1. Introduction
Volatile organic compounds (VOCs) are a group of diverse organic chemicals (including alcohols, amines, carboxylic acids, halogenated compounds, and nitroaromatics) that exhibit low boiling points and readily evaporate at room temperature. They are commonly found in indoor air as pollutants, as by-products of waste management and agriculture, as fuel vapors formed during its storage and transport, and in industrial emissions or released by bacteria and other living organisms including humans. Many of these compounds pose significant risks to the environment and human health [
1,
2]. Monitoring VOCs is also crucial in human health assessment or food control, as many products and living organisms emit specific odors [
3,
4]. Thus, there is a growing interest in developing efficient sensors for VOCs [
5,
6,
7].
The detection of various chemical compounds has developed within a vast and interdisciplinary field of knowledge, playing a crucial role in daily human life, and is applied to different types of analyses, ranging from qualitative recognition of target analytes to quantification and real-time monitoring. Many analytical laboratory instruments, such as Atomic Absorption Spectroscopy (AAS), Inductively Coupled Plasma Spectrometry (ICP), and Gas Chromatography-Mass Spectrometry (GC-MS), enable complex analyses to be performed within a reasonable timeframe. While these instruments are indispensable and irreplaceable for our daily comfort, they are also expensive, require experienced operators, and cannot be used in the field. In many cases, the target analysis does not necessitate all the capabilities these methods offer but instead requires a precise assessment of the presence or quantity of a target analyte in a routine manner. This has prompted numerous studies on simplified sensing devices based on various signal transduction schemes, such as chemiresistors, mass transducers, and electrochemical, photoelectrochemical and optical sensors.
Modern sensors are generally electronic devices that generate signals in response to the presence of an analyte. Such devices logically consist of two main components: a sensing compound or material (the receptor) and a transducer, which can involve complicated signal-processing software.
Optical sensors utilize light to gather information about the analyte [
8]. The majority of these devices deliver these data via the change in light absorption or luminescence, but sensors employing other spectroscopies as well as other optical parameters such as refractive index and reflectivity have also been developed [
9]. Optimized non-invasive optical sensors can achieve high sensitivity and selectivity, providing rapid responses and enabling remote sensing using portable devices. This allows for real-time monitoring and on-site measurements in various conditions. Sensing materials for these devices vary widely and can include metal oxides, polymers, or organic–inorganic hybrid materials.
Molecular chemistry contributes to this field by developing efficient receptors and sensitive transducers, which can sometimes be part of a single molecule. Although molecular compounds can be dissolved and used for detection as chemosensors in a solution, the solid-state sensors are more convenient due to their high robustness and reliability and ease of integration into electronic devices.
Among organic compounds suitable for the fabrication of optical sensors, porphyrins are of great value due to their specific structural and optical properties providing highly sensitive detection, albeit porphyrin-based chemosensors exhibit in general rather low selectivity. Porphyrins absorb light in different regions of the visible spectrum, primarily due to electronic transitions within their conjugated π-electron systems. Two distinct types of bands are typically observed: the Soret band (400–450 nm), corresponding to S0 → S2 transitions, and the Q-bands (500–700 nm), corresponding to S0 → S1 transitions. Additionally, charge transfer bands are permitted in complexes where electronic interactions occur between the d-orbitals of metal atoms (such as Fe and Mn) and the conjugated π-electron system of the porphyrin ring. Many of free-base porphyrins and their complexes are emissive. This spectral diversity enables multichannel transduction schemes. The high sensitivity of these chemosensors results from their exceptional molar absorptivity, particularly in the Soret region, and their moderate fluorescence. This enables us to increase the detection limits, which is particularly important for optical sensors that are typically less sensitive compared to electrochemical devices. Moreover, many free-base porphyrins and their metal complexes (such as Pd, Sn, and In) produce long-lived triplet states through an excited-state process known as intersystem crossing (ISC) being irradiated by visible light. The triplet state of porphyrins is prone to reacting with reagents (such as oxygen), which can be utilized in the sensing of these compounds.
There are also numerous ways in which free-base porphyrins in the ground state can interact with an analyte, primarily due to the presence of a large aromatic macrocycle in these molecules. These macrocycles often engage in π–π stacking interactions, as well as other weak interactions such as hydrogen bonding, van der Waals forces, quadrupole–dipole and other nonspecific interactions with the analyte. The periphery of the macrocycle can be easily modified with functional groups or more complex binding residues, allowing for specific responses to target analytes [
10]. Unfortunately, the common synthetic strategy for developing chemosensors based on the covalent binding of a receptor and signaling units is relatively inefficient for porphyrin derivatives. Porphyrin conjugates are typically prepared by functionalizing the
meso(5,10,15,20)-positions of the macrocycle using 1,4-phenylene linkers [
11], which are tilted with respect to the macrocycle’s main plane. As a result, the degree of conjugation in such molecules is relatively low, leading to a reduced optical response to analyte binding compared to other chromogenic chemosensors. For this reason, many studies in sensing focus on metalloporphyrins, which can bind analytes via axial coordination to metal centers located in the macrocycle cavity. The binding of a ligand or ligand exchange results in a change in the symmetry of molecules that induces the spectral changes observed. This approach is effective for the development of VOC sensors, as many VOCs are Lewis bases that readily bind to metal ions.
Another interesting feature of bulky porphyrins is that when organized into supramolecular aggregates in a solution or on solid supports, they do not lose their high absorptivity and often remain emissive. While non-structured aggregates generally absorb and emit less light compared to their molecular counterparts and partially lose their ability to bind an analyte, structurally ordered porphyrin aggregates obtained under specific conditions can display intriguing sensing properties not observed in their molecular precursors [
12,
13,
14].
The use of porphyrins and related compounds for chemical sensor applications was reviewed in the past [
15,
16,
17]. The aim of this review is to draw attention to the use of porphyrin molecules in VOCs detection
in the gaseous phase. We describe optical chemical sensors that are based on these derivatives, emphasizing various strategies for their immobilization. This is a key step in the fabrication of optical sensors for gaseous analytes, as chemosensors that demonstrate good performance in a solution are often not practical for detecting gaseous analytes. First, sensing films composed exclusively of porphyrin molecules are discussed, followed by the materials obtained by their incorporation into organic and inorganic polymer matrices. Considering growing interest in multianalyte analysis with porphyrin-based sensor arrays, special attention is devoted to this area. The detection of other gaseous compounds, such as oxygen, nitrogen oxides, and ammonia, in which porphyrins are also widely explored, is outside the scope of this review. Readers interested in these topics are encouraged to consult numerous other reviews for a comprehensive understanding [
15,
18,
19,
20,
21,
22,
23,
24,
25]. Structures of all porphyrins discussed in this review are shown in
Figure S1.
2. Sensors Based on Single Porphyrin Sensing Element
Porphyrins have garnered significant interest in the detection of VOCs partly because they are highly sensitive transductors and partly because many metalloporphyrins can axially coordinate Lewis bases yielding stable complexes. Among VOCs, Lewis bases are prevalent and metalloporphyrins are an excellent host for these compounds.
In sensing applications, thin films of porphyrin are often fabricated to increase the number of binding sites available for interaction with analytes. These films exhibit varying degrees of structural order, depending on the preparation method. The supramolecular aggregation of porphyrin molecules is commonly observed in these materials, regardless of the synthesis strategy employed. The optical and coordination properties of these supramolecular aggregates are influenced by their structure. Consequently, the selectivity and sensitivity of optical sensors are highly dependent on the method used to prepare the sensing material. Unfortunately, comparative studies where the same host molecules are immobilized according to different synthetic methods are scarce. Most research focuses on sensor sensitivity to one or a few VOCs; however, some studies do report multi-analyte analyses, offering varying levels of insight into the selectivity of these sensors.
Most commonly employed transduction schemes in porphyrin-based sensors involve absorption and fluorescence spectroscopy, although altering other physical properties have also been explored. Surface plasmon resonance (SPR), which assesses changes in thickness and swelling of the thin film upon exposure to the analyte, has garnered increasing attention. Additionally, change in the refractive index at a metal–dielectric interface and within waveguide configurations have also been utilized for analyte detection [
26,
27]. Crystalline porous materials based on porphyrins enable the use of specific optical methods for signal transduction, which will be discussed below.
2.1. Thin Film Formed from Only Porphyrin Molecules
Porphyrins can be deposited as thin films on solid supports using various experimental techniques, which significantly influence their structural organization and sensing efficiency. Many of these methods do not require specific functionalization of the porphyrin molecules, and commercially available derivatives are typically investigated.
2.1.1. Dip-Coating and Drop-Casting
Dip-coating is a well established technique for fabricating thin films, offering some control over the deposition of porphyrin onto solid substrates to produce films with relatively uniform thickness. The process involves immersing a solid support into a porphyrin solution and then withdrawing it at a controlled speed. The thickness of the resulting film depends on factors such as solution concentration, withdrawal speed, solution viscosity, and temperature. The self-assembly of porphyrin molecules on the solid surface can occur due to electrostatic or other specific non-covalent weak interactions, such as van der Waals forces, hydrogen bonds, π–π stacking, metal coordination, enhancing the uniformity and overall morphology of the film. In this context, highly charged meso-tetraaryl- or meso-tetrakis(methylpyridinium-4-yl)porphyrins (anionic and cationic species, respectively) are of particular interest for this deposition method.
Salleh and co-workers obtained a dense self-assembled monolayer (SAM) by immersing a quartz substrate in an aqueous solution of Cu(II)
meso-tetrakis(4-sulfonatophenyl)porphyrin (CuTSPP) (the structures of all porphyrins discussed in this section are shown in
Figure 1) for 30 min, followed by lifting the slide at a constant speed of 15 mm min
–1 [
28]. The deposition process was controlled by electrostatic interactions between the negatively charged sulfonate groups and the crystalline support. Due to the repulsion among the negatively charged porphyrin molecules, the formation of multilayers was prevented, and a monolayer film was obtained (
Figure 2a).
The sensing properties of this film were studied in a closed chamber equipped with a gas inlet, a two-arm fiber reflectance probe, and a green LED light source (λ = 514 nm). This sensor demonstrated sensitivity to ethanol (EtOH), 2-propanol (iPrOH), and cyclohexane vapors, yielding a fast optical response with good reproducibility, despite only small optical changes being observed upon exposure to the analytes.
Dip-coating can also be used for the preparation of sol–gel films [
30]. Such sensor films are discussed in
Section 2.2. This technique is also useful for the deposition of porphyrin H
2TSPP onto optical fibers when combined with UV irradiation of the porphyrin solution in dichloromethane (CH
2Cl
2) [
31]. Such fibers have potential applications in the remote detection of VOCs [
32].
A simpler method for preparing sensors can sometimes be advantageous. A drop of a porphyrin solution can be deposited onto a solid support and left to evaporate in air, forming a thin film material [
33]. Ding, Peng, and their co-workers used this deposition technique, commonly known as drop-casting, to prepare a fluorescent “ON-OFF” sensor for the detection of diethylchlorophosphate (DCP), a simulant of the nerve agent Sarin [
34]. They deposited a dichloromethane solution of 5,10-(4-ethynylphenyl)porphyrin (H
2DAcPP) onto silica plates using a syringe, followed by air-drying for 5 h. The response of the fluorescence to the present of DCP was investigated using a homemade laminated film-based fluorescence sensing platform. The sensor based on this composite material demonstrated excellent sensitivity, with a linear response to DCP concentration in the range of 100 ppb to 1 ppm. A variation in fluorescence intensity of 1.2% was observed when the DCP concentration decreased to 10 ppt, and this value was reported as the actual detection limit of the sensor. The sensor was also reversible and photostable.
2.1.2. Spin-Coating
Spin-coating is the most widely used method for depositing porphyrins onto solid supports, thanks to its low-cost equipment and the ability to control film thickness by adjusting spin speed, the duration of the process, and the solution concentration. Moreover, spin-coating can be applied to a wide range of solid substrates enabling the optimization of the sensing properties and the adaptation of the sensing material to various signal transduction methods. Typically, for the preparation of the optical sensors, diluted solutions (10
−4–10
−5 M) of porphyrins in chlorinated solvents are deposited onto transparent supports such as glass, quartz, or Au-covered glass substrates. The resulting films can be dried at elevated temperatures to remove solvent traces, which may improve molecular packing in the films and optimize the sensors’ performance and robustness [
35]. This technique can be used for the deposition of molecular compounds, nanoparticles, or in the preparation of the doped polymer matrices and sensor arrays. The sensing materials prepared according to this strategy are listed in
Table 1; the structures of porphyrins, which were deposited by this technique, are shown in
Figure 3.
The thickness of the films obtained by spin-coating commonly exceeded 100 nm (
Figure 2b). Such films are often composed of porphyrin aggregates formed during solvent evaporation, which can significantly reduce its efficiency by hindering analyte penetration and its interaction with the host porphyrin molecules [
29]. Their sensing properties can be improved by tailoring the molecular structure of porphyrins to minimize aggregation within the films. This can be achieved by using metalloporphyrins with axial ligands (Fe(III), Mn(III), In(III), Rh(III)), in which π–π stacking is less pronounced [
36,
37]. Introducing bulky functional groups or alkyl chains at the macrocycle periphery also diminishes the aggregation of chromophores, providing improved sensor characteristics [
38,
39]. Such functionalization of the porphyrins can also create specific binding sites for target VOCs and enhance the solubility of these aromatic compounds in organic solvents thereby facilitating material preparation [
40,
41].
Another approach to reducing porphyrin aggregation was investigated by Roales, Pedrosa, and co-workers, who synthesized a triphenylmethane analogs featuring three porphyrinyl residues (H
2Triad and ZnTriad) [
42]. In these bulky molecules, π–π stacking is reduced, leading to better accessibility of the Zn centers of ZnTriad for the guest molecules. The sensing properties of the thin films composed of ZnTPP and ZnTriad were compared by studying the volatile amines as analytes. A total of five primary amines were examined: three linear primary amines of increasing length, one bulky primary amine (
tert-butylamine), and one aromatic compound (
Table 1). The exposure of the ZnTriad films to these analytes resulted in specific responses toward each of the amine studied, allowing for the discrimination of analytes due to the difference in their size and basicity.
In a later related work, the authors compared the behavior of spin-coated films composed of H
2TPP, 5-(3-hydroxyphenyl)-10,15,20-triphenylporphyrin (H
2TPPOH), and H
2Triad (
Figure 3) in sensing nitrated explosives [
43]. Porphyrins are widely studied for fluorescence sensing of these analytes [
44] as they form stable charge transfer complexes with these compounds in which fluorescence is quenched due to electron transfer or other non-radiative processes. Thus, these explosives are commonly used as model analytes in the development of new nano-structured porphyrin-based sensors. UV–vis spectroscopy studies of these three films revealed that aggregation could not be completely prevented, even when using the hindered compound with three porphyrinyl residues. In the evaluation of the sensing properties of these films with respect to 1,3-dinitrobenzene (DNB), 2,4-dinitrotoluene (DNT), and 2,4,6-trinitrotoluene (TNT), the highest performance was observed for the H
2TPPOH film. However, all the films demonstrated efficient sensing, with 50–75% fluorescence quenching by the saturated vapors of the nitrated explosives within 1–2 min (
t50). These studies revealed that the sensing properties of spin-coated films are difficult to control, and trial experiments are required to optimize sensor performance.
As discussed above, the axial coordination of analytes to the metal centers within the macrocyclic cavity typically enhances sensor sensitivity, as the resulting complexes are stable and exhibit specific spectral properties. However, a contrasting situation arises for analytes that are not strong Lewis bases. Sensors that utilize metalloporphyrins may exhibit lower selectivity for some of these analytes compared to those that use free-base porphyrins, as such VOCs can likely be more easily bound through the van der Waals or π–π interactions with the large non-metalated aromatic tetrapyrrolic macrocycles, which are also weak Brønsted bases and bear additional centers for hydrogen bonding (NH). Moreover, different aggregation behaviors of free-base porphyrins and metalloporphyrins may influence sensor efficiency. This was observed when acetone and chloroform (CHCl
3) were detected using spin-coated films fabricated from octaethyl-substituted porphyrins H
2OEP and ZnOEP [
45]. These data nicely illustrate why both free-base porphyrins and their metal complexes are employed in sensing materials and in particular sensor arrays based on porphyrins.
As shown in
Table 1, most reported sensors register changes in light absorbance in the UV–vis region upon exposure of the sensing materials to VOCs. The changes in both the positions and intensities of the bands are relatively small and less pronounced compared to studies conducted in a solution. This was attributed to the strong tendency of porphyrin molecules to aggregate within these materials and the low diffusion rate of gaseous analytes in non-porous films. Consequently, considerable attention has been directed toward developing efficient methods of signal treatment. The simplest approach involves the comparative integration of the Soret band before and after exposure of the sensor to analyte vapors [
36]. To perform dynamic analyses of alcohols using a spectrophotometer with the spin-coating film of MgTPP, Kerdcharoen’s group developed in-house software based on Principal Component Analysis (PCA), one of the most widely used pattern recognition methods for analyzing gas sensors [
35]. The acquired data were analyzed in real-time for the identification of methanol (MeOH), EtOH, and
iPrOH. This sensor exhibited varying sensitivities for these analytes, with the highest response observed for MeOH.
Spadavecchia and co-workers quantified analytes by comparing the relative variations in the absorbance integral within specific wavelength intervals [
29,
39]. When the spectral data were treated in this manner, a linear dependence of the ZnTPP-based sensor response on 4-aminophenol concentrations was observed in the 5–40 ppb concentration range of the analyte [
29]. The remarkable sensitivity, reversibility, and reproducibility of this sensor highlight the advantages of spin-coating for the immobilization of porphyrins.
To increase sensitivity using this signal treatment method, two chromophores (porphyrin CuPBPP and phthalocyanine ZnPc (
Figure 3)) that absorb light at different wavelengths were mixed into the same film [
38]. Dynamic responses to the presence of VOCs were recorded using four channels in the 300–700 nm range, each covering 50–100 nm. This method enabled the identification of organic compounds within the complex matrices of the VOCs (
Table 1).
Further development of this approach was reported by Seesaard and co-workers, who developed optical sensors capable of discriminating among the odors of three pathogenic bacteria [
46]. This was achieved by preparing a sensing film composed of two porphyrins (ZnTPP and MgTPP) and phthalocyanine ZnPC1 (
Figure 3). An in-house optical artificial nose system based on light-emitting diodes and a photodetector was fabricated using commercially available components. It consisted of a hybrid optical sensor and a data acquisition algorithm involving multichannel signal registration.
The development of optical waveguide sensing is of great importance for performing remote measurements [
47]. In a sensor operating according to this principle, a MnTPP film was used, which was obtained by spin-coating a porphyrin solution onto the surface of a K
+ exchanged glass optical waveguide (OWG) with a thickness of 1–2 mm [
48]. Linear response to the presence of triethylamine (NEt
3) was observed in the concentration range of 0.1–1000 ppm of the analyte. The response time was only 1.5 s, while the recovery time was 50 s.
The same strategy was employed in the development of sensors for the ethylenediamine (EDA) vapors [
49,
50,
51]. The OWG was prepared using a spin-coated film of H
2TCPP. This sensor was studied for the detection of eighteen VOCs and exhibited a high response only in the presence of EDA vapors, with a detection limit (DL) of 0.1 ppm [
49]. When H
2TSPP was immobilized using spin-coating, the resulting H
2TSPP-magnetite film exhibited higher selectivity compared to the film containing only porphyrin [
52]. Recently, H
2THPP-based films were prepared by the same method for further optimization of EDA sensing [
51].
Surface plasmon resonance (SPR), which has garnered significant attention in the development of optical sensors due to its high sensitivity [
53], is still rarely employed in the development of porphyrin-based sensors [
45]. Tonezzer and co-workers developed planar metal-cladding leaky waveguides, where a thin porphyrin film serves as the guiding layer [
54]. The detection of analytes was achieved by monitoring changes in the refractive index of the guiding layer upon interaction with the analyte. Using a film composed of H
2TPP molecules, a very fast (
t90 < 30 s) linear optical response was observed as a function of EtOH vapor concentration in the air, within the range of 375–3000 ppm, the concentration range of the analyte.
Table 1.
Spin-coated film for VOCs sensing.
Table 1.
Spin-coated film for VOCs sensing.
Porphyrin
Precursor a | Solid Support | Matrix | Optical Response | VOCs | Sensor Metrics | Ref. |
|---|
| Sensitivity b | DL b | Response Time (s) |
|---|
| H2TPP or CoTPP or FeTPP | P-doped (100) silicon wafer | – | UV–vis
spectroscopy | EtOH
MeOH, iPrOH | 3000
500–8000 | – | 67 c
60–120 | [37] |
| H2TPP | Silica | – | UV–vis
spectroscopy | EtOH | 1100–4500 | – | 71 c | [55] |
| FeTPP | Silica | – | UV–vis
spectroscopy | EtOH | 1100–4500 | – | 63 c | [56] |
FeTPP
or MnTPP | Glass | – | UV–vis
spectroscopy | Py, NEt3, Me2NH | Saturated
vapors | – | – | [36] |
| CoTPP | Silica | – | UV–vis
spectroscopy | EtOH | 1100–4500 | – | 60 c | [57] |
ZnTPP
ZnTPP–NO2
ZnTPP=O | Quartz | – | UV–vis
spectroscopy | Py, MeOH,
ethyl acetate | 1 | – | – | [40] |
| MgTPP | Glass | – | UV–vis
spectroscopy | MeOH,
EtOH,
iPrOH | – | 0.15 d
27.72 d
22.03 d | – | [35] |
ZnTPP–NO2
ZnTPP=O | Quartz | – | UV–vis
spectroscopy | Py, NEt3, MeOH,
ethyl acetate | 1 | – | – | [41] |
| ZnTriad | Glass | – | UV–vis
spectroscopy | PrNH2,
BuNH2,
HexNH2,
PhNH2,
tBuNH2 | 90
90–57,000
90
90
90 | –
50 f
–
–
– | 45–58 e
45–47 e
37–45 e
68–88 e
77–79 e | [42] |
H2TPP
H2TPPOH
H2Triad
| Glass | – | Fluorescence
spectroscopy
UV–vis
spectroscopy | DNB,
DNT,
TNT
DNT | 1 g
0.290 g
0.005 g
– | –
–
–
– | 60, 55, 105 h
105, 55, 115 h
30, 88, 110 h
2040, 480, 600 h
| [43] |
| MnTPP | K+-exchanged glass (optical waveguide(OWG)) | – | UV–vis
spectroscopy | NEt3 | 0.1–1000 | 0.1 | 1.5 | [48] |
| H2TCPP | K+-exchanged glass (OWG) | – | UV–vis
spectroscopy | ethylenediamine | 0.1–1000 | 0.1 | 5 | [49] |
| H2TMPP | K+-exchanged glass (OWG) | – | UV–vis
spectroscopy | ethylenediamine | 1–1000 | 1 | 13 | [50] |
| H2TSPP | maghemite-covered glass (OWG) | – | UV–vis
spectroscopy | ethylenediamine | 0.1–1000 | 0.1 | – | [52] |
| H2THPP | TiO2-covered glass (OWG) | – | UV–vis
spectroscopy | ethylenediamine | 0.001–1 | 0.001 | 1 | [51] |
H2OEP
| Au-covered glass | – | SPR | CHCl3
acetone | Saturated vapors | – | 5
2 | [45] |
| ZnOEP | Au-covered glass | – | SPR | CHCl3
acetone | Saturated vapors | – | 3
2 | [45] |
| H2TPP | Au-covered glass | – | Reflectance spectroscopy | EtOH | 375–3000 | – | 30 c | [54] |
H2TPP or
ZnTPP or
CdTPP | Glass | SiO2 | Fluorescence spectroscopy | TNT,
DNT, NB | 0.01 g
– | –
– | 10
– | [58] |
H2TMPyPP
H2DPyPP | Glass | PMMA
PVP
PMMA
PVP | UV–vis
spectroscopy | benzene
benzene
benzene
benzene | –
–
45–1000 i
45–1000 i | –
–
–
45 | –
–
120
4800 | [59]
[59] |
CuTBPP
+ZnPC j | Quartz | – | UV–vis
spectroscopy | MeOH, EtOH, iPrOH, acetone | Saturated vapors | – | – | [39] |
CuTBPP
+ZnPC j | Quartz | – | UV–vis
spectroscopy | MeOH, EtOH, iPrOH, PhNEt2, Py,
2-bromopyridine, Hex, acetone, tBuNH2 | Saturated vapors | – | 180 | [38] |
ZnTPP
+MnTPP
+ZnPC1 j | Glass | – | UV–vis
spectroscopy | EtOH, acetone, MeCO2H, acetone, ethyl acetate, formaldehyde | – | – | – | [46] |
array of M-PCN-222 k
M=Ag, Zn, Fe, Cu, Co | Glass | PDMS | UV–vis
spectroscopy | acetone, CHCl3, CH2Cl2, EtOH, hexanal, BuNH2, tetrahydrofuran, toluene,
2,4-dinitrotoluene | Saturated vapors | – | – | [60] |
array of MTPP
M=Mg, Zn | Glass | – | UV–vis
spectroscopy | MeOH, EtOH, iPrOH, acetone, MeCO2H, methyl benzoate | – | – | – | [61] |
2.1.3. Vacuum Evaporation
In the vacuum evaporation technique, solid porphyrin is evaporated at a high temperature (300 °C) in a high vacuum (10
−4 Pa), and the vapors are deposited on solid substrates, enabling the formation of thin films on various substrates. This method is suitable for the deposition of porphyrins with low solubility in organic solvents and ensures that the resulting films have high purity and uniformity. Depending on the application, post-deposition treatments such as annealing may be performed to improve film quality, crystallinity, or adhesion. This method has been used relatively rarely for the preparation of VOC-sensing materials because it requires expensive equipment, the film preparation process is time-consuming, and the films obtained exhibit low porosity. Thin films of H
2TPP, FeTPP, and CoTPP porphyrins were deposited on P-doped (100) silicon wafers [
37]. These three sensors were compared to the corresponding films obtained by spin-coating in the detection of alcohols (MeOH, EtOH, and
iPrOH). The vacuum-evaporated films were significantly more sensitive and exhibited much faster responses for all alcohols compared to the spin-coated films. This increased sensitivity was attributed to the high purity of the vacuum-evaporated films, which did not contain traces of any solvent, resulting in greater reactivity toward analyte molecules.
2.1.4. Glow Discharge-Induced Sublimation
Glow discharge-induced sublimation (GDIS) is an alternative to vacuum evaporation, which is particularly useful for depositing compounds that are difficult to evaporate using traditional thermal methods. In this process, sublimation is triggered by a low ionized glow discharge produced in standard radio frequency magnetron sputtering equipment within a vacuum chamber evacuated to approximately 10
−4 Pa. The glow discharge occurs when a gas, typically an inert gas like argon or helium, at a pressure of about 20 Pa is ionized by applying a high voltage, creating a plasma that can be sustained at low pressure. As the sublimated material interacts with the substrate in the presence of the glow discharge, a thin film forms on the solid surface. The energy from the plasma also enhances the adhesion and orientation of molecules in the deposited films. Materials obtained through GDIS are typically very pure and possess a small thickness—two features that are highly desirable in sensing applications [
62].
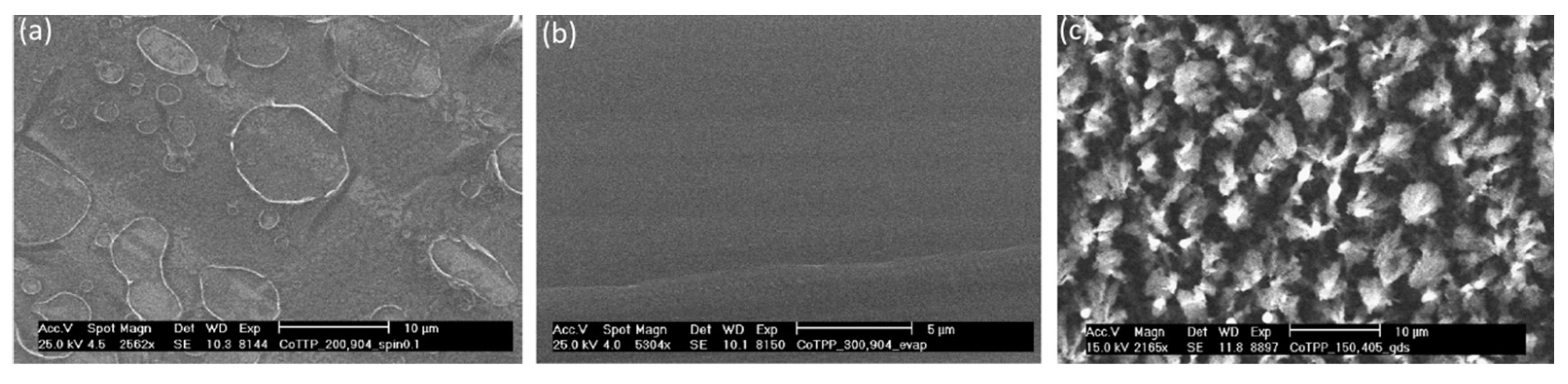
Tonezzer and co-workers used this method to deposit CoTPP onto a silica substrate [
57]. CoTPP was also immobilized on the silica substrates using spin-coating (CoTPP-SC) and vacuum evaporation (CoTPP-VE). The three films exhibited very different morphologies, as shown in
Figure 4. Specifically, CoTPP-VE displayed a flat surface, while CoTPP-SC contained shallow holes formed due to solvent evaporation. In contrast, the CoTPP-GD film was composed of microsized particles and exhibited a rough surface. The different supramolecular organization of the films was also confirmed by studying the films using UV–vis spectroscopy. Unfortunately, the sensing properties of these materials were investigated only in relation to EtOH. The CoTPP-GD film demonstrated significantly higher sensitivity (more than ten times intense) compared to CoTPP-SC and CoTPP-VE, exhibiting an optical response that was both rapid (
t50 = 12 s and
t90 = 72 s) and reversible.
It is worth noting that rough films can be obtained by this method for structurally diverse porphyrins and porphyrinoids. UV–vis studies of all films deposited by this method have shown that porphyrin aggregates form on the solid substrate. Nevertheless, this structural organization of the sensing film generally enhances the sensor’s performance compared to the films obtained by spin-coating. This was confirmed by studying the films prepared from 5,10,15,20-tetraphenylporphyrin (H
2TPP) and 5,10,15,20-tetraphenylporphyrin iron(III) chloride (FeTPP) [
55,
56]. Both sensors prepared by GDIS demonstrated higher sensitivity than those obtained by spin-coating, showing a linear response to EtOH vapors in the range of 1100–4500 ppm, with a fast response time (
t50~10 s and
t90~40 s) and full reversibility.
2.1.5. Langmuir–Blodgett/Langmuir–Schäfer Films
Langmuir–Blodgett (LB) and Langmuir–Schäfer (LS) techniques [
63,
64] rely on the supramolecular organization of molecules at gas–liquid (generally air–water) interfaces applying surface pressure and the subsequent transfer of the monolayers formed onto solid supports oriented perpendicularly (LB) or parallel (LS) to the liquid surface [
65]. Both hydrophilic and lipophilic solid supports can be covered by molecular monolayers, and the thickness of the film obtained can be precisely controlled by changing the number of transferred layers. Recent studies have demonstrated that these techniques are not only valuable for producing thin films from traditional amphiphilic molecules with long alkyl chains but also yield excellent results in the deposition of complex functional molecules and nanoparticles [
66,
67,
68,
69,
70]. When the LB technique is employed to fabricate thin films from porphyrins, the π–π stacking of the tetrapyrrolic macrocycles often serves as a key driving force for the supramolecular organization of the films. This organization is further influenced by various weak interactions between the peripheral substituents and the macrocycle. The structure of porphyrin molecules should be tailored because not all of these compounds form stable Langmuir monolayers on the water surface. Moreover, even when the compression isotherm at the air–water interface can be obtained, three-dimensional aggregates with ill-defined shapes are usually formed both at water and on solid surfaces [
71,
72,
73]. However, LB/LS techniques offer greater control over the structural and optical characteristics of molecular films compared to spin-coating since these methods allow for the precise organization of molecules in the monolayer at the water surface before transferring it onto the solid substrate. Moreover, these techniques enable control of the thickness of the sensor films by choosing the number of transferred monolayers. This controlled structural organization commonly affords enhanced sensitivity, selectivity, and functionality of the sensors. While complete control over the monolayer structures is not yet possible, practical guidelines are available, and monitoring their structure using compression isotherms and fiber-optic spectroscopy (UV–vis and fluorescence) simplifies the investigation and structural optimization of the films obtained [
64,
69,
74]. Another advantage of these methods is the possibility to form mixed monolayers in which porphyrin molecules are separated by typical amphiphiles such as fatty acids or phospholipids. The aggregation of porphyrin molecules can be reduced in such mixed films, which could positively impact the sensor parameters.
LB/LS porphyrin-based films are widely explored as gas sensors [
75], and research related to VOCs analysis is summarized in
Table 2. As shown in the table, most sensors were prepared using vertical deposition (LB film), while the LS method was employed much less frequently. UV–Vis absorption and reflectance spectroscopy are commonly used in signal transduction schemes due to the exceptional absorption properties of porphyrin molecules. These methods are compatible with a fiber setup [
3,
76] reflectance anisotropy spectroscopy, which has recently gained significant interest [
77,
78,
79], and enables not only the detection of analytes but also provides valuable insights into the structural organization of the films before and after analyte binding.
Recent research has also focused on the use of SPR in signal transduction [
80,
81]. SPR-readout has been shown to be effective for detecting aromatic compounds [
81]. Comparative studies on the binding of acetic acid (MeCO
2H) and methyl amine (MeNH
2) with LS films composed of H
2OPP molecules, utilizing UV–Vis spectroscopy and SPR, have demonstrated that sensor sensitivity is largely independent of the signal transduction method in these setups [
80].
Magneto-optical SPR combines the principles of magneto-optical effects with surface plasmon resonance, allowing for analyte detection by analyzing changes in the refractive index of films as well as the magneto-optical properties of materials near the surface. This signal transduction technique was employed to prepare a sensor using LS films of porphyrin dimer Co-H(OEP)
2 (
Figure 5).
This sensor demonstrated remarkable sensitivity to all three investigated alcohols (MeOH, EtOH, and iPrOH), providing a linear response within the range of 1 × 10−4–14 × 10−4 ppm of the analytes despite the fact that its sensing performance varied depending on the structure of the alcohol.
Porphyrin-based LB/LS films allowing naked-eye detection of VOCs were reported only recently [
82]. In this study, a 15-layer LS film of ZnTPP on a glass substrate enables the detection of Py visually, despite a relatively small red-shift of only 5 nm being observed after exposing the film to the analyte vapors for 1 min. The sensitivity of the film to vapors of other amines was not investigated.
Preparation of the highly ordered LB/LS films is essential for developing optical sensors and remains a primary focus in this field. Despite understanding that the structure of these films is influenced by the nature of substituents on the periphery of the tetrapyrrolic macrocycle and the metal centers within it, preparation of the well-structured monolayer films is still challenging [
73,
82,
83,
84,
85]. Several guidelines have been reported to reduce the aggregation of porphyrin molecules in the monolayers.
The oldest and simplest method involves preparing mixed porphyrin–fatty acid monolayers [
3,
76,
86,
87]. Fatty acids such as arachidic acid (
Figure 6) also facilitate the transfer of the monolayer onto solid substrates, as fatty acids are well known for their ability to form stable films on solid substrates.
Another strategy is based on the preparation of LB films using mixtures of porphyrins with an amphiphilic calix[8]arene derivative (
Figure 6) [
83,
88,
89]. Comparative studies of LB films prepared from MEHO (M = Zn, Mn) with and without calix[8]arene have shown that the separation of porphyrin molecules by these macrocyclic molecules decreases the size of nanoaggregates in the LB films, which induces an increase in both the magnitude and rate of the optical sensor response. The binding of amines by these two sensors is reversible and produces spectral changes, which depended on the amine structure and temperature.
In the case of free-base porphyrins, the formation of dense aggregates in the Langmuir monolayer can be decreased by adding carboxylic acids to the water subphase. Partial protonation of the tetrapyrrolic macrocycle significantly changes the supramolecular organization of the monolayer, yielding rarified layers in which analytes diffuse easily [
90].
Metal centers play a crucial role in sensor design, as clearly demonstrated by Dunbar and co-workers, who compared the optical response of LB films formed by the free-base porphyrin H
2EHO and six metal complexes with this ligand in the presence of ten VOCs (
Table 2) [
88,
91]. Metalloporphyrins with axial ligands form rarified films due to steric hindrance imposed by the ligand, which enhances diffusion and improves analyte binding [
36]. However, films composed of complexes with very strong axial ligands, such as the two hydroxyl ligands in SnEHO, did not show any response to the analytes [
88,
91]. The highest optical responses were observed for the Co(II) complex, which can alter its oxidation state in the presence of certain analytes, resulting in Co(III) complexes with different light absorption properties [
91]. The Mg complex produced the largest optical response in the presence of MeCO
2H among the ten studied VOCs, likely due to its transformation into the free-base porphyrin H
2EHO under acidic conditions, followed by the chemisorption of this analyte [
88].
The aggregation of porphyrin molecules in LB/LS films can also be decreased by introducing alkyl chains at the periphery of this macrocyclic molecule. This approach was widely investigated and applied to VOC detection [
77,
81,
92]. For instance, Richardson and co-workers compared the sensitivity properties of the LB films obtained from two
N-alkyl substituted derivatives of [5,10,15,20-tetrakis(3-amidophenyl)porphinato]zinc(II) ZnTmBuPP and ZnTmRPP (
Figure 5) [
92]. The porous film formed from the bulky compound ZnTmRPP exhibited a faster and higher response compared to its analog ZnTmBuPP in the detection of a series of primary, secondary, and tertiary amines.
Interestingly, the rate of porphyrin deposition on solid substrates influences the morphologies of the films and their sensing properties, due to the increased porosity of non-homogeneous films obtained at ultra-fast deposition rates (1000 mm min
−1) [
93]. This was used in the development of portable sensors for 2-methylbutan-2-ol, which is shown in
Figure 7 and represents a prototype of a useful portable toxic gas sensors [
94]. The device consists of a commercial blue LED that emits light detection by a phototransistor. The phototransistor is coated with an LS film of MgEHO obtained by the ultra-fact deposition. Upon exposure to 2-methylbutan-2-ol, the film undergoes a subtle shift in its absorbance characteristics, which varies the light intensity received by the phototransistor. This results in a change in the voltage across the phototransistor when the sensor is exposed to the analyte. Under dynamic conditions, the optical response of this device stabilizes after approximately 300 s. The absorbance changes are initially rapid, reaching about half of the maximum output in under 30 s. The highly reproducible response increases linearly with the concentration of the analyte below 41 ppt concentration of the analyte.
It has been reported that the sensing selectivity of LB/LS films can be increased by covering these films with thin protective layers containing specific molecules capable of providing size-selective diffusion. Evyapan and Dunbar have shown that such a layer can be obtained by performing the deposition of PMMA and carboxylic acid-substituted calix[8]arene (
Figure 6) on top of the H
2EHO LS film [
90]. This protective LS film served as a barrier to the diffusion of the bulky carboxylic acids, enabling an increase of MeCO
2H selectivity.
Sometimes sensing properties of LB/LS films can be improved by their annelation at 50–100 °C [
86,
95]. The surface of the film obtained after annealing was found to be smoother, indicating a significant structural reorganization of the aggregated species transferred onto the glass surface during the heating process.
Thus, despite some degree of structural organization in the LB/LS films, the selective detection of VOCs has not been yet achieved, although the optical signals of different analytes were distinguishable and useful for their identification [
83,
89].
Table 2.
Optical sensors based on porphyrins LB/LS films for VOCs determination.
Table 2.
Optical sensors based on porphyrins LB/LS films for VOCs determination.
Porphyrin
Precursor a | Method of Deposition/Substrate | Optical Response | VOCs | Sensor Metrics | Ref. |
|---|
Sensitivity
(ppm) | DL
(ppm) | Response Time (s) |
|---|
| ZnTPP | LS/glass | UV–vis
spectroscopy | Py | – | – | 60 | [82] |
| MnTPP | LB/glass | Reflectance
spectroscopy | EtOH | Saturated vapor | – | 5–10 | [95] |
RuTPP+
arachidic acid b | LB/glass
substrate | Reflectance
spectroscopy | MeOH, EtOH, iPrOH | Saturated vapors | – | 20 | [86] |
| H2THOPP | LB/oxidized
Si(001) | Reflectance
anisotropy
spectroscopy | EtOH | 100–2000 | – | 150 | [77] |
| ZnTHOPP | LS/quartz | Reflectance
anisotropy
spectroscopy | EtOH,
Hex,
NMe3 | 2314–13,887
593–3559
90–270 | –
–
– | 180
180
180 | [78] |
| ZnTHOPP | LB/quartz | Reflectance
anisotropy
spectroscopy | EtOH,
Hex,
BuNH2 | 10,656
27,180
1475 | –
–
– | 180
180
180 | [79] |
ZnEHO
or ZnEHO
+
calix[8]arene b | LB/HMDS-
covered glass | UV–vis
spectroscopy | MeCO2H, butanone, ethyl acetate, HexSH, HexNH2, HepCHO, OctOH, OctNH2, NEt3, P(OMe)3 | – | – | 300 | [83] |
| H2EHO | LS c/HMDS-
covered glass | UV–vis
spectroscopy | MeCO2H,
PrCO2H,
PeCO2H | 855
846
822 | 16
47
110 | 33
180
159 | [90] |
H2EHO,
or MgEHO
or SnEHO
or ZnEHO
or ZnEHO +
calix[8]arene b | LB/HMDS-
covered glass | UV–vis
spectroscopy | MeCO2H,
butanone,
ethyl acetate, HexSH,
HexNH2,
HepCHO,
OctOH,
OctNH2,
NEt3,
P(OMe)3 | –
–
–
–
2,000
–
–
200
14,000 | –
–
–
–
–
–
–
–
– | –
–
–
–
11
–
–
–
– | [88] |
MEHO
M = Mg, Sn, Zn, Au, Co,
Mn | LB/HMDS-covered glass | UV–vis
spectroscopy | MeCO2H,
butanone,
ethyl acetate,
HexSH,
HexNH2,
HepCHO,
OctOH,
OctNH2,
NEt3,
P(OMe)3 | 2600
32,000
32,000
940
2200
670
3
180
15,000
5900 | – | – | [91] |
MnEHO +
calix[8]arene b
ZnEHO +
calix[8]arene b | LB/hydrophobic glass | UV–vis
spectroscopy | PrNH2, BuNH2, PeNH2, HexNH2, HepNH2, OctNH2, NonNH2, NHEt2, NHPr2, NHBu2, NHHex2, NEt3,
NPr3 | – | – | 5–90 | [89] |
ZnTmBuPP
ZnTmRPP | LB/hydrophobic glass | UV–vis
spectroscopy | MeCO2H, butanone, ethyl acetate, HexSH, HepCHO, OctOH, P(OMe)3, PrNH2, BuNH2, NHPr2, NHBu2, NPr3, NBu3 | – | – | 25–300 | [92] |
| MgEHO | LS/HMDS-covered glass | Phototransistor | 2-methyl-butan-2-ol
EtOH,
MeOH,
OctOH,
PrOH,
MeBuOH | 2.5 × 10−5–
5 × 10−5
1.76 × 10−4
3.5 × 10−4
0.46 × 10−6
6.8 × 10−5
5 × 10−5 | – | – | [94] |
| H2EHO | LS/glass or Au
(Au-coated glass
SPR chip) | SPR and UV–vis
spectroscopy | MeCO2H,
MeNH2 | 855
900 | – | 300
499 | [80] |
| Co-H(OEP)2 | LS/Au
(Au/Co/Au coated SPR chip) | Magneto-optical SPR | MeOH,
EtOH,
iPrOH,
NMe3
butanone | 2.13 × 10−6
5.96 ×10−6
8.82 ×10−6
1.55 × 10−5
8.77 × 10−7 | – | 1200 | [96] |
| ZnTDPP | LB/gold coated SPR chip | SPR | benzene, toluene, ethyl benzene, xylene | Saturated vapors | – | 120 | [81] |
array of
ZnTPP, ZnPc2 d | LB/quartz | UV–vis
spectroscopy | Py, MeOH | 1 | – | 300 | [97] |
array of
FeTPP, FeOEP,
each mixed with arachidic acid | LB/glass | UV–vis
spectroscopy | EtOH, iPrOH, acetone, cyclohexane | – | – | – | [76] |
array of
MnOEP, FeOEP, CoOEP, RuOEP, each mixed with arachidic acid | LB/glass | UV–vis
spectroscopy | EtOH, iPrOH, acetone, cyclohexane | Saturated vapors | – | 17 | [87] |
array of
FeTPP, MnTPP, CoTMPP, CoOEP, each mixed with arachidic acid | LB/glass | UV–vis
spectroscopy | fresh capsicum annum, dried capsicum annum, fresh capsicum minimum | – | – | 10 | [3] |
array of
H2DmRDAPP,
H2TmOPP, H2EHO,
H2TmRPP, H2A2BCP, H2TZPP, H2TXPP, H2TYPP
H2TPP-Br,
H2A2B2P | LB/HMDS-
covered glass | UV–vis
spectroscopy | MeCO2H, butanone, ethyl acetate, HexSH, HexNH2, HepCHO, OctOH, OctNH2, NEt3, P(OMe)3 | – | – | – | [98] |
2.2. Materials Based on Oxide Matrices
The immobilization of chemosensors into transparent silica matrices is a promising strategy for preparing sensing materials, owing to the versatility of the sol–gel process. When organosils are prepared, this process involves the hydrolysis and polycondensation of a siloxane solution in the presence of chemosensors, which may or may not be functionalized with anchoring siloxane groups. Three-dimensional or 2D materials with different porosity are formed under these conditions, within which chemosensor molecules are either encapsulated or covalently bonded to a micro- or mesoporous silica matrix, allowing analyte molecules to diffuse easily through the film. The accessible range of chemosensor loading and the porosity of the matrix are highly dependent on experimental conditions, which should be optimized to achieve the best sensing performance of the material prepared. Functionalized optical fibers for remote spectroscopic detection can also be obtained using this technique. The immobilization of porphyrins through the sol–gel process is quite challenging compared to other organic compounds due to their low solubility in the polar protic solvents (such as alcohols and water) generally required for this process. The structures of porphyrins, which were incorporated in oxide matrices using this technique, are shown in
Figure 8.
The free-base porphyrin H
2TCPP incorporated into a periodic mesoporous organosilica matrix, obtained from 1,4-bis(trimethoxysilylethyl)benzene, exhibited a low fluorescence response in the presence of the saturated vapors of cyclohexane and toluene. These two analytes produced very similar responses, making it impossible to distinguish between them using this material [
99].
Silica monolite containing Co porphyrin Co(MSiTPP) have also shown low efficiency in the detection of pyridine (Py) due to low diffusion of the vapors in this monolithic material [
100]. This work highlights the particular importance of 2D materials (thin films) in the detection of gaseous analytes.
To prepare transparent mesoporous silica films on glass slides, spin-coating is a convenient method because this technique allows for the fine-tuning of the structural parameters of the films.
Bayindir and co-workers prepared sensing films with macro- and meso-porosity by performing a two-step polymerization of methyltrimethoxysilane in the presence of H
2TCPP [
101]. The hydrolysis step was conducted under acidic conditions, followed by initiating the condensation reaction by basifying the reaction mixture. The mixture was kept for 2 days to form a gel, which was subsequently sonicated to prepare a sol suitable for spin-coating. This sol was deposited onto glass substrates and dried. The porphyrin embedded in this silica matrix (ORGANOSIL) was emissive and showed a relatively low degree of fluorescence quenching (28%) after 2 min of exposure of the film to saturated TNT vapors. These data seem to indicate the low accessibility of the physisorbed porphyrin molecules within this matrix, despite its rather high porosity.
Li and co-workers synthesized sensing films on glass substrates from the porphyrin H
2TSiPP functionalized with siloxane anchoring groups (
Scheme 1) and tetraethoxysilane (TEOS) in the absence and presence of small molecular (cetyltrimethylammonium bromide (CTAB)) and polymeric (Pluronic F127 (EO
106PO
70EO
106) and P123 (EO
20PO
70EO
20)) surfactants using spin-coating [
58]. The films obtained by spin-coating these sols exhibited different mesostructures, which were, respectively, non-porous, hexagonal, and worm-like (
Scheme 1).
The insertion of zinc and cadmium ions into the incorporated tetrapyrrolic macrocycles occurs rapidly, yielding the corresponding complexes. Only the mesoporous films revealed a fast fluorescence response to the trace amounts of nitro-containing aromatics (TNT and DNT). The best results (quenching the efficiency of the saturated vapors of TNT (10 ppb) close to 60% after 10 s of exposure) were achieved in the detection of TNT with the CdTSiPP-based worm-like film, which enables rapid diffusion of TNT molecules to the metalloporphyrin residues that can bind them due to coordinative binding of nitro groups to metal atoms and/or π–π stacking between the aromatic macrocycle and this electron-deficient analyte. The energy-level matching between the porphyrin molecules and TNT is also important for efficient fluorescence quenching in the presence of the analyte.
In related work, the authors prepared similar sol–gel films from the free-base porphyrin H
2TSiPP using the dip-coating technique to compare two immobilization techniques. The results obtained for sensing the saturated vapors of TNT (10 ppb) and DNT (280 ppb) by films with different morphology were comparable to those discussed above, however, the quenching efficiency after 10 s was higher for the film obtained by dip-coating (27% compared to 7%). Interestingly, the aged films (stored for 4 days at room temperature and for 5 h at 100 °C) also demonstrated similar sensitivity to these analytes with fluorescence quenching ranging from 10% to 40% [
102].
This group also demonstrated the advantages of combining the sol–gel process with electrospinning for the deposition of porphyrin-based chemosensors [
103]. Electrospinning enables the cost-efficient fabrication of fibers with diameters ranging from nanometers to micrometers by applying a strong electric field to polymer solutions or melts. The sol obtained in a sol–gel process can also be used for the preparation of fiber membranes. For instance, such fibers were deposited onto glass slides using a sol prepared from TEOS and H
2TSiPP, with CTAB added as a porogen agent. The morphology and porosity of the fiber films were strongly dependent on the experimental conditions of their preparation. When the film characteristics were optimized, fluorescence quenching of 80% was observed when the sensor was exposed to saturated TNT vapors for 1 h.
A much more rapid response to saturated TNT vapors was observed when this sol was deposited onto a glass substrate covered with monodisperse PS spheres (450 nm in diameter) using spin-coating [
104]. The film obtained by this procedure exhibited bimodal macro-/meso-porosity. These structural characteristics allowed for the combination of high permeability and a high density of host molecules in the film, resulting in significant fluorescence quenching (55%) in the presence of saturated TNT vapors after just 10 s of exposure to the analyte. It is worth noting that the film obtained by spin-coating H
2TPPOH, as discussed in
Section 2.1.2, displayed similar sensing characteristics while being simpler to prepare. However, in this silica-based material, almost quantitative fluorescence quenching was achieved after 2 min of exposure to TNT vapors, whereas the residual fluorescence was significantly higher for the spin-coated film under consideration. These data reveal an increase in the accessibility of host molecules to the analyte in this ordered silica-based film. Interestingly, the incorporation of zinc and cadmium ions into the embedded tetrapyrrolic macrocycles enhanced the sensing performance of the film only in the case of the zinc ions. The unexpected decrease in the efficiency of the films containing CdTSiPP residues was attributed to the partial loss of its bimodal structure during treatment of the film with the metal salt.
PS beads can be replaced by monodisperse PMMA spheres, which can be deposited onto a glass surface via drop-casting [
105]. Silica-based films obtained using the same procedure as discussed above also exhibited bimodal macro-/meso-porosity and were efficient in the fluorescence sensing of explosives, achieving 55% fluorescence quenching after 10 s of exposure to TNT. The bimodal porosity significantly increases sensor sensitivity, as demonstrated in comparative studies of analogous films with different morphologies.
To overcome the solubility problems associated with the traditional sol–gel process, the incorporation of porphyrin into a silica matrix using atmospheric pressure dielectric barrier discharge (AP-DBD) was investigated by Boscher’s group [
106]. In this method, plasma is generated and maintained at atmospheric pressure using high voltage, eliminating the need for expensive high vacuum systems. However, controlling film thickness may be more complex compared to the preparation of films under vacuum. AP-DBD, which generates low-temperature plasmas, offers the opportunity to work with heat-sensitive compounds and can be easily adapted for industrial production. Using this technique, a solution of the porphyrin CrTPP and hexamethyldisiloxane (HMDS) in CH
2Cl
2 was sprayed through an ultrasonic atomizing nozzle onto the transparent polyethylene terephthalate foils placed on the moving stage of an AP-DBD reactor. This setup allows for the prompt exposure of the deposited liquid layer to the plasma discharge, facilitating the polymerization of the siloxane precursor. SEM images of the resulting material have shown that the film was porous and composed of agglomerated nano-spheres. Unfortunately, the sensing ability of the film was explored only briefly despite their promising morphology. A UV–vis response (a 5 nm hypsochromic shift in the Soret band) was observed in the presence of triethylamine (NEt
3), but it was too small to be visible by the naked eye. The reported experimental data do not allow for a conclusion regarding whether all metal centers in the film were accessible to the analyte molecules.
This porphyrin was also incorporated into the polyvinylsiloxane matrix, replacing HMDS with vinyltrimethoxysilane [
4]. The resulting films were tested as sensors for trimethylamine (NMe
3), NEt
3, and dimethylamine (NHMe
2) under both static and dynamic flow conditions. Notably, the sensor was able to detect concentrations as low as 10 ppm of NEt
3 under dynamic conditions and was successful in monitoring fish spoilage. However, once again, the color changes were too subtle for detection by the naked eye.
To optimize the film porosity, a mixture of isomers of sterically bulky CrDMDP porphyrins was embedded onto the HMDS matrix using the AP-DBD technique [
107]. The porous films based on CrDMDP were sensitive to NEt
3; however, the ultimate objective of naked-eye detection has not yet been achieved.
Siloxane matrices could also be used for the immobilization of porphyrin-based nanostructures. Porphyrin nanotubes prepared by Wang’s method, which involves the ionic self-assembly of
meso-tetrakis(4-sulfophenyl)porphyrin (H
2TSPP) into tubular supramolecular assemblies [
108], were embedded in polydimethylsiloxane to prepare a thin film containing spatially separated nanotubes suitable for sensing [
13]. This film was investigated as a sensing material for NEt
3, EtOH, toluene, and acetic acid. The polymeric matrix demonstrated good permeability to the studied analytes, and the response of the sensing layer was measured using the differential absorbance method. The analytes were successfully identified; however, they interacted with the porphyrin molecules through a variety of ways rather than being incorporated into the inner cavity of the nanotubes.
TiO
2 matrices are also transparent in the UV–vis region, and Yusoff and co-workers developed a procedure for the deposition of metalloporphyrins on titania nanoparticles covering a glass surface. For instance, a thin film composed of TiO
2 nanoparticles covered by iron(III)
meso-tetraphenylporphyrin chloride (FeTPP) was obtained on the surface of a quartz slide coated with Poly-L-Lysine [
109,
110]. In comparison to sensing films prepared using a similar procedure from nanoparticles formed by only porphyrin molecules, the composite material showed higher sensitivity to EtOH,
iPrOH, and acetone, as investigated by fluorescence spectroscopy. The morphology of the film and its sensitivity also depended on the amount of porphyrin deposited on the nanoparticles. The best sensing results were obtained for the films formed by the smallest nanoparticles. When FeTPP was replaced with MnTPP, the film was also sensitive to EtOH,
iPrOH, and acetone but the shifts in the emission bands were less pronounced [
30].
Carboxylate-substituted porphyrins can also be adsorbed on TiO
2 support. Although carboxylate anchoring groups yield less stable materials compared to phosphonate anchors [
111], their stability is adequate for sensing gaseous analytes. Zn(II) 5,10,15,20-tetrakis(3-carboxyphenyl)porphyrin (ZnTmCPP) and Zn(II) 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin (ZnTCPP) were anchored onto porous microcolumnar TiO
2 thin films (with a thickness of 150−400 nm), yielding structurally different materials [
112,
113]. FTIR analysis revealed that ZnTmCPP molecules are bound to the support by all carboxylic groups, in contrast to those of ZnTCPP where only one or two of these groups participate in material formation. As a result, the optical properties of the two materials differ. The Soret band of the
para-substituted derivative in the film is hypsochromically shifted compared to the solution spectrum, indicating formation of H-aggregates in the film obtained. In contrast, grafted ZnTmCPP remained monomeric due to its planar orientation on the TiO
2 surface. Both thin films exhibited colorimetric responses in the presence of acetone, acetonitrile (MeCN), butylamine (BuNH
2), CHCl
3, EtOH, and tetrahydrofuran. The optical response of the ZnTmCPP/TiO
2 was found to be more pronounced and faster than that of the ZnTCPP/TiO
2 film. The ZnTmCPP/TiO
2 film was also more sensitive to the nature of the studied analytes.
2.3. Hybrid Materials Based on Organic Polymers
Polymer matrices are widely used to immobilize chemosensors. They offer many advantages and compare well with sol–gel materials in the detection of gaseous analytes. The most commonly used polymer supports are polyvinyl chloride (PVC), polystyrene (PS), polymethyl methacrylates (PMMA), and cellulose derivatives. Dyes can be chemisorbed on polymer surfaces, encapsulated within the polymer matrix, or co-polymerized with inert monomers. Functionalization of the tetrapyrrolic macrocycle is commonly required to obtain molecular precursors that are soluble in reaction mixtures. The structure of porphyrins, which was used to prepare VOC sensors by these methods, are shown in
Figure 9.
Carturan and co-workers have nicely demonstrated the importance of the polymer matrix on the sensing properties of
meso-tetraphenylporphyrin (H
2TPP) [
114]. In this study, the free-base porphyrin was embedded using spin-coating technique into two different soluble fluorinated polyimides that differ only in their diamine residues (
Figure 9). Both the morphology and optical properties of the films were dependent on the polymer structure, and only the one containing 2,3,5,6-tetramethyl-
p-phenylenediamine residues was suitable for EtOH sensing by fluorescence spectroscopy. The film containing 1% H
2TPP, with a thickness of 118 nm, was able to detect this analyte in the range of 1700–106,000 ppm, with a response time (
t90) of 35 s. The sensor was recovered after exposure to nitrogen for 158 s.
The sensing materials prepared by impregnating WypAll X60 or 100% woven cotton with various metalloporphyrins were used for the development of portable sensors for EtOH [
115]. No significant differences in color changes were observed between the two supports; however, the nature of the porphyrin ligand played a key role in the sensing process. Chemisorbed complexes of Deuteroporphyrin IX bis ethyleneglycol (H
2DIX) showed a stronger response compared to those of
meso-tetrakis(4-aminophenyl)porphyrin (H
2TAPP).
The encapsulation of H
2TPP in PVC matrices is performed by dissolving PVC, a plasticizer, the dye, and sometimes specific additives in THF, then casting this solution onto glass plates. This process yielded films with a thickness that exceeded 5 μm. H
2TPP encapsulated in a PVC matrix is sensitive to EtOH over a wide range of concentrations [
116]. After optimizing the plasticizer, the doped polymer enabled a linear response in the range of 1 to 75% of saturated vapor pressure and was applied to the determination of EtOH in various types of wines and whisky. The detection limit was as low as 0.05 vol%, which is rarely achieved with other previously reported optical sensors.
Films of 5,10,15,20-tetrakis(methylpyridinium-4-yl)porphyrin tetrachloride (H
2TMPyPP) and 2,3,7,8,12,13,17,18-octamethyl-5,15-bis-(4-trimethylammoniumylphenyl)porphyrin dichloride (H
2DPyPP) in PMMA and polyvinyl pyrrolidone (PVP) were prepared by spin-coating. The sensing properties of these films with a thickness of ca. 1 mm were investigated with respect to benzene. Membranes containing encapsulated H
2DPyPP were more sensitive but could not be used for monitoring benzene in the air where this toxic compound is acceptable in the level of ppb [
59].
To increase the optical response of the sensor, Meyerhoff and co-workers proposed to use the monomer–dimer equilibrium of hydroxo(2,3,7,8,12,13,17,18-octaethylporphyrinato)indium(III) (
Scheme 2). This complex was obtained in a PVC membrane performing the polymerization in the presence of sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate [
117]. This salt with a lipophilic anion enables dimerization of the porphyrin within polymers. The sensing mechanism involves axial coordination of amines to metal centers in the polymeric matrix, accompanied by the formation of two monomer species (
Scheme 2). This process induces a red shift in the Soret band of up to 16 nm due to the significant structural difference between the bridged dimer and the two monomeric complexes formed after amine binding. The best sensitivity was observed for the film prepared using
o-nitrophenyl octyl ether as a plasticizer. Depending on the relative partition coefficient in the polymer film and the ligating properties, different degrees of monomer formation were observed for the eight primary alkylamines and Py studied in this work. With optimized film composition, BuNH
2 was detected at a level of 0.1 ppm, while the detection limit for less lipophilic primary amines did not exceed 10 ppm.
A serious drawback of the functionalized polymers discussed above is their low porosity. To increase the surface area of the sensing material, O’Donnell and co-workers prepared crosslinked polymer membranes by performing the polymerization of free-base 5,10,15,20-tetrakis(4-hydroxyphenyl)porphyrin (H
2THPP) and succinyl chloride at THF–CH
2Cl
2 interfaces, modifying previously reported methods [
118,
119] for the preparation of microporous membranes [
120]. Unfortunately, the porosity and morphology of the films obtained were not characterized, but their investigation by UV–vis spectroscopy showed a high degree of dye aggregation in this polymeric matrix. The membranes were then deposited onto glass supports and transformed into materials containing metalloporphyrin residues by treating them with Zn(II), Cu(II), and Co(II) salts. These metalloporphyrin membranes were exposed to alcohols (MeOH, EtOH, and
iPrOH), ketones (acetone, butanone, and 2-pentanone), and toxic chlorinated compound (CH
2Cl
2, CHCl
3, and 1,2-dichloroethane) vapors to test their efficiency as vapochromic materials using colorimetry. The spectral changes were rather small, and the absorbance of the films was monitored at two wavelengths (425 nm and 550 nm) before and after exposure to the analytes. The percentage difference in the absorbance at each wavelength was calculated and averaged to draw final conclusions. The CuTHPP-based membrane showed the best discrimination among the three alcohol vapors, while the thin film containing ZnTHPP enabled the efficient detection of MeOH vapors. Sensors based on H
2THPP, CuTHPP, and ZnTHPP exhibited reversible responses toward ketones, and membranes containing H
2THPP and ZnTHPP could distinguish between different ketones. In contrast, the CoTHPP-based membrane was efficient only in the discrimination of chlorinated compounds.
Liu and co-workers investigated the fabrication of porous sensing films using the electrospinning process [
121]. The films prepared by depositing a DMF solution of polystyrene (PS) and 5,10,15,20-tetrakis(4-methoxyphenyl)porphyrin (H
2TMPP) onto glass slides were emissive, in contrast to the films obtained from the same solution using a casting procedure. The porosity of the fibers was enhanced by adding a porogen (Triton X-100) to the electrospun solution. The emission of the film composed of fibers with diameters of 300–400 nm was sensitive to the presence of saturated vapors of DNT (38% quenching), TNT (4% quenching), 2,4-dinitrophenol (DNP, 17% quenching), and picric acid (PA, 2% quenching) after exposure to these analytes for 1 h. The sensor did not produce an optical response to a wide range of common organic VOCs and could be recovered by prolonged heating (more than 20 min) at 60 °C after exposure to DNT. The sensitivity of this film was further enhanced by adding dodecylamine to the electrospun solution. However, the long response time and low sensitivity to saturated TNT vapors could not be overcome, regardless of the procedural modifications explored.
Lv, Wang, and co-workers prepared a nanoporous membrane by copolymerizing Zn(II) 5,10-bis(4-aminophenyl)-15,20-diphenylporphyrin (ZnDADPP) with pyromellitic dianhydride (PMDA) and oxydianiline (ODA) using an electrospinning technique, followed by heating the nanofibers up to 250 °C [
122]. The structure of the resulting polymer is shown in
Figure 10.
The membrane, consisting of these fibers, enables the rapid and reversible detection of pyridine vapor, with a detection limit of 0.041 ppm, achieved due to a color change visible to the naked eye. Notably, this membrane demonstrated excellent selectivity for Py over common amines (NHEt2, NEt3, pyrrole, and cyclohexylamine) and other potential gaseous compounds (CO2 and H2O). The selectivity of the membrane for Py was attributed to differences in the basicity of the analytes. However, it is also important to consider the high stability of the zinc porphyrin complexes with Py, as well as the low accessibility of the metal centers within this polymeric matrix.
A similar polymer was prepared for the detection of nitrated explosives by replacing PMDA with 4,4′-hexafluoroisopropylidenediphthalic anhydride (6FDA) [
123]. Fluorescence quenching of more than 90% was achieved after just 2 min of exposure of the film to saturated TNT vapors. The sensor was also sensitive to the presence of saturated DNT, nitrobenzene (NB), and picric acid (PA) vapors, although it exhibited smaller emission changes compared to TNT.
An interesting approach to VOCs sensing using materials incorporating porphyrins is the fabrication of fiber-optic sensors, which are small in size, capable of remote sensing, and resilient to electromagnetic interference, while being cost-effective to produce. Sensing fibers based on organic polymers are less expensive and exhibit higher fracture toughness and greater flexibility in bending compared to conventional silica fibers. Yin, Gu, and co-workers proposed a procedure for spinning and drawing PMMA fibers doped with 5,10,15,20-tetrakis(4-aminophenyl)porphyrin (H
2TAPP) for DNT sensing [
124]. A decrease in fluorescence in the presence of DNT was observed, enabling the detection of DNT with a limit of detection around 120 ppb and a response time of approximately 3 min.
Zhang and co-workers reported the modification of PMMA fiber surface with a porous silica-based film containing covalently bonded porphyrin molecules [
125]. Their strategy relied on the photoinduced thiol-ene polymerization of 5,10,15,20-tetrakis[4-(allyloxy)phenyl]porphyrin (H
2TAlPP), vinyl-functionalized polyhedral oligomeric silsesquioxanes (POSS-V8), and 1,6-hexanedimercaptan on the surface of U-bent polymer optical fibers (POF) with core diameters of 980 μm and cladding diameters of 20 μm. This form of POF was chosen to enhance sensor sensitivity and compatibility with existing instrumental configurations. The photoinduced polymerization was initiated by passing 365 nm light through the POF while immersing the fiber in a solution containing the dithiol and two allyl-substituted monomers. UV–vis spectroscopy studies of the resulting films, which had thicknesses exceeding 120 nm, revealed a low degree of porphyrin aggregation in this matrix. Despite the porous structure of the fabricated film, only partial fluorescence quenching was observed in the presence of saturated TNT vapors (38% at 30 s and 62% at 300 s). The sensor also demonstrated sensitivity to DNT and nitrobenzene (NB), albeit with a lower optical response. The sensing film was recoverable by ultrasonic washing in MeOH.
2.4. Supramolecular Assemblies and Metal–Organic Frameworks
Metal–organic frameworks (MOFs) are porous crystalline materials composed of metal ions or clusters coordinated with organic ligands. These metal–organic frameworks offer exceptional tunability in their structural, physical, and chemical properties by varying the metal centers, organic linkers, or using post-synthesis modification, making them highly promising for various applications, including gas storage, separation, and catalysis. Their high surface area, abundance of binding sites, and adjustable pore sizes are particularly advantageous for sensing applications and the removal of VOCs. MOFs have already found their specific role as optical sensors for gaseous analytes [
126,
127,
128], but their use in the detection of VOCs remains relatively rare [
129,
130,
131]. Reported studies demonstrate that these materials hold significant potential for developing optical sensors for VOCs with various signal transduction mechanisms, including light absorption, emission, and refractive index changes. MOFs could show specific selectivity towards certain VOCs, often based on size exclusion or a correspondence of hydrophilic–lipophilic balance between MOF and an analyte. However, the main challenge in this field lies in the fabrication of sensing materials that are compatible with the conditions of target signal transduction schemes, as these materials are often difficult to integrate with existing devices due to their 3D character, and the technology for growing MOFs on solid supports is still underdeveloped.
MOFs based on porphyrin ligands not only exhibit high porosity but also demonstrate strong light absorption in the visible spectrum. Additionally, they efficiently generate singlet oxygen, which can be utilized for the removal of VOCs through performing photocatalytic oxidation reactions [
132]. However, porphyrin-based MOFs have been scarcely investigated as VOCs sensors. They have been reported as sensing elements in optical sensor arrays, which are discussed in the following section.
Coordination-based supramolecular chemistry also offers a pathway to create a wide variety of rigid, discrete multi-metal complexes featuring well-defined nanoscale cavities. These compounds hold significant promise for small-molecule sensing. In optimized structures, analyte retention can be achieved by adapting the size of the cavity to match the dimensions of the analyte. In such materials, optical sensing transduction is typically achieved by changes in color or luminescence upon analyte binding. However, they also enable more sophisticated signal transduction schemes. Hupp and co-workers reported a proof-of-concept study showing that light diffraction could serve as a transduction mechanism in sensing gaseous analytes (the photonic lattice method) [
132]. The self-assembly of the porphyrin ZnDPyMBP (
Figure 11) and [ReCl(CO)
5] resulted in the formation of a tetra-metallic complex defining an open-ended nanoscale box with a cavity with the approximate dimensions of 18 × 18 × 18 Å. In crystal form, these cavities align to create one-dimensional channels, and this structural organization can be easily achieved by evaporating solutions of this complex. To prepare a sensing material, a micropatterned elastomeric stamp was covered with this porphyrin-based tetramer. This resulted in a square perforated grid covering a few square millimeters and containing several thousand perforations. The presence of benzene, dioxane, and Py within the pores of this material was detected and the analytes were distinguished using photonic lattice diffraction measurements.
3. VOCs Sensing Based on Porphyrin-Based Sensor Arrays
The challenge of detecting VOCs arises from the limited number of binding sites in their molecules, which generally results in their weak interactions with host molecules. These interactions are predominantly governed by Brønsted or Lewis acid/base forces, making the development of selective sensors a difficult task. In nature, mammals use around four hundred different receptors to distinguish among thousands of odorants [
133]. Inspired by these biological olfactory systems, an artificial array-based sensing strategy was developed. This strategy employs multiple sensing elements, each providing a specific yet non-selective response, which work in unison to produce a unique, combined response for each analyte in a complex mixture. Artificial noses that modulate optical signals have emerged in this century as promising, low-cost, environmentally friendly, and portable sensors. Optical arrays based on color changes in dyes, photonic crystals, and fluorescence have been developed. Recently, sensor arrays utilizing near-infrared reflectance have also gained increasing interest [
134].
Colorimetric sensor arrays (CSAs) operate by relying on color changes produced by each component of the array in response to specific analytes. Commonly sensor arrays include different classes of dyes, such as Brønsted acidic or basic dyes, Lewis acidic or basic compounds, and chromophores with large permanent dipoles [
22,
135,
136]. This structural diversity of components enables cross-selectivity of non-selective optical responses and enhances the differentiation of VOCs. CSAs often require specific equipment to analyze the color changes, such as flatbed scanners, digital cameras (including photo cameras), or specialized optical detectors. These devices capture images of the colorimetric array, allowing for the analysis of color and its intensity, often with the aid of sophisticated software.
One of the earliest and simplest opto-electronic noses developed by Di Natale and co-workers is illustrated in
Figure 12 [
33].
This homemade device consisted of a low-cost blue LED and an array of photodiodes. Sensing films were deposited on one of the internal surfaces of a Plexiglass chamber equipped with gas inlets and outlets, with each porphyrin film positioned along a specific light path. Four sensing films were prepared by drop-casting Co(II) and Rh(III) porphyrin complexes (CoTNPP and RhTPP), as well as two other porphyrinoids: Mn(III) 2,3,7,8,12,13,17,18-octamethylcorrole and 3,17-diethyl-2,3,7,8,12,18,22,23-octamethylsapphyrin (
Figure 13).
In preliminary studies, light absorption changes in sensor components due to the presence of various VOCs such as hexane (Hex), propanal, MeOH, EtOH, acetone, MeCO2H, and NEt3 were evaluated. The highest optical responses were recorded in the presence of carboxylic acids and amines, although most of the analytes were detectable with these films. Consequently, these complexes were selected as working components for the development of an opto-electronic nose, with the output signals analyzed using the Self-Organizing Map (SOM). Notably, this CSA was composed solely of porphyrinoids, whereas recent practical colorimetric arrays typically include other organic dyes.
This and other early works, particularly from Suslick’s group [
137,
138,
139,
140], highlighted both the interest and complexity of developing opto-electronic noses, prompting further extensive research to optimize each component of detection systems and analysis methods. This research was recently discussed in excellent reviews [
15,
22]. Readers interested in these devices are encouraged to consult these and other reviews [
141,
142,
143], which detail all the steps involved in developing opto-electronic noses. In our review, we focus specifically on various strategies for preparing sensing materials for these devices, as well as highlighting selected remarkable results obtained using sensor arrays with principally porphyrins as dyes. Porphyrin-based CSAs reported recently are summarized in
Table 3.
Given that free-base porphyrins and their metal complexes offer specific responses to target analytes as discussed above, sensor arrays frequently include different metal complexes of the same tetrapyrrolic ligand, with H2TPP often being a commonly used choice.
The importance of the substitution pattern of the tetrapyrrolic macrocycle for developing CSAs was clearly demonstrated by Brittle and co-workers [
98]. They explored the sensing properties of two arrays based on free-base porphyrins, which were deposited on glass substrates using the LB technique. The analytes they employed for discrimination included octanol (OctOH), hexylamine (HexNH
2), octylamine (OctNH
2), NEt
3, octanal, MeCO
2H, 1-hexanethiol, 2-butanone, ethyl acetate, and P(OMe)
3. Two arrays, comprising six and four sensing elements, respectively, exhibited distinct sensing behaviors influenced by both the molecular structure of dyes and their packing within the films. Notably, the LB films of porphyrins containing only electron-donating substituents on the macrocycle periphery produced a significantly stronger response compared to those with both electron-donating and electron-withdrawing groups, contrasting their performance in solution detection. This finding underscores the key role of molecular packing in LB films, suggesting that a porphyrin exhibiting a robust chemosensory response in a solution might not perform similarly when incorporated into a thin film. This study also indicated that the fabrication of CSAs based on porphyrins with electron-donating substituents could be a crucial strategy for developing highly sensitive optical sensors for VOCs. Nevertheless, this conclusion requires further experimental validation as the complexity of these sensor systems and the limited number of examples reported previously may not completely represent the behavior of all porphyrins in the LB/LS sensing films.
The methods of porphyrin immobilization used in the preparation of colorimetric arrays are generally similar to those discussed in the previous section, with drop-casting being a commonly utilized technique. Porphyrins were immobilized onto solid supports using diverse substrates such as reverse-phase silica gel plates [
40,
41,
136,
137,
138,
139,
147,
148,
149,
150], silica gel plates [
146], polyethylene terephthalate (PET) foils [
151], polyvinylidene fluoride (PVDF) membrane [
152,
153,
154], poly(methyl methacrylate) (PMMA) plate [
144], and filter paper [
155]. Efficient CSAs were also prepared according to the LB/LS technique (
Table 2) and spin-coating (
Table 1).
Dyes can also be embedded in polymeric matrices to serve as sensing materials in CSAs. Hou and co-workers incorporated a mixture of chemosensors and 2,4-dinitrophenylhydrazine into a PEG-100 matrix [
156]. The 16-channel CSA thus prepared demonstrated excellent discrimination of nine aldehydes in a low concentration range (40 ppb to 10 ppm).
The importance of the porosity of the sensing materials was highlighted long ago. Suslick and co-workers utilized the incorporation of dyes into various silica matrices to obtain porous organosil materials [
151]. By combining this sol–gel technique with a rational molecular design of the sensing components and improving data analysis methods, this group developed numerous advanced CSAs. For example, they achieved sensitivities below 1 ppm for detecting seven different amines (NHPr
2, HexNH
2, cyclohexylamine, OctNH
2, pyridine, pyrrolidine, homopiperidine) and DMF, using a CSA with 24 components, of which 18 were porphyrin derivatives [
139]. In another study, they developed a 36 channel system capable of differentiating among 19 toxic industrial chemicals, including 4 VOCs (NHEt
2, EtNH
2, NH
2NHMe, NMe
3), after just 2 min of exposure to analytes at concentrations considered hazardous to health [
151]. Moreover, this group developed a hand-held analyzer and proposed colorimetric arrays for gaseous mixtures analysis which can be used for monitoring meat freshness, differentiation of explosives and human pathogenic bacteria [
15].
Functionalized nanomaterials have recently emerged as promising candidates to improve sensor arrays. Coating nanoparticles with dyes enhances the surface area of materials, which in turn improves the accessibility of the receptor sites. Gu and co-workers used colloidal crystalline beads (CCB) (
Figure 14) as a solid support for chromophores in developing a fluorescent sensor [
157,
158]. Such colloidal crystals composed of three-dimensional ordered arrays of submicrometer particles, often formed by self-assembly. In this work, these species were prepared from nanosized silica synthesized according to a modified Stöber method in a microfluidic device. The CCBs, with diameters of about 300 µm, were initially hydrophobized using trimethoxy(octadecyl)silane and then functionalized with a series of porphyrins (ZnTPP, SnTPP, ZnTAPP, H
2TPP, H
2TCPP, and H
2TAPP) using a dip-coating process [
158]. This sensor generated fluorescent responses, which were utilized for calculating the absolute difference between the RGB colors of the porphyrins during exposure to gaseous analytes. Carboxylic acids, ketones, and amines (cyclohexane, ethyl acetate, MeCO
2H, acetone, MeOH, EtOH) were distinguished by this sensor array. Notably, the fluorescent response differed for MeOH and EtOH, allowing for the semi-quantitative analysis of EtOH in the concentration range of 10–60 ppm by fluorescence color difference (R values in RGB spectrum).
Pedrosa and co-workers utilized a TiO
2 thin film prepared by glancing angle physical vapor deposition (GAPVD) as host material for carboxylate-substituted porphyrins [
112]. This solid support is transparent in the visible spectrum, non-dispersive, and porous, making it suitable for gas sensing via UV–visible and IR spectroscopies. Metalloporphyrins were deposited from EtOH solutions using impregnation, a technique that did not prevent dye aggregation. Porphyrin/TiO
2 films, each containing 1 of 11 different porphyrins, were exposed to 12 VOCs. Spectral changes observed in the Soret band region were explored using imaging spectroscopy, generating a recognition pattern that enables the easy identification of each VOC. This sensor exhibited a rapid (within a few seconds), reversible, and reproducible response that was attributed to significant porosity of the TiO
2 film with a columnar structure and open pores.
Recently, Pedrosa, Carrilo-Carrión, and co-workers developed a rapid and straightforward microwave-assisted method to synthesize nano-sized Zr-based MOFs incorporating
meso-tetrakis(4-carboxyphenyl)porphyrin residues (PCN-222) [
60]. The insertion of various metal ions (M = Fe, Co, Cu, Zn, Ag) into the tetrapyrrolic macrocycles within this matrix proceeded smoothly, resulting in the materials labeled as PCN-222(M) (
Figure 15).
These modified MOFs were then used to prepare a CSA. The nano-sized MOFs were dispersed into poly(dimethylsiloxane) (PDMS) to prepare transparent and flexible membranes. The sensing properties of these materials were tested against nine different VOCs, including DNT, acetone, CHCl3, CH2Cl2, THF, BuNH2, hexanal, toluene, and EtOH, as well as gasses like hydrogen sulfide, hydrogen chloride, and ammonia using UV–vis spectroscopy. The analytes produced distinct barcode-like identification patterns following 30 min of exposure, enabling easy differentiation between them. The membranes also demonstrated good stability, maintaining their functional properties for at least three months after fabrication.
Opto-electronic noses hold significant societal importance across various fields. As discussed earlier, they can be effectively utilized for the quantification of toxic industrial compounds, playing a critical role in environmental safety and pollution control. They are also crucial for monitoring human health [
15,
144]. For instance, Hou, Huo, and co-workers developed a CSA specifically designed to detect lung cancer-related VOCs [
144,
152,
156]. The reported CSA was based on a combination of free-base porphyrins (H
2TPP, H
2TF
5PP, H
2TS
1TPP), two metalloporphyrins (ZnTPP, EuTPP), and sodium fluorescein. This CSA successfully discriminated among key VOCs such as
p-xylene, styrene, isoprene, and hexanal, which are associated with lung cancer, when their concentrations were varied within the 50–500 ppb range [
144]. This level of sensitivity is essential for the early diagnosis and continuous monitoring of health conditions.
In the recent years, CSAs are becoming increasingly popular for the control of food freshness during storage [
15,
145,
146,
147,
150,
154,
155,
159]. The porphyrin dyes are particularly appealing here because being incorporated in sensing materials means they can detect a wide range of specific VOCs released during food spoilage, including amines, aldehydes, 2,4,5-trimethyloxazole, and other degradation products, even at very low concentrations. Since VOCs emitted by food products often exhibit complex compositions, detecting target analytes in these mixtures is essential. Porphyrin-based CSAs facilitate the analyses of complex gaseous mixtures and are suitable for creating portable devices that are compatible with smartphone cameras.
4. Conclusions
The detection of volatile organic compounds (VOCs) is a rapidly expanding research field due to their significance in environmental monitoring, human health assessment, food quality control, and homeland security. Expanding our knowledge of biosystems and the progress of the industry are significantly broadening the scope and applications of VOCs analysis.
Porphyrins and related compounds hold considerable promise for the optical sensing of small molecules. Porphyrins, known for their exceptional light absorption properties, are widely used as sensing materials in UV–visible and reflectance spectroscopy-based signal transduction, and these materials hold great promise for the development of portable devices using photodiodes and smartphone cameras. Although porphyrins have moderate emissions, their high light absorption afforded relatively high brightness, making them valuable for fluorescent sensor development. However, most fluorescent porphyrin-based sensors currently operate in an ON-OFF mode, which presents serious limitations for practical applications.
There are numerous ways in which porphyrins can interact with gaseous analytes, primarily due to the presence of a large aromatic macrocycle and metal centers within their macrocyclic cavity. These host molecules often participate in axial coordination, π–π stacking interactions, hydrogen bonding, and dipole interactions with an analyte. They are particularly useful for sensing a wide range of VOCs in a gaseous phase. In particular, free-base porphyrins have been widely studied as fluorescent sensors for explosive nitroaromatics due to the efficient formation of charge transfer complexes with these analytes. Another widely studied class of analytes includes Lewis bases, such as alcohols and amines, which can coordinate axially to the metal centers.
Furthermore, many free-base porphyrins and their metal complexes exhibit outstanding thermal, photochemical, and chemical stability, which surpasses that of most other organic dyes. This stability provides a solid background for developing robust devices that deliver reliable and reproducible responses over extended periods of use.
Porphyrins are also excellent building blocks for the preparation of porous nanomaterials that can be employed in the design of devices utilizing diverse optical signal transduction mechanisms, including methods based on changes in refractive index.
The use of porphyrin arrays in VOCs detection has advanced significantly in this century, with key contributions coming primarily from physicists and physical chemists. Modern miniature sensors can now feature up to 36 channels, enabling the discrimination and quantification of target VOCs in complex mixtures. However, many of these sensors still rely on readily available porphyrin derivatives that are not specifically designed to interact with the target analytes. Enhancing the sensing materials, both at the molecular level and through improved synthetic techniques, is anticipated to make significant strides in the progress of this field.
This review underscores that VOCs sensing remains in its early stages of development compared to the progress achieved with porphyrinoid-based sensors for oxygen, nitrogen oxides, and ammonia. Practical applications in fields such as medicine and food control are notably limited. With growing societal demand, we expect this area to progress rapidly in the near future.