Coffee Biomass-Based Carbon Material for the Electrochemical Determination of Antidepressant in Synthetic Urine
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Solutions and Reagents
2.3. Synthesis of HDC
2.4. Synthesis of HDC-CuNPs
2.5. Electrode Preparation
2.6. Sample Preparation and Analysis of ESC in Synthetic Urine
3. Results and Discussion
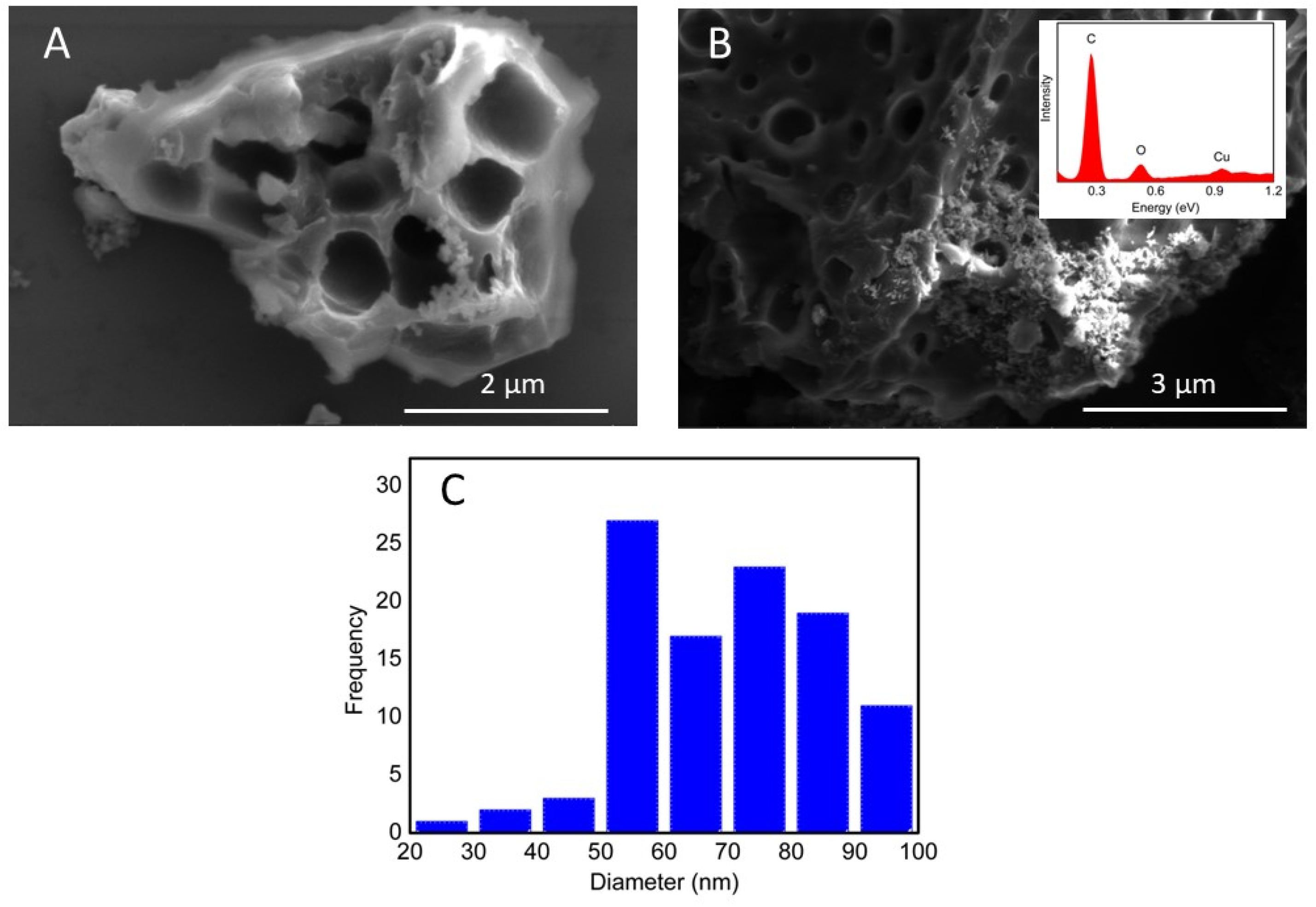
3.1. Morphological and Electrochemical Characterization of the Nanocomposites
3.2. Evaluation of Different Working Electrodes in Presence of a Redox Probe
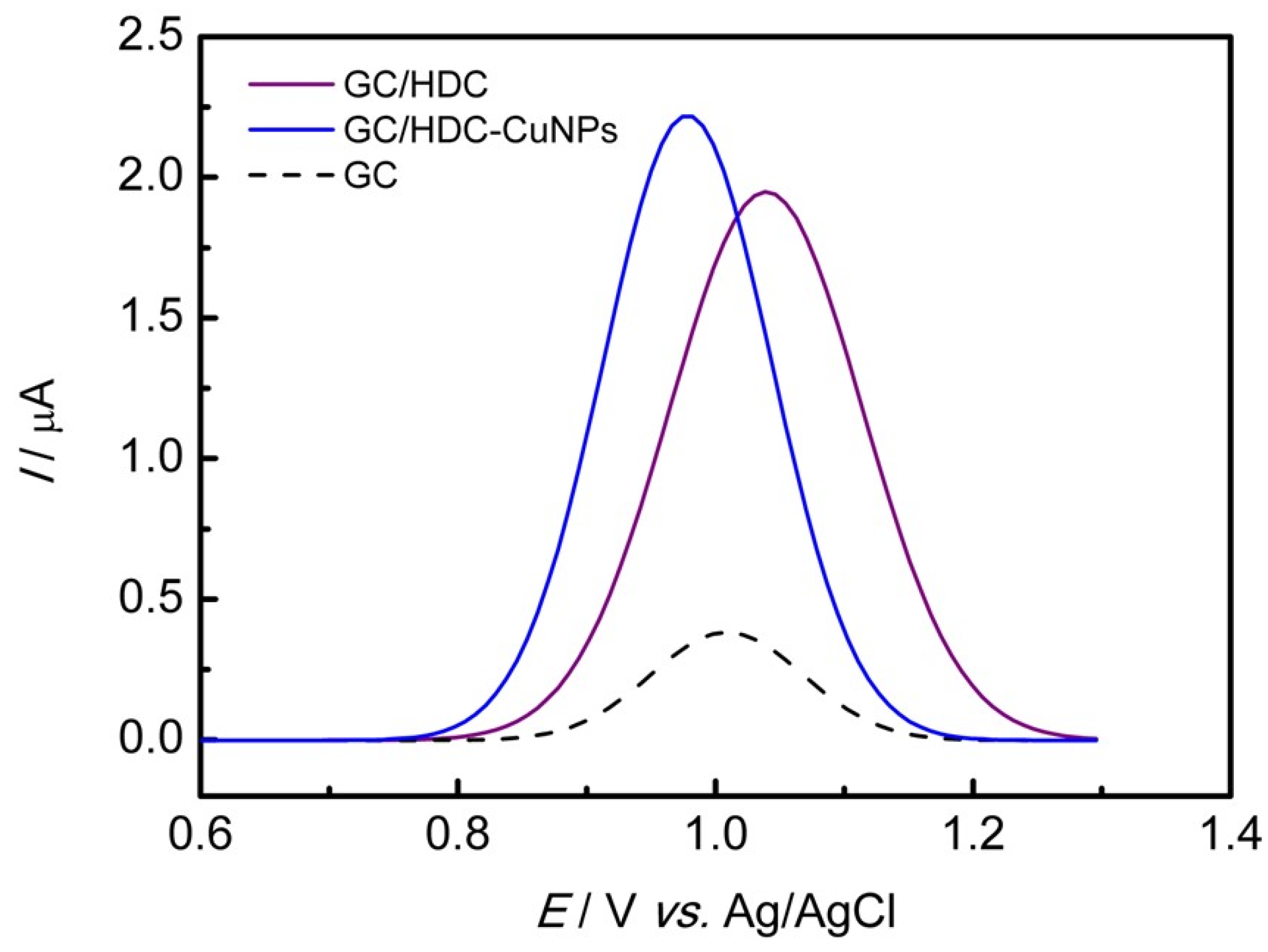
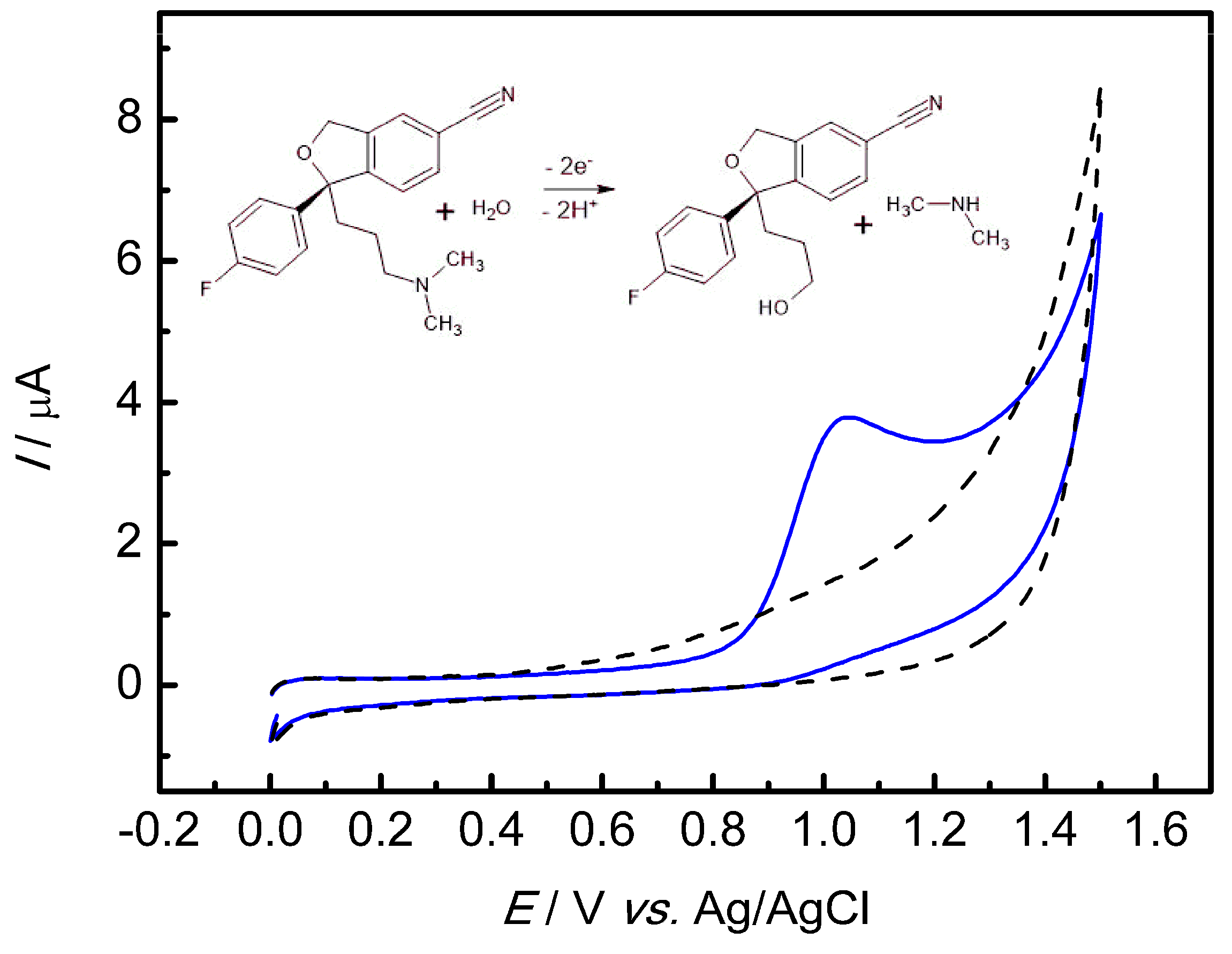
3.3. Electrochemical Oxidation of ESC
3.4. Optimization of Parameters
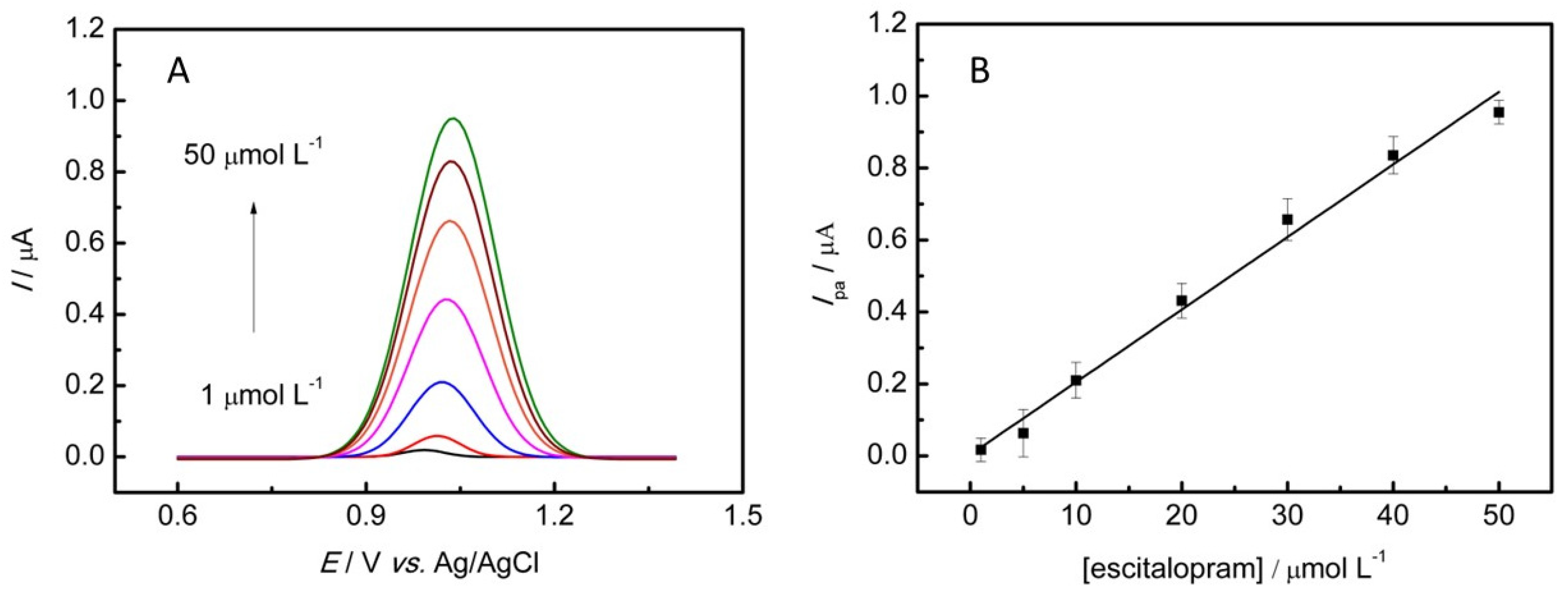
3.5. Calibration Curve
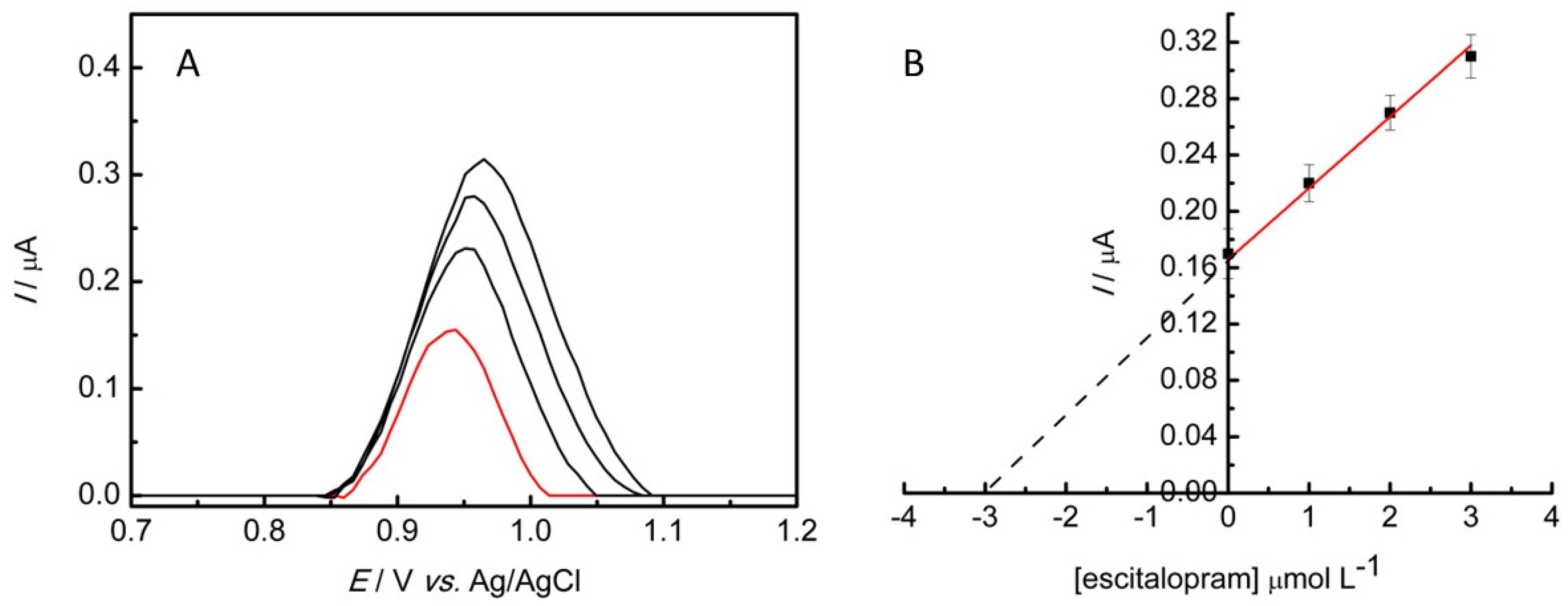
3.6. Determination of ESC in Synthetic Urine
3.7. Study with Interferents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castillo-Zacarías, C.; Barocio, M.E.; Hidalgo-Vázquez, E.; Sosa-Hernández, J.E.; Parra-Arroyo, L.; López-Pacheco, I.Y.; Barceló, D.; Iqbal, H.N.M.; Parra-Saldívar, R. Antidepressant Drugs as Emerging Contaminants: Occurrence in Urban and Non-Urban Waters and Analytical Methods for Their Detection. Sci. Total Environ. 2021, 757, 143722. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, X.; Liu, H.; Hashimoto, K. Treatment Concerns for Psychiatric Symptoms in Patients with COVID-19 with or without Psychiatric Disorders. Br. J. Psychiatry 2020, 217, 351. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Siddique, R.; Li, H.; Ali, A.; Shereen, M.A.; Bashir, N.; Xue, M. Impact of Coronavirus Outbreak on Psychological Health. J. Glob. Health 2020, 10, 010331. [Google Scholar] [CrossRef] [PubMed]
- Burke, W.J. Escitalopram. Expert Opin. Investig. Drugs 2002, 11, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Akay, S.; Yang, Y.; Kayan, B. Investigation on the Solubility of the Antidepressant Drug Escitalopram in Subcritical Water. J. Chem. Eng. Data 2021, 66, 2550–2560. [Google Scholar] [CrossRef]
- Bareggi, S.R.; Mundo, E.; Dell’Osso, B.; Altamura, A.C. The Use of Escitalopram beyond Major Depression: Pharmacological Aspects, Efficacy and Tolerability in Anxiety Disorders. Expert. Opin. Drug Metab. Toxicol. 2007, 3, 741–753. [Google Scholar] [CrossRef]
- Badulla, W.F.; Özcan, S.; Atkoşar, Z.; Arli, G. Study of Electrochemical Behavior of Escitalopram Oxalate Using Hanging Mercury Drop Electrode and Its Determination in Human Urine and Pharmaceuticals. J. Iran. Chem. Soc. 2021, 18, 739–750. [Google Scholar] [CrossRef]
- Kumari, M.; Kumar, A. Can Pharmaceutical Drugs Used to Treat COVID-19 Infection Leads to Human Health Risk? A Hypothetical Study to Identify Potential Risk. Sci. Total Environ. 2021, 778, 146303. [Google Scholar] [CrossRef]
- Thompson, W.A.; Vijayan, M.M. Antidepressants as Endocrine Disrupting Compounds in Fish. Front. Endocrinol. 2022, 13, 895064. [Google Scholar] [CrossRef]
- Al-Amri, F.M.G.; Alarfaj, N.A.; Aly, F.A. Development of New Sensors for Determination of Escitalopram Oxalate in Dosage Forms and Biological Fluids. Int. J. Electrochem. Sci. 2013, 8, 10044–10058. [Google Scholar] [CrossRef]
- Attia, A.K.; Mohamed, M.A.; Fekry, A.M. Electroanalytical Determination of Escitalopram Oxalate Using Nickel Nanoparticles Modified Carbon Paste Sensor. Acta Chim. Slov. 2017, 64, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Ravi, P.V.; Maharajan, A.; Pattabiraman, A.; Pichumani, M. Straightforward Paper Sensors for the Detection of SSRI Drugs Using Tyrosine Functionalized GQDs: Fluorescence ‘Turn-off’ Turns on the Crucial Dosage Monitoring. Diam. Relat. Mater. 2023, 139, 110407. [Google Scholar] [CrossRef]
- Barry, S.C.L.; Franke, C.; Mulaudzi, T.; Pokpas, K.; Ajayi, R.F. Review on Surface-Modified Electrodes for the Enhanced Electrochemical Detection of Selective Serotonin Reuptake Inhibitors (SSRIs). Micromachines 2023, 14, 1334. [Google Scholar] [CrossRef] [PubMed]
- Solanki, D.K.; Patel, S.P.; Surati, J.; Akabari, A.; Shah, K.; Sahu, D.; Patel, S. Simultaneous Determiantion of Aripiprazole and Escitalopram Oxalate by HPLC. J. Chem. Metrol. 2023, 17, 128–138. [Google Scholar] [CrossRef]
- Perumal, D.; Krishnan, M.; Lakshmi, K.S. Eco-Friendly Based Stability-Indicating HPTLC Technique for the Determination of Escitalopram and Etizolam by Employing Quality by Design Approach. Acta Chromatogr. 2023, 1, 156–167. [Google Scholar] [CrossRef]
- Canbolat, F.; Tasdemir Erinç, D.M.; Evrensel, A.; Aydın, A.; Tarhan, K.N. Quantitation of Escitalopram and Its Metabolites by Liquid Chromatography-Tandem Mass Spectrometry in Psychiatric Patients: New Metabolic Ratio Establishment. Basic Clin. Pharmacol. Toxicol. 2019, 124, 285–297. [Google Scholar] [CrossRef]
- Hu, Z.; Li, J.; Xiao, A.; Zheng, J.; Guan, S.; Guo, J.; Huang, M. Development and Validation of UHPLC-MS/MS Method for Simultaneous Quantification of Escitalopram and Its Major Metabolites in Human Plasma and Its Application in Depressed Patients. J. Pharm. Biomed. Anal. 2022, 217, 114810. [Google Scholar] [CrossRef]
- Das, S.; Chakravorty, A.; Raj, A.; Luktuke, S.; Appu Mini, A.; Awasthi, S.; Sankar Sana, S.; Kumar Pandey, S.; Raghavan, V. Graphene/MWCNT/Copper-Nanoparticle Fabricated Printed Electrode for Diclofenac Detection in Milk and Drinking Water: Electrochemical and in-Silico Analysis. J. Mol. Liq. 2024, 411, 125750. [Google Scholar] [CrossRef]
- Barreto, F.C.; Ito, E.Y.; Mounienguet, N.K.; Dal’ Evedove Soares, L.; Yang, J.; He, Q.; Cesarino, I. Electrochemical Sensor Based on Spent Coffee Grounds Hydrochar and Metal Nanoparticles for Simultaneous Detection of Emerging Contaminants in Natural Water. Chemosensors 2023, 11, 562. [Google Scholar] [CrossRef]
- Moussa, T.; Maalouf, C.; Bliard, C.; Abbes, B.; Badouard, C.; Lachi, M.; do Socorro Veloso Sodré, S.; Bufalino, L.; Bogard, F.; Beaumont, F.; et al. Spent Coffee Grounds as Building Material for Non-Load-Bearing Structures. Materials 2022, 15, 1689. [Google Scholar] [CrossRef]
- Andrade, C.; Perestrelo, R.; Câmara, J.S. Bioactive Compounds and Antioxidant Activity from Spent Coffee Grounds as a Powerful Approach for Its Valorization. Molecules 2022, 27, 7504. [Google Scholar] [CrossRef] [PubMed]
- Mutalib, A.A.A.; Jaafar, N.F.; Miskam, M.; Ratvijitvech, T.; Holilah, H.; Torlaema, T.A.M. Utilizing Phytochemical-Rich Spent Coffee Ground Extract for Eco-Friendly ZnO Electrochemical Synthesis: Assessing Photocatalytic Efficacy in 2,4-Dichlorophenol Degradation. Sep. Purif. Technol. 2025, 354, 129208. [Google Scholar] [CrossRef]
- Mahmood Al-Nuaimy, M.N.; Azizi, N.; Nural, Y.; Yabalak, E. Recent Advances in Environmental and Agricultural Applications of Hydrochars: A Review. Environ. Res. 2024, 250, 117923. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, M.; Bissessur, R.; Ahmed, M.; Hsiao, A.; He, Q.S.; Hu, Y. A Review: Hydrochar as Potential Adsorbents for Wastewater Treatment and CO2 Adsorption. Sci. Total Environ. 2024, 914, 169823. [Google Scholar] [CrossRef]
- Santos Santana, M.; Pereira Alves, R.; da Silva Borges, W.M.; Francisquini, E.; Guerreiro, M.C. Hydrochar Production from Defective Coffee Beans by Hydrothermal Carbonization. Bioresour. Technol. 2020, 300, 122653. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, S.; Borugadda, V.B.; Nanda, S.; Dalai, A.K. Hydrochar: A Review on Its Production Technologies and Applications. Catalysts 2021, 11, 939. [Google Scholar] [CrossRef]
- Welch, C.M.; Compton, R.G. The Use of Nanoparticles in Electroanalysis: A Review. Anal. Bioanal. Chem. 2006, 384, 601–619. [Google Scholar] [CrossRef]
- Feng, S.; Xing, X.; Hou, W. Copper Oxide Nanoparticles Modified Electrodes for High-Sensitivity Detection of Uric Acid in Athletes. Alex. Eng. J. 2024, 101, 1–7. [Google Scholar] [CrossRef]
- Zohaa; Arif, D.; Hassan, M.; Abdullah, M.; Miran, W.; Nasir, M.A.; Batool, S.; Baig, M.A.; Liaqat, U. An Electrochemical Sensor Based on Copper Oxide Nanoparticles Loaded on a Mesoporous MCM-41 for Non-Enzymatic Detection of Glucose. Ceram. Int. 2024, 50, 12614–12620. [Google Scholar] [CrossRef]
- Laube, N.; Mohr, B.; Hesse, A. Laser-Probe-Based Investigation of the Evolution of Particle Size Distributions of Calcium Oxalate Particles Formed in Artificial Urines. J. Cryst. Growth 2001, 233, 367–374. [Google Scholar] [CrossRef]
- Afolabi, O.O.D.; Sohail, M.; Cheng, Y.-L. Optimisation and Characterisation of Hydrochar Production from Spent Coffee Grounds by Hydrothermal Carbonisation. Renew. Energy 2020, 147, 1380–1391. [Google Scholar] [CrossRef]
- Çalışkan, M.; Akay, S.; Kayan, B.; Baran, T.; Kalderis, D. Preparation and Application of a Hydrochar-Based Palladium Nanocatalyst for the Reduction of Nitroarenes. Molecules 2021, 26, 6859. [Google Scholar] [CrossRef] [PubMed]
- Baccarin, M.; Cervini, P.; Cavalheiro, E.T.G. Comparative Performances of a Bare Graphite-Polyurethane Composite Electrode Unmodified and Modified with Graphene and Carbon Nanotubes in the Electrochemical Determination of Escitalopram. Talanta 2018, 178, 1024–1032. [Google Scholar] [CrossRef]
- Trindade, C.M.B.; Silva, M.K.L.; Cesarino, I. Copper Nanostructures Anchored on Renewable Carbon as Electrochemical Platform for the Detection of Dopamine, Fluoxetine and Escitalopram. Sens. Actuators Rep. 2022, 4, 100107. [Google Scholar] [CrossRef]
- Salomone, A.; Di Corcia, D.; Gerace, E.; Vincenti, M. A Fatal Case of Simultaneous Ingestion of Mirtazapine, Escitalopram, and Valproic Acid. J. Anal. Toxicol. 2011, 35, 519–523. [Google Scholar] [CrossRef][Green Version]
- Johannesson, N.; Bergquist, J. Rapid On-Line Extraction and Quantification of Escitalopram from Urine Using Sol–Gel Columns and Mass Spectrometric Detection. J. Pharm. Biomed. Anal. 2007, 43, 1045–1048. [Google Scholar] [CrossRef]
- Kanyong, P.; Rawlinson, S.; Davis, J. A Voltammetric Sensor Based on Chemically Reduced Graphene Oxide-Modified Screen-Printed Carbon Electrode for the Simultaneous Analysis of Uric Acid, Ascorbic Acid and Dopamine. Chemosensors 2016, 4, 25. [Google Scholar] [CrossRef]
- Manjunatha, J.G. Electroanalysis of Estriol Hormone Using Electrochemical Sensor. Sens. Bio-Sens. Res. 2017, 16, 79–84. [Google Scholar] [CrossRef]
- Barreto, F.C.; Silva, M.K.L.; Cesarino, I. An Electrochemical Sensor Based on Reduced Graphene Oxide and Copper Nanoparticles for Monitoring Estriol Levels in Water Samples after Bioremediation. Chemosensors 2022, 10, 395. [Google Scholar] [CrossRef]

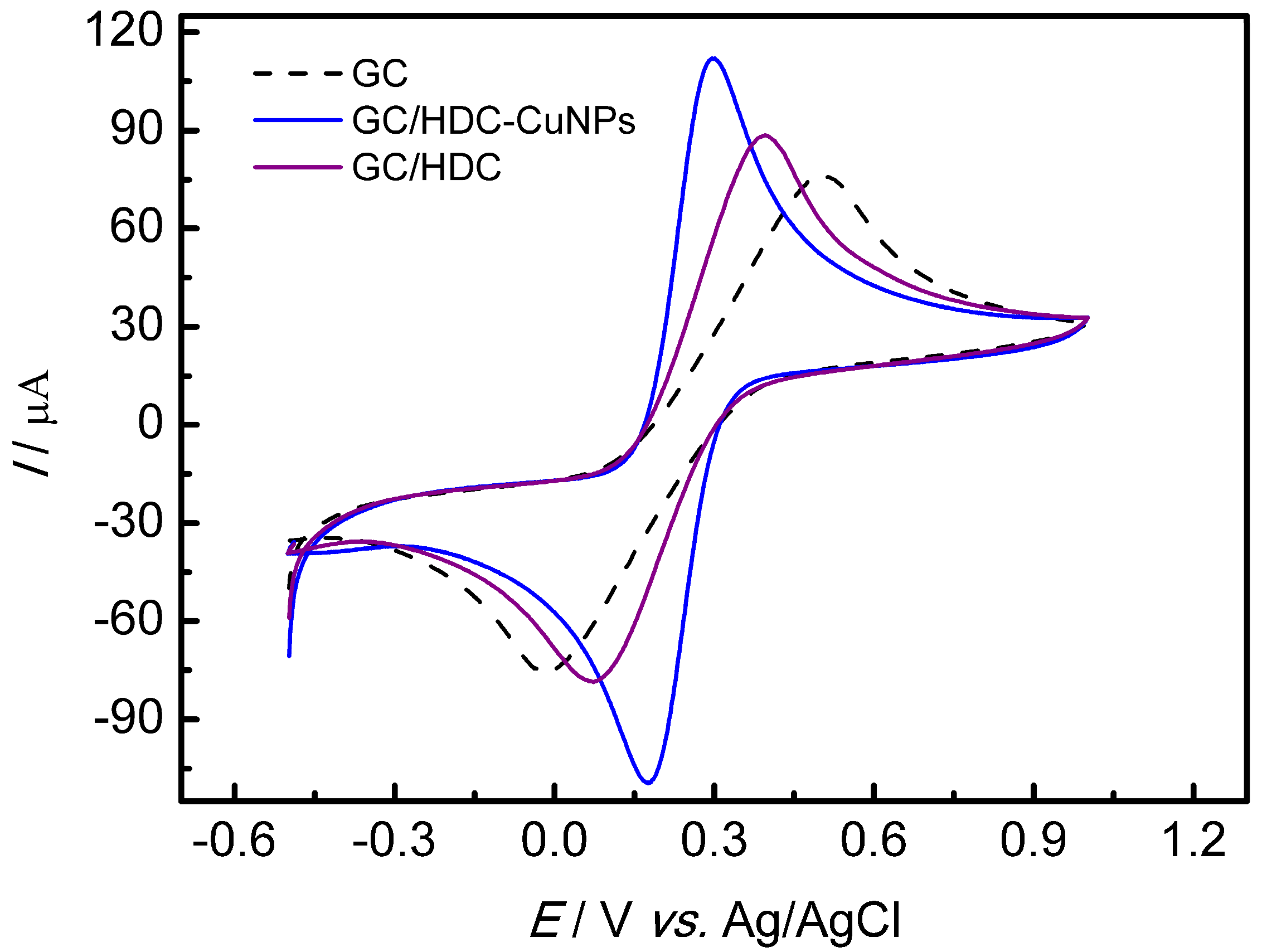

| Modified Electrode | Epa (mV) | Epc (mV) | ∆Ep (mV) | Ipa (µA) | Ipc (µA) | Ipa/Ipc |
|---|---|---|---|---|---|---|
| GC | 510 | 5 | 505 | 67.32 | −66.64 | 1.01 |
| GC/HDC | 400 | 82 | 318 | 88.01 | −70.19 | 1.25 |
| GC/HDC-CuNPs | 295 | 181 | 114 | 115.51 | −109.47 | 1.06 |
| Parameters | Tested Range | Optimized Values |
|---|---|---|
| Cu/HDC proportion in the synthesis (%) | 20–40 | 25% |
| Frequency (Hz) | 20–45 | 30 |
| Modulation amplitude (V) | 0.01–0.07 | 0.05 |
| Step potential (V) | 0.001–0.010 | 0.007 |
| pH | 5–9 | 7 |
| Repetition | ESC (µmol L−1) | Relative Errors (%) |
|---|---|---|
| 1 | 2.83 | −5.7% |
| 2 | 2.75 | −8.3% |
| 3 | 2.77 | −7.7% |
| Mean ± SD | 2.78 ± 0.03 | - |
| Interferents | Concentration (µmol L−1) | % ESC Signal |
|---|---|---|
| Dopamine | 2.5 | 95.8 |
| 5 | 92.0 | |
| 10 | 86.2 | |
| Estriol | 2.5 | 103.1 |
| 5 | 107.5 | |
| 10 | 109.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreto, F.C.; Mounienguet, N.K.; Ito, E.Y.; He, Q.; Cesarino, I. Coffee Biomass-Based Carbon Material for the Electrochemical Determination of Antidepressant in Synthetic Urine. Chemosensors 2024, 12, 205. https://doi.org/10.3390/chemosensors12100205
Barreto FC, Mounienguet NK, Ito EY, He Q, Cesarino I. Coffee Biomass-Based Carbon Material for the Electrochemical Determination of Antidepressant in Synthetic Urine. Chemosensors. 2024; 12(10):205. https://doi.org/10.3390/chemosensors12100205
Chicago/Turabian StyleBarreto, Francisco Contini, Naelle Kita Mounienguet, Erika Yukie Ito, Quan He, and Ivana Cesarino. 2024. "Coffee Biomass-Based Carbon Material for the Electrochemical Determination of Antidepressant in Synthetic Urine" Chemosensors 12, no. 10: 205. https://doi.org/10.3390/chemosensors12100205
APA StyleBarreto, F. C., Mounienguet, N. K., Ito, E. Y., He, Q., & Cesarino, I. (2024). Coffee Biomass-Based Carbon Material for the Electrochemical Determination of Antidepressant in Synthetic Urine. Chemosensors, 12(10), 205. https://doi.org/10.3390/chemosensors12100205








