Advanced NIR-II Fluorescence Imaging Technology for Precise Evaluation of Nanomedicine Delivery in Cancer Therapy
Abstract
1. Introduction
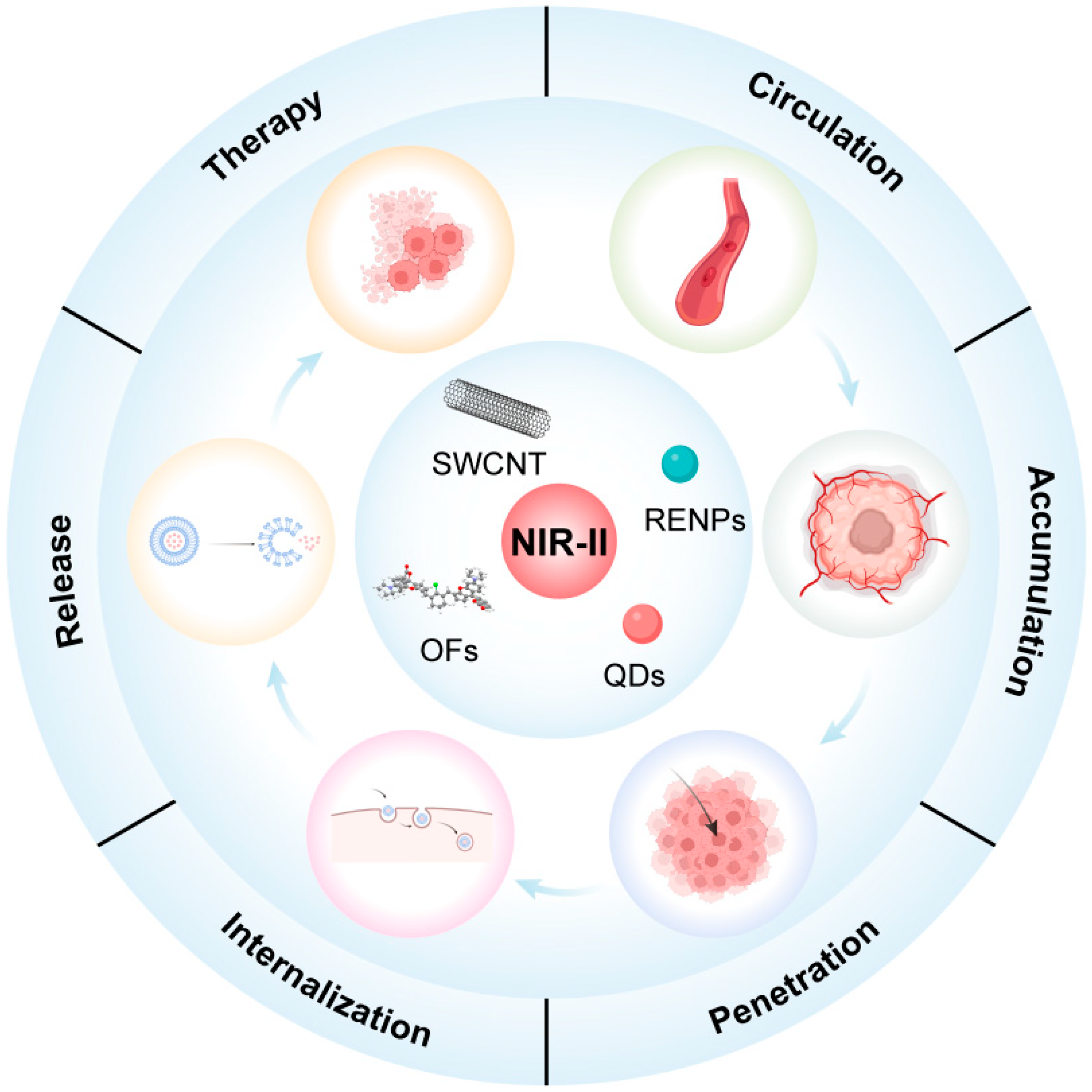
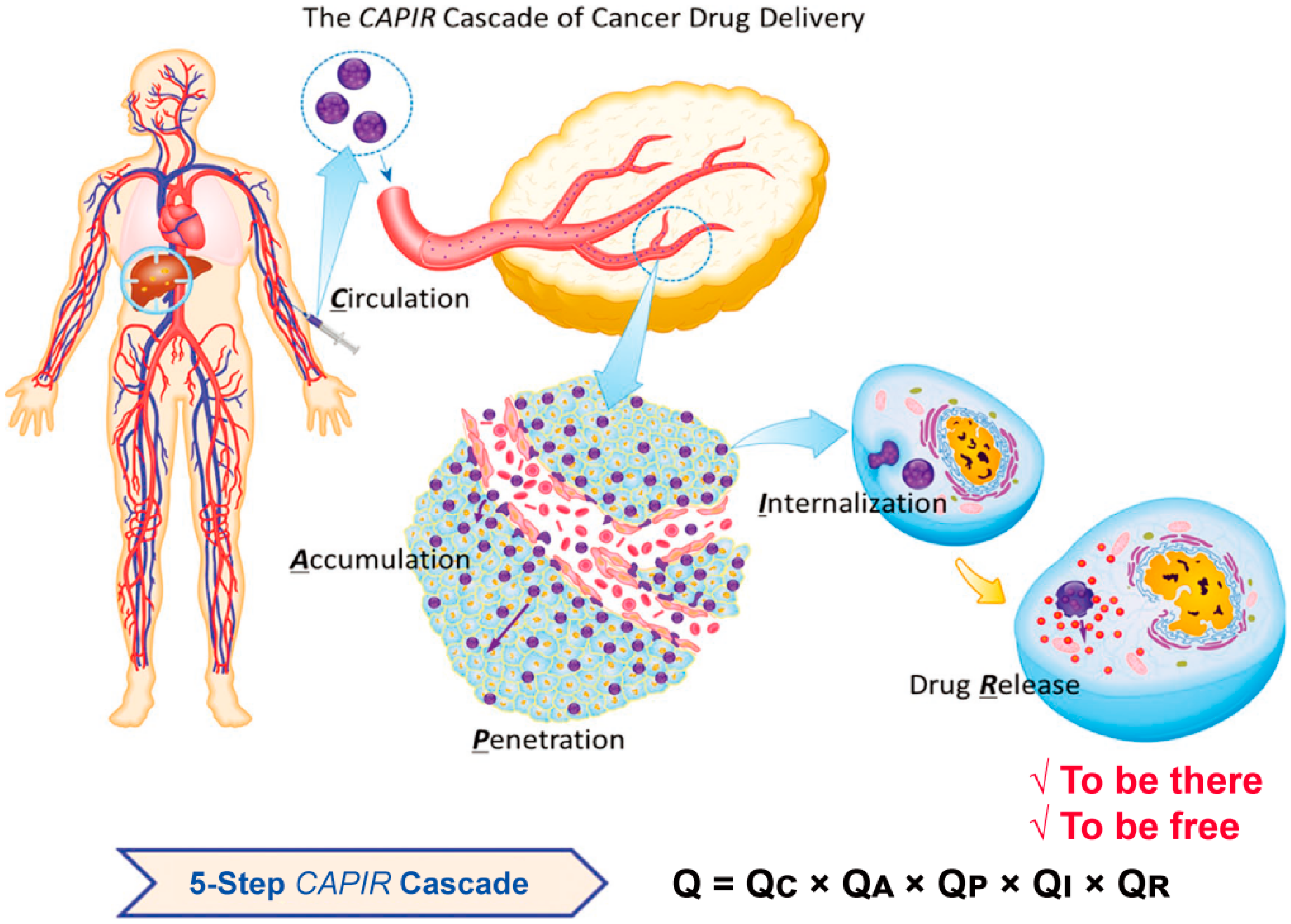
2. The Biological Barriers during the Nanomedicine Delivery Process
2.1. Blood Clearance
2.2. Tumor Accumulation
2.3. Intratumoral Penetration
2.4. Cellular Internalization
2.5. Intracellular Drug Release
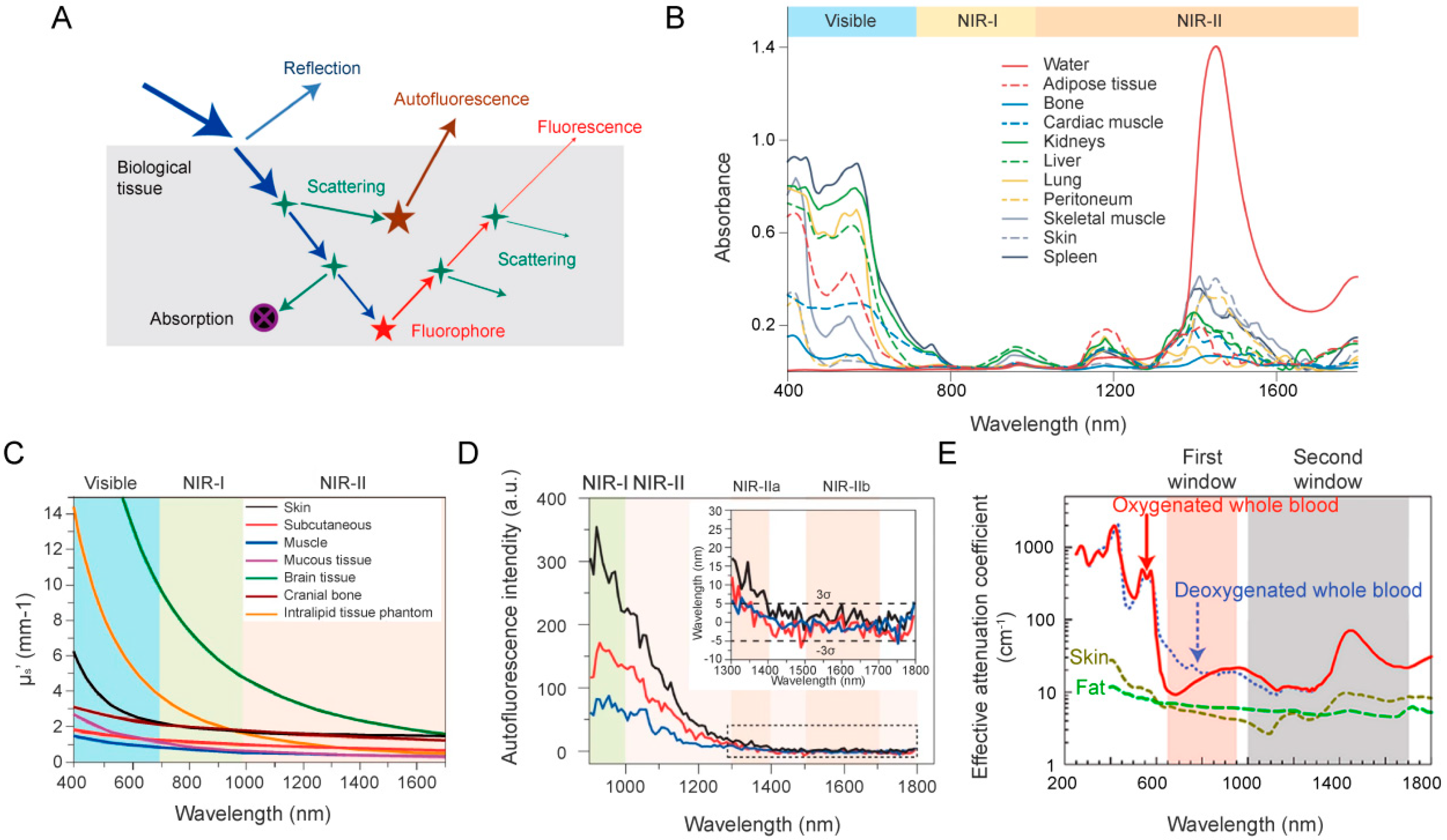
3. NIR-II Fluorescence Imaging Technologies
3.1. NIR-II Imaging Probes
3.2. NIR-II Fluorescence Imaging Systems
4. NIR-II Fluorescence Imaging for the Precise Evaluation of Nanomedicine Delivery in Cancer Therapy
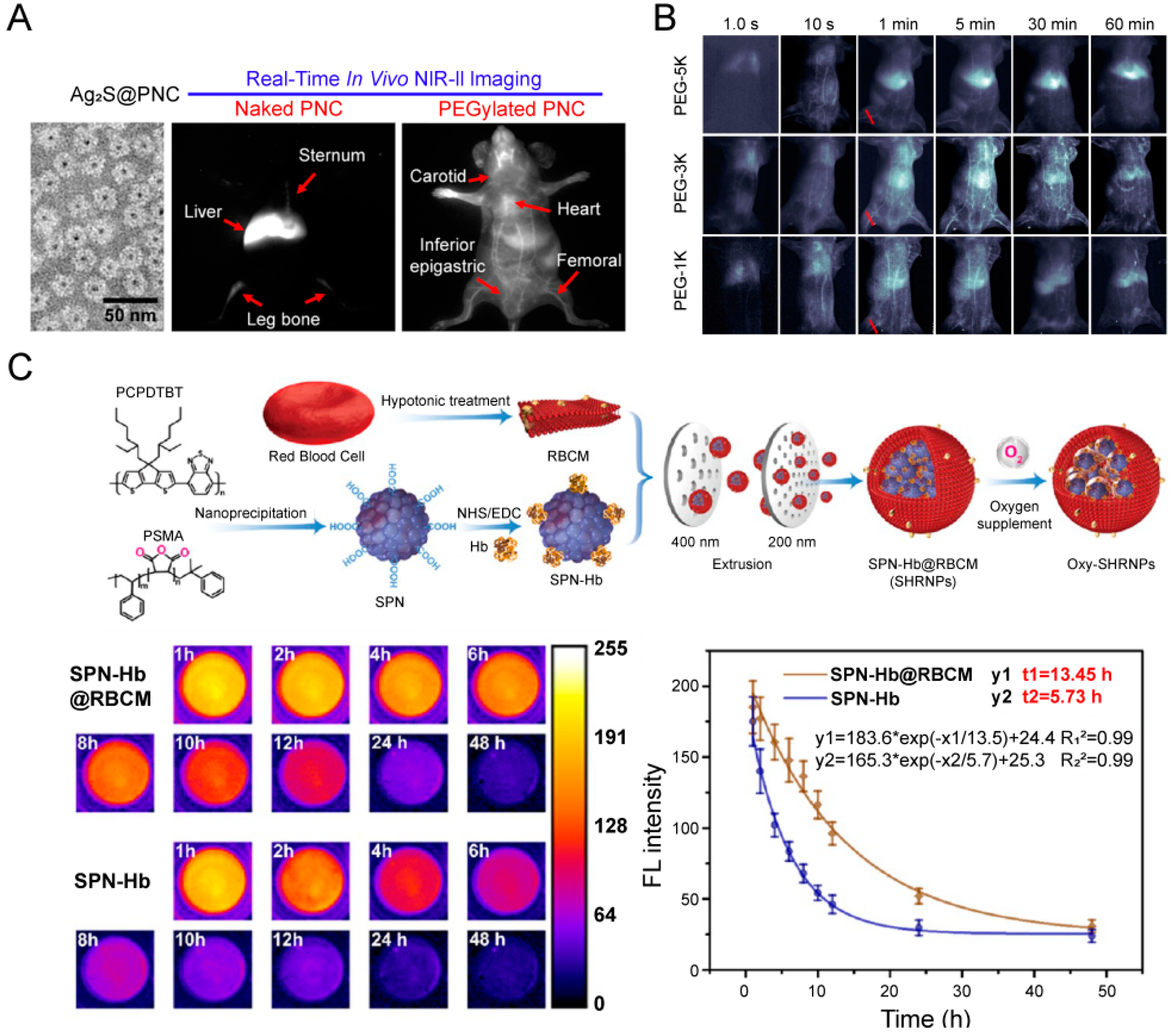
4.1. Evaluation of Blood Circulation
4.2. Evaluation of Tumor Accumulation
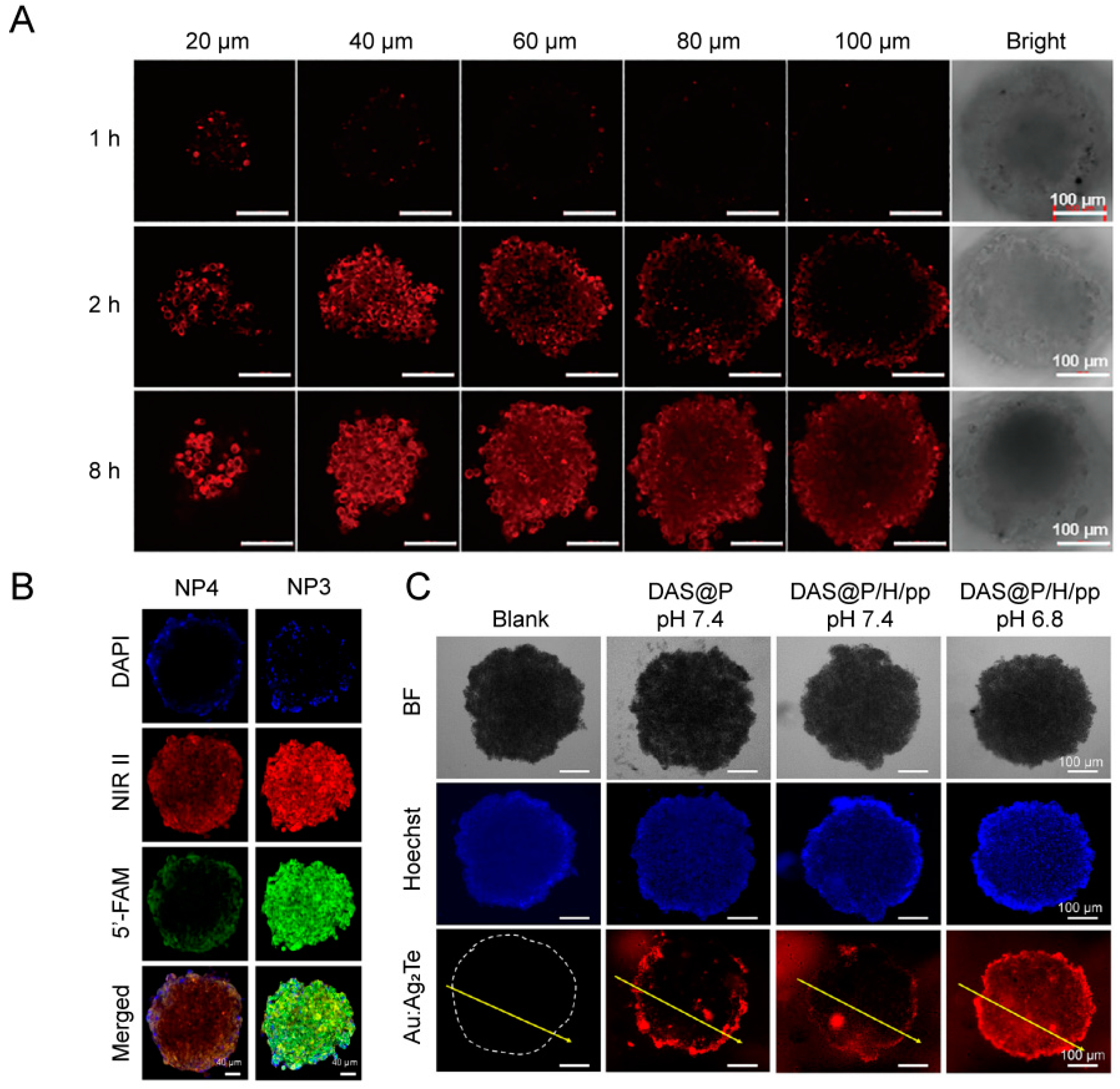
4.3. Evaluation of Tumor Tissue Penetration
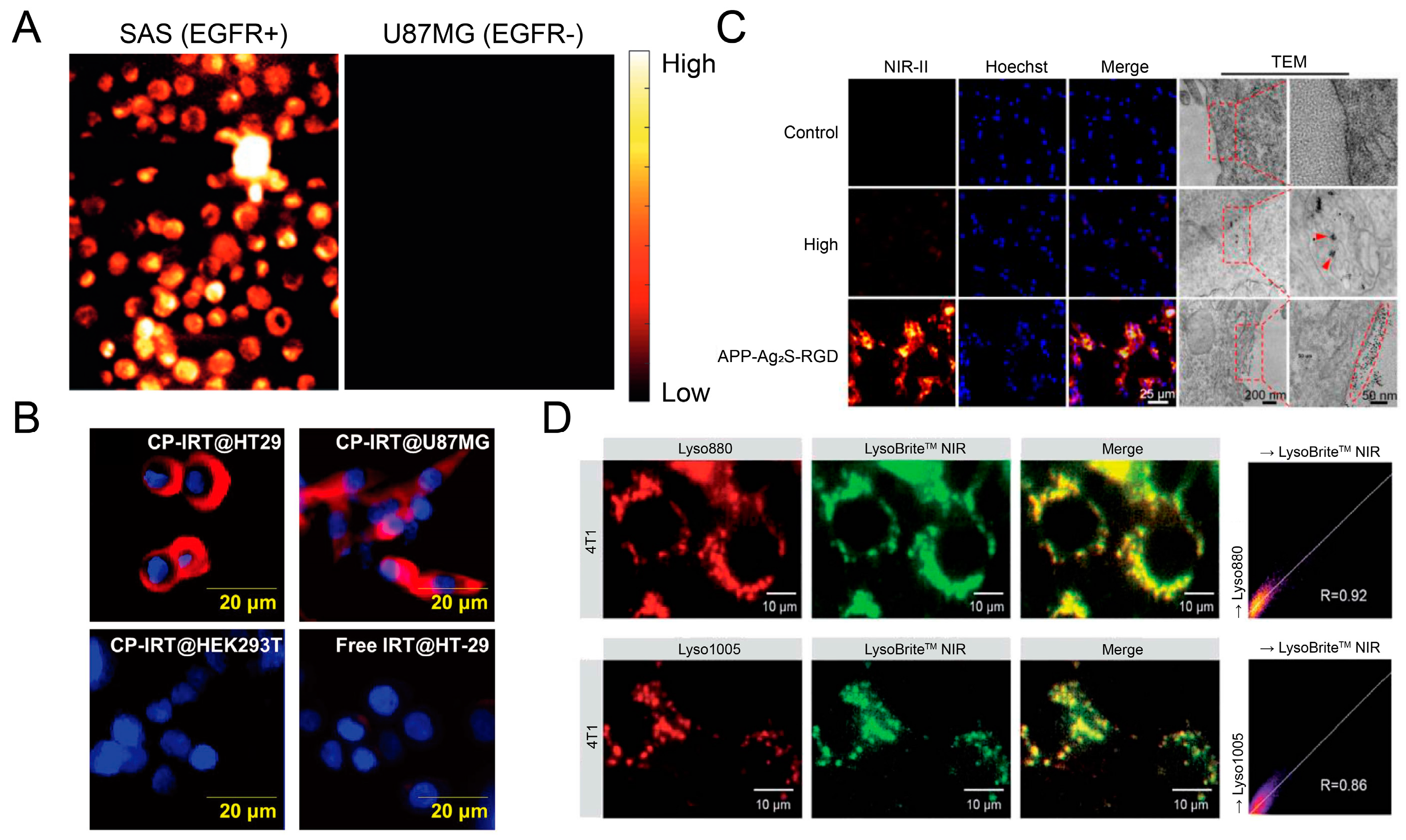
4.4. Evaluation of Cellular Internalization
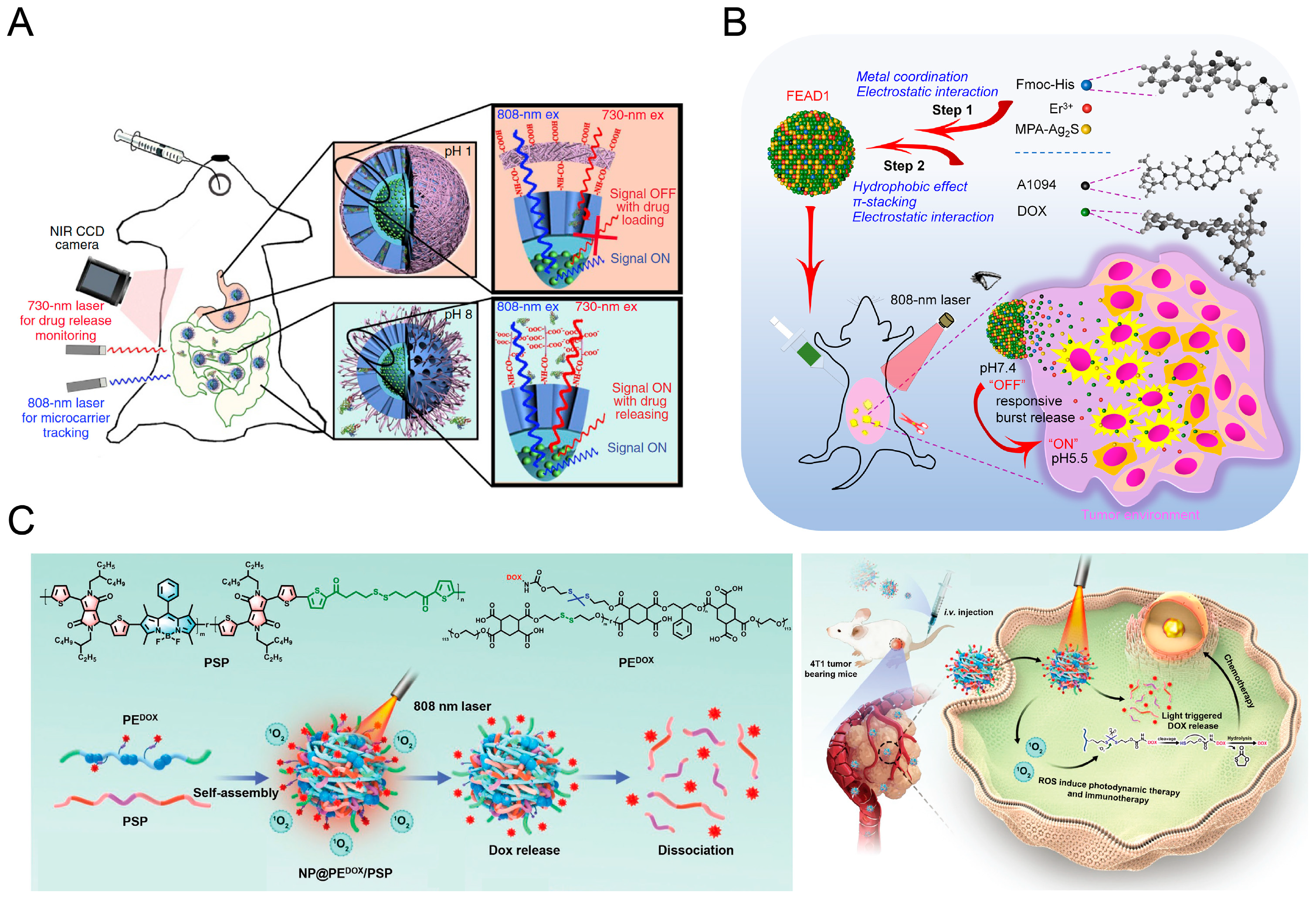
4.5. Evaluation of Drug Release
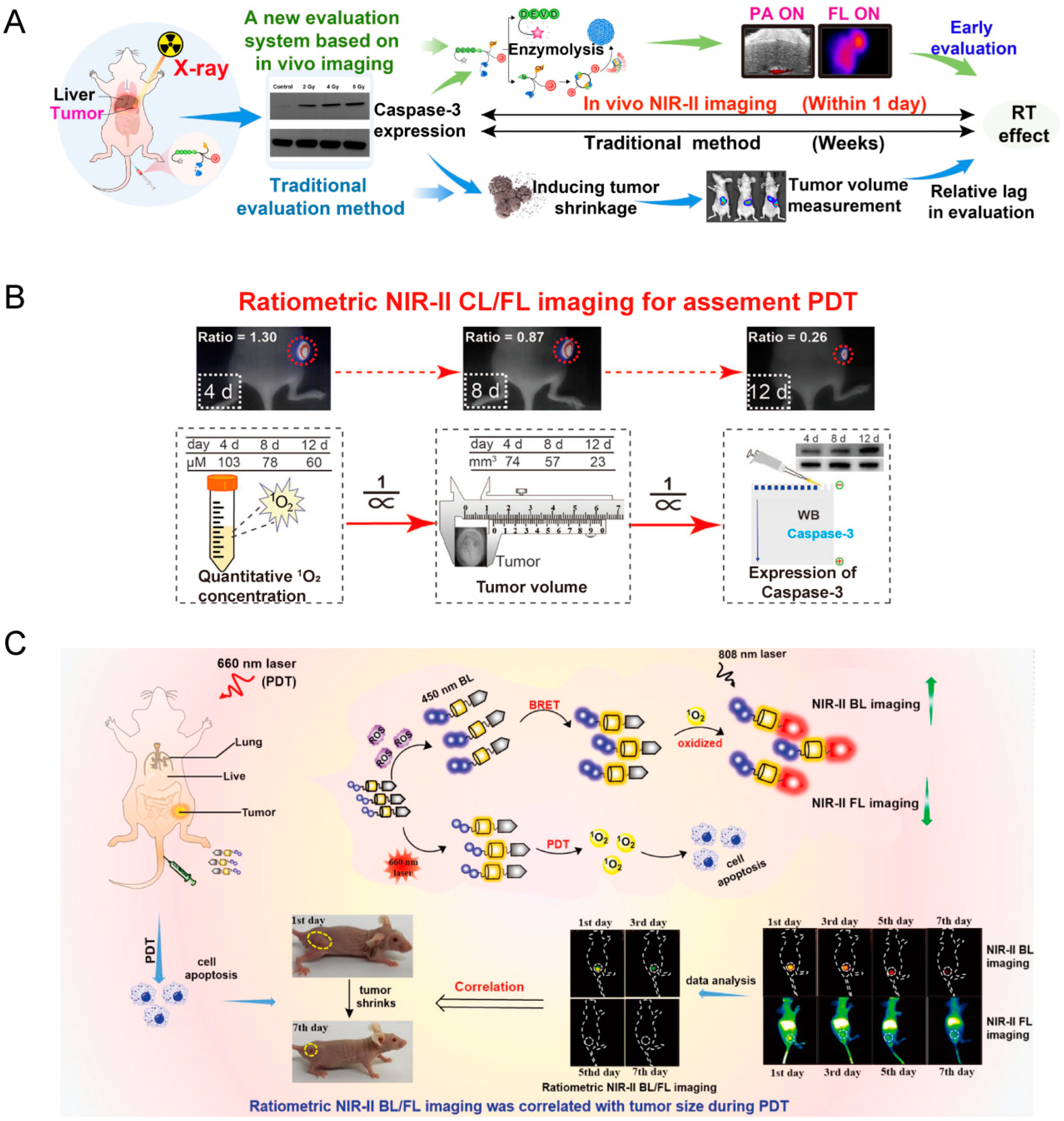
4.6. Evaluation of Therapeutic Effects
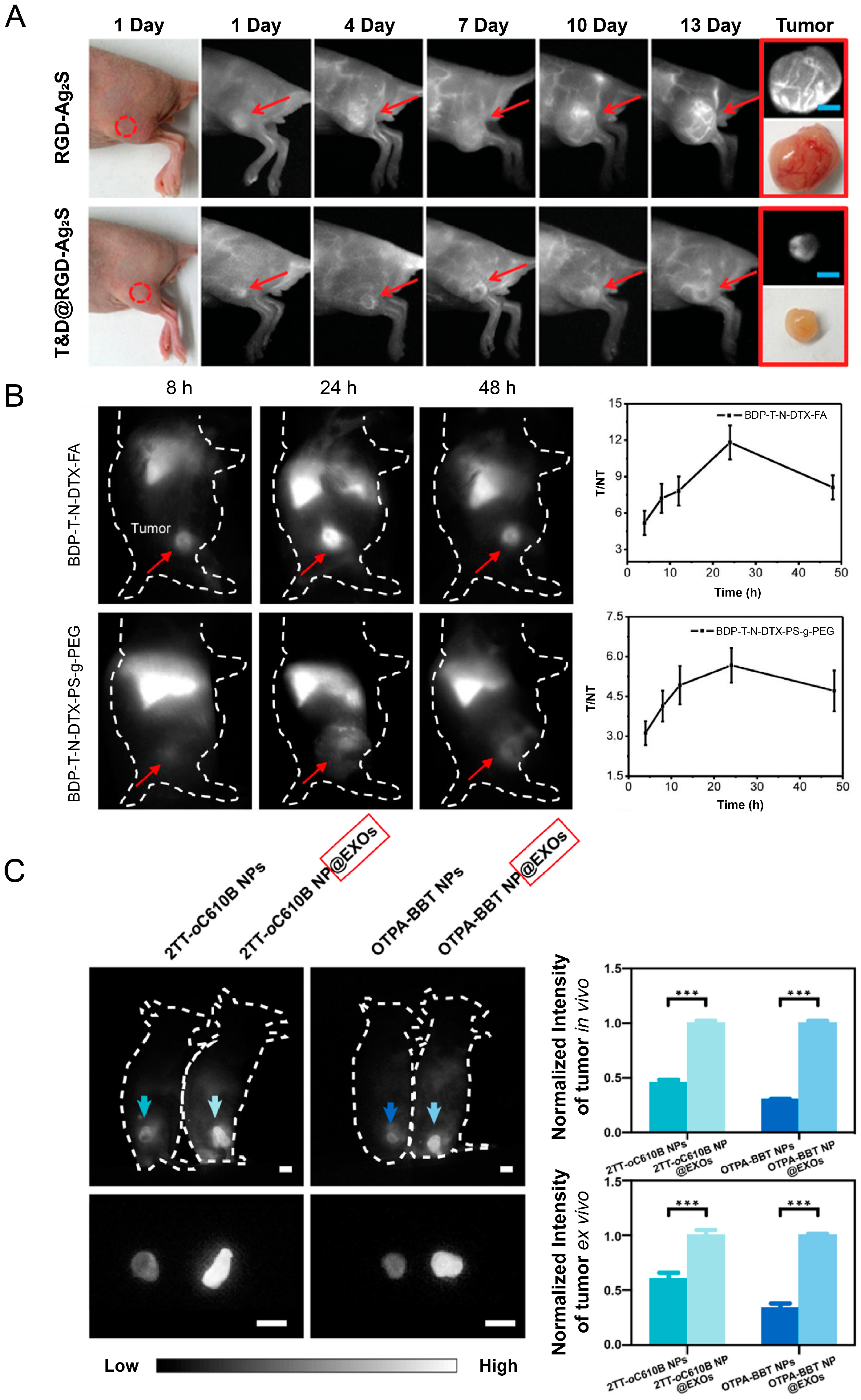
4.7. Evaluation of Targeted Tumor Delivery
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Basu, P.; Kramer, B.S.; Li, H.; Qu, C.; Yu, X.Q.; Canfell, K.; Qiao, Y.; Armstrong, B.K.; Chen, W. Cancer Screening in China: A Steep Road from Evidence to Implementation. Lancet Public Health 2023, 8, e996–e1005. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.W. Principles of Nanoparticle Delivery to Solid Tumors. BME Front. 2023, 4, 0016. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Bao, Z.; Wang, P.; Deng, Y.; Fan, J.; Zhu, X.; Xia, X.; Song, Y.; Yao, H.; Li, D. Gelatin-Functionalized Carbon Nanotubes Loaded with Cisplatin for Anti-Cancer Therapy. Polymers 2023, 15, 3333. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T. Nanomedicine Tumor Targeting. Adv. Mater. 2024, 2312169. [Google Scholar] [CrossRef]
- Ta, W.; Li, X.; Song, J.; Hua, R.; Zheng, Y.; Lu, W. Customizable Dual-Fluorescent Nanoparticles for Tracing and Quantifying of Cell Transport. Int. J. Nanomed. 2023, 18, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.B.; Decuzzi, P. Harnessing Endogenous Stimuli for Responsive Materials in Theranostics. ACS Nano 2021, 15, 2068–2098. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Qian, J.; Meng, C.; Liu, Y.; Ding, Q.; Wu, H.; Li, P.; Ran, F.; Liu, G.-Q.; Wang, Y.; et al. TME-Targeting Theranostic Agent Uses NIR Tracking for Tumor Diagnosis and Surgical Resection and Acts as Chemotherapeutic Showing Enhanced Efficiency and Minimal Toxicity. Theranostics 2022, 12, 2535–2548. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, F.-R.; Wang, L.; Hu, J. Second Near-Infrared (NIR-II) Window for Imaging-Navigated Modulation of Brain Structure and Function. Small 2023, 19, 2206044. [Google Scholar] [CrossRef]
- Meng, X.; Pang, X.; Zhang, K.; Gong, C.; Yang, J.; Dong, H.; Zhang, X. Recent Advances in Near-Infrared-II Fluorescence Imaging for Deep-Tissue Molecular Analysis and Cancer Diagnosis. Small 2022, 18, 2202035. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Antaris, A.L.; Dai, H. Near-Infrared Fluorophores for Biomedical Imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Zhang, F. Near-Infrared Luminescence High-Contrast in Vivo Biomedical Imaging. Nat. Rev. Bioeng. 2023, 1, 60–78. [Google Scholar] [CrossRef]
- Smith, A.M.; Mancini, M.C.; Nie, S. Second Window for in Vivo Imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; He, C.; Riviere, J.E.; Monteiro-Riviere, N.A.; Lin, Z. Meta-Analysis of Nanoparticle Delivery to Tumors Using a Physiologically Based Pharmacokinetic Modeling and Simulation Approach. ACS Nano 2020, 14, 3075–3095. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhou, Z.; Qiu, N.; Shen, Y. Rational Design of Cancer Nanomedicine: Nanoproperty Integration and Synchronization. Adv. Mater. 2017, 29, 1606628. [Google Scholar] [CrossRef] [PubMed]
- Wayteck, L.; Dewitte, H.; De Backer, L.; Breckpot, K.; Demeester, J.; De Smedt, S.C.; Raemdonck, K. Hitchhiking Nanoparticles: Reversible Coupling of Lipid-Based Nanoparticles to Cytotoxic T Lymphocytes. Biomaterials 2016, 77, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Kydd, J.; Jadia, R.; Velpurisiva, P.; Gad, A.; Paliwal, S.; Rai, P. Targeting Strategies for the Combination Treatment of Cancer Using Drug Delivery Systems. Pharmaceutics 2017, 9, 46. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance Properties of Nano-Sized Particles and Molecules as Imaging Agents: Considerations and Caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef]
- Efremova, M.V.; Naumenko, V.A.; Spasova, M.; Garanina, A.S.; Abakumov, M.A.; Blokhina, A.D.; Melnikov, P.A.; Prelovskaya, A.O.; Heidelmann, M.; Li, Z.-A.; et al. Magnetite-Gold Nanohybrids as Ideal All-in-One Platforms for Theranostics. Sci. Rep. 2018, 8, 11295. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, X.; Chen, X.; Zhou, M.; Xu, R.; Zhang, X. Smart Surface Coating of Drug Nanoparticles with Cross-Linkable Polyethylene Glycol for Bio-Responsive and Highly Efficient Drug Delivery. Nanoscale 2016, 8, 8118–8125. [Google Scholar] [CrossRef] [PubMed]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Junyaprasert, V.B.; Thummarati, P. Innovative Design of Targeted Nanoparticles: Polymer-Drug Conjugates for Enhanced Cancer Therapy. Pharmaceutics 2023, 15, 2216. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, S.M.; Azizov, S.; Elmasry, M.R.; Sharipov, M.; Lee, Y.-I. Recent Advances in Nanomicelles Delivery Systems. Nanomaterials 2020, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Masarapu, H.; Gu, Y.; Zhang, Y.; Yu, X.; Steinmetz, N.F. Physalis Mottle Virus-like Nanoparticles for Targeted Cancer Imaging. ACS Appl. Mater. Interfaces 2019, 11, 18213–18223. [Google Scholar] [CrossRef] [PubMed]
- Shipunova, V.O.; Sogomonyan, A.S.; Zelepukin, I.V.; Nikitin, M.P.; Deyev, S.M. PLGA Nanoparticles Decorated with Anti-HER2 Affibody for Targeted Delivery and Photoinduced Cell Death. Molecules 2021, 26, 3955. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.; Assaraf, Y.G.; Livney, Y.D. Novel Selectively Targeted Multifunctional Nanostructured Lipid Carriers for Prostate Cancer Treatment. Pharmaceutics 2021, 14, 88. [Google Scholar] [CrossRef]
- Li, C.; Xiao, C.; Zhan, L.; Zhang, Z.; Xing, J.; Zhai, J.; Zhou, Z.; Tan, G.; Piao, J.; Zhou, Y.; et al. Wireless Electrical Stimulation at the Nanoscale Interface Induces Tumor Vascular Normalization. Bioact. Mater. 2022, 18, 399–408. [Google Scholar] [CrossRef]
- Zhu, M.; Zhuang, J.; Li, Z.; Liu, Q.; Zhao, R.; Gao, Z.; Midgley, A.C.; Qi, T.; Tian, J.; Zhang, Z.; et al. Machine-Learning-Assisted Single-Vessel Analysis of Nanoparticle Permeability in Tumour Vasculatures. Nat. Nanotechnol. 2023, 18, 657–666. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, Q.; Dou, J.; Zhou, H.; Zeng, C.; Pan, H.; Shen, Y.; Li, Q.; Liu, Y.; Leong, D.T.; et al. Breaking through the Basement Membrane Barrier to Improve Nanotherapeutic Delivery to Tumours. Nat. Nanotechnol. 2023, 19, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Jang, H.; Gupta, B.; Jeong, J.-H.; Ge, Y.; Yong, C.S.; Kim, J.O.; Bae, J.-S.; Song, I.-S.; Kim, I.-S.; et al. Tie2-Mediated Vascular Remodeling by Ferritin-Based Protein C Nanoparticles Confers Antitumor and Anti-Metastatic Activities. J. Hematol. Oncol. 2020, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Miao, Y.; Chen, M.; Chen, X.; Li, F.; Zhang, X.; Gan, Y. Stepwise Targeting and Responsive Lipid-Coated Nanoparticles for Enhanced Tumor Cell Sensitivity and Hepatocellular Carcinoma Therapy. Theranostics 2020, 10, 3722–3736. [Google Scholar] [CrossRef] [PubMed]
- Bugno, J.; Poellmann, M.J.; Sokolowski, K.; Hsu, H.; Kim, D.-H.; Hong, S. Tumor Penetration of Sub-10 Nm Nanoparticles: Effect of Dendrimer Properties on Their Penetration in Multicellular Tumor Spheroids. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102059. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Zhang, Q.; Li, J. Tumor Extracellular Matrix Modulating Strategies for Enhanced Antitumor Therapy of Nanomedicines. Mater. Today Bio 2022, 16, 100364. [Google Scholar] [CrossRef] [PubMed]
- Durymanov, M.O.; Rosenkranz, A.A.; Sobolev, A.S. Current Approaches for Improving Intratumoral Accumulation and Distribution of Nanomedicines. Theranostics 2015, 5, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Gao, L.; Chen, K.; Zhang, W.; Zhang, Q.; Li, Q.; Hu, K. Nanoparticles: A New Approach to Upgrade Cancer Diagnosis and Treatment. Nanoscale Res. Lett. 2021, 16, 88. [Google Scholar] [CrossRef] [PubMed]
- Bourquin, J.; Milosevic, A.; Hauser, D.; Lehner, R.; Blank, F.; Petri-Fink, A.; Rothen-Rutishauser, B. Biodistribution, Clearance, and Long-Term Fate of Clinically Relevant Nanomaterials. Adv. Mater. 2018, 30, 1704307. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ni, Q.; Wang, Y.; Zhang, Y.; He, H.; Gao, D.; Ma, X.; Liang, X.-J. Nanoscale Drug Delivery Systems for Controllable Drug Behaviors by Multi-Stage Barrier Penetration. J. Control. Release 2021, 331, 282–295. [Google Scholar] [CrossRef]
- Cheng, Q.; Shi, X.-L.; Li, Q.-L.; Wang, L.; Wang, Z. Current Advances on Nanomaterials Interfering with Lactate Metabolism for Tumor Therapy. Adv. Sci. 2024, 11, e2305662. [Google Scholar] [CrossRef]
- Wu, W.; Pu, Y.; Shi, J. Nanomedicine-Enabled Chemotherapy-Based Synergetic Cancer Treatments. J. Nanobiotechnol. 2022, 20, 4. [Google Scholar] [CrossRef]
- Ng, T.S.C.; Garlin, M.A.; Weissleder, R.; Miller, M.A. Improving Nanotherapy Delivery and Action through Image-Guided Systems Pharmacology. Theranostics 2020, 10, 968–997. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xue, X.; He, Y.; Lu, Z.; Jia, B.; Wu, H.; Yuan, Y.; Huang, Y.; Wang, H.; Lu, H.; et al. Novel Redox-Responsive Polymeric Magnetosomes with Tunable Magnetic Resonance Property for in Vivo Drug Release Visualization and Dual-Modal Cancer Therapy. Adv. Funct. Mater. 2018, 28, 1802159. [Google Scholar] [CrossRef]
- Wu, L.; Wu, I.-C.; DuFort, C.C.; Carlson, M.A.; Wu, X.; Chen, L.; Kuo, C.-T.; Qin, Y.; Yu, J.; Hingorani, S.R.; et al. Photostable Ratiometric Pdot Probe for in Vitro and in Vivo Imaging of Hypochlorous Acid. J. Am. Chem. Soc. 2017, 139, 6911–6918. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Ma, H.; Zhu, S.; Lau, J.; Ma, R.; Liu, Y.; Lin, L.; Chandra, S.; Wang, S.; Zhu, X.; et al. Multiplexed NIR-II Probes for Lymph Node-Invaded Cancer Detection and Imaging-Guided Surgery. Adv. Mater. 2020, 32, 1907365. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Feng, L.; Yang, P. 2, 1, 3-Benzothiadiazole Derivative Small Molecule Fluorophores for NIR-II Bioimaging. Adv. Funct. Mater. 2024, 34, 2310818. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Koo, S.; Sun, Y.; Liu, Y.; Liu, X.; Pan, Y.; Zhang, Z.; Du, M.; Lu, S.; et al. Versatile Types of Inorganic/Organic NIR-IIa/IIb Fluorophores: From Strategic Design toward Molecular Imaging and Theranostics. Chem. Rev. 2022, 122, 209–268. [Google Scholar] [CrossRef]
- Li, C.; Chen, G.; Zhang, Y.; Wu, F.; Wang, Q. Advanced Fluorescence Imaging Technology in the Near-Infrared-II Window for Biomedical Applications. J. Am. Chem. Soc. 2020, 142, 14789–14804. [Google Scholar] [CrossRef]
- Welsher, K.; Liu, Z.; Sherlock, S.P.; Robinson, J.T.; Chen, Z.; Daranciang, D.; Dai, H. A Route to Brightly Fluorescent Carbon Nanotubes for Near-Infrared Imaging in Mice. Nat. Nanotechnol. 2009, 4, 773–780. [Google Scholar] [CrossRef]
- Welsher, K.; Sherlock, S.P.; Dai, H. Deep-Tissue Anatomical Imaging of Mice Using Carbon Nanotube Fluorophores in the Second Near-Infrared Window. Proc. Natl. Acad. Sci. USA 2011, 108, 8943–8948. [Google Scholar] [CrossRef]
- Hong, G.; Lee, J.C.; Robinson, J.T.; Raaz, U.; Xie, L.; Huang, N.F.; Cooke, J.P.; Dai, H. Multifunctional in Vivo Vascular Imaging Using Near-Infrared II Fluorescence. Nat. Med. 2012, 18, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Majdinasab, M.; Mitsubayashi, K.; Marty, J.L. Optical and Electrochemical Sensors and Biosensors for the Detection of Quinolones. Trends Biotechnol. 2019, 37, 898–915. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Lai, W.; Man, T.; Chang, B.; Li, L.; Chandrasekaran, A.R.; Pei, H. Rationally Engineered Nucleic Acid Architectures for Biosensing Applications. Chem. Rev. 2019, 119, 11631–11717. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, P.; Cong, R.; Xu, N.; Tan, Y.; Tan, C.; Jiang, Y. Sensitive Conjugated-Polymer-Based Fluorescent ATP Probes and Their Application in Cell Imaging. ACS Appl. Mater. Interfaces 2016, 8, 3567–3574. [Google Scholar] [CrossRef]
- Du, Y.; Xu, B.; Fu, T.; Cai, M.; Li, F.; Zhang, Y.; Wang, Q. Near-Infrared Photoluminescent Ag2S Quantum Dots from a Single Source Precursor. J. Am. Chem. Soc. 2010, 132, 1470–1471. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, G.; Zhang, Y.; Chen, G.; Li, F.; Dai, H.; Wang, Q. Ag2S Quantum Dot: A Bright and Biocompatible Fluorescent Nanoprobe in the Second Near-Infrared Window. ACS Nano 2012, 6, 3695–3702. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Robinson, J.T.; Zhang, Y.; Diao, S.; Antaris, A.L.; Wang, Q.; Dai, H. In Vivo Fluorescence Imaging with Ag2S Quantum Dots in the Second Near-Infrared Region. Angew. Chem. Int. Ed. Engl. 2012, 51, 9818–9821. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Huang, H.; Ma, X.; Zhang, Y.; Yang, X.; Yu, M.; Sun, Z.; Li, C.; Wu, F.; Wang, Q. Au-Doped Ag2Te Quantum Dots with Bright NIR-IIb Fluorescence for In Situ Monitoring of Angiogenesis and Arteriogenesis in a Hindlimb Ischemic Model. Adv. Mater. 2021, 33, 2103953. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Li, C.; Chen, G.; Zhang, Y.; Zhang, Y.; Deng, M.; Wang, Q. Facile Synthesis of Highly Photoluminescent Ag2Se Quantum Dots as a New Fluorescent Probe in the Second Near-Infrared Window for in Vivo Imaging. Chem. Mater. 2013, 25, 2503–2509. [Google Scholar] [CrossRef]
- Bruns, O.T.; Bischof, T.S.; Harris, D.K.; Franke, D.; Shi, Y.; Riedemann, L.; Bartelt, A.; Jaworski, F.B.; Carr, J.A.; Rowlands, C.J.; et al. Next-Generation in Vivo Optical Imaging with Short-Wave Infrared Quantum Dots. Nat. Biomed. Eng. 2017, 1, 0056. [Google Scholar] [CrossRef]
- Yang, M.; Gui, R.; Jin, H.; Wang, Z.; Zhang, F.; Xia, J.; Bi, S.; Xia, Y. Ag2Te Quantum Dots with Compact Surface Coatings of Multivalent Polymers: Ambient One-Pot Aqueous Synthesis and the Second Near-Infrared Bioimaging. Colloids Surf. B Biointerfaces 2015, 126, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, G.; Tong, Y.; Zhang, Y.; Sun, S.-K.; Yu, C. Noninvasive Gastrointestinal Tract Imaging Using BSA-Ag2Te Quantum Dots as a CT/NIR-II Fluorescence Dual-Modal Imaging Probe in Vivo. ACS Biomater. Sci. Eng. 2023, 9, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, S.; Hao, J. Non-Invasive Optical Guided Tumor Metastasis/Vessel Imaging by Using Lanthanide Nanoprobe with Enhanced Down-Shifting Emission beyond 1500 Nm. ACS Nano 2019, 13, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Li, T.; Yao, T.; Chen, G.; Li, C.; Wang, Q. Near-Infrared-II Fluorophores for In Vivo Multichannel Biosensing. Chemosensors 2023, 11, 433. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, P.; Lu, Y.; Wang, R.; Zhou, L.; Zheng, X.; Li, X.; Piper, J.A.; Zhang, F. Lifetime-Engineered NIR-II Nanoparticles Unlock Multiplexed In Vivo Imaging. Nat. Nanotechnol. 2018, 13, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Huang, H.; Yang, H.; Xia, B.; Ma, Z.; Zhang, Y.; Wu, F.; Li, C.; He, T.; Wang, Q. Tailoring Near-Infrared-IIb Fluorescence of Thulium(III) by Nanocrystal Structure Engineering. Nano Lett. 2023, 23, 10058–10065. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, X.; Zhang, H.; Zhang, F. Luminescence Lifetime Imaging Based on Lanthanide Nanoparticles. Angew. Chem. Int. Ed. Engl. 2022, 61, e202209378. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Sun, L.-D.; Feng, W.; Gu, Y.; Li, F.; Yan, C.-H. Versatile Spectral and Lifetime Multiplexing Nanoplatform with Excitation Orthogonalized Upconversion Luminescence. ACS Nano 2017, 11, 3289–3297. [Google Scholar] [CrossRef]
- Su, Y.; Yu, B.; Wang, S.; Cong, H.; Shen, Y. NIR-II Bioimaging of Small Organic Molecule. Biomaterials 2021, 271, 120717. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Wu, F.; Chen, G.; Li, C.; Wang, Q. Rational Design of NIR-II Ratiometric Fluorescence Probes for Accurate Bioimaging and Biosensing In Vivo. Small Methods 2024, 2400132. [Google Scholar] [CrossRef]
- Jiang, Z.; Ding, Y.; Lovell, J.F.; Zhang, Y. Design and Application of Organic Contrast Agents for Molecular Imaging in the Second Near Infrared (NIR-II) Window. Photoacoustics 2022, 28, 100426. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, B.; Shen, Y.; Cong, H. Design of NIR-II High Performance Organic Small Molecule Fluorescent Probes and Summary of Their Biomedical Applications. Coordin. Chem. Rev. 2022, 468, 214609. [Google Scholar] [CrossRef]
- Zhang, N.; Lu, C.; Chen, M.; Xu, X.; Shu, G.; Du, Y.; Ji, J. Recent Advances in Near-Infrared II Imaging Technology for Biological Detection. J. Nanobiotechnol. 2021, 19, 132. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ma, H. Design Principles of Spectroscopic Probes for Biological Applications. Chem. Sci. 2016, 7, 6309–6315. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Lin, S.; Wang, Q.; Zhang, Y.; Li, C.; Ji, R.; Wang, M.; Chen, G.; Wang, Q. An NIR-II Fluorescence/Dual Bioluminescence Multiplexed Imaging for In Vivo Visualizing the Location, Survival, and Differentiation of Transplanted Stem Cells. Adv. Funct. Mater. 2019, 29, 1806546. [Google Scholar] [CrossRef]
- Hu, Z.; Fang, C.; Li, B.; Zhang, Z.; Cao, C.; Cai, M.; Su, S.; Sun, X.; Shi, X.; Li, C.; et al. First-in-Human Liver-Tumour Surgery Guided by Multispectral Fluorescence Imaging in the Visible and Near-Infrared-I/II Windows. Nat. Biomed. Eng. 2020, 4, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Diao, S.; Chang, J.; Antaris, A.L.; Chen, C.; Zhang, B.; Zhao, S.; Atochin, D.N.; Huang, P.L.; Andreasson, K.I.; et al. Through-Skull Fluorescence Imaging of the Brain in a New Near-Infrared Window. Nat. Photonics 2014, 8, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Sun, C.; Zebibula, A.; Zhang, H.; Kwok, R.T.K.; Zhao, X.; Xi, W.; Lam, J.W.Y.; Qian, J.; Tang, B.Z. Real-Time and High-Resolution Bioimaging with Bright Aggregation-Induced Emission Dots in Short-Wave Infrared Region. Adv. Mater. 2018, 30, e1706856. [Google Scholar] [CrossRef]
- Chen, G.; Lin, S.; Huang, D.; Zhang, Y.; Li, C.; Wang, M.; Wang, Q. Revealing the Fate of Transplanted Stem Cells In Vivo with a Novel Optical Imaging Strategy. Small 2018, 14, 1702679. [Google Scholar] [CrossRef]
- Wang, F.; Wan, H.; Ma, Z.; Zhong, Y.; Sun, Q.; Tian, Y.; Qu, L.; Du, H.; Zhang, M.; Li, L.; et al. Light-Sheet Microscopy in the Near-Infrared II Window. Nat. Methods 2019, 16, 545–552. [Google Scholar] [CrossRef]
- Zhu, S.; Herraiz, S.; Yue, J.; Zhang, M.; Wan, H.; Yang, Q.; Ma, Z.; Wang, Y.; He, J.; Antaris, A.L.; et al. 3D NIR-II Molecular Imaging Distinguishes Targeted Organs with High-Performance NIR-II Bioconjugates. Adv. Mater. 2018, 30, 1705799. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ma, Z.; Zhong, Y.; Salazar, F.; Xu, C.; Ren, F.; Qu, L.; Wu, A.M.; Dai, H. In Vivo NIR-II Structured-Illumination Light-Sheet Microscopy. Proc. Natl. Acad. Sci. USA 2021, 118, e2023888118. [Google Scholar] [CrossRef] [PubMed]
- Mi, C.; Guan, M.; Zhang, X.; Yang, L.; Wu, S.; Yang, Z.; Guo, Z.; Liao, J.; Zhou, J.; Lin, F.; et al. High Spatial and Temporal Resolution NIR-IIb Gastrointestinal Imaging in Mice. Nano Lett. 2022, 22, 2793–2800. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Mu, X.; Zhang, X.-D.; Ming, D. The Near-Infrared-II Fluorophores and Advanced Microscopy Technologies Development and Application in Bioimaging. Bioconjug. Chem. 2020, 31, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cai, C.; Wang, H.; Ma, X.; Shao, A.; Sheng, J.; Yu, C. Drug Delivery Strategy in Hepatocellular Carcinoma Therapy. Cell. Commun. Signal. 2022, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.-V.; Lee, J.; Lee, H.; Shim, G.; Oh, Y.-K. Cell Membrane-Derived Vesicles for Delivery of Therapeutic Agents. Acta Pharm. Sin. B 2021, 11, 2096–2113. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, F.; Zhang, Y.; Zhang, W.; Zhang, X.-E.; Wang, Q. Real-Time Monitoring Surface Chemistry-Dependent In Vivo Behaviors of Protein Nanocages via Encapsulating an NIR-II Ag2S Quantum Dot. ACS Nano 2015, 9, 12255–12263. [Google Scholar] [CrossRef]
- Li, Y.; Gao, J.; Wang, S.; Du, M.; Hou, X.; Tian, T.; Qiao, X.; Tian, Z.; Stang, P.J.; Li, S.; et al. Self-Assembled NIR-II Fluorophores with Ultralong Blood Circulation for Cancer Imaging and Image-Guided Surgery. J. Med. Chem. 2022, 65, 2078–2090. [Google Scholar] [CrossRef]
- Li, S.; Chen, H.; Liu, H.; Liu, L.; Yuan, Y.; Mao, C.; Zhang, W.; Zhang, X.; Guo, W.; Lee, C.-S.; et al. In Vivo Real-Time Pharmaceutical Evaluations of Near-Infrared II Fluorescent Nanomedicine Bound Polyethylene Glycol Ligands for Tumor Photothermal Ablation. ACS Nano 2020, 14, 13681–13690. [Google Scholar] [CrossRef]
- Ding, L.; Wu, Y.; Wu, M.; Zhao, Q.; Li, H.; Liu, J.; Liu, X.; Zhang, X.; Zeng, Y. Engineered Red Blood Cell Biomimetic Nanovesicle with Oxygen Self-Supply for Near-Infrared-II Fluorescence-Guided Synergetic Chemo-Photodynamic Therapy against Hypoxic Tumors. ACS Appl. Mater. Interfaces 2021, 13, 52435–52449. [Google Scholar] [CrossRef]
- Yang, C.; Merlin, D. Nanoparticle-Mediated Drug Delivery Systems for The Treatment Of IBD: Current Perspectives. Int. J. Nanomed. 2019, 14, 8875–8889. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Naqvi, S.; Shams, S.; Sheikh, R.A.; Al-Abbasi, F.A.; Asseri, A.H.; Baig, M.R.; Kumar, V. Nanomedicines: Intervention in Inflammatory Pathways of Cancer. Inflammopharmacology 2023, 31, 1199–1221. [Google Scholar] [CrossRef] [PubMed]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor Targeting via EPR: Strategies to Enhance Patient Responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Yuan, H.; Song, Y.; Hu, H.; Wen, B.; He, M.; Zhang, H.; Li, Y.; Li, F.; Shu, P.; et al. Reappraisal of Anticancer Nanomedicine Design Criteria in Three Types of Preclinical Cancer Models for Better Clinical Translation. Biomaterials 2021, 275, 120910. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhang, Y.; Li, C.; Chen, G.; Kang, X.; Wang, Q. Enhanced Nanodrug Delivery to Solid Tumors Based on a Tumor Vasculature-Targeted Strategy. Adv. Funct. Mater. 2016, 26, 4192–4200. [Google Scholar] [CrossRef]
- Wang, M.; Yan, D.; Wang, M.; Wu, Q.; Song, R.; Huang, Y.; Rao, J.; Wang, D.; Zhou, F.; Tang, B.Z. A Versatile 980 Nm Absorbing Aggregation-Induced Emission Luminogen for NIR-II Imaging-Guided Synergistic Photo-Immunotherapy Against Advanced Pancreatic Cancer. Adv. Funct. Mater. 2022, 32, 2205371. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, J.; Tian, Y.; Sun, Q.; Sun, D.; Liu, D.; Wang, F.; Xu, H.; Ying, G.; Wang, J.; et al. Thiophene Donor for NIR-II Fluorescence Imaging-Guided Photothermal/Photodynamic/Chemo Combination Therapy. Acta Biomater. 2021, 127, 287–297. [Google Scholar] [CrossRef]
- Li, Y.; Fan, X.; Li, Y.; Zhu, L.; Chen, R.; Zhang, Y.; Ni, H.; Xia, Q.; Feng, Z.; Tang, B.Z.; et al. Biologically Excretable AIE Nanoparticles Wear Tumor Cell-Derived “Exosome Caps” for Efficient NIR-II Fluorescence Imaging-Guided Photothermal Therapy. Nano Today 2021, 41, 101333. [Google Scholar] [CrossRef]
- Betancur, P.A.; Abraham, B.J.; Yiu, Y.Y.; Willingham, S.B.; Khameneh, F.; Zarnegar, M.; Kuo, A.H.; McKenna, K.; Kojima, Y.; Leeper, N.J.; et al. A CD47-Associated Super-Enhancer Links pro-Inflammatory Signalling to CD47 Upregulation in Breast Cancer. Nat. Commun. 2017, 8, 14802. [Google Scholar] [CrossRef]
- Corma, A.; Botella, P.; Rivero-Buceta, E. Silica-Based Stimuli-Responsive Systems for Antitumor Drug Delivery and Controlled Release. Pharmaceutics 2022, 14, 110. [Google Scholar] [CrossRef]
- Chen, M.; Cui, Y.; Hao, W.; Fan, Y.; Zhang, J.; Liu, Q.; Jiang, M.; Yang, Y.; Wang, Y.; Gao, C. Ligand-Modified Homologous Targeted Cancer Cell Membrane Biomimetic Nanostructured Lipid Carriers for Glioma Therapy. Drug Deliv. 2021, 28, 2241–2255. [Google Scholar] [CrossRef]
- Zhang, X.; He, S.; Ding, B.; Qu, C.; Zhang, Q.; Chen, H.; Sun, Y.; Fang, H.; Long, Y.; Zhang, R.; et al. Cancer Cell Membrane-Coated Rare Earth Doped Nanoparticles for Tumor Surgery Navigation in NIR-II Imaging Window. Chem. Eng. J. 2020, 385, 123959. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Lin, Y.; Zhou, H.; He, H.; Ma, J.-J.; Luo, M.-Y.; Zhang, Z.-L.; Pang, D.-W. Cell Membrane-Camouflaged NIR II Fluorescent Ag2Te Quantum Dots-Based Nanobioprobes for Enhanced In Vivo Homotypic Tumor Imaging. Adv. Healthc. Mater. 2019, 8, e1900341. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shou, K.; Chen, S.; Qu, C.; Wang, Z.; Jiang, L.; Zhu, M.; Ding, B.; Qian, K.; Ji, A.; et al. Smart Self-Assembly Amphiphilic Cyclopeptide-Dye for Near-Infrared Window-II Imaging. Adv. Mater. 2021, 33, 2006902. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Liu, X.; Tian, Y.; Cheng, Y.; Tang, L.; Lin, H. Smart NIR-II Croconaine Dye-Peptide for Enhanced Photo-Sonotheranostics of Hepatocellular Carcinoma. Theranostics 2022, 12, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.U. Nanomedicine Penetration to Tumor: Challenges, and Advanced Strategies to Tackle This Issue. Cancers 2022, 14, 2904. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Chen, Q.; Liu, T.; Li, X.; Pei, P.; Zhou, L.; Zhou, S.; Zhang, R.; Liang, K.; Dong, J.; et al. Site-Selective Superassembly of Biomimetic Nanorobots Enabling Deep Penetration into Tumor with Stiff Stroma. Nat. Commun. 2023, 14, 4628. [Google Scholar] [CrossRef]
- Bugno, J.; Hsu, H.-J.; Pearson, R.M.; Noh, H.; Hong, S. Size and Surface Charge of Engineered Poly(Amidoamine) Dendrimers Modulate Tumor Accumulation and Penetration: A Model Study Using Multicellular Tumor Spheroids. Mol. Pharm. 2016, 13, 2155–2163. [Google Scholar] [CrossRef]
- Han, Y.; Liu, H.; Fan, M.; Gao, S.; Fan, D.; Wang, Z.; Chang, J.; Zhang, J.; Ge, K. Near-Infrared-II Photothermal Ultra-Small Carbon Dots Promoting Anticancer Efficiency by Enhancing Tumor Penetration. J. Colloid. Interf. Sci. 2022, 616, 595–604. [Google Scholar] [CrossRef]
- Wei, D.; Yu, Y.; Huang, Y.; Jiang, Y.; Zhao, Y.; Nie, Z.; Wang, F.; Ma, W.; Yu, Z.; Huang, Y.; et al. A Near-Infrared-II Polymer with Tandem Fluorophores Demonstrates Superior Biodegradability for Simultaneous Drug Tracking and Treatment Efficacy Feedback. ACS Nano 2021, 15, 5428–5438. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, C.; Yang, H.; Li, T.; Ling, S.; Zhang, Y.; Wu, F.; Liu, X.; Liu, S.; Fan, C.; et al. Programmed Remodeling of the Tumor Milieu to Enhance NK Cell Immunotherapy Combined with Chemotherapy for Pancreatic Cancer. Nano Lett. 2024, 24, 3421–3431. [Google Scholar] [CrossRef]
- Jia, R.; Teng, L.; Gao, L.; Su, T.; Fu, L.; Qiu, Z.; Bi, Y. Advances in Multiple Stimuli-Responsive Drug-Delivery Systems for Cancer Therapy. Int. J. Nanomed. 2021, 16, 1525–1551. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dai, W.; He, B.; Zhang, H.; Wang, X.; Wang, Y.; Zhang, Q. Current Multistage Drug Delivery Systems Based on the Tumor Microenvironment. Theranostics 2017, 7, 538–558. [Google Scholar] [CrossRef]
- Khawar, I.A.; Kim, J.H.; Kuh, H.-J. Improving Drug Delivery to Solid Tumors: Priming the Tumor Microenvironment. J. Control. Release 2015, 201, 78–89. [Google Scholar] [CrossRef]
- Haider, T.; Sandha, K.K.; Soni, V.; Gupta, P.N. Recent Advances in Tumor Microenvironment Associated Therapeutic Strategies and Evaluation Models. Mater. Sci. Eng. C 2020, 116, 111229. [Google Scholar] [CrossRef]
- Loria, R.; Giliberti, C.; Bedini, A.; Palomba, R.; Caracciolo, G.; Ceci, P.; Falvo, E.; Marconi, R.; Falcioni, R.; Bossi, G.; et al. Very Low Intensity Ultrasounds as a New Strategy to Improve Selective Delivery of Nanoparticles-Complexes in Cancer Cells. J. Exp. Clin. Cancer Res. 2019, 38, 1. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.K.; Chung, K.H.; Yoon, T.H. Heterogeneity in Biodistribution and Cytotoxicity of Silver Nanoparticles in Pulmonary Adenocarcinoma Human Cells. Nanomaterials 2019, 10, 36. [Google Scholar] [CrossRef]
- Choi, G.; Rejinold, N.S.; Piao, H.; Choy, J.-H. Inorganic-Inorganic Nanohybrids for Drug Delivery, Imaging and Photo-Therapy: Recent Developments and Future Scope. Chem. Sci. 2021, 12, 5044–5063. [Google Scholar] [CrossRef]
- Azizi, M.; Jahanban-Esfahlan, R.; Samadian, H.; Hamidi, M.; Seidi, K.; Dolatshahi-Pirouz, A.; Yazdi, A.A.; Shavandi, A.; Laurent, S.; Be Omide Hagh, M.; et al. Multifunctional Nanostructures: Intelligent Design to Overcome Biological Barriers. Mater. Today Bio. 2023, 20, 100672. [Google Scholar] [CrossRef]
- Sohrabi Kashani, A.; Packirisamy, M. Cancer-Nano-Interaction: From Cellular Uptake to Mechanobiological Responses. Int. J. Mol. Sci. 2021, 22, 9587. [Google Scholar] [CrossRef]
- Antaris, A.L.; Chen, H.; Cheng, K.; Sun, Y.; Hong, G.; Qu, C.; Diao, S.; Deng, Z.; Hu, X.; Zhang, B.; et al. A Small-Molecule Dye for NIR-II Imaging. Nat. Mater. 2016, 15, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, Z.; Zhu, S.; Wan, H.; Yue, J.; Ma, H.; Ma, R.; Yang, Q.; Wang, Z.; Li, Q.; et al. Molecular Cancer Imaging in the Second Near-Infrared Window Using a Renal-Excreted NIR-II Fluorophore-Peptide Probe. Adv. Mater. 2018, 30, 1800106. [Google Scholar] [CrossRef]
- Wen, Q.; Zhang, Y.; Li, C.; Ling, S.; Yang, X.; Chen, G.; Yang, Y.; Wang, Q. NIR-II Fluorescent Self-Assembled Peptide Nanochain for Ultrasensitive Detection of Peritoneal Metastasis. Angew. Chem. Int. Ed. Engl. 2019, 58, 11001–11006. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Diao, S.; Ni, X.; Zhang, D.; Yi, W.; Jian, C.; Hu, X.; Li, D.; Yu, A.; Zhou, W.; et al. Peptide-Based Semiconducting Polymer Nanoparticles for Osteosarcoma-Targeted NIR-II Fluorescence/NIR-I Photoacoustic Dual-Model Imaging and Photothermal/Photodynamic Therapies. J. Nanobiotechnol. 2022, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wan, J.; Mo, F.; Tang, D.; Xiao, H.; Li, Z.; Jia, J.; Liu, T. Targeting Bone Tumor and Subcellular Endoplasmic Reticulum via Near Infrared II Fluorescent Polymer for Photodynamic-Immunotherapy to Break the Step-Reduction Delivery Dilemma. Adv. Sci. 2022, 9, 2201819. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, S.; Yu, P.; Yan, K.; Ming, J.; Yao, C.; He, Z.; El-Toni, A.M.; Khan, A.; Zhu, X.; et al. NIR-II Cell Endocytosis-Activated Fluorescent Probes for in Vivo High-Contrast Bioimaging Diagnostics. Chem. Sci. 2021, 12, 10474–10482. [Google Scholar] [CrossRef]
- Wei, X.; Song, M.; Jiang, G.; Liang, M.; Chen, C.; Yang, Z.; Zou, L. Progress in Advanced Nanotherapeutics for Enhanced Photodynamic Immunotherapy of Tumor. Theranostics 2022, 12, 5272–5298. [Google Scholar] [CrossRef]
- Dasgupta, A.; Biancacci, I.; Kiessling, F.; Lammers, T. Imaging-Assisted Anticancer Nanotherapy. Theranostics 2020, 10, 956–967. [Google Scholar] [CrossRef]
- Mishra, S.; Bhatt, T.; Kumar, H.; Jain, R.; Shilpi, S.; Jain, V. Nanoconstructs for Theranostic Application in Cancer: Challenges and Strategies to Enhance the Delivery. Front. Pharmacol. 2023, 14, 1101320. [Google Scholar] [CrossRef]
- Liu, K.; Yao, Y.; Xue, S.; Zhang, M.; Li, D.; Xu, T.; Zhi, F.; Liu, Y.; Ding, D. Recent Advances of Tumor Microenvironment-Responsive Nanomedicines-Energized Combined Phototherapy of Cancers. Pharmaceutics 2023, 15, 2480. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, L.; Wang, W.; Li, X.; Zhang, F. In Vivo Gastrointestinal Drug-Release Monitoring through Second Near-Infrared Window Fluorescent Bioimaging with Orally Delivered Microcarriers. Nat. Commun. 2017, 8, 14702. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Yang, X.; Li, C.; Zhang, Y.; Yang, H.; Chen, G.; Wang, Q. Tumor Microenvironment-Activated NIR-II Nanotheranostic System for Precise Diagnosis and Treatment of Peritoneal Metastasis. Angew. Chem. Int. Edit. 2020, 59, 7219–7223. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Yu, Y.; Zhang, J.; Dong, X.; Liu, C.; Xiao, H. Self-Sacrificially Degradable Pseudo-Semiconducting Polymer Nanoparticles That Integrate NIR-II Fluorescence Bioimaging, Photodynamic Immunotherapy, and Photo-Activated Chemotherapy. Adv. Mater. 2022, 34, 2203820. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, J.; Chen, B.; Ge, X.; Zhang, X.; Gao, S.; Ma, Q.; Song, J. NIR-II Fluorescent Activatable Drug Delivery Nanoplatform for Cancer-Targeted Combined Photodynamic and Chemotherapy. ACS Appl. Bio Mater. 2022, 5, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, F.; Chen, K.; Sun, Z.; Wang, Z.; Xue, Y.; Li, M.; Fan, Q.; Shen, Q.; Zhao, Q. An Activatable Phototheranostic Nanoplatform for Tumor Specific NIR-II Fluorescence Imaging and Synergistic NIR-II Photothermal-Chemodynamic Therapy. Small 2023, 19, 2206053. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Hou, P.; Zhang, Y.; Ao, R.; Su, L.; Jiang, Y.; Zhang, Y.; Cai, H.; Wang, J.; Chen, Q.; et al. Singlet Oxygen Generation in Dark-Hypoxia by Catalytic Microenvironment-Tailored Nanoreactors for NIR-II Fluorescence-Monitored Chemodynamic Therapy. Angew. Chem. Int. Ed. Engl. 2021, 60, 15006–15012. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Feng, H.; Su, L.; Zhang, X.; Liu, L.; Fu, F.; Yang, H.; Song, J. An Activatable Hybrid Organic–Inorganic Nanocomposite as Early Evaluation System of Therapy Effect. Angew. Chem. Int. Ed. Engl. 2022, 61, e202112237. [Google Scholar] [CrossRef]
- Su, L.; Chen, Y.; Huo, H.; Liao, N.; Wu, Y.; Ge, X.; Guo, Z.; Chen, Z.; Zhang, X.; Song, J. NIR-II Ratiometric Chemiluminescent/Fluorescent Reporters for Real-Time Monitoring and Evaluating Cancer Photodynamic Therapy Efficacy. Small 2022, 18, 2202551. [Google Scholar] [CrossRef]
- Yuan, M.; Fang, X.; Liu, J.; Yang, K.; Xiao, S.; Yang, S.; Du, W.; Song, J. NIR-II Self-Luminous Molecular Probe for In Vivo Inflammation Tracking and Cancer PDT Effect Self-Evaluating. Small 2023, 19, 2206666. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, D.Y. Nanomedicine in Clinical Photodynamic Therapy for the Treatment of Brain Tumors. Biomedicines 2022, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Lin, L.; Chao, Z.; Shao, C.; Chen, Z.; Wei, Z.; Lu, J.; Huang, Y.; Li, L.; Liu, Q.; et al. Organic Spherical Nucleic Acids for the Transport of a NIR-II-Emitting Dye Across the Blood-Brain Barrier. Angew. Chem. Int. Ed. Engl. 2020, 59, 9702–9710. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Y.; Chen, G.; Hu, F.; Zhao, K.; Wang, Q. Engineered Multifunctional Nanomedicine for Simultaneous Stereotactic Chemotherapy and Inhibited Osteolysis in an Orthotopic Model of Bone Metastasis. Adv. Mater. 2017, 29, 1605754. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Li, T.; Wu, F.; Ren, F.; Xue, S.; Li, C. Advanced NIR-II Fluorescence Imaging Technology for Precise Evaluation of Nanomedicine Delivery in Cancer Therapy. Chemosensors 2024, 12, 113. https://doi.org/10.3390/chemosensors12060113
Li M, Li T, Wu F, Ren F, Xue S, Li C. Advanced NIR-II Fluorescence Imaging Technology for Precise Evaluation of Nanomedicine Delivery in Cancer Therapy. Chemosensors. 2024; 12(6):113. https://doi.org/10.3390/chemosensors12060113
Chicago/Turabian StyleLi, Meng, Tuanwei Li, Feng Wu, Feng Ren, Sumei Xue, and Chunyan Li. 2024. "Advanced NIR-II Fluorescence Imaging Technology for Precise Evaluation of Nanomedicine Delivery in Cancer Therapy" Chemosensors 12, no. 6: 113. https://doi.org/10.3390/chemosensors12060113
APA StyleLi, M., Li, T., Wu, F., Ren, F., Xue, S., & Li, C. (2024). Advanced NIR-II Fluorescence Imaging Technology for Precise Evaluation of Nanomedicine Delivery in Cancer Therapy. Chemosensors, 12(6), 113. https://doi.org/10.3390/chemosensors12060113




