water sources. In addition, they developed a filtration method that uses the PASP-ENHM design to detect Fe3+ in aqueous solutions and reported their results. The PASP-ENHM sensor exhibited colorimetric responses for Fe3+ detection. This indicates high sensitivity and selectivity. The sensor turned yellow upon Fe3+ injection, and its linear range extended from 0.01 × 10−3 to 10 × 10−3 g/L. The sensor detection limit was found to be 0.1 × 10−3 g/L. Although the PASP-ENHM absorbs very small amounts of Fe3+, it tends to form iron-hydroxyl micelles with a wider diameter, thus inhibiting the diffusion of Fe3+ ions. Upon the addition of Fe3+ ions, the solution color changed from white to yellow and was visible to the naked eye, aiding in the development of chemometric sensors. The optimized PASP-ENHM thickness has been fixed at 0.1 mm. From the reflectance spectra at ~430 nm, the reflectance intensity decreased upon the addition of higher concentrations, resulting in a light yellow and then a deep yellow that could be differentiated with the naked eye. Owing to its high sensitivity, wide detection range, and reusability, the PASP-ENHM-based Fe3+ sensor is a feasible, affordable, and simple Fe3+ sensor with potential for widespread application in the detection of Fe3+ pollution in drinking water [74]. El-Naggar et al. developed an innovative, straightforward, rapid, cost-effective, specific, highly sensitive, and portable colorimetric sensor using anthocyanin-activated nanofibrous PVA (polyvinyl alcohol) for Fe3+ ion detection. The detection mechanism uses anthocyanins extracted from red cabbage as a spectroscopic colorimetric agent embedded in a PVA nanofiber substrate. The PVA/AC sensor exhibited a detection limit as low as 1 mg/L with an effective detection range of up to 350 mg/L. This high sensitivity is attributed to the anthocyanin extract, which serves as an effective spectroscopic probe. The sensor showed excellent selectivity for Fe3+ ions over other metal ions such as Hg2+, Mg2+, Cu2+, Co2+, Ba2+, Zn2+, Cr3+, Ni2+, Ca2+, Al3+, K+, Cd2+, Na+, and Mn2+. This selectivity is crucial for practical applications because it ensures that other ions do not interfere with the detection of Fe3+. The optimal pH range for the sensor’s operation is between 3.75 and 6.0. This pH sensitivity is consistent with the color changes of anthocyanins [75]. Gouda et al. demonstrated the potential of xanthohumol(XNS)-encapsulated electrospun PVA nanofibers (XNS@PVA) as viable colorimetric sensors for real-time Fe3+ detection. The nanofibrous film underwent a color change from yellow to off-white upon binding with ferric ions because of the coordination between Fe3+ and the phenolic groups in the xanthohumol. The sensor has a detection limit ranging from 0.1 to 300 ppm showing high sensitivity and selectivity. The XNS@PVA nanofibers detected Fe3+ over other transition metal ions such as K+, Ba2+, Na+, Hg2+, Zn2+, Cd2+, Co2+, Mn2+, Mg2+, Cu2+, Ca2+, Cr3+, Ni2+, and Al3+. The optimal pH range for detection is between 5 and 7.75 [76].
7. Detection of Hg Metal Ions
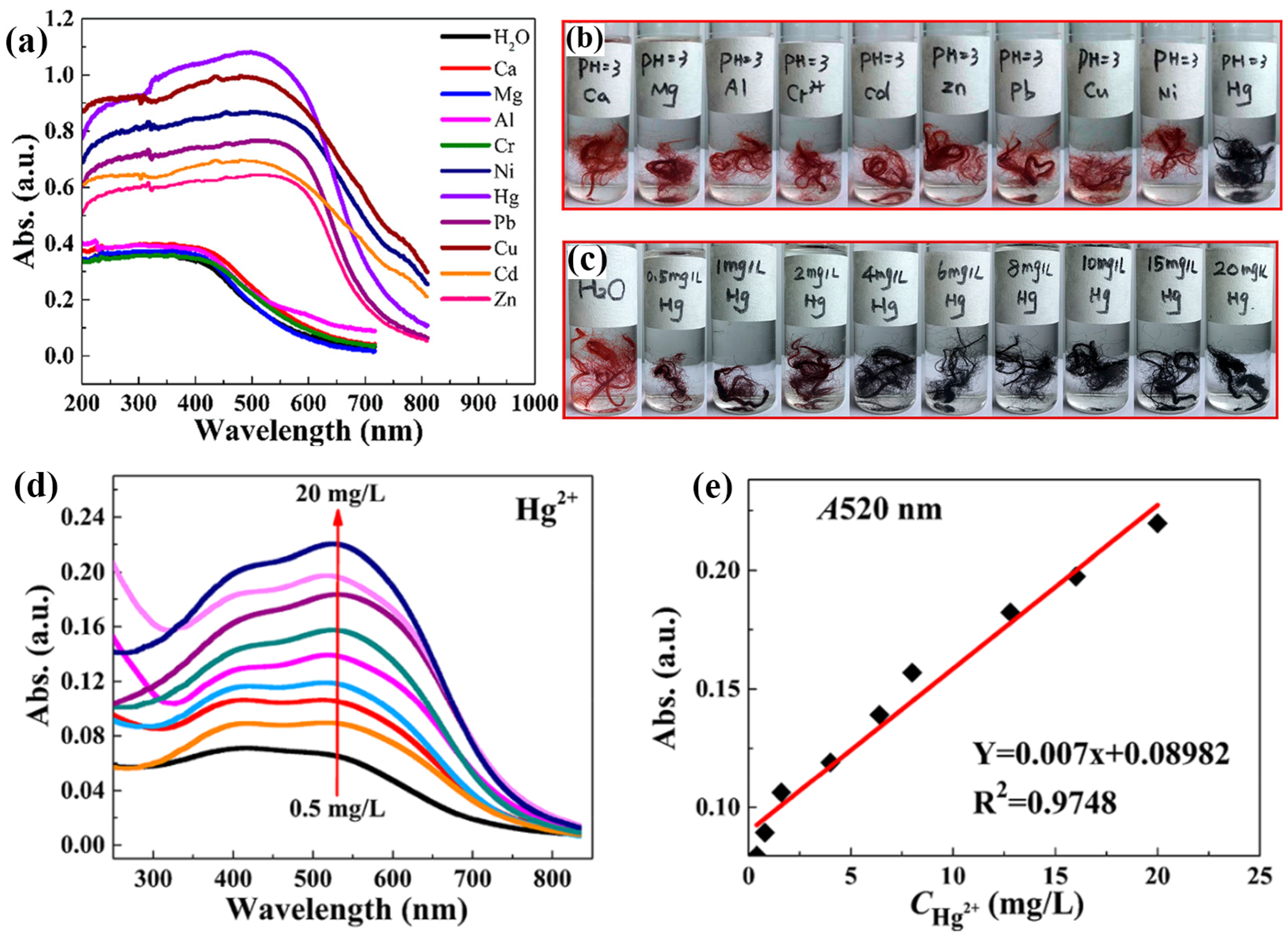
Mercury (Hg) poses a significant threat because of its high toxicity and environmental persistence. Mercuric ions (Hg2+) are of particular concern because they bioaccumulate and damage the nervous system [77,78]. Efficient and reliable methods of Hg2+ detection are required to safeguard human health and the environment. The development of efficient and reliable methods for Hg2+ detection is required for environmental monitoring and health protection. Colorimetric-based sensors are particularly promising for this purpose owing to their simplicity, rapidity, and visual detection capability. These sensors allow onsite and real-time monitoring without requiring sophisticated instrumentation, making them highly suitable for practical applications in diverse environmental settings [79]. Recent advancements in this field have focused on enhancing the sensitivity and selectivity of these sensors, often through the use of nanomaterials that provide clear visual indicators of Hg2+ presence [80,81]. Deng et al. demonstrated colorimetric detection using microwave irradiate 4-(2-pyridylazo)-resorcinol (PAR), onto polyacrylonitrile (PAN) (MWPAN/PAR) fibers as the platform for detecting Hg2+ ions. Interestingly, at a concentration of 0.05 mg/mL, the fabricated material exhibited a color change from red to black when exposed to various metal ions. However, in the presence of Hg2+, the MWPAN/PAR fibers exhibited an absorption peak shift to 520 nm, as shown in Figure 7a. This observation suggests that a coordination effect occurs between the azo nitrogen and ortho-hydroxyl groups in the PAR molecules and the unoccupied orbitals of the heavy metal ions. The color of the MWPAN/PAR fibers changed from red to black only when Hg2+ was present at pH 3, whereas the functionalized fibers did not change color when exposed to other heavy metal ions. In highly acidic solutions, the fibers still detected Hg2+ with high selectivity, as shown in the optical image in Figure 7b. At the lowest concentration, the Hg2+ with MWPAN/PAR fibers appeared dark purple, as the concentration increased, the fiber color transformed to black, as shown in the optical image in Figure 7c. Simultaneously, as depicted in Figure 7d,e, there is a gradual increase in the absorbance intensity at 520 nm with increasing Hg2+ from 0.5 to 20 mg/L. Five consecutive analyses determined the spectrometric limit of detection to be 35 g/L. The high reversibility of the MWPAN/PAR fibers for Hg2+ recognition enabled the functionalized fibers to effectively detect Hg2+ in specific waste effluents with high Hg2+ concentrations. In addition, the synthesized nanostructured fibers are not only capable of detecting heavy metal ions at low concentrations but also efficiently absorbing them. Ultimately, these bifunctional nanostructured fibers can significantly enhance ecological remediation [56]. Similarly, Rao et al. developed a fluorescent and colorimetric chemosensor using rhodamine 6G and an N-methylimidazole nucleus to detect Hg2+ with high selectivity. The addition of Hg2+ to the receptor quickly changed its color from colorless to pink. Researchers have examined the turn-on fluorescence response to Hg2+ among different cations. A Job’s plot revealed that the receptor and Hg2+ had a stoichiometric ratio of 1:1. The spirolactam ring-opening mechanism triggered a color change and activated a fluorescence response upon the addition of Hg2+ ions. Furthermore, the colorimetric sensing behavior of the electrospun polyurethane nanofibers mixed with a receptor in a 100% water medium was studied. Immediately after the addition of Hg2+, a noticeable change in the pink color was observed. The nanofiber strips remain unchanged despite the addition of other cations. This experiment greatly enhanced the efficiency of the receptor in quickly detecting Hg2+ onsite [58]. In another study, Izadi et al. developed a green and cost-effective chemosensor using gallic acid-capped AuNPs (GA/AuNP) for Hg2+ ion detection. Their study investigated key factors, including pH, buffer concentration, GA/AuNP volume, and incubation time. Optimal synergy between GA/AuNP and Hg2+ ions was observed at pH 8.5 within the pH range of 7.0–10.0. At pH 8.5, the utmost ratio (A670/A515) was recorded at a 100.0 mM tris buffer concentration, with concentrations above 100.0 mM diminishing sensor response due to increased ionic strength. Increasing GA/AuNPs volume up to 200.0 μL enhanced sensor response, while volumes exceeding 300.0 μL reached equilibrium in the AuNPs–Hg2+ ions reaction. The highest response occurred after the addition of 7.0 × 10−6 M Hg2+ ions. Additionally, to evaluate the repeatability and reusability of the sensor, five samples containing Hg2+ ions at a concentration of 7.0 × 10−6 M were independently assembled and added to the GA/AuNP solution under optimal conditions; the results were found to be 1.64% and 3.36%, respectively, indicating excellent repeatability and reproducibility for the detection of Hg2+ ions. The efficacy of the developed sensor was assessed by determining the Hg2+ ions in a water sample to demonstrate its systematic utility. Water samples sourced from the Fahlian River in Nourabad, Fars, Iran, and the Ghallat Fountain in Shiraz, Fars, Iran, were used in this study. The analysis of the spiked samples revealed recovery rates ranging from 96.79% to 103.87%. These results confirmed the notable capability of the constructed sensor to determine and quantify ultra-trace level concentrations of Hg2+ in real potable water samples [82].
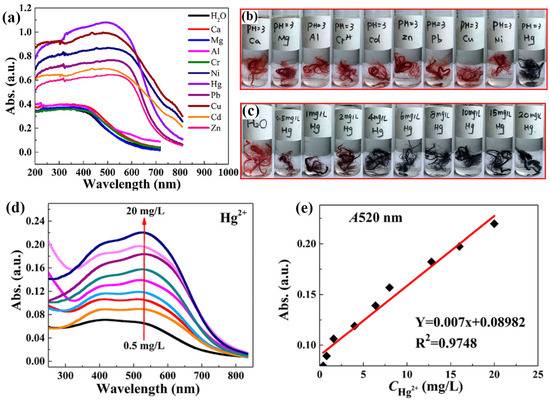
Figure 7.
(a) After being immersed in various metal ions solutions, MWPAN/PAR fibers display a different UV–vis adsorption spectrum; (b) the presence of various metal ions at a concentration of 0.05 mg/mL (50 mg/L) and optical images of MWPAN/PAR fibers at pH 3 are shown. The interaction between Hg2+ and the MWPAN/PAR fibers in (c) optical images, (d) UV–vis absorption spectra, and (e) absorption linear fit at 520 nm for the MWPAN/PAR fibers. (a–e) Reprinted with permission from Ref. [56].
8. Detection of Cr, Pb, Ag, and Cs Metal Ions
Kumar and Talreja developed an Ni-CNF-RhB-based colorimetric probe for the detection of Cr (Cr3+) and Pb (Pb2+) in aqueous systems. Their study showed that the developed Ni/CNF-RhB colorimetric probe could quickly and accurately detect Cr3+ and Pb2+ in a linear range of 100 to 1 × 106 ppb, depending on the pH of the solution. The probe exhibited a pH-dependent colorimetric response, enabling the determination of metal ion selectivity. Cr3+ and Pb2+ were detected with detection limits of 0.203 and 0.132 μM, respectively. CNF and NiNPs enhanced the binding capacity of the RhB dye and carbon fine particle-supported CNF to enhance probe detection, showing a rapid response time (30 s) and reusability (six cycles). In addition, a plausible mechanism for this sensor involves its ability to bind RhB with metal ions, binder support, and solution pH. RhB enables the transfer of charge from the donor to the acceptor moiety by facilitating significant binding affinity with a vacant orbital of the heavy metal ion. The opening of the spirolactam ring can be triggered by a heavy metal ion, which interferes with the carbonyl oxygen and nitrogen atoms present in the amide nitrogen. When the nitrogen atom joins the metal ions, it prevents the RhB from receiving electron donations. Because the N atom inhibits electron transfer to the RhB system, charges are transferred from the nitrogen atom to the metal ions, resulting in bright color changes upon the addition of heavy metal-contaminated effluents. In addition, at a lower pH, the solution exhibits a higher intensity as the concentration of metal ions increases for the protonation of RhB, which aids the generation of an open-chain structure as well as a higher-intensity color. This study clearly shows that Ni NPs supported on micron-sized carbon fine particles increase the CNF, and can be utilized as a sustainable, unsophisticated, handheld, and efficient calorimetric-based probe to promptly detect toxic metal ions in wastewater effluents [59]. Patel et al. effectively synthesized bovine serum albumin-functionalized silver nanostructures (BSA@AgNSs), which were employed as colorimetric sensors for the detection of metal ions such as Cr3+. The sensitivity of BSA@AgNSs for the colorimetric-based detection of Cr3+ ions was assessed by adjusting the concentrations of specific metal ions. It was noted that with an increase in the concentration of Cr3+ ions from 500 nm to 400 μM, a distinct color shift from yellow to orange was observed. To assess the selectivity, the UV–visible spectra of the BSA@AgNSs were examined in the presence of various chemical constituents (i.e., cations, anions, and pesticides). The absorption spectra of BSA@AgNSs remained identical in the presence of the chemical species. This suggests that these chemical substances do not cause aggregation of BSA@AgNSs. However, the addition of Cr3+ ions resulted in noticeable changes in both the color and SPR (surface plasmon resonance) band of BSA@AgNSs, even when present in a mixture with the chemical species. This indicates that the agglomeration of BSA@AgNSs was specifically triggered by Cr3+ ions [83]. Sheikhzadeh et al. devised a straightforward colorimetric sensor using curcumin-modified bacterial cellulose nanofiber sheets to detect Pb2+ ions. The experiment involved testing three different concentrations of curcumin (0.05, 0.1, and 0.2% w/v), which resulted in yellow, orange, and red bCNF-CU sheets, respectively. The analysis revealed that the orange bCN-CU sheet was the most distinguishable. Therefore, we used a 0.1% w/v curcumin loading concentration for subsequent analyses. The distinguishable color of the bCNF-CU strip appears when it is exposed to different concentrations of Pb2+ (i.e., ranging from 0.9 to 9000 μM). It is evident that the ΔE values of the bCNF-CU strips increase with the rise in Pb2+ concentration. Furthermore, image-based investigation was conducted to determine the ΔE values. The selectivity of the bCNF-CU sensor was assessed by exposing the bCNF-CU sheets to various metal ions (Cd2+, Ni2+, Ba2+, Mg2+, Zn2+, and Ca2+) at a concentration of 300 μM for 1 h. A distinct color difference distinguished between the interfering ions and Pb2+ was observed, with none of the other metal ions inducing any color change. The color variation of bCNF-CU in the presence of Pb2+ was attributed to the development of a curcumin–Pb2+ complex. The bCNF-CU sensor successfully demonstrated its ability to detect Pb2+ in rice samples [84].
9. Non-Metal Ion Sensing Using Nanostructured Fibers
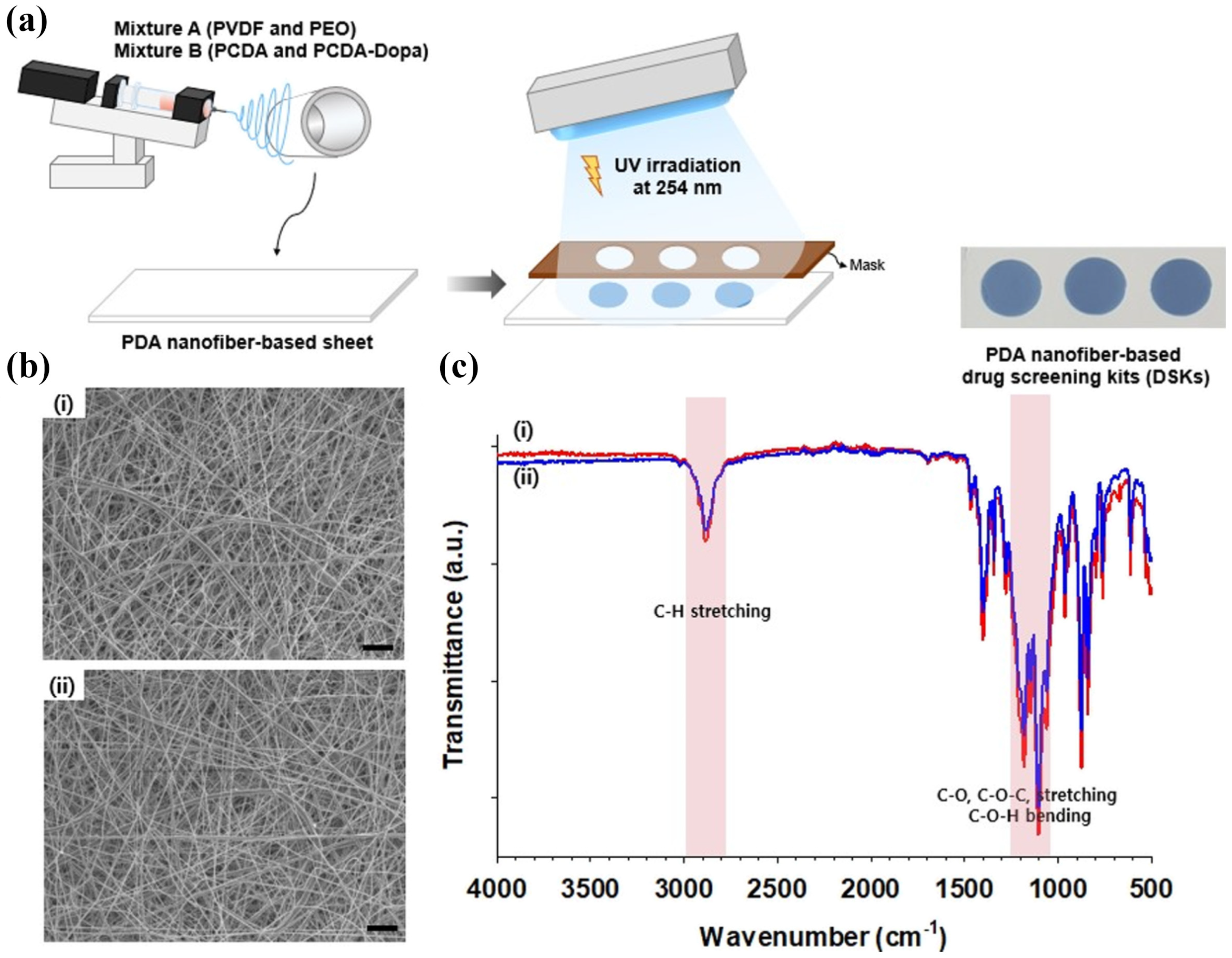
Jang et al. reported that a sheet of drug screening kits (DSKs) made with polydopamine (PDA) nanofibers was produced by electrospinning, as illustrated in Figure 8a. FTIR spectroscopy was used to analyze the chemical bonds in DSK. As shown in Figure 8b,c, strong absorption bands were observed in the FTIR spectra of DSK associated with the C-H, C-O-C, and C–O–C-O-C bending vibrations of PEO (i.e., poly (ethylene oxide)) at 2700–3000 and 900–1200 cm−1. SEM revealed the morphologies of the DSK nanofibers, showing that the sheets contained fine fibers with identical thicknesses and diameters. Moreover, the morphology was unaffected by the composition ratio (<7.5–8 mg) of 10,12 pentacosadiynoic acid (PCDA) and PCDA-Dopamine. Due to their facile fabrication on a paper-based platform, the DSK had the advantages of being lightweight and able to be stored for future use [85]. Hyun et al. developed an innovative colorimetric meta-aramide/dye nanofiber-based sensor for ammonia (NH3) gas detection. The developed sensor exhibited a clear color change from orange to dark brown upon exposure to NH3 gas, which was attributed to a bathochromic shift in the absorption band of dye 3 owing to deprotonation. The reaction duration was notably shorter, primarily within 10 s, for the NH3 exposure at a concentration of 1 ppm. The sensor demonstrated a maximum color difference (ΔE value) with the highest concentration of dye at 10 ppm NH3 exposure, indicating a high sensitivity. This study highlighted a detection limit of 1 ppm, with significant color changes observable within 10 s. Following a 30 min wash test with standard soap, the sensor retained its colorimetric functionality, though with a slight decrease in the ΔE value. This indicated that the sensor could be reused multiple times, maintaining distinguishable color changes even after washing [86]. Hwang et al. developed a flexible nanofibrous composite membrane, PdO@ZnO/PAN, using an electrospinning method, which could detect 4 × 106 ppb (4000 ppm) of hydrogen (H2) gas within two minutes at ambient temperature. The membrane undergoes a visible color shift from brown to dark gray, is easily detectable by the naked eye, and shows high selectivity for H2 gas. Flexible nanofibrous membranes are essential for the visual detection of gas leaks and provide timely warnings. Quick color changes upon exposure to gas render colorimetric gas sensors highly effective for accurately detecting and monitoring gas generation or leakage [87]. Zhang et al. developed a highly receptive, colorimetric, and fluorometric detection system for Escherichia coli (E. coli) using functionalized nanofiber membranes (NFMs). The prepared NFMs were loaded with the target molecules via chemical modification, resulting in functional NFMs (NFM/MUG and NFM/XG). These membranes respond to β-glucuronidase, an enzyme secreted by E. coli that triggers a color change that facilitates both qualitative and quantitative detection. The nanofiber-based platforms exhibited a broad detection range (102–107 CFU/mL−1) and impressive limit of detection (LOD) for NFM/MUG and NFM/XG of 26 and 69 CFU/mL−1, respectively. Additionally, the sensing times were efficient, with NFM/MUG and NFM/XG requiring 15 and 30 min, respectively. The NFMs demonstrated high stability and specificity, with minimal restriction of ionic compounds and pH variation. Robustness is critical for reliable field applications. Integration of the assay with a smartphone application underscores its portability and ease of use, making it a practical tool for real-time monitoring [88]. Additionally, emerging electrospun aggregation-induced emission (AIE) material-based sensors present a promising approach for detection due to their enhanced sensitivity and selectivity. AIE materials are a class of luminogenic materials that exhibit enhanced emission upon aggregation. Unlike traditional luminophores that suffer from aggregation-caused quenching (ACQ), AIE materials become more luminescent when aggregated, making them suitable for sensor applications. Li et al. fabricated an electrospun membrane 5-(N-carbazole styryl)-1,3-dimethyl-barbituric acid and polystyrene (CB-PS) using the electrospinning technique from a solution containing the aggregation-induced emission (AIE) active compound in a DMF/THF solvent mixture [89]. The membrane was electrospun onto amino-functionalized glass (G-NH2), creating a fluorescent sensor ([CB-PS]/[G-NH2]) for the detection of picric acid (PA). The AIE-active compound demonstrated high photostability, and the sensor ([CB-PS]/[G-NH2]) showed excellent selectivity and sensitivity for detecting PA over other nitroaromatics (NACs) in an aqueous phase. The quenching constant (KSV) for PA was determined to be 3.29 × 104 M−1 in the aqueous phase, with a LOD of up to 228 ppb. Selectivity studies indicated that common interferents had a negligible impact on the emission intensity of the fluorescent nanofibers in the aqueous phase. Reusability tests showed that the fluorescent nanofibers could be regenerated effectively. Testing with real water samples revealed an insignificant matrix effect on explosives detection. The fluorescence quenching by PA was attributed to the photo-induced electron transfer (PET) mechanism during the quenching process. This research provides new insights into the application of AIE-active materials in fluorescent nanofibrous sensors for explosives detection. Emerging developments in fiber technology have enabled the production of nanostructured fibers containing specific functional building blocks. Therefore, this advancement paves the way for the design and development of innovative colorimetric sensors and the comparison is given in Table 2.
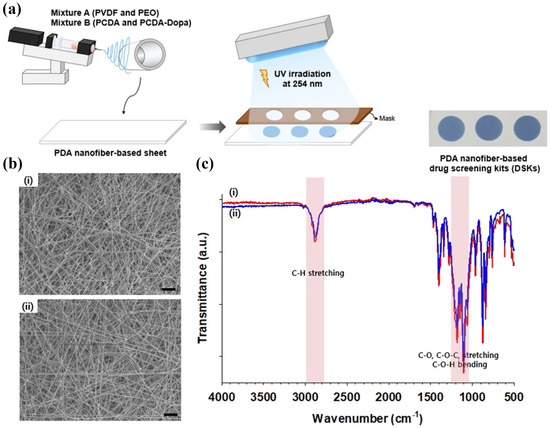
Figure 8.
(a) Schematic illustration of the development of DSKs, (b) SEM images, and (c) FT–IR spectra of (i) DSK-1 and (ii) DSK-2. (a–c) Reprinted with permission from Ref. [85].

Table 2.
Colorimetric detection of various heavy metal ions using nanostructured fibers.
10. Summary
This review article offers a comprehensive overview of recent advancements in nanostructured fiber-based colorimetric detection methods for heavy metal ions. These nanostructured fibers are notable for their exceptional advantages such as high surface area, enhanced sensitivity and specificity, rapid response times, and durability in harsh environments. These attributes render these fibers particularly suitable for applications in environmental observation and remediation. The key to the effectiveness of these fibers is innovative fabrication methods and functionalization strategies that significantly improve their performances. This review details how these methods are employed to optimize the fiber properties, enabling the precise and efficient recognition of heavy metal ions. The unique properties of these nanostructured fibers allow for effective naked-eye detection, even in challenging environments, highlighting their practical utility. Rather than Raman and fluorescence spectroscopy, the colorimetric method has received great attention due to its good sensitivity and selectivity, rapid response, and affordability. This paper discusses the mechanisms underlying the colorimetric detection process. This review elucidates how these fibers achieve high sensitivity and selectivity, by providing detailed insights into the design principles of these sensors. This understanding is critical for the development of efficient and effective colorimetric sensors. Several case studies are presented in this review to showcase the practical applications of these nanostructured fibers. Specific examples illustrate the detection of heavy metal ions, such as Hg2+, Cu2+, Fe2+, and Fe3+. These case studies demonstrate the high selectivity and sensitivity of the fibers, underscoring their potential for real-world applications. The performance of the fibers in these studies highlights their role in creating sustainable and accessible solutions for environmental monitoring. This review emphasizes the transformative potential of nanostructured fibers in environmental monitoring. Their ability to reliably and efficiently detect toxic metal ions can significantly advance current environmental remediation practices. The continued development and refinement of these fibers are essential to address the increasing environmental challenges posed by industrial activities and heavy metal contamination. The challenges and remedies in real-time and on-site analysis are not adequately described. In sensor research reports, the scientific community should mandate the inclusion of the following key sensor attributes: (i) validating the authenticity of the samples; (ii) pretreating a complex of the samples, including diluting factors and their duration; (iii) determining how analytical performance differs between the sequestered samples and the complexity of real sample matrices; and (iv) vigorous specific validation and correlation techniques suitable for the intended purpose of the real-time analysis.
11. Conclusions and Future Prospective
Recent research has demonstrated that hybrid materials prepared through electrospinning offer significant advantages, including a facile preparation process, high specific surface area, and diverse applications. Nanostructured fibers have emerged as a highly promising platform for the colorimetric-based detection of heavy metal ions, providing an efficient, cost-effective, and user-friendly approach for environmental monitoring. Advancements in fabrication techniques and functionalization strategies have notably enhanced the performance of these fibers, making them well-suited for detecting a wide array of heavy metals across different environmental contexts. The capacity of the fibers to reliably and efficiently detect toxic metal ions highlights their potential for environmental monitoring. These developments have paved the way for more sustainable environmental remediation efforts, emphasizing the necessity for ongoing research to tackle the increasing environmental challenges due to industrial activities and heavy metal contamination. Despite this promising futuristic progress, several challenges remain. One significant issue is the embrittlement of the nanofibers, which affects their sustainability, reproducibility, and recycling performance in colorimetric sensor applications. Therefore, appropriate preparation methods are essential to produce flexible and wearable nanofibrous hybrid-structured materials that can withstand the preparation process without self-destruction. Developing a sustainable and flexible nanofibrous hybrid-structured material will enhance the reaction hotspots and create environmentally friendly fiber materials. Additionally, the development of a flexible and wearable sensor component could extend the application of nanofibrous hybrid-structured materials for colorimetric sensors in real-time and industrial operations. Ensuring the uniform apportionment of nanomaterials on the fiber surface is crucial for achieving a stable and consistent colorimetric response. Future efforts should focus on developing high-performance nanofibrous hybrid materials that provide flexible and controllable substrates for functional recognition devices. The mechanism of colorimetric detection using nanofibrous hybrid-structured materials requires further refinement, aiming for intelligent progress from individual color and solitary-analyte detection to multiple colors and the simultaneous recognition of multiple substances. Moreover, there is a need for a standardized color recognition and concentration-based detection system for nanostructured fibrous material-based colorimetric sensors, particularly for smartphone recognition systems. Establishing a unified standard for these methods is crucial for the future development and widespread application of these sensors.
Author Contributions
M.J.—Conceptualization and writing, original draft; R.K.Y.—Writing, review and editing; and S.C.—Review, editing, funding acquisition, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF-2020M3A9E4104385, 2023R1A2C1003669) and the Korea Environment Industry & Technology Institute (KEITI) through the “Technology Development Project for Biological Hazards Management in Indoor Air” project, funded by the Korea Ministry of Environment (MOE) (G232021010381).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hara, T.O.; Singh, B. Electrochemical biosensors for detection of pesticides and heavy metal toxicants in water: Recent trends and progress. ACS ES T Water 2021, 1, 462–478. [Google Scholar] [CrossRef]
- Riu, J.; Giussani, B. Electrochemical biosensors for the detection of pathogenic bacteria in food. TrAC Trends Anal. Chem. 2020, 126, 115863. [Google Scholar] [CrossRef]
- Kirchhain, A.; Bonini, A.; Vivaldi, F.; Poma, N.; Di Francesco, F. Latest developments in non-faradic impedimetric biosensors: Towards clinical applications. TrAC Trends Anal. Chem. 2020, 133, 116073. [Google Scholar] [CrossRef]
- Dincer, C.; Bruch, R.; Costa-Rama, E.; Fernández-Abedul, M.T.; Merkoçi, A.; Manz, A.; Urban, G.A.; Güder, F. Disposable sensors in diagnostics, food, and environmental monitoring. Adv. Mater. 2019, 31, 1806739. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Q.; Lv, M.; Chen, L. Microorganism remediation strategies towards heavy metals. Chem. Eng. J. 2019, 360, 1553–1563. [Google Scholar] [CrossRef]
- Miranda, L.S.; Deilami, K.; Ayoko, G.A.; Egodawatta, P.; Goonetilleke, A. Influence of land use class and configuration on water-sediment partitioning of heavy metals. Sci. Total Environ. 2022, 804, 150116. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, X.; Chen, Z.; Zhou, X.; Lu, X.; Liu, J. Pollution characteristics and source analysis of heavy metals in surface sediments of Luoyuan Bay, Fujian. Environ. Res. 2022, 203, 111911. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.; Qi, J.; You, J.; Ma, J.; Chen, L. Colorimetric detection of heavy metal ions with various chromogenic materials: Strategies and applications. J. Hazard. Mater. 2023, 441, 129889. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Mustafa, F.S.; Omer, K.M.; Hama, S.; Hamarawf, R.F.; Rahman, K.O. Heavy metal pollution in the aquatic environment: Efficient and low-cost removal approaches to eliminate their toxicity: A review. RSC Adv. 2023, 13, 17595–17610. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Thai, V.-P.; Tran, D.N.; Kosugi, K.; Takahashi, K.; Sasaki, T.; Kikuchi, T. One-Step Synthesis of N-Doped Graphene Quantum Dots via Plasma Contacting Liquid for Multiple Heavy Metal Ion Detection. ACS Appl. Nano Mater. 2024, 7, 12664–12672. [Google Scholar] [CrossRef]
- Jagannathan, M.; Badhulika, S. Graphitic carbon nitride and its nanocomposites-based sensors for detection of pharmaceutical effluents in food, ecological and biological samples: A mini-review. Sens. Actuators Rep. 2023, 7, 100183. [Google Scholar] [CrossRef]
- Peivasteh-Roudsari, L.; Barzegar-Bafrouei, R.; Sharifi, K.A.; Azimisalim, S.; Karami, M.; Abedinzadeh, S.; Asadinezhad, S.; Tajdar-Oranj, B.; Mahdavi, V.; Alizadeh, A.M. Origin, dietary exposure, and toxicity of endocrine-disrupting food chemical contaminants: A comprehensive review. Heliyon 2023, 9, e18140. [Google Scholar] [CrossRef]
- Abd Elnabi, M.K.; Elkaliny, N.E.; Elyazied, M.M.; Azab, S.H.; Elkhalifa, S.A.; Elmasry, S.; Mouhamed, M.S.; Shalamesh, E.M.; Alhorieny, N.A.; Abd Elaty, A.E. Toxicity of heavy metals and recent advances in their removal: A review. Toxics 2023, 11, 580. [Google Scholar] [CrossRef] [PubMed]
- Marghade, D.; Mehta, G.; Shelare, S.; Jadhav, G.; Nikam, K.C. Arsenic contamination in Indian groundwater: From origin to mitigation approaches for a sustainable future. Water 2023, 15, 4125. [Google Scholar] [CrossRef]
- Sazakli, E. Human Health Effects of Oral Exposure to Chromium: A Systematic Review of the Epidemiological Evidence. Int. J. Environ. Res. Public Health 2024, 21, 406. [Google Scholar] [CrossRef]
- Mulenga, M.; Ouma, K.O.; Monde, C.; Syampungani, S. Aquatic mercury pollution from artisanal and small-scale gold mining in sub-Saharan Africa: Status, Impacts, and Interventions. Water 2024, 16, 756. [Google Scholar] [CrossRef]
- Brown, M.; Patel, P.; Nash, E.; Dikid, T.; Blanton, C.; Forsyth, J.; Fontaine, R.; Sharma, P.; Keith, J.; Babu, B. Prevalence of elevated blood lead levels and risk factors among children living in Patna, Bihar, India 2020. PLOS Glob. Public Health 2022, 2, e0000743. [Google Scholar] [CrossRef] [PubMed]
- Mendil, D. Mineral and trace metal levels in some cheese collected from Turkey. Food Chem. 2006, 96, 532–537. [Google Scholar] [CrossRef]
- Perelonia, K.B.S.; Benitez, K.C.D.; Banicod, R.J.S.; Tadifa, G.C.; Cambia, F.D.; Montojo, U.M. Validation of an analytical method for the determination of cadmium, lead and mercury in fish and fishery resources by graphite furnace and Cold Vapor Atomic Absorption Spectrometry. Food Control 2021, 130, 108363. [Google Scholar] [CrossRef]
- Xue, J.; Gong, S.; Wang, X.; Fan, Y.; Li, X. Determination of Hg, As, Pb, and Cd in orchard soils by sequential injection vapor generation atomic fluorescence spectrometry. Anal. Lett. 2012, 45, 2257–2268. [Google Scholar] [CrossRef]
- Ding, R.; Cheong, Y.H.; Ahamed, A.; Lisak, G. Heavy metals detection with paper-based electrochemical sensors. Anal. Chem. 2021, 93, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, M.; Dhinasekaran, D.; Rajendran, A.R.; Cho, S. A Review of Electroactive Nanomaterials in the Detection of Nitrogen-Containing Organic Compounds and Future Applications. Biosensors 2023, 13, 989. [Google Scholar] [CrossRef] [PubMed]
- Karthik, D.; Vijayarekha, K. Chemometric identification of a few heavy metals, pesticides and plasticides in edible sunflower oil for health risk assessment. Int. J. Food Prop. 2018, 21, 1442–1448. [Google Scholar] [CrossRef]
- Ichinoki, S.; Kitahata, N.; Fujii, Y. Selective determination of mercury (II) ion in water by solvent extraction followed by reversed-phase HPLC. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 1785–1798. [Google Scholar] [CrossRef]
- Hassan, A.H.; Zeinhom, M.M.; Shaban, M.; Korany, A.M.; Gamal, A.; Abdel-Atty, N.S.; Al-Saeedi, S.I. Rapid and sensitive in situ detection of heavy metals in fish using enhanced Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 313, 124082. [Google Scholar] [CrossRef] [PubMed]
- Makam, P.; Shilpa, R.; Kandjani, A.E.; Periasamy, S.R.; Sabri, Y.M.; Madhu, C.; Bhargava, S.K.; Govindaraju, T. SERS and fluorescence-based ultrasensitive detection of mercury in water. Biosens. Bioelectron. 2018, 100, 556–564. [Google Scholar] [CrossRef]
- Sarfo, D.K.; Izake, E.L.; O’Mullane, A.P.; Ayoko, G.A. Molecular recognition and detection of Pb (II) ions in water by aminobenzo-18-crown-6 immobilised onto a nanostructured SERS substrate. Sens. Actuators B Chem. 2018, 255, 1945–1952. [Google Scholar] [CrossRef]
- Cheng, F.; Xu, H.; Wang, C.; Gong, Z.; Tang, C.; Fan, M. Surface enhanced Raman scattering fiber optic sensor as an ion selective optrode: The example of Cd2+ detection. RSC Adv. 2014, 4, 64683–64687. [Google Scholar] [CrossRef]
- Sun, X.; Li, B.; Qi, A.; Tian, C.; Han, J.; Shi, Y.; Lin, B.; Chen, L. Improved assessment of accuracy and performance using a rotational paper-based device for multiplexed detection of heavy metals. Talanta 2018, 178, 426–431. [Google Scholar] [CrossRef]
- Sener, G.; Uzun, L.; Denizli, A. Colorimetric sensor array based on gold nanoparticles and amino acids for identification of toxic metal ions in water. ACS Appl. Mater. Interfaces 2014, 6, 18395–18400. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Meng, G.; Tang, H.; Ye, Y.; Sun, R.; Chen, M.; Wang, K.-M. A novel cationic iridium (iii) complex with a thiorhodamine-based auxiliary ligand: Application for luminescent and colorimetric detection of Hg2+ in an aqueous solution. New J. Chem. 2017, 41, 8312–8319. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Zou, C.; Zhou, J.; Yang, M.; Zhang, S.; Huo, D.; Hou, C. Colorimetric detection of Cr6+ ions based on surface plasma resonance using the catalytic etching of gold nano-double cone@ silver nanorods. Anal. Chim. Acta 2021, 1149, 238141. [Google Scholar] [CrossRef] [PubMed]
- Senthamizhan, A.; Balusamy, B.; Uyar, T. Recent progress on designing electrospun nanofibers for colorimetric biosensing applications. Curr. Opin. Biomed. Eng. 2020, 13, 1–8. [Google Scholar] [CrossRef]
- Soundharraj, P.; Jagannathan, M.; Dhinasekaran, D.; Thiruvarasu, P. Fluorescent zinc titanate as an effective sensing platform for urea detection. Mater. Today Proc. 2022, 50, 101–106. [Google Scholar] [CrossRef]
- Jagannathan, M.; Dhinasekaran, D.; Soundharraj, P.; Rajendran, S.; Vo, D.-V.N.; Prakasarao, A.; Ganesan, S. Green synthesis of white light emitting carbon quantum dots: Fabrication of white fluorescent film and optical sensor applications. J. Hazard. Mater. 2021, 416, 125091. [Google Scholar] [CrossRef]
- Andersson, M.; Jia, Q.; Abella, A.; Lee, X.-Y.; Landreh, M.; Purhonen, P.; Hebert, H.; Tenje, M.; Robinson, C.V.; Meng, Q. Biomimetic spinning of artificial spider silk from a chimeric minispidroin. Nat. Chem. Biol. 2017, 13, 262–264. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C. One-Dimensional Nanostructures: Electrospinning Technique and Unique Nanofibers; Springer: Berlin/Heidelber, Germany, 2013. [Google Scholar]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Mercante, L.A.; Pavinatto, A.; Pereira, T.S.; Migliorini, F.L.; dos Santos, D.M.; Correa, D.S. Nanofibers interfaces for biosensing: Design and applications. Sens. Actuators Rep. 2021, 3, 100048. [Google Scholar] [CrossRef]
- Ram Thimmiah, B.; Nallathambi, G. Thiol Functionalized Aloe Vera Fibre Filter: A Simple Portable Water Filter For Heavy Metal Ion Removal. ChemistrySelect 2023, 8, e202203747. [Google Scholar] [CrossRef]
- Fernández-Ramos, M.D.; Bastida-Armesto, M.; Blanc-García, R.; Capitán-Vallvey, L.F.; Medina-Castillo, A.L. Design of colorimetric nanostructured sensor phases for simple and fast quantification of low concentrations of acid vapors. Microchim. Acta 2023, 190, 160. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Song, Y.; Xu, L.; Wang, J.; Yang, Z.; Zhao, Y.; Chen, F.; Liu, B.; Liu, L.; Chen, D. Functional Electrospun Nanofibrous Hybrid Materials for Colorimetric Sensors: A Review. ACS Omega 2024, 9, 5157–5174. [Google Scholar] [CrossRef] [PubMed]
- Terra, I.A.; Mercante, L.A.; Andre, R.S.; Correa, D.S. Fluorescent and colorimetric electrospun nanofibers for heavy-metal sensing. Biosensors 2017, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Balusamy, B.; Senthamizhan, A.; Uyar, T. Functionalized electrospun nanofibers as a versatile platform for colorimetric detection of heavy metal ions in water: A review. Materials 2020, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Balusamy, B.; Senthamizhan, A.; Uyar, T. Functionalized electrospun nanofibers as colorimetric sensory probe for mercury detection: A review. Sensors 2019, 19, 4763. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Rowland, C.E.; Schaller, R.D.; Korgel, B.A. Synthesis and ligand exchange of thiol-capped silicon nanocrystals. Langmuir 2015, 31, 6886–6893. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.S.; Jaimes, R.F.; Borysow, W.; Gomes, O.F.; Salcedo, W.J. Portable multispectral colorimeter for metallic ion detection and classification. Sensors 2017, 17, 1730. [Google Scholar] [CrossRef] [PubMed]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, X.; Zhang, M.; Ge, M.; Wang, J.; Tang, Y.; Zhang, Y.; Mi, J.; Cai, W.; Lai, Y. Rational design of electrospun nanofibers for gas purification: Principles, opportunities, and challenges. Chem. Eng. J. 2022, 446, 137099. [Google Scholar] [CrossRef]
- Mitchell, G.R. Electrospinning: Principles, Practice and Possibilities; Royal Society of Chemistry: Cambridge, UK, 2015. [Google Scholar]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Xu, X.; Lu, T.; Wang, X.; Yang, L.; Jing, X. BCNU-loaded PEG–PLLA ultrafine fibers and their in vitro antitumor activity against Glioma C6 cells. J. Control. Release 2006, 114, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Razak, S.; Wahab, I.; Fadil, F.; Dahli, F.; Md Khudzari, A.; Adeli, H. A review of electrospun conductive polyaniline based nanofiber composites and blends: Processing, features, applications, and future directions. Adv. Mater. Sci. Eng. 2005, 2015, 356286. [Google Scholar]
- Katsogiannis, K.A.G.; Vladisavljević, G.T.; Georgiadou, S. Porous electrospun polycaprolactone (PCL) fibres by phase separation. Eur. Polym. J. 2015, 69, 284–295. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, G.; Wang, P. Visualized fibrous adsorbent prepared by the microwave-assisted method for both detection and removal of heavy metal ions. ACS Sustain. Chem. Eng. 2018, 7, 1159–1168. [Google Scholar] [CrossRef]
- Abedalwafa, M.A.; Li, Y.; Li, D.; Sanbhal, N.; Yang, J.; Wang, L. Aminated polyacrylonitrile nanofibers with immobilized gold-silver core-shell nanoparticles for use in a colorimetric test strip for copper (II). Microchim. Acta 2018, 185, 402. [Google Scholar] [CrossRef]
- Rao, P.G.; Saritha, B.; Siva Rao, T. Colorimetric and turn-on fluorescence Chemosensor for Hg2+ ion detection in aqueous media. J. Fluoresc. 2019, 29, 353–360. [Google Scholar] [CrossRef]
- Kumar, D.; Talreja, N. Nickel nanoparticles-doped rhodamine grafted carbon nanofibers as colorimetric probe: Naked eye detection and highly sensitive measurement of aqueous Cr3+ and Pb2+. Korean J. Chem. Eng. 2019, 36, 126–135. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Yu, Q.; Jia, L.; Wan, L.Y. Poly (aspartic acid) electrospun nanofiber hydrogel membrane-based reusable colorimetric sensor for Cu (II) and Fe (III) detection. ACS Omega 2019, 4, 14633–14639. [Google Scholar] [CrossRef]
- Shah, K.J.; Imae, T. Selective gas capture ability of gas-adsorbent-incorporated cellulose nanofiber films. Biomacromolecules 2016, 17, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Rahmawati, A.; Shih, C.-F.; Imae, T. Film sensor of a ligand-functionalized cellulose nanofiber for the selective detection of copper and cesium ions. Polym. J. 2020, 52, 1235–1243. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, S.Y.; Jung, J.M.; Kim, C. A new Schiff-base chemosensor for selective detection of Cu2+ and Co2+ and its copper complex for colorimetric sensing of S2− in aqueous solution. Photochem. Photobiol. Sci. 2017, 16, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- ReddyPrasad, P.; Imae, T. Selective detection of copper ion in water by tetradentate ligand sensor. J. Taiwan Inst. Chem. Eng. 2017, 72, 194–199. [Google Scholar] [CrossRef]
- Kim, K.B.; Kim, H.; Song, E.J.; Kim, S.; Noh, I.; Kim, C. A cap-type Schiff base acting as a fluorescence sensor for zinc (II) and a colorimetric sensor for iron (II), copper (II), and zinc (II) in aqueous media. Dalton Trans. 2013, 42, 16569–16577. [Google Scholar] [CrossRef]
- Shao, H.; Yin, D.; Li, D.; Ma, Q.; Yu, W.; Dong, X. Simultaneous visual detection and removal of Cu2+ with electrospun self-supporting flexible amidated polyacrylonitrile/branched polyethyleneimine nanofiber membranes. ACS Appl. Mater. Interfaces 2021, 13, 49288–49300. [Google Scholar] [CrossRef]
- Gao, R.; Xu, G.; Zheng, L.; Xie, Y.; Tao, M.; Zhang, W. A highly selective and sensitive reusable colorimetric sensor for Ag+ based on thiadiazole-functionalized polyacrylonitrile fiber. J. Mater. Chem. C 2016, 4, 5996–6006. [Google Scholar] [CrossRef]
- Wang, L.; Guo, W.; Zhu, H.; He, H.; Wang, S. Preparation and properties of a dual-function cellulose nanofiber-based bionic biosensor for detecting silver ions and acetylcholinesterase. J. Hazard. Mater. 2021, 403, 123921. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Metal–organic frameworks supported on nanofibers to remove heavy metals. J. Mater. Chem. A 2018, 6, 4550–4555. [Google Scholar] [CrossRef]
- Sherlala, A.; Raman, A.; Bello, M.; Asghar, A. A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere 2018, 193, 1004–1017. [Google Scholar] [CrossRef]
- Fakhre, N.A.; Ibrahim, B.M. The use of new chemically modified cellulose for heavy metal ion adsorption. J. Hazard. Mater. 2018, 343, 324–331. [Google Scholar] [CrossRef]
- Kaur, B.; Kaur, N.; Kumar, S. Colorimetric metal ion sensors–a comprehensive review of the years 2011–2016. Coord. Chem. Rev. 2018, 358, 13–69. [Google Scholar] [CrossRef]
- Parizadeh, P.; Moeinpour, F.; Mohseni-Shahri, F.S. Anthocyanin-induced color changes in bacterial cellulose nanofibers for the accurate and selective detection of Cu (II) in water samples. Chemosphere 2023, 326, 138459. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Kumaresan, S. Coumarin Xanthene Combined Probe for the Multi-Color Detection of Metal Ions and Electrospun Fibers Developed for Real-Time Monitoring. J. Fluoresc. 2023, 33, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.E.; El-Newehy, M.H.; Aldalbahi, A.; Salem, W.M.; Khattab, T.A. Immobilization of anthocyanin extract from red-cabbage into electrospun polyvinyl alcohol nanofibers for colorimetric selective detection of ferric ions. J. Environ. Chem. Eng. 2021, 9, 105072. [Google Scholar] [CrossRef]
- Gouda, M.; Abd El-Lateef, H.M.; Abou Taleb, M.F.; Khalaf, M.M. Immobilization of natural extract from Humulus lupulus L. in electrospun polyvinyl alcohol nanofibrous membrane for colorimetric determination of ferric. J. Mol. Liq. 2024, 399, 124447. [Google Scholar] [CrossRef]
- Wu, Z.; Shen, H.; Hu, J.; Fu, Q.; Yao, C.; Yu, S.; Xiao, W.; Tang, Y. Aptamer-based fluorescence-quenching lateral flow strip for rapid detection of mercury (II) ion in water samples. Anal. Bioanal. Chem. 2017, 409, 5209–5216. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Yang, X. Colorimetric biosensing of mercury (II) ion using unmodified gold nanoparticle probes and thrombin-binding aptamer. Biosens. Bioelectron. 2010, 25, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, M.A.; Gruünhut, M.; Pistonesi, M.F.; Di Nezio, M.S.; Centurión, M.E. Automatic flow-batch system for cold vapor atomic absorption spectroscopy determination of mercury in honey from Argentina using online sample treatment. J. Agric. Food Chem. 2012, 60, 4812–4817. [Google Scholar] [CrossRef] [PubMed]
- Diviš, P.; Reichstädter, M.; Gao, Y.; Leermakers, M.; Křikala, J. Determination of mercury in fish sauces by thermal decomposition gold amalgamation atomic absorption spectroscopy after preconcentration by diffusive gradients in thin films technique. Foods 2020, 9, 1858. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chi, E.-Z.; Zhao, X.-H.; Zhang, Q. A simple and rapid “signal on” fluorescent sensor for detecting mercury (II) based on the molecular beacon aptamer. Foods 2022, 11, 1847. [Google Scholar] [CrossRef]
- Izadi, S.; Tashkhourian, J.; Hafshejani, S.A.H. Ecofriendly ratiometric colorimetric determination of mercury (II) ion in environmental water samples using gallic acid-capped gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 308, 123778. [Google Scholar] [CrossRef]
- Patel, M.R.; Upadhyay, M.D.; Ghosh, S.; Basu, H.; Singhal, R.K.; Park, T.J.; Kailasa, S.K. Synthesis of multicolor silver nanostructures for colorimetric sensing of metal ions (Cr3+, Hg2+ and K+) in industrial water and urine samples with different spectral characteristics. Environ. Res. 2023, 232, 116318. [Google Scholar] [CrossRef] [PubMed]
- Sheikhzadeh, E.; Naji-Tabasi, S.; Verdian, A.; Kolahi-Ahari, S. Equipment-free and visual detection of Pb2+ ion based on curcumin-modified bacterial cellulose nanofiber. J. Iran. Chem. Soc. 2022, 19, 283–290. [Google Scholar] [CrossRef]
- Jang, S.; Son, S.U.; Kang, B.; Kim, J.; Lim, J.; Seo, S.; Kang, T.; Jung, J.; Lee, K.-S.; Kim, H. Electrospun nanofibrous membrane-based colorimetric device for rapid and simple screening of amphetamine-type stimulants in drinks. Anal. Chem. 2022, 94, 3535–3542. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Yeang, B.J.; Park, Y.K.; Choi, H.J.; Kim, J.H.; Kang, Y.S.; Bae, Y.; Kim, J.Y.; Lim, S.J.; Lee, W. Washable colorimetric nanofiber nonwoven for ammonia gas detection. Polymers 2020, 12, 1585. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-H.; Kim, Y.K.; Jeong, S.M.; Choi, C.; Son, K.Y.; Lee, S.-K.; Lim, S.K. Wearable colorimetric sensing fiber based on polyacrylonitrile with PdO@ ZnO hybrids for the application of detecting H2 leakage. Text. Res. J. 2020, 90, 2198–2211. [Google Scholar] [CrossRef]
- Zhang, J.; Hurren, C.; Lu, Z.; Wang, D. Nanofiber-based colorimetric platform for point-of-care detection of E. coli. Chem. Eng. J. 2023, 463, 142357. [Google Scholar] [CrossRef]
- Li, K.; Yu, R.-H.; Shi, C.-M.; Tao, F.-R.; Li, T.-D.; Cui, Y.-Z. Electrospun nanofibrous membrane based on AIE-active compound for detecting picric acid in aqueous solution. Sens. Actuators B Chem. 2018, 262, 637–645. [Google Scholar] [CrossRef]
- Wang, Y.; He, J.; Sun, H.; Zou, L.; Li, Y.V. Preparation of Polyvinyl Alcohol Fluorescent Nanofiber Mats Modified by Hyperbranched Poly (Phenylalanine-Lysine) via Electrospinning for Metal Ions Detection. J. Polym. Environ. 2023, 31, 131–148. [Google Scholar] [CrossRef]
- Dewi, I.R.; Rujiralai, T.; Putson, C.; Cheewasedtham, W. A novel double metal-dithizone functionalized polyurethane electrospun nanofiber and film for colorimetric determination of hexavalent chromium. RSC Adv. 2023, 13, 2852–2859. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).