Abstract
This paper aims to provide a large coverage of recent developments regarding environmental monitoring using metal oxide-based sensors. Particular attention is given to the detection of gases such as H2, COx, SOx, NOx, and CH4. The developments and analyses of the design of sensors and types of metal oxide sensing materials are emphasized. The sensing mechanisms and peculiarities of metal oxides used in chemoresistive sensors are provided. The main parameters that affect the sensitivity and selectivity of metal oxide sensors are indicated and their significance to the sensor signal is analyzed. Modern data processing algorithms, employed to optimize the measurement process and processing of the sensor signal, are considered. The existing sensor arrays/e-nose systems for environmental monitoring are summarized, and future prospects and challenges encountered with metal oxide-based sensor arrays are highlighted.
1. Introduction
Monitoring of the environment is becoming an urgent need of our time, as its quality affects human and biota life. Industrialization, automobile exhausts, and the burning of fossil fuels are the most significant factors causing environmental pollution, including atmospheric pollution. This problem can be successfully solved with the help of gas sensors that rapidly and reliably detect a wide range of harmful gases in the ambient air environment. Among the different types of gas sensors, such as electrochemical, catalytic combustion, thermal conductivity, infrared absorption, and semiconductor metal oxide sensors (SMOs), the latter is the most common due to their high sensitivity, ease of manufacture, ultrasmall size, and cost effectiveness [1,2].
Research in the field of metal oxide gas sensors has developed rapidly in recent years. Thanks to the progress made in the miniaturization of chemical sensors, the latter can be integrated into low-cost small drones, which will exponentially increase the use of gas sensors for online environmental sensing. The versatility of the chemical sensor and drone combination will allow the rapid deployment of metal oxide-based sensors for gas monitoring, for example, in atmospheric research, industrial emissions detection, and environmental compliance control [3]. Also, metal oxide gas sensors are being widely investigated in the areas of healthcare, food safety and quality, and agriculture. In this case, e-nose systems are being developed to increase the sensitivity and selectivity of sensor devices [4,5,6].
There are a number of factors that affect the sensitivity, selectivity, and stability of a semiconductor metal oxide gas sensor, which can vary in each case. As a rule, dopants improve the gas-sensing properties of SMOs by changing the microstructure or morphology, forming a stoichiometric solid solution, changing the activation energy, generating oxygen vacancies, or changing the electronic structure/band gap.
Different morphologies of metal oxides, such as 0D, 1D, and 2D [7], are used as sensing layers of gas sensors. The 0D morphology includes nanoparticles that are nanoscale in all three dimensions. At the same time, 1D and 2D particles are nanoscale in one and two dimensions, respectively. The inherent morphologies of 1D particles are wires, needles, whiskers, belts, rods, etc., while the 2D morphology includes metal oxide nanosheets.
Synthesis methods of metal oxides that allow the achievement of different particle morphologies include hydrothermal synthesis, the sol–gel method, thermal oxidation, electrolysis, electrospinning, chemical vapor deposition, and vapor-liquid-solid [8]. Varying the morphology during the wet-chemical synthesis of metal oxides is achieved by influencing the pH of the medium, temperature, addition of templates, etc. [9]. Depending on the process conditions, the CVD method can also produce different morphologies. For example, the work of [10] shows that by varying the precursor type, it is possible to obtain either 0D or 1D metal oxide particles, while the authors of [11] point out that the presence or absence of catalysts has a significant effect on the resulting morphology.
The morphology of the metal oxide (MOx) particles themselves such as nanowires, nanotubes, core-shell nanostructures, nanoneedles, nanosheets, and nanofibers contributes significantly to the sensing mechanism and thus to the performance of the gas sensor [12]. Therefore, recently, the research on chemical sensors based on one-dimensional (1D) semiconductor nanostructures of metal oxides with individual geometry has significantly intensified. Chemically sensitive 1D metal oxide nanostructures are usually considered resistors, whose conductivity changes during the charge transfer process, or field-effect transistors, whose properties are controlled by applying an appropriate potential to the gate. This suggests that 1D MOx nanostructures have significant potential for use in the next generation of chemical sensors that will be very small, low power, low cost, and more sensitive than existing commercial sensors [13,14].
The main issue in the usage of sensors based on metal oxide 1D nanostructures is ensuring the stable morphology of Mox particles, which directly affects the characteristics of the sensors. Many new strategies, in particular in-synthesis approaches, have been explored to solve this problem. For instance, nanomaterials synthesized by electrospinning have unprecedented advantages, including catalyst introduction, morphological control, thermodynamic stability, unique physicochemical properties, and composition control, and are attractive for the development of highly sensitive and selective gas sensors [15,16]. The formation of SMO-based nanoheterojunctions can effectively increase the response value of a sensing element due to the increased number of oxygen vacancies, active sites, and higher catalytic activity, resulting in a shorter detection time [17].
Despite the significant progress in the development of metal oxide-based gas sensors, their low selectivity and necessity to heat the sensing element for effective operation remain major problems. In this case, gas sensitivity can be improved by changing the particle size, nanoarchitecture, porous or hierarchical structure, doping or defect engineering, and design of nanocomposite and graphene hybrid sensor materials [18,19]. In turn, the development of gas sensors based on metal oxide nanostructures that operate at room temperature will reduce energy consumption, simplify device manufacturing, and improve the safety and stability of the sensors.
In terms of environmental monitoring, the most common gases to be detected are H2, COx, NOx, SOx, and CH4. Hydrogen is flammable over a very wide range of air concentrations (4–75%) and explosive over a wide range of concentrations (15–59%) at standard atmospheric temperatures [20]. Moreover, hydrogen produced by steam reforming of natural gas is not considered environmentally friendly [21].
COx causes the greenhouse effect, photochemical smog, and haze, threatening the urban atmosphere and human health [22]. CO is a colorless, odorless gas that is released during combustion. Outdoors, it contributes to air pollution, and indoors, inhaling large amounts is very dangerous to human health, causing dizziness, confusion, loss of consciousness, and ultimately, death. A fairly large amount of CO2 is emitted into the atmosphere as a result of burning fossil fuels, solid waste, and during the production of, for example, cement. Carbon dioxide in the atmosphere causes climate change by warming the planet but also affects the O2 movement in blood.
Nitrogen oxides are the main greenhouse effect gases and harm the ozone layer. Thus, N2O emissions lead to the destruction of the stratospheric ozone through nitrogen oxide-catalyzed processes [23]. The emission of NOx (NO, NO2, and N2O) from vehicle exhaust and industry leads to nitrogen deposition, causing over-fertilization. Moreover, increased NOx concentrations may result in respiratory and cardiovascular sickness in human beings.
SO2 is produced due to the fossil fuel combustion and power generation process. It is a greenhouse gas and causes the greenhouse effect (atmospheric warming) [24]. In high concentrations, gaseous SOx can harm trees and plants by damaging leaves and slowing growth. Sulfur dioxide and other sulfur oxides can contribute to acid rain, which can harm sensitive ecosystems. The long-term effect of SO2 on human beings creates breathing problems, respiratory disorders, and visibility impairment [25].
CH4 is a colorless, odorless, and flammable gas. It is also a potent greenhouse gas, which means it affects climate change by contributing to increased warming, according to the U.S. Environmental Protection Agency. Methane is released during coal, natural gas, and ore mining, at solid waste landfills, and on livestock farms [26].
Despite significant progress in the development of gas sensors based on metal oxides, achieving high rates of efficient gas detection is a very challenging task. Although they work effectively at high operating temperatures, the development of highly sensitive sensors capable of operating at ambient temperatures is essential to reduce power consumption. In general, it is believed that the development of low-temperature sensors should use materials with a high surface area, which is a combination of metal oxides with other metal oxides, either carbon or polymeric materials [27]. At the same time, the combination of modern equipment and the latest materials allows researchers to create new technological solutions incredibly quickly, which was the main reason for the creation of this work.
The purpose of this work is to highlight important advances in the field of metal oxide gas sensors, to distinguish important factors that complicate the development of gas sensors, and to review the development of old and new solutions to these problems. Particular attention is paid to the use of the latest developments in the context of environmental monitoring, which is becoming more relevant every year. We hope that this work will help to demonstrate the extent of the advances made in recent years, the current status of metal oxide gas sensors, and the main trends and direction of future development.
2. Environmental Monitoring by Metal Oxides Sensors
Semiconductor metal oxides have been widely used as the sensing layers of chemoresistive gas sensors for a variety of practical applications, with the main ones being real-time environmental monitoring, exhaled air diagnostics, and food freshness analysis. Nevertheless, chemoresistive metal oxide gas sensors have some disadvantages, such as low selectivity and high operating temperatures [28]. In order to overcome these disadvantages, metal oxide sensors are either decorated [29,30] or nanomaterials/nanocomposites are created [31,32]. In addition, chemoresistive metal oxide devices often suffer from nonlinear responses, signal fluctuations, and cross-sensitivity to different gases, which limits their use for air quality monitoring. To overcome these shortcomings, it has been proposed [33] to use the impedance method, which allows measuring dielectric excitation and provides sensors with a linear gas response, a wide dynamic range of gas detection, and high baseline stability, and also reduces the effect of humidity and ambient temperature on the output signal. The following are advances in metal oxide-based sensors for the environmental monitoring of gases such as H2, COx, SOx, NOx, and CH4.
2.1. H2
Monitoring the presence of hydrogen in the air, which is generated during the steam reforming of natural gas, is an important task from the point of view of environmental protection. Furthermore, to ensure the safe use of renewable hydrogen energy sources, there is an urgent need to develop fast and sensitive sensors for leak detection.
For hydrogen detection, sensitive layers based on SnO2, ZnO, TiO2, Nb2O5, In2O3, FeO, Fe2O3, NiO, Ga2O3, Sb2O5, MoO3, V2O5, and WO3 have been investigated [34], among which SnO2- and ZnO-based layers are the most promising. For instance, investigations into the morphology effect of SnO2 and ZnO on sensitivity have demonstrated that nanobelts significantly outdo the other types of 1D nanostructures. Herewith, sensors based on ZnO are the most attractive due to their low cost, ease of preparation, and thermal/chemical stability [35,36].
In addition to variation in the morphology, the sensitivity of SnO2 and ZnO to hydrogen can be increased by combining two metal oxides [34,37], creating composites with carbon materials [38], metal decorating [39], and creating core-shell nanoparticles [40]. For example, the researchers in [41] developed highly sensitive, stable, and selective sensors for gaseous hydrogen through the use of core-shell PdAu@ZnO nanoparticles and demonstrated that the core-shell sensors exhibit greater hydrogen sensitivity compared to pure ZnO.
Metal oxide sensors used for hydrogen detection mainly work as transducers that convert changes in physical or chemical properties into electrical signals. These sensors are classified into resistance-based, work function-based, optical, and acoustic sensors based on their detection mechanism [42].
Nanostructured thin-film sensors are generally used for hydrogen sensor fabrication due to their advantages, such as ease of implementation, good compatibility with integrated circuits, high sensitivity, and short response/recovery time. However, elevated working temperatures are still required for most thin-film sensors, which can lead to poor long-term stability and high power consumption [43].
To enhance gas selectivity and improve responses at lower temperatures, scientists have extensively studied the impact of grain size, porosity, orientation, surface doping, decorating, and device architecture on hydrogen sensor sensitivity. The selectivity problem is not as easy to solve because metal oxide semiconductor sensors cannot discriminate between H2 and other combustible gases.
Research has demonstrated that hydrogen sensors employing single SMO 1D nanostructures can attain ultra-sensitivity, a fast response, high selectivity towards low concentrations of hydrogen, and long-term stability [43]. The metal oxide semiconductor sensing material must have high sensitivity, selectivity, and linear response. Sensitivity can be enhanced by surface functionalization, nanostructure engineering, or special signal processing. In addition, as more green hydrogen becomes available, the fabrication of hydrogen sensors with both acceptable sensitivity and fast responses at or near room temperature remains an important issue [44].
2.2. COx
Carbon oxide detection using a MOx sensor is widely presented in the literature [45,46,47]. Most commonly, ZnO, SnO2, and TiO2 are discussed as key oxides for COx detection. However, sensitive materials based on CeO2, In2O3, WO3, CdO, CuO, composite oxides, and yttria-stabilized zirconia are also considered [48].
As the chemical inertness of CO2 makes it difficult to detect, to date, only a few CO2-sensitive materials have been found. Among these materials, various ZnO nanostructures showed high sensitivity. For example, sensitivity at the level of 0.1 ppm with an ultrafast response of <20 s is observed for zinc oxide nanoflakes [47]. Also, ZnO nanostructures obtained via hydrothermal synthesis have demonstrated responses in the range of 16–65% for COx detection at low temperatures [45].
For carbon monoxide, the situation is more predictable. In this case, various synthesis/manufacturing methods, including CVD, hydrothermal, screen printing, sputtering, spray pyrolysis, sol–gel, co-precipitation, electrospinning, the Peccini method, etc., are described along with their advantages and limitations [49,50]. Due to the alteration of the material’s morphological and compositional characteristics, these methods allow the production of metal oxide-based films that are highly sensitive to CO. Thus, in the case of the CVD method, the sensitivity and other performance parameters can be enhanced due to the casting of surface conditions, pore/grain sizes, and morphology. The sensitivity of MOx, synthesized via the hydrothermal method, depends mainly on the materials’ structure and morphology, such as nanorods, nanoparticles, nanowires, nanosheets, nanotubes, and wall coatings. In spray and spray pyrolysis approaches, the sensitivity and other performance parameters can be improved due to the usage of alloying metals, in particular, Pd, Pt, Al, Cu, Au, Ga, and Co, among others [48].
Zhang et al. investigated the effect of the synthesis method, doping effect, and morphology on the performance of CO gas sensors based on the selected key oxides, such as ZnO, SnO2, and TiO2. For instance, authors have demonstrated that among ZnO materials, synthesized using various methods, powder obtained via the hydrothermal method has shown a 16.3% response to 0.8% CO2 under UV light with a 19 s response time [51]. In turn, high sensitivity at low operating temperatures has been achieved for sensors based on SnO2, synthesized via various synthesis methods, such as screen printing, solvothermal synthesis, sol–gel, the chemical method, sputtering, etc. [45].
Despite significant progress in the development of CO sensors, the issues of sensitivity, selectivity, low operating temperature, and low detection limit, as well as miniaturization, low price, etc., remain the major challenges to date. In order to obtain even more sensitive and selective COx sensors, researchers continue to work on improving the key operating parameters of gas sensing—the so-called 4S parameters—Sensitivity, Selectivity, Stability, and Speed. Finally, metal oxide-based sensors are expected to be in high demand for air quality monitoring in the near future. Flexible sensors are also expected to be integrated into smart devices for various applications.
2.3. SOx
Available studies on sulfur dioxide detection also indicate the promising use of metal oxide nanostructures. For instance, the perspectives of NiO, NiO-modified metal oxide films, NiO-ZnO composites [52], and NiO-doped SnO2 [53,54] have been explored for the manufacturing of effective gas sensors for toxic and dangerous gases. In their work, Chaitra et al. synthesized Al-doped ZnO thin films, which exhibited a sensor response of 71% towards SO2 at concentrations below the threshold level value [55]. Tyagi et al. demonstrated that among different metal oxide catalysts (PdO, CuO, NiO, MgO, and V2O5) deposited on the SnO2 surface, the SnO2 film with NiO nanoclusters exhibited the maximum sensitivity of ∼56 at a low operating temperature of 180 °C to 500 ppm SO2 gas with a fast response time of 80 s [56]. The usage of NiO for SO2 detection has also been proven to be promising in other studies [52,57,58].
However, currently, only electrochemical sensors are commercially available for the measurement of SOx gases. The NASICON-based sensor is the most sensitive to this gas and also remains stable for a long time even in a highly corrosive atmosphere [25]. Therefore, the development of SOx-sensitive metal oxide sensors remains an important task to be addressed in the near future.
2.4. NOx
Numerous studies have shown the prospects of using SnO2, WO3, and ZnO metal oxides for NOx gas detection [59,60,61,62,63,64]. For example, in [65], SnO2 nanowires responded well to 5 ppm NOx at a 200 °C operating temperature. One-dimensional ZnO particles modified with In2O3 showed response of 55 at a NO2 concentration of 1 ppm, and a sufficiently high response (∼18) was observed at a relatively low NO2 concentration of 250 ppb [66].
Researchers are currently focusing on the development of low-temperature, stable, and highly selective NOx sensors. The most extensive research is being conducted on the fabrication of ZnO-based nanocomposites and heterostructures [61,67]. The decoration of ZnO with precious metals such as gold, platinum, and others is also quite popular [68]. In addition, the influence of morphology, porosity, defects, and optical properties on the sensory properties of ZnO-based sensors are also being actively investigated [69].
In summary, NOx sensors based on SnO2, WO3, and ZnO metal oxides have already demonstrated high sensitivity of up to 430% at room temperature [70,71,72] and can be easily embedded into instrumental platforms, for example, into the SOGS (Small Open General-purpose Sensor) platform. However, the reliability and sensitivity of ready-made sensor devices during real-time monitoring of the environment for several gases at the same time remain unresolved issues. In this sense, the main goal of further research should be related to ways to improve the reliability of sensor technology for measuring pollution in urban or field environments [73].
2.5. CH4
The development of effective sensor materials with superior performance for the selective, fast, and sensitive detection of organic gases is important not only for environmental protection but also for human health. CH4 can be successfully detected by various types of sensors, but MOx-based methane sensors are generally inexpensive, lightweight, reliable, durable, and poison-resistant. Nanocrystalline metal oxide CH4 sensors are characterized by sufficient sensitivity; however, they have a number of inherent disadvantages, such as low selectivity, low and high operating temperature ranges, slow recovery, and significant dependence on temperature and humidity [74].
To increase CH4 sensor selectivity, additional modification/doping of MOx nanostructures is required [75,76]. For instance, studies on the doping of metal oxides with precious and non-precious compounds exhibit the prospects of creating methane sensors based on ZnO thin films modified with non-precious metals, such as cobalt or Pn nanowires [77,78,79]. Also, researchers are proposing the use of metal-organic frameworks (MOFs) for the functionalization of metal oxides [80]. The latter have been widely investigated for their potential use as high-performance sensors for the detection of many different organic gases due to their large surface area, tunable pore size, functionalized sites, and interesting properties such as electrical conductivity, magnetism, ferroelectricity, luminescence, and chromism. The high porosity of MOFs allows them to interact closely with a variety of organic analytes, including CH4, resulting in easily measurable responses to various physical and chemical parameters.
The main problem with the detection of organic analytes in general and for CH4 in particular is the simultaneous presence of multiple gases. A previous paper [81] proposes a new detection method based on ZnO metal oxide sensors that can selectively detect different types and concentrations of organic gases, including CH4. The method is based on signal processing under temperature modulation using a generalized regression neural network (GRNN).
2.6. Summary of Semiconductor Metal Oxide Gas Sensors to H2, COx, NOx, SOx, and CH4 Gases
The performances of gas-sensitive properties of various SMO materials, along with particle morphology and the synthesis technique, are presented in Table 1.

Table 1.
Gas-sensing performance of various SMO materials.
Analysis of the available literature shows that different variations of metal oxide compounds are employed to fabricate layers sensitive to H2, COx, NOx, SOx, and CH4 gases: from simple metal oxides to nanocomposites and/or heterostructures based on them. For instance, the decoration of metal oxide layers and the production of 1D and flower-like structures are popular techniques (Figure 1). The range of sensory properties of the metal oxide layers under consideration indicates that the final properties of the obtained selective layers rely on the structure, morphology, phase, and chemical composition of the metal oxides, which in turn can be alternated via the synthesis method and processing parameters. Numerous studies confirmed these observations [90,126,127]. The morphology and structure of SMO particles also have an impact on the electrical properties of metal oxide materials [113,128,129]. According to data presented in Table 1, the hydrothermal method is the most widely used synthesis method due to its simplicity and the potential to vary the particle morphology across a broad range. Nonetheless, other methods including sol–gel, CVD, electrospinning, and magnetron sputtering have also been extensively studied.
Sensor configuration is another significant aspect, which shall be considered for the development of high-performance sensing devices. Thus, in the case of NO detection at room and low temperatures, memristor-based sensors (Figure 1d) [130,131] appear to be the most promising candidates due to their miniature sizes, simple operation, low-cost manufacturing, and easy integration [132].
Furthermore, the data in Table 1 indicates that researchers primarily focus on identifying the aforementioned gases within the following concentration intervals: H2: 5 to 100 ppm; COx: 25 to 1000 ppm; NOx: 1 to 1000 ppm; SOx: 5 to 600 ppm; CH4: 50 to 5000 ppm. According to the literature, feasible responses for these gases of 586, 54, 430, 100, and 65.6, respectively, have been achieved so far. Regrettably, the selectivity of metal oxide sensors is not often considered by scientists, remaining mostly understudied. However, the information available in the literature shows a wide range of selectivity values depending on the nature of associated gases. For instance, the selectivity of hydrogen sensors ranges from 1.3–156 [85], carbon monoxide sensors from 25–37 [92], and methane sensors from 6.3–68 [79,96]. The variation in metal oxide morphologies, doping, and the creation of heterostructures allowed a reduction in response/recovery time of 10–20 s. Moreover, it can be noted that there is a trend towards gas sensors operating at room temperature, which implies researchers’ efforts to minimize energy consumption and reduce costs. This indicates that researchers are increasingly making progress in creating highly sensitive, selective, miniaturized, and energy-efficient sensors based on metal oxides. Thus, to date, metal oxide sensors have demonstrated sufficiently high responses, sensitivity, and repeatability at room temperature. However, some challenges remain in their development. These often include low selectivity, insufficient lifetime, and problems in creating optimal geometric characteristics of the sensors [31]. Overcoming these shortcomings requires additional in-depth theoretical (e.g., DFT modeling) and experimental studies, as well as the use of modern micro-industrial approaches (e.g., 3D printing) to create the desired design and reduce the time for sensor fabrication.
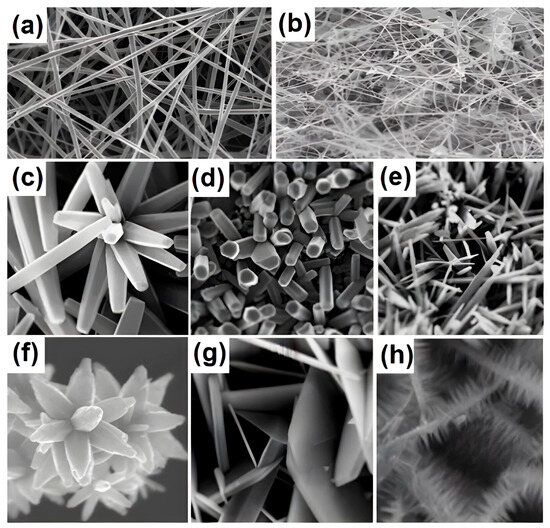
Figure 1.
Typical nanostructures of metal oxides: (a,b) 1D structures. Reprinted with permission from [131]. Copyright Creative Common CC BY license. (c–h) flower-like structures. Reprinted with permission from [130]. Copyright Creative Common CC BY license.
3. Multisensor Systems/e-Nose Based on Metal Oxides for Environmental Monitoring
Most commercial gas analyzers have a wide range for the detection of gases but usually employ different sensor types (metal oxide, electrochemical, optical, thermal, etc.) in one device, which leads to increased costs, bulky systems, and reduced life span. According to the data presented in Section 2, metal oxides are promising materials for creating affordable sensors based on them; however, they suffer from insufficient sensitivity and selectivity. The low-selectivity problem of SMO gas sensors can be solved by creating so-called e-nose devices. Such systems are based on sensor arrays, which do not exhibit high selectivity but rather require special statistical data processing, as well as the creation of gas signature databases, which also leads to an increase in the costs and a narrowing of their application scope [22,133]. The e-nose system overcomes the limitations of single SMO gas sensors by collecting response patterns of the gas sensors in different conditions with different driving temperature parameters [133].
Considering the fact that most air pollution has a complex composition, such multisensory systems are essential for environmental monitoring. For instance, COx, hydrocarbons, and suspended particles are the main components in automobile exhausts [22]. COx, NOx, and SOx are emitted from combustion processes of coal, oil, and waste plants. Nitrogen oxide can be attributed to fertilizer production. NOx and CH4 are released by the digestive processes in livestock waste management. Besides human activities, natural disasters are other sources of air pollution. Thus, CO and NOx gasses are formed during wildfires, and volcanic eruptions cause emissions of nitrogen compounds and SO2 [134]. In the area of environmental monitoring, e-nose systems can replace traditional analytical methods, providing real-time detection instead of costly analysis tools that often require long processing time [135]. For instance, in contradistinction to the determination of odor concentrations in gas samples by dynamic olfactometry [136], electronic noses can be used for continuous air monitoring and are able not only to measure the gas concentration but also to provide information regarding odor type [135].
Some relevant parameters for the development of gas monitoring systems for environmental applications are listed in Table 2. Furthermore, the greatest attention should be paid to the set of characteristics, such as simplicity, rapidity, robustness, reliability, and sensitivity [137].

Table 2.
Requirements for environmental gas-monitoring systems [137].
To date, multiple research studies have been conducted on the detection of gas compositions using sensor arrays. Betty et al. demonstrated the real-time detection of NH3, H2S, and NO2 gases at room temperature using a SnO2 nanocrystalline thin film. The developed sensor showed not only sufficient selectivity to the detected gasses in the presence of other interfering gasses but also high reliability with no changes in the response at high humidity up to 98% [138]. In their work, Kang et al. confirmed the significance of deep learning algorithms for the precise pattern recognition of sensor responses. The authors fabricated a sensor array based on SnO2, In2O3, WO3, and CuO nanocolumnar films for real-time selective CO, NH3, NO2, CH4, and C3H6O detection. The obtained sensor responses of the gas sensor array to the target gasses overlapped in the specific concentration ranges. In turn, it was possible to perform pattern recognition of the sensor signals by employing a convolutional neural network with a learning algorithm matrix (Figure 2). As a result, a high accuracy of 98% for gas classification with a very short response time was achieved. In addition, the authors confirmed the possibility of simultaneous gas concentration predictions with an average error of 10% [133].
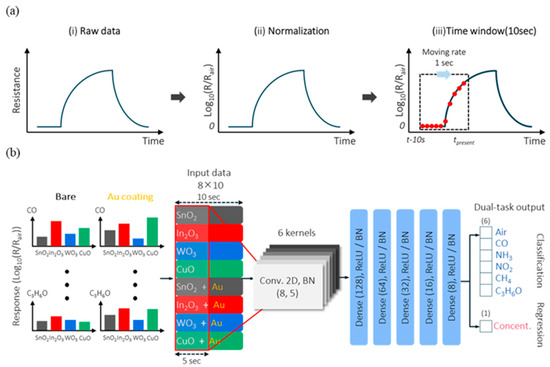
Figure 2.
(a) Schematic illustration of the sensing data preprocessing; (b) structure of the convolutional neural network for gas species recognition. Reprinted with permission from [133]. Copyright © 2022, American Chemical Society.
In general, multisensor systems are promising devices for air quality assessment, gas detection, the quantification of specific compounds, and control processes at lower costs. However, for real-time continuous environmental monitoring, specifically developed and fine-tuned e-nose devices able to produce accurate and reliable results are required. Simple instruments for the detection of gas leakage and the evaluation of gas concentration are not suitable for these purposes [135]. The challenges of e-nose usage in the field of environmental monitoring are mostly related to the adjustment of process parameters for outdoor applications due to the instability of the sensor signal with variations in temperature and humidity [139,140], cross-sensitivity to interfering substances [141], and the problem of sensor response drift over time [142,143,144]. On the other hand, sensor array systems are able to provide reliable results with high accuracy when the temperature and humidity are kept at constant values [139], and the same is related to the presence of other interfering gases [141]. The problem of sensor drift over time significantly affects the device’s reliability. Thus, an investigation of sensor array performance in a long-term period after sensor calibration demonstrated a radical deterioration of gas recognition over time from 98% to 20% after 3 years [145]. To avoid sensor drift over time, periodic re-calibration, as well as the use of specific software or specific equipment, is proposed [146,147].
4. Configuration and Geometry of Metal Oxide Sensors
The typical architecture of metal oxide-based gas sensors is generally represented by a sensitive layer deposited on the substrate with electrodes. Electrodes are one of the main elements of sensor construction and are used for the readout of the conductivity change in the sensing surface layer depending on the presence of gas in the environment. To date, several configurations of the electrodes of MOx sensors, such as cylindrical, disk-type, interdigitated, and memristor-type, have been proposed (Figure 3). In cylindrical gas sensors, the electrodes are deposited on or wound up around the cylindrical alumina substrate and covered with a layer of sensitive material (Figure 3a) [148,149]. To produce disk-type electrodes, the sensing material is sintered in pellet form and then the electrodes are deposited on both sides of the pellet (Figure 3b) [148,149,150]. Two-finger electrodes that comb parallel to each other with a fixed space between them form the interdigitated (IDE) sensor configuration pellet (Figure 3c) [148]. In the memristor type or so-called top–bottom (TBE) sensors, the sensing metal oxide material is sandwiched between the top and bottom electrodes (Figure 3d) [151].
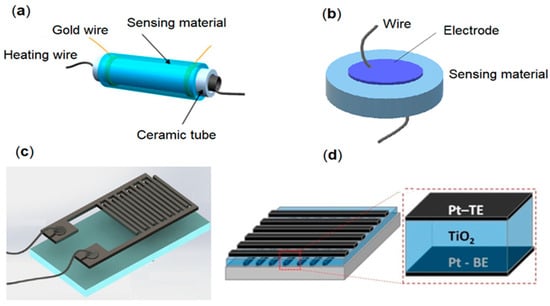
Figure 3.
Configurations of the electrodes used in metal oxide-based gas sensors: (a) Cylindrical. Reprinted with permission from [152]. Copyright Creative Common CC BY license; (b) disk-type; (c) interdigitated; Reprinted with permission from [16]. Copyright Creative Commons Attribution 4.0 International License; (d) memristor-type. Reprinted with permission from [153]. Copyright @ 2017 Elsevier B.V.
The interdigitated and memristor-type sensors are the most widely accepted electrode configurations of metal oxide-based gas sensors. IDE has an easy-to-produce stable geometry, which ensures a large active area between the electrodes, resulting in enhanced electron conduction and reduced resistance of the sensitive film [148,154,155]. To fabricate an interdigitated sensor, IDE electrodes are deposited onto the desired substrate (Si, glass, or Al2O3) using the microfabrication process, such as pattering and lift-off, and then the sensing material is coated on the top of electrodes [149]. The deposition of the sensing material on already-fabricated electrodes allows one to avoid any damage to the sensitive layer [150]. In the TBE geometry, one of the significant advantages is the equally small thickness of the sensitive film and lower distance between the electrodes (below 2 μm to as low as 40 nm) [156]. The thickness of the MOx layer of only several tens of nanometers can be achieved even without using sophisticated lithography methods, which in turn results in a very small interelectrode distance and, therefore, a high electric field even at small voltages [157]. Furthermore, the size of memristor-based sensors ranges from the nano- to micro-scale, while a typical gas sensor is usually a few micrometers in size [132].
As the gas-sensing characteristics depend strongly on electrode spacing, including the width of the digits and spacing between digits [148,158], an improvement in sensor performance can be achieved by altering the electrodes’ geometry. For instance, a change in the electrode gap size influences the resistance value, and consequently, the sensitivity. A better gas sensor response is achieved when the spacing between electrodes is less than the electrode’s width [159]. This is explained by the increased current conductivity of the sensing film for the closed electrode spacing due to the short path for current conduction. The wide gap between electrodes in turn results in current flow in both horizontal and vertical directions, thereby sampling a wider area [150]. However, the choice of the electrode gap size may also depend on the gas concentration. In their work, Shaalan et al. investigated the effect of electrode spacing on NO2 gas-sensing properties and concluded that higher sensitivity to lower NO2 concentrations was obtained for the short gap size of 1 μm. On the other hand, the authors observed that sensors with larger electrode gaps (30 μm) became highly sensitive to high NO2 concentrations [160]. A study of the effect of micro-gap electrodes with gap sizes in the range of 0.1–1.5 μm on sensing performance demonstrated that the sensitivity to diluted NO2 was almost unchanged for gap sizes larger than 0.8 μm but increased significantly with the decreasing gap size. The enhanced sensing behavior for the smallest gap size of electrodes is explained by the resistance change at two boundary types—the grain boundary and the electrode–grain interface [161].
Nguyen Minh et al. designed nanogap IDE electrodes for gas-sensing applications with controllable gaps in the range of 50–250 nm (Figure 4) and demonstrated that decreasing the IDE’s gap from the micro- to nano-range leads to an improvement in sensitivity of up to 35 times. The authors confirmed that the developed nanogap IDE can be used for the detection of very small analyte amounts (as low as 6 ppm) and sensitivity could be further enhanced by increasing the electrode area [162].
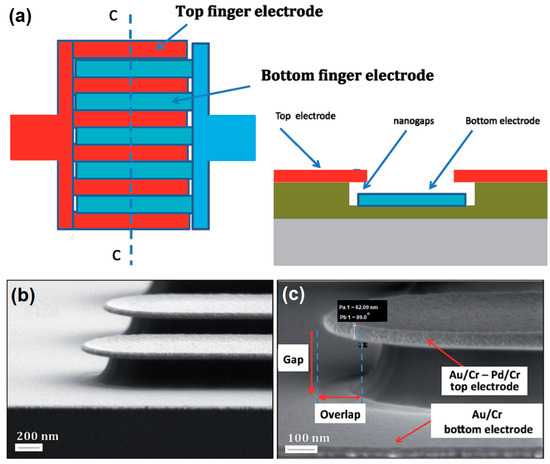
Figure 4.
Interdigitated nanogap electrodes: (a) Overview and cross-section; (b,c) SEM images of the bottom and top electrodes. Reprinted with permission from [162]. Copyright @ CC BY 3.0.
Similar to electrode spacing, electrode width contributes significantly to the electrode–grain interface, and as a result, affects the sensitivity of gas sensors. Miyaji et. al. investigated the sensitivity of WO3 microsensors with different electrode widths (10 and 50 μm) to NO2 and found that the sensitivity values were 2–3 times higher for the sensor with a 50 μm electrode width compared to the 10 μm sensor [163].
Besides the electrode spacing and electrode width, the electrode position can also contribute to the response of gas sensors. It has been assumed that the deposition of the sensitive layer above the electrode provides a larger surface area for interaction with gas molecules [154]. Nevertheless, the research work of Prajesh et al. on the positioning of interdigitated electrodes with respect to the sensing layer demonstrated that the sensor shows a significantly higher response to ammonia when the electrodes are placed above the sensing film (45% sensor response with top placement of electrodes in contrast to 25% sensor response for bottom electrode positioning). In this case, the enhanced gas sensor response is related to the fact that the majority of reactions between the gas and the sensing film occur on the surface of the sensing layer, therefore the top electrode provides a more accurate reading of the resistance changes compared to bottom-placed electrodes. The electrode position becomes even more notable when the thickness of the sensing film is higher than 100 nm since, in this case, the overall sensor response is highly dependent on the surface changes [164].
In this manner, interdigital and top–bottom electrode designs with controlled electrode widths and electrode gaps in the nano-range are the preferable sensor configurations to obtain high-sensitivity semiconductor metal oxide sensors. The variation in sensor geometry influences the active area of the sensitive film, leading to the enhancement of electron conduction and a reduction in resistance values, which in turn plays a major role in sensitivity improvement.
5. Detection Mechanism and Parameters Affecting the Sensitivity/Selectivity of the Sensors
5.1. Detection Models of Gas-Sensing Processes
Gas-sensing mechanisms for semiconductor metal oxide sensors have been extensively studied and are generally well-understood. For SMO sensors, the interaction between gas molecules and the surface and/or bulk of the oxide leads to a change in its electrical properties, which can be registered and interpreted by appropriate measuring equipment. The most commonly referred to theories are the electron depletion layer (EDL) theory and the hole accumulation layer (HAL) theory, which describes microscopic changes in the material itself, as well as the bulk resistance control mechanism and the gas diffusion control mechanism, which describe the physical and chemical interactions between the gas and the sensing material [165]. These theories and mechanisms are often related and used in combination for better modeling of the gas-sensing process.
The oxygen adsorption model is one of the most common models used to describe gas-sensing processes for both n-type and p-type SMOs. This model suggests that the gas-sensing properties of the SMO originate from the presence of point defects—oxygen vacancies on the surface of the oxide, which eventually adsorb atmospheric oxygen to form negative O2−, O−, and O2− ions, taking electrons from the conduction band. This process leads to the formation of a depletion zone near the surface and an increase in the potential barrier at the oxide grain boundary. As a result of these phenomena, the oxide surface behaves like an n-doped semiconductor, especially at high temperatures [166]. The interaction with a reducing gas leads to a reduction-oxidation reaction with sorbed oxygen anions, returning electrons to the conductivity band, shrinking the depletion zone, and restoring electrical conductivity. Interaction with an oxidizing gas typically results in a further decrease in conductivity. In p-type semiconductors, due to the metal-ion-deficient defects, hole charge carriers are predominant. The hole accumulation zone is present, which grows with the adsorption of molecular oxygen and the formation of oxygen anions, leading to a decrease in electrical resistance. Interaction with the oxidizing gas leads to a reduction in oxygen anions, its replacement by oxidizing gas atoms, enlargement of the hole accumulation zone, and a further decrease in electrical resistance. Conversely, exposure to a reducing gas narrows the hole accumulation zone and causes an increase in electrical resistance [147].
In the case of the memristor-type sensors, the non-stoichiometry of the sensitive layer plays an important role in the generation of oxygen vacancies, thus affecting the overall sensing mechanism. Haidry et al. demonstrated that annealing of the Pt/TiO2/Pt sensor structure results in the formation of two different zones in the sensitive layer: TiO2 composition near stoichiometry and non-stoichiometric TiO2-x at the bottom electrode. In turn, the thickness of the non-stoichiometric TiO2-x zone plays a dominant role in the sensing mechanism and may result in higher sensitivity due to the changes in the depletion layer [153].
Another phenomenon that must be taken into account when considering the mechanisms of gas sensitivity is chemical absorption/desorption itself. This process usually has a small but measurable effect on the sensor response. The chemical adsorption of gases on the surface of the oxide can lead to the formation of new compounds with different electrical properties, which will affect the electrical conductivity of the sensor material, such as the formation of SnS2 on the surface of SnO2 when sensing H2S gas [167]. In addition, chemisorption can, in some cases, improve the sensitivity of the sensor in a vacuum or inert atmosphere, as the gas can be adsorbed directly to oxygen vacancies, as described in [168] for certain concentrations of Cl2 gas. In [82], the improved response of the SnO2 sensor to H2 in a vacuum is also demonstrated, which was attributed to the chemical adsorption of hydrogen and the subsequent shift in the valence band.
5.2. Parameters Affecting Sensor’s Performance
Surface morphology and particle size naturally affect the sensitive characteristics of SMOs. Given the crystalline nature of metal oxides, they can form a variety of micro- and nanostructures of different dimensions, densities, and geometries, dramatically changing their electrical characteristics and interaction with different gases [169,170]. At a more basic level, it is known that reducing the size of oxide particles can improve the sensing properties of the sensor by increasing the specific surface area and reducing the surface charge density [10,171]. With a sufficiently small particle size (less than two Debye lengths), the electron depletion zone or hole accumulation zone extends over the entire thickness of the oxide particle, resulting in the strongest response [166]. Considering the processes described in the oxygen adsorption model, a high surface-area-to-volume ratio and the presence of exposed planes with crystal defects improve the sensing characteristics of metal oxides. The type of the exposed crystal facet directly affects the rate of chemisorption and electron transfer processes [82,166,172]. Changing the surface morphology can not only alter the sensitivity of the sensor to a particular gas [83,101,173,174] but also enables the sensitivity of the same material to different gases—in [175], the change in the sensitivity mechanism depending on the surface morphology and the resulting detection of H2S and NO2 using dispersed nanoparticles and SnO2 clusters is demonstrated. For instance, previous authors have shown that a SnO2 small nanoparticle film (Figure 5a) has more efficient contact with plenty of conducting channels, leading to a larger current enhancement signal. In the case of the SnO2 big cluster film (Figure 5b), fewer conducting channels among clusters result in a limited sensing signal. However, the morphology effect is strongly dependent on the gas nature. Thus, the SnO2 big cluster demonstrated better sensitivity towards NO2 down to ppb concentrations [169]. Xu et al. demonstrated control over the number of oxygen vacancies on the surface of CeO2 nanowires by controlling the composition of the atmosphere during annealing [176]. With the help of precise control over the synthesis conditions and modern approaches, such as refined GO and rGO synthesis and modification procedures [177], and extensive use of organic precursors [178,179], it is possible to obtain quite complex and exotic structures with defined morphologies. An example of this is the CuO-ZnO cubic nanostructures with excellent sensitivity to acetone and a detection limit of 9 ppb: such a structure promotes the formation of a large number of p-n heterojunctions, which improves the sensor’s performance [179]. In [180], the authors developed a method for the controlled synthesis of hollow GaFeO3 microcubes, which allows modulation of the size and microstructure of such micro-assemblies. Tests conducted with this sensor with such a morphology have shown an optimum response of 7.4 for 200 ppm of triethylamine with a 9 s response time at 200 °C.
Figure 5.
Schematic of SnO2 films of different morphologies: (a) small nanoparticles film; (b) big cluster film. Adopted from [175].
Operating temperature is one of the key factors that directly affect the performance of metal oxide sensors. For the majority of metal oxides, the normal operating temperature is in the range of 150–500 °C, but depending on the type of oxide, surface morphology, and target gas, it can be as low as room temperature or as high as 1100 °C [12,181,182,183]. The process of chemisorption of oxygen and the formation of negative ions at a sufficient rate usually occurs at higher temperatures [12,165], which, as mentioned above, is one of the key elements of the gas-sensing process. In terms of chemical kinetics, an elevated temperature accelerates the reactions that occur on the surface of the oxide [184]. The variety of effects that the operating temperature has on SMO opens up interesting opportunities for gas sensors. Particularly, at different temperatures, the response and sensing range of the sensor can change as observed in [168], where, at temperatures of 300–400 °C, a WO3 nanowire sensor demonstrated a decrease in response with an increase in the Cl2 gas concentration; at temperatures of 250 °C or lower, such a behavior was no longer observed. Alternative sensitivities can occur at different temperatures, as shown in [185], where a CuO thin-film sensor exposed to different VOCs demonstrated an enhanced response to 2-propanol at 225 °C, while at 250 °C, the response to ethanol was significantly higher. Even the changes at an ambient temperature in combination with humidity variations can lead to false predictions of the gas source location when employing gas distribution mapping algorithms [186]. The response speed can be altered significantly with the change in operating temperature; a SnO2/rGO sensor in [187] demonstrated a decrease in response time from ~400 s to almost ~200 s and faster recovery with an increase in the working temperature from 200 °C to 250 °C. Taking advantage of the known patterns of temperature effects on metal oxides, Yuan et al. achieved the detection of various VOC components using a single ZnO sensor using temperature modulation and a general regression neural network (GRNN) statistical model [81].
One of the important challenges constantly being faced by researchers is the efficient operation of sensors at room temperature without sacrificing sensor performance. Room temperature operation greatly expands the possible use of metal oxide sensors for wearable and medical applications, in explosive atmospheres, and in low-power applications. In recent years, much effort has been invested into solving this problem, opening up many new possibilities. Particular attention has been given to photoactivated metal oxide sensors: the use of UV radiation can improve the response and recovery time of sensors based on unmodified ZnO, SnO2, and In2O3 at RT [84,102,103,188]. The change in the sensing properties of SMO sensors in the presence of UV radiation is usually associated with optoelectric and photocatalytic processes that can occur on the surface of the oxide. UV radiation that has energies higher than that of the band gap of the metal oxide causes the generation of electron–hole pairs in the sensing material that ultimately increases the number of reactive adsorbed oxygen species [18,189]. Photoactivation with UV radiation also intensifies adsorption/desorption processes [105] and may cause photocatalytic processes to occur on the surface of metal oxide [76]. In [190], a polyaniline/NiO-loaded TiO2 sensor utilizing UV radiation showed high sensitivity to acetone (a minimum concentration of 176.2 ppb) and excellent stability at RT. The authors of [70] demonstrated the use of WS2/SnO2 2D/0D heterostructures with UV activation to achieve an improved NO2 response and fast response/recovery. Reduced graphene oxide and CeO2 (rGO-CeO2) heterostructures have demonstrated improved NO2 sensitivity and response/recovery time at RT in the presence of UV radiation [104]. Numerous methods have also been developed to improve the performance of SMO sensors at room temperature without photoactivation. Pedowitz et al. proposed a δ-MnO2/epitaxial graphene/silicon carbide heterostructure that demonstrates selective sensitivity to NO2 and NH3 at room temperature [191]. The use of rGO fibers coated with MoSxSe2-x with an increased Se content in [192] allowed the rapid recovery of the sensor surface with no additional heating. In [31], the authors exhaustively covered the latest advances in the use of hybrid polymer–metal oxide mixtures as gas sensors operating at RT. The use of bimetallic Pt-Au particles on ZnO nanorods, as demonstrated in [85], allows one to achieve a sensitivity of up to 250 ppm H2 with fairly high selectivity. Decoration with noble metals typically improves the sensing properties of SMOs by increasing the sensitivity and response speed to certain gases (H2, NO, NO2, H2S, and CO), as discussed in detail in [29].
Humidity is one of the factors that affect the sensitivity and selectivity of metal oxide sensors in real-world applications. In low-humidity conditions, water molecules are chemically adsorbed at active sites on semiconductor metal oxide surfaces, resulting in the formation of hydroxyl groups and mobile protons. At high humidity levels, all active sites are occupied by the previously chemisorbed water molecules, leading to the formation of physically adsorbed layers on top of the chemisorbed layer. This results in the formation of hydronium ions (H3O+), and electrical conductance occurs due to proton H+ hopping between neighboring water molecules. Higher humidity levels also lead to a reduction in chemisorbed oxygen anions and target gas adsorption due to competition with water molecules [193]. This typically leads to reduced sensitivity and baseline resistance drift [194]. Various strategies can be employed to negate the impact of humidity on SMO sensors. Utilizing higher operation temperatures of up to 450 °C leads to the removal of hydroxyl ions from the sensor surface, resulting in a full signal recovery in several minutes [181]. Nair et al. demonstrated that the formation of ZnO@MOF heterostructures is promising for a reduction in humidity cross-sensitivity. In this case, the ZnO/MOF interface acts as a diffusion barrier, resulting in the “trapping” of water molecules [195]. The formation of solid stoichiometric solutions of different oxides, for instance, Sn/Ti or Sn/Ti/Nb, was found to decrease the cross-sensitivity to humidity due to the lowering of the interaction strength with water by surface modification [196]. Abdullah et al. proposed a mathematical correction model to predict the corrected sensor response regardless of humidity changes throughout the experiment. The developed model allowed them to obtain more uniform sensor performances for more stable and reliable gas-sensing operations [194].
5.3. Methods of Processing Sensor Signals to Improve Sensitivity/Selectivity of the Sensors
With the development of SMO sensor technologies, requirements for sensor performance and efficiency have gradually increased, and new applications and tasks have emerged. A logical consequence of recent advances in microelectronics, algorithms, statistics, and artificial intelligence is the integration of these technologies into the field of SMO sensors. New ways to improve sensor performance have been discovered, which now include not only modifying the sensor structure or its chemical composition but also optimizing the measurement process and processing of the sensor output. The classical method of measuring the response of a metal oxide sensor is the measurement of resistance change [182], but measuring changes in complex resistance [197], capacitance [198,199], and measurements under alternating current conditions [200] allows us to enhance and optimize the efficiency of sensors from multiple angles. This amount of data that can be measured in real time requires adequate processing for its effective interpretation. Modern equipment allows us to achieve such goals while offering low power consumption and a compact form factor. The development of an integrated system consisting of a high-electron-mobility transistor (HEMT) H2 sensor and a readout-integrated circuit is described in detail in [201]. HEMT’s performance typically suffers from process, temperature, and voltage variations during the manufacturing process, which affects the repeatability of measurements and limits their application. The proposed readout circuit can be integrated with the sensor on the same chip and implements mechanisms of sensitivity nonlinearity correction, noise suppression, and calibration at the hardware level with a power consumption of only 3.1 mW and a resolution of 1 ppm. Another paper [202] demonstrated the use of a Kalman filter and absolute-deadband sampling to process signals from four different commercial gas sensors and determine the direction of the isopropyl alcohol “odor” source. These processing techniques allow us to compensate for the relatively slow response and recovery times of SMO sensors and detect quick changes in target gas concentrations. In [203], Burgués et al. utilized similar algorithms, a quadcopter, and two commercial SMO sensors to detect ethanol vapor sources in closed rooms and created a 3D map of gas concentration with a special resolution of 1.38 m. Notably, the measurements were conducted during continuous “sweeps” across the rooms, demonstrating the ability of the proposed algorithm to be utilized effectively and interpret data from relatively slow SMO sensors. The authors of [110] demonstrated the ability to effectively detect and distinguish between three different gases (O2, SO2, and NO2) in a mixture with a single ZnO-based sensor. To achieve this functionality with an error of under 2%, resistance and impedance measurements were used, followed by data processing by an artificial neural network. Another previous paper [204] showed the use of conventional machine learning algorithms (random forest, naïve Bayes, support vector machine, and multilayer perceptron) to prepare a dataset of various gas concentrations and interpret data from a single SnO2 sensor, which made it possible to distinguish formaldehyde, 2-propanol, toluene, and methanol and estimate their concentrations.
However, the application of specific signal-processing approaches, for instance, machine learning algorithms and artificial neural networks, also introduces certain limitations and challenges specific to these technologies. The utilization of artificial intelligence processing introduces additional inaccuracies due to the inherently imprecise nature of AI algorithms. The application of even relatively basic machine learning techniques also adds to the issues of algorithmic bias and the quality of the initial dataset used to train the algorithm [205]. The initial dataset, known as the training dataset, generally comprises various parameters including electrical conductivity, impedance, and relative humidity for various concentrations of the target gas or a mixture of gases [159]. Calibrating metal oxide sensors to account for physical variations in individual samples can be significantly more difficult when using complex data processing algorithms and may require a new training dataset [160,206]. These factors increase the complexity and hinder the use of these technologies in cases where high reliability and confidence in the accuracy of the data are required [205].
Despite these challenges, such technologies offer a new angle on improving the performance of gas sensors. Clever use of the effects of nonlinearity and cross-selectivity, which were previously considered undesirable, and modern data processing algorithms have opened up new prospects for metal oxide gas sensors in non-critical applications. It is safe to assume that the use of such approaches will continue to develop and will bring the creation of full-fledged “electronic nose” systems and “smart sensors” closer than ever before.
6. Conclusions
This article highlights recent advances in the field of semiconductor metal oxide gas sensors for environmental monitoring (especially H2, COx, NOx, SOx, and CH4 gases), underlines important factors that complicate the development of gas sensors, and discusses the development of old and new solutions to these problems. The paper demonstrates the extent of recent advances in the most common SMO sensors due to their high sensitivity, ease of fabrication, ultra-small size, and cost effectiveness. In particular, it is emphasized that 1D MOx nanostructures have significant potential for use in the next generation of chemical sensors that will be very small, low power, inexpensive, and more sensitive than existing commercial sensors. It is noted that recent research has aimed to develop low-temperature sensors based on materials with high surface area and composites of metal oxides with other metal oxides, carbon or polymeric materials, MOFs, etc.
For instance, according to the literature review, semiconducting metal oxides like SnO2, ZnO, TiO2, and NiO are frequently studied as sensing layers for chemoresistive gas sensors to monitor gases such as H2, COx, SOx, NOx, and CH4 in the environment. Herewith, the increase in sensitivity and the reduction in the operating temperature can be achieved via metal oxide decoration or creating metal oxide-based nanomaterials/nanocomposites. Hydrothermal synthesis is the most frequently used approach to produce metal oxides. Research on room-temperature sensors based on metal oxides is increasing. The sensors can achieve a response of 100 or more with short response and recovery times of up to 10 to 20 s, respectively. This shows that researchers are making significant progress in developing highly sensitive, selective, miniaturized, and energy-efficient sensors based on metal oxides. However, there are still some challenges in the development of metal oxide gas sensors. For instance, the selectivity issue is often overlooked by researchers and not often considered in scientific articles, remaining one of the main drawbacks of metal oxide sensors. Furthermore, insufficient lifetimes and challenges in creating optimal geometric characteristics require further in-depth studies to develop sensor devices with the desired design and at reduced fabrication times. In addition to the material’s properties, the electrode spacing, electrode width, and electrode position can also significantly contribute to the response of gas sensors. Accordingly, the configurations and geometry of SMO sensors have been analyzed in the manuscript. It has been concluded that among different electrode configurations, including cylindrical, disk-type, interdigitated, and memristor-type, interdigitated and memristor-type are the most widely accepted due to the possibility of enhancing sensor performance by altering the electrode’s geometry.
The sensitivity mechanisms of sensors and the factors that affect their performance are broad topics that are difficult to cover concisely. Therefore, only the main aspects of the gas-sensing process in metal oxide sensors have been identified and briefly described, such as the oxygen adsorption and chemisorption model, and the influence of operating temperature, humidity, and surface morphology. Despite being researched for a long time, there is still much effort being made to ensure metal oxide sensors work efficiently at room temperature and obtain the most optimal surface structure for sensitive oxides. Some of the studies reviewed in this paper have shown excellent results, demonstrating that significant progress is being made in this direction. Sophisticated nanostructures and sensors that effectively sense a wide range of gases at low temperatures have been developed.
The clever utilization of nonlinearity and cross-selectivity effects, previously considered undesirable, along with modern data processing algorithms have brought new opportunities for metal oxide gas sensors. It is reasonable to assume that the use of these approaches will continue to evolve and will bring us closer than ever to creating fully fledged “electronic nose” systems and “smart sensors”. Considering the complex composition of most air pollutants, multisensory systems are promising devices for environmental monitoring, including air quality assessment, gas detection, the quantification of specific compounds, and control processes. The employment of such systems allows us to overcome the insufficient selectivity of single-semiconductor metal oxide sensors; however, specifically developed and fine-tuned data-processing tools are required to obtain reliable results with high accuracy for real-time continuous environmental monitoring.
Author Contributions
M.T.: writing—original draft preparation; T.D.: writing—original draft preparation; visualization; writing—review and editing; supervision; B.S.: supervision, project administration, validation, visualization, writing—review and editing; S.K.: writing—original draft preparation; visualization; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Uma, S.; Shobana, M.K. Metal oxide semiconductor gas sensors in clinical diagnosis and environmental monitoring. Sens. Actuators A Phys. 2023, 349, 114044. [Google Scholar] [CrossRef]
- Dontsova, T.A.; Nahirniak, S.V.; Astrelin, I.M. Metaloxide nanomaterials and nanocomposites of ecological purpose. J. Nanomater. 2019, 2019, 5942194. [Google Scholar] [CrossRef]
- Burgués, J.; Marco, S. Environmental chemical sensing using small drones: A review. Sci. Total Environ. 2020, 748, 141172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zheng, Z. Electronic noses based on metal oxide semiconductor sensors for detecting crop diseases and insect pests. Comput. Electron. Agric. 2022, 197, 106988. [Google Scholar] [CrossRef]
- Nahirniak, S.; Dontsova, T.; Lapinsky, A.; Tereshkov, M.; Singh, R. Soil and soil breathing remote monitoring: A short review. Biosyst. Divers. 2020, 28, 350–356. [Google Scholar] [CrossRef]
- Nanotechnology-Based E-Noses: Fundamentals and Emerging Applications; Woodhead Publishing Series in Electronic and Optical Materials; Elsevier: Cambridge, UK, 2023; 457p.
- An, Y.; Tian, Y.; Wei, C.; Tao, Y.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Dealloying: An effective method for scalable fabrication of 0D, 1D, 2D, 3D materials and its application in energy storage. Nano Today 2021, 37, 101094. [Google Scholar] [CrossRef]
- Galstyan, V.; Moumen, A.; Kumarage, G.W.C.; Comini, E. Progress towards chemical gas sensors: Nanowires and 2D semiconductors. Sens. Actuators B Chem. 2022, 357, 131466. [Google Scholar] [CrossRef]
- Ortiz-Casas, B.; Galdámez-Martínez, A.; Gutiérrez-Flores, J.; Ibañez, A.B.; Panda, P.K.; Santana, G.; de la Vega, H.A.; Suar, M.; Rodelo, C.G.; Kaushik, A.; et al. Bio-acceptable 0D and 1D ZnO nanostructures for cancer diagnostics and treatment. Mater. Today 2021, 50, 533–569. [Google Scholar] [CrossRef]
- Nagirnyak, S.V.; Lutz, V.A.; Dontsova, T.; Astrelin, I.M. The effect of the synthesis conditions on morphology of tin (IV) oxide obtained by vapor transport method. In Nanophysics, Nanophotonics, Surface Studies, and Applications; Fesenko, O., Yatsenko, L., Eds.; Springer Proceedings in Physics; Springer: Berlin/Heidelberg, Germany, 2016; Volume 183, pp. 331–341. [Google Scholar]
- Masuda, Y. Recent advances in SnO2 nanostructure based gas sensors. Sens. Actuators B Chem. 2022, 364, 131876. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, M.; Comini, E. One-dimensional nanostructured oxide chemoresistive sensors. Langmuir 2020, 36, 6326–6344. [Google Scholar] [CrossRef] [PubMed]
- Nagirnyak, S.V.; Lutz, V.A.; Dontsova, T.A. Synthesis and characterization of tin (IV) oxide obtained by chemical vapor deposition method. Nanoscale Res. Lett. 2016, 11, 343. [Google Scholar] [CrossRef]
- Chen, L.; Yu, Q.; Pan, C.; Song, Y.; Dong, H.; Xie, X.; Li, Y.; Liu, J.; Wang, D.; Chen, X. Chemiresistive gas sensors based on electrospun semiconductor metal oxides: A review. Talanta 2022, 246, 123527. [Google Scholar] [CrossRef]
- Dontsova, T.A.; Nagirnyak, S.V.; Zhorov, V.V.; Yasiievych, Y.V. SnO2 nanostructures: Effect of processing parameters on their structural and functional properties. Nanoscale Res. Lett. 2017, 12, 332. [Google Scholar] [CrossRef][Green Version]
- Paul, R.; Das, B.; Ghosh, R. Novel approaches towards design of metal oxide based hetero-structures for room temperature gas sensor and its sensing mechanism: A recent progress. J. Alloys Compd. 2023, 941, 168943. [Google Scholar] [CrossRef]
- Wang, J.; Shen, H.; Xia, Y.; Komarneni, S. Light-activated room-temperature gas sensors based on metal oxide nanostructures: A review on recent advances. Ceram. Int. 2021, 47, 7353–7368. [Google Scholar] [CrossRef]
- Latif, U.; Dickert, F.L. Graphene hybrid materials in gas sensing applications. Sensors 2015, 15, 30504–30524. [Google Scholar] [CrossRef]
- Calise, F.; D’Accadia, M.D.; Santarelli, M.; Lanzini, A.; Ferrero, D. (Eds.) Solar Hydrogen Production: Processes, Systems and Technologies; Academic Press: Cambridge, MA, USA, 2019; 560p. [Google Scholar]
- Nowotny, J.; Veziroglu, T.N. Impact of hydrogen on the environment. Int. J. Hydrogen Energy 2011, 36, 13218–13224. [Google Scholar] [CrossRef]
- Xu, M.; Peng, B.; Zhu, X.; Guo, Y. Multi-gas detection system based on non-dispersive infrared (NDIR) spectral technology. Sensors 2022, 22, 836. [Google Scholar] [CrossRef] [PubMed]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- OECD. Environment at a Glance 2013: OECD Indicators; OECD Publishing: Paris, France, 2013; 108p. [Google Scholar] [CrossRef]
- Dhall, S.; Mehta, B.R.; Tyagi, A.K.; Sood, K. A review on environmental gas sensors: Materials and technologies. Sens. Int. 2021, 2, 100116. [Google Scholar] [CrossRef]
- Kholod, N.; Evans, M.; Pilcher, R.C.; Roshchanka, V.; Ruiz, F.; Coté, M.; Collings, R. Global methane emissions from coal mining to continue growing even with declining coal production. J. Clean. Prod. 2020, 256, 120489. [Google Scholar] [CrossRef]
- Sachin, N.; Sanjit, M.; Ali, M.; Sang, К. Metal oxide ceramic gas sensors. Encycl. Mater. Electron. 2023, 1, 452–462. [Google Scholar]
- Nikolić, M.; Milovanović, V.; Vasiljevic, Z.; Stamenkovic, Z. Semiconductor gas sensors: Materials, technology, design and application. Sensors 2020, 2020, 6694. [Google Scholar] [CrossRef]
- Zhu, L.-Y.; Ou, L.-X.; Mao, L.-W.; Wu, X.; Liu, Y.; Lu, H.-L. Advances in noble metal-decorated metal oxide nanomaterials for chemiresistive gas sensors: Overview. Nano-Micro Lett. 2023, 15, 89. [Google Scholar] [CrossRef]
- Sohal, M.K.; Mahajan, A.; Gasso, S.; Nahirniak, S.V.; Dontsova, T.A.; Singh, R.C. Rare earth-tuned oxygen vacancies in gadolinium-doped tin oxide for selective detection of volatile organic compounds. J. Mater. Sci. Mater. Electron. 2020, 31, 8446–8455. [Google Scholar] [CrossRef]
- Zegebreal, L.T.; Tegegne, N.A.; Hone, F.G. Recent progress in hybrid conducting polymers and metal oxide nanocomposite for room-temperature gas sensor applications: A Review. Sens. Actuators A Phys. 2023, 359, 114472. [Google Scholar] [CrossRef]
- Bhati, V.S.; Kumar, M.; Banerjee, R. Gas sensing performance of 2D nanomaterials/metal oxide nanocomposites: A review. J. Mater. Chem. C 2021, 9, 8776–8806. [Google Scholar] [CrossRef]
- Potyrailo, R.A.; Go, S.; Sexton, D.; Li, X.; Alkadi, N.; Kolmakov, A.; Amm, B.; St-Pierre, R.; Scherer, B.; Nayeri, M.; et al. Extraordinary performance of semiconducting metal oxide gas sensors using dielectric excitation. Nat. Electron. 2020, 3, 280–289. [Google Scholar] [CrossRef]
- Gu, H.; Wang, Z.; Hu, Y. Hydrogen gas sensors based on semiconductor oxide nanostructures. Sensors 2012, 12, 5517–5550. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Cao, Y.-Q.; Arulraj, D.; Liu, C.; Wu, D.; Li, W.; Li, A. Review—Resistive-type hydrogen sensors based on zinc oxide nanostructures. J. Electrochem. Soc. 2020, 167, 067528. [Google Scholar] [CrossRef]
- Hossain, M.K.; Drmosh, Q.A. Noble metal-decorated nanostructured zinc oxide: Strategies to advance chemiresistive hydrogen gas sensing. Chem. Rec. 2022, 22, e202200090. [Google Scholar] [CrossRef] [PubMed]
- Dontsova, T.; Yanushevska, O.; Nahirniak, S.; Kutuzova, A.; Krymets, G.; Smertenko, P. Characterization of commercial TiO2 P90 modified with ZnO by the impregnation method. J. Chem. 2021, 2021, 9378490. [Google Scholar] [CrossRef]
- Nahirniak, S.V.; Dontsova, T.A.; Chen, Q. Sensing properties of SnO2-MWCNTs nanocomposites towards H2. Mol. Cryst. Liq. Cryst. 2018, 674, 48–58. [Google Scholar] [CrossRef]
- Chang, C.W.; Liu, I.P.; Yao, P.C.; Lin, K.W.; Hsu, W.C.; Liu, W.C. Hydrogen detecting characteristics of a palladium nanoparticle/indium gallium oxide based sensor. Sens. Actuators B Chem. 2023, 393, 134240. [Google Scholar] [CrossRef]
- Nguyen, T.T.D.; Dao, D.V.; Kim, D.S.; Lee, H.L.; Oh, S.Y.; Lee, I.H.; Yu, Y.T. Effect of core and surface area toward hydrogen gas sensing performance using Pd@ZnO core-shell nanoparticles. J. Colloid Interface Sci. 2021, 587, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Le, H.J.; Dao, V.D.; Yu, Y.T. Superfast and efficient hydrogen gas sensor using PdAualloy@ZnO core–shell nanoparticles. J. Mater. Chem. A 2020, 8, 12968–12974. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, V.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Kim, S.H.; Yun, K.S. Room-temperature hydrogen gas sensor composed of palladium thin film deposited on NiCo2O4 nanoneedle forest. Sens. Actuators B Chem. 2023, 376, 132958. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, C.; Zheng, B.; Geng, X.; Debliquy, M. Hydrogen sensors based on noble metal doped metal-oxide semiconductor: A review. Int. J. Hydrogen Energy 2017, 42, 20386–20397. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, K.; Liu, K.; Xu, J.; Zheng, Z. Metal oxide resistive sensors for carbon dioxide detection. Coord. Chem. Rev. 2022, 472, 214758. [Google Scholar] [CrossRef]
- Lupan, O.; Chow, L.; Shishiyanu, S.; Monaico, E.; Shishiyanu, T.; Sontea, V.; Cuenya, B.R.; Naitabdi, A.; Park, S.; Schulte, A. Nanostructured zinc oxide films synthesized by successive chemical solution deposition for gas sensor applications. Mater. Res. Bull. 2009, 44, 63–69. [Google Scholar] [CrossRef]
- Kanaparthi, S.; Singh, V. Chemiresistive sensor based on zinc oxide nanoflakes for CO2 detection. ACS Appl. Nano Mater. 2019, 2, 700–706. [Google Scholar] [CrossRef]
- Mahajan, S.; Jagtap, S. Metal-oxide semiconductors for carbon monoxide (CO) gas sensing: A review. Appl. Mater. Today 2020, 18, 100483. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Tereshkov, M.; Dontsova, T.; Yanushevska, O.; Dusheiko, M.; Smertenko, P. Solution composition and temperature impact on physicochemical properties of synthesized zinc oxide. Appl. Nanosci. 2022, 12, 2523–2532. [Google Scholar] [CrossRef]
- Bagheri, F.; Haratizadeh, H. UV-activated CO2 sensor based on ZnO nanoparticles at low temperatures. Mater. Sci. Semicond. Process. 2022, 141, 106422. [Google Scholar] [CrossRef]
- Zhou, Q.; Zeng, W.; Chen, W.; Xu, L.; Kumar, R.; Umar, A. High sensitive and low-concentration sulfur dioxide (SO2) gas sensor application of heterostructure NiO-ZnO nanodisks. Sens. Actuators B Chem. 2019, 298, 126870. [Google Scholar] [CrossRef]
- Tyagi, P.; Sharma, A.; Tomar, M.; Gupta, V. Metal oxide catalyst assisted SnO2 thin film based SO2 gas sensor. Sens. Actuators B Chem. 2016, 224, 282–289. [Google Scholar] [CrossRef]
- Pan, Q.; Yang, Z.; Wang, W.; Zhang, D. Sulfur dioxide gas sensing at room temperature based on tin selenium/tin dioxide hybrid prepared via hydrothermal and surface oxidation treatment. Rare Met. 2020, 40, 1588–1596. [Google Scholar] [CrossRef]
- Chaitra, U.; Ali, A.; Viegas, A.E.; Kekuda, D.; Rao, K.M. Growth and characterization of undoped and aluminium doped zinc oxide thin films for SO2 gas sensing below threshold value limit. Appl. Surf. Sci. 2019, 496, 143724. [Google Scholar] [CrossRef]
- Tyagi, P.; Sharma, A.; Tomar, M.; Gupta, V. Efficient detection of SO2 gas using SnO2 based sensor loaded with metal oxide catalysts. Procedia Eng. 2014, 87, 1075–1078. [Google Scholar] [CrossRef]
- Kang, X.; Deng, N.; Yan, Z.; Pan, Y.; Sun, W.; Zhang, Y. Resistive-type VOCs and pollution gases sensor based on SnO2: A review. Mater. Sci. Semicond. Process. 2022, 138, 106246. [Google Scholar] [CrossRef]
- Fu, Y.; Li, J.; Xu, H. SnO2 recycled from tin slime for enhanced SO2 sensing properties by NiO surface decoration. Mater. Sci. Semicond. Process. 2020, 114, 105073. [Google Scholar] [CrossRef]
- Stoycheva, T.; Vallejos, S.; Blackman, C.S.; Moniz, S.J.A.; Calderer, J.; Correig, X. Important considerations for effective gas sensors based on metal oxide nanoneedles films. Sens. Actuators B Chem. 2012, 161, 406–413. [Google Scholar] [CrossRef]
- Liu, H.; Wan, J.; Fu, Q.; Li, M.; Luo, W.; Zheng, Z.; Cao, H.; Hu, Y.; Zhou, D. Tin oxide films for nitrogen dioxide gas detection at low temperatures. Sens. Actuators B Chem. 2013, 177, 460–466. [Google Scholar] [CrossRef]
- Liu, T.; Jia, X.; Qiao, L.; Yang, J.; Wang, S.; Li, Y.; Shao, D.; Feng, L.; Song, H. Conductometric NO2 gas sensors achieved by design of Ti3C2Tx @ZnO heterostructures for application around 80 °C. Sens. Actuators B Chem. 2023, 390, 133908. [Google Scholar] [CrossRef]
- Afzal, A.; Cioffi, N.; Sabbatini, L.; Torsi, L. NOx sensors based on semiconducting metal oxide nanostructures: Progress and perspectives. Sens. Actuators B Chem. 2012, 171–172, 25–42. [Google Scholar] [CrossRef]
- Sohal, M.K.; Mahajan, A.; Gasso, S.; Nahirniak, S.; Dontsova, T. Modification of SnO2 surface oxygen vacancies through Er doping for ultralow NO2 detection. Mater. Res. Bull. 2020, 133, 111051. [Google Scholar] [CrossRef]
- Srivastava, S. Study of gas sensor detection for NOX Gas: A review. Mater. Today Proc. 2021, 37, 3709–3712. [Google Scholar] [CrossRef]
- Trung, T.D.; Toan, N.V.; Tong, P.V.; Duy, N.V.; Hoa, N.D.; Hieu, N.V. Synthesis of single-crystal SnO2 nanowires for NOx gas sensors application. Ceram. Int. 2012, 38, 6557–6563. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Zhou, P.; Hao, F.; Xu, X.; Gao, S.; Wei, D.; Ao, Y.; Shen, Y. Enhanced NO2 sensing performance of ZnO nanowires functionalized with ultra-fine In2O3 nanoparticles. Sens. Actuators B Chem. 2020, 308, 127729. [Google Scholar] [CrossRef]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-based nanomaterials in gas sensors. Solid State Ion. 2021, 360, 115544. [Google Scholar] [CrossRef]
- Lux, K.C.; Hot, J.; Fau, P.; Bertron, A.; Kahn, M.L.; Ringot, E.; Fajerwerg, K. Nano-gold decorated ZnO: An alternative photocatalyst promising for NOx degradation. Chem. Eng. Sci. 2023, 267, 118377. [Google Scholar]
- Nundy, S.; Eom, T.; Kang, J.; Suh, J.; Cho, M.; Park, J.S.; Lee, H.J. Flower-shaped ZnO nanomaterials for low-temperature operations in NOX gas sensors. Ceram. Int. 2020, 46, 5706–5714. [Google Scholar] [CrossRef]
- Xia, Y.; Xu, L.; He, S.; Zhou, L.; Wang, M.; Wang, J.; Komarneni, S. UV-activated WS2/SnO2 2D/0D heterostructures for fast and reversible NO2 gas sensing at room temperature. Sens. Actuators B Chem. 2022, 364, 131903. [Google Scholar] [CrossRef]
- Chakrabarty, P.; Banik, M.; Gogurla, N.; Santra, S.; Ray, S.K.; Mukherjee, R. Light trapping-mediated room-temperature gas sensing by ordered ZnO nano structures decorated with plasmonic Au nanoparticles. ACS Omega 2019, 4, 12071–12080. [Google Scholar] [CrossRef]
- Chen, R.; Wang, J.; Xia, Y.; Xiang, L. Near infrared light enhanced room-temperature NO2 gas sensing by hierarchical ZnO nanorods functionalized with PbS quantum dots. Sens. Actuators B Chem. 2018, 255, 2538–2545. [Google Scholar] [CrossRef]
- Peterson, P.J.D.; Aujla, A.; Grant, K.H.; Brundle, A.G.; Thompson, M.R.; Hey, J.V.; Leigh, R.J. Practical use of metal oxide semiconductor gas sensors for measuring nitrogen dioxide and ozone in urban environments. Sensors 2017, 17, 1653. [Google Scholar] [CrossRef]
- Aldhafeeri, T.R.; Fowler, M.; Vrolyk, R.; Pope, M.A.; Fowler, M. A review of methane gas detection sensors: Recent developments and future perspectives. Inventions 2020, 5, 28. [Google Scholar] [CrossRef]
- Basu, S.; Basu, P.K. Nanocrystalline metal oxides for methane sensors: Role of noble metals. J. Sens. 2009, 2009, 861968. [Google Scholar] [CrossRef]
- De Lacy Costello, B.P.J.; Ewen, R.J.; Ratcliffe, N.M.; Richards, M. Highly sensitive room temperature sensors based on the UV-LED activation of zinc oxide nanoparticles. Sens. Actuators B Chem. 2008, 134, 945–952. [Google Scholar] [CrossRef]
- Aghagoli, Z.; Ardyanian, M. Synthesis and study of the structure, magnetic, optical and methane gas sensing properties of cobalt doped zinc oxide microstructures. J. Mater. Sci. Mater. Electron. 2018, 29, 7130–7141. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Basu, P.K.; Saha, H.; Basu, S. Fast response methane sensor using nanocrystalline zinc oxide thin films derived by sol–gel method. Sens. Actuators B Chem. 2007, 124, 62–67. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, X.; Yao, M.; Sun, G.; Zhang, Z. Enhanced CH4 sensing properties of Pd modified ZnO nanosheets. Ceram. Int. 2019, 45, 13150–13157. [Google Scholar] [CrossRef]
- Li, H.Y.; Shu-Na, Z.; Zang, S.Q.; Li, J. Functional metal–organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef]
- Yuan, Z.; Han, E.; Meng, F.; Zuo, K. Detection and identification of volatile organic compounds based on temperature-modulated ZnO sensors. IEEE Trans. Instrum. Meas. 2020, 69, 4533–4544. [Google Scholar] [CrossRef]
- Luan, Z.; Zeng, W.; Li, Y. A non-oxygen adsorption mechanism for hydrogen detection of nanostructured SnO2 based sensors. Mater. Res. Bull. 2019, 109, 108–116. [Google Scholar]
- Choi, K.-S.; Chang, S.-P. Effect of structure morphologies on hydrogen gas sensing by ZnO nanotubes. Mater. Lett. 2018, 230, 48–52. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, R.; Rajamani, S.; Ranwa, S.; Fanetti, M.; Valant, M.; Kumar, M. Efficient room temperature hydrogen sensor based on UV-activated ZnO nano-network. Nanotechnology 2017, 28, 365502. [Google Scholar] [CrossRef]
- Fan, F.; Zhang, J.; Li, J.; Zhang, N.; Hong, R.; Deng, X.; Tang, P.; Li, D. Hydrogen sensing properties of Pt-Au bimetallic nanoparticles loaded on ZnO nanorods. Sens. Actuators B Chem. 2017, 241, 895–903. [Google Scholar] [CrossRef]
- Geng, X.; Luo, Y.; Zheng, B.; Zhang, C. Photon assisted room-temperature hydrogen sensors using PdO loaded WO3 nanohybrids. Int. J. Hydrogen Energy 2017, 42, 6425–6434. [Google Scholar] [CrossRef]
- Mobtakeran, S.; Habashyani, S.; Çoban, Ö.; Budak, H.F.; Kasapoğlu, A.E.; Gür, E. Effect of growth pressure on sulfur content of RF-magnetron sputtered WS2 films and thermal oxidation properties of them toward using Pd decorated WO3 based H2 gas sensor. Sens. Actuators B Chem. 2023, 381, 133485. [Google Scholar] [CrossRef]
- Li, G.; Du, K.; Wang, X.; Wang, X.; Chen, B.; Qiu, C.; Xu, J. Pd nanoparticles decorated SnO2 ultrathin nanosheets for highly sensitive H2 sensor: Experimental and theoretical studies. Int. J. Hydrogen Energy 2023, 50, 761–771. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, L.; Zhou, Q.; Zhang, Z.; Dong, Z.; Nie, L.; Liu, Q.; Pan, G. In-Situ synthesis of needle-like PdO-decorated NiO thin films on Al2O3 substrates for high-performance H2 sensors. Ceram. Int. 2022, 48, 31746–31754. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Sharma, A.; Vikraman, D.; Lee, Y.A.; Sivakumar, P.; Korvink, J.G.; Kim, H.S.; Sharma, B. Room-temperature response of MOF-derived Pd@PdO core shell/γ-Fe2O3 microcubes decorated graphitic carbon based ultrasensitive and highly selective H2 gas sensor. J. Colloid Interface Sci. 2023, 652, 692–704. [Google Scholar] [CrossRef] [PubMed]
- More, M.S.; Bodkhe, G.A.; Ingle, N.N.; Singh, F.; Tsai, M.L.; Kim, M.; Shirsat, M.D. Metal-organic framework (MOF)/reduced graphene oxide (rGO) composite for high performance CO sensor. Solid-State Electron. 2023, 204, 108638. [Google Scholar] [CrossRef]
- Krivetskiy, V.; Efitorov, A.; Arkhipenko, A.; Vladimirova, S.; Rumyantseva, M.; Dolenko, S.; Gaskov, A. Selective detection of individual gases and CO/H2 mixture at low concentrations in air by single semiconductor metal oxide sensors working in dynamic temperature mode. Sens. Actuators B Chem. 2018, 254, 502–513. [Google Scholar] [CrossRef]
- Fort, A.; Mugnaini, M.; Rocchi, S.; Vignoli, V.; Comini, E.; Faglia, G.; Ponzoni, A. Metal-oxide nanowire sensors for CO detection: Characterization and modeling. Sens. Actuators B Chem. 2010, 148, 283–291. [Google Scholar] [CrossRef]
- Brosha, E.L.; Mukundan, R.; Brown, D.R.; Garzon, F.H.; Visser, J.H.; Zanini, M.; Zhou, Z.; Logothetis, E.M. CO/HC sensors based on thin films of LaCoO3 and La0.8Sr0.2CoO3−δ metal oxides. Sens. Actuators B Chem. 2000, 69, 171–182. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, D.; Pan, Q.; Wang, T.; Chen, F. Gas sensing performance of carbon monoxide sensor based on rod-shaped tin diselenide/MOFs derived zinc oxide polyhedron at room temperature. Sens. Actuators B Chem. 2022, 371, 132481. [Google Scholar] [CrossRef]
- Wu, R.; Tian, L.; Li, H.; Liu, H.; Luo, J.; Tian, X.; Hua, Z.; Wu, Y.; Fan, S. A selective methane gas sensor based on metal oxide semiconductor equipped with an on-chip microfilter. Sens. Actuators B Chem. 2022, 359, 131557. [Google Scholar] [CrossRef]
- Shwetha, H.R.; Sharath, S.M.; Guruprasad, B.; Rudraswamy, S.B. MEMS based metal oxide semiconductor carbon dioxide gas sensor. Micro Nano Eng. 2022, 16, 100156. [Google Scholar] [CrossRef]
- Keerthana, S.; Rathnakannan, K. Room temperature operated carbon dioxide sensor based on silver doped zinc oxide/cupric oxide nanoflowers. Sens. Actuators B Chem. 2023, 378, 133181. [Google Scholar] [CrossRef]
- Thomas, T.; Kumar, Y.; Ramón, J.A.R.; Agarwal, V.; Guzmán, S.S.; Pushpan, R.R.S.; Loredo, S.L.; Sanal, K.C. Porous silicon/α-MoO3 nanohybrid based fast and highly sensitive CO2 gas sensors. Vacuum 2021, 184, 109983. [Google Scholar] [CrossRef]
- Miao, F.; Wu, H.; Tao, B.; Zang, Y. A passive-chipless LC carbon dioxide sensor with non-contact ZnO/CuO/RGO nanocomposites at room temperature. Vacuum 2023, 215, 112261. [Google Scholar] [CrossRef]
- Wang, X.; Su, J.; Chen, H.; Li, G.; Shi, Z.; Zou, H.; Zou, X. Ultrathin In2O3 nanosheets with uniform mesopores for highly sensitive nitric oxide detection. ACS Appl. Mater. Interfaces 2017, 9, 16335–16342. [Google Scholar] [CrossRef]
- Liu, B.; Luo, Y.; Li, K.; Wang, H.; Gao, L.; Duan, G. Room-temperature NO2 gas sensing with ultra-sensitivity activated by ultraviolet light based on SnO2 monolayer array film. Adv. Mater. Interfaces 2019, 6, 1900376. [Google Scholar] [CrossRef]
- Shen, Y.; Zhong, X.; Zhang, J.; Li, T.; Zhao, S.; Cui, B.; Wei, D.; Zhang, Y.; Wei, K. In-situ growth of mesoporous In2O3 nanorod arrays on a porous ceramic substrate for ppb-level NO2 detection at room temperature. Appl. Surf. Sci. 2019, 498, 143873. [Google Scholar] [CrossRef]
- Hu, J.; Zou, C.; Su, Y.; Li, M.; Ye, X.; Cai, B.; Kong, E.S.-W.; Liu, Y. Light-assisted recovery for a highly-sensitive NO2 sensor based on RGO-CeO2 hybrids. Sens. Actuators B Chem. 2018, 270, 119–129. [Google Scholar] [CrossRef]
- Chinh, N.D.; Quang, N.D.; Lee, H.; Hien, T.T.; Hieu, N.M.; Kim, D.; Kim, C.; Kim, D. NO gas sensing kinetics at room temperature under UV light irradiation of In2O3 nanostructures. Sci. Rep. 2016, 6, 3506. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, G.; Xu, M.; Su, Y.; Tai, H.; Du, H.; Jiang, Y. Visible light-assisted room temperature gas sensing with ZnO-Ag heterostructure nanoparticles. Sens. Actuators B Chem. 2018, 259, 269–281. [Google Scholar] [CrossRef]
- Wang, C.Y.; Hong, Z.S.; Wu, R.J. Promotion effect of Pt on a SnO2–WO3 material for NOx sensing. Phys. E Low-Dimens. Syst. Nanostructures 2015, 69, 191–197. [Google Scholar] [CrossRef]
- Cao, Z.; Gao, Q.; Zhou, M.; Li, X.; Wang, Q. LaNiTiO3-SE-based stabilized zirconium oxide mixed potentiometric SO2 gas sensor. Ceram. Int. 2022, 48, 9269–9276. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, Y.; Zhang, Y.; Li, Y.; Tang, C.; Li, K.; Sun, J.; Deng, T. A novel room temperature SO2 gas sensor based on TiO2/rGO buried-gate FET. Microelectron. Eng. 2022, 263, 111841. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, J.; Gao, P.-X. Single bimodular sensor for differentiated detection of multiple oxidative gases. Adv. Mater. Technol. 2020, 5, 1901152. [Google Scholar] [CrossRef]
- Manikandan, V.; Vigneselvan, S.; Petrila, L.; Mane, R.S.; Singh, A.; Sobczak, K.; Chandrasekaran, J. Long-lasting stability and low-concentration SO2 gas detection aptitude of Sn-doped alumina sensors. Mater. Chem. Phys. 2022, 291, 126691. [Google Scholar]
- Li, R.; Wang, S.; Li, S.; Zhao, F.; Dong, T.; He, P.; Yu, L.; Miao, J.; Fan, X. Cu-doped flower-like SnO2 architecture toward promoting SO2 detection: Fast equilibrium and low trace monitoring. Sens. Actuators B Chem. 2023, 390, 133953. [Google Scholar] [CrossRef]
- Boudiba, A.; Zhang, C.; Bittencourt, C.; Umek, P.; Olivier, M.G.; Snyders, R.; Debliquy, M. SO2 Gas Sensors based on WO3 nanostructures with different morphologies. Procedia Eng. 2012, 47, 1033–1036. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Luong, N.A.; Nguyen, V.T.; Pham, A.T.; Le, A.T.; To, T.L.; Nguyen, V.Q. Effect of the phase composition of iron oxide nanorods on SO2 gas sensing performance. Mater. Res. Bull. 2021, 134, 111087. [Google Scholar] [CrossRef]
- Das, S.; Girija, K.G.; Debnath, A.K.; Vatsa, R.K. Enhanced NO2 and SO2 sensor response under ambient conditions by polyol synthesized Ni doped SnO2 nanoparticles. J. Alloys Compd. 2021, 854, 157276. [Google Scholar] [CrossRef]
- Thangamani, G.J.; Pasha, S.K.K. Titanium dioxide (TiO2) nanoparticles reinforced polyvinyl formal (PVF) nanocomposites as chemiresistive gas sensor for sulfur dioxide (SO2) monitoring. Chemosphere 2021, 275, 129960. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ying, Z.; Wen, F.; Li, L.; Zheng, X.; Zheng, P.; Wang, G. MoS2-doped spherical SnO2 for SO2 sensing under UV light at room temperature. Mater. Sci. Semicond. Process. 2021, 134, 105997. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Y.; Meng, X.; Zhang, Z.; Cao, J. A gas sensor based on Ag-modified ZnO flower-like microspheres: Temperature-modulated dual selectivity to CO and CH4. Surf. Interfaces 2021, 24, 101110. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Sun, X.; Wang, Y.; Li, M.; Cao, J.; Qin, С. Enhanced CH4 sensing performances of g-C3N4 modified ZnO nanospheres sensors under visible-light irradiation. Mater. Res. Bull. 2023, 165, 112290. [Google Scholar] [CrossRef]
- Tshabalala, Z.P.; Swart, H.C.; Motaung, D.E. Fabrication of TiO2 nanofibers based sensors for enhanced CH4 performance induced by notable surface area and acid treatment. Vacuum 2021, 187, 110102. [Google Scholar] [CrossRef]
- Han, L.; Zhang, S.; Zhang, B.; Zhang, B.; Wang, Y.; Bala, H.; Zhang, Z. Dual-selective detection of CO and CH4 based on hierarchical porous In2O3 nanoflowers with Pd modification. J. Mater. 2022, 8, 545–555. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Han, L.; Zhang, B.; Wang, Y.; Zhang, Z. Preparation of porous NiO/In2O3 nanoflower-like composites and their dual selectivity for CO/CH4. Mater. Res. Bull. 2023, 165, 112332. [Google Scholar] [CrossRef]
- Vatandoust, L.; Habibi, A.; Naghshara, H.; Sajedeh Mohammadi Aref, S.M. Fabrication and investigation of TiO1.5/ZnO nanocomposite nanosensor for detection of CO and CH4 gases. Surf. Interfaces 2022, 31, 102001. [Google Scholar] [CrossRef]
- Mishra, V.N.; Agarwal, R.P. Sensitivity, response and recovery time of SnO2 based thick-film sensor array for H2, CO, CH4 and LPG. Microelectron. J. 1998, 29, 861–874. [Google Scholar] [CrossRef]
- Mounasamy, V.; Mani, G.K.; Ponnusamy, D.; Tsuchiya, K.; Reshma, P.R.; Prasad, A.K.; Madanagurusamy, S. Investigation on CH4 sensing characteristics of hierarchical V2O5 nanoflowers operated at relatively low temperature using chemiresistive approach. Anal. Chim. Acta 2020, 1106, 148–160. [Google Scholar] [CrossRef]
- Nahirniak, S.; Dontsova, T.; Astrelin, I. Directional synthesis of SnO2-based nanostructures for use in gas sensors. In Nanochemistry, Biotechnology, Nanomaterials, and Their Applications. NANO 2017; Springer Proceedings in Physics; Springer: Berlin/Heidelberg, Germany, 2018; Volume 214, pp. 233–245. [Google Scholar]
- Dontsova, T.; Nahirniak, S.; Linyucheva, O.; Tereshkov, M.; Mahajan, A.; Singh, R.C. Physicochemical properties of Tin (IV) oxide synthesized by different methods and from different precursors. Appl. Nanosci. 2022, 12, 1155–1168. [Google Scholar] [CrossRef]
- Sviderskyi, A.; Nahirniak, S.; Yashchenko, T.; Dontsova, T.; Kalinowski, S. Properties of TiO2 and SnO2 in a state of different dispersion and morphology. In Proceedings of the NAP-2018, 2018 IEEE 8th International Conference on Nanomaterials: Applications and Properties (NAP), Zatoka, Ukraine, 9–14 September 2018; 01NNPT13-1-4. [Google Scholar]
- Yashchenko, T.; Sviderskyi, A.; Nahirniak, S.; Dontsova, T.; Kalinowski, S. Electrical properties and sensitivity of SnO2 nanostructures to organic compounds. Nanosistemi Nanomater. Nanotehnologii 2021, 19, 53–70. [Google Scholar]
- Korotcenkov, G. Current trends in nanomaterials for metal oxide-based conductometric gas sensors: Advantages and limitations. Part 1: 1D and 2D Nanostructures. Nanomaterials 2020, 10, 1392. [Google Scholar] [CrossRef]
- Xue, S.; Cao, S.; Huang, Z.; Yang, D.; Zhang, G. Improving gas-sensing performance based on MOS nanomaterials: A Review. Materials 2021, 14, 4263. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Chase, M.; Jung, J.; Kim, H.-D. Correlation between sensing accuracy and read margin of a memristor-based NO gas sensor array estimated by neural network analysis. ACS Sens. 2023, 8, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Cho, I.; Park, J.; Jeong, J.; Lee, K.; Lee, B.; Henriquez, D.D.O.; Yoon, K.; Park, I. High accuracy real-time multi-gas identification by a batch-uniform gas sensor array and deep learning algorithm. ACS Sens. 2022, 7, 430–440. [Google Scholar] [CrossRef]
- The Main Sources of Air Pollution. Available online: https://www.breeze-technologies.de/blog/main-sources-of-air-pollution/ (accessed on 31 July 2023).
- Capelli, L.; Sironi, S.; Del Rosso, R. Electronic noses for environmental monitoring applications. Sensors 2014, 14, 19979–20007. [Google Scholar] [CrossRef]
- EN 13725:2003; Air Quality: Determination of Odor Concentration by Dynamic Olfactometry. Comitee Europeen de Normalisation: Brussels, Belgium, 2007.
- Bourgeois, W.; Romain, A.-C.; Nicolas, J.; Stuetz, R.M. The use of sensor arrays for environmental monitoring: Interests and limitations. J. Environ. Monit. 2003, 5, 852–860. [Google Scholar] [CrossRef]
- Betty, C.A.; Choudhury, S.; Girija, K.G. Reliability studies of highly sensitive and specific multi-gas sensor based on nanocrystalline SnO2 film. Sens. Actuators B 2014, 193, 484–491. [Google Scholar] [CrossRef]
- Abbas, M.N.; Moustafa, G.A.; Gopel, W. Multicomponent analysis of some environmentally important gases using semiconductor tin oxide sensors. Anal. Chim. Acta 2001, 431, 181–194. [Google Scholar] [CrossRef]
- Özmet, A.; Dogan, E. Design of a portable e-nose instrument for gas classifications. IEEE Trans. Instrum. Meas. 2009, 58, 3609–3618. [Google Scholar]
- Helli, O.; Siadat, M.; Lumbreras, M. Qualitative and quantitative identification of H2S/NO2 gaseous components in different reference atmospheres using a metal oxide sensor array. Sens. Actuators B Chem. 2004, 103, 403–408. [Google Scholar] [CrossRef]
- Lee, D.S.; Jung, J.K.; Lim, J.W.; Huh, J.S.; Lee, D.D. Recognition of volatile organic compounds using SnO2 sensor array and pattern recognition analysis. Sens. Actuators B Chem. 2001, 77, 228–236. [Google Scholar] [CrossRef]
- Barisci, J.N.; Wallace, G.G.; Andrews, M.K.; Partridge, A.C.; Harris, P.D. Conduction polymer sensors for monitoring aromatic hydrocarbons using an electronic nose. Sens. Actuators B Chem. 2002, 84, 252–257. [Google Scholar] [CrossRef]
- De Vito, S.; Massera, E.; Piga, A.; Martinotto, L.; di Francia, G. On field calibration of an electronic nose for benzene estimation in an urban pollution monitoring scenario. Sens. Actuators B Chem. 2008, 129, 750–757. [Google Scholar] [CrossRef]
- Romain, A.-C.; Andre, P.; Nicolas, J. Three years experiment with the same tin oxide sensor array for identification of malodorous sources in the environment. Sens. Actuators B 2002, 84, 271–277. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, Y.W.; Na, K.J.; Jeon, G.J. Wireless electronic nose system for real-time quantitative analysis of gas mixture using micro-gas sensor array and neuro-fuzzy network. Sens. Actuators B Chem. 2008, 134, 104–111. [Google Scholar] [CrossRef]
- Dentoni, L.; Capelli, L.; Sironi, S.; del Rosso, R.; Zanetti, S.; Della Torre, M. Development of an electronic nose for environmental odour monitoring. Sensors 2012, 12, 14363–14381. [Google Scholar] [CrossRef]
- Reddy, B.K.; Borse, P.H. Review—Recent material advances and their mechanistic approaches for room temperature chemiresistive gas sensors. J. Electrochem. Soc. 2021, 168, 057521. [Google Scholar] [CrossRef]
- Dadkhan, M.; Tulliani, J.-M. Nanostructured metal oxide semiconductors towards greenhouse gas detection. Chemosensors 2022, 10, 57. [Google Scholar] [CrossRef]
- Lee, S.P. Electrodes for semiconductor gas sensors. Sensors 2017, 17, 683. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, A.; Mathew, J.; Jabir, A.; Di Natele, C.; Martinelli, E.; Ottavi, M. Efficient sensing approaches for high-density memristor sensor array. J. Comput. Electron. 2018, 17, 1285–1296. [Google Scholar] [CrossRef]
- Liu, J.; Han, T.; Sun, B.; Kong, L.; Jin, Z.; Huang, X.; Liu, J.; Meng, F. Catalysis-based cataluminiscent and conductometric gas sensors: Sensing nanomaterials, mechanism, applications and perspectives. Catalysis 2016, 6, 210. [Google Scholar]
- Haidry, A.A.; Ebach-Stahl, A.; Saruhan, B. Effect of Pt/TiO2 interface on room temperature hydrogen sensing performance of memristor type Pt/TiO2/Pt structure. Sens. Actuators B Chem. 2017, 253, 1043–4054. [Google Scholar] [CrossRef]
- Bhat, P.; Kumar, S.K.N. Evaluation of IDE-based flexible thin film ZnO sensor for VOC sensing in a custom designed gas chamber at room temperature. J. Mater. Sci. Mater. Electron. 2022, 33, 1529–1541. [Google Scholar] [CrossRef]
- Mikolasek, M.; Meri, J.; Chymo, F.; Ondrejka, P.; Rehacek, V.; Predanocy, M.; Kostic, I.; Hotovy, I. Novel Cu2O gas sensor prepared by potentiostatic electrodeposition on IDE electrodes. J. Physics Conf. Ser. 2019, 1319, 012009. [Google Scholar] [CrossRef]
- Plecenik, T.; Moško, M.; Haidry, A.A.; Ďurina, P.; Truchlý, M.; Grančič, B.; Gregor, M.; Roch, T.; Satrapinskyy, L.; Mošková, A.; et al. Fast high-sensitive room-temperature semiconductor gas sensor based on nanoscale Pt-TiO2-Pt sandwich. Sens. Actuators B 2015, 207, 351–361. [Google Scholar] [CrossRef]
- Vidiš, M.; Plecenik, T.; Moškp, M.; Tomašec, S.; Roch, T.; Satrapinskyy, L.; Grančič, B.; Plecenik, A. Gasistor: A memristor based gas-triggered switch and gas sensor with memory. Appl. Phys. Lett. 2019, 115, 093504. [Google Scholar] [CrossRef]
- Nagirnyak, S.V.; Dontsova, T.A. Gas sensor device creation. In Proceedings of the NAP-2017, 2017 IEEE 7th International Conference on Nanomaterials: Applications and Properties (NAP), Odessa, Ukraine, 10–15 September 2017. 01NNPT13-1-4. [Google Scholar]
- Feng, S.; Farha, F.; Li, Q.; Wan, Y.; Xu, Y.; Zhang, T.; Ning, H. Review on smart gas sensing technology. Sensors 2019, 19, 3760. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Yamazaki, T.; Kikuta, T. Effect of micro-electrode geometry on NO2 gas-sensig characteristics of one-dimensional tin dioxide nanostructure microsensors. Sens. Actuators B 2011, 156, 784–790. [Google Scholar] [CrossRef]
- Tamaki, J.; Mayaji, A.; Makinodan, J.; Ogura, S.; Konishi, S. Effect of micro-gap electrode on detection of dilute NO2 using WO3 thin film microsensors. Sens. Actuators B Chem. 2005, 108, 202–206. [Google Scholar] [CrossRef]
- Nguyen Mihn, Q.; Tong, H.D.; Kuijk, A.; van de Bent, F.; Beekman, P.; van Rijn, C.J.M. Gas sensing performance at room temperature of nanogap interdigitated electrodes for detection of acetone at low concentration. RSC Adv. 2017, 7, 50279. [Google Scholar]
- Miyaji, A.; Ogura, S.; Konishi, S.; Tamaki, J. Effect of micro-gap electrode in nitrogen dioxide sensor using tungsten oxide thin film. Electrochem. Soc. Proc. 2004, 2004-09, 201–204. [Google Scholar]
- Prajesh, R.; Goyal, V.; Saini, V.; Bhargava, J.; Sharma, A.; Agarwal, A. Effect of IDE placement on response in metal oxide gas sensors. Mater. Res. Express 2018, 5, 096415. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef]
- Li, T.; Zeng, W.; Long, H.; Wang, Z. Nanosheet-assembled hierarchical SnO2 nanostructures for efficient gas-sensing applications. Sens. Actuators B Chem. 2016, 231, 120–128. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, L.; Ma, J.; Ren, Y.; Cheng, X.; Zhu, Y.; Zhao, D.; Elzatahry, A.A.; Alghamdi, A.; Deng, Y. Ordered mesoporous tin oxide semiconductors with large pores and crystallized walls for high-performance gas sensing. ACS Appl. Mater. Interfaces 2018, 10, 1871–1880. [Google Scholar] [CrossRef]
- Van Dang, T.; Hoa, N.D.; Van Duy, N.; Van Hieu, N. Chlorine gas sensing performance of on-chip grown ZnO, WO3, and SnO2 nanowire sensors. ACS Appl. Mater. Interfaces 2016, 8, 4828–4837. [Google Scholar] [CrossRef]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B Chem. 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Ansari, S.G.; Boroojerdian, P.; Sainkar, S.R.; Karekar, R.N.; Aiyer, R.C.; Kulkarni, S.A. Grain size fffects on H2 gas sensitivity of thick film resistor using SnO2 nanoparticles. Thin Solid Film. 1997, 295, 271–276. [Google Scholar] [CrossRef]
- Baraton, M.-I.; Merhari, L. Influence of the particle size on the surface reactivity and gas sensing properties of SnO2 nanopowders. Mater. Trans. 2001, 42, 1616–1622. [Google Scholar] [CrossRef]
- Das, S.; Mojumder, S.; Saha, D.; Pal, M. Influence of major parameters on the sensing mechanism of semiconductor metal oxide based chemiresistive gas aensors: A review focused on personalized healthcare. Sens. Actuators B Chem. 2022, 352, 131066. [Google Scholar] [CrossRef]
- Hwang, S.-H.; Kim, Y.K.; Hong, S.H.; Lim, S.K. Effect of the morphology and electrical property of metal-deposited ZNO nanostructures on CO gas sensitivity. Nanomaterials 2020, 10, 2124. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, P.G.; Itoh, T.; Masuda, Y. Morphology control technique of ZnO by hydroxide ion supply rate and its effect on the ppt-level gas sensors. J. Am. Ceram. Soc. 2023, 106, 5944–5954. [Google Scholar] [CrossRef]
- Yao, K.; Caruntu, D.I.; Cao, B.; O’Connor, C.J.; Zhou, W. Investigation of gas-sensing performance of SnO2 nanoparticles with different morphologies. IEEE Trans. Nanotechnol. 2010, 9, 630–633. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C. Oxygen vacancy engineering on cerium oxide nanowires for room-temperature linalool detection in rice aging. J. Adv. Ceram. 2022, 11, 1559–1570. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Liu, Y.; Zhao, B. Progress in preparation, characterization, surface functional modification of graphene oxide: A review. J. Saudi Chem. Soc. 2022, 26, 101560. [Google Scholar] [CrossRef]
- Bae, K.L.; Kim, J.; Lim, C.K.; Nam, K.M.; Song, H. Colloidal zinc oxide-copper(I) oxide nanocatalysts for selective aqueous photocatalytic carbon dioxide conversion into methane. Nat. Commun. 2017, 8, 1156. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lim, C.K.; Park, H.J.; Song, H.; Choi, S.-Y.; Lee, D.-S. ZnO–CuO core-hollow cube nanostructures for highly sensitive acetone gas sensors at the ppb level. ACS Appl. Mater. Interfaces 2020, 12, 35688–35697. [Google Scholar] [CrossRef]
- Yu, Q.; Kong, W.; Zhang, Y.; Li, X.; Xu, Y. Morphology-controllable synthesis of hierarchical hollow GaFeO3 microcubes with selective triethylamine gas-sensing properties. Ceram. Int. 2022, 48, 4554–4562. [Google Scholar] [CrossRef]
- Wang, C.-X.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Neri, G. First fifty years of chemoresistive gas sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Meixner, H.; Lampe, U. Metal oxide sensors. Sens. Actuators B Chem. 1996, 33, 198–202. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, Z.; Shen, J.; Zheng, C. Improvement and mechanism for the fast response of a Pt/TiO2 gas sensor. Sens. Actuators B Chem. 2010, 148, 87–92. [Google Scholar] [CrossRef]
- Ghosh, A.; Maity, A.; Banerjee, R.; Majumder, S.B. Volatile organic compound sensing using copper oxide thin films: Addressing the cross sensitivity issue. J. Alloys Compd. 2017, 692, 108–118. [Google Scholar] [CrossRef]
- Kamarudin, K.; Bennetts, V.H.; Mamduh, S.H.; Visvanathan, R.; Yeon, A.S.A.; Shakaff, A.Y.M.; Zakaria, A.; Abdullah, A.; Kamarudin, L.M. Cross-sensitivity of metal oxide gas sensor to ambient temperature and humidity: Effects on gas distribution mapping. Am. Inst. Phys. 2017, 1808, 020025. [Google Scholar]
- Neri, G.; Leonardi, S.G.; Latino, M.; Donato, N.; Baek, S.; Conte, D.E.; Russo, P.A.; Pinna, N. Sensing behavior of SnO2/reduced graphene oxide nanocomposites toward NO2. Sens. Actuators B Chem. 2013, 179, 61–68. [Google Scholar] [CrossRef]
- Fan, S.; Srivastava, A.K.; Dravid, V.P. UV-activated room-temperature gas sensing mechanism of polycrystalline ZnO. Appl. Phys. Lett. 2009, 95, 142106. [Google Scholar] [CrossRef]
- Kumar, R.; Liu, X.; Zhang, J.; Kumar, M. Room-temperature gas sensors under photoactivation: From metal oxides to 2D materials. Nano-Micro Lett. 2020, 12, 164. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.-Y.; Mirzaei, A.; Nam, M.-S.; Kim, H.W.; Kim, S.S. Room-temperature detection of acetone gas by PANI/NiO-loaded TiO2 nanoparticles under UV irradiation. Sens. Actuators B Chem. 2023, 374, 132850. [Google Scholar] [CrossRef]
- Pedowitz, M.; Kim, S.; Lewis, D.I.; Uppalapati, B.; Khan, D.; Bayram, F.; Koley, G.; Daniels, K.M. Fast selective sensing of nitrogen-based gases utilizing δ-MnO2-epitaxial graphene-silicon carbide heterostructures for room temperature gas sensing. J. Microelectromech. Syst. 2020, 29, 846–852. [Google Scholar] [CrossRef]
- Shin, D.H.; Choi, Y.S.; Park, S.-J.; Yeo, C.-S.; Park, Y.Y.; Jun, S.; Lee, S.-K.; Kim, T.-W.; Bae, S.; Hong, B.H. Fast and complete recovery of TMDs-decorated RGO fiber gas sensors at room temperature. Appl. Surf. Sci. 2022, 578, 151832. [Google Scholar] [CrossRef]
- Moumen, A.; Kumarage, W.G.C.; Comini, E. P-type metal oxide semiconductor thin films: Synthesis and chemical sensor applications. Sensors 2022, 22, 1359. [Google Scholar] [CrossRef]
- Abdullah, A.N.; Kamarudin, L.M.; Adom, A.H.; Zakaria, S.A.; Juffry, Z.H.M.; Bennetts, V.H. Correction model for metal oxide sensor drift caused by ambient temperature and humidity. Sensors 2022, 22, 3301. [Google Scholar] [CrossRef]
- Nair, S.S.; Illyaskutty, N.; Tam, B.; Yazaydin, A.O.; Emmerich, K.; Steudel, A.; Hashem, T.; Schöttner, L.; Wöll, C.; Kohler, H.; et al. ZnO@ZIF-8: Gas sensitive core-shell hetero-structures show reduced cross-sensitivity to humidity. Sens. Actuators B Chem. 2020, 304, 127184. [Google Scholar] [CrossRef]
- Spagnoli, E.; Fabbri, B.; Gaiardo, A.; Valt, M.; Ardit, M.; Krik, S.; Cruciani, G.; Della Ciana, M.; Vanzetti, L.; Vola, G.; et al. Design of a metal-oxide solid solution for selective detection of ethanol with marginal influence by humidity. Sens. Actuators B Chem. 2022, 370, 132426. [Google Scholar] [CrossRef]
- Schipani, F.; Miller, D.B.; Ponce, M.A.; Aldao, C.M.; Akbar, S.A.; Morris, P.A. Electrical characterization of semiconductor oxide-based gas sensors using impedance spectroscopy: A review. Rev. Adv. Sci. Eng. 2016, 5, 86–105. [Google Scholar] [CrossRef]
- Ratan, S.; Kumar, C.; Kumar, A.; Jarwal, D.K.; Mishra, A.K.; Upadhyay, R.K.; Singh, A.P.; Jit, S. Room temperature high hydrogen gas response in Pd/TiO2/Si/Al capacitive sensor. Micro Nano Lett. 2020, 15, 632–635. [Google Scholar] [CrossRef]
- Marimuthu, G.; Nguyen, B.S.; Pham, V.T.; Nguyen, V.H.; Tran, V.M.; Sivashanmugan, K.; Pazhanivel, T. Novel NiCo2O4/MWCNTs nanocomposite with flake-like architecture as room temperature capacitive-type NH3 gas sensor. Mater. Lett. 2021, 283, 128814. [Google Scholar] [CrossRef]
- Schipani, F.; Miller, D.B.; Ponce, M.A.; Aldao, C.M.; Akbar, S.A.; Morris, P.A.; Xu, J. Conduction mechanisms in SnO2 single-nanowire gas sensors: An impedance spectroscopy study. Sens. Actuators B Chem. 2017, 241, 99–108. [Google Scholar] [CrossRef]
- Lee, S.; Jin, J.; Baek, J.; Lee, J.; Chae, H. Readout integrated circuit for small-sized and low-power gas sensor based on HEMT device. Sensors 2021, 21, 5637. [Google Scholar] [CrossRef] [PubMed]
- Drix, D.; Schmuker, M. Resolving fast gas transients with metal oxide sensors. ACS Sens. 2021, 6, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Burgués, J.; Hernandez, V.; Lilienthal, A.J.; Marco, S. Smelling nano aerial vehicle for gas source localization and mapping. Sensors 2019, 19, 478. [Google Scholar] [CrossRef]
- Acharyya, S.; Jana, B.; Nag, S.; Saha, G.; Guha, P.K. Single resistive sensor for selective detection of multiple VOCs employing SnO2 hollowspheres and machine learning algorithm: A proof of concept. Sens. Actuators B Chem. 2020, 321, 128484. [Google Scholar] [CrossRef]
- Liu, T.; Guo, L.; Wang, M.; Su, C.; Wang, D.; Dong, H.; Chen, J.; Wu, W. Review on algorithm design in electronic noses: Challenges, status, and trends. Intell. Comput. 2023, 2, 0012. [Google Scholar] [CrossRef]
- Casey, J.G.; Collier-Oxandale, A.; Hannigan, M. Performance of artificial neural networks and linear models to quantify 4 trace gas species in an oil and gas production region with low-cost sensors. Sens. Actuators B Chem. 2019, 283, 504–514. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).