Defect Engineering in Transition Metal Dichalcogenide-Based Gas Sensors
Abstract
1. Introduction
2. Defects in TMDs
2.1. Defect Types
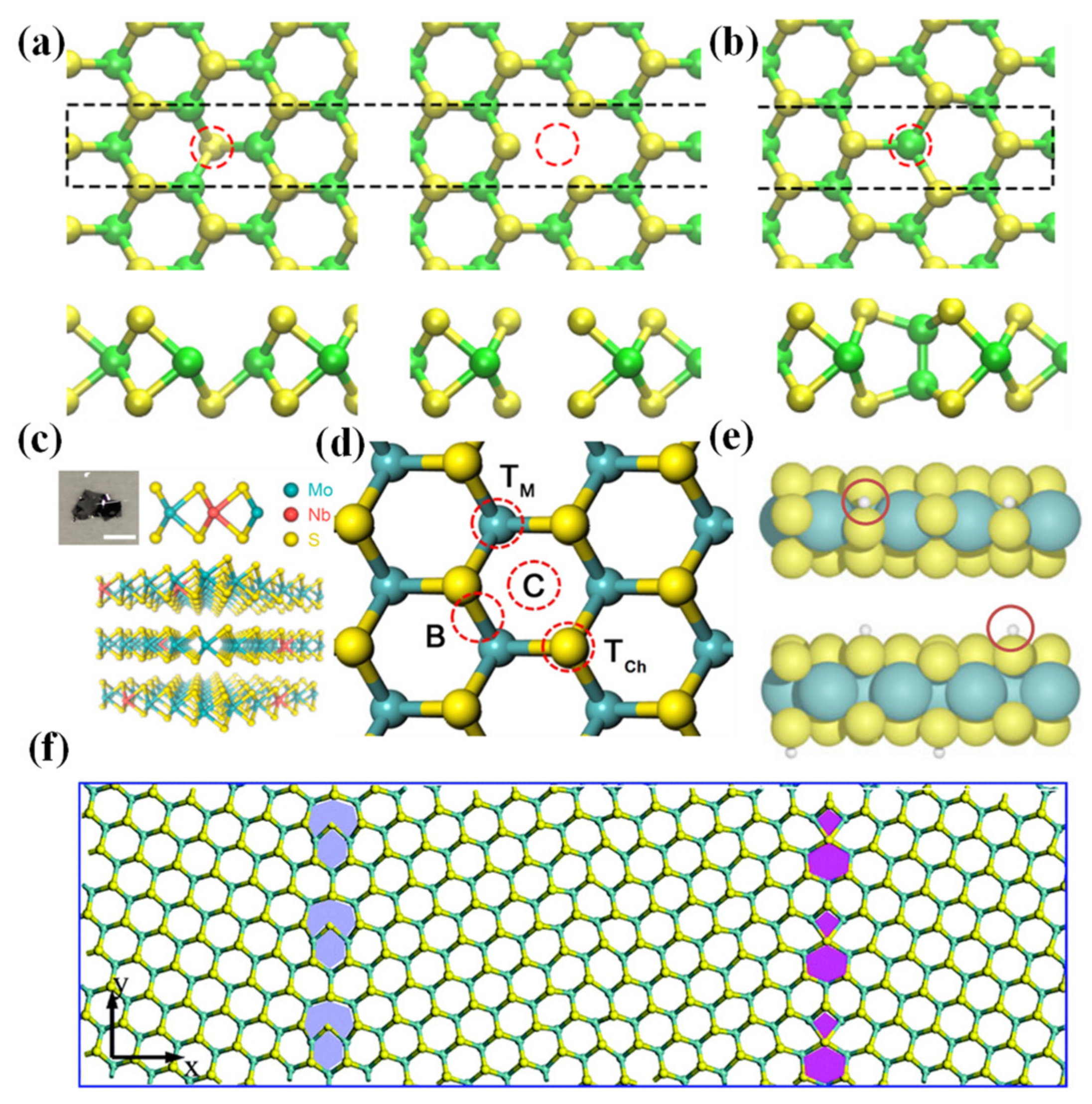
2.1.1. Point Defects
2.1.2. Edge Sites
2.1.3. Grain Boundaries
2.2. Characterization
2.2.1. Microscope
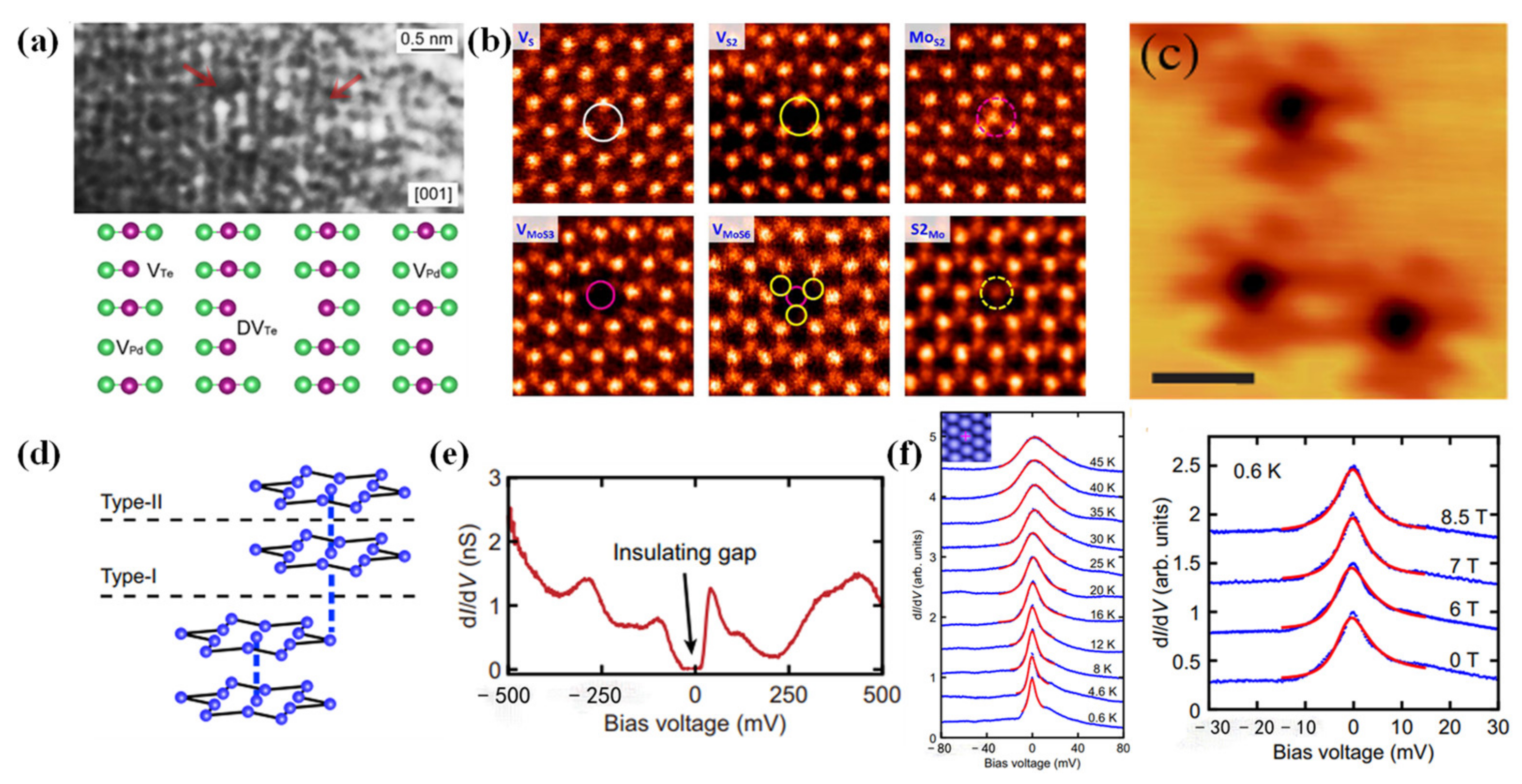
2.2.2. Raman Spectrum
2.2.3. EPR
2.2.4. PL Spectroscopy

2.3. Controllable Introduction of Defects into TMDs
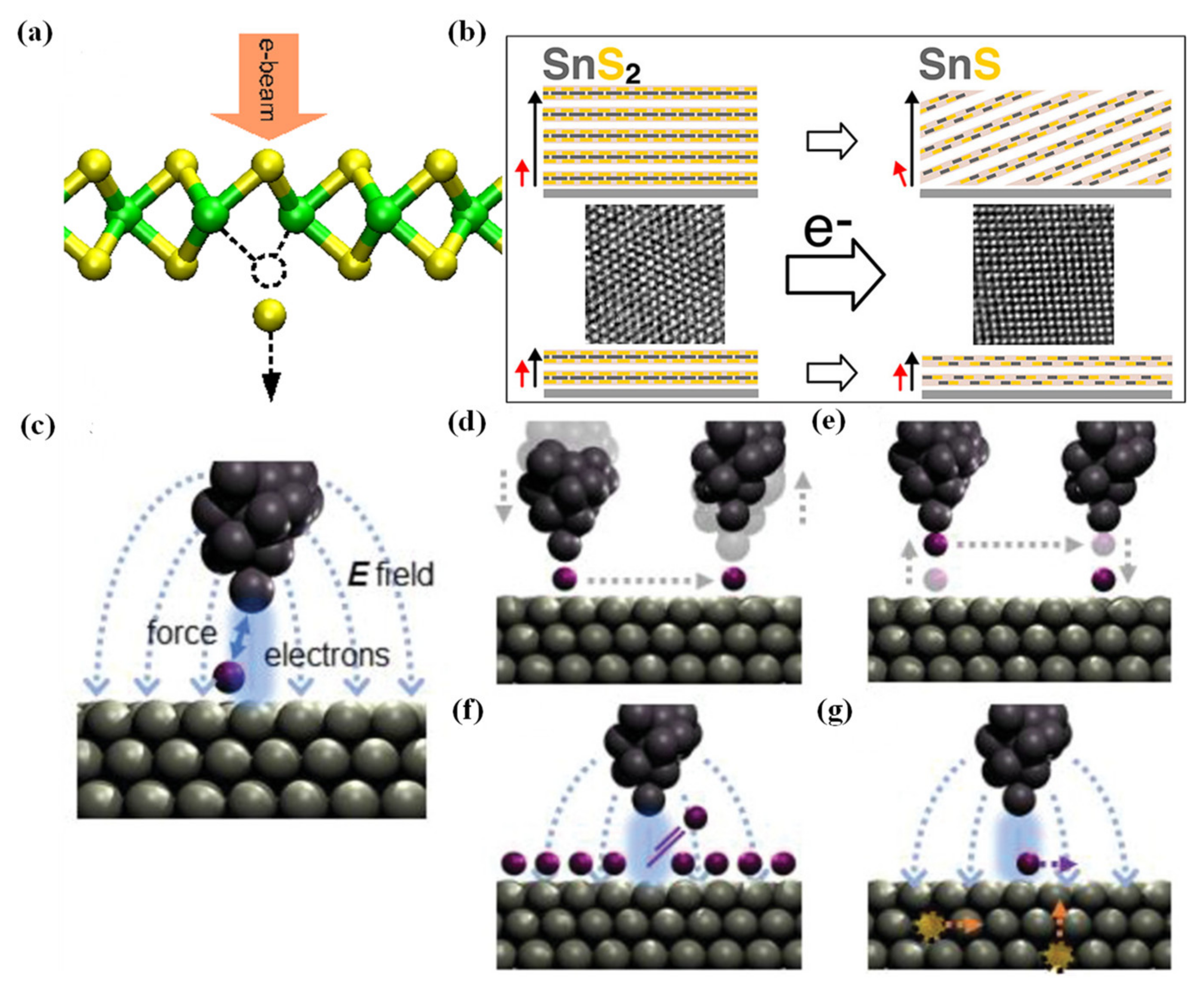
2.3.1. Electron Beam Irradiation
2.3.2. Scanning Tunneling Microscope (STM) Realized Atomic Manipulation
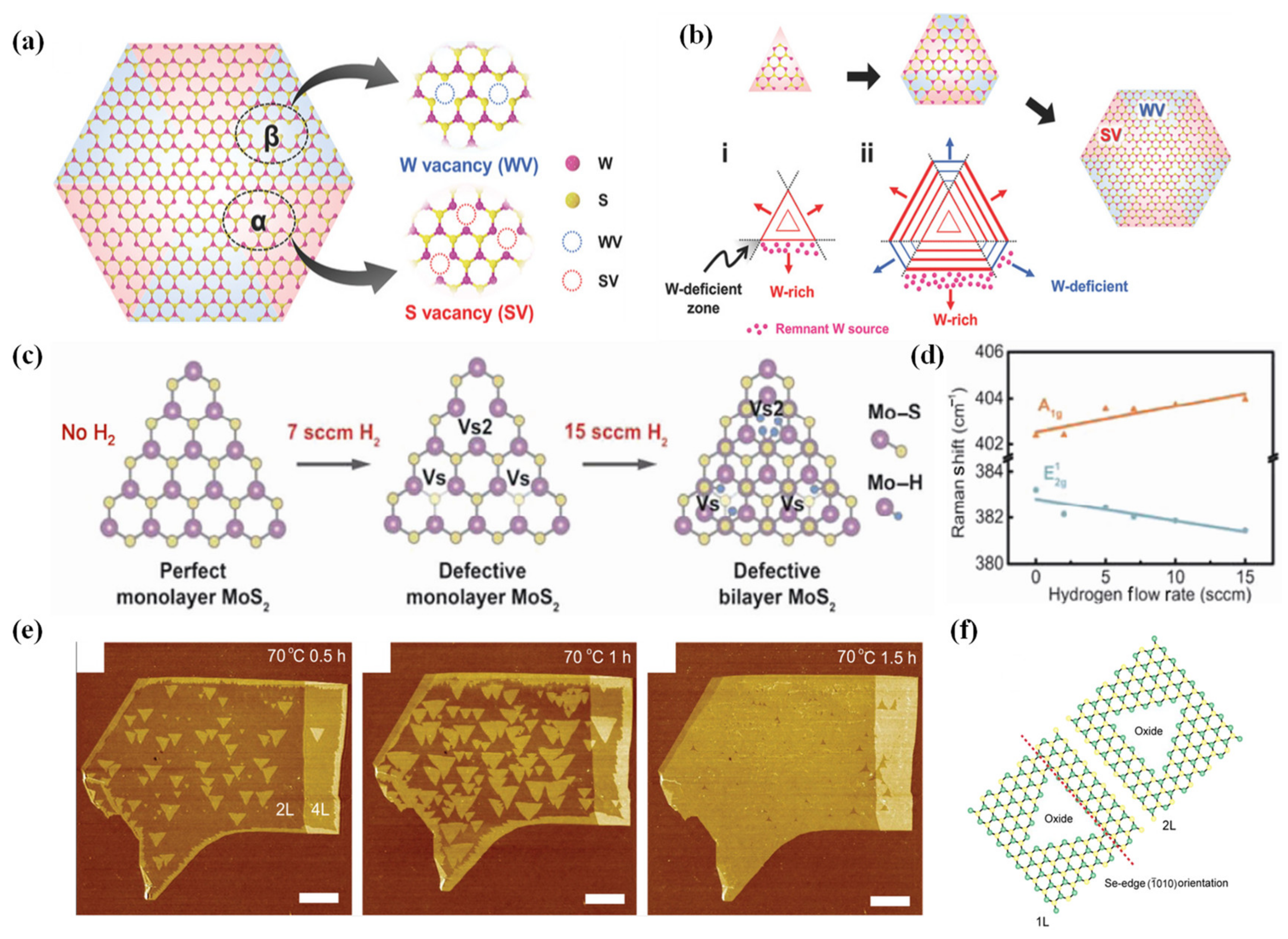
2.3.3. Bottom-Up Synthesis Method to Engineer Defects
2.3.4. Oxidation by Ozone Treatment
3. Gas Sensing Performance and Mechanism
3.1. Introduction of Gas Sensing Mechanism
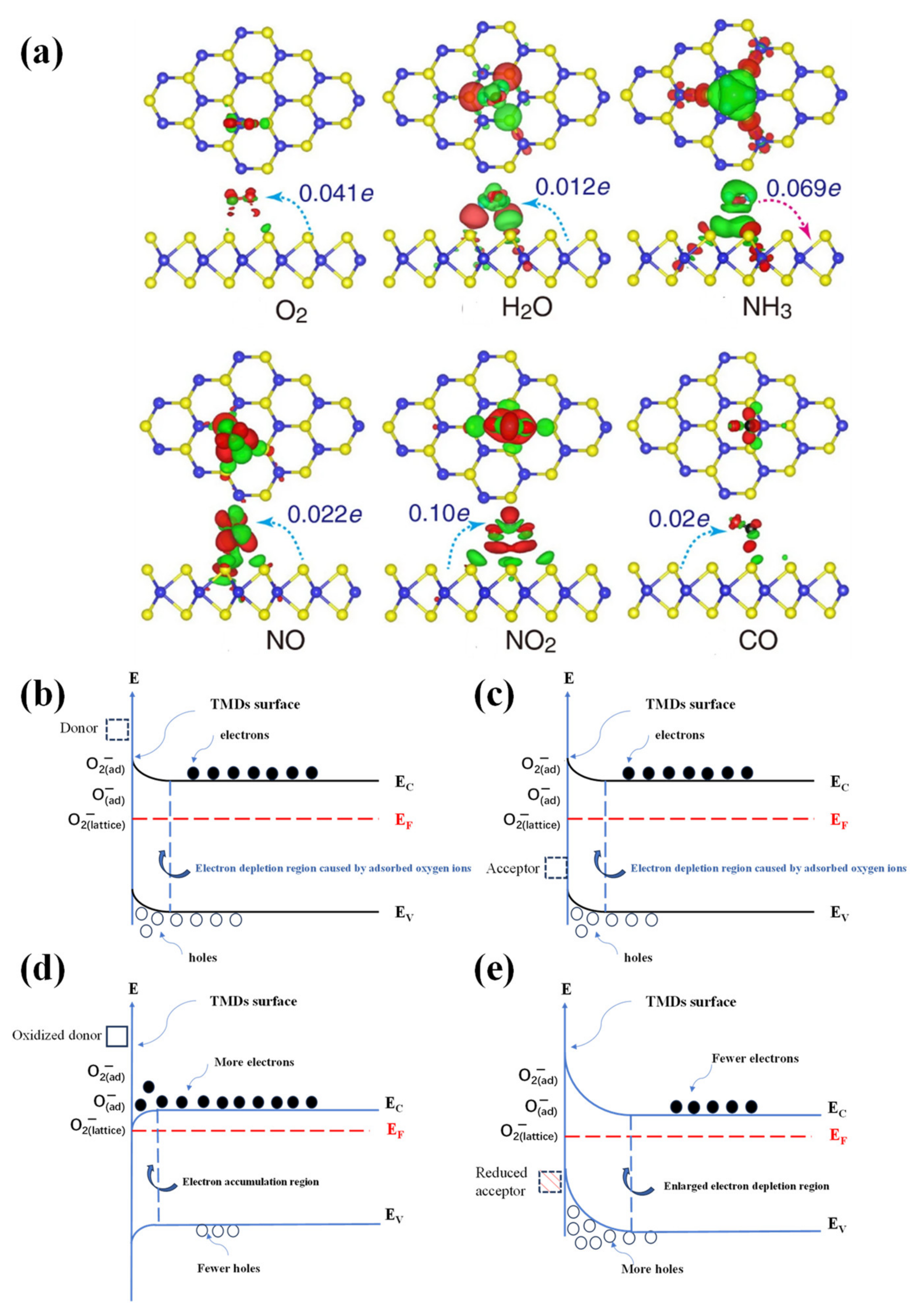
3.2. Connection between Defects and Sensing Mechanism
3.2.1. Vacancies
3.2.2. Substitution
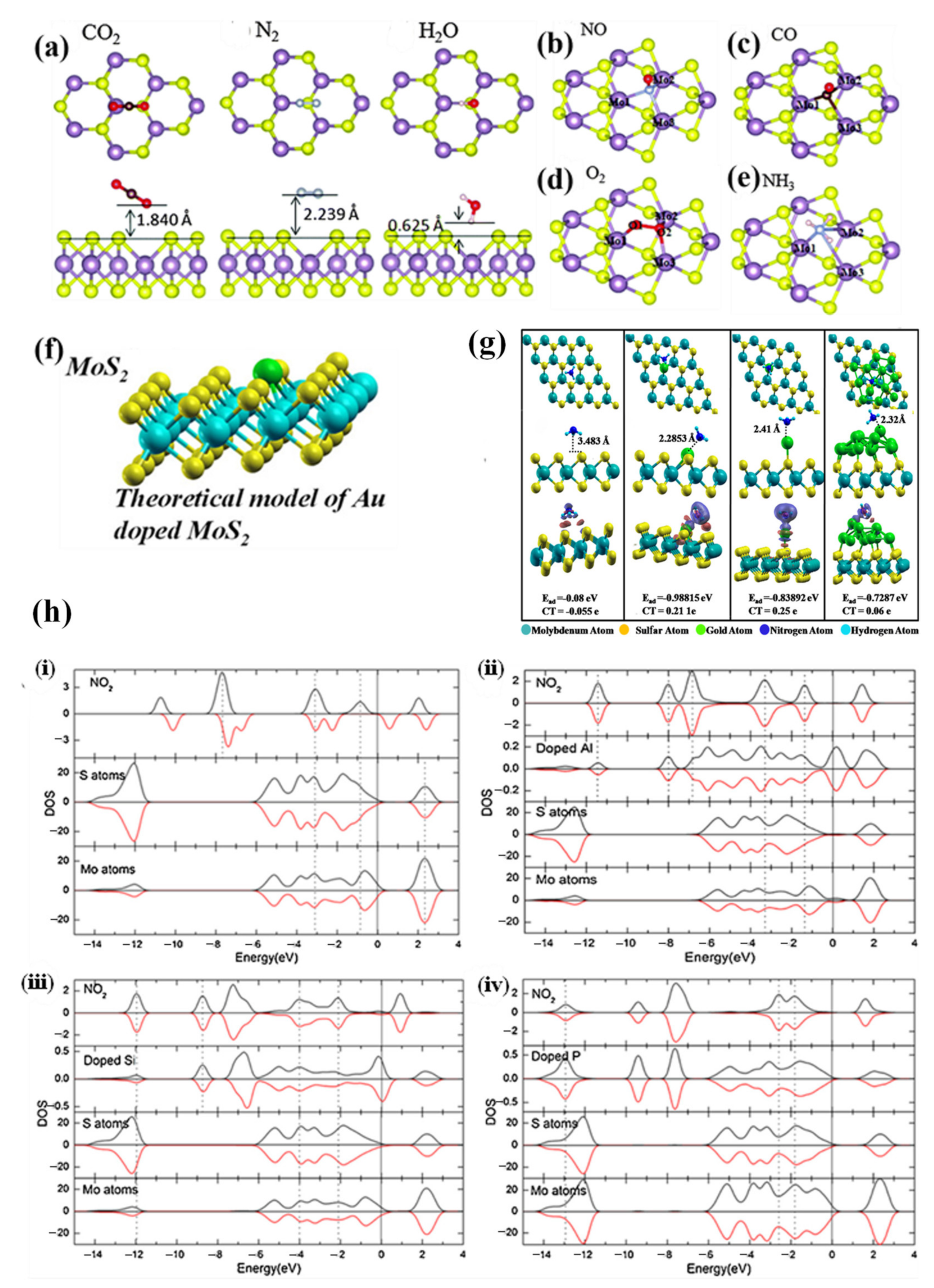
3.2.3. Grain Boundaries
3.2.4. Edge Site

4. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Khan, K.; Zou, J.; Zhang, H.; Li, Y. Recent advances in emerging 2D material-based gas sensors: Potential in disease diagnosis. Adv. Mater. Interfaces 2019, 6, 1901329. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, C.; Wei, S.-H. Gas sensing in 2D materials. Appl. Phys. Rev. 2017, 4, 021304. [Google Scholar] [CrossRef]
- Liu, Z.; Qiao, Z.; Li, C.-Y.; Sun, Y. Recent progress in multifunctional gas sensors based on 2D materials. Chemosensors 2023, 11, 483. [Google Scholar] [CrossRef]
- Sun, J.; Giorgi, G.; Palummo, M.; Sutter, P.; Passacantando, M.; Camilli, L. A scalable method for thickness and lateral engineering of 2D materials. ACS Nano 2020, 14, 4861–4870. [Google Scholar] [CrossRef]
- Wu, F.; Tian, H.; Shen, Y.; Hou, Z.; Ren, J.; Gou, G.; Sun, Y.; Yang, Y.; Ren, T.-L. Vertical MoS2 transistors with sub-1-nm gate lengths. Nature 2022, 603, 259–264. [Google Scholar] [CrossRef]
- Liu, L.; Kong, L.; Li, Q.; He, C.; Ren, L.; Tao, Q.; Yang, X.; Lin, J.; Zhao, B.; Li, Z. Transferred van der Waals metal electrodes for sub-1-nm MoS2 vertical transistors. Nat. Electron. 2021, 4, 342–347. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, H.; Cai, Y.; Zhao, J.; Gao, Z.; Song, Y.-Y. The Challenges and Opportunities for TiO2 Nanostructures in Gas Sensing. ACS Sens. 2024, 9, 1644–1655. [Google Scholar] [CrossRef]
- Aftab, S.; Hegazy, H.H. Emerging Trends in 2D TMDs Photodetectors and Piezo-Phototronic Devices. Small 2023, 19, 2205778. [Google Scholar] [CrossRef]
- Lee, E.; Yoon, Y.S.; Kim, D.-J. Two-dimensional transition metal dichalcogenides and metal oxide hybrids for gas sensing. ACS Sens. 2018, 3, 2045–2060. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, D.; Li, X.; Lin, S.; Zhao, S.; Rumyantseva, M.N.; Gaskov, A.M. Reduced graphene oxide hybridized with WS2 nanoflakes based heterojunctions for selective ammonia sensors at room temperature. Sens. Actuators B—Chem. 2019, 282, 290–299. [Google Scholar] [CrossRef]
- Agrawal, A.V.; Kumar, R.; Venkatesan, S.; Zakhidov, A.; Yang, G.; Bao, J.; Kumar, M.; Kumar, M. Photoactivated mixed in-plane and edge-enriched p-type MoS2 flake-based NO2 sensor working at room temperature. ACS Sens. 2018, 3, 998–1004. [Google Scholar] [CrossRef]
- Zhang, S.; Nguyen, T.H.; Zhang, W.; Park, Y.; Yang, W. Correlation between lateral size and gas sensing performance of MoSe2 nanosheets. Appl. Phys. Lett. 2017, 111, 161603. [Google Scholar] [CrossRef]
- Seo, S.-Y.; Yang, D.-H.; Moon, G.; Okello, O.F.; Park, M.Y.; Lee, S.-H.; Choi, S.-Y.; Jo, M.-H. Identification of point defects in atomically thin transition-metal dichalcogenide semiconductors as active dopants. Nano Lett. 2021, 21, 3341–3354. [Google Scholar] [CrossRef]
- Edelberg, D.; Rhodes, D.; Kerelsky, A.; Kim, B.; Wang, J.; Zangiabadi, A.; Kim, C.; Abhinandan, A.; Ardelean, J.; Scully, M. Approaching the intrinsic limit in transition metal diselenides via point defect control. Nano Lett. 2019, 19, 4371–4379. [Google Scholar] [CrossRef]
- Komsa, H.-P.; Krasheninnikov, A.V. Native defects in bulk and monolayer MoS2 from first principles. Phys. Rev. B 2015, 91, 125304. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, Y.; Guo, M.; Niu, C.; Lu, J.; Huang, B. Electronic and magnetic properties of perfect, vacancy-doped, and nonmetal adsorbed MoSe2, MoTe2 and WS2 monolayers. Phys. Chem. Chem. Phys. 2011, 13, 15546–15553. [Google Scholar] [CrossRef]
- Onofrio, N.; Guzman, D.; Strachan, A. Novel doping alternatives for single-layer transition metal dichalcogenides. J. Appl. Phys. 2017, 122, 185102. [Google Scholar] [CrossRef]
- Suh, J.; Park, T.-E.; Lin, D.-Y.; Fu, D.; Park, J.; Jung, H.J.; Chen, Y.; Ko, C.; Jang, C.; Sun, Y. Doping against the native propensity of MoS2: Degenerate hole doping by cation substitution. Nano Lett. 2014, 14, 6976–6982. [Google Scholar] [CrossRef]
- Chen, J.-W.; Lo, S.-T.; Ho, S.-C.; Wong, S.-S.; Vu, T.-H.-Y.; Zhang, X.-Q.; Liu, Y.-D.; Chiou, Y.-Y.; Chen, Y.-X.; Yang, J.-C. A gate-free monolayer WSe2 pn diode. Nat. Commun. 2018, 9, 3143. [Google Scholar] [CrossRef]
- Mouri, S.; Miyauchi, Y.; Matsuda, K. Tunable photoluminescence of monolayer MoS2 via chemical doping. Nano Lett. 2013, 13, 5944–5948. [Google Scholar] [CrossRef]
- Li, M.; Yao, J.; Wu, X.; Zhang, S.; Xing, B.; Niu, X.; Yan, X.; Yu, Y.; Liu, Y.; Wang, Y. P-type doping in large-area monolayer MoS2 by chemical vapor deposition. ACS Appl. Mater. Interfaces 2020, 12, 6276–6282. [Google Scholar] [CrossRef]
- Yang, L.; Majumdar, K.; Liu, H.; Du, Y.; Wu, H.; Hatzistergos, M.; Hung, P.; Tieckelmann, R.; Tsai, W.; Hobbs, C. Chloride molecular doping technique on 2D materials: WS2 and MoS2. Nano Lett. 2014, 14, 6275–6280. [Google Scholar] [CrossRef]
- Seo, S.-Y.; Park, J.; Park, J.; Song, K.; Cha, S.; Sim, S.; Choi, S.-Y.; Yeom, H.W.; Choi, H.; Jo, M.-H. Writing monolithic integrated circuits on a two-dimensional semiconductor with a scanning light probe. Nat. Electron. 2018, 1, 512–517. [Google Scholar] [CrossRef]
- Ataca, C.; Ciraci, S. Functionalization of single-layer MoS2 honeycomb structures. J. Phys. Chem. C 2011, 115, 13303–13311. [Google Scholar] [CrossRef]
- Lin, Z.; Carvalho, B.R.; Kahn, E.; Lv, R.; Rao, R.; Terrones, H.; Pimenta, M.A.; Terrones, M. Defect engineering of two-dimensional transition metal dichalcogenides. 2D Mater. 2016, 3, 022002. [Google Scholar] [CrossRef]
- Dolui, K.; Rungger, I.; Pemmaraju, C.D.; Sanvito, S. Possible doping strategies for MoS2 monolayers: An ab initio study. Phys. Rev. B 2013, 88, 075420. [Google Scholar] [CrossRef]
- Zhou, W.; Zou, X.; Najmaei, S.; Liu, Z.; Shi, Y.; Kong, J.; Lou, J.; Ajayan, P.M.; Yakobson, B.I.; Idrobo, J.-C. Intrinsic structural defects in monolayer molybdenum disulfide. Nano Lett. 2013, 13, 2615–2622. [Google Scholar] [CrossRef]
- Wang, H.; Tsai, C.; Kong, D.; Chan, K.; Abild-Pedersen, F.; Nørskov, J.K.; Cui, Y. Transition-metal doped edge sites in vertically aligned MoS2 catalysts for enhanced hydrogen evolution. Nano Res. 2015, 8, 566–575. [Google Scholar] [CrossRef]
- Zou, X.; Liu, Y.; Yakobson, B.I. Predicting dislocations and grain boundaries in two-dimensional metal-disulfides from the first principles. Nano Lett. 2013, 13, 253–258. [Google Scholar] [CrossRef]
- Lehtinen, O.; Komsa, H.-P.; Pulkin, A.; Whitwick, M.B.; Chen, M.-W.; Lehnert, T.; Mohn, M.J.; Yazyev, O.V.; Kis, A.; Kaiser, U. Atomic scale microstructure and properties of Se-deficient two-dimensional MoSe2. ACS Nano 2015, 9, 3274–3283. [Google Scholar] [CrossRef]
- Lin, J.; Pantelides, S.T.; Zhou, W. Vacancy-induced formation and growth of inversion domains in transition-metal dichalcogenide monolayer. ACS Nano 2015, 9, 5189–5197. [Google Scholar] [CrossRef]
- Azizi, A.; Zou, X.; Ercius, P.; Zhang, Z.; Elías, A.L.; Perea-López, N.; Stone, G.; Terrones, M.; Yakobson, B.I.; Alem, N. Dislocation motion and grain boundary migration in two-dimensional tungsten disulphide. Nat. Commun. 2014, 5, 4867. [Google Scholar] [CrossRef]
- Van Der Zande, A.M.; Huang, P.Y.; Chenet, D.A.; Berkelbach, T.C.; You, Y.; Lee, G.-H.; Heinz, T.F.; Reichman, D.R.; Muller, D.A.; Hone, J.C. Grains and grain boundaries in highly crystalline monolayer molybdenum disulphide. Nat. Mater. 2013, 12, 554–561. [Google Scholar] [CrossRef]
- Liu, X.; Yu, Z.G.; Zhang, G.; Zhang, Y.-W. Remarkably high thermal-driven MoS2 grain boundary migration mobility and its implications on defect healing. Nanoscale 2020, 12, 17746–17753. [Google Scholar] [CrossRef]
- Zuo, Y.; Antonatos, N.; Děkanovský, L.; Luxa, J.; Elliott, J.D.; Gianolio, D.; Šturala, J.; Guzzetta, F.; Mourdikoudis, S.; Regner, J. Defect engineering in two-dimensional layered PdTe2 for enhanced hydrogen evolution reaction. ACS Catal. 2023, 13, 2601–2609. [Google Scholar] [CrossRef]
- Meng, Y.; Ling, C.; Xin, R.; Wang, P.; Song, Y.; Bu, H.; Gao, S.; Wang, X.; Song, F.; Wang, J. Repairing atomic vacancies in single-layer MoSe2 field-effect transistor and its defect dynamics. NPJ Quantum Mater. 2017, 2, 16. [Google Scholar] [CrossRef]
- Patra, T.K.; Zhang, F.; Schulman, D.S.; Chan, H.; Cherukara, M.J.; Terrones, M.; Das, S.; Narayanan, B.; Sankaranarayanan, S.K. Defect dynamics in 2-D MoS2 probed by using machine learning, atomistic simulations, and high-resolution microscopy. ACS Nano 2018, 12, 8006–8016. [Google Scholar] [CrossRef]
- Chow, P.K.; Jacobs-Gedrim, R.B.; Gao, J.; Lu, T.-M.; Yu, B.; Terrones, H.; Koratkar, N. Defect-induced photoluminescence in monolayer semiconducting transition metal dichalcogenides. ACS Nano 2015, 9, 1520–1527. [Google Scholar] [CrossRef]
- Liu, J. Scanning transmission electron microscopy and its application to the study of nanoparticles and nanoparticle systems. Microscopy 2005, 54, 251–278. [Google Scholar] [CrossRef]
- Zhang, H.; Poh, E.T.; Lim, S.X.; Zhang, Y.; Qin, H.; Xie, H.; He, C.; Sow, C.H. In situ strain-induced phase transition and defect engineering in CVD-synthesized atomically thin MoS2. 2D Mater. 2023, 10, 035018. [Google Scholar] [CrossRef]
- Gao, J.; Kim, Y.D.; Liang, L.; Idrobo, J.C.; Chow, P.; Tan, J.; Li, B.; Li, L.; Sumpter, B.G.; Lu, T.-M. Transition-metal substitution doping in synthetic atomically thin semiconductors. Adv. Mater. 2016, 28, 0935–9648. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lin, Y.-H.; Chang, S.-H.; Tai, C.-D.; Liu, S.-J.; Chu, Y.; Wang, C.-J.; Hsu, M.-Y.; Chang, H.; Chang, G.-J. Local sustained delivery of acetylsalicylic acid via hybrid stent with biodegradable nanofibers reduces adhesion of blood cells and promotes reendothelialization of the denuded artery. Int. J. Nanomed. 2014, 9, 311–326. [Google Scholar]
- Santosh, K.; Longo, R.C.; Addou, R.; Wallace, R.M.; Cho, K. Impact of intrinsic atomic defects on the electronic structure of MoS2 monolayers. Nanotechnology 2014, 25, 375703. [Google Scholar]
- Lauritsen, J.V.; Kibsgaard, J.; Helveg, S.; Topsøe, H.; Clausen, B.S.; Lægsgaard, E.; Besenbacher, F. Size-dependent structure of MoS2 nanocrystals. Nat. Nanotech. 2007, 2, 53–58. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, C.-G.; Li, M.-Y.; Huang, D.; Li, L.-J.; Ji, W.; Wu, S. Defect structure of localized excitons in a WSe2 monolayer. Phys. Rev. Lett. 2017, 119, 046101. [Google Scholar] [CrossRef]
- Shen, S.; Wen, C.; Kong, P.; Gao, J.; Si, J.; Luo, X.; Lu, W.; Sun, Y.; Chen, G.; Yan, S. Inducing and tuning Kondo screening in a narrow-electronic-band system. Nat. Commun. 2022, 13, 2156. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, L.; Zhao, W.; Liang, Z.; Wu, X.; Nan, H.; Wu, Z.; Huang, S.; Sun, L.; Wang, J. Raman mapping investigation of chemical vapor deposition-fabricated twisted bilayer graphene with irregular grains. Phys. Chem. Chem. Phys. 2014, 16, 21682–21687. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Wu, Z.T.; Zhao, W.W.; Chen, W.Y.; Jiang, J.; Nan, H.Y.; Guo, X.T.; Liang, Z.; Chen, Y.M.; Chen, Y.F.; Ni, Z.H. The influence of chemical solvents on the properties of CVD graphene. J. Raman Spectrosc. 2015, 46, 21–24. [Google Scholar] [CrossRef]
- Parkin, W.M.; Balan, A.; Liang, L.; Das, P.M.; Lamparski, M.; Naylor, C.H.; Rodríguez-Manzo, J.A.; Johnson, A.C.; Meunier, V.; Drndic, M. Raman shifts in electron-irradiated monolayer MoS2. ACS Nano 2016, 10, 4134–4142. [Google Scholar] [CrossRef] [PubMed]
- Mignuzzi, S.; Pollard, A.J.; Bonini, N.; Brennan, B.; Gilmore, I.S.; Pimenta, M.A.; Richards, D.; Roy, D. Effect of disorder on Raman scattering of single-layer MoS2. Phys. Rev. B 2015, 91, 195411. [Google Scholar] [CrossRef]
- Nipane, A.; Karmakar, D.; Kaushik, N.; Karande, S.; Lodha, S. Few-layer MoS2 p-type devices enabled by selective doping using low energy phosphorus implantation. ACS Nano 2016, 10, 2128–2137. [Google Scholar] [CrossRef]
- He, Z.; Zhao, R.; Chen, X.; Chen, H.; Zhu, Y.; Su, H.; Huang, S.; Xue, J.; Dai, J.; Cheng, S. Defect engineering in single-layer MoS2 using heavy ion irradiation. ACS Appl. Mater. Interfaces 2018, 10, 42524–42533. [Google Scholar] [CrossRef]
- Windom, B.C.; Sawyer, W.; Hahn, D.W. A Raman spectroscopic study of MoS2 and MoO3: Applications to tribological systems. Tribol. Lett. 2011, 42, 301–310. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Si, H.; Zhang, Q.; Wu, J.; Gao, L.; Wei, X.; Sun, Y.; Liao, Q.; Zhang, Z. Single-atom vacancy defect to trigger high-efficiency hydrogen evolution of MoS2. J. Am. Chem. Soc. 2020, 142, 4298–4308. [Google Scholar] [CrossRef]
- Li, L.; Qin, Z.; Ries, L.; Hong, S.; Michel, T.; Yang, J.; Salameh, C.; Bechelany, M.; Miele, P.; Kaplan, D. Role of sulfur vacancies and undercoordinated Mo regions in MoS2 nanosheets toward the evolution of hydrogen. ACS Nano 2019, 13, 6824–6834. [Google Scholar] [CrossRef]
- Ding, X.; Liu, T.; Ahmed, S.; Bao, N.; Ding, J.; Yi, J. Enhanced ferromagnetism in WS2 via defect engineering. J. Alloys Compd. 2019, 772, 740–744. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Zhang, Y.; Sun, Y.; Ma, K.; Xie, Y.; Zheng, W.; Tian, Z.; Kang, Z.; Zhang, Y. Vacancy defects in 2D transition metal dichalcogenide electrocatalysts: From aggregated to atomic configuration. Adv. Mater. 2023, 35, 2206576. [Google Scholar] [CrossRef]
- Li, W.; Qiao, Z.; Liu, Z. Behind the gas sensors: Revealing sensing mechanism with advanced magnetic resonance technology. J. Mater. Chem. A 2023, 11, 19281–19297. [Google Scholar] [CrossRef]
- Yang, H.; Du, Z.; Yang, Y.; Li, X.; Wu, Q.; Tang, J.; Wang, X.; Zeng, D. Ag intercalated SnS2 with S vacancy and expanded interlayer for enhancing NO2 sensing. Sens. Actuators B—Chem. 2023, 393, 134140. [Google Scholar] [CrossRef]
- Wu, R.; Hao, J.; Wang, T.; Zheng, S.; Wang, Y. Carbon-doping-induced energy-band modification and vacancies in SnS2 nanosheets for room-temperature ppb-level NO2 detection. Inorg. Chem. Front. 2021, 8, 5006–5015. [Google Scholar] [CrossRef]
- Tang, H.; Gao, C.; Yang, H.; Sacco, L.; Sokolovskij, R.; Zheng, H.; Ye, H.; Vollebregt, S.; Yu, H.; Fan, X. Room temperature ppt-level NO2 gas sensor based on SnOx/SnS nanostructures with rich oxygen vacancies. 2D Mater. 2021, 8, 045006. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Huang, B.; Liu, H.; Li, X. Layered SnSe2 microflakes and SnSe2/SnO2 heterojunctions for low-temperature chemiresistive-type gas sensing. J. Mater. Chem. C 2020, 8, 15804–15815. [Google Scholar] [CrossRef]
- Guo, X.; Shi, Y.; Ding, Y.; He, Y.; Du, B.; Liang, C.; Tan, Y.; Liu, P.; Miao, X.; He, Y. Indium-doping-induced selenium vacancy engineering of layered tin diselenide for improving room-temperature sulfur dioxide gas sensing. J. Mater. Chem. A 2022, 10, 22629–22637. [Google Scholar] [CrossRef]
- Kumar, R.R.; Habib, M.R.; Khan, A.; Chen, P.-C.; Murugesan, T.; Gupta, S.; Anbalagan, A.K.; Tai, N.-H.; Lee, C.-H.; Lin, H.-N. Sulfur monovacancies in liquid-exfoliated MoS2 nanosheets for NO2 gas sensing. ACS Appl. Nano Mater. 2021, 4, 9459–9470. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Yao, B.; Wang, S.; Li, H.; Chen, T.; Xiao, X.; Wang, Y. Ultrasensitive room-temperature flexible ammonia gas sensor based on Au-functionalized polypyrrole wrapped enriched edge sulfur vacancies MoS2 nanosheets. Sens. Actuators B—Chem. 2023, 395, 134449. [Google Scholar] [CrossRef]
- Zheng, S.; Yin, D.; Zhang, S.; Wang, Y.; Li, J.; Wang, Z.; Yuan, Y.; Tsai, H.-S.; Hao, J. Tailoring selenium vacancies in MoSe2 through oxygen passivation for room-temperature NO2 sensing enhancement. J. Mater. Chem. A 2023, 11, 18755–18764. [Google Scholar] [CrossRef]
- Ross, J.S.; Wu, S.; Yu, H.; Ghimire, N.J.; Jones, A.M.; Aivazian, G.; Yan, J.; Mandrus, D.G.; Xiao, D.; Yao, W. Electrical control of neutral and charged excitons in a monolayer semiconductor. Nat. Commun. 2013, 4, 1474. [Google Scholar] [CrossRef]
- Wu, Z.; Luo, Z.; Shen, Y.; Zhao, W.; Wang, W.; Nan, H.; Guo, X.; Sun, L.; Wang, X.; You, Y. Defects as a factor limiting carrier mobility in WSe2: A spectroscopic investigation. Nano Res. 2016, 9, 3622–3631. [Google Scholar] [CrossRef]
- Mak, K.F.; He, K.; Lee, C.; Lee, G.H.; Hone, J.; Heinz, T.F.; Shan, J. Tightly bound trions in monolayer MoS2. Nat. Mater. 2013, 12, 207–211. [Google Scholar] [CrossRef]
- Sun, L.; Banhart, F.; Warner, J. Two-dimensional materials under electron irradiation. MRS Bull. 2015, 40, 29–37. [Google Scholar] [CrossRef]
- Xu, T.; Yin, K.; Sun, L. In-situ study of electron irradiation on two-dimensional layered materials. Chin. Sci. Bull. 2017, 62, 2919–2930. [Google Scholar] [CrossRef]
- Rummeli, M.H.; Ta, H.Q.; Mendes, R.G.; Gonzalez-Martinez, I.G.; Zhao, L.; Gao, J.; Fu, L.; Gemming, T.; Bachmatiuk, A.; Liu, Z. New frontiers in electron beam–driven chemistry in and around graphene. Adv. Mater. 2019, 31, 1800715. [Google Scholar] [CrossRef] [PubMed]
- Komsa, H.-P.; Kotakoski, J.; Kurasch, S.; Lehtinen, O.; Kaiser, U.; Krasheninnikov, A.V. Two-dimensional transition metal dichalcogenides under electron irradiation: Defect production and doping. Phys. Rev. Lett. 2012, 109, 035503. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.G.; Pang, J.; Bachmatiuk, A.; Ta, H.Q.; Zhao, L.; Gemming, T.; Fu, L.; Liu, Z.; Rümmeli, M.H. Electron-driven in situ transmission electron microscopy of 2D transition metal dichalcogenides and their 2D heterostructures. ACS Nano 2019, 13, 978–995. [Google Scholar] [CrossRef] [PubMed]
- Sutter, E.; Huang, Y.; Komsa, H.-P.; Ghorbani-Asl, M.; Krasheninnikov, A.; Sutter, P. Electron-beam induced transformations of layered tin dichalcogenides. Nano Lett. 2016, 16, 4410–4416. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Dumcenco, D.O.; Huang, Y.-S.; Suenaga, K. Atomic mechanism of the semiconducting-to-metallic phase transition in single-layered MoS2. Nat. Nanotech. 2014, 9, 391–396. [Google Scholar] [CrossRef]
- Bartels, L.; Meyer, G.; Rieder, K.-H.; Velic, D.; Knoesel, E.; Hotzel, A.; Wolf, M.; Ertl, G. Dynamics of electron-induced manipulation of individual CO molecules on Cu (111). Phys. Rev. Lett. 1998, 80, 2004. [Google Scholar] [CrossRef]
- Stroscio, J.A.; Celotta, R.J. Controlling the dynamics of a single atom in lateral atom manipulation. Science 2004, 306, 242–247. [Google Scholar] [CrossRef]
- Ko, W.; Ma, C.; Nguyen, G.D.; Kolmer, M.; Li, A.P. Atomic-scale manipulation and in situ characterization with scanning tunneling microscopy. Adv. Funct. Mater. 2019, 29, 1903770. [Google Scholar] [CrossRef]
- Oyedele, A.D.; Yang, S.; Liang, L.; Puretzky, A.A.; Wang, K.; Zhang, J.; Yu, P.; Pudasaini, P.R.; Ghosh, A.W.; Liu, Z. PdSe2: Pentagonal two-dimensional layers with high air stability for electronics. J. Am. Chem. Soc. 2017, 139, 14090–14097. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, K.A.; Lee, J.-H.; Kastl, C.; Haber, J.B.; Zhang, T.; Kozhakhmetov, A.; Robinson, J.A.; Terrones, M.; Repp, J.; Neaton, J.B. Spin-dependent vibronic response of a carbon radical ion in two-dimensional WS2. Nat. Commun. 2021, 12, 7287. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Liu, H.; Park, K.; Li, Z.; Losovyj, Y.; Starr, M.; Werbianskyj, M.; Fertig, H.; Zhang, S. p-Type transition-metal doping of large-area MoS2 thin films grown by chemical vapor deposition. Nanoscale 2017, 9, 3576–3584. [Google Scholar] [CrossRef]
- Han, H.-V.; Lu, A.-Y.; Lu, L.-S.; Huang, J.-K.; Li, H.; Hsu, C.-L.; Lin, Y.-C.; Chiu, M.-H.; Suenaga, K.; Chu, C.-W. Photoluminescence enhancement and structure repairing of monolayer MoSe2 by hydrohalic acid treatment. ACS Nano 2016, 10, 1454–1461. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Jin, Y.; Yun, S.J.; Zhao, J.; Baik, J.; Keum, D.H.; Lee, H.S.; Lee, Y.H. Heterogeneous defect domains in single-crystalline hexagonal WS2. Adv. Mater. 2017, 29, 1605043. [Google Scholar] [CrossRef]
- Wu, K.; Li, Z.; Tang, J.; Lv, X.; Wang, H.; Luo, R.; Liu, P.; Qian, L.; Zhang, S.; Yuan, S. Controllable defects implantation in MoS2 grown by chemical vapor deposition for photoluminescence enhancement. Nano Res. 2018, 11, 4123–4132. [Google Scholar] [CrossRef]
- Yamamoto, M.; Dutta, S.; Aikawa, S.; Nakaharai, S.; Wakabayashi, K.; Fuhrer, M.S.; Ueno, K.; Tsukagoshi, K. Self-limiting layer-by-layer oxidation of atomically thin WSe2. Nano Lett. 2015, 15, 2067–2073. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Yang, H.I.; Choi, W. Oxidation of WS2 and WSe2 monolayers by ultraviolet-ozone treatment. J. Phys. D Appl. Phys. 2019, 52, 505105. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, Q.; Gou, J.; Song, T.; Arramel; Chen, H.; Yang, M.; Lim, S.X.; Wang, Q.; Zhu, R. Performance improvement by ozone treatment of 2D PdSe2. ACS Nano 2020, 14, 5668–5677. [Google Scholar] [CrossRef]
- Kim, Y.; Sohn, I.; Shin, D.; Yoo, J.; Lee, S.; Yoon, H.; Park, J.; Chung, S.-m.; Kim, H. Recent Advances in Functionalization and Hybridization of Two-Dimensional Transition Metal Dichalcogenide for Gas Sensor. Adv. Eng. Mater. 2024, 26, 2301063. [Google Scholar] [CrossRef]
- Ou, J.Z.; Ge, W.; Carey, B.; Daeneke, T.; Rotbart, A.; Shan, W.; Wang, Y.; Fu, Z.; Chrimes, A.F.; Wlodarski, W. Physisorption-based charge transfer in two-dimensional SnS2 for selective and reversible NO2 gas sensing. ACS Nano 2015, 9, 10313–10323. [Google Scholar] [CrossRef] [PubMed]
- Leenaerts, O.; Partoens, B.; Peeters, F. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 2008, 77, 125416. [Google Scholar] [CrossRef]
- Zeng, Y.; Lin, S.; Gu, D.; Li, X. Two-dimensional nanomaterials for gas sensing applications: The role of theoretical calculations. Nanomaterials 2018, 8, 851. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Shao, Z.; Chang, S.; Li, J. Adsorption of gas molecules on monolayer MoS2 and effect of applied electric field. Nanoscale Res. Lett. 2013, 8, 425. [Google Scholar] [CrossRef] [PubMed]
- Annanouch, F.E.; Alagh, A.; Umek, P.; Casanova-Chafer, J.; Bittencourt, C.; Llobet, E. Controlled growth of 3D assemblies of edge enriched multilayer MoS2 nanosheets for dually selective NH3 and NO2 gas sensors. J. Mater. Chem. C 2022, 10, 11027–11039. [Google Scholar] [CrossRef]
- Kim, J.-H.; Sakaguchi, I.; Hishita, S.; Ohsawa, T.; Suzuki, T.T.; Saito, N. Self-heated CO gas sensor based on Au-decorated Sb-implanted WS2 nanosheets. Sens. Actuators B—Chem. 2023, 382, 133501. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.; Song, J.G.; Ko, K.Y.; Woo, W.J.; Lee, S.W.; Park, M.; Lee, H.; Lee, Z.; Choi, H. 2D transition metal dichalcogenide heterostructures for p-and n-type photovoltaic self-powered gas sensor. Adv. Funct. Mater. 2020, 30, 2003360. [Google Scholar] [CrossRef]
- Sik Hwang, W.; Remskar, M.; Yan, R.; Protasenko, V.; Tahy, K.; Doo Chae, S.; Zhao, P.; Konar, A.; Seabaugh, A.; Jena, D. Transistors with chemically synthesized layered semiconductor WS2 exhibiting 105 room temperature modulation and ambipolar behavior. Appl. Phys. Lett. 2012, 101, 013107. [Google Scholar] [CrossRef]
- Wu, Y.; Joshi, N.; Zhao, S.; Long, H.; Zhou, L.; Ma, G.; Peng, B.; Oliveira, O.N., Jr.; Zettl, A.; Lin, L. NO2 gas sensors based on CVD tungsten diselenide monolayer. Appl. Surf. Sci. 2020, 529, 147110. [Google Scholar] [CrossRef]
- Ko, K.Y.; Park, K.; Lee, S.; Kim, Y.; Woo, W.J.; Kim, D.; Song, J.-G.; Park, J.; Kim, H. Recovery improvement for large-area tungsten diselenide gas sensors. Acs. Appl. Mater. Inter. 2018, 10, 23910–23917. [Google Scholar] [CrossRef]
- Pitt, I.G.; Gilbert, R.G.; Ryan, K.R. Application of transition-state theory to gas-surface reactions: Barrierless adsorption on clean surfaces. J. Phys. Chem. 1994, 98, 13001–13010. [Google Scholar] [CrossRef]
- Wang, B.; Gu, Y.; Chen, L.; Ji, L.; Zhu, H.; Sun, Q. Gas sensing devices based on two-dimensional materials: A review. Nanotechnology 2022, 33, 252001. [Google Scholar] [CrossRef]
- Zhao, S.; Xue, J.; Kang, W. Gas adsorption on MoS2 monolayer from first-principles calculations. Chem. Phys. Lett. 2014, 595, 35–42. [Google Scholar] [CrossRef]
- Li, H.; Huang, M.; Cao, G. Markedly different adsorption behaviors of gas molecules on defective monolayer MoS2: A first-principles study. Phys. Chem. Chem. Phys. 2016, 18, 15110–15117. [Google Scholar] [CrossRef]
- Cui, Z.; Yang, K.; Shen, Y.; Yuan, Z.; Dong, Y.; Yuan, P.; Li, E. Toxic gas molecules adsorbed on intrinsic and defective WS2: Gas sensing and detection. Appl. Surf. Sci. 2023, 613, 155978. [Google Scholar] [CrossRef]
- Shen, J.; Yang, Z.; Wang, Y.; Xu, L.-C.; Liu, R.; Liu, X. The gas sensing performance of borophene/MoS2 heterostructure. Appl. Surf. Sci. 2020, 504, 144412. [Google Scholar] [CrossRef]
- Raad, N.H.; Manavizadeh, N.; Frank, I.; Nadimi, E. Gas sensing properties of a two-dimensional graphene/h-BN multi-heterostructure toward H2O, NH3 and NO2: A first principles study. Appl. Surf. Sci. 2021, 565, 150454. [Google Scholar] [CrossRef]
- Qin, Z.; Xu, K.; Yue, H.; Wang, H.; Zhang, J.; Ouyang, C.; Xie, C.; Zeng, D. Enhanced room-temperature NH3 gas sensing by 2D SnS2 with sulfur vacancies synthesized by chemical exfoliation. Sens. Actuators B—Chem. 2018, 262, 771–779. [Google Scholar] [CrossRef]
- Norouzzadeh, E.; Mohammadi, S.; Moradinasab, M. First principles characterization of defect-free and vacancy-defected monolayer PtSe2 gas sensors. Sens. Actuators A—Phys. 2020, 313, 112209. [Google Scholar] [CrossRef]
- Wang, S.; Robertson, A.; Warner, J.H. Atomic structure of defects and dopants in 2D layered transition metal dichalcogenides. Chem. Soc. Rev. 2018, 47, 6764–6794. [Google Scholar] [CrossRef]
- Burman, D.; Raha, H.; Manna, B.; Pramanik, P.; Guha, P.K. Substitutional doping of MoS2 for superior gas-sensing applications: A proof of concept. ACS Sens. 2021, 6, 3398–3408. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Cao, Y.; Zhou, J.; Feng, J.; Cao, J.; Guo, H. Adsorption of NO2, NH3 on monolayer MoS2 doped with Al, Si, and P: A first-principles study. Chem. Phys. Lett. 2016, 643, 27–33. [Google Scholar] [CrossRef]
- Panigrahi, P.; Hussain, T.; Karton, A.; Ahuja, R. Elemental substitution of two-dimensional transition metal dichalcogenides (MoSe2 and MoTe2): Implications for enhanced gas sensing. ACS Sens. 2019, 4, 2646–2653. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, M.; Carotta, M.; Guidi, V.; Martinelli, G.; Ronconi, F.; Richard, O.; Van Dyck, D.; Van Landuyt, J. Structural characterization of Nb–TiO2 nanosized thick-films for gas sensing application. Sens. Actuators B—Chem. 2000, 68, 140–145. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, Y.; Chung, H.-S.; Kim, A.R.; Kwon, J.-D.; Park, J.; Kim, Y.L.; Kwon, S.-H.; Hahm, M.G.; Cho, B. Effect of Nb doping on chemical sensing performance of two-dimensional layered MoSe2. ACS Appl. Mater. Interfaces 2017, 9, 3817–3823. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Park, J.C.; Choi, S.Y.; Kim, Y.; Seo, S.Y.; Park, T.E.; Kwon, S.H.; Cho, B.; Ahn, J.H. Self-formed channel devices based on vertically grown 2D materials with large-surface-area and their potential for chemical sensor applications. Small 2018, 14, 1704116. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-Y.; Kim, S.J.; Lee, Y.; Kim, J.-S.; Jung, W.-B.; Yoo, H.-W.; Kim, J.; Jung, H.-T. Highly enhanced gas adsorption properties in vertically aligned MoS2 layers. ACS Nano 2015, 9, 9314–9321. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Feng, S.; Zhang, K.; Qiu, J.; Zhang, S. Vertical few-layer WSe2 nanosheets for NO2 sensing. Acs. Appl. Nano. Mater. 2021, 4, 12043–12050. [Google Scholar] [CrossRef]
- Shim, Y.-S.; Kwon, K.C.; Suh, J.M.; Choi, K.S.; Song, Y.G.; Sohn, W.; Choi, S.; Hong, K.; Jeon, J.-M.; Hong, S.-P. Synthesis of numerous edge sites in MoS2 via SiO2 nanorods platform for highly sensitive gas sensor. Acs Appl. Mater. Interfaces 2018, 10, 31594–31602. [Google Scholar] [CrossRef]
- Suh, J.M.; Kwon, K.C.; Lee, T.H.; Kim, C.; Lee, C.W.; Song, Y.G.; Choi, M.-J.; Choi, S.; Cho, S.H.; Kim, S. Edge-exposed WS2 on 1D nanostructures for highly selective NO2 sensor at room temperature. Sens. Actuators B—Chem. 2021, 333, 129566. [Google Scholar] [CrossRef]
- Cha, J.-H.; Choi, S.-J.; Yu, S.; Kim, I.-D. 2D WS2-edge functionalized multi-channel carbon nanofibers: Effect of WS2 edge-abundant structure on room temperature NO2 sensing. J. Mater. Chem. A 2017, 5, 8725–8732. [Google Scholar] [CrossRef]
- Koo, W.T.; Cha, J.H.; Jung, J.W.; Choi, S.J.; Jang, J.S.; Kim, D.H.; Kim, I.D. Few-layered WS2 nanoplates confined in Co, N-doped hollow carbon nanocages: Abundant WS2 edges for highly sensitive gas sensors. Adv. Funct. Mater. 2018, 28, 1802575. [Google Scholar] [CrossRef]
- Yang, H.; Li, X.; Wu, Q.; Su, H.; Ma, C.; Wang, X.; Xie, C.; Zeng, D. Synergetic effect of highly active Ce sites and interlayer engineering induced by Ce doping of SnS2 to enhance gas sensing of NO2. Sens. Actuators B—Chem. 2023, 376, 133033. [Google Scholar] [CrossRef]
- Sibi, S.L.; Rajkumar, M.; Manoharan, M.; Mobika, J.; Priya, V.N.; Kumar, R.R. Humidity activated ultra-selective room temperature gas sensor based on W doped MoS2/RGO composites for trace level ammonia detection. Anal. Chim. Acta 2024, 1287, 342075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liang, Y.; Yu, L.; Wang, H.; Yin, M. Activating and modifying the basal planes of MoS2 for superior NO2 sensing at room temperature. Sens. Actuators B—Chem. 2022, 359, 131539. [Google Scholar] [CrossRef]
- Alagh, A.; Annanouch, F.E.; Umek, P.; Bittencourt, C.; Sierra-Castillo, A.; Haye, E.; Colomer, J.F.; Llobet, E. CVD growth of self-assembled 2D and 1D WS2 nanomaterials for the ultrasensitive detection of NO2. Sens. Actuators B—Chem. 2021, 326, 128813. [Google Scholar] [CrossRef]
- Alagh, A.; Annanouch, F.E.; Al Youssef, K.; Bittencourt, C.; Gueell, F.; Martínez-Alanis, P.R.; Reguant, M.; Llobet, E. PdO and PtO loaded WS2 boosts NO2 gas sensing characteristics at room temperature. Sens. Actuators B—Chem. 2022, 364, 131905. [Google Scholar] [CrossRef]
- Jaiswal, J.; Das, A.; Chetry, V.; Kumar, S.; Chandra, R. NO2 sensors based on crystalline MoSe2 porous nanowall thin films with vertically aligned molecular layers prepared by sputtering. Sens. Actuators B—Chem. 2022, 359, 131552. [Google Scholar] [CrossRef]

| Sensing Materials | Target Gas | Types of Defects | EPR Signal | Ref. |
|---|---|---|---|---|
| Ag-doped SnS2 | NO2 | S vacancy | g = 2.004 | [60] |
| SnS2 | NO2 | S vacancy | g = 2.003 | [61] |
| SnOx/SnS | NO2 | O vacancy | g = 2.000 | [62] |
| SnSe2 | NO2 | Se vacancy | g = 2.002 | [63] |
| In-doped SnSe2 | SO2 | Se vacancy | g = 2.003 | [64] |
| MoS2 | NO2 | S vacancy | g = 2.004 | [65] |
| MoS2/PPy | NH3 | S vacancy | g = 2.002 | [66] |
| MoSe2 | NO2 | Se vacancy | g = 2.0019 | [67] |
| Sensing Materials | Target Gas | LOD | Response | Mechanism | Ref. |
|---|---|---|---|---|---|
| W-MoS2/RGO | 50 ppm NH3 | 1.32 ppm | 42.3% a | Charge transfer | [124] |
| Zn -MoS2 | 5 ppm NO2 | 8.1 ppb | 368% b | Charge transfer | [125] |
| Edge-enriched MoS2 | 10 ppm NO2 | 1 ppm | 6600% b | Charge transfer Surface-adsorbed oxygen ions | [94] |
| Au-MoS2 | 500 ppm NH3 | / | 150% a | Charge transfer | [111] |
| Au-decorated Sb-WS2 NSs | 50 ppm CO | 41 ppb | 3.9 c | Surface-adsorbed oxygen ions | [95] |
| Edge-enriched WS2 | 0.8 ppm NO2 | <5 ppb | ~5600% b | Charge transfer | [126] |
| Edge-enriched WS2 | 1 ppm NO2 | 100 ppb | 18% b | Charge transfer | [122] |
| Grain boundaries-enriched WS2 | 100 ppm H2 | 5 ppb | 30% b | Charge transfer | [127] |
| Edge-enriched MoSe2 | 10 ppm NO2 | / | −78.3% b | Charge transfer | [128] |
| Grain boundaries-enriched MoSe2 | 3 ppm NO2 | / | 8% b | Charge transfer | [115] |
| Edge-exposed WSe2 | 1 ppm NO2 | 4 ppb | 34.6% b | Charge transfer | [118] |
| S vacancies SnS2 | 500 ppm NH3 | / | 4.2 d | Charge transfer | [108] |
| Ce-SnS2 | 500 ppb NO2 | / | 1.67 c | Surface-adsorbed oxygen ions | [123] |
| Grain boundaries-enriched SnSe2 | 10 ppm NO2 | 300 ppb | 2000% b | Charge transfer | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Qiao, Z.; Zhou, H.; Xie, D. Defect Engineering in Transition Metal Dichalcogenide-Based Gas Sensors. Chemosensors 2024, 12, 85. https://doi.org/10.3390/chemosensors12060085
Fu X, Qiao Z, Zhou H, Xie D. Defect Engineering in Transition Metal Dichalcogenide-Based Gas Sensors. Chemosensors. 2024; 12(6):85. https://doi.org/10.3390/chemosensors12060085
Chicago/Turabian StyleFu, Xiaqing, Zirui Qiao, Hangyu Zhou, and Dan Xie. 2024. "Defect Engineering in Transition Metal Dichalcogenide-Based Gas Sensors" Chemosensors 12, no. 6: 85. https://doi.org/10.3390/chemosensors12060085
APA StyleFu, X., Qiao, Z., Zhou, H., & Xie, D. (2024). Defect Engineering in Transition Metal Dichalcogenide-Based Gas Sensors. Chemosensors, 12(6), 85. https://doi.org/10.3390/chemosensors12060085







