Response Surface Modelling of Six Organic Acids from Pinellia ternata (Thunb.) Breit by Ultrasound-Assisted Extraction and Its Determination by High-Performance Liquid Chromatography Coupled to Triple Quadrupole Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Standard Solutions Preparation
2.3. Single Factor Ultrasonic Extraction Assay
2.4. Experimental Design and Statistical Analysis of Response Surface Methodology
2.5. Method of Application of Different Production Regions on P. ternata
2.6. HPLC-QqQ-MS/MS Analysis
2.7. Method Validation
3. Results
3.1. Optimization of Chromatographic Conditions
3.2. Establishment of Mass Spectrometry Conditions
3.3. Linearity, Limit of Detection, and Limit of Quantification of the Established HPLC-QqQ-MS/MS Method
3.4. One-Variable-at-a-Time (OVAT) Experiment
3.5. Response Surface Methodology Optimization
3.6. Practical Application of the Optimized Method for Determining OA Content of P. ternata from Different Regions
3.7. Precision, Reproducibility, Stability, and Recovery Rates of the Established Ultrasonic Extraction and HPLC-QqQ-MS/MS Methods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, W.; Li, N.; Jiang, E.; Zhang, C.; Huang, Y.; Tan, L.; Chen, R.; Wu, C.; Huang, Q. A review of traditional and current processing methods used to decrease the toxicity of the rhizome of Pinellia ternata in traditional Chinese medicine. J. Ethnopharmacol. 2022, 299, 115696. [Google Scholar] [CrossRef]
- Chen, C.; Sun, Y.; Wang, Z.; Huang, Z.; Zou, Y.; Yang, F.; Hu, J.; Cheng, H.; Shen, C.; Wang, S. Pinellia genus: A systematic review of active ingredients, pharmacological effects and action mechanism, toxicological evaluation, and multi-omics application. Gene 2023, 870, 147426. [Google Scholar] [CrossRef]
- Yang, L.; Gao, H.; Luo, W.; He, D. Research progress and prospect of Pinellia ternata. Anhui Agric. Sci. Bull. 2023, 02, 33–38. [Google Scholar]
- Yang, B.Y.; Lin, M.; Wu, F.M.; Xia, Q.; Zhou, H.; Liang, P. Methodological study on quality evaluation of crude and processed Pinelliae Rhizoma based on antitussive bioassay. Chin. Tradit. Herb. Drugs 2015, 46, 2586–2592. [Google Scholar]
- Yang, B.Y.; Lin, M.; Ren, M.; Zhao, C.Y.; Wei, H.; Peng, L. Spectrum-effect relationship of antitussive effect by total organic acids in crude and four processed Pinellia ternata based on gray relative analysis method. Chin. Tradit. Herb. Drugs 2016, 47, 2301–2307. [Google Scholar]
- Wen, Q.; Zhang, Y.; Zhang, J.; Zhao, H. Simultaneous determination of 6 organic acids, 3 nucleosides, and ephedrine in Pinellia ternata by HPLC. J. Chin. Pharm. Sci. 2016, 25, 906–913. [Google Scholar]
- Lee, J.Y.; Park, N.H.; Lee, W.; Kim, E.H.; Jin, Y.H.; Seo, E.K.; Hong, J. Comprehensive chemical profiling of Pinellia species tuber and processed Pinellia tuber by gas chromatography–mass spectrometry and liquid chromatography–atmospheric pressure chemical ionization–tandem mass spectrometry. J. Chromatogr. A 2016, 1471, 164–177. [Google Scholar] [CrossRef]
- Uclés, S.; Lozano, A.; Sosa, A.; Vázquez, P.P.; Valverde, A.; Fernández-Alba, A.R. Matrix interference evaluation employing GC and LC coupled to triple quadrupole tandem mass spectrometry. Talanta 2017, 174, 72–81. [Google Scholar] [CrossRef]
- Dong, M.Y.; Wang, Y.L.; Tang, H.R.; Huang, Q.X.; Lei, H.H. Quantitative analysis of 10 classes of phospholipids by ultrahigh-performance liquid chromatography tandem triple-quadrupole mass spectrometry. Analyst 2019, 144, 3980–3987. [Google Scholar]
- Ji, W.; Xu, P.; Yin, J.; Chen, J. Effect of extraction methods on the chemical components and taste quality of green tea extract. Food Chem. 2018, 248, 146–154. [Google Scholar]
- Díaz-de-Cerio, E.; Tylewicz, U.; Verardo, V.; Fernández-Gutiérrez, A.; Segura-Carretero, A.; Romani, S. Design of sonotrode ultrasound-assisted extraction of phenolic compounds from Psidium guajava L. leaves. Food Anal. Method 2017, 10, 2781–2791. [Google Scholar] [CrossRef]
- Seo, S.; Kim, K. Antioxidant activities of Aster glehni extracted with different solvent. Iran. J. Public Health 2019, 48, 176–178. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.; Li, J.; Zhang, C.; Li, G. Rectification extraction of Chinese herbs’ volatile oils and comparison with conventional steam distillation. Sep. Purif. Technol. 2010, 77, 261–268. [Google Scholar]
- Fernando, G.S.N.; Wood, K.; Papaioannou, E.H.; Marshall, L.J.; Sergeeva, N.N.; Boesch, C. Application of an Ultrasound-Assisted Extraction Method to Recover Betalains and Polyphenols from Red Beetroot Waste. ACS Sustain. Chem. Eng. 2021, 9, 8736–8747. [Google Scholar] [CrossRef]
- Fernandes, J.B.; Palma, M.; Nebo, L.; Varela, R.M. Microwave-Assisted Extraction of Ricinine from Ricinus communis Leaves. Antioxidants 2019, 8, 438. [Google Scholar]
- Atwi-Ghaddar, S.; Lesellier, E.; Destandau, E. Optimization of supercritical fluid extraction of polar flavonoids from Robinia pseudoacacia L. heartwood. J. CO2 Util. 2023, 20, 102440. [Google Scholar] [CrossRef]
- Liu, H.; Yan, Y.; Wang, X.; Liu, X.; Qin, G. Emulsifying and structural properties of polysaccharides extracted from Chinese yam by an enzyme-assisted method. LWT 2019, 111, 242–251. [Google Scholar] [CrossRef]
- Belokurov, S.S.; Narkevich, I.A.; Flisyuk, E.V.; Kaukhova, I.E.; Aroyan, M.V. Modern extraction methods for medicinal plant raw material. Pharm. Chem. J. 2019, 53, 559–563. [Google Scholar] [CrossRef]
- Zhi, W.W.; Wei, T.C.; Jen, Y.W.; Long, W.H.; Lin, C.C.; Der, C.J.; Kuang, L.M.; Tung, L.W. Comparative study on the physicochemical and functional properties of the mucilage in the carpel of Nymphaea odorata using ultrasonic and classical heating extractions. Int. J. Biol. Macromol. 2018, 117, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, F.; Tan, J.K.; Faudzi, S.M.M.; Rahman, M.B.A.; Ashari, S.E. Ultrasound-assisted extraction conditions optimisation using response surface methodology from Mitragyna speciosa (Korth.) Havil leaves. Ultrason. Sonochem. 2021, 81, 105851. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Stachniuk, A.; Szmagara, A.; Czeczko, R.; Fornal, E. Determination of six kinds on organic in fruits and vegetables by LC-MS method. Food Sci. Technol. 2016, 41, 275–279. [Google Scholar]
- Li, D.M.; Zhang, S.W.; Gao, X.N.; Sun, J.Y.; Li, Y.J.; Li, W.P. Research progress on main chemical constituents and detection methods of Pinellia ternata. Sci. Tech. Informat. Gansu 2023, 52, 37–41. [Google Scholar]
- Zhang, Y.Q.; Yi, X.H.; Yan, G.M.; Yuan, J.L. Determination of total organic acids in different Pinellia ternata by potentiometric titration. Jiangxi J. Tradit. Chin. Med. 2019, 50, 64–65. [Google Scholar]
- Fu, S.; Zhang, J.; Li, T.; Wang, S.; Ding, W.; Zhao, M.; Du, Y.F.; Wang, Q.; Jia, J. Multi-responses extraction optimization based on response surface methodology combined with polarity switching HPLC–MS/MS for the simultaneous quantitation of 11 compounds in Cortex Fraxini: Application to four species of Cortex Fraxini and its 3 confusable species. J. Pharmaceut Biomed. 2014, 91, 210–221. [Google Scholar]
- Daneshvand, B.; Ara, K.M.; Raofie, F. Comparison of supercritical fluid extraction and ultrasound-assisted extraction of fatty acids from quince (Cydonia oblonga Miller) seed using response surface methodology and central composite design. J. Chromatogr. A 2012, 1252, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.M.; Li, S.Q.; Niu, X.Y.; Shen, L.W.; Li, L.; Qin, W. Optimizing ultrasonic extraction of polysaccharides from Chuanminshen violaceum based on orthogonal experiments design. Sci. Technol. Food Indust. 2014, 35, 306–309,322. [Google Scholar]
- Liu, Y.; Guo, Y.Y.; Xu, Y.L.; Zhang, Z.F.; Wei, Y.; Feng, S.X.; Yan, Z.G. Optimization in ultrasonic extraction process of rubusoside by response surface methodology. Chem. Bioeng. 2023, 40, 34–38,63. [Google Scholar]
- Wu, S.Z.; Wang, C.; Gui, S.Y.; Peng, C.; Luo, J.P.; Li, Q.M. Optimization of vacuum-coupled uitrasonic extraction process for Ganderma lucidum triterpenoids and their anti-parkinsonian activity. Chin. Tradit. Pat. Med. 2023, 45, 2822–2827. [Google Scholar]
- Zhang, D.; Jiang, X. Extraction and antibacterial activity of Fructus mume organic acids. Chin. J. Bioprocess. Eng. 2018, 16, 47–52. [Google Scholar]
- Hatambeygi, N.; Abedi, G.; Talebi, M. Method development and validation for optimised separation of salicylic, acetyl salicylic and ascorbic acid in pharmaceutical formulations by hydrophilic interaction chromatography and response surface methodology. J. Chromatogr. A 2011, 1218, 5995–6003. [Google Scholar] [CrossRef]
- Sun, L.M.; Zhang, B.; Wang, Y.C.; He, H.K.; Chen, X.G.; Wang, S.J. Metabolomic analysis of raw Pinelliae Rhizoma and its alum-processed products via UPLC–MS and their cytotoxicity. Biomed. Chromatogr. 2019, 33, e4411. [Google Scholar] [CrossRef]
- Lv, L.L.; Huang, W.; Huang, Y.Y.; Wang, L.; Sun, R. Experimental Research on Influences on the Content of Toxical Substances and Acute Toxicity of Different Producing Areas’ Rhizoma pinelliae. Chin. J. Pharmacovigil. 2010, 7, 649. [Google Scholar]
- Lai, Y.Y.; Jing, Y.; Li, H.Y.; Liu, J.L.; Li, M. A study on the effect of sodium metabisulfite on the quality of Pinelliae Rhizoma. Chin. Tradit. Pat. Med. 2020, 42, 965–968. [Google Scholar]
- Liao, F.J.; Liu, Y.Y.; He, H.; Chen, L.; Chen, H.P.; Wang, F.; Hu, Y.; Liu, Y.P. Comparison on chemical constituents and irritant toxicity of Banxia of different diameters. Pharmacol. Clin. Chin. Mater. Medica 2023, 39, 88–95. [Google Scholar]
- Jing, Y.; Lai, Y.; Chen, H.; Li, M.; Zhou, J.; Lan, Z. Study on the identification of Pinelliae Rhizoma and Pinelliae pedatisectae rhizoma based on the characteristic component triglochinic acid. RSC Adv. 2019, 9, 11774–11780. [Google Scholar] [CrossRef]




| Independent Variables | Range and Level | |||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| A | Solid–liquid ratio | 1:10 | 1:50 | 1:80 |
| B | Ultrasonic time (min) | 20 | 60 | 100 |
| C | Ultrasonic temperature (°C) | 20 | 50 | 80 |
| Solid–Liquid Ratio (g/mL) | Extraction Time (min) | Extraction Temperature (°C) | |
|---|---|---|---|
| 1 | 100 | 60 | 55 |
| 2 | 100 | 60 | 55 |
| 3 | 100 | 100 | 80 |
| 4 | 150 | 60 | 30 |
| 5 | 150 | 60 | 80 |
| 6 | 100 | 20 | 30 |
| 7 | 150 | 100 | 55 |
| 8 | 100 | 60 | 55 |
| 9 | 50 | 60 | 30 |
| 10 | 50 | 100 | 55 |
| 11 | 150 | 20 | 55 |
| 12 | 100 | 20 | 80 |
| 13 | 50 | 60 | 80 |
| 14 | 100 | 60 | 55 |
| 15 | 50 | 20 | 55 |
| 16 | 100 | 60 | 55 |
| 17 | 100 | 100 | 30 |
| Compound | Structural Formula | MF | MW | Precursor Ion/(m/z) | DP/V | Product Ion/(m/z) | CE/eV | tR/min |
|---|---|---|---|---|---|---|---|---|
| Malic acid |  | C4H6O5 | 134.09 | 133.1 | −67 | 115.1 | 9 | 1.685 |
| 71.2 | 13 | |||||||
| Succinic acid |  | C4H6O4 | 118.09 | 117.1 | −62 | 73.1 | 9 | 3.277 |
| 99.1 | 5 | |||||||
| Citric acid | 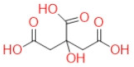 | C6H8O7 | 192.13 | 191.1 | −72 | 111.1 | 9 | 2.612 |
| 87.1 | 17 | |||||||
| Cis-aconite acid | 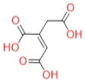 | C6H6O6 | 174.11 | 173.1 | −60 | 85 | 9 | 2.782 |
| 129.1 | 5 | |||||||
| Fumaric acid | 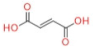 | C4H4O4 | 116.07 | 115.1 | −48 | 71.1 | 1 | 2.982 |
| 32.1 | 13 | |||||||
| Oxalic acid | 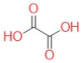 | H2C2O4 | 90.3 | 89 | −48 | 61 | 5 | 1.289 |
| 45 |
| Organic Acid Species | Number | N: Sample Content/mg | M: Add the Amount of Standard/mg | O: Detected Content/mg | Recovery/% | Average Recovery/% | RSD/% |
|---|---|---|---|---|---|---|---|
| Malic acid | 1 | 4.37 | 4.42 | 8.83 | 100.90 | 98.91 | 2.95 |
| 2 | 4.39 | 8.85 | 100.90 | ||||
| 3 | 4.38 | 8.73 | 98.42 | ||||
| 4 | 4.34 | 8.63 | 97.06 | ||||
| 5 | 4.35 | 8.65 | 97.29 | ||||
| 6 | 4.29 | 8.92 | 104.75 | ||||
| Oxalic acid | 1 | 0.95 | 0.98 | 1.97 | 104.08 | 107.14 | 4.41 |
| 2 | 0.91 | 1.99 | 110.20 | ||||
| 3 | 0.96 | 2.03 | 109.18 | ||||
| 4 | 0.99 | 2.03 | 106.12 | ||||
| 5 | 0.91 | 1.95 | 106.12 | ||||
| 6 | 0.92 | 2.07 | 117.35 | ||||
| Citric acid | 1 | 13.75 | 13.75 | 27.63 | 100.95 | 101.50 | 1.67 |
| 2 | 13.73 | 27.75 | 101.96 | ||||
| 3 | 13.74 | 27.66 | 101.24 | ||||
| 4 | 12.96 | 26.92 | 101.53 | ||||
| 5 | 13.64 | 27.64 | 101.82 | ||||
| 6 | 13.76 | 27.16 | 97.45 | ||||
| Cis-aconite acid | 1 | 0.84 | 0.8 | 1.63 | 98.75 | 97.75 | 3.91 |
| 2 | 0.83 | 1.59 | 95.00 | ||||
| 3 | 0.82 | 1.65 | 103.75 | ||||
| 4 | 0.85 | 1.62 | 96.25 | ||||
| 5 | 0.82 | 1.58 | 95.00 | ||||
| 6 | 0.79 | 1.61 | 102.50 | ||||
| Cis-aconite acid | 1 | 0.48 | 0.45 | 0.96 | 106.67 | 103.56 | 4.50 |
| 2 | 0.44 | 0.90 | 102.22 | ||||
| 3 | 0.43 | 0.91 | 106.67 | ||||
| 4 | 0.44 | 0.88 | 97.78 | ||||
| 5 | 0.49 | 0.96 | 104.44 | ||||
| 6 | 0.46 | 0.89 | 95.56 | ||||
| Succinic acid | 1 | 0.63 | 0.63 | 1.24 | 96.83 | 97.78 | 2.39 |
| 2 | 0.61 | 1.22 | 96.83 | ||||
| 3 | 0.65 | 1.27 | 98.41 | ||||
| 4 | 0.59 | 1.21 | 98.41 | ||||
| 5 | 0.64 | 1.26 | 98.41 | ||||
| 6 | 0.57 | 1.22 | 103.17 |
| Compound | Linear Equation | R2 | Linear Range (ng/mL) | LLOD (ng/mL) | LLOQ (ng/mL) |
|---|---|---|---|---|---|
| Oxalic acid | Y = 0.06188X + 7.774199 | R2 = 0.9991 | 1.872–7488 | 0.604 | 1.872 |
| Malic acid | Y = 1.422647X + 123.057655 | R2 = 0.9946 | 0.614–12624 | 0.138 | 0.614 |
| Citric acid | Y = 2.357504X − 46.699725 | R2 = 0.9998 | 0.819–89136 | 0.327 | 0.819 |
| Cis-aconite acid | Y = 1.098471X − 153.403601 | R2 = 0.9923 | 1.252–56064 | 0.398 | 1.252 |
| Fumaric acid | Y = 0.725715X + 4.070693 | R2 = 0.9998 | 0.724–5792 | 0.284 | 0.724 |
| Succinic acid | Y = 2.157399X + 2.342711 | R2 = 0.9998 | 0.508–5080 | 0.194 | 0.508 |
| Region | Malic Acid (mg/g) | Oxalic Acid (mg/g) | Citric Acid (mg/g) | Cis-Aconite Acid (mg/g) | Fumaric Acid (mg/g) | Succinic Acid (mg/g) | Total Acid (mg/g) | |
|---|---|---|---|---|---|---|---|---|
| 1 | Huangshan City, Anhui Province | 6.58 ± 0.19 | 0.49 ± 0.02 | 14.56 ± 0.47 | 0.74 ± 0.03 | 0.52 ± 0.01 | 0.54 ± 0.01 | 23.42 ± 0.63 |
| 2 | Chongqing City | 2.36 ± 0.36 | 0.45 ± 0.02 | 7.68 ± 0.35 | 0.29 ± 0.04 | 0.40 ± 0.01 | 0.55 ± 0.03 | 11.56 ± 0.37 |
| 3 | Xinyang City, Henan Province | 4.40 ± 0.28 | 1.12 ± 0.05 | 14.67 ± 0.33 | 0.51 ± 0.04 | 0.50 ± 0.01 | 0.45 ± 0.03 | 21.65 ± 0.28 |
| 4 | Baoding City, Hebei Province | 1.36 ± 0.13 | 0.82 ± 0.06 | 3.50 ± 0.12 | 0.11 ± 0.01 | 0.13 ± 0.01 | 0.26 ± 0.01 | 6.18 ± 0.11 |
| 5 | Qianjiang City, Hubei Province | 3.39 ± 0.21 | 0.56 ± 0.04 | 15.44 ± 1.16 | 0.27 ± 0.01 | 0.33 ± 0.01 | 2.57 ± 0.03 | 22.55 ± 1.26 |
| 6 | Qianjiang City, Hubei Province | 4.47 ± 0.35 | 0.95 ± 0.05 | 13.75 ± 0.34 | 0.84 ± 0.04 | 0.48 ± 0.01 | 0.63 ± 0.01 | 21.11 ± 0.54 |
| 7 | Tianmen City, Hubei Province | 4.07 ± 0.23 | 1.58 ± 0.29 | 16.65 ± 0.55 | 0.78 ± 0.01 | 0.44 ± 0.02 | 0.57 ± 0.02 | 24.09 ± 0.42 |
| 8 | Yichang City, Hubei Province | 4.26 ± 0.41 | 0.85 ± 0.11 | 12.61 ± 0.23 | 0.72 ± 0.05 | 0.52 ± 0.03 | 0.49 ± 0.01 | 19.45 ± 0.65 |
| 9 | Shiyan City, Hubei Province | 5.47 ± 0.28 | 0.24 ± 0.02 | 15.89 ± 0.11 | 0.66 ± 0.01 | 0.45 ± 0.01 | 0.76 ± 0.01 | 23.47 ± 0.29 |
| 10 | Huaihua City, Hunan Province | 4.90 ± 0.05 | 1.01 ± 0.07 | 16.84 ± 0.37 | 0.84 ± 0.05 | 0.56 ± 0.01 | 0.78 ± 0.01 | 24.93 ± 0.53 |
| 11 | Yingtan City, Jiangxi Province | 3.28 ± 0.23 | 0.21 ± 0.01 | 9.33 ± 0.42 | 0.47 ± 0.01 | 0.21 ± 0.01 | 0.95 ± 0.02 | 14.45 ± 0.68 |
| 12 | Shangluo City, Shanxi Province | 2.10 ± 0.03 | 0.36 ± 0.02 | 4.35 ± 0.09 | 0.19 ± 0.01 | 0.19 ± 0.01 | 0.76 ± 0.02 | 7.95 ± 0.12 |
| 13 | Wenshan Prefecture, Yunnan Province | 3.47 ± 0.10 | 0.92 ± 0.05 | 11.55 ± 0.32 | 0.52 ± 0.01 | 0.44 ± 0.01 | 0.51 ± 0.01 | 17.40 ± 0.60 |
| Compound | Repeatability RSD (%) | Precision RSD (%) | Stability RSD (%) | Recovery | |
|---|---|---|---|---|---|
| Average Recovery Rate (%) | RSD (%) | ||||
| Oxalic acid | 1.66 | 1.91 | 1.92 | 98.91 | 2.95 |
| Malic acid | 1.75 | 0.89 | 1.33 | 107.14 | 4.41 |
| Citric acid | 2.28 | 1.2 | 1.3 | 101.5 | 1.67 |
| Cis-aconite acid | 2.36 | 1.07 | 2.65 | 97.75 | 3.91 |
| Fumaric acid | 2.41 | 1 | 2.27 | 103.56 | 4.5 |
| Succinic acid | 1.68 | 1.05 | 2.82 | 97.78 | 2.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.; Li, J.; Zhang, J.; Qu, K.; Wang, M.; Ni, T.; Miao, Y.; Luo, M.; Feng, S.; Liu, D. Response Surface Modelling of Six Organic Acids from Pinellia ternata (Thunb.) Breit by Ultrasound-Assisted Extraction and Its Determination by High-Performance Liquid Chromatography Coupled to Triple Quadrupole Mass Spectrometry. Chemosensors 2024, 12, 47. https://doi.org/10.3390/chemosensors12030047
Wei L, Li J, Zhang J, Qu K, Wang M, Ni T, Miao Y, Luo M, Feng S, Liu D. Response Surface Modelling of Six Organic Acids from Pinellia ternata (Thunb.) Breit by Ultrasound-Assisted Extraction and Its Determination by High-Performance Liquid Chromatography Coupled to Triple Quadrupole Mass Spectrometry. Chemosensors. 2024; 12(3):47. https://doi.org/10.3390/chemosensors12030047
Chicago/Turabian StyleWei, Lu, Jinxin Li, Jingyi Zhang, Kaili Qu, Mingxing Wang, Tingting Ni, Yuhuan Miao, Ming Luo, Shumin Feng, and Dahui Liu. 2024. "Response Surface Modelling of Six Organic Acids from Pinellia ternata (Thunb.) Breit by Ultrasound-Assisted Extraction and Its Determination by High-Performance Liquid Chromatography Coupled to Triple Quadrupole Mass Spectrometry" Chemosensors 12, no. 3: 47. https://doi.org/10.3390/chemosensors12030047
APA StyleWei, L., Li, J., Zhang, J., Qu, K., Wang, M., Ni, T., Miao, Y., Luo, M., Feng, S., & Liu, D. (2024). Response Surface Modelling of Six Organic Acids from Pinellia ternata (Thunb.) Breit by Ultrasound-Assisted Extraction and Its Determination by High-Performance Liquid Chromatography Coupled to Triple Quadrupole Mass Spectrometry. Chemosensors, 12(3), 47. https://doi.org/10.3390/chemosensors12030047




